Abstract
For some intermetallic compounds containing lanthanides, structural transitions can result in intermediate electronic states between trivalency and tetravalency; however, this is rarely observed for praseodymium compounds. The dominant trivalency of praseodymium limits potential discoveries of emergent quantum states in itinerant 4f1 systems accessible using Pr4+-based compounds. Here, we use in situ powder x-ray diffraction and in situ electron energy-loss spectroscopy (EELS) to identify an intermetallic example of a dominantly Pr4+ state in the polymorphic system Pr2Co3Ge5. The structure-valence transition from a nearly full Pr4+ electronic state to a typical Pr3+ state shows the potential of Pr-based intermetallic compounds to host valence-unstable states and provides an opportunity to discover previously unknown quantum phenomena. In addition, this work emphasizes the need for complementary techniques like EELS when evaluating the magnetic and electronic properties of Pr intermetallic systems to reveal details easily overlooked when relying on bulk magnetic measurements alone.
An example of a dominantly Pr4+ intermetallic compound is reported using complementary in situ techniques.
INTRODUCTION
Crystalline structure plays a key role in controlling thermal decomposition in batteries, improving catalyst efficiency, and the development of quantum materials due to its explicit relationship with electronic structure and chemical behavior. In the latter group, electron-electron interactions result in the formation of strongly correlated electronic states that can be linked to emergent quantum phenomena depending on the presence of specific structural elements (1). For example, in combination with strong electron interactions, nonsymmorphic symmetry elements give rise to topological electronic states (2, 3) and geometrically frustrated lattices to magnetic spin-liquid behavior (4–6), both of which find applications in spintronic and quantum computing (7).
Often, materials obtain their strongly correlated nature from the presence of lanthanide elements with 4f electrons at the limit between local moment and itinerant behavior (8–12). Valence instabilities in lanthanide compounds can have substantial impacts on crystal structure and physical properties (13–16). Targeting systems with structural phase transformations can provide an exciting path forward for strongly correlated materials, specifically in identifying valence-unstable states in dominantly trivalent lanthanides like Pr with the opportunity to access technologically relevant quantum states (17–20). Still, Pr compounds are typically trivalent, with only a small number of dominantly tetravalent Pr-containing oxides and fluorides reported (21). Critically, there are no examples of dominantly tetravalent intermetallic compounds leaving much more to explore regarding the impact of Pr4+ ions on their magnetic properties. Given the link between structural and valence transitions, one avenue for identifying an intermetallic system capable of hosting tetravalent Pr is to target structure types where instabilities in electronic structure and valence are already present.
A particularly deep reservoir for studies of interesting electronic states are compounds with the formula A2M3X5 (A = lanthanide, actinide, Y, Sc; M = transition metal; X = main group metal) where various examples exhibit quantum critical points, unstable valence states, superconductivity, topological semimetallic states, charge density waves, and other phenomena (22). These compounds crystallize in eight known structure types and can be considered polyanionic three-dimensional frameworks of transition and main group metals that are most commonly filled with lanthanide or actinide cations (23). Polymorphism and structural phase transformations between the U2Co3Si5 (Ibam) (24), Lu2Co3Si5 (C2/c or the nonstandard equivalent I2/c) (25), and Sc2Fe3Si5 (P4/mnc) (26) structure types have opened avenues of investigation as the formation of metastable structures often leads to emergent physical properties (27–30).
Intrigued by the unstable electronic states and polymorphism in the group 9 germanides (31–35), we recently showed that large single crystals of both m-Pr2Co3Ge5 (Lu2Co3Si5 type, I2/c) (36) and o-Pr2Co3Ge5 (U2Co3Si5 type, Ibam) (37) can be stabilized at room temperature. In this work, we reveal a second-order structural phase transition between two polymorphs of Pr2Co3Ge5 through complementary in situ powder x-ray diffraction (PXRD) and in situ electron energy-loss spectroscopy (EELS). We demonstrate a relationship between the structural phase transformation and a change in Pr valence from Pr3.80(5)+ to Pr3.00(5)+, the first of its kind for an intermetallic compound. We discuss the hallmarks of valence changes and delocalization evident in the Pr and Co electron energy-loss spectra and show how the anisotropic magnetic properties are affected with this change in structure and valence. This work demonstrates how minor changes in crystal structure can be indicative of substantial reconfigurations in electronic and magnetic interactions leading to unexplored and exciting avenues for quantum materials investigations.
RESULTS
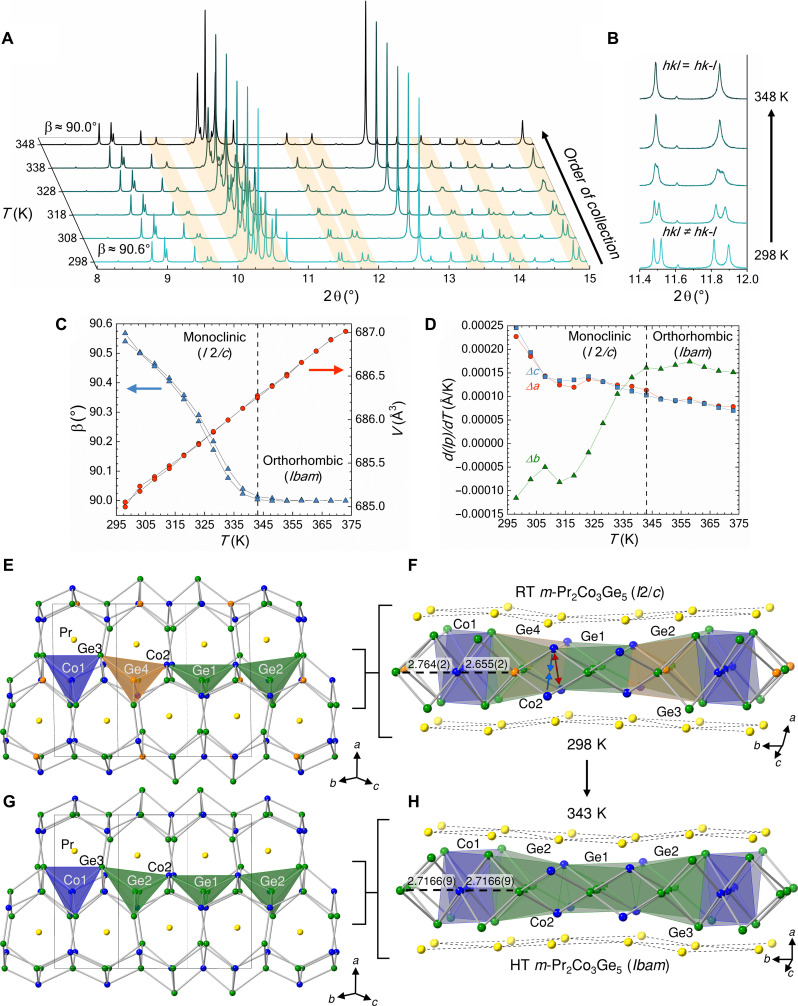
Figure 1A shows the temperature-dependent PXRD patterns from 298 to 348 K taken for m-Pr2Co3Ge5. The convergence of the hkl and hk-l reflections (Fig. 1B) demonstrates that the structure of m-Pr2Co3Ge5 undergoes a second-order structural phase transformation from the space group I2/c (Lu2Co3Si5 structure type) to Ibam (U2Co3Si5 structure type). The β angle, the angle between the a and c lattice directions, of m-Pr2Co3Ge5 begins converging homogeneously toward 90° immediately upon heating from room temperature indicating the beginning of the structural phase transformation and concludes at approximately 343 K. The high-temperature diffraction pattern of m-Pr2Co3Ge5 matches exceedingly well with the room-temperature powder diffraction pattern of o-Pr2Co3Ge5. For comparison, Rietveld refinements of synchrotron PXRD for room-temperature m-Pr2Co3Ge5 and o-Pr2Co3Ge5 are shown in fig. S1, and atomic position and bond distance tables for all structures are provided in tables S1 and S2. Figure 1C shows that while the β angle converges to 90° upon heating, the volume increases linearly with temperature giving no clear indication of a valence change. The first derivative of the change in lattice parameters of m-Pr2Co3Ge5 (Fig. 1D) shows nonlinear change in the b lattice direction in contrast to the a and c directions corresponding with a transformation to the orthorhombic structure type. The length of the b axis is directly related to the β angle of m-Pr2Co3Ge5. As the β angle approaches 90° across the phase transition, the b axis must elongate to compensate for the change since the volume of the Pr2Co3Ge5 unit cell of changes minimally as a function of temperature.
