Abstract
Mammalian cells contain thousands of metalloproteins and evolved systems to correctly incorporate metal cofactors into their designated sites. Among the transient metals in living cells, iron is the most abundant element that present as an iron sulfur cluster, mono- and dinuclear iron centers or heme for catalytic reactions. Iron homeostasis is tightly regulated by intestinal iron absorption in mammals owing to the lack of an iron excretive transport system, apart from superficial epithelial cell detachment and urinary outflow reabsorptive impairment. In mammals, the central site for iron absorption is in the duodenum, where the divalent metal transporter 1 is essential for iron uptake. The most notable manifestation of mutated divalent metal transporter 1 presents as iron deficiency anemia in humans. In contrast, the mutation of ferroportin, which exports iron, causes iron overload by either gain or loss of function. Furthermore, hepcidin secretion from the liver suppresses iron efflux by internalizing and degrading ferroportin; thus, the hepcidin/ferroportin axis is extensively investigated for its potential as a therapeutic target to treat iron overload. This review focuses on the divalent metal transporter 1-mediated intestinal iron uptake and hepcidin/ferroportin axis that regulate systemic iron homeostasis.
Keywords: iron, divalent metal transporter 1 (DMT1), ferroportin, hepcidin, oxidative stress
Introduction
In mammalian cells, >30% of proteins require a transition metal cofactor that binds to the protein at the active site; thus, a lack of micronutrients (including transition metals) causes clinical symptoms.(1) Among the micronutrients, iron, zinc, and vitamin A are essential in preschool-aged children, while iron, zinc, and folate are required by women of reproductive age due to a high prevalence of deficiency.(2) A worldwide survey revealed the wide spectrum of the micronutrient deficiency or overload, especially iron across different countries, warranting periodical reassessments. In Japan, the recommended iron daily intake is 10 mg for men aged >20 years and menopausal women and 15 mg for menstruating women.(3) On an average, 3–5 g of iron is stored in the adult body, while approximately 25 mg iron/day is released from the reticuloendothelial system. Subsequently, iron is incorporated into the erythron and 1–2 mg iron/day is absorbed to equilibrate the lost iron by detached epithelial cells in the digestive tract and skin, minor blood loss, sweat, and urine.(4) However, the excretory mechanism of active iron transport is lacked; thus, iron balance is tightly regulated by intestinal absorption. These results indicate iron recycling is considered a “semi-closed system”.
Iron catalyzes DNA synthesis, metabolic energy production, organic compound biosynthesis, oxygen transport, and reactive oxygen species (ROS) generation, making it an essential transition metal in nearly all organisms.(5) In cells, >95% of iron is protein-bound, either directly by protein residues or iron-containing groups, such as heme or iron sulfur clusters. In cytosolic and organellar components, various states and forms of iron are present with millimolar concentrations of organophosphates, carboxylates, amides, thiolates, and hydroxylates.(6) Iron has two common oxidation states: ferrous (Fe2+) and ferric (Fe3+). Furthermore, more high-valent iron-oxo species, such as ferryl (Fe4+; Fe4+O2+) and ferrates (Fe5+; Fe5+O43−, Fe6+; Fe6+O42−), are also present, and these oxidize compounds rapidly, becoming the most stable ferric ions.(7–9) Ferryl intermediates are transient versatile complexes involved in substrate catalysis via peroxidases, catalases, and the cytochrome P450 family(7) or molecule deterioration via heme ferryl species or the Fenton reaction.(10,11) Furthermore, iron overload triggers cellular injury, exacerbates atherosclerosis, and dysregulates organ function, ultimately causing fetal impairment or carcinogenesis in rodents and humans. Therefore, appropriate iron management is essential to avoid oxidative damage and iron-mediated lipid oxidation-dependent cell death, namely, ferroptosis.(7,12–16)
In mammals, the intestine was identified as an entry site for iron uptake in the 1930s.(17) Enterocytes have a relatively short lifespan of <4 days in humans,(5) and once detached (spontaneously or cytotoxically), all intracellular contents are lost into the gut lumen; thus, orchestrated transcytosis from the apical side to the bloodstream via enterocytes is critical for iron uptake. Dietary iron is mostly present in the ferric state, which is reduced to ferrous ions by duodenal cytochrome b reductase (Dcytb)(18) or dietary reductants, such as ascorbate or microbe-produced short-chain fatty acids,(5) and subsequently absorbed from the duodenum by divalent metal transporter 1 (DMT1). Herein, the mechanisms of intestinal iron absorption, which tightly regulates iron homeostasis, are discussed to clarify the role of iron in the body.
DMT1
Hypochromic microcytic anemia is a common symptom of iron deficiency in the body. Iron deficiency anemia (IDA), caused by the loss of iron due to gastrointestinal or genital bleeding or malnutrition, is treated with oral or intravenous iron replenishment. In contrast, iron refractory iron deficiency anemia (IRIDA) is rarely observed. Mammalian iron influx transporter has been identified using two methods. In 1997, a divalent cation transporter (DCT1) was identified by forced expression of intestinal cDNA in Xenopus laevis.(19) In the same year, a positional cloning approach identified the natural resistance-associated macrophage protein 2 (Nramp2), which was originally cloned by cross-hybridization to the Nramp1 gene,(20–22) as a causative gene to develop autosomal recessive hypochromic microcytic anemia in mk mouse.(23) The following year, a point mutation (G185R) in Nramp2 was identified as the causative mutation for impaired duodenal iron uptake in Belgrade (b) rats, which also developed inherited autosomal recessive hypochromic microcytic anemia.(24) Surprisingly, G185R mutation in Nramp2 was commonly detected in mk mice.
