Abstract
Background and Aims:
The purpose of this Third Stroke Recovery and Rehabilitation Roundtable (SRRR3) was to develop consensus recommendations to address outstanding barriers for the translation of preclinical and clinical research using the non-invasive brain stimulation (NIBS) techniques Transcranial Magnetic Stimulation (TMS) and Transcranial Direct Current Stimulation (tDCS) and provide a roadmap for the integration of these techniques into clinical practice.
Methods:
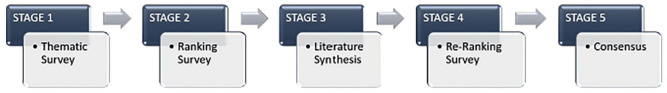
International NIBS and stroke recovery experts (N = 18) contributed to the consensus process. Using a nominal group technique, recommendations were reached via a five-stage process, involving a thematic survey, two priority ranking surveys, a literature review and an in-person meeting.
Results and Conclusions:
Results of our consensus process yielded five key evidence-based and feasibility barriers for the translation of preclinical and clinical NIBS research, which were formulated into five core consensus recommendations. Recommendations highlight an urgent need for (1) increased understanding of NIBS mechanisms, (2) improved methodological rigor in both preclinical and clinical NIBS studies, (3) standardization of outcome measures, (4) increased clinical relevance in preclinical animal models, and (5) greater optimization and individualization of NIBS protocols. To facilitate the implementation of these recommendations, the expert panel developed a new SRRR3 Unified NIBS Research Checklist. These recommendations represent a translational pathway for the use of NIBS in stroke rehabilitation research and practice.
Keywords: Stroke, rehabilitation, non-invasive brain stimulation, transcranial magnetic stimulation, transcranial direct current stimulation, stroke recovery and rehabilitation roundtable
Introduction
Numerous studies have explored non-invasive brain stimulation (NIBS) technologies as a method of modulating human brain activity to gain a deeper understanding of neural circuitry and function in healthy individuals. 1 They have led to the development of new therapeutic approaches to promote recovery for various neurological conditions, including stroke. 2 In stroke survivors, transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) are the two most common NIBS methods, with established safety profiles. 3 tDCS delivers weak (about 0.5–2.0 mA) currents to the cortex via two polarizing (anodal, cathodal) electrodes to modulate cortical excitability, 4 while rTMS delivers repetitive magnetic pulses at varying rates, intensities, and frequencies to induce changes in the stimulated neurons and remotely, in interconnected brain regions. 5 The present recommendations focus on evidence from both preclinical (animal stroke model and healthy adults) and clinical (stroke populations) research to support the therapeutic use of tDCS and rTMS.
Over 30 years of research using NIBS in animal models and healthy adults has established that both tDCS and rTMS induce controllable synaptic changes akin to long-term potentiation and depression 6 and can generate lasting alterations in cortical excitability, promoting brain plasticity. 7 In stroke populations, hundreds of interventional studies and randomized controlled trials have examined the efficacy of NIBS interventions to optimize rehabilitation treatment outcome for multiple post-stroke deficits, including motor impairment, aphasia, dysphagia and neglect at various phases of recovery. 8 – 10 While these findings have influenced clinical guidelines (e.g. Level B evidence, tDCS for motor rehabilitation; 11 Level A evidence, low-frequency rTMS (LF-rTMS) for hand function; Level C evidence, tDCS for post-stroke aphasia), 12 they have been insufficient to change rehabilitation practice. Although NIBS is an approved stand-alone therapeutic intervention for major depression and pain in several jurisdictions, 13 stroke rehabilitation guidelines are just beginning to acknowledge the potential of NIBS for post-stroke recovery.14,15 There are thus several major barriers to advancement of TMS and tDCS as therapeutic tools that urgently need to be addressed to accelerate translation. Foremost, we need to identify critical evidence-based and feasibility barriers that have limited the development of optimized protocols and the ability to conduct large, definitive phase 3 trials. The purpose of this Third Stroke Recovery and Rehabilitation Roundtable (SRRR3) was to develop consensus recommendations to identify and address outstanding translational barriers and provide a roadmap for the use of TMS and tDCS for stroke rehabilitation.
Methodology
A total of 18 basic and clinical scientists with expertise in NIBS and stroke recovery contributed to the consensus process from January 2022 to June 2023. In accordance with SRRR guidelines, panelist selection included approximately equal sex representation, with individuals across career stages and broad geographical locations. Each expert was also encouraged to include one trainee with experience in NIBS to support consensus activities. A full description of the methodology is provided in Supplemental Appendix 1a. Consensus recommendations were reached following a five-stage nominal group process 16 (Figure 1). In a thematic survey, experts first identified up to 10 evidence gaps, barriers, and needs for the translation of preclinical and clinical NIBS research (Supplemental Appendix 1b). Evidence gaps and barriers were then combined and ranked by priority and feasibility to address the needs. To ensure consensus recommendations reflected current knowledge and prevent unconscious bias, we conducted a review of recent (last 10 years) preclinical and clinical NIBS research (Supplemental Appendix 1c–g; Figure S1). After this review, barriers were re-ranked and the five highest were brought to the consensus discussion (Supplemental Table S1).
Figure 1.
Five-stage consensus building process.
Results summary and recommendations
We identified 12 evidence-based and seven feasibility barriers for the translation of preclinical and clinical findings (Supplemental Table S1). As several barriers were common across fields, they were grouped into five major knowledge gaps. Sections below describe the evidence-based and feasibility barriers for each knowledge gap, and the five consensus recommendations formulated to address them (Supplemental Table S2).
Knowledge gap 1: NIBS mechanisms
The greatest knowledge gap limiting the translation of preclinical NIBS findings was a lack of mechanistic understanding of NIBS, and this remained the highest priority after re-ranking. This includes potential effects on mechanisms such as gene expression, neurotransmission and cellular excitability that can be affected locally at the stimulation site, but also distally, across areas of the targeted network (e.g. sensorimotor or language). The literature review revealed two main evidence-based translational barriers for this gap, including a lack of (1) studies systematically comparing stimulation parameters within and across stimulation modalities and, as such, a lack of evidence for the mechanisms of response to NIBS interventions and (2) studies providing justification for the stimulation target within a neurobiological framework and target engagement.
