Abstract
Background
Recent trials suggest that programmed cell death 1 (PD-1)-directed immunotherapy may be beneficial for some patients with anal squamous cell carcinoma and biomarkers predictive of response are greatly needed.
Methods
This multicenter phase II clinical trial (NCT02919969) enrolled patients with metastatic or locally advanced incurable anal squamous cell carcinoma (n=32). Patients received pembrolizumab 200 mg every 3 weeks. The primary endpoint of the trial was objective response rate (ORR). Exploratory objectives included analysis of potential predictive biomarkers including assessment of tumor-associated immune cell populations with multichannel immunofluorescence and analysis of circulating tumor tissue modified viral-human papillomavirus DNA (TTMV-HPV DNA) using serially collected blood samples. To characterize the clinical features of long-term responders, we combined data from our prospective trial with a retrospective cohort of patients with anal cancer treated with anti-PD-1 immunotherapy (n=18).
Results
In the phase II study, the ORR to pembrolizumab monotherapy was 9.4% and the median progression-free survival was 2.2 months. Despite the high level of HPV positivity observed with circulating TTMV-HPV DNA testing, the majority of patients had low levels of tumor-associated CD8+PD-1+ T cells on pretreatment biopsy. Patients who benefited from pembrolizumab had decreasing TTMV-HPV DNA scores and a complete responder’s TTMV-HPV DNA became undetectable. Long-term pembrolizumab responses were observed in one patient from the trial (5.3 years) and three patients (2.5, 6, and 8 years) from the retrospective cohort. Long-term responders had HPV-positive tumors, lacked liver metastases, and achieved a radiological complete response.
Conclusions
Pembrolizumab has durable efficacy in a rare subset of anal cancers. However, despite persistence of HPV infection, indicated by circulating HPV DNA, most advanced anal cancers have low numbers of tumor-associated CD8+PD-1+ T cells and are resistant to pembrolizumab.
Keywords: Immunotherapy; Lymphocytes, Tumor-Infiltrating; Gastrointestinal Neoplasms; Programmed Cell Death 1 Receptor
WHAT IS ALREADY KNOWN ON THIS TOPIC
Programmed cell death 1 (PD-1) inhibitor monotherapy can be beneficial in some patients with advanced anal cancer. Biomarkers of response are greatly needed.
WHAT THIS STUDY ADDS
Most patients with advanced anal cancer had resistance to pembrolizumab, and the response rate was 9.4%. Multichannel immunofluorescence revealed that the majority of trial patients had a low level of tumor-associated CD8+PD-1+ T cells, perhaps suggestive of the resistance mechanism. Biomarkers associated with clinical benefit to pembrolizumab included human papillomavirus (HPV) and programmed cell death ligand 1 positive tumors, no liver metastases, and low baseline HPV cell-free DNA.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
While a rare subset of patients with advanced anal cancer have durable responses to pembrolizumab, therapeutic strategies that increase T-cell tumor-infiltration are needed to overcome the resistance to PD-1-directed monotherapy seen in most patients with advanced anal cancer.
Introduction
The pathogenesis of virally-driven cancers presents unique opportunities and challenges in the development of immunotherapy. Anal cancer is a prototypical virally-driven malignancy in which 80%–90% of cases are caused by the human papillomavirus (HPV).1 2 The E6 and E7 HPV proteins catalyze the oncogenesis of anal cancer by inhibiting the function of the p53 and retinoblastoma tumor suppressors.3–6 While viral proteins are antigenic targets for the immune system, the immunosuppressive properties of HPV promote their persistence within anal epithelial cells.5–7 Mechanisms of HPV-mediated suppression of the antitumor immune response include inhibition of the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway and decreased antigen presentation by the major histocompatibility complex (MHC) class I and II systems.8 9 In addition, HPV promotes immune evasion by upregulating expression of the programmed cell death ligand 1 (PD-L1) checkpoint protein, resulting in PD-L1 being expressed in approximately 70% of anal cancers.10 11
Therapeutic targeting of the programmed cell death 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) immune checkpoint can release T cells from negative inhibition and is effective therapy in multiple cancers.12–15 Though not yet approved by the Food and Drug Administration, PD-1-directed immunotherapy has been recognized as a potentially beneficial therapy in a subset of patients with anal cancer, and its use is endorsed by the National Cancer Comprehensive Network guidelines.11 16–21 To date, trials evaluating single agent PD-1-directed therapy have reported response rates of 11%–24% and a median progression-free survival (PFS) of 2.0–4.1 months.10 11 16 17 Whether patients can have sustained long-term responses to PD-1-directed therapy remains unclear. In addition, identifying patients that could benefit from PD-1-directed therapy is a major clinical challenge. Correlative studies have suggested that PD-L1 positivity and biomarkers indicative of increased intratumoral inflammation, such as baseline tumor-associated CD8+T cells, may be predictive of response to PD-1-directed therapy.16 17
Cell-free circulating DNA derived from circulating tumor cells is a predictive biomarker of treatment response in multiple tumor types.22–25 A challenge in the development of cell-free DNA assays for virally driven cancers is distinguishing between viral DNA produced by chronic viral infection and tumor-associated viral DNA.26 Tumor tissue modified viral (TTMV)-HPV DNA is specific for HPV-associated viral DNA because it is produced during the fragmentation of integrated and/or episomal HPV DNA of malignant epithelial cells.27 28 In HPV-positive squamous cell carcinoma of the head and neck, changes in TTMV-HPV DNA scores correlate with treatment response, and the persistence of TTMV-HPV DNA following treatment is a poor prognostic indicator.26–29 Similarly, persistence of HPV cell-free DNA is a poor prognostic sign following chemoradiation in locally advanced anal cancer as well as first-line palliative chemotherapy in advanced anal cancer.22 30–32
Herein, we report the results of a phase II trial of pembrolizumab in 32 patients with advanced anal squamous cell carcinoma, along with a 19-patient retrospective cohort, in which we examined the efficacy of pembrolizumab, long-term responders, and biomarkers of response and resistance.
Materials and methods
Patient population and trial design
This was a multicenter, open label, single arm phase II clinical trial of pembrolizumab in advanced anal cancer (ClinicalTrials.gov identifier: NCT02919969). Eligible patients had an incurable locally advanced or metastatic anal squamous cell carcinoma. Patients who received previous treatment or who had newly diagnosed incurable disease were eligible for protocol therapy. Additional eligibility requirements included age 18 years, measurable disease according to Response Evaluation Criteria in Solid Tumors, V.1.1 (RECIST V.1.1) criteria, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Key exclusion criteria included active autoimmune disease requiring systemic immunosuppressive treatment, known active central nervous system metastases, and prior PD-1/PD-L1-directed immunotherapy. Patients with medically controlled hepatitis B virus or hepatitis C virus were eligible. HIV-positive patients were eligible if their CD4+ count ≥300 cells/µL, if they had undetectable viral load, and were receiving highly active antiretroviral therapy. The study protocol and all amendments were approved by the participating sites’ institutional review board (IRB) (online supplemental file 2). All patients provided written informed consent. This investigator-initiated trial was funded by Merck, who approved the final manuscript.
jitc-2023-008436supp004.pdf (44MB, pdf)
A separate cohort of patients with advanced anal cancer treated with PD-1-directed therapy at Dana-Farber Cancer Institute (DFCI), who consented to an IRB-approved biobanking protocol from 2017 to 2022, were retrospectively identified and their clinical features and outcomes were recorded by chart review.
Treatment and assessments
Pembrolizumab was administered intravenously at 200 mg once every 3 weeks for up to 2 years. Response was assessed every 9 weeks (three cycles) until cycle 7. After cycle 12, restaging scans were performed every 3–4 cycles at the discretion of the treating investigator. Toxicity was assessed with the Common Toxicity Criteria for Adverse Events, V.4.0.
Outcomes
The primary endpoint was objective response rate (ORR), based on the RECIST V.1.1 criteria. Enrollment of 32 patients provided 90% power to differentiate between an unacceptable 5% ORR and a desirable ORR of 20% at a one-sided 10% alpha error rate. Secondary endpoints included PFS and overall survival (OS) in an intention-to-treat analysis. OS was calculated as the time from treatment initiation until death, and PFS was calculated as the time from treatment initiation until disease progression or death. The Kaplan-Meier method was used to estimate PFS and OS. The clinical benefit rate (CBR) was defined as the percentage of patients with a complete response (CR), partial response (PR) (confirmed and unconfirmed), or stable disease (SD) for at least 6 months’ duration. Wilcoxon and Mann-Whitney tests were used for statistical comparisons and the Spearman coefficient was used to analyze correlation of continuous variables using Prism V.8 (GraphPad), Stata, or R software.
