Abstract
Introduction
Knee osteoarthritis represents the prevalent and incapacitating disease. Acupuncture, a widely used clinical treatment for knee osteoarthritis, has been shown to ameliorate pain and enhance joint function in affected individuals. However, there is a lack of evidence comparing different courses of acupuncture for knee osteoarthritis. In this trial, we will assess the effect of 4 weeks vs 8 weeks of acupuncture in patients with knee osteoarthritis.
Methods and analysis
The protocol is a pragmatic, parallel, two-arm randomised controlled trial, with the data analyst and assessor being blinded. 148 eligible patients with knee osteoarthritis will be randomly allocated in a 1:1 ratio to receive 4-week or 8-week acupuncture. Electroacupuncture will be administered three times per week for 4 or 8 weeks, respectively. Patients with knee osteoarthritis in both groups will be followed up to 26 weeks. The primary outcome is the response rate at week 26, and secondary outcomes include knee joint pain, knee joint function, knee joint stiffness, quality of life, patient global assessment, the Osteoarthritis Research Society International response rate and rescue medicine. A cost-effectiveness analysis will be carried out over 26 weeks.
Ethics and dissemination
The protocol has been approved by the Medical Ethical Committee of Beijing University of Chinese Medicine (2023BZYL0506). The study findings will be disseminated through presentation in a medical journal. Additionally, we plan to present them at selected conferences and scientific meetings.
Trial registration number
Chinese Clinical Trials Registry (ChiCTR2300073383; https://www.chictr.org.cn/showproj.html?proj=199310).
Keywords: COMPLEMENTARY MEDICINE, Protocols & guidelines, Musculoskeletal disorders
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Different courses of acupuncture for knee osteoarthritis will be compared head-to-head in this randomised controlled trial.
During the trial, various researchers will be responsible for the generation of the random number sequence, allocation concealment, patients’ recruitment, acupuncture treatment and outcome measure assessment to control bias.
Cost-effectiveness of acupuncture will be assessed over 26 weeks in both 4-week and 8-week groups.
The nature of the intervention precludes the possibility of blinding both acupuncturists and patients, which may bring bias.
The trial will only compare two common treatment courses of acupuncture, 4 weeks and 8 weeks, without any comparison with other courses.
Background
Osteoarthritis, a degenerative joint disease, is a prevalent affliction among adults globally, with the knee joint being the most commonly affected.1 2 Estimates published in 2020 suggest that 300 million people worldwide are affected by hip and knee osteoarthritis (KOA).3 KOA is characterised by pain and functional limitations, which impair patients’ quality of life seriously,2 4 with age, gender, obesity, excessive physical activities and previous knee trauma being some of the associated risk factors.1 5 Of note, female patients are more likely to suffer from KOA than male patients.1 6 In addition, the incidence of KOA increases with age, making it a major contributor to disability in the elderly population.1 6 The annual sick leave costs due to knee and hip osteoarthritis are about €40 million for the Dutch workforce, and KOA costs approximately twice as much as hip osteoarthritis.7 KOA reduces the employability of patients and raises the cost of healthcare, which places a heavy financial burden on individuals and society.
Exercise therapy and weight loss have been shown to be effective in KOA,1 but sustained maintenance of these methods persists as a challenge2 due to depending heavily on patient compliance.1 Cognitive–behavioural therapy may reduce pain of patients with KOA; however, evidence is limited.8 Acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) are strongly recommended for patients with KOA in the guideline.9 Nonetheless, using acetaminophen alone is not much of a role.10 Taking into consideration the potential gastrointestinal and cardiovascular side effects, NSAIDs should be used for shortest duration possible.11 Intra-articular corticosteroid injections may be an effective management strategy for patients with KOA, but prolonged usage increases the risk of joint deterioration.12 13 Opioid has limited benefits for KOA and an elevated risk of the gastrointestinal adverse event or somnolence.14–17 Over 20% of patients had a poor prognosis for knee replacement.18 Despite this, the demand for this treatment continues to grow on a global scale, and young people account for an increasing proportion.18 Opioid misuse and considerable growth of joint replacement requirements lead to overstretched healthcare systems. Urgent research is required to develop new treatment options, evaluate the effect of existing treatments or enhance current approaches.19
As an integral component of the long-standing practice of traditional Chinese medicine spanning over 4000 years, acupuncture has emerged as a safe and low-risk physical therapy with demonstrated cost-effectiveness.19–22 Acupuncture is widely used in clinical practice to treat KOA, resulting in notable improvements in pain and joint function.19 23 24 In light of these findings, the American College of Rheumatology (ACR) and American Academy of Orthopaedic Surgeons have conditionally recommended the implementation of acupuncture for KOA.8 25 The effect of acupuncture is inherently intertwined with the treatment course.20 26 A cumulative course of treatment is required to produce and maintain the effect of acupuncture.20
Currently, there is a paucity of evidence on head-to-head comparison of diverse acupuncture courses, with regard to their effect on treating KOA. The 8-week acupuncture courses resulted in pain relief and improved joint function among patients with KOA,27 which was consistent with our own previous research.28 A meta-analysis has shown that a minimum of 4-week acupuncture treatment was needed to alleviate symptoms in patients with KOA.29 Additionally, other studies also have shown that 4-week acupuncture for KOA can improve the patients’ pain and dysfunction.30–34 Four to 8 weeks of acupuncture for KOA is recommended by the latest clinical practice guideline.35 However, it is unclear which course of acupuncture is more effective, 8 weeks or 4 weeks, for KOA.36 More evidence is required to explain the effect of acupuncture on KOA between different treatment courses. In order to address this, we conduct a trial to evaluate the effect of 4 weeks vs 8 weeks of acupuncture on KOA.
