Abstract
Owing to rising drug-resistant Helicobacter species infections in people and animals, currently therapies are losing their efficacy; therefore, regimens efficacious in the presence of drug resistance are needed. This study assessed the efficacy and safety of a 14-day quadruple Helicobacter species therapy in cats with naturally acquired infection. Thirteen asymptomatic adult stray cats with Helicobacter species infection (identified by analysis of gastric biopsies using polymerase chain reaction and Helicobacter-specific primers) received omeprazole 0.7 mg/kgq 8 h plus amoxicillin 20 mg/kgq 12 h, metronidazole 20 mg/kgq 12 h and clarithromycin 7.5 mg/kgq 12 h, for 14 days. Second molecular analysis of gastric biopsies revealed persistence of Helicobacter species DNA in four cats that were negative on quantitative urease testing, cytology and histopathology. Our results suggest that antibiotic regimens that are effective against Helicobacter pylori in people cannot eradicate Helicobacter species in cats with naturally acquired infection, although transient suppression may occur.
Helicobacters are spiral-shaped or curved Gram-negative bacteria that inhabit the gastric mucosa. 1 The main Helicobacter species identified in the stomach of cats are Helicobacter heilmannii and Helicobacter felis. 2–4 The total prevalence of gastric Helicobacter species has been reported as 40% and 100% in healthy and sick cats, respectively. 2 Polymerase chain reaction (PCR) amplification of 16S rRNA detected Helicobacter species colonisation in 56.7% and 100% of stray and domestic cats in Tehran, Iran, respectively. 5 Some studies have shown significant association between colonisation of Helicobacter species and gastric histological changes in cats. 6,7 However, the lack of knowledge of the pathogenicity of gastric Helicobacter species has meant that veterinarians are faced with the dilemma of either treating or ignoring spiral organisms observed in biopsies from cats or dogs with chronic vomiting and gastritis. 2 In light of their pathogenicity in man and other animals it would seem reasonable that eradication of gastric Helicobacter species should be considered prior to initiating treatment with immunosuppressive agents to control gastritis in dogs and cats. 2
The eradication of Helicobacter pylori infection has been reported to relieve symptoms and gastric abnormalities in different species including humans. 8,9 However, in human medicine, the success rate for eradication of Helicobacter species particularly H pylori has decreased steadily. 10 In clinical practice, the regimen of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin or a PPI plus clarithromycin and metronidazole/tinidazole standard triple therapies generally produce a lower than acceptable eradication rate that ranges from 60 to 80%. 11–13 In one veterinary study, clinical signs in 90% of 63 dogs and cats responded to treatment with a combination of metronidazole, amoxicillin and famotidine, and 74% of 19 animals reassessed had no evidence of Helicobacter species in gastric biopsies. 15 However, controlled therapeutic studies in asymptomatic cats suggest that it is difficult to eradicate gastric Helicobacter species in cats with azithromycin, tinidazole, bismuth and ranitidine, or clarithromycin, metronidazole, bismuth and ranitidine for 4 or 7 days. 1
The variability of treatment success with an individual regimen has been related to the presence of antimicrobial resistance, compliance with the drug regimen, and duration of therapy. 14 For example, a recent study from the United States compared the efficacy of 3-, 7-, and 10-day triple therapies and found that they were equally poor with none achieving a cure rate as high as 80%. 14 Success is best with at least 14 days of therapy which provides a higher eradication rate than does 7- or 10-day therapy. 10,11
The objective of this study was to assess the efficacy and safety of a long-term (14 days) four-drug regimen in eradicating Helicobacter species in asymptomatic cats with naturally acquired infection.
Material and methods
Animals and sampling procedure
This study has been approved by the Iranian laboratory animal ethics framework under the supervision of the Iranian Society for the Prevention of Cruelty to Animals. Twenty asymptomatic (revealed by physical examination, complete blood count (CBC), serum biochemistry profile and evaluation of faecal parasite egg count) adult domestic shorthair stray cats (Felis catus) (13 male and seven female), 1–3 years of age (median: 1.7±0.35), were isolated from different locations of Tehran, Iran. Cats were housed in separate cages for 4 weeks prior to initiation of study. All cats were individually housed during the entire course of study and fed the same diet. In order to evaluate the status of Helicobacter species infection, gastroscopy was performed with 4.9 mm diameter pediatric bronchoscope (Vet-Vu, Switzerland). For this purpose, fasting cats were anaesthetised with acepromazine (Neurotranq, 0.1 mg/kg, IM) and thiopental sodium (Nesdonal; Sanofi-Aventis, 25–30 mg/kg, IV given until effective). Biopsy forceps (2 mm) were used to prepare pinch biopsies (four from each) of the following areas: gastric cardia, body (greater curvature) and antrum (incisura to pyloric sphincter).