Fig. 1. In situ x-ray diffraction and crystal structure of m-Pr2Co3Ge5 and o-Pr2Co3Ge5.
(A and B) Temperature-dependent x-ray diffraction of m-Pr2Co3Ge5 (λ = 0.458977 Å) highlighting the structural phase transformation from I2/c to Ibam. The change in angle β as related to the change in volume and the change in lattice parameters (lp) are given in (C) and (D), respectively. Dashed lines indicate the conclusion of the second-order structural phase transformation. Crystal structure of room-temperature (E and F) and 343-K (G and H) m-Pr2Co3Ge5 obtained from synchrotron PXRD where Pr, Co, and Ge are represented with yellow, blue, and green/orange spheres, respectively. Select Co-Ge distances (Å) are shown to illustrate the distortion of the basal atoms of the tetrahedral slab along the crystallographic b direction. Red and blue arrows indicate the separation and contraction of the Co2 dimerization, respectively.
The structural differences of room-temperature and high-temperature m-Pr2Co3Ge are shown in Fig. 1 (E to H). Notably, the five coordinate Co2 atoms that were dimerized along the c direction at room temperature undergo the largest change in bond distance within the framework, expanding from dCo2-Co2 = 3.464(2) Å to dCo2-Co2 = 3.5838(7) Å at 343 K. The distortion of the [Co3Ge5] framework across the phase transition leads to an increase in Pr local environment symmetry from C1 to Cs as the Pr-Ge2/4 and Pr-Co2 distances undergo contractions ranging from ΔPr-Ge4 = −0.029(1) Å to ΔPr-Ge2 = −0.019(1) Å and elongations ranging from ΔPr-Ge2 = 0.010(1) Å to ΔPr-Ge4 = 0.028(1) Å. With the existence of a structural phase transformation confirmed in m-Pr2Co3Ge5, temperature-dependent EEL spectra were obtained to determine the structure-valence relationship of the two polymorphs.
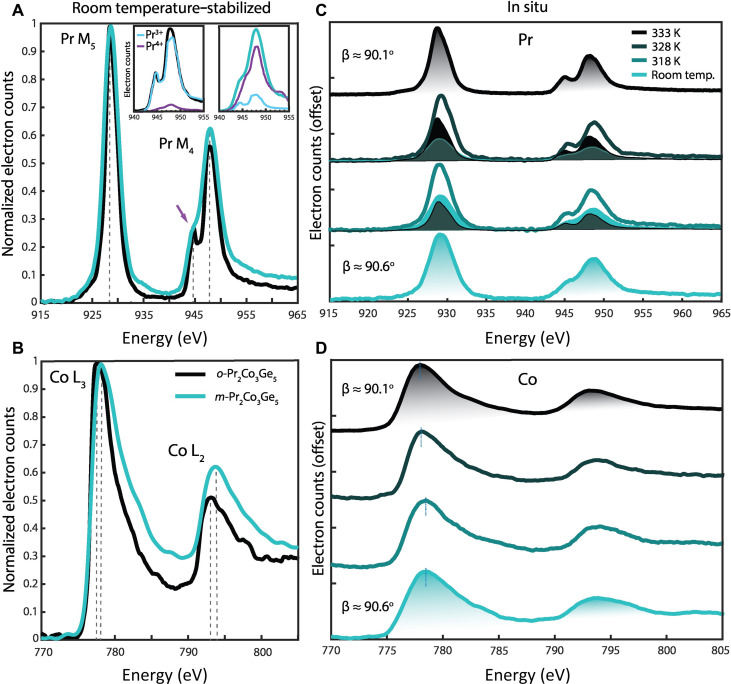
In situ EELS collects quantitative information regarding the 4f electronic states of the lanthanide without the influence of sample contributions like thickness and compositional inhomogeneities. Monochromated EELS data were acquired during in situ heating of m-Pr2Co3Ge5 within the TEAM I S/TEM from room temperature to 333 K with an energy resolution of 0.25 eV. The final state of the Pr and Co edges after heating (shown in black in Fig. 2, C and D, respectively) were compared to a sample produced in the room-temperature orthorhombic phase (shown in black in Fig. 2, A and B, respectively) to confirm that the sample had been fully transformed in situ under vacuum. A description of the room-temperature EELS for each polymorph can be found in the Supplementary Materials. During the heating process, the Pr M4,5 and Co L2,3 edges representing the excitation of core electrons to the partially filled higher energy 4f and 3d orbitals, respectively, were recorded at two intermediate temperature steps.
Fig. 2. Room-temperature and in situ EEL spectra of m-Pr2Co3Ge5 and o-Pr2Co3Ge5.
Core-loss EELS of the Pr M4,5 (A) and Co L2,3 edges (B) for room-temperature m-Pr2Co3Ge5 (blue) and o-Pr2Co3Ge5 (black). Dashed lines indicate the peak maxima, highlighting differences in the distribution of density of states between the two structures. Pr valency was determined by fitting reference M4,5 spectra for Pr3+ and Pr4+ (see Methods) to the acquired data, as shown in the inset of (A). The M4 edge feature at 945 eV (marked with an arrow) is a signature of Pr3+. This feature increases in prominence when the sample undergoes transformation. Temperature-dependent EEL spectra across the structural phase transformation are shown in (C and D). The Pr M4,5 edges correspond to 4f orbital occupancy and the Co L2,3 edges to 3d occupancy. The shading in (C) shows the linear fit contributions of the initial and final structures’ M4,5 edges to the spectra of the intermediate structures, indicating a gradual transition in Pr valency with structural transformation. The total integrated area of the spectra taken at room temperature and 333 K are shaded beneath their respective curves. Data were normalized on the basis of the M5 or L3 edge maxima.
Upon heating, several changes are observed in the Pr EEL spectra. A lower-energy satellite edge emerged on the Pr M4 edge of m-Pr2Co3Ge5 (see arrow in Fig. 2A), indicating a change in Pr valence from a dominantly Pr4+ state to a dominantly Pr3+ state most likely due to an increase in electron filling of the 4f orbitals and reconfiguration of the Pr 4f electronic states. In addition, the edge onset, defined as 10% the peak maxima, increases from 925.02 to 925.65 eV, indicating that the ionization energy required for an electron to transition from the core level to the 4f valence band is greater at 333 K. We also observe an increase in both the M5:M4 edge height ratio and a decrease in total integrated area (see Supplementary Materials) upon heating. The reduction in unoccupied 4f states indicated by the decrease in integrated area implies a greater filling of the 4f orbitals in the high-temperature state of m-Pr2Co3Ge5. Multiple linear least squares (MLLS) fits (see Methods) of room-temperature m-Pr2Co3Ge5 reveals a valence state of Pr3.80(5)+ compared to Pr3.00(5)+ in room-temperature o-Pr2Co3Ge5. At 333 K, the MLLS fits of m-Pr2Co3Ge5 yield a valence of Pr3.16(5)+, in good agreement with the in situ PXRD, which indicate a β angle slightly greater than 90°. It is important to note that the MLLS method relies on the precision of the reference spectra and does not account for differences in coordination environment or data collection methods. While the exact values of the Pr oxidation state may differ from the determined values, the change from tetravalent to trivalent with heating is clear.
