Abstract
Background
Patients suspected of platelet function defects represent a diagnostic challenge for the clinical laboratory, mainly due to the complexity and poor standardization of screening methods. We compared a new flow-based chip-equipped point-of-care (T-TAS) device with lumi-aggregometry and other specific tests.
Materials and methods
The study included 96 patients suspected of platelet function defects and 26 patients referred to hospital for an evaluation of residual platelet function while on antiplatelet therapy.
Results
Forty-eight of 96 patients displayed abnormal platelet function by lumi-aggregometry, and 10 of them had defective granule content and were classified as δ-storage pool disease (δ-SPD). T-TAS compared favorably with lumi-aggregometry in detecting the most severe forms of platelet function defects (i.e., δ-SPD) [test agreement (lumi-light transmission aggregometry [lumi-LTA] vs T-TAS) for the δ-SPD subgroup was 80% and K CHOEN 0.695. T-TAS was less sensitive to milder platelet function defects (i.e., primary secretion defects [PSD]). Concerning patients on antiplatelets, test agreement (lumi-LTA vs T-TAS) in detecting patients who were responders to this therapy was 54%; K CHOEN 0.150.
Discussion
The results indicate that T-TAS can detect the more severe forms of platelet function defects such as δ-SPD. There is limited agreement of T-TAS with lumi-aggregometry in identifying responders to antiplatelets. However, this poor agreement is commonly shared by lumi-aggregometry and other devices owing to the lack of test specificity and of prospective data from clinical trials linking platelet function with therapeutic efficacy.
Keywords: primary hemostasis, platelet aggregation, coagulation, antiplatelet drugs, laboratory investigation
INTRODUCTION
Hemostasis is a complex tightly regulated cellular/humoral mechanism which serves to maintain blood flow in the normal circulation and to stop bleeding at the site of vessel wall injury. Among the cellular components, a crucial role is played by platelet-vessel wall interactions, collectively known as primary hemostasis, whereby platelet adherence to the subendothelial matrix at the site of vessel wall injury is followed by aggregation. Adhesion and aggregation mediated by von Willebrand factor (VWF) and either VWF or fibrinogen lead to thromboxane (TXA2) and ADP formation. Both are potent platelet pro-aggregatory substances which favor release from dense and alpha platelet granules of stored substances that stabilize aggregation. Besides primary hemostasis, platelets play a crucial role in secondary hemostasis or coagulation by exposing negatively charged phospholipids on their surface that act as cellular receptors for vitamin K-dependent coagulation factors, thus localizing thrombin generation and fibrin formation at the site of the vessel wall injury.
Congenital and acquired abnormalities of platelet function are associated with bleeding. Hence, platelet function tests are frequently included in the laboratory workup of patients experiencing bleeding episodes. However, while blood coagulation can be investigated even in less specialized laboratories, primary hemostasis testing requires a level of expertise that is not usually available in non-specialized laboratories. Platelet function tests are also prescribed to identify platelet hyperactivity as a possible predictor of thrombosis recurrence in patients who, owing to past episodes of ischemic or thrombotic events of the coronary or cerebral vascular districts, are on antiplatelet medication. Therefore, investigation of primary hemostasis is an unmet need and new simple methods that could be used in clinical laboratories need to be found and evaluated. This article aims to compare the laboratory results obtained in a relatively large number of well-characterized patients who were referred to hospital either because of suspected platelet function defects or for residual platelet activity testing while on antiplatelet therapy. Laboratory tests used for the comparative evaluation were the lumi-light transmission aggregometry (lumi-LTA) and the Total Thrombus Analysis System (T-TAS) that was designed to investigate overall primary hemostasis function.
MATERIALS AND METHODS
Study design and patient population
The primary objective of the study was the evaluation of the efficacy of T-TAS to detect congenital or acquired disorders of platelet function in patients referred to our hospital because of unexplained bleeding. We investigated a group of 96 consecutive outpatients (27 men, 69 women, median [min–max] age 40 [7–78] years) over the period October 2019–July 2021. They presented a mild to moderate bleeding diathesis of variable severity, characterized by mucocutaneous bleeding, menorrhagia, and/or excessive post-surgical and post-traumatic blood loss. The study cohort all had the following laboratory parameters within normal limits: platelet count and size, prothrombin time (PT) and activated partial thromboplastin time (APTT), VWF (antigen and ristocetin co-factor activity) and factor (F)VIII activity.
A secondary objective was to evaluate the ability of T-TAS to monitor the effects of drugs in patients with coronary artery disease on chronic treatment with antiplatelet drugs (No.=16: clopidogrel 75 mg; No.=6: clopidogrel 75 mg+ASA 100 mg No.=4: ASA 100 mg). Fifty healthy subjects with no personal or family history of hemorrhage/thrombosis, who over the previous 10 days had not taken antithrombotic medication or any drug known to interfere with platelet function were investigated as controls. Results from controls were also used to determine the limits of the reference range for T-TAS; limits of the reference range for lumi-LTA were those historically determined in the laboratory.
The study was approved by the institutional review board and all study participants gave informed consent to donate small amounts of blood for the study in addition to those needed for their routine investigation.
Blood sampling
Blood was drawn with plastic syringes. The first three mL were collected into K-EDTA tubes (Sarstedt, Verona, Italy) for blood count, the next 30mL were collected in non-evacuated home-made prepared tubes containing trisodium citrate (109 mM) for testing with lumi-LTA. To analyze hemostatic plug formation (T-TAS), blood was anticoagulated with benzilsulfonil-D-Arg-Pro-4-amidinobenzilamide1,2 provided by the manufacturer.
