Abstract
In this study we examined seven queens with normal oestrous cycles and a history of infertility after normal matings. We performed clinical examination, vaginal cytology, evaluation of oestradiol, progesterone and total T4 levels, vaginal bacterial culture, ultrasonography, and serum analyses for detection of antibodies against chlamydia and feline leukaemia virus (FeLV) antigenemia. Ovariohysterectomy (OHE) was recommended for 1/7 queens because of pathological uterine changes detected at ultrasonography and clinical examination. Four out of seven queens were treated with antibiotics and two of these had more litters. One of the queens that were treated was not mated again and one was mated without conceiving and was at a later OHE found to have degenerative uterine changes. No treatment was given and no diagnosis could be established in 2/7 queens. Both of them were later ovariohysterectomised and one showed degenerative uterine changes while the uterus of the other queen could not be obtained for follow-up. In summary, 4/7 cats were diagnosed with uterine pathology and no definitive diagnosis could be established for 3/7 queens.
Several aspects of feline health have to be taken into consideration for successful breeding. Proper nutrition and management, including control of infectious diseases, are essential (Lawler and Bebiak 1986). Individual queens that fail to deliver kittens despite normal ovarian activity, reflected as normal oestrous behaviour and a normal mating behaviour, present a diagnostic challenge for the clinician. An infectious or endocrinological pathology is often suggested in these cases.
Many infectious agents have been described to have a potential negative effect on fertility. Feline leukaemia virus (FeLV) and the feline immunodeficiency virus (FIV) are known causative agents of pregnancy failure (Cotter et al 1975, Goldsmith 1975, Weaver et al 2005). Chlamydophila felis has been isolated from the vagina of infected queens and has been suggested as a cause of infertility although a causal relation has never been confirmed (Wills et al 1984, Sykes et al 1999a,b).
The incidence of low-grade uterine bacterial infections in queens is not known, as these are not associated with obvious clinical signs. Vaginal bacterial samples cannot be used to diagnose uterine infections because bacteria that are found in the uterus of queens suffering from uterine infections are typically the same species that constitute the normal flora of the vagina (Clemetson and Ward 1990, Lawler et al 1991, Ström Holst et al 2003). Bacteria are usually not present in the uterus but have been found in the uteri of a small proportion of apparently healthy queens during oestrus (Clemetson and Ward 1990). During oestrus the cervix is open and may, therefore, allow bacteria to enter from the vagina (Chatdarong et al 2002). In humans, abnormal vaginal bacterial flora has been suggested to cause infertility (Tan et al 1987, Spandorfer et al 2001), but no such cases have been described in cats.
Several endocrinological disturbances have a potentially negative effect on reproduction. Thyroid disorders are known to have an impact on fertility in humans (Trokoudes et al 2006), but their relevance to feline reproductive problems is unknown. Cats are generally induced ovulators, and anovulatory cycles are a potential cause of infertility. Ovulation depends on both the timing of mating and the number of matings during oestrus. Allowing cats to mate several times during mid-oestrus will usually result in ovulation while matings too early in oestrus may not result in ovulation. Even during mid-oestrus a single mating may not be sufficient to induce enough luteinising hormone (LH) release for ovulation to occur (Concannon et al 1980, Banks and Stabenfeldt 1982, Glover et al 1985, Donoghue et al 1993). Naturally, infertility in the male cat may also be a cause of unsuccessful matings (Axnér et al 1996). Cystic endometrial hyperplasia (CEH) is not unusual in queens and the incidence increases with age (Dow 1962, Perez et al 1999). Frequent periods of oestrus, spontaneous ovulation and exogenous administration of progestagens such as medroxyprogesterone acetate (MPA) probably predispose to CEH (Perez et al 1999, Chatdarong et al 2005). Ultrasound can be used to diagnose CEH but there is no clear definition of ultrasound findings that distinguish between pathological lesions and normal cyclic changes in the endometrium.
The aim of this study was to evaluate queens with a history of infertility despite normal ovarian activity and normal mating behaviour resulting in ovulation. The clinical and diagnostic imaging findings together with results of investigations regarding infectious and endocrinological status in seven cases of suspected infertility in queens are reported.
Materials and methods
Cats – inclusion criteria
Seven queens with a history of infertility were examined at the Swedish University of Agricultural Sciences (SLU). The inclusion criteria were that they should be >18 months old and should have been mated at least three times with previously proven fertile males without giving birth. The last mating should have occurred during the previous 12 months. Post-coital reactions, signs of pseudopregnancy and/or prolonged interoestrous interval should have been observed to avoid studying queens that had not been mated. All owners reported that the queens had been kept with the male for several days during oestrus. The exact number of days varied between queens and cycles but was at least 3 days for all queens from day 1 to day 3 of oestrus. Some of the queens had been treated with MPA (standard dose is 5 mg/cat PO once a week).
A detailed reproductive and medical history of each queen was recorded. The case history for each cat included data on clinical signs, including signs not attributed to the reproductive organs, and, in addition, vaccinations and previous diseases, medications, feeding, the social status of the examined cat and the health status of the other cats in the cattery. A general clinical examination was performed for each cat. Vaginal swabs were taken for vaginal cytology and bacterial culture. The bacteriological samples were transported in Amies medium with charcoal (Venturi Transystem, Copan, Brescia, Italy) and cultured on the same day, with the exception of samples for two cats (cat 6, cultured after 72 h, and cat 7, cultured within 24 h). Vaginal swabs were also collected for detection of chlamydial DNA using polymerase chain reaction (PCR). Swabs were stored at −20°C until analysis. Blood samples were collected by venepuncture. Serum was stored at −20°C until analysis.
