Abstract
Background and Aims: The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has fundamentally reshaped the landscape of global public health, with some people suffering more adverse clinical outcomes than others. The aim of this study is to deepen our understanding of the specific impact of acute kidney injury (AKI) on the in-hospital mortality in octogenarian patients with COVID-19. Methods: This is a prospective observational cohort study, which involved 23 COVID-19 hospital units in the Campania Region, Italy. Exposure variables were collected during hospital admission and at discharge. Only patients aged ≥80 years were deemed eligible for the study. Results: 197 patients were included in the study (median age 83.0 [82.0–87.0] years; 51.5% men), with a median duration of hospitalization of 15.0 [8.0–25.0] days. From the multivariable Cox regression analysis, after the application of Šidák correction, only the respiratory rate (HR 1.09, 95% CI: 1.04 to 1.14; p < 0.001) and AKI development (HR: 3.40, 95% CI: 1.80 to 6.40; p < 0.001) were independently associated with the primary outcome. Moreover, the Kaplan–Meier analysis showed a significantly different risk of in-hospital mortality between patients with and without AKI (log-rank: <0.0001). Conclusions: In our investigation, we identified a significant association between AKI and mortality rates among octogenarian patients admitted for COVID-19. These findings raise notable concerns and emphasize the imperative for vigilant monitoring of this demographic cohort.
Keywords: acute kidney injury, octogenarian, COVID-19, in-hospital mortality, SARS-CoV-2
1. Introduction
The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has fundamentally reshaped the landscape of global public health. This highly transmissible virus manifests with diverse clinical presentations, ranging from mild respiratory symptoms to severe systemic complications [1,2]. It has been reported by the World Health Organization that the global COVID-19 pandemic has resulted in a total mortality of 6,981,263 deaths [3]. As our understanding of COVID-19 has evolved, it has become increasingly evident that certain demographic groups face unique challenges, and among them, octogenarian individuals have emerged as a particularly vulnerable population [4,5]. In fact, frailty, identified as a geriatric condition, is characterized by physiological alterations affecting the musculoskeletal, neuroendocrine, and immune systems [6]. These changes not only contribute to an accelerated trajectory of functional decline but also intersect with a myriad of comorbidities commonly observed in octogenarians, including cardiovascular diseases, diabetes, and compromised immune function. This convergence of factors renders octogenarians more susceptible to severe health outcomes [6,7].
Recent epidemiological reports have underscored the elevated mortality rates and heightened risk of complications among octogenarians diagnosed with COVID-19 [7]. Additionally, individuals with pre-existing chronic kidney disease (CKD) may face heightened risks and challenges when infected with COVID-19. In addition, COVID-19 can exacerbate existing renal conditions, leading to a decline in kidney function [8]. Acute kidney injury (AKI), a critical renal complication, has garnered increased attention in the context of COVID-19. The virus’s impact on renal health extends beyond exacerbating pre-existing conditions, with emerging evidence suggesting a direct link between COVID-19 infection and the development of AKI. This raises concerns about the potential implications of AKI for patient outcomes, especially in vulnerable populations such as octogenarians [9]. Despite the recognized importance of renal health in the context of COVID-19, the specific consequences of AKI for certain demographic groups, especially octogenarians, remain less explored. The aim of this study is to deepen our understanding of the specific impact of AKI on in-hospital mortality in octogenarian patients with COVID-19.
2. Methods and Materials
2.1. Study Design and Participants
COVOCA (observational study on the COVID-19 population hOspitalized in the CAmpania Region) is a prospective observational cohort study that engaged 23 COVID-19 centers within hospitals across the Campania Region, Italy. The study centered on adult patients (≥18 years old) hospitalized due to SARS-CoV-2 infection between 1 November 2020 and 30 June 2021. Individuals with missing or incomplete laboratory and clinical data at the commencement or conclusion of their hospitalization were excluded from the study. The primary sources of data for the study comprised electronic records and clinical charts of each hospitalized subject. From the COVOCA dataset of 1403 hospitalized subjects with a positive swab for SARS-CoV-2, 209 individuals aged ≥80 years were deemed eligible for the study. Following the exclusion of 12 patients due to missing data, a total of 197 patients were included in the present analysis. All patients discharged alive were phone-called to confirm their 30-day survival. All patients provided written informed consent. The study received approval from the local Ethics Committees (Universita’ degli studi della Campania “Luigi Vanvitelli”, Azienda Ospedaliera Universitaria, “Luigi Vanvitelli”, and Azienda Ospedaliera di Rilievo Nazionale “Ospedali dei colli”; ID 10879/I; approval date: 11 May 2020) and aligned with the principles outlined in the 1976 Declaration of Helsinki and its subsequent amendments.
2.2. Variables (Outcome and Exposure)
The diagnosis of SARS-CoV-2 infection was established through real-time polymerase chain reaction (RT-PCR) analysis of specimens obtained via nasal–pharyngeal swabs. In assessing in-hospital mortality and length of stay, either death certificates or discharging letters were utilized. Upon admission and discharge of subjects, details of the following exposure variables were collected: (a) Anthropometric and demographic characteristics; (b) Anamnestic data, incorporating the number of vaccinated individuals, type of vaccine received, COVID-19 positive cases in the family, and the duration between diagnosis and hospitalization; (c) Symptoms and signs experienced by patients, including cough, anosmia, fever, diarrhea, chest and abdominal pain, dysgeusia (including the onset day), dyspnea, and altered consciousness; (d) Information on pre-existing comorbidities, such as diabetes, smoking habits, chronic cardiac disease, hypertension, chronic liver disease (CLD), chronic kidney disease (CKD), chronic respiratory disease, cancers, and chronic neurological disorders; (e) Details about drugs administered at the beginning and during hospitalization for infection treatment; (f) Regarding laboratory data collected for the COVOCA registry, specific attention was directed to creatinine, and the estimated glomerular filtration rate (eGFR) was calculated using the CKD-Epi formula.
