Abstract
Objectives
Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex, can infect cats and has proven zoonotic risks for owners. Infected cats typically present with a history of outdoor lifestyle and hunting behaviour, and cutaneous granulomas are most commonly observed. The aim of this study is to describe an outbreak of tuberculous disease commencing with six young cats, living exclusively indoors in five different households across England, being presented to separate veterinarians across the UK with a variety of clinical signs.
Methods
Investigations into the pyogranulomatous lesions, lymphadenopathy and/or pulmonary disease of these cases consistently identified infection with M bovis. Infection was confirmed by PCR, where possible, or was indicated with a positive interferon-gamma release assay (IGRA), where material for PCR was unavailable. In-contact, cohabiting cats were screened by IGRA and follow-up testing was undertaken/advised where results were positive. A lifestyle investigation was undertaken to identify the source of infection.
Results
Six clinically sick cats and seven in-contact cats were identified with evidence of M bovis infection. Five clinical cases were either too sick to treat or deteriorated despite therapy, giving a mortality rate of 83%. Lifestyle investigations revealed the common factors between clusters to be that affected cats had mycobacterial infections speciated to M bovis, were exclusively indoor cats and were fed a commercially available raw food product produced by a single manufacturer. The Food Standards Agency, Animal & Plant Health Agency, Public Health England and the food manufacturer concerned have been notified/informed. Other possible sources of exposure for these cats to M bovis were explored and were excluded, including wildlife contact, access to raw milk, the presence of rodent populations inside the buildings in which the cats lived and exposure to known infectious humans.
Conclusions and relevance
Upon investigations, our results provide compelling, if circumstantial, evidence of an association between the commercial raw diet of these cats and their M bovis infections.
Keywords: Tuberculosis, infectious disease, outbreak, raw food diet
Introduction
The increasing importance of mycobacterial infections in companion animals in the UK has become apparent in recent years.1,2 It has been demonstrated that approximately 1% of all feline biopsies submitted for histo-pathological analysis show changes consistent with mycobacteriosis (pyo/granulomatous inflammation dominated by epithelioid macrophages) and at least one-third of these have demonstrable Ziehl–Neelsen (ZN)-positive organisms when stained, with a thin rod-like appearance, indicative of mycobacteria; these are also referred to as acid- or alcohol-fast bacilli (AFB).3–5
Clinically, about a third of feline mycobacteriosis cases in the UK are caused by Mycobacterium tuberculosis complex (MTBC) pathogens, with Mycobacterium microti being cultured from 19% of all cases of feline mycobacteriosis, and a further 15% caused by Mycobacterium bovis.6,7
There is a strong geographical predisposition to feline infections with members of the MTBC. 6 M bovis infections are strongly coincident with where there are high levels of endemic infection in local bovine and wildlife populations, such as in the south-west of England.6–8 Feline MTBC infections, particularly those caused by M bovis, pose a potential zoonotic risk to owners; 9 they may also act as a potential source of environmental contamination, which is critically important, as M bovis is a pathogen of major animal health significance in the UK and other countries as it is the causative agent of bovine tuberculosis (TB). 10
In contrast to human TB, the majority of feline TB cases present with localised nodular cutaneous disease, frequently with a degree of ulceration and occasionally with draining sinus tracts.1,2,6,7,11,12 The lesions are typically distributed around the face, extremities and tail base: the so-called ‘fight and bite’ sites.1,2,6,7,11,12 Skin lesions may be accompanied by a localised or occasionally generalised lymphadenopathy, or lymphadenopathy (usually of the submandibular, prescapular or popliteal lymph nodes) may be the only presenting sign, termed an incomplete primary complex.1,2,6,7,11,12
Primary pulmonary lesions do occur in cats, but rarely.1,6 They can result from bacteria being inhaled and causing tubercle formation in the lungs and hilar lymph nodes.1,2,6,7,11,12 However, much more commonly, pulmonary disease is secondary to the putative haematogenous spread of bacteria from the site of inoculation in the skin.1,2,6,7,11,12 This generates a diffuse interstitial pattern of disease that eventually becomes bronchial, and is clinically observable as progressive dyspnoea followed eventually by a soft productive cough.1,2,6,7,11,12 Disseminated disease can cause a range of clinical signs, including hepatosplenomegaly, pleural and pericardial effusions, generalised lymphadenopathy and weight loss.1,2,6,7,11,12 Historically, TB in cats commonly presented as alimentary disease, caused by cats drinking tuberculous cow’s milk, but following the introduction of milk pasteurisation this form of TB is now seen extremely rarely.1,2,6,7,11,12
Owing to the putative transmission of M bovis and M microti to cats by hunting infected prey, TB is most frequently diagnosed in adult cats, with a history of hunting reported in almost every instance. 6 Male cats are over-represented, possibly secondary to contamination of fight-inflicted wounds. 6 The median age of infection is 3 years for M bovis and 8 years for M microti.1,2,6,7,11,12 There appears to be no link between MTBC infection and classical immunosuppression (ie, feline immunodeficiency virus [FIV] and feline leukaemia virus [FeLV] infection) in cats. 6
The diagnosis of mycobacterial disease in cats is challenging; the traditional tuberculin skin-testing technique has been shown to be ineffective in domestic cats. 13 Molecular techniques such as PCR and DNA hybridisation1,14,15 are available through human reference laboratories in the UK, but remain expensive, in some circumstances prohibitively so. These techniques also require the organism to be present within the sample being tested, and are most sensitive when performed on a cultured isolate. Where this is not possible for a cat, then an interferon-gamma (IFN-γ) release assay (IGRA) is available.16,17 This test uses specific mycobacterial proteins to stimulate peripheral immune cells in order to determine if a patient has been infected with an organism that either contains or secretes those peptides.16–18 The production of IFN-γ in response to protein purified derivative (PPD) from M bovis (PPDB), at a greater concentration than that produced in response to PPD from Mycobacterium avium (PPDA), confirms infection with an MTBC organism.16,17 A further peptide cocktail of 6 kDa early secreted antigenic target (ESAT-6) and 10 kDA culture filtrate protein (CFP-10) can be used to determine if a patient has been infected with an MTBC organism that encodes the genetic region RD-1; M tuberculosis and M bovis do encode this region, while M microti does not.19,20 Experimental challenge studies have shown that M tuberculosis does not cause clinical disease in cats, so a positive response to ESAT-6/CFP-10 and PPDB in a feline patient indicates infection with M bovis. 21 This is not the case for canine patients, which can present with disease due to M tuberculosis. 22
Contrary to the common presentation of feline TB as cutaneous lesions on non-pedigree adult cats that hunt, we recently published our findings regarding three highly unusual systemic/abdominal cases of TB caused by M bovis that had occurred in young pedigree cats with no outdoor access in two households in England.23,24 Of particular note, both of these households are located well within areas of the country deemed to be low risk for M bovis in cattle and other animal species. However, we noted that all three cats had been fed a commercially available complete raw food diet and that the epidemiological significance of this was unclear at the time. Following the publication of letters in the Veterinary Record and Veterinary Times,23,24 we have now identified and investigated a total of five households infected with M bovis involving 13 cats. Here we report on all of the households and show that the raw food diet is epidemiologically implicated as a possible source of these infections. Further to these cases, we have investigated a continually growing number of cases, which has allowed us to begin a more extensive epidemiological investigation into this outbreak that will be published separately upon its completion.