The maxima of the Co L2,3 edges in m-Pr2Co3Ge5, shown with dotted lines in Fig. 2D, shift to lower energy upon heating while the edge onsets remain relatively constant or shift slightly higher in energy. The L3:L2 edge height ratio remains constant across the phase transition. Given the alteration to the Co interatomic distances during the phase transition, it is not unexpected that we observe corresponding energy shifts in the Co L2,3 edge maxima and smaller peaks in the edge tails. Specifically, we observe a broader energy-loss range for electron transitions in the room-temperature m-Pr2Co3Ge5 Co spectra compared to those measured at 333 K, indicating an increased diversity in energy transition pathways and, consequently, a greater variety of potentially unoccupied final 3d states.
EEL spectra of Pr valence transitions in cobalt containing oxides indicate nearly identical modifications of Pr M4,5 edges as reported here; however, large changes in the Co L2,3 edges were not observed despite O K edge modifications indicating alterations to the Co oxidation state (38). Ultimately, in situ EELS conclusively demonstrates that the observed variations in the electronic states of Co and Pr are inherent to the structural differences of the Pr2Co3Ge5 polymorphs.
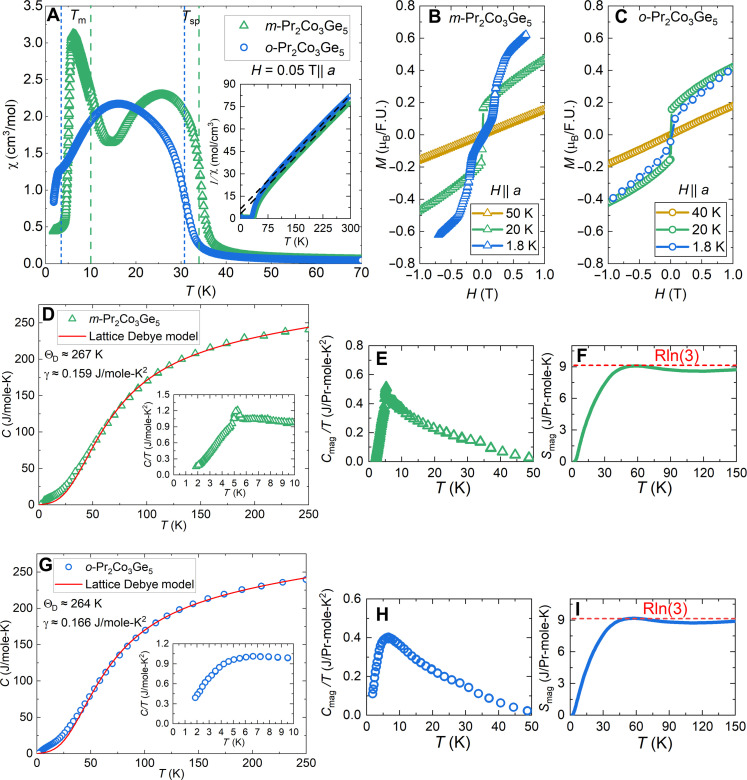
While we would expect a reduction in effective magnetic moment, magnetic saturation moment, and magnetic entropy associated with the nearly tetravalent Pr state of m-Pr2Co3Ge5 when compared to the room-temperature stabilized o-Pr2Co3Ge5, magnetic and transport measurements of both polymorphs indicate that this is not the case. The temperature-dependent magnetic susceptibility is shown in Fig. 3 and fig. S5 and are summarized in table S3. The differences in the magnetic ordering of the two polymorphs is most visible with applied fields of H = 0.05 T parallel to the a crystallographic direction, where two ordering events, Tsp = 30.8 K and TM = 3.4 K, are visible for o-Pr2Co3Ge5 that shift to higher temperatures, Tsp = 35.3 K and TM = 10 K, for m-Pr2Co3Ge5 (Fig. 3A).
Fig. 3. Magnetic and transport properties of m-Pr2Co3Ge5 and o-Pr2Co3Ge5.
(A) Zoomed temperature-dependent magnetic susceptibility of m-Pr2Co3Ge5 and o-Pr2Co3Ge5 at H = 0.05 T||a. The inset shows the inverse magnetic susceptibility and Curie-Weiss fit for the entire temperature range. (B and C) Zoomed field-dependent magnetization with H||a per formula unit of m-Pr2Co3Ge5 (triangles) and o-Pr2Co3Ge5 (circles) at T = 1.8, 20, and 40, or 50 K (blue, green, and yellow, respectively). Magnetic data for o-Pr2Co3Ge5 were obtained from prior work (37). (D and G) Temperature-dependent heat capacity with a Lattice Debye fit for m-Pr2Co3Ge5 and o-Pr2Co3Ge5, respectively. The insets feature the low-temperature behavior of the heat capacity. (E and H) Magnetic contribution to the heat capacity. (F and I) Temperature-dependent magnetic entropy.
The inverse susceptibility data indicates a linear Curie-Weiss dependence. Both polymorphs have an effective magnetic moment μeff ~ 4.0 μB/Pr, elevated from the calculated value of a Pr3+ ion, μcalc = 3.58μB/Pr3+, implying a Co contribution to the magnetic moment. Weiss constants for o-Pr2Co3Ge5 and m-Pr2Co3Ge5, summarized in table S3, are consistent from H = 0.05 to 0.5 T and indicate dominant antiferromagnetic interactions along the a direction and dominant ferromagnetic interactions along the c direction. The potential for Co magnetic contribution prevents an unambiguous quantification of Pr moment between the two polymorphs through bulk magnetic measurement and will require magnetic structure determination (e.g., using neutron scattering). However, the magnetic contribution of Co can be estimated using the oxidation states determined from EELS (see Methods), resulting in μcalc = 2.32 μB/Co for Pr3.80(5)+ in m-Pr2Co3Ge5 and μcalc = 1.35 μB/Co for Pr3.00(5)+ in o-Pr2Co3Ge5. On the basis of these calculations, the Co contribution to the magnetic moment is decreased in o-Pr2Co3Ge5, countering the increased Pr magnetic contribution from the trivalent oxidation state.
From field-dependent magnetization applied along the a direction at 20 K (Fig. 3, B and C, and figs. S6 and S7), o-Pr2Co3Ge5 attains a saturation moment of μsat = 1.36 μB/F.U. and m-Pr2Co3Ge5 a value of μsat = 1.66 μB/F.U., far below the expected saturation moment for Pr3+ (μsat = 6.40μB/F.U.) or Pr4+ (μsat = 4.28 μB/F.U.) assuming no Co contribution. When measured along the c direction at 1.8 K (figs. S6 and S7), the magnetic saturation is higher, μsat = 3.66 and 2.60 μB/F.U. for o-Pr2Co3Ge5 and m-Pr2Co3Ge5, respectively, but this is still less than half the expected value based on the oxidation states determined from EELS. In both cases, it is likely that the reduced magnetic saturation value can be attributed to crystal electric field splitting of the full J multiplets; however, additional measurements such as inelastic neutron scattering are needed to understand the crystal electric field splitting. The monoclinic polymorph exhibits multiple metamagnetic transitions at fields less than 0.7 T applied along the a direction while the orthorhombic polymorph does not. In addition, m-Pr2Co3Ge5 saturates more rapidly up to 0.7 T than o-Pr2Co3Ge5, attaining magnetizations of 0.60 and 0.30 T, respectively. Similar behavior is observed with magnetic field applied along the c-direction, where the monoclinic polymorph is more responsive to weak magnetic fields.