Vaginal cytology
Cytological smears were obtained by introducing a cotton swab into the caudal vagina, and stained with Hemacolor (Merck, Darmstadt, Germany) and investigated to determine stage of the oestrous cycle. A smear with a proportion of superficial cells of ≥80% was considered an oestrous smear (Chatdarong et al 2002). Vaginal smears were also examined for presence of neutrophil leukocytes or other signs of infection.
Bacterial culture procedures
The vaginal specimens were streaked on agar plates, incubated at 37°C and examined after 24 and 48 h. One 5% horse blood agar plate and one lactose bromcresol purple agar plate were incubated aerobically. One blood agar plate with a streak of Staphylococcus aureus, to provide growth-enhancing factors for fastidious bacteria, was incubated in 5% CO2 atmosphere and one fastidious anaerobe agar (LabM, Bury, United Kingdom) with 5% defibrinated horse blood was incubated anaerobically. All agar plates were manufactured at the National Veterinary Institute (SVA), Uppsala, Sweden.
Bacterial growth was classified as sparse (n<20), moderate (n=20–100) or profuse (n>100), according to the number of colonies growing on the plate. When only single colonies of different bacterial species were found, growth was described as mixed culture. Otherwise, each of the isolated organisms was classified into genus or species, according to colony morphology, Gram-staining and biochemical characteristics using standard methods (Quinn et al 2000). Lancefield group-G streptococci were assumed to be Streptococcus canis without further evaluation.
Detection of chlamydiae
A real-time PCR developed by Everett et al (1999) and using an internal control to monitor inhibition in the sample material (Bölske et al 2006) was used. This PCR detects all chlamydiae belonging to the family Chlamydiaceae.
Serum analysis
For analysis of FeLV antigenemia, an enzyme-linked immunosorbent assay (ELISA) against FeLV p27 protein was used (ViraCHEK/FeLV; Synbiotics Corporation, San Diego, CA, USA). Antibodies against Chlamydophila species were analysed using an in-house ELISA, with antigen extracted from elementary bodies of Chlamydophila psittaci (Ström Holst et al 2006a). Titres ≥1:200 were considered positive. Serum progesterone levels were measured by a solid-phase radioimmunoassay (Coat-A-Count Progesterone; Diagnostic Products Corporation, Los Angeles, CA, USA) evaluated for feline serum and used according to the manufacturer's recommendations. Serum concentration of oestradiol-17β was determined by a radioimmunoassay (Double Antibody Estradiol; Diagnostic Products Corporation, Los Angeles, CA, USA), used according to the manufacturer's recommendations, with modification according to Mwanza et al (2000), and validated for feline serum. Serum concentration of total T4 was measured using a chemiluminescence method (canine Total T4 Immulite 2000; Diagnostic Products Corporation, Los Angeles, CA, USA) validated for feline serum.
Ultrasound
Two-dimensional ultrasound of the uterus and ovaries was performed on all cats with a 5–8MHz-phased array linear transducer (8L5) using an Acuson/Siemens Sequoia 512. The maximum diameter of the uterus, appearance of the wall (even and uneven echogenicity), presence of peristalsis, presence of cysts in the wall and presence of fluid in the lumen were recorded. It was also recorded whether the entire uterus or only parts of it could be seen. When the ovaries could be visualised, the size and presence of follicles/corpora lutea or cysts seen as rounded, anechoic areas were noted. With four of the cats in this study, a fertile cat of the same breed was used as a control during the ultrasound evaluation. All ultrasound examinations were performed by the same sonographer.
Case reports
Results of the examinations are summarised in Tables 1 and 2. None of the serum samples from the queens were positive for FeLV, and chlamydial DNA was not detected in any of the vaginal samples. There was no growth of anaerobic bacteria in any of the cultures. Levels of total T4 were within the normal range for all cats. Unless stated otherwise, no abnormal clinical findings were observed.
Table 1.
Summary of findings in the cases and controls included in the study
| Queen number | Breed | Age (months) | Cycle stage/MPA | Ultrasound | Vaginal discharge | T4 (nmol/l) | Prog (nmol/l) | E2 (pmol/l) | Preliminary diagnosis | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uterine diameter (mm) | Appearance of the wall and size of lumen | ||||||||||
| 1 | Maine Coon | 92 | Not in oestrus | 8 | Uneven, no cysts, 1 mm fluid | Purulent | 19 | 1.0 | ne | Endometritis/pyometra | Recommended OHE |
| 1C | Maine Coon control | 59 | MPA | 4 | Uneven, no cysts, no fluid | ne | ne | ne | ne | – | Seven kittens 14 months later |
| 2 I | Siamese | 22 | MPA | 5 | Even, no cysts, 1 mm fluid | No | 32 | <0.6 | 2.0 | Not established | – |
| 2 II | Siamese | 24 | In oestrus | 5 | Even, no cysts, no fluid | No | ne | ne | ne | Not established | Three litters |
| 2C | Siamese control | 32 | MPA | 4.5 | Uneven, 0.5 mm anechoic areas (cysts?), no fluid | ne | ne | ne | ne | – | OHE 7 weeks later |
| 3 | Persian | 20 | End of oestrus | 4 | Even, no cysts, small amount of fluid | Small amount of clear fluid | 26 | 1.2 | 29 | Not established | OHE, CEH |
| 3C | Persian control | 16 | In oestrus | 5 | Even, no cysts, no fluid | ne | ne | ne | ne | Not established | Not used for breeding |
| 4 | Siamese | 48 | Dioestrus | 7 | Uneven, no cysts, 1 mm fluid in corpus | Small amount dried black | 26 | 20.6 | ne | Endometritis | Mated again without conception, OHE, CEH |
| 5 | Ragdoll | 29 | Not in oestrus | 4 | Even, no cysts, no fluid | Small amount red-brown | 35 | <0.6 | 10 | Mild endometritis | Not mated again, OHE |
| 5C | Ragdoll control | 52 | Not in oestrus | 3 | Even, no cysts, no fluid | ne | ne | ne | ne | – | Died from kidney failure 9 months later |
| 6 | Abyssinian | 34 | MPA | 4 | Even, no cysts, no fluid | No | 32 | 0.7 | 26 | Not established | Four litters (and two matings without result) |
| 7 | Burmese | 20 | Dioestrus | 2.3–3 | – | No | 25 | 37.5 | ne | Not established | Not mated again, OHE |
MPA=medroxyprogesterone acetate-treated; tT4=total T4, reference range 7–35 nmol/l; Prog=progesterone; E2=oestradiol; ne=not evaluated; OHE = ovariohysterectomy; CEH = cystic endometrial hyperplasia.