Quantitative urease test
One biopsy specimen from each location was placed into 5 ml of urea broth media (Difco, USA) and incubated at 37°C for 24 h. Conversion to a pink–red colour within 24 h was considered as positive result. No colour change in the course of 24 h was considered as negative.
Cytology
Impression smears from the second pair of gastric biopsies were imprinted on glass slides and the slides were then fixed with methanol and stained with Giemsa for detection of Helicobacter species. The degree of Helicobacter species colonisation (the mean of 10 microscopic fields at ×400) was recorded as follows: (0) No organism, (1) mild: less than 10 bacteria in each field, (2) moderate: between 10 and 50 bacteria in each field, (3) severe: more than 50 bacteria in each field.
Histopathology
Gastric biopsy specimens were carefully laid flat between layers of absorbent paper and immersed in neutral buffered 10% formalin. The strips were trimmed, processed by standard methods, embedded in paraffin, sectioned at 5 μm and stained with Warthin–Starry silver (WSS) stain and haematoxylin and eosin stain (H&E). All biopsies were evaluated by one pathologist who was blind to the results of all other tests. For the histopathological assessment, the presence of lymphocyte aggregates, the number of leukocytes, and the degree of Helicobacter species colonisation were recorded. Grading of leukocytic infiltration was as follows: for inflammatory cells (mean of three fields at ×400), absent, mild (<10), moderate (10–50), or severe (>50). Gastritis was defined as follows: ‘no gastritis’, no lymphocyte aggregates or leukocytes; (grade 1): ‘mild gastritis’, no lymphocyte aggregates and <10 leukocyte per field; (grade 2): ‘moderate gastritis’, lymphocyte aggregates and/or 10–50 leukocytes per field; (grade 3): ‘severe gastritis’, lymphocyte aggregates and >50 leukocytes per field.
DNA extraction and PCR assays
Gastric mucosal biopsy specimens were frozen at −70°C until DNA extraction. The extraction was performed with DNeasy tissue DNA extraction kit (Qiagen, Germany) according to the manufacturer's instructions. PCR amplifications were performed in a final volume of 20 μl containing 100 ng of extracted DNA, 2 μl of 10× PCR buffer (Fermentas, Lithuania), 0.2 mM of dNTP, 1.5 mM MgCl2, 25 pM/μl of each primer and 0.5 U of Taq DNA polymerase (Fermentas, Lithuania) under conditions previously described. 16 Gastric biopsies were first tested against genus-specific primers (16S rRNA), then assessed for colonisation with H pylori, H felis, or H heilmannii via strain-specific primers (Table 1). 17–19 The resulting PCR products underwent gel electrophoresis (1.5% (w/v) agarose gel with 0.3% ethidium bromide in 10% Tris–borate ethylenediaminetetraacetic acid (EDTA) buffer (TBE)) and visualised under an ultraviolet transilluminator.
Table 1.
Oligonucleotide primers used in this study, reference source, primer sequence and length of amplified fragment (in base pairs).