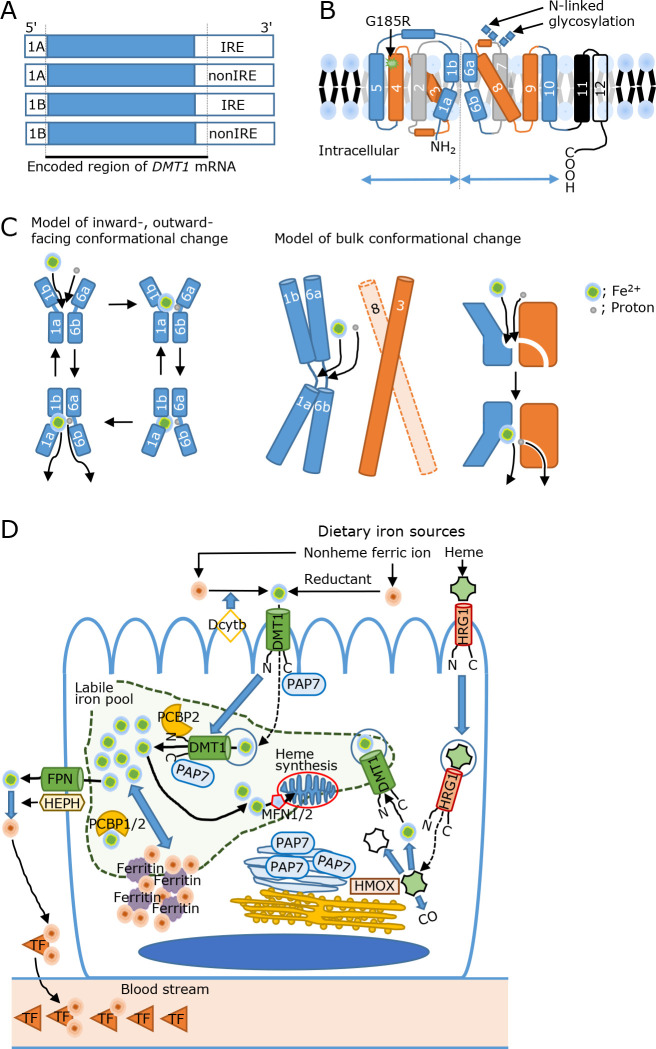
In the 3' untranslated regions (UTR) of Nramp2 mRNA, two isoforms, that contain an iron responsive element (IRE) or do not contain the IRE (nonIRE), were identified.(19,20) The Dmt1-IRE isoform is predominantly expressed in the duodenal mucosa,(25) and the expression of duodenal iron regulatory protein 1 (Irp1), Irp2, and Dmt1-IRE mRNA and protein axis was more activated in anemic homozygous (b/b) rats than in phenotypically normal heterozygous (+/b) rats, suggesting G185R mutated Dmt1-IRE was induced to compensate iron uptake.(26) Furthermore, two variants in 5' DMT1 mRNA were identified, which revealed four DMT1 mRNA and protein isoforms to date (Fig. 1A).(27)
Fig. 1.
Divalent metal transporter 1 (DMT1) structure and DMT1-mediated iron transport mechanisms. (A) Four transcriptional variants, which have unique amino acid in each N- or C-terminal, are spliced. (B) The mammalian DMT1 has 12 transmembrane (TM) domains, indicated by number, and both of N- and C-termini are located in intracellular side. Two N-glycosylation sites are located in the extracellular loop between TM 7 and 8. The secondary structure of DMT1 comprises two pseudosymmetric inverted repeats, which are separated by intertwining domains to TMs 1–5 and 6–10 in the tertiary structure. (C) The proposed mechanism of DMT1-mediated iron transport via conformational change. The two hinges between TM 1a and 1b, as well as 6a and 6b, contains an iron-binding site, while the hinge between 6a and 6b contains a proton (H+)-binding site. The left side model indicates iron transport by the movement of the inward-outward-facing conformation.(28) In contrast, the right side model indicates bulk conformational change to the inward-open state.(31) (D) Intestinal iron absorption. Dietary nonheme ferric ion is reduced by Dcytb to a divalent state. The ferrous ion is then uptaken by endocytosis with a DMT1-containing vesicle or transported by membrane-bound DMT1 at the brush border membrane. DMT1 travels with guiding proteins, such as N-terminus interacting PCBP2 or C-terminus interacting PAP7, which is usually located at the Golgi apparatus. The ferrous iron is designated to supply cytoplasmic iron storage, which is termed the labile iron pool (LIP). At the LIP, iron is shuttled into mitochondria for heme synthesis by MFN1/2 or to ferritin for intracytoplasmic iron homeostasis by PCBP1/2. From basolateral membrane, ferrous ion is exported by FPN and subsequently oxidized to ferric ion by HEPH. The ferric ion is bound onto TF and delivered to iron-deficient organs via the blood stream. In contrast, heme is absorbed and transported by HRG1. Heme is degraded by heme oxygenase on the endoplasmic reticulum (ER) and released as ferrous ion. The internalization of DMT1 after heme feeding for 2 h suggests that DMT1 mediates orchestrated iron transport. Dcytb, duodenal cytochrome b reductase; DMT1, divalent metal transporter 1; FPN, ferroportin; HEPH hephastin, HMOX; heme oxygenase; HRG1, heme responsive gene 1; MFN1/2, mitoferrin 1/2; PAP7, peripheral-type benzodiazepine receptor-associated protein 7; PCBP2; poly r(C)-binding protein 2; TF, transferrin.