Diabetes mellitus was identified following the guidelines outlined by the American Diabetes Association (ADA). The diagnosis was derived from anamnestic records, coupled with laboratory examinations performed upon admission [10]. In a similar manner, hypertension diagnosis adhered to the guidelines set by the European Society of Hypertension and the European Society of Cardiology, supplemented by anamnestic data [11]. Chronic cardiac conditions, including heart failure, previous acute myocardial infarction (AMI), ischemic cardiopathy, atrial fibrillation, and valvulopathy, were diagnosed based on medical history and clinical investigation. For other conditions such as chronic liver disease (CLD), chronic respiratory diseases, chronic kidney disease (CKD), malignancies, and neurologic disorders, the diagnosis relied on the anamnesis of each subject.
AKI was diagnosed according to KDIGO guidelines: (1) an increase in serum creatinine of ≥0.3 mg/dL (≥26.5 µmol/L) within 48 h; (2) an increase in serum creatinine to ≥1.5 times the baseline within the previous 7 days; (3) urine volume ≤ 0.5 mL/kg/h for 6 h [12].
2.3. Statistical Analysis
Categorical data were summarized as frequencies in absolute and relative percentages. Continuous variables were expressed as either the mean and standard deviation (SD) or the median and interquartile range (IQR), depending on their distribution, as assessed using the Shapiro–Wilk test. Regarding missing data, categorical variables were categorized as ”Missing”, representing a specific category for each variable. For continuous variables, no imputation methods were applied, and missing information was denoted as not applicable (N/A) in the dataset.
Population data were stratified into two groups based on AKI development during the in-hospital stay. Group differences were assessed using p values, calculated through ANOVA or Kruskal–Wallis tests for continuous data and chi-squared or Fisher’s exact tests for categorical data, depending on the distribution and sample size. We considered p values less than 5% as statistically significant in our analyses. Additionally, a logistic regression analysis was conducted on the medications administered during the in-hospital stay to investigate whether they could be associated with the development of AKI.
Our primary endpoint, in-hospital mortality, was evaluated using Kaplan–Meier survival analysis, with log-rank tests to compare survival curves between patients with or without AKI. The Kaplan–Meier curve further allowed us to visually depict survival estimates over time. The follow-up time lasted from hospitalization until the discharge (or death) date to ensure inclusion of all enrolled patients.
To identify potential prognostic factors influencing survival outcomes, univariable and multivariable Cox proportional hazards regression models were utilized. These models provided hazard ratios (HR) and their corresponding 95% confidence intervals (CI), assessing the strength and significance of factors in relation to the primary endpoint. Šidák correction was applied to address the issue of multiple testings in the context of multivariable Cox regression analysis. Šidák correction was used to counteract the problem of multiple comparisons (α < 0.007).
All statistical analyses were performed using RStudio (RStudio Team (2016); RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, USA; URL http://www.rstudio.com/, accessed on 3 August 2023).
3. Results
We included 197 patients with positive swabs for SARS-CoV-2 in the study (median age 83.0 [82.0–87.0] years; 51.5% men) followed at our referral centers for a median hospitalization period of 15.0 [8.0–25.0] days. Throughout the observation period, 91 patients (53.1%) experienced a primary outcome event; 38 (74.5%) in the AKI subgroup and 53 (37.1%) in the non-AKI group (p < 0.001). Of note, the causes of mortality included 48 cases (52.7%) due to ARDS, 17 cases (18.7%) due to septic shock, 3 cases (3.3%) due to acute coronary syndrome, 1 case (1.1%) related to complications of multiple myeloma, 1 case (1.1%) following diarrhea from clostridium difficile, 18 cases (19.8%) of sudden death, and 3 cases (3.3%) due to ab ingestis pneumonia. The AKI group exhibited a higher prevalence of moderate/severe impaired consciousness, along with lower levels of eGFR and a shorter duration of hospitalization. All discharged patients were alive 30 days after discharge. All the baseline clinical characteristics of the study population are summarized in Table 1.
Table 1.
Baseline characteristics of the study population presented as overall data and stratified according to AKI development.