Correlative analysis
Exploratory objectives include analysis of potential predictive biomarkers on pretreatment tumor biopsies and serial blood samples. Samples were collected every cycle for the first three cycles and then every other cycle thereafter until disease progression or treatment discontinuation. Plasma samples were analyzed with a commercially available assay (NavDx, Naveris) for circulating TTMV-HPV DNA.27 28 Samples were assessed for overall DNA integrity, as well as for interpretation of TTMV-HPV DNA score. An indeterminate result indicated consistently low values of TTMV-HPV DNA below the positive threshold value of 5. The PFS landmark time point for TTMV-HPV DNA score change was set at the date of the second blood draw used to calculate the score change.
Multichannel immunofluorescence with an institutional platform (DFCI ImmunoProfile) was used to analyze the tumor microenvironment.33–36 FFPE tumor samples were stained and analyzed with cytokeratin (AE1/AE3), CD8, PD-L1 (E1L3N), PD-1, and FOXP3. Regions of interest (ROIs, minimum five per case) were defined for each image as the regions with the greatest immune cell infiltrates. Within each ROI, InForm Image Analysis software (PerkinElmer/Akoya) was run to phenotype and score cells based on each biomarker expression. Each ROI was divided into one of two defined regions: intratumoral, which was defined as the region of the slide consisting of tumor beyond the tumor-stroma interface; tumor-stroma interface, which was defined as the region within 40 microns to either side of the defined border between tumor and stroma. The total number of tumor-associated cells included cells in the intratumoral space or tumor-stroma interface. Cell count was calculated per ROI and averaged (unweighted) across ROIs, reported as count per millimeter squared±SE. Methodological descriptions of multicolor flow cytometry, ELISA of E6 HPV antibody plasma levels, and central laboratory assessment of PD-L1 immunohistochemistry are included in the online supplemental methods.37
jitc-2023-008436supp003.pdf (183.1KB, pdf)
Results
Patient population and trial design
The demographic and disease characteristics of the patients at baseline are reported in table 1. Between October 12, 2016 and July 9, 2021, 32 eligible patients at three institutions were enrolled (online supplemental figure S1). All registered patients (n=32) received at least one infusion of pembrolizumab and were included in both the safety and efficacy analysis. Patients on the trial were treated for a median of 2 months (range: 0–23 months). The median age was 61 years (range: 36–83 years), 66% of patients were women (n=21), and most patients were white (n=29 (91%)). Most patients (91%) had received prior anticancer therapy with a median number of lines of treatment of 1 (range: 0–3); three patients were treatment naïve. Baseline liver metastases were present in 41% of patients (n=13). As of the data cut-off (September 7, 2022), all 32 patients had discontinued study treatment. The most common reason for removal from the trial was progressive disease (69%). The median follow-up period was 12.6 months (range=0.6–51.6 months).
Table 1.
Demographic and clinical characteristics of patients who started protocol treatment
| Basic demographics | |
| Age (years) (median; range) |
61 (36–83) |
| Sex (n, %) | (n=32) |
| Female | 21 (66) |
| Male | 11 (34) |
| Race (n, %) | (n=32) |
| Asian | 1 (3) |
| White | 29 (91) |
| Other | 2 (6) |
| Ethnicity (n, %) | (n=32) |
| Hispanic | 1 (3) |
| Non-Hispanic | 27 (84) |
| Unknown | 4 (13) |
| ECOG (n, %) | (n=32) |
| 0 | 13 (41) |
| 1 | 19 (59) |
| HIV status (n, %) | (n=32) |
| Positive | 1 (3) |
| Negative | 31 (97) |
| HPV status (n, %) | (n=32) |
| Positive | 27 (84) |
| Negative | 3 (9) |
| Unknown | 2 (6) |
| T stage at diagnosis (n, %) | (n=32) |
| T1 | 4 (13) |
| T2 | 12 (38) |
| T3 | 7 (22) |
| T4 | 4 (13) |
| Unknown | 5 (16) |
| N stage at diagnosis (n, %) | (n=32) |
| N0 | 8 (25) |
| N1 | 14 (44) |
| N2 | 1 (3) |
| N3 | 3 (9) |
| Unknown | 6 (19) |
| M stage at diagnosis (n, %) | (n=32) |
| M0 | 15 (47) |
| M1 | 16 (50) |
| Unknown | 1 (3) |
| Tumor mutational burden (median mut/Mb, range) | 6.083 (3.042–14.45) |
| Mismatch repair status | (n=32) |
| Proficient | 11 (34) |
| Indeterminate | 1 (3) |
| Unknown | 20 (63) |
| PD-L1 combined positive score | (n=27) |
| <1 | 4 (15) |
| ≥1 | 23 (85) |
| Metastases | (n=32) |
| Liver | 13 (41) |
| Lungs | 17 (53) |
| Lymph nodes | 25 (78) |
| Bone | 2 (6) |
| Prior treatment | (n=32) |
| Radiation (n, %) | 25 (78) |
| Systemic therapy | |
| Yes (n, %) | 29 (91) |
| No (n, %) | 3 (9) |
| Therapy type | (n=29) |
| Capecitabine | 3 (10) |
| Carboplatin/paclitaxel | 2 (7) |
| Fluoropyrimidine/cisplatin | 14 (48) |
| Fluoropyrimidine/mitomycin C | 21 (72) |
| FOLFIRI | 1 (3) |
| FOLFOX | 1 (3) |
ECOG, Eastern Cooperative Oncology Group; FOLFIRI, 5-fluorouracil, folinic acid, and irinotecan; FOLFOX, 5-fluorouracil, folinic acid, and oxaliplatin; HPV, human papillomavirus; mut/Mb, mutations/megabase; PD-L1, programmed cell death ligand 1.
jitc-2023-008436supp002.pdf (2.4MB, pdf)
Pembrolizumab efficacy
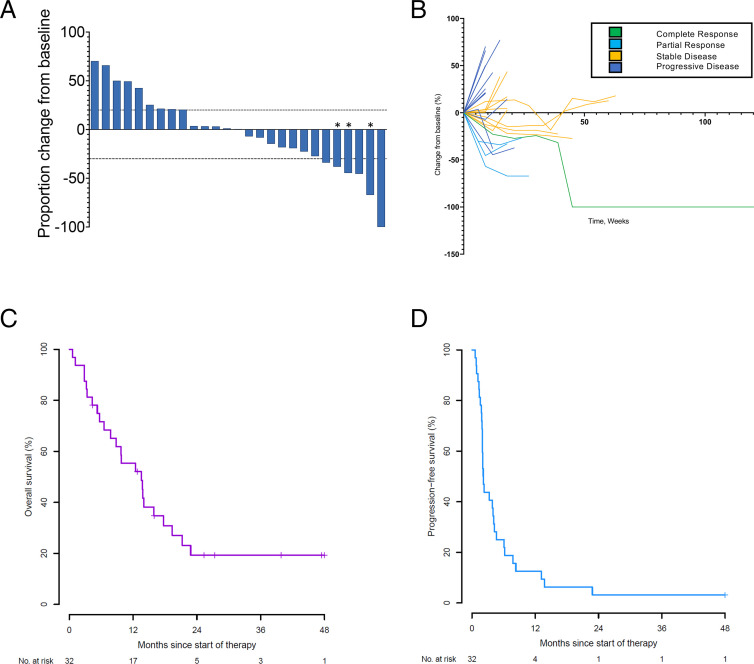
The ORR was 9.4% (95% CI: 2.0% to 25.0%), thus the primary endpoint was not met (figure 1A,B and online supplemental table 1). The ORR in patients whose tumors expressed PD-L1 with a combined positive score (CPS) of 1 or greater was 13.0% (95% CI: 2.8% to 33.6%). The CBR was 21.9% (95% CI: 9.3% to 40.0%). The median OS was 13.6 months (95% CI: 6.5 to 17.7) (figure 1C). The 6-month OS rate was 71.6% (95% CI: 52.5% to 84.1%), and the 12-month OS rate was 55.3% (95% CI: 36.5% to 70.7%). The median PFS was 2.2 months (95% CI: 1.9 to 4.1) (figure 1D). The 6-month PFS rate was 25.0% (95% CI: 11.8% to 40.7%), and the 12-month PFS rate was 12.5% (95% CI: 3.9% to 26.2%). In HPV-positive patients, the median PFS was 2.2 months (95% CI: 1.9 to 4.3), and the median OS was 13.8 months (95% CI: 9.7 to 21.3). None of the patients with HPV-negative anal squamous cell carcinoma (n=3) benefited from pembrolizumab as all these patients discontinued the study within 2 months. Patients with liver metastases had significantly shorter PFS than patients who did not have liver metastases (median PFS 1.8 months vs 4.1 months, log-rank p=0.0006), along with significantly shorter OS (median OS 6.5 months vs 17.7 months, log-rank p=0.0309).