Table 2.
Vaginal bacterial flora, titres against Chlamydophila vaginal cytology, and antibiotic treatment
| Queen number | Vaginal bacterial flora | Antibody titres against Chlamydophila psittaci | Vaginal cytology | Antibiotic treatment |
|---|---|---|---|---|
| 1 | Pasteurella multocida, Haemophilus species | 1:200 | Not in oestrus, few leukocytes | NT |
| 2 I | E coli, β-haemolytic Streptococcus species | ND | Not in oestrus, occasional leukocytes | NT |
| 2 II | Pasteurella species in mixed flora with >5 species | not determined | In oestrus | Amoxycillin with clavulanic acid |
| 3 | Haemolytic E coli | ND | Not in oestrus | NT |
| 4 | β-Haemolytic Streptococcus species, coagulase-negative Staphylococcus species | 1:200 | Not in oestrus | Amoxycillin |
| 5 | Pasteurella multocida in mixed flora | ND | Not in oestrus | Amoxycillin with clavulanic acid |
| 6 | Haemolytic E coli, β-haemolytic Streptococcus species | 1:800 | Not in oestrus | Amoxycillin |
| 7 | Micrococcus species | ND | Not in oestrus | NT |
ND=not detected; NT=not treated.
During the ultrasound examinations, both ovaries could be seen in eight out of the 11 examined cats (ie, the seven cats included in the study, and four controls). One ovary could be identified in two cats (cats 5 and 6) and no ovary was identified in one cat (cat 2). No abnormalities were detected in the examined ovaries. The uterus could be identified with ultrasound in all cats and the entire uterus could be seen in nine of the cats. In two of the cats only parts of the uterus could be seen (cat 5 and its control).
Case 1
Case 1 was a Maine Coon, 92 months old. The cat had had two normal litters in Belgium before being imported to Sweden where she had two further litters. The last litter was born 3.5 years before the queen was examined for the present study. This female was mated during three oestrous periods with a fertile male without producing kittens. The interval between the last two oestrous periods was approximately 70 days. The owner reported that the cat had had an intermittent vaginal discharge since the last pseudopregnancy. She had been treated with progestins previously but not during the previous year.
The clinical examination revealed a small amount of purulent vaginal discharge. There were also a few leukocytes. Vaginal bacteriology revealed sparse growth of Pasteurella multocida and Haemophilus species. Vaginal cytology showed a mixture of cell types indicative of interoestrus. An enlarged uterus was found on abdominal palpation. On ultrasound the uterus measured 8 mm. The echogenicity of the wall was uneven and the lumen was 1 mm wide, containing anechoic fluid. In addition, this cat had a cyst in one mammary gland.
A diagnosis of endometritis/pyometra with possible CEH was made. Because of this cat's age and clinical findings, ovariohysterectomy (OHE) was recommended. The reproductive organs were not obtained for follow-up.
Case 2
Case 2, a Siamese, 22 months old, had been mated at 8 months of age. At week 5–6 of the pregnancy vaginal bleeding and straining had been observed. According to the owner, the cat had been treated with amoxycillin (Vetrimoxin; CEVA Vetpharma) but when the bleeding had not stopped enrofloxacin (Baytril; Bayer) had been prescribed. The vaginal discharge had, however, become purulent and a bacterial culture had shown growth of β-haemolytic streptococci. Treatment with amoxicillin combined with clavulanic acid (Synolux; Orion, Animal Health) had been successful and the queen had delivered three healthy kittens after a subsequent mating. After this she had been mated during three oestrous periods with a fertile male, without producing kittens. The interoestrous interval was, according to the owner, prolonged after the matings (1–1.5 months) compared with oestrous cycles when no mating occurred (2 days to 1 week). The cat had been treated with oral MPA for 3 weeks.
There was no vaginal discharge. Vaginal bacteriology showed sparse growth of haemolytic Escherichia coli in almost pure culture and one single colony of β-haemolytic streptococci. Vaginal cytology showed no sign of oestrus, and very few neutrophil leukocytes. The uterus was of normal size, as evaluated by ultrasound, but the lumen contained anechoic fluid (0.5–1 mm).
After the first examination, treatment with MPA was withdrawn. Two months later, the queen was evaluated again. This time she was in oestrus, according to behavioural signs and vaginal cytology. The uterus was of the same size as at the previous investigation, but no fluid was detected at the ultrasound examination and the ovaries had small anechoic structures. A hysterographic evaluation confirmed the normality of the endometrial lining. No endocrinological samples were taken. Vaginal bacteriology showed moderate to profuse growth of a mixed flora with Pasteurella species.
