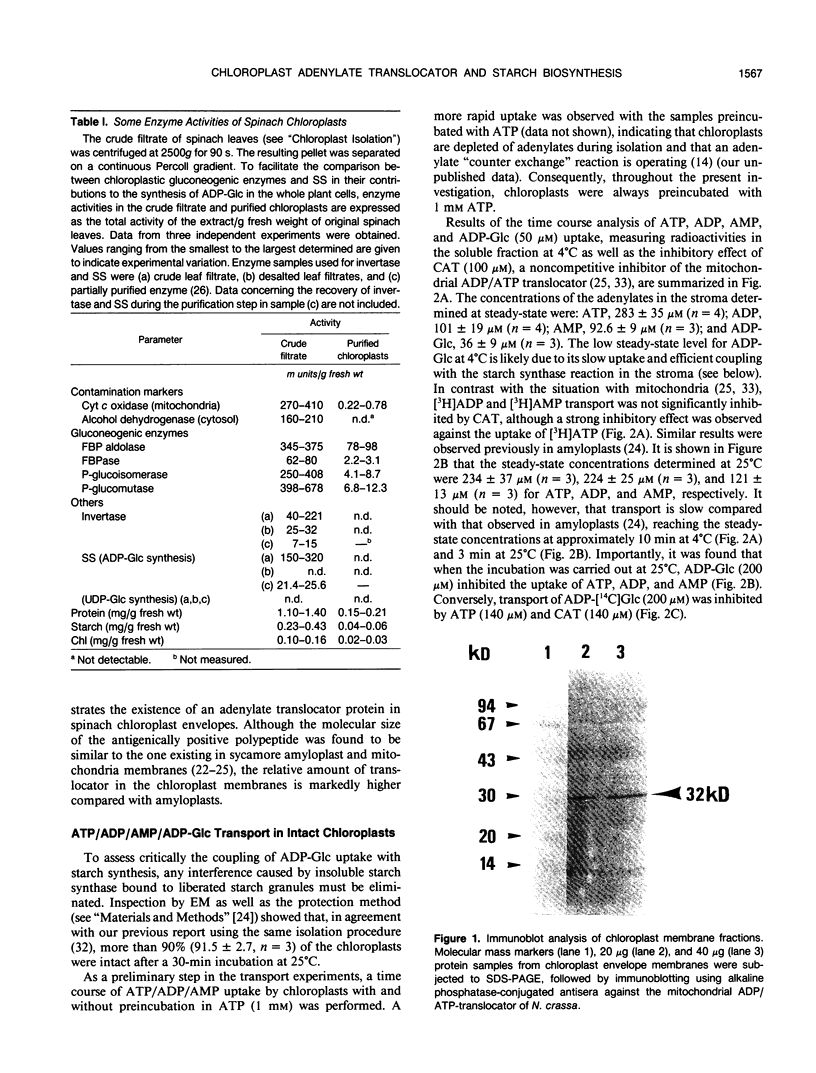
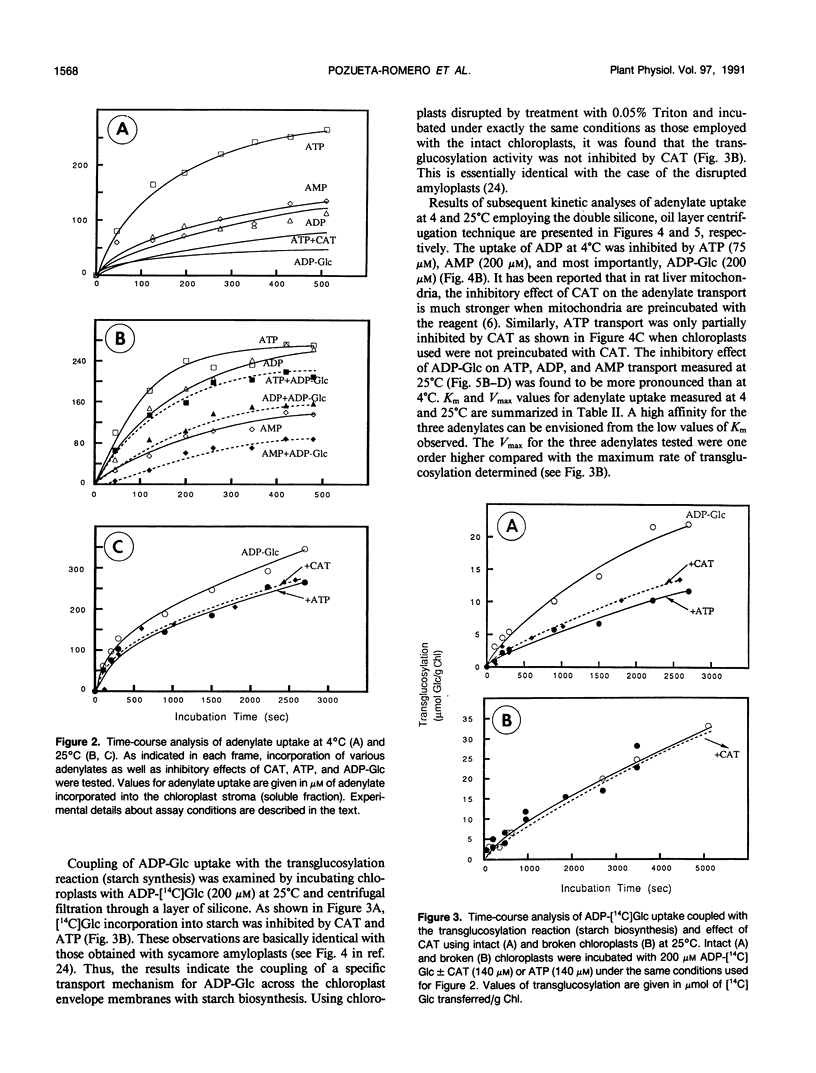
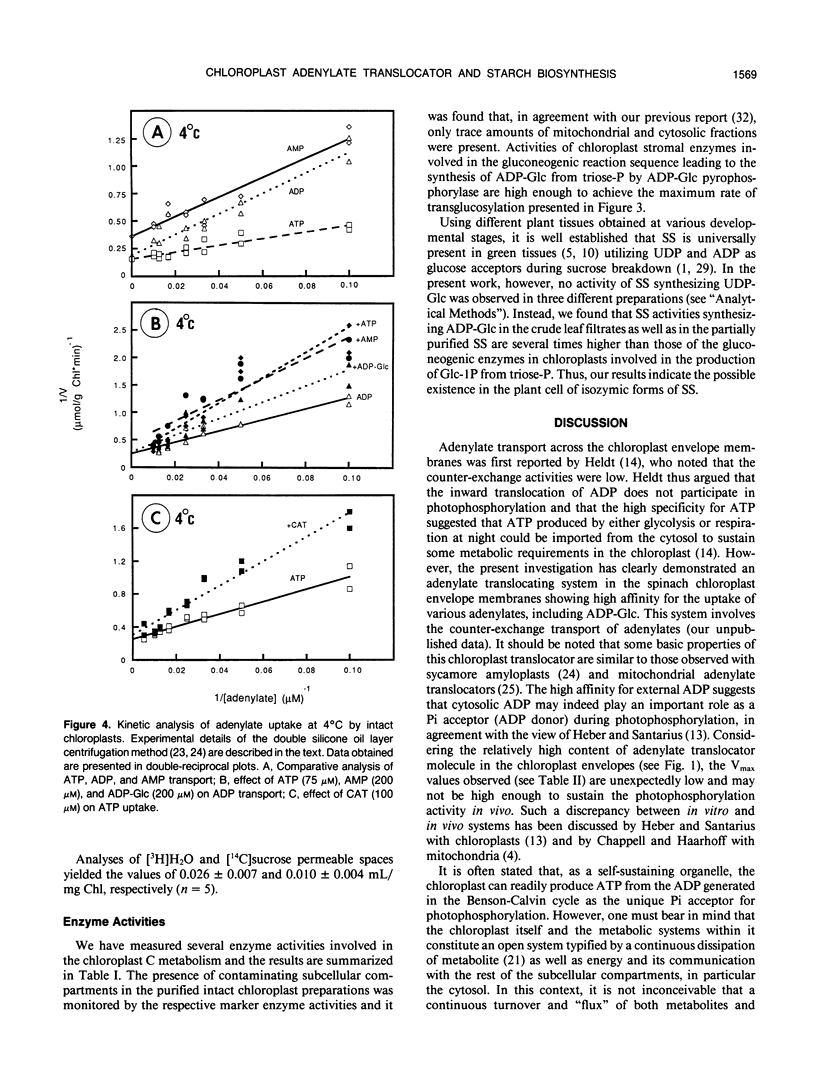
Abstract
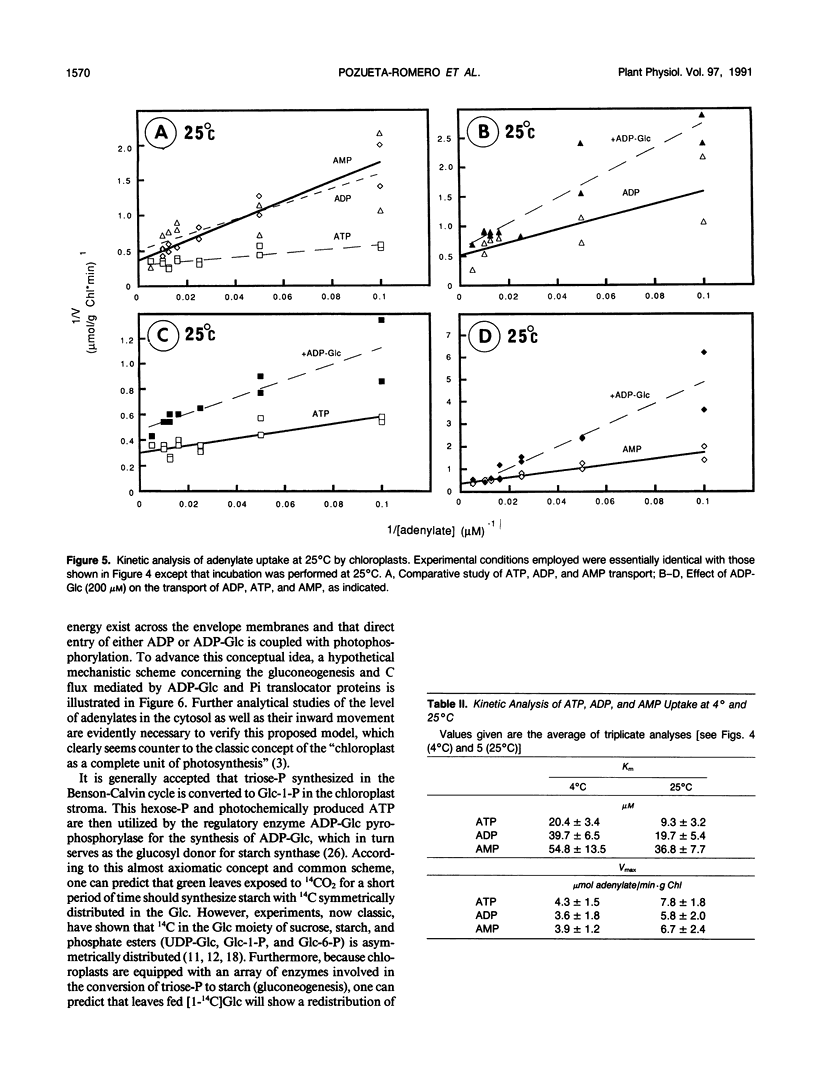
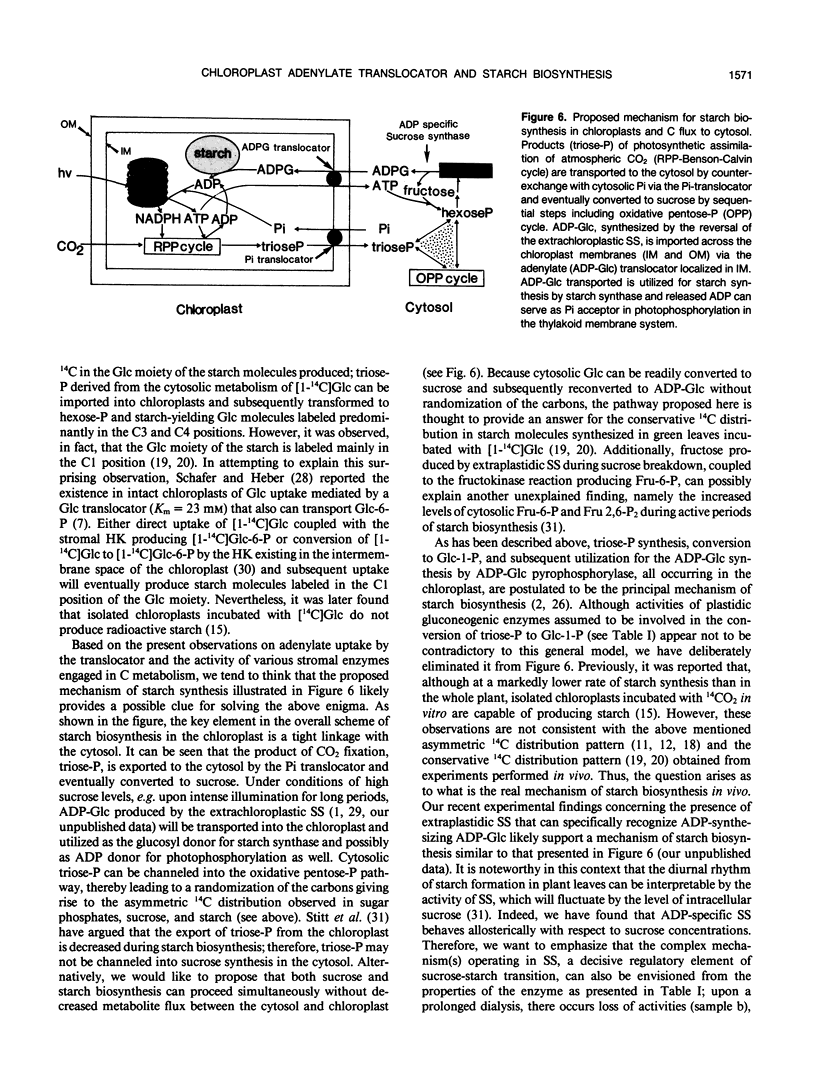
In organello starch biosynthesis was studied using intact chloroplasts isolated from spinach leaves (Spinacia oleracea). Immunoblot analysis using a specific antiserum against the mitochondrial adenylate (ADP/ATP) translocator of Neurospora crassa shows the presence of an adenylate translocator protein in the chloroplast envelope membranes, similar to that existing in mitochondria and amyloplasts from cultured cells of sycamore (Acer pseudoplatanus). The double silicone oil layer-filtering centrifugation technique was employed to study the kinetic properties of adenylate transport in the purified chloroplasts; ATP, ADP, AMP, and most importantly ADP-Glc were shown to be recognized by the adenylate translocator. Similar to the situation with sycamore amyloplasts, only ATP and ADP-Glc uptake was inhibited by carboxyatractyloside, an inhibitor of the mitochondrial adenylate translocator. Evidence is presented to show that the ADP-Glc transported into the chloroplast stroma is utilized for starch