Abstract
The stress response is evolutionarily conserved across vertebrates to maintain homeostasis, but in excess can be damaging for body functions. We review the most recent research from basic sciences and epidemiology linking stress to the development and progression of metabolic disorders across the life course. Findings from rodents demonstrate that stress can affect features of metabolic dysfunction, such as insulin resistance, glucose and lipid homeostasis, as well as fundamental ageing processes such as cellular senescence and telomere length shortening. In human studies, stressors in the home, workplace and neighbourhood are associated with accelerated biological ageing and metabolic and immune alterations, both directly as well as indirectly via behavioural risks, including low physical activity, sleep disturbances, and alcohol abuse. The likelihood of developing clinical conditions, such as diabetes and fatty liver disease is increased in individuals with adverse childhood experiences or long-term stress at work or in private life – often as part of disease trajectories associated with other stress-related disorders, such as mental health problems, cardiovascular disease and increased susceptibility to infections. Among people with a metabolic disorder, stress may worsen disease prognosis. As favourable modifications in stressors are associated with reductions in incidence of metabolic disorders, the therapeutic value of targeting stress in personalised medicine merits further investigation.
The age-standardised burden of chronic diseases has declined world-wide over the past 30 years, but this has not been the case for metabolic disorders1,2. Between 1990 and 2019, obesity increased by 13% in women and 27% in men3, while diabetes increased by 24%1. A similar trend has been observed for non-alcoholic fatty liver disease (NAFLD), which is strongly associated with obesity2. These trends combined with the fact that hyperglycaemia and obesity now rank as the third and fifth leading risk factors for the global burden of disease, respectively, underscore the need to refine our understanding of metabolic physiology and to identify additional targets for prevention of metabolic disorders1,2.
One emerging risk factor for metabolic disorders is life stress, the topic of this Review. The physiological and behavioural responses to everyday challenges are evolutionarily conserved across mammals, birds, fish, reptiles, and amphibians. Most of the physiological responses activated to maintain homeostasis are short-lasting and adaptive with no harmful impact on body functions4,5. However, chronic or very intensive stressors characterised by uncontrollability and unpredictability can be damaging for body functions increasing disease susceptibility, particularly in vulnerable individuals5. Such stressors can also modify the course of disease among those already living with a disease.
In this Review, we evaluate the evidence on life stress as a risk and prognostic factor for metabolic disorders. Our focus is on a broad spectrum of stressors, ranging from adverse childhood experiences and major life events to chronic adulthood stressors and disadvantage. Our overview is organised according to the disease process. We begin with stress-related changes in metabolic traits and the phenomenon of accelerated ageing. Following these preclinical impacts, we evaluate the significance of life stress as a risk factor for clinical endpoints, with particular focus on obesity, type 2 diabetes and liver disease. To describe the role of stress in disease progression, we review clinical studies on patients with pre-existing metabolic disease. We conclude the Review with a discussion of the implications of managing stress for prevention and treatment of metabolic diseases.
Stress measurement in human and animal studies
Rather than being a monolithic concept, “life stress” is considered a process that involves interactions between individual and environmental factors, current and past events, allostatic states, and psychological and physiological reactivity4-6. Accordingly, different approaches to measure stress and quantify the stress response and putative biomarkers have been developed. Measurement of stress has focused on one or more of 3 components: the conditions that elicit stress (i.e., stressors); stress appraisal; and the stress response (table 1).
Table 1.
Assessment methods of life stress by measurement focus, indicators and mode
| Focus | Indicator | Measurement mode* |
|---|---|---|
| Exposure to stressors | Community level | |
| Neighbourhood socioeconomic disadvantage | S or M | |
| Catastrophical events (e.g. earth quake, 9/11 terror attack) | M | |
| Individual level | ||
| Trier Social Stress Test | E | |
| Adverse childhood experiences | S | |
| Major life events | S or M | |
| Socioeconomic disadvantage | S or M | |
| Work stress, parental stress, caregiver stress | S | |
| Loneliness, social isolation, lack of social contacts | S | |
| Animals in naturalistic settings | ||
| Subordinate status in stable social hierarchies | M + B | |
| Overall social rank instability | M + B | |
| Social conflict, aggression | M + B | |
| Animal models in laboratory | ||
| Chronic subordination stress | E | |
| Social instability stress | E | |
| Single prolonged stress (e.g. restraint) | E | |
| Stress response | Acute stress | |
| Blood/saliva/faecal/urine stress hormones (glucocorticoids, catecholamines) | B | |
| Implantable cardioverter–defibrillator recording + eDiary | M + S | |
| Chronic or repeated stress | ||
| Hair cortisol, allostatic load index | B | |
| Genetic variants of stress chemistry (Mendelian randomisation) | B | |
| Stress reactivity trait | E + B | |
| Post-traumatic stress disorder | D | |
| Cushing's syndrome (a stress hormone disorder) | D, B | |
| Stress appraisal | Retrospective and online reporting | |
| Psychological distress, symptom check lists | S | |
| Perceived stress, daily hassles | S | |
| Mobile monitoring (smart phone applications) | S |
Abbreviations: B, measurement of stress biomarkers (e.g. cortisol); E, external manipulation (e.g. Trier Social Stress Test); D, diagnosed condition; M, measurement of stressor (e.g. record of widowhood, death of child or neighbourhood disadvantage from national registries); S, self-report (e.g. questionnaire, interview, eDiary)
Stressors.
In animal models, several different stressors (i.e. agents causing a stress response) have been studied, including physical (e.g. foot shock), psychological (e.g. immobilisation) and psychosocial (social defeat, social subordination) factors. Among those stressors, social defeat in an aggressive encounter and subordinate status within a group or dyads have been studied extensively5,7,8.
In human stress research, tasks which involve a social-evaluative threat (in which task performance could be negatively judged by others) have been found to elicit a measurable neuroendocrine stress response. One of the most widely used standardised stress induction protocols of this type is the Trier Social Stress Test in which subjects undergo a public speaking task followed by mental arithmetic9.
Real-life stressors in human studies include adverse childhood experiences (e.g. family financial problems, psychological/physical abuse, death of parent), low socioeconomic status (e.g., low occupational position, living in socioeconomically disadvantaged neighbourhoods), major life events (e.g. divorce, job loss), natural catastrophes (war, earthquake, terrorist attack) and chronic adversity (e.g., daily hassles, work stress, caregiver stress, racial micro-aggressions and social isolation)10,11. Several of these real-life stressors have been reproduced in animal studies as well7.
Assessment of stress perception.
A variety of individual factors are thought to affect stress perception (or stress appraisal), including personality, cognitive style, earlier stress exposure, behaviours, concurrent physiological responses and genetic vulnerability10. Stress appraisal is assumed to occur when an individual perceives that environmental demands tax or exceed their regulatory homeostatic range or adaptive capacity4-6. Thus, the same exposure can be viewed as stressful or not, depending on individual appraisal12.
While stress appraisal (also referred to as cognitive stress response10) and stress-related emotions and behaviours (i.e., affective and behavioural stress responses) are typically assessed using self-administered questionnaires, more recent approaches have used eDiaries, health applications on mobile telephones, and wearable devices. These technologies allow for momentary assessment of how stressors are being perceived and enable these perceptions to be linked to physiologic changes using real time biometric data collection. This is a promising approach for integrated multidimensional data collection, although to date few large-scale studies have been completed13.
Physiological stress response.
The purpose of the homeostatic systems is to maintain optimal physiological functions under conditions of objective or perceived challenge4. In terms of health, a favourable profile consists of low basal stress-related hormone levels, acute activation in the presence of stressors, followed by rapid recovery. This physiological response is common to most daily responses to a variety of challenges, including mild stressors. In contrast, maladaptive processes potentially leading to clinical disorders, particularly when experienced during periods of rapid brain development, can result from intensive, repeated and frequent stressors, lack of adaptation, or inadequate responses that lead to compensatory hyperactivity of other physiological mediators4,14,15.
The physiological stress response involves changes in brain neurocircuitry (e.g. the limbic forebrain, the hypothalamus and the brainstem16), which affect vigilance to sensory stimuli, produce emotional responses of fear and avoidance and can be imaged using functional MRI (fMRI) or 18F-fluoro deoxyglucose PET/CT17. It has been shown that limbic regions intersect with circuits that are responsible for memory and reward, providing a means to tailor the stress response with respect to prior experience and anticipated outcomes16.
The acute physiological stress response is well characterized and consists of the activation of several neuroendocrine circuits, including the hypothalamus–pituitary–adrenal (HPA) axis, the sympatho-medullary axis (SAM), the autonomic nervous system and other systems (Figure 1; for reviews of the neuroendocrine systems implicated in the stress response, see e.g.14-16).
Figure 1. Mechanisms of stress-induced metabolic changes.