A diagnosis was not established.
After the second evaluation at the SLU this queen was treated with amoxicillin combined with clavulanic acid (Synolux; Orion, Animal Health) for 3 weeks starting during oestrus, and subsequently delivered two kittens. After this she had two more litters with five kittens each.
Case 3
Case 3, a Persian, 20 months old, had been mated with three different proven fertile males during four oestrous periods without conceiving. According to the owner, post-coital reactions and bite wounds in the neck had been observed. The interoestrous intervals had, however, been approximately 25, 44, 22 and 60 days, indicating that the cat had not ovulated after two of the matings. The oestrous periods usually lasted around 2 weeks and this female had been mated at each oestrus. She had been put with a male on days 2–3 of oestrus and stayed with this male for 4–8 days. The cats in the cattery had all been treated with azithromycin (Azitromax) 15 months prior to the examination because chlamydia had been diagnosed in a kitten. The queen had not been treated with progestins.
At the time of examination, the queen was reaching the end of behavioural oestrus and had sparse, clear vaginal discharge. Vaginal cytology showed a mixed pattern typical of post-oestrus (mixed with large intermediate cells and superficial cells). Vaginal bacteriology showed profuse growth of haemolytic E coli, almost in pure culture. The uterus was of normal size, according to the ultrasound, with peristalsis of the uterine wall and a small amount of fluid in the lumen. The right ovary contained an anechoic area 2 mm in diameter, most likely a degenerating follicle, which is a normal finding towards the end of oestrus.
A diagnosis was not established.
This female was ovariohysterectomised at another clinic less than 1 month after the visit. The uterus was subjected to histopathological examination at the National Veterinary Institute. The endometrium was hyperplastic, with microcysts, polyps and fibrosis. There was sporadic infiltration of lymphocytes between the endometrial glands.
Case 4
Case 4, a Siamese, 48 months, had been mated during six oestrous periods with three different fertile males but had failed to produce kittens. She had an interoestrous interval of at least 7 weeks whether or not she was mated. Oestrous signs usually lasted for 5 days. Apart from the infertility this queen also had a food allergy presenting as pruritus, which is why she had to be on special low-allergenic food. In addition, she had been treated with an MPA (Depo-Medrol) injection 8 days before the second mating (and 2.5 months before the third mating). The last oestrus, during which this queen was not mated, was 51 days before the examination. She had not been treated with progestins.
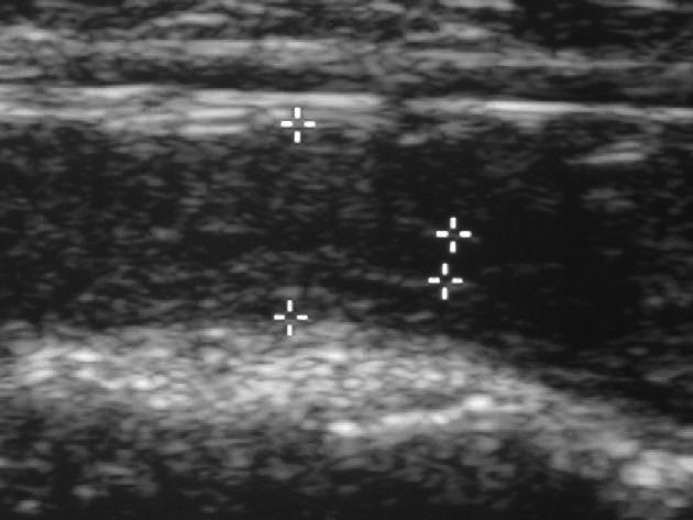
Clinical examination revealed a small amount of dry discharge in the fur around the vulva. Vaginal cytology showed no cornified cells, indicating that the queen was not in oestrus. Progesterone levels indicated that she was in dioestrus (20.6 nmol/l). Vaginal bacteriology showed sparse growth of β-haemolytic streptococci and coagulase-negative staphylococci. The wall of the uterus was uneven in echogenicity and the uterine diameter measured 7 mm at the ultrasound examination (Fig. 1). There was anechoic fluid in the lumen within the body of the uterus (1 mm).
Fig 1.
Cat 4. Uterus measuring 7 mm in diameter with uneven echogenicity of the wall and anechoic fluid in the lumen (between the pointers). This cat had a tentative diagnosis of endometritis based on the vaginal discharge. After OHE 29 months later the uterus was found to have undergone chronic degenerative changes.
A tentative differential diagnosis of endometritis/pyometra, mucometra/hydrometra, CEH was indicated.
Because of the vaginal discharge and the history of infertility, and based on bacterial culture, this queen was treated with amoxicillin (Vetrimoxin; CEVA, Vetpharma) for 4 weeks from the first day of oestrus. She was mated on two more occasions after the visit without conceiving and was ovariohysterectomised at another clinic 29 months after the examination. The uterus was fixed in formalin and sent to us. The gross uterine diameter was enlarged, varying from 9 to 12 mm. Histological examination revealed that the ovaries had corpora lutea and there was focal cystic dilation of the uterine glands (Fig. 2) and focal subepithelial and intraglandular infiltration of lymphocytes and plasma cells, indicative of chronic degenerative changes of CEH.
Fig 2.