Platelet aggregation and secretion
Platelet function disorders (PFD) were assessed in platelet-rich plasma (PRP) by lumi-LTA (Chronolog, Mascia-Brunelli, Milan, Italy). Since the pattern and extent of aggregation abnormalities vary among individuals, it is essential to evaluate secretion and aggregation to determine whether PFD are present3. PRP or platelet-poor plasma (PPP) were prepared by centrifugation of citrated blood at 200 or at 1,400 g for 10 or 15 minutes (min), respectively. The original PRP platelet counts were used to measure platelet function4. After incubation with luciferin/luciferase to evaluate ATP secretion, the following agonists were used: adenosine-diphosphate (ADP) 4 μM, collagen 2 μg/mL, U46619 (TXA2 analog) 1 μM, 14-residue-thrombin-receptor-activator peptide (TRAP-14) 10 μM and arachidonic acid (AA) 1 mM. Results were expressed as maximal percentage increase in light-transmission (the smaller the percentage of light-transmission, the more likely the impairment of the platelet function). A standard curve using ATP doses added in vitro to PPP was used to quantify secretion expressed as nmol/108 platelets.
To determine if the platelet function abnormalities were primary defects of platelet biogenesis delta storage pool diseases (δ-SPD) or signaling pathway disorders leading to secretion defects of platelet granule (PSD), we also measured substances contained in delta granules. Total platelet ADP and ATP were measured with luminometer (LKB 1250, Bio-Orbit Oy, Turku, Finland) and the firefly luciferin/luciferase method5. Platelet serotonin was measured by the o-phthaldialdehyde method6.
Flow-based thrombus formation
To analyze flow-based thrombus formation, the modified T-TAS (Zacros, Fujimori Kogyo, Tokyo, Japan) was used. T-TAS is a flow-chamber device that analyzes platelet plug formation by type 1 collagen-coated microchips. Blood was perfused into the 26-capillary path of the microchips with a precision pump and parameters were automatically recorded. Briefly, whole blood (350 μL) was applied to the platelet microchip at a flow rate of 24μL•min−1, corresponding to 2000s−1 wall shear rate (equivalent to an approximate arteriolar shear stress). The flow pressure curves were assessed for 10 min and platelet plug formation was evaluated using the following parameters: (i) occlusion start time (OST), defined as the time (min) needed to reach the occlusion start-pressure of 10 kPa; (ii) area under the flow pressure curve (AUC) below 60 kPa for 10 min, which quantifies plug stability (min•kPa); (iii) occlusion time (OT), which defines the time (min) at which 60kPa fixed occlusion pressure is reached. Prolonged OST, OT and low AUC are considered indexes of platelet function defects.
Platelet aggregation in patients on antiplatelet drugs
Antiplatelet therapy was assessed by lumi-LTA and results were expressed as maximal increase in light transmission when tested with the agonists ADP (4 and 20 μM) and AA (1 mM). A maximal percentage increase in light transmission smaller than the 5th percentile of the distribution of results from controls (<35%, <49%, <55% with ADP-4 μM, ADP-20 μM, AA-1 mM) was taken as an indication of poor residual platelet reactivity and therefore as good response to antiplatelet therapy; high residual platelet reactivity was considered poor or absent response.
Statistical analysis
Quantitative variables for parameters of lumi-LTA and T-TAS are reported as median (min–max). Differences between groups were analyzed by the non-parametric Mann-Whitney U test. Receiver operating characteristic (ROC) curves were used to assess which of the T-TAS parameters better discriminated the more severe from milder defects. Areas under ROC curves (AUC) were used to estimate the predictive value of the method. Two-sided p <0.05 was considered statistically significant. Analyses were performed by SPSS, version 27.0, and the statistical software R, release 4.0.0.
