Abstract
Background
Patient support programmes (PSPs) allow patients with chronic diseases to receive treatment and support at home. This study describes the Connect 360 PSP delivery and impact on patient-reported outcomes, satisfaction and adherence/persistence among benralizumab-treated patients with severe eosinophilic asthma (SEA).
Methods
A non-interventional retrospective cohort study using data collected during routine care in the Connect 360 PSP. All consenting enrollees (≥18 years) were included in the study.
Results
746 patients formed the study cohort. Mean (SD) age was 53.7 (14.5) years on PSP entry; 38.3% were female (38.7% unknown). 79.6% of patients were experienced biological therapy users. Oral corticosteroid (OCS) use was reported in 48.4% of patients at baseline and 34.8% at 48 weeks. 8.2% of patients reported asthma hospitalisation in the previous 6 months at 24 weeks vs 3.0% at 48 weeks. Mean (SD) 6-item Asthma Control Questionnaire (ACQ-6) scores were 2.7 (1.5) at baseline vs 1.6 (1.3) at 48 weeks. Mean (SD) patient satisfaction scores remained high (4.5 of 5 (1.0) at baseline; 4.7 of 5 (0.6) at 48 weeks). 28.3% of patients were considered adherent at 24 weeks, increasing to 98.3% when supplemented with sales/delivery data (sensitivity analysis). Discontinuation from PSP/benralizumab was low at 24 (3.4%/3.0%) and 48 (12.6%/5.8%) weeks.
Conclusions
Connect 360 PSP achieved high levels of satisfaction and persistence, with indications of positive outcomes including OCS use, hospitalisation and ACQ-6. The study was conducted during COVID-19, so it provides reassurance that patients with SEA receiving benralizumab may be supported safely and effectively at home.
Keywords: Asthma, COVID-19, Patient Outcome Assessment
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patient support programmes (PSPs) have been successful in helping to manage chronic illness such as inflammatory bowel disease, idiopathic pulmonary fibrosis, psoriasis and diabetes. However, to date, there are limited data on the impact of PSPs on management of disease in patients with severe asthma.
The Connect 360 PSP supports those with severe eosinophilic asthma receiving benralizumab in the home setting.
WHAT THIS STUDY ADDS
The patients in the Connect 360 PSP demonstrated high levels of satisfaction, favourable clinical outcomes and increased persistence to the programme as well as the treatment.
The Connect 360 PSP allowed for continued care and better management of these patients in the home setting during the COVID-19 pandemic.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study shows how a PSP can successfully support the use of benralizumab to manage severe asthma in the home setting, and extends the evidence base for potential wider implementation of these programmes.
Introduction
Severe asthma has been defined by the American Thoracic Society and European Respiratory Society as ‘asthma which requires treatment with high-dose inhaled corticosteroids plus a second controller (and/or systemic corticosteroids) to prevent it from becoming ‘uncontrolled’ or which remains uncontrolled despite this therapy’.1 2 Of the 340 million people thought to have asthma worldwide, 5–10% are estimated to have severe asthma.3 Severe asthma has been reported to affect approximately 3.6% of the UK population, around 200 000 people in total,4 although this is likely to be an underestimate.
Monoclonal antibody therapies that target the interleukin (IL)-5 pathway (mepolizumab and reslizumab) and benralizumab (target IL-5 or the alpha subunit of the IL-5 receptor pathway) have demonstrated benefits as an adjunct to standard care in people with severe eosinophilic asthma (SEA) and poor symptom control.5 These agents significantly reduce exacerbation rates in SEA,6 7 whereas benralizumab and mepolizumab also reduce dependency on oral corticosteroids (OCS) without loss of asthma control.8–10 Mepolizumab and reslizumab are both injected 4-weekly,11 12 whereas benralizumab is given as a 4-weekly injection for the first three doses and 8-weekly thereafter.13
Numerous real-world studies have confirmed the benefits of benralizumab in patients with SEA in terms of reduced OCS use,9 14–18 improved asthma control9 14 16–18 and enhanced lung function.15 19 One UK study reported an 85% reduction in exacerbation rates, substantial OCS sparing, and improved asthma control and quality of life following 48 weeks of benralizumab therapy.20
Benralizumab is typically administered by a healthcare professional (HCP),13 although patients with no known history of anaphylaxis may self-administer benralizumab with the agreement of their physician.13 However, the shielding requirements from the COVID-19 pandemic for extremely clinically vulnerable patients triggered the rapid transition of large patient numbers from clinic-based care to self-administration at home. In April 2020, the National Institute of Health and Care Excellence published the COVID-19 Rapid Guideline: Severe Asthma,21 which recommended that patients already initiated on biological treatment should be considered for self-administration training, or be treated at a clinic or home. It also suggested routine remote monitoring of biological treatment, if possible.
