Abstract
We have developed an adoptive cell transfer model in mice to study the ability of a glycoprotein conjugate vaccine to induce immunologic memory for the polysaccharide moiety. We used type III capsular polysaccharide from the clinically relevant pathogen group B streptococci conjugated to tetanus toxoid (GBSIII-TT) as our model vaccine. GBS are a major cause of neonatal infections in humans, and type-specific antibodies to the capsular polysaccharide protect against invasive disease. Adoptive transfer of splenocytes from mice immunized with the GBSIII-TT conjugate vaccine conferred anti-polysaccharide immunologic memory to naive recipient mice. The transfer of memory occurred in a dose-dependent manner. The observed anamnestic immune response was characterized by (i) more rapid kinetics, (ii) isotype switching from immunoglobulin M (IgM) to IgG, and (iii) 10-fold-higher levels of type III-specific IgG antibody than for the primary response in animals with cells transferred from placebo-immunized mice. The adoptive cell transfer model described in this paper can be used for at least two purposes: (i) to evaluate conjugate vaccines with different physicochemical properties for their ability to induce immunologic memory and (ii) to study the cellular interactions required for an immune response to these molecules.
Immunologic memory following the first encounter with an antigen is specific and can persist for years. Recall of immunologic memory induced by an immunizing event, either by vaccination or by an encounter with the naturally occurring antigen as presented on the pathogen, can result in an anamnestic immune response characterized by more rapid kinetics and a greater magnitude than the primary response.
Activation of B cells by their being turned into antibody-secreting cells by protein antigens has been demonstrated by adoptive cell transfer experiments to be T-cell dependent (21). These classic studies showed that transfer of both B cells (bone marrow cells) and T cells (thymocytes) was needed for a significant antibody response to sheep erythrocytes in irradiated naive recipient mice (9). A hapten-carrier system for studies of the transfer of immunologic memory has been used to examine B- and T-cell collaborations (18, 20). A hapten, which is not immunogenic alone, can induce an immune response if coupled to an immunogenic carrier. Interactions between hapten-carrier-primed B cells and carrier-primed T cells were found to be necessary for transfer of anti-hapten immunologic memory to irradiated naive recipient mice.
In experimental animals, immune responses to bacterial polysaccharide (PS) antigens are thymus independent and fail to induce classic immunologic memory (6, 12). The antibody response to a PS alone is typically of the immunoglobulin M (IgM) isotype, and repeated immunizations do not result in increased levels of PS-specific antibodies (6, 12). Covalent coupling of a PS to an immunogenic protein carrier results in an enhanced immune response with (i) high levels of PS-specific antibodies, (ii) rapid kinetics, and (iii) isotype switching of the PS-specific antibodies (IgM to IgG) upon booster vaccination or subsequent encounter with the PS as presented on the bacteria (12). The enhanced immune response to a PS conjugated to a protein with respect to the response to the PS alone indicates a shift from a thymus-independent to a thymus-dependent response (12, 28). The first glycoconjugate vaccine (Haemophilus influenzae type b PS coupled to a protein carrier) was licensed in the United States in 1990. Such vaccines have been proven to be efficacious in preventing invasive disease (11, 23) and in lowering the prevalence of H. influenzae type b colonization in the immunized population (5, 23). Even though the principle that glycoconjugate vaccines can protect against invasive disease with encapsulated bacteria is established, the immunological mechanisms altering the immune response to the conjugated compared with the unconjugated PS are only partially understood (27).
To examine the induction and recall of immunologic memory by a glycoconjugate vaccine, we chose the clinically relevant group B streptococcal type III (GBSIII) PS conjugated to tetanus toxoid (TT) as our model conjugate vaccine (17, 30). There is a strong clinical need for conjugate vaccines to GBS (8), which are a major cause of neonatal infections in humans (2). Maternal type-specific IgG antibodies to the capsular PS are transported across the placenta and protect the newborn against invasive disease (3, 4). Repeated immunizations with unconjugated GBSIII PS do not induce GBSIII-specific IgG in mice (30). Conjugation of GBSIII PS to TT enhances the immunogenicity of the PS moiety (17, 30). To investigate whether the conjugation of capsular PS to carrier proteins confers immunologic memory for the PS moiety, we developed an adoptive cell transfer model in mice.
MATERIALS AND METHODS
Animals.
SJL/J, CBA/J, and BALB/cByJ (BALB/c) mice were purchased from The Jackson Laboratory, Bar Harbor, Maine, and C3H/HeNCrlBR (C3H) mice were purchased from Charles River Laboratories, Wilmington, Mass. All experiments began with 10-week-old female mice.
Vaccines.
