Abstract
Osteoarthritis (OA), as a degenerative disease, leads to high socioeconomic burdens and disability rates. The knee joint is typically the most affected and is characterized by progressive destruction of articular cartilage, subchondral bone remodeling, osteophyte formation and synovial inflammation. The current management of OA mainly focuses on symptomatic relief and does not help to slow down the advancement of disease. Recently, mesenchymal stem cells (MSCs) and their exosomes have garnered significant attention in regenerative therapy and tissue engineering areas. Preclinical studies have demonstrated that MSC-derived exosomes (MSC-Exos), as bioactive factor carriers, have promising results in cell-free therapy of OA. This study reviewed the application of various MSC-Exos for the OA treatment, along with exploring the potential underlying mechanisms. Moreover, current strategies and future perspectives for the utilization of engineered MSC-Exos, alongside their associated challenges, were also discussed.
Keywords: exosomes, osteoarthritis, mesenchymal stem cells, tissue engineering, cartilage regeneration
1 Introduction
Osteoarthritis (OA) is the most common chronic degenerative joint disease, marked by gradual deterioration of articular cartilage, subchondral bone remodeling, osteophyte formation and synovial inflammation (Jiang, 2022). With an ageing population, OA is emerging as a major health issue, impacting over 300 million people worldwide, more than 40% of whom are over the age of 70 (Hunter and Bierma-Zeinstra, 2019; Kolasinski et al., 2020). An increasing number of research have indicated that articular cartilage and subchondral bone form a functional unit that has a coherent and reciprocal effect on the development of OA (Hu et al., 2021).
Articular cartilage consists of chondrocytes and extracellular matrix (ECM). As the primary cellular constituents of cartilage, chondrocytes play a fundamental role in synthesizing and maintaining ECM to preserve the structural integrity of articular cartilage (Sophia Fox et al., 2009). Specifically, chondrocytes secrete various ECM components, including lubricin, glycoproteins and type II collagen (COL2) fibers to maintain a stable environment within articular cartilage (Gilbert et al., 2021). Chondrocyte function is intricately regulated by multiple factors. Physiological loading from joint movement and exercise is beneficial, stimulating chondrocytes to maintain cartilage integrity (Deng et al., 2023). However, abnormal mechanical loading can lead to cartilage degeneration. Additionally, inflammatory mediators like interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) negatively impact chondrocyte function and accelerate cartilage degradation (Schuerwegh et al., 2003). During the development of OA, the homeostasis within the articular cartilage is disrupted. Chondrocytes undergo hypertrophic changes and abnormally secrete multiple cartilage matrix-degrading enzymes, such as a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), matrix metalloproteinase-3 (MMP-3) and matrix metalloproteinase-13 (MMP-13) (Cho et al., 2021). These matrix-degrading enzymes sequentially degrade the cartilage matrix, leading to articular cartilage degeneration. However, cartilage has a limited regenerative capacity compared to other tissues such as skin or blood vessels due to its avascular nature and low cell turnover rate (Gilbert et al., 2021). Cartilage regeneration is a complex process involving chondrocytes proliferation and differentiation. When the injury occurs, mesenchymal stem/stromal cells (MSCs) can be recruited to the specific site, which have the potential to differentiate into chondrocytes to replace damaged tissue and are responsible for producing the ECM of cartilage (Jablonski et al., 2019).
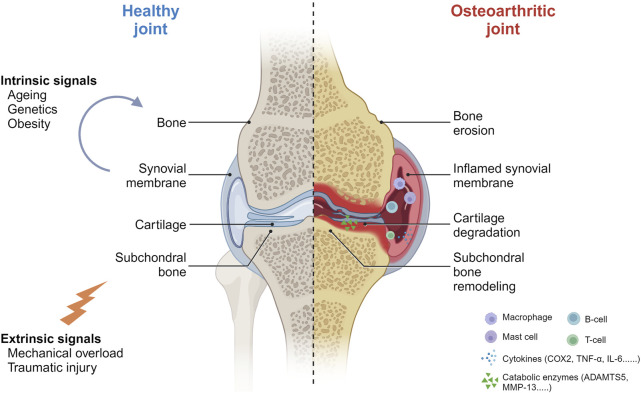
Subchondral bone supplies mechanical support to cartilage and undergoes dynamic remodeling to adapt to microenvironmental changes (Lu et al., 2023). Compared to articular cartilage, subchondral bone exhibits a greater capacity in response to surrounding mechanical stress (Hu et al., 2021). In early-stage OA, accelerated bone resorption and reduced subchondral bone plate thickness precede obvious cartilage degeneration (Kazemi and Williams, 2021; Hu et al., 2022). Subsequently, cartilage destruction occurs primarily in areas where the subchondral bone plate thickness is decreased. As OA progresses, subchondral bone resorption rate is significantly reduced, resulting in uncoupled remodeling of subchondral bone, which is manifested by an abnormal thickening of the subchondral bone growth plates (Kazemi and Williams, 2021). This is also one of the significant pathological signs of the late stage of OA (Figure 1).
FIGURE 1.
The pathobiological network of osteoarthritis. Ageing, genetics, obesity, mechanical overload and traumatic injury are reported to be mainly risk factors that may improve the susceptibility to OA. OA comes with various symptoms like cartilage degradation and subchondral bone remodeling. Numerous cytokines and catabolic enzymes are associated during OA progression (Created with BioRender.com).
Currently, OA treatment can be categorized into two main groups. One is early medication, including non-steroidal anti-inflammatory drugs (NSAIDs), which are used primarily to relieve symptoms, or glucosamine, hyaluronic acid and chondroitin sulphate, which help protect cartilage. However, drug treatment merely decelerates the progression of OA and may augment the probability of adversities towards the gastrointestinal tract and cardiovascular system (Richette et al., 2015). Surgical treatment, such as subchondral bone microfracture, autologous chondrocyte implantation and knee arthroplasty, is considered when conservative treatment is unsatisfactory (Rahmani Del Bakhshayesh et al., 2020). Nonetheless, it is not only imposing a heavy economic burden on individuals but also to their families and even the whole society. Therefore, intervening early in the disease process and enhancing damaged cartilage reconstruction are currently the primary goals of OA treatment.
Over the past decade, cell-based therapies have rapidly emerged as a promising approach to articular cartilage repair. Numerous preclinical studies have shown that injecting MSCs into joint cavity can enhance cartilage regeneration and reduce synovial inflammation to alleviate OA progression (Desando et al., 2013). Although a systematic review reported that MSC-based therapy could significantly reduce pain symptoms and repair joint function (Wei et al., 2021), there are still some challenges to clinical implementation. For example, potential pro-tumorigenic effects, lack of standardized cell production and ethical audit, which have led researchers to investigate alternative approaches in the field of MSC-based biological tissue engineering (Lukomska et al., 2019). Recently, a growing number of evidence supports that MSC-derived extracellular vesicles (MSC-EVs) play a crucial role in intercellular communication and retain valuable properties of parental cells (Thakur et al., 2022). Compared to cell-based therapy, EVs show great advantages such as low immunogenicity, good stability, no ethical controversy, easy storage and direct fusion with target cells (Zhou et al., 2022). These attributes make EV-based therapy as a potential substitute for MSC-based cell therapy.
EVs comprise various subtypes such as microvesicles, apoptotic bodies and exosomes (Exos), each of which plays a unique role in several biological processes (Srinivasan and Sundar, 2021). Among them, exosomes have received more attention than other EVs due to their outstanding performance (Samanta et al., 2018; Gurunathan et al., 2019). Exosomes are membrane-bound vesicles characterized by nanoscale dimensions (typically in the range of 30–150 nm). They can be isolated from various bodily fluids, including blood, plasma and saliva, and derived from a diverse range of cell varieties such as fibroblasts, immune cells, tumour cells, chondrocytes and MSCs (Zhu et al., 2020). Exosomes possess the capacity to deliver a wide range of bioactive molecules, making them a potent tool for intercellular communication and therapeutic applications. Importantly, exosomes play a critical role in various physiological and pathological processes, including maintaining cellular homeostasis, regulating apoptosis and modulating inflammation (Kalluri and LeBleu, 2020; Kim, 2022).
Recently, numerous investigations have shown that MSC-derived exosomes (MSC-Exos) can be effectively used for tissue repair and immunomodulation (Wang et al., 2018; Yu et al., 2022). In addition, several systematic reviews have mentioned that MSC-Exos, as a potential strategy for OA, can attenuate OA progression by mitigating cartilage degradation and enhancing chondrocyte phenotype (To et al., 2020; Zhang et al., 2021; Tan et al., 2021). A completed clinical trial reported that 6 months after the injection of 2 mL ExoFlo (a BM-MSC-Exos product), pain was significantly reduced and joint function improved, indicating BMMSC-Exos was safe and effective for the treatment of OA (Dordevic M., 2020). In this review, we summarized the applications of various MSC-Exos for OA treatment and the underlying mechanisms. Moreover, current methods and future perspectives for the utilization of engineered MSC-Exos, alongside their associated challenges, were also discussed.