RESULTS
Platelet aggregation and secretion in bleeding patients
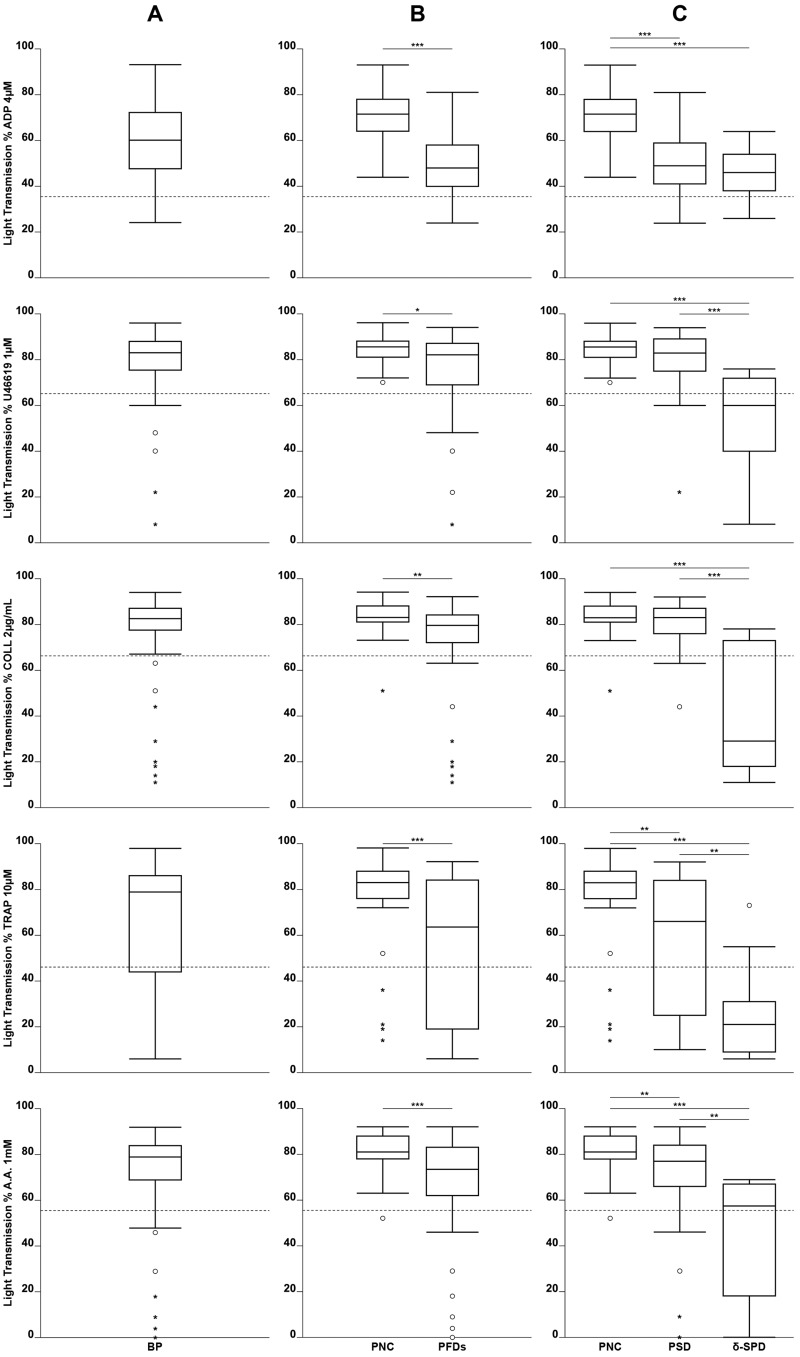
Laboratory data on PFD were available for 96 patients with a bleeding diathesis and suspected PFD were initially evaluated by lumi-LTA. The values of maximal percent light transmission are shown in Figure 1. Forty-eight of 96 patients showed normal aggregation and are identified hereafter as patients with a non-confirmed diagnosis (PNC). In contrast, the remaining 48 showed significantly (p<0.05 or p<0.001) reduced percent light transmission (irrespective of the agonist used) and were, therefore, diagnosed as having PFD owing to impaired aggregation, such as an absent secondary wave, decreased slope, or reversible aggregation. These abnormalities resulted primarily from impaired ATP release from delta granules, as shown by the finding that ATP secretion expressed as nmol/108 platelets was significantly reduced with all agonists for PFD compared to PNC (p<0.01 or p<0.001) (Table I). To determine whether the defective secretion was due to an abnormal platelet biogenesis or to signaling pathway disorders, we measured delta granule contents. Median concentrations of delta granule content (serotonin (0.19 vs 0.38nmol/108platelets); ADP (0.87 vs 2.01nmol/108 platelets) were lower in 10 of 48 patients with PFD, so that these patients were classified as δ-SPD. In contrast, the 38 patients with a defective aggregation/secretion with normal platelet delta granule contents were classified as having PSD (Table II).
Figure 1.
Distribution of percent light transmission in patients with normal coagulation and a history of bleeding
(A) BP: all 96 bleeding patients (irrespective of the final diagnosis based on lumi-light transmission aggregometry [lumi-LTA]). (B) PNC: 48 bleeding patients without confirmed platelet defect based on lumi-LTA; PFD: 48 bleeding patients with confirmed platelet defect based on lumi-LTA. (C) PNC: 48 bleeding patients without confirmed platelet defect based on lumi-LTA; PSD: 38 bleeding patients diagnosed as having platelet secretion defect; δ-SPD: 10 bleeding patients diagnosed as having δ-storage pool disease. Dashed line indicates 5th percentile of distribution of healthy controls. *p<0.05, **p<0.01, ***p<0.001.
Table I.
Median (min–max) of released ATP (nmol/108 platelets) for patients with normal coagulation and a history of bleeding, when induced by ADP, COLL, U46619, TRAP, AA
| PNC (No.=48) | PFDs (No.=48) | p-value PNC vs PFDs | PSD (No.=38) | p-value PNC vs PSD | δ-SPD (No.=10) | p-value PNC vs δ-SPD | p-value PSD vs δ-SPD | |
|---|---|---|---|---|---|---|---|---|
| ADP 4μM (0.022–0.982 nmol/10 8 plt) * | 0.081 (0–0.561) | 0.000 (0–0.209) | <0.001 | 0 (0–0.116) | <0.001 | 0 (0–0.209) | <0.001 | n.s. |
| COL 2μg/mL (0.168–0.932 nmol/10 8 plt) * | 0.504 (0.209–0.950) | 0.377 (0–0.869) | <0.01 | 0.427 (0.196–0.869) | <0.05 | 0.072 (0–0.406) | <0.001 | <0.001 |
| U46619 1μM (0.100–1.030 nmol/10 8 plt) * | 0.315 (0.097–0.673) | 0.197 (0–0.903) | <0.001 | 0.208 (0–0.903) | <0.01 | 0.043 (0–0.231) | <0.001 | <0.001 |
| TRA P 10μM (0.012 –1.074 nmol/10 8 plt) * | 0.472 (0–1.032) | 0.176 (0–0.785) | <0.001 | 0.249 (0–0.785) | <0.001 | 0.000 (0–0.289) | <0.001 | <0.01 |
| AA 1mM (0.201 –1.020 nmol/10 8 plt) * | 0.540 (0.140–0.920) | 0.409 (0–1.061) | <0.01 | 0.427 (0–1.061) | <0.05 | 0.127 (0–0.580) | <0.001 | <0.05 |
Laboratory reference range.