Capsular PS from GBSIII M781 was isolated and purified as described previously (30). The structure and purity of the GBSIII PS were verified by 1H nuclear magnetic resonance spectroscopy (31). GBSIII PS in which 25% of the sialic acid residues had been oxidized by periodate treatment was conjugated to monomeric TT (North American Vaccine Inc., Beltsville, Md.) (GBSIII-TT) by reductive amination (30). The degree of oxidation of sialic acid residues was verified by gas chromatography-mass spectrometry (30). The GBSIII-TT conjugate vaccine was composed of 66% (wt/wt) carbohydrate and 34% (wt/wt) protein.
Immunogenicity in different inbred mouse strains.
The mouse strain for an adoptive transfer model was selected by examining the immunogenicity of GBSIII vaccines in inbred mice of each of four strains: SJL (Ias), CBA (lak), C3H (lak), and BALB/c (lad). Groups of six mice were immunized intraperitoneally (i.p.) three times (days 1, 26, and 54) with GBSIII-TT, GBSIII PS (2 μg of PS per dose), or saline with Al(OH)3 as adjuvant (1.75 mg of Al/dose). The mice were bled before and 14 days after each immunization, and the sera were stored at −20°C until analyzed for the presence of specific antibodies.
Immunization.
The benefit of an adjuvant was examined by immunizing groups of six BALB/c mice i.p. with two doses of GBSIII-TT (days 1 and 14) either with or without Al(OH)3 at doses ranging from 0.1 to 1.75 mg of Al. The optimal dose of GBSIII-TT was determined by immunizing groups of six BALB/c mice three times with GBSIII-TT mixed with 0.5 mg of Al (days 1, 21, and 49), with doses ranging from 0.031 to 8 μg of PS. The kinetics of the IgG antibody response to GBSIII PS in the GBSIII-TT vaccine mixed with 0.5 mg of Al was examined after either one or two priming doses (2 μg of PS per dose 2 weeks apart) followed by a booster dose with GBSIII-TT either 2 or 4 weeks later. The mice were bled before each immunization and 7, 14, and 28 days after the last immunization unless otherwise specified, and sera were stored at −20°C until analyzed for the presence of specific antibodies.
Antibodies used.
The following monoclonal antibodies (MAbs) were used in flow cytometry analysis: anti-murine B220-R–phycoerythrin (rat IgG2a, Ly5, clone RA3-6B2; Caltag Laboratories, South San Francisco, Calif.), anti-class II major histocompatibility complex-lad–fluorescein isothiocyanate (FITC) (mouse IgG2b, clone AMS-32.1; PharMingen, San Diego, Calif.), anti-Thy1.2–FITC (rat IgG2a, clone 53-2.1; PharMingen), anti-CD4–FITC (rat IgG2b, clone YTS191.1; Caltag Laboratories), anti-CD8–FITC (rat IgG2a, clone CT-CD8a; Caltag Laboratories), anti-Mac-1–FITC (rat IgG2b, clone M1/70.15; Caltag Laboratories), anti-CD3–FITC (hamster IgG, clone 500-A2; Caltag Laboratories), and rat IgG-FITC (rat IgG2a isotype control, clone UC8-4B3; PharMingen).
Lymphocyte isolation.
Single-cell suspensions were obtained by pressing spleens through a stainless steel grid (mesh size, 80 μm) followed by filtration through a nylon mesh (mesh size, 95 μm). The cells were harvested by centrifugation, washed in balanced salt solution (pH 7.4), and depleted of erythrocytes by treatment with 0.15 M NH4Cl in 17 mM Tris (pH 7.2) and centrifugation over fetal calf serum (1). Dead cells were removed from the cell suspensions by centrifugation over a density gradient (Ficoll Hypaque; Sigma) (1), and live cells were suspended in Dulbecco’s modified Eagle’s medium (pH 7.4) with heat-inactivated 10% fetal calf serum (HyClone Laboratories, Inc., Logan, Utah), 50 μM 2-mercaptoethanol, 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml.
Flow cytometry evaluation.
The expression of B- and T-lymphocyte surface antigens was studied by flow cytometric analysis of single-cell suspensions with a FACScan apparatus (Becton Dickinson, San Jose, Calif.) (1). Data were analyzed with CELL QUEST FACS analysis software (Becton Dickinson). The splenocytes of immune and naive animals were aliquoted and incubated with the anti-B- or anti-T-cell surface ligand fluorochrome-conjugated MAbs mentioned above by standard methods (1a). Propidium iodide was used to check the viability of the cell suspensions. FITC-labeled rat IgG was used as an isotype control to determine the nonspecific binding to the cell suspensions.
Adoptive cell transfer.