Previous recommendations: Prior SRRR2 trial development recommendations highlighted the need for systematic preclinical and clinical dose studies stroke recovery interventions; 17 however, no prior recommendations have addressed systematic testing or target engagement specifically for NIBS and this gap has previously been noted in tDCS guidelines. 11
Evidence-based translational barriers: (a) Systematic NIBS parameter testing: For both tDCS and rTMS, the combination of parameter settings is large. To date, systematic studies of stimulation parameters have been mostly limited to healthy young adults, targeting M1, and using only physiological outcome measures (e.g. motor outputs). 18 In the post-stroke population, there are numerous considerations that limit the extrapolation of findings from the young healthy literature directly, including aging, post-lesion plasticity, vascular burden, lesion characteristics (size/location), post-stroke inflammatory processes or medication. In our literature synthesis, the most studied parameters were electrode polarity for tDCS and pulse frequency for rTMS. However, less than 10% of animal studies and clinical studies/trials compared stimulation parameters or dosing within or across modalities. The investigation of the effects of parameters on the molecular and cellular responses in the brain (e.g. neuronal excitability, inflammation, etc.) and to behavioral outcomes was scarce. This lack of systematic testing limits our understanding of the effect of specific parameters on the targeted brain area, precluding comparisons across studies using different parameters and the optimization of stimulation protocols.
(b) Target engagement: The second major translational barrier is related to the choice/location of the stimulation target. Most studies (>90%) targeted a single cortical area, without confirmation of its involvement in impairment or recovery. For example, most post-stroke motor recovery trials targeted either the ipsilesional or contralesional M1, regardless of the level of impairment, lesion characteristics or confirmation of atypical activity in this area during behavior. However, these factors can affect the pattern of brain activation during behavior, and accordingly the NIBS target and after-effects. To enable the design of effective RCTs in stroke populations, preclinical studies (both in animals and healthy adults) must justify the selection of the cortical target using evidence-based biological frameworks that allow for primary hypothesis testing and include evaluation of causal links between stimulation, target engagement and behavioral effects. For future clinical trials, inclusion of lesion characteristics (e.g. volume, structures and pathways involved) is critical. Ideally, trials should include physiological/imaging measures (e.g. motor evoked potentials (MEPs), functional magnetic resonance imaging (fMRI) or electroencephalography (EEG)) to quantify target engagement beyond behavioral effects. As these data accumulate, for example through shared repositories allowing retrospective studies, we will be able to refine our mechanistic hypotheses about the effects of the lesion on brain reorganization and individualize the choice of target brain area and stimulation parameters. Although practically, this must be balanced with effective recruitment and integration of stimulation into clinical workflow. Multi-center trials may need to focus either on mechanisms using physiological/imaging methods in more moderate sample sizes or clinical outcomes and treatment efficacy in larger phase-III trials, each of which has a different purpose and design considerations.
Feasibility translational barriers: Under the current peer-reviewed funding system, studies systematically testing NIBS parameters and providing incremental knowledge about mechanisms and dose/effect relationships have difficulty achieving a high priority. Thus, consistent with prior general stroke recovery recommendations, 17 we also strongly recommend that funding agencies offer increased and dedicated support for these studies in NIBS to provide key evidence for the design of effective large RCTs.
| Recommendation 1 |
| Preclinical and clinical studies/trials should: (a) systematically compare stimulation parameters within and across modalities and quantify the effects of these parameters on the brain and behavior, and; (b) use an evidence-based biological framework for target selection and confirm the intervention effect at the level of the target. |
Knowledge gap 2: methodological rigor
The second highly ranked gap concerned the methodological rigor of existing studies. The main translational barriers regarding study methodology were: (1) limitations in design, (2) a lack of adequate sample sizes and statistical power to test the therapeutic benefits of NIBS, and (3) a lack of transparency in reporting. Notably, while initially receiving a lower priority ranking, this item increased after the literature synthesis, with major concerns regarding clinical trial methodology emerging.
Previous recommendations: Prior SRRR recommendations emphasized the importance of methodological rigor and adequate reporting in stroke recovery research 19 including the use of TIDierR 20 for interventions and CONSORT 21 flow diagrams for reporting, and ARRIVE guidelines 22 for preclinical studies. However, there are no recommendations specific to the use of NIBS in preclinical research. There is only one prior set of recommendations for methodology and reporting of NIBS for clinical research, 23 with an accompanying checklist for single or paired pulse TMS that was also adapted to tDCS. 24 However, there is no comprehensive, unified cross-modality checklist for both preclinical and clinical NIBS research.
Evidence-based translational barriers: (a) NIBS methodology: For preclinical studies, almost all were single center and very few involved pre-registration to ensure the internal and external validity of findings. In addition, across studies, there was a lack of inclusion of sham stimulation (~1/3 of studies) and poor reporting of critical stimulation parameters. For clinical trials, most trials (>85%) were single center, and while almost half of tDCS trials were pre-registered, only ~20% of TMS trials reported pre-registration. Many trials reported eligibility criteria, but the inclusion of important clinical selection criteria (i.e. physiology, lesion location/size, recovery phase) was highly variable and almost none reported how patients were excluded based on neurophysiological findings. Although most trials included some form of blinding, the majority were single blinded (65%) and very few reported testing the efficacy of blinding. The majority of tDCS trials involved the use of sham stimulation. However, only ~75% of TMS trials included a sham. Across modalities there was a complete lack of standardization of sham procedures, with tDCS sham durations ranging from 5 s to 2 min and TMS sham involving a broad range of methods including varying coil types, orientation, and distance from the target location. Finally, while many trials reported pairing stimulation with some form of therapy, almost 1/3 did not and the details of the rehabilitation therapy were poorly reported. These limitations represent a major translational barrier that aside from a small number of well reported trials 25 resulted in an overall low quality of evidence and substantial heterogeneity in results.12,14
(b) Sample size and statistical power: Preclinical studies with animal models largely comprised multiple different subgroups, each with small sample sizes. Consequently, very few studies (<5%) were sufficiently powered to test the primary hypothesis, with many reporting non-significant effects or small effects in only one or few subgroups, based on very limited data, and none reporting power analyses. In humans, the majority of RCTs were small, with over 70% reporting sample sizes of <N = 50 and only a few trials of N > 100 across all modalities and deficit types. Critically, less than 1/3 of trials reported power analyses to test the efficacy of primary endpoints. Reporting for both the statistical methodology used and observed effect sizes was very sparse.
