Abstract
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) have emerged as promising therapeutic agents with potent anti-inflammatory properties and diverse clinical implications. This in-depth review article explores the mechanisms behind the anti-inflammatory actions of GLP-1RAs and assesses their prospective applicability in a wide range of disease scenarios. The current review establishes the significance of comprehending the anti-inflammatory role of GLP-1RAs and identifies pertinent research gaps. A concise overview of inflammation and its clinical consequences underscores the critical need for effective anti-inflammatory interventions. Subsequently, the article elucidates the intricate mechanisms through which GLP-1RAs modulate immune cell signaling and regulate the nuclear factor-kappa B (NF-κB) pathway. Detailed discussions encompass their impact on inflammatory responses, cytokine production, and attenuation of oxidative stress. The exposition is substantiated by a collection of pertinent examples and an extensive array of references from both preclinical and clinical investigations. The historical trajectory of GLP-1RA drugs, including exenatide, lixisenatide, liraglutide, and semaglutide, is traced to delineate their development as therapeutic agents. Moreover, the review emphasizes the therapeutic potential of GLP-1RAs in specific disease contexts like type 2 diabetes, a neurodegenerative disorder, and inflammatory bowel disease (IBD), shedding light on their anti-inflammatory effects through rigorous examination of preclinical and clinical studies. The article also provides an outlook on future perspectives for GLP-1RAs, encompassing the domains of diabetes, neurodegenerative diseases, and IBD. In conclusion, GLP-1RAs exhibit substantial anti-inflammatory effects, rendering them promising therapeutic agents with broad clinical implications. They are very useful in a wide variety of diseases because they regulate immunological responses, block NF-κB activation, and decrease production of pro-inflammatory cytokines. Ongoing research endeavors aim to optimize their therapeutic use, delineate patient-specific treatment paradigms, and explore novel therapeutic applications. GLP-1RAs represent a significant breakthrough in anti-inflammatory therapy, offering novel treatment options, and improved patient outcomes.
Keywords: Glucagon-like peptide 1 receptor agonists, inflammation, inflammatory bowel disease, neurodegenerative disorder, nuclear factor-kappa B, obesity, type-2 diabetes
Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RA), a category of medications frequently used to treat type 2 diabetes (T2D), comprise drugs such as exenatide, liraglutide, and semaglutide.1–4 These medications mimic the function of the naturally occurring hormone GLP-1, predominantly released by intestinal L-cells upon food consumption.5,6 GLP-1RA and dipeptidyl peptidase-4 (DPP-4) inhibitors (DPP-4i) regulate blood sugar levels by focusing on the incretin system. A gastrointestinal (GI) hormone, GLP-1, and a gastric inhibitory peptide, GIP, are secreted in response to food consumption; they may alter the pharmacological doses of GLP-1, thus lowering inactivated DPP-4 kinetics. These promote pancreatic-cell development and differentiation, decrease islet-cell activity, and increase insulin production. Human GLP-1 is broken down in circulation by DPP-4 in approximately 2–3 min. DPP-4 inhibition augments the levels of GLP-1 and GIP, reduces blood sugar levels, and boosts insulin production. 7 Besides their well-established impact on glucose balance, new evidence indicates that GLP-1RA may have anti-inflammatory properties beyond glycemic regulation.
Numerous GLP-1RAs have been constructed and sanctioned for therapeutic applications (Table 1). Among these, exenatide (Byetta) was initially authorized by the U.S. Food and Drug Administration (FDA) in 2005. 8 Scientists have chemically reproduced the hormone exendin-4 that is secreted by Gila monster lizards. 9 Exenatide is applied subcutaneously and initially necessitates twice-daily injections. However, an extended-release composition named Bydureon was subsequently created, facilitating once-weekly dosing. Exenatide has prominently enhanced glycemic regulation, weight reduction, and cardiovascular risk factors in therapeutic trials.5,10 Lixisenatide (Adlyxin) is a GLP-1RA derived from exendin-4. It attained FDA approval in 2016 for the management of T2D. It is administered via a daily injection. It has effectively reduced HbA1c levels and optimized postprandial glucose regulation. In clinical trials, Lixisenatide has also exhibited cardiovascular safety. 11 Liraglutide (Victoza) is a potent GLP-1RA that secured FDA approval in 2010. It is administered daily through subcutaneous injection. Liraglutide has been proven to ameliorate glycemic management, facilitate weight reduction, and lessen cardiovascular risk in individuals with T2D. Increased dosages of liraglutide (3 mg) have also been sanctioned for obesity treatment under Saxenda. 12 Semaglutide (Ozempic) modifies the natural glucagon-like peptide-1 molecule. For the treatment of T2D, the FDA gave their approval in 2017. Semaglutide is administered weekly through subcutaneous injection. It has displayed superior effectiveness in reducing HbA1c levels and facilitating weight reduction compared to other GLP-1RAs. Semaglutide has also demonstrated cardiovascular benefits in clinical trials. 13 GLP-1RAs have notably broadened the therapeutic possibilities for T2D patients by offering efficient glycemic regulation, weight reduction, and possible cardiovascular advantages. Evidence from a wide range of research shows that GLP1-RA therapy may also help with weight loss, fatty liver, and cardiovascular issues.14–16 Tirzepatide (LY3298176), a medication that merges GIP and GLP1-RA, is utilized to treat T2D. It has been observed that patients undergoing therapy with Tirzepatide often meet their weight objectives more frequently when compared to those who are either on placebos or dulaglutide. Alterations have also been noted regarding waist measurements.17,18 Despite some evidence of their benefit for adipocytes and lipids, their precise role in lipid balance needs further investigation. 19 A research conducted by Xu and his team in the year 2016 revealed exendin-4’s ability to stimulate fatty acid combustion through a pathway signaling dependent on sirtuin-1 (SIRT-1) present within 3T3L1 adipocytes. Their studies established that GLP-1 signaling enhances oxidant capacity, which successively escalates fatty acid oxidation within cultured adipocytes. 20 Our understanding suggests that drawing comparisons between different trials hints at a shared method of action. Yet, significant disparities concerning pharmacokinetic attributes exist (for instance, one daily lixisenatide injection does not extend throughout an entire 24-h cycle). The optimization of dosages based on the outcomes of phase II dose-discovery studies is likely to apply to a weekly intake of 2 mg exenatide. Moreover, the rates at which drugs are discontinued also influence the extent of cardiovascular advantage attainable with specific compounds or preparations, as Caruso et al. proposed. 21 Since the year 2005, with exenatide’s initial authorization, there has been swift progression leading to enhancements in GLP1-RAs pharmacokinetics. This progress has facilitated a transition from daily injections to a more manageable once-weekly dosage regimen. 5
Table 1.
List of clinically approved GLP-1RA and their dosing frequency.
| GLP-1RA name | Brand | Approved date by FDA | Dosing frequency | Time to peak |
|---|---|---|---|---|
| Exenatide | Byetta | April 2005 | 5–10 mcg, twice-daily or once-daily | 2.1 h |
| Bydureon | January 2012 | |||
| Lixisenatide | Lixumia | July 2016 | 10 mcg for 2 weeks, a once-daily injection | 1.3–5 h |
| Liraglutide | Victoza | January 2010 | 0.6, 1.2, or 1.8 mg, dose can be increased to 3 mg, once-daily | 8–12 h |
| Semaglutide | Ozempic | December 2017 | 0.25, 0.5, or 1 mg, dose can be increased to 1.8 mg, once weekly | 1–3 days |
| Rybelsus | September 2019 |
FDA, U.S. Food and Drug Administration.
