Opinion statement
Biliary tract cancers are molecularly and anatomically diverse cancers which include intrahepatic cholangiocarcinoma, extrahepatic (perihilar and distal) cholangiocarcinoma, and gallbladder cancer. While recognized as distinct entities, the rarer incidence of these cancers combined with diagnostic challenges in classifying anatomic origin has resulted in clinical trials and guideline recommended strategies being generalized patients with all types of biliary tract cancer. In this review, we delve into the unique aspects, subtype-specific clinical trial outcomes, and multidisciplinary management of patients with extrahepatic cholangiocarcinoma. When resectable, definitive surgery followed by adjuvant chemotherapy (sometimes with selective radiation/chemoradiation) is current standard of care. Due to high recurrence rates, there is growing interest in the use of upfront/neoadjuvant therapy to improve surgical outcomes and to downstage patients who may not initially be resectable. Select patients with perihilar cholangiocarcinoma are being successfully treated with novel approaches such as liver transplant. In the advanced disease setting, combination gemcitabine and cisplatin remains the standard base for systemic therapy and was recently improved upon with the addition of immune checkpoint blockade to the chemotherapy doublet in the recently reported TOPAZ-1 and KEYNOTE-966 trials. Second-line all-comer treatments for these patients remain limited in both options and efficacy, so clinical trial participation should be strongly considered. With increased use of molecular testing, detection of actionable mutations and opportunities to receive indicated targeted therapies are on the rise and are the most significant driver of improved survival for patients with advanced stage disease. Though these targeted therapies are currently reserved for the second or later line, future trials are looking at moving these to earlier treatment settings and use in combination with chemotherapy and immunotherapy. In addition to cross-disciplinary management with surgical, medical, and radiation oncology, patient-centered care should also include collaboration with advanced endoscopists, palliative care specialists, and nutritionists to improve global patient outcomes.
Keywords: Extrahepatic cholangiocarcinoma, Biliary tract cancer, Targeted therapy, Chemotherapy, Immunotherapy
Introduction
Biliary tract cancers (BTC) are a diverse group of pathologically distinct entities that can be further divided into intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), and gallbladder cancer (GBC), with the latter two of these comprising extrahepatic BTC (eBTC). Extrahepatic CCA is further divided into distal (dCCA) and perihilar cholangiocarcinoma (pCCA). Distal CCA includes the common bile duct and distal ducts whereas pCCA arises from the second-order bile ducts down to the cystic duct [1]. Given their heterogeneity, BTCs have unique pathophysiology and molecular fingerprints, which have both prognostic and treatment implications.
The mainstay of treatment for advanced disease, until the recent addition of adjunct immunotherapy, has been the chemotherapy doublet of gemcitabine (Gem) and cisplatin (Cis) [2]. With the improved detection and knowledge of actionable mutations, targeted therapy has significantly boosted the treatment armamentarium for BTCs. Herein, we review pathogenesis, molecular characterization, and treatment modalities for patients with eCCA in both early stage and advanced disease. We highlight the continued need for upfront molecular testing and clinical trial consideration across disease stages to determine the optimal timing of targeted therapy and immunotherapy and its role in combination with established therapies to improve outcomes.
Epidemiology and prognosis
Extrahepatic CCA makes up 20–30% of all BTC in the USA and globally and is associated with certain conditions that significantly increase risk of eCCA including non-alcoholic fatty liver disease (odds ratio [OR] 2.9), cirrhosis (OR 3.8), alcohol-related liver disease (OR 2.6), and primary sclerosing cholangitis (PSC; OR 40.8), with the latter carrying up to a 36% lifetime risk of developing CCA [3–5]. Hepatitis B (HBV) and C (HCV) are associated with iCCA (predominantly) and eCCA (HBV OR 2.38; HCV OR 3.18) [4–7]. Conditions predisposing to eCCA include chronic pancreatitis, cholangitis, and choledocholithiasis [5, 8] with the estimated incidence of around 1.02 cases per 100,000 annually [9]. The prognosis for eCCA remains poor with 5-year survival estimated at 11% (localized and regional: 18%; distant: 2%) and surgery remains the only curative option in non-metastatic eCCA [10, 11]. Patients with risk factors such as higher T-stage (p < 0.001, locoregional disease [LRD]; p = 0.005, distant disease [DD] in T1a vs T1b-T4 disease) and the presence of lymphovascular invasion (p = 0.004, LRD; p = 0.01, DD) or perineural invasion (p = 0.04, LRD; p = 0.006, DD) are at risk for local and distant recurrence following definitive resection [12].
Molecular pathogenesis and distinctive molecular characteristics of subtypes
Though classified together as BTCs, iCCA, eCCA, and GBC contain discrete molecular profiles. The differentiation between eCCA and GBC from iCCA can be traced to their cell of origin. Extrahepatic cholangiocytes derive from endoderm and are closely related to the pancreas and duodenum, whereas intrahepatic cholangiocytes arise from hepatoblasts, closely resembling hepatocytes [13]. Distal CCA can often be difficult to distinguish from ampullary and pancreatic ductal adenocarcinoma (PDAC) given their similar embryological development and overlapping mutational profile [14].
Extrahepatic CCA can be characterized into four distinct classes based on the most prevalent mutations: mesenchymal, metabolic, proliferation, and immune [15]. The mesenchymal class (47.3%) is characterized by abnormal transforming growth factor beta (TFG-β) activity and epithelial-to-mesenchymal transition leading to fibrosis and poor overall survival (OS) compared to the 3 other classes (OS HR 1.95; p = 0.018). Infiltration of cancer-associated fibroblasts occurs by aberrant activation of TFG-β which both suppresses immune cells in the tumor microenvironment and also promotes angiogenesis [16]. Increased cell signaling pathways such as MAPK/ERK and AKT/mTOR along with ERBB2 overexpression are increased in the proliferation class (22.5%). The metabolic class (18.7%) contains mutations associated with dysregulated bile acid metabolism (such as HDAC6 overexpression leading to ciliary loss), promoting proliferation and ultimately, metastasis [17]. Lastly, the immune class (11.5%) is characterized by infiltration of dysfunctional T-cells along with elevated PD-1 and PD-L1 expression which may represent a distinct cohort that could benefit from immunotherapy.
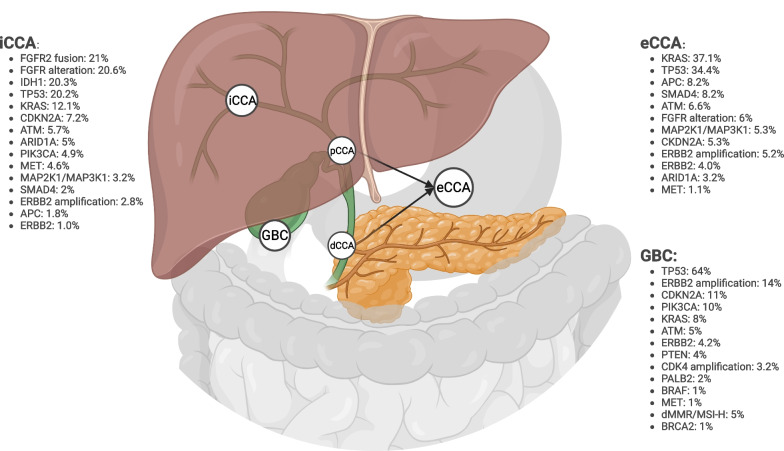
Similar to differences at the anatomic and cellular level, molecular profiles also differ across BTC subtypes (Fig. 1). Alterations in KRAS (36.7% vs 12.1%), TP53 (34.4% vs 20.2%), APC (8.2% vs 1.6), SMAD4 (10.4% vs 2%), and ERBB2 (9.7% vs. 4.2%) are significantly more common in eCCA than iCCA [18–23]. KRAS G12D variant is most common in dCCA and GBC, followed by G12V in pCCA. Though not quite approaching the >90% incidence of KRAS mutations seen in PDAC, dCCA has the most similar mutation profile to PDAC [14, 24]. Most notably, two of the most common actionable genomic alterations with FDA-approved targeted therapies, IDH1 and FGFR2 alterations, occurring approximately 18% and 15% of iCCA, respectively, are rarely found in eCCA [15, 25•, 26, 27]. Given that up to 50% of patients with BTC have an potentially actionable mutations [28, 29] with targeted agents, guideline-recommended therapy should always include upfront molecular testing on patients with unresectable BTC [2].
