Abstract
Practical relevance:
Stem cell therapy is an innovative field of scientific investigation with tremendous potential for clinical application in veterinary medicine. Based on the known desirable immunomodulatory properties of mesenchymal stem cells, this therapy holds promise for the treatment of a variety of inflammatory diseases in cats.
Aims:
This review details our current understanding of feline stem cell biology and proposed mechanism of action. Studies performed in feline clinical trials for diseases including gingivostomatitis, chronic enteropathy, asthma and kidney disease are summarized, with the goal of providing an overview of the current status of this treatment modality and its potential for the future.
Emergence of a new therapeutic strategy
Feline mesenchymal stem/stromal cells (MSCs) were first isolated and characterized from bone marrow (bMSCs) in 2002, 1 and later readily expanded from fat, 2 and fetal fluids and amniotic membranes. 3 Currently, adipose-derived MSCs (aMSCs) are most commonly used in clinical applications due to ease of attainment and their superior pro-liferative ability. 2 Feline MSCs meet the minimal defining criteria for multipotent stem cells as published over a decade ago 4 and ongoing research confirms that they also have similar immunomodulatory potency as more recently defined for human MSCs.5,6 In addition to culture-expanded MSCs, regenerative medicine therapies include commercially available patient-side therapies such as platelet-rich plasma, bone marrow concentrate and the stromal vascular fraction (SVF) of fat.
In cats, published data are focused exclusively on the use of culture-expanded MSCs. SVF has the advantage of a fast turnaround time; however, the product is heterogeneous and includes only small numbers of stem cells relative to a culture-expanded population of aMSCs. Thus, in this review, we discuss the biology, immunology and clinical application of culture-expanded MSCs in cats.
Feline MSC biology and immunology
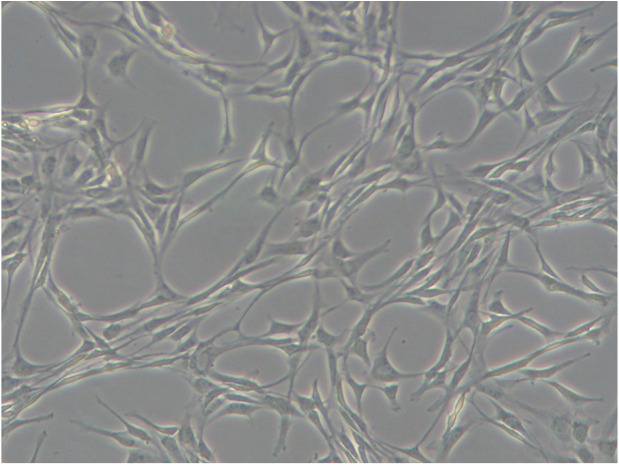
Like all MSCs, feline MSCs have a fibroblast morphology in culture (Figure 1) and they express CD44 and CD105, and variably express CD90 on their surface.1,2,6 MSCs do not express leukocyte antigens (CD18, CD4) or major histo-compatibility complex (MHC) II.1,2,6 Functionally in vitro, feline MSCs undergo trilineage differentiation, inhibit activated lymphocyte proliferation, and secrete a variety of mediators capable of modulating immune cell function.6,7
Figure 1.
Feline mesenchymal stem cells (MSCs) in culture are plastic-adherent and display a typical fibroblast-like appearance
Feline MSCs are similar to simian MSCs in that 20–50% of feline MSC lines can be infected by a foamy virus. 8 Feline foamy virus (FFV) is a member of the Retroviridae family, is present in 20–80% of cats and is not associated with a clinical disorder.9,10 FFV replication in cell lines is associated with syncytial cell formation, decreased proliferation rate and, finally, cell death. 11 MSC lines derived from specific pathogen-free (SPF) cats do not appear to be infected with FFV and may be a source of allogeneic MSCs for clinical application. FFV infection of MSC lines may hinder large-scale expansion of autologous MSCs for therapeutic use in feline patients. 8
Feline MSCs largely mimic the immuno-modulatory phenotype described for human, equine and canine MSCs – with a few notable differences. Numerous groups have demonstrated that feline MSCs inhibit activated T lymphocyte proliferation.6,7,18,19 Feline MSCs also reduce neutrophil production of reactive oxygen species. 20 The interaction of feline MSCs with other cells of the innate and humoral immune systems, including dendritic cells, monocytes, eosinophils, NK cells and B lymphocytes, and the mechanisms involved have not yet been detailed but will be important areas for future studies.