Inflammation is vital in developing chronic diseases [such as diabetes, cardiovascular disease, neurodegenerative disorders (ND)] and inflammatory bowel disease (IBD). 22 It is now acknowledged that inflammation exacerbates disease progression and symptoms in these conditions. 23 GLP-1RAs’ putative anti-inflammatory properties and therapeutic consequences have gained more attention. Although it is well established that GLP-1RA’s anti-inflammatory effects originate from their impact on immune cell signaling, additional in-depth investigation into the underlying molecular pathways is required. 24 The anti-inflammatory effects of GLP-1RAs are better understood when these molecular details are uncovered, which may lead to novel therapeutic options for inflammatory diseases. Several clinical and preclinical studies have shown the anti-inflammatory effect of GLP-1RAs. 25 GLP-1RA achieves anti-inflammatory results through various methods. 26 For example, in rheumatoid arthritis-induced mouse, liraglutide administration lessened synovial inflammation and decreased pro-inflammatory cytokine production, resulting in better outcomes of joint damage. 27 High-sensitivity C-reactive protein (hs-CRP), an indicator of systemic inflammation, was significantly reduced in people with T2D and cardiovascular disease treated with semaglutide. These results indicate that GLP-1RA may be able to regulate inflammation in various disease conditions. 28 Regulating immune signaling pathways prevents inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) from producing and releasing. 29 Second, GLP-1RAs encourage initiating anti-inflammatory mechanisms such as the AMP-activated protein kinase (AMPK) pathway, which helps suppress inflammation.30,31 Furthermore, GLP-1RA has demonstrated the ability to reduce the activation of nuclear factor-kappa B (NF-κB), a crucial inflammation controller, leading to a decrease in the generation of inflammatory agents. 32
Understanding the GLP-1RAs’ anti-inflammatory properties is very beneficial in clinical practice. These substances could enhance glucose regulation by focusing on inflammation and offer additional advantages in addressing coexisting inflammatory disorders. 33 Moreover, as chronic inflammation is associated with developing insulin resistance, GLP-1RA’s anti-inflammatory capabilities may contribute to their overall glucose-lowering benefits. 34 GLP-1RAs exhibit anti-inflammatory capabilities that go beyond their function in glucose management. The influence of GLP-1RAs on inflammatory pathways has consequences for various chronic inflammatory diseases, such as diabetes, heart disease, neurodegenerative diseases, and inflammatory bowel syndrome will be addressed in the current review. More study is needed to determine the full therapeutic potential of GLP-1RAs and understand the mechanisms behind their anti-inflammatory effects. Comprehensive, meticulously planned clinical trials that assess the outcomes of GLP-1RA-induced anti-inflammatory properties in various patient groups are essential. These research efforts can prove the efficacy, safety, and real-world implications of GLP-1RAs as anti-inflammatory agents, ultimately informing treatment choices and improving patient care.
Mechanisms of action
GLP-1RAs have become notable for their ability to reduce glucose levels and their emerging function as anti-inflammatory agents. It is critical to understand their fundamental mechanisms of action to establish the therapeutic potential of GLP-1RAs for various inflammatory conditions. Numerous essential mechanisms have been suggested, emphasizing the complex nature of GLP-1RA-mediated inflammation control.
Regulation of immune cell signals: GLP-1RAs are anti-inflammatory because they control the signals that immune cells send and receive. GLP-1RAs may modulate inflammatory responses since GLP-1 receptors are present in immune cells such as macrophages, monocytes, and lymphocytes.24,35 Through their interactions with GLP-1RAs, immune cell signals are modulated inside the cell. Pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, are suppressed by GLP-1R in immune cells.33,36 Furthermore, GLP-1RAs can boost the generation of anti-inflammatory cytokines such as IL-10, which helps to mitigate inflammation and restore immune balance. 33 However, there is strong evidence that central and peripheral immune responses undergo pathological alterations and develop with time. 37
Regulation of nuclear factor-kappa B pathway
Inflammation is tightly regulated by the NF-κB pathway, which regulates the expression of several pro-inflammatory genes. GLP-1RA has been demonstrated to reduce the generation of inflammatory mediators by inhibiting NF-κB activation. Effectively suppressing the production of pro-inflammatory cytokines, adhesion molecules, and chemokines, GLP-1RAs may attenuate the inflammatory response by decreasing NF-κB signaling. In the adipose tissue of GLP-1-treated ob/ob mice expressing recombinant adenovirus, for instance, GLP-1RA has been shown to decrease the production of TNF-α, IL-6, and IL-1β, all of which are significant mediators of inflammation. 38 The anti-inflammatory effects of GLP-1RA are facilitated by its ability to suppress pro-inflammatory cytokine production. Studies on kidney tissue revealed that exendin-4 suppressed NF-kB activity. 39 When administered to mice at 10 g/kg, exendin-4 effectively suppressed the binding activity of NF-Bp65 in individuals with T2D, and after 48 weeks of therapy, activation of NF-κB was reduced. 40 Human Umbilical Vein Endothelial Cells (HUVECs). There was a dose-response relationship between liraglutide and increased nitric oxide generation in. As a result of this factor, eNOS phosphorylation was also induced, and its activity was enhanced. Restoring NF-κB activity reversed cytokine-induced reductions in eNOS (NOS3) mRNA levels. 41
Signaling via AMP-AMPK: The energy-sensing kinase AMPK has major effects on cellular metabolism and inflammation. 42 Research has shown that GLP-1RA can trigger the AMPK pathway, resulting in anti-inflammatory outcomes. By increasing the synthesis of anti-inflammatory components, activating AMPK suppresses the production of pro-inflammatory cytokines and chemokines. By regulating AMPK signaling, GLP-1RAs can efficiently mitigate inflammation and re-establish metabolic equilibrium within cells. GLP-1RAs can directly initiate signaling processes within liver cells. These cells when grown in vitro may respond to GLP-1RA by increasing AMPK phosphorylation. 43
Prevention of reactive oxygen species formation
Excessive reactive oxygen species (ROS) may cause chronic inflammation and tissue damage. 44 GLP-1RAs decrease ROS generation, thus mitigating oxidative stress and inflammation. These agents protect cells from inflammatory harm by reducing oxidative stress and supporting tissue equilibrium. 45 GLP-1RA could hinder the development of asymmetric dimethylarginine through AGE-RAGE mediation by suppressing the expression of protein arginine methyltransferase-1 and inhibiting ROS production. 46 By enhancing HDAC6 through the GLP-1R-ERK1/2 pathway, GLP-1 treatment may inhibit the aberrant autophagy and inflammation caused by ROS. 47 GLP-1RAs not only regulate cardiomyocyte activity, but also improve cardiac fibroblast performance. For instance, they may mitigate cardiac fibroblast development and myocardial fibrosis by modulating the CD36-JNK-AP1 pathway, which reduces P4HA1 levels, blocking ROS production mediated by the Ang II type I receptor.48,49
Modulation of lipid metabolism pathway
Medications based on incretin can influence both the formation and breakdown of fat, or lipogenesis and lipolysis, respectively. 50 Research has shown that GLP-1 and its related compounds significantly affect these processes in rat fat cells. It is common for individuals afflicted with obesity to exhibit changes in the makeup of their circulating plasma fats, a condition known as dyslipidemia. This typically includes elevated levels of apolipoprotein B (apoB), diminished levels of high-density lipoprotein, and alterations in low-density lipoprotein’s particle composition (LDL). 51 Upon direct infusion of carbohydrates or fats into the ileum, healthy human bodies witness a swift rise in GLP-1 plasma concentrations. While this might be sufficient for triggering an early increase in circulating GLP-1 during food consumption, it is also suggested that due to relatively fewer L-cells present at proximal parts compared to distal parts within small intestines – seen across rodents and humans alike – some other neural or humoral signals may influence release patterns concerning GLP-1 during meals; particularly evident within initial secretion phases pertaining to GLP-1. 52
While the direct response of L-cells to secrete GLP-1 in reaction to luminal nutrients is acknowledged, it could be recognized as the principal process behind GLP-1 secretion. It is intriguing to note that a proposed function of GLP-1 includes acting as a controller for intestinal lipid absorption, mediated through downregulation of apoB. However, there has been no evaluation regarding its long-term impacts on lipid metabolism or the influence on plasma levels of apoB in nondiabetic individuals with obesity who have experienced weight reduction.53,54 Research by Ben-Shlomo et al. 43 established that an AMPK-dependent pathway promotes inhibition of lipogenesis by GLP-1 in rats fed a high-fat diet. They found evidence suggesting that therapy involving GLP-1 curtails lipogenic enzymes such as fatty acid synthase and carnitine palmitoyl transferase-1 within hepatic cells derived from experimental rats. 43 Subsequent research conducted by Parlevliet et al. 55 substantiates this point while also revealing both DPP-1i (exendin-4) and GLP1-RA result in decreased hepatic lipogenesis among mice consuming high-fat diets. They also discovered that treatment with GLP-1 suppresses the activity of genes participating in lipogenesis. 55
Reducing body weight from 5 to 10% enhances the regulation of blood glucose levels. It positively impacts risk factors associated blood pressure and lipid profile. 56 Proteins known as Sodium-dependent glucose cotransporter proteins 1 and 2 (SGLT1/2), particularly inhibitors of these proteins, stimulate a rise in the glucagon-to-insulin ratio, increasing lipids’ mobilization. This effect is especially notable with SGLT1i. In addition, inhibitors of SGLT-2 decrease leptin concentrations in serum while enhancing adiponectin concentrations – processes that promote fat breakdown, weight reduction, and diminishing fat accumulation within heart muscle tissue. 57 GIP or gastric inhibitory polypeptide directly impacts subcutaneous white fat tissue; it promotes improved insulin response capabilities and enhanced capacity for lipid buffering. Additionally, GIP improves blood flow and storage capacity while mitigating the infiltration by pro-inflammatory immune cells into tissues. 58 Studies have demonstrated that therapy involving teneligliptin activates AMPK pathway responses while curbing the expression of genes linked to lipogenesis, thereby ameliorating nonalcoholic fatty liver disease observed within mouse models. 59 Numerous studies have hinted at a signaling relationship between GLP-1 and lipogenesis, where incretin-based treatments improve lipogenesis through the AMPK activation pathway. 60 Research has extensively examined the combined agonists of GLP-1 and GIP1-RA for their potential impact on diabetes management and weight reduction. 61
It is important to note that the several mechanisms through which GLP-1RAs exert their anti-inflammatory effects are intertwined and contribute to overall effect. However, a deeper exploration of the specific molecular pathways and receptors responsible for these effects is necessary. In addition, both GLP-1R-dependent- and -independent pathways may be responsible for the anti-inflammatory effects of GLP-1RAs, illustrating the complexity of their activities. Gaining insight into the complex mechanisms through which GLP-1RAs produce their anti-inflammatory effects will improve our understanding of their therapeutic potential and facilitate the creation of new anti-inflammatory approaches. Further research is needed to fully understand GLP-1RA’s downstream targets, signaling sequences, and interactions with other inflammation-related pathways.