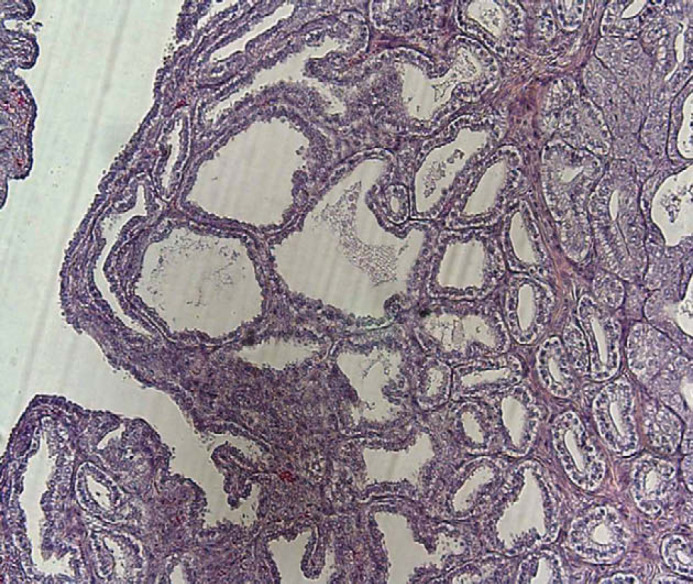
Cat 4, dilated uterine glands.
Case 5
Case 5, a Ragdoll, 29 months, had been mated with one fertile male during three oestrous periods. The owner had observed post-coital reactions after matings. Oestrus usually lasted for 10–12 days, with weak signs. According to the owner, the interoestrous intervals were 1–2 months when the queen was not mated and were prolonged after mating, although the exact length was uncertain. This cat had never been treated with progestins.
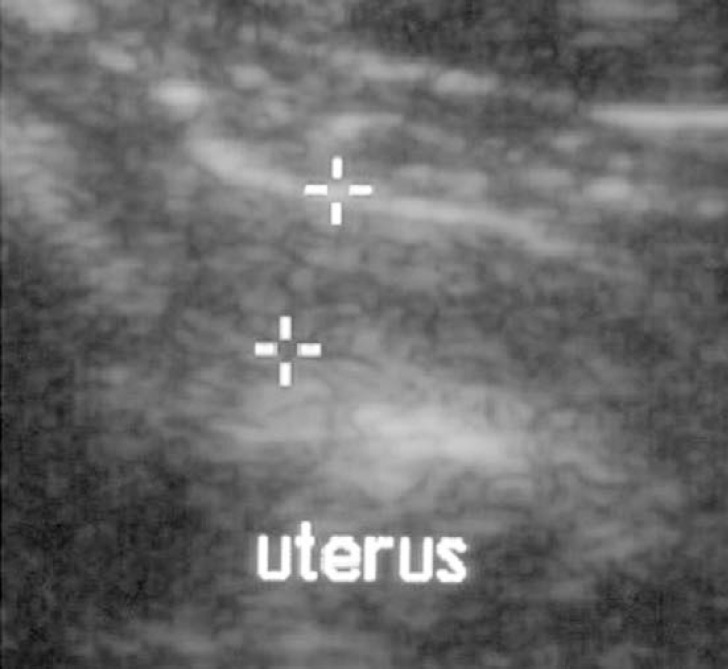
The clinical examination showed a small amount of brown-red vaginal discharge and the cotton swab for vaginal cytology was also coloured brown-red. Vaginal cytology showed a pattern typical of a cat not in oestrus (Fig. 3). Vaginal bacteriology showed a sparse growth of P multocida in a sparse mixed culture. The uterus had a normal appearance on ultrasound.
Fig 3.
Cat 5. Uterus measuring 4 mm in diameter with even echogenicity of the wall. This cat had a tentative diagnosis of mild endometritis based on the vaginal discharge but was ovariohysterectomised without being mated again. The uterus was lost to follow-up, which is why no definitive diagnosis could be made.
A tentative diagnosis of mild endometritis was made based on the vaginal discharge.
Amoxicillin with clavulanic acid (Synolux: Orion, Animal Health) was prescribed, according to the antimicrobial susceptibility testing of P multocida. This queen was, however, not used again for breeding and was ovariohysterectomised at another clinic. The reproductive organs were, unfortunately, not obtained for follow-up.
Case 6
Case 6, an Abyssinian, 34 months old, had been mated four times with two fertile males. Three weeks after the first mating she had had vaginal bleeding and been treated with antibiotics. Three matings after this had not resulted in kittens. Oestrous signs were usually weak but she accepted the male for 1 week. Interoestrous intervals after matings were 4–5 weeks. This cat had previously had skin problems that disappeared with a change in diet. The queen had been treated with MPA for 6 weeks before the examination. She had also been treated with oral MPA before the first mating.
Vaginal cytology showed a normal pattern of a cat not in oestrus. Vaginal bacteriology revealed a moderate growth of haemolytic E coli and a sparse growth of β-haemolytic streptococci. The clinical examination revealed no pathological changes. The uterus appeared normal on ultrasound.
After examination at the SLU this queen was prescribed amoxicillin (Vetrimoxin; CEVA, Vetpharma) for 4 weeks according to antimicrobial susceptibility of the E coli and β-haemolytic streptococci strains. The decision to put this female on antibiotics was based on the history of infertility after a previous resorption. One kitten was born 82 days after the visit to the SLU. After this she was mated five more times and had three more litters.
Case 7
Case 7, a Burmese, 20 months old, had been mated in Spain at 11 months of age and again before export to Sweden, so that she was expected to be pregnant when she came to Sweden at 16 months of age. She did, however, not deliver any kittens. The queen was mated on two subsequent occasions in Sweden with two different fertile males but only became pseudopregnant, with prolonged interoestrous intervals, increase in body weight and pinkening of the teats. Oestrus usually lasted for 1 week and was very intense. When this queen arrived in Sweden she had otodectes and otitis media, which were treated but recurred after treatment. This queen had been mated 30 days before the visit. She had not been treated with progestins.