ADP: adenosine diphosphate (ADP); COLL: Horm Collagen; U46619: Thromboxane A2 analog; TRAP: 14-residue thrombin receptor activator peptide; AA: arachidonic acid; PNC: bleeding patients without confirmed platelet defect based on lumi-light transmission aggregometry (lumi-LTA); PFD: bleeding patients with confirmed platelet function defect; PSD: bleeding patients diagnosed as having platelet secretion defect; δ-SPD: bleeding patients diagnosed as having δ-storage pool disease.
Table II.
Median (min–max) delta granules contents for 5HT, ADP and ATP for patients with normal coagulation and previous history of bleeding
| PNC (No.=48) | PFDs (No.=48) | p-value PNC vs PFDs | PSD (No.=38) | p-value PNC vs PSD | δ-SPD (No.=10) | p-value PNC vs δ-SPD | p-value PSD vs δ-SPD | |
|---|---|---|---|---|---|---|---|---|
| 5HT (0.23–0.58 nmol/10 8 plt) * | 0.47 (0.01–0.91) | 0.38 (0.02–1.03) | <0.05 | 0.40 (0.04–1.03) | n.s. | 0.17 (0.02–0.42) | <0.01 | <0.001 |
| ATP (3.86–7.82 nmol/10 8 plt) * | 5.31 (1.71–8.02) | 4.77 (2.53–10.62) | n.s. | 4.91 (3.85–10.62) | n.s. | 4.43 (2.53–7.52) | n.s. | n.s. |
| ADP (1.23–3.91 nmol/10 8 plt) * | 2.50 (1.50–3.49) | 2.01 (0.15–3.16) | <0.05 | 2.19 (1.32–3.16) | n.s. | 0.87 (0.15–1.22) | <0.001 | <0.001 |
| Ratio ATP/ADP (1.43 –3.26) * | 2.31 (1.63–3.21) | 2.84 (1.41–24.60) | n.s. | 2.36 (1.41–6.56) | n.s. | 7.16 (3.54–24.60) | <0.001 | <0.001 |
Laboratory reference range.
5HT: serotonin; ADP: adenosine diphosphate; ATP: adenosine triphosphate; PNC: bleeding patients without confirmed platelet defect based on lumi-light transmission aggregometry (lumi-LTA); PFD: bleeding patients with confirmed platelet function defect; PSD: bleeding patients diagnosed as having platelet secretion defect; δ-SPD: bleeding patients diagnosed as having δ-storage pool disease.
Flow-based thrombus formation
Results for the T-TAS parameters are shown in Figure 2. There was no significant difference in median T-TAS parameters between all investigated patients and controls (i.e., AUC [378 vs 379]; OST [2.2 vs 2.0 min] or OT [4.9 vs 4.9 min]).
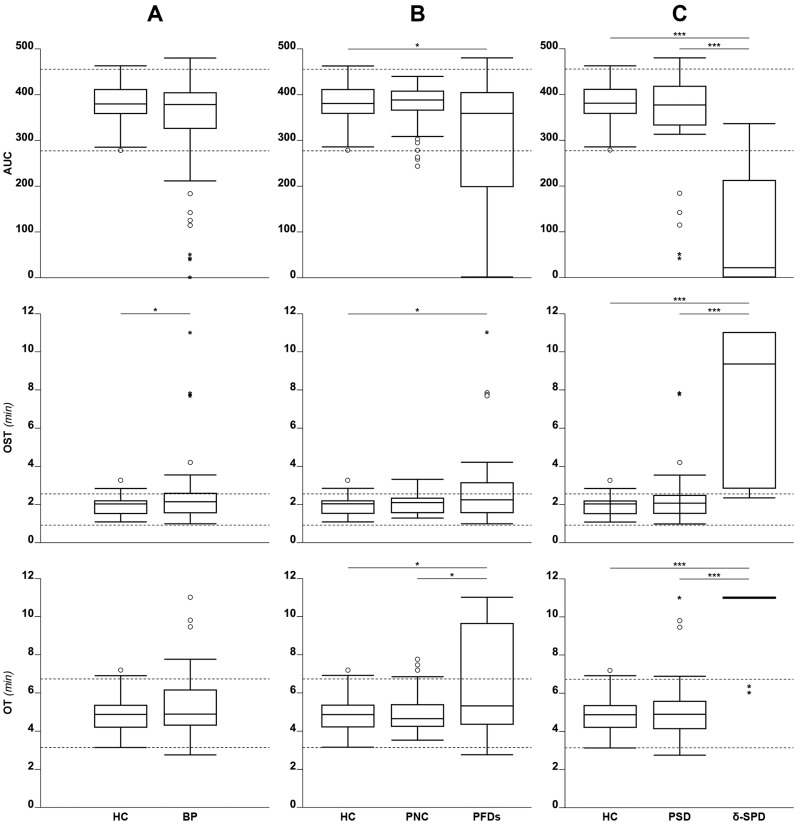
Figure 2.