| Parameter | Overall (n = 194) |
Non-AKI Development (n = 143) |
AKI Development (n = 51) |
p |
|---|---|---|---|---|
| Age, median [IQR] | 83.0 [82.0–87.0] | 83.0 [81.3–87.0] | 84.0 [82.0–87.0] | 0.562 |
| Sex, n (%) | ||||
| M | 100 (51.5) | 70 (49.0) | 30 (58.8) | 0.207 |
| F | 94 (48.5) | 73 (51.0) | 21 (41.2) | |
| Duration of hospitalization, median [IQR] | 15.0 [8.0–25.0] | 16.0 [9.0–25.0] | 10.0 [6.0–16.8] | 0.007 |
| Days before hospitalization, median [IQR] | 6.0 [3.0–9.0] | 5.0 [3.0–8.0] | 7.0 [1.3–12.8] | 0.688 |
| Body temp (°C), median [IQR] | 36.3 [36.0–37.0] | 36.3 [36.0–37.0] | 36.3 [36.0–37.1] | 0.956 |
| History of fever, n (%) | 109 (56.2) | 81 (56.6) | 28 (54.9) | 0.830 |
| Respiratory rate (apm), median [IQR] | 20.0 [18.0–25.0] | 20.0 [16.8–25.0] | 22.0 [18.0–28.0] | 0.056 |
| Heart rate (bpm), median [IQR] | 80.0 [73.0–93.0] | 80.0 [73.0–90.0] | 85.5 [73.5–100.0] | 0.255 |
| Blood pressure (mmHg), median [IQR] | ||||
| Systolic | 135.0 [120.0–145.0] | 135.0 [120.0–145.0] | 130.0 [120.0–153.0] | 0.928 |
| Diastolic | 73.5 [69.0–80.0] | 70.0 [66.0–80.0] | 77.5 [70.0–80.0] | 0.217 |
| Diarrhea, n (%) | 15 (7.7) | 12 (8.4) | 3 (5.9) | 0.566 |
| Oxygen saturation %, median [IQR] | 93.0 [88.0–96.0] | 93.0 [89.0–96.0] | 94.0 [87.0–97.0] | 0.566 |
| GCS/15, n (%) | ||||
| Mild/non-impaired consciousness | 167 (86.1) | 128 (89.5) | 39 (76.5) | 0.021 |
| Moderate/Severe impaired consciousness | 27 (13.9) | 15 (10.5) | 12 (23.5) | |
| Oxygen therapy, n (%) | 99 (51.0) | 70 (48.9) | 29 (56.9) | 0.134 |
| Chronic cardiac disease, n (%) | 92 (49.5) | 63 (44.1) | 29 (56.9) | 0.053 |
| CKD, n (%) | 37 (18.8) | 23 (16.1) | 14 (27.5) | 0.097 |
| eGFR, mL/min/1.73 m2, median [IQR] | 59.1 [34.7–75.7] | 63.0 [46.4–80.6] | 34.6 [20.4–57.7] | <0.001 |
| Hypertension, n (%) | 147 (75.8) | 108 (73.5) | 39 (83.0) | 0.187 |
| Diabetes, n (%) | 50 (25.8) | 35 (23.8) | 15 (31.9) | 0.223 |
| Smoking, n (%) | 15 (7.7) | 14 (9.5) | 1 (2.1) | 0.099 |
| CLD, n (%) | 12 (6.2) | 10 (7.0) | 2 (3.9) | 0.436 |
| Chronic Respiratory Disease, n (%) | 51 (26.3) | 34 (23.8) | 17 (33.3) | 0.184 |
| Chronic neurological disorder, n (%) | 42 (21.6) | 32 (22.4) | 10 (19.6) | 0.681 |
| Malign, n (%) | 24 (12.4) | 16 (10.9) | 8 (17.0) | 0.267 |
| In-hospital mortality, n (%) | 91 (53.1) | 53 (37.1) | 38 (74.5) | <0.001 |
| In-hospital Drugs | ||||
| Steroids, n (%) | 175 (90.2) | 131 (91.6) | 44 (86.3) | 0.209 |
| Monoclonal Abs, n (%) | 2 (1.0) | 1 (0.7) | 1 (2.0) | 0.445 |
| Antivirals, n (%) | 25 (12.9) | 21 (14.7) | 4 (7.8) | 0.212 |
| Antibiotics, n (%) | 166 (87.4) | 122 (85.3) | 44 (86.2) | 0.784 |
| NSAIDs, n (%) | 29 (14.9) | 24 (16.8) | 5 (9.8) | 0.244 |
| Anticoagulants, n (%) | 188 (96.9) | 137 (95.8) | 51 (100) | 0.138 |
| Diuretics, n (%) | 82 (42.3) | 45 (31.4) | 37 (72.5) | <0.001 |
Abbreviations: M: male; F: female; IQR: interquartile range; apm: acts per minute; bpm: beats per minute; Body temp: body temperature; GCS: Glasgow Coma Score; CKD: chronic kidney disease; CLD: chronic liver disease; Malign: malignancies; NSAID: nonsteroidal anti-inflammatory drug.
From the univariate logistic regression analysis, diuretics demonstrated a significant association with the development of AKI (OR 5.76, 95% CI 2.79 to 11.90; p < 0.001). No other drugs showed a significant association with AKI in this analysis.
From the multivariable Cox regression analysis (Table 2), after the application of Šidák correction, only respiratory rate (HR 1.09, 95% CI 1.04 to 1.14; p < 0.001) and AKI development (HR: 3.40, 95% CI 1.80 to 6.40; p < 0.001) were independently associated with the primary outcome.
Table 2.
Univariable and multivariable Cox’s regression model for the primary outcome among the study population.