2 The potential mechanisms of MSC-Exos for OA treatment
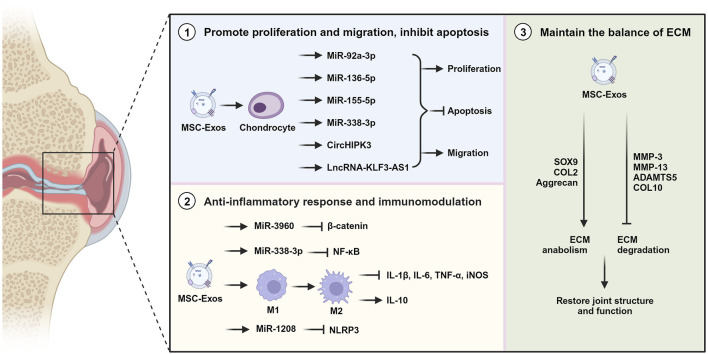
MSC-Exos, serving as vital messengers for cartilage regeneration and intercellular communication, have shown remarkable potential to mitigate the progression of OA by modulating various cellular processes (Kim et al., 2020; Wu et al., 2022). They were reported to promote chondrocyte proliferation, inhibit chondrocyte apoptosis, reduce pro-inflammatory cytokines, modulate immune responses and redeposit cartilage matrix (Figure 2). Exosomes derived from various types of MSCs were used in OA-related cell and animal experiments (Table 1). And the efficacy of exosomes is also influenced by different sources of tissues (Li et al., 2021; Wang et al., 2020).
FIGURE 2.
The potential mechanisms of MSC-Exos for OA treatment. MSC-Exos mitigate OA through stimulating cell proliferation, preventing apoptosis, triggering anti-inflammatory responses, modulating the immune system, and preserving the ECM equilibrium (Created with BioRender.com).
TABLE 1.
The impact of exosomes sourced from various types of mesenchymal stem cells on OA.
| Exosome source | Cargo | Biological effect | References |
|---|---|---|---|
| BM-MSCs | MiR-92a-3p | Promote proliferation while suppress degradation of cartilage in OA model | Mao et al. (2018) |
| MiR-136-5p | Promote chondrocyte migration, reduce the degeneration of cartilage extracellular matrix in OA model | Chen et al. (2020) | |
| MiR-320c | Promote osteoarthritis chondrocyte proliferation, downregulate MMP-13 and upregulate SOX9 expression | Sun et al. (2019) | |
| MiR-3960 | Decrease the degradation of ECM and reduce the ratio of apoptosis in chondrocytes | Ye et al. (2022) | |
| MiR-125a-5p | Alleviate chondrocytes degeneration while promote ECM secretion | Xia et al. (2021) | |
| MiR-361-5p | Mitigate the damage of chondrocytes | Tao et al. (2021b) | |
| CircHIPK3 | Induce migration and proliferation, inhibit apoptosis of chondrocytes | Li et al. (2021b) | |
| LncRNA NEAT1 | Activate the proliferation and autophagy of chondrocytes | Zhang and Jin (2022) | |
| LncRNA LYRM4 | Regulate chondrocyte growth and reduce inflammation in OA | Wang et al. (2021b) | |
| N.D. | Increase the repair of cartilage and the viability of chondrocytes | Yang et al. (2022) | |
| UC-MSCs | LncRNA H19 | Decrease pain level in early stage of OA via enhancing chondrocyte proliferation and matrix synthesis | Yan et al. (2021) |
| MiR-1208 | Suppress cartilage ECM degradation via decreasing level of pro-inflammatory factors | Zhou et al. (2022b) | |
| MiR-100-5p | Inhibit apoptosis and ROS production in chondrocytes | Li et al. (2021d) | |
| MiRNAs | Promote M2 macrophage polarization, lessen the progression of ACLT-induced OA | Li et al. (2022c) | |
| S-MSCs | MiR-140-5p | Promote chondrocyte proliferation and migration, delay early-stage OA progression | Tao et al. (2017) |
| MiR-26a-5p | Inhibit apoptosis and inflammation, ameliorate cartilage injury of OA | Lu et al. (2021) | |
| MiR-155-5p | Promote proliferation, inhibit apoptosis and regulate secretion of ECM | Wang et al. (2021b) | |
| MiR-129-5p | Decline chondrocyte apoptosis and inflammatory response | Qiu et al. (2021) | |
| CircRNA3503 | Preserve the equilibrium of ECM in chondrocytes | Tao et al. (2021a) | |
| N.D. | Inhibit ECM degradation | Duan et al. (2021) | |
| AT-MSCs | DKK-1 | Promote chondrogenesis and chondrocyte redifferentiation | Gorgun et al. (2021) |
| MiR-338-3p | Stimulate cell proliferation and inhibit cell apoptosis | Li et al. (2022d) | |
| N.D. | Attenuate inflammatory micro-environment | Cavallo et al. (2021) | |
| ESC-MSCs | N.D. | Improve the effect on cartilage repair via cell proliferation and apoptosis | Zhang et al. (2018) |
| N.D. | Induce cartilage repair in vivo | Zhang et al. (2016) | |
| N.D. | Maintain chondrocyte phenotype and alleviate cartilage destruction in vivo | Wang et al. (2017) | |
| IPFP-MSCs | MiR-100-5p | Suppress cartilage apoptosis, promote anabolism and prevent cartilage injury in cell and animal experiments | Wu et al. (2019) |
| Urine MSCs | MiR-140-5p | Increase ECM secretion and enhance the ability of cell proliferation | Liu et al. (2022) |
| MiR-26a-5p | Promote cell migration and proliferation | Wan et al. (2022) |
MSC, mesenchymal stem cells; OA, osteoarthritis; N.D., no data; BM-MSCs, bone marrow derived MSCs; UC-MSCs, umbilical cord derived MSCs; S-MSCs, synovial MSCs; IPFP-MSCs, infrapatellar fat pad derived MSCs; AT-MSCs, adipose tissue derived MSCs; ESC-MSCs, embryonic stem cell derived MSCs; ACLT, transection of the anterior cruciate ligament; DKK1, Dickkopf-1; MMP-13, matrix metallopeptidase-13; SOX9, (sex determining region Y)-box 9; COX2, cyclooxygenase-2; ECM, extracellular matrix; ROS, reactive oxygen species.
2.1 Effect on cartilage repair
Cartilage faces challenges in self-repair due to its avascular nature and limited exchange of signaling molecules, oxygen and nutrients (Carballo et al., 2017). MSC-Exos, which target biological processes such as proliferation and apoptosis of chondrocytes, exhibit great capability for the treatment of OA (Xiang et al., 2022).
A variety of MSC-Exos have been employed to enhance chondrocyte proliferation and migration, thereby promoting cartilage restoration (Charlier et al., 2016). Zhu et al. reported that exosomes derived from induced pluripotent stem cell-derived MSCs (iMSC-Exos) and synovial MSCs (SMSC-Exos) could enhance the proliferation and migration of chondrocytes, however iMSC-Exos showed a superior effect compared to SMSC-Exos (Zhu et al., 2017). One study found that exosomes derived from bone marrow-derived MSCs (BMMSC-Exos) affected chondrocyte viability, proliferation and migration by improving mitochondrial activity (Yang et al., 2022). In addition, Li et al. proved that MSC-EVs containing circHIPK3 could enhance chondrocyte proliferation and simultaneously suppress chondrocyte apoptosis via combining with miR-124-3p and then targeting the gene MYH9 (Li et al., 2021b). Furthermore, BMMSC-Exos, delivering the lncRNA LYRM4-AS1, modulated the viability of IL-1β-induced chondrocytes via the LYRM4-AS1/GRPR/miR-6515-5p axis (Wang et al., 2021). Another study confirmed that human umbilical cord-derived MSCs exosomes (UCMSC-Exos) could effectively promote chondrocytes proliferation and migration (Li et al., 2022). It was reported that MSC-Exos derived from embryonic stem cell (ESCMSC-Exos) promoted the proliferation and migration of chondrocytes without affecting matrix synthesis through adenosine-mediated activation of AKT and ERK signaling pathways (Zhang et al., 2018). Additionally, an in vitro study verified that human umbilical cord Wharton’s jelly MSCs-derived exosomes (WJMSC-Exos) can increase chondrocyte proliferation in a dose-dependent manner (Jiang et al., 2021).