| Target genes | References | Primer sequence (5′→3′) | Amplified fragment (bp) |
|---|---|---|---|
| 16S rRNA (Helicobacter) species | Germani et al (1997) 17 | (f): AAC GAT GAA GCT TCT AGC TTG CTA | 399 |
| (r): GTG CTT ATT CGT GAG ATA CCG TCA T | |||
| ureA and ureB (H felis) | Germani et al (1997) 17 | (f): GTG AAG CGA CTA AAG ATA AAC AAT | 241 |
| (r): GCA CCA AAT CTA ATT CAT AAG AGC | |||
| ureB (H heilmannii) | Neiger et al (1998) 18 | (f): GGG CGA TAA AGT GCG CTT G | 580 |
| (r): CTG GTC AAT GAG AGC AGG | |||
| ureB (H pylori) | Clayton (1992) 19 | (f): GCC AAT GGT AAA TTA GTT | 411 |
| (r): CTC CTT AAT TGT TTT TAC |
Anti-helicobacter quadruple therapy
The quadruple therapy regimen was adapted from similar antibiotic therapies successfully used in humans to eradicate H pylori 10 and dosages appropriate for cats were calculated. Each cat regardless of its status of Helicobacter species infection, received a 14-day oral course of quadruple therapy consisting of 0.7 mg/kg omeprazole q 8 h (Zahravi Pharmaceutical, Tabriz, Iran), 20 mg/kg amoxicillin q 12 h (Farabi Pharmaceutical, Isfahan, Iran), 20 mg/kg metronidazole q 12 h (Tehran Chemie Pharmaceutical, Tehran, Iran) and 7.5 mg/kg clarithromycin q 12 h (Tehran Chemie Pharmaceutical, Tehran, Iran). Bismuth subsalicylate was not used in the combination therapy because of the potential toxicity of subsalicylate in cats. 20
Assessment of the adverse effects of therapy
Gastrointestinal adverse effects of quadruple therapy were monitored using a numerical score proportional to severity of signs: (0) No change in appetite/no change in faecal consistency/no vomiting/no excessive saliva, (1) longer duration of eating meal/mild change in faecal consistency/occasion vomiting/moderately excessive saliva and (2) loss of appetite/watery faeces/severe vomiting/severe excessive saliva. In addition, dermatological and neurological side effects were assessed using visual analogue scales.
Statistical analysis
For assessment of the relationship between the intensity of Helicobacter species colonisation in the three gastric regions, the Fisher's exact test was used. Fisher's exact test was also used for the assessment of the correlation between Helicobacter species infection and gastritis occurrence. P values less than 0.05 were considered as statistically significant.
Results
Helicobacter species animals
The gross endoscopic appearance of gastric mucosa was unremarkable in all cats. Out of 20 study cats, PCR assay resulted in exclusion of seven cats due to failure of demonstration of Helicobacter species in the gastric samples.
Quantitative urease testing and cytology
Prior to commencing the study, the quantitative urease testing and cytology were positive for samples from one or more gastric sites for 10/13 and 8/13 cats, respectively (Table 2). Bacteria were present in surface mucus, gastric pits, and gland, and severity ranged from 1 (rare bacteria) to 3 (many bacteria packed in gland or in gastric mucus). Bacteria were most consistently present in antrum biopsy specimens (8/13 cats). Despite this finding, differences in the intensity of colonisation between the three gastric regions were not significant (P=0.72). Following 14-day anti-Helicobacter species quadruple therapy, none of the cats were found to be infected with Helicobacter species by quantitative urease testing, or cytological examination of gastric biopsies (Table 3).
Table 2.
Evaluation of Helicobacter species infection status by different tests prior to 14-day quadruple therapy.
| Prior to 14-day quadruple therapy | |||||||
|---|---|---|---|---|---|---|---|
| Cat | 16S rRNA | Hf | Hh | Hh/Hf | Cytology | RUT | WSS |
| 3 | + | + | + | + | + | + | + |
| 5 | + | + | + | + | + | + | + |
| 6 | + | + | + | + | − | + | − |
| 7 | + | + | + | + | − | + | − |
| 10 | + | + | − | − | + | + | + |
| 12 | + | + | − | − | + | + | + |
| 13 | + | + | + | + | + | + | + |
| 15 | + | + | − | − | + | + | + |
| 16 | + | + | − | − | − | − | − |
| 17 | + | + | − | − | + | + | − |
| 18 | + | + | + | + | − | − | − |
| 19 | + | + | + | + | − | − | − |
| 20 | + | + | − | − | + | + | − |
| Total | (13/13) | (13/13) | (7/13) | (7/13) | (8/13) | (10/13) | (6/13) |
Hf= Helicobacter felis; Hh= Helicobacter heilmanni; RUT=rapid urease test.
Table 3.
Evaluation of Helicobacter infection status by different tests following 14-day quadruple therapy.
