Abstract
Type G9 of group A rotavirus (GAR) was shown to be predominant in a survey of VP7 (G) and VP4 (P) genotypes among porcine GARs associated with outbreaks of diarrhea in young pigs in Japan between 2000 and 2002. Comparison of the G9 VP7 gene sequences showed that the porcine G9 strains were more closely related to human G9 strains reemerging globally since the mid-1990s than to those from the mid-1980s. The VP7 gene sequences of porcine G9 strains from different farms were divergent (6.1 to 7.2% difference in nucleotides), suggesting that these G9 VP7 genes were not the result of recent introduction into the porcine population. Regarding the P genotype specificities of porcine G9 strains, while the majority of strains were close to unusual porcine P types (P[13] and P[23]), two strains were of the P[6] type, which has closer sequence identity with the human AU19 strain than with the porcine Gottfried strain. These unexpected results suggest that G9 GARs in the porcine population have spread more widely than previously thought and that the VP7 genes of porcine G9 strains and those of some human G9 strains detected recently may have a common progenitor.
Group A rotaviruses (GARs), are the major cause of acute diarrhea in the young of many mammalian and avian species, including piglets (12, 29, 32). The two outer capsid proteins of GARs, VP7 and VP4, which independently elicit neutralizing antibody responses, form the basis for a dual classification system based on VP7 (G) and VP4 (P) types (12). There are at least 14 different G serotypes, which correlate with the classification of G genotypes determined by sequence analysis of the VP7 genes (12). Recently, genotype G15 in bovine GARs has been demonstrated (28). Because of difficulties in determining P serotypes, P genotyping based on VP4 gene sequences is conducted more frequently. At least 23 P genotypes have been reported thus far (12, 14, 15, 28). There is not a complete correlation between P serotypes and P genotypes; thus, P genotypes are designated in brackets for distinction from P serotypes.
Limited studies in several countries have identified at least four main G types of porcine GARs (G3, G4, G5, and G11) and two main P genotypes (Gottfried-like type P[6] and P[7]) (29, 33). In addition, three human G types (G1, G2, and G9), three bovine G types (G6, G8, and G10), three other porcine P genotypes (P[13], P[19], and P[23]), and two human P genotypes (M37-like type P[6] and P[8]) have been detected in porcine GARs (1, 3, 8, 9, 11, 14, 16, 24, 25). However, much remains unknown regarding porcine G9 GARs. In humans, G9 GARs have reemerged globally since the mid-1990s, and their VP7 genes have been shown not to be direct descendants of the VP7 genes of prototype human G9 strains from the mid-1980s (2, 13, 19, 20, 27). The origin of the VP7 genes in the reemerging human G9 strains is therefore unknown. In the present study, we show the predominance of the G9 genotype in porcine GARs associated with recent outbreaks of diarrhea in young pigs in Japan and compare their VP7 gene sequences and sequences of the VP8* fragment of VP4 genes with those of GAR strains available in databases.
A total of 223 fecal samples were collected from nursing and weanling pigs involved in 36 outbreaks of diarrhea at 18 farms in seven prefectures in Japan between 2000 and 2002 in a passive surveillance. At each farm, 500 or more sows were bred. Five to 10 fecal samples were obtained from each outbreak of diarrhea. Viral RNA was extracted from the feces using TRIzol LS (Invitrogen Corp., Carlsbad, Calif.) according to the manufacturer's instructions, and double-stranded RNAs were then examined by polyacrylamide gel electrophoresis, followed by silver staining using a commercially available kit (Bio-Rad Laboratories, Hercules, Calif.). Eighteen outbreaks of diarrhea were considered to be associated with GARs, because at least two fecal samples in these outbreaks were positive for GAR by polyacrylamide gel electrophoresis. The outbreaks of GAR-associated diarrhea were not related to the seasons, and these mortality rates ranged from 0 to 10%. RNA electrophoretic migration patterns of these GARs from the same outbreaks were similar to each other (data not shown). Therefore, 18 GAR-positive samples (one sample per outbreak) were examined for the G and P genotypes by reverse transcription-PCR and direct sequencing. For G typing, the full length of the VP7 gene was amplified with the primers End9 and Beg9 as described by Gouvea et al. (7). For P typing, an entire VP8* fragment (876 nucleotides [nt]) of the VP4 gene was amplified with the primers Con2 and Con3 as described by Gentsch et al. (5). The PCR products were then sequenced directly by cycle sequencing with an auto sequencer (ABI PRISM 310; Applied Biosystems, Foster City, Calif.). Viral isolation from selected fecal samples was conducted with MA104 cells in the presence of trypsin (31).