| Univariable Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | p | HR | 95% CI | p | ||
| Age | 1.01 | 0.96 | 1.06 | 0.637 | ||||
| Sex | ||||||||
| M (ref) | 1 | |||||||
| F | 0.86 | 0.56 | 1.30 | 0.464 | ||||
| Days before hospitalization | 1.03 | 0.92 | 1.14 | 0.650 | ||||
| Body temp (°C) | 1.11 | 0.86 | 1.12 | 0.429 | ||||
| History of fever | 0.93 | 0.61 | 1.41 | 0.724 | ||||
| Respiratory rate (apm) | 1.12 | 1.07 | 1.18 | <0.001 | 1.09 | 1.04 | 1.14 | <0.001 |
| Blood pressure (mmHg) | ||||||||
| Systolic blood pressure | 0.98 | 0.97 | 0.99 | 0.004 | 0.98 | 0.97 | 0.99 | 0.022 |
| Diastolic blood pressure | 0.99 | 0.97 | 1.01 | 0.205 | ||||
| Diarrhea | 0.40 | 0.13 | 1.28 | 0.123 | ||||
| Heart rate (bpm) | 1.02 | 1.01 | 1.03 | 0.017 | 1.00 | 0.99 | 1.02 | 0.161 |
| Oxygen saturation | 0.96 | 0.94 | 0.98 | <0.001 | 0.96 | 0.94 | 0.99 | 0.015 |
| GCS | 0.62 | 0.36 | 1.05 | 0.077 | ||||
| Oxygen therapy | 1.34 | 0.89 | 2.03 | 0.162 | ||||
| Chronic cardiac disease | 1.67 | 1.08 | 2.58 | 0.020 | 1.38 | 0.76 | 2.50 | 0.295 |
| Hypertension | 0.85 | 0.53 | 1.35 | 0.483 | ||||
| CKD | 1.38 | 0.84 | 2.26 | 0.203 | ||||
| Diabetes | 1.01 | 0.62 | 1.63 | 0.974 | ||||
| Smoking | 0.38 | 0.12 | 1.21 | 0.103 | ||||
| CLD | 0.51 | 0.16 | 1.60 | 0.248 | ||||
| Chronic respiratory disease | 1.22 | 0.77 | 1.93 | 0.388 | ||||
| Chronic neurological disorder | 0.87 | 0.52 | 1.43 | 0.574 | ||||
| Malign | 0.92 | 0.47 | 1.78 | 0.800 | ||||
| Steroids | 0.73 | 0.36 | 1.46 | 0.373 | ||||
| Antivirals | 0.51 | 0.24 | 1.10 | 0.087 | ||||
| Antibiotics | 1.07 | 0.69 | 1.68 | 0.757 | ||||
| NSAIDs | 0.42 | 0.19 | 0.93 | 0.031 | 0.79 | 0.24 | 2.60 | 0.704 |
| Diuretics | 1.84 | 1.19 | 2.82 | 0.006 | 0.79 | 0.42 | 1.49 | 0.470 |
| Anticoagulants | 0.39 | 0.14 | 1.07 | 0.068 | ||||
| AKI development | 2.60 | 1.70 | 3.97 | <0.001 | 3.96 | 1.87 | 8.41 | <0.001 |
| eGFR | 0.99 | 0.98 | 0.998 | 0.015 | 1.01 | 0.99 | 1.02 | 0.278 |
Abbreviations: HR: hazard ratio; M: male; F: female; GCS: Glasgow Coma Score; CKD: chronic kidney disease; NSAID: nonsteroidal anti-inflammatory drug.
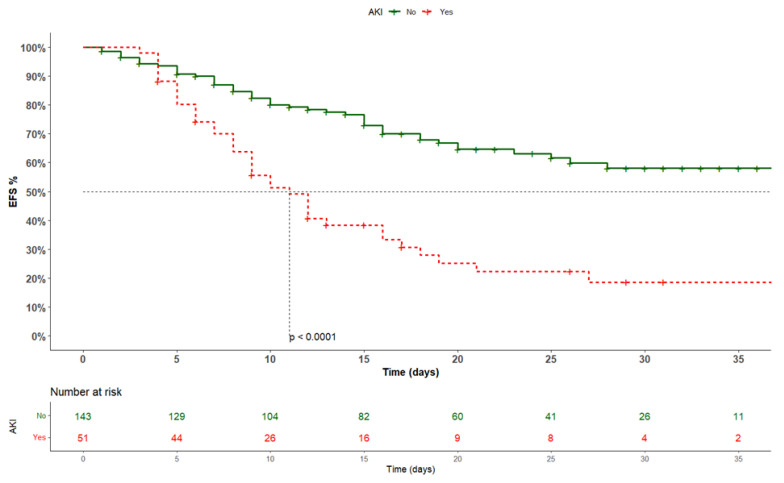
The Kaplan–Meier analysis showed a significantly different risk of the primary outcome event between the two subgroups (log-rank: <0.0001) (Figure 1).
Figure 1.
Kaplan–Meier analysis according to AKI development. AKI: acute kidney injury; EFS: event-free survival.
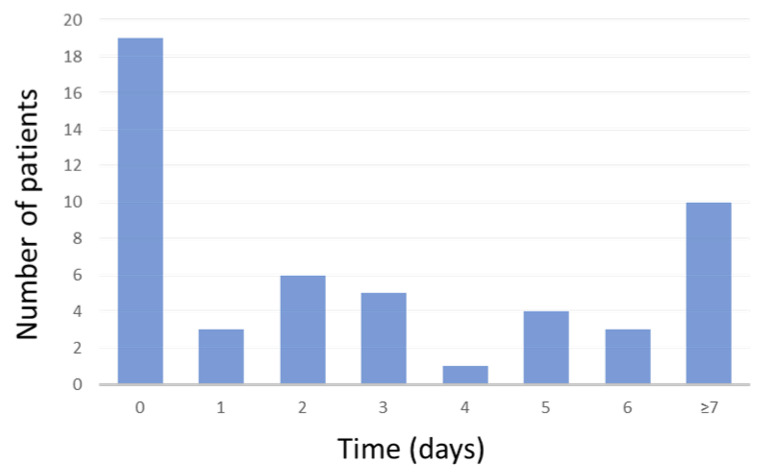
In 19 cases of AKI (37.3%), this was already present at in-hospital admission. The time to AKI development in our cohort study is shown in Figure 2.
Figure 2.
Time to AKI development in our cohort over days of hospitalization.