MSC-Exos have also been illustrated to inhibit chondrocyte apoptosis. The apoptosis of chondrocytes is associated with many signaling pathways, particularly those involving phosphorylation. Qi et al. noted that BMMSC-Exos promoted Akt phosphorylation while inhibited ERK and p38 phosphorylation, consequently suppressing mitochondrial-induced apoptosis in chondrocytes Qi et al. (2019). Besides, Jin et al. demonstrated that BMMSC-Exos containing lncRNA MEG-3 could mitigate IL-1β-induced chondrocyte senescence and apoptosis, effectively inhibiting OA progression Jin et al. (2021). Studies have shown that MSC-Exos are capable to activate the mTOR pathway, which promotes autophagy to inhibit apoptosis and improve chondrocyte performance (Shen et al., 2017; Wu et al., 2019). The ratio of the anti-apoptosis gene Bcl-2 to the apoptosis gene Bax can influence whether or not chondrocytes undergo apoptosis (KARALIOTAS et al., 2015). It was reported that ESCMSC-Exos elevated the levels of Survivin and Bcl-2 expression while reduced the proportion of cleaved caspase-3-positive apoptotic cells in vivo (Zhang et al., 2018). Additionally, a study demonstrated that UCMSC-Exos, including miR-100-5p, could directly target NOX4 to inhibit ROS production and apoptosis induced by cyclic strain in chondrocytes (Li et al., 2021). Lu et al. verified that SMSC-EVs containing miR-26a-5p mitigated cartilage damage via inhibiting cartilage apoptosis and directly targeting the PTEN gene in vivo Lu et al. (2021).
2.2 Anti-inflammatory response and immunomodulation
The progression of OA is positively correlated with the degree of inflammatory infiltration. Inflammatory cytokines are secreted, leading to induced immune responses that play a role in OA pathogenesis and progression. Several studies indicate that MSC-Exos possess the ability to regulate inflammatory responses by lowering concentrations of pro-inflammatory factors and promoting secretions of anti-inflammatory cytokines (Hassanzadeh et al., 2023).
Macrophages and synovial cells are closely associated with the initiation and progression of inflammation (Oishi and Manabe, 2018). Peng et al. found that MSC-Exos could prevent macrophage ferroptosis through the GOT1/CCR2/Nrf2/HO-1 signaling pathway and rescue cartilage injury in OA Peng et al. (2023). Shifting of synovial macrophages from a pro-inflammatory to an anti-inflammatory phenotype has the potential to significantly impact the development of the intra-articular microenvironment (Wang and He, 2022). It was reported that WJMSC-EVs effectively promoted the polarization of macrophages towards an M2 phenotype, thereby reducing the inflammatory response (Joo et al., 2021). In addition, Zhang et al. showed that ESCMSC-Exos induced a large number of M2 macrophages to infiltrate into the synovial fluid in vivo Zhang et al. (2018). Another study demonstrated that microRNAs (miRNAs) in human amniotic membrane-derived MSC-EVs, such as miR-24-3p, miR-222-3p, miR-146a-5p, miR-34a-5p and miR-181a-5p, could influence macrophage activation states, promote M2 macrophage polarization, and stimulate cartilage regeneration (Ragni et al., 2021). Furthermore, UCMSC-Exos, containing miR-100-5p, miR-let-7a-5p, miR-122-5p, miR-486-5p and miR-148a-3p, facilitate macrophage polarization towards an M2 phenotype and attenuate the deterioration of ACLT-induced OA (Li et al., 2022).
During the progression of OA, several pro-inflammatory factors, including tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β) and IL-6, are released to accelerate the degeneration of cartilage (Pourakbari et al., 2019; Lee et al., 2020). IL-4, IL-10 and transforming growth factor-beta (TGF-β), acting as anti-inflammatory cytokines, are secreted by M2 macrophages to repair the cartilage (Fernandes et al., 2020). A study showed BMMSC-Exos regulated the levels of IL-6 and TNF-α in chondrocytes and tissues (Jiang et al., 2021). Moreover, exosomal miR-9-5p derived from BMMSC was proved to inhibit SDC1 expression, further decreased IL-1 and TNF-α in ACLT-induced OA (Jin et al., 2020). It was proved that adipose tissue-derived MSCs exosomes (ATMSC-Exos), containing miR-145 and miR-221, upregulated the level of IL-10 while downregulated the expression of TNF-α and IL-6 (Zhao et al., 2020). Besides, it was shown that SMSC-derived exosomal miR-129-5p could decrease the inflammation in IL-1β-induced OA by inhibiting HMGB1 release (Qiu et al., 2021).
2.3 Maintain the balance of ECM
The gradual cartilage matrix deterioration is pivotal in OA pathology, triggering the breakdown of joint structure and consequent damage. To promote the redeposition of cartilage ECM and maintain cartilage integrity, it is essential to activate reparative responses in chondrocytes and enhance the expression of genes related to synthetic metabolism (Heard et al., 2015). The investigation into how MSC-Exos maintain ECM balance has been conducted.
Several studies indicated that MSC-Exos could downregulate ADAMTS-5, MMP-3 and MMP-13 expression, while upregulate the levels of tissue inhibitors of metalloproteinases (TIMPs), COL2, glycosaminoglycans (GAGs), and sex-determining region Y-Box 9 (SOX9) (Lozito and Tuan, 2011; Lozito et al., 2014). Cosenza et al. and Vonk et al. reported that BMMSC-Exos could promote the production of proteoglycan, COL2 and aggrecan, while inhibiting the expression of MMP-13 and ADAMTS5 and the activity of collagenase Cosenza et al. (2017), Vonk et al. (2018). Besides, ATMSC-Exos were demonstrated to effectively improve COL2 expression while reducing ADAMTS-5 and MMP-1, -3, -13 expression in chondrocytes, thereby attenuating cartilage matrix degradation in the monosodium iodoacetate (MIA)-induced OA model (Woo et al., 2020). Furthermore, Jammes et al. found that equine BMMSC-derived exosomes induced a greater improvement in hyaline-like matrix neosynthesis by modulating collagen levels, increasing PCNA, and decreasing Htra1 synthesis Jammes et al. (2023).
Exosomal RNAs have shown great potential in promoting cartilage ECM repair. It was reported that BMMSC-derived exosomal miR-320c increased chondrocyte proliferation by increasing the expression of SOX9 and decreasing MMP-13 levels (Sun et al., 2019). Another study showed that BMMSC-Exos could upregulate the levels of COL2 and aggrecan alongside downregulate ADAMTS-5 and MMP-13 expression by encapsulating miR-3960 (Ye et al., 2022). Moreover, BMMSC-derived exosomal miR-125a-5p was demonstrated to alleviate chondrocyte ECM degradation via inhibiting E2F2 in post-traumatic OA (Xia et al., 2021). Wang et al. found that SMSC-Exos containing miR-155-5p enhanced the secretion of ECM in chondrocytes by negatively regulating Runx2 expression to prevent OA Wang et al. (2021a). Zhou et al. showed that UCMSC-Exos suppressed the degradation of cartilage ECM in OA mouse models via miR-1208, which targeting METTL3 to decrease NLRP3 mRNA methylation in macrophages Zhou et al. (2022a).
3 Engineering strategies of MSC-Exos for OA treatment
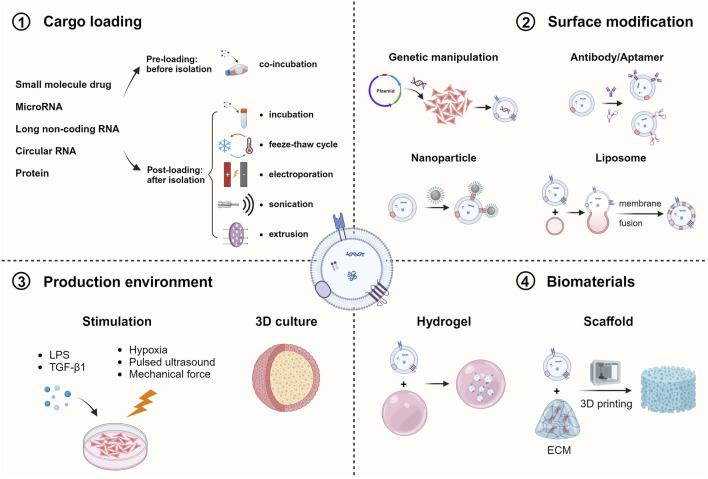
Despite natural exosomes have great potential for cartilage tissue repair, they still come with some limitations such as low yield, circulatory stability and inadequate targeting ability, making them insufficient for disease treatment (Kimiz-Gebologlu and Oncel, 2022). To overcome these challenges and advance the clinical application of exosome therapy, various engineering approaches have been developed, including cargo loading, surface modification, changing the production environment and combination of biomaterials, focusing on both parent cells and exosomes (Figure 3).
FIGURE 3.
Engineering Strategies of MSC-Exos for OA treatment. Various methods have been utilized to engineer ESC-Exos in order to elevate the therapeutic effect via cargo loading, surface modification, changing the production environment and combining with biomaterials (Created with BioRender.com).
3.1 Cargo loading strategies of MSC-Exos
Two main strategies for loading cargo into exosomes are pre-loading and post-loading (Xu et al., 2023). Pre-loading entails loading cargo into parent cells before isolating exosomes, resulting in the secretion of exosomes already loaded with cargo. However, post-loading involves loading cargo directly into exosomes using passive or active techniques after they have been isolated (Elsharkasy et al., 2020; Soekmadji et al., 2020).