Methods
Study design
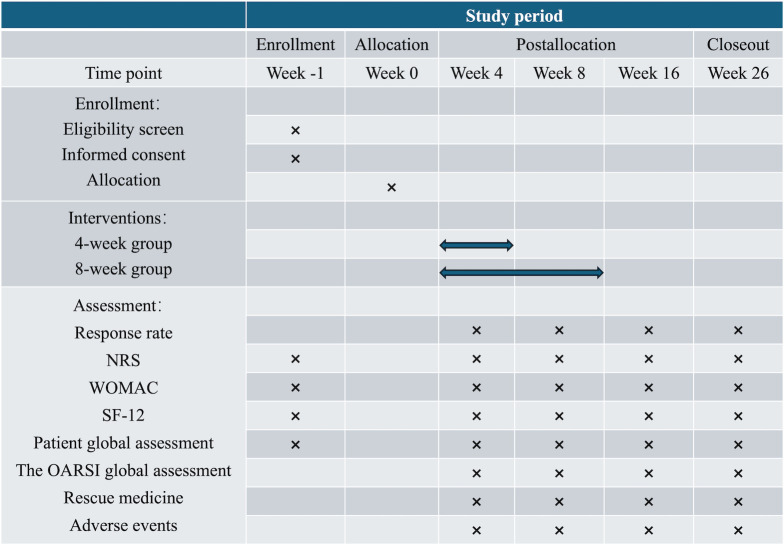
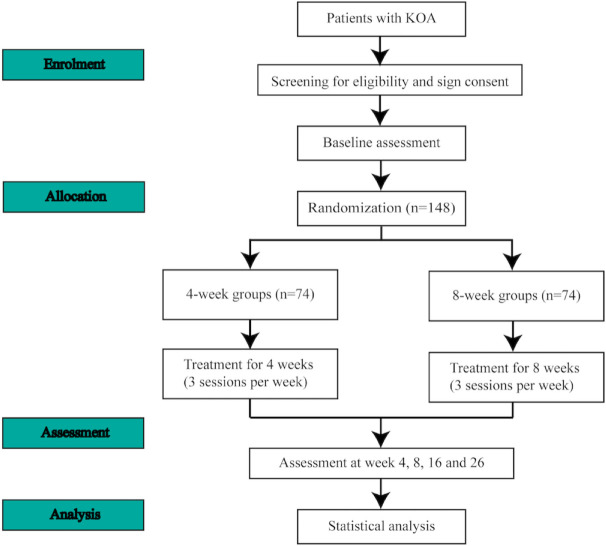
This is a pragmatic, parallel, two-arm randomised controlled research scheduled to take place at Dongzhimen Hospital and Beijing Liangxiang Hospital from August 2023 to June 2024. A total of 148 eligible patients will be assigned randomly in a 1:1 ratio to receive 4-week or 8-week acupuncture. Electroacupuncture (EA) treatment will be administered three times per week for 4 or 8 weeks. Patients with KOA in both groups will be followed up to 26 weeks. The schedule of the assessments completed by all patients is illustrated in figure 1 and the research flow diagram is presented in figure 2. The protocol adheres to the principles of the Declaration of Helsinki and will be reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials guidelines (online supplemental file 1).37
Figure 1.
The schedule of assessments. NRS, Numerical Rating Scale; OARSI, Osteoarthritis Research Society International; SF-12, 12-item Short Form Health Survey; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 2.