Revolutionizing diabetes management and inflammation with the power of GLP-1RA
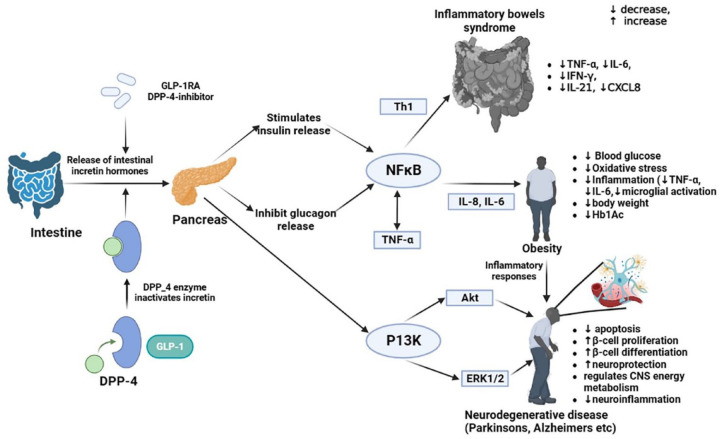
Upon ingestion of food, native GLP-1 is released into the bloodstream by the intestine. In addition to augmenting insulin secretion, it inhibits glucagon release, improving glycemic control. 62 Different tissues, such as the pancreas, the stomach, and the peripheral nerves, express GLP-1 receptors. Food intake and body weight are controlled by peripheral and central GLP-1-sensitive pathways 63 This peptide activates GLP-1 receptors by mimicking endogenous GLP-1. This hypothesized mechanism could enhance insulin secretion and suppress glucagon secretion in glucose-dependent manners (Figure 1). Additionally, they delay gastric emptying and reduce food intake by suppressing appetite, thereby reducing blood sugar levels. 64 Animals and humans with diabetes or obesity can reduce local or systemic inflammation by administering GLP-1-RA, regardless of their glycemic status or body weight. As a result, GLP-1-RA may possess anti-inflammatory properties that extend beyond glucose and weight control. 35
Figure 1.
A schematic representation of the mechanism of glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RA) inducing anti-inflammatory response while treating obesity, neurodegenerative disorders, and IBD. The role of GLP-1RA in obesity, neurodegenerative and IBD disorders is accomplished by NFκB and PI3K-Akt pathways, leading to the anti-inflammatory effect, by downregulation the pro-inflammatory cytokines and chemokines like IL-6, IL-8, TNF-α, IL-21, and IFNγ. As a result of these pathways, various tissues are more anti-oxidative, which reduces obesity, neurodegenerative, and IBD complications. In neurodegenerative conditions, β-cells undergo differentiation which enhances their neuroprotective capabilities and lessens the extent of neuroinflammation.
Akt, activating receptor tyrosine kinase; CXCL8, C-X-C motif chemokine ligand 8; DPP-4, dipeptidyl peptidase-4; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; GLP-1, Glucagon-like peptide-1; GLP-1RA, Glucagon-like peptide-1 receptor agonists; IBD, inflammatory bowel disease; IFN-γ, Interferon-γ; IL-8, interleukin-8; IL-6, interleukin-6; IL-21, interleukin-21; NFκB, Nuclear Factor-kappa B; PI3k, phosphoinositide-3-3kinase-protein kinase; Th1, T-helper-1; TNF-α, Tumor necrosis factor- α.
Impact of GLP1-RAs on diabetes and inflammation
Exendin administration in diabetic mice has been shown to attenuate inflammatory responses by upregulating regulatory T cells. 65 Exendin-4, a GLP-1 analog, showed cardioprotective properties in a diabetic animal model by preventing heart remodeling and diastolic dysfunction. In addition to these effects, a decline in macrophage infiltration, IL-1β, and IL-6 expression was observed, and an increase in IL-10 expression within the heart was reported. 66 Treatment with liraglutide substantially reduced the mean concentration of CRP in a retrospective study of obese individuals with T2DM, suggesting its potential as an anti-inflammatory drug. Baseline CRP and TNF-α levels were also significantly decreased due to using exenatide in combination with metformin. These results prove that GLP-1-based therapy may reduce inflammation and improve fatty liver disease in animal and human models. 67 Diabetic kidney inflammation is greatly aided by oxidative stress. Disturbances in the body’s oxidant/antioxidant balance trigger NF-κB signaling. 68 Renal pathology is seen in GLP-1 receptor knockout mice due to an increase in glomerular superoxide, an upregulation of renal nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase expression, and a decrease in renal cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) activity. By stimulating the cAMP-PKA pathway, GLP-1RA protects cells from oxidative stress by reducing the production of ROS. Treatment with liraglutide reduced NADPH oxidase activity, increased cAMP-PKA activity, and reduced mesangial expansion in mouse models.69,70 Liraglutide has been shown to modulate immune responses in individuals with diabetes. By treating patients with liraglutide, invariant natural killer (iNK) cells were significantly increased and monocytes secreting pro-inflammatory cytokines decreased significantly. Another research found that therapy with exenatide may decrease oxidative stress and upregulate anti-inflammatory responses in the peripheral lymphocytes of type 1 and T2D patients. 71 The functionality of GLP-1 in treating individuals with T2D has been thoroughly researched and validated. Its application in managing Type 1 diabetes mellitus (T1DM), however, remains less explored. GLP-1RAs may be viable for overweight or obese T1DM patients struggling to reach their blood sugar targets. GLP-1’s ability to lessen inflammation in the pancreas helps preserve beta cells, potentially slowing the evolution toward T1DM. Yet, a deeper understanding of GLP-1’s role in T1DM treatment demands further study. In the context of T2DM, GLP-1RAs have produced a range of beneficial pancreatic impacts, leading to significant enhancements in glycated hemoglobin (HbA1c), fasting and postprandial plasma glucose levels, and pancreatic beta-cell performance indicators.72–74 Drugs such as Liraglutide and Semaglutide have demonstrated superior blood sugar control compared to placebo or alternative antihyperglycemic treatments. 75 Recorded clinical data in 2019 demonstrated that liraglutide, a specific GLP-1RA, effectively diminishes adiposity and overall body fat by enhancing lipolysis among patients with T2DM who are obese. 76 A significant study conducted by Patel et al. 77 provided evidence that agonists of the GLP-1R can decrease cholesterol generation via inhibition of HMG-CoA reductase, a crucial enzyme involved in cholesterol synthesis, as well as SREBP-1C. The transcriptional impacts caused by GLP-1 have shown to be cardioprotective due to their ability to lessen the formation of atheroma plaque. 77 Current treatment protocols propose considering weight impact when choosing diabetes treatment strategies, and GLP-1RAs have shown a notably positive therapeutic profile for overweight or obese individuals with T2DM. 78 Recent rigorous scientific studies, specifically randomized controlled trials, have proven that GLP-1RAs consistently offer substantial weight loss advantages beyond the early stages of treatment. Furthermore, GLP-1RAs have been shown to positively influence multiple cardiometabolic risk elements, positioning them as crucial developments in weight management medication for medical professionals and their patients. 79 Liraglutide 3.0 mg (Saxenda®, produced by Novo Nordisk) is sanctioned for use in managing overweight and obesity in both the United States and Europe when combined with a reduced-calorie diet and physical exercise. The SCALE (Satiety and Clinical Adiposity Liraglutide Evidence) study assessed the safety and effectiveness of 3.0 mg liraglutide subcutaneous injections for diabetic and nondiabetic individuals. In this weight control initiative, those administered with 3.0 mg liraglutide demonstrated a dose-responsive weight reduction between 6.0 and 8.8 kg. Conversely, participants who were given a placebo (only diet and exercise) showed an average weight reduction between 0.2 and 3.0 kg. 12 (Table 2).