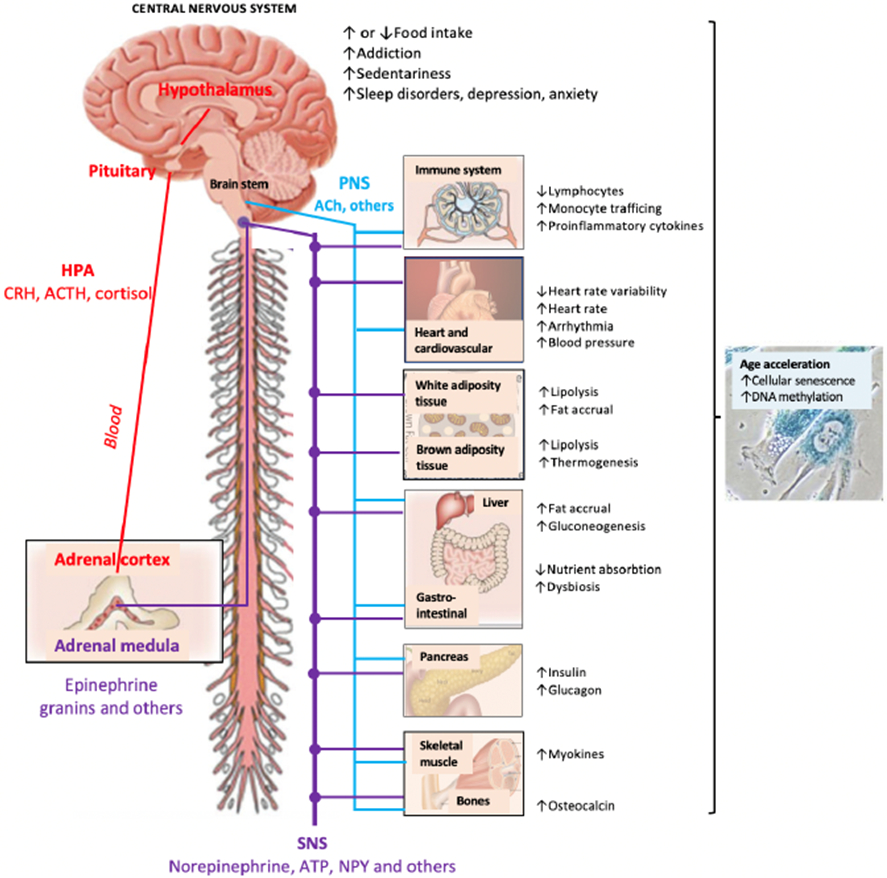
The figure summarizes major neuroendocrine pathways and behavioural disorders implicated in chronic stress, affecting (either directly or indirectly) metabolic functions, risk of metabolic disease and the pace of biological ageing. Major stress-related pathways include: The Hypothalamus Pituitary Adrenocortical (HPA) axis secreted hormones, such as corticotrophin releasing factor (CRH), adrenocorticotrophin hormone (ACTH) and cortisol; the Sympatho-Adreno-Medullary axis secreted catecholamines (epinephrine>>norepinephrine), granins and other hormones and neuropeptides34; the Sympathetic Nervous System (SNS) secreted neurotransmitters, including norepinephrine, ATP, Neuropetide Y (NPY) and other factors35,36; and the Parasympathetic Nervous System (PNS) secreted neurotransmitters, such as acetylcholine (ACh) and other factors. SNS activation and PNS withdrawal contribute to stress reactions whereas PNS activation facilitates recovery11,19. In addition to classical stress-associated metabolic regulators (such as insulin), glucagon and several cytokines, myokines and osteocalcin37 have been recently identified as regulators of the stress response. In addition, growing evidence suggest that stress can induce markers of immune senescence, DNA methylation and accumulation of senescent cells in multiple metabolic organs, including the liver, adipose and the brain20,38-41,74.
Activation of the HPA axis results in secretion of adrenal glucocorticoid cortisol which is one of the most widely assessed stress effector and is also often used as a stress biomarker11. Cortisol can be measured in saliva, urine and blood samples, but due to large diurnal variation, repeat measures are necessary for meaningful assessment18. Cortisol is easy to measure repeatedly from saliva allowing determination of morning levels, the cortisol awakening response, and the slope in cortisol decline across the day. However, collection of data over months and years required to observe transitions from a healthy stage to the development and progression of stress-related morbidity may be difficult at scale. Hair samples provide a summary cortisol measure over a longer exposure period (months), but they miss potential stress-related disturbances in the diurnal regulation of cortisol.
Other major systems activated during stress include the SAM resulting in secretion of catecholamine adrenaline (and other factors) from the adrenal medulla and the sympathetic nervous system (SNS) resulting in noradrenaline (and other factors) secretion from nerve terminals and spillover to the general circulation14-16. These stress hormones are secreted very rapidly (within fractions of a second after stress exposure) and are tightly controlled via synaptic reuptake or plasma clearance. Thus, to be useful as biomarkers, the blood collection needs to be carefully controlled and biofluids, including the urine, need to be preserved or frozen immediately after collection. For this reason, the secretion of catecholamines is often not measured directly, but rather determined indirectly via measures of heart rate variability, skin conductance or rarely direct nerve activation (activation of the SNS leads to increased heart rate, blood pressure, pulse pressure, and systemic vascular resistance). Activation of another branch of the autonomic nervous system, the parasympathetic nervous system (PNS, e.g. vagal tone) generally opposes SNS mediated effects and facilitates recovery from stress. Consistently, withdrawal of the PNS activity contributes to physiological stress reactions (e.g. reduced heart rate variability) and adversely affects the immune system19.
Challenges in interpreting the evidence.
Studies in laboratory animals complement research on humans as their shorter lives allow observation of the development of metabolic disorders across the entire lifespan20. Animal studies also allow for experimental manipulation and randomization of conditions which trigger stress (such as position in a status hierarchy), which would be either unethical or infeasible in human studies21.
However, generalisation of findings from animals to humans can be challenging since there are differences in stress responsiveness across species. While the general pattern of neuroendocrine response to stressors is conserved in rodents and humans, including responsiveness of the autonomic nervous system and other neuroendocrine mediators, rodents have a more reactive HPA axis than humans. In the Trier Social Stress test (one of the most stressful acute experimental protocols9), the elevation of cortisol is on average only moderate and due to adaptation, repeating this test in the same subjects may lead to no clear HPA axis activation. Furthermore, while a sustained increase in HPA axis activation is commonly observed in various social and non-social stress models in rodents5,16, in humans several highly stressful conditions results in reduced (rather than normal) HPA activation and cortisol14,15.
Another challenge involves extrapolations of the findings from neuroendocrine diseases (Cushing’s syndrome or pheocromocytoma) or post-traumatic stress disorder (PSTD) to the general stress response. Cushing’s syndrome (a condition characterised by excessive production of ACTH caused e.g. by a pituitary cancer, resulting in high cortisol) has been used as a model to study the possible long-term consequences of chronic hormone activation on function and health22,23. However, the excessive cortisol levels caused by Cushing’s disease occur in the absence of stressors or activation of hypothalamic corticotropin-releasing hormone (CRH). Likewise, pheochromocytoma (a rare, usually noncancerous tumour in adrenal gland) and PTSD (a condition that develops in some people who have experienced a shocking, frightening, or dangerous event) have been used as proxy to understand the mediators of the stress response. Pheocromocytoma manifests itself with chronically increased plasma catecholamine levels which in some studies have also been found to be associated with elevated cortisol levels24. PSTD is associated with high catecholamine levels (sympathetic nervous system overactivation), but, unlike Cushing`s disease, low cortisol levels. Overall, these three conditions do not have a shared biology or pathophysiology, hence they can be only informative about specific mechanisms subserving the physiological stress response25,26.
In observational studies, the technique of Mendelian randomization leverages randomly assigned genetic variation at conception as an instrument to examine the causal effect on disease onset27. The low levels of cortisol observed in PTSD and people with stressful life events14,28,29 and null findings in Mendelian randomization analyses27 suggest it is an oversimplification to treat high cortisol levels as a sole marker for harmful stress. Animal models confirm that HPA-axis activation per se is not a unique biomarker of chronic stress by demonstrating that high glucocorticoids exist in response to both stressful and pleasurable stimuli5. Indeed, both high and blunted physiological stress response may characterise harmful conditions in humans, with the latter pattern suggested to be indicative of “burnout” as a result of extended exposure to stressors4,30.
In terms of stress-related pathophysiology, disturbances in the 24-hour rhythm of circulating glucocorticoids (in addition to the levels of concentration) also play an important role31. Among all circulating factors showing a dynamic pattern of secretion, the circadian rhythm of plasma glucocorticoids is one of the largest (with values being generally highest in the mornings and lowest at night)31. In addition to a well-recognized circadian rhythm of cortisol secretion, significant knowledge is now available on ultradian rhythms and pulsatile cortisol secretion and their upstream mechanism (reviewed in15,18). When stress becomes chronic and cortisol levels stay elevated, the 24-hour glucocorticoid rhythm can be disrupted and part of the beneficial actions of cortisol are lost, particularly at the time of the usual nadir.
Because the physiological neuroendocrine stress response is variable and complex including many mediators, it is reductive to mechanistically explain the effect of stress only focusing on or measuring HPA axis activation31-33.
Stress-related mechanisms and preclinical changes
Research has linked life stress exposure with indicators of adverse glycaemic and anthropometric changes, deterioration of immune function, and accelerated biological ageing20,34-41 (figure 1). Many findings have been confirmed in studies which used different measurement methods or different stressors. Such verification of results across disparate lines of evidence — a technique called triangulation – strengthens validity, yet it does not necessarily prove causality. Studies have also highlighted that the associations between life stress and adverse biological changes may be partially attributable to (or even potentiated by) the higher prevalence of unhealthy behaviours and mental health problems among stressed individuals.
Changes in glucose and insulin metabolism.
In cells, actions of glucocorticoids are mediated by the glucocorticoid receptors (expressed throughout the body) and mineralocorticoid receptors (expressed e.g. in the kidney, heart, colon, and specific brain nuclei42). Glucocorticoids are essential for the regulation of glycaemia and lipid metabolism, but they also regulate immunity, inflammation, growth, reproduction, and cardiovascular function43. Metabolic processes regulated by glucocorticoid receptors include lipolysis, hepatic gluconeogenesis, amino acid mobilization, and reduced skeletal muscle glucose uptake. It has been observed that in certain chronic stress conditions, hypothalamic activation of the pituitary changes from a predominant CRH-dominant to arginine vasopressin (AVP)-dominant regulation, and cortisol levels may remain raised due to decreased cortisol metabolism (in addition HPA-axis dysregulation)15. These changes contribute to higher glucose levels and insulin resistance. States of excess secretion of stress-related hormones, such as Cushing syndrome and pheochromocytoma, and systemic administration of glucocorticoids also induce insulin resistance44,45. In insulin resistance, metabolic tissues that are sensitive to insulin (e.g. skeletal muscle, liver, and white adipose tissue) become less sensitive to insulin increasing the risk of diabetes and fatty liver disease46. This may be one of the major pathways linking stress to metabolic disorders.
Epidemiological evidence supports this concept. An observational cohort study of 3,000 human participants aged 6-18 years at baseline and followed up for over 30 years via eight repeated biomedical examinations provides population-level evidence measured life stress using indicators of disadvantaged residential neighbourhoods47 (figure 2a). While groups with high vs low life stress did not differ in metabolic traits at baseline, by early adulthood the high-stress group was characterised by increased blood lipid levels, decreased insulin sensitivity, and compensatory increases in insulin which, however, were insufficient to prevent elevated circulating glucose concentrations. By midlife, individuals consistently exposed to high stress were more likely to be obese, hypertensive, and have a fatty liver and diabetes compared with those who were consistently exposed to low stress. In a randomised trial for patients with type 2 diabetes, stress management training was associated with a small but significant improvement in long-term glycaemic control48.
Figure 2. Preclinical metabolic changes from childhood to adulthood in individuals exposed to stressors at individual level and in community and with stress appraisal.
