Abstract
Objectives
The aim of this study was to evaluate the results of serum allergen-specific IgE testing in cats with a clinical diagnosis of asthma and to determine if the number of allergens with positive IgE reactivity and magnitude of positive IgE responses would be associated with the severity of clinical signs or airway eosinophilia.
Methods
Medical records from 2008 to 2018 were retrospectively reviewed. Inclusion required a diagnosis of feline asthma based on consistent clinicopathologic features and bronchoalveolar lavage (BAL) cytology with >10% eosinophils; additionally, cats needed to have the results of serum allergen-specific IgE tests.
Results
Eighteen cases satisfied the inclusion criteria. Median age was 5 years and the most common presenting clinical sign was cough (n = 10/18). Most cats lived exclusively indoors (n = 13/18). The median percentage of BAL eosinophils was 47%. Serum allergen-specific IgE testing supported an underlying allergic etiology in 14/18 (78%) cats, with all but one having polysensitization. The severity of clinical signs and magnitude of airway eosinophilia did not correlate with the degree of positive IgE reactivity.
Conclusions and relevance
This study identified a strong association between the identification of allergen-specific IgE and cats with asthma, and the majority of these cats were polysensitized. However, larger numbers of allergens with positive IgE reactivity or magnitude of IgE reactivity were not significantly associated with clinical severity or airway eosinophilia. Knowledge of positive allergen-specific IgE results could guide allergen avoidance, regardless of the magnitude of IgE reactivity.
Keywords: Asthma, allergy, mucosal immunity, airway eosinophilia, allergen-specific IgE, bronchoalveolar lavage, inflammatory airway disease
Introduction
A suspected diagnosis of feline asthma, a common and serious respiratory disease, should prompt further investigation for an underlying cause as a means to advance management strategies. Triggers for asthma that have been documented in humans include pharmacologic products (eg, aspirin), environmental/air pollutants, exercise, emotional stress, infections and, most commonly, allergens.1,2 Allergic asthma is driven by an allergen-specific T helper 2 response with elaboration of cytokines, production of IgE and activation of eosinophils.1,3 Collectively, these immune changes result in recurring clinical signs, airway eosinophilia, airway hyper-responsiveness and airway remodeling.4,5 In pet cats, aeroallergens remain the best documented stimuli underpinning the asthmatic response.4,6–8 While there is ample evidence for aeroallergens as a trigger for feline asthma, no studies have evaluated cats with a clinical diagnosis of asthma to determine how commonly they have increases in serum allergen-specific IgE, a biomarker of allergen sensitization, or to determine if the magnitude of IgE reactivity corresponds to a more severe asthmatic phenotype or airway eosinophilia.
Recognition of increased allergen-specific IgE antibodies may have diagnostic and therapeutic implications in cats. Allergen-specific IgE testing has been used to document an allergic component, as well as to guide allergen avoidance and even select appropriate allergens for immunotherapy.9–11 Intradermal testing is considered to be the criterion standard for allergy testing in veterinary medicine, with a greater sensitivity in identifying allergens. 9 However, there is less research supporting intradermal testing in feline patients and it is still subject to false negatives and false positives. Intradermal testing also requires sedation, shaving a large portion of skin for injections and stocking of relevant allergens. Additionally, it is affected by concurrent administration of glucocorticoids. 12 For these reasons, intradermal testing is not commonly performed in general practice. In comparison, allergen-specific serum IgE has the advantage of requiring only a blood sample that is shipped to an outside laboratory, and is minimally affected by concurrent glucocorticoid therapy. 13 Though intradermal testing has a greater sensitivity, serum allergen-specific IgE determination is highly specific 9 and may be ideal for identifying clinically relevant allergens in asthmatic cats. However, it is important to consider that not all commercial serum allergen-specific IgE panels are created equally; one study showed that the enzyme immunometric assay provided unreliable results. 9
Clinically, diagnosis of asthma relies on a comprehensive approach, including an appropriate history, key clinicopathologic features, testing to rule out other disease mimics such as heartworm-associated respiratory disease, lungworms or chronic bronchitis, and documenting airway eosinophilia.1,14 Median age at diagnosis is 4–5 years, and the most common clinical signs include cough, wheeze or respiratory distress. A complete blood count (CBC) and biochemistry profile are often unremarkable, although a peripheral eosinophilia is identified in approximately 20% of affected asthmatic cats.14–17 Thoracic radiography frequently demonstrates a bronchial or bronchointerstitial pattern; approximately a quarter of cats can have normal thoracic radiographs. 14 Bronchoalveolar airway lavage (BAL) reveals eosinophilia with cytologic absence of infectious organisms. Once a clinical diagnosis of asthma is suspected, a positive response to glucocorticoid therapy is often used to support the diagnosis of feline asthma. However, it must be understood that many diseases mentioned previously that mimic asthma may also respond to glucocorticoids; so, while practical, response to therapy is an imperfect metric of diagnosis.