(c) Transparency of reporting: Across both preclinical and clinical studies and all NIBS modalities, reporting of patient characteristics (lesion size/location, severity), stimulation and sham parameters, paired rehabilitation, and statistical methods and power was very poor, limiting our ability to interpret and synthesize evidence for the efficacy of NIBS. The consistent use of reporting checklists specific to NIBS would facilitate replication of NIBS protocols and meta-analyses and improve translation of NIBS study results from preclinical to clinical domains. To address this gap, we developed a Unified Checklist for NIBS Research (Table 1) for use in either preclinical or clinical studies involving tDCS and rTMS and including a comprehensive reporting of stimulation parameters and targets. We strongly recommend the use of this checklist in conjunction with other recommended design and reporting checklists (i.e. ARRIVE, CONSORT, and TIDieR) in all future NIBS preclinical and clinical stroke studies.
Table 1.
SRRR3 unified checklist for NIBS research.
| Please indicate, for each item, if it has been thoroughly reported in your manuscript by writing a 1 if reported or 0 if not reported (shaded square are not applicable) | tDCS | TMS |
| I. Stimulation device | ||
| Brand | ||
| Model | ||
| Type (constant current, or constant voltage) | ||
| Range of plausible protocols (single pulses, paired pulses, repetitive stimulation) | ||
| Stimulation capacity (maximum frequency and output) | ||
| Pulse shapes produced (e.g. monophasic and biphasic) | ||
| Pulse duration (in µs) or range of plausible pulse durations (if applicable) | ||
| II. Electrode | ||
| Material | ||
| Contact medium | ||
| Size | ||
| Geometry | ||
| III. Stimulation coil | ||
| Type | ||
| Diameter | ||
| IV. Stimulation target | ||
| Target choice (local, network or area) | ||
| Target definition (anatomical, functional) | ||
| Tool for target definition (e.g. structural imaging and neurophysiology) | ||
| Rationale on the decision to use general or individualized targets | ||
| If individualized targets were chosen, the criteria (e.g. modeling and physiology) | ||
| Relationship of the target to any pathology (i.e. does the targeted area include a lesion) | ||
| If the target contains a lesion, the assessment of the viability of the stimulated tissue or connections to it via the target (e.g. MEP, the effect of TMS on remote brain areas, or other imaging or electrophysiological measures) | ||
| V. Stimulation parameters | ||
| Electrode placement in relation to target (e.g. single- and multi-electrode) | ||
| The rationale for electrode location (e.g. over the target or over nodes of the target network or target area) | ||
| If used, a description of the modeling performed for placement for the electrode location | ||
| Placement of return electrode(s) | ||
| Information about the direction, and magnitude of excitability alterations of the target based on standard arrangements, if applicable | ||
| The polarity of stimulation electrodes | ||
| Stimulation intensity delivered by each electrode (mA) | ||
| Current density under each electrode | ||
| If modeling was involved, the resulting current density at the target | ||
| Description of ramping up/down of intensity at the start and end of stimulation | ||
| Coil orientation (relative to anatomical landmarks) | ||
| Direction of the induced current (e.g. posterior to anterior and anterior to posterior) | ||
| Pulse amplitude (i.e. stimulation intensity, absolute and relative to a relevant threshold) | ||
| Pulse duration (in µs) (if applicable) | ||
| Nature of stimulation (e.g. single, paired, or repetitive pulses) | ||
| Pattern of stimulation | ||
| VI. Stimulation protocol | ||
| Stimulation duration per session (min) (tDCS only) | ||
| Number of pulses per session (rTMS only) | ||
| Total number of sessions | ||
| Rationale for patients or animals needed per group/arm (e.g. power estimation) | ||
| The interval between sessions (e.g. once/twice daily and including interval) | ||
| Information about the time(s) of the day the stimulation took place | ||
| Stand-alone stimulation or combined with task/rehabilitation | ||
| If combined stimulation and task; provided information about the task | ||
| If combined stimulation and task; provided information about the readouts (e.g. kinematics) | ||
| If combined stimulation and task; provided information about the stimulation timing relative to the task | ||
| If using a motor task; and forces were applied, their description | ||
| If using a motor task; the muscles involved | ||
| Control and monitoring of the alertness level | ||
| Addition of adjunctive interventions (e.g. pharmacological) | ||
| If there were muscle contraction requirements during the stimulation, how were they implemented? | ||
| If the subject applied a contraction, its nature (e.g. isometric and isotonic) and its monitoring | ||
| Number of pulses per outcome measure (if TMS is used as an outcome, e.g. changes in excitability) | ||
| If using a repetitive protocol, and burst trains are applied, number of pulses per burst | ||
| If using a repetitive protocol, and burst trains are applied, number of bursts | ||
| Interval between pulses (in ms) | ||
| If using a repetitive protocol, and burst trains are applied, interval between bursts | ||
| VII. Blinding/Sham/Control | ||
| Blinding approach and rationale (single, double, triple) | ||
| Blinding success exploration (e.g. questionnaires) | ||
| Sham usage and rationale | ||
| If sham is used, its description | ||
| Active control usage and rationale | ||
| If an active control is used, its description | ||
| VIII, Side effects and negative findings | ||
| Adverse events and tolerability monitored (e.g. by a questionnaire) | ||
| Report negative findings | ||
| IX. Clinical specific; Patient characteristics | ||
| Patient Demographics (age, sex, ethnicity) | ||
| Lesion characteristics (including lesion location, and size) supported by imaging, if applicable | ||
| Comorbidities | ||
| Clinical deficits and their dynamics | ||
| General therapeutic procedures | ||
| Pharmacological treatments at the time of stimulation | ||
| X. Preclinical specific | ||
| Species, age, sex, weight | ||
| Rationale for the species used | ||
| Lesion model, localization, extension, time elapsed from lesion to stimulation | ||
| Rationale on inclusion or not of behavioral outcome measures | ||
| Rationale on inclusion or not of follow-up to recovery | ||
TMS: transcranial magnetic stimulation; MEP: motor evoked potential.