The enrichment of therapeutic molecules in MSC-Exos is mainly accomplished through the overexpression of various non-coding RNAs, including miRNAs, long non-coding RNAs (lncRNAs), circular RNAs (circRNAs) and others. Among these, there is extensive evidence supports that miRNAs can promote Exos-mediated the regeneration of cartilage (Foo et al., 2021). One study showed that miR-92a-3p-overexpressing BMMSC-Exos promoted cartilage proliferation and reduced cartilage matrix synthesis by targeting WNT5A and inhibiting WNT signaling pathway (Mao et al., 2018). Zheng et al. reported that miR-212-5p-overexpressing SMSC-Exos reduced the degeneration, degradation and inflammation processes by targeting ELF3 in IL-1β-induced chondrocytes Zheng et al. (2022). Another study demonstrated that exosomes derived from miR-140-5p-overexpressing SMSCs enhanced cartilage tissue repair and mitigated OA progression in an animal model via the WNT signaling pathway (Tao et al., 2017). Wen et al. showed that exosomes derived from lncRNAs KLF3-AS1-overexpressing MSCs were involved in suppressing apoptosis and autophagy of chondrocytes via PI3K/Akt/mTOR signaling pathway (Wen et al., 2022). In addition, Li et al. reported that circHIPK3 was observed to directly sponge miR-124-3p and subsequently enhance the MYH9 expression, contributing to promoting chondrocyte proliferation while suppressing chondrocyte apoptosis mediated by MSC-Exos Li et al. (2021b). Furthermore, SMSC-derived exosomal circRNA3503, acting as a sponge for hsa-let-7b-3p and hsa-miR-181c-3p, ameliorated chondrocyte apoptosis induced by inflammation and regulated the balance of ECM synthesis and degradation (Tao et al., 2021). Shuai et al. showed that exosomal CircRNA0008365 enhanced the expression of SOX9 by sponging miR-338-3p, leading to inhibition of chondrocyte apoptosis and ECM degradation in OA Shuai et al. (2022).
Small molecule drugs and proteins can also be encapsulated using vairous methods. In a sheep OA model, MSC-Exos loaded with TGF-β3 and bone morphogenetic protein-6 (BMP-6) increased cartilage repair and chondrogenesis (Ude et al., 2018; Yoo et al., 2022). Thomas et al. revealed that exosomes loaded with WNT3a successfully initiated WNT signaling in cartilage, contributing to osteochondral defects repair in an OA model (Thomas et al., 2021). Besides, Qiu et al. showed that MSC-Exos loading with curcumin inhibited the apoptosis of OA cells via miR-143/ROCK1/TLR9 and miR-124/NF-kB signaling pathways Qiu et al. (2020).
3.2 Surface modification strategies of MSC-Exos
Enhancing the targeting capacity of exosomes by incorporating specific ligands on their surface enables the precise delivery of therapeutic cargo to the disease site, which is a critical factor for effective treatment of OA. Zhao et al. found that chondrocyte-binding peptide (CAP) binding subcutaneous fat MSC-derived exosomes could particularly send miR-199a-3p into targeting cells and deep articular tissues, which showed great effect on OA progression (Zhao et al., 2023). And CAP-exosomes had the potential to deliver miR-140 to chondrocytes and deep cartilage region in vitro and in vivo, alleviating OA progression by inhibiting cartilage-degrading proteases (Liang et al., 2020). Researchers found that the MSC-binding peptide E7 could be fused with exosomal membrane protein Lamp2b to construct functional exosomes (E7-SMSC-Exos) with SMSC targeting capability, which could efficiently induce cartilage differentiation when further combined with KGN (Xu et al., 2021). Another study showed that ATMSC-Exos binding with chitosan oligosaccharides (COS) facilitated regeneration of injury cartilage and protect chondrocytes from apoptosis by regulating vital pathways such as WNT and MAPK in OA progression (Li et al., 2021c). Nanoparticles combined with exosomes can have positive effects on functions. Li et al. reported that CD90-positive SMSC-Exos-coated nanoparticle could bind to injured chondrocytes, promote chondrocyte regeneration, and influence M2 macrophage polarization in a rat OA model Li et al. (2022c). In addition, by fusing CAP to Lamp2b on exosomal surfaces and subsequently merging with liposomes, Liang et al. found that the hybrid CAP-Exos could successfully deliver CRISPR/Cas9 sgMMP-13 plasmids to silence MMP-13 expression, thereby mitigating the hydrolytic degradation of ECM proteins in the deep regions of damaged cartilage in a rat model Liang et al. (2022).
3.3 Production environment of MSC-Exos
In addition to directly increasing the content of therapeutic molecules, altering the environment of production for MSC-Exos also presents a favorable engineering strategy.
An effective method for generating MSC-Exos in significant amounts is by expanding MSCs, which can be accomplished by enlarging the available surface area for cellular proliferation (Cheng et al., 2022). Rocha and others showed that MSC-Exos cultured using a three-dimensional (3D) approach generated a higher quantity of exosomes in comparison to the traditional two-dimensional (2D) method, illustrating the advantage of the 3D method for scaling up exosome production Rocha et al. (2019). Further study found that 3D-Exos exhibited a 7.5-time higher yield compared to 2D-Exos. In addition, UCMSC-Exos cultured in a 3D environment demonstrated a notably enhanced therapeutic efficacy than their 2D counterparts (Yan and Wu, 2020). Furthermore, Dias et al. found that a poly (ethylene glycol) (PEG)-based microcarrier could enhance the adhesion and expansion capabilities of human MSCs Dias et al. (2017). Another study showed that decellularized extracellular matrix (dECM) could provide a better microenvironment for MSC expansion, and significantly increased miR-3473b levels in dECM-BMMSC-Exos, which had a better ability to regenerate cartilage than BMMSC-Exos in vivo (Zhang et al., 2023).
To adapt to the environment, cells can transmit stress-related information by regulating the release of exosomes. Previous studies showed that the expression of miR-135b in BMMSC-Exos could be enhanced by TGF-β1 stimulation, leading to a decrease in the expression of Sp1, promoting the proliferation of chondrocytes (Wang et al., 2018). Rong et al. reported that exosomes derived from HIF-1α-induced hypoxic BMMSCs enhanced the chondrocyte proliferation while suppressed chondrocyte apoptosis compared to normal BMMSC-Exos Rong et al. (2021). Additionally, hypoxia-treated ATMSC-Exos increased collagen and proteoglycan expression in cartilage and normalized uncoupled bone remodeling in subchondral bone compared to the normal ADSC-Exo group in a murine OA model (Zhao et al., 2023). Chang et al. found that hypoxia-ATMSC-Exos improved articular chondrocyte function, alleviated articular chondrocyte inflammation and suppressed the OA progression in cell and animal experiments Chang et al. (2023). Another study demonstrated that mechanical stimulation from a rotary cell culture system could expand the exosome yield, and then enhance the repair of cartilage defect by up-regulating LncRNA H19 in UCMSC-Exos (Yan et al., 2021). Furthermore, Liao et al. showed that BMMSC-Exos treated with low-intensity pulsed ultrasound inhibited inflammation and further enhanced chondrocyte proliferation and ECM synthesis Liao et al. (2021).
3.4 Biomaterials for MSC-Exos retention and delivery
In OA treatment, the prevailing approach for exosome delivery is intra-articular injection (Bousnaki et al., 2020). An increasing number of researches are focusing on combing exosomes with biomaterials to prolong retention time and improve therapeutic effect.
Hydrogel is a favourable biomaterial for cartilage tissue engineering applications due to its injectability and cross-linking capability under UV exposure. Pang et al. reported that gelatin methacryloyl hydrogels (GelMA) facilitated the prolonged release of MSC-Exos and significantly enhanced their therapeutic impact on OA Pang et al. (2023). Wan et al. applied photocrosslinking spherical gelatin methacryloyl hydrogel to act as injectable carriers for LRRK2-IN-1-loaded exosomes Wan et al. (2023). The results indicated that engineered BMMSC-Exos had a superior effect on cartilage repair in vivo. In a previous investigation, researchers explored the application of an adhesive, injectable hydrogel inspired by mussels, which incorporated BMMSC-Exos. They were utilized to promote regeneration of cartilage defects and the remodeling of the ECM (Zhang et al., 2021).
Exosomes can collaborate with bioactive scaffolds, especially ECM-derived scaffolds, to improve capabilities of promoting cartilage repair (Cheng et al., 2022). Jiang et al. found the regenerative effect of WJMSC-Exos was amplified by the incorporation of the acellular cartilage ECM (ACECM) scaffold in a rabbit model Jiang et al. (2021a). Mechanically, the ACECM scaffold provided a cartilage-like microenvironment that facilitated the attachment of local cells (Sun et al., 2018). Using desktop-stereolithography technology, Chen et al. reported that they designed an innovative 3D-printed cartilage ECM/GelMA/exosome scaffold to deliver MSC-Exos, which had the ability to preserve exosomes for more than 7 days and significantly accelerated the process of cartilage regeneration in vivo Chen et al. (2019).