In asthmatic cats, an allergen-specific IgE panel can be used to investigate specific allergic triggers to assist in allergen avoidance strategies or potentially to guide allergen-specific immunotherapy. Serum allergen-specific IgE assays function to quantify the reactivity of the patient serum to individual allergen extracts. 18 IgE has a complex role in asthma and provides a bridge between recognition of the antigen by the adaptive immune system and subsequent activation of mast cells and eosinophils at mucosal sites of environmental exposure. 19 Serum IgE concentrations are increased in humans with atopic disorders and there is overwhelming evidence for the role of allergen-specific IgE in atopic asthma.20,21 Additionally, higher IgE concentrations are observed in people with moderate and severe asthma than in non-asthmatic or non-allergic populations. 22
The objectives of this study were to evaluate results of serum allergen-specific IgE testing in pet cats with a clinical diagnosis of asthma to determine: (1) how commonly positive results would be identified; (2) if larger numbers of positive IgE responses to tested aeroallergens would be associated with either an increased clinical score or magnitude of airway eosinophilia; and (3) if higher reactivity of IgE would be associated with either an increased clinical severity score or magnitude of airway eosinophilia. We hypothesized that allergen-specific IgE panels would identify mono- or polysensitization in the majority of cats with a clinical diagnosis of asthma, and that reactivity to multiple allergens and strong positive responses to allergens would correlate with increased severity of clinical signs and airway eosinophilia.
Materials and methods
Case selection
The electronic medical database of the Veterinary Health Center, University of Missouri, Columbia, was searched for cats that had a commercial serum allergen-specific IgE panel using FcεR1-based technology (Heska; Allercept Serum IgE testing) submitted between September 2008 and October 2018. Cats were included if they had a diagnosis of feline asthma based on compatible clinical signs and BAL cytology with airway eosinophilia >10%, 23 as well as an adequately detailed medical record. Medical records had to include history (including medications), physical examination findings, results of thoracic imaging if performed and any other diagnostic testing at the attending clinician’s discretion. Cats were excluded if the serum allergen-specific IgE panel was performed without a clinical diagnosis of feline asthma (eg, performed for a dermatologic condition). Cats were also excluded if there was evidence of a cause of respiratory disease other than feline asthma. Owing to differences in the IgE assay methodology that precluded comparison, all cats tested prior to 2013 were excluded from final analysis.
Review of medical records
Information obtained from the medical records included breed, sex, age, historical clinical signs, indoor/outdoor lifestyle, physical examination findings, results of diagnostic tests (CBC, fecal testing, empiric deworming course, heartworm antigen and/or antibody testing, thoracic radiographs, and/or CT, BAL cytology and culture). Thoracic imaging was interpreted by board-certified radiologists and BAL cytology was evaluated by board-certified clinical pathologists at the University of Missouri Veterinary Health Center. When administered at the time of submission of the serum allergy panel, corticosteroid (type and dose) was recorded.
Metrics for determination of allergen sensitization
The allergens selected for the serum IgE panels were determined for each cat based on the botanical zone using the zip code of residence (see Table 1 in the supplementary material for the selected allergens). Allergens were identified by major category as grasses, trees, weeds, mites, insects, epithelials and fungi, and within each of these major categories as individual allergens (the category of grasses included June grass, Meadow Fescue grass, Orchard grass, etc). Each allergen was reported as either negative, 1+ (allergen-specific IgE detected), 2+ (high levels of allergen-specific IgE detected) and 3+ (very high levels of allergen-specific IgE detected). Each cat was first assessed for IgE reactivity to any of the seven major categories of allergens. If any allergen within the major category was positive, it was assigned a value of 1 (positive) and if none of the allergens within the major category was positive, it was assigned a value of 0 (negative). Second, for cats in which allergen-specific IgE was noted, the number of cats with monosensitization (having a positive result for only a single allergen category) or polysensitization (having a positive result for >1 allergen category) was determined. A cumulative score reflective of all identified reactive allergens (out of a total of 83) was given to each cat based on addition of all positive scores across all allergen categories.