Figure 1.
Response to pembrolizumab in patients with anal cancer. (A) Waterfall plot of best radiological response to pembrolizumab. Best overall response in the 27 radiologically evaluable patients treated with pembrolizumab. *Signifies that a new lesion developed. (B) Spider plot of radiological response to pembrolizumab stratified by best radiological response. (C,D) Kaplan-Meier estimates of overall survival (C) and progression-free survival (D). NO, number.
jitc-2023-008436supp001.pdf (114.8KB, pdf)
Safety
The main reason for pembrolizumab discontinuation was progressive disease. Pembrolizumab was generally well tolerated, and its toxicity profile was similar to what has been reported in other malignancies.10–12 38 39 A total of 78% of patients experienced at least one grade ≥1 adverse event (table 2). Two patients (6%) experienced grade 3 toxicity attributable to pembrolizumab, including hypothyroidism, hyperthyroidism, and rash. No grade 4 or 5 adverse events related to trial therapy were observed. Notably, in agreement with prior reports, the one patient with HIV positive enrolled in the study, who had a CD4 count of 205 cells/microliter at study initiation, tolerated the pembrolizumab well and showed no grade ≥3 toxicities.40
Table 2.
Adverse events (possibly, probably, definitely)
| Any-grade adverse events occurring in ≥2 patients, n (%) N=25 | |
| Anemia | 2 (8) |
| Anorexia | 2 (8) |
| Arthralgia | 4 (16) |
| Cough | 2 (8) |
| Creatinine increase | 2 (8) |
| Diarrhea | 4 (16) |
| Dyspnea | 2 (8) |
| Edema | 3 (12) |
| Fatigue | 12 (48) |
| Hypothyroidism | 4 (16) |
| Liver function test* | 11 (44) |
| Alanine aminotransferase | 2 (8) |
| Alkaline phosphatase | 2 (8) |
| Aspartate aminotransferase | 6 (24) |
| Bilirubin | 1 (4) |
| Myalgia | 2 (8) |
| Nausea | 3 (12) |
| Pruritus | 5 (20) |
| Rash | 5 (20) |
| Grade 3–4 adverse events occurring in ≥1 patient, n (%) | |
| Rash | 2 (8) |
| Hypothyroidism | 1 (4) |
| Hyperthyroidism | 1 (4) |
| Headache | 1 (4) |
*Liver function test includes aspartate aminotransferase, alanine aminotransferase, bilirubin, and alkaline phosphatase elevations.
Analysis of the tumor immune microenvironment
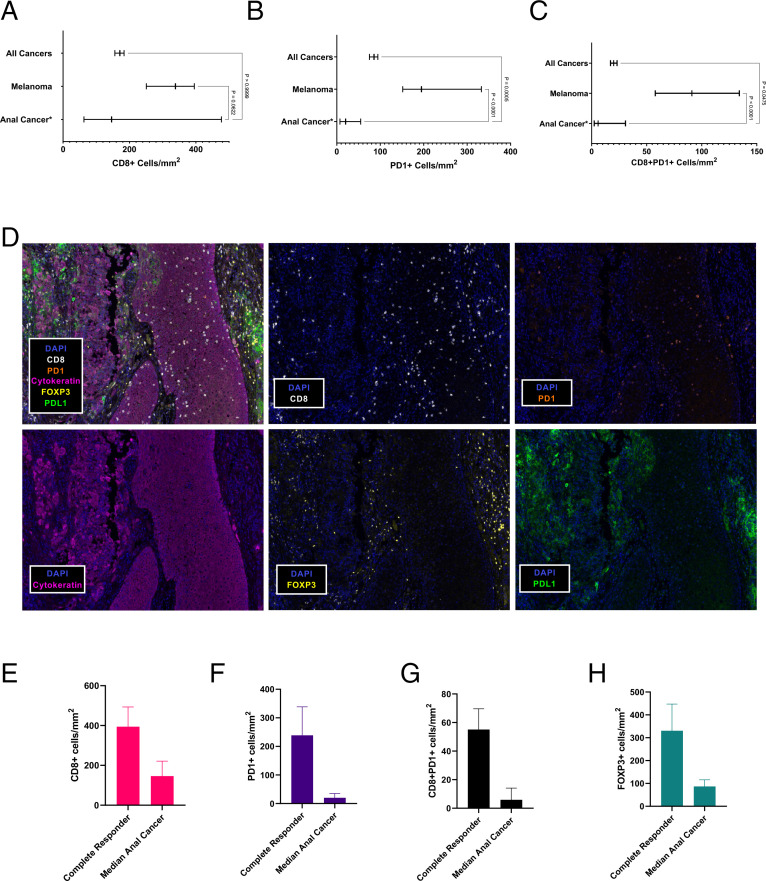
To understand the sensitivity and resistance of anal cancer to pembrolizumab, we examined the tumor immune microenvironment in patients (n=24) enrolled in the prospective clinical trial using pre-enrollment biopsy samples (n=8) or archival samples (n=16). These biopsy samples were obtained from either the primary anal tumor (n=5) or a metastatic site (n=19). We used the DFCI ImmunoProfile multichannel immunofluorescence assay, which has been used to profile over 1000 patient tumors of more than 20 cancer types, to characterize T-cell infiltration in the biopsy samples.34 41 To perform this analysis, we examined the total number of tumor-associated cells located intratumorally and at the tumor-stroma interface. Compared with the median number of tumor-associated PD-1+ and CD8+PD-1+ cells observed in the pancancer cohort of 1152 patients assayed by ImmunoProfile (online supplemental table 2), there were significantly fewer tumor-associated PD-1+cells (p=0.0005) and CD8+PD-1+ cells (p=0.0475) in biopsies obtained from patients with anal cancer on the prospective trial (n=24) (figure 2A–C). When compared with a cohort of patients with melanoma (n=69) assayed by ImmunoProfile, patients with anal cancer in the trial cohort (n=24) also had a significantly lower number of mean tumor-associated PD-1+cells (345 cells/mm2 vs 844 cells/mm2, p<0.0001, Kruskal-Wallis test) and median tumor-associated CD8+PD-1+ cells (441 cells/mm2 vs 916 cells/mm2, p<0.0001, Kruskal-Wallis test).
Figure 2.
Tumor immune microenvironment of anal cancer. (A,B,C) Multichannel immunofluorescence (mIF) measurement of median CD8+T cells (A) median PD-1+cells (B) and median CD8+PD-1+ cells (C) in all cancers tested, melanoma, and anal cancer (95% CI). (D) Tumor immune microenvironment by mIF (20× magnification) for patient 12 who experienced a complete response to pembrolizumab. Blue, DAPI; white, CD8; orange, PD-1; pink, cytokeratin; yellow, FOXP3; green, PD-L1;. (E, F, G, H) Median number of tumor-associated cells in the patient with a complete response compared with median number of cells tumor-associated cells in all other patients with anal cancer enrolled on the trial. (E) CD8+tumor-associated T cells (394.5 cells/mm2 vs 146.1 cells/mm2), (F) PD-1+tumor-associated cells (238 cells/mm2 vs 20.3 cells/mm2), (G) CD8+PD-1+ tumor-associated cells (55.1 cells/mm2 vs 5.9 cells/mm2), (H) FOXP3+cells (330.7 cells/mm2 vs 87.0 cells/mm2). DAPI, 4',6-diamidino-2-phenylindole; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Given the relatively small number of patients in this clinical trial, we were unable to discern any differences in the tumor immune microenvironment between patients with versus without clinical benefit (online supplemental figure 2A–E). Notably, the tumor-associated CD8+T cells and CD8+PD-1+ T cells were substantially higher in the patient (trial patient #12) who had a long-term CR. Compared with the median staining levels observed in the entire clinical trial, the long-term responder had 2.7-fold greater CD8+cells, 9-fold greater CD8+PD-1+ cells, and 3.8-fold greater FOXP3+cells (figure 2D–G, online supplemental figure S3).