The evidence gathered over the pandemic adds to the available literature evaluating use of patient support programmes (PSPs) for patients with chronic diseases treated at home. Studies in inflammatory bowel disease,22 idiopathic pulmonary fibrosis,23 psoriasis24 and diabetes25 demonstrate that PSPs allow greater adherence and improved quality of life, although there is a lack of evidence among patients with SEA.26 27
The Connect 360 PSP is designed to benefit patients with SEA initiated on benralizumab therapy and subsequently treated at home. Participants are able to choose from three service levels: (1) delivery of medication only; (2) delivery and nurse administration/self-injection training; (3) delivery and nurse administration/self-injection training with additional service support including completion of the 6-item Asthma Control Questionnaire (ACQ-6)28 and injection reminders/adherence tracking.
This study describes the demographics and clinical characteristics of patients enrolled in the Connect 360 PSP. Key patient-reported clinical outcomes and patient experience and satisfaction with benralizumab and the Connect 360 PSP are also presented, as well as adherence and persistence to benralizumab after enrolment.
Methods
Study design
This was a non-interventional retrospective cohort study based on data collected in the UK as part of routine patient care in the Connect 360 PSP. The design of the Connect 360 PSP is shown in online supplemental figure 1, and a Strengthening the Reporting of Observational Studies in Epidemiology checklist is provided.
bmjresp-2023-001734supp001.pdf (593.3KB, pdf)
Study population
All enrollees aged ≥18 years from the UK who consented to the use of personal data for research and publication were considered for inclusion in the study. There were no exclusion criteria.
Data source
Data were drawn from the Connect 360 service administration database which incorporates service information collected from: (1) Connect 360 PSP registration form (HCP completed; when service requested); (2) nurse visit form (nurse completed; if home visits requested); (3) adherence check call (nurse completed; if adherence follow-ups requested); (4) ongoing nurse support call (nurse completed; if full support service requested).
Patient data were collected through the PSP management system, HealthNet Clinical Platform, which records baseline data on enrolment, as provided by the referring asthma centre, and information collected through all patient encounters. Data from all participating centres were combined into a single anonymised dataset for analysis. To support analysis of treatment schedules and adherence, benralizumab sales and delivery data were also used.
Outcome measures
Data collected included patient characteristics at enrolment; patient-reported clinical outcomes (OCS use, hospitalisations, ACQ-6); service satisfaction (scale with 1–5 points, 5=most satisfied); treatment satisfaction (Treatment Satisfaction Questionnaire for Medication-9 items (TSQM-9)); and adherence/persistence to benralizumab. The primary measure of adherence was defined as taking the injection within a specified 24-hour window based on nurse interactions, with a sensitivity analysis using confirmed date of delivery of benralizumab also performed to allow greater numbers of patients to be analysed. For ACQ-6, a score of ≤0.75 indicated well-controlled asthma, 0.75–<1.5 indicated partly controlled asthma and ≥1.5 indicated not well-controlled asthma.28 TSQM-9 domain scores range from 0 to 100, with higher scores representing higher satisfaction.29
Study endpoints were assessed during a baseline period (0–4 weeks post-PSP enrolment) and at two landmarks post-PSP enrolment (24 (±4) and 48 (±8) weeks, as appropriate). Patients had to have been enrolled in the PSP for ≥20 or ≥40 weeks at time of data extraction to be included in the respective landmark analysis.
Statistical analysis
Data collected between 10 October 2019 and 27 September 2021 were extracted and analysed. Patients were observed from date of PSP enrolment to the last/most recently recorded activity.
All statistical analyses were descriptive. For numerical variables, mean and SD are reported. Frequencies and proportions are reported for categorical variables. Data were analysed based on observed cases only without imputation (complete case analysis). A sensitivity analysis was performed for adherence, in which injection data were supplemented with benralizumab delivery data. Where benralizumab delivery was within 9 weeks of the preceding delivery, it was assumed that the dose was administered and so adherence was maintained. Time-to-event outcomes were analysed descriptively using Kaplan-Meier estimates. All data analyses were executed using Stata V.17.0.30
Patient and public involvement
This study did not include patient or public involvement.