Scoring system for clinical signs and airway eosinophilia severity
To determine a correlation between the presence of allergens and the severity of clinical signs and airway eosinophilia, the following metrics were used. Cats were assigned a score for severity of clinical signs based upon a three-point scoring system: 1 = mild (cough alone); 2 = moderate (wheezing or exercise intolerance); or 3 = severe (respiratory distress episodes). Airway eosinophilia was reported as the percentage of BAL eosinophils from a cytospin with a 400-cell differential cell count, and a BAL eosinophilia >10% was considered abnormal. 23
Statistical analysis
Descriptive statistics were used when appropriate and one-way ANOVA on ranks was performed to compare the number of total positive allergens with the eosinophil severity score and clinical severity scores. A Pearson correlation test was used to evaluate if there was any correlation between the number of positive allergens and percentage of BAL eosinophils. A P value of <0.05 was considered significant. All analyses were performed on commercially available statistical software (SigmaPlot; Systat Software).
Results
Of the 29 cats identified during the study period, 18 cats met the criteria for study inclusion (Figure 1). Thirteen cats were domestic shorthair, and there was also one of each of the following breeds: domestic longhair, Bengal, Siamese, Sphynx and Himalayan. There were 11 female spayed and seven male neutered cats. The median age was 5 years old with a range of 1–10 years. The most common presenting clinical signs were cough (n = 10), acute or historical respiratory distress (n = 6), wheezing (n = 2), tachypnea (n = 1), sneezing (n = 1) and nasal discharge (n = 1). The duration of clinical signs ranged from acute onset (respiratory distress) to several years (cough). The majority of cats were housed exclusively indoors (n = 13), with both indoor and outdoor environments in five cats. Physical examination findings included increased bronchovesicular sounds (n = 7), respiratory distress (n = 4), mild tachypnea (n = 3), increased respiratory sounds including stridor (n = 1), referred upper airway noise (n = 2) and no abnormalities (n = 1).
Figure 1.
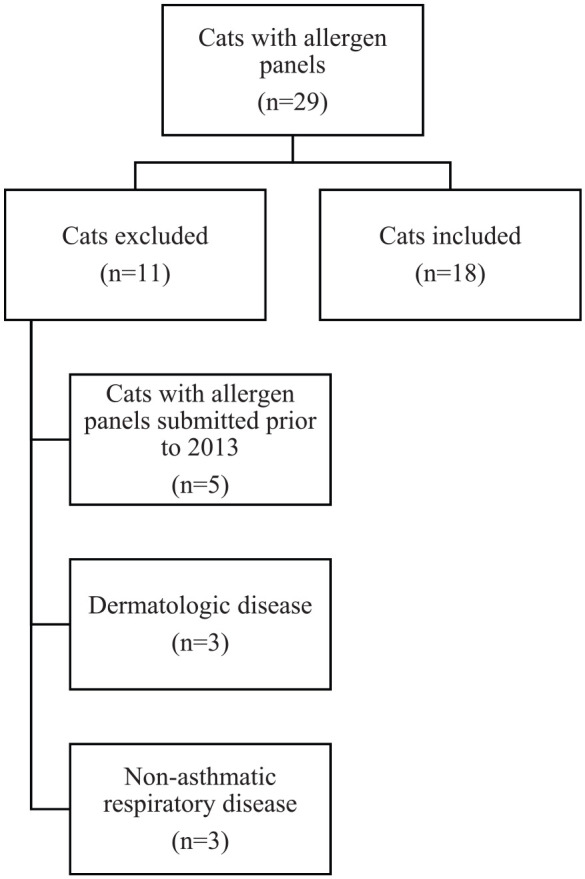
Flowchart for case selection
Diagnostic testing varied for each cat. None of the 15 cats that had a CBC performed had a peripheral eosinophilia. Only two cats had a fecal Baerman performed, but 10 cats were previously prescribed an empiric deworming course of fenbendazole and seven cats were noted in the records to have been receiving monthly flea/tick or heartworm preventative. Heartworm antigen and/or antibody tests were submitted for 13 cats, all of which were negative. Thoracic imaging included thoracic radiographs (n = 14) and thoracic CT scans (n = 11), with some cats having both imaging modalities. Thoracic radiographs revealed a diffuse bronchial pattern (n = 6), bronchointerstitial pattern (n = 5), interstitial pattern (n = 1) or unremarkable pulmonary parenchyma (n = 2). All 11 cats that underwent thoracic CT scan had evidence of peribronchial cuffing and bronchial wall thickening consistent with asthma. The median percentage of BAL eosinophils was 47% (range 11–93%).
Four cats were receiving a corticosteroid at the time of panel submission: prednisolone (n = 3; with one of these cats receiving inhaled fluticasone) and injectable methylprednisolone (n = 1). The oral prednisolone dosages were 0.5 mg/kg every other day, 1 mg/kg/day and 3 mg/kg/day. The cat that received the methylprednisolone injection received it 22 days prior to allergy testing at an unknown dose. The cat receiving 0.5 mg/kg prednisolone every other day was also receiving two actuations of fluticasone from a meter dose inhaler (220 µg/puff) twice daily. All of the cats receiving glucocorticoids at the time of allergy testing had at least one respiratory clinical sign despite therapy and their BAL fluid eosinophilia were all >50%.