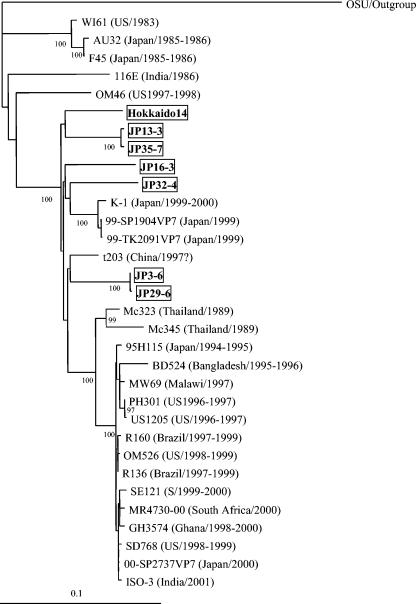
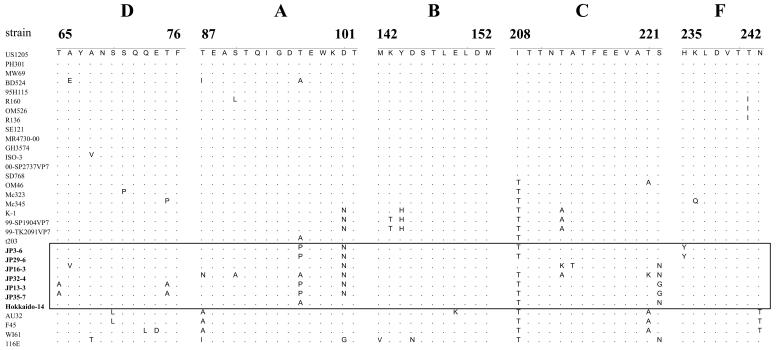
The VP7 genes from 18 GAR strains were 1,061 to 1,062 nt in length and encoded a polypeptide of 326 amino acids (aa). Sequence analysis of these VP7 genes with the BLASTN program (National Center for Biotechnology Information [http://www.ncbi.nlm.nih.gov/BLAST/]) led to classification of the 18 strains into seven G9, five G5, three G3, two G4, and one G1 (Table 1). To confirm the G9 specificities of the seven strains, their VP7 gene sequences were compared with those from reference GAR strains representing 15 G genotypes (Table 2) by the Clustal W method using Lasergene software (DNASTAR, Madison, Wis.). The VP7 gene sequences of seven strains, designated Hokkaido-14, JP3-6, JP13-3, JP16-3, JP29-6, JP32-4, and JP35-7, were most closely related to those of G9 strains (87 to 94% nucleotide and 91 to 97% amino acid identity) (Table 2). The seven strains had only 65 to 81% nucleotide and 56 to 88% amino acid identity with VP7 genes of other G types. Strains of the same G type generally share >91% VP7 amino acid identity (12). These G9 strains were detected from 1- to 60-day-old pigs in seven outbreaks at five farms in four prefectures in Japan. Figure 1 shows RNA electrophoretic migration patterns of five culture-adapted porcine G9 strains, Hokkaido-14, JP3-6, JP13-3, JP16-3, and JP29-6. These strains displayed “long” RNA patterns, which resembled that of strain OSU. In particular, the RNA patterns of strains JP3-6 and JP29-6 derived from the same farm closely resembled each other. The VP7 genes of G9 strains from different farms showed a sequence diversity of 6.1 to 7.2% in nucleotides and 3.1 to 5.8% in amino acids. In contrast, the VP7 genes of GARs from the same farms (JP3-6 and JP29-6, JP13-3 and JP35-7) showed high sequence identity (99.6 to 99.9% in nucleotides and 100% in amino acids), indicating that the VP7 genes from the same farms were of the same ancestry, despite having originated from different outbreaks occurring at intervals of 5 to 6 months. The VP7 genes of porcine G9 strains were compared to those of 25 human G9 strains selected from the GenBank database, including all the genetic variants of G9 strains reported previously (Fig. 2). The VP7 sequence identity of porcine G9 strains with human G9 strains that reemerged globally since the mid-1990s (recent strains; for example, US1205, 95H115, and R160) was generally higher (92 to 95% nucleotide and 93 to 98% amino acid identity) than with those isolated in the mid-1980s (old strains; for example, 116E, WI61, and AU32) (87 to 90% nucleotide and 91 to 95% amino acid identity), with one exception, namely, strain OM46 isolated in 1997 to 1998 (86 to 88% nucleotide and 95 to 96% amino acid identity) (13). Similarly, phylogenetic analysis showed that the VP7 genes of porcine G9 strains were more closely related to those of recent human G9 strains than to those of old human G9 strains. In particular, porcine strain JP32-4 was grouped together with Japanese strains K-1, 99-SP1904VP7, and 99-TK2091VP7, and porcine strains JP3-6 and JP29-6 were grouped together with Chinese strain t203 (Fig. 2). However, the lineages formed with these porcine and human strains were distinct from that composed of other recent human strains (Fig. 2). A similar relationship was observed in a phylogenetic tree based on VP7 amino acid sequences (data not shown). Antigenic regions (A, B, C, D, and F) of VP7 were compared among porcine and human G9 strains (Fig. 3). Three to eight amino acid substitutions in the antigenic regions were identified between recent human strain US1205 and porcine strains, and relatively conserved amino acid substitutions among porcine strains were identified in region A at positions 96 and 100 and in region C at positions 208 and 221. At three of these positions (96, 100, and 208), amino acid substitutions were also observed in Japanese strains K-1, 99-SP1904VP7, and 99-TK2091VP7 or in Chinese strain t203. In addition, a replacement of isoleucine with threonine at position 208 was also identified in strains OM46, Mc323, and Mc345 and in old strains AU32, F45, WI61, and 116E (Fig. 3). Six to 11 amino acid substitutions in the antigenic regions were identified between porcine strains and old human strains AU32, F45, WI61, and 116E.
TABLE 1.