To understand how feline MSCs respond to inflammatory stimuli, they have been incubated with allogeneic peripheral blood mono-nuclear cells (PBMCs) or stimulated with lectins (concanavalin A), interferon gamma (IFNγ), and/or tumor necrosis factor alpha (TNFα).6,7 In response to these stimuli, MSCs upregulate gene expression of immunomodulatory mediators including indoleamine 2,3-dioxygenase (IDO)1, programmed death ligand-1, interleukin (IL)-6, cyclooxygenase 2 and hepatocyte growth factor. 7 They also secrete IDO, prostaglandin E2 (PGE2), IL-6, vascular endothelial growth factor, IL-8 and transforming growth factor beta.6,7 The inhibition of lymphocyte proliferation depends on both soluble mediators and direct contact between the aMSCs and lymphocytes; however, the actual ligands and mediators have not been determined. 6 In other species, IDO and PGE2 have been implicated in the initiation of lymphocyte cell cycle arrest and/or lymphocyte apoptosis.21–23 Feline aMSCs potently inhibit the pro-inflammatory cytokine TNFα in vitro; however, their interaction with IFNγ is more complicated. 6 Feline aMSCs are able to inhibit lymphocyte proliferation and secrete immunomodulatory mediators in the presence of high concentrations of IFNγ. 6
The transcriptome of three feline aMSC lines has been defined and functional analysis of the most highly expressed genes revealed processes relating to: 1) the regulation of apoptosis; 2) cell adhesion; 3) response to oxidative stress; and 4) regulation of cell dif-ferentiation. 6 Together these data suggest that feline MSCs are highly similar in their functional and, notably, immunomodulatory capacities to MSCs of other species.
Clinical applications of MSCs in feline disease
Given the desirable immunomodulatory properties of MSCs, this therapy has potential for treatment of a variety of inflammatory diseases. In cats, MSCs have been evaluated as therapies for a number of inflammatory, degenerative and immune-mediated diseases including feline chronic gingivostomatitis (FCGS), 18 acute and chronic kidney disease (CKD),24–28 enteropathies 29 and asthma.30,31 An overview of clinical studies is provided in Table 1. MSCs could also be explored for feline osteoarthritis and cardiomyopathy, among other diseases.32,33 Additionally, many of these naturally occurring diseases have translational potential for similar diseases in people, including oral inflammatory diseases, chronic pancreatitis, idiopathic cystitis, CKD, inflammatory bowel disease, osteoarthritis, cardio-myopathy and asthma.32,34
Table 1.
Clinical applications of mesenchymal stem cells in feline disease
| Disease | Study type | MSC source | Interventions | Outcome | Reference |
|---|---|---|---|---|---|
| Gingivostomatitis | Open-label, baseline-controlled clinical trial | Autologous adipose-derived | Two doses IV aMSCs 30 days apart (7 cats) | Complete remission (3 cats), substantial improvement (2 cats), no response (2 cats) | Arzi et al 18 |
| Gingivostomatitis | Open-label, baseline-controlled clinical trial | Allogeneic adipose-derived | Two doses IV aMSCs 30 days apart (7 cats) | Complete remission (2 cats), substantial improvement (2 cats), no response (3 cats) | Arzi et al 8 |
| Acute experimental asthma | Randomized, placebo-controlled study | Allogeneic adipose-derived | Five doses IV aMSCs (4 cats), with 14, 14, 14, 70 and 30 days between each respective dose, or placebo (2 cats) | Decrease in airway eosinophilia, diminished airway hyper-responsiveness, decreased airway remodeling | Trzil et al 31 |
| Chronic experimental asthma | Randomized, placebo-controlled study | Allogeneic adipose-derived | Six doses IV aMSCs (5 cats) 14 days apart or placebo (4 cats) | Decreased airway remodeling | Trzil et al 30 |