Distribution of Total Thrombus Analysis System (T-TAS) results for patients with normal coagulation and a history of bleeding
(A) HC: 50 healthy controls; BP: all 96 bleeding patients (irrespective of the final diagnosis based on lumi-light transmission aggregometry [lumi- LTA]). (B) HC: 50 healthy controls; PNC: 48 bleeding patients without confirmed platelet defect based on lumi-LTA; PFD: 48 bleeding patients with confirmed platelet defect based on lumi-LTA. (C) HC: 50 healthy controls; PSD: 38 bleeding patients diagnosed as having platelet secretion defect; δ-SPD: 10 bleeding patients diagnosed as having δ-storage pool disease. Dashed lines indicate reference range (2.5th – 97.5th percentile of the distribution of HC). *p<0.05, **p<0.01, ***p<0.001.
Forty-eight patients diagnosed with lumi-LTA as having PFD showed median T-TAS parameters that differed slightly to the median of the 48 PNC (i.e., AUC [358 vs 388]; OST [2.2 vs 2.1 min] or OT [5.3 vs 4.6 min]). The analysis of the δ-SPD subgroup showed markedly reduced median AUC (22 vs 377) and markedly increased OST (9.3 vs 2.1 min) or OT (11 vs 5 min) when compared to the PSD subgroup, who, in contrast, presented with similar median AUC (377 vs 358); OST (2.1 vs 2.2 min) or OT (4.9 vs 5.3 min) to the 48 patients identified as PFD. The PSD cases had T-TAS median parameters similar to those of the 48 PNC (i.e., AUC [377 vs 388]; OST [2.1 vs 2.1 min] or OT [4.9 vs 4.6 min]). Test agreement (lumi-LTA vs T-TAS) for the δ-SPD cases, calculated on results from the T-TAS parameter OT>10 min was 80% (8/10); K CHOEN 0.695 (Online Supplementary Table SI).
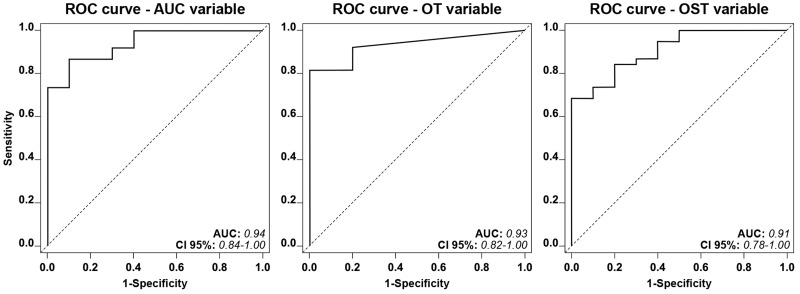
To see whether any of the cut-off value for the T-TAS parameters were potentially able to discriminate between the forementioned patient subgroups, ROC curve analyses were performed. Each of the T-TAS parameters (AUC, OST, OT) had a favorable capacity (i.e., area under the ROC curve >0.90) to differentiate between the 38 patients diagnosed as PSD and the 10 patients with the most severe platelet function defects diagnosed as δ-SPD (Figure 3). The diagnostic efficacy for any of the T-TAS parameters displayed no acceptable discriminatory capacity in distinguishing between the 48 patients with normal platelet function and the 48 patients diagnosed as PFD (i.e., area under the ROC curve <0.70) (data not shown).
Figure 3.
Receiver operating characteristic (ROC) curve showing the predictive ability of the full logistic model containing Total Thrombus Analysis System (T-TAS) parameters Area under Curve (AUC), occlusion time (OT), and occlusion start time (OST) for the discrimination of bleeding patients diagnosed as having δ-storage pool disease (δ-SPD) or bleeding patients diagnosed as having platelet secretion defect (PSD)
The dashed diagonal line represents the situation of predictive uncertainty (AUC=0.50).