| Following 14-day quadruple therapy | |||||||
|---|---|---|---|---|---|---|---|
| Cat | 16S rRNA | Hf | Hh | Hh/Hf | Cytology | RUT | WSS |
| 3 | − | − | − | − | − | − | − |
| 5 | − | − | − | − | − | − | − |
| 6 | − | − | − | − | − | − | − |
| 7 | − | − | − | − | − | − | − |
| 10 | + | + | − | − | − | − | − |
| 12 | − | − | − | − | − | − | − |
| 13 | + | + | + | + | − | − | − |
| 15 | + | + | − | − | − | − | − |
| 16 | − | − | − | − | − | − | − |
| 17 | − | − | − | − | − | − | − |
| 18 | − | − | − | − | − | − | − |
| 19 | + | + | − | − | − | − | − |
| 20 | − | − | − | − | − | − | − |
| Total | (4/13) | (4/13) | (1/13) | (1/13) | (0/13) | (0/13) | (0/13) |
Histopathology
Initially, WSS stain revealed only large spiral organisms, morphologically resembling H felis or H heilmannii but not H pylori, in six cats (Fig 1). Gastric inflammation was absent from eight cats, three cats had grade 1 inflammation in one or more sections, and two cats had grade 2 inflammation in at least one section (Figs 2 and 3). There were no significant differences in the severity of gastric inflammation among the three gastric regions (P=0.67). There was no correlation between the presence of histological lesions and the presence or intensity of colonisation with Helicobacter species for each of the three gastric lesions (P=0.63).
Fig 1.

Arrows indicate Helicobacter species with a spiral shape and dark brown colour. WSS.
Fig 2.
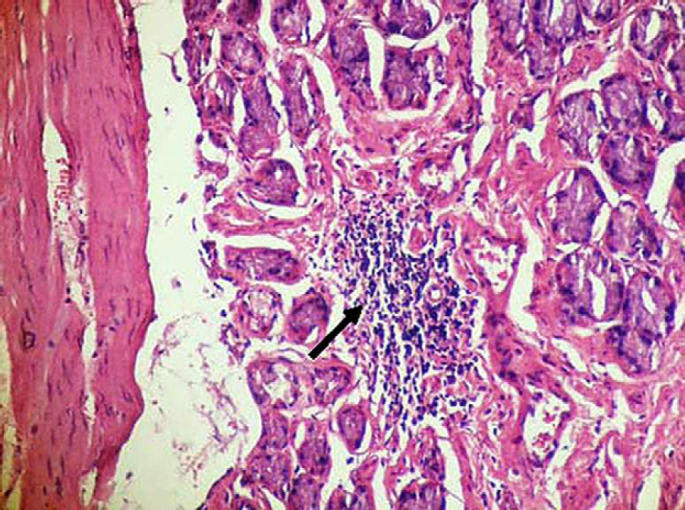
Antral lymphoid follicle with germinal center (arrow) in a cat with naturally acquired Helicobacter species infection. H&E stain ×200.
Fig 3.

Lymphocytic–plasmacytic gastritis in the gastric mucosa of a cat with naturally acquired Helicobacter species infection. H&E stain. ×200.
Following quadruple therapy, none of the cats was found to be infected with Helicobacter species by WSS staining. However, in two Helicobacter species-positive cats, grade 1 inflammation was observed.
PCR assays
Prior to commencing the study, using 16S rRNA PCR assay and Helicobacter species-specific primers, natural H felis and H heilmannii infection were detected in 13 and seven cats (out of 13 study cats), respectively. Mixed infection was detected in six cats (Table 2).
None of the cats was found to be naturally infected with H pylori.
Following 14-day anti-Helicobacter species quadruple therapy, PCR identified the presence of Helicobacter species in four cats considered negative by all other criteria (Table 3). H felis and H heilmannii infection were detected in 4/4 cats and 1/4 cats, respectively. Mixed infection was detected only in one cat. Therefore, Helicobacter species eradication therapy was not completely successful.
Assessment of adverse effects of therapy
During the 14-day quadruple therapy, all cats received all medications. There were no gastrointestinal, dermatological and neurological side effects during the quadruple therapy course and during the 4-week follow-up after the end of the treatment.