PD-L1, tumor mutational burden, and circulating immune cells as biomarkers of pembrolizumab response
Because PD-L1 staining and tumor mutational burden (TMB) are predictive biomarkers of PD-1-directed therapy in other malignancies, we analyzed whether these biomarkers correlated with objective response and clinical benefit to pembrolizumab in anal cancer.38 39 42 43 In the prospective trial, 12 of the 32 patients had genomic sequencing data that included TMB. The median TMB was 6.083 mutations/Mb (range: 3.042–14.448). We did not observe any significant differences in the TMB on the basis of response or clinical benefit (online supplemental figure S4A, B). Similar to other studies, we observed that 85% of anal cancers (n=22/26) from patients enrolled on the trial had tumors that expressed PD-L1 with a CPS of 1 or greater.10 11 The four patients with PD-L1 negative tumors all rapidly progressed on pembrolizumab (median PFS for PD-L1 negative cancers was 0.85 months; range: 0.59–4.27). All patients who derived clinical benefit (n=7), defined as CR, PR, or SD for at least 6 months’ duration, had PD-L1 positive tumors. Among PD-L1 positive tumors (n=22), the median CPS was 30 (range: 1–100). We did not observe an association between higher CPS and the ORR or the clinical benefit rate.
To understand whether pembrolizumab-induced changes in the peripheral circulating immune cell populations were associated with treatment response, we performed flow cytometry on peripheral blood mononuclear cells obtained from patients on the clinical trial at pretreatment and 6 weeks after pembrolizumab initiation. The decrease in PD-1 mean fluorescence intensity indicated target engagement by pembrolizumab, which binds to an epitope that overlaps with the anti-PD-1 antibody used for detection by flow cytometry (p=0.0001) (online supplemental figure S4C). Response to PD-1 blockade is associated with reinvigoration of antigen-specific CD8+T cells in peripheral blood.44 Six weeks after pembrolizumab initiation, we observed an expansion of CD8+CD45RO+HLA-DR+ CD38+ T cells (p=0.0058), in agreement with findings from previous studies showing that this population expands in patients with non small cell lung cancer (NSCLC) treated with PD-1 blockade (online supplemental figure S4D).44 These changes in PD-1 accessibility and expansion of HLA-DR+CD38+ CD8 T cells were observed across all patients exposed to pembrolizumab. No differences in circulating quantities of T cells were associated with clinical benefit, including CD4+T cells, peripheral helper T cells, CD8+T cells, and CD8+CX3CR1+T cells (online supplemental figures S5–S7). No significant differences in circulating quantities of B cells were associated with clinical benefit, including naïve B cells, antibody secreting B cells, or activated B cells (online supplemental figures S5–S7).
Circulating TTMV-HPV DNA is a biomarker of response to pembrolizumab
Given the strong association of HPV with anal cancer, we explored whether the presence of circulating anti-E6 antibody, as determined by ELISA, could be used as a blood-based biomarker.45 However, results from our ELISA assay did not correlate with the presence of HPV-positive tumors or therapy-related tumor volume changes (online supplemental figure S8).
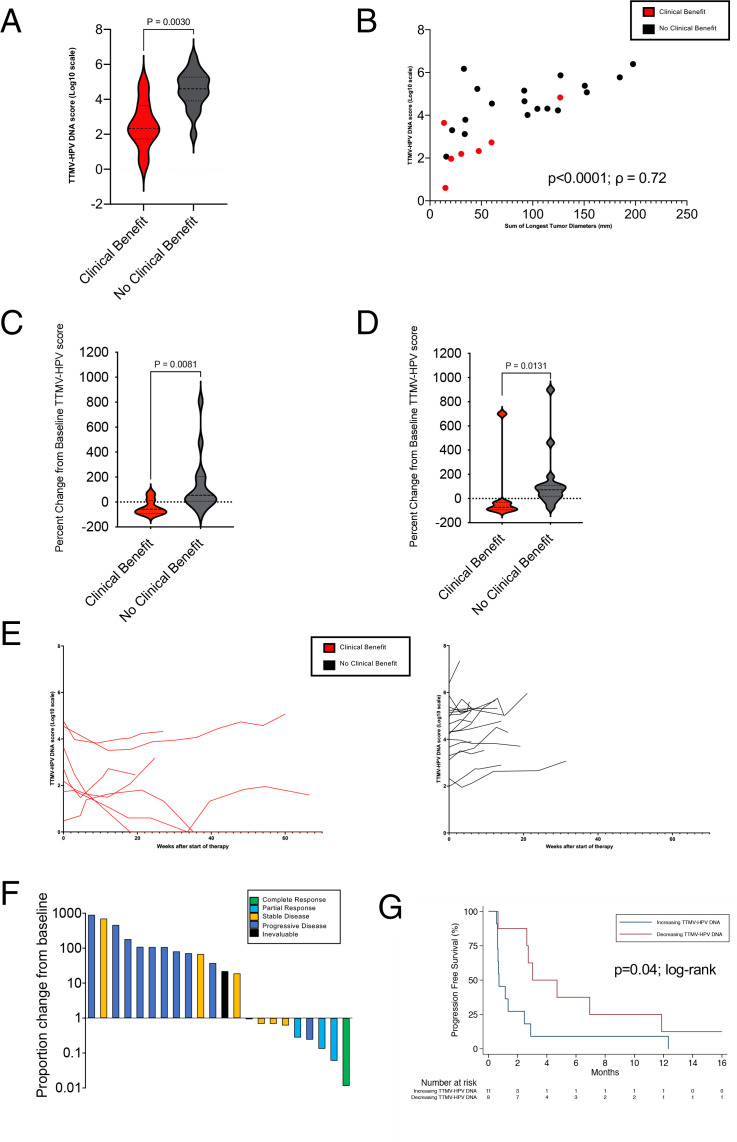
Since anti-E6 antibody titers were not an adequate biomarker of cancer detection or pembrolizumab response, we investigated whether the TTMV-HPV DNA score could be used as a biomarker in patients on the prospective trial using serial plasma samples. Twenty-nine of the 32 trial patients had a baseline plasma sample available, of which 26 had HPV-positive anal cancer and three patients had HPV-negative anal cancer, on the basis of p16 immunohistochemistry (IHC) and/or HPV genotyping (online supplemental table 3). Of the 26 patients with HPV-positive anal cancer, 24 were TTMV-HPV DNA positive and 1 was TTMV-HPV DNA indeterminate (later TTMV-HPV DNA positive). There was one anal cancer that was p16-positive, but serial plasma samples were TTMV-HPV DNA negative despite progressive disease, consistent with literature showing that p16 is not 100% specific for HPV.1 Compared with tumors identified as HPV-positive by p16 and/or HPV genotyping, the sensitivity of the pretreatment TTMV HPV-DNA score was 95% and the specificity was 100%. Twenty-seven patients had a positive TTMV-HPV DNA score at any time point during the trial, all of whom had the HPV-16 genotype, which is consistent with epidemiological studies indicating that HPV-16 is the most implicated genotype in anal cancer.1 3 4 At baseline, lower TTMV-HPV DNA scores were associated with clinical benefit (best response as CR, PR, or SD≥6 months) (p=0.003) (figure 3A). The median baseline TTMV-HPV DNA score was 376 versus 35,046 in patients who had clinical benefit compared with those without benefit. Baseline TTMV-HPV DNA scores are positively correlated with greater tumor burden, according to baseline RECIST measurements (Spearman coefficient, ρ=0.72, p<0.0001) (figure 3B). Notably, on multivariate analysis controlling for site of metastatic disease, PD-L1 CPS, and circulating immune cell ratios, baseline TTMV-HPV DNA score was not independently associated with an improvement in PFS.
Figure 3.