The mammalian DMT1 structure has 12 transmembrane (TM) domains with two N-glycosylation sites in the extracellular loop between TM 7 and 8 and intracytoplasmic N- and C-termini (Fig. 1B).(19) Among the TM domains, TMs 1–5 and 6–10 comprise two pseudosymmetric inverted repeats that intertwine in the tertiary structure, whereas TM12 is lacking in most prokaryotes.(28) An iron-binding site is located at two hinges between TM 1a and 1b, as well as 6a and 6b, which are also occupied by Mn2+, Co2+, Ni2+, Cd2+, and Pb2+, whereas the hinge between 6a and 6b contains a proton (H+)-binding site. Among the amino acid methionine substitution with alanine at the iron-binding site in TM6 suppresses Mn2+, Co2+, and Fe2+ transport, while Mg2+ and Ca2+ transport is accelerated, indicative of metal discrimination by the binding site.(29) Furthermore, DMT1, which harbors the G185R mutation in TM4, induces selective Ca2+ permeability, suggesting a gain of function phenotype in mk and b rodents.(30) As conformational changes are not detected in the presence or absence of divalent metals, two mechanisms for DMT1-mediated iron transport have been proposed. One model describes iron transport via the movement of the inward- outward-facing conformation.(28) Another model indicates a bulk conformational change to an inwardly open state (Fig. 1C).(31) However, the active transport mechanism of proton and metal symporters, including whether or how the substrates are thermodynamically coupled and released, remains unclear.(32) Nramp homologs are found throughout the tree of life, and the Nramp family is subdivided into several distinct evolutionary cascades. Two Nramp homologs have been identified in mice, rats, and humans. Nramp1 and Nramp2 share 78% sequence similarity with highly conserved primary sequence motifs and secondary structures.(21,25) Polymorphisms in NRAMP1, also known as SLC11A1 (solute carrier family 11 member 1), which is expressed in phagocytic cells to aid damage to engulfed microbes, are associated with different rates of bacterial infection, indicating the difference between NRAMP1 and NRAMP2 that specializes in innate immunity and metal acquisition.(32)
DMT1-Mediated Iron Absorption Molecular Mechanisms
Dietary iron starvation induces Dmt1-IRE at the brush border membrane (BBM) of the duodenal villi.(25) After bolus iron feeding, Dmt1-IRE rapidly migrates from the BBM to cytoplasmic vesicles in the duodenum in heterozygous (+/b) and homozygous (b/b) rats, indicating that the G185R mutation does not alter endocytosis signaling.(26) However, Dcytb-deficient mice exhibited no change in body iron stores and hematological parameters, suggesting that non-enzymatic reactions are functional in reducing dietary ferric iron.(33) Furthermore, systemic Dmt1 deletion caused IDA and ultimately death within 7 days, the lifespan of which could be extended by a red blood cell (RBC) transfusion.(34) The intestine-specific Dmt1 ablation induced IDA and decreased iron storage. However, these iron deficiencies were restored by an intraperitoneal iron injection.(35) In contrast, Dmt1-IRE systemic overexpression elevated duodenal iron absorption.(36)
In the intestine, apical BBM protein mislocation, which is partially maintained by intracellular sorting and trafficking of vesicular endocytosis, is associated with malnutrition, diarrheal disorders, inflammatory bowel diseases, and cancer development,(37) indicating that the orchestrated endocytosis machinery is indispensable for iron uptake. Polarized Caco-2 cells form a tight monolayer and enterocyte-like morphology with brush border enzymes or markers in a Transwell chamber. In these cells, BBM- or basolateral membrane (BLM)- originated endocytic vesicles are transported into specific subcellular compartments.(38,39) In this model, apo-transferin (TF) and ferri-TF undergo different subcellular processes, suggesting different endocytic cycles between apo- and ferri-TF.(40) Indeed, DMT1-IRE containing BBM-derived vesicles significantly co-localized with BLM-derived apo-TF following iron-ascorbate feeding into the apical chamber for 20 min, whereas no co-localization of BLM-derived ferri-TF was detected at 20 min, suggesting that the recycling endosome signal separates iron-containing vesicles.(41) Furthermore, iron supplementation into the apical chamber decreased DMT1 in the apical membrane fraction and, in turn, elevated DMT1 in the basolateral membrane fraction in polarized Caco-2 cells, indicating BBM- and BLM-derived vesicle fusion.(42) The interaction of the DMT1 N-terminal with poly r(C) binding protein 2 (PCBP2) (which also binds to iron)(43) and the C-terminal of DMT1-IRE with peripheral-type benzodiazepine receptor-associated protein 7 (PAP7) (which regulates cellular proliferation)(44) suggests that the DMT1-containing traveling vesicle supplies iron to the intracytoplasmic labile iron pool (LIP) using guiding proteins. In the LIP, the iron concentration was estimated to range between 1–7 μM, with the majority being Fe2+ rather than Fe3+, and forming a complex (1:1) with the cysteinyl residue of GSH. From this pool, a designated amount of iron is delivered to ferritin for storage by PCBP1 or 2.(1,45) When enterocytes sense iron requirements from the body, ferrous ions are exported by ferroportin (FPN, also called SLC40A1)(46) and are rapidly oxidized to ferric ions by hephastin (HEPH).(47) The spontaneous oxidation of ferrous to ferric ions in BLM may not be efficient as Heph was isolated from sex-linked anemia (sla) mice, which developed intestinal iron malabsorption and IDA at 6–7 weeks of age.(47) Indeed, systemic or intestine-specific knockout (KO) of Heph mice suppressed intestinal iron absorption, while Heph KO mice survived for up to 76–79 weeks and IDA was resolved at 10–12 weeks, similar to in sla mice, suggesting that Heph is important, but not essential, for optimal iron absorption.(48) Interactions between Fpn and Heph were transiently assembled by iron feeding, suggesting that a protein complex is required to enable iron export in rat enterocytes.(49,50) After export, ferric ions were loaded onto apo-TF and transported to iron-deficient locations (Fig. 1D). When Tf was ablated in mice, elevated systemic iron deposition with hypochromic microcytic anemia was observed,(51) which recapitulates congenital atransferrinemia.(52)
In addition to nonheme iron, which is usually associated with plants, heme, which is associated with animals, is also available as a dietary iron source. Heme is transported by heme-responsive gene 1 (HRG1, also known as SLC48A1) at the plasma membrane and phagolysosomes to maintain heme homeostasis.(53,54) However, the confirmation of heme importer remains debatable argument.(4,55) Hemin also elicited DMT1-IRE internalization in polarized Caco-2 cells after 2 h, suggesting absorbed hemin-initiated signal processing for DMT1 migration (Fig. 1D).(56) Notably, the extent to which heme is converted to nonheme iron within enterocytes or transferred in intact form to the plasma remains unknown.(57)