Fig. 1.
Biliary tract cancer anatomy and molecular alteration prevalence by subtype. Frequencies shown were extracted from recent BTC molecular profiling publications [23, 25•, 30, 31]. Created with Biorender.
Presentation, diagnosis, and multidisciplinary review
Most patients with eCCA present with de novo metastatic disease (up to 37%) [7], as initial non-specific symptoms of fatigue, abdominal pain, and weight loss may be overlooked until disease progresses and more pressing symptoms (e.g., jaundice, acholia, biliary obstruction) develop [9]. Biliary obstruction leads to imaging and subsequent diagnosis earlier in eCCA than iCCA due to external biliary compression [4]. Computed tomography (CT) is the imaging modality of choice except in pCCA where magnetic resonance cholangiopancreatogram (MRCP) can be especially useful to delineate biliary tree anatomy and determine local invasion [9]. Prior to obtaining pathology, PDAC may be difficult to distinguish from dCCA based on imaging alone [32].
After imaging is obtained, endoscopic retrograde cholangiopancreatography (ERCP) is often used to directly visualize the biliary tree, obtain brush cytology, biopsy, and relieve obstruction with biliary stenting, if needed [9]. Initial diagnosis of CCA based on ERCP (including brush cytology and biopsy via forceps) may be difficult as the sensitivity and specificity from ERCP are reported as 49% and 96%, respectively. EUS with FNA of biliary stricture has slightly improved sensitivity and specificity of 75% and 100%, respectively, but cannot exclude CCA due to low sensitivity [33]. Fluorescence in situ hybridization (FISH), an alternative method examining chromosomal and 9p21 abnormalities, can be combined with cytology to improve sensitivity (35 to 63% [with FISH]; p < 0.05) [34].
Patients should be presented in a multidisciplinary tumor board since treatment modalities range from surgery, locoregional treatment, systemic therapy, and can often involve a combinatorial approach. Implementation of multidisciplinary tumor boards has been shown to prolong life, improve patient-reported quality of life outcomes, and increase clinical trial awareness and evaluation [35]. Multidisciplinary patient care also should extend to involving dieticians for nutrition needs, palliative care for symptom management and serious illness conversations, and interventionalists for endobiliary decompression, as indicated.
Early-stage disease
Surgical considerations and recurrence risk factors
For patients who present with localized disease, definitive treatment with surgical resection can offer cure. Unfortunately, only 20% of patients have resectable disease at presentation, and recurrence rates up to 75% have been reported [6, 36].
If an anatomical and biological candidate, surgery is often extensive and usually requires a pancreaticoduodenectomy (Whipple procedure) for dCCA and extensive hepatectomy for pCCA to achieve R0 resection [37]. Underlying liver disease or bilateral eCCA can be contraindications to surgery. Liver function may need to be optimized by relieving biliary obstruction prior to patients undergoing resection. In those with underlying liver disease or bilateral biliary duct involvement by eCCA without lymph node (LN) or metastatic disease, liver transplant can be considered and will be discussed subsequently with neoadjuvant therapy [38]. Higher rates of recurrence after surgical resection are seen in patients with vascular invasion, higher tumor stage (5-year OS 28% [T2b/T3] vs 57% [T1–2a]; HR 2.23; 95% CI 1.24–4.01; p = 0.007), nodal spread (5-year OS 27% [Node +] 50% vs 27% [Node −], HR 2.07 [95% CI 1.16, 3.68], p = 0.014), R1 resection (5-year OS 13% [R1] vs 49% [R0], HR 2.09 [95% CI 1.17, 3.71], p = 0.012), and initial CA19-9 greater than 37 U/mL (HR 1.42) [39–42].
Carbohydrate antigen 19-9 (CA19-9) can offer predictive value as a surrogate marker for micrometastatic disease and post-operative recurrence risk. Elevated CA19-9 ( ≥37 U/mL) are found in 59.1% of CCA [43] with a reported sensitivity and specificity of 66% and 88%, respectively [44]. A retrospective review analyzed CA19-9 in patients with eCCA (N = 390) before and after curative-intent surgery who had either normal (<37 U/mL) or elevated (≥ 37 U/mL) values. In patients with normal values prior to surgery or elevated values that normalized postoperatively, the 5-year OS was significantly better than patients who had elevated CA19-9 both pre- and post-operatively (53%, 38%, 23%, respectively; p <0.001). Patients with CA19-9 <37 U/mL prior to surgery had a significantly better OS with R0 resection (5-year OS 59% vs 7%; p < 0.001). Even though the rates of local recurrence were similar, patients with normal CA19-9 serum level were less likely to have distant recurrence than patients with pre-operatively elevated (p = 0.003) or persistently elevated post-operative values (p < 0.001). CA19-9 should always be obtained prior to surgery given its use in evaluating micrometastatic disease and risk of recurrence after resection [42].
Adjuvant therapy
Adjuvant chemotherapy
Prior to 2019, adjuvant chemotherapy had not demonstrated a benefit over observation in resected BTC. The phase III PRODIGE12/ACCORD18 trial [41] comparing adjuvant GemOx to observation in resected BTC patients showed no significant differences in RFS and OS. However, with the reporting of the BILCAP trial [45], 6 months of adjuvant Cap became standard of care. This phase III study randomized 447 patients (n = 284 [64%] eCCA; n = 279 [62%] R0 resection) with resected BTC to either adjuvant Cap (D1–14, 21-day cycles) or observation for 6 months. On per protocol analysis, adjusting for disease grade and nodal status, Cap significantly improved OS (mOS 49.6 months [Cap] vs 36.1 months [obs]; HR 0.74, [95% CI 0.59, 0.94]) and DFS (mDFS 25.3 months [Cap] vs 16.8 months [obs]; DFS HR 0.77 [95% CI 0.61, 0.97]) compared to observation. Recently, the phase II STAMP trial [46] randomized patients with resected lymph node-positive (LN-positive) eCCA to either GemCis or Cap (n=101), but failed to demonstrate the benefit of GemCis over Cap in OS (HR 1.08 [95% CI 0.71, 1.64], p = 0.404) or DFS (HR 0.96 [95% CI 0.71, 1.3], p = 0.430) with GemCis having an 84% grade 3+ AE rate (vs 14% with Cap). The currently ongoing phase III ACTICCA-1 trial (NCT02170090) [47] will compare adjuvant GemCis with observation, the results of which are expected in 2024 (see Table 1 for a summary of seminal adjuvant therapy clinical trials for BTC).
Table 1.