Platelet reactivity in patients on antiplatelet drugs
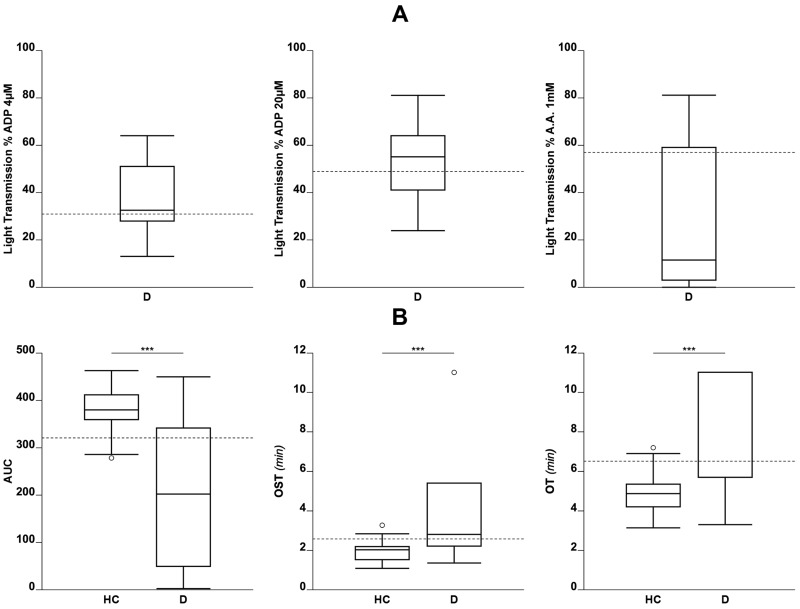
Results obtained for lumi-LTA or T-TAS in patients on antiplatelet drugs are shown in Figure 4. Patients were considered as having achieved a full antiplatelet response when results for lumi-LTA measured in the presence of the agonists were below the 5th percentile of the distribution of healthy controls (<35%, <49%, <55% with ADP 4 μM, ADP 20 μM, AA 1 mM, respectively); those above the 5th percentile were considered non-responders. Drug response when evaluated for T-TAS was based on the analysis of OST, OT or AUC taken as indexes of platelet response. Specifically, patients on treatment who had OST or OT values higher than 95th and AUC smaller than 5th percentiles of the distribution of controls were considered to have achieved complete antiplatelet response and those with OST or OT values below 95th and AUC values higher than 5th percentiles were considered non-responders. Of the 26 patients on antiplatelet therapy (16 clopidogrel, 4 ASA, 6 clopidogrel+ASA), the lumi-LTA identified 15 with complete response, 6 with no response, and 5 with partial response to therapy. T-TAS identified 17 patients with complete response, no patient with a partial response, and 9 with no response. Test agreement (lumi-LTA vs T-TAS) was 54% (14/26); K CHOEN 0.150 (Online Supplementary Table SII).
Figure 4.
Distribution of percent light transmission in patients on treatment with antiplatelet drugs when tested by lumi-light transmission aggregometry (lumi-LTA; ADP4 20μM, arachidonic acid 1mM) or by Total Thrombus Analysis System (T-TAS)
(A) Percent light transmission (D) in all patients on antiplatelet drugs. Dashed lines indicate 5th percentile of the distribution of healthy controls (HC). (B) T-TAS parameters. AUC: Area Under Curve; OST: occlusion start time; OT: occlusion time. Dashed lines indicate 5th and 95th percentile of the distribution of HC, respectively. *p<0.05, **p<0.01, ***p<0.001.
DISCUSSION
Patients with bleeding diathesis
Diagnosis of platelet function defects (PFD) requires accurate laboratory testing and many in vitro tests have been developed for this purpose. Among them, light transmission aggregometry (LTA) is still considered to be the most useful to screen patients suspected of having PFD. However, LTA is time consuming, technically demanding, and subject to many preanalytical and analytical variables. The modified LTA, lumi-LTA, is more sensitive to the less severe but more common platelet defects because, in parallel with platelet aggregation, it also measures platelet secretion. To help standardize aggregometry procedures across laboratories, the relevant Subcommittee of the International Society on Thrombosis and Haemostasis has issued guidelines to promote standardization7. However, despite these efforts, LTA and/or lumi-LTA results are not yet comparable across laboratories, suggesting that these tests should be run in specialized laboratories with high levels of expertise to perform testing and interpret results8. In addition, consideration should be made of the fact that aggregometry takes places in non-physiological conditions. For example, during testing, platelets in PRP or whole blood are exposed to shear forces that differ from those under in vivo conditions. Appropriate evaluation of platelet function would require laboratory investigation under conditions mimicking as closely as possible those in vivo, including blood flow and other blood cells that play crucial roles in platelet adhesion/aggregation. In this respect, flow chamber-based systems using whole blood have the potential to significantly improve the elucidation of platelet-related hemostatic mechanisms. However, these systems require special equipment and considerable technical expertise, which are often not available in non-specialized clinical laboratories9,10. Recently, the Total Thrombus System (T-TAS), a micro-automated system equipped with a disposable microchip flow-chamber has been made available. The device was designed to investigate platelet function in whole blood flowing through collagen-coated capillaries. While blood is flowing, platelets gradually adhere and aggregate to the surface of the capillary channels to form plugs. The system works with specimens under controlled flow and handling is straight forward, offering a system that could be useful to evaluate primary hemostasis11,12.
In this study, we chose to compare the diagnosis obtained using standard tests (lumi-LTA, and platelet granule content) with T-TAS in relatively large numbers of patients referred to hospital for investigation of a bleeding tendency. At the time of referral, they were suspected of having defective primary hemostasis owing to a previous history of bleeding with normal platelet count/size and traditional hemostasis tests (PT, APTT, VWF antigen and VWF ristocetin co-factor activity), and after exclusion of rare hemostasis abnormalities (i.e., FXIII or plasmin inhibitor deficiencies) and/or dysfibrinogenemia. Because patients suspected of having platelet function defects still represent a formidable diagnostic challenge for the non-specialized clinical laboratory, providing a comparative evaluation of the results for the new relatively simple T-TAS device vs more complex tests (i.e., lumi-LTA and granule content) might improve the diagnostic workup of platelet defects.