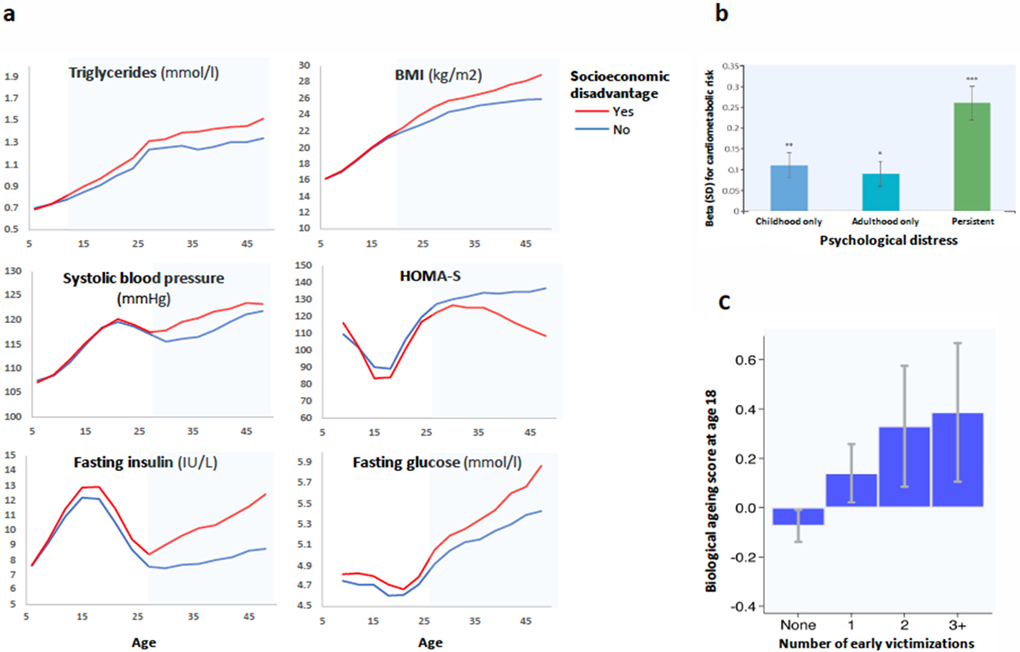
a Repeated measurements from childhood to adulthood in the Young Finns cohort show no difference in metabolic traits between groups with vs without socioeconomic disadvantage at age 6 but gradually increasing differences in trajectories of triglycerides, glycaemic traits and BMI between these groups until age 48. b In the 1958 British Birth Cohort study, the association between psychological distress and a composite cardiometabolic index incorporating inflammatory markers is significant in both childhood and adulthood. Supporting a dose-response pattern, persistent psychological distress across both childhood and adulthood is more strongly associated with the cardiometabolic index than psychological distress in just childhood or adulthood but not both. c In the E-Risk study, there is a graded relationship between a higher number of childhood victimisations (domestic violence, peer bullying, physical and sexual harm by an adult, and neglect) and accelerated biological ageing score at age 18. Figures have been adapted from references 39,47,51.
Immune dysfunction and inflammation.
There is a complex interaction between the physiological stress response and immune system activation8,32,49,50. One hypothesis is that exposure to chronic stress may induce glucocorticoid receptor resistance, a condition of reduced sensitivity of immune cells to glucocorticoids that normally terminate the inflammatory response50. According to this view, what matters is the way target tissue responds to stress hormones rather than the levels of the hormone per se. Glucocorticoid receptor resistance leads to increased duration and/or intensity of the inflammatory response contributing to the development and progression of insulin resistance, type 2 diabetes and cardiovascular disease. Experimental studies based on viral-challenge paradigm for groups with and without stressors, such as adverse life events, loneliness and severe disease of a child or spouse, support this hypothesis although assessment of glucocorticoid receptor resistance has relied on indirect data50.
PNS activation leads to release of neurotransmitter acetylcholine to target tissues. Acetylcholine has an anti-inflammatory function as it binds to macrophage surface receptors blocking release of inflammatory cytokines including IL-1, -2, and -6, and tumour necrosis factor alpha (TNF-α). Conversely, activation of the SNS-mediated release of norepinephrine tends to increase the secretion of those molecules. Thus, stressful conditions associated with sustained SNS activity or withdrawal of the PNS may elevate the levels of these proinflammatory proteins contributing to chronic inflammation19. There is also a bidirectional communication between the immune systems and stress neuroendocrine axes, as certain cytokines can trigger the activation of the HPA axis and the SNS. These include TNF-α, IL-1, and IL-6 produced at inflammatory sites and elsewhere in response to inflammation32.
Observational evidence from real-life settings supports associations between long-term stress, altered immune function and metabolic changes. Prospective life course studies, for example, have linked exposure to stressors at individual and community levels and stress appraisal with increased systemic inflammation (circulating C-reactive protein and glycoprotein acetyls) as well as worse glycaemic traits, high blood pressure and composite measures of adverse metabolic profile (allostatic load)39,47,51-56. In the 1958 British Birth Cohort study, psychological distress was more strongly associated with a composite metabolic index (C-reactive protein, glycosylated haemoglobin, fibrinogen, triglycerides, total cholesterol, high-density-lipoprotein cholesterol (reversed scored), blood pressure, resting heart rate) in childhood than adulthood51. Supporting a dose-response pattern, psychological distress across childhood and adulthood was more strongly associated with this metabolic index than psychological distress in either childhood or adulthood but not in both51 (figure 2b).
Evidence from clinical disorders is also converging (but see limitation to extrapolate these results to the general population discussed above). Cushing’s syndrome is associated with proinflammatory state (increased circulating cytokines) and immune suppression (e.g. reduced B- and T-cell count)23,57,58 and other metabolic alterations (hyperglycaemia, hypertension, dyslipidaemia). PSTD is also associated with alterations of inflammatory-immune activity (e.g. elevated levels of the proinflammatory cytokines interleukin 1β, interleukin 6, and interferon γ59,60) and elevated blood pressure25,61, although little research suggests dyslipidaemia as an important clinical feature of PTSD25,62. Furthermore, chronic catecholamine excess in pheochromocytoma is accompanied by an increase in inflammation markers which is reversed by the tumour removal63.
Finally, epidemiological evidence also confirms the link between life stress and declining immune function at older ages. In the Health and Retirement Study, adulthood socioeconomic adversity and unhealthy behaviours was associated with age-related metabolic and immune biomarkers (included albumin, creatinine, glucose, C-reactive protein, lymphocyte percent, mean cell volume, red cell distribution width, alkaline phosphatase, and white blood cell count)64.
Stress induced acceleration of biological ageing.
Similar to stress, ageing is associated with impaired metabolic function including increased insulin resistance, impaired unrestrained hepatic gluconeogenesis and adipose lipogenesis, and increasingly defective glycogen synthesis and glucose uptake in skeletal muscle65. Many components of the immune system also change with age, including a rise in circulating inflammatory mediators, such as proinflammatory cytokines (which interfere with insulin action) and the overall down-regulation of immune responsiveness66,67. Parallels in the effects of stress and ageing are in agreement with the stress-induced accelerated ageing hypothesis. This hypothesis relies on the assumption that ageing is characterised by a gradual accumulation of damage to cells and tissues, and that this effect can be affected by external factors such as environmental exposures, lifestyle, diet, and diseases. According to the stress-acceleration of ageing hypothesis, life stress is one of the factors that accelerate biological ageing68,69.
Supporting this notion, research suggests that stressors in childhood and adulthood stressors and chronically elevated stress biomarkers are not only linked to metabolic and immune dysfunction, but also other hallmarks of biological ageing70, such as telomere erosion69 and accumulation of senescent cells. The molecular mechanisms linking stress to senescence mechanisms are being actively investigated but this field is still in its infancy38,71. It has been observed that stress-induced glucocorticoid secretion generates reactive oxidative stress (ROS) via increased mitochondrial activity, potentially damaging telomeres and inhibiting telomerase activity, both contributing to cellular senescence72. Cellular senescence appears to accumulate under stress conditions in animal models and humans20,38, resulting in pro-oxidant, proinflammatory “senescence associated secretory phenotype” (SASP) which reinforces stress-induced systemic inflammation and cellular ageing72. A recent meta-analysis of nine human studies found an association between early life threat and biomarkers of cellular ageing, such as telomere length shortening in immune cells73, although there was heterogeneity in study-specific estimates which also included some null findings. Positive findings were reported at least for caregiver stress74. Another meta-analysis based on a different set of studies concluded that there is a link between exposure to childhood trauma, lifetime PTSD severity and accelerated ageing as indicated by accelerated DNA methylation ageing75. Findings from the Environmental Risk (E-Risk) Longitudinal Twin Study found that children raised in more socioeconomically disadvantaged neighbourhoods may enter young adulthood epigenetically distinct from their less disadvantaged peers76. A recent review of studies on socioeconomic disadvantage in childhood and adulthood confirmed an association with accelerated epigenetic ageing77. Lastly, the Northern Finland Birth Cohort 1966 Study suggested that working long hours may contribute to age acceleration, although such an association was not observed for other work-related stressors, such as job strain and effort-reward imbalance78.
Childhood could be a more sensitive period for adverse stress effects on biological ageing than adulthood. A systematic review including 13 animal and 27 human studies published since 2004 found consistent support for associations between early-life adversity and accelerated cellular ageing (89% of studies reported positive findings) whereas the evidence for parental stress and other adulthood psychosocial stressors was somewhat less consistent (67-70% positive findings)79. In the E-Risk study, there was a graded relationship between a higher number of childhood victimisations and more advanced biological ageing score at age 18 (figure 2c)39. In agreement with this observation, a meta-analysis suggested that early-life adversity characterized by threat is associated with accelerated pubertal development73.
Impact of stress on body composition.
Obesity is a major driver of metabolic diseases80,81. Computational tomography studies in humans and research on aged rodents show that subcutaneous fat decreases, whereas visceral fat (the sum of fat depots inside the abdominal cavity) increases with age65,82. Body weight tends to increase up to ~70 years of age in humans and ~22 month of age in laboratory mice, while values decline at older ages in both species with the weight loss being more pronounced in mice20,82. Osteoporosis and skeletal muscle loss (sarcopenia) represent further age-related changes, increasing the risk of frailty at older ages.