Discussion
In the present study, gastric Helicobacter species were detected in 65% (13/20 cats) which is the same as two previous studies in our area by Akhtardanesh et al 5 (56.7%) and Shojaee Tabrizi et al 21 (67.5%). Based on results of the present study and the studies by Neiger et al 18 and Akhtardanesh et al 22 there was no correlation between presence and degree of Helicobacter species colonisation and the occurrence of non-specific gastritis and its severity in cats. Controversially, other authors have shown an association between Helicobacter species infections and mild to moderate gastritis, especially in the feline gastric body. 23,24
In the present study, the four-drug regimen for 14 days failed to eliminate Helicobacter species infection in 4/13 study cats. This was revealed on the second molecular analysis of gastric biopsies. In another study, a combination of metronidazole, amoxicillin and famotidine for 7 days, reportedly led to complete eradication in 74% of Helicobacter species-infected cats. 15 Recent studies suggest that the success rate of triple regimens (PPI plus two antibiotics) can be improved if the duration is extended to 14 days or if an additional antibiotic is given. 10 In human medicine, 14-day triple therapy has an approximately 12% better cure rate than does 7-day therapy; therefore, shorter durations can no longer be recommended. 10
Our results suggest that antibiotic regimens that are effective against H pylori in people cannot eradicate Helicobacter species in cats with naturally acquired infection. This finding may indicate the resistance of these organisms to such intensive anti-Helicobacter species treatment or the possibility of recrudescence following treatment. It is unclear in the previous studies if antibiotic failure was due to reinfection or recrudescence, although the persistence of Helicobacter species by PCR suggests recrudescence is likely. These findings contrast markedly with studies in H pylori infected people where 80% cure rates with 1%/year reinfection are observed. 2 One approach is to use quadruple therapy but to substitute a new drug for the metronidazole. Probably the most effective approach is to substitute furazolidone (eg, 100 mg q 8 h). 25 Another approach is to substitute a new drug for the clarithromycin/metronidazole in legacy triple therapy (eg, furazolidone, rifabutin, a fluoroquinolone). 11 Vilaichone et al 10 prefer a sequential therapy using high-dose PPI and amoxicillin as the base. The outcome is predictably best if one chooses agents to which the organism is susceptible and where available, susceptibility testing is recommended. Van den Bulck et al 26 investigated the susceptibilities of Helicobacter species isolated from the gastric mucosa of different cats and dogs to 10 antimicrobial agents by determination of the minimal inhibitory concentration (MIC) using the agar dilution method. No consistent differences were noticed between the different Helicobacter species, which were all highly susceptible to ampicillin, clarithromycin, tetracycline, tylosin, enrofloxacin, gentamicin, and neomycin, as demonstrated by low MICs. 26
In the present study, histopathological assessment of four PCR-positive cats following eradication therapy, revealed no evidence of Helicobacter species infection in the gastric mucosa. It could be speculated that although these four cats did not have any living Helicobacter species after the therapy but fragments of Helicobacter species DNA left in the four treated cats that were PCR positive but undetectable by histopathological examination.
Our observations revealed that the ease with which owners would be able to administer the medications is very important for a successful drug programme. It seems that most owners might not have problems giving the anti-Helicobacter species regimen in this study.
Conclusions
Our results suggest that antibiotic regimens that are effective against H pylori in people may only cause transient suppression, rather than eradication of gastric Helicobacter species in many cats.
Further controlled trials of antibiotic therapy in infected cats, both with and without clinical signs of gastritis and Helicobacter species infection, are clearly required before guidelines regarding the treatment of gastric Helicobacter species in cats can be made.
Acknowledgements
The authors wish to thank the members of the Helicobacter pylori research group at the Biotechnology Research Center of Pasteur Institute of Iran for their kind assistance.
References
- 1.Simpson KW. Helicobacter in dogs and cats – what's new? In: Svoboda M, ed. Proceedings of 31st World Congress World Small Animal Veterinary Association (WSAVA), Czech Republic, 2006: 411–5. [Google Scholar]
- 2.Simpson K.W. Diseases of the stomach. Ettinger S.J., Feldman E.C. Textbook of veterinary internal medicine diseases of the dog and cat, 5th edn, 2005, Saunders: Philadelphia, 1322–1323. [Google Scholar]