Based on the classification established by lumi-LTA and measurement of granule content, we found that T-TAS was able to identify the patients with δ-SPD, who are among the most severe forms of platelet function defects, whereas those with milder forms such as PSD were undetectable. Our study, performed on a consecutive population of patients with previous bleeding, confirms the results of previous studies on a characterized δ-SPD family13. The reasons for the failure of T-TAS to detect patients with relatively mild platelet defects is still not known. As mentioned above, T-TAS works under flow, and if one considers that flow conditions are among the most important determinants in eliciting platelet adhesion/aggregation, it is possible that modification of the flow conditions may increase the diagnostic efficacy of T-TAS towards those mild forms of platelet defects. However, the fact that T-TAS was unaffected by milder platelet defects is not surprising. A comprehensive review of the challenges faced during the laboratory investigation of patients with inherited bleeding disorders highlighted the between-assay disagreement observed for results of platelet function14.
Platelet reactivity in patients on antiplatelet drugs
Aspirin has been historically used to prevent ischemic cardiovascular events15,16. However, over the last decades, new classes of antiplatelet drugs have become available including clopidogrel, prasugrel and ticagrelor17. These drugs have different biochemical/clinical characteristics that make them suitable for personalized treatment, for example, when some patients show drug resistance18,19. However, in spite of the number of devices that have been developed and the number of evaluation studies carried out, there is still no consensus on the best device to be used and/or the optimal effective/safe residual platelet activity to be reached and maintained in patients on treatment18,20. The present study aimed to evaluate T-TAS in comparison with LTA for a small group of patients on chronic antiplatelet drugs. Taken together, test agreement between T-TAS vs LTA was 54% (14/26); K CHOEN 0.150. These results suggest that T-TAS is less effective than lumi-LTA in assessing patients’ response to antiplatelet drugs. A previous study on T-TAS showed that the device has a relatively good capability of detecting drug response in patients on double antiplatelet drugs (i.e., ASA + clopidogrel) when compared with patients on single therapy (i.e., ASA)21. The post-hoc analysis in our cohort confirmed those results. The median (min–max) T-TAS AUC of patients on double therapy (i.e., 29[3–265]) was smaller than controls and patients on single therapy (i.e., ASA 320[107–389] or clopidogrel 240[3–449]). However, the sample size was relatively small and, therefore, no conclusions can be drawn from the above results.
Some limitations of the study should be recognized. We did not apply additional laboratory tools that can be used to detect and characterize platelet defects (i.e., genotyping by means of NGS, or exome analysis). However, recent articles in the literature argue that NGS-based diagnostic strategies for causal gene identification in such a heterogeneous clinical/laboratory phenotype as primary PFD may be ineffective22, and phenotyping analyses are ultimately needed to diagnose platelet defects. Furthermore, we did not compare T-TAS and lumi-LTA in our patient population with other devices designed to assess platelets functional defects and/or response to antiplatelet drugs (i.e., PFA-100® [Siemens Healthcare, Erlangen, Germany] Verify-Now® [Werfen, Barcelona, Spain], Multiplate® [Roche Diagnostics, Indianapolis, IN, USA], and others). However, there are so many devices available that a face-to-face comparison would have been prohibitive. In spite of this, we believe that the reported results and conclusions may be of value to non-specialized clinical laboratories engaged in the daily workup of patients suspected of platelet function defects.
CONCLUSIONS
This study reports results on an investigation of platelet function in patients with normal coagulation but a bleeding history. The study also reports on a group of patients referred to the laboratory for assessment of residual platelet hyperactivity while on chronic antiplatelet therapy.
Patients were tested using the current diagnostic test (i.e., lumi-LTA and granule content) and the relatively new flow-based chip-equipped point of care device T-TAS. Results showed that T-TAS compared favorably with lumi-LTA and granule content tests in detecting the most severe forms of platelet function defects (i.e., δ-SPD), whereas it showed poor sensitivity to those milder defects (i.e., PSD). These results are not unexpected because they reflect the complexity of the mechanisms of primary hemostasis coupled with the multifaceted role played by platelets, about which much is still not known. Its relatively simplicity in handling specimens, recording relevant parameters, and interpreting results makes the T-TAS an addition to aggregometry and a useful device for laboratory workup to investigate platelet function defects.
Supplementary Information
ACKNOWLEDGMENTS
We would like to thank Professor P.M. Mannucci for helpful criticism and advice during the preparation of this manuscript. We thank L. Ghilardini for his help in preparing the figures.
Footnotes
INFORMED CONSENT: Informed consent was obtained from all individuals included in this study.
ETHICAL APPROVAL: The study was approved by the institutional review board.
AUTHORS’ CONTRIBUTION: AL and AT conceived the study, reviewed the results, and wrote the manuscript. AL managed patients and collected data. SLM and LP performed laboratory testing and helped in the statistical analysis. MB performed the statistical analysis. FP reviewed the results. All Authors reviewed the data and approved the final version of the manuscript.
DISCLOSURE OF CONFLICT OF INTEREST: AT reports speaker’s fees from Werfen, Stago and Sobi outside the submitted work. FP reports personal fees from Bioverativ, Grifols, Roche, Sanofi, Sobi, Spark, and Takeda, outside the submitted work. The other Authors have nothing to declare.