The flow diagram of the trial. KOA, knee osteoarthritis.
bmjopen-2023-079709supp001.pdf (125.8KB, pdf)
Recruitment
Participants will be recruited from individuals diagnosed with KOA based on the ACR criteria.38 Announcements will be distributed through social media (WeChat), outpatient units and print advertisements. Interested patients can contact the clinical research coordinator (CRC) via telephone, email or WeChat to be enrolled in this research. The CRC will inform them of the study protocol in detail, including the purpose, procedures, time commitment, potential risks and benefits associated with participation in this trial. Following a preliminary screening process for inclusion and exclusion, potential candidates will be invited to undergo face-to-face screening by the CRC, who will advise patients to maintain their existing lifestyle. Confidentiality measures will be taken to safeguard patient privacy. Eligible participants will sign the informed consent form before randomisation.
Inclusion criteria
Aged 45–75 years (both genders).
Diagnosed with KOA according to the ACR criteria.
Unilateral/bilateral chronic knee pain for over 3 months.
Radiological confirmation of KOA within 6 months (Kellgren-Lawrence grade II or III).
The average score for knee pain during walking on flat ground in the last week was ≥4 out of 10 on a Numerical Rating Scale (NRS).39
Written informed consent.
Exclusion criteria
History of knee surgery or waiting for surgery (knee replacement or arthroscopic knee surgery).
Knee pain caused by other diseases (autoimmune diseases, infection, malignant tumours, trauma, fracture, joint loose bodies, severe effusion of joint cavity, lumbosacral vertebrae disease, gout, etc).
Arthroscopy within 1 year and intra-articular injection in the past 6 months.
Acupuncture treatment during the past 6 months.
Serious acute or chronic organ diseases or mental disorders.
Blood coagulation disorders.
Pregnancy and breast feeding.
Cardiac pacemaker and epilepsy.
Participation in another clinical study within 1 month.
Randomisation
All eligible patients will be randomly allocated to either 4-week group or 8-week group in a 1:1 ratio through a random number sequence, which will be generated by a professional statistician who is not involved in the assessment or treatment of participants using SAS V.9.3 software. This trial will use stratified block randomisation, stratified by centre, with variable block length. The random number sequence and treatment plans will be placed inside corresponding numbered opaque envelopes. After the baseline assessment of eligible participants by the CRC, an independent research assistant who is separated from the trial will assign the envelopes to acupuncturists. The acupuncturists will ensure the envelopes are sealed and will open them to determine which intervention should be performed. The CRC will be responsible for enrolling participants, obtaining informed consent and requesting randomisation.
Blinding
In consideration of the nature of the intervention, the acupuncturists and patients will not be blinded. The outcome assessor and data analyst will be blinded to group assignments. The allocation will remain undisclosed to the outcome assessor and data analyst until the completion of the statistical analysis.
Patient and public involvement
There is no patient or public involvement in the study design, recruitment or conduct of the study.
Interventions
The acupuncture prescription, derived from clinical practice and a prestudy,28 comprises five essential acupoints and three adjunct acupoints. The essential acupoints consist of dubi (ST35), neixiyan (EX-LE5), ququan (LR8), xiyangguan (GB33) and an ash point (the acupoint where the patient reports the most intense pain). The acupuncturist will select appropriate adjunct acupoints based on the patient’s lesion types, which are shown in table 1. The acupoints are localised according to the WHO Standard Acupuncture Locations and are presented in table 2 and figure 3. Licensed acupuncturists have a minimum of 5 years’ experience in acupuncture. For individuals afflicted with bilateral osteoarthritis, both knees will be subjected to acupuncture needling.27 Conversely, patients with unilateral osteoarthritis will receive acupuncture therapy on the affected knee.27 Throughout the course of the trial, patients with unbearable pain can obtain diclofenac sodium enteric-coated tablets (Beijing Novartis Pharma) from the CRC, who will record the use of medication. Diclofenac sodium enteric-coated tablets will be dispensed in sets of six tablets, 25 mg per tablet, with instructions for patients to ingest one orally three times a day.
Table 1.
The adjunct acupoints
| Meridian syndrome | Acupoints |
| Foot yangming (anterior side of leg) |
Liangqiu (ST34), zusanli (ST36), futu (ST32), fenglong (ST40), heding (EX-LE2) |
| Foot shaoyang (lateral side of the leg) |
Fengshi (GB31), waiqiu (GB36), yanglingquan (GB34), xuanzhong (GB39), zulinqi (GB41) |
| Foot taiyang (posterior side of the leg) |
Weiyang (BL39), weizhong (BL40), chengshan (BL57), kunlun (BL60) |
| Foot three-yin (medial side of the leg) |
Xiguan (LR7), yinlingquan (SP9), xuehai (SP10), yingu (KI10), gongsun (SP4), sanyinjiao (SP6), taichong (LR3), taixi (KI3) |
Table 2.