BALB/c mice were immunized i.p. twice (3 weeks apart) with GBSIII-TT (2 μg of PS per dose) or saline (placebo) mixed with alum (0.5 mg of Al per dose). The mice were sacrificed 4 weeks after the last priming dose, and their spleens were harvested. Single-cell suspensions of whole spleen cells from GBSIII-TT- or placebo-immunized mice were transferred intravenously via the tail vein to groups of five or six nonimmune, nonirradiated recipient mice at doses ranging from 2 × 105 to 2 × 108 splenocytes per transfer (200 μl). The recipient mice were injected i.p. 24 h later with GBSIII-TT or saline to determine whether immunologic memory to the GBSIII PS was transferred.
Immunoassays.
Quantitative enzyme-linked immunosorbent assays (ELISAs) with monomeric TT (North American Vaccine Inc.) or GBSIII PS covalently linked to human serum albumin (HSA) (Sigma, St. Louis, Mo.) as the sensitizing antigen were used to measure the amount of serum antibody specific for the carrier (TT) and the GBSIII PS (13), respectively. Briefly, microtiter plates (Nunc-Immuno Plates; Maxisorp, Roskilde, Denmark) were coated with 0.1 ml of coating antigen (5 μg/ml) in 0.1 M carbonate buffer (pH 9.8) (optimal concentrations were determined by checkerboard ELISAs). Unknown sera and standards were diluted in 10 mM phosphate-buffered saline–0.05% Brij 35–5% newborn calf serum (Whittaker Bioproducts, Walkesville, Md.) and titrated twofold across the plate. Alkaline phosphatase-labeled anti-IgG (total and subclass) (Southern Biotechnology Associates, Birmingham, Ala.) and anti-IgM conjugates (PharMingen) were used to detect specific antibodies of the different isotypes and subclasses. The levels of specific antibodies in various sera were determined by comparing the absorbance at 405 nm of the test serum with standard curves generated from separate ELISAs involving anti-mouse IgG (Sigma) or anti-mouse IgM (PharMingen) as capturing antibodies on microtiter wells and known concentrations of murine IgG (total or subclass) (Southern Biotechnology Associates) and IgM (PharMingen) standards, respectively (13, 15). The titration curves of the test sera and the Ig standards were parallel (22). The sum of the GBSIII-specific IgG subclass antibodies as determined by the subclass ELISAs was within 10% of the level of the total specific IgG antibodies obtained in the IgG ELISAs. The GBSIII-specific assays were highly specific for GBSIII PS as demonstrated by identical inhibition curves with either purified GBSIII PS or GBSIII-HSA, while no inhibition was seen with HSA or irrelevant polysaccharides (reference 13 and data not shown). The TT ELISAs were highly specific for TT as demonstrated by specific inhibition with purified TT and no inhibition with irrelevant antigens (data not shown). The detection limits for the specific IgG subclass and the IgG and IgM ELISAs were 10, 50, and 25 ng/ml, respectively, with an initial dilution of the test sera of 1:50 (IgG subclass and IgM assays) and 1:200 (total IgG assays).
Statistics.
Nonparametric statistics from InStat 2.0 software (Graphpad Software, San Diego, Calif.) were used, and the continuous variables were expressed as median values. The Mann-Whitney test was used to assess the statistical significance of differences between independent samples. A two-sided probability value of <0.05 was considered statistically significant (24).
RESULTS
Development of an adoptive lymphocyte transfer model.
The mouse strain and doses of adjuvant and vaccine used were chosen in a series of pilot experiments. None of the murine strains tested developed anti-GBSIII IgG after three doses of the vaccine containing unconjugated polysaccharide given with adjuvant. Immunization with GBSIII-TT conjugate vaccine with Al(OH)3 induced significant levels of anti-GBSIII IgG in BALB/c and SJL mice after one dose and anamnestic immune responses to subsequent vaccinations (≥10-fold increase), while CBA and C3H mice responded less well (data not shown). The well-characterized BALB/c mouse strain was chosen for all further experiments.
In mice immunized with GBSIII-TT either with or without Al(OH)3, the adjuvant greatly enhanced the GBSIII-specific IgG response (data not shown). The highest levels of GBSIII-specific IgG antibodies after both priming and recall vaccination were induced by the conjugate (2 μg of GBSIII PS) mixed with 0.5 mg of alum. The level of anti-GBSIII IgG antibodies after immunization with the GBSIII-TT conjugate vaccine was dose dependent. A 2-μg dose of PS induced the highest levels of GBSIII-specific IgG during both induction and recall of immunologic memory (Table 1). Any further increase of the vaccine dose did not result in a better response. All subsequent experiments were conducted with a 2-μg dose of GBSIII PS (given as GBSIII-TT) and 0.5 mg of alum in BALB/c mice.
TABLE 1.