The “mucosal block” hypothesis, which opposes transcytosis, is established by diminished intestinal iron absorption from orally administered iron to the body, likely due to exfoliative cell death in enterocytes.(42,58,59) Dietary iron overload significantly induced fetal gastrointestinal erosive hemorrhage in Dmt1-IRE transgenic (Tg) mice.(36) In contrast, in intestine-specific Irp KO mice, the mucosal block was withheld by a large excess of ferritin, but not by epithelial detachment.(60) Whole body- and intestine-specific Heph KO mice developed iron deposition in enterocytes contrastingly, decreased iron storage in hepatocytes was indicative of mucosal block.(48) Furthermore, double KO of Heph and Ceruloplasmin, which oxidize ferrous ions to ferric ions, developed hypochromic microcytic anemia with iron deposition in the enterocytes, liver, heart, and pancreas and died within 20–30 weeks of age.(61) When Fpn, the sole nonheme iron exporter identified in mammals to date, was deleted, embryonal lethality was induced in mice. Conditional Fpn deletion leads to iron deposition in enterocytes, which is indicative of the mucosal block phenotype.(62) Notably, not all DMT1 in BBM undergoes endocytosis with a bolus feeding of iron, suggesting a DMT1-mediated non-endocytic iron influx.(58)
Mutated DMT1 Causes Hypochromic Microcytic Anemia
In humans, 10 published cases of SLC11A2 mutation have been reported, presenting onset hypochromic microcytic anemia at fetal stage (1 case), birth (6 cases), infancy (1 case), 3 months (1 case), and 13 years of age (1 case), indicating that congenital SLC11A2 mutation is extremely rare and has an early onset.(63) A single patient, diagnosed with hypochromic microcytic anemia during metrorrhagia treatment, carried compound heterozygosity of G212V and N491S in SLC11A2 and homozygosity of H63D in HFE, while no mutation was detected in SLC40A1 (ferroportin), HJV (homojuvelin), HAMP (hepatic antimicrobial peptide; also known as hepcidin), or TfR2 (transferrin receptor 2).(64) The hyperferritinemia detection in this patient suggested an overlap of hereditary hemochromatosis (HH), which is a disorder of the iron store regulators and is caused by HFE, SLC40A1, HAMP, HJV, or TfR2 mutations.(52,65) Notably, hepatic iron overload is not necessarily observed in SLC11A2-mutated patients.(66) The phenotype of the homozygous C282Y HFE mutation, which is responsible for >90% of HH cases in people of North European descent, varies markedly from liver cirrhosis to subclinical HH. Previously, three SLC11A2 polymorphisms, 1245T>C, IVS4+44C>A, and IVS15Ex16-16C>G, were found not to be associated with clinical symptoms in patients carrying the homozygous C282Y HFE.(67) An intronic SLC11A2 polymorphism IVS4+44C>A genotype, which has been associated with an increased risk of type 2 diabetes mellitus,(68) Wilson’s disease, age-related macular degeneration, and Parkinson’s disease(69) elevated the four-fold risk of IDA, despite the degree of atrophy in patients with celiac disease. These results suggest that patients with celiac disease, who have impaired duodenal mucosal uptake due to reduced absorptive surface as a result of chronic inflammation-induced villous atrophy, may unmask the SLC11A2 IVS4+44C>A polymorphism-caused IDA.(70) Conclusively, the residual function of mutated SLC11A2 as an iron transporter and presence or absence of other mutated iron-related genes may modulate hematological parameters and present clinical symptoms.
Furthermore, a genome-wide meta-analysis yielded novel variants of DUOX2 (dual oxidase 2), F5 (factor V), and TMPRSS6 (transmembrane serine protease 6), which are associated with IDA onset, in addition to SLC11A2.(71) DUOX2 variants increase infection susceptibility by dysregulating innate immunity, whereas F5 variants may cause blood loss by hypercoagulable state-induced thrombosis. TMPRSS6 variants, primarily expressed in the liver and identified as a causative gene for IRIDA due to elevated hepcidin levels,(72) were also associated with IDA.
Hepcidin-Ferroportin Axis
Ferroportin is evolutionarily conserved and is found in plants and humans, whereas hepcidin was first discovered in fish when the hepcidin-binding site in ferroportin was simultaneously detected, indicating that ferroportin and hepcidin co-evolved.(73) During the unhygienic conditions of our evolutionary history, humans were under constant threat from a range of potentially fatal microbes. A host-defense mechanism, which confers extracellular pathogens by depriving iron, was recognized as “hypoferremia of infection” in the 1940s, because nearly all microorganisms are dependent on iron.(17) Hypoferremia of infection is largely mediated by hepcidin, which was invented based on the fact that hepcidin is highly expressed in the liver (hep-) and possesses microbicidal activity (-cidin).(17) Because, hepcidin was initially shown to be a member of the defensin antimicrobial peptide family.(5) In Hamp-deficient mice, elevated iron was deposited in liver, pancreas, and heart with high transferrin saturation, and contrastingly, splenic iron deposition was decreased.(74) Despite the common phenotype of iron overload, the mRNA levels of Dmt1-IRE in the duodenum were elevated in Hfe-null mice,(75) but not in Hamp-deficient mice.(74) In Hamp Tg mice, severe IDA and neonatal death were observed, which are indicative of negative regulators of intestinal iron absorption.(76) Indeed, among the causative molecules of HH, TfR2 interacts with HFE, which, in turn, interacts with bone morphogenetic protein receptors and the HJV complex in the plasma membrane of hepatocytes to regulate hepcidin secretion, indicating the critical role for the hepcidin-ferroportin axis in intestinal hyperabsorption of dietary iron and iron overload with consequent tissue injuries.(52,77)
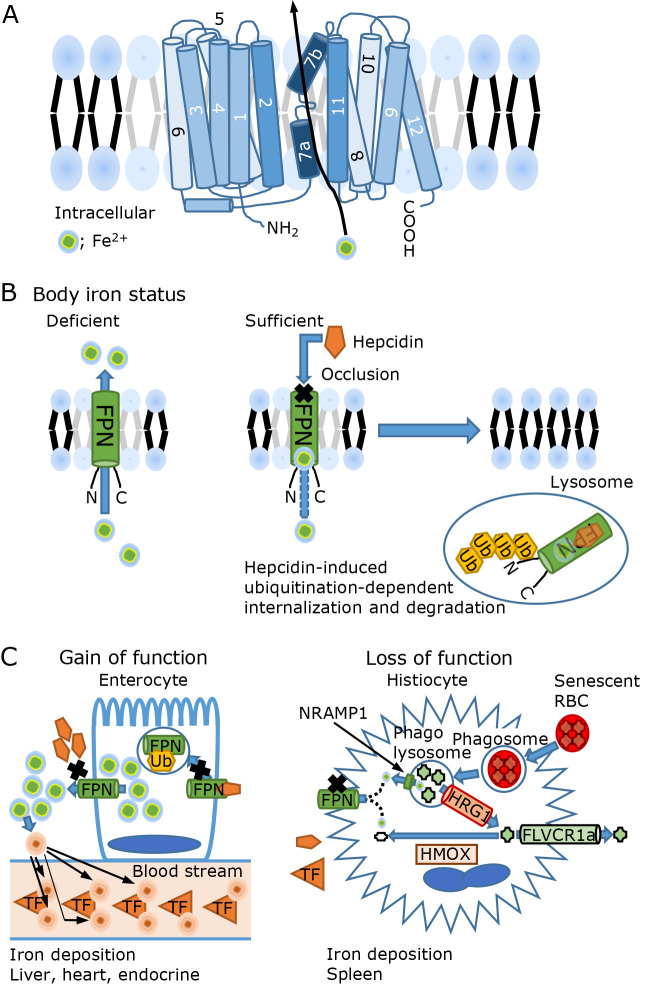
Iron efflux suppression by hepcidin is triggered by binding to ferroportin, whose topology firmly establishes that ferroportin is composed of 12 TM domains and intracellular locations of both the N- and C-termini (Fig. 2A), despite the initial controversial results.(78) After binding to ferroportin, hepcidin induces ferroportin occlusion, internalization, and degradation in many cell types (Fig. 2B),(79) including enterocytes, macrophages, erythrons, and hepatocytes, which are central regulators of iron homeostasis. Furthermore, K8R-mutant ferroportin, which inhibits N-terminal ubiquitination, and C326S-mutant ferroportin, which inhibits efflux occlusion, suppress hepcidin-induced internalization and degradation.(80) Furthermore, the binding affinity of hepcidin to ferroportin was elevated to a near 80-fold change in the presence of iron, indicating that hepcidin selectively confers iron-loaded ferroportin (Fig. 2B).(81)
Fig. 2.