Table 2.
Summary of some of the GLP-1RA-approved drugs in the treatment of diabetes and neurodegenerative disorders.
| Disease/Disorder | Drug | Patient | Dosage | Study design | Primary endpoint | Treatment length | Outcome | Follow-up (years) | Adverse effects | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary outcome | Secondary outcome | ||||||||||
| Exenatide BID (EUREXA) | T2D patients with cardiovascular disease (CVD) risk factors, including systolic blood pressure | 1–4 mmHg | Open-label, randomized controlled trial at 128 centers, phase III | Reduction in treatment failure for rosiglitazone or glyburide as monotherapy | 4 weeks | Time to inadequate glycemic control (HbA1C more than 9% in first 3 months of treatment) | Improved baseline, estimation of β-cell function, body weight, hypoglycemia, blood pressure and heart rate | 2–3 | Gallwitz et al. 80 | ||
| Exenatide | T2D patients with congestive heart failure | 73–94 bpm | Single-center, randomized, double-blind, two-period crossover study | Increase in cardiac index or decrease in pulmonary pressure (>20%) | 8 weeks | Reduced nausea feeling | Improved cardiac index, hemodynamics | 3 | No serious adverse effects | Nathanson et al. 81 | |
| Diabetes (Type-2) | Exenatide (ByettaTM) | T2D patients | 5 µg/dose, dose can be increased to 10 µg/dose after 1 month | Triple-blind, phase III trial | Mean changes in HbA1c | 30 weeks | Reduction in HbA1c (⩽7%), reduced plasma glucose levels | Improvement in baseline, DTSQ (diabetes treatment satisfaction questionnaire) between 26.9 and 29.0, Insulin glargine- 24.1 and 30.4 | - | Nausea most commonly reported in 39% of patients who received 5 µg of exenatide | Cvetkovi?? and Plosker 82 |
| Liraglutide (LEAN) | T2D patients with fatty liver disease | 1.8 mg | Multi-centered, phase II, double-blinded, randomized, placebo-controlled | Liver biopsy | 48 | Improved liver enzymes and hepatic steatosis, histological improvement, no fibrosis | Changes in NAFLD activity score (NAS = 8), lobular inflammation, steatosis, fibrosis, liver stiffness, insulin resistance, lipid profile, | 1 | – | Armstrong et al. 83 | |
| Liraglutide (SCALE) | Patients with T2D and obesity | 3.0 mg | Randomized, placebo-controlled trails, phase III | Changes in body weight among ITT at 20 weeks | 20 | Changes in apnea-hypopnea index (AHI) | Dose-dependent weight loss ranging from 6 to 8.8 kg (5% reduction) | – | gastrointestinal disturbance | Mehta et al. 12 | |
| Semaglutide | T2D patients | 1.6 mg/week | Randomized, single-centered | Decline in glucose, plasma nitrotyrosine, plasma 8-iso prostaglandin F2alpha, Il-6 | 4 | Drop in HbA1c and reduction in weight | Increase in IL-6, 8-iso-PGF2a, nitrotyrosine | No deleterious effects were noticed | Ceriello et al. 72 | ||
| Neurodegenerative disorders | |||||||||||
| Alzheimer’s disease | Liraglutide (ELAD) (NCT01843075) | Elderly patients with AD | 1.8 mg/day | Multi-center, randomized, double-blind, placebo-controlled, Phase IIb trial | MRI changes | 52 | Changes in cerebral glucose metabolic rate, Improvement in cognitive function | Changes in cognitive and functional abilities, improved β cell function and suppression of glucagon, changes in MRI spectra | decreased brain oxidative stress by reduction in glucose-6-phosphate dehydrogenase | 12 months | Femminella et al. 84 |
| Parkinson’s disease | Exenatide (NCT01971242) | Early-stage PD | 2 mg/week | Single-center, randomized, double-blinded, placebo-controlled | - | 12 | Slowing of disease progression | differences between exenatide and placebo in each subsection of the MDS-UPDRS in the on-medication state and the Mattis Dementia Rating Scale at weeks 48 and 60, changes in vital signs, weight, and clinical laboratory values | No changes | 48 weeks | Athauda et al. 85 |
| Lixisenatide (NCT01174810) | PD patients | 20–40 µg/week | Randomized, placebo-controlled, double-blinded | Changes in MDS-UPDRS Part 3 | Improvement in motor symptoms and cognition, limb, and neck rigidity | MDS-UPDRS part 3 in exenatide group 2.7 points | No relevant changes in ECG, hematological and biochemical indices, minimal changes in basal ganglia subregions | nausea, abdominal pain, loss of appetite, constipation, Sciatica, insomnia, ischemic attack, lymph node dissection, anxiety | Aviles-Olmos et al. 86 | ||
| Multiple sclerosis | Exenatide | Relapsing MS | 5 µg twice daily | Randomized | – | – | Reduction in brain atrophy and disability | elevated CSF NFL, enhanced MRI lesions, | - | Novakova et al. 87 | |
–, Not available; 8-iso-PGF2a, plasma 8-iso prostaglandin F2alpha; AD, Alzheimer’s disease; ITT, Intention-to-treat; MDS-UPDRS, Movement Disorders Society-Unified Parkinson’s Disease Rating Scale; NAS, NAFLD activity score; NFL, Neurofilament light; PD, Parkinson’s disease.
In a recent study by Lazzaroni et al., 61 they documented a higher (>5%) reduction in weight among T2D patients who were given liraglutide, semaglutide, and tirzepatide. Apovian et al. 88 discovered that the introduction of exenatide to an intensive lifestyle alteration program – one entailing a daily deficit of 600 kcal coupled with at least 2.5 h per week of physical exercise – spurred greater weight reduction compared to the lifestyle program combined with placebo (−6.2 ± 0.5 versus −4.0 ± 0.5 kg). A noteworthy decrease in weight was observed by Davies et al. 89 specifically in obese patients with T2D who were treated with varying doses of Liraglutide; after following this regimen for 12 weeks, average weight loss stood at 4.7% of initial body mass (−5 kg as an absolute value) and increased to 6% (−6.4 kg as an absolute value) when administered doses rose from 1.8 to 3 mg respectively, compared against a mere loss of about 2.2% body mass (which equates to −2.2 kg actual value) among those on placebo. Sandsdal et al. 90 revealed that structured exercise coupled with liraglutide treatment – which consists of consuming only 800 kcal/day over 8 weeks – effectively diminished metabolic syndrome severity, abdominal obesity prevalence, and inflammation levels one had priorly experienced. It also became evident that utilizing such combination therapy might result in a more effective strategy toward curtailing cardiometabolic risks rather than relying on individual treatments alone.