Antibody response elicited by various doses of GBSIII-TT conjugate vaccine in BALB/c micea
| Amt of GBSIII-TT (μg) | Median level of GBSIII IgG antibodies (range) (ng/ml)b afterc:
|
||
|---|---|---|---|
| Injection 1 | Injection 2 | Injection 3 | |
| 0.031 | 90 (50–100)* | 70 (50–90)* | 70 (50–100)* |
| 0.125 | 90 (50–160)* | 160 (50–570)* | 780 (80–2,260)* |
| 0.5 | 100 (50–170)* | 360 (130–710)* | 2,180 (440–13,440)* |
| 2 | 340 (90–1,220)** | 3,710 (1,740–21,940)** | 16,080 (5,380–188,700)** |
| 8 | 690 (310–2,460)*** | 5,100 (1,180–24,710)*** | 14,240 (1,770–107,700)*** |
Mice were injected i.p. with various doses of GBSIII-TT as described in Materials and Methods.
** and *** versus *, P < 0.05; ** versus ***, the differences in anti-GBSIII IgG levels were not statistically significant (Mann-Whitney test).
Mice were bled 21 days after first injection and 24 days after the second and third injections.
The GBSIII-specific IgM responses to immunization with unconjugated GBSIII PS or GBSIII-TT did not differ significantly. The median GBSIII-specific IgM levels in BALB/c mice after three doses of GBSIII, unconjugated or conjugated to TT, were 17.4 μg/ml (range, 11.0 to 57.2 μg/ml) and 32.7 μg/ml (range, 11.2 to 36.9 μg/ml), respectively (Mann-Whitney test; P = 0.24). Sera obtained 28 days after the third immunization with the conjugate vaccine had significantly higher levels of GBSIII PS-specific IgG antibodies (median, 16 μg/ml) than did sera obtained after immunization with GBSIII PS alone (undetectable levels of GBSIII PS-specific IgG [<50 ng/ml]), thus demonstrating that the GBSIII-TT vaccine induced an isotype switch.
The kinetics of the IgG antibody response to GBSIII PS in the GBSIII-TT vaccine was examined after either one or two priming doses (2 weeks apart) followed by a booster dose either 2 or 4 weeks later. The peak levels of GBSIII-specific IgG antibody following booster vaccination with the GBSIII-TT conjugate vaccine (secondary response) after two priming doses were more than 10-fold higher than after one priming dose. A 4-week interval between the second priming dose and the booster vaccination resulted in two- to threefold-higher GBSIII-specific IgG levels than did a 2-week interval.
Adoptive lymphocyte transfer study.
Donor BALB/c mice were primed with two doses of GBSIII-TT or saline 3 weeks apart. Four weeks after the second priming dose, the spleens were removed from the donor mice. The splenocytes (2 × 108) were transferred intravenously via the tail vein to groups of six nonimmune, nonirradiated recipient mice, which were injected i.p. 24 h later with GBSIII-TT or saline to determine whether immunologic memory for the GBSIII PS was transferred.
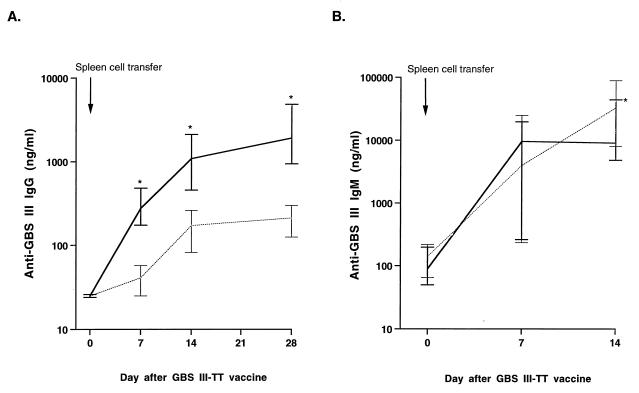
Adoptive transfer of splenocytes from donors primed with GBSIII-TT conjugate vaccine conferred memory of GBSIII PS to previously naive recipient mice, as demonstrated by an anamnestic immune response to one dose of GBSIII-TT (Fig. 1A). Naive mice with adoptively transferred splenocytes from GBSIII-TT-immunized mice had a rapid response to GBSIII-TT, with substantially elevated GBSIII-specific IgG antibody levels 7 days after immunization. In contrast, naive recipient mice with splenocytes transferred from mice immunized with placebo vaccine had at least a 7-day delay in the anti-GBSIII IgG response (Fig. 1A). The median level of anti-GBSIII IgG 7, 14, and 28 days after recall vaccination with GBSIII-TT was 10 times higher in mice with splenocytes transferred from GBSIII-TT-immunized mice than in mice receiving splenocytes from placebo-immunized animals (Mann-Whitney test, P = 0.002). The splenocyte cell suspensions from immune and naive mice contained equivalent numbers of B cells (47 to 55% labeled with B220 and class II [lad]) and T cells (35 to 40% labeled with Thy 1.2, of which approximately 80% were CD4-positive T helper cells).