Ferroportin structure, regulation, and mutation. (A) The mammalian ferroportin (FPN) has 12 transmembrane (TM) domains, indicated by number, and both of N- and C-termini are located on the intracellular side. Iron is exported from TM7a and 7b. (B) With sufficient iron, a hepcidin occludes FPN efflux and initiates N-terminus ubiquitination, subsequently, FPN is internalized and degraded. (C) The gain of function and loss of function in FPN causes hereditary hemochromatosis. The gain of function type is characterized by hyperferritinemia with high transferrin (TF) saturation and iron overload in the liver, heart, and endocrine organs. All clinical FPN mutants are functionally resistant to hepcidin, caused by impaired binding or ubiquitination that induces hepcidin overexpression. While the loss of function type is not clinically severe, with hyperferritinemia and iron overload in splenic histiocyte, which phagocytoses senescent red blood cells (RBCs) to recycle iron. The degraded RBC yields heme, which is then transported by the heme responsive gene 1 (HRG1). Some intracytoplasmic heme is degraded by heme oxygenase (HMOX) that yields nonheme iron, while heme is exported by feline leukemia virus subgroup C receptor 1a (FLVCR1a). In this type, the level of hepcidin ranges between low or normal.
Clinically detectable ferroportin (SLC40A1) mutations, which are inherited in an autosomal dominant manner, are heterogeneous and classified into two broad phenotypic categories with some overlap. A group with a gain of function ferroportin mutant is caused by partial or complete resistance to hepcidin-induced occlusion or internalization. Here, hyperferritinemia with high transferrin saturation and iron induced toxic damage to the liver, heart, and endocrine organs are often observed at young ages (Fig. 2C). Another group of loss of function ferroportin mutants is characterized by hyperferritinemia without high transferrin saturation or iron-induced tissue damage. Hyperferritinemia is caused by macrophages or histiocytes that phagocytose senescent RBCs to recycle heme and non-heme iron from heme-containing proteins. These cells regulate ferroportin synthesis transcriptionally and translationally, rapidly requiring iron export after erythropagocytosis and in response to infection.(57) They also develop a defense system against iron-induced ROS; thus, symptoms of organ damage are subclinical. Furthermore, systemic hetero Fpn KO (Fpn+/−), which recapitulates the inherited form in humans, demonstrated age-dependent erythropoiesis disruption and splenic iron overload. At 3 months of age, Fpn+/− mice were indistinguishable from their wild-type littermates, while at 6 months, Fpn+/− mice developed low hemoglobin levels and decreased erythrocyte volume without anemia or significant splenic iron deposition. Fpn+/− mice exhibited significantly decreased hemoglobin and elevated splenic iron deposition at 1 year of age.(62) In Dmt1-IRE Tg mice, elevated iron absorption from the duodenum without significant hepatosplenic iron deposition was observed at 3 months of age,(36) while Dmt1-IRE Tg mice >71 weeks of age exhibited increased hepatosplenic iron deposition.(82) Furthermore, nonheme iron and transferrin saturation in sera increased with aging in wild-type rats.(83) Taken together, aged rodents may have changed iron sensors to increase body iron storage, as also observed in humans.(16)
Inflammatory stimuli, such as lipopolysaccharides (LPSs), elevate DMT1 and hepcidin expressions, whereas systemic iron requirement by IDA, phlebotomy, and hypoxia suppresses hepcidin expression.(84) Furthermore, conditional KO of murine Hamp elevated mRNA and protein levels of Fpn, Dmt1, and Tfr1 via hypoxia inducible factor 2α (Hif2α) activation in duodenal mucosa, indicating that the liver controls intestinal iron uptake via the hepcidin/ferroportin/Hif2α axis.(85) In contrast, dextran sulfate sodium exposure to induce colitis did not elevate duodenal iron uptake, indicating that deletion of hepcidin and/or tissue iron deposition attenuated the physiological response to intestinal inflammation in Hamp-deleted rats.(83) However, microbiota and intestinal inflammation have been shown to regulate hepcidin expression and systemic iron metabolism.(5) Furthermore, another FPN mRNA transcript that lacks 5' IRE may modulate the ferroportin protein levels to regulate iron efflux in enterocytes and erythrons.(73) These results indicate that the degree of inflammation and its response may modulate cytokine-induced hepcidin secretion.
