1. Introduction
1.1. Quantitative sensory testing and norms
Quantitative Sensory Testing (QST) is commonly used to investigate somatosensory system functioning by analyzing temperature, touch, and pain sensitivity in specific body regions [49, 51]. QST can be used to assess descending pain inhibition and central sensitization, quantify pain perception, and determine variability in pain sensitivity and modulation [34]. Multi-center trials conducted by the German Research Network on Neuropathic Pain (DFNS) determined QST parameters across six body regions of the bilateral face, hand, and foot, which are age (17-75 years), race, ethnicity, and sex-dependent in a German population [41]. However, norms for the abdominal somatosensory region do not exist, which is problematic given the prevalence of diseases that may result in pain in this region.
1.2. Chronic abdominal pain
Chronic abdominal pain is defined as continuous or intermittent abdominal discomfort lasting at least six months [42]. Both pediatric and adult abdominal pain are increasingly common [9, 46]. The most recent cross-sectional survey of US adults with upper gastrointestinal complaints was conducted in 1999 and reported a prevalence of 21.8% in the general population, with women being more likely than men to report chronic abdominal pain [15, 42]. The prevalence of pediatric functional abdominal pain was last investigated in 2015 and was reported to be about 14% of children in the US [23].
Table 1 outlines potential diagnoses for the myriad of causes of chronic abdominal pain, classified by organ system [7].
Table 1.
List of potential diagnoses for various causes of chronic abdominal pain which are classified by organ system [8]. Adapted from Charles et al, 2019 [8]. Used with permission.
| PULMONARY | GENITOURINARY | NEUROLOGIC |
| Cystic Fibrosis | Nephrolithiasis | Abdominal Cutaneous Nerve |
| Entrapment Syndrome | ||
| GASTROINTESTINAL | GYNECOLOGIC | Herpes Zoster |
| Gastroesophageal Reflux | Ovarian Cyst | |
| Esophageal Cancer | Ovarian Cancer | |
| Hernias (ventral, hiatal) | Pelvic Inflammatory Disease | |
| Chronic Gastritis | Leiomyoma | |
| Gastric Cancer | Endometriosis | |
| Gastroparesis | ||
| Functional Dyspepsia | HEMATOLOGIC | |
| Peptic Ulcer Diseases | Sickle Cell Anemia | |
| Chronic Cholecystitis | ||
| Chronic Cholelithiasis | ||
| Chronic Hepatitis | PSYCHOLOGICAL | |
| Hepatocellular Cancer | Anxiety Disorders | |
| Chronic Pancreatitis | Adjustment Disorder | |
| Pancreatic Cancer | Somatic Symptom Disorder | |
| Celiac Disease | ||
| Irritable Bowel Syndrome | ||
| Lactase Deficiency/Intolerance | MISCELLANEOUS CAUSES | |
| Crohn’s Disease | Functional Abdominal Pain | |
| Ulcerative Colitis | Referred Pain from Extra-Abdominal Organ | |
| Colorectal Cancer | Drug/Medication Induced | |
| Chronic Mesenteric Ischemia | ||
| Post-Surgical Abdominal Adhesions | ||
| Chronic Abdominal Wall Pain | ||
| Narcotic Bowel Syndrome | ||
| Abdominal Migraine | ||
| Subacute Intestinal Obstruction |
Patients with abdominal visceral pain usually present with somatic referral pain to the abdominal wall described as tenderness subjectively and sensitivity to palpation by an examiner [28, 38, 50]. Visceral pain from the abdomen activates the sensory afferent fibers and second-order neurons in the dorsal horn of the spinal cord. Animal models show that activation of viscero-moter reflex produces abdominal muscle contraction and cutaneous hypersensitivity through hyperexcitability of the second-order neurons and/or mediated through supraspinal neurons. [21, 44]. This viscero-sensitive signaling converges with somatic neurons receiving nociceptive input from corresponding dermatomes and myotomes. Therefore, visceral pain is referred to deep somatic tissues, to the skin, and to other visceral organs resulting in spontaneous pain and mechanical hyperalgesia [19, 20].
1.3. QST norms for the somatosensory abdominal wall
Given the findings of differences in nociceptive somatosensory processing across age and biological sex [11, 26], establishing abdominal QST norms is necessary to better elucidate the pathophysiological mechanisms contributing to pain in patients with the myriad of diseases outlined in Table 1. Additionally, these norms provide a metric for who may be at risk for pain chronification, which could have important treatment implications.
This study aimed to establish QST reference values for the abdomen in a diverse sample of pain-free adolescent and adult males and females. A secondary aim was to explore group differences of age and sex on sensitivity in the abdominal quadrants. We hypothesize that age and sex-related differences will be present and, given prior research [3, 11] that young females will have the highest pain sensitivity.
2. Methods
2.1. Enrollment, inclusion, and exclusion criteria
Participants included 181 males and females (assigned sex at birth) recruited from Boston Children’s Hospital (BCH) and the community. Before consenting to the study, all participants were screened by assessing current and past medical history. Eligible participants were between the ages of 12-50 years, as 12 is the age when research has demonstrated the onset of sex differences in pain perception due to puberty [3, 4]. Exclusion criteria included: (1) Age older than 50 years; (2) Presence of medical or pain conditions (e.g., cancer, sickle-cell disease, juvenile idiopathic arthritis); (3) A history of hysterectomy or oophorectomy; (4) Menopause (defined as the cessation of menses for 12 consecutive months, unrelated to exogenous hormonal suppression); (5) Use of opioid analgesics within three months of the study visit; 6) Non-English speaking; and (6) Severe cognitive impairment (e.g., intellectual disability, traumatic brain injury, etc.). Participants were compensated for study participation.
2.2. Procedure
Recruitment fliers were distributed around Boston, MA (e.g., college campuses, coffee shops, train stations), online, and within the Adolescent and Young Adult Medicine Clinical Practice at BCH to obtain an inclusive and representative sample. The study was approved by the BCH Institutional Review Board and met the scientific and ethical guidelines for human pain research of the Helsinki Accord (http://ohsr.od.nih.gov/guidelines/helsinki.html) and the International Association for the Study of Pain. Each participant provided written informed consent or assent (with parental consent) on the day of testing. All testing occurred in one session at the Biobehavioral Pain Innovations Lab at BCH. Participants were compensated with $50 for their time.
2.2.1. Quantitative Sensory Testing
The QST protocol was designed to measure sensory detection and pain threshold of the four abdominal quadrants divided at the umbilicus. The protocol was continuous with natural breaks between the tests that were on average 3-5 minutes in duration. The abdominal wall and parietal peritoneum are innervated by somatic nerves T6-L1 dermatomes. The parietal peritoneum also receives innervation from visceral afferent nerves [sympathetic nerves]. The abdominal viscera are innervated by sympathetic chain nerves T6-S1. Dividing the abdomen into four quadrants at the umbilicus [T10] divides the abdominal visceral into above and below T10 dermatomes: (1) upper abdomen T6 -T12; stomach on the left, and caecum and small intestine in the middle, spleen, and liver on the right (2) lower abdomen colon (ascending on the right and descending on the left), and mesentery from T12-S1[47].