Fat tissue, skeletal muscle and bones are organs that respond to efferent signals from hormone systems and the central nervous system and are therefore plausibly affected by life stress. Supporting the hypothesis of stress-acceleration of ageing in humans, some studies suggest chronic stress and stress hormone hypersecretion are associated with increased fat mass, cellular dehydration, osteo-sarcopenia, and frailty83. In the Prospective Urban and Rural Epidemiological (PURE) study of 120,000 people, those with higher composite indicator of life stress (stress at work and home, major life events, and financial stress) had higher prevalence of abdominal obesity compared to participants with low life stress84. Similarly, long-term socioeconomic adversity has been related to small birth weight (a risk factor for diabetes) but subsequent weight gain and obesity in a number of birth cohort studies (e.g. British Birth Cohort 1958, UK, Dunedin cohort, New Zealand, the Southwest Finland Birth Cohort and the Young Finns Study, Finland, the Dutch perinatal registry study, the Netherlands)47,51-55,85,86. Stress-induced hyperphagia has been demonstrated in humans and some animal models of psychosocial stress87.
However, not all the studies of stress and weight change have been consistent88. There may be stable individual differences in response to stress such that stress appraisal and stressors contribute to upward BMI trajectories for some people, downward trajectories for others and no weight change in still others87,89. This is likely to be mediated by dietary behaviour. Some people eat more in response to stress (hyperphagic), craving foods with high fat-salt/sugar content89. Others become hypophagic and eat less89. Differences in response to stress are supported by longitudinal studies90 and a large cross-sectional individual-participant meta-analysis (IPD Work consortium) which reported increased prevalence of both obese and underweight individuals among those reporting work stress91. In animal models, physical and psychological stressors have been linked to negative energy balance, while psychosocial stressors appear to contribute to positive energy balance87,92.
Behavioural pathways.
Life stress can affect metabolic health directly through autonomic, neuroendocrine and immune responses, but also indirectly, through changes in health-related behaviours and mental health. Psychosocial stressors at work and in private life and PSTD have been linked to unhealthy behaviours which may partially mediate the stress-metabolic disease association. Large-scale cohort studies, such as PURE, and a meta-analysis of 61 cohorts found stressors at work stress and in private life to be associated with greater alcohol consumption84,93, a finding consistent with the observation of higher alanine transaminase and gamma-glutamyl transferase levels in stressed men in another large study94. Longitudinal analyses of IPD-Work suggest a link between stress and lower physical activity95 and PURE found greater smoking prevalence among participants with work stress84. Stress-related behavioural effects are also supported by research showing reduced physical activity and increased smoking in patients with PSTD96,97.
In addition, sleep disturbances and short sleep are common under stress contributing to dysregulated homeostasis97,98. Population studies have shown exposure to stressful events (such as major life events and daily hassles) may impair normal sleep function resulting in difficulty falling and staying asleep99. A review of major life events, life trauma and perceived chronic stress concluded that stress may be associated with impaired sleep100. A meta-analysis of 31 studies with polysomnography measurements found PTSD patients to have decreased total sleep time, slow wave sleep and sleep efficiency, and increased wake time after sleep onset compared with healthy controls97.
The mediating role of disturbed sleep in the associations between life stress and metabolic disorders is biologically plausible as disturbances in the circadian regulation of stress hormones, cortisol in particular, are considered to contribute to pathophysiologies31,101. Critically, abnormal glucocorticoid rhythm, sleep disturbances and sleep loss are risk factors for insulin resistance, chronic inflammation, high glucose and cardiometabolic disorders, such as type 2 diabetes and cardiovascular disease31,102-105.
Psychological mediators.
Indirect effects through changes in mental health are also likely. In the Twins Early Development Study, adverse childhood experiences assessed between 3 and 11 years of age were associated with elevated depressive symptoms at age 21106. Meta-analyses of prospective cohort studies have reported increased risk of depressive symptoms and disorders in individuals with personal or neighbourhood socioeconomic adversity and work-related stress107-109. Furthermore, Cushing’s syndrome is a risk factor for psychiatric disorders57 and anxiety is a common symptom in patients with pheochromocytoma110.
Similar findings have been reported from natural catastrophes. In a study from Iran, increased prevalence rates for anxiety and depressive symptoms, ranging between 40% and 65% were observed among individuals who were exposed to high-intensity warfare and chemical weapons in the 1980-1988 Iran-Iraq war111. The corresponding rates were lower, 6-18% among those exposed to low-intensity warfare. The prevalence of depression and PTSD increased among survivors in the aftermath of the 2011 Great East Japan Earthquake and Tsunami112. Disaster-related trauma was also associated with marked increases in obesity, as well as elevated blood pressure and lipids, based on linkage to individual medical records before and after the disaster. Cardiometabolic risk was also correlated with the extent of housing damage (e.g., minor damage versus total destruction) caused by the tsunami112.
Plausible biological pathways underlying the association between mental health problems and obesity include alterations in systems involved in HPA axis, immuno-inflammatory activation, neuroendocrine regulators of energy metabolism and brain circuitries integrating homeostatic and mood regulatory responses113,114. In a population-based register study of 5.9 million Danish people, those with mood or neurotic disorder, such as depression or anxiety, had approximately 1.5-fold increased risk of developing type 2 diabetes, liver disease or kidney disorders115. Other studies have identified a bi-directional association for type 2 diabetes and NAFLD, such that having these metabolic disorders also increase the risk of developing mental health problems113,116-119.
Stress and risk of clinical disease
In light of the above-reviewed evidence on cardiometabolic and immune dysfunction and stress-acceleration of ageing amplified by unhealthy behaviours and mental health problems, an association of life stress with a broad set of clinical conditions is expected. Supporting this hypothesis, life stress has been linked to metabolic disorders, such as diabetes7,22,25,120, fatty liver23,121,122, and composite morbidity indices123 in separate prospective studies across multiple stressors. This evidence is strengthened by the dose-response pattern observed between stress and risks of diabetes and liver disease across the life course: higher exposure to early life stress seems to increase adulthood disease risk in a graded fashion7.
Outcome-wide studies.
Outcome-wide studies allow an evaluation of the relative importance of stress in the aetiology of metabolic diseases compared to other health conditions91,109,124-133. As shown in table 2, individuals exposed to stressors at work and private life and at community level have increased risk of mental disorders and cardiometabolic disease. Stressors across the life course are associated with about 1.1 to 1.4-fold increased risk of diabetes124-129,131,132. In people with childhood adverse experiences, the risk of adulthood cardiovascular, liver and digestive diseases, sexually transmitted infections, illicit drug use and mental disorders is particularly marked, 2- to 6-fold compared to those with no such experiences124.
Table 2.
Relative risk of health outcomes for life stressors (exposed vs non-exposed) in selected large outcome-wide meta-analyses and outcome-wide cohort studies
| Relative risk for a disease (descending effect)* |
Stressor in private life | Stressor at work | ||
|---|---|---|---|---|
| Adverse childhood experiences | Neighbourhood socioeconomic disadvantage |
Long working hours | Job strain | |
| 2.00 or higher (strong effect) | Sexually transmitted infections | |||
| Illicit drug use | ||||
| Depression, anxiety | ||||
| Respiratory disease | ||||
| Liver or digestive disease | ||||
| Cancer, all sites | ||||
| Cardiovascular disease | ||||
| 1.50 to 1.99 | Stroke (intracerebral haemorrhage) | |||
| Obesity requiring hospital treatment | Early cardiovascular death | |||
| Self-harm | ||||
| COPD | ||||
| 1.20 to 1.49 | Overweight & obesity Diabetes | Lung cancer | Atrial fibrillation Haemorrhagic stroke Obesity requiring hospital treatment Self-harm |
Obesity (class II or III) Depressive disorder Coronary heart disease |
| Substance abuse | ||||
| Mood disorders | ||||
| Viral infections | ||||
| Stroke (cerebral infarction) | ||||
| Liver disease | ||||
| Ischaemic heart disease | ||||
| 1.10 to 1.19 | Osteoarthritis Bacterial infections |
COPD Lung cancer Substance abuse Heart failure |
Stroke (ischaemic) Lung cancer Diabetes COPD |
|
| <1.10 or no effect | Colorectal cancer | Mood disorders | Asthma | |
| Breast cancer | Viral infections | Stroke (haemorrhagic) | ||
| Prostate cancer | Diabetes | Any cancer | ||
Relative risks refer to hazard ratios for associations between life stressors (exposed vs non-exposed) and health outcomes from references91,109,124-133. The strongest links are between adverse childhood experiences and clinical diseases suggesting that childhood is a sensitive period, particularly in terms of risk of poor mental health. Moderate excess risk of metabolic diseases (obesity, diabetes, liver disease) is observed in individuals with adverse childhood experiences, neighbourhood socioeconomic disadvantage and job strain. Risk of cardiovascular diseases and infections is also elevated among those exposed to stressors.
Cushing’s syndrome presents with partially similar metabolic comorbidities and complications as seen in stress-related disorders, implicating ACTH and glucocorticoids in the sequalae of stress-induced metabolic changes. This notion is consistent with findings from studies on glucocorticoid receptor agonists, such as prednisone and cortisone, which are commonly used in the treatment of inflammatory diseases (e.g. rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, psoriasis) due their anti-inflammatory, immunosuppressive action. In line with findings for chronic stress, long-term use of oral glucocorticoids is associated with serious side effects, such as metabolic disease and increased risk of cardiovascular and inflammatory disease, and osteoporosis. However, caution should be taken because chronic steroid treatment leads to a blunted HPA axis activation, unlike chronic physiological stress. Additionally, the potency of oral glucocorticoids drugs is much higher than that observed for endogenous cortisol134.
The scale of the problem (a horizontal comparison).
Findings from large-scale studies and meta-analyses on various stressors in relation to obesity, type 2 diabetes and liver disease are summarised in table 323,96,124,125,131,132,135-150. The relative risk estimates have varied between 1.1 and 1.5 for markers of stress, such as PSTD, workplace bullying, psychological distress, job strain, long working hours, effort-reward imbalance and stressful life events. Relative risk of liver disease associated with adverse childhood experiences was higher, almost 3-fold. As expected, the strongest link involves a primary neuroendocrine disease, Cushing’s syndrome.
Table 3.
Strength of association between indicators of stress and risk of three metabolic diseases from large epidemiologic and clinical studies
| Obesity/overweight | Diabetes | Liver disease | |
|---|---|---|---|
| Cushing's syndrome | 2.00 | 9.40 | 4.30 |
| Adverse childhood experiences | 1.39 | 1.52 | 2.76 |
| Low occupational position | 1.82 | 1.31 | 1.21 |
| PSTD | 1.31 | 1.49 | – |
| Workplace bullying | 1.24 | 1.46 | – |
| Psychological distress | 1.26 | 1.33 | 1.40 |
| Job strain | 1.30 | 1.16 | – |
| Long working hours | 1.13 | 1.18 | 1.22 |
| Effort-reward imbalance | 1.09 | 1.24 | – |
| Stressful life events | 1.07 | 1.18 | – |
Numbers are hazard ratios for being exposed (versus not being exposed) to various stressors, stress appraisal and proxy measures of the stress response from references23,96,124,125,131,132,135-150. The strongest associations with obesity, diabetes and liver disease are observed for Cushing’s syndrome (a disease with high cortisol secretion), adverse childhood experiences, and low occupational position.