Hepcidin is suppressed by ineffective erythropoiesis, resulting in hepatic iron overload due to increased intestinal iron absorption. Urinary hepcidin levels in patients with DMT1 mutations are normal or moderately low, although hepatic iron is not deficient.(66) Recently, a hepcidin suppressor, erythroferrone, was identified which is induced by phlebotomy and/or erythropoietin treatment of erythroblasts. Indeed, erythroferrone antagonists are therapeutic targets that suppress intestinal iron absorption by increasing hepcidin in patients with hemoglobinopathies without regular transfusion and massive hepatosplenic iron overload.(77) Taken together, dyserythropoiesis-induced erythroferrone may contribute hepcidin suppression due to increased hepatic iron storage in patients with DMT1 mutation.
DMT1 is indispensable for intestinal iron absorption and recycling for tight iron homeostasis regulation. Recently, four amino acids, Asp, Gln, Glu, and Gly, were found to facilitate iron uptake in iron-deficient mice, indicating an improved oral iron supplementation formula to treat IDA.(86) Furthermore, the FePO4 nanoparticle bioavailability is not toxic to iron deficient women with anemia.(87) Indeed, oral intake of water-soluble iron alters the gut microbiota in patients with inflammatory backgrounds, such as obesity, inflammatory bowel disease, or colorectal cancer that may cause declined absorption.(12) To improve dietary iron supplementation, probiotics and prebiotics are explored to alter iron bioavailability, as impaired gut health-induced dysbiosis results in poor therapeutic effect.(5) Furthermore, microbiotal metabolites, such as 1,3-diaminopropane and reuterin, suppressed intestinal iron absorption via Hif2α suppression, but not Hif1α, indicating metabolic crosstalk between microbiota and the host enterocytes.(88)
Patients with β-thalassemia or sickle cell disease, who experience hemolysis and transfusion-dependent anemia, experience vasculotoxicity and atherosclerosis that may exacerbate ischemic change and cardiovascular disease, ultimately causing fatal heart failure.(13) Recently, a clinical trial on hepcidin-like peptide (LJPC-401) revealed that LJPC-401 significantly reduced the need for phlebotomy in patients with HH. Furthermore, another clinical trial on hepcidin agonists (PTG-300) for polycythemia vera effectively replaced phlebotomy for the hematocrit control.(89) N-acetylgalactosamine-modified antisense oligonucleotide, which targets TMPRSS6 mRNA (Sapablursen), has been analyzed in clinical trials in patients with non-transfusion-dependent β-thalassemia (NCT04059406) and is currently being assessed in an ongoing clinical study in patients with polycythemia vera (NCT05143957). Further studies on iron metabolism are required to develop novel therapeutic approaches.
Acknowledgments
This study was partially supported by the JSPS KAKENHI (grant numbers 15K08398 and 21K06968). YO was awarded the SFRR Japan Award of Scientific Excellent 2022. I would like to express my gratitude to the member of laboratory, collaborators and mentors, Emeritus Professor Shigeru Okada (Okayama University), Emeritus Professor Jonathan Glass (Louisiana State University in Shreveport) and Professor Shinya Toyokuni (Nagoya University) for their longstanding encouragements and supports. I would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- b
Belgrade
- BBM
brush border membrane
- BLM
basolateral membrane
- Dcytb
duodenal cytochrome b reductase
- DMT1
divalent metal transporter 1
- FPN
ferroportin
- HEPH
hephastin
- HIF2α
hypoxia inducible factor 2α
- HJV
hemojuvelin
- HRG1
heme responsive gene 1
- IDA
iron deficiency anemia
- IRE
iron responsive element
- IRIDA
iron refractory iron deficiency anemia
- IRP
iron responsive protein
- KO
knock out
- LIP
labile iron pool
- NRAMP2
natural resistance-associated macrophage protein 2
- PAP7
peripheral-type benzodiazepine receptor-associated protein 7
- PCBP2
poly r(C) binding protein 2
- RBC
red blood cell
- ROS
reactive oxygen species
- SLC11A2
solute carrier family 11 member 2
- TF
transferrin
- TfR2
Tranaferrin receptor 2
- Tg
transgenic
- TM
transmembrane
- TMPRSS6
transmembrane serine protease 6
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Philpott CC, Jadhav S. The ins and outs of iron: escorting iron through the mammalian cytosol. Free Radic Biol Med 2019; 133: 112–117. [DOI] [PubMed] [Google Scholar]
- 2.Stevens GA, Beal T, Mbuya MNN, Luo H, Neufeld LM; Global Micronutrient Deficiencies Research Group . Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health 2022; 10: e1590–e1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japanese BioIron Society. General Guidelines for the Appropriate Use of Iron Supplements in the Treatment of Anemia (3rd ed.). Kyobunsha, Sapporo, 2015. (in Japanese) [Google Scholar]
- 4.Knutson MD. Iron transport proteins: gateways of cellular and systemic iron homeostasis. J Biol Chem 2017; 292: 12735–12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa Gerós A, Simmons A, Drakesmith H, Aulicino A, Frost JN. The battle for iron in enteric infections. Immunology 2020; 161: 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol 2014; 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine (5th ed.). Oxford University Press, Croydon, 2015. [Google Scholar]
- 8.Kawabata T. Iron-induced oxidative stress in human diseases. Cells 2022; 11: 2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma VK. Ferrate(VI) and ferrate(V) oxidation of organic compounds: kinetics and mechanism. Coordin Chem Rev 2013; 257: 495–510. [Google Scholar]
- 10.Enami S, Sakamoto Y, Colussi AJ. Fenton chemistry at aqueous interfaces. Proc Natl Acad Sci U S A 2014; 111: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki Y, Ito N, Tanaka H, Hori M, Toyokuni S. Non-thermal plasma elicits ferrous chloride-catalyzed DMPO-OH. Free Radic Res 2022; 56: 595–606. [DOI] [PubMed] [Google Scholar]
- 12.Malesza IJ, Bartkowiak-Wieczorek J, Winkler-Galicki J, et al. The dark side of iron: the relationship between iron, inflammation and gut microbiota in selected diseases associated with iron deficiency anaemia—a narrative review. Nutrients 2022; 14: 3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinchi F. Non-transferrin-bound iron in the spotlight: novel mechanistic insights into the vasculotoxic and atherosclerotic effect of iron. Antioxid Redox Signal 2021; 35: 387–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okazaki Y. The role of ferric nitrilotriacetate in renal carcinogenesis and cell death: from animal models to clinical implications. Cancers (Basel) 2022; 14: 1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazaki Y. Asbestos-induced mesothelial injury and carcinogenesis: involvement of iron and reactive oxygen species. Pathol Int 2022; 72: 83–95. [DOI] [PubMed] [Google Scholar]