Analysis of TTMV-HPV DNA and correlation with treatment outcome. (A) Baseline TTMV-HPV DNA score in patients with clinical benefit (patients with best response as complete response, partial response, or stable disease ≥6 months) versus patients with no clinical benefit (patients with best tumor response as stable disease <6 months, progressive disease, or inevaluable). (B) Correlation between baseline TTMV-HPV DNA score and tumor diameter measurement by Response Evaluation Criteria in Solid Tumors (p<0.0001, ρ=0.72, Spearman coefficient). Each patient is represented by a colored dot that is based on clinical benefit and detailed in the upper-right. (C) TTMV-HPV DNA score per cent change from baseline after cycle 1 (range: 2–4 weeks after first dose of pembrolizumab) by clinical benefit. (D) TTMV-HPV DNA score percent change from baseline to cycle 2 (range: 5–7 weeks after first dose of pembrolizumab) by clinical benefit. (E) Changes in TTMV-HPV DNA score of trial participants over time (weeks). Red line indicates clinical benefit. Black line indicates no clinical benefit. (F) TTMV-HPV DNA score proportional change plotted in descending order, color coded by best objective response. X axis, bars represent individual patient data; Y axis, TTMV-HPV DNA score proportional change 6 weeks after start of pembrolizumab. (G) Patient progression-free survival segregated by proportional TTMV-HPV DNA score change 6 weeks after start of pembrolizumab (increasing vs decreasing TTMV-HPV DNA score). X axis, time from second plasma draw in months; Y axis, per cent of patients with progression-free survival. TTMV-HPV, tumor tissue modified viral-human papillomavirus.
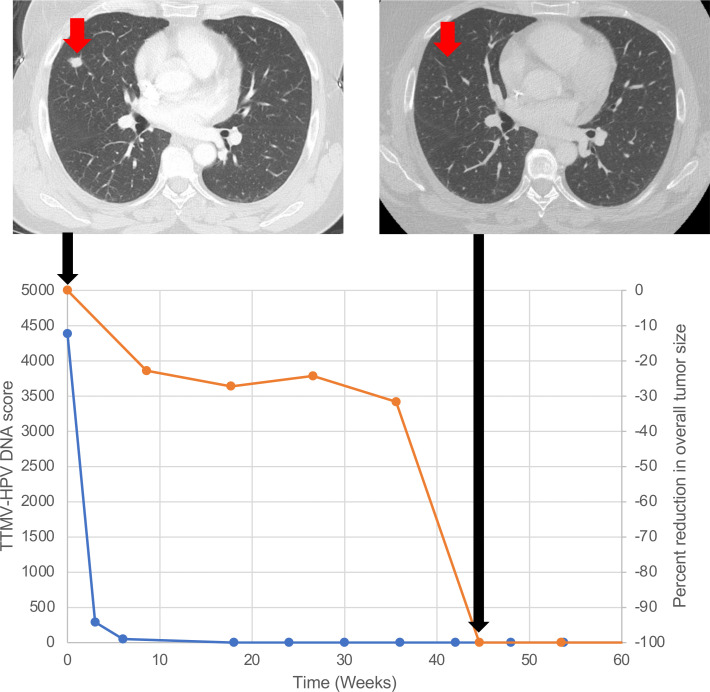
We next analyzed whether dynamic changes in serial TTMV-HPV DNA scores correlated with tumor response to pembrolizumab. No patient had stable TTMV-HPV DNA scores; all either increased or decreased. Decreasing TTMV-HPV DNA scores correlated with clinical outcomes as well; a decrease in TTMV-HPV DNA score from baseline to cycle 2 (3 weeks) was significantly associated with clinical benefit (median 58% decrease vs 54% increase, p=0.008) (figure 3D; online supplemental figure S9). Moreover, a decrease in TTMV-HPV DNA score from baseline to cycle 3 (6 weeks) was also significantly associated with clinical benefit from pembrolizumab (median 72% decrease vs 72% increase, p=0.01) (figure 3E). All patients with an objective response (CR or PR) had a decreasing TTMV-HPV DNA score at cycle 3 (6 weeks) (figure 3F). Furthermore, patients who had a decreasing TTMV-HPV DNA score at 6 weeks had a median PFS of 3.0 months (95% CI: 0.66 to 11.9), which was significantly higher than the 0.7 months median PFS (95% CI: 0.6 to 2.5) seen in patients with an increasing TTMV-HPV DNA score at 6 weeks (HR: 0.37; 95% CI: 0.14 to 0.99, log-rank p=0.04) (figure 3G). Notably, the patient (trial patient #12) who achieved a radiological CR at week 45, had undetectable TTMV-HPV DNA at 18 weeks (figure 4).
Figure 4.
Radiological and cell-free DNA changes in a trial patient with a radiological complete response. Chest CT scans of the trial patient (patient 12) with a complete response (top of the figure). The bottom of the figure graphs the complete responder’s TTMV-HPV DNA score (blue) and per cent reduction in overall tumor size (orange) over time. The TTMV-HPV DNA score became undetectable by 18 weeks after start of treatment. Radiographic resolution of disease was achieved 45 weeks after start of treatment. Black arrows correspond to time points when the CT scans were obtained. TTMV-HPV, tumor tissue modified viral-human papillomavirus.
Long-term response to PD-1-directed immunotherapy in anal cancer
On the prospective clinical trial, the patient with a CR (trial patient #12) had durable benefit to pembrolizumab with no evidence of cancer recurrence 5.3 years after starting treatment with pembrolizumab. The clinical characteristics of patients with advanced anal cancer who have a long-term response to PD-1-directed therapy is poorly understood. To further examine this question, we also analyzed a retrospective cohort of patients with advanced anal cancer (n=18) who enrolled in an institutional biobanking protocol and were treated with anti-PD-1/PD-L1 therapy. The clinical and demographic characteristics of this cohort are listed in online supplemental table 4. Over half of the patients (11/18, 61%) in this retrospective cohort presented with locally advanced disease and were treated with chemoradiation. The other 39% of patients (7/18) in this cohort presented with metastatic disease and all of these patients received first-line cytotoxic chemotherapy (7/7), with four patients receiving palliative radiation as well. The median number of prior systemic therapies (including chemoradiation) prior to anti-PD-1 immunotherapy for this cohort was 1 (range: 1–4).
Three of the patients in the retrospective cohort had a long-term CR to pembrolizumab (n=3/18). All three of these patients have ongoing responses that continue 2.5, 6, and 8 years after initiating pembrolizumab.46 Each of the long-term responders in the retrospective cohort initially presented with locally advanced disease. Two of these patients developed metastatic disease within 4 months of completing chemoradiation, while the third patient developed metastatic disease 1.5 years after having a clinical CR to chemoradiation. Trial patient #12, the one long-term responder (n=1/32) on the prospective trial, initially presented with metastatic disease and pembrolizumab was the second-line of therapy. Notably, all four of the complete responders stopped pembrolizumab after 2–3 years and have not needed any additional anticancer treatments.
To further explore the underlying characteristics of long-term responders, we examined the clinical features and molecular biomarkers of each patient’s cancer (table 3). All long-term responders achieved a complete radiological response and had tumors that were HPV-positive, based on P16 positivity as determined by IHC. Two patients with available HPV genotyping data had HPV genotype 16. The TMB of the long-term responder cohort was modest ranging from 6.1 to 12.2 mut/Mb. All tumors from long-term responders that could be tested (n=3/4) were PD-L1 positive. None of the long-term responding patients had liver metastases when they started pembrolizumab. Two patients had lymph node metastases alone, another patient had lung metastases alone, and the other patient had both lymph node and lung metastases.
Table 3.
Baseline characteristics of long-term responders to pembrolizumab
| Patient | Age (yrs) | Prior therapies | Sites of metastases at pembrolizumab initiation | Duration of pembrolizumab prior to treatment break (months)* | HPV status | TMB | MMR status | PD-L1 status | PFS from time of pembrolizumab initiation (yrs) |
| DFCI PT #1* | 60s | 5FU/MMC/RT, FOLFOX | Lymph node | 24 | Positive | 10.0 | Not reported | Positive | 8 |
| DFCI PT #2* | 70s | 5FU/MMC/RT | Lung, lymph node | 36 | Positive | 7.6 | MMR proficient | Unknown | 6 |
| DFCI PT #3* | 60s | 5FU/MMC/RT | Lymph node | 24 | Positive | 12.2 | MMR proficient | Positive | 2.5 |
| Trial PT #12 | 50s | CIS/5FU/RT | Lung only | 24 | Positive | 6.1 | MMR proficient | Positive | 5.3 |
TMB is measured in mutations/megabase.
*Indicates patients in the retrospective cohort.