In conclusion, GLP-1RAs have anti-inflammatory properties in diabetes treatment through modulation of immune cell signaling, inhibiting NF-κB activation, and reducing TNF-α production. These characteristics contribute to the potential advantages of GLP-1RAs, such as decreasing systemic inflammation, improving blood sugar control, and lowering the risk of diabetes-related complications. Additionally, findings from lipoprotein tracer kinetic research are set to guide us in understanding the influence of incretin-derived treatments on the creation and breakdown of lipoproteins. There is still a scarcity of substantial clinical proof concerning how these incretin-oriented therapies affect the likelihood of macrovascular and microvascular complications in diabetics. Lastly, it remains uncertain whether there may be benefits from merging standard lipid-reducing drugs with those based on incretins for individuals without diabetes. 91
GLP-1RA and the inflammatory link to ND
Cognitive impairment is highly linked with Diabetes mellitus, a primary hazard element. Neurodegeneration and cognitive deterioration can be influenced by the brain’s GLP-1 secretion and its peripheral in those affected by diabetes; however, external GLP-1 treatment may prove advantageous.92,93 Thus, the current article has also focused on ND, due to its association with diabetes mellitus (DM). Also, inflammation is involved in various neurological disorders such as Alzheimer’s disease (AD), dementia, multiple sclerosis (MS), and Parkinson’s disease (PD)24,94 (Table 2). Injured neurons and synapses produce chemicals, and inflammatory regulatory systems are disrupted, all of which contribute to the progression of these ND. 95 There is a lack of knowledge on GLP-1RA’s precise effect on neurogenesis. However, one theory suggests that this is due to an increase in the expression of mammalian achaete-scute homolog 1 (Mash1), a protein crucial for neuronal development that is thought to encourage hippocampus regeneration. 93 Akt activation increases Mash1 protein levels and transactivation activity are increased during Akt activation, which may indicate a role for the GLP-1-induced PI3K-AKT pathway 96 (Figure 1).
Many scientific investigations have explored the neuroprotective qualities of GLP-1RAs in diabetic animal subjects. GLP-1RA has been studied extensively regarding cerebral ischemia/reperfusion injuries. 92 In addition to regulating brain functions, GLP-1, and its receptor agonists have been shown to influence thermogenesis, blood pressure, neurogenesis, neurodegeneration, retinal repair, and energy balance. 97 Specifically, the precise localization of the GLP-1 receptors are yet unknown. GLP-1R mRNA has been found in various tissues and organs in rat models, including pancreatic islets, lungs, stomach, heart, ovaries, and kidneys. 24 There is substantial evidence that GPL-1R may improve signal transduction by enhancing the PI3K/AKT pathway due to the anti-apoptotic effects of GLP-1 through modulation of transcription factor cyclic AMP response-binding protein (CREB) and protein survival factors like Bcl-2 and Bcl-XL, including -arrestin-1 and phosphorylation of ERK1/2. As a consequence, activating the PI3K/AKT pathway can inhibit caspases and NF-κB, preventing the release of pro-inflammatory cytokines. 92
The nuclei containing GLP1-R are located in the circumventricular area postrema and median eminence, as well as in the nucleus of the solitary tract (NTS). 98 The transportation of GLP-1 or its ligands through the central nervous system (CNS) may be mediated by astrocytes and tanycytes. Incretin receptor agonists, such as ligand, semaglutide, and peptide, may infiltrate the brain when they are non-acylated or non-PEGylated.98,99 Given that GLP-1RA is a significant moderator of the gut-brain axis, it could potentially impact the brain indirectly through vagal nerve fibers in the enteric region, conveying metabolic information to NTS. 100 Timper et al. and his team discovered that the communication through GLP-1 receptor facilitates FA β-oxidation in astrocytes grown in a lab setting. This process subsequently enhances lipid regulation and memory performance. Their findings propose that the signaling of GLP-1 plays a central role in maintaining energy balance and brain functionality, by means of pathways dependent on FA β-oxidation. 101
Recent research has shown that the GLP-1 RA exenatide improves brain vascular health in an aged mice model, with transcriptome analysis revealing an enrichment of human AD genes from genome-wide association studies. 22 Studies have shown that gliptin may improve cognitive abilities in Alzheimer’s disease (AD). High-fat diet-induced insulin-resistant rats could benefit from both vildagliptin and sitagliptin to protect against mitochondrial complications. 102 DPP-4 inhibitors show potential in treating AD, but further research is required. Human studies have demonstrated that for those with obesity, prediabetes, or early-stage T2D, liraglutide slows a decline of memory function independent of weight reduction. 103 It is well established that mitochondria changes contribute to AD development. An increase in oxidative stress and ROS production play a role in mitochondrial DNA damage, respiratory impairment, and calcium imbalance, where all these symptoms of mitochondrial dysfunction are associated with AD. 93 Mitochondrial damage in AD may be caused by Aβ-production and tau phosphorylation. 104 Furthermore, a mitochondrial injury may play a role in transitioning from diabetes to AD, as exposing diabetic rats’ brain mitochondria to Aβ increases their vulnerability. 105
Based on this evaluation, although additional clinical evidence is required, existing information indicates the promising potential of employing GLP-1 and its analogs in future AD therapies. 106 It has been discovered that providing Liraglutide to AD patients for 6 months increases the ability to transfer blood-brain glucose, allowing the reestablishment of glucose transport, a vital initial step in improving brain alterations. 107 Furthermore, Gejl et al. 107 (ClinicalTrials.govNCT01469351) revealed that a 6-month Liraglutide treatment for AD patients helps prevent the decrease in the rate of cerebral glucose metabolism, a sign of cognitive deterioration, synaptic issues, and disease progression.
Parkinson’s disease (PD) is influenced by chronic inflammation. Microglia and astrocyte activation causes an increase in cytokines, which in turn activates the NF-κB pathway and causes oxidative damage to proteins. This phenomenon has been noted in the cerebrospinal fluid and brain tissue of living PD patients and in postmortem brain autopsies of those with PD. 37 Oxidative stress induced by inflammation and toxicity driven by cytokines may further the decay of the nigrostriatal pathway, thereby hastening the progression of idiopathic PD. 37 According to preliminary clinical trials, patients treated with exenatide improved by 2.7 points on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) after 12 months, compared to a decline of 2.2 points in control patients. 86 Exenatide has also exhibited the potential to lessen brain deterioration and disability in patients with MS. 87 Furthermore, lixisenatide has been tested for amyotrophic lateral sclerosis, showing promise in slowing disease advancement and enhancing survival. 24 When tested on patients with early-stage PD, exendin-4 was the first GLP-1 RA to show efficacy. By decreasing apoptosis, promoting mitogenesis, and increasing autophagy flux, exendin-4 and DA-CH5 have been demonstrated to protect SH-SY5Y cells against the cytotoxicity of 6-hydroxydopamine (6-OHDA). 108 Progress in 45 individuals with mild PD was evaluated in a pilot research utilizing a single-blind trial design. Subjects were either given exenatide injections under the skin for 12 months or served as controls.
The results indicate that GLP-1RAs improve neurodegenerative conditions through several mechanisms, such as decreasing inflammation, enhancing neuroprotection, and boosting cognitive performance. Nonetheless, additional investigation is required to comprehensively comprehend the action mechanisms and ascertain the ideal dosage and therapy duration for distinct neurodegenerative ailments. In general, GLP-1RAs present encouraging treatment alternatives for neurodegenerative diseases, providing novel pathways for managing these conditions and potential neuroprotective benefits.
Therapeutic potential of GLP-1RAs in IBD
Crohn’s disease (CD) and ulcerative colitis (UC) are under the broad category of IBD, which describes a group of chronic inflammatory gastrointestinal disorders. 23 Recent research points to the potential of GLP-1RAs as a therapeutic solution in treating IBD. One of the primary objectives of IBD treatment is to promote mucosal healing, an indicator of clinical remission. 109 There is evidence that GLP-1RA can reduce intestinal inflammation in IBD by regulating immune cell signaling and having anti-inflammatory properties. 110 GLP-1RA contributes to gut microbiota composition and intestinal barrier integrity, which are associated with IBD development. 111 Reduced production of pro-inflammatory cytokines, blocked NF-κB signaling, and increased regulatory T-cell activity are some of the mechanisms through which GLP-1RA protects against intestinal inflammation in early-stage IBD models 112 (Figure 1).