FIG. 1.
Transfer of immunologic memory for GBSIII PS. BALB/c mice were immunized twice with GBSIII-TT conjugate vaccine, and 4 weeks later 2 × 108 spleen cells were adoptively transferred into groups of six naive recipients (solid line). In control animals, 2 × 108 naive spleen cells were transferred (dotted line). All recipient mice were immunized 24 h later with GBSIII-TT, and the antibody response to the GBSIII PS in serum was measured 0, 7, 14, and 28 days after immunization. (A) GBSIII-specific IgG response; (B) GBSIII-specific IgM response. Lines represent the median levels, and error bars indicate the range. The results of two independent experiments are shown. ∗, P < 0.01 compared with recipients of splenocytes from placebo-immunized donors (Mann-Whitney test).
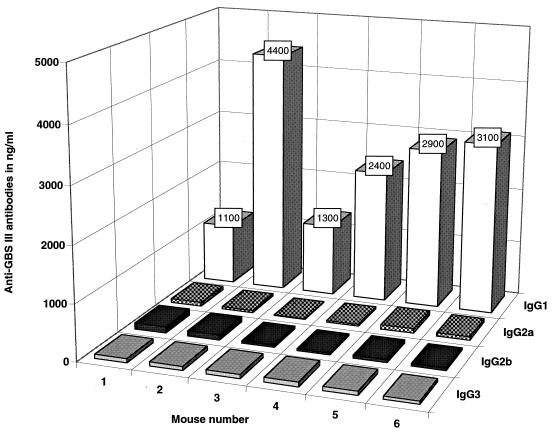
The subclass distribution of GBSIII PS-specific IgG antibodies was similar after immunization, whether the naive mice had received splenocytes from GBSIII-TT- or placebo-immunized donors. More than 75% of the GBSIII-specific IgG antibodies detected in recipients were IgG1 (Fig. 2). However, all the recipients developed GBSIII-specific antibodies of all IgG subclasses. Furthermore, the median levels of GBSIII-specific IgM antibodies 7 days after recall vaccination were not different in mice receiving immune cells (median anti-GBSIII IgM [lower and upper 95% confidence interval] of 9.5 μg/ml [4.9 to 12.8]) or naive cells (median anti-GBSIII IgM of 4.0 μg/ml [1.1 to 13.2]) (Mann-Whitney test; P = 0.43) (Fig. 1B).
FIG. 2.
GBSIII-specific IgG subclass antibodies in naive mice transferred with splenocytes from GBSIII-TT-immunized donors. BALB/c mice were immunized twice 3 weeks apart with GBSIII-TT conjugate vaccine; 4 weeks after the second immunization, 2 × 108 spleen cells were adoptively transferred into six naive recipients. All recipient mice were immunized 24 h later with GBSIII-TT, and the IgG subclass response to the GBSIII PS was measured in serum taken 28 days after immunization. The levels of GBSIII-specific IgG subclass antibodies are reported in nanograms per milliliter.
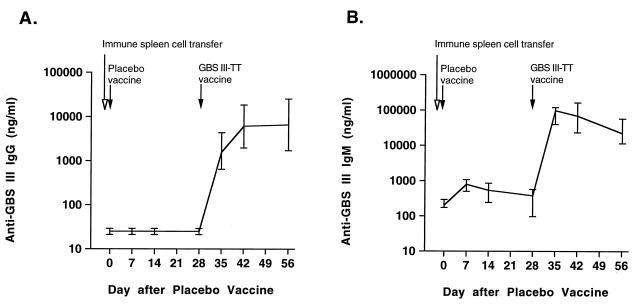
Mice with splenocytes adoptively transferred from GBSIII-TT-immunized animals did not develop increased anti-GBSIII IgG or IgM levels when a placebo vaccine (saline) was given within 24 h after the transfer, thus indicating that transfer of anti-GBSIII IgG- or IgM-producing plasma cells had not occurred. However, immunologic memory for GBSIII PS was conferred by the immune splenocytes transferred to these mice. A typical booster response was seen when GBSIII-TT conjugate vaccine was given 28 days after transfer of immune cells (Fig. 3).
FIG. 3.
Transfer of immunologic memory for GBSIII PS. BALB/c mice were immunized twice 3 weeks apart with GBSIII-TT conjugate vaccine, and 4 weeks after the second immunization, 2 × 108 spleen cells were adoptively transferred into six naive recipients. All recipient mice were immunized 24 h later with saline (first immunization) and 28 days later with GBSIII-TT (second immunization), and the antibody response to GBSIII PS was measured in serum taken 0, 7, 14, and 28 days after these two immunizations. (A) GBSIII-specific IgG response; (B) GBSIII-specific IgM response. Lines represent the median levels, and error bars indicate the range.