In the 18 cats with comprehensive clinical data supporting a diagnosis of feline asthma, 14 (78%) of the cats had a positive serum allergen-specific IgE test. Of the four cats with negative results to all tested aeroallergens, one was receiving concurrent glucocorticoids. The severity of clinical signs in these four cats were scored as 1 (n = 2) and 3 (n = 2), and they had 28%, 42%, 49% and 93% eosinophils in their BAL fluid. Of the cats with positive test results, one cat was monosensitized; in the remaining 13 cats, the number of aeroallergens with IgE reactivity ranged from 3 to 68, with a median of 17. The severity of clinical signs in the 14 cats with positive allergen testing were scored as 1 (n = 5), 2 (n = 4) or 3 (n = 5), with no correlation between the clinical severity score and magnitude of BAL eosinophilia (Figure 2). Of the 13 cats living in an environment described as exclusively indoors, nine had positive allergen-specific IgE panels. All nine were sensitized to indoor allergens, with eight sensitized to both indoor and outdoor allergens. Of the five cats having access to indoor and outdoor environments, four had positive allergen-specific IgE panels and all four were sensitized to both indoor and outdoor allergens. The indoor allergens include allergens within the family of mites, epithelials, fungi and insects. Outdoor allergens are grasses, weeds and trees. See Table 1 in the supplementary material for the full list of tested allergens within these categories.
Figure 2.
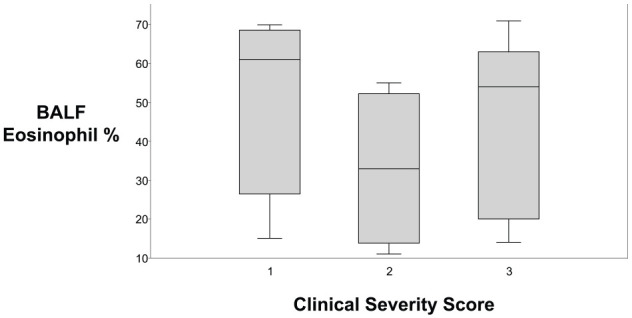
Eighteen cats with a clinical diagnosis of asthma were evaluated using a clinical severity score and percentage of bronchoalveolar lavage fluid (BALF) eosinophils, with data shown in box and whisker plots. There was no correlation between the severity of clinical signs and severity of airway eosinophilia (P = 0.941). The upper and lower boxes represent the 25th and 75th percentiles, respectively. The line represents the median and the upper and lower whiskers represent the maximum and minimum values, respectively
The number of cats having at least one positive reaction within each major category of allergen is summarized in Table 1. The most commonly implicated allergen out of 83 total was the mite Dermatophagoides farinae (n = 9 cats); the closely related mite Dermatophagoides pteronyssinus was frequently positive (n = 7). The most commonly implicated grass allergens were June grass and Timothy grass (n = 6 cats each). Among the weeds, ragweed, western, dog fennel and nettle were the most commonly identified allergens (n = 6 each). The most common tree allergen was mulberry (n = 6), and fleas were the most common allergen within the insects category (n = 6). The fungi and epithelials category contained the fewest positive allergens compared to the other groups.
Table 1.
Number of cats having at least one positive reaction to the specified category of allergen
| Grasses (13)* | Weeds (18) | Trees (33) | Mites (6) | Insects (3) | Epithelials (2) | Fungi (8) | |
|---|---|---|---|---|---|---|---|
| Total number of cats with a positive IgE result | 8 | 11 | 8 | 11 | 9 | 4 | 6 |
The number in parenthesis represents the total number of individual allergens tested in the panel within the broad allergen category
There was no significant difference in the median severity score depending on the number of allergens with positive results (P = 0.675; Figure 3) or number of 3 + positive reactions (P = 0.270; Figure 4). Additionally, there was no correlation between the total number of allergens with positive responses and clinical severity score (r = 0.0173; P = 0.675) or with the percentage of BAL eosinophils (r = 0.205; P = 0.414; Figure 5), or having one or more very high IgE responses (number of 3+ positive reactions) and the percentage of BAL eosinophils (r = −0.310; P = 0.211; Figure 6).
Figure 3.