synthesis catalyzed by starch synthase (ADP-Glc:1,4-α-d-glucan 4-α-d-glucosyltransferase). The high activity of sucrose synthase producing ADP-Glc observed in the extrachloroplastic fractions suggests that starch biosynthesis in chloroplasts may be coupled with the direct import of ADP-Glc from the cytosol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I. The chloroplast as a complete photosynthetic unit. Science. 1955 Jul 1;122(3157):9–16. doi: 10.1126/science.122.3157.9. [DOI] [PubMed] [Google Scholar]
- Erdelt H., Weidemann M. J., Buchholz M., Klingenberg M. Some principle effects of bongkrekic acid on the binding of adenine nucleotides to mitochondrial membranes. Eur J Biochem. 1972 Oct 17;30(1):107–122. doi: 10.1111/j.1432-1033.1972.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Frehner M., Pozueta-Romero J., Akazawa T. Enzyme Sets of Glycolysis, Gluconeogenesis, and Oxidative Pentose Phosphate Pathway Are Not Complete in Nongreen Highly Purified Amyloplasts of Sycamore (Acer pseudoplatanus L.) Cell Suspension Cultures. Plant Physiol. 1990 Oct;94(2):538–544. doi: 10.1104/pp.94.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Source and sink leaf metabolism in relation to Phloem translocation: carbon partitioning and enzymology. Plant Physiol. 