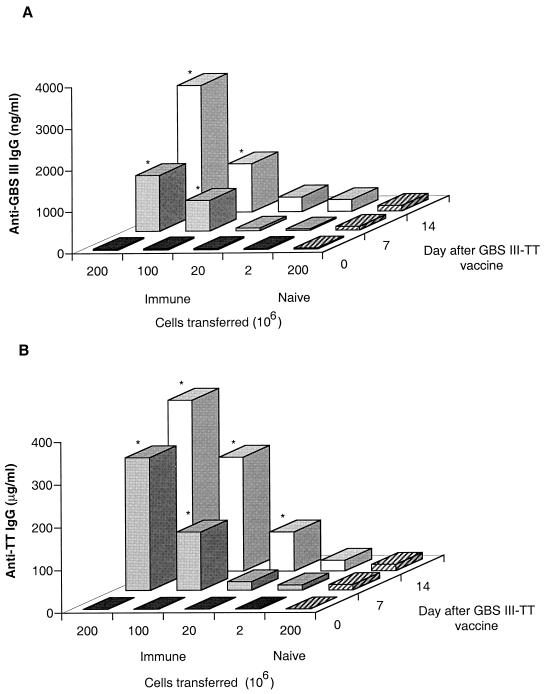
A dose-response study was used to determine the relationship between the number of splenocytes transferred and the levels of anti-GBSIII IgG and anti-TT IgG achieved in recipient mice after boosting with the GBSIII-TT conjugate vaccine (Fig. 4). Transfer of immunologic memory for both the GBSIII PS and TT appeared to be dose dependent, with the highest levels of specific IgG antibodies achieved when 2 × 108 cells were transferred (equivalent to the number of cells in an adult murine spleen). However, levels of anti-GBSIII IgG antibodies in animals receiving 2 × 107 or fewer splenocytes from immunized mice did not differ statistically from levels in control mice receiving splenocytes from placebo-immunized donors.
FIG. 4.
Dose response following adoptive transfer of spleen cells from mice primed with GBSIII-TT conjugate vaccine. BALB/c mice were immunized with GBSIII-TT conjugate vaccine, and 2 × 108, 1 × 108, 2 × 107, or 2 × 106 spleen cells from these primed mice were adoptively transferred into naive recipients (groups of six mice). In the control group, 2 × 108 naive spleen cells were transferred (hatched bars). All recipient mice were immunized with GBSIII-TT, and blood was taken 0, 7, and 14 days after immunization. (A) GBSIII PS-specific IgG antibodies; (B) TT-specific IgG antibodies. The levels of GBSIII-specific and carrier TT-specific IgG antibodies after the transfer of spleen cells are reported as medians. ∗, P < 0.01 compared with recipients of splenocytes from placebo-immunized donors (Mann-Whitney test).
The response to TT, used as the carrier in the conjugate vaccine, served as a control for T-cell dependence in the adoptive splenocyte transfer model. A primary type of antibody response to TT was observed in GBSIII-TT-immunized mice that received nonimmune splenocytes (Fig. 4B). In contrast, the response to TT in naive mice receiving a transfer of splenocytes from GBSIII-TT-immunized mice was even more rapid than the response to the GBSIII PS in the same mice, with higher levels of TT-specific IgG being achieved.
DISCUSSION
Immunologic memory is a hallmark of the immune system and is necessary for successful immunization (16). Memory for a T-cell-dependent antigen results in secondary responses to the antigen in vivo when the memory is recalled by booster vaccination or by encounter with the antigen as presented on the pathogenic organism. Assessment of the immunologic memory induced by priming of the immune system by vaccination should be an important factor in evaluating candidate vaccines (27).
To investigate whether the conjugation of capsular PS to carrier proteins confers immunologic memory to the PS moiety, we developed a murine adoptive cell transfer model to study the effect of recall vaccination in naive mice with splenocytes transferred from mice immunized with a conjugate vaccine. As our model vaccine, we used a clinically relevant glycoconjugate vaccine consisting of purified GBSIII capsular PS conjugated to TT by reductive amination.
Transfer of splenocytes from GBSIII-TT-immunized mice conferred immunologic memory for GBSIII PS following a single dose. The anamnestic immune response was characterized by (i) more rapid kinetics, (ii) isotype switching with a higher percentage of GBSIII-specific antibodies of the IgG isotype, and (iii) 10-fold-higher levels of GBSIII-specific IgG antibody levels than the primary response in animals with cells transferred from placebo-immunized mice (Fig. 1A). A lag phase of at least 7 days was observed before GBSIII-specific IgG antibodies were detected in the sera of mice receiving splenocytes from placebo-primed animals, typical of a primary response.