| Chronic enteropathy | Owner-blinded, randomized, placebo-controlled clinical trial | Allogeneic adipose-derived | Two doses IV aMSCs 14 days apart (7 cats) or placebo (4 cats) | Improved clinical signs in 5/7 aMSC-treated cats and 0/4 placebo cats | Webb and Webb 29 |
| CKD | Open-label, baseline-controlled clinical trial | Autologous adipose-derived or bone marrow-derived | Single unilateral intrarenal injection of aMSCs (3 cats) or bMSCs (3 cats) | Mild decrease in serum creatinine and increase in GFR in 2 cats that received aMSCs | Quimby et al 24 |
| CKD | Three open-label, baseline-controlled, pilot clinical trials | Allogeneic adipose-derived | Three doses IV aMSCs 14 days apart (16 cats) | Mild decrease in serum creatinine in 7/15 cats (1 cat excluded due to medication non-compliance) and increase in GFR in 4/16 cats. Side effects included vomiting and increased respiratory rate | Quimby et al 25 |
| CKD | Randomized, placebo-controlled clinical trial | Allogeneic adipose-derived | Three doses IV aMSCs (4 cats) 14 days apart or placebo (3 cats) | No significant improvement in creatinine or GFR | Quimby et al 26 |
| Ischemic AKI | Randomized, placebo-controlled study | Allogeneic adipose-derived or bone marrow-derived | Single dose IV aMSCs (5 cats), bMSCs (5 cats) or fibroblasts (5 cats) 1 h after unilateral ischemia | No difference in percentage of cats with AKI | Rosselli et al 27 |
| CKD | Open-label, baseline-controlled clinical trial | Allogeneic amnion-derived | Two doses IV amnion-derived MSCs 21 days apart (9 cats) | Mild decrease in serum creatinine | Vidane et al 28 |
IV = intravenous; MSC = mesenchymal stem cell; aMSCs = adipose-derived MSCs; bMSCs = bone marrow-derived MSCs; GFR = glomerular filtration rate; AKI = acute kidney injury; CKD = chronic kidney disease
Feline chronic gingivostomatitis
FCGS is a severe, painful, inflammatory oral disease that is often refractory to treatment and is estimated to affect 0.7–12% of cats presenting to veterinary practices.40–42 Clinical signs reflect oral discomfort, including inappetence, reduced grooming, weight loss and hypersalivation. FCGS can be debilitating and may result in euthanasia of severely affected cats. There is no cure and the current standard of care consists of full or near-full mouth tooth extraction and lifelong therapy with antibiotics, steroids and analgesics. 42 The pathogenesis of FCGS is complex and multifactorial, and may result from alterations in microbiota, loss of self-tolerance to antigens, alterations in innate immunity or underlying viral disease.43,44 Histologically, lesions in cats are characterized by lymphocyte-rich inflammation with a predominance of effector T cells and B cells. 40
Given the predominance of T cell activation in FCGS and the absence of a curative therapy, aMSCs have been utilized to treat FCGS in two clinical trials because of their potent ability to downregulate T cell activation. In both clinical trials, FCGS cats that were refractory to full mouth tooth extraction were enrolled. In addition, these cats had no known concurrent diseases (eg, feline immunodeficiency virus/feline leukemia virus negative).18,35 In each trial, seven FCGS cats received two intravenous (IV) injections of 2 × 107 aMSCs 3–4 weeks apart. In the first trial, the seven cats received autologous aMSCs; 18 in the second trial, the seven cats received unmatched, allogeneic aMSCs from SPF donor cats. 35 The outcome of these published studies, as well as other clinical trials, is summarized in the box below.
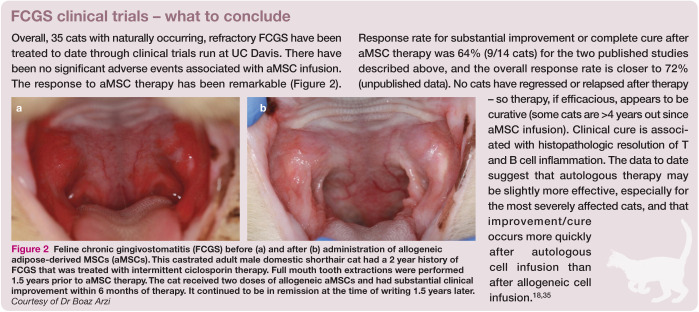
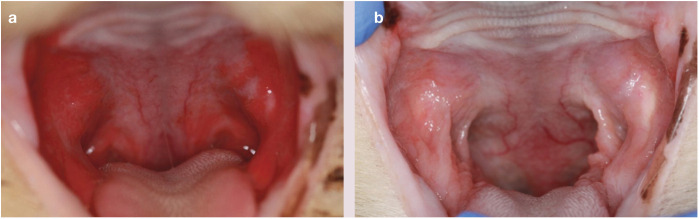
Figure 2.