Feasibility barriers: Many studies did not adhere to current reporting guidelines, resulting in major challenges with the interpretation and translation of findings. Stroke (https://www.ahajournals.org/journal/str) has established requirements to improve transparent reporting practices. We recommend that more journals require strict adherence to recommended design and reporting checklists before acceptance, for both preclinical studies and clinical trials. In addition, multiple feasibility challenges were identified that limit the integration of NIBS techniques into clinical workflow, including the cost of equipment, need and availability of trained operators, and protocol length and cheaper, automated systems may be required for widespread uptake of these technologies.
| Recommendation 2 |
| Preclinical and clinical studies/trials should: (a) include pre-registration and use of appropriate patient eligibility criteria, blinding and sham stimulation protocols, and appropriate paired therapies; (b) conduct and report prospective power analysis to determine samples sizes appropriate to test primary hypotheses, and; (c) use the SRRR3 Unified Checklist for NIBS Research and adhere to current recommended design and reporting guidelines. |
Knowledge gap 3: outcome standardization
The next highly ranked gap related to the standardization of outcomes measures. The main translational barriers to address this gap were a lack of: (1) preclinical studies including behavioral in addition to physiological and cellular-molecular outcome measures to provide a complete assessment across domains; (2) standardized preclinical outcome assessment tools for domains other than sensorimotor; and (3) studies/clinical trials that reported minimally important clinical differences for standardized outcomes that were aligned with International Classification of Functioning, Disability, and Health (ICF) categories or study hypotheses.
Previous recommendations: Previous SRRR recommendations have focused on the standardized measurement of core tissue and behavioral outcomes for preclinical stroke recovery research 26 and both sensorimotor recovery 27 and upper limb movement quality 28 in stroke recovery clinical trials. There are currently no recommendations specific to outcomes for NIBS research.
Evidence-based translational barriers: (a) Multi-domain and standardized preclinical outcomes. NIBS studies in animal models were largely biased toward post-mortem evaluations of the cellular and molecular impacts of stimulation (approximately 60%). The most commonly reported outcome across animal studies was the modified neurological severity score, a standardized tool that provides a global behavioral assessment. Other common but non-standardized measures were changes in neurogenesis, cell migration, and neuroprotection assessed by immunohistochemistry and western blot techniques. Very few studies provided detailed behavioral or physiological assessments. There is thus a lack of comparable outcome measures between animal and human studies, creating a major limitation for the translation of preclinical findings. Future studies in animal models should include behavioral assessments, such as kinematics and other biomarkers (e.g. brain imaging) that are paralleled in human studies and better characterize the physiological properties and NIBS-induced alterations of the stimulation target.
(b) Minimally important differences. Our review of human NIBS RCTs for motor recovery generally showed good use of standardized outcome measures. For studies targeting the therapeutic use of TMS or tDCS for recovery of the upper extremity, the Fugl-Meyer (FMA-UE) and Action Research Arm Test (ARAT) were the most used measures (>80%). Both have acceptable intra- and inter-rater variability and have been previously recommended. 29 They also have the important advantage of involving a standardized minimal clinically important difference. For the lower extremity, FMA-LE, another standardized measure, was most frequently reported (~50%). Few studies included activity outcomes for walking (e.g. 10-m walk test gait speed) and there was a lack of assessment of movement quality with kinetic and kinematic measures, despite its use to distinguish true neural restitution from behavioral compensation during recovery. 27 Finally, few studies reported selecting and aligning outcomes with ICF categories 30 and several studies did not use outcome measures that directly aligned with the study hypothesis (e.g. hypothesis specific to skilled hand function but outcome not a direct measure). 31
Unlike the motor domain, there was a striking lack of standardization of outcomes in studies in other domains, including aphasia, dysphagia, and cognition. Most trials included non-standardized measures with unclear psychometric properties (e.g. author-created questionnaires or tests), resulting in concerns about outcome utility and reduced generalizability. Finally, while studies included multiple behavioral outcomes (e.g. motor function, activity and kinematics), few included both physiological and behavioral measures, which is crucial to link stimulation protocols to physiological mechanisms and behavior.
Feasibility barriers: No feasibility barriers were identified with respect to outcomes.
| Recommendation 3 |
| (a) Preclinical studies should: (i) conduct complete outcome assessments across cellular-molecular, physiological and behavioral domains, and; (ii) include standardized behavioral outcomes common to human studies (b) Clinical studies/trials should: (i) use standardized assessments with established psychometric properties, and; (ii) report the minimal clinically important difference for outcomes that align with ICF categories and/or study hypotheses. |
Knowledge gap 4: clinically relevant preclinical animal models
The next gap emerging from the survey was the need for preclinical models with increased clinical relevance. The main translational barriers were a lack of (1) measures to test interactions between the effects of NIBS on the brain and behavior; (2) studies that directly align NIBS stimulation parameters with those commonly used in humans and that closely replicate the effects that commonly used protocols for humans have on the brain (e.g. considering brain size and/or cortical architecture); (3) studies that test NIBS effects according to lesion characteristics (i.e. lesion location and size); and (4) animal models with comorbidities (i.e. aged animals or animal strains with spontaneous diseases, such as vasculopathies and diabetes).