1978 Mar;61(3):380–385. doi: 10.1104/pp.61.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M., Kandler O. ASYMMETRIC DISTRIBUTION OF C IN SUGARS FORMED DURING PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1957 Jun 15;43(6):446–451. doi: 10.1073/pnas.43.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVIR E. A., GIBBS M. STUDIES ON THE REDUCTIVE PENTOSE PHOSPHATE CYCLE IN INTACT AND RECONSTITUTED CHLOROPLAST SYSTEMS. J Biol Chem. 1963 Oct;238:3183–3187. [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDLER O., GIBBS M. Untersuchungen über den Einfluss der Photosynthese auf die Austauschvorgänge innerhalb des Hexosemoleküls. Z Naturforsch B. 1959 Jan;14B(1):8–13. [PubMed] [Google Scholar]
- Kandler O., Gibbs M. Asymmetric Distribution of C in the Glucose Phosphates Formed During Photosynthesis. Plant Physiol. 1956 Sep;31(5):411–412. doi: 10.1104/pp.31.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLACHLAN G. A., PORTER H. K. Replacement of oxidation by light as the energy source for glucose metabolism in tobacco leaf. Proc R Soc Lond B Biol Sci. 1959 Sep 1;150:460–473. doi: 10.1098/rspb.1959.0035. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Takabe T., Akazawa T. Immunochemical Analysis Shows That an ATP/ADP-Translocator Is Associated with the Inner-Envelope Membranes of Amyloplasts from Acer pseudoplatanus L. Plant Physiol. 1989 Apr;89(4):1024–1027. doi: 10.1104/pp.89.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozueta-Romero J., Frehner M., Akazawa T. Filtering centrifugation through two layers of silicone oil: a method for the kinetic analysis of rapid metabolite transport in organelles. Cell Struct Funct. 1991 Oct;16(5):357–363. doi: 10.1247/csf.16.357. [DOI] [PubMed] [Google Scholar]
- Pozueta-Romero J., Frehner M., Viale A. M., Akazawa T. Direct transport of ADPglucose by an adenylate translocator is linked to starch biosynthesis in amyloplasts. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5769–5773. doi: 10.1073/pnas.88.13.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozueta-Romero J., Viale A. M., Akazawa T. Comparative analysis of mitochondrial and amyloplast adenylate translocators. FEBS Lett. 1991 Aug 5;287(1-2):62–66. doi: 10.1016/0014-5793(91)80016-v. [DOI] [PubMed] [Google Scholar]
- Rorem E. S., Walker H. G., McCready R. M. Biosynthesis of Sucrose and Sucrose-Phosphate by Sugar Beet Leaf Extracts. Plant Physiol. 1960 Mar;35(2):269–272. doi: 10.1104/pp.35.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G., Heber U. Glucose transport into spinach chloroplasts. Plant Physiol. 1977 Aug;60(2):286–289. doi: 10.1104/pp.60.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Snyder F. W. Comparative Enzymic Studies of Sucrose Metabolism in the Taproots and Fibrous Roots of Beta vulgaris L. Plant Physiol. 1979 Dec;64(6):1070–1073. doi: 10.1104/pp.64.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Bulpin P. V., ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978 Nov 15;544(1):200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Stitt M., Kürzel B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : II. Partitioning between Sucrose and Starch. Plant Physiol. 1984 Jul;75(3):554–560. doi: 10.1104/pp.75.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. V., Douce R., Lauquin G. J., Vignais P. M. Binding of radioactively labeled carboxyatractyloside, atractyloside and bongkrekic acid to the ADP translocator of potato mitochondria. Biochim Biophys Acta. 1976 Sep 13;440(3):688–696. doi: 10.1016/0005-2728(76)90051-7. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]