4. Discussion
The main finding of the current study demonstrates that AKI development in COVID-19 octogenarian patients seems to be a predictor of in-hospital mortality. In addition, we also report an AKI incidence of 26.3%, which was mostly already present at in-hospital admission.
AKI frequently complicates ARDS, arising from factors such as impaired oxygenation, fluid overload, cardiogenic shock, sepsis, or injurious mechanical ventilation [13]. In the context of COVID-19, the incidence of AKI has been observed in a range of 0.5% to 39% [14,15,16,17,18], similar to our findings. Despite its widespread occurrence, the exact pathophysiology of AKI in COVID-19 continues to be a subject of ongoing research. The multifaceted impact of the SARS-CoV-2 virus on the renal system is predominantly facilitated through the angiotensin-converting enzyme 2 (ACE2) pathway. The virus’s spike protein binds to ACE2 receptors in host cells, initiating a cascade of events that can result in diverse renal conditions [19]. Through this pathway, several pathological outcomes have been observed, including acute tubular necrosis, protein leakage in Bowman’s capsule, collapsing glomerulopathy, and mitochondrial impairment [20]. The direct impact of SARS-CoV-2 on renal cells is underscored by studies such as that by Puelles et al., which detected the virus in various kidney compartments. The evidence of RNA enrichment for ACE2 in diverse kidney cell types supports the hypothesis of a direct viral influence on renal cells [21]. Cytopathic effects on these cells, as evidenced by autopsy findings revealing acute tubular necrosis and inflammatory cell infiltration, further substantiate the intricate relationship between the virus and renal manifestations in COVID-19 [22,23]. The dysregulation of immune responses, triggered by SARS-CoV-2, indirectly contributes to AKI. In particular, cytokine storm, macrophage activation syndrome, and lymphopenia are among the immune-mediated mechanisms associated with renal damage. In fact, the release of various inflammatory mediators, such as interleukin (IL)-6, IL-1β, tumor necrosis factor-alpha, inducible protein-10, monocyte chemotactic protein 1, granulocyte-colony stimulating factor, and macrophage inflammatory protein-1α, can directly contribute to kidney injury through the resultant activation of the innate immune system [24,25,26]. This occurs against the backdrop of the hypotension associated with a cytokine storm and the superimposed sepsis, further elevating the risk of AKI [24]. The intricate interplay of these immune responses amplifies the complexity of renal involvement in COVID-19. Beyond immune dysregulation, other potential mechanisms of AKI have been identified. These include organ interactions, endothelial dysfunction, hypercoagulability, rhabdomyolysis, and sepsis. Additionally, a decrease in oxygen delivery to the kidneys may lead to ischemic injury, further exacerbating renal complications in COVID-19 patients [24,27]. Finally, COVID-19 treatment has included different drug therapies operating through distinct mechanisms, with certain medications carrying nephrotoxic implications [28,29,30]. Hydroxychloroquine, largely used during the first wave in Italy [31,32] and antiviral agents, including lopinavir, ritonavir, and remdesivir, also pose a potential risk of kidney injury [28,29], with documented instances of renal impairment reported in users of remdesivir [33]. Furthermore, intravenous immunoglobulin, in addition to antivirals, carries a potential risk of proximal tubular injury [28,29]. Although the underlying pathophysiology varies, the incidence of AKI in COVID-19 has consistently shown a strong association with increased mortality, as evident in both our data and the existing literature [34]. In our study, we have observed an association between diuretic use and the heightened risk of AKI, which aligns with the pharmacological effects of diuretics on renal physiology. Diuretics, by design, enhance urine production and electrolyte excretion to alleviate fluid overload and manage hypertension. However, this intensified diuresis can lead to intravascular volume depletion and subsequent reductions in renal blood flow, potentially compromising kidney function [12]. Our findings are consistent with those reported in a recent meta-analysis [9], emphasizing the importance for clinicians to be cautious about the potential nephrotoxic effects of diuretics, despite the absence of an association with the primary outcome.
A recent meta-analysis also revealed that advanced age independently increased the risk of AKI in COVID-19 patients, with an odds ratio of 3.53 [16]. In elderly patients infected with SARS-CoV-2, the increased mortality rates suggest a potential link to weakened immune system function and the aging of tissues, rendering them more susceptible to viral replication. This observation aligns with the broader context of age-related vulnerabilities, where the aging process contributes to physiological changes that may compromise the body’s defense mechanisms [35,36]. Intriguingly, this age-related susceptibility contrasts sharply with observations in the pediatric population, where, despite having an impaired immune system during infancy, children tend to exhibit less severe symptoms of COVID-19 [37]. It has been reported that older adults generally exhibit a reduction in ACE2-positive cells, primarily localized in the lower pulmonary tract, coupled with a concurrent decrease in lung progenitor cells, potentially influencing the severity of the disease and the recuperation process from pneumonia resulting from SARS-CoV-2 infection in elderly individuals [38]. Moreover, even in the absence of statistical differences in the number of days of infection before hospitalization, the significant incidence of AKI at admission (10%) may imply a more aggressive manifestation of the disease in these patients. Indeed, a recent meta-analysis reported that the occurrence of AKI is more than five times higher in severe cases and non-survivors compared to non-severe cases and survivors [39]. Likewise, this observation might partially substantiate the independent association between the respiratory rate at admission and adverse clinical outcomes, as revealed by the COX regression multivariate analysis. Unfortunately, the parameter of respiratory rate remains relatively underexplored in the context of COVID-19 patients. Specifically, in a recent meta-analysis, respiratory rate was identified as a risk factor in only a limited number of studies; specifically, five in total. Regrettably, amalgamation of the data and the derivation of risk estimates encountered challenges due to the inherent heterogeneity stemming from the disparate reported outcomes and risk measures [40].