Insulin resistance persists in people with IBD, despite attempts to suppress TNF. No significant differences were seen in plasma insulin, glucose, or insulin resistance between before and after infliximab treatment in children with CD. 102 Histological scores, the colon weight/length ratio, and inflammatory cytokines/chemokines (such as CCL20, IL-33, and IL-22) were all considerably improved in a study by Bang-Berthelsen et al. when the GLP-1 analog liraglutide was used. 113 Patients with active UC and CD had higher amounts of bioactive GLP-2 than healthy controls, according to a study by Xiao et al. 114 However, Schmidt et al. found no difference in meal-stimulated GLP-2 plasma or tissue concentrations between IBD and non-IBD individuals. 115 These results suggest that GLP-1RA might be beneficial as a therapeutic agent for treating IBD, providing a new strategy to target inflammation and improve clinical outcomes in affected patients.
Limitations and future perspective
The use of GLP-1RAs is predicted to increase in treating diabetes, neurological disorders, and IBD. Current research focuses on creating stronger and longer GLP-1RAs with improved effectiveness and safety. Combination treatments involving GLP-1RA and other antidiabetic substances, such as SGLT-2 inhibitors or DPP-4 inhibitors, are expected to become increasingly prevalent to improve glycemic control and address the complex nature of diabetes. Additionally, using personalized medicine strategies through biomarkers or genetic profiling can help pinpoint patients most likely to respond positively to GLP-1RA. This could guide the selection of treatment and maximize therapeutic results. Further studies are necessary to determine the best dosage schedules and treatment periods for GLP-1RA. Nevertheless, the type of response to GLP-1RAs and the extent of their anti-inflammatory effects may vary among different patient populations depending on the severity of the disease, the presence of comorbidities, and the characteristics of the individual patient. Despite this heterogeneity, the current review needed to include more in discussing how it might impact generalizability. There is limited data on the anti-inflammatory effects of GLP-1RAs in different diseases over the long term. These drugs have yet to be tested for their long-term effectiveness and safety in terms of inflammation.
GLP-1RA’s therapeutic benefits could be enhanced by combining them with other neuroprotective substances, such as antioxidants or drugs that reduce inflammation. Developing a GLP-1RA that crosses the blood-brain barrier and targets brain areas affected by neurodegeneration could provide a novel therapeutic possibility. Intense research should focus on revealing how GLP-1RAs display their anti-inflammatory actions in the gut and establishing the most effective dosage and treatment plans. Complementary treatments that include GLP-1RA and other IBD drugs, for example, biologics or immunomodulators, could provide combined benefits and enhance disease management. Safety studies and bigger clinical trials are needed to assess GLP-1RAs’ efficacy and safety in different IBD patient groups.
The potential of GLP-1RA to manage diabetes, ND, and IBD is optimistic. Subsequent studies will focus on improving treatment protocols, developing new agents, investigating combined treatment options, and customizing therapeutic strategies. With continued progress in our understanding of the workings and potential therapeutic advantages of GLP-1RA, these agents could radically alter the control of these conditions and improve the patient’s prognosis.
Conclusion
GLP-1RAs have emerged as potential therapeutic agents with unique anti-inflammatory capabilities and significant clinical implications. They reduce systemic inflammation and enhance disease outcomes by modulating immune cell signaling, decreasing NF-κB pathway activation, and reducing pro-inflammatory cytokines. The anti-inflammatory benefits of GLP-1RA go beyond their recognized function in blood sugar regulation and weight control. Clinical and experimental research shows it reduces inflammation in neurological disorders, IBD, and diabetic complications. These revelations indicate an expanded therapeutic scope for GLP-1RA, surpassing its conventional application in the management of diabetes. Furthermore, GLP-1RAs have several benefits as therapeutic agents. They demonstrate a commendable safety record, a low incident rate of hypoglycemia, and negligible side effects. Their multiple products, including improved endothelial function, antioxidant activity, and safeguarding beta-cell functionality, contribute to their comprehensive clinical advantages.
GLP-1RAs have demonstrated potential in managing coexisting conditions associated with chronic inflammation, including obesity, dyslipidemia, and factors contributing to cardiovascular risk. There has been substantial progress in understanding the anti-inflammatory function of GLP-1RA. More research is required to understand the core mechanisms and find the best treatments. This includes examining the possible synergistic results of combined therapies, investigating safety profiles over extended periods, and conducting more comprehensive clinical trials to assess their effectiveness in various patient groups. Positive results from using GLP-1RA in treating inflammation disorders are promising. GLP-1RAs introduce an innovative therapeutic method that enhances current treatment options by targeting inflammation in the cellular and molecular stages. They might enhance disease outcomes, chronic inflammation, and patient quality of life.
In general, GLP-1RAs constitute a substantial breakthrough in anti-inflammatory treatment. Their ability to regulate immune reactions, mitigate systemic inflammation, and influence the course of diseases emphasizes their prospective clinical applications beyond blood sugar regulation. As ongoing studies uncover the complex operations and therapeutic potential of GLP-1RA, it is evident that they could serve as an important instrument in managing inflammatory diseases.
Acknowledgments
None.
Footnotes
ORCID iD: Saleh Hadi Alharbi  https://orcid.org/0000-0002-9635-3307
https://orcid.org/0000-0002-9635-3307
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution: Saleh Hadi Alharbi: Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Visualization; Writing – original draft; Writing – review & editing.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
The author declares that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Janzen KM, Steuber TD, Nisly SA. GLP-1 agonists in Type 1 diabetes mellitus. Ann Pharmacother 2016; 50: 656–665. [DOI] [PubMed] [Google Scholar]
- 2. Burcelin R, Gourdy P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes Rev 2017; 18: 86–98. [DOI] [PubMed] [Google Scholar]
- 3. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr 2017; 30: 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunt B, Malkin SJP, Moes RGJ, et al. Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. BMJ Open Diabetes Res Care 2019; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nauck MA, Quast DR, Wefers J, et al. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab 2021; 46: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gourdy P, Darmon P, Dievart F, et al. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc Diabetol 2023; 22: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giugliano D, Sportiello L, Capuano A, et al. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapy – focus on alogliptin. Drug Des Devel Ther 2013; 7: 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bond A. Exenatide (byetta) as a novel treatment option for type 2 diabetes mellitus. Proc (Bayl Univ Med Cent) 2006; 19: 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Triplitt C, Chiquette E. Exenatide: from the Gila monster to the pharmacy. J Am Pharm Assoc 2006; 46: 44–55. [DOI] [PubMed] [Google Scholar]
- 10. Chakraborti CK. Exenatide: a new promising antidiabetic agent. Indian J Pharm Sci 2010; 72: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neumiller JJ, Campbell RK. Liraglutide: a once-daily incretin mimetic for the treatment of type 2 diabetes mellitus. Ann Pharmacother 2009; 43: 1433–1444. [DOI] [PubMed] [Google Scholar]
- 12. Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract 2017; 3: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocr Metab Disord 2022; 23: 521–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honigberg MC, Chang L-S, McGuire DK, et al. Use of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes and cardiovascular Disease: a review. JAMA Cardiol 2020; 5: 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhir G, Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-Alcoholic fatty liver disease: a novel therapeutic option. J Investig Med 2018; 66: 7–10. [DOI] [PubMed] [Google Scholar]