Acupoint manipulation
| Acupoints | Angle | Depth | Twisting and reinforcing | De Qi |
| ST35 | Oblique jab in the inner-up direction | 0.5–1 inch | Even reinforcing–reducing | Yes |
| EX-LE5 | Oblique jab in the external-superior direction | 0.5–1 inch | Even reinforcing–reducing | Yes |
| LR8 | Penetrating jab to GB33 | 1–1.5 inch | Reducing | Yes |
| GB33 | Penetrating jab to LR8 | 1–1.5 inch | Reducing | Yes |
| GB34 | Perpendicular inserting | 1–1.5 inch | Even reinforcing–reducing | Yes |
| EX-LE2 | Perpendicular inserting | 0.8–1 inch | Even reinforcing–reducing | Yes |
| ST34 | Perpendicular inserting | 1–1.2 inch | Even reinforcing–reducing | Yes |
| ST36 | Perpendicular inserting | 1–2 inch | Reinforcing | Yes |
| ST40 | Perpendicular inserting | 1–1.5 inch | Reducing | Yes |
| ST32 | Perpendicular inserting | 1–2 inch | Reinforcing | Yes |
| GB31 | Perpendicular inserting | 1–1.5 inch | Reinforcing | Yes |
| GB36 | Perpendicular inserting | 0.5–0.8 inch | Even reinforcing–reducing | Yes |
| GB39 | Perpendicular inserting | 0.5–0.8 inch | Even reinforcing–reducing | Yes |
| GB41 | Perpendicular inserting | 0.5–0.8 inch | Even reinforcing–reducing | Yes |
| BL39 | Perpendicular inserting | 1–1.5 inch | Reducing | Yes |
| BL40 | Perpendicular inserting | 1–1.5 inch | Even reinforcing–reducing | Yes |
| BL57 | Perpendicular inserting | 1.5–2 inch | Reinforcing | Yes |
| BL60 | Perpendicular inserting | 0.5–0.8 inch | Even reinforcing–reducing | Yes |
| KI3 | Perpendicular inserting | 0.5–0.8 inch | Reducing | Yes |
| KI10 | Perpendicular inserting | 1–1.5 inch | Even reinforcing–reducing | Yes |
| SP4 | Perpendicular inserting | 0.5–1.2 inch | Reducing | Yes |
| SP6 | Perpendicular inserting | 1–1.5 inch | Reinforcing | Yes |
| LR3 | Perpendicular inserting | 0.5–0.8 inch | Even reinforcing–reducing | Yes |
| LR7 | Perpendicular inserting | 1–1.5 inch | Even reinforcing–reducing | Yes |
| SP9 | Perpendicular inserting | 1.5–2 inch | Reducing | Yes |
| SP10 | Perpendicular inserting | 1–2 inch | Even reinforcing–reducing | Yes |
Reinforcing: angle <90°, frequency >120 r/min; even reinforcing–reducing: angle between 120° and 180°, frequency between 60 and 120 r/min; reducing: angle>180°, frequency <60 r/min.
Figure 3.
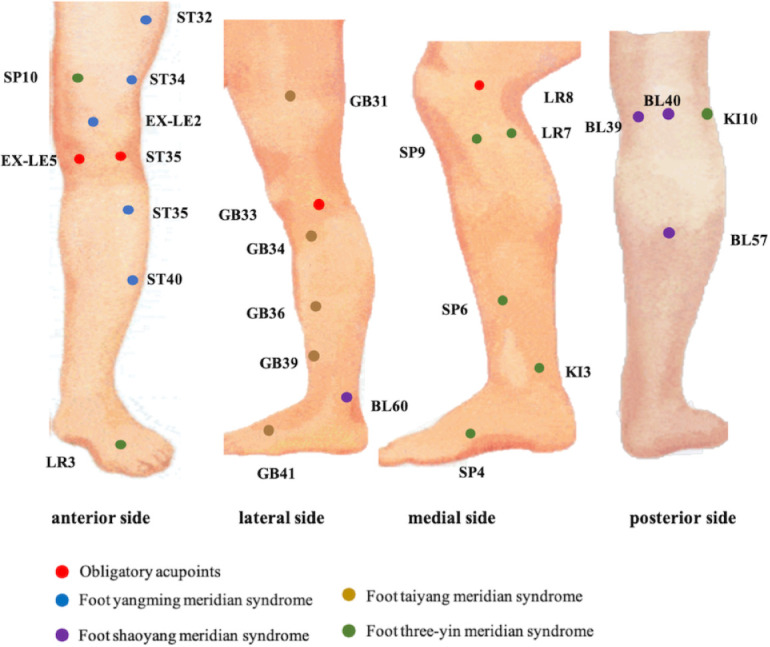
Meridian acupoint chart.