Distribution of G and P types of group A rotaviruses associated with 18 outbreaks of porcine diarrhea in Japan between 2000 and 2002
| Strain | Farm | Yr/mo detected | G type | P type |
|---|---|---|---|---|
| JPT8 | A | 2000/2 | G4 | P[7] |
| JPS8 | B | 2001/1 | G4 | P[23] |
| Hokkaido-14 | C | 2001/2 | G9 | P[23] |
| Kyusyu-14 | D | 2001/2 | G1 | P[7] |
| Tohoku-2 | A | 2001/2 | G5 | P[7] |
| JP3-6 | E | 2002/5 | G9 | P[6] |
| JP9-8 | F | 2002/5 | G5 | P[6] |
| JP10-5 | G | 2002/5 | G5 | P[13] or P[22]a |
| JP13-3 | H | 2002/5 | G9 | P[13] or P[22]a |
| JP16-3 | I | 2002/6 | G9 | P[23] |
| JP19-2 | J | 2002/7 | G3 | P[6] |
| JP24-12 | K | 2002/9 | G5 | P[23] |
| JP29-6 | E | 2002/10 | G9 | P[6] |
| JP31-1 | L | 2002/10 | G5 | P[23] |
| JP32-4 | M | 2002/10 | G9 | P[23] |
| JP33-3 | F | 2002/10 | G3 | P[6] |
| JP34-7 | N | 2002/11 | G3 | NDb |
| JP35-7 | H | 2002/11 | G9 | P[13] or P[22]a |
Tentatively classified until the full lengths of these VP4 genes are sequenced.
ND, the P type could not be determined.
TABLE 2.
Nucleotide and amino acid sequence identity of VP7 genes of seven porcine G9 GAR strains with those of GARs belonging to various G typesa
| Strain (origin) | G type | % Identity of VP7 gene with that of:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hokkaido-14
|
JP3-6
|
JP13-3
|
JP16-3
|
JP29-6
|
JP32-4
|
JP35-7
|
|||||||||
| nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | ||
| KU (human) | 1 | 78 | 83 | 78 | 82 | 78 | 82 | 77 | 82 | 78 | 82 | 78 | 82 | 78 | 82 |
| KUN (human) | 2 | 75 | 75 | 75 | 76 | 75 | 76 | 75 | 77 | 75 | 76 | 75 | 76 | 75 | 76 |
| SA11 (simian) | 3 | 81 | 88 | 79 | 87 | 80 | 87 | 79 | 88 | 79 | 87 | 80 | 87 | 80 | 87 |
| Gottfried (porcine) | 4 | 78 | 80 | 78 | 80 | 77 | 80 | 77 | 78 | 78 | 80 | 78 | 79 | 77 | 80 |
| OSU (porcine) | 5 | 79 | 83 | 79 | 82 | 79 | 82 | 78 | 83 | 79 | 82 | 78 | 83 | 79 | 82 |
| UK (bovine) | 6 | 78 | 84 | 77 | 84 | 77 | 84 | 77 | 84 | 77 | 84 | 77 | 84 | 77 | 84 |
| PO-13 (avian) | 7 | 67 | 57 | 66 | 56 | 68 | 57 | 67 | 56 | 66 | 56 | 65 | 58 | 68 | 57 |
| A5 (bovine) | 8 | 79 | 83 | 77 | 83 | 78 | 82 | 78 | 83 | 77 | 83 | 78 | 82 | 78 | 82 |
| 116E (human) | 9 | 89 | 93 | 87 | 91 | 88 | 92 | 88 | 92 | 87 | 91 | 88 | 91 | 89 | 92 |
| AU32 (human) | 9 | 90 | 95 | 88 | 94 | 89 | 94 | 90 | 94 | 88 | 94 | 88 | 93 | 89 | 94 |
| 95H115 (human) | 9 | 93 | 97 | 94 | 97 | 93 | 95 | 93 | 96 | 94 | 97 | 93 | 95 | 93 | 95 |
| KK3 (bovine) | 10 | 77 | 83 | 76 | 82 | 78 | 83 | 76 | 82 | 76 | 82 | 77 | 82 | 78 | 83 |
| YM (porcine) | 11 | 79 | 85 | 80 | 85 | 79 | 84 | 79 | 86 | 80 | 85 | 79 | 85 | 79 | 84 |
| L26 (human) | 12 | 76 | 82 | 75 | 82 | 77 | 82 | 77 | 81 | 75 | 82 | 76 | 82 | 77 | 82 |
| L338 (equine) | 13 | 76 | 79 | 75 | 79 | 76 | 79 | 76 | 79 | 75 | 79 | 77 | 79 | 76 | 79 |
| CH3 (equine) | 14 | 79 | 83 | 79 | 83 | 80 | 82 | 79 | 83 | 79 | 83 | 80 | 83 | 80 | 82 |
| Hg18 (bovine) | 15 | 76 | 81 | 75 | 80 | 76 | 81 | 76 | 80 | 75 | 80 | 76 | 79 | 76 | 81 |
The VP7 nucleotide sequences (1,061 to 1,062 nt in length and 326 aa) used were from the following accession numbers: for KU, D16343; for KUN, D50124; for SA11, V01546; for Gottfried, X06386; for OSU, X04613; for UK, X00896; for PO-13, D82979; for A5, D01054; for 116E, L14072; for AU32, AB045372; for 95H115, AB045373; for KK3, D01056; for YM, M23194; for L26, M58290; for L338, D13549; for CH3, D25229; and for Hg18, AF237666. Values for strains with the same G type as the seven porcine strains are shown in boldface type.