A horizontal comparison of these estimates to those from other metabolic risk factors suggests that life stress represents a moderate-size risk factor. Life stress is obviously associated with more modest excess risk of metabolic disease than major risk factors, such as obesity and intermediate hyperglycaemia (i.e. prediabetes). The relative risk of diabetes is approximately 7-fold for obesity and about 3-fold for overweight compared to normal weight151. The relative risk of type 2 diabetes for low versus high physical activity and for high vs normal triglycerides is ≈1.5-fold and thus of a similar or slightly higher order of magnitude than life stress152,153.
Support for causality.
While strong experimental evidence exists on mechanisms in cells and tissues activated by stress-mediators, these data may not fully inform processes that underlie the pathological effects of life stress. The physiological stress response is complex and includes both adaptive mechanisms to maintain homeostasis in a changing environment, and pathophysiological alterations that compromise health in relation to chronic and intensive stressors4-6. Many physiological stress mediators affect metabolic functions in different, sometimes opposite directions87. Additionally, the laboratory paradigm in humans does not allow extended time windows to explore chronic stress and randomization of people to different degree of real-life stressors is not possible or ethically acceptable. Given these limitations, the causal role of life stress in the development of clinical disease, such as metabolic disorders, cannot be proven by current evidence, although causality seems likely in light of the converging findings from multiple lines of research.
Stress and disease progression
It is important to evaluate evidence on risk of disease incidence separately from disease prognosis because the same exposure can increase the likelihood of developing a specific disease only marginally but affect progression of this disease substantially (or vice versa). While the same stress-related pathophysiological mechanisms that contribute to the development of metabolic disease may also worsen the outcome of the disease, additional effects of stress might also come into the play. For example, life stress is related to reduced self-care, both directly and via mental health problems, thereby posing a potential barrier for treatment adherence. Studies suggest that people with overweight and life stress benefit less from weight loss interventions than those without stress100 and that financial strain among older adults is associated with lower medication adherence154.
Adverse stress effects may be more pronounced among people with disease than healthy controls, exacerbating the development of complications and comorbidities. In an experimental study of 140 diabetic and 280 matched nondiabetic participants, for example, those with diabetes showed impaired post-stress recovery in systolic and diastolic blood pressure, heart rate and cholesterol and inadequate responses in other mediating pathways after a stress exposure (modified Stroop colour-word interference task and a mirror drawing task)155. Stress-induced elevation in cortisol and IL6 concentrations measured over the day were particularly marked in the diabetes group.
Disease complications and outcomes.
Findings from real-life settings support the notion that stress may accelerate the progression of disease. In IPD-Work, work-related stress was not associated with total mortality among healthy employees, but a 1.6-fold increased risk of death was observed among stressed men with cardiometabolic disease (diabetes, myocardial infarction or stroke), with the contribution of stress being clinically significant and independent of conventional risk factors and their treatment156. A similar interaction was observed in another large-scale study of over 485,000 adults who participated in the National Health Interview Survey157. During 8-year follow-up, severe psychological distress was associated with a 2-fold increased risk of death in individuals with diabetes but only 1.5-fold increased risk in non-diabetic people, while the corresponding relative risks were 2.0- and 1.4-fold for cardiovascular deaths. In other studies, psychological distress was associated with 1.4 to 1.8 times higher cardiovascular disease risk, all-cause mortality,158 and liver disease mortality144. These findings in humans are paralleled by observations in randomised mouse models in which subordination stress (a model of low socioeconomic status) induced an earlier onset of lesions in multiple organs, resulting in significantly shortened lifespan when compared to mice high in social rank20.
Disease cascades and multimorbidity.
At least one outcome-wide study has examined the temporal sequence of the onset of multiple diseases during adulthood125 (figure 3a). This multicohort study from Finland and the UK used linked electronic health records for disease ascertainment. The findings highlighted the importance of mental health and behavioural problems in setting in motion the development of a range of stress-related physical illnesses, such as liver and kidney diseases, ischaemic heart disease, cerebral infarction, and dementia among people with socioeconomic disadvantage. In contrast, the association of socioeconomic disadvantage with diabetes was largely independent of preceding mental health problems125.
Figure 3. Disease trajectories, multimorbidity and death in individuals exposed to socioeconomic stressors at individual and community levels.
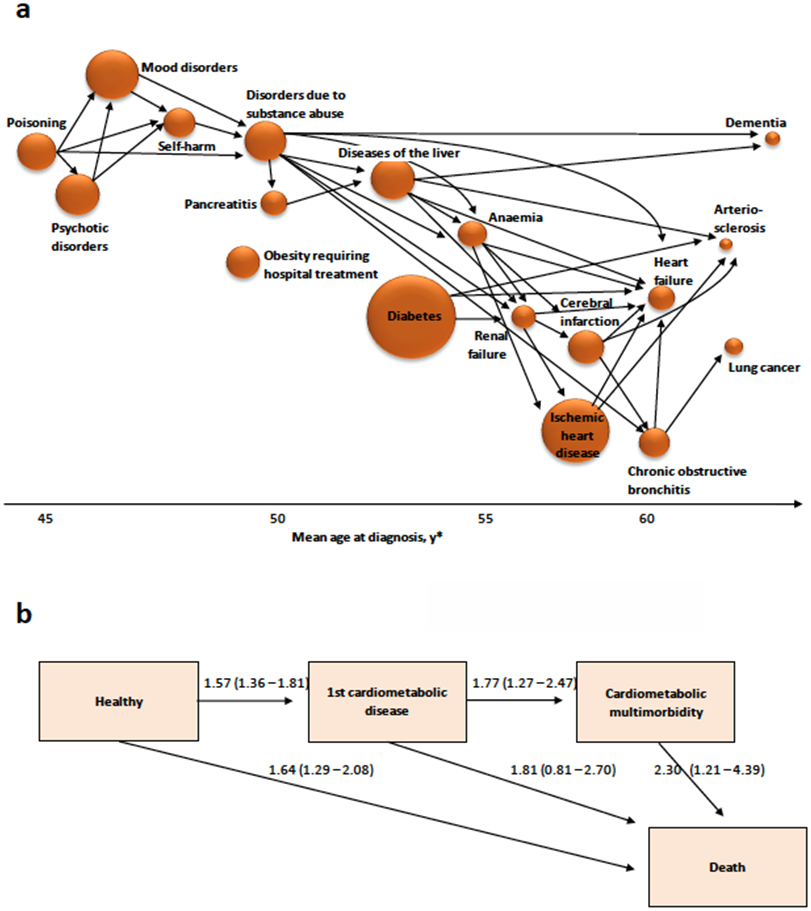
a Associations between diseases in participants with neighbourhood socioeconomic adversity, pooled data from the Health and Social Support study and the Finnish Public Sector study (reference125). Arrows link only pairs of diseases with hazard ratios greater than 3. Diseases along the x-axis are in order of increasing mean age at diagnosis or hospitalisation. The size of plots is proportional to the incidence of disease. b Numbers are hazard ratios (95% confidence intervals) from the British Whitehall II study for low vs high occupational position. They show increased risk of developing cardiometabolic disease (diabetes, coronary heart disease or stroke), multimorbidity and dying (reference170
Diabetes, liver and kidney diseases in the socioeconomically disadvantaged group were strongly linked to subsequent cardiovascular diseases, suggesting that life stress may increase the risk of complications and multimorbidity among those with metabolic disease125. While liver diseases were additionally associated with increased risk of dementia, this was not seen for diabetes in the study population which included a relatively low number of elderly people125. Nonetheless, the majority of research evidence suggests that type 2 diabetes increases the risk of dementia159 and also observational cohort studies have linked chronic work stress with increased risk of developing dementia in old age160.
Mortality.
Cumulative early life adversity and low social rank predict longevity in animal studies including mice, wild rabbits, meerkats, baboons, rhesus macaques, and long-tailed (cynomolgus) macaques baboons7,20,161. In humans, a range of stressors, including adverse childhood experiences162, socioeconomic adversity163, stressful life events (e.g. bereavement)164,165, work stress156, financial strain166 and perceived stress or distress167,168, particularly among those living with disease or multimorbidity, present risks for premature mortality169 (figure 3b). According to results from the Whitehall II study, socioeconomic disadvantage affects disease progression from the development of first cardiometabolic disease to multimorbidity, frailty (a state of impaired function across multiple physiologic systems compromising the ability to cope with stressors) and death170,171.
Impact of stress prevention
In the following section, we evaluate benefits and harms of selected interventions altering life stress in terms of metabolic health (table 2). In addition, we describe recommendations regarding stress reduction in current clinical guidelines for metabolic disease prevention.
Policies and interventions to reduce life stress.
Societal interventions to reduce the amount of stress in people’s lives lie outside the realm of health care at present. Examples of such structural interventions include the provision of income security through government safety nets, assuring access to the basic necessities of life (such as health care, housing, safe neighbourhoods, secure jobs), and improving the conditions of work (e.g. regulating over-time and work schedules, provision of paid sick leave and parental leave). Unfortunately, randomized trials evaluating the impact of social policies on population health remain sparse due to feasibility and cost, and the existing studies were under-powered to detect improvements in health outcomes172. Individual studies, such as the Moving to Opportunity (MTO) experiment in the United States provide some clue about the expected magnitude of health improvement as a result of improving people’s social circumstances. In the MTO, adults living in disadvantaged areas in five US cities were randomly given the opportunity to move to a less disadvantaged area173. Follow-up 10-15 years later showed that people who moved to a less disadvantaged area had lower prevalence of extreme obesity and diabetes than did members of the control group who were not given this opportunity. The incidence of having BMI≥35 at follow-up was 31.1% in the experimental group and 35.5% in controls, the corresponding incidence for BMI≥40 (morbid obesity) being 14.4% versus 17.7%.
In a large observational study from Finland (N=114,000), changes in health were observed among people who experienced improvements in residential neighbourhood conditions174. The risk of diabetes was 15% lower among residents of neighbourhoods where unemployment dropped from high to low compared to those whose neighbourhood unemployment rate remained high174. A further ‘natural experiment’ followed up 61,000 refugees who arrived in Sweden and were assigned to one of 4,833 neighbourhoods175. Being assigned to an area deemed low deprivation versus high deprivation was associated with a 18% lower relative risk of diabetes. Neighbourhood effects grew over time such that 5 years of additional exposure to lower deprivation neighbourhoods was associated with a 9% lower diabetes risk.