Materials and methods
Each cluster is designated by its regional geographical location, which is represented on the map (Figure 1); the locations given are not exact (county level) in order to comply with client data protection (Figure 1). Furthermore, a timetable of the key dates for the outbreak is given in Table 1.
Figure 1.
Map showing the approximate location of each of the clusters throughout England. Exact locations are withheld for client data protection purposes. The map is adapted from TBhub (https://www.tbhub.co.uk/, accessed 3 April 2019), a joint industry initiative supported by the Agriculture and Horticulture Development Board (AHDB), the Animal & Plant Health Agency (APHA), the British Cattle Veterinary Association (BCVA), the Department for Environment, Food & Rural Affairs (Defra), Landex and the National Farmers’ Union (NFU)
Table 1.
Timetable of the dates on which the first cat in each cluster was diagnosed with tuberculosis and when the relevant government authorities were alerted to our concerns
| Date (2018) | Event |
|---|---|
| 15 August | First cat in cluster 1 diagnosed by NR, Wear Referrals |
| 21 September | First cat in cluster 2 diagnosed by ED, Top Cat Veterinary Centre |
| 24 September | University of Edinburgh (DGM/COH) notify Animal & Plant Health Agency (APHA) and begin a collaborative epidemiological investigation |
| 29 September | University of Edinburgh (DGM/COH) open investigations with Public Health England |
| 10 October | University of Edinburgh (DGM/COH) contact food manufacturer with concerns |
| 29 October | First cat in cluster 3 diagnosed by RH, Wymondham Veterinary Clinic |
| 3 November | First cat in cluster 4 diagnosed by JG, City Vets |
| 14 November | First cat in cluster 5 diagnosed by SVP, Millennium Veterinary Practice |
| 11 December | Food manufacturer recalls suspect product |
Cluster 1: Durham
A 2-year-old male neutered Siamese cat was referred with lethargy, hyporexia and pyrexia (40.5°C) of 2 weeks’ duration. The cat had been acquired from a breeder at 12 weeks of age, had not received any vaccinations since the kitten course and was indoor-only with one other cat (see below). The cat had been fed only a single commercial frozen raw food diet since acquisition (Natural Instinct; https://www.naturalinstinct.com). Routine haematology and serum biochemistry (Table 2) documented a mild non-regenerative anaemia. The cat tested negative for FeLV antigen and FIV antibodies. An abdominal mass attributed to an enlarged mesenteric lymph node was palpated and surgically biopsied, with pyogranulomatous, necrotising lymphadenitis identified histopathologically.
Table 2.
Abnormalities identified on routine haematology and serum biochemistry analysis of the presenting cat in each cluster
| Analyte | Measured value | Reference interval | |
|---|---|---|---|
| Cluster 1 | Haematocrit (%) | 25.8 | 27.0–45.0 |
| Red blood cell count (× 1012/l) | 4.7 | 5.0–10.0 | |
| Neutrophil count (× 109/l) | 12.85 | 2.5–12.8 | |
| Glucose (mmol/l) | 8.1 | 4.5–8.0 | |
| Urea (mmol/l) | 15.8 | 2.8–11.0 | |
| Cluster 2 | Haematocrit (%) | 21.8 | 27.0–45.0 |
| Neutrophil count (× 109/l) | 25.00 | 1.48–10.29 | |
| Monocyte count (× 109/l) | 2.00 | 0.07–0.85 | |
| Cluster 3 | Packed cell volume (%) | 14 | 25–45 |
| Neutrophil count (× 109/l) | 14.34 | 1.48–10.29 | |
| SDMA (µg/dl) | 16 | <14 | |
| Cluster 4 | Haematocrit (%) | 25.2 | 30.3–52.3 |
| Total white blood cell count (× 109/l) | 18.16 | 2.87–17.02 | |
| Neutrophil count (× 109/l) | 13.49 | 1.48–10.29 | |
| Cluster 5 | Haematocrit (%) | 21.0 | 27.0–45.0 |
| Neutrophil count (× 109/l) | 24.32 | 1.48–10.29 |
SDMA = symmetric dimethylarginine
On presentation at the referral centre, a thin body condition was noted (body condition score [BCS] 3/9; weight 4.3 kg). The respiratory rate was elevated at 42 breaths/min, with wheezing detected on inspiration and expiration. An 8 cm firm mass was palpable in the mid-abdomen, without evidence of pain.
Repeated routine haematology and serum biochemistry (Table 2) identified marginal mature neutrophilia, mild hyperglycaemia and mildly elevated urea. A CT scan of the thorax showed a diffuse ground-glass appearance to the lung parenchyma, which also contained disseminated micronodular lesions (Figure 2). The sternal lymph node was mildly enlarged. A CT scan of the abdomen showed the enlarged and lobulated appearance of the mesenteric lymph nodes (Figure 3).
Figure 2.
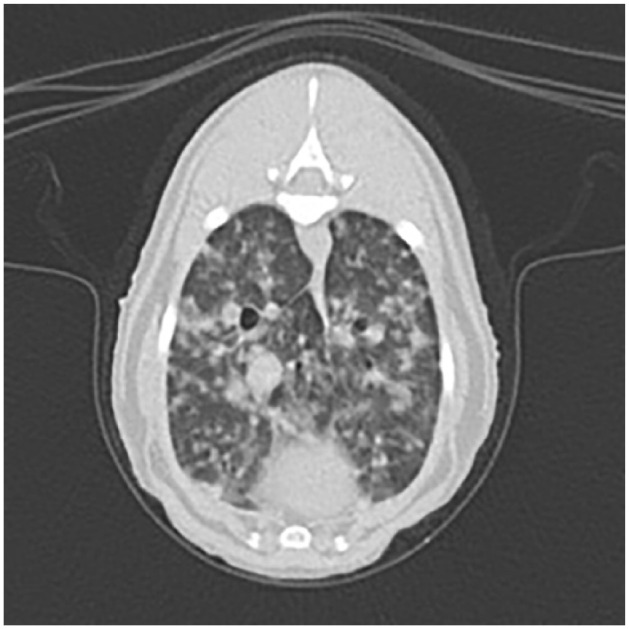
Transverse CT scan of the thorax of the first clinical case from cluster 1, showing an extensive multifocal nodular and alveolar lung pattern, consistent with mycobacteriosis
Figure 3.

Transverse CT scan of the abdomen of the first clinical case in cluster 1. The scan shows a large multinodular mass occupying approximately two-thirds of the abdominal cavity
Lymph node biopsies showed pyogranulomatous lymphadenitis, with no infectious organisms seen on ZN staining. To begin to investigate the differential diagnoses for pyogranulomatous lymphadenitis in the cat, feline coronavirus (FCoV) antibodies, serum alpha-1-acid glycoprotein and serum albumin:globulin ratio were tested; they were not suggestive of feline infectious peritonitis (FIP). Toxoplasma gondii serology was negative and Bartonella species serology was weakly positive (6; reference interval [RI] <5.5). On suspicion of mycobacterial infection, an IGRA was conducted at Biobest Laboratories and indicated infection with M bovis.