The clinical examination did not reveal any pathological changes. Teats were slightly pink. Vaginal cytology was normal for a cat not in oestrus, and progesterone levels were elevated (37.5 nmol/l), indicating that she had ovulated and was in dioestrus. Vaginal bacteriology showed very sparse growth of Micrococcus species in pure culture. The uterus seemed normal on ultrasound, with no signs of pregnancy or resorption.
No treatment was given. This female was never mated again and was ovariohysterectomised at another clinic. According to the owner, the surgeon had considered the uterus to appear normal.
Discussion
The results of the present study suggest that uterine pathologies are associated with infertility in cycling cats. In four of the seven cats with suspected infertility a uterine pathology was diagnosed: two cats had CEH (cats 3 and 4), one had pyometra/endometritis (cat 1) and one had a suspected mild endometritis (cat 5). In addition, two cats that were infertile after a previous probable resorption regained fertility after treatment with antibiotics (cats 2 and 6). In these two cases, a bacterial infection may have been the reason for previous pregnancy failure. The organs were not available for pathological examination, however, which is why a definitive diagnosis cannot be made. As both these queens had a history of resorption, it is possible that they either had a low-grade persistent uterine infection or that they had increased susceptibility to uterine infection.
A definitive diagnosis of low-grade endometritis can only be made on biopsies, unless the queen is hysterectomised. This is, however, an invasive procedure. The bacterial flora in the vagina may differ from bacteria in the uterus in a cat with endometritis. The vaginal bacterial flora of the cats in the present study did not differ from that described in clinically healthy cats (Clemetson and Ward 1990, Ström Holst et al 2003). Two cats had a profuse bacterial growth. One of these was in oestrus and the other had just been in oestrus. An increase in the relative numbers of bacteria during oestrus has previously been described (Clemetson and Ward 1990), as have changes in the vaginal flora during the oestrous cycle, as noted for cat 2 (Ström Holst et al 2003). It should also be noted that pure vaginal culture of haemolytic E coli, as was found in cat 3, is a normal finding (Ström Holst et al 2003).
An inflammatory response may indicate that there is an infection. Although there were leukocytes in the vaginal cytological sample of cat 1 they were too few to be a clear indication of infection. A low number of neutrophil leukocytes was also noted during cytological examination from the vaginal sample of cat 2. The histological evaluation of the uterus from cats 3 and 4 revealed some intrauterine inflammatory cells indicative of chronic endometritis. The absence of acute changes explains why no inflammatory cells were detected at vaginal cytological examination of these cats.
Clinical findings in cat 3 did not differ from those in healthy cats. In accordance with the prudent use of antibiotics policy in Sweden, this queen was not treated as the history, clinical and ultrasound findings did not suggest that she suffered from endometritis. Cat 4 was treated with amoxicillin but did not conceive again. It is unlikely that treatment with antibiotics would have cured the uterine pathological changes that were found in queens 3 and 4.
The uterine diameter of cat 4 was 7 mm. This is not different from the diameter seen in healthy queens during dioestrus (Chatdarong et al 2005). At OHE 29 months later, the gross uterine diameter was, however, clearly enlarged compared with healthy queens and histopathological evaluation revealed cystic dilation of the uterine glands. This queen had long interoestrous intervals whether or not she was mated and an elevated progesterone level at the examination, indicating that she experienced repeated spontaneous ovulations. The repeated periods of influence of progesterone can predispose to pathological growth of the endometrium (Lawler et al 1991, Perez et al 1999, Ström Holst et al 2002, Chatdarong et al 2005). In addition, this queen had been treated with a corticosteroid, which is not recommended for a queen used for breeding as it has been shown in other species to cause a decrease in serum progesterone and induction of abortion (Zone et al 1995).
To inhibit development of bacterial strains resistant to antibiotics, antibiotics should only be prescribed after ruling out that the absence of pregnancy is due to no mating having taken place, and preferably only when history and/or clinical signs give a well-founded reason to suspect infection. Because of the difficulty to diagnose low-grade endometritis, queens may in selected cases be treated with antibiotics without a definitive diagnosis. If there is pus in the uterine lumen the antibiotic treatment should be combined with prostaglandin F2α (PGF2α) and/or aglepristone (Davidson et al 1992, Hecker et al 2000). There is currently no efficient treatment for CEH.
Four of the females had a small amount of vaginal discharge. Vaginal discharge is not considered normal in a healthy queen except for a small amount of clear fluid during oestrus, as was seen in one queen at the end of behavioural oestrus. A vaginal discharge was also observed in cats 1, 4 and 5. In these cats it is likely that the discharge was an indication of a uterine abnormality.
As far as possible, we tried to avoid studying queens that failed to conceive because of poor breeding management by including only cats that had shown evidence of mating (post-coital behaviour) and/or a prolonged interoestrous interval after mating, indicating that ovulation had occurred. Breeding management seemed to be appropriate for all queens as they were allowed to be with a male for at least 3 days during oestrus. Nevertheless queen 3 had obviously not ovulated in two of the cycles in which mating occurred. In two cycles the interoestrous interval was, however, prolonged, suggesting that the degenerative uterine changes found after OHE were the most likely cause for this queen's infertility. In six of the cats the clinical findings or the regained fertility after treatment with antibiotics indicated that a uterine pathological condition was the likely cause of infertility although for cat 5, the diagnosis was somewhat uncertain. Cat 7 did not have any abnormal findings and was not bred again but the uterus was, unfortunately, not obtained for histological evaluation. Poor breeding management does, however, not seem likely for this cat as the owner reported that she had had obvious signs of pseudopregnancy after mating and her progesterone value was above basal values, 30 days after the last mating.