In summary, structural interventions hold the promise of addressing the root causes of life stress but they have proved difficult to evaluate due to feasibility and cost. Meantime, in the realm of health care, interventions have targeted individuals with the aim of reducing health disparities.
Individual level interventions and clinical guidelines.
Health care utilizes the high-risk individual approach in which individuals at high risk of disease are targeted for treatment. Stress management interventions to reduce stress perception and symptoms have shown relatively little effect on risk or prognosis of metabolic diseases. A Cochrane review of randomized controlled trials concluded that due to low quality of evidence from psychological interventions it remains unclear whether they can improve self-efficacy and glycaemic control, although psychological interventions added to usual care probably did not result in significant harm176. A subsequent systematic review and meta-analysis suggested minimal, if any, clinical benefit for psychological interventions in improving glycaemic control177. This result contrasts with findings on policy interventions, suggesting that psychological interventions to relieve stress are not addressing the root causes of stress-induced metabolic diseases.
Except for alcohol-associated liver diseases178, there are only vague recommendations regarding stress reduction in clinical guidelines for metabolic disease prevention. This is appropriate given the current state of evidence, viz., modest findings from psychological interventions. The International Diabetes Federation (IDF), for example, recommends the following: “Be alert to signs of cognitive, emotional, behavioural and/or social problems which may negatively impact quality of life and complicate self-care, particularly where diabetes outcomes are sub-optimal” and that “Screening for depression with a validated tool should be encouraged in primary care diabetes clinics”. The recommendation by the American Diabetes Association is to follow a standardised diabetes prevention programme. The 16-session core curriculum includes sections on lowering calories, increasing physical activity, self-monitoring, maintaining healthy lifestyle behaviours, and psychological, social, and motivational challenges179. In the real world, however, patient adherence to treatment advice remains a significant hurdle in achieving health-care targets. According to personalised medicine, this should be addressed with more tailored management of patients, including, as necessary, psychological support as an adjunct to treatment180, but to date, development of these approaches is still in progress.
Further approaches.
A further suggested approach to mitigating the harmful effects of stress is through strengthening resilience. Benefits of building stress resilience by strengthening social relations are supported by animal and human evidence. Research on wild macaques suggests that responses to social and environmental stress are attenuated by strong male bonds181. In chimpanzees, consolation has been shown to reduce behavioural measures of stress in recipients of aggression182. Even in rats, the adverse behavioural and physiological consequences of an aggressive interaction are more severe if the subject is subsequently housed in isolation rather than returned to its sibling group183. In humans, neighbourhood social capital and social cohesion have been associated with reduced risk of obesity, although the findings vary depending on the measures and covariates used184,185.
Conclusions
Life stress is linked to a broad range of stressors in the home, workplace, and neighbourhood and studies have discussed life stress in terms of a general “predisposing risk factor”, a source of “accumulated wear and tear”, and a disturbed physiological state. In this narrative review of animal models, epidemiological research, and experimental and genetic studies, converging findings from many lines of investigation supported three general conclusions:
First, observational evidence suggests that life stress is an important prognostic factor for metabolic diseases, adversely affecting the course of disease. Long-term follow-ups highlight the importance of metabolic diseases as part of stress-related disease cascades during the lifecourse in which behavioural and mental health problems set in motion the development of a range of physical illnesses. These studies show that life stress does not only increase the risk of first metabolic disease, but also contributes to subsequent multimorbidity.
Second, several large-scale studies show that people with life stress are at increased risk of clinical metabolic disorders, including obesity, type 2 diabetes and fatty liver disease. The excess disease risk is not specific to metabolic pathologies but is also related to other non-communicable diseases and severe infections, with the link between life stress and cardiovascular and mental disorders being slightly stronger than that with diabetes. In terms of effect size, horizontal comparisons suggest life stress represents a moderate size risk factor for metabolic diseases.
Third, there is evidence to suggest that life stress results in cumulative acceleration of biological age and senescence, including unfavourable changes in metabolism and immune function and exacerbation of genetic disease. In addition to these direct pathophysiological alterations, life stress may contribute to increased risk of metabolic disorders indirectly via mental health problems and unhealthy behaviours. Childhood appears to be a sensitive period during which long-term trajectories of adverse stress effects are likely to become established.
Further translational research is needed because population trends in metabolic disorders suggest that national prevention programmes, lifestyle interventions (weight reduction, increased physical activity, healthy diet, smoking cessation, moderation of alcohol consumption) and preventive medications (blood pressure-, lipid- and glucose-lowering therapies) have not been sufficient in reversing the continual growth in the burden of metabolic disease. Emerging evidence from structural interventions suggests that policies that reduce sources of life stress (e.g., via improving socioeconomic circumstances or strengthening social safety nets with the aim of reducing uncertainty in people’s lives and increasing controllability of the social and physical environment) might complement individually-targeted approaches to metabolic disease prevention. Once metabolic disorder has developed, patient adherence to lifestyle and treatment advice poses a major barrier in achieving treatment targets and may be further complicated by the presence of daily stress. Additional evaluation in the context of personalised medicine is warranted to determine whether more individualised treatment regimes that take into account the patient’s life situation could provide improved, cost-effective treatment options.
Table 4.
Approaches to reduce life stress and prevent metabolic disease
| Intervention | Description | Evidence |
|---|---|---|
| Structural interventions | Improvements in government safety nets, socioeconomic circumstances and the conditions of work reduce a wide range of stressors. | Reduced risk of obesity and diabetes demonstrated in real-life randomised and natural experiments and longitudinal observational studies on change in neighbourhoods. |
| Increase in resilience | Higher social capital and social cohesion in community may relate to better emotional and instrumental social support. | Animal models show attenuated responses to social and environmental stress. Comparisons of human communities suggest social capital and social cohesion may be associated with reduced risk of obesity. |
| Psychological interventions | Stress management training, meditation and other interventions to alter stress perception and relieve symptoms | Randomized controlled trials suggest that psychological interventions alone have little benefit in terms of metabolic disease prevention or treatment. |
| Precision medicine | Aims to develop tailored treatments centred on the patient’s needs and circumstances, including as necessary stress management | Limited evidence on effectiveness |
Key points.
Both animal and human research suggests that stress and related changes in sympathetic-parasympathetic balance as well as hypothalamic-pituitary-adrenal axis can accelerate biological ageing, including unfavourable changes in metabolism and immune function.
Childhood appears to be a sensitive period to stress exposure. The adverse impact on metabolic disease risk in adults with a history of childhood adversity is potentiated by mental disorders and behavioural risks and can be over 2-fold compared to those without childhood adversity.
In relation to stress in adulthood, the excess risk of obesity, diabetes and liver disease is 1.1 to 1.4-fold. The excess risk of mental disorders, such as depression, and cardiovascular disease among individuals with stress in adulthood is slightly higher.
Life stress is also a prognostic factor in patients, accelerating the transition of metabolic diseases towards multimorbidity, frailty and death.
Acknowledgements
M.K.’s work was supported by the UK Medical Research Council (S011676), Wellcome Trust, UK (221854/Z/20/Z), National Institute on Aging (NIH), US (R01AG056477), and the Academy of Finland (329202, 350426). A.B.’s work was supported by NIH/NIDDK (DK117504, DK118150, DK102496), NIH/NHLBI (HL151740), NIH/NIA (AG043972) and MN Partnership for Biotechnology and Molecular Genomic #18.4. I.K’s work was supported by National Institutes of Aging (R01 AG042463).