The cat began treatment with the recommended antimycobacterial triple antibiotic therapy,1,2 comprising pradofloxacin (Veraflox; Bayer, 3 mg/kg PO q24h), azithromycin (Zithromax; Pfizer, 9 mg/kg PO q24h) and rifampicin (generic, 11 mg/kg PO q24h). Two months after starting medication the abdominal mass was no longer palpable and the cat’s weight had increased to 5.12 kg. Treatment is ongoing at the time of writing.
A 2-year-old female neutered Oriental cat that resided in the same household as the case described above was referred to the same centre with a chronic intermittent cough, and a 3 week history of increased respiratory rate and effort, lethargy and hyporexia. The cat was acquired from a different breeder at the same time as the above cat, had not received any vaccinations since the kitten course and was indoor-housed only. The cat was fed exclusively on the same commercial complete raw diet as the above cat (Natural Instinct). Prior to referral, pyrexia (39.8°C) had been recorded and routine serum biochemistry had identified a significant hyperbilirubinaemia (64 μmol/l; RI 0–12 μmol/l), while haematology was unremarkable.
On presentation at the referral centre, the cat’s respiratory rate was 100 breaths/min, with bilateral moist rales on thoracic auscultation and a tracheal wheeze on auscultation of the cervical neck. A thoracic CT scan showed a marked diffuse nodular lung pattern with areas of alveolar infiltrate and an IGRA test confirmed infection with M bovis.
This cat was not amenable to medication for behavioural reasons, and the evident respiratory signs increased the risk of transmission to the owners, so this cat was euthanased. No post-mortem examination was conducted.
Cluster 2: Sussex
A 12-month-old female neutered indoor-only domestic shorthair cat was presented with a history of lethargy, hyporexia and significant weight loss (reduced by 12.5% over 4 months, BCS 2/5). This cat, and three other co-habiting cats, had been fed on the same commercial raw food (Natural Instinct) as those in cluster 1 since acquisition as kittens. On presentation, this cat was pyrexic (41.1°C) and an abdominal mass was palpable cranial to the bladder. The cat was referred for further investi-gation, which revealed mild generalised peripheral lymphadenopathy and diffuse bilateral wheezes and crackles on lung auscultation. Routine haematology and serum biochemistry (Table 2) showed non-regenerative anaemia, a mature neutrophilia and monocytosis. Serum biochemistry revealed elevated globulin levels. As for cluster 1, the differential diagnoses included FIP, a lymphoproliferative disease, mycobacterial disease or other infectious disease. The cat tested negative for FeLV antigen and FIV antibodies. A chest and abdominal CT scan revealed multifocal pulmonary nodular-like lesions, alveolar foci and partially consolidated left and right cranial lung lobes, generalised severe lymphadenopathy, a multilobulated multicavitated right cranial abdominal mass around the ileum with perilesional peritonitis, and moderate splenomegaly.
A fine-needle aspirate from the enlarged nodes revealed extensive pyogranulomatous inflammation, containing numerous AFB with typical mycobacterial morphology (Figure 4). Unstained sections were submitted to Leeds Teaching Hospital for mycobacterial PCR, which identified MTBC DNA; subsequent speciation using GenoType MTBC kit (Hain LifeScience) confirmed infection with M bovis.
Figure 4.
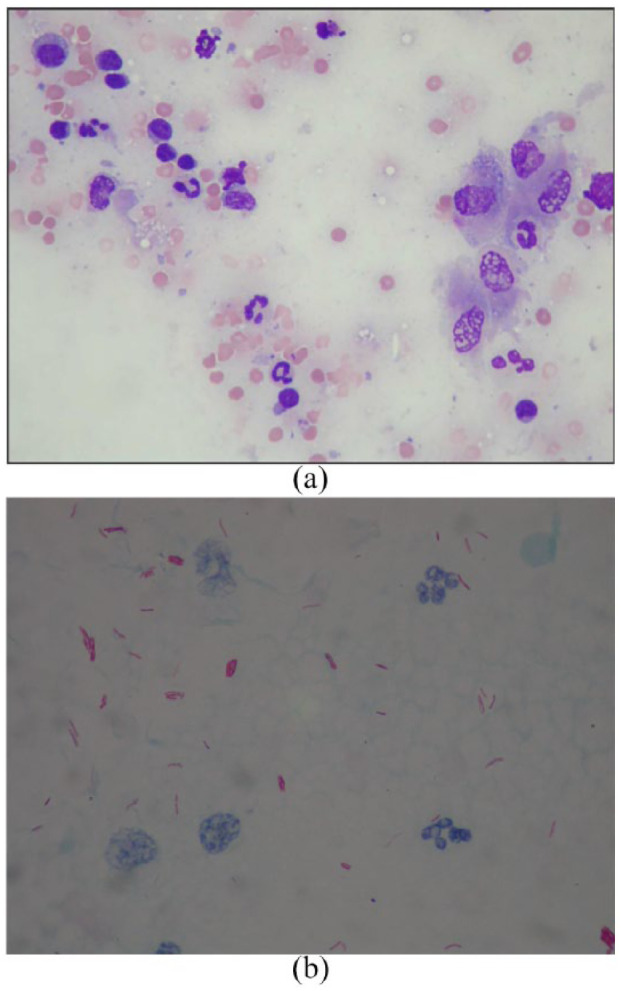
(a) Fine-needle aspirate from the inguinal lymph node of the clinically affected cat in cluster 2. The slide is stained with Wright’s stain (× 50 magnification). It shows reactive, vacuolated ‘foamy’ macrophages and mature neutrophils. (b) Ziehl–Neelsen stain of a second slide made from a fine-needle aspirate (as shown in Figure 4a) viewed under oil immersion (× 100 magnification). Similarly, the slide shows reactive, vacuolated ‘foamy’ macrophages. Throughout the slide there are positively stained (fuchsia-coloured) extracellular rod-shaped bacilli with typical mycobacterial morphology
Given the severity of disease the cat was euthanased on welfare grounds. On IGRA testing, the three cohabiting cats had results indicative of infection with M bovis. They remain clinically well, although owing to financial restrictions further investigation (eg, diagnostic imaging) has not been undertaken at the time of writing.
Cluster 3: Norfolk
A 15-month-old male neutered domestic longhair cat that had been exclusively indoor-housed since acquisition as a kitten along with a female sibling was presented. Both cats had been fed exclusively the same brand of raw food (Natural Instinct) as the cats in the other clusters from the time that they were kittens.
The cat was presented with diarrhoea and on examination had pale mucous membranes, abdominal distension, poor muscle condition and marked generalised lymphadenomegaly involving the submandibular, pre-scapular and popliteal lymph nodes, in addition to an abdominal mass assumed to be an enlarged mesenteric lymph node(s). The cat was borderline pyrexic (39.5°C). Routine haematology and serum biochemistry (Table 2) revealed anaemia, a mature neutrophilia and a mild increase in symmetric dimethylarginine. Fine-needle aspirates of the abdominal mass/lymph node showed large numbers of macrophages (Figure 5) and intracytoplasmic AFB; confirmatory mycobacterial PCR and speciation at Leeds on the remaining slide confirmed that MTBC DNA was present, but as the slide had been previously stained the DNA was of insufficient quality to differentiate between MTBC organisms (ie, M bovis or M microti). An IGRA test showed a weakly positive result suggestive of M bovis infection.