Chlamydial DNA was not present in vaginal swabs from any of the queens. Antibodies to Chlamydophila species were detected in three of the seven queens, but titres were low or moderate. In a previous study, antibodies to chlamydia were seen in 11% of Swedish cats, with a tendency for a higher prevalence among purebred cats (Ström Holst et al 2006b). Although this organism has been suggested as a reproductive tract pathogen (Wills et al 1984), there was no link between Chlamydophila felis and reproductive problems in other studies (Sykes et al 1999a, 1999b), and the results of the present study do not indicate any role for chlamydia in the present infertility problems.
FeLV has been considered a common cause of infertility in queens (Goldsmith 1975, Cotter et al 1975) but is a rare cause nowadays due to routine testing of breeding cats. All queens in the present study tested negative for FeLV. All cats also had normal levels of total T4, and reproductive disorders caused by thyroid disease are considered uncommon in cats (Johnson 2002).
Uneven echogenicity of the uterine wall was seen in four cats, all of which were either being treated with MPA or which had a later diagnosis of CEH. Therefore, it is possible that uneven echogenicity may be seen as a reflection of a physiological endometrial response to progesterone. However, two cats which were being treated with MPA and one cat with CEH had an even echogenicity of the wall, indicating that an even echogenicity of the uterine wall does not exclude hyperplasia of the endometrium. Three of the cats (cats 1, 2 and 4) had fluid in the uterine lumen at ultrasound examination. One of them had a purulent vaginal discharge and an enlarged and uneven uterus, indicating a uterine infection. Cat 4 was in dioestrus and cat 2 under MPA treatment. It cannot be concluded from our results whether uterine fluid may be a normal finding related to a hormonal influence on the uterus or whether it is always an indication of uterine pathology.
Considering the cost–benefit value of different evaluations, a thorough clinical evaluation, ultrasound of the uterus and ovaries and, if infection is suspected, a vaginal bacterial culture and cytology sample are likely to be the most rewarding routine evaluations of infertile queens. In addition, serology against FeLV and, depending on husbandry conditions, also FIV is recommended.
In conclusion, our findings suggest that degenerative uterine changes and low-grade uterine infections are causes of infertility in normally cycling queens. It can also be concluded that it is often difficult to make a definitive diagnosis of the cause of infertility with routine evaluations.
Acknowledgements
The authors would like to thank Professor Anne-Marie Dalin for help with histological evaluation of case 4. We also wish to thank the cat owners for their co-operation.
References
- Axnér E., Ström B., Linde-Forsberg C., Gustavsson I., Lindblad K., Wallgren M. Reproductive disorders in 10 domestic male cats, Journal of Small Animal Practice 37, 1996, 394–401. [DOI] [PubMed] [Google Scholar]
- Banks D.H., Stabenfeldt G. Luteinizing hormone release in the cat in response to coitus on consecutive days of estrus, Biology of Reproduction 26, 1982, 603–611. [DOI] [PubMed] [Google Scholar]
- Bölske G, Ström Holst B., Stillesjö A, Englund S. (2006) Detection of Chlamydiaceae in Swedish cats with a real-time PCR with internal control. In: Proceedings of the 4th Annual Workshop of COST Action 855 Animal Chlamydioses and Zoonotic Implications, Edinburgh, UK. pp. 74–75.
- Chatdarong K., Kampa N., Axnér E., Forsberg C. Linde. Investigation of cervical patency and uterine appearance in domestic cats by fluoroscopy and scintigraphy, Reproduction in Domestic Animals 37, 2002, 275–281. [DOI] [PubMed] [Google Scholar]
- Chatdarong K., Rungsipipat A., Axnér E., Forsberg C. Linde. Hysterographic appearance and uterine histology at different stages of the reproductive cycle and after progestagen treatment in the domestic cat, Theriogenology 64, 2005, 12–29. [DOI] [PubMed] [Google Scholar]
- Clemetson L.L., Ward A.C.S. Bacterial flora of the vagina and uterus of healthy cats, Journal of the American Veterinary Medical Association 15, 1990, 902–906. [PubMed] [Google Scholar]
- Concannon P., Hodgson B., Lein D. Reflex LH release in estrous cats following single and multiple copulations, Biology of Reproduction 23, 1980, 111–117. [DOI] [PubMed] [Google Scholar]
- Cotter S.M., Hardy W.D., Essex M. Association of feline leukemia virus with lymphosarcoma and other disorders in the cat, Journal of the American Veterinary Medical Association 166, 1975, 449–454. [PubMed] [Google Scholar]
- Davidson A.P., Feldman E.C., Nelson R.W. Treatment of pyometra in cats, using prostaglandin F2 alpha: 21 cases (1982–1990), Journal of the American Veterinary Medical Association 200, 1992, 825–828. [PubMed] [Google Scholar]
- Donoghue A.M., Johnston L.A., Goodrowe K.L., O'Brien S.J., Wildt D.E. Influence of day of oestrus on egg viability and comparative efficiency of in vitro fertilization in domestic cats in natural and gonadotrophin-induced oestus, Journal of Reproduction and Fertility 68, 1993, 85–90. [DOI] [PubMed] [Google Scholar]