4 Conclusion and perspective
MSC-Exos, as a cell-free therapy, provides an advanced strategy for alleviating the progression of OA (Boulestreau et al., 2021; Fan et al., 2022). The role of MSC-Exosomes in chondrocyte regeneration, immunomodulation and ECM balance has been extensively studied. However, current studies on MSC-Exos for the treatment of OA are still in early stages. Most studies are based on small animal models, necessitating validation through large animal models before advancing to clinical research (Yu et al., 2022). Currently, there is great variability in the preparation of MSC-Exos, which may be affected by different MSC sources, culture conditions, and exosomes harvesting strategies (Gimona et al., 2021). Owing to the diverse contents and function of exosomes, it is essential to explore the characterization of MSC-Exos in different subpopulations and accurately determine the content of their cargo, which may alter the impact on the target tissue (Forsberg et al., 2020). Therefore, more attention needs to be paid to make standardized, convenient, and strictly controlled methods in the future. In addition, the shortage and strict selection of MSC donors need to be taken into account. For example, BM-MSCs are difficult to isolate and obtain due to the surprisingly low content (less than 0.01% of the cells in the bone marrow) (Yang et al., 2018). And bone marrow collection is an invasive and painful procedure for donors, which may lead them to abandon donation. As for UC-MSCs, the infectious and familial genetic disease of pregnant woman need to be considered (Tang et al., 2022). So, the challenge of eliminating or inactivating pathogens while retaining the properties of exosomes also needs to be addressed (Burnouf et al., 2019). In order to achieve a therapeutic effect, it is necessary for MSC-Exos to carry bioactive factors like proteins or miRNAs at a sufficient dosage and with functional activity to elicit biological responses in target cells (Toh et al., 2018). However, Chevillet et al. found that most exosomes did not carry biologically significant amounts of miRNAs Chevillet et al. (2014). Therefore, it is particularly important to increase miRNA content by loading methods such as electroporation.
In recent years, although a variety of MSC-Exos engineering strategies have been developed to improve therapeutic efficacy, challenges remain. Large-scale production of MSC-Exos is still a big challenge to be solved for clinical application. And homogenous and high-purity exosomes are hard to obtain by existing time-consuming and low-yield isolation techniques (Charoenviriyakul et al., 2017). Recently, bioreactors or microfluidic platforms have been used to increase the production of exosomes. It was reported that a microfluidic cell culture platform was developed that could harvest large-scale and antigen-modify exosomes in one workflow (Zhao et al., 2019). Furthermore, it should be noted exosomes contain some functional proteins and immune molecules, so the use of engineered exosomes may trigger a strong response by the host immune system and be rapidly eliminated (Lim et al., 2019). Meanwhile, many factors including storage conditions and time, administrate path and dose affect the biological activity and therapeutic efficacy of MSC-Exos.
To our delight, there are several clinical trials underway to evaluate MSC-Exos therapy for OA, and another one has been completed. The current results have shown that the use of MSC-Exos for OA treatment is effective and safe, and has the potential to be an alternative to joint replacement surgery. In summary, MSC-Exos is a promising cell-free therapy for knee OA and deserves more attention.
Acknowledgments
All the figures were created with BioRender.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing (Wuhan University of Technology, Grant No. 2022-KF-29), the Natural Science Foundation of China (Grant No. 82202673 and 81974350).
Author contributions
DL: Conceptualization, Methodology, Writing–original draft, Writing–review and editing. HZ: Writing–original draft, Funding acquisition. SL: Writing–original draft. ZW: Writing–review and editing, Conceptualization, Visualization. JX: Funding acquisition, Project administration, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Bousnaki M., Bakopoulou A., Kritis A., Koidis P. (2020). The efficacy of stem cells secretome application in osteoarthritis: a systematic review of in vivo studies. Stem Cell Rev. Rep. 16 (6), 1222–1241. 10.1007/s12015-020-09980-x [DOI] [PubMed] [Google Scholar]
- Burnouf T., Agrahari V., Agrahari V. (2019). Extracellular vesicles as nanomedicine: hopes and hurdles in clinical translation. Int. J. Nanomedicine 14, 8847–8859. 10.2147/IJN.S225453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo C. B., Nakagawa Y., Sekiya I., Rodeo S. A. (2017). Basic science of articular cartilage. Clin. Sports Med. 36 (3), 413–425. 10.1016/j.csm.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Cavallo C., Merli G., Borzì R. M., Zini N., D’Adamo S., Guescini M., et al. (2021). Small Extracellular Vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-κB dependent inflammatory/catabolic environment of osteoarthritis. Sci. Rep. 11 (1), 1053. 10.1038/s41598-020-80032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.-H., Wu S.-C., Chen C.-H., Chen J.-W., Huang W.-C., Wu C.-W., et al. (2023). Exosomes derived from hypoxia-cultured human adipose stem cells alleviate articular chondrocyte inflammaging and post-traumatic osteoarthritis progression. Int. J. Mol. Sci. 24 (17), 13414. 10.3390/ijms241713414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier E., Relic B., Deroyer C., Malaise O., Neuville S., Collée J., et al. (2016). Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int. J. Mol. Sci. 17 (12), 2146. 10.3390/ijms17122146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenviriyakul C., Takahashi Y., Morishita M., Matsumoto A., Nishikawa M., Takakura Y. (2017). Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur. J. Pharm. Sci. Official J. Eur. Fed. Pharm. Sci. 96, 316–322. 10.1016/j.ejps.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., et al. (2019). Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9 (9), 2439–2459. 10.7150/thno.31017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Shi Y., Xue P., Ma X., Li J., Zhang J. (2020). Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 22 (1), 256. 10.1186/s13075-020-02325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Sun Y., Ma Y., Ao Y., Hu X., Meng Q. (2022). Engineering of MSC-derived exosomes: a promising cell-free therapy for osteoarthritis. Membranes 12 (8), 739. 10.3390/membranes12080739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillet J. R., Kang Q., Ruf I. K., Briggs H. A., Vojtech L. N., Hughes S. M., et al. (2014). Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. 111 (41), 14888–14893. 10.1073/pnas.1408301111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Jeong S., Kim H., Kang D., Lee J., Kang S.-B., et al. (2021). Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp. Mol. Med. 53 (11), 1689–1696. 10.1038/s12276-021-00710-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noël D. (2017). Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 7 (1), 16214. 10.1038/s41598-017-15376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Li Z., Lu L., Zhang H., Chen R., Liu Y., et al. (2023). Sophisticated magneto-mechanical actuation promotes in situ stem cell assembly and chondrogenesis for treating osteoarthritis. ACS Nano 17 (21), 21690–21707. 10.1021/acsnano.3c06909 [DOI] [PubMed] [Google Scholar]
- Desando G., Cavallo C., Sartoni F., Martini L., Parrilli A., Veronesi F., et al. (2013). Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res. Ther. 15 (1), R22. 10.1186/ar4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A. D., Elicson J. M., Murphy W. L. (2017). Microcarriers with synthetic hydrogel surfaces for stem cell expansion. Adv. Healthc. Mater. 6 (16). 10.1002/adhm.201700072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordevic M. (2020). IRB approved pilot safety study of an extracellular vesicle isolate product evaluating the treatment of osteoarthritis in combat-related injuries. Stem Cell Res. 1(2). 11. 10.52793/JSCR.2020.1(2)-09 [DOI] [Google Scholar]
- Duan A., Shen K., Li B., Li C., Zhou H., Kong R., et al. (2021). Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res. Ther. 12 (1), 427. 10.1186/s13287-021-02507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsharkasy O. M., Nordin J. Z., Hagey D. W., de Jong O. G., Schiffelers R. M., Andaloussi S. E. L., et al. (2020). Extracellular vesicles as drug delivery systems: why and how? Adv. Drug Deliv. Rev. 159, 332–343. 10.1016/j.addr.2020.04.004 [DOI] [PubMed] [Google Scholar]