The immune response to TT was studied in the recipient mice to reflect the immune response to a T-cell-dependent antigen. Splenocytes from GBSIII-TT-immunized mice also conferred immunologic memory for TT in naive nonirradiated recipient mice, as demonstrated by an anamnestic anti-TT response to one dose of GBSIII-TT. The similar humoral response pattern to the carbohydrate moiety and the protein moiety of the conjugate vaccine used in our adoptive transfer model provides experimental data supporting the hypothesis that conjugation of a PS to a carrier converts the PS from a T-cell-independent to a T-cell-dependent antigen in vivo.
A large number of spleen cells were required for transfer of memory for both the GBSIII PS and the T-cell-dependent control (Fig. 4), which suggests that a relatively small number of the splenocytes carried memory specific for our vaccine antigens, in agreement with the findings of Spear et al. (26), who demonstrated that less than 0.1% of the splenocytes were specific for the immunizing antigen. In addition, only a small fraction of splenocytes are memory cells (21, 25). The memory for the GBSIII PS is long lasting. Splenocytes from immunized donors 20 weeks after the last priming dose conferred memory for GBSIII PS (14).
Many of the classic studies from the 1960s and 1970s on T-cell dependence and induction of immunologic memory for haptens conjugated to carrier proteins were performed by adoptive cell transfer (18, 20). Most of these studies used synthetic haptens such as dinitrophenyl, trinitrophenyl, or 4-hydroxy-3-iodo-5-nitrophenylacetic acid conjugated to carrier proteins such as hen ovalbumin, keyhole limpet hemocyanin, HSA, bovine serum albumin, and bovine gamma globulin as model vaccines. Considerations of safety, component hypersensitivity, innate immunostimulatory activity (29), and potential cross-reactivity with human tissue exclude many of the strictly experimental vaccines used previously in animal models to demonstrate a T-cell-dependent immune response to hapten components in human use (10). Our confirmation of transfer of immunologic memory in vivo for a clinically relevant capsular PS conjugated to a carrier protein (GBSIII-TT) represents an extension of these classic studies.
A common design featured in the classic studies on immunologic memory was the absolute requirement for irradiation of the recipient mice before adoptive cell transfer to obtain a detectable antibody response upon recall vaccination. This phenomenon has been referred to as “the radiosensitive barrier to syngeneic transplantation” by Celada (7). A recent study by Kündig et al. (19) describes what they call a “qualitative change” of memory T cells after adoptive transfer into irradiated recipients compared with nonirradiated recipients, possibly suggesting that irradiation of the recipients activates transferred memory T cells. In contrast to the classic studies, which used less sensitive immunoassays to measure antibodies, we have used a highly sensitive ELISA to detect PS-specific antibodies (13). We observed 10-fold more GBSIII IgG antibodies after transfer of splenocytes from GBSIII-TT-immunized donors than from placebo-immunized donors when we used nonirradiated recipient mice. This sensitive assay allowed us to circumvent the need for irradiation of the recipient mice and made it possible to avoid the confounding factors that irradiation might introduce.
While extensive research has demonstrated that a PS conjugated to an immunogenic carrier protein can elicit anti-PS antibody in a T-cell-dependent fashion and protect against invasive disease (11, 23), the cellular mechanisms involved are not defined (12, 27). The adoptive cell transfer model described here will be a useful tool to study in vivo which cells are responsible for the transfer of immunologic memory for the PS moiety of glycoconjugate vaccines. The model will also be helpful to evaluate conjugate vaccines with different physicochemical properties for their ability to induce immunologic memory.
ACKNOWLEDGMENTS
We thank April Blodgett, Julieanne Pinel, and Barbara G. Reinap (Channing Laboratory) for polysaccharide production and purification and conjugate production; Yu Ho (The Maxwell Finland Laboratory for Infectious Diseases) for invaluable technical assistance with the splenocyte preparation and evaluation; Jeanie H. Kwon (Channing Laboratory) for assistance with analysis of antibody responses; and Harold J. Jennings (National Research Council of Canada) for gas chromatography-mass spectrometry analysis.
This work was supported by National Institutes of Health, National Institutes of Allergy and Infectious Diseases grant AI 23339, training grant T32 AI-07061 (to H.-K. Guttormsen), and the Edward and Amalie Kass Fellowship (to H.-K. Guttormsen).