REFERENCES
- 1.Mani H, Hellis M, Lindhoff-Last E. Platelet function testing in hirudin and BAPA anticoagulated blood. Clin Chem Lab Med. 2011;49:501–507. doi: 10.1515/CCLM.2011.074. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser AFC, Endres HG, Mügge A, Neubauer H. BAPA, a synthetic dual inhibitor of Factor Xa and Thrombin, extends the storage-time to a maximum of 12 hours in ADP- and 24 hours in arachidonic acid-induced impedance aggregometry. Scand J Clin Lab Invest. 2011;71:253–256. doi: 10.3109/00365513.2011.559554. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo M. Light transmission aggregometry and ATP release for the diagnostic assessment of platelet function. Semin Thromb Hemost. 2009;35:158–167. doi: 10.1055/s-0029-1220324. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo M, Lecchi A, Zighetti ML, Lussana F. Platelet aggregation studies: autologous platelet-poor plasma inhibits platelet aggregation when added to platelet-rich plasma to normalize platelet count. Haematologica. 2007;92:694–697. doi: 10.3324/haematol.10999. [DOI] [PubMed] [Google Scholar]
- 5.Dangelmaier CA, Holmsen H. Platelet dense granule and lysosome content. In: Harker LA, Zimmerman TS, editors. Methods in hematology: measurement of platelet function. Vol. 92. Edinburgh: Churchill Livingstone; 1983. pp. 92–114. [Google Scholar]
- 6.Drummond AH, Gordon JL. Rapid sensitive microassay for platelet 5ht. Thromb Diathes Haemorrh. 1974;31:366–367. [PubMed] [Google Scholar]
- 7.Cattaneo M, Cerletti C, Harrison P, Hayward CP, Kenny D, Nugent D, et al. Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of ssc/isth. J Thromb Haemost. 2013 doi: 10.1111/jth.12231. [Online ahead of print.] [DOI] [PubMed] [Google Scholar]
- 8.Szanto T, Zetterberg E, Ramström S, Leinøe EB, Holme PA, Antovic JP, et al. Nordic Haemophilia Council. Platelet function testing: current practice among clinical centres in Northern Europe. Haemophilia. 2022;28:642–648. doi: 10.1111/hae.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savion N, Varon D. Impact--the cone and plate(let) analyzer: testing platelet function and anti-platelet drug response. Pathophysiol Haemost Thromb. 2006;35:83–88. doi: 10.1159/000093548. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji S, Sugimoto M, Miyata S, Kuwahara M, Kinoshita S, Yoshioka A. Real-time analysis of mural thrombus formation in various platelet aggregation disorders: distinct shear-dependent roles of platelet receptors and adhesive proteins under flow. Blood. 1999;94:968–975. [PubMed] [Google Scholar]
- 11.Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, et al. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost. 2011;9:2029–2037. doi: 10.1111/j.1538-7836.2011.04464.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaikita K, Hosokawa K, Dahlen JR, Tsujita K. Total Thrombus- Formation Analysis System (T-TAS): clinical application of quantitative analysis of thrombus formation in cardiovascular disease. Thromb Haemost. 2019;119:1554–1562. doi: 10.1055/s-0039-1693411. [DOI] [PubMed] [Google Scholar]
- 13.Minami H, Nogami K, Ogiwara K, Furukawa S, Hosokawa K, Shima M. Use of a microchip flow-chamber system as a screening test for platelet storage pool disease. Int J Hematol. 2015;102:157–162. doi: 10.1007/s12185-015-1819-8. [DOI] [PubMed] [Google Scholar]
- 14.Pereira J, Quiroga T, Mezzano D. Laboratory assessment of familial, non-thrombocytopenic mucocutaneous bleeding: a definitive diagnosis is often not possible. Semin Thromb Hemost. 2008;34:654–662. doi: 10.1055/s-0028-1104544. [DOI] [PubMed] [Google Scholar]
- 15.Born GV. Anti-thrombotic drugs in the treatment of coronary heart disease: the present situation with aspirin. Z Kardiol. 1990;79(Suppl 3):147–150. [PubMed] [Google Scholar]
- 16.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Meyer SF, Vanhoorelbeke K, Broos K, Salles II, Deckmyn H. Antiplatelet drugs. Br J Haematol. 2008;142:515–528. doi: 10.1111/j.1365-2141.2008.07233.x. [DOI] [PubMed] [Google Scholar]
- 18.Cattaneo M. Resistance to antiplatelet drugs: molecular mechanisms and laboratory detection. J Thromb Haemost. 2007;5(Suppl 1):230–237. doi: 10.1111/j.1538-7836.2007.02498.x. [DOI] [PubMed] [Google Scholar]
- 19.De Gregorio MG, Marcucci R, Migliorini A, Gori AM, Giusti B, Vergara R, et al. Clinical implications of “tailored” antiplatelet therapy in patients with chronic total occlusion. J Am Heart Assoc. 2020;9:e014676. doi: 10.1161/JAHA.119.014676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattaneo M. Response variability to clopidogrel: is tailored treatment, based on laboratory testing, the right solution? J Thromb Haemost. 2012;10:327–336. doi: 10.1111/j.1538-7836.2011.04602.x. [DOI] [PubMed] [Google Scholar]
- 21.Arima Y, Kaikita K, Ishii M, Ito M, Sueta D, Oimatsu Y, et al. Assessment of platelet-derived thrombogenicity with the total thrombus-formation analysis system in coronary artery disease patients receiving antiplatelet therapy. J Thromb Haemost. 2016;14:850–859. doi: 10.1111/jth.13256. [DOI] [PubMed] [Google Scholar]
- 22.Gorski MM, Lecchi A, Eti AF, La Marca S, Cairo A, Pappalardo E, et al. Complications of whole-exome sequencing for causal gene discovery in primary platelet secretion defects. Haematologica. 2019;104:2084–2090. doi: 10.3324/haematol.2018.204990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.