Previous recommendations: The first set of SRRR recommendations stressed the urgency for the development of robust and diverse preclinical models that more accurately reflect patient profiles. 32 There are currently no recommendations specific to preclinical models for NIBS research.
Evidence-based translational barriers: (a) Brain–behavior Interactions. In our literature review, no study involving preclinical stroke models specifically tested interactions between the effects of NIBS on the brain and behavioral outcomes. This is a missed opportunity, considering that relevant post-stroke variables (i.e. lesion location, age, and comorbidities) can be more easily controlled in these models and facilitate the evaluation of interactions. This evidence is critical to delineate potential joint and cumulative effects of NIBS on behavior across different lesion profiles and clinical phenotypes.
(b) Aligning stimulation parameters and replicating effects of NIBS in humans. Other key limitations of NIBS preclinical animal studies included the divergence from stimulation parameters typically used in human studies and the high degree of variability in stimulation equipment. For example, many tDCS studies have reported neuroprotective or anti-inflammatory effects using stimulation parameters not currently used in practice in human studies. 33 Several rTMS studies in rodents use large commercial coils designed for humans 34 or small custom-made coils with limited information about the relative volume of the stimulation in relation to brain size. 35 Yet, there is evidence that small coils still result in relatively non-focal stimulation 36 and thus the modulation of very large areas in proportion to the size of small rodent brains.
To facilitate direct comparisons with humans some neuromodulation studies with other modalities (e.g. deep brain stimulation (DBS)) are increasingly using pig models. The pig brain is gyrencephalic, has a similar white to gray matter ratio and is large enough to accommodate human DBS electrodes, increasing the translational value of findings, such as the characterization of the effects of different stimulation protocols on local excitability or circuit function. 37 For NIBS, our literature review highlighted that most animal NIBS studies used rodent models (75%), with only a small proportion in other mammals with larger brains, including non-human primates (NHP). Alternative animal models with cortical architecture more similar to humans (e.g. pigs, sheep, or NHP) should be considered for experimental questions regarding NIBS efficacy or mechanisms of action. To facilitate translation to clinical populations, care should be taken in all models to align and approximate effects that can be safely delivered in human stroke population.
(c) Lesion characteristics and models with comorbidities. The majority of studies investigating the effects of NIBS on preclinical models of stroke focused on otherwise healthy young adult male rodents (~70%) after middle cerebral artery occlusion. There is thus a lack of studies testing the effects of NIBS across various lesions profiles, including in aged animals, animals with and without comorbidities, and across sexes. 26
Feasibility barriers: Although there are a few readily available TMS coil technologies suitable for animal models adjusted to the relative size of the brain, there is still a lack of accessibility of specifications to inform researchers on how these compare to human coils in regard to the characteristics of the electric fields, ideally using both modeling and physiological assessments. This poses a major translational barrier for the interpretation of preclinical NIBS research. There is an urgent need for standardized equipment and stimulation protocols in animals that are better aligned with those used in human studies. We recommend that neurostimulation equipment manufacturers prioritize the development of coil models and the sharing of their coil validation processes using models or simulations with appropriate brain morphologies and topographies. 38 As these new, well-characterized tools become more accessible, they should be prioritized in animal studies designed to study the effects of TMS in the brain.
| Recommendation 4 |
| Preclinical studies should use stroke animal models that: (a) include head-equipment size relationship, aged animals, comorbidities, behavioral assessments, and the clinical trajectory of recovery in humans. |
Knowledge gap 5: optimized and individualized NIBS protocols
The final gap identified was a need to accelerate the identification of NIBS stimulation parameters/protocols with high potential for efficacy, NIBS treatment response phenotypes, and biomarkers for the development of individualized NIBS protocols. The main translational barriers for this gap were a lack of (1) animal studies testing the effect of NIBS on functional recovery across domains; (2) clinical studies with sufficiently broad inclusion/exclusion criteria and sufficient sample sizes to enable subgroup analyses for the identification of NIBS treatment response phenotypes; and (3) evidence for biomarkers predictive of NIBS treatment response.
Previous recommendations: The first SRRR made recommendations to advance preclinical to clinical pipelines for stroke recovery research 26 and identify biomarkers of stroke recovery. 39 However, to date, there are no recommendations for protocol optimization or biomarker identification specific to NIBS.
Evidence-based translational barriers: (a) Preclinical response domains. Most preclinical studies (approximately 60%) focused on the use of tDCS or rTMS to promote neuroprotection and plasticity after stroke in relation to motor recovery, 40 with few studies testing functional recovery across multiple domains. These studies have provided crucial information about glial differentiation, neuronal gene regulation, and other molecular changes induced by NIBS. However, more preclinical evidence specifically linking the effects of NIBS to behavior across recovery domains (e.g. motor, sensory, cognition, etc.) is required to improve clinical translation.
(b) Identification of NIBS response phenotypes: As previously highlighted, most trials included in our literature synthesis were small (over 70% with N < 50), precluding the analysis of treatment benefits for subgroups with varying characteristics. While some trials included broad criteria with both cortical and subcortical patients from 6 months to many years post-stroke and even sometimes a mixture of ischemic and hemorrhagic etiologies, the majority were too small to enable meaningful subgroup analyses. Conversely, other trials reported narrow inclusion criteria, enrolling patients only within certain age ranges, time window post-stroke, or with specific deficit severity, resulting in low recruitment potential and again low sample sizes. This latter approach can reduce variability due to heterogeneous patient characteristics, but also limits the disaggregation and generalizability of findings by subgroups. Importantly, meta-analyses based on numerous small trials cannot mitigate biases inherent in the original trials. Thus, larger trials across a broad range of inclusion criteria sufficiently powered for subgroup analyses are needed for the identification of response phenotypes.