Feline chronic gingivostomatitis (FCGS) before (a) and after (b) administration of allogeneic adipose-derived MSCs (aMSCs). This castrated adult male domestic shorthair cat had a 2 year history of FCGS that was treated with intermittent ciclosporin therapy. Full mouth tooth extractions were performed 1.5 years prior to aMSC therapy. The cat received two doses of allogeneic aMSCs and had substantial clinical improvement within 6 months of therapy. It continued to be in remission at the time of writing 1.5 years later. Courtesy of Dr Boaz Arzi
Clinical trials are ongoing and have expanded to include a multicenter investigation with a crossover design to ensure that control cats are recruited. Ongoing work includes: 1) pursuing mechanism of action and biomarker studies (focused on immunomodulation, and blood and lymph node CD4 and CD8 T cells); 6 2) refining clinical trial parameters to increase the therapeutic efficacy of aMSC infusion; 3) determining whether aMSCs can be used prior to full mouth tooth extraction; and 4) enrolling cats with concurrent diseases to determine efficacy in a broader range of diseased cats.
Asthma
Feline asthma is a component of feline lower respiratory disease that is characterized by airway eosinophilia, airway hyper-responsiveness and remodeling. Clinical management to target inflammation and open airways consists of oral or inhaled corticosteroid and bron-chodilator therapy. However, these therapies are associated with side effects and are typically lifelong, and may not result in a therapeutic response or prevent progression of disease.
The application of MSC therapy to this disease process would be ideal given the immunomodulatory capabilities demonstrated by MSCs and their passage through the lung when administered intravenously. In rodent models, improvement in airway eosinophilia, airway hyper-responsiveness and remodeling has been demonstrated following MSC therapy. 30 Two placebo-controlled pilot studies have evaluated aMSC therapy in cats in an experimental model of feline asthma; both involved sensitization to Bermuda grass allergen, which results in development of an asthmatic phenotype including airway eosinophilia and airway hyper-responsiveness.30,31
The first study involved administration of allogeneic aMSCs to cats in which asthma had been induced a relatively short time period before treatment. 31 A total of five IV injections of allogeneic aMSCs were administered (four cats) or saline placebo (two cats). Due to difficulties with expansion of cells to obtain adequate quantities for the study, the number of cells administered varied between 2 × 106 and 1 × 107 per cat; initially aMSCs were from cryopreservation and subsequently they were cultured fresh from cryopreserved adipose.
The second study assessed the effects of aMSC infusion on cats that had chronic experimentally induced asthma (induced 9 months prior to study initiation). 30 Cats were randomized to receive a total of six IV injections of fresh allogeneic aMSCs cultured from cryo-preserved adipose (five cats) at a dose of 3.6 × 106 to 2.5 × 107 per cat (average 1.4 × 107 per cat) or saline placebo (four cats).
The results of these two clinical trials are described in the box below.
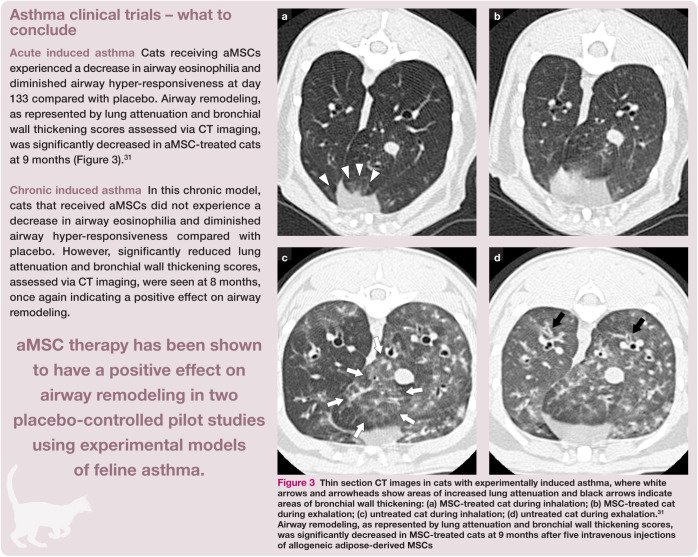
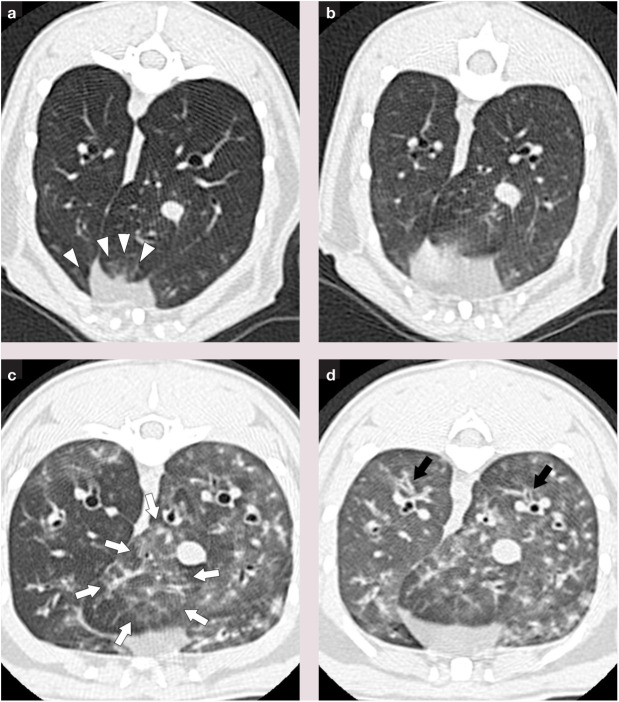
Figure 3.
Thin section CT images in cats with experimentally induced asthma, where white arrows and arrowheads show areas of increased lung attenuation and black arrows indicate areas of bronchial wall thickening: (a) MSC-treated cat during inhalation; (b) MSC-treated cat during exhalation; (c) untreated cat during inhalation; (d) untreated cat during exhalation.31 Airway remodeling, as represented by lung attenuation and bronchial wall thickening scores, was significantly decreased in MSC-treated cats at 9 months after five intravenous injections of allogeneic adipose-derived MSCs