Summary of select biliary tract cancer clinical trials conducted in the adjuvant, first-line advanced stage, or second-line advanced stage treatment setting
| NCT | Trial | Study agent | Study population | Primary outcome | mPFS, RFS, or DFS (months) | HR (95% CI) p-value |
mOS (months) | HR (95% CI) p-value |
ORR (%) |
|---|---|---|---|---|---|---|---|---|---|
| Adjuvant therapy | |||||||||
| NCT00363584 | BILCAP [48] | Cap vs obs | Resected BTC | OS | DFS: 25.9 vs 17.4 |
0.75 (0.58, 0.98) p = 0.033 |
49.6 vs 36.1 |
0.71 (0.55, 0.92) p = 0.010 |
|
|
GBC: 0.84 (0.43, 1.63) dCCA: 0.70 (0.47, 1.06) pCCA: 1.08 (0.68, 1.71) | |||||||||
| NCT03712605 | STAMP [46] | GemCis vs Cap | Resected, LN-positive eCCA | DFS | DFS: 14.3 vs 11.1 |
0.96 (0.71–1.30) p = 0.86 |
35.7 vs 35.7 |
1.08 (0.71, 1.64) p = 0.404 |
|
|
pCCA: 1.38 (CI 0.91, 2.11) dCCA: 0.63 (0.42, 0.96) |
pCCA: 1.50 (0.84, 2.65) dCCA: 0.75 (CI 0.41, 1.38) |
||||||||
| NCT01313377 | PRODIGE 12-ACCORD 18-UNICANCER GI [41] | GemOx vs obs | Resected BTC | RFS | RFS: 30.4 vs 18.5 |
0.88 (0.62, 1.25) p = 0.48 |
75.8 vs 50.8 |
1.08 (CI 0.79, 1.66) p = 0.74 |
|
|
GBC: 2.56 (CI 1.04, 6.23) eCCA: 0.60 (CI 0.34, 1.1) |
GBC: 3.4 (1.17, 9.8) eCCA: 0.70 (0.34, 1.44) |
||||||||
| NCT02170090 | ACTICCA-1 [47] | GemCis vs observation | Resected BTC | DFS | Ongoing | ||||
| NCT00789958 | SWOG S0809 [49••] | GemCap followed by CRT with Cap | Resected eCCA or GBC with pT2-4, N1, or R1 disease | 2-yr OS |
2-yr DFS: 52% R0: 54% R1: 48% GBC: 48% eCCA: 54% |
2-yr OS: 65% R0: 67% R1: 60% GBC: 56% eCCA: 68% |
|||
| Advanced first line (chemotherapy) | |||||||||
| NCT00262769 | ABC-02 [50] | GemCis vs Gem | 1st line, advanced BTC | OS | mPFS: 8.0 vs 5.0 |
0.63 (0.51, 0.77) p < 0.001 |
11.7 vs 8.1 |
0.64 (0.51, 0.8) p < 0.001 |
81.4% vs 71.8% p = 0.049 |
| NCT03768414 | SWOG-1815 [51] | GAP vs GemCis | 1st line, advanced BTC | OS | mPFS: 8.2 vs 6.4 |
0.92 (85% CI 0.72, 1.16) p = 0.47 |
14 vs 12.7 |
0.93 (0.74, 1.19) p = 0.58 |
34% vs 25% p = 0.11 |
| GemOx[52] | 1st line, advanced BTC | ORR |
mPFS: 3.4 GBC: 2.5 CCA: 3.8 |
(2.5, 4.6) (1.6, 4.3) (2.7, 5.6) |
8.8 GBC: 6.1 CCA: 11 |
(6.9, 11.1) (3.0, 10.8) (7.5, 14.1) |
GBC: 4.3% CCA: 20.5% |
||
| GemCap[53] | 1st line, advanced BTC | ORR, DOR, OS |
mPFS: 7.0 GBC: 4.4 CCA: 9.0 |
(4.6, 11.8) | 14.0 | (7.3 to NE) |
31% GBC: 27% CCA: 34% |
||
| NCT02591030 | PRODIGE 38 AMEBICA [54] | mFOLFIRINOX vs GemCis | 1st line, advanced BTC | 6-mo PFS | mPFS: 6.2 vs 7.4 | 11.7 vs 13.8 | 25% vs 19.4% | ||
| CRTI/2010/091/001406 [55] | mGemOx vs GemCis | 1st line, advanced GBC | OS | mPFS: 5.0 vs 4.0 | p = 0.047 | 9 vs 8.3 |
0.057 (0.6, 1.01) p = 0.057 |
25.2% vs 23.4% | |
| Advanced first line (chemoimmunotherapy) | |||||||||
| NCT03875235 | TOPAZ-1 [56••] | GemCis+durva vs Gemcis+placebo | 1st line, advanced BTC | OS | mPFS: 7.2 vs 5.7 |
0.75 (0.63, 0.89) p = 0.001 |
12.8 vs 11.5 |
0.80 (0.66, 0.97) p = 0.021 |
26.7% vs 18.7% |
| NCT04003636 | KEYNOTE-966 [57••] | GemCis+pembro vs GemCis | 1st line, advanced BTC | OS | mPFS: 6.5 vs 5.6 |
0.86 (0.75, 1.0) p = 0.023 |
12.7 vs 10.9 |
0.83 (0.72, 0.95) p = 0.0034 |
29% vs 29% |
| NCT 04677504 | IMbrave-151 [58] | GemCis+atezo + bev vs GemCis+atezo | 1st line, advanced BTC | PFS | mPFS: 8.4 vs 7.9 | 0.76 (0.51, 1.14) | Ongoing | 24% vs 25% | |
| Advanced second line | |||||||||
| NCT01926236 | ABC-06 [59••] | FOLFOX vs ACS | 2nd line, advanced BTC | OS | mPFS: 4.0 (FOLFOX) | 6.2 vs 5.3 |
0.69 (0.5, 0.97) p = 0.031 |
5% (FOLFOX) | |
| NCT03524508 | NIFTY[60] | FOLFIRI vs 5FU | 2nd line, advanced BTC | PFS |
mPFS: 7.1 vs 1.4 GBC: 9 vs 1.4 eCCA: 8.8 vs 1.6 |
HR 0.56 (0.39, 0.81) p = 0.0019 GBC: 0.3 (0.15, 0.61) eCCA: 0.79 (0.38, 1.66) |
8.6 vs 5.5 GBC: 12.4 vs 4.7 eCCA: 8.8 vs 7.6 |
0.68 (CI 0.48, 0.98) p = 0.035 GBC: 0.29 (0.14, 0.58) eCCA: 1.06 (0.53, 2.16) |
14.8% vs 5.8% |
| NCT03043547 | NALIRICC [61] | FOLFIRI vs 5FU | 2nd line, advanced BTC | PFS | mPFS: 2.76 vs 2.3 | 6.9 vs 8.21 | 14.3% vs 3.9% | ||
Abbreviations: BTC, biliary tract cancer; CCA, cholangiocarcinoma; DOR, duration of response; eCCA, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; ORR, objective response rate; mOS, median overall survival; mPFS, median progression-free survival; NE, not evaluable
Adjuvant chemoradiation
In a meta-analysis of 20 studies comparing outcomes between BTC patients who received adjuvant chemotherapy (CT), chemoradiotherapy (CRT), or radiotherapy (RT) after resection, overall, patients who received CT (OR 0.39) or CRT (OR 0.61) had improved OS compared to those who received RT alone (OR 0.98) [62]. When analyzed by margin-positivity and LN-positive status, most patients with R1 resections received RT alone (63% of patients; OR 0.33 [95% CI 0.14, 0.81], p = 0.01) which was associated with significantly improved OS, whereas no benefit was seen with RT in R0 resections (OR 1.26 [95% CI 0.88, 1.79], p = 0.20) [62]. In LN-positive disease, 77% of patients received adjuvant CT while the remaining received CRT in which showed an overall OS benefit with adjuvant therapy (OR 0.49 [95% CI 0.3, 0.8], p = 0.002) [62]. A more recent meta-analysis evaluated 5-year OS in patients who received adjuvant RT compared to observation and found significantly improved OS (OR 0.63 [95% CI 0.5, 0.81], p = 0.0002) and lower rate of local recurrence (OR 0.54 [95% CI 0.38, 0.76], p = 0.0004) in LN-positive disease (OR 0.15 [95% CI 0.07, 0.35], p < 0.00001) and R1 resection (OR 0.4 [95% CI 0.19, 0.85], p = 0.0002) with RT [63]. Additionally, a National Cancer Database analysis including patients with R1 resection or LN-positive disease who received adjuvant CT had significantly improved OS with the addition of adjuvant RT (mOS 34 vs 27 months; p < 0.001) [64].