FIG. 1.
Electrophoretic migration patterns of viral RNAs from the cultured porcine G9 strains Hokkaido-14, JP3-6, JP13-3, JP16-3, and JP29-6. OSU is a reference porcine G5 strain with a long RNA pattern.
FIG. 2.
Phylogenetic tree for the VP7 genes of human and porcine G9 GAR strains constructed by the Clustal W method and drawn using the TreeView program (22). All of the G9 strains were rooted to strain OSU (G5). Bootstrap values of greater than 700 in 1,000 pseudoreplicates are shown as percentages. The porcine G9 strains presented in this study are boxed. The VP7 nucleotide sequences of human G9 strains used were taken from the following accession numbers or reference: for WI61, reference 10; for AU32, AB045372; for F45, reference 10; for 116E, L14072; for OM46, AJ491181; for K1, AB045374; for 99-SP1904VP7, AB091754; for 99-TK2091VP7, AB091756; for t203, AY003871; for Mc323, D38053; for Mc345, D38055; for BD524, AJ250543; for MW69, AJ250545; for PH301, AJ491184; for US1205, AF060487; for 95H115, AB045373; for R160, AF274971; for OM526, AJ491182; for R136, AF438228; for SE121, AJ491192; for MR4730-00, AY262749; for GH3574, AY211068; for ISO-3, AF501580; for 00-SP2737VP7, AB091752; and for SD768, AJ491191. Countries and years in which human strains were detected are shown in parentheses.
FIG. 3.
Comparison of the deduced amino acid sequences in five antigenic regions (D, aa 65 to 76; A, aa 87 to 101; B, aa 142 to 152; C, aa 208 to 221; and F, aa 235 to 242) of the VP7 of human and porcine G9 GAR strains. Sequences of porcine G9 strains presented in this study are boxed. For accession numbers of the nucleotide sequences used, refer to the legend of Fig. 2.
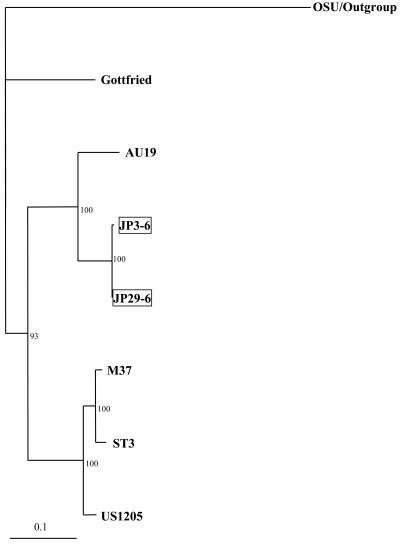
Sequence analysis of the VP8* fragment of VP4 genes with the BLASTN program classified the 17 porcine strains into five P[6], three P[7], three P[13] or P[22], and six P[23] (Table 1). P[23] has recently been reported to be a novel P genotype in a porcine strain (14). To confirm the P genotype specificity of seven G9 strains, 710-nt (236-aa) sequences of the VP8* fragment were compared with those from reference GAR strains representing 23 P genotypes (Table 3). The sequences of two strains, JP13-3 and JP35-7, were most closely related to that of porcine strain MDR13 of P[13] (81% nucleotide and 82% amino acid identity) and that of lapine strain 160/01 of P[22] (82% nucleotide and 83% amino acid identity). The identity of these strains with other P genotypes was 49 to 67% in nucleotides and 34 to 62% in amino acids. While strains of the same P genotype generally show >89% VP4 amino acid identity (6), we tentatively classify these strains as P[13] or P[22] until the full lengths of these VP4 genes are sequenced. The VP8* sequence identity between strains JP13-3 and JP35-7 was 99.9% in nucleotides and 100% in amino acids. Combined with the high level of VP7 sequence identity and the common source (these strains were from the same farm), the result suggests that strains JP13-3 and JP35-7 may be descendants of a single strain. Interestingly, porcine strains A46 and ICB2212, which were registered in the GenBank database as novel P types with the accession numbers AY050274 and AY124576, respectively, shared 83% nucleotide and 83% amino acid identity with strains JP13-3 and JP35-7, 81 to 83% nucleotide and 82% amino acid identity with strain 160/01 of P[22], and 79 to 80% nucleotide and 80 to 81% amino acid identity with strain MDR13 of P[13] in the VP8* fragments. The sequences of three strains, Hokkaido-14, JP16-3, and JP32-4, were most closely related to that of porcine strain A34 of P[23] (87 to 91% nucleotide and 95% amino acid identity). The identity of these strains with those of other P genotypes was 49 to 72% in nucleotides and 37 to 78% in amino acids. Thus, the P genotype of the three G9 strains was determined to be P[23]. The VP8* fragment sequences among strains Hokkaido-14, JP16-3, and JP32-4 showed 85 to 89% nucleotide and 95% amino acid identity. The sequences of strains JP3-6 and JP29-6 were most closely related to those of P[6] strains M37 and Gottfried (79 to 82% nucleotide and 84 to 86% amino acid identity) (Table 3). To date, three subtypes among P[6] VP4 strains, two human subtypes (P2A[6] [M37-like type] and P2C[6] [AU19-like type]), and 1 porcine subtype (P2B[6] [Gottfried-like type]) have been reported (18). When the 501-nt (166-aa) sequences of the VP8* fragments of strains JP3-6 and JP29-6 were compared with those of P[6] subtypes, these porcine strains showed the closest identity with P2C[6] human strain AU19 (89% nucleotide and 89 to 90% amino acid identity). Similarly, a phylogenetic tree of the VP8* gene sequences among P[6] GARs showed that strains JP3-6 and JP29-6 formed a cluster with AU19, suggesting that the VP4 gene of JP3-6 and JP29-6 might be of human origin (Fig. 4). Alternatively, the VP4 gene of human strain AU19 might originally be from a porcine strain. The sequences of the VP8* fragment between strains JP3-6 and JP29-6 were 99.9% identical in nucleotides and 99.6% identical in amino acids. Taken together with the high level of VP7 sequence identity, the close resemblance of the RNA electrophoretic patterns, and the sources of these strains, this result indicated that JP3-6 and JP29-6 may be descendants from a single strain, as with strains JP13-3 and JP35-7 as mentioned above.