While our study contributes to the existing body of evidence, it also highlights the ongoing need for research in this field. Further investigations into the specific mechanisms linking AKI to in-hospital mortality, the long-term consequences of renal complications, and the efficacy of targeted therapeutic interventions are crucial. Collaborative efforts across research institutions may accelerate the pace of discovery and contribute to more effective strategies for managing AKI in the context of COVID-19.
The present study is subject to several limitations that warrant consideration. Firstly, while our study included a substantial number of participants, the sample size may still limit the generalizability of our findings to broader populations. Larger, more diverse cohorts are necessary to validate and extend the external validity of our results. Secondly, a limitation arises from the lack of data collection throughout the entire hospitalization period spanning from admission to discharge. Such comprehensive data collection would have been instrumental in identifying forms of AKI that manifested within the initial 48 h of hospitalization, offering a more detailed temporal perspective. Thirdly, the absence of body mass index (BMI) and related data presents another limitation. The inclusion of BMI as a variable in the analysis could have contributed to a more nuanced understanding of its potential association with AKI in the context of COVID-19. Fourthly, the study lacks pre-hospital admission creatinine values. Inclusion of these values would have provided valuable baseline information regarding the patients’ kidney function before the onset of the infection, offering insights into the dynamics of renal changes during the course of COVID-19. Fifthly, the timeframe of enrollment predates the widespread vaccination campaign against SARS-CoV-2 in Italy. Consequently, the study lacks complete data on the vaccination status of participants, preventing the incorporation of this variable into the statistical analysis. This limitation highlights the evolving nature of the pandemic and the need for ongoing research to capture the impact of vaccination on COVID-19 outcomes. Lastly, there was an absence of laboratory data on urinary protein levels, sediment measurements, and hematuria, which could have provided valuable insights into the phenotype of renal damage, thus enhancing our understanding of the renal implications of COVID-19 [41]. In addition, the absence of biopsy data restricts our ability to elucidate the specific histological renal damage that occurred in COVID-19 patients. While such data would have been valuable for a comprehensive understanding, the emergency status induced by the pandemic and the characteristics of the patients made it logistically challenging to perform renal biopsies during the study period.
5. Conclusions
In our investigation, we identified a significant association between AKI and mortality rates among octogenarian patients admitted with COVID-19. These findings raise notable concerns and emphasize the imperative for vigilant monitoring of this demographic cohort.
Acknowledgments
COVOCA Study Group: Alfredo Caturano, Raffaele Galiero, Erica Vetrano, Giulia Medicamento, Maria Alfano, Domenico Beccia, Chiara Brin, Sara Colantuoni, Jessica Di Salvo, Raffaella Epifani, Ferdinando Carlo Sasso (Unit of Internal Medicine, Department of Advanced Medical and Surgical Sciences, University of Campania “Luigi Vanvitelli”, Naples), Nicola Coppola, Caterina Monari, and Giulia De Angelis (Centro COVID A.O.U. Vanvitelli, Department of Mental Health and Public Medicine, Naples); Cecilia Calabrese, Paola Maria Medusa, Gaia Pugliese, and Nicola Carro (Pneumologia Vanvitelli-AORN Ospedali Ospedali dei Colli); Roberto Parrella and Gianfranco Gaglione (Cotugno Hospital—U.O.C. Respiratory Infectious Diseases); Fiorentino Fraganza (Cotugno Hospital—U.O.C. Anestesia and Intensive Care Unit); Costanza Sbreglia and Serena Parente (Cotugno Hospital—U.O.C. Infectious Diseases of the Elderly); Nicola Maturo and Paolina Lumino (Cotugno Hospital—U.O.S.D. Infectious Diseases Emergency and Acceptance); Vincenzo Esposito and Annamaria Rossomando (Cotugno Hospital—IVth Division of Immunodeficiency and Gender Infectious Diseases); Giosuele Calabria, Mario Catalano, and Laurenza Paradiso (Cotugno Hospital—IXth Division of Infectious Diseases and Interventional Ultrasound); Carolina Rescigno and Raffaella Pisapia (Cotugno Hospital—U.O.C. Infectious Diseases and Neurology); Carolina Bologna (Internal Medicine Unit, Ospedale Del Mare, Naples); Antonio Pagano and Fabio Giuliano Numis (Emergency and Acceptance Unit, “Santa Maria delle Grazie” Hospital, Pozzuoli); Laura Vocciante, Antonio Asti, and Michele Langella (General Medicine Unit—Loreto Mare Hospital, Naples); Riccardo Nevola (Sant’Ottone Frangipane Hospital, Ariano Irpino (Avellino)); Maria Amitrano, Sara Mangiacapra, and Mariangela Raimondo (U.O.C. Internal Medicine—Moscati Hospital, Avellino); Annamaria Romano (U.O.C. Pneumology—Moscati Hospital, Avellino); Carmine Coppola and Ferdinando Scarano (COVID Center “S. Anna e SS. Madonna della Neve” Hospital, Boscotrecase); Alessandro Perrella, Andrea Del Mastro, and Rodolfo Nasti (Cardarelli Hospital, Naples); Benedetto Polverino (“Giovanni da Procida” Hospital, Salerno); Pierpaolo di Micco (Ospedale Buonconsiglio Fatebenefratelli-Napoli); Paolo Maggi, Alessio Codella, and Paolo Cirillo (U.O.C. Infectious and Tropical diseases, S. Anna e S. Sebastiano Hospital, Caserta); Pellegrino De Lucia Sposito (COVID Center—Maddaloni Hospital). The authors thank all the colleagues, nurses, and workers who are facing the COVID-19 disease.