The single-arm phase II trial, SWOG S0809 [49••], explored the role of four adjuvant cycles of GemCap followed by concurrent CRT (systemic Cap with 45 Gy to regional lymphatics; 54 to 59.4 Gy to tumor bed) in resected eCCA and GBC (N = 78; n = 54 [eCCA]). This demonstrated a mOS of 35 months with 2-year RFS of 54% and 11% local relapse rate. The 2-year OS of 65% (67% [R0]; 60% [R1]) was significantly higher than the rates expected based on historical controls. The mOS of 35 months was similar between patients with R0 and R1 resections which suggests that this approach could bring the risk of recurrence with R1 resection closer to that associated with R0 resections. Although not powered for comparison, the similar OS may suggest efficacy of the treatment regimen, particularly in patients with R1 resections [36]. Reported grade 3/4 AEs included neutropenia (35%), hand-foot syndrome (13%), diarrhea (8%), and lymphopenia (8%) [49••]. Based on these findings, the NCCN recommends adjuvant CT in R0 resection and either CT and/or CRT in R1 or LN-positive disease under the guidance of a multidisciplinary team [2].
Locally advanced disease
Upfront/perioperative systemic therapy
For patients with locally advanced/potentially resectable disease, the use of upfront therapy prior to surgery to increase curative resection rates has been increasing over time, but its role remains unclear [65, 66]. The benefits of upfront/neoadjuvant therapy include downstaging tumor burden, systemic therapy treatment prior to major surgery and potential post-operative recovery complications/functional setbacks, and testing disease biology (in terms of pathological assessment of treatment response and conversely avoiding an unnecessary surgery for patients whose cancer progresses while upfront therapy) [67]. A retrospective review of resectable dCCA patients who underwent either neoadjuvant therapy followed by resection or upfront resection showed more R0 resection rates (83% vs 76%; p = 0.04) and improved mOS (38.4 vs 25.6 months; p < 0.001) in patients who received neoadjuvant therapy compared to upfront surgery [68]. Another review including 45 patients with eCCA (n = 33 [adjuvant RT]; n = 12 [neoadjuvant CT]) found that 11 (91%) patients treated neoadjuvantly had R0 resections and numerically improved 5-year OS, trending towards significance (53% vs 23%; p = 0.16) compared to those who received adjuvant treatment [69].
The ongoing phase III GAIN trial [70] in Germany is comparing the use of perioperative GemCis (3 cycles before and after surgery) in patients with incidentally discovered GBC and resectable or borderline resectable eCCA/iCCA to upfront surgery followed by adjuvant therapy (physician’s choice). The primary outcome of OS is anticipated in 2024 as this will be the first phase III trial comparing perioperative therapy to upfront surgery [71]. In patients with locally advanced or borderline resectable disease, the use of upfront therapy for downstaging, improving R0 resection rates, and allowing response to therapy should be considered, but prospective data is needed [69].
Chemoradiotherapy and liver transplantation
Upfront CRT followed by liver transplantation may be considered in a select pCCA cases without nodal or metastatic disease and who are not candidates for standard resection [72]. In a series of 11 pCCA patients who subsequently underwent liver transplant as their definitive therapy, mOS was 25 months (range 4–174) [73]. A larger study then included 56 pCCA patients who received neoadjuvant RT, brachytherapy, and fluorouracil (5FU) prior to transplant. Of the 28 patients who underwent transplantation, 1-year OS was 88% and 5-year OS was 82% [74]. A recent review found that patients with LN-negative, unresectable pCCA who received upfront CRT followed by transplant had significantly better 5-year DFS when compared to matched LN-negative, resectable patients who underwent upfront resection (50.2% vs 17.4%; p < 0.001) [75••]. Several high-volume transplant centers have implemented institutional protocols [76] to identify and treat select pCCA patients with this high-impact intervention.
Advanced stage disease
In addition to the discussion below, the highlighted clinical trials in advanced-stage BTC (all-comers population) are summarized in Table 1.
First line — chemotherapy
Gemcitabine and cisplatin
Until recently, standard-of-care (SOC) first-line therapy for unresectable eCCA was based on the results of the seminal phase III ABC-02 trial which randomized 410 patients with locally advanced or metastatic BTC to Gem (N = 204) or GemCis (N = 206) [50]. Compared to patients who received Gem alone, treatment with the GemCis doublet significant improved both OS (mOS 11.7 months [GemCis] vs. 8.1 months [Gem]; HR 0.64 [95% CI 0.51, 0.8], p < 0.001) and PFS (mPFS 8.0 months [GemCis] vs. 5.0 months [Gem]; HR 0.63 [95% CI 0.51, 0.77], p < 0.001). Observed ORR favored GemCis (26.1% vs. 15.5%). The clinical benefits of the chemotherapy doublet were observed across all BTC subtypes including eCCA (eCCA OS HR = 0.73 [95% CI 0.43, 1.23]) and were similar to the overall population. Despite a numerically higher incidence of grade 3+ neutropenia in the doublet group (25.3% [GemCis] vs. 16.6% [Gem]), incidences of grade 3+ infection were similar between the two arms (18.2% [GemCis] vs. 19.1% [Gem]). Based on these results, GemCis became SOC first-line (1L) therapy for BTC in 2010 [2].
Gemcitabine, cisplatin, and albumin-bound paclitaxel
After promising results in a single-arm phase II trial [77•], SWOG 1815 [51] examined whether 1L GemCis could be improved with the addition of a third chemotherapeutic agent, albumin-bound (nab) paclitaxel (GAP). Study participants were randomized 2:1 to GAP (n=294) or standard GemCis (n=147). Treatment with GAP did not provide a statistically significant benefit in OS (mOS 14.0 [GAP] vs. 12.7 months [GemCis]; HR 0.93 [95% CI 0.74, 1.19], p = 0.58), PFS (mPFS 8.2 months [GAP] vs. 6.4 months [GemCis], HR 0.92 [95% CI 0.72, 1.16], p = 0.47), or ORR (31% [GAP] vs. 22% [GemCis]) compared to the standard doublet. Notably, GAP treatment had significantly more grade 3+ hematologic toxicity compared to the GemCis (60% vs. 45%, p = 0.003) and had numerically higher discontinuation rates due to toxicity (24% [GAP] vs. 19% [GemCis]). For now, GemCis continues to be the chemotherapy backbone of choice for eCCA.
Gemcitabine and oxaliplatin
For patients with chronic kidney disease where treatment with cisplatin would be contraindicated due to the potential for cisplatin-related nephrotoxicity, GemOx is a suitable alternative. GemOx (dosed as Gem 1000mg/m2 + Ox 100mg/m2 [D1, q14 days]) was studied in a single-arm phase II trial [52] in 67 patients with unresectable BTC (n = 13 [eCCA]) with a mOS of 11.0 months for non-GBC BTC. GemOx was overall well-tolerated and has been associated with less neutropenia and thrombocytopenia than GemCis although grade 3/4 AEs of thrombocytopenia (14.9%) and neutropenia (12%) were reported [52]. Modified (m)GemOx (Gem 900 mg/m2 + Ox 80 mg/m2 × 6 cycles) has been found to significantly improve clinical outcomes in unresectable GBC patients compared to best supportive care (BSC) or 5FU (425mg/m2 weekly bolus): median OS of 4.5, 4.6, and 9.5 months for BSC, 5FU, and mGemOx, respectively (p=0.039); mPFS of 2.8, 3.5, and 8.5 months for BSC, 5FU, and mGemOx, respectively (p < 0.001) [78]. A subsequent phase III study in 1L unresectable GBC (N = 243) compared mGemOx (Gem 900 mg/m2 + Ox 80 mg/m2 [days 1 and 8, q21 days, max 6 cycles]) with GemCis (Gem 1000mg/m2 + Cis 25 mg/m2 8 cycles [days 1 and 8, q21 days, max 8 cycles]). OS was similar between the two cohorts at 9 and 8.3 months in the mGemOx and GemCis groups, respectively (HR 0.78 [95% CI 0.60, 1.01], p = 0.057). However, this study was not powered to assess for superiority. Significantly less grade 3+ nephrotoxicity was reported with mGemOx (0% [mGemOx] vs. 7% [GemCis], p = 0.01), numerically less grade 3+ neutropenia (18% [mGemOx] vs. 26% [GemCis], p = 0.12) but with the tradeoff of increased grade 3+ neuropathy (8% [mGemOx] vs. 1% [GemCis], p = 0.02) [55].