TABLE 3.
Nucleotide and amino acid sequence identities of the VP8* fragment of VP4 genes of seven G9 porcine strains with those of GARs belonging to various P typesa
| Strain (origin) | P genotype | P serotype | % identity of VP8* fragment with that of:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hokkaido-14
|
JP3-6
|
JP13-3
|
JP16-3
|
JP29-6
|
JP32-4
|
JP35-7
|
||||||||||
| nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | |||
| A5 (bovine) | 1 | 6 | 70 | 76 | 61 | 58 | 62 | 58 | 71 | 76 | 61 | 58 | 71 | 76 | 63 | 58 |
| SA11 (simian) | 2 | 5B | 72 | 75 | 61 | 54 | 64 | 62 | 71 | 73 | 61 | 54 | 71 | 74 | 64 | 62 |
| K9 (canine) | 3 | 5A | 72 | 75 | 60 | 57 | 65 | 60 | 72 | 75 | 60 | 57 | 73 | 75 | 65 | 60 |
| RRV (simian) | 3 | 5B | 72 | 76 | 61 | 56 | 65 | 61 | 71 | 75 | 61 | 56 | 70 | 75 | 65 | 61 |
| RV-5 (human) | 4 | 1B | 63 | 59 | 69 | 65 | 57 | 52 | 62 | 59 | 69 | 66 | 65 | 60 | 57 | 52 |
| UK (bovine) | 5 | 7 | 64 | 64 | 58 | 54 | 58 | 52 | 64 | 65 | 58 | 54 | 65 | 66 | 58 | 52 |
| M37 (human) | 6 | 2A | 66 | 62 | 82 | 86 | 58 | 51 | 63 | 61 | 82 | 86 | 65 | 60 | 58 | 51 |
| Gottfried (porcine) | 6 | 2B | 64 | 64 | 79 | 84 | 59 | 52 | 63 | 63 | 79 | 84 | 65 | 64 | 58 | 52 |
| OSU (porcine) | 7 | 9 | 70 | 74 | 59 | 54 | 67 | 62 | 71 | 74 | 59 | 54 | 70 | 74 | 67 | 62 |
| KU (human) | 8 | 1A | 64 | 60 | 69 | 67 | 58 | 51 | 62 | 59 | 70 | 67 | 64 | 59 | 58 | 51 |
| K8 (human) | 9 | 3 | 57 | 56 | 51 | 48 | 54 | 50 | 57 | 55 | 51 | 49 | 56 | 54 | 54 | 50 |
| 69M (human) | 10 | 4 | 72 | 78 | 62 | 58 | 63 | 60 | 69 | 76 | 62 | 58 | 71 | 76 | 63 | 60 |
| KK3 (bovine) | 11 | 8 | 49 | 37 | 46 | 31 | 49 | 34 | 49 | 37 | 46 | 32 | 50 | 37 | 49 | 34 |
| FI23 (equine) | 12 | 4 | 71 | 74 | 63 | 58 | 63 | 59 | 69 | 73 | 63 | 58 | 70 | 72 | 63 | 59 |
| MDR13 (porcine) | 13 | 66 | 61 | 57 | 51 | 81 | 82 | 65 | 61 | 57 | 52 | 65 | 61 | 81 | 82 | |
| Mc35 (human) | 14 | 11 | 58 | 59 | 53 | 48 | 55 | 52 | 57 | 57 | 53 | 48 | 57 | 57 | 55 | 52 |
| Lp14 (ovine) | 15 | 71 | 74 | 61 | 56 | 63 | 59 | 68 | 74 | 61 | 56 | 70 | 74 | 63 | 59 | |
| Eb (murine) | 16 | 10 | 63 | 60 | 57 | 48 | 57 | 50 | 63 | 59 | 57 | 48 | 63 | 60 | 57 | 50 |
| 993/83 (bovine) | 17 | 49 | 37 | 46 | 32 | 52 | 34 | 49 | 37 | 46 | 33 | 49 | 37 | 52 | 34 | |
| L338 (equine) | 18 | 72 | 72 | 63 | 56 | 65 | 56 | 69 | 70 | 63 | 57 | 72 | 72 | 65 | 56 | |
| Mc345 (human) | 19 | 12 | 68 | 64 | 72 | 75 | 59 | 52 | 66 | 64 | 72 | 75 | 67 | 64 | 59 | 52 |
| EHP (murine) | 20 | 13 | 68 | 75 | 59 | 56 | 60 | 60 | 67 | 74 | 59 | 57 | 68 | 75 | 60 | 60 |
| Hg18 (bovine) | 21 | 68 | 66 | 63 | 59 | 64 | 59 | 63 | 67 | 63 | 59 | 66 | 66 | 64 | 59 | |
| 160/01 (lapine) | 22 | 64 | 61 | 56 | 53 | 82 | 83 | 65 | 63 | 56 | 53 | 64 | 59 | 82 | 83 | |
| A34 (porcine) | 23 | 14 | 87 | 95 | 63 | 60 | 64 | 60 | 89 | 95 | 63 | 60 | 91 | 95 | 64 | 60 |
The fragment analyzed encompasses 710-nt (236-aa) sequences of the VP8* fragment of the VP4 genes. The VP4 nucleotide sequence used were from the following accession numbers: for A5, D13395; for SA11, M23188; for K9, D14725; for RRV, M18736; for RV-5, M32559; for UK, M22306; for M37, L20877; for Gottfried, M33516; for OSU, X13190; for KU, M21014; for K8, D90260; for 69M, M60600; for KK3, D13393; for FI23, D16342; for MDR13, L07886; for Mc35, D14032; for Lp14, L11599; for Eb, L18992; for 993/83, D16352; for L338, D13399; for Mc345, D38054; for EHP, U08424; for Hg18, AF237665; for 160/01, AF526374; and for A34, AY174094. Values for strains with the same P type as that of the porcine strains analyzed herein are given in boldface type.
FIG. 4.
Phylogenetic tree for the VP8* fragment of VP4 genes from P[6] strains constructed by the Clustal W method and drawn using the TreeView program (22). All the P[6] strains were rooted to strain OSU (P[7]). Bootstrap values of greater than 700 in 1,000 pseudoreplicates are shown as percentages. The porcine G9P[6] strains presented in this study are boxed. The VP4 nucleotide sequences of P[6] strains used were taken from the following accession numbers: M33516 (Gottfried), AB017917 (AU19), L20877 (M37), L33895 (ST3), and AF079356 (US1205).