Figure 5.

Ziehl–Neelsen stain of a slide made from a fine-needle aspirate of the abdominal mass from the case in cluster 3, viewed under oil immersion (× 100 magnification) and containing high numbers of acid-fast bacilli
Treatment was attempted with pradofloxacin (Veraflox; Bayer, 3 mg/kg PO q24h), azithromycin (Zithromax; Pfizer, 9 mg/kg PO q24h) and doxycycline (generic; Summit, 10 mg/kg PO q24h). Unfortunately, the cat continued to deteriorate and was euthanased on welfare grounds. The sibling remains clinically well at the time of writing and screening by IGRA test gave a positive result for infection with a non-MTBC mycobacterial species.
Cluster 4: Devon
A 6-year-old female neutered Bengal-cross cat was presented to its primary veterinary surgeon with lethargy and hyporexia. The cat was an indoor-only cat and had been in the owner’s possession since it was 7 weeks old. This cat lived with another exclusively indoor-housed cat, a 4-year-old male neutered Abyssinian cat that had asthma; both had been fed the same commercial raw food diet (Natural Instinct) as the cats in the other clusters since they were acquired, although they were also given occasional meals of commercial cooked processed fish-based foods (Sainsbury’s Delicious Recipes 1+ Adult Cat Food Tuna in Jelly; Whiskas 1+ Years Complete Dry Cat Food with Tuna 340 g).
On presentation, the cat was pyrexic (39.9°C) with an enlarged inguinal lymph node and localised overlying cellulitis. Routine haematology (Table 2) revealed non-regenerative anaemia, leukocytosis and mature neutrophilia. The cat tested negative for FeLV antigen and FIV antibodies. Histopathology of the lesion biopsy revealed marked necrotising panniculitis containing AFB with mycobacterial morphology. Unstained sections were submitted to Leeds for mycobacterial PCR and speciation, as described above, and confirmed M bovis.
Pending these results, the necrotic area of the lesion was surgically debrided and the cat was given potentiated-amoxicillin (Clavaseptin; Vetoquinol, 18 mg/kg PO q12h) and marbofloxacin (Marbocare; Animalcare, 2 mg/kg PO q24h). Unfortunately, the wound broke down irreparably; the cat was euthanased on welfare grounds. A post-mortem examination was conducted within the Containment Level 3 facility of the Roslin Institute, University of Edinburgh; there was a surgical defect in the right inguinal area where the affected skin, fat and lymph node had been removed, as well as pathological tissue breakdown (Figure 6). On examination of the abdomen, many small white, well-demarcated hard lesions were present throughout the spleen on both the visceral and cut surfaces. Within the thorax, similar lesions were visible in the caudal lung lobes and pericardium; there was a moderate degree of pericardial effusion.
Figure 6.

(a) Post-mortem examination of a 6-year-old female neutered Bengal-cross cat (cluster 4) shows evidence of the extensive nature of surgical resection required to remove the granulomatous panniculitis present. (b) Evidence of the breakdown of the surgical wound and surrounding tissue damage, which ultimately led to the euthanasia of this patient on welfare grounds
Grossly pathological tissues were taken from the lung and spleen, homogenised in 1% collagenase solution containing 10% penicillin-G, decontaminated with 4% NaOH for 30 mins and sown onto OADC-supplemented 7H11 (Middlebrook) slopes, Löwenstein–Jensen medium with pyruvate and Stonebrink slopes in order to isolate and genotype the causative strain. After 9 weeks, small colonies were visible on the plates with the typical morphological appearance of M bovis. Initial in-house PCR testing at the University of Edinburgh (protocol unpublished, manuscript in preparation) followed by PCR performed at Leeds (as described above) confirmed the colonies to be M bovis; the Animal & Plant Health Agency (APHA) was notified and sent a second sample for independent analysis, which is pending.
The in-contact cat remains clinically well at the time of writing, but the results of a screening IGRA test indicate M bovis infection. Thoracic radiographs and abdominal ultrasound have identified no pathology. However, this cat has experienced significant recent weight loss (~12% body weight), so the owner has elected to treat it with standard antimycobacterial therapy (drug dosages as given above in cluster 1).
Cluster 5: Essex
A 15-month-old indoor-only male neutered Maine Coon cat was presented with lethargy and constipation of approximately 48 h duration. The cat had been fed exclusively on the same diet (Natural Instinct) as the cats in the other clusters since it was acquired at 12 weeks of age. Physical examination revealed pyrexia (40.0°C) and a palpable, mobile, non-painful abdominal mass in the region of the mesentery. Ultrasound examination confirmed the presence of an 8 × 13 cm mass adjacent to an enlarged mesenteric lymph node and a small amount of free fluid. Fine-needle aspirates of the mass revealed large, foamy macrophages, mesenchymal cells and histiocytic infiltration. Routine haematology and serum biochemistry (Table 2) revealed non-regenerative anaemia and marked mature neutrophilia.
An exploratory laparotomy was performed and the fluid, mass and mesenteric lymph node were resected (Figure 7) and submitted for histological examination. Large numbers of macrophages were observed and a presumptive diagnosis of FIP was made. However, further investigation to confirm FIP, including immunohistochemistry on the biopsy material, quantitative PCR for mutated FCoV on the abdominal fluid, and serum FCoV titre quantification and alpha-1-acid glycoprotein, was not compatible with FIP. During this investigation, retrospective staining of the original biopsy revealed individual AFB with mycobacterial morphology scattered in large areas of necrosis.
Figure 7.

The Maine Coon in cluster 5 had a large abdominal mass (centre) measuring 8 × 13 cm removed during an exploratory laparotomy. The local draining mesenteric lymph node (left) was also removed during surgery, as was the ~12 ml ascitic fluid (right). The fluid was tested for mutated feline coronavirus by reverse transcription quantitative PCR but was negative. Retrospective histopathological examination of the mass (centre) showed scattered mycobacteria
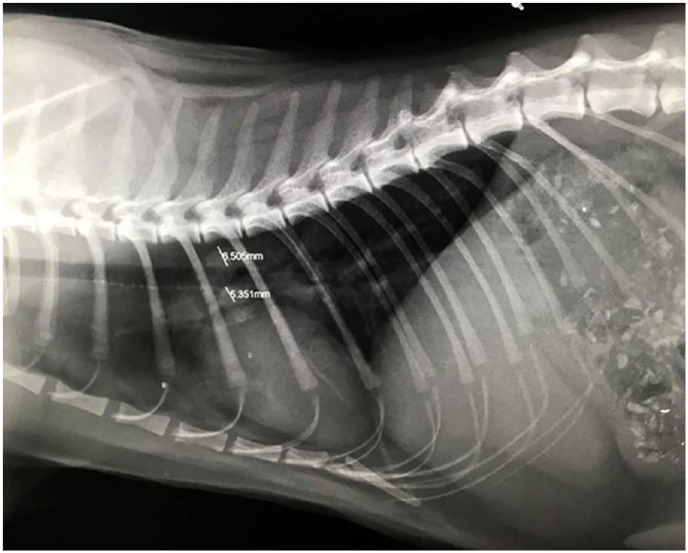
The cat improved clinically postoperatively but 6 weeks later developed a cough; thoracic radiography revealed a mass dorsal to the carina (Figure 8), while a repeat abdominal ultrasound revealed a 2 cm mid-ventral mass caudal to the spleen. Cytology of the abdominal mass again revealed macrophage infiltration with intracellular non-staining rods present. Cytology with ZN staining revealed AFB, initial PCR testing at the University of Edinburgh (as described above) indicated infection with M bovis and the remaining slides were submitted for mycobacterial PCR. Analysis at Leeds was unable to isolate sufficient mycobacterial DNA for definitive speciation. IGRA screening tests on the two cohabiting domestic shorthair cats, which ate mainly commercial dry food, separately from the Maine Coon cat, showed positive responses indicative of M bovis infection, although they were clinically well.