Author Contributions
Concept and design: A.C. and F.C.S.; Acquisition of data: A.C., L.R., E.V., G.M., M.A., D.B., C.B. (Chiara Brin), S.C., J.D.S., R.E., R.N. (Riccardo Nevola), C.C. (Carmine Coppola), F.S., P.M., C.C. (Cecilia Calabrese), P.D.L.S., C.R., C.S. (Costanza Sbreglia), F.F., R.P., A.R., G.C., B.P., A.P. (Antonio Pagano), F.G.N., C.B. (Carolina Bologna), M.N., V.E., N.C., N.M., R.N. (Rodolfo Nasti), P.D.M., C.S. (Celestino Sardu), M.D.D., G.D., R.R. and A.P. (Alessandro Perrella); Supervision: R.M., L.E.A., M.M., V.R. and F.C.S.; Data analysis: A.C.; Interpretation of data: A.C. and F.C.S.; Drafting of the manuscript: A.C. and F.C.S.; Critical revision of the manuscript for important intellectual content: A.C., R.G. and F.C.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (Universita’ degli studi della Campania “Luigi Vanvitelli”, Azienda Ospedaliera Universitaria, “Luigi Vanvitelli”, and Azienda Ospedaliera di Rilievo Nazionale “Ospedali dei colli”; ID 10879/I; approval date: 11 May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sharma A., Ahmad Farouk I., Lal S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses. 2021;13:202. doi: 10.3390/v13020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdenassi L., Franzini M., Ricevuti G., Rinaldi L., Galoforo A.C., Tirelli U. Potential mechanisms by which the oxygen-ozone (O2-O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4059–4061. doi: 10.26355/eurrev_202004_20976. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. [(accessed on 25 November 2023)]. Available online: https://covid19.who.int/
- 4.Vianello A., De Vita N., Scotti L., Guarnieri G., Confalonieri M., Bonato V., Molena B., Maestrone C., Airoldi G., Olivieri C., et al. Clinical Outcomes in Patients Aged 80 Years or Older Receiving Non-Invasive Respiratory Support for Hypoxemic Acute Respiratory Failure Consequent to COVID-19. J. Clin. Med. 2022;11:1372. doi: 10.3390/jcm11051372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galiero R., Pafundi P.C., Simeon V., Rinaldi L., Perrella A., Vetrano E., Caturano A., Alfano M., Beccia D., Nevola R., et al. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: Findings from COVOCA study. PLoS ONE. 2020;15:e0243700. doi: 10.1371/journal.pone.0243700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 7.Capdevila-Reniu A., Pellice M., Prieto-González S., Ventosa H., Ladino A., Naval J., Rodriguez-Nuñez O., César Milisenda J., Moreno-Lozano P.J., Soriano A., et al. Clinical characteristics and outcome of patients aged over 80 years with COVID-19. Medicine. 2021;100:e24750. doi: 10.1097/MD.0000000000024750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiero R., Simeon V., Loffredo G., Caturano A., Rinaldi L., Vetrano E., Medicamento G., Alfano M., Beccia D., Brin C., et al. Association between Renal Function at Admission and COVID-19 in-Hospital Mortality in Southern Italy: Findings from the Prospective Multicenter Italian COVOCA Study. J. Clin. Med. 2022;11:6121. doi: 10.3390/jcm11206121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J., Pang Q., Zhou T., Meng J., Dong X., Wang Z., Zhang A. Risk factors for acute kidney injury in COVID-19 patients: An updated systematic review and meta-analysis. Ren. Fail. 2023;45:2170809. doi: 10.1080/0886022X.2023.2170809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44((Suppl. S1)):S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 11.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D., Coca A., De Simone G., Dominiczak A., et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018;36:2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 12.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W., He J.C. Mechanisms and treatment of COVID-19-associated acute kidney injury. Mol. Ther. 2023;31:306–307. doi: 10.1016/j.ymthe.2023.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali H., Daoud A., Mohamed M.M., Salim S.A., Yessayan L., Baharani J., Murtazaf A., Rao V., Soliman K.M. Survival rate in acute kidney injury superimposed COVID-19 patients: A systematic review and meta-analysis. Ren. Fail. 2020;42:393–397. doi: 10.1080/0886022X.2020.1756323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., Hazzan A.D., Fishbane S., Jhaveri K.D. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin L., Wang X., Ren J., Sun Y., Yu R., Li K., Zheng L., Yang J. Risk factors and prognosis for COVID-19-induced acute kidney injury: A meta-analysis. BMJ Open. 2020;10:e042573. doi: 10.1136/bmjopen-2020-042573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shchepalina A., Chebotareva N., Akulkina L., Brovko M., Sholomova V., Androsova T., Korotchaeva Y., Kalmykova D., Tanaschuk E., Taranova M., et al. Acute Kidney Injury in Hospitalized Patients with COVID-19: Risk Factors and Serum Biomarkers. Biomedicines. 2023;11:1246. doi: 10.3390/biomedicines11051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan L., Chaudhary K., Saha A., Chauhan K., Vaid A., Zhao S., Paranjpe I., Somani S., Richter F., Miotto R., et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magrone T., Magrone M., Jirillo E. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin-converting Enzyme 2 as a Potential Drug Target—A Perspective. Endocr. Metab. Immune Disord. Drug Targets. 