The heat capacity data of the two polymorphs is shown in Fig. 3, D and G. Above 50 K, the two polymorphs are indistinguishable, which is expected given that the similarities between the lattices are likely to produce similar phonon behaviors. The broad magnetic contributions below 50 K in both polymorphs are expected from the ferromagnetic ordering observed in susceptibility measurements and show that the initial ordering for both polymorphs is not abrupt, possibly because of magnetic frustration. The distinct lambda feature at 5 K in m-Pr2Co3Ge5 (Fig. 3D, inset, and E) is absent in o-Pr2Co3Ge5 (Fig. 3, G, inset, and H) and corresponds to the prominent spin reorientation observed at 10 K in temperature-dependent magnetic susceptibility measurements. To obtain the magnetic contribution to the specific heat and the magnetic entropy of m-Pr2Co3Ge5 and o-Pr2Co3Ge5, we fit the heat capacity to a lattice-Debye model (Methods) and subtracted it from the measured heat capacities of each polymorph (Fig. 3, F and I, respectively). For both polymorphs, the experimental magnetic entropy is reduced from a theoretical value of either Rln(9) for Pr3+ or Rln(6) for Pr4+ each attaining a value of Rln(3) at 50 K. This is consistent with the effects of crystal field splitting but may be difficult to interpret because of the presence of Co magnetism. The Sommerfeld coefficients are approximately γ = 0.159 J/mol-K2 for m-Pr2Co3Ge5 and γ = 0.166 J/mol-K2 for o-Pr2Co3Ge5 indicating a moderately correlated system that, when considered with the broad curvature in electrical resistivity measurements observed in prior work (36, 37), could suggest Kondo lattice behavior.
DISCUSSION
The large change in Pr valence is notable given the unit cell volume of room-temperature m-Pr2Co3Ge5 sees a contraction of only ΔV = −0.52(1) Å3 when compared to the high-temperature transformed m-Pr2Co3Ge5. In addition, neither the Pr-Ge, nor the Pr-Co interatomic distances undergo substantial contraction to accommodate the smaller atomic radii of the Pr4+ dominant state in room-temperature m-Pr2Co3Ge5 relative to the Pr3+ state at 343 K. However, lattice volume contraction does not always directly correlate with the degree of valence transition (39). Upon heating, Pr transforms from an 8-coordinate system in m-Pr2Co3Ge5 to a 12-coordinate system in o-Pr2Co3Ge5, which could motivate a change in interaction of the Pr 4f and conduction electrons. The effects of local point group symmetry on the valence behavior of Pr are well known, where loss of cubic symmetry typically generates a singlet ground state and lack of strong Kondo interactions unless a low-lying singlet state can form (40–43). The increase in point symmetry from m-Pr2Co3Ge5 to o-Pr2Co3Ge5 could tune the crystal electric field of Pr from a quasi-doublet state to a singlet state that, given strong enough hybridization with neighboring p and d electrons, could motivate a transition from an intermediate valent state like what has been described for Pr-based skutterudites (44) to a fully trivalent sate. The largely reduced saturation magnetic moments and experimental magnetic entropies for both polymorphs suggest strong crystal electric field splitting of the Pr J multiplets. However, in this case, larger differences between the two polymorphs’ experimental magnetic entropies and Sommerfeld coefficients would be expected, as these metrics are strongly tied to the crystal field splitting schemes. It is possible that the Co contribution to the magnetic moment is adding additional complexity to the interpretation of ΔSmag and γ.
The loss of Co dimerization across the structural phase transformation of m-Pr2Co3Ge5 suggests that the structural dimorphism could be motivated by an electronic instability due to an unfilled transition metal valence shell, as previously shown for many binary intermetallic compounds (45). To account for this lack of electrons, transition metal–transition metal (M─M) bonding occurs, effectively reducing the required electron count from 18 e−/M to some 18-n e−/M, where n is the number of electron pairs formed via M─M bonding. While more complex bonding analysis is required to fully grasp the influence of Co─Co bonding on the oxidation state of Pr in Pr2Co3Ge5, it would not be an isolated case. On the basis of spectral and structural evidence, however, the behavior of m-Pr2Co3Ge5 is distinct from these previous observations in that both the lanthanide and transition metal become more oxidized without marked changes in bond lengths or unit cell size. The lack of electron transfer between the lanthanide and transition metals, as observed in other systems like CeRhGe (46), suggests that the Ge atoms are withdrawing electrons. The determination of electron transfer and bonding in lanthanide–transition metal–germanides is a complex issue that is still under investigation due to the similar electronegativities of group 8 to 10 transition metals and Ge (47). Still, Ge has been determined to participate in strong polar-covalent interactions with alkali metals and La (48). It is possible that the strong lanthanide–transition metal interactions exhibited in the 4d transition metal lanthanide germanide systems (49, 50) departs from what is observed for Pr2Co3Ge5 because of the lower electronegativities of the 3d transition metals. While the role of the transition metal and tetrel elements in the formation of tetravalent Pr are not presently clear, we can broadly state that further investigations should be focused toward 3d transition metal germanides that likely have electronic instabilities, i.e., structural phase transformations. Naturally, this demonstrates the importance of future temperature-dependent spectroscopic and structural investigations in the A2M3X5 family of compounds, but other tetragonal to monoclinic deformations have been found in systems of the ThCr2Si2 (51), BaNiSn3 (52), and CaBe2Ge2 structure types (53) which may also host Pr intermediate valence behavior.
While a combination of computational and inelastic scattering experiments would be required to fully elucidate the mechanism responsible for the nearly Pr4+ valence state in m-Pr2Co3Ge5, it is most likely that a combination of changing symmetry and 4f hybridization across the structural phase transformation are responsible. Tangentially, lattice vibrations could also be playing a role in the structural phase transformation of m-Pr2Co3Ge5. The configuration of bonds within the constraints of the 18-n rule can undergo rearrangement due to electron-phonon interactions as illustrated by the temperature-dependent phase transformation of IrIn3 (54). The presence of charge density waves in compounds isostructural to Pr2Co3Ge5 seems to suggest a complicated interplay between valence, vibrational, and electronic states.
We have shown a marked hidden valence change in a Pr-based intermetallic compound linked to a monoclinic to orthorhombic second-order phase transformation in Pr2Co3Ge5. The nature of the Pr valence state in m-Pr2Co3Ge5 and o-Pr2Co3Ge5 was confirmed through in situ PXRD and in situ EELS across the structural phase transformation of m-Pr2Co3Ge5. While the origin of the Pr valence transition is not clear on the basis of bulk heat capacity and magnetic measurements, its implications for understanding the duality between local/itinerant 4f states and the moderately correlated nature of Pr2Co3Ge5 make it a promising platform for future investigations of Pr-based mixed valent intermetallic compounds. Given the ubiquity of the U2Co3Si5 structure type, many more examples of structural phase transformations might be found in the A2M3X5 system. Developing our understanding of the relationship between valence and structure in Pr-based intermetallic compounds enables design of strongly correlated materials capable of accessing presently unfound quantum states based on magnetic lanthanides and actinides with unstable valences.
METHODS
Synthesis
o-Pr2Co3Ge5 and m-Pr2Co3Ge5 were synthesized using the flux growth method, where Sn was the low–melting metal flux and elements were weighed out in the stoichiometric ratio of 3 Pr:2 Co:7 Ge:50 Sn. The reactants were transferred into an alumina Canfield crucible set (55) and sealed in a fused silica tube with ~1/3-atm argon gas. For o-Pr2Co3Ge5, the ampoule was placed in a furnace set to 300°C, raised to 1175°C at a rate of 150°C/hour, and dwelled for 6 hours, whereas for m-Pr2Co3Ge5, the ampoule was raised to 1175°C at a slower rate of 100°C/hour and dwelled for longer (24 hours). In both cases, the samples were cooled to 815°C at a rate of 3°C/hour before being removed, inverted, and centrifuged at 815°C to separate excess Sn from the large (1 to 5 mm in length) polyhedral crystals. The remaining Sn flux was etched using 1:1 HCl:H2O, with a yield of approximately 80%. Almost all reactions produced members of the Prn+1ConGe3n+1 homologous series (56) as a side product, but this phase was easily removed because of its plate like morphology and size (~2 mm by ~2 mm). In addition, trace amounts of PrCoGe3 are evident in high-resolution PXRD.