Figure 8.
Left laterolateral radiograph of the thorax of the Maine Coon cat from cluster 5. There is a mass (total diameter 1.2 cm) at the level of the carina. A cough had become clinically evident at the time this radiograph was taken. It resolved within 3 days of antimycobacterial triple-antibiotic therapy
The Maine Coon was treated with the recommended antimycobacterial triple-antibiotic therapy,1,2 comprising marbofloxacin (Marbocyl; Vetquinol, 2 mg/kg PO q24h), azithromycin (Zithromax; Pfizer, 9 mg/kg PO q24h) and rifampicin (generic, 11 mg/kg PO q24h). Two months after starting medication the abdominal mass was no longer palpable and the cat’s weight had increased. However, following owner-led cessation of therapy at this stage, the cat subsequently relapsed and was euthanased on welfare grounds and has been submitted to APHA officials for post-mortem examination, the results of which are pending at the time of writing. Owing to the owner being immunocompromised, the two in-contact IGRA-positive cats were also euthanased.
Follow-up investigations
The current legislation applicable to England (The Tuberculosis [England] Order 2014) Article 6 25 states that suspicion of TB in the carcase of any pet mammal is notifiable, and as such the Secretary of State has been duly notified of all of the cases presented here via the APHA. However, there is no statutory obligation for investigation in non-bovine animals and so current investigations have been led by the University of Edinburgh and largely financed, where conducted, by the university and/or owners.
Public Health England (PHE) has similarly been informed of the cases and all owners have been offered the opportunity to discuss the zoonotic aspects that these cases present should they wish to do so with PHE. PHE is currently adopting a precautionary public health approach similar to that which has been described previously. 26 The owners of two of the affected cats were found to be IGRA and Mantoux (tuberculin skin test) positive, respectively; one has required the instigation of antituberculosis medical therapy.
Potential sources of exposure to M bovis were explored with the owners; all except the raw commercial food were excluded. Excluded sources included wildlife contact (all cats were solely indoor cats), the presence of rodent populations inside the buildings in which the cats lived, access to raw milk and exposure to any known infectious humans.
As the only common factors between all clusters are that they were diagnosed with the same infection (M bovis), were exclusively indoor cats and were fed a commercially available raw food product produced by a single manufacturer (Natural Instinct), the Food Standards Agency (FSA) has similarly been informed and is investigating appropriately.
The authors at the University of Edinburgh have been sent detailed batch information and photographs and/or actual samples of the diet from 3/5 clusters, as was being fed to the cats at the time the diagnoses were made (Figure 9). Notably, all three of these samples were the venison product produced by the same company. On questioning the owners, the venison product from the single manufacturer comprised ~80% of the ration of one cat (cluster 4), the sole intake of another (cluster 5) and the ‘vast majority’ of the diet of a third (cluster 2). The remaining two clusters (clusters 1 and 3) were fed a variety of the products from the same company, including the venison product.
Figure 9.
For three of the five clusters, owners had retained the food product that the affected cats were being fed at the time that they became ill. (a) One owner sent photos taken by themselves; (b,c) the other two owners sent the food to the University of Edinburgh by refrigerated courier and the photos were taken inside the Containment Level 3 facility prior to further testing
As such, the evidence we have collected during our investigations of these cases provides compelling, if only circumstantial, evidence of an association between the diet of these cats and their M bovis infections.
The company was alerted, and an internal investigation by it led to the voluntary withdrawal of the venison version of their food from sale, ‘because some of the ingredients were not inspected in line with EU requirements. The absence of inspection means the safety of the product cannot be confirmed and may therefore carry a potential risk’. The batches withdrawn are those dated as best before between March 2019 and August 2019, and pre-date those submitted for testing at the University of Edinburgh. The FSA is now undertaking this area of the outbreak investigation independently.
Discussion
This outbreak currently includes 13 cats, in five separate clusters, that were found to be infected with M bovis; six cats were clinically unwell at diagnosis (five of these are now dead), while seven were found to be positive by IGRA (ie, they had evidence that they have been infected with M bovis), although how many of these latter cats are likely to become ill is unclear. Three have not been assessed further by their veterinarian (cluster 2), and one (cluster 4) has already lost a great deal of weight, potentially related to (this cat has been treated for M bovis infection and has since recovered all its previously lost weight). While still circumstantial, the only probable source of the infection is that the cats were all exposed to M bovis via the same commercially available raw food. New cases are still occurring and further data collection is in progress at the time of writing, but the authors intend to make readers aware of the details and the scale of this problem as soon as practically possible. The cases reported here appear to be limited to cats that have been fed this feline-specific food (ie, no dogs have yet been affected) and no owners are self-reporting as unwell.
The feeding of commercial raw meat-based diets (RMBDs) to companion animals has increased substantially in popularity in recent years, not just in the UK, but globally.27,28 In the USA, sales of RMBDs doubled in the 5 years to October 2017 and have expanded by 15.9% in the past year alone, making the industry worth an estimated US$195 million to the US economy. 29 In Europe, a study from the Netherlands found that 51% of dog owners fed their animals either completely or partially an RMBD. 30 A similar increase has been seen in the UK, with what were only a handful of companies registered as producers of RMBDs a few years ago having increased to a current excess of 80 registered with the Department for Environment, Food & Rural Affairs. 27 Shapiro et al 31 found in a survey of Australian cat breeders that raw meat was fed as an integral constituent of the diet by 89% of respondents. The reasons behind so many people adopting RMBD feeding are numerous and complex. Some owners feel that it is physiologically more ‘natural’ for their pet to consume food similar to that they would have eaten at an earlier stage in their evolution (ie, pre-domestication).31–33 There have been speculative suggestions that RMBD feeding may be beneficial in preventing the onset of dental disease, food-responsive inflammatory bowel disease and atopy,31–33 and a recent paper has shown that the microbiome of dogs fed RMBDs is more diverse than when fed processed diets. 34
The question of what the veterinary profession should be advising clients with respect to RMBDs is highly emotive on both sides of the debate, and the evidence for and against is frequently either low quality or simply anecdotal reports with a lack of rigorous, properly controlled trials.31–35 There is, however, consensus that home-prepared RMBDs should be generally avoided as it is extremely difficult to make these nutritionally balanced. 35 This concern has been overcome by the introduction of commercially available complete RMBDs that comply with the strict legislative requirements, overseen by the European Pet Food Industry Federation, to ensure nutritional and energy requirements are met if any given product was provided as the sole ration for the lifetime of the animal in question.36–38
Unfortunately, statutory regulations governing RMBDs only relate to the presence or absence of a limited number of infectious organisms. 39 It is one of the few areas regarding RMBDs on which there is agreement: that there is a significant increase in the risk of infectious diseases to both pets and owners through the consumption and handling of such products on a regular basis.40–46
Almost all RMBDs are sold frozen in order to extend their expiry dates and, in the case of some organisms (eg, nematodes such as Trichinella species), reduce or eliminate contamination. 47 In studies of the zoonotic infectious agents present within thawed RMBDs, Salmonella species have received the most attention.44–46 Although subclinical infections occur frequently in animals, Salmonella species can cause gastroenteritis and even septicaemia.48–50 For example, Stiver et al reported fatal septicaemic salmonellosis after RMBD feeding in two cats. 51 Other studies have successfully recovered a variety of viable zoonotic pathogens, including Escherichia coli serotype O157:H7, Listeria monocytogenes, Sarcocystis species, Campylobacter species and T gondii, from RMBDs.44–46,52 At least one such RMBD contaminant, Shiga toxin-releasing E coli, has been linked to human deaths in the UK, and PHE is attempting to educate owners about the risks associated with RMBDs. 53 In the USA, raw cat food has similarly had to be recalled due to contamination with L monocytogenes. 54
At this stage, as cases are continuing to be diagnosed, there is insufficient evidence to ascertain if this is a single, one-off event or an ongoing problem. However, in either instance this outbreak raises concerns over the strength of current regulations with regard to meat inspection procedures and may necessitate an increase in trained veterinary meat inspection of a carcase before it is passed as fit for animal consumption. With particular reference to wild venison, one such development could be that the entire gralloch (all viscera) be brought to the slaughterhouse for veterinary inspection.