Chronic enteropathy
Chronic enteropathies such as inflammatory bowel disease are common in cats and likely result from alterations in gastro-intestinal mucosal immunity and loss of tolerance to intestinal antigens.29,45 Clinical management is typically lifelong and includes dietary therapy and corticosteroid administration. Patient and/or owner compliance can be a concern with chronic therapies and not all cats experience a treatment response. Therefore, alternative approaches are needed.
The efficacy of allogeneic aMSCs for the treatment of cats with clinical signs of chronic enteropathy (diarrhea and/or vomiting for >3 months) was assessed in a randomized, single-blinded, placebo-controlled clinical trial. 29 Two IV injections of 2 × 106/kg aMSCs fresh cultured from cryopreserved adipose (seven cats) or saline placebo (four cats) were administered 2 weeks apart and cats were followed for 1–2 months. Owners answered a questionnaire at the beginning and end of the study about medications, supplements, diet, appetite and clinical signs, including quantification of the frequency and consistency of diarrhea, and the presence and frequency of vomiting. A Texas A&M gastrointestinal panel (feline pancreatic lipase immunoreactivity, feline trypsin-like immunoreactivity, folate and cobalamin) was performed at the beginning of the study and 2 weeks after the second injection. No other changes in diet or medications were allowed during the study period. Results of the trial are reported in the box below.

Kidney disease
A series of pilot studies assessing the safety and efficacy of administration of MSCs for treatment of cats with CKD has been conducted.24–26
The first MSC study in cats with CKD was designed to assess the safety and feasibility of autologous intrarenal MSC therapy. 24 Six cats (two healthy, four with CKD) received a single unilateral intrarenal injection of autologous bMSCs or aMSCs via ultrasound guidance. Two IRIS stage 3 CKD cats that received aMSCs experienced modest improvement in glomerular filtration rate (GFR) and a mild decrease in serum creatinine concentration. Intrarenal injection of MSCs did not induce immediate or longer term adverse effects but it was concluded that the number of sedations and interventions required to implement this approach made it unattractive for clinical application. In the course of conducting this study it was also determined that expanding sufficient numbers of autologous MSCs in culture from elderly diseased patients was very difficult and time-consuming. A more recent study in which one healthy cat received an intrarenal injection of amniotic-derived allogeneic MSCs documented hematuria and significant stress as a result of the procedure, and this study also determined the technique not to be clinically feasible. 28
The feasibility of IV administration of allogeneic aMSCs to cats with CKD has also been investigated. 25 Stable CKD cats with no concurrent illness were enrolled in a series of pilot studies and received every 2 weeks an IV infusion of allogeneic aMSCs collected and cryopreserved from healthy young SPF cats. Six cats in pilot 1 received 2 × 106 cryopre-served aMSCs per infusion, five cats in pilot 2 received 4 × 106 cryopreserved aMSCs per infusion, and five cats in pilot 3 received 4 × 106 aMSCs cultured from cryopreserved adipose. Cats in pilot 1 had few adverse effects from the aMSC infusions and there was a statistically significant decrease in serum creatinine concentrations during the study period. However, the degree of decrease was judged not to represent a clinically relevant improvement.