CIS, cisplatin; FOLFOX, folinic acid, 5FU, and oxaliplatin; 5FU, 5-fluorouracil; HPV, human papillomavirus; MMC, mitomycin C; MMR, mismatch repair; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; DFCI PT, Dana-Farber Cancer Institute patient; RT, radiation therapy; TMB, tumor mutational burden; yrs, years.
Discussion
For decades, treatment options for patients with advanced anal cancer have been limited to cytotoxic chemotherapy. Results reported here, and in other studies, demonstrate that PD-1-directed therapy has efficacy in a subset of patients with advanced anal cancer.10 11 16 17 We observed an ORR of 9.4% and a CBR of 25.9%. These data are consistent with those from other trials of PD-1 inhibition in previously treated advanced anal squamous cell carcinoma (SCC) with ORR ranging from 11% to 24%.10 11 16 17 The median PFS (2.2 months) and median OS (13.6 months) herein were also similar to those in the KEYNOTE-028 (3.0 months PFS and 9.3 months OS) and KEYNOTE-158 (2.0 months PFS and 11.9 months OS) trials.
Although pembrolizumab has activity in only a subset of patients with anal cancer, in the analysis of our clinical trial and retrospective cohort, we observed durable long-term responses, of 2.5, 5.3, 6, and 8 years, in a rare subset of patients treated with PD-1-directed therapy. Our data indicate that long-term responders shared several characteristics. All of these patients were HPV-positive, lacked liver metastases, and achieved a radiological CR. The tumor microenvironment before pembrolizumab treatment in one long-term responder had substantially more tumor-associated CD8+T cells and CD8+PD-1+ T cells than other patients enrolled on the trial. This is consistent with findings from the NCI9673 trial showing that anal cancers sensitive to nivolumab had higher levels of tumor-infiltrating CD8+PD-1+ T cells compared with anal cancers resistant to nivolumab.16
While a small number of patients with anal cancer can have a durable response to pembrolizumab, a critical question is why most anal cancers are refractory to PD-1-directed therapy. The 9.4% ORR observed in our trial is consistent with the ORR of single-agent PD-1-directed therapy in other advanced HPV-associated malignancies such as cervical cancer (12%) and head and neck cancer (19%).38 47 48 In addition to upregulating PD-L1, HPV also inhibits the c-GAS/STING pathway and interferes with antigen presentation by MHC class I and MHC class II, making it less surprizing that PD-1-directed monotherapy is insufficient therapy in most tumors. Both c-GAS/STING pathway inhibition and decreased antigen presentation can result in decreased T-cell infiltration. We observed low levels of tumor-associated CD8+PD-1+ T cells in patients enrolled on this trial, which contrasts with malignancies known for PD-1-inhibitor responsiveness like melanoma.49 Instead, these findings suggest that most patients with advanced anal cancer have an “immunologically cold” tumor microenvironment which may explain the resistance to anti-PD-1 immunotherapy that is commonly seen in this population. This finding is consistent with other reports which have described low levels of tumor infiltrating T cells in patients with anal cancer with chemoradiation-refractory and/or advanced disease.50–52 While these data suggest that most patients with advanced anal cancer have low levels of T-cell infiltration, interestingly high levels of T-cell infiltration portends a positive prognosis in localized anal cancer suggesting important biological differences in these two disease subgroups.50 51
In addition to T-cell infiltration, our study examined other potential predictive biomarkers of pembrolizumab sensitivity in anal cancer. All responding patients had PD-L1-positive and HPV-positive tumors. Although the TMB of responding patients was modest (median=5.703 mut/MB), virally-driven tumors do not require a high mutational burden to be antigenic. For instance, viral-associated Merkel cell carcinomas have a relatively modest TMB while still being sensitive to PD-1 inhibition likely because viral proteins act as sensitizing antigens.13 53
Similar to other malignancies, we observed that liver metastases were associated with resistance to PD-1-directed therapy in anal cancer.54 None of the long-term responders in either cohort had liver metastases. Similarly, in the POD1UM-202 trial, the only patient with a CR to the anti-PD-1 antibody retifanlimab had lymph node metastases alone without liver metastases.17 Unfortunately, liver metastases are common in anal cancer and were present in 40% of patients enrolled on our trial. Preclinical studies have suggested that liver metastases promote resistance to immunotherapy by downregulating the function and number of CD8+T cells, perhaps due to the known upregulation of monocyte-derived macrophages found in tumor-bearing livers.54 55
Since anal cancers have a range of responses to PD-1-directed therapy, biomarkers that can predict and track response to therapy are greatly needed. In patients with anal cancer treated with docetaxel, cisplatin, and 5-fluorouracil, circulating tumor HPV DNA was prognostic of progression if still detectable after completion of chemotherapy.22 56 57 In our analysis of circulating tumor cell derived HPV cell-free DNA, patients with lower baseline circulating TTMV-HPV DNA scores were more likely to derive clinical benefit from immunotherapy, similar to reported findings in patients with HPV-positive head and neck cancer.58 Furthermore, we demonstrated a strong positive correlation with TTMV-HPV DNA scores and overall tumor burden.22 27 28 In our study, patients with clinical benefit were also significantly more likely to show a decrease in TTMV-HPV DNA score within the first 6 weeks of therapy than patients who did not show clinical benefit to pembrolizumab. Notably, circulating TTMV-HPV DNA became undetectable in a patient with a CR 6 months before the radiologic CR was achieved.
The presence of circulating TTMV-HPV DNA in all patients with HPV-positive tumors indicates that viral antigens are likely still being expressed by these cancers. Therefore, the low level of T-cell infiltration and the poor efficacy of immunotherapy in most patients with advanced anal cancer are unlikely due to lack of antigen and more likely attributable to other factors. Loss of antigen presentation on MHC class I or blunting of the type I interferon response are both strategies for HPV-mediated immune evasion.6 8 9 Two recent negative randomized trials, the NCI9673 study (NCT02314169) evaluating nivolumab/ipilimumab as well as the SCARCE-PRODIGE 60 study (NCT03519295) evaluating atezolizumab combined with first-line docetaxel, cisplatin, and 5-fluorouracil chemotherapy, highlight the difficulty of designing next generation PD-1-directed immunotherapy strategies in anal cancer.59 60 Ongoing anal cancer research to overcome this immune evasion includes trials that combine PD-1 immunotherapy with carboplatin/paclitaxel chemotherapy (NCT04444921) as well as incorporating PD-1 blockade with chemoradiation in patients with high risk locally advanced disease (NCT03233711). Since HPV is known to inhibit the cGAS-STING pathway, one rational combination for future clinical trials would be an anti-PD-1 antibody combined with a STING agonist.
This study has several limitations. Our correlative analyses were limited by the small number of patients in the trial. Therefore, correlative analysis of tumor-associated lymphocytes was limited by power. Given the rarity of advanced anal cancer and the difficulty identifying long-term responders to PD-1-directed therapy, we combined the prospective clinical trial cohort (n=32) and with a retrospective cohort (n=18). The potential shortcoming of this approach is that the retrospective cohort might have introduced a selection bias which may overestimate the number of long-term responders. Additionally, despite up to 10% of patients with anal cancer having HIV, our trial only enrolled one patient with HIV.61 Consistent with prior work on PD-1 inhibition being well tolerated in patients with HIV, our patient tolerated the pembrolizumab well and did not experience any grade ≥3 toxicity.40 Continued efforts need to be made to ensure that patients with HIV receive appropriate cancer-directed treatment and are also enrolled into clinical trials.
In summary, a small subset of anal cancers are responsive to pembrolizumab immunotherapy, including a rare subgroup of patients who achieve a durable, long-term, CR. While the complete responder in our trial had a high level of tumor-associated CD8+PD-1+ T cells, most advanced anal cancers, despite having persistent HPV infection and antigen production, have low levels of tumor-associated CD8+PD-1+ T cells. The low level of CD8+PD-1+ T-cell infiltration likely drives resistance to PD-1 blockade in most patients with advanced anal cancer, and future immunotherapeutic strategies should explore ways of increasing CD8+PD-1+ T-cell infiltration.
Acknowledgments
We thank the patients and their families for participating in this clinical trial. We thank Dana-Farber/Harvard Cancer Center in Boston, Massachusetts, USA, for the use of the Specialized Histopathology Core, which provided multiplex immunofluorescence and IHC staining services.
Footnotes
Twitter: @bhuffmanmd, @vanallenlab, @aparna1024, @sunildavv, @labrat3
SR, SKD and JMC contributed equally.