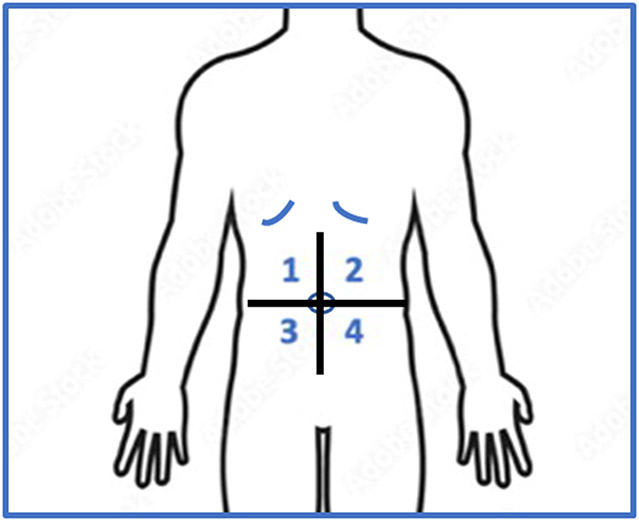
Each test was performed in the center of the upper left and right quadrants and lower left and right quadrants of the abdomen. The QST protocol was conducted in numerical order of quadrants 1 (upper right), 2 (upper left), 3 (lower right), and 4 (lower left). Each test was conducted on each quadrant before moving on to the next test (Figure 1). The QST measures were conducted in the following order [41]: (1) Dynamic Mechanical Allodynia, (2) Mechanical Detection, (3) Mechanical Pain Threshold, (4) Temporal Summation of Pain, (5) Pressure Pain Threshold, (6) Thermal Detection, and (7) Thermal Pain Threshold. Regarding training of the experimenters, a standardized protocol, modified from the DFNS protocol [41] was devised for this study. Of note, one of the research assistants on the study received a pain certification after completing the QST training course by the German Research Network on Neuropathic Pain. A detailed script was then created that all experimenters had to read verbatim for every study visit. Experimenters were not allowed to test on a research participant until they demonstrated competency in as many mock sessions as needed and proficiency in the protocol was established.
Figure 1.
Figure one describes the four abdominal quadrants used to administer quantitative sensory testing. The upper abdomen included quadrants 1 and 2. The lower abdomen included quadrants 3 and 4.
2.2.2. Mechanical Stimuli
a. Dynamic Mechanical Allodynia.
While participants closed their eyes, a 2-gram brush was run vertically across 3 cm/second over 5 inches of distance for each quadrant of the abdomen. Participants were then asked to describe what they felt after applying the stimulus. If the participant described a feeling other than soft, they were asked to clarify whether the brush felt “soft” or “harsh”. If “harsh” was endorsed (which was infrequent among our healthy controls), the participant provided a pain rating on a scale of 0 (no pain) to 10 (worst pain imaginable). A description of “soft” was rated as a zero. The test was repeated three times, and the average pain score of the three trials was used for data analysis.
b. Mechanical Detection.
We used a modified version of the DFNS protocol (ascending stimuli until the participant perceived the touch) to determine mechanical detection. We applied Von Frey (Ugo Basile Semmes-Weinstein) filaments perpendicularly to the skin three times each. We started at 0.008 grams and increased (i.e., filament size) to maximum force of 300 grams. The threshold was determined as the lowest force the participant could detect for at least two trials. This was repeated in each abdominal quadrant. Participants were instructed to close their eyes so they would not be visually aware of the filament size touching the skin.
c. Mechanical Pain Threshold.
Using the von Frey filament determined to be the mechanical detection threshold from the Mechanical Detection tests, filaments were gradually applied in an ascending strength until the patient reported pain. The pain was rated on a numerical rating scale of 0-10 points. The force, in grams, was then determined to be the mechanical pain threshold. Participants were then asked to give a pain score on a scale of 0-10. The test was repeated three times and the average pain score of the three trials was used for the data analysis.
2.2.3. Pressure Stimuli
a. Pressure Pain Threshold.
An electronic pressure algometer (Algomed, Wagner) was used to gradually apply increasing pressure (measured in Newtons/second) until the participant reported pain. The maximum amount of pressure able to be applied was 100 Newtons. This test was repeated three times in each quadrant, with 30 seconds between each trial, and their final score was calculated by averaging the force required to produce pain across the three trials.
2.2.4. Temporal Summation of Pain
a. Temporal Summation of Pain.
The lowest force von Frey filament that each participant reported as painful from the mechanical pain detection task was applied ten times with eyes closed, using a rate of one application per second. The participant was then asked to give a pain score on a scale of 0-10. This test was repeated three times in each quadrant. There were ten seconds between each trial. Temporal summation was calculated by subtracting the average pain rating provided from the mechanical pain threshold task (2.2.2.c) from the average pain rating provided after the tenth stimulus application.
2.2.5. Thermal Stimuli
Using a Medoc Thermal Sensory Analyzer (TSA) II, a thermode was placed on one abdominal quadrant at a time. The thermode baseline temperature was programmed to 32°C with a set range of temperatures between 0-50°C. The TSA II was programmed to enter a safety mode, where the thermode returned to baseline temperature when the thermode reached the lower limit of 0°C or the upper limit of 50°C. This was to ensure the safety of each participant and avoid potential thermal injury. The thermode was strapped to the participants using a Velcro strap.
a. Thermal Detection.
Warm detection was assessed by starting at the baseline temperature of 32°C and increasing the temperature of the thermode at a rate of either 1°C per second until the participant detected warmth. A similar method was used to decrease the thermode temperature at a rate of 1°C per second until the participant detected cool. The participant signalled the detection of warmth and cool thresholds by pressing a button synced to the TSA II, resulting in the temperature returning to baseline. Three trials were completed on each abdominal quadrant. Detection thresholds were determined as the average of differences between the baseline and detection thermal temperatures
b. Thermal Pain Detection.
Heat and cold pain were assessed using a method of limits in which the thermode increased or decreased by 1°C/second for heat or cold pain detection, respectively. The participant indicated the first pain sensation of either hot or cold. Participants provided a numerical pain score on a scale of 0-10. Three heat and cold pain trials were conducted on each abdominal quadrant. Outcomes included the average temperature at which pain was detected and average pain intensity at detection.
3. Data Analyses
The participants were grouped by age into (1) adolescents: 12-19 years, n= 48; (2) young adults: 20-30 years, n= 87; (3) adults: 31-50 years, n= 46. Participants were also grouped by sex, males, n= 63; females, n= 118.
QST measurements from quadrants 1 and 2 (upper abdomen) and quadrants 3 and 4 (lower abdomen) (Figure 1) were compared for all QST measures using t-tests. There were no significant differences found. Thus, all further analyses were grouped as upper vs. lower abdomen. Kolmogorov-Smirnov tests indicated that the QST measures did not follow a normal distribution.
We calculated the normative values as medians for each QST measure for the upper and lower abdomen by age group and sex. The Kruskal Wallis H Test, for age categories, and the Mann-Whitney U Test, for sex, were used to assess differences in QST measures. The significance level was set to p<.05 to reduce the possibility of Type I error. Statistical analyses were performed using SPSS software ver. 28.0 (IBM, Armonk, NY, USA).
4. Results
4.1. Demographics (Table 2)
Table 2.