This study is the first to report that genotype G9 was predominant among porcine GARs. This result was unexpected because G3, G4 and G5 types of porcine GARs have been predominant in the United States and Canada (29, 33) and G9 was an unusual type in pigs. P genotypes of the present porcine G9 strains were also classified into unusual types, including human types (18). Although the previous relative frequencies of VP7 and VP4 genotypes among porcine GARs in Japan are unknown, the present results indicate that the epidemiological situation regarding porcine GAR infection might be changing (32) and that VP7 and VP4 genotypes, which were previously regarded as unusual types in pigs, might now be more common. In addition, interspecies transmission of GARs between humans and pigs may occur frequently as pointed out in other recent reports (8, 9, 17, 23, 30).
The present study revealed the moderate sequence divergence of VP7 genes of porcine G9 strains, suggesting that these G9 VP7 genes were not recently introduced into the porcine population. It is of note that a porcine G9 strain, ISU-64, had been isolated in Iowa in 1988 (24). Recent reports have indicated the presence of G9 strains from pigs in several countries, although little has been described about their VP7 genes (26, 30, 32). Together with these results, the present data suggest that G9 GARs in the porcine population have spread more widely than previously thought.
The VP7 genes of porcine G9 strains from the present study were genetically more closely related to recent human G9 strains that have emerged since the mid-1990s than they are to classical human G9 strains that were first detected in the mid-1980s. The majority of porcine G9 strains possessed porcine-specific P types, although these types were unusual. In addition, all porcine G9 strains showed long RNA electrophoretic migration patterns, a characteristic common to nonhuman GARs. Therefore, it is difficult to consider that most porcine G9 strains were of human origin as whole virions.
Some reports have suggested that recent human G9 strains have been introduced into the human population recently by reassortment events (13, 27). Most novel rotaviruses may derive from reassortment between progenitor viruses from different species (23). In the present study and other phylogenetic analyses (13, 27, 34), several G9 lineages distinct from the major G9 lineage have been demonstrated to exist in recent human G9 strains. The VP7 genes of the Japanese porcine G9 strains discussed here were more closely related to some of these variant G9 lineages, which comprise human Japanese (K-1, 99-SP1904VP7, and 99-TK2091VP7) and Chinese (t203) G9 strains, than to the major G9 lineage. Thus, the VP7 genes of porcine G9 strains and at least those of recent human G9 strains belonging to the variant G9 lineages may have a common progenitor. Interestingly, the same amino acid substitution in the VP7 antigenic regions was identified at position 208 (isoleucine to threonine) among most porcine strains, recent human G9 strains of the variant G9 lineages, and old human G9 strains, suggesting that these VP7 genes may have an evolutionary relationship. Of note, the VP7 genes of human G9 strains Mc323 and Mc345 detected in Thailand between 1987 and 1989 were classified into the recent G9 lineages (13, 31) but were distinct from the porcine lineages of the present study. Strain Mc323 had subgroup I specificity and a long RNA electropherotype. Strain Mc345 had the same antigenic specificity as Ms323 (31). In addition, the VP4 genes of strains Mc323 and Mc345 belonged to P[19], as was previously reported for a porcine strain (3, 21), and RNA-RNA hybridization tests showed that these strains were genetically more related to porcine than to human GARs (31). Thus, strains Mc323 and Mc345 are likely to be of porcine origin. Considering the data presented in this study, we cannot completely rule out the possibility that the VP7 genes of human G9 strains that have shown a global spread since the mid-1990s might have been introduced from porcine GARs by genetic reassortment between porcine and human rotaviruses. It is of note that an ovine G9 GAR was identified in Scotland in 1995 (4), and to clarify whether interspecies transmission has been associated with the reemergence of G9 human strains, further surveys of G9 GARs in pigs and other animals are needed.