2020;20:807–811. doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadian E., Hosseiniyan Khatibi S.M., Razi Soofiyani S., Abediazar S., Shoja M.M., Ardalan M., Zununi Vahed S. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021;31:e2176. doi: 10.1002/rmv.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Eng. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., Yi F., Yang H.C., Fogo A.B., Nie X., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diao B., Wang C., Wang R., Feng Z., Zhang J., Yang H., Tan Y., Wang H., Wang C., Liu L., et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Nat. Commun. 2021;12:2506. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozzolino D., Sessa G., Salvatore T., Sasso F.C., Giugliano D., Lefebvre P.J., Torella R. The involvement of the opioid system in human obesity: A study in normal weight relatives of obese people. J. Clin. Endocrinol. Metab. 1996;81:713–718. doi: 10.1210/jcem.81.2.8636293. [DOI] [PubMed] [Google Scholar]
- 26.Caturano A., Acierno C., Nevola R., Pafundi P.C., Galiero R., Rinaldi L., Salvatore T., Adinolfi L.E., Sasso F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes. 2021;9:135. doi: 10.3390/pr9010135. [DOI] [Google Scholar]
- 27.Caturano A., D’Angelo M., Mormone A., Russo V., Mollica M.P., Salvatore T., Galiero R., Rinaldi L., Vetrano E., Marfella R., et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023;45:6651–6666. doi: 10.3390/cimb45080420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassanein M., Radhakrishnan Y., Sedor J., Vachharajani T., Vachharajani V.T., Augustine J., Demirjian S., Thomas G. COVID-19 and the kidney. Cleve. Clin. J. Med. 2020;87:619–631. doi: 10.3949/ccjm.87a.20072. [DOI] [PubMed] [Google Scholar]
- 29.Hassanein M., Thomas G., Taliercio J. Management of acute kidney injury in COVID-19. Cleve. Clin. J. Med. 2020:1–3. doi: 10.3949/ccjm.87a.ccc034. [DOI] [PubMed] [Google Scholar]
- 30.Salvatore T., Galiero R., Caturano A., Vetrano E., Rinaldi L., Coviello F., Di Martino A., Albanese G., Colantuoni S., Medicamento G., et al. Dysregulated Epicardial Adipose Tissue as a Risk Factor and Potential Therapeutic Target of Heart Failure with Preserved Ejection Fraction in Diabetes. Biomolecules. 2022;12:176. doi: 10.3390/biom12020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pafundi P.C., Galiero R., Simeon V., Rinaldi L., Perrella A., Vetrano E., Caturano A., Alfano M., Beccia D., Nevola R., et al. Lack of effect on in-hospital mortality of drugs used during COVID-19 pandemic: Findings of the retrospective multicenter COVOCA study. PLoS ONE. 2021;16:e0256903. doi: 10.1371/journal.pone.0256903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoudi J., Sadigh-Eteghad S., Salehi-Pourmehr H., Gharekhani A., Ziaee M. Nephrotoxicity of Chloroquine and Hydroxychloroquine in COVID-19 Patients. Tabriz Univ. Med. Sci. 2021;7:113–117. doi: 10.34172/apb.2021.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F., Liang Y. Potential risk of the kidney vulnerable to novel coronavirus 2019 infection. Am. J. Physiology. Ren. Physiol. 2020;318:F1136–F1137. doi: 10.1152/ajprenal.00085.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheruiyot I., Henry B., Lippi G., Kipkorir V., Ngure B., Munguti J., Misiani M. Acute Kidney Injury is Associated with Worse Prognosis In COVID-19 Patients: A Systematic Review and Meta-analysis. Acta Biomed. 2020;91:e2020029. doi: 10.23750/abm.v91i3.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhler C., Shivashankar G.V. Mechano-genomic regulation of coronaviruses and its interplay with ageing. Nat. Rev. Mol. Cell Biol. 2020;21:247–248. doi: 10.1038/s41580-020-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhler C., Shivashankar G.V. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat. Rev. Mol. Cell Biol. 2017;18:717–727. doi: 10.1038/nrm.2017.101. [DOI] [PubMed] [Google Scholar]
- 37.Chou J., Thomas P.G., Randolph A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022;23:177–185. doi: 10.1038/s41590-021-01123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z., Guo L., Huang L., Zhang C., Luo R., Zeng L., Liang H., Li Q., Lu X., Wang X., et al. Distinct Disease Severity Between Children and Older Adults With Coronavirus Disease 2019 (COVID-19): Impacts of ACE2 Expression, Distribution, and Lung Progenitor Cells. Clin Infect Dis. 2021;73:e4154–e4165. doi: 10.1093/cid/ciaa1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X., Jin Y., Li R., Zhang Z., Sun R., Chen D. Prevalence and Impact of Acute Renal Impairment on COVID-19: A Systematic Review and Meta-analysis. Crit. Care. 2020;24:356. doi: 10.1186/s13054-020-03065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth A., Reed A.B., Ponzo S., Yassaee A., Aral M., Plans D., Labrique A., Mohan D. Population Risk Factors for Severe Disease and Mortality in COVID-19: A Global Systematic Review and Meta-analysis. PLoS ONE. 2021;16:e0247461. doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Generalić A., Davidović M., Kos I., Vrljičak K., Lamot L. Hematuria as an Early Sign of Multisystem Inflammatory Syndrome in Children: A Case Report of a Boy With Multiple Comorbidities and Review of Literature. Front. Pediatr. 2021;9:760070. doi: 10.3389/fped.2021.760070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.