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.GBD Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paik JM et al. The growing burden of disability related to nonalcoholic fatty liver disease: Data from the Global Burden of Disease 2007-2017. Hepatol Commun 4, 1769–1780 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai H. et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: An analysis of the Global Burden of Disease Study. PLoS medicine 17, e1003198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS Protective and damaging effects of stress mediators. N Engl J Med 338, 171–179 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Koolhaas JM et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35, 1291–1301 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Epel ES et al. More than a feeling: A unified view of stress measurement for population science. Front Neuroendocrinol 49, 146–169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder-Mackler N. et al. Social determinants of health and survival in humans and other animals. Science 368, eaax9553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartolomucci A. Social stress, immune functions and disease in rodents. Front Neuroendocrinol 28, 28–49 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Kirschbaum C, Pirke KM & Hellhammer DH The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiol 28, 76–81 (1993). [DOI] [PubMed] [Google Scholar]
- 10.Harkness KL & Monroe SM The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. J Abnorm Psychol 125, 727–745 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Kivimaki M & Steptoe A Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 15, 215–229, (2018). [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Janicki-Deverts D & Miller GE Psychological stress and disease. JAMA 298, 1685–1687 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Lampert R. et al. Triggering of symptomatic atrial fibrillation by negative emotion. J Am Coll Cardiol 64, 1533–1534 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Gunnar M & Quevedo K The neurobiology of stress and development. Annu Rev Psychol 58, 145–173 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Russell G & Lightman S The human stress response. Nat Rev Endocrinol 15, 525–534 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Ulrich-Lai YM & Herman JP Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10, 397–409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawakol A. et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389, 834–84, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lightman SL, Birnie MT & Conway-Campbell BL Dynamics of ACTH and cortisol secretion and implications for disease. Endocr Rev 41, bnaa002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sara JDS et al. Mental stress and its effects on vascular health. Mayo Clin Proc 97, 951–990 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzoli M. et al. Social stress shortens lifespan in mice. Aging Cell 17, e12778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koert A. et al. The social instability stress paradigm in rat and mouse: a systematic review of protocols, limitations, and recommendations. Neurobiol Stress 5, 100410 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacroix A, Feelders RA, Stratakis CA & Nieman LK Cushing’s syndrome. Lancet 386, 913–927 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Ferrau F & Korbonits M Metabolic comorbidities in Cushing’s syndrome. Eur J Endocrinol 173, M133–157 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Constantinescu G. et al. Glucocorticoid excess in patients with pheochromocytoma compared with paraganglioma and other forms of hypertension. J Clin Endocrinol Metabol 105, dgaa423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell CJ et al. Posttraumatic stress disorder and cardiovascular disease: State of the science, knowledge gaps, and research opportunities. JAMA Cardiol 6, 1207–1216 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Wingenfeld K, Whooley MA, Neylan TC, Otte C & Cohen BE Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinol 52, 83–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok MK, Kawachi I, Rehkopf D & Schooling CM The role of cortisol in ischemic heart disease, ischemic stroke, type 2 diabetes, and cardiovascular disease risk factors: a bi-directional Mendelian randomization study. BMC Med 18, 363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X, Wang Z, Wu X, Wen SW & Liu A Salivary cortisol in post-traumatic stress disorder: a systematic review and meta-analysis. BMC Psychiat 18, 324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP & Olff M Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry 191, 387–392 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Stalder T. et al. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinol 77, 261–274 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Oster H. et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev 38, 3–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrousos GP Stress, chronic inflammation, and emotional and physical well-being: concurrent effects and chronic sequelae. J Allergy Clin Immunol 106, S275–291 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Ramamoorthy S & Cidlowski JA Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin North Am 42, 15–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartolomucci A. et al. The extended granin family: structure, function, and biomedical implications. Endocr Rev 32, 755–797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch D & Zukowska Z NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol 32, 645–659 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Possenti R. et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J 441, 511–522 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Berger JM et al. Mediation of the acute stress response by the skeleton. Cell Metab 30, 890–902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rentscher KE et al. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16(INK4a). Psychoneuroendocrinol 102, 139–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belsky DW et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife 9, 54870 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noren Hooten N, Pacheco NL, Smith JT & Evans MK The accelerated aging phenotype: The role of race and social determinants of health on aging. Ageing Res Rev 73, 101536 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder-Mackler N. et al. Social status alters immune regulation and response to infection in macaques. Science 354, 1041–1045 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH & Joels M Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol 49, 124–145 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Fine NHF et al. Glucocorticoids reprogram beta-cell signaling to preserve insulin secretion. Diabetes 67, 278–290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogawa A. et al. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest 90, 497–504 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiesner TD, Bluher M, Windgassen M & Paschke R Improvement of insulin sensitivity after adrenalectomy in patients with pheochromocytoma. J Clin Endocrinol Metabol 88, 3632–3636 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Utzschneider KM & Kahn SE Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metabol 91, 4753–4761 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Kivimaki M. et al. Neighbourhood socioeconomic disadvantage, risk factors, and diabetes: a cohort study from childhood to middle age. Lancet Public Health 3, e365–e373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surwit RS et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care 25, 30–34 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Takahashi A, Flanigan ME, McEwen BS & Russo SJ Aggression, social stress, and the immune system in humans and animal models. Front Behav Neurosci 12, 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen S. et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. PNAS 109, 5995–5999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winning A, Glymour MM, McCormick MC, Gilsanz P & Kubzansky LD Psychological distress across the life course and cardiometabolic risk: Findings from the 1958 British Birth Cohort study. J Am Coll Cardiol 66, 1577–1586 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Deighton S, Neville A, Pusch D & Dobson K Biomarkers of adverse childhood experiences: A scoping review. Psychiatry Res 269, 719–732 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Crick DCP et al. Associations between adverse childhood experiences and the novel inflammatory marker glycoprotein acetyls in two generations of the Avon Longitudinal Study of Parents and Children Birth Cohort. Brain Behav Immun 100, 112–120 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berger E. et al. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat Commun 10, 773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danese A & McEwen BS Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 106, 29–39 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro AI et al. Neighbourhood socioeconomic deprivation and allostatic load: a multi-cohort study. Sci Rep 9, 8790 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pivonello R. et al. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol 4, 611–629 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Hasenmajer V. et al. The Immune System in Cushing's Syndrome. Trends Endocrinol Metab 31, 655–669 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Passos IC et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiat 2, 1002–1012 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Olff M & van Zuiden M Neuroendocrine and neuroimmune markers in PTSD: pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Curr Opin Psychol 14, 132–137 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Sumner JA et al. Post-traumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychol Med 46, 3105–3116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shalev A, Liberzon I & Marmar C Post-traumatic stress disorder. N Engl J Med 376, 2459–2469 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Zelinka T. et al. Elevated inflammation markers in pheochromocytoma compared to other forms of hypertension. Neuroimmunomodulation 14, 57–64 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Liu Z. et al. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: Evidence from the Health and Retirement Study. PLoS Med 16, e1002827 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barzilai N, Huffman DM, Muzumdar RH & Bartke A The critical role of metabolic pathways in aging. Diabetes 61, 1315–1322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alpert A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med 25, 487–495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrucci L & Fabbri E Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15, 505–522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crimmins EM Social hallmarks of aging: Suggestions for geroscience research. Ageing Res Rev 63, 101136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Epel ES The geroscience agenda: Toxic stress, hormetic stress, and the rate of aging. Ageing Res Rev 63, 101167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez-Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Childs BG, Durik M, Baker DJ & van Deursen JM Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21, 1424–1435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin J & Epel ES Stress and telomere shortening: Insights from cellular mechani. Ageing Res Rev 73, 101507 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colich NL, Rosen ML, Williams ES & McLaughlin KA Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol Bull 146, 721–764 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Epel ES et al. Accelerated telomere shortening in response to life stress. PNAS 101, 17312–17315 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf EJ et al. Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology 92, 123–134, doi: 10.1016/j.psyneuen.2017.12.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reuben A. et al. Association of neighborhood disadvantage in childhood with DNA methylation in young adulthood. JAMA Netw Open 3, e206095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raffington L & Belsky DW Integrating DNA methylation measures of biological aging into social determinants of health research. Curr Environ Health Rep 9, 196–210 (2022). [DOI] [PubMed] [Google Scholar]
- 78.Freni-Sterrantino A. et al. Work-related stress and well-being in association with epigenetic age acceleration: A Northern Finland Birth Cohort 1966 Study. Aging 14, 1128–1156 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turecki G & Meaney MJ Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biol Psychiat 79, 87–96 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng Y, Ley SH & Hu FB Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14, 88–98 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Carlsson LM et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 367, 695–704 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Pontzer H. et al. Daily energy expenditure through the human life course. Science 373, 808–812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stefanaki C, Pervanidou P, Boschiero D & Chrousos GP Chronic stress and body composition disorders: implications for health and disease. Hormones 17, 33–43 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Santosa A. et al. Psychosocial risk factors and cardiovascular disease and death in a population-based cohort from 21 low-, middle-, and high-income countries. JAMA Netw Open 4, e2138920 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rautava S. et al. Neighborhood socioeconomic disadvantage and childhood body mass index trajectories from birth to 7 years of age. Epidemiol 33, 121–130 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ochoa LB et al. Association of neighbourhood socioeconomic trajectories with preterm birth and small-for-gestational-age in the Netherlands: a nationwide population-based study. Lancet Reg Health Eur 10, 100205 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Razzoli M & Bartolomucci A The dichotomous effect of chronic stress on obesity. Trends Endocrinol Metab 27, 504–515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosengren A. et al. Psychosocial factors and obesity in 17 high-, middle- and low-income countries: The Prospective Urban Rural Epidemiologic (PURE) study. Int J Obes 39, 1217–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oliver G & Wardle J Perceived effects of stress on food choice. Physiol Behav 66, 511–515 (1999). [DOI] [PubMed] [Google Scholar]
- 90.Kivimäki M. et al. Work stress, weight gain and weight loss: evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. Int J Obes 30, 982–7 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Nyberg ST et al. Job strain in relation to body mass index: pooled analysis of 160 000 adults from 13 cohort studies. J Int Med 272, 65–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Razzoli M, Pearson C, Crow S & Bartolomucci A Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci Biobehav Rev 76, 154–162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Virtanen M. et al. Long working hours and alcohol use: systematic review and meta-analysis of published studies and unpublished individual participant data. BMJ 350, g7772 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Magnusson-Hanson L. et al. Work stress, anthropometry, lung function, blood pressure, and blood-based biomarkers: a cross-sectional study of 43,593 French men and women. Sci Rep 7, 9282 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fransson EI et al. Job strain as a risk factor for leisure-time physical inactivity: An individual-participant meta-analysis of up to 170 000 men and women - The IPD-Work Consortium. Am J Epidemiol 176, 1078–1089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van den Berk-Clark C. et al. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: A systematic review and meta-analysis. Health Psychol 37, 407–416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y. et al. Sleep in posttraumatic stress disorder: A systematic review and meta-analysis of polysomnographic findings. Sleep Med Rev 48, 101210 (2019). [DOI] [PubMed] [Google Scholar]