Four-week group
Acupuncture treatments will consist of 12 sessions, each lasting 30 min, and will be administered three times per week over 4 weeks. Single-use aseptic needles (length: 25–40 mm, diameter: 0.25 mm, Hwato, Suzhou, China) will be used. Before needle insertion, both the acupuncturist’s hands and acupuncture site will be strictly disinfected with 75% alcohol. Then, the acupuncturist will proceed with inserting the needles through the skin, selecting the appropriate acupuncture angle, depth and manipulation technique as per table 2. In order to achieve the sensation of ‘De Qi’ (a composite sensation characterised by soreness, numbness, distention or heaviness), needles will be manually stimulated for the duration of 10 s. Paired electrodes from an EA apparatus (HANS-200A acupoint nerve stimulator; Jisheng, Nanjing, China) will be attached to needle holders at LR8–GB33 and two adjunct acupoints. The EA stimulation will be a dilatation wave of 2/100 Hz, depending on the patient’s comfort level.
Eight-week group
Acupuncture treatments will consist of 24 sessions, each lasting 30 min, and will be administered three times per week over 8 weeks. All other procedures will remain consistent with the 4-week group. The differences and similarities between two groups are shown in online supplemental table 1.
bmjopen-2023-079709supp002.pdf (55.6KB, pdf)
Outcomes
If patients have unilateral osteoarthritis, the evaluation of outcomes will pertain exclusively to that knee. For bilateral osteoarthritis, the knee with worse symptoms at baseline will be assessed.
Primary outcome
The primary outcome is the response rate at week 26, which is defined as the proportion of patients achieving a minimal clinically important improvement (MCII).40 Specifically, the MCII is a ≥2-point improvement on the NRS and a ≥6-point improvement on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function score compared with baseline.40 An 11-point NRS ranges from ‘absence of pain’ (0) to ‘most intense pain imaginable’ (10). The WOMAC function subscale scored from 0 to 68 will be used to evaluate the mean knee discomfort experienced over the preceding week, with lower scores indicating better functional ability.
Secondary outcomes
Response rate at other time points
The response rate will be assessed at weeks 4, 8 and 16.
Knee joint pain
The mean pain experienced within the preceding week will be measured using an 11-point NRS and the WOMAC pain subscale at weeks 0, 4, 8, 16 and 26. The WOMAC pain subscale that ranges from 0 to 20 comprises five items.41 Higher scores are indicative of increased pain.
Knee joint function
The average function of the last 7 days will be measured using the WOMAC function subscale at weeks 0, 4, 8, 16 and 26. The tool has a range of 0–68, encompassing 17 items. Lower scores on this subscale are indicative of superior physical function.41
Knee joint stiffness
The mean stiffness throughout the preceding week will be assessed via the WOMAC stiffness subscale at weeks 0, 4, 8, 16 and 26. The WOMAC stiffness subscale ranges from 0 to 8, including two items, with higher scores indicating increased stiffness.41
Quality of life
The evaluation for the standard of living will be performed at weeks 0, 4, 8, 16 and 26, following the randomisation process, by mean of the 12-item Short Form Health Survey (SF-12).42 The SF-12 comprises of both mental and physical domains, and each domain is calibrated from 0 to 100. Higher scores on the scale indicate an improved quality of life.
Patient global assessment
The singular item of the patient global assessment concerns the knee symptoms experienced by participants during the preceding week. Using the Visual Analogue Scale, which ranges from 0 to 100, the severity of the disease is positively correlated with the magnitude of the score. This inquiry will be administered at weeks 0, 4, 8, 16 and 26.
The Osteoarthritis Research Society International response rate
The proportion of subjects with improvement in pain or function ≥50% and absolute change ≥20, or enhancement in a minimum of two of the following: pain ≥20% and absolute change ≥10, function ≥20% and absolute change ≥10, and patient global assessment ≥20% and absolute change ≥10.43 The Osteoarthritis Research Society International response rate will be assessed at weeks 4, 8, 16 and 26.
Rescue medicine
Any use of diclofenac sodium enteric-coated tablets will be quantified at weeks 4, 8, 16 and 26.