Our study is the first to report infection of companion animals with an MTBC pathogen, which appears to be associated with feeding an RMBD, with 13 cats in five households infected, as identified to date. Not only does this imply that the owners were handling contaminated material, but the gastrointestinal presentation means that the cats may have been shedding M bovis into their home environment, creating the potential for even greater exposure to owners. Of further concern, the Pet Food Manufacturers’ Association guidance to owners handling RMBDs is that they should clean surfaces, utensils and animal food bowls with ‘soap and hot water’. 55 Pasteurisation requires milk to be heated to 71.5°C and held at that temperature for a minimum of 15 s to inactivate M bovis; 56 it is highly unlikely that owners would be able to achieve this in a domestic setting, so M bovis is very likely to be able to persist on kitchen surfaces even after the owners feel that the surfaces have been ‘cleaned’. Furthermore, M bovis is able to survive for at least 60 days in standing water, 57 such as might be found in or around a kitchen sink, over which time further organisms may be added with each prepared meal, making it highly plausible that the small dose needed to infect a human could be reached.
The severity of clinical disease that was seen in the cats we report here is much greater than we would typically expect to see through infections acquired from hunting infected rodents. The clinical signs of pyrexia, anaemia and leukocytosis (caused by a mature neutrophilia) are not typically present in feline TB cases and the young age of many of the cats affected is also striking, as is the fact that the cats were indoor only, and not from areas of England known to have endemic M bovis in rodents, badgers, other wildlife and cattle.58–60 These factors may mislead clinicians presented with future cases to not consider TB as a potential differential diagnosis, delaying the time until correct diagnosis and thus exposing owners to possible infection for extended periods.
Many of the cats presented in these clusters were either too sick to attempt treatment, or clinically deteriorated despite attempted therapies, giving a case fatality rate, to date, of 83% compared with our more usual 70–80% successful treatment rate. 2 The severity of infection was similar to that which we previously reported in an outbreak of M bovis TB in a pack of working Foxhounds, where it was concluded that the source of infection was most likely the feeding of contaminated raw meat. 61 That outbreak resulted in a higher-than-typical clinical attack rate for TB (approximately 10% of the group) and when the hounds started to display clinical signs, they deteriorated so rapidly that euthanasia was required on welfare grounds within hours to days. 61 Additionally, feline abdominal M bovis infections acquired in a nosocomial setting and cats with disseminated M bovis infection that appeared to have followed extensive ingestion also displayed rapid clinical progression and severe signs such that euthanasia on welfare grounds was quickly required.62,63
The parallels between these fulminant outbreaks suggest that there may be an underlying difference in the immunopathogenesis of the disease, whereby gastrointestinal/peritoneal challenge of companion animals with M bovis results in more severe disease that is less amenable to treatment. Thus there may be implications for the clinical management of gastrointestinal TB cases in comparison with the more typical dermal presentation. Alternatively, or additionally, it may be that strains of M bovis that are capable of establishing infections in companion animals following gastrointestinal/peritoneal challenge may have or utilise additional virulence factors which make these infections clinically more aggressive. The latter scenario would have profound implications as regards the zoonotic risk to owners if they were being exposed to M bovis with inherently greater virulence.
The IGRA was used to detect M bovis infection in all six of the unwell and a further six of the in-contact cats. However, one in-contact cat (cluster 3) was IGRA tested and found to have a greater response to PPDA than PPDB (ie, a pattern not consistent with that expected of M bovis infection). It may be that this cat has not been challenged with M bovis but has coincidentally and/or previously been sensitised to antigens of the M avium–intracellulare complex. However, studies in calves sensitised to environmental mycobacteria and infected with M bovis have concluded that M bovis infection may be concealed for some time in this situation. 64 It is therefore possible that this cat may develop a different response (ie, that typical of M bovis) if it were to be retested in the future.
Work by the team at the University of Edinburgh is ongoing to develop a rapid PCR assay, which we hope can be performed on tissue lysates, (unstained) cytology slides and formalin-fixed biopsy tissues. Where this was used in these cases it proved extremely useful; however, its use outwith a research capacity will require more extensive evaluation before this is made available to clinicians. Similarly, the team is continuing to work with Biobest Laboratories to validate the use of the feline IGRA in the detection and monitoring of TB in cats.
Urgent work is now needed to establish the extent of any contamination of the RMBD pet food chain in the UK, and a full epidemiological investigation is under way to establish where lessons can be learned in order to safeguard animals being fed RMBDs.
Management of suspect cases
The team at the University of Edinburgh is always keen to hear from clinicians who may have, or have had, suspicions about similar cases, and can offer our experience in both diagnosing and managing these patients. Currently, we are advising that animals known to have been exposed to the recalled food, or which have been exposed to a confirmed case but are displaying no clinical signs, should be tested by IGRA at least 4 weeks after the last known exposure. If these tests are positive then diagnostic imaging should be undertaken to assess for structural (ie, active) disease (full-body CT or radiographs and, ideally, abdominal ultrasonography).
Where present, active disease should be treated appropriately, as outlined in O’Halloran and Gunn-Moore. 1 There is currently no evidence on which to base recommendations for the appropriate course of action for animals that are IGRA test positive but lack evidence of structural disease. It may be appropriate in these cases to closely monitor body weight, BCS and resting respiratory rate, and to investigate further if these decline. Alternatively, some owners may elect to give prophylactic therapy; isoniazid (which is occasionally used in humans for this purpose) can be used for 6 months but experience of its use in cats is limited and toxicities may occur. Instead, some owners may choose to treat using the triple combination antituberculosis therapy for 3 months, given that the use of this protocol is well established and potential side effects are well documented.