- 3.Scanziani E., Simpson K.W., Monestiroli S., Soldati S., Strauss-yali D., Del Piero F. Histological and immunohistochemical detection of different Helicobacter species in the gastric mucosa of cats, J Vet Diagn Invest 13, 2001, 3–12. [DOI] [PubMed] [Google Scholar]
- 4.Lecoindre P., Cherallier M., Peyrol S., Boude M., Ferrero R.L., Labigna A. Gastric helicobacters in cats, J Feline Med Surg 2, 2000, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhtardanesh B., Mohammadi M., Jamshidi S., Sasani F., Bokaie S. Clinical significance of Helicobacter infection in gastric mucosa of cats, Online J Vet Res 10, 2006, 177–201. [Google Scholar]
- 6.Erginsoy S.D., Sozmen M. Gastric Helicobacter – like organisms in stray cats, Acta Vet Brno 75, 2006, 91–98. [Google Scholar]
- 7.Takemura L.S., Camargo P.L., Alfieri A.A., Bracarense A.P. Helicobacter spp in cats: association between infecting species and epithelial proliferation within the gastric lamina propria, J Comp Pathol 141, 2009, 127–134. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P., Sipponen P., Naumann M., et al. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique, Am J Gastroenterol 100, 2005, 2100–2115. [DOI] [PubMed] [Google Scholar]
- 9.Romano M., Cuom A. Eradication of Helicobacter pylori: a clinical update, Med Gen Med 6, 2004, 19. [PMC free article] [PubMed] [Google Scholar]
- 10.Vilaichone R.K., Mahachai V., Graham D.Y. Helicobacter pylori diagnosis and management, Gastroenterol Clin North Am 35, 2006, 229–247. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama Y., Graham D.Y. Helicobacter pylori infection: diagnosis and treatment, Expert Rev Anti Infect Ther 2, 2004, 599–610. [DOI] [PubMed] [Google Scholar]
- 12.Fischbach L.A., van Zanten S., Dickason J. Meta analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies, Aliment Pharmacol Ther 20, 2004, 1071–1082. [DOI] [PubMed] [Google Scholar]
- 13.Ford A., Moayyedi P. How can the current strategies for Helicobacter pylori eradication therapy be improved?, Can J Gastroenterol 17 (suppl B), 2003, 36B–40. [DOI] [PubMed] [Google Scholar]
- 14.Vakil N., Lanza F., Schwartz H., Barth J. Seven-day therapy for Helicobacter pylori in the United States, Aliment Pharmacol Ther 20, 2004, 99–107. [DOI] [PubMed] [Google Scholar]
- 15.DeNovo RC, Magne ML. Current concepts in the management of Helicobacter-associated gastritis. In: Proceedings 13th Annual ACVIM Veterinary Forum, Lake Buena Vista, FL, 1995: 57–61.
- 16.Camargo P.L., Alfieri A.A., Bracarense A.P., Menoli R., Spinosa S.R., Hagiwara M.K. Use of polymerase chain reaction and enzymatic cleavage in the identification of Helicobacter spp in gastric mucosa of human beings from North Paraná, Brazil, Mem Inst Oswaldo Cruz 98, 2003, 265–268. [DOI] [PubMed] [Google Scholar]
- 17.Germani C., Duval D.P., Huerre M., et al. Strategy for the detection of Helicobacter species by amplification of 16S rRNA genes and identification of H felis in a human gastric biopsy, Res Microbiol 148, 1997, 315–326. [DOI] [PubMed] [Google Scholar]
- 18.Neiger R., Dieterich C., Burnens A., et al. Detection and prevalence of Helicobacter infection in pet cats, J Clin Microbiol 36, 1998, 634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton C.L., Kleanthous H., Coates P.J., Morgan D.D., Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction, J Clin Microbiol 30, 1992, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins S.E., Yan L.L., Shen Z., Hayward A., Murphy J.C., Fox J.G. Use of PCR and culture to detect Helicobacter pylori in naturally infected cats following triple antimicrobial therapy, Antimicrob Agents Chemother 40 (341), 1996, 1486–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabrizi A. Shojaee, Jamshidi Sh, Oghalaeia A., Salehi T. Zahraei, Eshkaftakie A. Bayati, Mohammadi M. Identification of Helicobacter spp in oral secretions vs. gastric mucosa of stray cats, Vet Microbiol 140, 2010, 142–146. [DOI] [PubMed] [Google Scholar]
- 22.Akhtardanesh B., Jamshidi S., Sasani F., Mohammadi M., Bokaie S., Salehi T. Zahraie. Helicobacter spp infection and gastric lesions in domestic and stray cats, Veterinarski Arhiv 76, 2006, 479–488. [Google Scholar]
- 23.Happonen I., Saari S., Castren L., Tyni O., Henninen M., Westermarch E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats, J Vet Med A 43, 1996, 305–315. [DOI] [PubMed] [Google Scholar]
- 24.Otto G., Hazell S.L., Fox J.G., et al. Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms, J Clin Microbiol 32, 1994, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham D.Y., Osato M.S., Hoffman J., Opekun A.R., Anderson S.Y., El-Zimaity H.M. Furazolidone combination therapies for Helicobacter pylori infection in the United States, Aliment Pharmacol Ther 14, 2000, 211–215. [DOI] [PubMed] [Google Scholar]
- 26.Van den Bulck K., Decostere A., Gruntar I., et al. In vitro antimicrobial susceptibility testing of Helicobacter felis, H bizzozeronii, and H salomonis, Antimicrob Agents Chemother 49, 2005, 2997–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]