Results
Study population
As of September 2021, data were available from 2318 patients within the PSP from 38 UK hospital sites. Of the 746 patients who consented, 186 (24.9%) had available clinical data and comprise the baseline clinical cohort (0–4 weeks from enrolment). Clinical cohorts at 24 and 48 weeks post-enrolment include 231 (31.0%) and 302 (40.5%) patients, respectively.
The number of patients with specific nurse interactions at key time points is summarised in online supplemental table 1.
Patient demographic and baseline characteristics
Mean (SD) patient age at enrolment was 53.7 (14.5) years. In total, 22.9% patients were male and 38.3% were female, with gender data unknown for 38.7% of patients (not mandatory on registration form). Key patient characteristics at enrolment are summarised in table 1.
Table 1.
Key patient characteristics at enrolment
| Characteristics at enrolment | Study cohort (n=746)* |
| Age at enrolment (years), mean (SD) | 53.7 (14.5) |
| Gender, n (%) | |
| Male | 171 (22.9) |
| Female | 286 (38.3) |
| Unknown | 289 (38.7) |
| Benralizumab injections received in hospital prior to PSP enrolment | |
| N (%) | |
| 0 | 1 (0.1) |
| 1–3 | 257 (34.5) |
| 4+ | 213 (28.6) |
| Unknown | 275 (36.8) |
| Mean (SD) | |
| Service registration status, n (%) | |
| Biologic naïve | 123 (16.5) |
| Biologic experienced | 573 (76.8) |
| Existing–transfer | 8 (1.1) |
| Change of therapy | 4 (0.5) |
| Unknown | 38 (5.1) |
| Service choice at enrolment, n (%) | |
| Delivery only | 30 (4.0) |
| Delivery and nurse administration service | 4 (0.5) |
| Delivery and self-injection training | 209 (28.0) |
| Additional service support including ACQ-6 | 477 (63.9) |
*All patients enrolled in PSP and consenting.
ACQ-6, 6-item Asthma Control Questionnaire; PSP, patient support programme.
Clinical outcomes
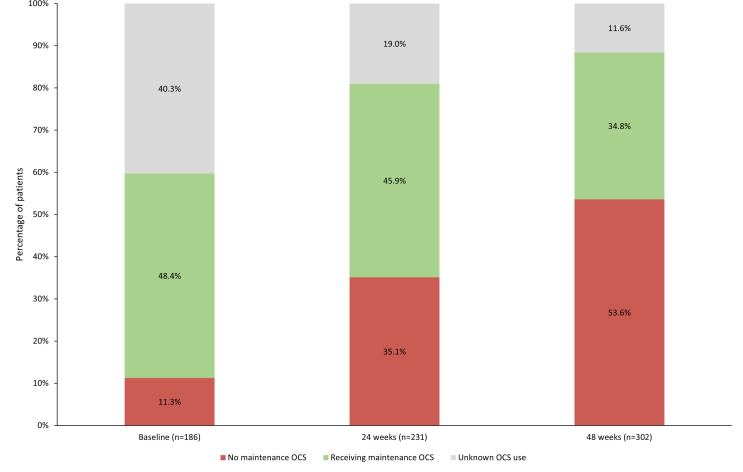
Patient-reported clinical outcomes are summarised in table 2. In the clinical cohort, the proportion of patients reporting maintenance OCS (mOCS) use was similar at baseline and 24 weeks but had fallen by 48 weeks (substantial proportion of unknown data) (figure 1). Of those who reported mOCS at each time point, 21 (19.8%) had increased OCS dose since their previous injection at 24 weeks and 32 (30.5%) had increased dose by 48 weeks (data unknown for 31.1% and 11.4% of patients, respectively). Mean daily dose of OCS remained relatively stable between time points (10.9 mg/day at 24 weeks and 10.0 mg/day at 48 weeks); however, dosage information was only captured in 48 and 30 patients, respectively.
Table 2.
Patient-reported clinical outcomes at baseline, 24 and 48 weeks post-enrolment
| Clinical cohort* | |||
| Patient-reported clinical outcomes | Baseline (N=186) | 24 weeks (n=231) | 48 weeks (n=302) |
| Maintenance OCS use, n (%) | |||
| No | 21 (11.3) | 81 (35.1) | 162 (53.6) |
| Yes | 90 (48.4) | 106 (45.9) | 105 (34.8) |
| Unknown | 75 (40.3) | 44 (19.0) | 35 (11.6) |
| Hospitalised due to severe asthma in preceding 6 months, n (%) | |||
| No | – | 119 (51.5) | 200 (66.2) |
| Yes | – | 19 (8.2) | 9 (3.0) |
| Unknown | – | 93 (40.3) | 93 (30.8) |
| ACQ-6 score | |||
| n† | 104 | 163 | 258 |
| Mean score (SD) | 2.7 (1.5) | 1.9 (1.5) | 1.6 (1.3) |
*At least one nurse interaction in study time frame.