Notably, adverse effects of aMSC infusion were observed in the majority of cats enrolled in the second pilot study following treatment with MSCs taken directly from cryopreservation. Vomiting occurred in 2/5 cats during infusion, and increased respiratory rate and effort was noted in 4/5 cats. In contrast, cats in pilot study 3 that received aMSCs cultured from cryopreserved adipose did not experience any adverse side effects. Serum creatinine concentrations, urinary cytokines and GFR did not change significantly in cats in these latter two studies. Based on the accumulated results of the three pilot studies, it appeared that use of higher doses of aMSCs taken directly from cryopreservation was the source of the treatment-related adverse effects. The most likely explanation for this reaction is an instant blood-mediated inflammatory reaction, which results in clumping of the cells as they contact the blood and potential subsequent micropulmonary thromboembolism. 46
A placebo-controlled, blinded, one-way, crossover clinical study assessing the efficacy of allogeneic MSCs expanded from cryopre-served adipose with repeated administrations has also been performed. 26 Four cats were randomized to receive 2 × 106 aMSCs/kg intravenously at 2, 4 and 6 weeks and three cats were randomized to receive saline placebo. While administration of aMSCs was not associated with adverse effects, significant improvement in renal function (as determined by serum creatinine and GFR by nuclear scintigraphy) was not observed in the weeks following administration.
The IV administration of allogeneic MSCs derived from amniotic membrane has been assessed in nine cats with CKD that received two injections of 2 × 106 MSCs 21 days apart. 28 One cat experienced vomiting during the first administration, but otherwise the MSCs were well tolerated. A statistically significant but mild decrease in serum creatinine was seen, with stable body weight over the course of the study. Mild improvements in proteinuria and urine specific gravity were also seen. However, studies with a control group are necessary to determine if changes are attributable to MSC therapy or normal variation in values.
Application of aMSCs for acute kidney injury (AKI) in an ischemic kidney model has also been investigated. 27 Adult research cats underwent unilateral renal ischemia for 60 mins. One hour after reperfusion, 4 × 106 of one of the following cell types were administered via jugular catheter: aMSCs (five cats), bMSCs (five cats) or fibroblasts (five cats). Three historical control cats that had previously undergone ischemia as part of this model were used for comparison. No difference in the percentage of cats that developed AKI and no difference in urine specific gravity, protein-uria, GFR or histopathology were noted as a result of MSC administration in this model. 27
Key Points
Although MSC therapy potentially has great applicability to feline disease, there are still many questions to be answered regarding the logistics of its use.
The optimal route of MSC administration, the ideal source of MSCs (allogeneic vs autologous; culture-expanded vs SVF) and the impact of tissue donor status (eg, age, disease status, sex) on MSC function remain to be determined.
Studies are currently under way investigating many of these aspects and additional information is eagerly awaited.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Funding for MSC studies performed by the authors was provided by the Winn Feline Foundation; the Morris Animal Foundation; Frankie’s Fund for Feline Stem Cell Therapy; and by an NIH Grant (1R21DE024711-01).
Contributor Information
Jessica M Quimby, The Ohio State University, Department of Veterinary Clinical Sciences, Columbus, OH 43210, USA.
Dori L Borjesson, University of California–Davis, Veterinary Institute for Regenerative Cures, and Department of Pathology, Microbiology and Immunology, Davis, CA 95616, USA.