Safety
For adverse events that occur during the trial, researchers should record the appearance time, duration, classification, severity, remedial actions taken, remedial process, final resolution, etc on the case report form (CRF). Moreover, researchers will assess the correlation between the intervention and the adverse event, in conjunction with other potential causative and confounding factors, in a comprehensive manner. In the event of serious adverse occurrences, researchers should immediately address and report to the principal investigator. Adverse events comprise subcutaneous haematoma, unrelenting post-injection pain, pruritus at the needle puncture location, etc. It is imperative that all adverse events are meticulously tracked until resolution or stabilisation is achieved.
Sample size
Drawing upon the team’s prior research, the anticipated response rates for the 4-week and 8-week groups are 35% and 60%.28 The sample size of 59 patients in each group was calculated to provide 80% power, with a two-tailed α level of 0.05, using PASS V.15.05 software. This requires 74 patients per group allowing for 20% dropout.
Data analysis
Using IBM SPSS V.26.0 software for statistical analysis, p<0.05 will be deemed statistically significant. Measurement data will be tested for normality. For those conforming to a normal distribution, the mean and SD will be calculated. For those that do not, median (IQR) will be calculated, and percentages will be used for enumeration data. All randomised cases will be included into intention-to-treat analysis, and missing data will be filled in using multiple imputation. Per-protocol analysis will only include the cases that fully complied with the trial protocol and will be used for the primary outcome as sensitivity analysis. If measurement data conform to a normal distribution, it will undergo t-test analysis. On the other hand, if it does not conform to normal distribution, it will be subjected to Mann-Whitney test. Enumeration data will be analysed via χ2 test or Fisher’s exact test. NRS and WOMAC scores at multiple time points will be compared using a mixed-effects model with repeated measure as sensitivity analysis. Subgroup analysis will be used for potential confounders such as age, gender and weight, and affected knee. Cost-effectiveness of acupuncture will be assessed over 26 weeks in both 4-week and 8-week groups.
Data management
The data collector and entry clerks, data manager, statistician and outcome assessor will undergo training in data management. At the end of the treatment phase, all participant data will be completed and recorded on the original CRFs. The data will be entered into Excel spreadsheets by the data entry clerks in time, following which the accuracy of the two datasets will be compared by the data manager. Any discrepancies will be corrected in accordance with the original CRFs.
All paper files related to the research will be preserved, and electronic documents will be securely stored on a password-protected computer. These research documents, whether in paper or electronic form, will be retained for a minimum of 5 years following publication. If readers and reviewers have any questions regarding our published data, they can contact the corresponding author to request for the original data. Patient private information, including their name and telephone number, will be safeguarded. The trial has a low risk of safety and is not designed for interim analyses. Therefore, a Data Monitoring Committee will not be established.
Quality control
Patients will be included in strict accordance with diagnosis, inclusion and exclusion criteria. Standard operating procedures will be developed for each aspect of the trial to ensure uniformity in its implementation and provide a reference in case of disagreements. Researchers will undergo uniform training that covers the study objectives, treatment standards, random allocation, acupuncture techniques and assessment forms. Moreover, they should strictly follow the subject design plan, fill in the CRF carefully and objectively, and record truthfully all kinds of problems that arise in the clinical trial. Compliance control will be reinforced by registering participants’ contact information and then making an appointment in advance for them to come for the treatment. The inspector will regularly check the trial records. Any problems encountered will be reported to the supervisor promptly, and strict measures will be taken to identify and solve them. During the trial, various researchers will be responsible for the generation of the random number sequence, allocation concealment, patients’ recruitment, acupuncture treatment and outcome measure assessment to control bias.
Ethics and dissemination
This protocol has been approved by the Medical Ethical Committee of Beijing University of Chinese Medicine (2023BZYL0506) and registered on Chinese Clinical Trials Registry (ChiCTR2300073383). The participants’ information will be kept anonymous and confidential. They can make the decision to withdraw from the trial at any time. All information will be encrypted and only the designated researcher can access it. The study findings will be disseminated through presentation in a medical journal. Additionally, we plan to present them at selected conferences and scientific meetings.
Synthesis
Our clinical trial incorporates rigorous scientific methodologies and comprehensive outcome measures to assess treatment response, and we will provide a high-quality report on the difference in acupuncture treatment effect between 4 and 8 weeks. It is an innovative and practical approach, and the research will provide strong clinical evidence on the effect of various acupuncture treatment courses. All participants will receive acupuncture treatment throughout the trial, without a placebo control group, which will promote their compliance.
These findings will enhance the high-quality evidence on the effect of different acupuncture treatment courses. Furthermore, this research will provide invaluable insights into developing effective and practical treatment plans for patients with KOA. It will help make medical decisions, improve health insurance reimbursement programmes and ensure efficient allocation of precious healthcare resources.