(c) Biomarkers of NIBS treatment response: There is currently a lack of evidence for biomarkers predictive of NIBS response. In the reviewed studies, patient enrollment was rarely based on a specific biomarker. However, some trials included patients based on neurophysiological characteristics, such as the presence or absence of MEPs. Demographic factors, such as age and sex, do not show clear utility in predicting response to NIBS protocols 41 and while time post-stroke may influence response magnitude, 42 most clinical studies identified in our literature synthesis involved either chronic or mixed and not acute/subacute patient populations. White matter connectivity or measures of neuronal oscillations have previously been identified as biomarkers with potential for stroke recovery39,43 and prior tDCS work suggests that EEG connectivity may explain variability in corticospinal excitability changes. 44 Furthermore, the presence of Val66Met brain-derived neurotrophic factor (BDNF) polymorphisms influence motor cortical excitability in stroke patients, 45 as well as responses to TMS outside motor cortex. 46
A major limitation of existing biomarker studies is the variable use of statistical methods appropriate to establish predictive utility and the lack of calibration and internal validation of prediction models. For example, prior predictive algorithms for NIBS response based on a combination of motor strength, corticospinal tract integrity and imaging parameters have been proposed. 47 However, subsequent simulation studies have shown that scale properties dramatically impact model fit 48 and these algorithms did not involve methods for model calibration and validation. 49 To facilitate the development of individualized NIBS protocols, future studies assessing the predictive utility of potential biomarkers should employ best practices for model development and calibration and follow TRIPOD guidelines for reporting. 50
Feasibility barriers: A major challenge with reproducibility is a lack of data repositories to consolidate existing preclinical and clinical evidence that enable meta-analyses. Large-scale repositories, such as VISTA-Rehab 51 are good initial forays into large-scale repositories. However, they are not focused on NIBS interventions. Increased international coordination for publicly available harmonized NIBS repositories is required.
The identification of response phenotypes and predictive biomarkers is limited by the design of traditional clinical trials, of which few have sufficient sample sizes to support analyses of response subtypes. Increased funding for and use of innovative adaptive trial designs 52 with flexibility to integrate new knowledge of protocols with high potential for efficacy and individualized NIBS prescriptions is a promising new translational avenue for NIBS. These designs are particularly advantageous when considering the number of experimental surrogates needed to account for the stratification of stroke patient subpopulations, and the need to differentiate putative contributions of multiple response predictors in NIBS stroke models, ranging from electrophysiology, to imaging, behavior, and omics. The adoption of adaptive designs can prove critical, as preclinical studies involving recovery might otherwise become unfeasible. Long-lasting behavioral and electrophysiological testing necessary to assess functional changes after NIBS could be performed in late phases of mechanistic studies and only with small subsets of animals. This would broaden the comprehension of protocol efficacy without compromising the viability of the study’s execution.
| Recommendation 5 |
| Preclinical and clinical studies/trials should: (a) test multi-domain NIBS response; (b) have sufficient sample sizes to identify response phenotypes; and (c) use appropriate statistical methodology to identify predictive biomarkers. |
Conclusion
Non-invasive brain stimulation technologies, and specifically rTMS and tDCS as the focus of this current consensus exercise, have a long history of experimental and clinical evidence supporting they are safe and can induce rapid and reproducible effects on the brain. Importantly, they show promising potential therapeutic benefits for the improvement of multiple post-stroke deficits. However, several major translational barriers have limited their advancement as a clinical tool for stroke recovery. The consensus recommendations and SRRR3 Unified NIBS Checklist developed by this roundtable are designed to address these outstanding barriers and provide a roadmap for the integration of TMS and tDCS technologies into clinical practice for stroke rehabilitation.
Supplemental Material
Supplemental material, sj-docx-1-wso-10.1177_17474930231203982 for A translational roadmap for transcranial magnetic and direct current stimulation in stroke rehabilitation: Consensus-based core recommendations from the third stroke recovery and rehabilitation roundtable by Jodi D Edwards, Adan Ulises Dominguez-Vargas, Charlotte Rosso, Meret Branscheidt, Lisa Sheehy, Fanny Quandt, Simon A Zamora, Melanie K Fleming, Valentina Azzollini, Ronan A Mooney, Charlotte J Stagg, Chiristian Gerloff, Simone Rossi, Leonardo G Cohen, Pablo Celnik, Michael A Nitsche, Cathrin M Buetefisch and Numa Dancause in International Journal of Stroke
Supplemental material, sj-docx-2-wso-10.1177_17474930231203982 for A translational roadmap for transcranial magnetic and direct current stimulation in stroke rehabilitation: Consensus-based core recommendations from the third stroke recovery and rehabilitation roundtable by Jodi D Edwards, Adan Ulises Dominguez-Vargas, Charlotte Rosso, Meret Branscheidt, Lisa Sheehy, Fanny Quandt, Simon A Zamora, Melanie K Fleming, Valentina Azzollini, Ronan A Mooney, Charlotte J Stagg, Chiristian Gerloff, Simone Rossi, Leonardo G Cohen, Pablo Celnik, Michael A Nitsche, Cathrin M Buetefisch and Numa Dancause in International Journal of Stroke
Supplemental material, sj-pdf-3-wso-10.1177_17474930231203982 for A translational roadmap for transcranial magnetic and direct current stimulation in stroke rehabilitation: Consensus-based core recommendations from the third stroke recovery and rehabilitation roundtable by Jodi D Edwards, Adan Ulises Dominguez-Vargas, Charlotte Rosso, Meret Branscheidt, Lisa Sheehy, Fanny Quandt, Simon A Zamora, Melanie K Fleming, Valentina Azzollini, Ronan A Mooney, Charlotte J Stagg, Chiristian Gerloff, Simone Rossi, Leonardo G Cohen, Pablo Celnik, Michael A Nitsche, Cathrin M Buetefisch and Numa Dancause in International Journal of Stroke
Acknowledgments
We acknowledge Dr. Kathryn Hayward and Dr. Gert Kwakkel for co-chairing and convening the third Stroke Recovery and Rehabilitation Roundtable (SRRRIII) and Dr. Emily Dalton for supporting SRRRIII conduct and organization of the in-person meeting. We also acknowledge the contributions of Dr. Randolph Nudo, Dr. Christopher Pack and Dr. Brian Corneil to the thematic survey, Ms. Sarah Visintini, medical librarian at the University of Ottawa Heart Institute for the literature searches, and members of the other SRRRIII for feedback.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Canadian Partnership for Stroke Recovery, NHMRC Center of Research Excellence to Accelerate Stroke Trial Innovation and Translation (GNT2015705), and unrestricted educational grants provided by Ipsen Pharma and Moleac.