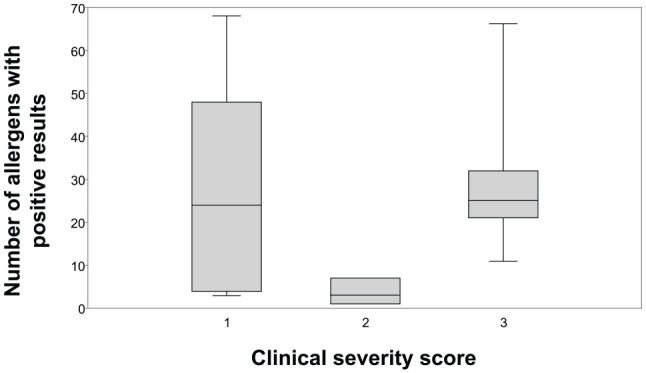
For the 18 asthmatic cats included in the study, the clinical severity score and number of allergens (out of 83 total allergens) with positive responses were compared using box and whisker plots. The whiskers extend to the highest and lowest observations and the ends of the boxes are the upper and lower quartiles. The median is marked by the line inside the box. There was no significant difference between the clinical severity score when compared with the total number of allergen IgE reactivity (P = 0.675)
Figure 4.
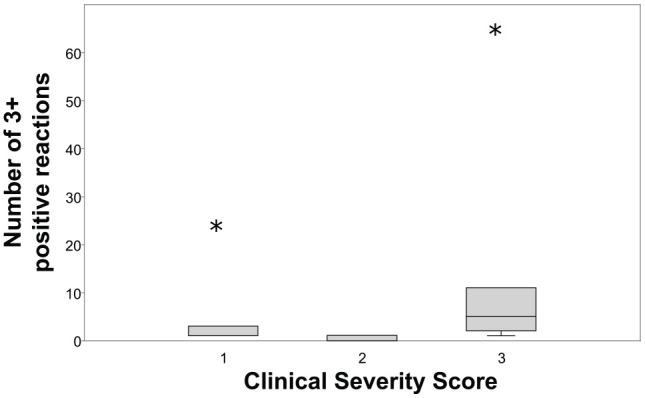
Box and whisker plot evaluating the number of allergens with 3+ positive results and the clinical severity score of asthmatic cats. When present, the whiskers extend to the highest and lowest observation and the ends of the boxes are the upper and lower quartiles. The median is marked by the line inside the box. Extreme outliers are denoted by the asterisks. There was no significant difference in clinical severity score depending on the number of 3+ positive reactions (P = 0.270)
Figure 5.
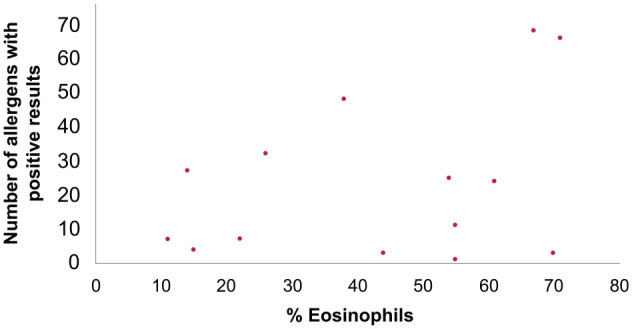
Dot plot showing the number of allergens with positive results and percentage of bronchoalveolar lavage fluid eosinophils for the 18 asthmatic cats. Each dot represents data from an individual cat. There was no correlation between the total number of positive responses to individual aeroallergens and increased airway eosinophils (r = 0.205; P = 0.414)
Figure 6.
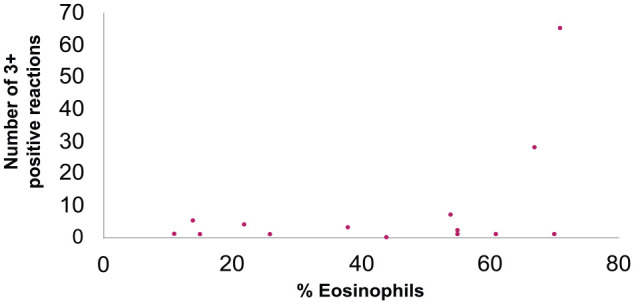
Dot plot showing the number of allergens with 3+ positive results and percentage of bronchoalveolar lavage (BAL) fluid eosinophils. Each dot represents data from an individual cat. There was no correlation between cats with a strongly positive (3+) IgE response and absolute percentage of BAL eosinophils (P = 0.829)
Discussion
This study retrospectively evaluated the results of serum allergen-specific IgE testing in cats with a clinical diagnosis of asthma and investigated if the number and magnitude of positive IgE responses was associated with severity of clinical signs and airway eosinophilia. There was a strong association between identification of allergen-specific IgE and cats with a clinical asthmatic phenotype. However, larger numbers of allergens with positive IgE reactivity or magnitude of IgE reactivity were not significantly associated with clinical severity or airway eosinophilia. These data provide important insights to our feline asthmatic patients in understanding the propensity for an underlying allergic stimulus as a causative mechanism of disease. It also highlights future opportunities to evaluate allergen-specific IgE testing as an incorporated diagnostic in feline asthmatics during their clinical evaluation, which could have relevance for allergen avoidance strategies or other novel therapies targeting the allergic inflammatory cascade.