Nucleotide sequence accession numbers.
The nucleotide sequences described in this paper have been submitted to the DDBJ nucleotide sequence database and are retrievable from GenBank. The accession numbers for the VP7 sequences are as follows: for Hokkaido-14, AB176677; for JP3-6, AB176678; for JP13-3, AB176679; for JP16-3, AB176680; for JP29-6, AB176681; for JP32-4, AB176682; and for JP35-7, AB176683. The accession numbers for the VP8* fragment of the VP4 sequences are as follows: for Hokkaido-14, AB176684; for JP3-6, AB176685; for JP13-3, AB176686; for JP16-3, AB176687; for JP29-6, AB176688; for JP32-4, AB176689; and for JP35-7, AB176690.
Acknowledgments
This research was supported in part by grants from the Japan International Cooperation Agency and the National Institute of Animal Health, Tsukuba, Japan.
We thank M. Kamiyama, T. Shouji, and T. Onodera for technical assistance.
REFERENCES
- 1.Bellinzoni, R. B., N. M. Mattion, D. O. Matson, J. Blackhall, J. L. La Torre, E. A. Scodeller, S. Urasawa, K. Taniguchi, and M. K. Estes. 1990. Porcine rotaviruses antigenically related to human rotavirus serotypes 1 and 2. J. Clin. Microbiol. 28:633-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bok, K., G. Palacios, K. Sijvarger, D. Matson, and J. Gomez. 2001. Emergence of G9 P[6] human rotaviruses in Argentina: phylogenetic relationships among G9 strains. J. Clin. Microbiol. 39:4020-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke, B., M. A. McCrae, and U. Desselberger. 1994. Sequence analysis of two porcine rotaviruses differing in growth in vitro and in pathogenicity: distinct VP4 sequences and conservation of NS53, VP6 and VP7 genes. J. Gen. Virol. 75:2205-2212. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald, T. A., M. Munoz, A. R. Wood, and D. R. Snodgrass. 1995. Serological and genomic characterisation of group A rotaviruses from lambs. Arch. Virol. 140:1541-1548. [DOI] [PubMed] [Google Scholar]
- 5.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorziglia, M., G. Larralde, A. Z. Kapikian, and R. M. Chanock. 1990. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc. Natl. Acad. Sci. USA 87:7155-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z. Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gouvea, V., N. Santos, and M. D. C. Timenetsky. 1994. VP4 typing of bovine and porcine group A rotaviruses by PCR. J. Clin. Microbiol. 32:1333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouvea, V., N. Santos, and M. D. C. Timenetsky. 1994. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 32:1338-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green, K. Y., Y. Hoshino, and N. Ikegami. 1989. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology 168:429-433. [DOI] [PubMed] [Google Scholar]
- 11.Huang, J. A., H. S. Nagesha, and I. H. Holmes. 1993. Comparative sequence analysis of VP4s from five Australian porcine rotaviruses: implication of an apparent new P type. Virology 196:319-327. [DOI] [PubMed] [Google Scholar]
- 12.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833, In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 13.Laird, A. R., J. R. Gentsch, T. Nakagomi, O. Nakagomi, and R. I. Glass. 2003. Characterization of serotype G9 rotavirus strains isolated in the United States and India from 1993 to 2001. J. Clin. Microbiol. 41:3100-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liprandi, F., M. Gerder, Z. Bastidas, J. A. Lopez, F. H. Pujol, J. E. Ludert, D. B. Joelsson, and M. Ciarlet. 2003. A novel type of VP4 carried by a porcine rotavirus strain. Virology 315:373-380. [DOI] [PubMed] [Google Scholar]
- 15.Martella, V., M. Ciarlet, A. Camarda, A. Pratelli, M. Tempesta, G. Greco, A. Cavalli, G. Elia, N. Decaro, V. Terio, G. Bozzo, M. Camero, and C. Buonavoglia. 2003. Molecular characterization of the VP4, VP6, VP7, and NSP4 genes of lapine rotaviruses identified in Italy: emergence of a novel VP4 genotype. Virology 314:358-370. [DOI] [PubMed] [Google Scholar]
- 16.Martella, V., A. Pratelli, G. Greco, M. Tempesta, M. Ferrari, M. N. Losio, and C. Buonavoglia. 2001. Genomic characterization of porcine rotaviruses in Italy. Clin. Diagn. Lab. Immunol. 8:129-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagomi, O., and T. Nakagomi. 2002. Genomic relationships among rotaviruses recovered from various animal species as revealed by RNA-RNA hybridization assays. Res. Vet. Sci. 73:207-214. [DOI] [PubMed] [Google Scholar]