Gemcitabine and capecitabine
For patients with existing neuropathy, e.g., end organ complications from diabetes mellitus, Gem in combination with capecitabine (GemCap) can be considered. In 2005, a phase II trial evaluated GemCap in 45 patients with advanced BTC (n=23 [CCA]) in the 1L with reported mOS of 14 months (19 months [CCA] vs. 6.6 months [GBC], p = 0.011), mPFS of 7 months (9.0 months [CCA] vs. 4.8 months [GBC], p = 0.026), ORR 31% [34% [CCA] vs. 28% [GBC]), and DOR 13.8 months. There was a significant OS (HR 3.61 [95% CI 1.35, 9.67], p = 0.11) and PFS (HR 2.37 [95% CI 1.11, 5.06], p = 0.026) benefit seen in CCA compared to GBC [53]. This combination was generally well tolerated, and grade 3/4 AEs included neutropenia (34%), thrombocytopenia (11%), and hand-foot rash (9%) [53].
Modified FOLFIRINOX
The 2022 phase II/III PRODIGE 38 AMEBICA trial [54] compared GemCis with modified (no bolus) 5FU, leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX) in 191 treatment-naïve advanced BTC patients. Because the phase II portion did not meet its 6-month PFS goal (44.6% [mFOLFIRINOX] vs 47.3% [GemCis]), it was not expanded to phase III. There was no significant overall difference in mPFS (6.2 months [mFOLFIRINOX] vs 7.4 months [GemCis]) or mOS (11.7 months [mFOLFIRINOX] vs 13.8 months [GemCis]). In a sub-group analysis, there was a trend towards better 6-month PFS with mFOLFIRINOX in patients with eCCA (HR 1.29 [95% CI 0.65, 2.57], p = 0.47) compared to iCCA patients suggesting that mFOLFIRINOX may have more benefit in eCCA. Taking into consideration the similar molecular profiles of dCCA and PDAC, mFOLFIRINOX could be considered in fit patients with eCCA; however, further prospective data in this select patient population are needed.
First line — chemoimmunotherapy
Gemcitabine, cisplatin, and durvalumab
Despite underwhelming clinical outcomes with immunotherapy-exclusive regimens in BTC [79–82], immune checkpoint inhibitor (ICI) therapy, when added to first-line chemotherapy, has recently led to the first change in SOC systemic therapy in BTC in more than a decade. The phase III TOPAZ-1 trial [56••] randomized 685 patients (n = 383 [iCCA], n = 131 [eCCA], n = 171 [GBC]) to GemCis with or without the programmed cell death ligand 1 (PD-L1) inhibitor, durvalumab (Durva). Patients received GemCis ± Durva (21-day cycles, max 8 cycles) and then were continued on either Durva monotherapy or placebo monthly until progression or intolerance. Compared to standard GemCis, patients treated with GemCis + Durva had significantly improved OS (mOS 12.8 months [GemCis + Durva] vs. 11.5 months [GemCis], HR 0.80 [95% CI 0.66, 0.97], p = 0.21), PFS (mPFS 7.2 months [GemCis + Durva] vs. 5.7 months [GemCis], HR 0.75 [95% CI 0.63, 0.89], p = 0.001), and ORR (26.7% [GemCis + Durva] vs. 18.7% [GemCis]) with a small amount of grade 3+ immune-related toxicity (2.4%). When broken down by tumor site, OS benefit of the GemCis + Durva in eCCA patients (OS HR 0.61 [95% CI 0.41, 0.91]) was similar to iCCA and more pronounced than GBC patients. Benefit was also independent of PD-L1 status with CPS <1% and ≥1% having similar outcomes. Based on these significant results, GemCis + Durva was approved by the FDA as a 1L option in 2022 for advanced or metastatic BTC.
Gemcitabine, cisplatin, and pembrolizumab
Shortly following the TOPAZ-1 trial and its 1L FDA-approval, the phase III KEYNOTE-966 trial [57••], assessing GemCis with or without programmed cell death protein 1 (PD-1) inhibitor, pembrolizumab (Pembro), was reported (n =1069 [19% eCCA]). In contrast to TOPAZ-1, KEYNOTE-966 allowed for the continuation of Gem as part of the maintenance regimen alongside Pembro or placebo, which was a critique of TOPAZ-1 which only compared Durva vs placebo in the maintenance setting. Additionally, KEYNOTE-966 had better representation of sites outside of East Asia (55%). KEYNOTE-966 met its primary endpoint with a mOS of 12.7 months with GemCis + Pembro vs 10.9 months in the SOC arm (HR 0.83 [95% CI 0.72, 0.95], p = 0.0034). This benefit was similarly independent of PD-L1 status (CPS of <1% or ≥1%). In the prespecified subgroup analysis for OS, GemCis and GemCis + Pembro had the most benefit in iCCA compared to eCCA (HR 0.99 [95% CI 0.73, 1.35]). Unlike TOPAZ-1, KEYNOTE-966 did not demonstrate a significant additive benefit of Pembro when it came to PFS (mPFS 6.5 months [GemCis + Pembro] vs. 5.6 months [GemCis]; HR 0.86 [0.75, 1], p = 0.23) or ORR (29%, both arms) [57••]. GemCis + Pembro is under accelerated FDA review for a 1L indication. Taken together, TOPAZ-1 and KEYNOTE-966 have solidified the role of immune checkpoint blockade as an adjunct to standard chemotherapy in the 1L management strategy of advanced BTCs. However, the benefit remains modest and more optimal predicative biomarkers are needed to identify patients and tumors that will benefit most from the addition of ICI.
Gemcitabine, cisplatin, atezolizumab, and bevacizumab
The immunosuppressive effect of tumor cells can be attributed in part to aberrant vascular endothelial growth factor (VEGF) expression. Abnormal VEGF activity causes dysfunctional angiogenesis which prevents proper T-cell infiltration and creates a tumor microenvironment exempt from immune regulation [83]. The addition of VEGF inhibitors, like bevacizumab (Bev), to ICI has been postulated to further increase immune response by creating neo-antigens to enhance T-cell recognition and anti-cancer activity. Based on this, the IMbrave151 phase II trial [58] randomized 162 patients (19% eCCA) to GemCis + Atezo ± Bev (21-day cycles). GemCis + Atezo ± Bev was given for a maximum of 8 cycles and Atezo ± Bev was subsequently continued until disease progression or toxicity. Initial results showed similar rates of grade 3+ AEs, and a modest improvement in PFS of 8.4 months with GemCis + Atezo + Bev compared to 7.9 months with GemCis + Atezo. While ORR was similar between the arms (24% and 25%), 89% of patients treated with Bev had a duration of response (DOR) ≥6 months compared to 47% without Bev, suggesting there is potentially a more durable anti-cancer effect with the addition of VEGF inhibition to ICI [58]. However, further follow-up and mature OS outcomes are needed.