Demographic information for the sample across the decade age distribution by sex.
| Demographics | ||||
|---|---|---|---|---|
| Female (n = 118) |
Male (n = 63) |
Total Sample (n = 181) |
||
| Age, years [M (SD)] | 23 (7) | 30 (9) | 25 (9) | |
| Age Decade [n, %] | 12-19 y | 58, 49% | 10, 16% | 68, 38% |
| 20-30 y | 38, 32% | 24, 38% | 62, 34% | |
| 31-50 y | 9, 8% | 19, 30% | 28, 15% | |
| Race [n, %] | White | 56, 47% | 28, 44% | 84, 46% |
| Asian | 14, 12% | 13, 21% | 7, 15% | |
| Black | 23, 20% | 8, 13% | 31, 17% | |
| Other | 25, 21% | 14, 22% | 39, 22% | |
| Ethnicity [n, %] | Hispanic | 10, 9% | 7, 11% | 17, 9% |
| Non-Hispanic | 108, 91% | 56, 89% | 164, 91% | |
| Education [n, %] | Middle School | 4, 3% | 0, 0% | 4, 2% |
| High School | 13, 11% | 4, 6% | 17, 9% | |
| College | 70, 60% | 16, 25% | 86, 48% | |
| Graduate School | 4, 3% | 3, 5% | 7, 4% | |
| Did Not Report | 27, 23% | 40, 63.5% | 67, 37% | |
| Work Status [n, %] | Full-time Student | 76, 65% | 17, 27% | 93, 51% |
| Employed | 11, 9% | 3, 5% | 14, 8% | |
| Homemaker/Caregiver | 1, 1% | 0, 0% | 1, 1% | |
| Did Not Report | 30, 25% | 43, 68% | 73, 40% | |
Participants included 181 healthy, pain-free participants ages 12 to 50 years with a mean age of 25 years (SD = 9 years). While the ages for the developmental transition from adolescence to emerging or young adulthood to adulthood have been debated [22, 27], these age cut-offs fall within the ranges used in other studies of adolescents, young adults, and adults [22]. There were no statistically significant differences (p > .05) for any QST measures between ages 31- 40 years and 41- 50 years. Therefore, these two groups were combined.
75% of participants ranged in age from 12-30 years. Sixty-five percent of the participants were female, 46% identified as White, and 9% reported being Hispanic. Although a proportion of participants did not report their highest level of education (37%), more than 80% of those who did report their education endorsed completing at least some college, with more than half of the participants identifying as full-time students.
4.2. QST reference values (Tables 3-9)
Table 3.
Results from Mechanical Detection
*IQR= Interquartile Range
| Mechanical Detection – (in Grams) | |||||||
|---|---|---|---|---|---|---|---|
| Upper Ab | Sex | Median Grams |
IQR | Lower Ab | Sex | Median Grams |
IQR |
| Female, n = 115 | .24 | .45 | Female, n = 115 | .22 | .45 | ||
| Male, n = 63 | .16 | .46 | Male, n = 63 | .12 | .37 | ||
| Decade Age | Decade Age | ||||||
| 12-19 years, n = 48 | .26 | .45 | 12-19 years, n =48 | .19 | .55 | ||
| 20-30 years, n = 86 | .21 | .35 | 20-30 years, n =86 | .12 | .40 | ||
| 31-50 years, n =44 | .32 | .43 | 31-50 years, n =44 | .28 | .33 | ||
| Decade Age – Female | Decade Age - Female | ||||||
| 12-19 years, n =39 | .24 | .45 | 12-19 years, n =39 | .22 | .55 | ||
| 20-30 years, n =60 | .21 | .35 | 20-30 years, n =60 | .16 | .43 | ||
| 31-50 years, n =16 | .45 | .39 | 31-50 years, n =16 | .28 | .41 | ||
| Decade Age - Males | Decade Age - Males | ||||||
| 12-19 years, n =9 | .40 | .59 | 12-19 years, n =9 | .12 | .61 | ||
| 20-30 years, n =26 | 0.14 | .34 | 20-30 years, n =26 | .06 | .40 | ||
| 31-50 years, n = 28 | 0.22 | .46 | 31-50 years, n =28 | .25 | .33 | ||
Table 9.
Results from Hot Pain Threshold
| Hot Pain Threshold – (in Celsius) | |||||||
|---|---|---|---|---|---|---|---|
| Upper Ab | Sex | Median Celsius |
IQR | Lower Ab | Sex | Median Celsius |
IQR |
| Female, n = 112 | 43.58 | 5.01 | Female, n = 112 | 44.70 | 6.30 | ||
| Male, n = 61 | 45.65 | 5.98 | Male, n = 61 | 45.60 | 5.50 | ||
| Decade Age | Decade Age | ||||||
| 12-19 years, n =46 | 43.25 | 4.38 | 12-19 years, n =46 | 43.13 | 3.93 | ||
| 20-30 years, n =80 | 45.15 | 5.51 | 20-30 years, n =80 | 45.03 | 5.54 | ||
| 31-50 years, n =45 | 45.80 | 6.35 | 31-50 years, n =45 | 46.40 | 5.00 | ||
| Decade Age – Female | Decade Age – Female | ||||||
| 12-19 years, n =38 | 42.95 | 4.49 | 12-19 years, n =38 | 43.08 | 5.77 | ||
| 20-30 years, n =57 | 45.10 | 5.07 | 20-30 years, n =57 | 45.50 | 5.25 | ||
| 31-50 years, n =17 | 45.25 | 8.57 | 31-50 years, n =17 | 45.40 | 7.70 | ||
| Decade Age - Males | Decade Age - Males | ||||||
| 12-19 years, n =8 | 43.73 | 6.02 | 12-19 years, n =8 | 43.43 | 3.48 | ||
| 20-30 years, n =25 | 44.02 | 6.17 | 20-30 years, n =25 | 44.75 | 6.32 | ||
| 31-50 years, n =28 | 47.00 | 4.15 | 31-50 years, n =28 | 46.90 | 4.34 | ||
QST reference values are described using medians separated by age group and sex for the upper and lower abdominal quadrants as shown in Tables 3-9. It is important to note that while no participant stopped a QST test for safety concerns or due to intolerance or refusal of a test there was some missing data for each QST test. Each table represents the sample size of those who completed that measure. Missing QST data was due to issues including equipment malfunction on the day of the study visit and participants arriving either late or needing to leave early resulting in a shortened protocol. Given that the data for this study focuses on variables, which are either the outcome or the exposure, we did not impute missing values for these exposures and outcomes in the analyses.
4.3. Differences in QST by age category and sex
4.3.1. Dynamic Stimuli
a. Dynamic Mechanical Allodynia (0-10 Pain Scores).
In our sample, 162 participants endorsed no pain or a “soft” feeling from the brush used, which was expected from a sample of healthy, pain-free individuals. Eighteen participants (female, n=13, median age = 21 years; male, n=5, median age = 25 years) reported a “harsh” feeling with a median pain score of 0.42 (range = 0 to 3.5) on a scale of 0-10. As expected from a pain-free sample, this group did not experience allodynia overall. Given that only 18 participants a “harsh” feeling from the brush, we did not conduct group comparisons.
b. Mechanical Detection (Grams) (Table 3).
There were no differences in grams between the age groups for the upper (χ2(2) = 1.843, p = 0.398) nor lower abdomen (χ2(2) =1.805, p = 0.406). Similarly, there were no sex differences on the upper (U = 3306.5, p = 0.336) or the lower (U = 3152.5, p = 0.152) abdomen.
c. Mechanical Pain Threshold (Grams) (Table 4).
Table 4.