- Fernandes T. L., Gomoll A. H., Lattermann C., Hernandez A. J., Bueno D. F., Amano M. T. (2020). Macrophage: a potential target on cartilage regeneration. Front. Immunol. 11, 111. 10.3389/fimmu.2020.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo J. B., Looi Q. H., How C. W., Lee S. H., Al-Masawa M. E., Chong P. P., et al. (2021). Mesenchymal stem cell-derived exosomes and MicroRNAs in cartilage regeneration: biogenesis, efficacy, miRNA enrichment and delivery. Pharmaceuticals 14 (11), 1093. 10.3390/ph14111093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. J., Bonnet C. S., Blain E. J. (2021). Mechanical cues: bidirectional reciprocity in the extracellular matrix drives mechano-signalling in articular cartilage. Int. J. Mol. Sci. 22 (24), 13595. 10.3390/ijms222413595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M., Brizzi M. F., Choo A. B. H., Dominici M., Davidson S. M., Grillari J., et al. (2021). Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 23 (5), 373–380. 10.1016/j.jcyt.2021.01.001 [DOI] [PubMed] [Google Scholar]
- Gorgun C., Palamà M. E. F., Reverberi D., Gagliani M. C., Cortese K., Tasso R., et al. (2021). Role of extracellular vesicles from adipose tissue- and bone marrow-mesenchymal stromal cells in endothelial proliferation and chondrogenesis. Stem Cells Transl. Med. 10 (12), 1680–1695. 10.1002/sctm.21-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S., Kang M.-H., Jeyaraj M., Qasim M., Kim J.-H. (2019). Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 8 (4), 307. 10.3390/cells8040307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanzadeh A., Vousooghi N., Rahimnia R., Razeghian E., Rajaeian S., Seyhoun I., et al. (2023). Recent advances in mesenchymal stem/stromal cells (MSCs)‐based approaches for osteoarthritis (OA) therapy. Cell Biol. Int. 47 (6), 1033–1048. 10.1002/cbin.12008 [DOI] [PubMed] [Google Scholar]
- Heard B. J., Barton K. I., Chung M., Achari Y., Shrive N. G., Frank C. B., et al. (2015). Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J. Orthop. Res. 33 (12), 1826–1834. 10.1002/jor.22972 [DOI] [PubMed] [Google Scholar]
- Hu W., Cai C., Li Y., Kang F., Chu T., Dong S. (2022). Farnesoid X receptor agonist attenuates subchondral bone osteoclast fusion and osteochondral pathologies of osteoarthritis via suppressing JNK1/2/NFATc1 pathway. FASEB J. 36 (4), e22243. 10.1096/fj.202101717R [DOI] [PubMed] [Google Scholar]
- Hu W., Chen Y., Dou C., Dong S. (2021). Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheumatic Dis. 80 (4), 413–422. 10.1136/annrheumdis-2020-218089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. J., Bierma-Zeinstra S. (2019). Osteoarthritis. Lancet 393 (10182), 1745–1759. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- Jablonski C. L., Leonard C., Salo P., Krawetz R. J. (2019). CCL2 but not CCR2 is required for spontaneous articular cartilage regeneration post-injury. J. Orthop. Res. Official Publ. Orthop. Res. Soc. 37 (12), 2561–2574. 10.1002/jor.24444 [DOI] [PubMed] [Google Scholar]
- Jammes M., Cassé F., Velot E., Bianchi A., Audigié F., Contentin R., et al. (2023). Pro-inflammatory cytokine priming and purification method modulate the impact of exosomes derived from equine bone marrow mesenchymal stromal cells on equine articular chondrocytes. Int. J. Mol. Sci. 24 (18), 14169. 10.3390/ijms241814169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K., Jiang T., Chen Y., Mao X. (2021a). Mesenchymal stem cell-derived exosomes modulate chondrocyte glutamine metabolism to alleviate osteoarthritis progression. Mediat. Inflamm., 2021, 1–10. 10.1155/2021/2979124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Tian G., Yang Z., Gao X., Wang F., Li J., et al. (2021b). Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 6 (9), 2711–2728. 10.1016/j.bioactmat.2021.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. (2022). Osteoarthritis year in review 2021: biology. Osteoarthr. Cartil. 30 (2), 207–215. 10.1016/j.joca.2021.11.009 [DOI] [PubMed] [Google Scholar]
- Jin Y., Xu M., Zhu H., Dong C., Ji J., Liu Y., et al. (2021). Therapeutic effects of bone marrow mesenchymal stem cells‐derived exosomes on osteoarthritis. J. Cell. Mol. Med. 25 (19), 9281–9294. 10.1111/jcmm.16860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Ren J., Qi S. (2020). Exosomal miR-9-5p secreted by bone marrow–derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 381 (1), 99–114. 10.1007/s00441-020-03193-x [DOI] [PubMed] [Google Scholar]
- Joo H., Oh M.-K., Kang J. Y., Park H. S., Chae D.-H., Kim J., et al. (2021). Extracellular vesicles from thapsigargin-treated mesenchymal stem cells ameliorated experimental colitis via enhanced immunomodulatory properties. Biomedicines 9 (2), 209. 10.3390/biomedicines9020209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., LeBleu V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaliotas G. I., Mavridis K., Scorilas A., Babis G. C. (2015). Quantitative analysis of the mRNA expression levels of BCL2 and BAX genes in human osteoarthritis and normal articular cartilage: an investigation into their differential expression. Mol. Med. Rep. 12 (3), 4514–4521. 10.3892/mmr.2015.3939 [DOI] [PubMed] [Google Scholar]
- Kazemi M., Williams J. L. (2021). Properties of cartilage–subchondral bone junctions: a narrative review with specific focus on the growth plate. CARTILAGE 13 (2_Suppl. l), 16S–33S. 10.1177/1947603520924776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. B. (2022). Function and therapeutic development of exosomes for cancer therapy. Archives Pharmacal Res. 45 (5), 295–308. 10.1007/s12272-022-01387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. G., Choi J., Kim K. (2020). Mesenchymal stem cell‐derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnol. J. 15 (12), e2000082. 10.1002/biot.202000082 [DOI] [PubMed] [Google Scholar]
- Kimiz-Gebologlu I., Oncel S. S. (2022). Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 347, 533–543. 10.1016/j.jconrel.2022.05.027 [DOI] [PubMed] [Google Scholar]
- Kolasinski S. L., Neogi T., Hochberg M. C., Oatis C., Guyatt G., Block J., et al. (2020). 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care & Res., 72(2), 149–162. 10.1002/acr.24131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-H., Park H.-K., Auh Q.-S., Nah H., Lee J. S., Moon H.-J., et al. (2020). Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int. J. Mol. Sci. 21 (4), 1541. 10.3390/ijms21041541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Li W., Pu G., Wu J., Qin F. (2022a). Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. J. Orthop. Surg. Res. 17 (1), 567. 10.1186/s13018-022-03437-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Yan G., Huang H., Zheng M., Ma K., Cui X., et al. (2022b). Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnology 20 (1), 38. 10.1186/s12951-021-01236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Lv S., Jiang W., Si L., Liao B., Zhao G., et al. (2022c). Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann. Transl. Med. 10 (18), 976. 10.21037/atm-22-3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yu H., Sun M., Yang P., Hu X., Ao Y., et al. (2021a). The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 125, 253–266. 10.1016/j.actbio.2021.02.039 [DOI] [PubMed] [Google Scholar]
- Li S., Liu J., Liu S., Jiao W., Wang X. (2021b). Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J. Nanobiotechnology 19 (1), 343. 10.1186/s12951-021-01086-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Liu J., Liu S., Jiao W., Wang X. (2021c). Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnology 19 (1), 194. 10.1186/s12951-021-00940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang Y., Cai Z., Zhou Q., Li L., Fu P. (2021d). Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR‐100‐5p/NOX4 axis. Cell Biol. Int. 45 (10), 2096–2106. 10.1002/cbin.11657 [DOI] [PubMed] [Google Scholar]
- Li Y., Tu Q., Xie D., Chen S., Gao K., Xu X., et al. (2022d). Triamcinolone acetonide-loaded nanoparticles encapsulated by CD90+ MCSs-derived microvesicles drive anti-inflammatory properties and promote cartilage regeneration after osteoarthritis. J. Nanobiotechnology 20 (1), 150. 10.1186/s12951-022-01367-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., et al. (2020). Chondrocyte-Targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces 12 (33), 36938–36947. 10.1021/acsami.0c10458 [DOI] [PubMed] [Google Scholar]
- Liang Y., Xu X., Xu L., Iqbal Z., Ouyang K., Zhang H., et al. (2022). Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics 12 (11), 4866–4878. 10.7150/thno.69368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q., Li B. J., Li Y., Xiao Y., Zeng H., Liu J. M., et al. (2021). Low-intensity pulsed ultrasound promotes osteoarthritic cartilage regeneration by BMSC-derived exosomes via modulating the NF-κB signaling pathway. Int. Immunopharmacol. 97, 107824. 10.1016/j.intimp.2021.107824 [DOI] [PubMed] [Google Scholar]
- Lim S., Park J., Shim M. K., Um W., Yoon H. Y., Ryu J. H., et al. (2019). Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 9 (25), 7906–7923. 10.7150/thno.38425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zeng Y., Si H.-B., Tang L., Xie H.-Q., Shen B. (2022). Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am. J. Sports Med. 50 (4), 1088–1105. 10.1177/03635465221073991 [DOI] [PubMed] [Google Scholar]
- Lozito T. P., Jackson W. M., Nesti L. J., Tuan R. S. (2014). Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 34, 132–143. 10.1016/j.matbio.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Lozito T. P., Tuan R. S. (2011). Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiology 226 (2), 385–396. 10.1002/jcp.22344 [DOI] [PubMed] [Google Scholar]