†Not all patients in clinical cohort completed ACQ-6.
ACQ-6, 6-item Asthma Control Questionnaire; OCS, oral corticosteroid.
Figure 1.
Proportion of patients using maintenance OCS at baseline, 24 and 48 weeks post-enrolment. OCS, oral corticosteroid.
Numerically, fewer patients reported being hospitalised for asthma in the previous 6 months at 48 weeks vs 24 weeks (table 2). The number of hospital admissions since the previous injection was similar at 24 and 48 weeks (3.5% and 2.7%, respectively), although hospitalisation status was not known for a little over half the sample.
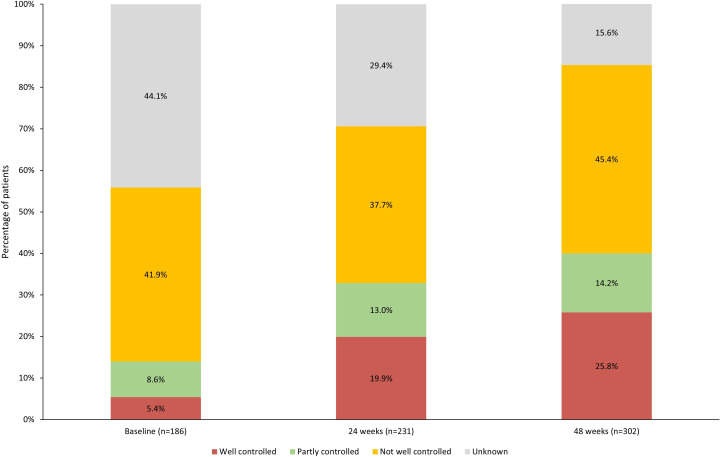
ACQ-6 scores improved from baseline to 24 weeks and 48 weeks (table 2); however, there was a high proportion of unknown ACQ-6 scores. The proportion of patients with ACQ-6 scores ≤0.75 (well-controlled asthma) increased from baseline to 24 and 48 weeks (figure 2). From baseline to 48 weeks, 26.8% of patients reported a reduction in ACQ-6 of ≥0.5 (minimal clinically important difference (MCID)) (data not shown).
Figure 2.
Categorised 6-item Asthma Control Questionnaire scores at baseline, 24 and 48 weeks post-enrolment.
Satisfaction
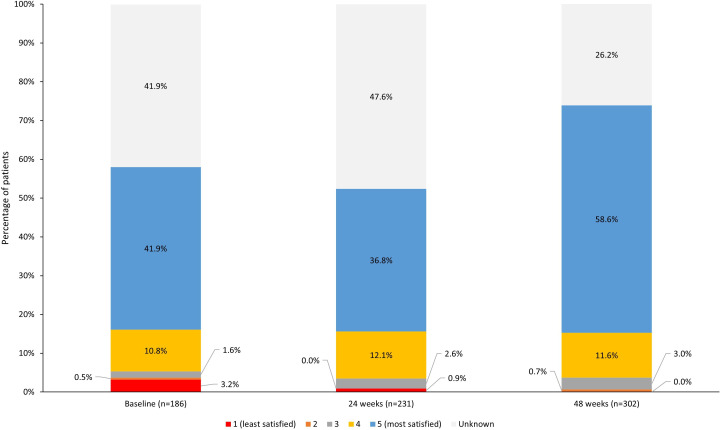
Patient satisfaction with the Connect 360 PSP was generally high, with mean (SD) scores of 4.5 (1.0) out of 5 at baseline, increasing to 4.7 (0.6) at 48 weeks (scores unknown for 26.2–41.9% of patients). The proportion of patients reporting a score of 4 out of 5 increased from 52.7% (baseline) to 70.2% (48 weeks), although a large proportion of data were unknown at each time point (baseline: 41.9%; 24 weeks: 26.2%; 48 weeks: 26.2%) (figure 3).
Figure 3.
Patient satisfaction with the patient support programme at baseline, 24 and 48 weeks post-enrolment (linear scale).