References
- 1. Martin DR, Cox NR, Hathcock TL, et al. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol 2002; 30: 879–886. [DOI] [PubMed] [Google Scholar]
- 2. Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg 2012; 14:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iacono E, Cunto M, Zambelli D, et al. Could fetal fluid and membranes be an alternative source for mesenchymal stem cells (MSCs) in the feline species? A preliminary study. Vet Res Commun 2012; 36:107–118. [DOI] [PubMed] [Google Scholar]
- 4. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchy-mal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 5. Galipeau J, Krampera M, Barrett J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016; 18: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark KC, Fierro FA, Ko EM, et al. Human and feline adipose-derived mesenchymal stem cells have comparable phenotype, immunomodulatory functions, and transcrip-tome. Stem Cell Res Ther 2017; 8: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parys M, Kruger JM, Yuzbasiyan-Gurkan V. Evaluation of immunomodulatory properties of feline mesenchymal stem cells. Stem Cells Dev 2017; 26: 776–785. [DOI] [PubMed] [Google Scholar]
- 8. Arzi B, Kol A, Murphy B, et al. Feline foamy virus adversely affects feline mesenchymal stem cell culture and expansion: implications for animal model development. Stem Cells Dev 2015; 24: 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bleiholder A, Muhle M, Hechler T, et al. Pattern of seroreactivity against feline foamy virus proteins in domestic cats from Germany. Vet Immunol Immunopathol 2011; 143: 292–300. [DOI] [PubMed] [Google Scholar]
- 10. Winkler IG, Lochelt M, Flower RL. Epidemiology of feline foamy virus and feline immunodeficiency virus infections in domestic and feral cats: a seroepidemiological study. J Clin Microbiol 1999; 37: 2848–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikeda Y, Itagaki S, Tsutsui S, et al. Replication of feline syncytial virus in feline T-lymphoblas-toid cells and induction of apoptosis in the cells. Microbiol Immunol 1997; 41: 431–435. [DOI] [PubMed] [Google Scholar]
- 12. Carrade DD, Borjesson DL. Immuno -modulation by mesenchymal stem cells in veterinary species. Comp Med 2013; 63: 207–217. [PMC free article] [PubMed] [Google Scholar]
- 13. Fu Y, Karbaat L, Wu L, et al. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng Part B Rev 2017; 23: 515–528. [DOI] [PubMed] [Google Scholar]
- 14. Glenn JD, Whartenby KA. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014; 6: 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 2015; 40: 82–88. [DOI] [PubMed] [Google Scholar]
- 16. Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodu-latory therapeutic strategy for autoimmune diseases. Autoimmun Rev 2011; 10: 410–415. [DOI] [PubMed] [Google Scholar]
- 17. Le Blanc K, Davies LC. Mesenchymal stro-mal cells and the innate immune response. Immunol Lett 2015; 168:140–146. [DOI] [PubMed] [Google Scholar]
- 18. Arzi B, Mills-Ko E, Verstraete FJ, et al. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med 2016; 5: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zajic LB, Webb TL, Webb P, et al. Comparison of proliferative and immunomodulatory potential of adipose-derived mesenchymal stem cells from young and geriatric cats. J Feline Med Surg 2017; 19:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mumaw JL, Schmiedt CW, Breidling S, et al. Feline mesenchymal stem cells and supernatant inhibit reactive oxygen species production in cultured feline neutrophils. Res Vet Sci 2015; 103: 60–69. [DOI] [PubMed] [Google Scholar]
- 21. Carrade DD, Lame MW, Kent MS, et al. Comparative analysis of the immunomodula-tory properties of equine adult-derived mes-enchymal stem cells. Cell Med 2012; 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carrade Holt DD, Wood JA, Granick JL, et al. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev 2014; 23: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 23. Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103: 4619–4621. [DOI] [PubMed] [Google Scholar]
- 24. Quimby JM, Webb TL, Gibbons DS, et al. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: a pilot study. J Feline Med Surg 2011; 13: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quimby JM, Webb TL, Habenicht LM, et al. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther 2013; 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quimby JM, Webb TL, Randall E, et al. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: a randomized, placebo-controlled clinical trial in eight cats. J Feline Med Surg 2016; 18:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosselli DD, Mumaw JL, Dickerson V, et al. Efficacy of allogeneic mesenchymal stem cell administration in a model of acute ischemic kidney injury in cats. Res Vet Sci 2016; 108:18–24. [DOI] [PubMed] [Google Scholar]