Second line — chemotherapy
FOLFOX
The phase III ABC-06 trial [59••] prospectively compared FOLFOX (5FU [bolus + extended infusion], leucovorin, Ox) plus active symptom control (ASC) to ASC alone in advanced BTC with prior progression on 1L Gem-based chemotherapy (N = 162; n = 45 [eCCA]). The addition of FOLFOX to ASC modestly improved OS (mOS 6.2 months vs 5.3 months; HR 0.69 [95% CI 0.5, 0.97], p = 0.031) which was less pronounced in eCCA (HR 0.84 [95% CI 0.45–1.56]) compared to the overall population, though not statistically significant. The mPFS was 4 months and ORR was 5% with FOLFOX; however, the incidence of grade 3/4 infection (16%) was greater with FOLFOX. Six-month and 1-year survival rates were 50.6% and 25.9% for the FOLFOX arm compared to 35.5% and 11.4% for ASC [59••].
Liposomal irinotecan/5-FU and FOLFIRI
The South Korean phase IIb NIFTY trial [84] evaluated 5FU-leucovorin with nanoliposomal irinotecan (nal-IRI) compared to 5FU alone after progression on GemCis (N = 174; n = 47 [eCCA]). Unlike ABC-06, NIFTY was powered for PFS as the primary endpoint. Updated analysis revealed a significantly improved OS (mOS 8.6 months [nal-IRI + 5FU] vs 5.3 months [5FU], HR 0.68 [95% CI 0.48, 0.95], p = 0.02), PFS (mPFS 4.2 months [nal-IRI + 5FU] vs 1.7 months [5FU], HR 0.61 [95% CI 0.44, 0.86], p = 0.004), and ORR (12.5% [nal-IRI + 5FU] vs 3.5% [5FU]) favoring the study doublet. There were no significant differences in outcomes of eCCA patients compared to other subgroups. There was more grade 3/4 neutropenia (24% [nal-IRI + 5FU] vs 1% [5FU]) and anemia (9% [nal-IRI + 5FU] vs 3% [5FU]) in study doublet arm [84].
The German phase II NALIRICC [61] trial (N = 100, n = 19 [eCCA]) similarly compared nal-IRI + 5FU vs 5FU but did not find the same improvement in OS (mOS 8.21 months [5FU] vs 6.9 months [5FU + nal-IRI]; HR 1.08 [95% CI 0.68, 1.72]) or PFS (mPFS 2.3 months [5FU] vs 2.76 months [5FU + nal-IRI]; HR 0.87 [95% CI 0.68, 1.72]) [61]. These discordant results may be explained by different patient populations (NALIRICC in a European population, NIFTY in an Asian population) along with more iCCA patients included in NALIRICC than NIFTY (64% vs 43%) [85].
Standard irinotecan + 5FU (FOLFIRI) was compared against mFOLFOX in a phase II trial [86] in 2L advanced BTC patients (N = 118; n = 29 [eCCA]). Both arms had similar OS (mOS 6.3 months [mFOLFOX] vs 5.7 months [mFOLFIRI], HR 1.1 [95% CI 0.7, 0.16], p = 0.677), PFS (mPFS 2.8 months [mFOLFOX] vs 2.1 months [mFOLFIRI], HR 1.0 [95% CI 0.7, 1.5], p = 0.974), and ORR (5.9% [mFOLFOX] vs 4.0% [mFOLFIRI]). Patients who received mFOLFOX had more grade 3+ thrombocytopenia (10.7% vs 8.6%) and peripheral neuropathy (3.6% vs 1.7%), and thus, mFOLFIRI is a reasonable option given comparable efficacy for patients who have residual chemo-induced neuropathy or thrombocytopenia from previous therapy [86]. 5FU monotherapy is also an option with lower rates of grade 3/4 AEs as shown in the NALIRICC study (70.8% [5FU + nal-IRI] vs 50% [ 5FU]), though with limited efficacy [61]. FOLFOX, FOLFIRI, nal-IRI + 5FU, and 5FU are all recommended by the NCCN in the 2L given their comparable efficacies and unique side effect profiles [2]. Overall, second-line therapy chemotherapy options are available, but the magnitude of clinical benefit is limited (Table 1), highlighting the importance of molecular sequencing to identify potentially actionable mutations (Fig. 1, Table 2) and need for impactful clinical trials in this space.
Table 2.
Summary of select clinical trials evaluating targeted therapy in biliary tact cancer patients harboring actionable genetic alterations
| NCT | Trial | Study agent | Study population | Primary outcome | mPFS (months) | HR (95% CI) p-value |
mOS (months) | HR (95% CI) p-value |
ORR (%) |
|---|---|---|---|---|---|---|---|---|---|
| ERBB2/HER2 | |||||||||
| NCT01953926 | SUMMIT [87] | Neratinib | HER2 expressing, advanced BTC | ORR | 2.8 | (1.1, 3.7) | 5.4 | (3.7, 11.7) | 16% |
| NCT02091141 | MyPathway [88] | Pertuzumab, trastuzumab | HER2 expressing, 2nd line, advanced BTC | ORR |
4.0 GBC: 4.2 eCCA: 6.8 |
(1.8, 5.7) (1.8, 8.2) (1.3, 13.5) |
10.9 GBC: 14.2 eCCA: 8 |
(5.2, 15.6) (4.2, NE) (2.0, NE) |
23% |
| JMA-IIA00423 | HERB [89] | Trastuzumab-deruxtecan | HER2 expressing, 2nd line, advanced BTC | ORR | 4.4 | (2.8, 8.3) | 7.1 | (4.7, 14.6) |
36.4% p = 0.01 |
| NCT04482309 | DESTINY-PanTumor02 [90] | Trastuzumab-deruxtecan | HER2 expressing, advanced, solid tumors | ORR | Ongoing |
37.1% BTC: 22% |
|||
| NCT04466891 | HERIZON-BTC-01 [91••] | Zanidatamab | HER2 expressing, 2nd line, advanced BTC | ORR | 5.5 | (3.7, 7.2) | Ongoing |
41.3% GBC: 46.3 eCCA: 43.8% |
|
| NCT04579380 | SGNTUC-019 [92] | Tucatinib + trastuzumab | HER2 expressing, 2nd line, advanced BTC | ORR | 5.5 | 90% CI 3.9, 8.1 | Ongoing | 46.7% | |
| BRAFV600E | |||||||||
| NCT02034110 | ROAR [93] | Dabrafenib + trametinib | BRAFV600E mutated solid tumors | ORR | 8.9 | (5.6, 13.7) | 13.5 | (10.4, 17.6) | 47% |
| KRASG12C | |||||||||
| NCT03785249 | KRYSTAL-1 [94] | Adagrasib | KRASG12C mutated solid tumors | ORR | 8.6 | (2.7, 11.3) | 15.1 | (8.6, NE) | 41.7% |
| EGFR | |||||||||
| NCT00552149 | BINGO [95] | GemOx + cetuximab vs GemOx | 1st line, advanced BTC | 4-mo PFS | 6.1 vs 5.5 | 11 vs 12.4 | 18% vs 17% | ||
| NCT01149122 [96] | GemOx vs GemOx + erlotinib | 1st line, advanced BTC | PFS | 4.2 vs 5.8 |
0.80 (0.61, 1.03) p = 0.087 |
9.5 vs 9.5 |
0.93 (0.69, 1.25) p = 0.611 |
16% vs 30% | |
| MSI-H/dMMR | |||||||||
| NCT02628067 | KEYNOTE-158 [97] | Pembrolizumab | MSI-H/dMMR advanced solid tumor, 2nd line | ORR | CCA: 4.2 | (2.1–NE) | CCA: 24.3 | (6.5, NE) | CCA: 40.9% |
| TMB-H | |||||||||
| NCT03668119 | CHECKMATE-848 [98] | Ipi-nivo | TMB-H advanced solid tumors, 2nd line stratified by blood or tumor tissue TMB-H | ORR | 4.1 | ( 2.8, 11.3) | 14.5 | (7.7, NE) | 35.3% |
| LAT1 | |||||||||
| UMIN000034080 [99••] | Nanvuranlat | 2nd line or later, advanced BTC | PFS |
0.56 (0.34, 0.90) p = 0.016 LAT1-h: 0.44 (0.23, 0.85) p = 0.013 LAT1-l: 1.44 (0.56, 3.69) p = 0.444 |
0.85 (0.53, 1.36) p = 0.493 LAT1-h: 0.67 (0.35, 1.30) p = 0.231 LAT1-l: 1.5 (052, 4.34) p = 0.454 |
||||