Statement of endorsement: The World Stroke Organization (WSO) endorses the goals of the Stroke Recovery and Rehabilitation Roundtable research activity which are consistent with the mission of the WSO.
ORCID iDs: Jodi D Edwards  https://orcid.org/0000-0002-9275-4787
https://orcid.org/0000-0002-9275-4787
Adan Ulises Dominguez-Vargas  https://orcid.org/0000-0003-1991-9044
https://orcid.org/0000-0003-1991-9044
Fanny Quandt  https://orcid.org/0000-0001-7042-6785
https://orcid.org/0000-0001-7042-6785
Chiristian Gerloff  https://orcid.org/0000-0002-6484-8882
https://orcid.org/0000-0002-6484-8882
Supplemental material: Supplemental material for this article is available online.
References
- 1. Siebner HR, Funke K, Aberra AS, et al. Transcranial magnetic stimulation of the brain: what is stimulated?—a consensus and critical position paper. Clin Neurophysiol 2022; 140: 59–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guggisberg AG, Koch PJ, Hummel FC, Buetefisch CM. Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol 2019; 130: 1098–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossi S, Antal A, Bestmann S, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol 2021; 132: 269–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nitsche MA, Doemkes S, Karaköse T, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 2007; 97: 3109–3117. [DOI] [PubMed] [Google Scholar]
- 5. Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118. [DOI] [PubMed] [Google Scholar]
- 6. Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil 2009; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol 2014; 592: 4115–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He Y, Li K, Chen Q, Yin J, Bai D. Repetitive transcranial magnetic stimulation on motor recovery for patients with stroke: a PRISMA compliant systematic review and meta-analysis. Am J Phys Med Rehabil 2020; 99: 99–108. [DOI] [PubMed] [Google Scholar]
- 9. Kielar A, Patterson D, Chou YH. Efficacy of repetitive transcranial magnetic stimulation in treating stroke aphasia: systematic review and meta-analysis. Clin Neurophysiol 2022; 140: 196–227. [DOI] [PubMed] [Google Scholar]
- 10. Wang T, Dong L, Cong X, et al. Comparative efficacy of non-invasive neurostimulation therapies for poststroke dysphagia: a systematic review and meta-analysis. Neurophysiol Clin 2021; 51: 493–506. [DOI] [PubMed] [Google Scholar]
- 11. Fregni F, El-Hagrassy MM, Pacheco-Barrios K, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 2021; 24: 256–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lefaucheur J-P, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol: Off J Int Feder Clin Neurophysiol 2020; 131: 474–528. [DOI] [PubMed] [Google Scholar]
- 13. Antal A, Alekseichuk I, Bikson M, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol 2017; 128: 1774–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teasell R, Salbach NM, Foley N, et al. Canadian stroke best practice recommendations: rehabilitation, recovery, and community participation following stroke—part one: rehabilitation and recovery following stroke; 6th edition update 2019. Int J Stroke 2020; 15: 763–788. [DOI] [PubMed] [Google Scholar]
- 15. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: e98–e169. [DOI] [PubMed] [Google Scholar]
- 16. McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016; 38: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernhardt J, Hayward KS, Dancause N, et al. A stroke recovery trial development framework: consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2019; 14: 792–802. [DOI] [PubMed] [Google Scholar]
- 18. Ngomo S, Leonard G, Moffet H, Mercier C. Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. J Neurosci Methods 2012; 205: 65–71. [DOI] [PubMed] [Google Scholar]
- 19. Walker MF, Hoffmann TC, Brady MC, et al. Improving the development, monitoring and reporting of stroke rehabilitation research: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair 2017; 31: 877–884. [DOI] [PubMed] [Google Scholar]
- 20. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 21. Hopewell S, Clarke M, Moher D, et al. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet 2008; 371: 281–283. [DOI] [PubMed] [Google Scholar]
- 22. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr Cartil 2012; 20: 256–260. [DOI] [PubMed] [Google Scholar]
- 23. Chipchase L, Schabrun S, Cohen L, et al. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clin Neurophysiol 2012; 123: 1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buch ER, Santarnecchi E, Antal A, et al. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol 2017; 128: 589–603. [DOI] [PubMed] [Google Scholar]
- 25. Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial direct current stimulation vs Sham stimulation to treat Aphasia after stroke: a randomized clinical trial. JAMA Neurology 2018; 75: 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corbett D, Carmichael ST, Murphy TH, et al. Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable translational working group. Int J Stroke 2017; 12: 462–471. [DOI] [PubMed] [Google Scholar]
- 27. Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017; 12: 451–461. [DOI] [PubMed] [Google Scholar]
- 28. Kwakkel G, Van Wegen E, Burridge JH, et al. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2019; 14: 783–791. [DOI] [PubMed] [Google Scholar]
- 29. Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 2002; 16: 232–240. [DOI] [PubMed] [Google Scholar]
- 30. International classification of functioning, disability and health 2001. https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health
- 31. Chen G, Lin T, Wu M, et al. Effects of repetitive transcranial magnetic stimulation on upper-limb and finger function in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Front Neurol 2022; 13: 940467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernhardt J, Borschmann K, Boyd L, et al. Moving rehabilitation research forward: developing consensus statements for rehabilitation and recovery research. Neurorehabil Neural Repair 2017; 31: 694–698. [DOI] [PubMed] [Google Scholar]