Limitations
First, the nature of the intervention and a lack of completely inert sham needles preclude the possibility of blinding both acupuncturists and patients.44 However, data analysts and outcome assessors will be blinded, and acupuncturists will receive training in how to minimise bias by interacting less with patients. Second, only patients with Kellgren-Lawrence grade II or III will be included in this trial and results of the research will not be generalised to patients with Kellgren-Lawrence grade IV, who might require longer treatment courses. Third, the trial will only compare two common treatment courses of acupuncture, 4 weeks and 8 weeks, without any comparison with other courses.
Trial status
This trial is currently recruiting participants.
Supplementary Material
Acknowledgments
We appreciate the researchers and institutions who will collaborate with us in this study, as well as all participants who will cooperate with our research.
Footnotes
Contributors: J-FT conceived the research. J-FT and YY initiated the research design. YY, J-FT and C-ZL drafted and critically revised the manuscript for important intellectual content. X-ZW participated in methodological improvements of the protocol. Y-WX coordinated the study. C-ZL and Y-WX sought ethical approval. Y-MF assisted in manuscript revision. J-FT, C-ZL and B-HM sought funding. Y-MF and B-HM contributed significantly to the editing of this manuscript. All authors contributed to the refinement of the research protocol and approved the final manuscript.
Funding: This research is supported by the National Science Fund for Distinguished Young Scholars (no. 81825024), the National Natural Science Foundation of China (no. 82104693) and the Fundamental Research Funds for the Central Universities (2023-JYB-JBQN-028).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 2021;325:568–78. 10.1001/jama.2020.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 3.Safiri S, Kolahi A-A, Smith E, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis 2020;79:819–28. 10.1136/annrheumdis-2019-216515 [DOI] [PubMed] [Google Scholar]
- 4.Chen AT, Shrestha S, Collins JE, et al. Estimating contextual effect in nonpharmacological therapies for pain in knee osteoarthritis: a systematic analytic review. Osteoarthritis Cartilage 2020;28:1154–69. 10.1016/j.joca.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverwood V, Blagojevic-Bucknall M, Jinks C, et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:507–15. 10.1016/j.joca.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 6.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 7.Hardenberg M, Speklé EM, Coenen P, et al. The economic burden of knee and hip osteoarthritis: absenteeism and costs in the Dutch workforce. BMC Musculoskelet Disord 2022;23:364. 10.1186/s12891-022-05306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolasinski SL, Neogi T, Hochberg MC, et al. American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020;72:220–33. 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum 2014;43:701–12. 10.1016/j.semarthrit.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 10.da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2017;390:e21–33. 10.1016/S0140-6736(17)31744-0 [DOI] [PubMed] [Google Scholar]
- 11.da Costa BR, Reichenbach S, Keller N, et al. RETRACTED: effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2016;387:2093–105. 10.1016/S0140-6736(16)30002-2 [DOI] [PubMed] [Google Scholar]
- 12.Jüni P, Hari R, Rutjes AWS, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015;2015:CD005328. 10.1002/14651858.CD005328.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orchard JW. Is there a place for intra-articular corticosteroid injections in the treatment of knee osteoarthritis BMJ 2020;368:l6923. 10.1136/bmj.l6923 [DOI] [PubMed] [Google Scholar]
- 14.Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis: a systematic review and metaanalysis. J Rheumatol 2007;34:543–55. [PubMed] [Google Scholar]
- 15.da Costa BR, Nüesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2014;2014:CD003115. 10.1002/14651858.CD003115.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Costa BR, Pereira TV, Saadat P, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ 2021;375:n2321. 10.1136/bmj.n2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osani MC, Lohmander LS, Bannuru RR. Is there any role for opioids in the management of knee and hip osteoarthritis? A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2021;73:1413–24. 10.1002/acr.24363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet 2018;392:1672–82. 10.1016/S0140-6736(18)32344-4 [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ, Harris RE. Acupuncture and knee osteoarthritis: does dose matter. Arthritis Rheumatol 2021;73:371–3. 10.1002/art.41583 [DOI] [PubMed] [Google Scholar]