Results from Mechanical Pain Threshold
| Mechanical Pain Threshold – (in Grams) | |||||||
|---|---|---|---|---|---|---|---|
| Upper Ab | Sex | Median Grams |
IQR | Lower Ab | Sex | Median Grams |
IQR |
| Female, n = 116 | 7.00 | 298.00 | Female, n = 116 | 7.00 | 297.85 | ||
| Male, n = 63 | 6.00 | 155.50 | Male, n = 63 | 6.00 | 238.60 | ||
| Decade Age | Decade Age | ||||||
| 12-19 years, n =48 | 3.85 | 93.28 | 12-19 years, n =48 | 4.00 | 227.08 | ||
| 20-30 years, n =86 | 8.00 | 183.08 | 20-30 years, n =86 | 7.08 | 251.48 | ||
| 31-50 years, n =45 | 6.00 | 297.00 | 31-50 years, n =45 | 6.00 | 298.40 | ||
| Decade Age – Female | Decade Age - Female | ||||||
| 12-19 years, n =39 | 3.70 | 55.80 | 12-19 years, n =39 | 4.00 | 12.50 | ||
| 20-30 years, n =60 | 9.50 | 272.00 | 20-30 years, n =60 | 8.00 | 296.00 | ||
| 31-50 years, n =17 | 153.00 | 296.75 | 31-50 years, n =17 | 150.70 | 296.75 | ||
| Decade Age - Males | Decade Age - Males | ||||||
| 12-19 years, n =9 | 8.00 | 227.85 | 12-19 years, n =9 | 3.00 | 299.10 | ||
| 20-30 years, n =26 | 6.00 | 151.30 | 20-30 years, n =26 | 7.00 | 239.10 | ||
| 31-50 years, n =28 | 5.50 | 172.75 | 31-50 years, n =28 | 4.85 | 201.75 | ||
There were no differences in mechanical pain threshold (measured in grams) between age groups for the upper (χ2(2) = 3.983, p = 0.137) nor the lower (χ2(2) =3.048, p = 0.218) abdomen. Similarly, there were no sex differences between the upper (U = 3463.5, p = 0.562) nor the lower (U = 3388.0, p = 0.418) abdomen.
d. Temporal Summation of Pain.
Less than half (43%) of the sample met the criteria for temporal summation of pain (average of upper and lower quadrants together). Upper abdomen pain scores differed between the age groups (χ2(2) = 13.284, p = 0.001), with a mean rank pain score of 105.89 for adolescents, 92.56 for young adults, and 68.16 for adults. The corresponding medians and IQR to mean rank pain scores for adolescents were .5 (.98), .5 (1) for young adults, and 0 (.5) for adults. Similarly, age group differences in pain scores for the lower abdomen were found, χ2(2) = 10.799, p = 0.005, with a mean rank pain score of 101.34 for adolescents, 94.75 for young adults, and 68.97 for adults. The corresponding medians and Interquartile Range (IQR) to mean rank pain scores for adolescents were 1 (.98), .5 (1) for young adults, and 0 (1) for adults. When compared to adults, adolescent participants reported a higher pain score on their lower (U = 617.5, p < 0.001) and upper abdomen (U = 672, p = 0.002). Additionally, young adult participants reported a higher pain score for their lower (U=1414.5, p=0.009) and upper (U= 1396.5, p=0.006) abdomen when compared to adults. No sex differences were detected for the upper (U = 3404.0, p = 0.440) nor the lower (U = 3623.0, p = 0.924) abdomen.
4.4.2. Pressure Stimuli:
Pressure Pain Detection (Newtons) (Table 5).
Table 5.
Results from Pressure Pain Detection Threshold
| Pressure Pain Detection – (in Newtons) | |||||||
|---|---|---|---|---|---|---|---|
| Upper Ab | Sex | Median Newtons |
IQR | Lower Ab |
Sex | Median Newtons |
IQR |
| Female, n = 117 | 16.90 | 12.25 | Female, n = 117 | 16.80 | 13.40 | ||
| Male, n = 61 | 33.40 | 24.38 | Male, n = 61 | 32.60 | 25.83 | ||
| Decade Age | Decade Age | ||||||
| 12-19 years, n = 48 | 15.35 | 14.83 | 12-19 years, n = 48 | 15.70 | 12.85 | ||
| 20-30 years, n = 87 | 20.40 | 13.80 | 20-30 years, n = 87 | 19.70 | 13.00 | ||
| 31-50 years, n = 43 | 35.06 | 21.30 | 31-50 years, n = 43 | 36.20 | 22.40 | ||
| Decade Age – Female | Decade Age - Female | ||||||
| 12-19 years, n = 39 | 14.00 | 9.30 | 12-19 years, n =39 | 14.00 | 11.40 | ||
| 20-30 years, n = 61 | 17.50 | 10.50 | 20-30 years, n =61 | 16.60 | 12.40 | ||
| 31-50 years, n = 17 | 26.40 | 16.50 | 31-50 years, n =17 | 26.50 | 16.85 | ||
| Decade Age - Males | Decade Age - Males | ||||||
| 12-19 years, n =9 | 27.10 | 11.90 | 12-19 years, n =9 | 26.20 | 15.00 | ||
| 20-30 years, n = 26 | 24.05 | 27.55 | 20-30 years, n =26 | 24.60 | 24.51 | ||
| 31-50 years, n =26 | 40.00 | 18.96 | 31-50 years, n =26 | 42.30 | 28.00 | ||
Differences in the amount of pressure (Newtons) needed to trigger a pain sensation on the upper abdomen (χ2(2) = 45.580, p <0 .001) were found between the age groups, with a mean rank pressure of 62.78 for adolescents, 82.58 for young adults, and 133.33 adults. Similarly, differences were detected for the lower abdomen (χ2(2) =45.007, p < 0.001) between the age groups, with a mean rank pressure of 63.81 for adolescents, 81.97 for young adults, and 133.41 for adults. Table 5 presents the corresponding upper and lower medians and IQR to the mean rank pressure values. Less pressure was needed to trigger pain for adolescents when compared to adults on both the lower (U = 254.5, p < 0.001) and upper abdomen (U = 236, p <0 .001), suggesting heightened sensitivity for adolescents compared to adults. Similarly, less pressure was needed for young adults when compared to adults on both the lower (U=760, p < 0.001) and upper abdomen (U= 782, p <0 .001). Sex differences in pressure pain detection were significant. More pressure was needed to trigger pain for the male participants when compared to the female participants for both the upper (U = 1261.0, p < 0.001) and lower (U = 1427.0, p < 0.001) abdomen, demonstrating increased sensitivity to pressure pain for the females.
4.4.3. Thermal Stimuli
Thermal Detection for Cold (0Celsius) (Table 6).
Table 6.
Results from Cold Touch Detection Threshold
| Cold Touch Detection – (in Celsius) | |||||||
|---|---|---|---|---|---|---|---|
| Upper Ab | Sex | Median Celsius |
IQR | Lower Ab | Sex | Median Celsius |
IQR |
| Female, n = 112 | 29.83 | 1.81 | Female, n = 112 | 29.90 | 2.66 | ||
| Male, n = 61 | 28.60 | 3.00 | Male, n = 61 | 27.30 | 3.80 | ||
| Decade Age | Decade Age | ||||||
| 12-19 years, n = 46 | 29.83 | 2.04 | 12-19 years, n =46 | 29.73 | 2.63 | ||
| 20-30 years, n =82 | 29.70 | 1.82 | 20-30 years, n =82 | 27.35 | 3.08 | ||
| 31-50 years, n =44 | 28.45 | 3.08 | 31-50 years, n =44 | 27.35 | 4.47 | ||
| Decade Age – Female | Decade Age - Female | ||||||
| 12-19 years, n =38 | 30.15 | 1.52 | 12-19 years, n =38 | 30.03 | 1.85 | ||
| 20-30 years, n =57 | 29.80 | 1.78 | 20-30 years, n =57 | 29.85 | 2.45 | ||
| 31-50 years, n =17 | 29.35 | 2.92 | 31-50 years, n =17 | 28.95 | 5.10 | ||
| Decade Age - Males | Decade Age - Males | ||||||
| 12-19 years, n =8 | 27.85 | 2.18 | 12-19 years, n =8 | 27.2 | 3.44 | ||
| 20-30 years, n =25 | 29.10 | 3.18 | 20-30 years, n =25 | 27.40 | 4.27 | ||
| 31-50 years, n =28 | 28.23 | 2.63 | 31-50 years, n =28 | 27.23 | 4.16 | ||
Temperature differences (Celsius) were found for the upper abdomen between the age groups, χ2(2) = 10.347, p = 0.006, with a mean rank temperature of 96.45 for adolescents, 93.74 for young adults, and 66.79 for adults. However, no age differences in temperature were found for the lower abdomen, χ2(2) = 4.295, p = 0.117. Table 6 presents the corresponding upper and lower medians and IQR to the mean rank cool temperature values. Adolescents were more sensitive to cool temperatures on the upper abdomen; they endorsed a higher temperature for cool detection when compared to adults (U = 722, p < 0.009). No sex differences in temperature were detected on the upper (U = 1842.0, p = 0.677) nor the lower (U = 1812.5, p = 0.870) abdomen.