While the synthetic conditions presented above are different for each polymorph, the formation of o-Pr2Co3Ge5 is challenging. We initially attempted to reproduce o-Pr2Co3Ge5 by varying the duration of the high-temperature dwell step ranging from 4 to 48 hours but obtained only the monoclinic polymorph. From here, we investigated the possibility that o-Pr2Co3Ge5 was a metastable high-temperature polymorph. Reheating and annealing of m-Pr2Co3Ge5 sealed in an evacuated quartz ampoule followed by quenching in either ice water, icy brine, or liquid nitrogen were also unsuccessful in stabilizing o-Pr2Co3Ge5, but this could be due to the reduced rate of heat transfer caused by the quartz ampoule. We then moved to altering the postsynthetic conditions directly from the final dwell temperature of 815°C including varying the centrifugation speed, precooling the centrifuge with dry ice, and inverting the reaction ampoule directly into liquid nitrogen but were unsuccessful in obtaining the orthorhombic polymorph. While some reactions have been successful in synthesizing o-Pr2Co3Ge5, m-Pr2Co3Ge5 is the more common product.
Powder x-ray diffraction
High-resolution in situ PXRD data (λ = 0.458977 Å) were collected in the 2θ range from 0.5° to 28° on a ground single crystal of m-Pr2Co3Ge5 at the 11-BM beamline at the Advanced Photon Source of Argonne National Laboratory. TOPAS was used to perform a sequential Rietveld refinement, identifying m-Pr2Co3Ge5 as the dominant phase with PrCoGe3 (less than 1 wt %) and Sn (less than 4 wt %) as impurity phases. The PrCoGe3 and residual Sn from flux growth most likely originate from excess flux that remained on the surface of the crystal after acid etching. For in situ experiments, samples were placed in Kapton capillaries and heated using a hot gas blower in 5-K increments from 298 to 373 K and back. For each measurement, the sample was held for 30 min at temperature before diffraction data were collected to ensure the sample was at equilibrium.
Anisotropic susceptibility and transport measurements
Magnetic properties measurements were performed on oriented single crystals of m-Pr2Co3Ge5 and o-Pr2Co3Ge5 using a Quantum Design VSM magnetic property measurement system. For m-Pr2Co3Ge5, all anisotropic measurements were performed with crystal oriented relative to the I-centered monoclinic unit cell to allow direct comparison between the two polymorphs. The magnetization measurements were performed at constant temperatures of T = 1.8, 10, 20, 30, 40, and 50 K for o-Pr2Co3Ge5 and T = 1.8 K under applied magnetic fields (H) from −7 to 7 T. For the zero field–cooled and field-cooled magnetic susceptibility measurements, the sample was cooled down to 1.8 K with no applied field before heating to 300 K and cooling back down to 1.8 K with an applied field of H = 0.2 T. In both cases, single crystals were mounted such that the magnetic fields were parallel to a, b, and c axes. Heat capacity (C) was measured from 1.8 to 250 K using a Quantum Design physical property measurement system.
The magnetic contribution of Co can be estimated using Eq. 1
| (1) |
where μeff is the effective magnetic moment per formula unit determined from Curie-Weiss fit of the inverse magnetic susceptibility (Fig. 3A, inset), μcalc is the calculated Pr or Co contribution based on valences determined from EELS, nPr is the number of moles of Pr per formula unit, and nCo is the number of moles of Co per formula unit. For m-Pr2Co3Ge5 with μeff = 5.58 μB/F.U. (H = 0.05 T||a) and μcalc = 2.74 μB/Pr for a valence of Pr3.80(5)+, then the expected Co contribution to the effective magnetic moment is μcalc = 2.32 μB/Co. Following the same procedure for Pr3+ in o-Pr2Co3Ge5, we obtain a Co contribution of μcalc = 1.35 μB/Co.
The heat capacity was fit to a lattice-Debye model shown in Eq. 2
| (2) |
where x = hω/κBT, ω is the Debye frequency, θD is the Debye temperature, and CD is a constant containing numbers of oscillators and degrees of freedom. To obtain the magnetic contribution to the specific heat and the magnetic entropy, these fits were subtracted from the measured heat capacities of each polymorph.
Energy-dispersive x-ray spectroscopy
Elemental maps were collected in a FEI ThemIS operated at 300 kV with a Super-X energy-dispersive x-ray spectroscopy (EDS) detector. The energy resolution of the system is 140 eV. The composition of m-Pr2Co3Ge5 was measured as 17.9–atomic % (at %) Pr, 35.2–at % Co, and 46.9–at % Ge (Pr2Co3.9Ge5.2). The composition of o-Pr2Co3Ge5 was measured as 17.8–at % Pr, 31.5–at % Co, and 50.7–at % Ge (Pr2Co3.5Ge5.7). EDS maps were acquired over 5 min from bulk regions of the sample with drift correction enabled. No other elements, including Sn, which is used during flux synthesis, were detected in quantities above their fit errors.
Electron energy-loss spectroscopy
The TEAM I, an aberration-corrected transmission electron microscope, was used to collect dual EELS spectral images in probe-corrected scanning transmission electron microscopy mode that include the zero-loss peak and either the L2,3 edges (2p → 3d) of Co or the M4,5 edges (3d → 4f) of Pr. The following acquisition parameters were used: a 17-mrad convergence angle, 400-mm camera length, 90-meV dispersion, and 2.5-mm energy filtering slit. Core-loss EELS was collected from a 40-nm-thick region of an electron-transparent lamella of m-Pr2Co3Ge5. The lamella was tilted off zone axis to avoid orientation-specific channeling effects.
The room-temperature orthorhombic and monoclinic Pr M4,5 edges were fit using MLLS to determine the Pr oxidation state. MLLS fits were performed using spectra obtained from Pr2O3 and BaPrO3 as references for Pr3+ and Pr4+, respectively (57, 58). Fitting the edges indicates that the orthorhombic sample is 90 to 100% Pr3+ and 0 to 10% Pr4+ (95% +/− 5% Pr3+). As for the monoclinic sample, the valence states are 75 to 85% Pr4+ and 15 to 25% Pr3+ (20% +/− 5% Pr3+). The MLLS fits require a trade-off between fitting the fine features and fitting the general edge shapes. The fine features were weighted more heavily, particularly those visible in the M4 edge, in determination of oxidation state because of the broadening issues that would influence peak shapes and the dependency of location on the energy axis on crystal structure.
Using a Gatan model 652 double-tilt heating holder, the temperature of the lamella was raised from room temperature (approximately 297 K) until changes in the Pr were first observed. This occurred around 318 K. The temperature was held for 2 min, and then EELS images from both the Co L2,3 and Pr M4,5 energy regimes were collected. The temperature was then raised to 328 K, and spectral images were collected again following the same procedure. Last, the holder temperature was raised to 333 K, held at that temperature for 2 min, and then allowed to cool back to room temperature before EELS was performed. There is an estimated 5-K systematic error in the temperature control of the heating holder within this temperature range.
Acknowledgments
We are grateful to S. Lapidus for helpful suggestions and conversation.