Patient satisfaction with treatment was also high among 194 of 290 patients who completed the TSQM-9. Mean (SD) scores were 80.7 (18.9) out of 100 for global satisfaction, 81.9 (17.3) for effectiveness and 83.1 (12.7) for convenience (data not shown).
Adherence and persistence
When adherence was defined as taking the injection on time (within 24 hours of expected administration) based on nurse interactions, patients took <55% of their benralizumab injections on time at 24 weeks, decreasing to 34.9% by 48 weeks. Based on this stringent definition, only 28.3% (n=84) of patients were considered adherent at 24 weeks, reducing to just 3.0% (n=6) of patients at 48 weeks. Adherence could not be estimated for 31.3% and 46.5% of patients at 24 and 48 weeks, respectively, using this definition because the relevant information was not captured.
On supplementing data from nurse interactions with benralizumab delivery data, the percentage of patients considered adherent was substantially higher (98.3% (n=632) at 24 weeks and 95.0% (n=421) at 48 weeks). Furthermore, the proportion of patients for whom adherence could not be estimated via this method was reduced versus nurse interaction data alone to 1.6% and 2.9% at 24 and 48 weeks, respectively. However, benralizumab may have continued beyond the PSP. Only 3.0% and 5.8% of evaluable patients could be confirmed as having discontinued benralizumab at 24 and 48 weeks (online supplemental figure 2). As most patients in the PSP were existing benralizumab users (mean (SD) of 4.3 (3.1) injections prior to enrolment; table 1), this study may underestimate benralizumab persistence, as doses prior to enrolment are not included in the analysis.
Discussion
This study indicates that the Connect 360 PSP is likely to support patients with SEA taking benralizumab, with patient-reported outcomes showing numerical improvement over the programme, as well as high satisfaction and persistence rates. As the study took place during the COVID-19 pandemic, the data also offer insights into the transition from hospital-based care to a home-care regimen. Like other studies conducted over the same period, this analysis suggests that the sudden implementation of home care was achieved without detriment to the patients’ clinical status or disease-related quality of life.31–33
In terms of clinical outcomes, the proportion of patients reporting mOCS decreased from 48.4% at baseline to 34.8% at 48 weeks. In comparison, 52–56% of patients receiving benralizumab for severe asthma in a previous study achieved a 100% reduction in final OCS dose over 28 weeks in a hospital setting, with a concomitant reduction in exacerbation rates.8 The proportion of patients reporting asthma hospitalisation in the preceding 6 months also decreased between the 24-week and 48-week assessments, from 8.2% to 3.0%, although hospitalisation in the earlier time point may have been experienced prior to PSP enrolment. There was a small difference in number of hospital admissions between the last injection at 24 and 48 weeks (3.5% and 2.7%, respectively), although hospitalisation status was not known for a little over half the sample. In comparison, a hospital-based study reported hospitalisation rate of 22–27% over 28 weeks for benralizumab-treated patients vs 35% for patients receiving placebo.8 These observations suggest patients in our study maintained good disease control over the duration observed.
Mean ACQ-6 scores improved between baseline and 48 weeks by 1.1, more than twice the MCID of 0.5 for this measure. For a subset of patients, changes in ACQ-6 were observed that also suggest clinically meaningful improvements in control.
High and sustained levels of satisfaction were reported by patients who remained in the PSP from baseline to 48 weeks using the 5-point scale (ranging from 4.5 to 4.7). TSQM-9 data also showed high levels of satisfaction, with mean global satisfaction, mean effectiveness and convenience scores of 80.7, 81.9 and 83.1, respectively. In the original validation of the TSQM-9, similar high scores were shown to correlate with high adherence scores according to a modified Moritsky scale29; thus, the high TSQM-9 scores coupled with high satisfaction observed here may be associated with the impressive persistence observed in this study. The satisfaction scores are particularly striking given that most data were collected during the COVID-19 pandemic, at a time of uncertainty and anxiety among patients.34 35
It was originally planned that adherence would be defined as taking the injection on time (within 24 hours of expected administration), as recorded during nurse calls. However, this definition meant considerable missing data from patients no longer requesting adherence check calls or additional support from nurses, possibly due to confidence or familiarity with the device, but opting instead for services such as Short Messaging Service support (for which no data were available). A sensitivity analysis also using medication delivery data was therefore undertaken to allow more patients to be assessed (97%), in a similar approach to that adopted previously in a comparable study.36 This analysis indicated high levels of adherence (24 weeks: 98.3%; 48 weeks: 95.0%), although it should be noted that delivery of benralizumab does not guarantee on-schedule administration of injections.