- 16. Patoulias D, Michailidis T, Dimosiari A, et al. Effect of glucagon-like peptide-1 receptor agonists on cardio-metabolic risk factors among obese/overweight individuals treated with antipsychotic drug classes: an updated systematic review and meta-analysis of randomized controlled trials. Biomedicines 2023; 11: 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 355–366. [DOI] [PubMed] [Google Scholar]
- 18. Bendotti G, Montefusco L, Pastore I, et al. The anti-inflammatory and immunological properties of SGLT-2 inhibitors. J Endocrinol Invest 2023; 46: 2445–2452. [DOI] [PubMed] [Google Scholar]
- 19. Farr S, Taher J, Adeli K. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states. Cardiovasc Hematol Disord Drug Targets 2014; 14: 126–136. [DOI] [PubMed] [Google Scholar]
- 20. Xu F, Lin B, Zheng X, et al. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 2016; 59: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 21. Caruso I, Cignarelli A, Giorgino F. Heterogeneity and similarities in GLP-1 receptor agonist cardiovascular outcomes trials. Trends Endocrinol Metab 2019; 30: 578–589. [DOI] [PubMed] [Google Scholar]
- 22. Zhao X, Wang M, Wen Z, et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol 2021; 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt JE, Holst JJ, Jeppesen PB, et al. GLP-1 and intestinal diseases. Biomedicines 2021; 9: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diz-Chaves Y, Mastoor Z, Spuch C, et al. Anti-inflammatory effects of GLP-1 receptor activation in the brain in neurodegenerative diseases. Int J Mol Sci 2022; 23: 9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma X, Liu Z, Ilyas I, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci 2021; 17: 2050–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiernan MC, Vucic S, Cheah BC, et al. Pain in amyotrophic lateral sclerosis. Lancet 2017; 16: 144–157. [Google Scholar]
- 27. Meurot C, Jacques C, Martin C, et al. Targeting the GLP-1/GLP-1R axis to treat osteoarthritis: a new opportunity? J Orthop Translat 2022; 32: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balogh DB, Wagner LJ, Fekete A. An overview of the cardioprotective effects of novel antidiabetic classes: focus on inflammation, oxidative stress, and fibrosis. Int J Mol Sci 2023; 24: 7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new Insights and opportunities for cardiovascular protection. Adv Exp Med Biol 2021; 1307: 193–212. [DOI] [PubMed] [Google Scholar]
- 30. Krasner NM, Ido Y, Ruderman NB, et al. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One 2014; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei R, Ma S, Wang C, et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab 2016; 310: E947–E957. [DOI] [PubMed] [Google Scholar]
- 32. Tang ST, Zhang Q, Tang HQ, et al. Effects of glucagon-like peptide-1 on advanced glycation endproduct-induced aortic endothelial dysfunction in streptozotocin-induced diabetic rats: possible roles of rho kinase- and AMP kinase-mediated nuclear factor κB signaling pathways. Endocrine 2016; 53: 107–116. [DOI] [PubMed] [Google Scholar]
- 33. Pang J, Feng JN, Ling W, et al. The anti-inflammatory feature of glucagon-like peptide-1 and its based diabetes drugs – therapeutic potential exploration in lung injury. Acta Pharm Sin B 2022; 12: 4040–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sazgarnejad S, Yazdanpanah N, Rezaei N. Anti-inflammatory effects of GLP-1 in patients with COVID-19. Expert Rev Anti-Infect Ther 2022; 20: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bendotti G, Montefusco L, Lunati ME, et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol Res 2022; 182: 106320. [DOI] [PubMed] [Google Scholar]
- 36. Ferreira ST. Brain insulin, insulin-like growth factor 1 and glucagon-like peptide 1 signalling in Alzheimer’s disease. J Neuroendocrinol 2021; 33: 1–11. [DOI] [PubMed] [Google Scholar]
- 37. Bettcher BM, Tansey MG, Dorothée G, et al. Peripheral and central immune system crosstalk in Alzheimer disease – a research prospectus. Nat Rev Neurol 2021; 17: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee Y-S, Park M-S, Choung J-S, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012; 55: 2456–2468. [DOI] [PubMed] [Google Scholar]
- 39. Kodera R, Shikata K, Kataoka HU, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011; 54: 965–978. [DOI] [PubMed] [Google Scholar]
- 40. Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010; 139: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hattori Y, Jojima T, Tomizawa A, et al. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 2010; 53: 2256–2263. [DOI] [PubMed] [Google Scholar]
- 42. Gupta NA, Mells J, Dunham RM, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010; 51: 1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ben-Shlomo S, Zvibel I, Shnell M, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 2011; 54: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 44. Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014; 20: 1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Q, Tuo X, Li B, et al. Semaglutide attenuates excessive exercise-induced myocardial injury through inhibiting oxidative stress and inflammation in rats. Life Sci 2020; 250: 117531. [DOI] [PubMed] [Google Scholar]
- 46. Ojima A, Ishibashi Y, Matsui T, et al. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am J Pathol 2013; 182: 132–141. [DOI] [PubMed] [Google Scholar]
- 47. Cai X, Gao X, Yang W, et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: a meta-analysis. J Diabetes Invest 2018; 9: 850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao T, Chen H, Cheng C, et al. Liraglutide protects high-glucose-stimulated fibroblasts by activating the CD36-JNK-AP1 pathway to downregulate P4HA1. Biomed Pharmacother 2019; 118: 1–11. [DOI] [PubMed] [Google Scholar]
- 49. Chen P, Yang F, Wang W, et al. Liraglutide attenuates myocardial fibrosis via inhibition of AT1R-Mediated ROS production in hypertensive mice. J Cardiovasc Pharmacol Ther 2021; 26: 179–188. [DOI] [PubMed] [Google Scholar]
- 50. Sancho V, Trigo MV, González N, et al. Effects of glucagon-like peptide-1 and exendins on kinase activity, glucose transport and lipid metabolism in adipocytes from normal and type-2 diabetic rats. J Mol Endocrinol 2005; 35: 27–38. [DOI] [PubMed] [Google Scholar]
- 51. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013; 5: 1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eissele R, Göke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 1992; 22: 283–291. [DOI] [PubMed] [Google Scholar]
- 53. Hsieh J, Longuet C, Baker CL, et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 2010; 53: 552–561. [DOI] [PubMed] [Google Scholar]
- 54. Engelbrechtsen L, Lundgren J, Wewer Albrechtsen NJ, et al. Treatment with liraglutide may improve markers of CVD reflected by reduced levels of apoB. Obes Sci Pract 2017; 3: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parlevliet ET, Wang Y, Geerling JJ, et al. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS One 2012; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Montefusco L, D’Addio F, Loretelli C, et al. Anti-inflammatory effects of diet and caloric restriction in metabolic syndrome. J Endocrinol Invest 2021; 44: 2407–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu P, Wen W, Li J, et al. Systematic Review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Horm Metab Res 2019; 51: 487–494. [DOI] [PubMed] [Google Scholar]
- 58. Samms RJ, Coghlan MP, Sloop KW. How may GIP enhance the therapeutic efficacy of glp-1? Trends Endocrinol Metab 2020; 31: 410–421. [DOI] [PubMed] [Google Scholar]
- 59. Ideta T, Shirakami Y, Miyazaki T, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice. Int J Mol Sci 2015; 16: 29207–29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen J, Zhao H, Ma X, et al. GLP-1/GLP-1R signaling in regulation of adipocyte differentiation and lipogenesis. Cell Physiol Biochem 2017; 42: 1165–1176. [DOI] [PubMed] [Google Scholar]
- 61. Lazzaroni E, Ben Nasr M, Loretelli C, et al. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res 2021; 171: 1–26. [DOI] [PubMed] [Google Scholar]
- 62. Ryan D, Acosta A. GLP-1 receptor agonists: nonglycemic clinical effects in weight loss and beyond. Obesity 2015; 23: 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Larsen PJ. Mechanisms behind GLP-1 induced weight loss. Br J Diabetes Vasc Dis 2008; 8: S34–S41. [Google Scholar]
- 64. Yang F, Zeng F, Luo X, et al. GLP-1 receptor: a new target for sepsis. Front Pharmacol 2021; 12: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xue S, Wasserfall CH, Parker M, et al. Exendin-4 therapy in NOD mice with new-onset diabetes increases regulatory T cell frequency. Ann N Y Acad Sci 2008; 1150: 152–156. [DOI] [PubMed] [Google Scholar]