Thermal Detection for Warm (0Celsius) (Table 7).
Table 7.
Results from Warm Touch Detection Threshold
| Warm Touch Detection – (in Celsius) | |||||||
|---|---|---|---|---|---|---|---|
| Upper Ab | Sex | Median Celsius |
IQR | Lower Ab | Sex | Median Celsius |
IQR |
| Female, n = 112 | 35.20 | 1.72 | Female, n = 112 | 35.18 | 2.14 | ||
| Male, n = 61 | 36.35 | 2.17 | Male, n = 61 | 36.65 | 3.15 | ||
| Decade Age | Decade Age | ||||||
| 12-19 years, n =46 | 35.58 | 1.90 | 12-19 years, n =46 | 35.40 | 2.40 | ||
| 20-30 years, n =82 | 35.48 | 2.23 | 20-30 years, n =82 | 35.53 | 2.46 | ||
| 31-50 years, n =45 | 36.10 | 2.60 | 31-50 years, n =45 | 36.25 | 4.28 | ||
| Decade Age – Female | Decade Age - Female | ||||||
| 12-19 years, n =38 | 35.25 | 1.34 | 12-19 years (n=38) | 34.88 | 2.13 | ||
| 20-30 years, n =57 | 35.10 | 1.83 | 20-30 years (n=57) | 35.30 | 1.83 | ||
| 31-50 years, n =17 | 35.40 | 2.27 | 31-50 years (n=17) | 36.45 | 4.15 | ||
| Decade Age - Males | Decade Age - Males | ||||||
| 12-19 years, n =8 | 36.83 | .96 | 12-19 years, n =8 | 37.55 | 3.06 | ||
| 20-30 years, n =25 | 36.30 | 2.72 | 20-30 years, n =25 | 36.90 | 2.90 | ||
| 31-50 years, n =28 | 36.25 | 2.40 | 31-50 years, n =28 | 36.05 | 3.61 | ||
There were no differences in temperature for the upper (χ2(2) = 2.670, p = 0.263) nor the lower (χ2(2) = 4.295, p =0 .117) abdomen between the age groups. However, females were more sensitive to warm detection compared to males. Females detected a lower temperature on both the upper (U =2146.5, p < 0.001) and lower (U =2173.5, p = <0.001) abdomen suggesting heightened sensitivity.
Thermal Pain Threshold for Cold (0Celsius) (Table 8).
Table 8.
Results from Cold Pain Threshold
| Cold Pain Threshold – (in Celsius) | |||||||
|---|---|---|---|---|---|---|---|
| Upper Ab | Sex | Median Celsius |
IQR | Lower Ab | Sex | Median Celsius |
IQR |
| Female, n = 112 | 20.83 | 21.26 | Female, n = 112 | 19.63 | 21.54 | ||
| Male, n = 61 | 18.85 | 21.90 | Male, n = 61 | 21.05 | 20.58 | ||
| Decade Age | Decade Age | ||||||
| 12-19 years, n =46 | 22.40 | 12.46 | 12-19 years, n =46 | 21.98 | 13.48 | ||
| 20-30 years, n = 82 | 19.25 | 22.90 | 20-30 years, n =82 | 19.25 | 23.85 | ||
| 31-50 years, n = 45 | 14.15 | 20.95 | 31-50 years, n =45 | 19.30 | 17.48 | ||
| Decade Age – Female | Decade Age - Female | ||||||
| 12-19 years, n =38 | 22.05 | 13.30 | 12-19 years, n =38 | 21.15 | 13.69 | ||
| 20-30 years, n =57 | 18.80 | 24.15 | 20-30 years, n =57 | 18.90 | 26.60 | ||
| 31-50 years, n =17 | 19.65 | 23.98 | 31-50 years, n =17 | 22.35 | 15.40 | ||
| Decade Age - Males | Decade Age - Males | ||||||
| 12-19 years, n =8 | 22.80 | 7.88 | 12-19 years, n =8 | 23.88 | 12.08 | ||
| 20-30 years, n =25 | 23.80 | 20.50 | 20-30 years, n =25 | 23.50 | 22.53 | ||
| 31-50 years, n =28 | 11.33 | 19.56 | 31-50 years, n =28 | 18.30 | 20.09 | ||
There were no differences in the temperature needed to trigger pain on the upper (χ2(2) = 5.569, p = 0.062) nor lower (χ2(2) = 2.272, p = 0.321) abdomen between the age groups. Additionally, there were no sex differences on the upper (U = 3009.0, p = 0.224) nor the lower (U = 3282, p = 0.878) abdomen.
Thermal Pain Threshold for Hot (0 Celsius) (Table 9).
The temperature needed to trigger pain for the upper abdomen differed between the age groups, χ2(2) = 11.735, p =0 .003, with a mean rank temperature of 66.53 for adolescents, 86.63 for young adults, and 102.35 for adults. Age group differences were also found for the lower abdomen, χ2(2) = 13.130, p = 0.001, with a mean rank Celsius of 64.77 for adolescents and 86.14 for young adults, and 102.35 for adults. Table 9 presents the corresponding upper and lower medians and IQR to the mean rank hot temperature values. Adolescents needed a lower temperature to trigger pain when compared to adults for both the upper (U = 579.5, p =0 .001) and lower abdomen (U = 540, p <0 .001), indicating increased sensitivity. Additionally, males required a higher temperature to trigger pain only on the upper abdomen (U =2554.0, p = 0.018), indicating less sensitivity compared to females. No sex differences were found for the lower abdomen (U =2690.5, p = 0.096).
A snapshot summary of these results can be found in Table 10.
Table 10.