This study did not specifically investigate those aspects of the PSP that were particularly successful, or the drivers behind the success of the model. However, previous studies of home administration of biologics in SEA suggest that quality of self-administration training and ease of use of the device were key in the success of similar programmes.37 38 This would be an interesting avenue to pursue to optimise the effectiveness of PSPs.
The success of the Connect 360 PSP adds to the evidence of the effectiveness of targeted patient support in patients with chronic diseases.22–25 Previous programmes have also demonstrated improved adherence,22 high levels of patient satisfaction24 25 and improvements in all aspects of patient quality of life.24
Given the dearth of information on use of PSPs in SEA,26 our findings are particularly pertinent and provide validity for this approach, while mandating need for further studies in this area. While COVID-19 was instrumental in accelerating the transition to home care for many patients with chronic disease, the success of this programme and other trials conducted over the same period31–33 suggests that this model is likely to persist in the future, allowing patients to enjoy the greater flexibility of home administration without detriment to their clinical status. The high levels of satisfaction and adherence/persistence in this study also demonstrate the enthusiasm of patients for this approach.
Limitations
Several limitations of this study must be acknowledged. Most notably, data were limited to information available in the service database as collected by the Connect 360 PSP vendor at the time of contact with the patient, resulting in noticeable gaps in the data. As the quality and accuracy of data recorded in the PSP database were not monitored by reference to any source data, any gaps or inconsistences were difficult to check and correct. In addition, it was not possible to extract all data provided in free-text responses, thus limiting the sample sizes (and potentially the interpretability) of outcomes reliant on free-text extractions.
Although this study captures some information on hospitalisation, there were no checks against patient medical records, meaning that hospitalisation may be under-reported. Furthermore, patients were unable to see a healthcare professional during the pandemic, potentially resulting in reduced hospitalisation rates, healthcare contacts or other medication prescriptions such as OCS during this period.
The study also has limited information on benralizumab utilisation and clinical outcomes before initiation of benralizumab or Connect 360 PSP enrolment. This presents difficulties in interpreting the results of the study, particularly clinical outcomes, since there is no comparative arm and limited baseline data. In addition, benralizumab therapy is inextricably linked to the PSP experience, as patients only treated with benralizumab can participate in the programme. Furthermore, a large proportion of patients were on benralizumab prior to enrolment, and duration of follow-up may be different.
Adherence to benralizumab was defined in relation to injections taken according to schedule. Patients who were more confident in administering the injections may have declined to be contacted again for adherence checks, potentially resulting in underestimation of adherence. It is for this reason that the sensitivity analysis was performed, supplementing the injections data with delivery data.
Other limitations include potential recall bias associated with patient-reported healthcare events as captured during nurse interactions. Also, as patients were referred by severe asthma centres, it is possible that only well-managed patients were included, leaving those with poorer outcomes to be seen in the asthma centres. However, from March 2020, patients with all severities of asthma were likely moved onto home-care programmes due to the COVID-19 pandemic. Finally, this analysis only includes patients who were willing to participate in the PSP and consent to use of personal and service data, reducing the representativeness of the study population.
Conclusions
This study demonstrates the success of the Connect 360 PSP in terms of high satisfaction and persistence, as well as indicating a positive impact on clinical outcomes including use of maintenance OCS, hospitalisation and ACQ-6 score. The use of programmes of this kind during the COVID-19 pandemic allowed patients to receive high-quality care at home without apparent loss of asthma control and demonstrates how patients with SEA receiving benralizumab may continue to be supported safely and effectively within the context of home care.
Acknowledgments
The co-authors of this paper, Robert Wood, Mark Silvey, Christina Diomatari, of Adelphi Real World, provided medical writing and editorial support funded by AstraZeneca, UK, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3)
Footnotes
Contributors: TM has made substantial contributions to the conception and design of the study. TM is the guarantor. TM, SM and JL contributed to the analysis and interpretation of data, drafting and revising the manuscript for important intellectual content. RW, MS and CD performed data collection, analyses, interpretation of data, drafting and revising of the manuscript. All authors have contributed to the development of this manuscript and agree with the presented findings. All authors agree that the work has not been published before nor is being considered for publication in another journal. All authors agree to be accountable for all aspects of the work.
Funding: This study was funded by AstraZeneca UK (grant number: n/a).