- 16.Toyokuni S, Kong Y, Zheng H, Maeda Y, Motooka Y, Akatsuka S. Iron as spirit of life to share under monopoly. J Clin Biochem Nutr 2022; 71: 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012; 338: 768–772. [DOI] [PubMed] [Google Scholar]
- 18.McKie AT, Barrow D, Latunde-Dada GO, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001; 291: 1755–1759. [DOI] [PubMed] [Google Scholar]
- 19.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997; 388: 482–488. [DOI] [PubMed] [Google Scholar]
- 20.Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics 1995; 25: 514–525. [DOI] [PubMed] [Google Scholar]
- 21.Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 1993; 73: 469–485. [DOI] [PubMed] [Google Scholar]
- 22.Kishi F. Isolation and characterization of human Nramp cDNA. Biochem Biophys Res Commun 1994; 204: 1074–1080. [DOI] [PubMed] [Google Scholar]
- 23.Fleming MD, Trenor CC 3rd, Su MA, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 1997; 16: 383–386. [DOI] [PubMed] [Google Scholar]
- 24.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A 1998; 95: 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 1999; 93: 4406–4417. [PubMed] [Google Scholar]
- 26.Yeh KY, Yeh M, Watkins JA, Rodriguez-Paris J, Glass J. Dietary iron induces rapid changes in rat intestinal divalent metal transporter expression. Am J Physiol Gastrointest Liver Physiol 2000; 279: G1070–G1079. [DOI] [PubMed] [Google Scholar]
- 27.Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc Natl Acad Sci U S A 2002; 99: 12345–12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrnstorfer IA, Geertsma ER, Pardon E, Steyaert J, Dutzler R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol 2014; 21: 990–996. [DOI] [PubMed] [Google Scholar]
- 29.Bozzi AT, Bane LB, Weihofen WA, et al. Conserved methionine dictates substrate preference in Nramp-family divalent metal transporters. Proc Natl Acad Sci U S A 2016; 113: 10310–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Jin J, DeFelice LJ, Andrews NC, Clapham DE. A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway. PLoS Biol 2004; 2: E50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozzi AT, Zimanyi CM, Nicoludis JM, Lee BK, Zhang CH, Gaudet R. Structures in multiple conformations reveal distinct transition metal and proton pathways in an Nramp transporter. Elife 2019; 8: e41124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozzi AT, Gaudet R. Molecular mechanism of nramp-family transition metal transport. J Mol Biol 2021; 433: 166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunshin H, Starr CN, Direnzo C, et al. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood 2005; 106: 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 2005; 115: 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shawki A, Anthony SR, Nose Y, et al. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am J Physiol Gastrointest Liver Physiol 2015; 309: G635–G647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirase T, Mori K, Okazaki Y, et al. Suppression of SLC11A2 expression is essential to maintain duodenal integrity during dietary iron overload. Am J Pathol 2010; 177: 677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klunder LJ, Faber KN, Dijkstra G, van IJzendoorn SCD. Mechanisms of cell polarity-controlled epithelial homeostasis and immunity in the intestine. Cold Spring Harb Perspect Biol 2017; 9: a027888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Hernandez X, Smith M, Glass J. Apo-transferrin is internalized and routed differently from Fe-transferrin by caco-2 cells: a confocal microscopy study of vesicular transport in intestinal cells. Blood 2000; 95: 721–723. [PubMed] [Google Scholar]
- 39.Hughson EJ, Hopkins CR. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol 1990; 110: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Núñez MT, Núñez-Millacura C, Beltrán M, Tapia V, Alvarez-Hernandez X. Apotransferrin and holotransferrin undergo different endocytic cycles in intestinal epithelia (Caco-2) cells. J Biol Chem 1997; 272: 19425–19428. [DOI] [PubMed] [Google Scholar]
- 41.Ma Y, Specian RD, Yeh KY, Yeh M, Rodriguez-Paris J, Glass J. The transcytosis of divalent metal transporter 1 and apo-transferrin during iron uptake in intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 2002; 283: G965–G974. [DOI] [PubMed] [Google Scholar]
- 42.Núñez MT, Tapia V, Rojas A, Aguirre P, Gómez F, Nualart F. Iron supply determines apical/basolateral membrane distribution of intestinal iron transporters DMT1 and ferroportin 1. Am J Physiol Cell Physiol 2010; 298: C477–C485. [DOI] [PubMed] [Google Scholar]
- 43.Yanatori I, Yasui Y, Tabuchi M, Kishi F. Chaperone protein involved in transmembrane transport of iron. Biochem J 2014; 462: 25–37. [DOI] [PubMed] [Google Scholar]
- 44.Okazaki Y, Ma Y, Yeh M, et al. DMT1 (IRE) expression in intestinal and erythroid cells is regulated by peripheral benzodiazepine receptor-associated protein 7. Am J Physiol Gastrointest Liver Physiol 2012; 302: G1180–G1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanatori I, Richardson DR, Toyokuni S, Kishi F. The new role of poly (rC)-binding proteins as iron transport chaperones: proteins that could couple with inter-organelle interactions to safely traffic iron. Biochim Biophys Acta Gen Subj 2020; 1864: 129685. [DOI] [PubMed] [Google Scholar]
- 46.McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 2000; 5: 299–309. [DOI] [PubMed] [Google Scholar]
- 47.Vulpe CD, Kuo YM, Murphy TL, et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 1999; 21: 195–199. [DOI] [PubMed] [Google Scholar]
- 48.Fuqua BK, Lu Y, Darshan D, et al. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS One 2014; 9: e98792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh KY, Yeh M, Glass J. Interactions between ferroportin and hephaestin in rat enterocytes are reduced after iron ingestion. Gastroenterology 2011; 141: 292–299.e1. [DOI] [PubMed] [Google Scholar]
- 50.Yeh KY, Yeh M, Mims L, Glass J. Iron feeding induces ferroportin 1 and hephaestin migration and interaction in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 2009; 296: G55–G65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercadante CJ, Prajapati M, Parmar JH, et al. Gastrointestinal iron excretion and reversal of iron excess in a mouse model of inherited iron excess. Haematologica 2019; 104: 678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med 2019; 133: 46–54. [DOI] [PubMed] [Google Scholar]