Funding: J.Y.C. and T.M.K. acknowledge support from the Welch Foundation under award no. AA-2056-20220101 and the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, under award no. DE-SC0022854. This material is based on work supported by the U.S. DOE, Office of Science, Office of Workforce Development for Teachers and Scientists, Office of Science Graduate Student Research (SCGSR) program. The SCGSR program is administered by the Oak Ridge Institute for Science and Education for the DOE under contract no. DE-SC0014664. This research used resources of the Advanced Photon Source; a U.S. DOE Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under contract nho. DE-AC02-06CH11357. M.C.S. was supported by National Science Foundation STROBE grant DMR-1548924. Work at the Molecular Foundry is supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. DOE under contract no. DE-AC02-05CH11231. R.E.B. was supported by the National Science Foundation through NSF DMR-1904361. The National High Magnetic Field Laboratory is supported by the National Science Foundation through NSF DMR-1644779 and the State of Florida.
Author contributions: T.M.K. synthesized m-Pr2Co3Ge5 and o-Pr2Co3Ge5 and collected and analyzed the PXRD data. G.T.M. assisted in the interpretation of the crystallography. E.R.K. collected and analyzed the EEL spectra. J.G.-C. collected and analyzed the magnetic and transport data. T.M.K. and J.Y.C. conceived the study. J.Y.C., R.E.B., and M.C.S. are the principal investigators.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in this paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S7
Tables S1 to S3
References
REFERENCES AND NOTES
- 1.Yang Y.-F., An emerging global picture of heavy fermion physics. J. Phys. Condens. Matter 35, 103002 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Grefe S. E., Lai H.-H., Paschen S., Si Q., Weyl-Kondo semimetals in nonsymmorphic systems. Phys. Rev. B 101, 075138 (2020). [Google Scholar]
- 3.Chen L., Setty C., Hu H., Vergniory M. G., Grefe S. E., Fischer L., Yan X., Eguchi G., Prokofiev A., Paschen S., Cano J., Si Q., Topological semimetal driven by strong correlations and crystalline symmetry. Nat. Phys. 18, 1341–1346 (2022). [Google Scholar]
- 4.Witczak-Krempa W., Chen G., Kim Y. B., Balents L., Correlated quantum phenomena in the strong spin-orbit regime. Annu. Rev. Condens. Matter Phys. 5, 57–82 (2014). [Google Scholar]
- 5.Zhao H., Zhang J., Lyu M., Bachus S., Tokiwa Y., Gegenwart P., Zhang S., Cheng J., Yang Y.-F., Chen G., Isikawa Y., Si Q., Steglich F., Sun P., Quantum-critical phase from frustrated magnetism in a strongly correlated metal. Nat. Phys. 15, 1261–1266 (2019). [Google Scholar]
- 6.Kavai M., Friedman J., Sherman K., Gong M., Giannakis I., Hajinazar S., Hu H., Grefe S. E., Leshen J., Yang Q., Nakatsuji S., Kolmogorov A. N., Si Q., Lawler M., Aynajian P., Inhomogeneous Kondo-Lattice in geometrically frustrated Pr2Ir2O7. Nat. Commun. 12, 1377 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giustino F., Lee J. H., Trier F., Bibes M., Winter S. M., Valentí R., Son Y.-W., Taillefer L., Heil C., Figueroa A. I., Plaçais B., Wu Q., Yazyev O. V., Bakkers E. P. A. M., Nygård J., Forn-Díaz P., De Franceschi S., McIver J. W., Torres L. E. F. F., Low T., Kumar A., Galceran R., Valenzuela S. O., Costache M. V., Manchon A., Kim E.-A., Schleder G. R., Fazzio A., Roche S., The 2021 quantum materials roadmap. J. Phys. Mater. 3, 042006 (2020). [Google Scholar]
- 8.Yuan H. Q., Grosche F. M., Deppe M., Geibel C., Sparn G., Steglich F., Observation of two distinct superconducting phases in CeCu2Si2. Science 302, 2104–2107 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Macaluso R. T., Nakatsuji S., Kuga K., Thomas E. L., Machida Y., Maeno Y., Fisk Z., Chan J. Y., Crystal structure and physical properties of polymorphs of LnAlB4 (Ln = Yb, Lu). Chem. Mater. 19, 1918–1922 (2007). [Google Scholar]
- 10.Riseborough P. S., Lawrence J. M., Mixed valent metals. Rep. Prog. Phys. 79, 084501 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Kuga K., Matsumoto Y., Okawa M., Suzuki S., Tomita T., Sone K., Shimura Y., Sakakibara T., Nishio-Hamane D., Karaki Y., Takata Y., Matsunami M., Eguchi R., Taguchi M., Chainani A., Shin S., Tamasaku K., Nishino Y., Yabashi M., Ishikawa T., Nakatsuji S., Quantum valence criticality in a correlated metal. Sci. Adv. 4, eaao3547 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prochaska L., Li X., MacFarland D. C., Andrews A. M., Bonta M., Bianco E. F., Yazdi S., Schrenk W., Detz H., Limbeck A., Si Q., Ringe E., Strasser G., Kono J., Paschen S., Singular charge fluctuations at a magnetic quantum critical point. Science 367, 285–288 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Jayaraman A., Narayanamurti V., Bucher E., Maines R. G., Continuous and discontinuous semiconductor-metal transition in samarium monochalcogenides under pressure. Phys. Rev. Lett. 25, 1430–1433 (1970). [Google Scholar]
- 14.Jarrige I., Yamaoka H., Rueff J. P., Lin J. F., Taguchi M., Hiraoka N., Ishii H., Tsuei K. D., Imura K., Matsumura T., Ochiai A., Suzuki H. S., Kotani A., Unified understanding of the valence transition in the rare-earth monochalcogenides under pressure. Phys. Rev. B 87, 115107 (2013). [Google Scholar]
- 15.Liao Y.-F., Tegomo Chiogo B., Clausse T., Mazet T., Tsuei K.-D., Malterre D., Chainani A., Dual kondo effect charge ordering and zero thermal expansion in a correlated intermetallic. Commun. Mater. 3, 23 (2022). [Google Scholar]
- 16.Ye M., von Westarp M. J. G., Souliou S.-M., Peters M., Möller R., Kliemt K., Merz M., Heid R., Krellner C., Le Tacon M., Strong electron-phonon coupling and enhanced phonon Grüneisen parameters in valence-fluctuating metal EuPd2Si2. Phys. Rev. B 107, 195111 (2023). [Google Scholar]
- 17.Jang S.-H., Sano R., Kato Y., Motome Y., Antiferromagnetic Kitaev interaction in f-electron based honeycomb magnets. Phys. Rev. B 99, 241106 (2019). [Google Scholar]