As hypothesized, this study supported an allergic etiology in the majority of tested cats with an asthmatic phenotype. However, the number of positive allergen responses and degree of IgE reactivity did not correlate with more severe clinical signs or greater airway eosinophilia. A cat with a serum allergen-specific IgE panel documenting weaker sensitization with very few allergens could have a more severe asthmatic phenotype than a cat with a polysensitization and strong positive responses. Identification of patient serum IgE binding the diagnostic reagent FcεR1 is the basis for the commercial allergen panel used herein. It is important to consider that only serum and not tissue IgE was assayed via the allergen panels. After allergen exposure, activation of allergen-specific T helper cells leads to the production of IgE. In the blood, IgE has a short half-life of approximately 2–3 days, and in the absence of ongoing allergenic exposure, IgE blood levels are low.24,25 Rather than staying in circulation, IgE binds to the high affinity FcεR1 on mast cells in tissue; the half-life of bound IgE can be up to several months. 24 Serum IgE can be considered a biomarker of disease; if present, it likely reflects a sensitizing allergen. However, if absent, additional diagnostics such as intradermal testing may be required to confirm and identify the sensitizing allergen(s).
Serum allergen-specific IgE testing identified an underlying allergic etiology in 14/18 cats. Intradermal testing is more sensitive than serum testing, although both are equally specific.9,26 It is possible that intra-dermal testing would have identified positive allergens in some of the four cats with negative allergen-specific IgE with serum testing. However, referral to a veterinary dermatologist is often required for intradermal testing, along with sedation and aggressive shaving of the haircoat. Intradermal testing is technically challenging both to do and read. There is a lot of room for error without proper training for interpretation, whereas there is much less for serum testing. Importantly, corticosteroids can cause false-negative results with intradermal testing. 12 Thus, intradermal testing might not have offered an advantage in the single cat that had a negative serum IgE test after treatment with corticosteroids. Neither oral nor inhaled glucocorticoids have been shown to interfere with serum allergen-specific IgE testing in experimental feline asthma. 12 Withdrawal of glucocorticoids may be challenging in cats with a severe asthmatic phenotype; however, withdrawal is not necessary prior to serum testing. 12 Collectively, these attributes make serum allergen-specific IgE testing an appealing, safe, simple, and widely available alternative. In the current study, 3/4 cats receiving glucocorticoids at the time of serum allergen-specific IgE testing had positive results.
Polysensitization was documented in all but one cat with positive allergen responses in this study. While polysensitization is common, the results suggest the allergen(s) with stronger IgE reactivity may not be the primary allergen(s) responsible for the severity of the clinical signs or magnitude of airway eosinophilia. Thus, when feasible, pet owners should be advised to modify environmental exposure to an avoidable allergen, even if the IgE reactivity was reportedly low. For example, the most commonly implicated allergen in this study was the common house dust mite, D farinae. In a cat with any level of positive IgE reactivity to this mite, efforts should be made to ensure identification and avoidance of this allergen (eg, using impermeable covers for pillows and mattresses; washing bedding weekly at high temperatures; use of HEPA-type filters in the vacuum; controlling ambient humidity).
Of nine cats living in an indoor-only environment with positive allergen responses, eight were sensitized to both indoor and outdoor allergens. Major sources of indoor allergens include dust mite, cockroach, fungi and dander. 27 Outdoor aeroallergens such as grasses can disperse into the indoor air from an open door, window or fomite (eg, via the owner’s clothes, skin or hair). The common sensitization of indoor-only cats to outdoor allergens underscores the challenge of allergen avoidance. Simply attempting to switch the primary environment is unlikely to be an effective sole management strategy. Allergen-specific immunotherapy for feline asthma would likely be a more effective and potentially curative treatment measure compared with allergen avoidance but awaits a randomized clinical trial. Although immunotherapy in asthmatic cats is still considered somewhat experimental, the current study raises the question of whether picking allergens with the strongest reactivity for use in allergen-specific immunotherapy is an appropriate strategy.