Contributors: All authors satisfy the International Committee of Medical Journal Editors (ICMJE) criteria for authorship, and no person or persons other than the authors listed have contributed significantly to its preparation. The contents of this manuscript are our original work and have not been previously presented in whole or in part. JMC is responsible for the overall content as guarantor.
Funding: This trial was funded by Merck. This study was also supported by the Donna V. Toelke Fund for Cancer Research, the Margaret Anthony Family Fund, and the Maggie and Don Swift Fund. The work of JMC is supported by Stand Up to Cancer, the Lustgarten Foundation, Hale Family Center for Pancreatic Cancer Research and Breakthrough Cancer. The work was also supported by a grant from the National Institutes of Health (P50CA127003). MTH was funded by a Baruj Benacerraf Fellowship. SKD was funded by NIH 1R01AI158488-01, U01 CA274276-01 and the Ludwig Center at Harvard.
Competing interests: BMH has received honorarium from TD Cowen for consulting. JMC has received funding to her institution for research from Lilly and Sanofi. She has served as a consultant or on advisory boards for Novartis/AAA, Ipsen, Curium, and TerSera. She has received royalties from UpToDate. She owns stock in Merck. JAM received an honorarium for being on the advisory board of Merck. NJM received funding to her institution for research from Bristol Myers Squibb. MBY received research funding from Janssen Pharmaceuticals and fees for peer review services from UpToDate. BR has institutional patents filed on methods for clinical interpretation. EMVA has served in an advisory/consulting role for Tango Therapeutics, Genome Medical, Genomic Life, Enara Bio, Manifold Bio, Monte Rosa, Novartis Institute for Biomedical Research, Riva Therapeutics, and Serinus Bio. He receives research support from Novartis, BMS, and Sanofi. He has investment equity in Tango Therapeutics, Genome Medical, Genomic Life, Syapse, Enara Bio, Manifold Bio, Microsoft, Monte Rosa, Riva Therapeutics, Serinus Bio. He holds institutional patents filed on chromatin mutations and immunotherapy response, and methods for clinical interpretation; intermittent legal consulting on patents for Foaley & Hoag. AP has equity in C2i Genomics XGenomes and Parithera and in the last 36 months, has served as an advisor/consultant for Eli Lilly, Pfizer, Inivata, Biofidelity, Checkmate Pharmaceuticals, FMI, Guardant, Abbvie, Bayer, Delcath, Taiho, CVS, Value Analytics Lab, Seagen, Saga, AZ, Scare, Illumina and Science For America. She receives fees from Up to Date. She has received travel fees from Karkinos Healthcare. She has been on the DSMC for a Roche study and on Steering Committee for Exilixis. She has received research funding to the Institution from PureTech, PMV Pharmaceuticals, Plexxicon, Takeda, BMS, Mirati, Novartis, Erasca, Genentech, and Daiichi Sankyo. KN has received research funding to her institution from Pharmavite, Evergrande Group, Janssen, and Revolution Medicines. She has consulted or served on advisory boards for Bayer, Pfizer, and GSK. SK, CDVF, and CK are employed by Naveris, Inc. and may hold equity or stock options in Naveris, Inc. ALC received research funding unrelated to this project from Novocure, Astrazeneca, Amgen, Nucana, Gilead, Tanabe, Seagen. DAR has served in a consulting or advisory role for Boston Scientific, Instylla, Taiho, and AxialTx. SKD received research funding unrelated to this project from Eli Lilly and Company, Novartis Pharmaceuticals, Genocea, and Bristol-Myers Squibb and is a founder, science advisory board member (SAB) and equity holder in Kojin. JMC receives research funding to his institution from Merus, Roche, Servier, and Bristol Myers Squibb. He receives research support from Merck, AstraZeneca, Esperas Pharma, Bayer, Tesaro, Arcus Biosciences, and Apexigen; he has also received honoraria for being on the advisory boards of Incyte and Blueprint Medicines. The remaining authors report no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Dana-Farber Harvard Cancer Center # 16-301. Participants gave informed consent to participate in the study before taking part.
References
- 1. Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, et al. Human papillomavirus genotyping and P16 expression as prognostic factors for patients with American joint committee on cancer stages I to III carcinoma of the anal canal. J Clin Oncol 2014;32:1812–7. 10.1200/JCO.2013.52.3464 [DOI] [PubMed] [Google Scholar]
- 2. Rödel F, Wieland U, Fraunholz I, et al. Human papillomavirus DNA load and P16INK4A expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy . Int J Cancer 2015;136:278–88. 10.1002/ijc.28979 [DOI] [PubMed] [Google Scholar]
- 3. Carr RM, Jin Z, Hubbard J. Research on anal squamous cell carcinoma: systemic therapy strategies for anal cancer. Cancers (Basel) 2021;13:2180. 10.3390/cancers13092180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med 2000;342:792–800. 10.1056/NEJM200003163421107 [DOI] [PubMed] [Google Scholar]
- 5. Ciardiello D, Guerrera LP, Maiorano BA, et al. Immunotherapy in advanced anal cancer: is the beginning of a new era. Cancer Treat Rev 2022;105:102373. 10.1016/j.ctrv.2022.102373 [DOI] [PubMed] [Google Scholar]
- 6. Shamseddine AA, Burman B, Lee NY, et al. Tumor immunity and immunotherapy for HPV-related cancers. Cancer Discov 2021;11:1896–912. 10.1158/2159-8290.CD-20-1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jácome AA, Morris VK, Eng C. The role of immunotherapy in the treatment of anal cancer and future strategies. Curr Treat Options Oncol 2022;23:1073–85. 10.1007/s11864-022-00939-3 [DOI] [PubMed] [Google Scholar]
- 8. Luo X, Donnelly CR, Gong W, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest 2020;130:1635–52. 10.1172/JCI129497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dos Santos LV, Abrahão CM, William WN. Overcoming resistance to immune checkpoint inhibitors in head and neck squamous cell carcinomas. Front Oncol 2021;11:596290. 10.3389/fonc.2021.596290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marabelle A, Cassier PA, Fakih M, et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol Hepatol 2022;7:446–54. 10.1016/S2468-1253(21)00382-4 [DOI] [PubMed] [Google Scholar]
- 11. Ott PA, Piha-Paul SA, Munster P, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol 2017;28:1036–41. 10.1093/annonc/mdx029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maio M, Ascierto PA, Manzyuk L, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol 2022;33:929–38. 10.1016/j.annonc.2022.05.519 [DOI] [PubMed] [Google Scholar]
- 13. Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med 2016;374:2542–52. 10.1056/NEJMoa1603702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 15. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–21. 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 16. Morris VK, Salem ME, Nimeiri H, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:446–53. 10.1016/S1470-2045(17)30104-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rao S, Anandappa G, Capdevila J, et al. A phase II study of retifanlimab (INCMGA00012) in patients with squamous carcinoma of the anal canal who have progressed following platinum-based chemotherapy (POD1UM-202). ESMO Open 2022;7:100529. 10.1016/j.esmoop.2022.100529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benson AB, Venook AP, Al-Hawary MM, et al. Anal carcinoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:852–71. 10.6004/jnccn.2018.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eng C, Ciombor KK, Cho M, et al. Anal cancer: emerging standards in a rare disease. J Clin Oncol 2022;40:2774–88. 10.1200/JCO.21.02566 [DOI] [PubMed] [Google Scholar]
- 20. Sclafani F, Rao S. Systemic therapies for advanced squamous cell anal cancer. Curr Oncol Rep 2018;20:53. 10.1007/s11912-018-0698-6 [DOI] [PubMed] [Google Scholar]
- 21. Dhawan N, Afzal MZ, Amin M. Immunotherapy in anal cancer. Curr Oncol 2023;30:4538–50. 10.3390/curroncol30050343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernard-Tessier A, Jeannot E, Guenat D, et al. Clinical validity of HPV circulating tumor DNA in advanced anal carcinoma: an ancillary study to the epitopes-HPV02 trial. Clin Cancer Res 2019;25:2109–15. 10.1158/1078-0432.CCR-18-2984 [DOI] [PubMed] [Google Scholar]