- 18.Chaturvedi V., Ghosh S., Gautreau D., Postiglione W. M., Dewey J. E., Quarterman P., Balakrishnan P. P., Kirby B. J., Zhou H., Cheng H., Huon A., Charlton T., Fitzsimmons M. R., Korostynski C., Jacobson A., Figari L., Barriocanal J. G., Birol T., Mkhoyan K. A., Leighton C., Room-temperature valence transition in a strain-tuned perovskite oxide. Nat. Commun. 13, 7774 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanathan A., Kaplan J., Sergentu D.-C., Branson J. A., Ozerov M., Kolesnikov A. I., Minasian S. G., Autschbach J., Freeland J. W., Jiang Z., Mourigal M., La Pierre H. S., Chemical design of electronic and magnetic energy scales of tetravalent praseodymium materials. Nat. Commun. 14, 3134 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramanathan A., Walter E. D., Mourigal M., La Pierre H. S., Increased crystal field drives intermediate coupling and minimizes decoherence in tetravalent praseodymium qubits. J. Am. Chem. Soc. 145, 17603–17612 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gompa T. P., Ramanathan A., Rice N. T., La Pierre H. S., The chemical and physical properties of tetravalent lanthanides: Pr, Nd, Tb, and Dy. Dalton Trans. 49, 15945–15987 (2020). [DOI] [PubMed] [Google Scholar]
- 22.K. W. Brown, M. A. Plata, M. E. Raines, J. Y. Chan, Structural and Physical Properties of R2M3X5 Compounds, in Handbook on the Physics and Chemistry of Rare Earth Elements, J.-C. G. Bünzli, S. M. Kauzlarich, Eds. (Elsevier, 2023), vol. 64, chap. 330, pp. 1–92. [Google Scholar]
- 23.Bugaris D. E., Malliakas C. D., Bud'ko S. L., Calta N. P., Chung D. Y., Kanatzidis M. G., Flux crystal growth of the RE2Ru3Ge5 (RE = La, Ce, Nd, Gd, Tb) series and their magnetic and metamagnetic transitions. lnorg. Chem. 56, 14584–14595 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Aksel’rud L. G., Yarmolyuk Y. P., Gladyshevskij E. I., Kristallicheskaya structure soedineniya U2Co3Si5. Kristallografiya 22, 861 (1977). [Google Scholar]
- 25.Chabot B., Parthé E., Dy2Co3Si5, Lu2Co3Si5, Y2Co3Si5 and Sc2Co3Si5 with a monoclinic Structural deformation variant of the orthorhombic U2Co3Si5 structure type. J. Less-Common Met. 106, 53–59 (1985). [Google Scholar]
- 26.Bodak O. I., Kotur B. Y., Yarovets V. I., Gladyshevskii E. I., Kristallicheskaya struktura soedinenij Sc2Fe3Si5 i Dy2Fe3Si5. Kristallografiya 22, 385–388 (1977). [Google Scholar]
- 27.Bugaris D. E., Malliakas C. D., Han F., Calta N. P., Sturza M., Krogstad M. J., Osborn R., Rosenkranz S., Ruff J. P. C., Trimarchi G., Bud’ko S. L., Balasubramanian M., Chung D. Y., Kanatzidis M. G., Charge density wave in the new polymorphs of RE2Ru3Ge5 (RE = Pr, Sm, Dy). J. Am. Chem. Soc. 139, 4130–4143 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Kuo C. N., Hsu C. J., Tseng C. W., Chen W. T., Lin S. Y., Liu W. Z., Kuo Y. K., Lue C. S., Charge density wave like behavior with magnetic ordering in orthorhombic Sm2Ru3Ge5. Phys. Rev. B 101, 155140 (2020). [Google Scholar]
- 29.Sokkalingam R., Lingannan G., Sundaramoorthy M., Lue C. S., Kuo C. N., Joseph B., Arumugam S., Evidence of structural modulations induced by a charge density wave transition in orthorhombic Sm2Ru3Ge5. Solid State Commun. 372, 115293 (2023). [Google Scholar]
- 30.Li Z., Chen X., Liu X., Yu Z., Su N., Liu Z., Xia W., Jiao J., Zhou C., Zhang L., Dong Z., Wang X., Yu N., Zou Z., Ma J., Cheng J., Zhong Z., Guo Y., Suppressed charge-density-wave, robust ferromagnetism and lifshitz transition in Sm2Ru3Ge5 crystal under high pressure. J. Alloys Compd. 937, 168337 (2023). [Google Scholar]
- 31.Hossain Z., Ohmoto H., Umeo K., Iga F., Suzuki T., Takabatake T., Takamoto N., Kindo K., Antiferromagnetic kondo-lattice systems Ce2Rh3Ge5 and Ce2Ir3Ge5 with moderate heavy-fermion behavior. Phys. Rev. B 60, 10383–10387 (1999). [Google Scholar]
- 32.Layek S., Anand V. K., Hossain Z., Valence fluctuation in Ce2Co3Ge5 and crystal field effect in Pr2Co3Ge5. J. Magn. Magn. Mater. 321, 3447–3452 (2009). [Google Scholar]
- 33.Ramakrishnan S., Schönleber A., Rekis T., van Well N., Noohinejad L., van Smaalen S., Tolkiehn M., Paulmann C., Bag B., Thamizhavel A., Pal D., Ramakrishnan S., Unusual charge density wave transition and absence of magnetic ordering in Er2Ir3Si5. Phys. Rev. B 101, 060101 (2020). [Google Scholar]
- 34.Ramakrishnan S., Schönleber A., Bao J.-K., Rekis T., Kotla S. R., Schaller A. M., van Smaalen S., Noohinejad L., Tolkiehn M., Paulmann C., Sangeetha N. S., Pal D., Thamizhavel A., Ramakrishnan S., Modulated crystal structure of the atypical charge density wave state of single-crystal Lu2Ir3Si5. Phys. Rev. B 104, 054116 (2021). [Google Scholar]
- 35.Ramakrishnan S., Bao J., Eisele C., Patra B., Nohara M., Bag B., Noohinejad L., Tolkiehn M., Paulmann C., Schaller A. M., Rekis T., Kotla S. R., Schönleber A., Thamizhavel A., Singh B., Ramakrishnan S., van Smaalen S., Coupling between charge density wave ordering and magnetism in Ho2Ir3Si5. Chem. Mater. 35, 1980–1990 (2023). [Google Scholar]
- 36.Kyrk T. M., Scheifers J. P., Thanabalasingam K., McCandless G. T., Young D. P., Chan J. Y., It runs in the BaAl4 family: relating the structure and properties of middle child Ln2Co3Ge5 (Ln = Pr, Nd, and Sm) to its siblings LnCo2Ge2 and LnCoGe3. lnorg. Chem. 60, 15343–15350 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Kyrk T. M., Galeano-Cabral J., Kennedy E. R., Wei K., McCandless G. T., Scott M. C., Baumbach R. E., Chan J. Y., Anisotropic magnetic and transport properties of orthorhombic o-Pr2Co3Ge5. J. Phys. Mater. 5, 044007 (2022). [Google Scholar]
- 38.Gulec A., Phelan D., Leighton C., Klie R. F., Simultaneous first-order valence and oxygen vacancy order/disorder transitions in (Pr0.85Y0.15)0.7Ca0.3CoO3−δ via analytical transmission electron microscopy. ACS Nano 10, 938–947 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Weidner P., Keulerz K., Löhe R., Roden B., Röhler J., Wittershagen B., Wohlleben D., High temperature susceptibility, valence and volume anomaly of some Ce-compounds. J. Magn. Magn. Mater. 47–48, 75–78 (1985). [Google Scholar]
- 40.White R. M., Fulde P., Excitonic mass enhancement in praseodymium. Phys. Rev. Lett. 47, 1540–1542 (1981). [Google Scholar]
- 41.Anand V. K., Hossain Z., Geibel C., Magnetic order in Pr2Pd3Ge5 and possible heavy-fermion behavior in Pr2Rh3Ge5. Phys. Rev. B 77, 184407 (2008). [Google Scholar]
- 42.Sayles T. A., Baumbach R. E., Yuhasz W. M., Maple M. B., Bochenek Ł., Wawryk R., Cichorek T., Pietraszko A., Henkie Z., Ho P.-C., Superconductivity and crystalline electric field effects in the filled skutterudite PrRu4As12. Phys. Rev. B 82, 104513 (2010). [Google Scholar]
- 43.Sakai A., Nakatsuji S., Kondo effects and multipolar order in the cubic PrTr2Al20 (Tr=Ti, V). J. Phys. Soc. Jpn. 80, 063701 (2011). [Google Scholar]