- 18.Nakagomi, T., Y. Horie, Y. Koshimura, H. B. Greenberg, and O. Nakagomi. 1999. Isolation of a human rotavirus strain with a super-short RNA pattern and a new P2 subtype. J. Clin. Microbiol. 37:1213-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagomi, T., and O. Nakagomi. 2002. Genogroup characterization of reemerging serotype G9 human rotavirus strain 95H115 in comparison with earlier G9 and other human prototype strains. Microbiol. Immunol. 46:575-578. [DOI] [PubMed] [Google Scholar]
- 20.Oka, T., T. Nakagomi, and O. Nakagomi. 2000. Apparent re-emergence of serotype G9 in 1995 among rotaviruses recovered from Japanese children hospitalized with acute gastroenteritis. Microbiol. Immunol. 44:957-961. [DOI] [PubMed] [Google Scholar]
- 21.Okada, J., T. Urasawa, N. Kobayashi, K. Taniguchi, A. Hasegawa, K. Mise, and S. Urasawa. 2000. New P serotype of group A human rotavirus closely related to that of a porcine rotavirus. J. Med. Virol. 60:63-69. [PubMed] [Google Scholar]
- 22.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 23.Palombo, E. A. 2002. Genetic analysis of Group A rotaviruses: evidence for interspecies transmission of rotavirus genes. Virus Genes 24:11-20. [DOI] [PubMed] [Google Scholar]
- 24.Paul, P. S., Y. S. Lyoo, J. I. Andrews, and H. T. Hill. 1988. Isolation of two new serotypes of porcine rotavirus from pigs with diarrhea. Arch. Virol. 100:139-143. [DOI] [PubMed] [Google Scholar]
- 25.Pongsuwanna, Y., K. Taniguchi, M. Chiwakul, T. Urasawa, F. Wakasugi, C. Jayavasu, and S. Urasawa. 1996. Serological and genomic characterization of porcine rotavirus in Thailand: detection of a G10 porcine rotavirus. J. Clin. Microbiol. 34:1050-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Racz, M. L., S. S. Kroeff, V. Munford, T. A. Caruzo, E. L. Durigon, Y. Hayashi, V. Gouvea, and E. A. Palombo. 2000. Molecular characterization of porcine rotaviruses from the southern region of Brazil: characterization of an atypical genotype G[9] strain. J. Clin. Microbiol. 38:2443-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran, M., C. D. Kirkwood, L. Unicomb, N. A. Cunliffe, R. L Ward, M. K. Bhan, H. F. Clark, R. I. Glass, and J. R. Gentsch. 2000. Molecular characterization of serotype G9 rotavirus strains from a global collection. Virology 278:436-444. [DOI] [PubMed] [Google Scholar]
- 28.Rao, D. C., K. Gowda, and B. S. Reddy. 2000. Sequence analysis of VP4 and VP7 genes of nontypeable strains identifies a new pair of outer capsid proteins representing novel P and G genotypes in bovine rotaviruses. Virology 276:104-113. [DOI] [PubMed] [Google Scholar]
- 29.Rosen, B. I., A. V. Parwani, S. Lopez, J. Flores, and L. J. Saif. 1994. Serotypic differentiation of rotaviruses in field samples from diarrheic pigs by using nucleic acid probes specific for porcine VP4 and human and porcine VP7 genes. J. Clin. Microbiol. 32:311-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos, N., R. C. Lima, C. M. Nozawa, R. E. Linhares, and V. Gouvea. 1999. Detection of porcine rotavirus type G9 and of a mixture of types G1 and G5 associated with Wa-like VP4 specificity: evidence for natural human-porcine genetic reassortment. J. Clin. Microbiol. 37:2734-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urasawa, S., A. Hasegawa, T. Urasawa, K. Taniguchi, F. Wakasugi, H. Suzuki, S. Inouye, B. Pongprot, J. Supawadee, S. Suprasert, P. Rangsiyanond, S. Tonusin, and Y. Yamasi. 1992. Antigenic and genetic analyses of human rotaviruses in Chiang Mai, Thailand: evidence for a close relationship between human and animal rotaviruses. J. Infect. Dis. 166:227-234. [DOI] [PubMed] [Google Scholar]
- 32.Winiarczyk, S., P. S. Paul, S. Mummidi, R. Panek, and Z. Gradzki. 2002. Survey of porcine rotavirus G and P genotype in Poland and the United States using RT-PCR. J. Vet. Med. B 49:373-378. [DOI] [PubMed] [Google Scholar]
- 33.Zaberezhny, A. D., Y. S. Lyoo, and P. S. Paul. 1994. Prevalence of P types among porcine rotaviruses using subgenomic VP4 gene probes. Vet. Microbiol. 39:97-110. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, Y., L. Li, S. Okitsu, N. Maneekarn, and H. Ushijima. 2003. Distribution of human rotaviruses, especially G9 strains, in Japan from 1996 to 2000. Microbiol. Immunol. 47:591-599. [DOI] [PubMed] [Google Scholar]