Snapshot Summary of Significant Results on QST Measures by Age and Biological Sex
| QST Measure | Significant Differences for Upper Abdomen |
Significant Differences for Lower Abdomen |
|---|---|---|
| Dynamic Mechanical Allodynia | None | None |
| Mechanical Detection | None | None |
| Mechanical Pain Threshold | None | None |
| Temporal Summation of Pain | • Adolescents endorsed more pain than Adults • Young adults endorsed more pain than Adults |
• Adolescents endorsed more pain than Adults • Young adults endorsed more pain than Adults |
| Pressure Pain Detection | • Adolescents endorsed more pain than Adults • Young adults endorsed more pain than Adults • Females more sensitive than Males |
• Adolescents endorsed more pain than Adults • Young adults endorsed more pain than Adults • Females more sensitive than Males |
| Cold Thermal Detection | • Adolescents more sensitive compared to adults. | None |
| Warm Thermal Detection | • Females more sensitive than males | • Females more sensitive than males |
| Cold Thermal Pain | None | None |
| Hot Thermal Pain | • Adolescents more sensitive than Adults • Females more sensitive than males |
None |
5. Discussion
Chronic abdominal pain is prevalent and can be challenging to evaluate and treat due to a multitude of functional and visceral causes (Table 1). Abdominal pain is the most common gastrointestinal symptom reported in ambulatory clinics [25], accounting for approximately 10% of emergency room visits [36]. A recent 20-year longitudinal study found that 20% of children with chronic abdominal pain still suffered from abdominal pain as adults [48]. This study found that children with chronic abdominal pain also present with health-related anxiety in adulthood and endorse greater emotional problems in offspring, suggesting potentially important intergenerational pathways of risk and resilience for chronic abdominal pain [48]. Chronic abdominal pain is also implicated in many intraabdominal and systemic diseases (e.g., digestive, reproductive, muscular) [7]. Given the long-term and deleterious impact of chronic abdominal pain, evaluating sensory functioning of the abdomen and characterizing ranges on QST measures is an essential first step in understanding and monitoring the clinical course of sensory abnormalities of the abdomen in patients with underlying diseases that can cause chronic abdominal pain. The present study aimed to establish QST reference values for the upper and lower abdomen in a diverse sample of pain-free adolescent and adult males and females. A secondary aim was to explore group differences of age and sex on pain sensitivity in the abdominal quadrants.
Age and sex-related differences were found. We hypothesized that younger participants would exhibit more significant pain and heightened pain sensitivity than adults, and this was the case for some of the tests. Both adolescents and young adults reported a significantly higher pain score for both their lower and upper abdomen during temporal summation of pain when compared to adults. Additionally, the younger two cohorts were more sensitive to pressure pain detection on both the upper and lower abdomen compared to adults. Adolescents were also more sensitive to heat pain in the upper and lower abdomen compared to adults, as well as more sensitive to cold temperatures in the upper abdomen. While more research is warranted, these age-related differences could reflect potential developmental neurophysiological differences in central pain processing of the developing central nervous system.
Additionally, some significant sex differences emerged, as expected. Females demonstrated heightened sensitivity to both pressure pain and heat pain detection in both the upper and lower quadrants compared to males, as well as lower heat pain threshold of the upper abdomen. These results are consistent with existing literature demonstrating female sex as a risk factor for chronic pain [3, 11]. Previous studies also reported that female patients endorsed more pain than male patients in conditions associated with abdominal pain, like irritable bowel syndrome and inflammatory bowel disease, which may indicate sex differences in visceral pain perception [5, 6, 12].
Our results, coupled with others, clearly underscore the need to consider sex differences when developing diagnostics and therapeutics for those affected by chronic abdominal pain. In the present study, while participants in menopause were excluded, we did not control for the menstrual cycle, which is a limitation. While more research is warranted, pain perception and intensity vary across the menstrual cycle, with significantly greater pain and body temperature changes in the luteal phase [16]. Additionally, given that many conditions causing chronic abdominal pain are found exclusively in people assigned female at birth (e.g., endometriosis, vulvodynia, ovarian cysts, uterine fibroids) or are over-represented in females, this preliminary study provides objective biological norms that could help lessen the dismissal of women’s pain and the overall disenfranchisement of women, which is a too common occurrence in medicine [8, 43].
Although we had a racially diverse sample, we did not report QST norms based on racial or ethnic groups. There is evidence to suggest differences in response to pain across racial and ethnic groups [30, 31]; however, the differences in response to pain among Black, Indigenous, and People of Color (BIPOC), which are often misattributed to biological mechanisms [17, 18], are the complex result of systemic racism [29, 33]. In other words, BIPOC experience discrimination, oppression, and lack of access to care [32], which can manifest as changes in cardiovascular, metabolic, inflammatory, psychological, and pain responses [35]. As such, we must not contribute to a literature that continues to identify such differences without helping to understand and change the complex mechanisms by which racial and ethnic disparities in pain persist. Instead, future studies should aim to include groups that have historically been underrepresented in pain research to identify mechanisms of inter- and intra-group differences in pain responses, as well as interventions for reducing racial and ethnic pain disparities.
To our knowledge, this study is the first report of QST norms of the four abdominal quadrants, which can be used as reference values in the clinic setting to better understand pain and pain perception in the many patient populations who experience chronic abdominal pain. Additionally, the results speak to the need for interdisciplinary pain management as an integral component for treating these wide-ranging diseases. Sensory profiles of the abdomen by age and sex may provide a metric of underlying pain pathophysiological mechanisms, which could inform therapeutic interventions [24]. Unfortunately, applying QST in clinical practice has remained logistically challenging [1]. However, the promotion of validated and simple “bedside” QST has recently gained momentum to advance personalized pain medicine [10, 13, 24, 39, 40]. One important consideration for researchers and clinicians performing QST on the abdomen is that abdominal skin can be sensitive to light touch, particularly stroking the skin in different quadrants, which may provoke abdominal muscle contraction (i.e., a normal superficial neurological reflex, which is not painful). Therefore, patient education about relaxing the muscles is important, akin to clinical instructions given to patients during deep palpation of the abdomen when the patient is relaxed in the supine position.
While this study has several notable strengths, including a large and diverse sample and the use of rigorous, validated QST measures, there are some limitations. First, participants were assessed at a single study visit, so we lacked data on reliability; however, each measure was conducted several times within one study visit. Also, as aforementioned, the menstrual cycle was not accounted for and could have impacted the observed variations. Additionally, while including participants ranging in age from adolescence to adulthood is a strength, young children and older adults were excluded allowing us to focus on an age range where we most frequently see sex-related differences in pain processing. Future research to establish abdominal QST norms in an expanded age range is warranted. Further, there are gender biases present in pain treatment, including in abdominal pain [8], leading to diagnostic and treatment delays that can have deleterious consequences for pain and functional outcomes [53]. Thus, studying not only sex but also gender differences in the different QST modalities is an important future direction. Finally, it should be noted that the current study did not involve a substantial sample of adolescent males. Therefore, future research should aim to replicate the study with a larger sample size that includes more adolescent males.
Regarding the QST protocol, our measure of mechanical allodynia asked participants to describe what they felt after applying the stimulus. If the participant described a feeling other than soft, they were asked to clarify whether the brush felt “soft” or “harsh”. If “harsh” was endorsed, an NRS pain rating was obtained. For mechanical allodynia, the response to light touch is normally felt as soft contact, which is expected for healthy, pain-free people. Still, some patients report rough or harsh contact but not painful (i.e., an abnormal sensation similar to dysesthesia, an unpleasant sensation of touch) [14]. However, while it seems unlikely that a participant who describes the brush a “soft” would perceive that sensation as painful, we acknowledge that this could be described as painful by people with chronic pain, especially neuropathic pain who experience allodynia, and future QST protocols assessing this should be mindful of assessing both sensation and pain ratings for mechanical allodynia tasks. Lastly, QST was only assessed somatically on the four abdominal wall quadrants, which is presumed visceromotor responses to visceral nociceptor activation and neural convergence of visceral and somatic stimuli at the spinal cord [37]. There may be utility to establish visceral QST norms given our recent findings that high pelvic floor tenderness may be a marker of heightened pain sensitivity [45] to quantify autonomic and behavioral response. Comparing sex, gender, and age-related differences on external and internal QST paradigms could be an important area of inquiry further to inform personalized pain treatment for chronic abdominal pain.