Conclusions
This report follows our initial alert regarding an ongoing outbreak of M bovis TB in pet cats across the UK. It describes six clinically sick cats and seven in-contact cats with a mortality rate of 83%. Lifestyle investigation revealed the common factors between clusters were that the cats were exclusively indoor only and fed a commercially available raw food product produced by a single manufacturer. The FSA, APHA, PHE and the food manufacturer concerned have been notified/informed. Our results provide compelling, if circumstantial, evidence of an association between the commercial raw diet of these cats and their M bovis infections.
Acknowledgments
We wish to thank all of the veterinarians and owners of these cats for facilitating the investigation and giving permission for the findings to be published.
Footnotes
Accepted: 11 April 2019
Addendum: Since submitting this article in November 2018, the outbreak has continued across the UK. The number of cases has now increased to include at least 30 clusters involving >90 cats. Information about the continuing outbreak will be published as soon as is practically possible.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Conor O’Halloran is supported by a Biotechnology and Biological Sciences Research Council (BBSRC) studentship (BB/M014894/1). Jordan Mitchell is supported by a BBSRC studentship (BB/M010996/1). Jayne Hope is funded by BBSRC Institute Strategic Programme funding (BB/P013740/1 & BBS/E/D/20002174).
ORCID iD: Conor O’Halloran  https://orcid.org/0000-0002-4921-2907
https://orcid.org/0000-0002-4921-2907
Olympia Ioannidi  https://orcid.org/0000-0003-0274-8373
https://orcid.org/0000-0003-0274-8373
Jordan Mitchell  https://orcid.org/0000-0001-6755-3214
https://orcid.org/0000-0001-6755-3214
References
- 1. O’Halloran C, Gunn-Moore D. Mycobacteria in cats: an update. In Pract 2017; 39: 399–406. [Google Scholar]
- 2. O’Halloran C, Dobromylskyj M. Clinical mycobacterial diseases of companion animals: part 2. Management of companion animal mycobacteriosis. Comp Anim 2017; 22: 652–657. [Google Scholar]
- 3. Gunn-Moore DA, Gaunt C, Shaw DJ. Incidence of mycobacterial infections in cats in Great Britain: estimate from feline tissue samples submitted to diagnostic laboratories. Transbound Emerg Dis 2013; 60: 338–344. [DOI] [PubMed] [Google Scholar]
- 4. O’Brien CR, Malik R, Globan M, et al. Feline leprosy due to Candidatus ‘Mycobacterium tarwinense’: further clinical and molecular characterisation of 15 previously reported cases and an additional 27 cases. J Feline Med Surg 2017; 19: 498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunn-Moore DA, McFarland SE, Schock A, et al. Mycobacterial disease in a population of 339 cats in Great Britain: II. Histopathology of 225 cases, and treatment and outcome of 184 cases. J Feline Med Surg 2011; 13: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunn-Moore DA, McFarland SE, Brewer JI, et al. Mycobacterial disease in cats in Great Britain: I. Culture results, geographical distribution and clinical presentation of 339 cases. J Feline Med Surg 2011; 13: 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gunn-Moore DA. Feline mycobacterial infections. Vet J 2014; 201: 230–238. [DOI] [PubMed] [Google Scholar]
- 8. Pesciaroli M, Alvarez J, Boniotti MB, et al. Tuberculosis in domestic animal species. Res Vet Sci 2014; 97 Suppl: S78–S85. [DOI] [PubMed] [Google Scholar]
- 9. Roberts T, O’Connor C, Nuñez-Garcia J, et al. Unusual cluster of Mycobacterium bovis infection in cats. Vet Rec 2014; 174: 326. DOI: 10.1136.vr.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollock JM, Neill SD. Mycobacterium bovis infection and tuberculosis in cattle. Vet J 2002; 163: 115–127. [DOI] [PubMed] [Google Scholar]
- 11. Laprie C, Duboy J, Malik R, et al. Feline cutaneous mycobacteriosis: a review of clinical, pathological and molecular characterization of one case of Mycobacterium microti skin infection and nine cases of feline leprosy syndrome from France and New Caledonia. Vet Dermatol 2013; 24: 561–569. [DOI] [PubMed] [Google Scholar]
- 12. Gibbens N. Mycobacterium bovis infection in cats. Vet Rec 2014; 174: 331–332. [DOI] [PubMed] [Google Scholar]
- 13. Broughan JM, Downs SH, Crawshaw TR, et al. Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 2: a review of diagnostic methods. Vet J 2013; 198: 346–351. [DOI] [PubMed] [Google Scholar]
- 14. Warren RM, Gey van Pittius NC, Barnard M, et al. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int J Tuberc Lung Dis 2006; 10: 818–822. [PubMed] [Google Scholar]
- 15. Hussain A, et al. Comparison of Fastsure TB DNA and MGIT 960 for the detection of Mycobacterium tuberculosis complex in clinical specimens. Pak Armed Forces Med J 2013; 63: 26–28. [Google Scholar]
- 16. Rhodes SG, et al. Adaptation of IFN-gamma ELISA and ELISPOT tests for feline tuberculosis. Vet Immunol Immunopathol 2008; 124: 379–384. [DOI] [PubMed] [Google Scholar]
- 17. Rhodes SG, Gruffydd-Jones T, Gunn-Moore D, et al. Interferon-γ test for feline tuberculosis. Vet Rec 2008; 162: 453–455. [DOI] [PubMed] [Google Scholar]
- 18. Adams LG, et al. In vivo and in vitro diagnosis of Mycobacterium bovis infection. Rev Sci Tech 2001; 20: 304–324. [DOI] [PubMed] [Google Scholar]
- 19. Mostowy S, Cousins D, Behr MA. Genomic interrogation of the dassie bacillus reveals it as a unique RD1 mutant within the Mycobacterium tuberculosis complex. J Bacteriol 2004; 186: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith NH, Crawshaw T, Parry J, et al. Mycobacterium microti: more diverse than previously thought. J Clin Microbiol 2009; 47: 2551–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jennings AR. The distribution of tuberculous lesions in the dog and cat with reference to the pathogenesis. Vet Rec 1949; 61: 380–384. [Google Scholar]
- 22. Parsons SDC, Warren RM, Ottenhoff TH, et al. Detection of Mycobacterium tuberculosis infection in dogs in a high-risk setting. Res Vet Sci 2012; 92: 414–419. [DOI] [PubMed] [Google Scholar]
- 23. O’Halloran C, Gunn-Moore D, Reed N, et al. Mycobacterium bovis in pet cats. Vet Rec 2018; 183: 510. [DOI] [PubMed] [Google Scholar]
- 24. Anon. Investigations under way in wake of feline TB outbreak. https://www.vettimes.co.uk/news/investigations-under-way-in-wake-of-feline-tb-outbreak/ (2018, accessed April 19, 2019).
- 25. The Tuberculosis (England) Order 2014 . http://www.legislation.gov.uk/uksi/2014/2383/made (2014, accessed November 6, 2018).