REFERENCES
- 1.Anonymous. In vitro assays for mouse lymphocyte function. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 3.1.2–3.6.4. [DOI] [PubMed] [Google Scholar]
- 1a.Anonymous. Immunofluorescence and cell sorting. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 5.3.1–5.4.13. [Google Scholar]
- 2.Baker C J, Edwards M S. Group B streptococcal infections. Perinatal impact and prevention methods. Ann N Y Acad Sci. 1988;549:193–202. doi: 10.1111/j.1749-6632.1988.tb23972.x. [DOI] [PubMed] [Google Scholar]
- 3.Baker C J, Rench M A, Edwards M S, Carpenter R J, Hays B M, Kasper D L. Immunization of pregnant women with a polysaccharide vaccine of group B Streptococcus. N Engl J Med. 1988;319:1180–1220. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- 4.Baker C J, Rench M A, Kasper D L. Response to type III polysaccharide in women whose infants have had invasive group B streptococcal infection. N Engl J Med. 1990;322:1857–1860. doi: 10.1056/NEJM199006283222606. [DOI] [PubMed] [Google Scholar]
- 5.Barbour M L, Mayon-White R T, Coles C, Crook D W M, Moxon E R. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J Infect Dis. 1995;171:93–98. doi: 10.1093/infdis/171.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Bishop C T, Jennings H J. Immunology. In: Aspinall G O, editor. The polysaccharides. Vol. 1. New York, N.Y: Academic Press, Inc.; 1982. pp. 292–328. [Google Scholar]
- 7.Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966;124:1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: a public health perspective. Morbid Mortal Weekly Rep. 1996;45(RR-7):1–25. [PubMed] [Google Scholar]
- 9.Claman H N, Chaperon E A, Triplett R F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966;122:1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- 10.Dick W E J, Beurret M. Glycoconjugates of bacterial carbohydrate antigens. A survey and consideration of design and preparation factors. In: Cruise J M, Lewis R E Jr, editors. Conjugate vaccines. Vol. 10. Basel, Switzerland: Karger; 1989. pp. 48–114. [PubMed] [Google Scholar]
- 11.Eskola J, Peltola H, Takala A K, Kayhty H, Hakulinen M, Karanko V, Kela E, Rekola P, Ronnberg P R, Samuelsen J S, et al. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N Engl J Med. 1987;317:717–722. doi: 10.1056/NEJM198709173171201. [DOI] [PubMed] [Google Scholar]
- 12.Garner C V, Pier G B. Immunologic considerations for the development of conjugate vaccines. In: Cruise J M, Lewis R E Jr, editors. Conjugate vaccines. Vol. 10. Basel, Switzerland: Karger; 1989. pp. 11–17. [PubMed] [Google Scholar]
- 13.Guttormsen H-K, Baker C J, Edwards M S, Paoletti L C, Kasper D L. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J Infect Dis. 1996;173:142–150. doi: 10.1093/infdis/173.1.142. [DOI] [PubMed] [Google Scholar]
- 14.Guttormsen, H.-K., and D. L. Kasper. Unpublished data.
- 15.Guttormsen H-K, Wetzler L M, Næss A. Humoral immune response to the class 3 outer membrane protein during the course of meningococcal disease. Infect Immun. 1993;61:4734–4742. doi: 10.1128/iai.61.11.4734-4742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janeway C A J, Travers P. Immunobiology: the immune system in health and disease. New York, N.Y: Current Biology Ltd./Garland Publishing Inc.; 1994. [Google Scholar]
- 17.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H-K, Carey V J, Jennings H J, Baker C J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz D H, Benacerraf B. The regulatory influenze of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- 19.Kündig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchison N A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurements of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971;1:10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- 21.Paul W E. Fundamental immunology. 2nd ed. New York, N.Y: Raven Press; 1989. [Google Scholar]
- 22.Plikytaris B D, Holder P F, Pais L B, Maslanka S E, Gheesling L L, Carlone G M. Determination of parallelism and nonparallelism in bioassay dilution curves. J Clin Microbiol. 1994;32:2441–2447. doi: 10.1128/jcm.32.10.2441-2447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbins J B, Schneerson R, Anderson P, Smith D H. Prevention of systemic infections, especially meningitis, caused by Haemophilus influenzae type b. JAMA. 1996;276:1181–1185. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 24.Rosner B. Fundamentals of biostatistics. 3rd ed. Boston, Mass: PWS-Kent; 1990. [Google Scholar]
- 25.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 26.Spear P G, Wang A-L, Rutishauser U, Edelman G M. Characterization of splenic lymphoid cells in fetal and newborn mice. J Exp Med. 1973;138:557–573. doi: 10.1084/jem.138.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein K E. Glycoconjugate vaccines. What next? Int J Technol Assess Health Care. 1994;10:167–176. doi: 10.1017/s0266462300014094. [DOI] [PubMed] [Google Scholar]
- 28.Stein, K. E. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. S49–S52. [DOI] [PubMed]
- 29.Ward K N. Helper activity of T lymphocytes which have been stimulated by keyhole limpet haemocyanin in vitro. Immunology. 1981;43:111–116. [PMC free article] [PubMed] [Google Scholar]
- 30.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wessels M R, Pozsgay V, Kasper D L, Jennings H J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987;262:8262–8267. [PubMed] [Google Scholar]