- 33. Kaviannejad R, Karimian SM, Riahi E, Ashabi G. Using dual polarities of transcranial direct current stimulation in global cerebral ischemia and its following reperfusion period attenuates neuronal injury. Metab Brain Dis 2022; 37: 1503–1516. [DOI] [PubMed] [Google Scholar]
- 34. Kloosterboer E, Funke K. Repetitive transcranial magnetic stimulation recovers cortical map plasticity induced by sensory deprivation due to deafferentiation. J Physiol 2019; 597: 4025–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zong X, Li Y, Liu C, et al. Theta-burst transcranial magnetic stimulation promotes stroke recovery by vascular protection and neovascularization. Theranostics 2020; 10: 12090–12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parthoens J, Verhaeghe J, Servaes S, Miranda A, Stroobants S, Staelens S. Performance characterization of an actively cooled repetitive transcranial magnetic stimulation coil for the rat. Neuromodulation 2016; 19: 459–468. [DOI] [PubMed] [Google Scholar]
- 37. Chang SJ, Santamaria AJ, Sanchez FJ, et al. Deep brain stimulation of midbrain locomotor circuits in the freely moving pig. Brain Stimul 2021; 14: 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boonzaier J, Petrov PI, Otte WM, Smirnov N, Neggers SFW, Dijkhuizen RM. Design and evaluation of a rodent-specific transcranial magnetic stimulation coil: an in silico and in vivo validation study. Neuromodulation 2020; 23: 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boyd LA, Hayward KS, Ward NS, et al. Biomarkers of stroke recovery: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017; 12: 480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buetefisch CM, Wei L, Gu X, Epstein CM, Yu SP. Neuroprotection of low-frequency repetitive transcranial magnetic stimulation after ischemic stroke in rats. Ann Neurol 2023; 93: 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hildesheim FE, Silver AN, Dominguez-Vargas AU, et al. Predicting individual treatment response to rTMS for motor recovery after stroke: a review and the CanStim perspective. Front Rehabil Sci 2022; 3: 795335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Lieshout ECC, van der Worp HB, Visser-Meily JMA, Dijkhuizen RM. Timing of repetitive transcranial magnetic stimulation onset for upper limb function after stroke: a systematic review and meta-analysis. Front Neurol 2019; 10: 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bönstrup M, Krawinkel L, Schulz R, et al. Low-frequency brain oscillations track motor recovery in human stroke. Ann Neurol 2019; 86: 853–865. [DOI] [PubMed] [Google Scholar]
- 44. Hordacre B, Moezzi B, Ridding MC. Towards targeted brain stimulation in stroke: connectivity as a biomarker of response. J Exp Neurosci 2018; 12: 809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di Lazzaro V, Pellegrino G, Di Pino G, et al. Val66Met BDNF gene polymorphism influences human motor cortex plasticity in acute stroke. Brain Stimul 2015; 8: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abellaneda-Perez K, Martin-Trias P, Casse-Perrot C, et al. BDNF Val66Met gene polymorphism modulates brain activity following rTMS-induced memory impairment. Sci Rep 2022; 12: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012; 135: 2527–2535. [DOI] [PubMed] [Google Scholar]
- 48. Hawe RL, Scott SH, Dukelow SP. Taking proportional out of stroke recovery. Stroke 2018; 50: 204–211. [DOI] [PubMed] [Google Scholar]
- 49. Steyerberg EW, Harrell FE., Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol 2016; 69: 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015; 350: g7594. [DOI] [PubMed] [Google Scholar]
- 51. Ali M, Ashburn A, Bowen A, et al. VISTA-Rehab: a resource for stroke rehabilitation trials. Int J Stroke 2010; 5: 447–452. [DOI] [PubMed] [Google Scholar]
- 52. Pallmann P, Bedding AW, Choodari-Oskooei B, et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med 2018; 16: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wso-10.1177_17474930231203982 for A translational roadmap for transcranial magnetic and direct current stimulation in stroke rehabilitation: Consensus-based core recommendations from the third stroke recovery and rehabilitation roundtable by Jodi D Edwards, Adan Ulises Dominguez-Vargas, Charlotte Rosso, Meret Branscheidt, Lisa Sheehy, Fanny Quandt, Simon A Zamora, Melanie K Fleming, Valentina Azzollini, Ronan A Mooney, Charlotte J Stagg, Chiristian Gerloff, Simone Rossi, Leonardo G Cohen, Pablo Celnik, Michael A Nitsche, Cathrin M Buetefisch and Numa Dancause in International Journal of Stroke
Supplemental material, sj-docx-2-wso-10.1177_17474930231203982 for A translational roadmap for transcranial magnetic and direct current stimulation in stroke rehabilitation: Consensus-based core recommendations from the third stroke recovery and rehabilitation roundtable by Jodi D Edwards, Adan Ulises Dominguez-Vargas, Charlotte Rosso, Meret Branscheidt, Lisa Sheehy, Fanny Quandt, Simon A Zamora, Melanie K Fleming, Valentina Azzollini, Ronan A Mooney, Charlotte J Stagg, Chiristian Gerloff, Simone Rossi, Leonardo G Cohen, Pablo Celnik, Michael A Nitsche, Cathrin M Buetefisch and Numa Dancause in International Journal of Stroke
Supplemental material, sj-pdf-3-wso-10.1177_17474930231203982 for A translational roadmap for transcranial magnetic and direct current stimulation in stroke rehabilitation: Consensus-based core recommendations from the third stroke recovery and rehabilitation roundtable by Jodi D Edwards, Adan Ulises Dominguez-Vargas, Charlotte Rosso, Meret Branscheidt, Lisa Sheehy, Fanny Quandt, Simon A Zamora, Melanie K Fleming, Valentina Azzollini, Ronan A Mooney, Charlotte J Stagg, Chiristian Gerloff, Simone Rossi, Leonardo G Cohen, Pablo Celnik, Michael A Nitsche, Cathrin M Buetefisch and Numa Dancause in International Journal of Stroke