- Dow C. The cystic hyperplasia–pyometra complex in the cat, Veterinary Record 74, 1962, 141–146. [Google Scholar]
- Everett K.D.E., Hornung L.J., Andersen A.A. Rapid detection of the Chlamydiaceae and other families in the order Chlamydiales: three PCR tests, Journal of Clinical Microbiology 37, 1999, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover T.E., Watson P.F., Bonney R.C. Observations on variability in LH release and fertility during oestrus in the domestic cat (Felis catus), Journal of Reproduction and Fertility 75, 1985, 145–152. [DOI] [PubMed] [Google Scholar]
- Goldsmith F.G. Habitual abortion and FeLV, Feline Practice 5, 1975, 4. [Google Scholar]
- Hecker B.R., Wehrend A., Bostedt H. Treatment of pyometra in cats with the progesterone-antagonist aglepristone, Kleintierpraxis 45, 2000, 845–848. [Google Scholar]
- Johnson C.A. Thyroid issues in reproduction, Clinical Techniques in Small Animal Practice 17, 2002, 129–132. [DOI] [PubMed] [Google Scholar]
- Lawler D.F., Bebiak D.M. Nutrition and management of reproduction in the cat, Veterinary Clinics of North America. Small Animal Practice 16, 1986, 495–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler D.F., Evans R.H., Reimers T.J., Colby E.D., Monti K.L. Histopathologic features, environmental factors, and serum estrogen, progesterone, and prolactin values associate with ovarian phase and inflammatory uterine disease in cats, American Journal of Veterinary Research 52, 1991, 1747–1753. [PubMed] [Google Scholar]
- Mwanza A.M., Madej A., Kindahl H., Lundeheim N., Einarsson S. Plasma levels of cortisol. Progesterone, oestradiol-17β and prostaglandin F2α metabolite after ACTH (Synacthen® depot) administration in ovariectomised gilts, Journal of Veterinary Medicine. A, Physiology, Pathology, Clinical Medicine 47, 2000, 193–200. [DOI] [PubMed] [Google Scholar]
- Perez J.F., Conley A.J., Dieter J.A., Sanz-Ortega J., Lasley B.L. Studies on the origin of ovarian interstitial tissue and the incidence of endometrial hyperplasia in domestic and feral cats, General and Comparative Endocrinology 116, 1999, 10–20. [DOI] [PubMed] [Google Scholar]
- Quinn P.J., Carter M.E., Markey B.K., Carter G.R. Clinical Veterinary Microbiology, 2000, Mosby International: London, pp. 1–366 [Google Scholar]
- Spandorfer S.D., Neuer A., Giraldo P.C., Rosenwaks Z., Witkin S.S. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF, Journal of Reproductive Medicine 46, 2001, 806–810. [PubMed] [Google Scholar]
- Ström Holst B., Karlstam E, Bergström A, Båverud V, Englund L, Lagerstedt A-S. (2002) Uterine pathology in routinely ovariohysterectomised cats. In: 3rd EVSSAR Congress on Reproduction in Companion, Exotic and Laboratory Animals, Liège, Belgium. pp. 178–179 (Abstract).
- Holst B. Ström, Bergström A., Lagerstedt A.S., Karlstam E., Englund L., Båverud V. Characterization of the bacterial population of the genital tract of adult cats, American Journal of Veterinary Research 64, 2003, 963–968. [DOI] [PubMed] [Google Scholar]
- Holst B. Ström, Englund L., Palacios S., Renström L., Berndtsson L.T. Prevalence of antibodies against feline coronavirus and Chlamydophila in Swedish cats, Journal of Feline Medicine and Surgery 8, 2006b, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ström Holst B., Gruffydd-Jones T, Bölske G. (2006a) Prevalence of antibodies to Chlamydophila felis in Swedish cats assayed with three different tests. In: Proceedings of the 4th Annual Workshop of COST Action 855 Animal chlamydiosis and zoonotic implications, Edinburgh, UK. pp. 76–77.
- Sykes J.E., Anderson G.A., Studdert V.P., Browning G.F. Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease, Journal of Veterinary Internal Medicine 13, 1999a, 153–162. [DOI] [PubMed] [Google Scholar]
- Sykes J.E., Studdert V.P., Browning G.F. Comparison of the polymerase chain reaction and culture for the detection of feline Chlamydia psittaci in untreated and doxycycline-treated experimentally infected cats, Journal of Veterinary Internal Medicine 13, 1999b, 146–152. [DOI] [PubMed] [Google Scholar]
- Tan S.L., Scammell G., Houang E. The midcycle microbial flora as studied by the weighed-swab method, and its possible correlation with results of sperm cervical mucus penetration tests, Fertility and Sterility 47, 1987, 941–946. [DOI] [PubMed] [Google Scholar]
- Trokoudes K.M., Skordis N., Picolos M.K. Infertility and thyroid disorders, Current Opinion in Obstetrics and Gynecology 18, 2006, 446–451. [DOI] [PubMed] [Google Scholar]
- Weaver C.C., Burgess S.C., Nelson P.D., Wilkinson M., Ryan P.L., Nail K.A., Kelly-Quagliana K.A., May M.L., Reeves R.K., Boyle C.R., Coats K.S. Placental immunopathology and pregnancy failure in the FIV-infected cat, Placenta 26, 2005, 138–147. [DOI] [PubMed] [Google Scholar]
- Wills J., Gruffydd-Jones T.J., Richmond S., Paul I.D. Isolation of Chlamydia psittaci from cases of conjunctivitis in a colony of cats, Veterinary Record 114, 1984, 344–346. [DOI] [PubMed] [Google Scholar]
- Zone M., Wanke M., Rebuelto M., Loza M., Mestre J., Duchene A., Concannon P. Termination of pregnancy in dogs by oral administration of dexamethasone, Theriogenology 43, 1995, 487–494. [DOI] [PubMed] [Google Scholar]