- 66. Tate M, Robinson E, Green BD, et al. Exendin-4 attenuates adverse cardiac remodelling in streptozocin-induced diabetes via specific actions on infiltrating macrophages. Basic Res Cardiol 2016; 111: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee Y-S, Jun H-S. Anti-inflammatory effects of GLP-1-Based therapies beyond glucose control. Mediators Inflamm 2016; 11: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jha JC, Banal C, Chow BSM, et al. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal 2016; 25: 657–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mehdi SF, Pusapati S, Anwar MS, et al. Glucagon-like peptide-1: a multi-faceted anti-inflammatory agent. Front Immunol 2023; 14: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fujita H, Morii T, Fujishima H, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 2014; 85: 579–589. [DOI] [PubMed] [Google Scholar]
- 71. He L, Wong CK, Cheung KK, et al. Anti-inflammatory effects of exendin-4, a glucagon-like peptide-1 analog, on human peripheral lymphocytes in patients with type 2 diabetes. J Diabetes Invest 2013; 4: 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ceriello A, Novials A, Ortega E, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013; 36: 2346–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stonehouse A, Walsh B, Cuddihy R. Exenatide once-weekly clinical development: safety and efficacy across a range of background therapies. Diabetes Technol Ther 2011; 13: 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab 2014; 16: 673–688. [DOI] [PubMed] [Google Scholar]
- 75. Huthmacher JA, Meier JJ, Nauck MA. Efficacy and safety of short- and long-acting glucagon-like peptide 1 receptor agonists on a background of basal insulin in type 2 diabetes: a meta-analysis. Diabetes Care 2020; 43: 2303–2312. [DOI] [PubMed] [Google Scholar]
- 76. Anholm C, Kumarathurai P, Samkani A, et al. Effect of liraglutide on estimates of lipolysis and lipid oxidation in obese patients with stable coronary artery disease and newly diagnosed type 2 diabetes: a randomized trial. Diabetes Obes Metab 2019; 21: 2012–2016. [DOI] [PubMed] [Google Scholar]
- 77. Patel V, Joharapurkar A, Kshirsagar S, et al. Coagonist of GLP-1 and glucagon decreases liver inflammation and atherosclerosis in dyslipidemic condition. Chem Biol Interact 2018; 282: 13–21. [DOI] [PubMed] [Google Scholar]
- 78. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2022.. Diabetes Care 2022; 45: S125–S143. [DOI] [PubMed] [Google Scholar]
- 79. Taha MB, Yahya T, Satish P, et al. Glucagon-like peptide 1 receptor agonists: a medication for obesity management. Curr Atheroscler Rep 2022; 24: 643–654. [DOI] [PubMed] [Google Scholar]
- 80. Gallwitz B, Guzman J, Dotta F, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet 2012; 379: 2270–2278. [DOI] [PubMed] [Google Scholar]
- 81. Nathanson D, Ullman B, Löfström U, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia 2012; 55: 926–935. [DOI] [PubMed] [Google Scholar]
- 82. Cvetkovi?? RS, Plosker GL. Exenatide. Drugs 2007; 67: 935–954. [DOI] [PubMed] [Google Scholar]
- 83. Armstrong MJ, Barton D, Gaunt P, et al.; LEAN trial team. Liraglutide efficacy and action in non-alcoholic steatohepatitis (LEAN): study protocol for a phase II multicentre, double-blinded, randomised, controlled trial. BMJ Open 2013; 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Femminella G, Frangou E, Love S, et al. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study). Trials 2019; 20: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Investig 2013; 123: 2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89: 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Apovian CM, Bergenstal RM, Cuddihy RM, et al. Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes. Am J Med 2010; 123: 468.e9–468.e17. [DOI] [PubMed] [Google Scholar]
- 89. Davies MJ, Bergenstal R, Bode B, et al.; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes. JAMA 2015; 314: 687. [DOI] [PubMed] [Google Scholar]
- 90. Sandsdal RM, Juhl CR, Jensen SBK, et al. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: a randomized controlled trial. Cardiovasc Diabetol 2023; 22: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yaribeygi H, Maleki M, Butler AE, et al. The Impact of incretin-based medications on lipid metabolism. J Diabetes Res 2021; 2021: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Grieco M, Giorgi A, Gentile MC, et al. Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front Neurosci 2019; 13: 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cheng D, Yang S, Zhao X, et al. The role of glucagon-like Peptide-1 receptor agonists (GLP-1 RA) in diabetes-related neurodegenerative diseases. Drug Des Devel Ther 2022; 16: 665–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gilhus NE, Deuschl G. Neuroinflammation – a common thread in neurological disorders. Nat Rev Neurol 2019; 15: 429–430. [DOI] [PubMed] [Google Scholar]
- 95. Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease – a double-edged sword. Neuron 2002; 35: 419–432. [DOI] [PubMed] [Google Scholar]
- 96. Oishi K, Watatani K, Itoh Y, et al. Selective induction of neocortical GABAergic neurons by the PDK1-Akt pathway through activation of mash1. Proc Natl Acad Sci USA 2009; 106: 13064–13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rowlands J, Heng J, Newsholme P, et al. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol 2018; 9: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020; 5: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Salameh TS, Rhea EM, Talbot K, et al. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem Pharmacol 2020; 180: 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Burcelin R, Gourdy P, Dalle S. GLP-1-Based strategies: a physiological analysis of differential mode of action. Physiology 2014; 29: 108–121. [DOI] [PubMed] [Google Scholar]
- 101. Timper K, del Río-Martín A, Cremer AL, et al. GLP-1 receptor signaling in astrocytes regulates fatty acid oxidation, mitochondrial integrity, and function. Cell Metab 2020; 31: 1189–1205.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pipatpiboon N, Pintana H, Pratchayasakul W, et al. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci 2013; 37: 839–849. [DOI] [PubMed] [Google Scholar]
- 103. Vadini F, Simeone PG, Boccatonda A, et al. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes 2020; 44: 1254–1263. [DOI] [PubMed] [Google Scholar]
- 104. Corsetti V, Florenzano F, Atlante A, et al. NH2-truncated human tau induces deregulated mitophagy in neurons by aberrant recruitment of Parkin and UCHL-1: implications in Alzheimer’s disease. Hum Mol Genet 2015; 24: 3058–3081. [DOI] [PubMed] [Google Scholar]
- 105. Moreira PI, Santos MS, Moreno AM, et al. Increased vulnerability of brain mitochondria in diabetic (Goto-Kakizaki) rats with aging and amyloid-beta exposure. Diabetes 2003; 52: 1449–1456. [DOI] [PubMed] [Google Scholar]
- 106. Boccardi V, Murasecco I, Mecocci P. Diabetes drugs in the fight against Alzheimer’s disease. Ageing Res Rev 2019; 54: 1–36. [DOI] [PubMed] [Google Scholar]
- 107. Gejl M, Gjedde A, Egefjord L, et al. In alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, Placebo-Controlled, double-blind clinical trial. Front Aging Neurosci 2016; 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang L-Y, Jin Q-Q, Hölscher C, et al. Glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide dual receptor agonist DA-CH5 is superior to exendin-4 in protecting neurons in the 6-hydroxydopamine rat Parkinson model. Neural Regen Res 2021; 16: 1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012; 61: 1619–1635. [DOI] [PubMed] [Google Scholar]
- 110. Anbazhagan AN, Thaqi M, Priyamvada S, et al. GLP-1 nanomedicine alleviates gut inflammation. Nanomed 2017; 13: 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lebrun LJ, Lenaerts K, Kiers D, et al. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep 2017; 21: 1160–1168. [DOI] [PubMed] [Google Scholar]
- 112. Zietek T, Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Front Immunol 2016; 7: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bang-Berthelsen CH, Holm TL, Pyke C, et al. GLP-1 induces barrier protective expression in Brunnerʼs Glands and regulates colonic inflammation. Inflamm Bowel Dis 2016; 22: 2078–2097. [DOI] [PubMed] [Google Scholar]
- 114. Xiao Q, Boushey RP, Cino M, et al. Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol 2000; 278: 1–7. [DOI] [PubMed] [Google Scholar]
- 115. Schmidt PT, Ljung T, Hartmann B, et al. Tissue levels and post-prandial secretion of the intestinal growth factor, glucagon-like peptide-2, in controls and inflammatory bowel disease: comparison with peptide YY. Eur J Gastroenterol Hepatol 2005; 17: 207–212. [DOI] [PubMed] [Google Scholar]