- 44.Otsuki J., Kusunose H., Kuramoto Y., Theory of crystalline electric field and Kondo effect in Pr skutterudites. J. Physical Soc. Jpn. 74, 200–208 (2005). [Google Scholar]
- 45.Yannello V. J., Fredrickson D. C., Generality of the 18-n rule: Intermetallic structural chemistry explained through isolobal analogies to transition metal complexes. Inorg. Chem. 54, 11385–11398 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Svitlyk V., Hermes W., Chevalier B., Matar S. F., Gaudin E., Voßwinkel D., Chernyshov D., Hoffmann R.-D., Pöttgen R., Change of the cerium valence with temperature – Structure and chemical bonding of HT-CeRhGe. Solid State Sci. 21, 6–10 (2013). [Google Scholar]
- 47.Zhang D., Oliynyk A. O., Mar A., Three Rh-rich ternary germanides in the Ce–Rh−Ge system. J. Solid State Chem. 304, 122585 (2021). [Google Scholar]
- 48.Freccero R., Solokha P., De Negri S., Saccone A., Grin Y., Wagner F. R., Polar-covalent bonding beyond the zintl picture in intermetallic rare-earth germanides. Chemistry 25, 6600–6612 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Freccero R., De Negri S., Rogl G., Binder G., Michor H., Rogl P. F., Saccone A., Solokha P., La2Pd3Ge5 and Nd2Pd3Ge5 compounds: Chemical bonding and physical properties. Inorg. Chem. 60, 3345–3354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freccero R., Pereira L. C. J., Solokha P., De Negri S., Flux growth, crystal structure, and chemical bonding of Yb2PdGe3, an AlB2 superstructure within the rare-earth series. lnorg. Chem. 62, 1988–1999 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai Y., Wei K., Chappell G., Diaz J., Siegrist T., Moll P. J. W., Graf D., Baumbach R. E., Tuning the structural and antiferromagnetic phase transitions in UCr2Si2: Hydrostatic pressure and chemical substitution. Phys. Rev. Mater. 4, 075003 (2020). [Google Scholar]
- 52.Subbarao U., Peter S. C., Crystal growth and properties of YbCuGa3: First monoclinic system in the RETX3 family. Cryst. Growth Des. 13, 953–959 (2013). [Google Scholar]
- 53.Imre A., Hellmann A., Mewis A., LaPt2Ge2 und EuPt2Ge2 –Neubestimmung der Kristallstrukturen. Z. Anorg. Allg. Chem. 632, 2217–2221 (2006). [Google Scholar]
- 54.Lim A., Fredrickson D. C., Entropic control of bonding, guided by chemical pressure: Phase transitions and 18-n+m isomerism of IrIn3. Inorg. Chem. 62, 10833–10846 (2023). [DOI] [PubMed] [Google Scholar]
- 55.Canfield P., Kong T., Kaluarachchi U., Jo N. H., Use of Frit-disc crucibles for routine and exploratory solution growth of single crystalline samples. Philos. Mag. 96, 84–92 (2016). [Google Scholar]
- 56.Kyrk T. M., Bravo M., McCandless G. T., Lapidus S. H., Chan J. Y., Investigating the An+1BnX3n+1 homologous series: A new platform for studying magnetic praseodymium based intermetallics. ACS Omega 7, 19048–19057 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrero-Martín J., García-Muñoz J. L., Valencia S., Frontera C., Blasco J., Barón-González A. J., Subías G., Abrudan R., Radu F., Dudzik E., Feyerherm R., Valence change of praseodymium in Pr0.5Ca0.5CoO3 investigated by X-ray absorption spectroscopy. Phys. Rev. B 84, 115131 (2011). [Google Scholar]
- 58.Lu Q., Vardar G., Jansen M., Bishop S. R., Waluyo I., Tuller H. L., Yildiz B., Surface defect chemistry and electronic structure of Pr0.1Ce0.9O2−δ revealed in operando. Chem. Mater. 30, 2600–2606 (2018). [Google Scholar]
- 59.Solokha P., Freccero R., De Negri S., Proserpio D. M., Saccone A., The R2Pd3Ge5 (R = La–Nd, Sm) Germanides: Synthesis, crystal structure and symmetry reduction. Struct. Chem. 27, 1693–1701 (2016). [Google Scholar]
- 60.Chabot B., Parthé E., Ce2Co3Si5 and R2Ni3Si5 (R = Ce, Dy, Y) with the orthorhombic U2Co3Si5-type structure and the structural relationship with the tetragonal Sc2Fe3Si5-type structure. J. Less-Common Met. 97, 285–290 (1984). [Google Scholar]
- 61.Chevalier B., Roisnel T., Etourneau J., Magnetic structure of U2(Ru0.65Rh0.35)3Si5 silicide. J. Magn. Magn. Mater. 134, 88–94 (1994). [Google Scholar]
- 62.Kawai T., Muranaka H., Endo T., Duc Dung N., Doi Y., Ikeda S., Matsuda T. D., Haga Y., Harima H., Settai R., Ōnuki Y., Split fermi surface properties of LaTGe3 (T: Transition Metal) and PrCoGe3 with the non-centrosymmetric crystal structure. J. Phys. Soc. Jpn. 77, 064717 (2008). [Google Scholar]
- 63.Szytula A., Leciejewicz J., Bińczycka H., Crystal and magnetic structures of PrCo2Ge2 and HoCo2Ge2. Phys. Stat. Sol. A 58, 67–70 (1980). [Google Scholar]
- 64.Rahm M., Hoffmann R., Ashcroft N. W., Atomic and ionic radii of elements 1–96. Chem. A Eur. J. 22, 14625–14632 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Fukuoka H., Baba K., Yoshikawa M., Ohtsu F., Yamanaka S., High-pressure synthesis and structures of lanthanide germanides of LnGe5 (Ln=Ce, Pr, Nd, and Sm) isotypic with LaGe5. J. Solid State Chem. 182, 2024–2029 (2009). [Google Scholar]
- 66.Fukuoka H., Yoshikawa M., Baba K., Yamanaka S., Preparation and structures of lanthanoid germanides, PrGe3.36, NdGe3.25, and TmGe3 with double square Ge-Mesh structures. Bull. Chem. Soc. Jpn. 83, 323–327 (2010). [Google Scholar]
- 67.Liu J., Smetana V., Jr K. A. G., Miller G. J., Pecharsky V. K., The crystal structure and magnetic properties of Pr117Co56.7Ge112. J. Appl. Phys. 113, 17E120 (2013). [Google Scholar]
- 68.Skanthakumar S., Lynn J. W., Mazumdar C., Nagarajan R., Gupta L. C., Magnetic phase transitions in R2Ni3Si5. Physica B 241–243, 693–695 (1997). [Google Scholar]
- 69.Gil A., Kolenda M., Baran S., Penc B., Hofmann M., Szytuła A., Neutron diffraction studies of R2Co3Si5 (R = Tb,Dy) and Tb2Ir3Si5 compounds. Physica B Condens. Matter 276-278, 742–743 (2000). [Google Scholar]
- 70.Durivault L., Bourée F., Chevalier B., André G., Etourneau J., Magnetic ordering in the ternary germanide Ce2Ni3Ge5 as studied by neutron powder diffraction. J. Magn. Magn. Mater. 246, 366–374 (2002). [Google Scholar]
- 71.Anand V. K., Adroja D. T., Ritter C., Das D., Nair H. S., Bhattacharyya A., Liborio L., Sturniolo S., Pratt F. L., Le D., Andre G., Luetkens H., Hillier A. D., Hossain Z., Magnetic structure and crystal field states of Pr2Pd3Ge5: μSR and neutron scattering investigations. Phys. Rev. B 107, 104412 (2023). [Google Scholar]
- 72.Efremova N. N., Praseodymium valence state in PrFe10Mo2, PrNi5, and PrNi4M intermetallic compounds (M = Cu, Al, Ga). Phys. Solid State 47, 424–428 (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S7
Tables S1 to S3
References