Competing interests: MS, RW and CD are employees of Adelphi Real World, which received payment funding from AstraZeneca as part of this research in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). TM, JL and SM are employees and shareholders of AstraZeneca.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and did not require ethics approval as it involved secondary data collection and will not have had an influence on prescribing behaviour of physicians or on the outcomes of patients in the programme. All patients provided consent for use of personal data for research and publication. All data were anonymised by HealthNet and contained no identifiable information.
References
- 1.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343–73. 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 2.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European respiratory society/American Thoracic society guideline. Eur Respir J 2020;55:1900588. 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 3.Varsano S, Segev D, Shitrit D. Severe and non-severe asthma in the community: A large electronic database analysis. Respir Med 2017;123:131–9. 10.1016/j.rmed.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 4.Asthma UK . Slipping through the net: The reality facing patients with difficult and severe asthma. Available: https://www.asthma.org.uk/6fc29048/globalassets/get-involved/external-affairs-campaigns/publications/severe-asthma-report/auk-severe-asthma-gh-final.pdf [accessed Jul 2022]. [Google Scholar]
- 5.Farne HA, Wilson A, Milan S, et al. Anti-IL-5 therapies for asthma. Cochrane Database Syst Rev 2022;7:CD010834. 10.1002/14651858.CD010834.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of Benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting Β(2)-Agonists (SIROCCO): a randomised, Multicentre, placebo-controlled phase 3 trial. Lancet 2016;388:2115–27. 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 7.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-Interleukin-5 receptor Α Monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128–41. 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 8.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of Benralizumab in severe asthma. N Engl J Med 2017;376:2448–58. 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 9.Jackson DJ, Burhan H, Menzies-Gow A, et al. Benralizumab effectiveness in severe asthma is independent of previous biologic use. J Allergy Clin Immunol Pract 2022;10:1534–44. 10.1016/j.jaip.2022.02.014 [DOI] [PubMed] [Google Scholar]
- 10.Pilette C, Canonica GW, Chaudhuri R, et al. REALITI-A study: real-world oral corticosteroid-sparing effect of Mepolizumab in severe asthma. J Allergy Clin Immunol Pract 2022;10:2646–56. 10.1016/j.jaip.2022.05.042 [DOI] [PubMed] [Google Scholar]
- 11.Nucala Summary of Product Characteristics . Glaxosmithkline Https://Www.medicines.org.UK/Emc/Product/1938#Gref2022. 2022. Available: https://www.medicines.org.uk/emc/product/1938#gref
- 12.AstraZeneca . Cinqaero Summary of Product Characteristics. Available: https://www.medicines.org.uk/emc/product/4370#gref [accessed Jul 2022]. [Google Scholar]
- 13.AstraZeneca . Fasenra Summary of Product Characteristics. Available: https://www.medicines.org.uk/emc/product/8918/smpc#gref [accessed Jul 2022]. [Google Scholar]
- 14.Menzella F, Fontana M, Galeone C, et al. Real world effectiveness of Benralizumab on respiratory function and asthma control. Multidiscip Respir Med 2021;16:785. 10.4081/mrm.2021.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayser MZ, Drick N, Milger K, et al. Real-world multicenter experience with Mepolizumab and Benralizumab in the treatment of uncontrolled severe eosinophilic asthma over 12 months. J Asthma Allergy 2021;14:863–71. 10.2147/JAA.S319572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura Y, Suzukawa M, Inoue N, et al. Real-world benefits of Biologics for asthma: exacerbation events and systemic corticosteroid use. World Allergy Organ J 2021;14. 10.1016/j.waojou.2021.100600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Moragón E, García-Moguel I, Nuevo J, et al. Real-world study in severe eosinophilic asthma patients refractory to anti-Il5 biological agents treated with Benralizumab in Spain (ORBE study). BMC Pulm Med 2021;21:417. 10.1186/s12890-021-01785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Moguel I, Rosado A, Gómez-Cardeñosa A, et al. Reliability, satisfaction and effectiveness of Benralizumab home self-administration in patients with severe eosinophilic asthma in real-world practice: the auto-Benra study. J Asthma Allergy 2022;15:623–32. 10.2147/JAA.S358738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic Rhinosinusitis with nasal polyps: A real-world multicenter study. J Allergy Clin Immunol Pract 2021;9:4371–80. 10.1016/j.jaip.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of Benralizumab in severe eosinophilic asthma. Chest 2021;159:496–506. 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence (NICE) . COVID-19 rapid guideline: severe asthma. NICE guideline [NG166], Available: https://www.nice.org.uk/guidance/ng166 [accessed Jul 2022]. [PubMed] [Google Scholar]