- Lu H., Wei J., Liu K., Li Z., Xu T., Yang D., et al. (2023). Radical-scavenging and subchondral bone-regenerating nanomedicine for osteoarthritis treatment. ACS Nano 17 (6), 6131–6146. 10.1021/acsnano.3c01789 [DOI] [PubMed] [Google Scholar]
- Lu L., Wang J., Fan A., Wang P., Chen R., Lu L., et al. (2021). Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A‐26a‐5p ameliorate cartilage damage of osteoarthritis. J. Gene Med. 23 (11), e3379. 10.1002/jgm.3379 [DOI] [PubMed] [Google Scholar]
- Lukomska B., Stanaszek L., Zuba-Surma E., Legosz P., Sarzynska S., Drela K. (2019). Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int., 2019, 1, 10. 10.1155/2019/9628536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Zhang Z., Hu S., Zhang Z., Chang Z., Huang Z., et al. (2018). Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 9 (1), 247. 10.1186/s13287-018-1004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y., Manabe I. (2018). Macrophages in inflammation, repair and regeneration. Int. Immunol. 30 (11), 511–528. 10.1093/intimm/dxy054 [DOI] [PubMed] [Google Scholar]
- Pang L., Jin H., Lu Z., Xie F., Shen H., Li X., et al. (2023). Treatment with mesenchymal stem cell-derived nanovesicle-containing gelatin methacryloyl hydrogels alleviates osteoarthritis by modulating chondrogenesis and macrophage polarization. Adv. Healthc. Mater. 12 (17), e2300315. 10.1002/adhm.202300315 [DOI] [PubMed] [Google Scholar]
- Peng S., Sun C., Lai C., Zhang L. (2023). Exosomes derived from mesenchymal stem cells rescue cartilage injury in osteoarthritis through Ferroptosis by GOT1/CCR2 expression. Int. Immunopharmacol. 122, 110566. 10.1016/j.intimp.2023.110566 [DOI] [PubMed] [Google Scholar]
- Pourakbari R., Khodadadi M., Aghebati-Maleki A., Aghebati-Maleki L., Yousefi M. (2019). The potential of exosomes in the therapy of the cartilage and bone complications; emphasis on osteoarthritis. Life Sci. 236, 116861. 10.1016/j.lfs.2019.116861 [DOI] [PubMed] [Google Scholar]
- Qi H., Liu D.-P., Xiao D.-W., Tian D.-C., Su Y.-W., Jin S.-F. (2019). Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. Vitro Cell. Dev. Biol. - Animal 55 (3), 203–210. 10.1007/s11626-019-00330-x [DOI] [PubMed] [Google Scholar]
- Qiu B., Xu X., Yi P., Hao Y. (2020). Curcumin reinforces MSC‐derived exosomes in attenuating osteoarthritis via modulating the miR‐124/NF‐kB and miR‐143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 24 (18), 10855–10865. 10.1111/jcmm.15714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M., Liu D., Fu Q. (2021). MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 269, 118987. 10.1016/j.lfs.2020.118987 [DOI] [PubMed] [Google Scholar]
- Ragni E., Papait A., Perucca Orfei C., Silini A. R., Colombini A., Viganò M., et al. (2021). Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl. Med. 10 (7), 1044–1062. 10.1002/sctm.20-0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Del Bakhshayesh A., Babaie S., Tayefi Nasrabadi H., Asadi N., Akbarzadeh A., Abedelahi A. (2020). An overview of various treatment strategies, especially tissue engineering for damaged articular cartilage. Artif. Cells, Nanomedicine, Biotechnol. 48 (1), 1089–1104. 10.1080/21691401.2020.1809439 [DOI] [PubMed] [Google Scholar]
- Richette P., Latourte A., Frazier A. (2015). Safety and efficacy of paracetamol and NSAIDs in osteoarthritis: which drug to recommend? Expert Opin. Drug Saf. 14 (8), 1259–1268. 10.1517/14740338.2015.1056776 [DOI] [PubMed] [Google Scholar]
- Rocha S., Carvalho J., Oliveira P., Voglstaetter M., Schvartz D., Thomsen A. R., et al. (2019). 3D cellular architecture affects MicroRNA and protein cargo of extracellular vesicles. Adv. Sci. 6 (4), 1800948. 10.1002/advs.201800948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y., Zhang J., Jiang D., Ji C., liu W., Wang J., et al. (2021). Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 122, 325–342. 10.1016/j.actbio.2020.12.034 [DOI] [PubMed] [Google Scholar]
- Samanta S., Rajasingh S., Drosos N., Zhou Z., Dawn B., Rajasingh J. (2018). Exosomes: new molecular targets of diseases. Acta Pharmacol. Sin. 39 (4), 501–513. 10.1038/aps.2017.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerwegh A. J., Dombrecht E. J., Stevens W. J., Van Offel J. F., Bridts C. H., De Clerck L. S. (2003). Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 11 (9), 681–687. 10.1016/s1063-4584(03)00156-0 [DOI] [PubMed] [Google Scholar]
- Shen T., Alvarez-Garcia O., Li Y., Olmer M., Lotz M. K. (2017). Suppression of Sestrins in aging and osteoarthritic cartilage: dysfunction of an important stress defense mechanism. Osteoarthr. Cartil. 25 (2), 287–296. 10.1016/j.joca.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai S., Cai Q., Ou Y. (2022). Circular RNA circ_0008365 regulates SOX9 by targeting miR-338-3p to inhibit IL-1β-induced chondrocyte apoptosis and extracellular matrix degradation. J. Orthop. Surg. Res. 17 (1), 452. 10.1186/s13018-022-03240-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekmadji C., Li B., Huang Y., Wang H., An T., Liu C., et al. (2020). The future of Extracellular Vesicles as Theranostics – an ISEV meeting report. J. Extracell. Vesicles 9 (1), 1809766. 10.1080/20013078.2020.1809766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophia Fox A. J., Bedi A., Rodeo S. A. (2009). The basic science of articular cartilage: structure, composition, and function. Sports Health A Multidiscip. Approach 1 (6), 461–468. 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Sundar I. K. (2021). Recent updates on the role of extracellular vesicles in the pathogenesis of allergic asthma. Extracell. Vesicles Circulating Nucleic Acids 2, 127–147. 10.20517/evcna.2021.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Hu S., Zhang Z., Lun J., Liao W., Zhang Z. (2019). Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J. Cell. Biochem. 120 (1), 171–181. 10.1002/jcb.27289 [DOI] [PubMed] [Google Scholar]
- Sun X., Yin H., Wang Y., Lu J., Shen X., Lu C., et al. (2018). In situ articular cartilage regeneration through endogenous reparative cell homing using a functional bone marrow-specific scaffolding system. ACS Appl. Mater. Interfaces 10 (45), 38715–38728. 10.1021/acsami.8b11687 [DOI] [PubMed] [Google Scholar]
- Tan S. S. H., Tjio C. K. E., Wong J. R. Y., Wong K. L., Chew J. R. J., Hui J. H. P., et al. (2021). Mesenchymal stem cell exosomes for cartilage regeneration: a systematic review of preclinical in vivo studies. Tissue Eng. Part B Rev. 27 (1), 1–13. 10.1089/ten.teb.2019.0326 [DOI] [PubMed] [Google Scholar]
- Tang Y., Wu P., Li L., Xu W., Jiang J. (2022). Mesenchymal stem cells and their small extracellular vesicles as crucial immunological efficacy for hepatic diseases. Front. Immunol. 13, 880523. 10.3389/fimmu.2022.880523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S.-C., Huang J.-Y., Gao Y., Li Z.-X., Wei Z.-Y., Dawes H., et al. (2021a). Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 6 (12), 4455–4469. 10.1016/j.bioactmat.2021.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S.-C., Yuan T., Zhang Y.-L., Yin W.-J., Guo S.-C., Zhang C.-Q. (2017). Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7 (1), 180–195. 10.7150/thno.17133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Zhou J., Wang Z., Tao H., Bai J., Ge G., et al. (2021b). Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg. Chem. 113, 104978. 10.1016/j.bioorg.2021.104978 [DOI] [PubMed] [Google Scholar]
- Thakur A., Parra D. C., Motallebnejad P., Brocchi M., Chen H. J. (2022). Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 10, 281–294. 10.1016/j.bioactmat.2021.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. L., Eldridge S. E., Nosrati B., Alvarez M., Thorup A., Nalesso G., et al. (2021). WNT3A‐loaded exosomes enable cartilage repair. J. Extracell. Vesicles 10 (7), e12088. 10.1002/jev2.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K., Romain K., Mak C., Kamaraj A., Henson F., Khan W. (2020). The treatment of cartilage damage using human mesenchymal stem cell-derived extracellular vesicles: a systematic review of in vivo studies. Front. Bioeng. Biotechnol. 8, 580. 10.3389/fbioe.2020.00580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh W. S., Lai R. C., Zhang B., Lim S. K. (2018). MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 46 (4), 843–853. 10.1042/BST20180079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ude C. C., Shamsul B. S., Ng M. H., Chen H. C., Ohnmar H., Amaramalar S. N., et al. (2018). Long-term evaluation of osteoarthritis sheep knee, treated with TGF-β3 and BMP-6 induced multipotent stem cells. Exp. Gerontol. 104, 43–51. 10.1016/j.exger.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Vonk L. A., van Dooremalen S. F. J., Liv N., Klumperman J., Coffer P. J., Saris D. B. F., et al. (2018). Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro . Theranostics 8 (4), 906–920. 10.7150/thno.20746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J., He Z., Peng R., Wu X., Zhu Z., Cui J., et al. (2023). Injectable photocrosslinking spherical hydrogel-encapsulated targeting peptide-modified engineered exosomes for osteoarthritis therapy. J. Nanobiotechnology 21 (1), 284. 