- 20.Chon TY, Lee MC. Acupuncture. Mayo Clin Proc 2013;88:1141–6. 10.1016/j.mayocp.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 21.Witt CM, Reinhold T, Jena S, et al. Cost-effectiveness of acupuncture treatment in patients with headache. Cephalalgia 2008;28:334–45. 10.1111/j.1468-2982.2007.01504.x [DOI] [PubMed] [Google Scholar]
- 22.MacPherson H, Thomas K, Walters S, et al. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ 2001;323:486–7. 10.1136/bmj.323.7311.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vickers AJ, Vertosick EA, Lewith G, et al. Acupuncture for chronic pain: update of an individual patient data meta-analysis. J Pain 2018;19:455–74. 10.1016/j.jpain.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei F, Yao M, Wang Y, et al. Acupuncture for knee osteoarthritis: a systematic review and meta-analysis. J Evid Based Med 2023;16:138–40. 10.1111/jebm.12532 [DOI] [PubMed] [Google Scholar]
- 25.Brophy RH, Fillingham YA. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty). J Am Acad Orthop Surg 2022;30:e721–9. 10.5435/JAAOS-D-21-01233 [DOI] [PubMed] [Google Scholar]
- 26.Sun N, Tu JF, Lin LL, et al. Correlation between acupuncture dose and effectiveness in the treatment of knee osteoarthritis: a systematic review. Acupunct Med 2019;37:261–7. 10.1136/acupmed-2017-011608 [DOI] [PubMed] [Google Scholar]
- 27.Witt C, Brinkhaus B, Jena S, et al. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet 2005;366:136–43. 10.1016/S0140-6736(05)66871-7 [DOI] [PubMed] [Google Scholar]
- 28.Tu J-F, Yang J-W, Shi G-X, et al. Efficacy of intensive acupuncture versus sham acupuncture in knee osteoarthritis: a randomized controlled trial. Arthritis Rheumatol 2021;73:448–58. 10.1002/art.41584 [DOI] [PubMed] [Google Scholar]
- 29.Chen N, Wang J, Mucelli A, et al. Electro-acupuncture is beneficial for knee osteoarthritis: the evidence from meta-analysis of randomized controlled trials. Am J Chin Med 2017;45:965–85. 10.1142/S0192415X17500513 [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Jia Y-J, LÜ J-H, et al. Comparision of therapertic effect of different acupuncture methods for knee osteoarthritis. Zhen Ci Yan Jiu 2020;45:569–73. 10.13702/j.1000-0607.191015 [DOI] [PubMed] [Google Scholar]
- 31.Bao F, Zhang Y, Wu Z-H, et al. Efficacy observation on knee osteoarthritis treated with electroacupuncture and its influence on articular cartilage with T2 mapping. Zhongguo Zhen Jiu 2013;33:193–7. [PubMed] [Google Scholar]
- 32.Sangdee C, Teekachunhatean S, Sananpanich K, et al. Electroacupuncture versus diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. BMC Complement Altern Med 2002;2:3. 10.1186/1472-6882-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang D-E, Qin Y, Lin M-N, et al. Clinical efficacy of different waves of electroacupuncture on knee osteoarthritis and its effect on TGF-Beta1 in joint fluid. Zhongguo Zhen Jiu 2020;40:370–4. 10.13703/j.0255-2930.20190422-0005 [DOI] [PubMed] [Google Scholar]
- 34.Lü N, Cheng P, Xia J-X, et al. Effect of acupuncture combined with moxibustion on serum matrix metalloproteinase-9 and matrix metalloproteinase inhibitor-1 in patients with knee osteoarthritis. Zhen Ci Yan Jiu 2022;47:262–7. 10.10372/j.1000-0607.20210079 [DOI] [PubMed] [Google Scholar]
- 35.Luo X, Liu J, Li Q, et al. Acupuncture for treatment of knee osteoarthritis: a clinical practice guideline. J Evid Based Med 2023;16:237–45. 10.1111/jebm.12526 [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Kang S-B, Tu J-F, et al. Effect of acupuncture duration in patients with knee osteoarthritis: a secondary analysis of a multi-center randomized controlled trial. World Journal of Acupuncture - Moxibustion 2023;33:372–6. 10.1016/j.wjam.2023.10.004 [DOI] [Google Scholar]
- 37.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. osteoarthritis of the knee. Arthritis Rheum 1995;38:1541–6. 10.1002/art.1780381104 [DOI] [PubMed] [Google Scholar]
- 39.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain 1986;27:117–26. 10.1016/0304-3959(86)90228-9 [DOI] [PubMed] [Google Scholar]
- 40.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 2005;64:29–33. 10.1136/ard.2004.022905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- 42.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 43.Pham T, Van Der Heijde D, Lassere M, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol 2003;30:1648–54. [PubMed] [Google Scholar]
- 44.Lee B, Kim T-H, Birch S, et al. Comparative effectiveness of acupuncture in sham-controlled trials for knee osteoarthritis: a systematic review and network meta-analysis. Front Med (Lausanne) 2022;9:1061878. 10.3389/fmed.2022.1061878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-079709supp001.pdf (125.8KB, pdf)
bmjopen-2023-079709supp002.pdf (55.6KB, pdf)