- 22.Srulovici E, Garg V, Ghilai A, et al. Is patient support program participation associated with longer persistence and improved adherence among new users of Adalimumab? A retrospective cohort study. Adv Ther 2018;35:655–65. 10.1007/s12325-018-0706-0 [DOI] [PubMed] [Google Scholar]
- 23.Near AM, Burudpakdee C, Viswanathan S, et al. Effect of a patient support program for idiopathic pulmonary fibrosis patients on medication persistence: A retrospective database analysis. Adv Ther 2021;38:3888–99. 10.1007/s12325-021-01768-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Argenziano G, Amerio P, Aragone MG, et al. Assessing the beneficial impact of a patient support program in Secukinumab-treated patients with psoriasis in Italy. Patient Prefer Adherence 2021;15:2551–62. 10.2147/PPA.S326498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natalicchio A, Sculco C, Belletti G, et al. Patient-support program in diabetes care during the COVID-19 pandemic: an Italian Multicentric experience. Patient Prefer Adherence 2022;16:113–22. 10.2147/PPA.S343949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabe A, Loke W, Heaney L, et al. Diversity of patient support programs for severe asthma treated with biologic therapies – a systematic literature review. Value in Health 2022;25:S393. 10.1016/j.jval.2022.09.1955 [DOI] [Google Scholar]
- 27.Impact of patient support programs on outcomes among patients with severe asthma treated with biologic therapies – a systematic literature review (accepted). In: British Thoracic Society Winter Meeting. London: QEII Centre, 2022. [Google Scholar]
- 28.Juniper EF, Bousquet J, Abetz L, et al. “Identifying 'well-controlled' and 'not well-controlled' asthma using the asthma control questionnaire”. Respir Med 2006;100:616–21. 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 29.Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated treatment satisfaction questionnaire for medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009;7:36. 10.1186/1477-7525-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.StataCorp . Stata Statistical Software: Release 17. College Station TSL, 2021. [Google Scholar]
- 31.d’Ancona G, Stewart-Kelcher N, Bains S, et al. P105 Does asthma control change when patients transition to home administration of mepolizumab? British Thoracic Society Winter Meeting, Wednesday 17 to Friday 19 February 2021, Programme and Abstracts; February 2021:A144–5 10.1136/thorax-2020-BTSabstracts.250 [DOI] [Google Scholar]
- 32.d’Ancona G, Bains S, Stewart-Kelcher N, et al. P104 Does asthma control change following transition to home benralizumab administration? British Thoracic Society Winter Meeting, Wednesday 17 to Friday 19 February 2021, Programme and Abstracts; February 2021:A144–A44 10.1136/thorax-2020-BTSabstracts.249 [DOI] [Google Scholar]
- 33.D’Ancona G, Stewart-Kelcher N, Bains S, et al. A multi-disciplinary teams’ collaborative approach to transition Benralizumab dependent severe eosinophilic asthmatic patients to self-administration in response to the COVID-19 pandemic. 25th anniversary EAHP Congress - hospital Pharmacy 50 - the future of patient care. 2021.
- 34.Shah SMA, Mohammad D, Qureshi MFH, et al. Prevalence, psychological responses and associated correlates of depression, anxiety and stress in a global population, during the Coronavirus disease (COVID-19) pandemic. Community Ment Health J 2021;57:101–10. 10.1007/s10597-020-00728-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santomauro DF, Mantilla Herrera AM, Shadid J, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. The Lancet 2021;398:1700–12. 10.1016/S0140-6736(21)02143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fendrick AM, Brixner D, Rubin DT, et al. Sustained long-term benefits of patient support program participation in immune-mediated diseases: improved medication-taking behavior and lower risk of a hospital visit. J Manag Care Spec Pharm 2021;27:1086–95. 10.18553/jmcp.2021.20560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guarnieri G, Caminati M, Achille A, et al. Severe asthma, Telemedicine, and self-administered therapy: listening first to the patient. J Clin Med 2022;11:960. 10.3390/jcm11040960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flokstra-de Blok B, Kocks J, Wouters H, et al. Perceptions on home-administration of Biologics in the context of severe asthma: an international qualitative study. J Allergy Clin Immunol Pract 2022;10:2312–23. 10.1016/j.jaip.2022.04.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001734supp001.pdf (593.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.