10.1186/s12951-023-02050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Bao D., Li J., Lin K., Huang Q., Li Q., et al. (2022). Extracellular vesicles from hypoxic pretreated urine-derived stem cells enhance the proliferation and migration of chondrocytes by delivering miR-26a-5p. Cartilage 13 (2), 194760352210774. 10.1177/19476035221077401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., He C. (2022). Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 13, 967193. 10.3389/fimmu.2022.967193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Xu B., Xu H. (2018a). TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17 (24), 2756–2765. 10.1080/15384101.2018.1556063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li Z., Cui Y., Cui X., Chen C., Wang Z. (2021a). Exosomes isolated from bone marrow mesenchymal stem cells exert a protective effect on osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front. Cell Dev. Biol. 9, 644380. 10.3389/fcell.2021.644380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Omar O., Vazirisani F., Thomsen P., Ekström K. (2018b). Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLOS ONE 13 (2), e0193059. 10.1371/journal.pone.0193059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yu D., Liu Z., Zhou F., Dai J., Wu B., et al. (2017). Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 8 (1), 189. 10.1186/s13287-017-0632-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., He Z., Liang S., Yang Q., Cheng P., Chen A. (2020). Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 11 (1), 511. 10.1186/s13287-020-02032-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yan K., Ge G., Zhang D., Bai J., Guo X., et al. (2021b). Exosomes derived from miR-155-5p–overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 37 (1), 85–96. 10.1007/s10565-020-09559-9 [DOI] [PubMed] [Google Scholar]
- Wei Z.-J., Wang Q.-Q., Cui Z.-G., Inadera H., Jiang X., Wu C.-A. (2021). Which is the most effective one in knee osteoarthritis treatment from mesenchymal stem cells obtained from different sources? —a systematic review with conventional and network meta-analyses of randomized controlled trials. Ann. Transl. Med. 9 (6), 452. 10.21037/atm-20-5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Lin L., Zou R., Lin F., Liu Y. (2022). Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell CycleGeorget. Tex.) 21 (3), 289–303. 10.1080/15384101.2021.2019411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C. H., Kim H. K., Jung G. Y., Jung Y. J., Lee K. S., Yun Y. E., et al. (2020). Small extracellular vesicles from human adipose‐derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 9 (1), 1735249. 10.1080/20013078.2020.1735249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Kuang L., Chen C., Yang J., Zeng W.-N., Li T., et al. (2019). miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206, 87–100. 10.1016/j.biomaterials.2019.03.022 [DOI] [PubMed] [Google Scholar]
- Wu Y., Li J., Zeng Y., Pu W., Mu X., Sun K., et al. (2022). Exosomes rewire the cartilage microenvironment in osteoarthritis: from intercellular communication to therapeutic strategies. Int. J. Oral Sci. 14 (1), 40. 10.1038/s41368-022-00187-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q., Wang Q., Lin F., Wang J. (2021). miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered 12 (2), 11225–11238. 10.1080/21655979.2021.1995580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X.-N., Zhu S.-Y., He H.-C., Yu X., Xu Y., He C.-Q. (2022). Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 13 (1), 14. 10.1186/s13287-021-02689-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., et al. (2021). Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 269, 120539. 10.1016/j.biomaterials.2020.120539 [DOI] [PubMed] [Google Scholar]
- Xu X., Xu L., Wen C., Xia J., Zhang Y., Liang Y. (2023). Programming assembly of biomimetic exosomes: an emerging theranostic nanomedicine platform. Mater. Today. Bio 22, 100760. 10.1016/j.mtbio.2023.100760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Liu G., Wu X. (2021). Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J. Orthop. Transl. 26, 111–120. 10.1016/j.jot.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Wu X. (2020). Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 36 (2), 165–178. 10.1007/s10565-019-09504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Cong M., Huang W., Chen J., Zhang M., Gu X., et al. (2022). The effect of human bone marrow mesenchymal stem cell-derived exosomes on cartilage repair in rabbits. Stem Cells Int., 2022, 1–12. 10.1155/2022/5760107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-H. K., Ogando C. R., Wang See C., Chang T.-Y., Barabino G. A. (2018). Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro . Stem Cell Res. Ther. 9 (1), 131. 10.1186/s13287-018-0876-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P., Mi Z., Wei D., Gao P., Ma M., Yang H. (2022). miR-3960 from mesenchymal stem cell-derived extracellular vesicles inactivates SDC1/wnt/β-catenin Axis to relieve chondrocyte injury in osteoarthritis by targeting PHLDA2. Stem Cells Int., 2022, 1–18. 10.1155/2022/9455152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo K., Thapa N., Chwae Y., Yoon S., Kim B., Lee J., et al. (2022). Transforming growth factor-β family and stem cell-derived exosome therapeutic treatment in osteoarthritis (Review). Int. J. Mol. Med. 49 (5), 62. 10.3892/ijmm.2022.5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.-W., Wan Q.-Q., Wei Y., Li Y.-T., Li Q.-H., Ye T., et al. (2022). Engineered extracellular vesicles: regulating the crosstalk between the skeleton and immune system. Eng. Regen. 3 (3), 270–282. 10.1016/j.engreg.2022.06.004 [DOI] [Google Scholar]
- Zhang B., Tian X., Qu Z., Liu J., Yang L., Zhang W. (2021a). Efficacy of extracellular vesicles from mesenchymal stem cells on osteoarthritis in animal models: a systematic review and meta-analysis. Nanomedicine 16 (15), 1297–1310. 10.2217/nnm-2021-0047 [DOI] [PubMed] [Google Scholar]
- Zhang F.-X., Liu P., Ding W., Meng Q.-B., Su D.-H., Zhang Q.-C., et al. (2021b). Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 278, 121169. 10.1016/j.biomaterials.2021.121169 [DOI] [PubMed] [Google Scholar]
- Zhang S., Chu W. C., Lai R. C., Lim S. K., Hui J. H. P., Toh W. S. (2016). Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 24 (12), 2135–2140. 10.1016/j.joca.2016.06.022 [DOI] [PubMed] [Google Scholar]
- Zhang S., Chuah S. J., Lai R. C., Hui J. H. P., Lim S. K., Toh W. S. (2018). MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16–27. 10.1016/j.biomaterials.2017.11.028 [DOI] [PubMed] [Google Scholar]
- Zhang S., Jin Z. (2022). Bone mesenchymal stem cell-derived extracellular vesicles containing long noncoding RNA NEAT1 relieve osteoarthritis. Oxidative Med. Cell. Longev., 2022, 1–21. 10.1155/2022/5517648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qi G., Yan Y., Wang C., Wang Z., Jiang C., et al. (2023). Exosomes derived from bone marrow mesenchymal stem cells pretreated with decellularized extracellular matrix enhance the alleviation of osteoarthritis through miR-3473b/phosphatase and tensin homolog axis. J. Gene Med. 25 (8), e3510. 10.1002/jgm.3510 [DOI] [PubMed] [Google Scholar]
- Zhao C., Chen J., Peng W., Yuan B., Bi Q., Xu Y. (2020). Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 21, 1881–1889. 10.3892/mmr.2020.10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Sun Y., Sheng X., Xu J., Dai G., He R., et al. (2023a). Hypoxia-treated adipose mesenchymal stem cell-derived exosomes attenuate lumbar facet joint osteoarthritis. Mol. Med. 29 (1), 120. 10.1186/s10020-023-00709-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Xiu G., Wang J., Wen Y., Lu J., Wu B., et al. (2023b). Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J. Nanobiotechnology 21 (1), 341. 10.1186/s12951-023-02086-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., McGill J., Gamero-Kubota P., He M. (2019). Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab a Chip 19 (10), 1877–1886. 10.1039/c8lc01279b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T., Li Y., Zhang X., Xu J., Luo M. (2022). Exosomes derived from miR-212-5p overexpressed human synovial mesenchymal stem cells suppress chondrocyte degeneration and inflammation by targeting ELF3. Front. Bioeng. Biotechnol. 10, 816209. 10.3389/fbioe.2022.816209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Shen X., Yan C., Xiong W., Ma Z., Tan Z., et al. (2022a). Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res. Ther. 13 (1), 322. 10.1186/s13287-022-03005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Cao H., Guo J., Yuan Y., Ni G. (2022b). Effects of BMSC-derived EVs on bone metabolism. Pharmaceutics 14 (5), 1012. 10.3390/pharmaceutics14051012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Sun H.-T., Wang S., Huang S.-L., Zheng Y., Wang C.-Q., et al. (2020). Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 13 (1), 152. 10.1186/s13045-020-00987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., et al. (2017). Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 8 (1), 64. 10.1186/s13287-017-0510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]