- 98.Kim EJ & Dimsdale JE The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med 5, 256–278 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kalmbach DA, Anderson JR & Drake CL The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res 27, e12710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geiker NRW et al. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes Rev 19, 81–97 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Meier-Ewert HK et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43, 678–683 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Kumari M, Shipley M, Stafford M & Kivimaki M Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J Clin Endocrinol Metabol 96, 1478–1485 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hackett RA, Kivimaki M, Kumari M & Steptoe A Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the Whitehall II Cohort Study. J Clin Endocrinol Metabol 101, 619–625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Briancon-Marjollet A et al. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr 7, 25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iyegha ID, Chieh AY, Bryant BM & Li L Associations between poor sleep and glucose intolerance in prediabetes. Psychoneuroendocrinol 110, 104444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iob E, Baldwin JR, Plomin R & Steptoe A Adverse childhood experiences, daytime salivary cortisol, and depressive symptoms in early adulthood: a longitudinal genetically informed twin study. Transl Psychiatry 11, 420 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorant V. et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol 157, 98–112 (2003). [DOI] [PubMed] [Google Scholar]
- 108.Richardson R, Westley T, Gariepy G, Austin N & Nandi A Neighborhood socioeconomic conditions and depression: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 50, 1641–1656 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Madsen IEH et al. Job strain as a risk factor for clinical depression: systematic review and meta-analysis with additional individual participant data. Psychol Med 47, 1342–1356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsirlin A. et al. Pheochromocytoma: a review. Maturitas 77, 229–238 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Hashemian F. et al. Anxiety, depression, and posttraumatic stress in Iranian survivors of chemical warfare. JAMA 296, 560–566 (2006). [DOI] [PubMed] [Google Scholar]
- 112.Kawachi I, Aida J, Hikichi H & Kondo K Disaster resilience in aging populations: Lessons from the 2011 Great East Japan Earthquake & Tsunami. J R Soc N Z 50, 263–278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Milaneschi Y, Simmons WK, van Rossum EFC & Penninx BW Depression and obesity: evidence of shared biological mechanisms. Mol Psychiat 24, 18–33 (2019). [DOI] [PubMed] [Google Scholar]
- 114.Ulrich-Lai YM & Ryan KK Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metab 19, 910–925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Momen NC et al. Association between mental disorders and subsequent medical conditions. N Engl J Med 382, 1721–1731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tabak AG, Akbaraly TN, Batty GD & Kivimaki M Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol 2, 236–245 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Moulton CD, Pickup JC & Ismail K The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol 3, 461–471 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Lindekilde N. et al. Prevalence of type 2 diabetes in psychiatric disorders: An umbrella review with meta-analysis of 245 observational studies from 32 systematic reviews. Diabetol 65, 440–456 (2022). [DOI] [PubMed] [Google Scholar]
- 119.Soto-Angona O. et al. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med 18, 261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Warrier V. et al. Gene-environment correlations and causal effects of childhood maltreatment on physical and mental health: a genetically informed approach. Lancet Psychiat 8, 373–386 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Laitinen TT et al. Childhood socioeconomic disadvantage and risk of fatty liver in adulthood: the Cardiovascular Risk in Young Finns Study. Hepatology 71, 67–75 (2019). [DOI] [PubMed] [Google Scholar]
- 122.Rahimi L, Rajpal A & Ismail-Beigi F Glucocorticoid-induced fatty liver disease. Diabetes Metab Syndr Obes 13, 1133–1145 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Crowe CL et al. Associations of loneliness and social isolation with health span and life span in the U.S. Health and Retirement Study. J Gerontol A Biol Sci Med Sci 76, 1997–2006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hughes K. et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2, e356–366 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Kivimaki M. et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 5, e140–e149 (2020). [DOI] [PubMed] [Google Scholar]
- 126.Heikkila K. et al. Job strain and COPD exacerbations: an individual-participant meta-analysis. Eur Resp J 44, 247–251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heikkila K. et al. Job strain and the risk of severe asthma exacerbations: a meta-analysis of individual-participant data from 100 000 European men and women. Allergy 69, 775–783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heikkila K. et al. Work stress and risk of cancer: meta-analysis of 5700 incident cancer events in 116,000 European men and women. BMJ 346, f165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nyberg ST et al. Job strain as a risk factor for type 2 diabetes: A pooled analysis of 124 808 men and women. Diabetes Care 37, 2268–2275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fransson EI et al. Job strain and the risk of stroke: an individual-participant data meta-analysis. Stroke 46, 557–559 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Kivimaki M. et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet 380, 1491–1497 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ervasti J. et al. Long working hours and risk of 50 health conditions and mortality outcomes: a multicohort study in four European countries. Lancet Reg Health Eur 11, 100212 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Virtanen M. et al. Long working hours and change in body weight: analysis of individual-participant data from 19 cohort studies. Int J Obes 44, 1368–1375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Daley-Yates PT Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol 80, 372–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sharma ST, Nieman LK & Feelders RA Comorbidities in Cushing’s disease. Pituitary 18, 188–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dekkers OM et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metabol 98, 2277–2284 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Pigeyre M. et al. How obesity relates to socio-economic status: identification of eating behavior mediators. Int J Obes 40, 1794–1801 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Agardh EE et al. Burden of type 2 diabetes attributed to lower educational levels in Sweden. Popul Health Metr 9, 60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vancampfort D. et al. Type 2 diabetes among people with posttraumatic stress disorder: Systematic review and meta-analysis. Psychosom Med 78, 465–473 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Xu T. et al. Onset of workplace bullying and risk of weight gain: A multicohort longitudinal study. Obesity 28, 2216–2223 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Xu T. et al. Workplace bullying and violence as risk factors for type 2 diabetes: a multicohort study and meta-analysis. Diabetol 61, 75–83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ul-Haq Z, Mackay DF, Fenwick E & Pell JP Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity 21, E322–327 (2013). [DOI] [PubMed] [Google Scholar]
- 143.Mommersteeg PM, Herr R, Zijlstra WP, Schneider S & Pouwer F Higher levels of psychological distress are associated with a higher risk of incident diabetes during 18 year follow-up: results from the British household panel survey. BMC Public Health 12, 1109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Russ TC et al. Association between psychological distress and liver disease mortality: A meta-analysis of individual study participants. Gastroenterol 148, 958–966 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Pena-Gralle APB et al. Job strain and effort-reward imbalance as risk factors for type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Scand J Work Environ Health 48, 5–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li D & Zou Y Causal effects of life course adiposity on chronic kidney disease: a Mendelian randomization study. Ann Palliat Med 10, 10861–10869 (2021). [DOI] [PubMed] [Google Scholar]
- 147.Cuevas AG et al. Stressful life events and obesity in the United States: The role of nativity and length of residence. Am J Health Promotion 36, 190–193 (2022). [DOI] [PubMed] [Google Scholar]
- 148.Wang M. et al. Associations between stressful life events and diabetes: Findings from the China Kadoorie Biobank study of 500,000 adults. J Diabetes Investig 10, 1215–1222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nordentoft M. et al. Effort-reward imbalance at work and weight changes in a nationwide cohort of workers in Denmark. Am J Ind Med 63, 634–643 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kouvonen A, Kivimäki M, Cox SJ, Cox T & Vahtera J Relationship between work stress and body mass index among 45,810 female and male employees. Psychosom Med 67, 577–583 (2005). [DOI] [PubMed] [Google Scholar]
- 151.Abdullah A, Peeters A, de Courten M & Stoelwinder J The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 89, 309–319 (2010). [DOI] [PubMed] [Google Scholar]
- 152.Aune D, Norat T, Leitzmann M, Tonstad S & Vatten LJ Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 30, 529–542 (2015). [DOI] [PubMed] [Google Scholar]
- 153.Zhao J. et al. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: a prospective study with 8-year follow-ups in two cohorts. J Transl Med 17, 403 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khera R. et al. Cost-related medication nonadherence in adults with atherosclerotic cardiovascular disease in the United States, 2013 to 2017. Circulation 140, 2067–2075 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Steptoe A et al. Disruption of multisystem responses to stress in type 2 diabetes: investigating the dynamics of allostatic load. PNAS 111, 15693–15698 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kivimaki M. et al. Work stress and risk of death in men and women with and without cardiometabolic disease: a multicohort study. Lancet Diabetes Endocrinol 6, 705–713 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang W. et al. Psychological distress and all-cause, cardiovascular disease, cancer mortality among adults with and without diabetes. Clin Epidemiol 13, 555–565 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dalsgaard EM et al. Psychological distress, cardiovascular complications and mortality among people with screen-detected type 2 diabetes: follow-up of the ADDITION-Denmark trial. Diabetologia 57, 710–717 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Livingston G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fratiglioni L, Marseglia A & Dekhtyar S Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference? Lancet Neurol 19, 533–543 (2020). [DOI] [PubMed] [Google Scholar]
- 161.Tung J, Archie EA, Altmann J & Alberts SC Cumulative early life adversity predicts longevity in wild baboons. Nat Commun 7, 11181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bellis MA et al. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. J Public Health (Oxf) 37, 445–454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stringhini S et al. Socioeconomic status and the 25 x 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 389, 1229–1237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Prior A. et al. Bereavement, multimorbidity and mortality: a population-based study using bereavement as an indicator of mental stress. Psychol Med 48, 1437–1443 (2018). [DOI] [PubMed] [Google Scholar]
- 165.Rutters F. et al. The association between psychosocial stress and mortality is mediated by lifestyle and chronic diseases: the Hoorn Study. Soc Sci Med 118, 166–172 (2014). [DOI] [PubMed] [Google Scholar]
- 166.Falvey JR, Hajduk AM, Keys CR & Chaudhry SI Association of financial strain with mortality among older US adults recovering from an acute myocardial infarction. JAMA Intern Med 182, 445–448 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Prior A. et al. The Association Between Perceived Stress and Mortality Among People With Multimorbidity: A Prospective Population-Based Cohort Study. Am J Epidemiol 184, 199–210 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Batty GD, Hamer M & Gale CR Life-course Psychological Distress and Total Mortality by Middle Age: The 1970 Birth Cohort Study. Epidemiol 32, 740–743 (2021). [DOI] [PubMed] [Google Scholar]
- 169.Emerging Risk Factors C. et al. Association of cardiometabolic multimorbidity with mortality. JAMA 314, 52–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singh-Manoux A. et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: A cohort study. PLoS Med 15, e1002571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brunner EJ et al. Midlife contributors to socioeconomic differences in frailty during later life: a prospective cohort study. Lancet Public Health 3, e313–e322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Courtin E, Kim S, Song S, Yu W & Muennig P Can socialpolicies improve health? A systematic review and meta-analysis of 38 randomized trials. Milbank Q 98, 297–371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ludwig J. et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med 365, 1509–1519 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kivimaki M. et al. Modifications to residential neighbourhood characteristics and risk of 79 common health conditions: a prospective cohort study. Lancet Public Health 6, e396–e407 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.White JS et al. Long-term effects of neighbourhood deprivation on diabetes risk: quasi-experimental evidence from a refugee dispersal policy in Sweden. Lancet Diabetes Endocrinol 4, 517–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chew BH, Vos RC, Metzendorf MI, Scholten RJ & Rutten GE Psychological interventions for diabetes-related distress in adults with type 2 diabetes mellitus. Cochrane Database Syst Reviews 9, CD011469 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Winkley K. et al. Psychological interventions to improve glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. BMJ Open Diabetes Res Care 8, 001150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Crabb DW, Im GY, Szabo G, Mellinger JL & Lucey MR Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 71, 306–333 (2020). [DOI] [PubMed] [Google Scholar]
- 179.American Diabetes, A. 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 44, S34–S39 (2021). [DOI] [PubMed] [Google Scholar]
- 180.Chatterjee S, Khunti K & Davies MJ Type 2 diabetes. Lancet 389, 2239–2251 (2017). [DOI] [PubMed] [Google Scholar]
- 181.Young C, Majolo B, Heistermann M, Schulke O & Ostner J Responses to social and environmental stress are attenuated by strong male bonds in wild macaques. PNAS 111, 18195–18200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fraser ON, Stahl D & Aureli F Stress reduction through consolation in chimpanzees. PNAS 105, 8557–8562 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ruis MA et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinol 24, 285–300 (1999). [DOI] [PubMed] [Google Scholar]
- 184.Carrillo-Alvarez E, Kawachi I & Riera-Romani J Neighbourhood social capital and obesity: a systematic review of the literature. Obes Rev 20, 119–141 (2019). [DOI] [PubMed] [Google Scholar]
- 185.Perez E. et al. Neighbourhood community life and health: A systematic review of reviews. Health Place 61, 102238 (2020). [DOI] [PubMed] [Google Scholar]