- 28. Vidane AS, Pinheiro AO, Casals JB, et al. Transplantation of amniotic membrane-derived multipotent cells ameliorates and delays the progression of chronic kidney disease in cats. Reprod Dottiest Anim 2017; 52 Suppl 2: 316–326. [DOI] [PubMed] [Google Scholar]
- 29. Webb TL, Webb CB. Stem cell therapy in cats with chronic enteropathy: a proof-of-concept study. J Feline Med Surg 2015; 17: 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trzil JE, Masseau I, Webb TL, et al. Long-term evaluation of mesenchymal stem cell therapy in a feline model of chronic allergic asthma. Clin Exp Allergy 2014; 44:1546–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trzil JE, Masseau I, Webb TL, et al. Intravenous adipose-derived mesenchymal stem cell therapy for the treatment of feline asthma: a pilot study. J Feline Med Surg 2016; 18: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffman AM, Dow SW. Concise review: stem cell trials using companion animal disease models. Stem Cells 2016; 34:1709–1729. [DOI] [PubMed] [Google Scholar]
- 33. Taghavi S Sharp TE 3rd Duran JM et al. Autologous c-Kit+ mesenchymal stem cell injections provide superior therapeutic benefit as compared to c-Kit+ cardiac-derived stem cells in a feline model of isoproterenol-induced cardiomyopathy. Clin Fransl Sci 2015; 8: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kol A, Arzi B, Athanasiou KA, et al. Companion animals: translational scientist’s new best friends. Sci Fransl Med 2015; 7: 308ps21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arzi B, Clark KC, Sundaram A, et al. Therapeutic efficacy of fresh, allogeneic mesenchymal stem cells for severe refractory feline chronic gingivostomatitis. Stem Cells Fransl Med 2017; 6: 1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klinkhammer BM, Kramann R, Mallau M, et al. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS One 2014; 9: e92115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Idziak M, Pedzisz P, Burdzinska A, et al. Uremic toxins impair human bone marrow-derived mesenchymal stem cells functionality in vitro. Exp Foxicol Pathol 2014; 66: 187–194. [DOI] [PubMed] [Google Scholar]
- 38. Parys M, Nelson N, Koehl K, et al. Safety of intraperitoneal injection of adipose tissue-derived autologous mesenchymal stem cells in cats. J Vet Intern Med 2016; 30:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quimby JM, Webb TL, Randall E, et al. Retroperitoneal delivery of mesenchymal stem cells for the treatment of feline chronic kidney disease. J Vet Intern Med 2015; 29:1216. [Google Scholar]
- 40. Harley R, Gruffydd-Jones TJ, Day MJ. Immunohistochemical characterization of oral mucosal lesions in cats with chronic gingivo-stomatitis. J Comp Pathol 2011; 144: 239–250. [DOI] [PubMed] [Google Scholar]
- 41. Healey KA, Dawson S, Burrow R, et al. Prevalence of feline chronic gingivostomatitis in first opinion veterinary practice. J Feline Med Surg 2007; 9: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winer JN, Arzi B, Verstraete FJ. Therapeutic management of feline chronic gingivostomati-tis: a systematic review of the literature. Front Vet Sci 2016; 3: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lommer MJ, Verstraete FJ. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis. Oral Microbiol Immunol 2003; 18: 131–134. [DOI] [PubMed] [Google Scholar]
- 44. Dowers KL, Hawley JR, Brewer MM, et al. Association of Bartonella species, feline cali-civirus, and feline herpesvirus 1 infection with gingivostomatitis in cats. J Feline Med Surg 2010; 12: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Janeczko S, Atwater D, Bogel E, et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol 2008; 128: 178–193. [DOI] [PubMed] [Google Scholar]
- 46. Moll G, Rasmusson-Duprez I, von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012; 30: 1565–1574. [DOI] [PubMed] [Google Scholar]
- 47. Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 2009; 27: 3063–3073. [DOI] [PubMed] [Google Scholar]
- 48. Villanueva S, Ewertz E, Carrion F, et al. Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci 2011; 121: 489–499. [DOI] [PubMed] [Google Scholar]
- 49. Lee SR, Lee SH, Moon JY, et al. Repeated administration of bone marrow-derived mesenchy-mal stem cells improved the protective effects on a remnant kidney model. Ren Fail 2010; 32: 840–848. [DOI] [PubMed] [Google Scholar]
- 50. Cavaglieri RC, Martini D, Sogayar MC, et al. Mesenchymal stem cells delivered at the sub-capsule of the kidney ameliorate renal disease in the rat remnant kidney model. Fransplant Proc 2009; 41: 947–951. [DOI] [PubMed] [Google Scholar]