In sum, we provide normative values for abdominal QST among healthy male and female adolescents and adults. This study is an important first step in better understanding sensory perception in the abdominal area, an area associated with numerous acute and chronic painful conditions. These reference values will be important in helping to identify patient phenotypes and guide personalized treatment of conditions associated with abdominal pain.
Acknowledgments:
Thank you to Yashoda Dhole, Emily Mosher, Margaret Aiken, Madison LaCasse, Isha Jha, Whitney Sandford, Trevor Coles, Rose Eiduson, and Camden Waterhouse who assisted with recruitment and data collection for this study.
Footnotes
Funding/Disclosures: CBS was funded by a grant from the Office of Faculty Development at BCH and a Boston Center for Endometriosis/Marriott Family Foundation Investigator Grant for this work. None of the authors have a conflict of interest.
Data Sharing Statement:
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Adler M, & Taxer B (2022). [Quantitative sensory testing for neuropathic pain and its relevance for physiotherapy]. Schmerz (Berlin, Germany), 36(6). 10.1007/S00482-021-00576-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alfvén G. The pressure pain threshold (PPT) of certain muscles in children suffering from recurrent abdominal pain of non-organic origin. An algometric study. Acta Paediatr. 1993;82(5):481–3. [DOI] [PubMed] [Google Scholar]
- [3].Bartley EJ, & Fillingim RB (2013). Sex differences in pain: a brief review of clinical and experimental findings. BJA: British Journal of Anaesthesia, 111(1), 52. 10.1093/BJA/AET127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blankenburg M, Meyer D, Hirschfeld G, Kraemer N, Hechler T, Aksu F, Krumova EK, Magerl W, Maier C, & Zernikow B (2011). Developmental and sex differences in somatosensory perception--a systematic comparison of 7- versus 14-year-olds using quantitative sensory testing. Pain, 152(11), 2625–2631. 10.1016/J.PAIN.2011.08.007 [DOI] [PubMed] [Google Scholar]
- [5].Camilleri M. (2020). Sex as a biological variable in irritable bowel syndrome. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society, 32(7). 10.1111/NMO.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chang L, Mayer EA, Labus JS, Schmulson M, Oh YL, Olivas TI, Stains J, & Naliboff BD (2006). Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 291(2). 10.1152/AJPREGU.00729.2005 [DOI] [PubMed] [Google Scholar]
- [7].Charles G, Chery M, & King Channell M (2019). Chronic Abdominal Pain: Tips for the Primary Care Provider ∣ Osteopathic Family Physician. OFP , 11(1). https://ofpjournal.com/index.php/ofp/article/view/596 [Google Scholar]
- [8].Chen EH, Shofer FS, Dean AJ, Hollander JE, Baxt WG, Robey JL, Sease KL, & Mills AM (2008). Gender disparity in analgesic treatment of emergency department patients with acute abdominal pain. Academic Emergency Medicine: Official Journal of the Society for Academic Emergency Medicine, 15(5), 414–418. 10.1111/J.1553-2712.2008.00100.X [DOI] [PubMed] [Google Scholar]
- [9].Colucciello S. (2019). Assessing abdominal pain in adults: a rational, cost-effective, and evidence-based strategy - PubMed. Emerg Med Pract, 21(6), 1–32. https://pubmed.ncbi.nlm.nih.gov/31124641/ [PubMed] [Google Scholar]
- [10].Cruz-Almeida Y, & Fillingim RB (2014). Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Medicine (Malden, Mass.), 15(1), 61–72. 10.1111/PME.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, & Riley JL (2009). Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. The Journal of Pain: Official Journal of the American Pain Society, 10(5), 447. 10.1016/J.JPAIN.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Francis-Malavé AM, Gonzalez SM, Pichardo C, Wilson TD, Rivera-García LG, Brinster LR, & Carrasquillo Y (2023). Sex differences in pain-related behaviors and clinical progression of disease in mouse models of colonic pain. Pain, 164(1). 10.1097/J.PAIN.0000000000002683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gierthmü Hlen J, Schneider U, Seemann M, Freitag-Wolf S, Maihö Fner C, Enax-Krumova EK, Azad SC, Ü Ç Eyler N, Birklein F, Maier C, Tö Lle T, Treede RD, & Barona R (2019). Can self-reported pain characteristics and bedside test be used for the assessment of pain mechanisms? An analysis of results of neuropathic pain questionnaires and quantitative sensory testing. Pain, 160(9), 2093–2104. 10.1097/J.PAIN.0000000000001601 [DOI] [PubMed] [Google Scholar]
- [14].Gylfadottir SS, Itani M, Kristensen AG, Karlsson P, Krøigård T, Bennett DL, Tankisi H, Andersen NT, Jensen TS, Sindrup SH, Finnerup NB. The characteristics of pain and dysesthesia in patients with diabetic polyneuropathy. PLoS One. 2022. Feb 17;17(2):e0263831. doi: 10.1371/journal.pone.0263831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heading R. (1999). Prevalence of upper gastrointestinal symptoms in the general population: a systematic review - PubMed. Scand J Gastroenterol Suppl, 231(3-8). https://pubmed.ncbi.nlm.nih.gov/10565617/ [PubMed] [Google Scholar]
- [16].Hellström B, & Anderberg UM (2003). Pain perception across the menstrual cycle phases in women with chronic pain. Perceptual and Motor Skills, 96(1), 201–211. 10.2466/PMS.2003.96.1.201 [DOI] [PubMed] [Google Scholar]
- [17].Hoffman KM, Trawalter S, Axt JR, & Oliver MN (2016). Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences of the United States of America, 113(16), 4296–4301. 10.1073/PNAS.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Janevic MR, Mathur VA, Booker SQ, Morais C, Meints SM, Yeager KA, & Meghani SH (2022). Making Pain Research More Inclusive: Why and How. The Journal of Pain, 23(5), 707–728. 10.1016/J.JPAIN.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jänig W. Neurobiologie viszeraler Schmerzen [Neurobiology of visceral pain]. Schmerz. 2014. Jun;28(3):233–51. German. doi: 10.1007/s00482-014-1402-x [DOI] [PubMed] [Google Scholar]
- [20].Jänig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996. Jan 5;42(1-2):29–51. doi: 10.1016/0301-0511(95)05145-7 [DOI] [PubMed] [Google Scholar]
- [21].Kamp EH, Jones RCW, Tillman SR, & Gebhart GF (2003). Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. American Journal of Physiology - Gastrointestinal and Liver Physiology, 284(3 47-3), 434–444. [DOI] [PubMed] [Google Scholar]
- [22].Kleinert S, & Horton R (2016). Adolescent health and wellbeing: a key to a sustainable future. Lancet (London, England), 387(10036), 2355–2356. 10.1016/S0140-6736(16)30297-5 [DOI] [PubMed] [Google Scholar]
- [23].Korterink JJ, Diederen K, Benninga MA, & Tabbers MM (2015). Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS ONE, 10(5). 10.1371/JOURNAL.PONE.0126982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koulouris AE, Edwards RR, Dorado K, Schreiber KL, Lazaridou A, Rajan S, White J, Garcia J, Gibbons C, & Freeman R (2020). Reliability and Validity of the Boston Bedside Quantitative Sensory Testing Battery for Neuropathic Pain. Pain Medicine (Malden, Mass.), 21(10), 2336–2347. 10.1093/PM/PNAA192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lakhoo K, Almario C. v., Khalil C, & Spiegel BMR (2021). Prevalence and Characteristics of Abdominal Pain in the United States. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association, 19(9), 1864–1872.e5. 10.1016/J.CGH.2020.06.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lautenbacher S, Peters JH, Heesen M, Scheel J, & Kunz M (2017). Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neuroscience and Biobehavioral Reviews, 75, 104–113. 10.1016/J.NEUBIOREV.2017.01.039 [DOI] [PubMed] [Google Scholar]