Limitations of this study include its retrospective nature, lack of a control group for comparison and the relatively small sample size. Importantly, testing for IgE can be problematic in its own regard. While an important part of the diagnostic evaluation for allergic disease, there is a lack of standardization in allergen testing in veterinary medicine. In humans, allergenic extracts are tightly controlled, and the quality and performance of these extracts have been studied extensively.28,29 Much of our knowledge and use of allergens in veterinary patients is extrapolated from the human field. 26 Veterinary laboratories performing serum IgE testing within the USA divide regions into aerobiological zones and select allergens implicated in that zone based on many factors; 30 however, the methods for detection of IgE and the numbers and types of allergens between laboratories varies greatly. 31 For example, serum from cats that were experimentally sensitized to either house dust mite or Bermuda grass allergen were divided and sent as blinded samples to two commercial laboratories with different methods for IgE testing, with dramatically discrepant results. 9 Future studies are needed to determine the optimal means of allergen-specific IgE identification.
Although there were only a few negative serum IgE allergen panels (n = 4/18) within this study, there are many possibilities for failure to document an allergen reactivity. These include drug interference, not using relevant sensitizing allergens, low test concentration of allergens, immunopathologic pathways outside of IgE, asthma that is not caused by an inciting aeroallergen or an incorrect diagnosis of asthma. 29 There are hundreds, if not thousands, of potential allergens in a cat’s environment and commercial allergy panels only test a small subset of aeroallergens. Another factor that may lead to false-negative results is serum IgE testing performed too long after peak allergy season or exposure to specific allergen(s). 31 Owing to its short half-life, serum IgE concentrations could have declined by the time of panel submission in cats with negative panels.
Conclusions
This study used serum allergen-specific IgE panels to document an underlying allergic etiology involving multiple allergens in the majority of cats with a clinical diagnosis of asthma. The study showed that a larger number of sensitizing allergens was not associated with a more severe clinical asthmatic phenotype or airway eosinophilia. Similarly, more strongly positive allergen-specific IgE responses were not predictive of a more severe clinical asthmatic phenotype or airway eosinophilia. Positive allergen-specific IgE results can be used to guide avoidance or elimination of sensitizing allergens, regardless of the magnitude of IgE reactivity, to the extent possible. Whether serum allergen-specific IgE testing can guide allergen-specific immunotherapy to treat pet cats with allergic asthma remains to be determined in a clinical trial.
Supplemental Material
A list of allergens used for serum allergen-specific IgE testing.
Footnotes
Accepted: 25 January 2020
Supplementary material: The following file is available online:
Supplementary Table 1: A list of allergens used for serum allergen-specific IgE testing
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are individually identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Megan E van Eeden  https://orcid.org/0000-0003-1908-5473
https://orcid.org/0000-0003-1908-5473
Leah A Cohn  https://orcid.org/0000-0002-7785-2696
https://orcid.org/0000-0002-7785-2696
References
- 1. Reinero CR. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet J 2011; 190: 28–33. [DOI] [PubMed] [Google Scholar]
- 2. McCarty JC, Ferguson BJ. Identifying asthma triggers. Otolaryngol Clin North Am 2014; 47: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deo SS, Mistry KJ, Kakade AM, et al. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India 2010; 27: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reinero CR, DeClue AE, Rabinowitz P. Asthma in humans and cats: is there a common sensitivity to aeroallegens in shared environments? Environ Res 2009; 109: 634–640. [DOI] [PubMed] [Google Scholar]
- 5. Russell RJ, Brightling C. Pathogenesis of asthma: implications for precision medicine. Clin Sci (Lond) 2017; 131: 1723–1735. [DOI] [PubMed] [Google Scholar]
- 6. Norris Reinero CR, Decile KC, Berghaus RD, et al. An experimental model of allergic asthma in cats sensitized to house dust mite or bermuda grass allergen. Int Arch Allergy Immunol 2004; 135: 117–131. [DOI] [PubMed] [Google Scholar]
- 7. Moriello KA, Stepien RL, Henik RA, et al. Pilot study: prevalence of positive aeroallergen reactions in 10 cats with small-airway disease without concurrent skin disease. Vet Dermatol 2007; 18: 94–100. [DOI] [PubMed] [Google Scholar]