| FGFR2 fusion, rearrangement | |||||||||
| NCT02924376 | FIGHT-202 [100] | Pemigatinib | 2nd line or later, advanced CCA with or without FGFR2 fusions, rearrangement, or alteration | ORR with FGFR2 fusions, rearrangement | 6.9 | (6.2, 9.6) | 21.1 | (14.8, NE) | 35.5% |
| NCT02052778 | FEONIX-CCA2 [101] | Futibatinib | 2nd line or later, advanced iCCA with FGFR2 fusions, rearrangement | ORR | 9.0 | (6.9, 13.1) | 21.7 | (14.5, NE) | 42% |
| NCT04083967 | RAGNAR [102] | Erdafitinib | 2nd line or later, advanced solid tumors with FGFR1-4 alterations | ORR | 5.2 | (4, 5.6) | 10.9 | (7.9, 14.3) | CCA: 41.9% |
| NCT04526106 | ReFocus [103] | RLY-4008 | 2nd line or later, advanced CCA with FGFR2 fusion, rearrangement | ORR | Ongoing | 88.2% | |||
| IDH1 | |||||||||
| NCT02989857 | ClarIDHy [104, 105] | Ivosidenib vs placebo | 2nd line or later, advanced CCA with IDH1 mutation | PFS | 6.9 vs 2.7 |
0.37 (0.25, 0.54) p < 0.0001 |
10.3 vs 5.1 |
0.49 (0.34, 0.70) p = <0.001 |
2% vs 0% |
| NTRK | |||||||||
|
EudraCT 2012-000148-88 |
STARTRK-1/2, ALKA-372-002 [106] | Entrectinib | 2nd line or later, advanced solid tumors, NTRK-fusion positive | ORR, DOR | 13.8 | (10.1, 20.0) | 37.1 | (27.2, NE) | 61.3% |
| LOXO-TRK-14001, SCOUT, NAVIGATE [107] | Larotrectinib | 2nd line or later, advanced solid tumors, NTRK-fusion positive | ORR | 28.3 | (22.1, NE) | 44.4 | (36.5, NE) | 79% | |
| RET | |||||||||
| NCT03157128 | LIBRETTO-001 [108] | Selpercatinib | 2nd line or later, advanced solid tumors, RET-fusion positive | ORR | 13.2 | (7.4, 26.2) | 18.0 | (10.7, NE) | 43.9% |
| NCT03037385 | ARROW [109] | Pralsetinib | 2nd line or later, advanced solid tumors, RET-fusion positive | ORR | 7.4 | (5.1, 13.6) | 13.6 | (7.5, NE) | 57.% |
Abbreviations: BTC, biliary tract cancer; CCA, cholangiocarcinoma; CRT, chemoradiotherapy; DOR, duration of response; eCCA, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; ORR, objective response rate; mOS, median overall survival; mPFS, median progression-free survival; NE, not evaluable
Second line — targeted systemic therapy
The development of targeted therapeutic agents with the potential for less systemic side effects and improved efficacy have expanded treatment options for patients with advanced eCCA. Figure 1 illustrates the most frequent actionable mutations found in BTC and Table 2 summarizes key clinical trials in target-selected populations. Herein, we will address the most frequently found mutations in eCCA, acknowledging that rare genetic alternations found in eCCA such as IDH1/2 mutations and FGFR2, NTRK, and RET fusions have targetable therapeutic options but are found almost exclusively in iCCA and will not be discussed in depth below but are included as part of Table 2 [6].
HER2/HER3
Human epidermal growth factor receptor 2 (HER2[ERBB2]) amplifications or mutations are found in 10–15% of eBTC with HER2 overexpression reported at even higher frequencies [23, 30, 110]. At this time, this represents the most frequently altered molecular target with effective therapeutic options that is unique to eBTC subtypes compared to iCCA.
In the phase II SUMMIT basket trial [111], eCCA patients harboring HER2 mutations received neratinib, an oral pan-HER small molecule tyrosine kinase inhibitor (TKI), monotherapy daily (28-day cycles). Among cholangiocarcinoma patients, PFS (mPFS 1.4 months, [95% CI 0.5, 9.1]), OS (mOS 5.4 months, [95% CI 0.8, 16.2]), and ORR (16%) were modest in this pretreated population [87]. The most notable grade 3+ AE was diarrhea in 24% of patients [111].
Dual HER2 inhibition with trastuzumab and pertuzumab (anti-HER2 antibodies) has also been studied in advanced BTC with HER2 amplification/overexpression. In the phase IIa MyPathway [88] HER2-targeted BTC study (N = 39: n = 12 [eCCA/ampullary cancer]), BTC patients with HER2 amplification and/or overexpression (measured by next-generation sequencing, IHC ± FISH) who received pertuzumab + trastuzumab (21-day cycles) had mOS of 10.9 months (95% CI 5.2, 15.6) and mPFS of 4.0 months (95% CI 1.8, 5.7). ORR was 23%, and mDOR was 10.8 months. Patients tolerated therapy well with minimal AEs (grade 3/4 of AST elevation [13%], ALT elevation [13%], and hyperbilirubinemia [11%]) [88].
The antibody-drug conjugate trastuzumab-deruxtecan (T-DXd) showed improvement in ORR (36.4%; 90% CI 19.6, 56.1; p = 0.01) in patients with HER2-expressing BTC (n = 6 [eCCA]) in the phase II HERB trial [89]. Median PFS and mOS were 4.4 months (95% CI 2.8, 8.3) and 7.1 months (95% CI 4.7, 14.6), respectively. It is significant to note that 81.3% of patients had at least grade 3 AEs (most commonly anemia, neutropenia, leukopenia), and 25% developed interstitial lung disease. The DESTINY-Pantumor02 trial [90] is currently in progress, but interim results show a promising ORR of 22% in BTC with DOR of 8.6 months (95% CI 2.1, NE) with T-DXd in patients with prior HER-2 therapy exposure. No new grade 3/4 AEs were reported, and the most common were neutropenia (19.1%), anemia (8.6%), and fatigue (6%) [89]. The final PFS and OS results are still maturing.
The ongoing phase II HERIZON-BTC-01 trial [91••] is evaluating zanidatamab, a HER2 bispecific antibody targeting multiple HER2 domains, in 87 BTC patients (18% eCCA) with either IHC 2+/3+ (N = 80 patients) or IHC 0/1+ (N = 7 patients). The ORR in HER2+/3+ patients was 41.3% (79.4% and 11.8% in HER 3+ and 2+ patients, respectively) with a median DOR of 12.9 months. Median PFS was 5.5 months (95% CI 3.7, 7.2) with OS not yet reported. Grade 3/4 AEs were reported in 18% of patients, most commonly diarrhea (5%) and decreased ejection fraction (3%) [91••]. With no responses in patients with HER2 0/+1, this suggests no current role for HER2-directed therapy in HER2-low BTC.
The combination of the HER2-selective TKI, tucatinib, with trastuzumab was studied in HER2+ amplified/overexpressing BTC post 1L GemCis in the phase 2 SGNTUC-019 study [92]. The ORR of 46.7% is promising in this pre-treated population with reported DOR of 6 months (90% CI 5.5, NE). The PFS of 5.5 months (90% CI 3. 9, 8.1) is similar to the PFS benefit from zanidatamab. Grade 3/4 AEs of cholangitis, anorexia, and nausea were reported in 10% of patients [92].