- 53.Rajagopal A, Rao AU, Amigo J, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 2008; 453: 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White C, Yuan X, Schmidt PJ, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab 2013; 17: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiening M, Lange N. A recap of heme metabolism towards understanding protoporphyrin IX selectivity in cancer cells. Int J Mol Sci 2022; 23: 7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Y, Okazaki Y, Glass J. A fluorescent metal-sensor study provides evidence for iron transport by transcytosis in the intestinal epithelial cells. J Clin Biochem Nutr 2018; 62: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drakesmith H, Nemeth E, Ganz T. Ironing out ferroportin. Cell Metab 2015; 22: 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y, Yeh M, Yeh KY, Glass J. Iron Imports. V. Transport of iron through the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 2006; 290: G417–G422. [DOI] [PubMed] [Google Scholar]
- 59.Cegarra L, Colins A, Gerdtzen ZP, Núñez MT, Salgado JC. Mathematical modeling of the relocation of the divalent metal transporter DMT1 in the intestinal iron absorption process. PLoS One 2019; 14: e0218123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galy B, Ferring-Appel D, Becker C, et al. Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell Rep 2013; 3: 844–857. [DOI] [PubMed] [Google Scholar]
- 61.Fuqua BK, Lu Y, Frazer DM, et al. Severe iron metabolism defects in mice with double knockout of the multicopper ferroxidases hephaestin and ceruloplasmin. Cell Mol Gastroenterol Hepatol 2018; 6: 405–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 2005; 1: 191–200. [DOI] [PubMed] [Google Scholar]
- 63.Romero-Cortadellas L, Hernández G, Ferrer-Cortès X, et al. New cases of hypochromic microcytic anemia due to mutations in the SLC11A2 gene and functional characterization of the G75R mutation. Int J Mol Sci 2022; 23: 4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bardou-Jacquet E, Island ML, Jouanolle AM, et al. A novel N491S mutation in the human SLC11A2 gene impairs protein trafficking and in association with the G212V mutation leads to microcytic anemia and liver iron overload. Blood Cells Mol Dis 2011; 47: 243–248. [DOI] [PubMed] [Google Scholar]
- 65.Sandhu K, Flintoff K, Chatfield MD, et al. Phenotypic analysis of hemochromatosis subtypes reveals variations in severity of iron overload and clinical disease. Blood 2018; 132: 101–110. [DOI] [PubMed] [Google Scholar]
- 66.Blanco E, Kannengiesser C, Grandchamp B, Tasso M, Beaumont C. Not all DMT1 mutations lead to iron overload. Blood Cells Mol Dis 2009; 43: 199–201. [DOI] [PubMed] [Google Scholar]
- 67.Kelleher T, Ryan E, Barrett S, O′Keane C, Crowe J. DMT1 genetic variability is not responsible for phenotype variability in hereditary hemochromatosis. Blood Cells Mol Dis 2004; 33: 35–39. [DOI] [PubMed] [Google Scholar]
- 68.Ozbayer C, Kurt H, Nur Kebapci M, Veysi Gunes H, Colak E, Degirmenci I. The genetic variants of solute carrier family 11 member 2 gene and risk of developing type-2 diabetes. J Genet 2018; 97: 1407–1412. [PubMed] [Google Scholar]
- 69.He Q, Du T, Yu X, et al. DMT1 polymorphism and risk of Parkinson’s disease. Neurosci Lett 2011; 501: 128–131. [DOI] [PubMed] [Google Scholar]
- 70.Tolone C, Bellini G, Punzo F, et al. The DMT1 IVS4+44C>A polymorphism and the risk of iron deficiency anemia in children with celiac disease. PLoS One 2017; 12: e0185822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell S, Rigas AS, Magnusson MK, et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun Biol 2021; 4: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet 2008; 40: 569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nemeth E, Ganz T. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int J Mol Sci 2021; 22: 6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A 2001; 98: 8780–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleming RE, Migas MC, Zhou X, et al. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci U S A 1999; 96: 3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A 2002; 99: 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ganz T. Erythropoietic regulators of iron metabolism. Free Radic Biol Med 2019; 133: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonaccorsi di Patti MC, Polticelli F, Cece G, et al. A structural model of human ferroportin and of its iron binding site. FEBS J 2014; 281: 2851–2860. [DOI] [PubMed] [Google Scholar]
- 79.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306: 2090–2093. [DOI] [PubMed] [Google Scholar]
- 80.Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018; 131: 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Billesbølle CB, Azumaya CM, Kretsch RC, et al. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature 2020; 586: 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Funahashi S, Okazaki Y, Nishiyama T, et al. Global overexpression of divalent metal transporter 1 delays crocidolite-induced mesothelial carcinogenesis in male mice. Free Radic Res 2018; 52: 1030–1039. [DOI] [PubMed] [Google Scholar]
- 83.Flores SRL, Nelson S, Woloshun RR, et al. Intestinal iron absorption is appropriately modulated to match physiological demand for iron in wild-type and iron-loaded Hamp (hepcidin) knockout rats during acute colitis. PLoS One 2021; 16: e0252998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002; 110: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwartz AJ, Das NK, Ramakrishnan SK, et al. Hepatic hepcidin/intestinal HIF-2alpha axis maintains iron absorption during iron deficiency and overload. J Clin Invest 2019; 129: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woloshun RR, Yu Y, Xu X, et al. Four AAs increase DMT1 abundance in duodenal brush-border membrane vesicles and enhance iron absorption in iron-deprived mice. Blood Adv 2022; 6: 3011–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baumgartner J, Winkler HC, Zandberg L, et al. Iron from nanostructured ferric phosphate: absorption and biodistribution in mice and bioavailability in iron deficient anemic women. Sci Rep 2022; 12: 2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Das NK, Schwartz AJ, Barthel G, et al. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab 2020; 31: 115–130.e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ganz T, Nemeth E, Rivella S, et al. TMPRSS6 as a therapeutic target for disorders of erythropoiesis and iron homeostasis. Adv Ther 2023; 40: 1317–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]