- 8. Dudley DJ. The immune system in health and disease. Baillieres Clin Obstet Gynaecol 1992; 6: 393–416. [DOI] [PubMed] [Google Scholar]
- 9. Lee-Fowler TM, Cohn LA, DeClue AE, et al. Comparison of intradermal skin testing (IDST) and serum allergen-specific IgE determination in an experimental model of feline asthma. Vet Immunol Immunopathol 2009; 132: 46–52. [DOI] [PubMed] [Google Scholar]
- 10. Norris CR, Decile KC, Byerly JR, et al. Production of polyclonal antisera against feline immunoglobulin E and its use in an ELISA in cats with experimentally induced asthma. Vet Immunol Immunopathol 2003; 96: 149–157. [DOI] [PubMed] [Google Scholar]
- 11. Prost C. Treatment of allergic feline asthma with allergen avoidance and specific immunotherapy: experience with 20 cats. Rev Franc Allerg Immunol Clin 2008; 48: 409–413. [Google Scholar]
- 12. Chang CH, Lee-Fowler TM, Declue AE, et al. The impact of oral versus inhaled glucocorticoids on allergen specific IgE testing in experimentally asthmatic cats. Vet Immunol Immunopathol 2011; 144: 437–441. [DOI] [PubMed] [Google Scholar]
- 13. Chang CH, Cohn LA, DeClue AE, et al. Oral glucocorticoids diminish the efficacy of allergen-specific immunotherapy in experimental feline asthma. Vet J 2013; 197: 268–272. [DOI] [PubMed] [Google Scholar]
- 14. Trzil JE, Reinero CR. Update on feline asthma. Vet Clin North Am Small Anim Pract 2014; 44: 91–105. [DOI] [PubMed] [Google Scholar]
- 15. Corcoran BM, Foster DJ, Fuentes VL. Feline asthma syndrome: a retrospective study of the clinical presentation in 29 cats. J Small Anim Pract 1995; 36: 481–488. [DOI] [PubMed] [Google Scholar]
- 16. Adamama-Moraitou KK, Patsikas MN, Koutinas AF. Feline lower airway disaese: a retrospective study of 22 naturally occurring cases from Greece. J Feline Med Surg 2004; 6: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clercx C. Diseases of the trachea and small airways. In: Ettinger SJ, Feldman EC, Cote E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2017, pp 2718–2729. [Google Scholar]
- 18. DeBoer DJ, Hillier A. The ACVD Task Force on Canine Atopic Dermatitis (XVI): laboratory evaluation of dogs with atopic dermatitis with serum-based ‘allergy’ tests. Vet Immunol Immunopathol 2001; 81: 277–287. [DOI] [PubMed] [Google Scholar]
- 19. Matucci A, Vultaggio A, Maggi E, et al. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir Res 2018; 19: 113. DOI: 10.1186/s12931-018-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dottorini T, Sole G, Nunziangeli L, et al. Serum IgE reactivity profiling in an asthma affected cohort. PLoS One 2011; 6: e22319. DOI: 10.1371/journal.pone.0022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menz G, Ying S, Durham SR, et al. Molecular concepts of IgE-initiated inflammation in atopic and nonatopic asthma. Allergy 1998; 53: 15–21. [DOI] [PubMed] [Google Scholar]
- 22. Borish L, Chipps B, Deniz Y, et al. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol 2005; 95: 247–253. [DOI] [PubMed] [Google Scholar]
- 23. Shibly S, Klang A, Galler A, et al. Architecture and inflammatory cell composition of the feline lung with special consideration of eosinophil counts. J Comp Pathol 2014; 150: 408–415. [DOI] [PubMed] [Google Scholar]
- 24. Tizard IR. Veterinary immunology. St Louis, MO: Elsevier, 2018. [Google Scholar]
- 25. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 2010; 125: S73–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hillier A, DeBoer DJ. The ACVD Task Force on Canine Atopic Dermatitis (XVII): intradermal testing. Vet Immunol Immunopathol 2001; 81: 289–304. [DOI] [PubMed] [Google Scholar]
- 27. Kelly LA, Erwin EA, Platts-Mills TA. The indoor air and asthma: the role of cat allergens. Curr Opin Pulm Med 2012; 18: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loewenstein C, Mueller RS. A review of allergen-specific immunotherapy in human and veterinary medicine. Vet Dermatol 2009; 20: 84–98. [DOI] [PubMed] [Google Scholar]
- 29. Ipsen H, Larsen JN, Niemeijer NR, et al. Allergenic extracts. In: Cox L, Nelson H, Lockey R. (eds). Allergy principles and practice. 5th ed. St Louis, MO: Mosby Year Book, 1998, pp 404–416. [Google Scholar]
- 30. Plant JD, Neradilek MB. Effectiveness of regionally-specific immunotherapy for the management of canine atopic dermatitis. BMC Vet Res 2017; 13: 4. DOI: 10.1186/s12917-016-0917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hensel P, Santoro D, Favrot C, et al. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet Res 2015; 11: 196. DOI: 10.1186/s12917-015-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of allergens used for serum allergen-specific IgE testing.


