Abstract
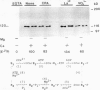
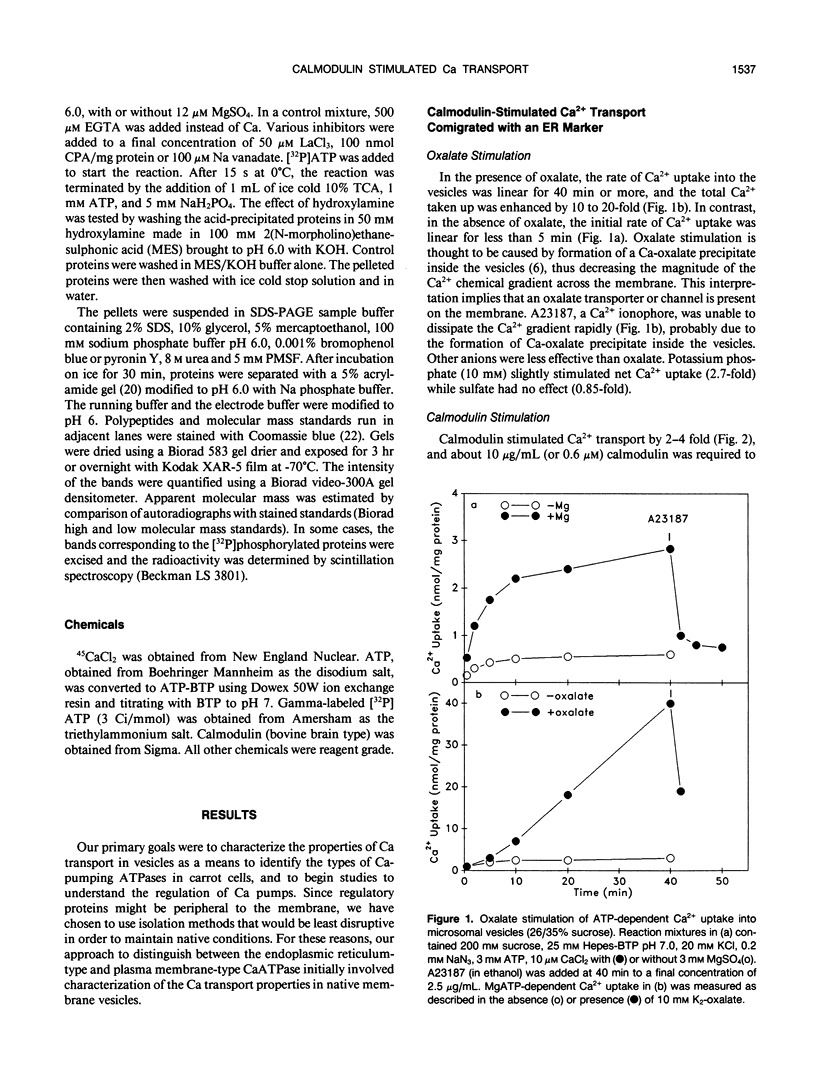
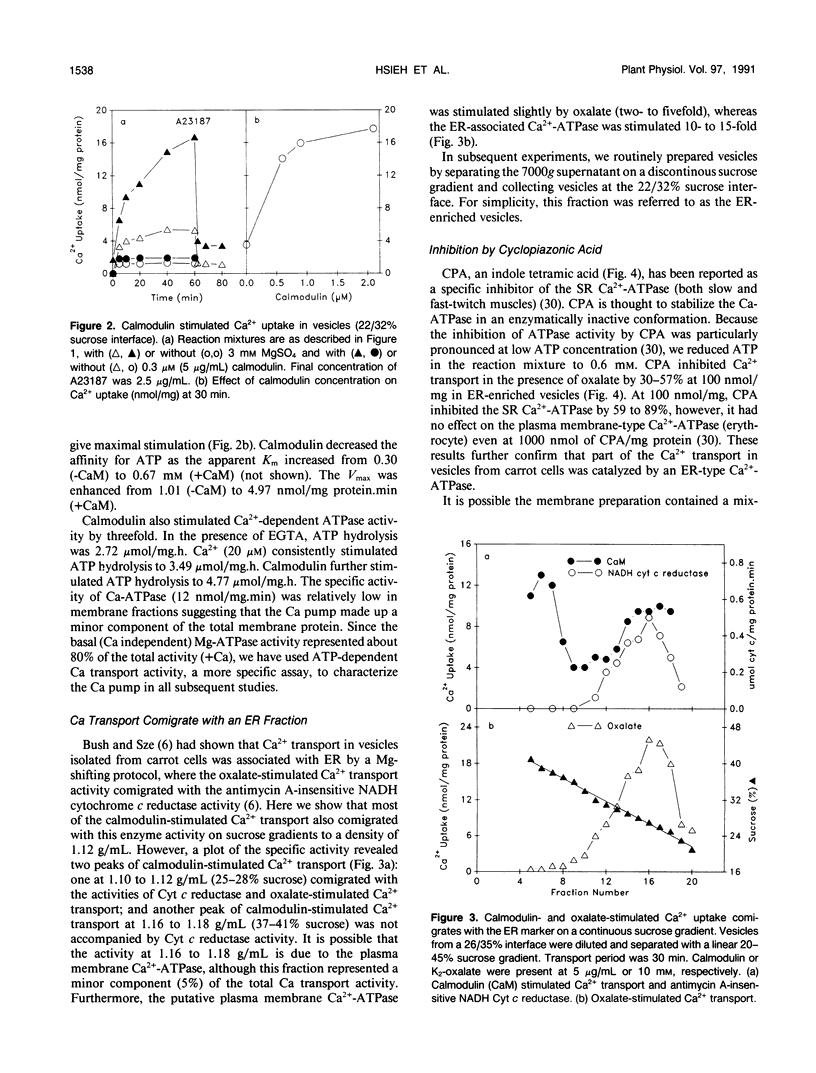
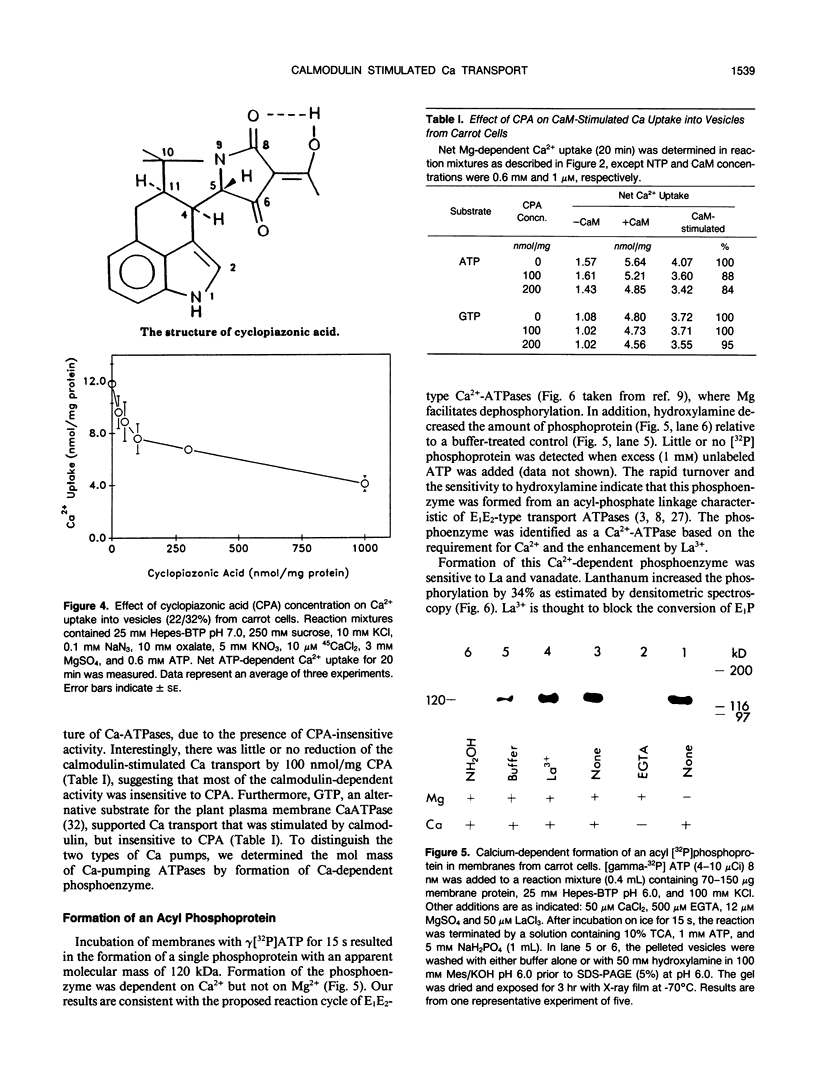
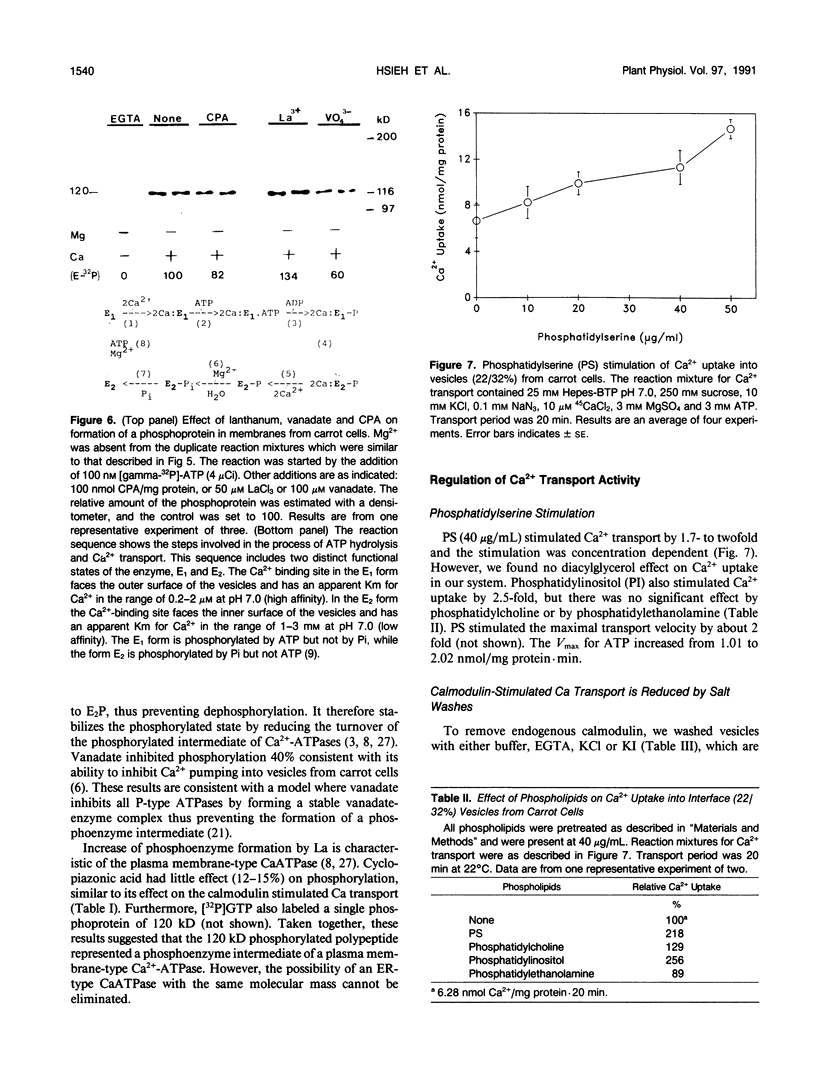
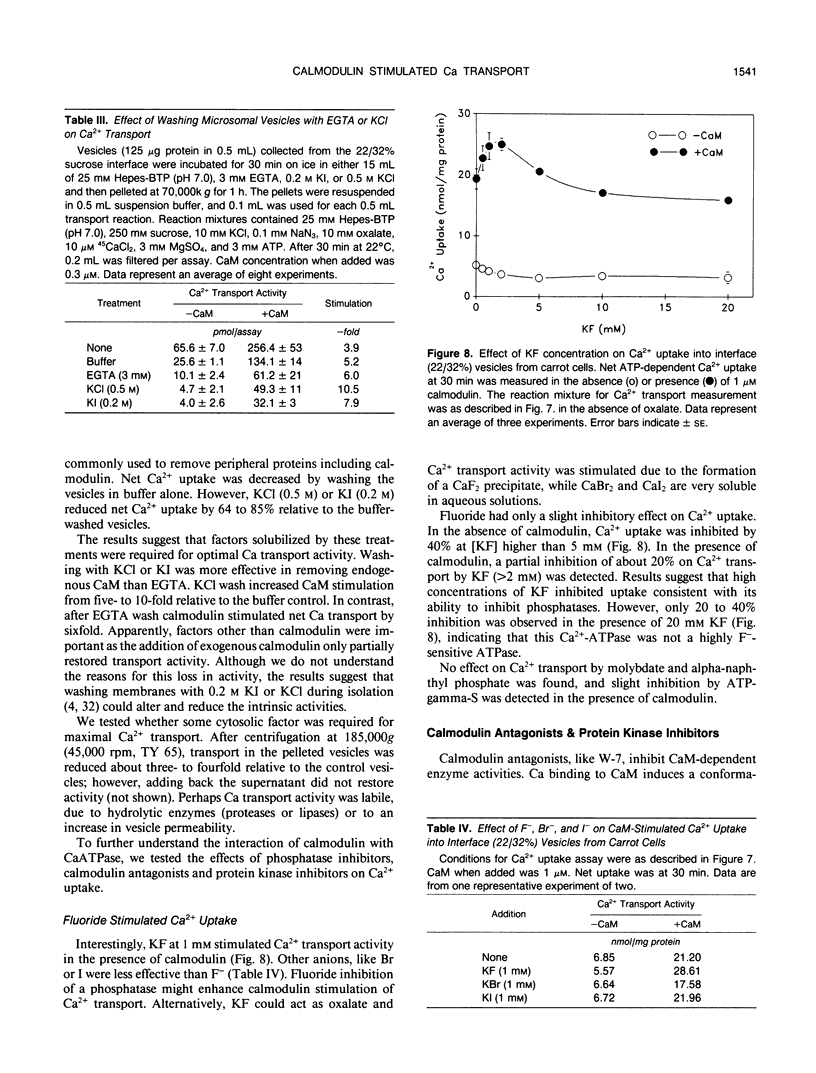
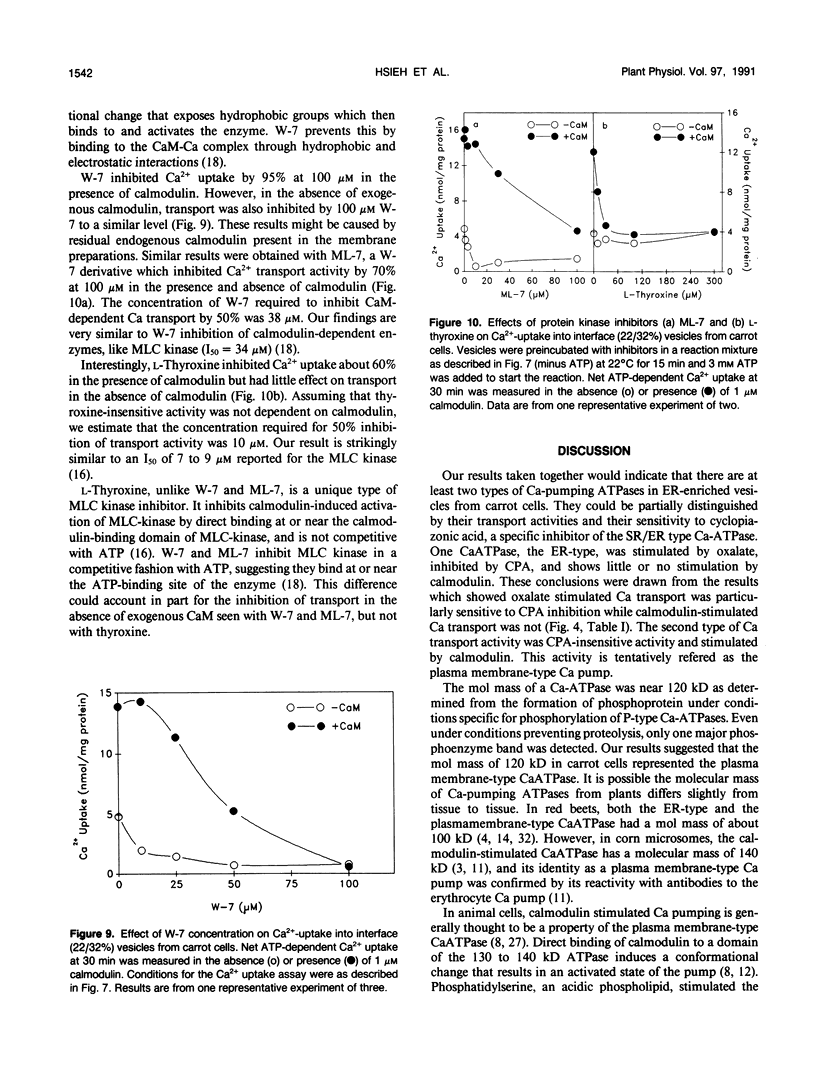
Ca2+-ATPases keep cytoplasmic [Ca2+] low by pumping Ca2+ into intracellular compartments or out of the cell. The transport properties of Ca2+-pumping ATPases from carrot (Daucus carota cv Danvers) tissue culture cells were studied. ATP-dependent Ca2+ transport in vesicles that comigrated with an endoplasmic reticulum marker, was stimulated three- to fourfold by calmodulin. Cyclopiazonic acid (a specific inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) partially inhibited oxalate-stimulated Ca2+ transport activity; however, it had no effect on calmodulin-stimulated Ca2+ uptake driven by ATP or GTP. The results would suggest the presence of two types of Ca2+-ATPases, an endoplasmic reticulum- and a plasma membrane-type. Interestingly, incubation of membranes