- [27].Lunde CE, Fisher E, Donovan E, Serbic D, & Sieberg CB (2022). Cutting the cord? Parenting emerging adults with chronic pain. Paediatric & Neonatal Pain, 4(3), 136–147. 10.1002/PNE2.12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luz LL, Fernandes EC, Miklos Sivado M, Kokai E, Szucs P, Safronov BV (2015) Monosynaptic convergence of somatic and visceral C-fiber afferents on projection and local circuit neurons in lamina I: a substrate for referred pain. Pain 156(10):2042–2051. 10.1097/j.pain.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mathur VA, Richeson JA, Paice JA, Muzyka M, & Chiao JY (2014). Racial bias in pain perception and response: experimental examination of automatic and deliberate processes. The Journal of Pain, 15(5), 476–484. 10.1016/J.JPAIN.2014.01.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meints SM, Cortes A, Morais CA, & Edwards RR (2019). Racial and ethnic differences in the experience and treatment of noncancer pain. Pain Management, 9(3), 317. 10.2217/PMT-2018-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Meints SM, Mosher C, Rand KL, Ashburn-Nardo L, & Hirsh AT (2018). An experimental investigation of the relationships among race, prayer, and pain. Scandinavian Journal of Pain, 18(3), 545–553. 10.1515/SJPAIN-2018-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Michael Byrd W, Clayton LA, Boston M, & Byrd M (2001). Race, medicine, and health care in the United States: a historical survey. Journal of the National Medical Association, 93(3 Suppl), 11S. /pmc/articles/PMC2593958/?report=abstract [PMC free article] [PubMed] [Google Scholar]
- [33].Morais CA, Aroke EN, Letzen JE, Campbell CM, Hood AM, Janevic MR, Mathur VA, Merriwether EN, Goodin BR, Booker SQ, & Campbell LC (2022). Confronting Racism in Pain Research: A Call to Action. The Journal of Pain, 23(6), 878. 10.1016/J.JPAIN.2022.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mücke M, Cuhls H, Radbruch L, Baron R, Maier C, Tölle T, Treede RD, & Rolke R (2021). Quantitative sensory testing (QST). English version. Schmerz (Berlin, Germany), 35(Suppl 3), 153–160. 10.1007/S00482-015-0093-2 [DOI] [PubMed] [Google Scholar]
- [35].Myers HF, Wyatt GE, Ullman JB, Loeb TB, Chin D, Prause N, Zhang M, Williams JK, Slavich GM, & Liu H (2015). Cumulative Burden of Lifetime Adversities: Trauma and Mental Health in Low-SES African Americans and Latino/as. Psychological Trauma: Theory, Research, Practice and Policy, 7(3), 243. 10.1037/A0039077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Natesan S, Lee J, Volkamer H, & Thoureen T (2016). Evidence-Based Medicine Approach to Abdominal Pain. Emergency Medicine Clinics of North America, 34(2), 165–190. 10.1016/J.EMC.2015.12.008 [DOI] [PubMed] [Google Scholar]
- [37].Ness TJ, Metcalf AM, & Gebhart GF (1990). A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain, 43(3), 377–386. 10.1016/0304-3959(90)90035-C [DOI] [PubMed] [Google Scholar]
- [38].Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005. Jun;128(7):1953–64. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- [39].Reimer M, Sachau J, Forstenpointner J, & Baron R (2021). Bedside testing for precision pain medicine. Current Opinion in Supportive and Palliative Care, 15(2), 116–124. 10.1097/SPC.0000000000000556 [DOI] [PubMed] [Google Scholar]
- [40].Reimer M, Forstenpointner J, Hartmann A, Otto JC, Vollert J, Gierthmühlen J, Klein T, Hüllemann P, & Baron R (2020). Sensory bedside testing: a simple stratification approach for sensory phenotyping. Pain Reports, 5(3). 10.1097/PR9.0000000000000820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, … Wasserka B (2006). Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain, 123(3), 231–243. 10.1016/j.pain.2006.01.041 [DOI] [PubMed] [Google Scholar]
- [42].Sabo CM, Grad S, & Dumitrascu DL (2021). Chronic Abdominal Pain in General Practice. Digestive Diseases (Basel, Switzerland), 39(6), 606–614. 10.1159/000515433 [DOI] [PubMed] [Google Scholar]
- [43].Samulowitz A, Gremyr I, Eriksson E, & Hensing G (2018). “Brave Men” and “Emotional Women”: A Theory-Guided Literature Review on Gender Bias in Health Care and Gendered Norms towards Patients with Chronic Pain. Pain Research & Management, 2018. 10.1155/2018/6358624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sengupta JN (2009). Visceral Pain: The Neurophysiological Mechanism. Handbook of Experimental Pharmacology, 194(194), 31. 10.1007/978-3-540-79090-7_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shafrir AL, Martel E, Missmer SA, Clauw DJ, Harte SE, As-Sanie S, & Sieberg CB (2021). Pelvic floor, abdominal and uterine tenderness in relation to pressure pain sensitivity among women with endometriosis and chronic pelvic pain. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 264, 247–253. 10.1016/J.EJOGRB.2021.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sjölund J, Uusijärvi A, Tornkvist NT, Kull I, Bergström A, Alm J, Törnblom H, Olén O, & Simrén M (2021). Prevalence and Progression of Recurrent Abdominal Pain, From Early Childhood to Adolescence. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association, 19(5), 930–938.e8. 10.1016/J.CGH.2020.04.047 [DOI] [PubMed] [Google Scholar]
- [47].Struller F, Weinreich FJ, Horvath P, Kokkalis MK, Beckert S, Königsrainer A, Reymond MA. Peritoneal innervation: embryology and functional anatomy. Pleura Peritoneum. 2017. Dec 1;2(4):153–161. doi: 10.1515/pp-2017-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stone AL, Epstein I, Bruehl S, Garber J, Smith CA, & Walker LS (2023). Twenty-year Outcomes of a Pediatric Chronic Abdominal Pain Cohort: Early Adulthood Health Status and Offspring Physical and Behavioral Health. The Journal of Pain, 24(1). 10.1016/J.JPAIN.2022.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Treede RD (2019). The role of quantitative sensory testing in the prediction of chronic pain. Pain, 160 Suppl 1(1), S66–S69. 10.1097/J.PAIN.0000000000001544 [DOI] [PubMed] [Google Scholar]
- [50].Vecchiet L, Vecchiet J, Giamberardino MA. Referred Muscle Pain: Clinical and Pathophysiologic Aspects. Curr Rev Pain. 1999;3(6):489–498. doi: 10.1007/s11916-999-0077-y. [DOI] [PubMed] [Google Scholar]
- [51].Weaver KR, Griffioen MA, Klinedinst NJ, Galik E, Duarte AC, Colloca L, Resnick B, Dorsey SG, & Renn CL (2021). Quantitative Sensory Testing Across Chronic Pain Conditions and Use in Special Populations. Frontiers in Pain Research, 2, 779068. 10.3389/FPAIN.2021.779068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zondervan KT, Becker CM, & Missmer SA (2020). Endometriosis. The New England Journal of Medicine, 382(13), 1244–1256. 10.1056/NEJMRA1810764 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.