- 23. Rao S, Sclafani F, Eng C, et al. International rare cancers initiative multicenter randomized phase II trial of cisplatin and fluorouracil versus carboplatin and paclitaxel in advanced anal cancer: InterAAct. J Clin Oncol 2020;38:2510–8. 10.1200/JCO.19.03266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huffman BM, Aushev VN, Budde GL, et al. Analysis of circulating tumor DNA to predict risk of recurrence in patients with esophageal and gastric cancers. JCO Precis Oncol 2022;6:e2200420. 10.1200/PO.22.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris VK, Strickler JH. Use of circulating cell-free DNA to guide precision medicine in patients with colorectal cancer. Annu Rev Med 2021;72:399–413. 10.1146/annurev-med-070119-120448 [DOI] [PubMed] [Google Scholar]
- 26. Berger BM, Hanna GJ, Posner MR, et al. Detection of occult recurrence using circulating tumor tissue modified viral HPV DNA among patients treated for HPV-driven oropharyngeal carcinoma. Clin Cancer Res 2022;28:4292–301. 10.1158/1078-0432.CCR-22-0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chera BS, Kumar S, Beaty BT, et al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during Chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin Cancer Res 2019;25:4682–90. 10.1158/1078-0432.CCR-19-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chera BS, Kumar S, Shen C, et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol 2020;38:1050–8. 10.1200/JCO.19.02444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gunning A, Kumar S, Williams CK, et al. Analytical validation of Navdx, a cfDNA-based fragmentomic profiling assay for HPV-driven cancers. Diagnostics (Basel) 2023;13:725. 10.3390/diagnostics13040725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim S, Meurisse A, Spehner L, et al. Pooled analysis of 115 patients from updated data of epitopes-HPV01 and epitopes-HPV02 studies in first-line advanced anal squamous cell carcinoma. Ther Adv Med Oncol 2020;12:1758835920975356. 10.1177/1758835920975356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morris VK. Circulating tumor DNA in advanced anal cancer: a blood biomarker goes viral. Clin Cancer Res 2019;25:2030–2. 10.1158/1078-0432.CCR-18-3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cabel L, Jeannot E, Bieche I, et al. Prognostic impact of residual HPV ctDNA detection after chemoradiotherapy for anal squamous cell carcinoma. Clin Cancer Res 2018;24:5767–71. 10.1158/1078-0432.CCR-18-0922 [DOI] [PubMed] [Google Scholar]
- 33. Alessi JV, Wang X, Elkrief A, et al. Impact of aneuploidy and chromosome 9p loss on tumor immune microenvironment and immune checkpoint inhibitor efficacy in NSCLC. J Thorac Oncol 2023;18:1524–37. 10.1016/j.jtho.2023.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ricciuti B, Wang X, Alessi JV, et al. Association of high tumor Mutation burden in non-small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol 2022;8:1160–8. 10.1001/jamaoncol.2022.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ricciuti B, Alessi JV, Elkrief A, et al. Dissecting the clinicopathologic, genomic, and immunophenotypic correlates of KRAS(G12D)-mutated non-small-cell lung cancer. Ann Oncol 2022;33:1029–40. 10.1016/j.annonc.2022.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alessi JV, Ricciuti B, Alden SL, et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J Immunother Cancer 2021;9:11. 10.1136/jitc-2021-003536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bankhead P, Loughrey MB, Fernández JA, et al. Qupath: open source software for digital pathology image analysis. Sci Rep 2017;7:16878. 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burtness B, Rischin D, Greil R, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol 2022;40:2321–32. 10.1200/JCO.21.02198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfister DG, Haddad RI, Worden FP, et al. Biomarkers predictive of response to pembrolizumab in head and neck cancer. Cancer Med 2023;12:6603–14. 10.1002/cam4.5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer-a phase 1 study. JAMA Oncol 2019;5:1332–9. 10.1001/jamaoncol.2019.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ademuyiwa FO, Gao F, Street CR, et al. A randomized phase 2 study of neoadjuvant carboplatin and paclitaxel with or without atezolizumab in triple negative breast cancer (TNBC). NPJ Breast Cancer 2022;8:134. 10.1038/s41523-022-00500-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li H, van der Merwe PA, Sivakumar S. Biomarkers of response to PD-1 pathway blockade. Br J Cancer 2022;126:1663–75. 10.1038/s41416-022-01743-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu Q, Wang J, Sun Y, et al. Efficacy and safety of sintilimab plus anlotinib for PD-L1-positive recurrent or metastatic cervical cancer: a multicenter, single-arm. J Clin Oncol 2022;40:1795–805. 10.1200/JCO.21.02091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114:4993–8. 10.1073/pnas.1705327114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wieland A, Patel MR, Cardenas MA, et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021;597:274–8. 10.1038/s41586-020-2931-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mouw KW, Cleary JM, Reardon B, et al. Genomic evolution after chemoradiotherapy in anal squamous cell carcinoma. Clin Cancer Res 2017;23:3214–22. 10.1158/1078-0432.CCR-16-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung HC, Ros W, Delord J-P, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2019;37:1470–8. 10.1200/JCO.18.01265 [DOI] [PubMed] [Google Scholar]
- 48. Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 2021;385:1856–67. 10.1056/NEJMoa2112435 [DOI] [PubMed] [Google Scholar]
- 49. Yamazaki N, Kiyohara Y, Uhara H, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci 2017;108:1022–31. 10.1111/cas.13226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balermpas P, Martin D, Wieland U, et al. Human papilloma virus load and PD-1/PD-L1, CD8(+) and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapy. Oncoimmunology 2017;6:e1288331. 10.1080/2162402X.2017.1288331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu W-H, Miyai K, Cajas-Monson LC, et al. Tumor-infiltrating CD8(+) T lymphocytes associated with clinical outcome in anal squamous cell carcinoma. J Surg Oncol 2015;112:421–6. 10.1002/jso.23998 [DOI] [PubMed] [Google Scholar]
- 52. Hernandez S, Das P, Holliday EB, et al. Differential spatial gene and protein expression associated with recurrence following chemoradiation for localized anal squamous cell cancer. Cancers 2023;15:1701. 10.3390/cancers15061701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 2013;1:54–63. 10.1158/2326-6066.CIR-13-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152–64. 10.1038/s41591-020-1131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kumagai S, Koyama S, Itahashi K, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 2022;40:201–218. 10.1016/j.ccell.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 56. Kim S, François E, André T, et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): a multicentre, single-arm, phase 2 study. Lancet Oncol 2018;19:1094–106. 10.1016/S1470-2045(18)30321-8 [DOI] [PubMed] [Google Scholar]
- 57. Kim S, Jary M, André T, et al. Docetaxel, cisplatin, and 5-fluorouracil (DCF) chemotherapy in the treatment of metastatic or unresectable locally recurrent anal squamous cell carcinoma: a phase II study of French interdisciplinary GERCOR and FFCD groups (Epitopes-HPV02 study). BMC Cancer 2017;17:574. 10.1186/s12885-017-3566-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chung CH, Li J, Steuer CE, et al. Phase II multi-institutional clinical trial result of concurrent cetuximab and nivolumab in recurrent and/or metastatic head and neck squamous cell carcinoma. Clin Cancer Res 2022;28:2329–38. 10.1158/1078-0432.CCR-21-3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morris V, Ciombor K, Polite B, et al. O-12 NCI9673 (part B): a multi-institutional ETCTN randomized phase II study of nivolumab with or without Ipilimumab in refractory, metastatic squamous cell carcinoma of the anal canal. Ann Oncol 2023;34:S185–6. 10.1016/j.annonc.2023.04.027 [DOI] [Google Scholar]
- 60. Kim S, Ghiringhelli F, De La Fouchardiere C, et al. Atezolizumab plus modified DCF (docetaxel, cisplatin, and 5-fluorouracil) as first-line treatment for metastatic or locally advanced squamous cell anal carcinoma: a SCARCE-PRODIGE 60 randomized phase II study. JCO 2022;40:3508. 10.1200/JCO.2022.40.16_suppl.3508 [DOI] [Google Scholar]
- 61. Shing JZ, Engels EA, Austin AA, et al. Survival by sex and HIV status in patients with anal cancer in the USA between 2001 and 2019: a retrospective cohort study. Lancet HIV 2024;11:e31–41. 10.1016/S2352-3018(23)00257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-008436supp004.pdf (44MB, pdf)
jitc-2023-008436supp003.pdf (183.1KB, pdf)
jitc-2023-008436supp002.pdf (2.4MB, pdf)
jitc-2023-008436supp001.pdf (114.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request.