with [gamma32P]ATP resulted in the formation of a single acyl [32P]phosphoprotein of 120 kilodaltons. Formation of this phosphoprotein was dependent on Ca2+, but independent of Mg2+. Its enhancement by La3+ is characteristic of a phosphorylated enzyme intermediate of a plasma membrane-type Ca-ATPase. Calmodulin stimulated Ca2+ transport was decreased by W-7 (a calmodulin antagonist), ML-7 (myosin light chain kinase inhibitor) or thyroxine. Acidic phospholipids, like phosphatidylserine, stimulated Ca2+ transport, similar to their effect on the erythrocyte plasma membrane Ca2+-ATPase. These results would indicate that the calmodulin-stimulated Ca2+ transport originated in large part from a plasma membrane-type Ca2+ pump of 120 kilodaltons. The possibility of calmodulin-stimulated Ca2+-ATPases on endomembranes, such as the endoplasmic reticulum and secretory vesicles, as well as the plasma membrane is suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briars S. A., Evans D. E. The calmodulin-stimulated ATPase of maize coleoptiles forms a phosphorylated intermediate. Biochem Biophys Res Commun. 1989 Feb 28;159(1):185–191. doi: 10.1016/0006-291x(89)92421-2. [DOI] [PubMed] [Google Scholar]