- 26. Phipps E, et al. Bovine tuberculosis in working foxhounds: lessons learned from a complex public health investigation. Epidemiol Infect. Epub ahead of print 9 October 2018. DOI: 10.1017/S0950268818002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Waters A. Raw diets: are we at a turning point? Vet Rec 2017; 181: 384. DOI: 10.1136/vr.j4709. [DOI] [Google Scholar]
- 28. Finley R, et al. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin Infect Dis 2006; 42: 686–691. [DOI] [PubMed] [Google Scholar]
- 29. Sprinkle D. US pet food market to reach US$27 billion in 2018. https://www.petfoodindustry.com/articles/6826-us-pet-food-market-to-reach-us27-billion-in-2018?v=preview (2017, accessed November 6, 2018).
- 30. Corbee RJ, Breed RD, Hazewinkel HAW. Feeding practice of dog owners active on internet forums. Poster session presented at the 17th European Society of Veterinary and Comparative Nutrition Congress; 2013. Sept 19–21; Ghent, Belgium. [Google Scholar]
- 31. Shapiro AJ, Norris JM, Bosward KL, et al. Q fever (Coxiella burnetii) knowledge and attitudes of Australian cat breeders and their husbandry practices. Zoonoses Public Health 2017; 64: 252–261. [DOI] [PubMed] [Google Scholar]
- 32. Handl S. The “BARF” trend – advantages, drawbacks and risks. Vet Focus 2014; 24: 16–23. [Google Scholar]
- 33. Schlesinger D, Joffe D. Raw food diets in companion animals: a critical review. Can Vet J 2011; 52: 50–54. [PMC free article] [PubMed] [Google Scholar]
- 34. Sandri M, Dal Monego S, Conte G, et al. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet Res 2017; 13: 65. DOI: 10.1186/s12917-017-0981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weeth LP. Home-prepared diets for dogs and cats. Vetlearn 2012; 35. [PubMed] [Google Scholar]
- 36. EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J 2015; 13: 4329. [Google Scholar]
- 37. European Commission. EC Regulation 1069/2009 . https://ec.europa.eu/food/safety/animal-by-products_en (accessed November 6, 2018).
- 38. Pet Food Association: legislation . https://www.pfma.org.uk/uk-pet-food-legislation (accessed November 6, 2018).
- 39. Commission of the European Communities. Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. https://eur-lex.europa.eu/eli/reg/2005/2073/oj (2005, accessed April 19, 2019).
- 40. Bojanić K, Midwinter AC, Marshall JC, et al. Isolation of Campylobacter spp. from client-owned dogs and cats, and retail raw meat pet food in the Manawatu, New Zealand. Zoonoses Public Health 2017; 64: 438–449. [DOI] [PubMed] [Google Scholar]
- 41. Baede VO, Broens EM, Spaninks MP, et al. Raw pet food as a risk factor for shedding of extended-spectrum beta-lactamase-producing Enterobacteriaceae in household cats. PLoS One 2017; 12: e0187239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stogdale L and Diehl G, et al. Just how pathogenic to pets and humans are the bacteria in the concentrations found in raw pet foods [multiple letters]. Can Vet J 2005; 46: 967–971. [PMC free article] [PubMed] [Google Scholar]
- 43. Anon. Study finds listeria in raw pet foods. JAVMA News. https://www.avma.org/News/JAVMANews/Pages/141015j.aspx (2014, accessed May 1, 2019). [PubMed]
- 44. Anon. Raw feeding of pets: safe and nutritious – or reckless and irresponsible? Vet Rec 2017; 181: 386–387. [DOI] [PubMed] [Google Scholar]
- 45. Finley R, et al. The occurrence and antimicrobial susceptibility of salmonellae isolated from commercially available canine raw food diets in three Canadian cities. Zoonoses Public Health 2008; 55: 462–469. [DOI] [PubMed] [Google Scholar]
- 46. Nemser S, Doran T, Grabenstein M, et al. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog Dis 2014; 11: 706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Centers for Disease Controls and Prevention. Parasitology – Trichinellosis. https://www.cdc.gov/parasites/trichinellosis/prevent.html (accessed November 6, 2018).
- 48. Tauni MA, Osterlund A. Outbreak of Salmonella typhimurium in cats and humans associated with infection in wild birds. J Small Anim Pract 2000; 41: 339–341. [DOI] [PubMed] [Google Scholar]
- 49. Wall PG, Davis S, Threlfall EJ, et al. Chronic carriage of multidrug resistant Salmonella typhimurium in a cat. J Small Anim Pract 1995; 36: 279–281. [DOI] [PubMed] [Google Scholar]
- 50. Fauth E, Freeman LM, Cornjeo L, et al. Salmonella bacteriuria in a cat fed a Salmonella-contaminated diet. J Am Vet Med Assoc 2015; 247: 525–530. [DOI] [PubMed] [Google Scholar]
- 51. Stiver SL, Frazier KS, Mauel MJ, et al. Septicemic salmonellosis in two cats fed a raw-meat diet. J Am Anim Hosp Assoc 2003; 39: 538–542. [DOI] [PubMed] [Google Scholar]
- 52. Tenter AM. Toxoplasma gondii: from animals to humans. Int J Parastiol 2000; 30: 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Limb M. Human deaths linked to raw pet food. Vet Rec 2018; 183: 519. [Google Scholar]
- 54. Quality Assurance & Food Safety. Raw pet food recalled due to Listeria, E. Coli contamination concerns. https://www.qualityassurancemag.com/article/radagast-food-recalled-listeria-e-coli/ (accessed November 6, 2018).
- 55. Pet Food Manufacturers’ Association. Raw feeding factsheet. https://www.pfma.org.uk/raw-feeding-factsheet (accessed November 6, 2018).
- 56. The Dairy Products (Hygiene) Regulations 1995. http://www.legislation.gov.uk/uksi/1995/1086/made (1995, accessed April 19, 2019).
- 57. Vaerewijck MJM, Huys G, Palomino JC, et al. Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol Rev 2005; 29: 911–934. [DOI] [PubMed] [Google Scholar]
- 58. Jenkins HE, Morrison WI, Cox DR, et al. The prevalence, distribution and severity of detectable pathological lesions in badgers naturally infected with Mycobacterium bovis. Epidemiol Infect 2008; 136: 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anon. Online map shows TB breakdowns. Vet Rec 2015; 177: 32. [DOI] [PubMed] [Google Scholar]
- 60. Cavanagh R, Begon M, Bennett M, et al. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J Clin Microbiol 2002; 40: 3281–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O’Halloran C, Hope JC, Dobromylskyj M, et al. An outbreak of tuberculosis due to Mycobacterium bovis infection in a pack of English Foxhounds (2016–2017). Transbound Emerg Dis 2018; 65: 1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murray A, Dineen A, Kelly P, et al. Nosocomial spread of Mycobacterium bovis in domestic cats. J Feline Med Surg 2015; 17: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Černá P, O’Halloran C, Sjatkovska JO, et al. Outbreak of tuberculosis caused by Mycobacterium bovis in a cattery of Abyssinian cats in Italy. Transbound Emerg Dis 2019; 66: 250–258. [DOI] [PubMed] [Google Scholar]
- 64. Amadori M, Tagliabue S, Lauzi S, et al. Diagnosis of Mycobacterium bovis infection in calves sensitized by mycobacteria of the avium/intracellulare group. J Vet Med B Infect Dis Vet Public Health 2002; 49: 89–96. [DOI] [PubMed] [Google Scholar]