- Briskin D. P. Ca-translocating ATPase of the plant plasma membrane. Plant Physiol. 1990 Oct;94(2):397–400. doi: 10.1104/pp.94.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R., Sze H. Calcium transport in tonoplast and endoplasmic reticulum vesicles isolated from cultured carrot cells. Plant Physiol. 1986 Feb;80(2):549–555. doi: 10.1104/pp.80.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A., Vorherr T., James P., McCormick D. J., Filoteo A. G., Carafoli E., Penniston J. T. The calmodulin binding domain of the plasma membrane Ca2+ pump interacts both with calmodulin and with another part of the pump. J Biol Chem. 1989 Jul 25;264(21):12313–12321. [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Reynolds-Niesman I., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : I. Characterization of a Ca-Pumping ATPase Associated with the Endoplasmic Reticulum. Plant Physiol. 1987 Dec;85(4):1129–1136. doi: 10.1104/pp.85.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs E. M., Lienhard G. E., Appleman J. R., Lane M. D., Frost S. C. Insulin stimulates fluid-phase endocytosis and exocytosis in 3T3-L1 adipocytes. J Biol Chem. 1986 Mar 25;261(9):3944–3951. [PubMed] [Google Scholar]
- Hagiwara M., Mamiya S., Ochiai M., Hidaka H. Thyroid hormones inhibit the Ca2+ calmodulin-induced activation of myosin light chain kinase. Biochem Biophys Res Commun. 1988 Apr 15;152(1):270–276. doi: 10.1016/s0006-291x(88)80710-1. [DOI] [PubMed] [Google Scholar]
- Kasai M., Muto S. Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from corn leaves. J Membr Biol. 1990 Mar;114(2):133–142. doi: 10.1007/BF01869094. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+ - ATPase. J Biol Chem. 1981 Aug 25;256(16):8588–8592. [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., Olivari C., De Michelis M. I. Identification and Characterization of the Ca-ATPase which Drives Active Transport of Ca at the Plasma Membrane of Radish Seedlings. Plant Physiol. 1989 Aug;90(4):1429–1434. doi: 10.1104/pp.90.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J. The calcium pump of the surface membrane and of the sarcoplasmic reticulum. Annu Rev Physiol. 1989;51:473–485. doi: 10.1146/annurev.ph.51.030189.002353. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Thuleau P. Ca2+ Channels in Higher Plant Cells. Plant Cell. 1991 Jun;3(6):555–559. doi: 10.1105/tpc.3.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Tada M., Kadoma M., Inui M., Fujii J. Regulation of Ca2+-pump from cardiac sarcoplasmic reticulum. Methods Enzymol. 1988;157:107–154. doi: 10.1016/0076-6879(88)57073-8. [DOI] [PubMed] [Google Scholar]
- Williams L. E., Schueler S. B., Briskin D. P. Further Characterization of the Red Beet Plasma Membrane Ca-ATPase Using GTP as an Alternative Substrate. Plant Physiol. 1990 Mar;92(3):747–754. doi: 10.1104/pp.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meis L. Approaches to studying the mechanisms of ATP synthesis in sarcoplasmic reticulum. Methods Enzymol. 1988;157:190–206. doi: 10.1016/0076-6879(88)57075-1. [DOI] [PubMed] [Google Scholar]