Overall, there are several exciting options for HER2-overexpressing BTC. Given the proportion of eCCA patients with HER2 overexpression or amplification, early molecular testing is paramount as ongoing trials are needed to evaluate the benefit of targeted treatment in the front-line setting. Further standardization of HER2-positive criteria, along with prospective data on 1L use and in patients previously exposed to HER2 therapy to evaluate sequencing of these agents, will be important areas of inquiry going forward.
BRAF
Downstream of EGFR and HER2, the MAPK pathway consisting of the RAS-RAF-MEK-ERK signal cascade participates in a series of activation events ultimately leading to cellular proliferation and when disrupted, tumorigenesis [112]. BRAF mutations, more commonly found in iCCA (2–10%), are present in just 1–2% of eCCA and tend to be a poor prognostic indicator [19, 113]. The phase II ROAR basket trial [93] evaluated the efficacy of dual BRAF/MEK inhibition with dabrafenib (oral BRAF inhibitor) and trametinib (oral MEK inhibitor) in 43 (20.9% of study population) patients with BTC and BRAFV600E mutations. All patients in the BTC cohort had been on prior therapy. The medication was very well tolerated with grade 3/4 AEs of elevated γ-glutamyl transferase and leukopenia in 3% of patients. The ORR in the BTC cohort was 47% with a DOR of 8.9 months (95% CI 5.6, 13.7). The mPFS of 9 months (95% CI 5.5, 9.4) and mOS of 13.5 months (95% CI 10.4, 17.6) are promising results in heavily pre-treated patients [93].
KRAS
Though RAS mutations have been reported in 37% of patients with eCCA, actionable RAS mutations such as KRASG12C are much rarer, occurring in 1–2% of BTC [25•, 114]. The phase II KRYSTAL-1 trial [94] evaluated adagrasib, an oral KRASG12C irreversible inhibitor, in 12 patients (21% of study population) with BTC. The BTC cohort had been on a median of 1.5 lines of prior therapy and showed promising results in ORR (41.7%; 95% CI 15.2, 72.3), mPFS (8.6 months; 95% CI 2.7, 11.3), and mOS (15.1 months; 95% CI 8.6, NE). The DOR for the entire BTC cohort was 5.3 months (95% CI 2.8, 7.3). Reported grade 3/4 AEs were fatigue (11.1%), anemia (7.9%), and QT prolongation on EKG (6.3%) [94]. A phase I/II clinical trial is underway evaluating the new KRASG12D inhibitor, MRTX1133, in solid tumors [115]. Recently, a novel pan-KRAS inhibitor (BI-2493) showed efficacy in animal models and its appearance in clinical trials is highly anticipated for KRAS-mutated tumors [116].
EGFR
EGFR dysregulation, reported in up to 15% of eCCA, allows growth and metastasis by aberrant signaling through the MAPK pathway [19, 117]. Though EGFR inhibition seems promising, studies have failed to produce significant differences in outcomes with EGFR-targeted therapies. The phase II BINGO [95] trial in 2014 compared GemOx ± cetuximab (anti-EGFR monoclonal antibody) in 150 patients with advanced BTC (n = 22 [eCCA]). ORR was similar between the 2 cohorts (18% [GemOx + cetuximab] vs 17% [GemOx]) with median DOR of 5.7 months (GemOx + cetuximab) vs 8.4 months (GemOx). There was no significant difference in OS (mOS 12.4 months [GemOx + cetuximab] vs 11 months [GemOx]). Interestingly, KRAS, BRAF mutation, or EGFR overexpression were not prognostic. Subsequently, patients were stratified by KRAS mutation status to GemOx ± cetuximab. While there was a numerical trend towards improved PFS in the GemOx + cetuximab KRAS-wild type arm, this was not significant (mPFS 7.1 months [GemOx + cetuximab] vs 5.6 months [GemOx]; p = 0.06) [118]. Similarly, a phase III study [96] evaluating GemOx ± erlotinib (oral EGFR TKI) failed to produce a significant difference in OS (mOS 9.5 months in both arms, HR 0.93 [95% CI 0.69, 1.25], p = 0.611) or PFS (mPFS 4.2 months [GemOx] vs 5.8 months [GemOx + erlotinib], HR 0.80 [85% CI 0.61, 1.03], p = 0.087). A subgroup analysis showed improvement in PFS for patients with CCA who received GemOx + erlotinib (5.9 months [GemOx + erlotinib] vs 3 months [GemOx]; HR 0.73 [95% CI 0.53, 1.0], p = 0.049). Unfortunately, lack of significant benefit has also been shown in studies evaluating panitumumab. Currently, there is no role for EGFR-inhibition in the treatment of eCCA [119, 120].
BRCA1/2
BRCA mutations leading to DNA damage repair deficiency have been reported in 2–5% of eCCA (similar BRCA1/2 incidence) [121]. Though studies evaluating the therapeutic implications of BRCA mutations are ongoing in BTC [122], their response to platinum chemotherapy and poly-ADP ribose polymerase inhibitors (PARPi) has been shown in several other cancer types, including pancreas cancer [123]. Since platinum agents are currently recommended in both the 1L and 2L setting for advanced BTC, further studies evaluating PARPi, immunotherapy, and their combination are needed for patients following platinum-based therapy. Genomic instability from BRCA mutations has been significantly associated with higher tumor mutational burden (TMB), regardless of deficient or proficient mismatch repair (dMMR or pMMR) [121]. Given that BRCA mutations are often associated with dMMR/MSI-H, the combination of PARPi + ICI could aid in the prevention of PARPi resistance and improve outcomes [124]. This combination is currently being studied in advanced BTC in the phase II trial combining rucaparib (PARPi) with nivolumab after proven platinum sensitivity (no radiographic or clinical progression after 4–6 months of platinum-based systemic chemotherapy), the results of which are anticipated in 2024 [125].
Microsatellite instable (MSI-H)/mismatch repair deficient (dMMR)
The prevalence of dMMR is estimated to be 5–13% in eCCA [126, 127]. The phase II KEYNOTE-158 [128] trial evaluated 22 (9.4% of total study population) patients with MSI-H CCA to receive pembrolizumab for up to 2 years or disease progression. The ORR of 40.9% (95% CI 20.7, 63.6%) in the CCA cohort with a DOR for all cancer types of 47.5 months (95% CI 2.1+, 51.1+, indicating no PD at the time of final analysis) in the updated analysis is especially promising in the 2L setting [97]. In the CCA group, the mPFS was 4.2 months (95% CI 2.1, NE) and mOS 24.3 months (95% CI 6.5, NE) [97]. Though grade 3/4 immune-related adverse events (IRAEs) of pneumonitis (1.3%), skin reaction (1.3%), colitis (0.9%), and hepatitis (0.9%) were reported, ICI in patients with dMMR/MSI-H CCA can improve survival by years for patients with an otherwise dismal prognosis on 2L therapy [128]. Because of this, pembrolizumab was given a tumor-agnostic FDA indication in 2017 for dMMR/MSI-H solid tumors.
High tumor mutation burden (TMB-H)
TMB has emerged with sequencing to quantify the number of mutations per megabase (mt/MB) of DNA to predict response to certain therapies and serve as a prognostic indicator. Because a higher mutational burden produces neoantigens that can be recognized by the immune system, those with very high TMB (>50 mt/MB) tend to have a better prognosis than patients with intermediate TMB given improved immune response and tumor infiltration [129]. Similarly, a linear correlation has been observed in a wide range of solid tumors between TMB and response to ICI such that as TMB increases, response to ICI increases [130]. High TMB (TMB-H; ≥10 mt/MB) has been reported in up to 8.2% of CCA [131]. In BTC, TMB-H (>20 mt/MB) often co-exists with TP53 (58%) and DNA damage repair mutations such as BRCA1/2 and dMMR (78%) [132]. In TMB-H CCA patients who received ICI, ORR were significantly improved (25% vs 13.5%; p = 0.048) [131]. In the Checkmate-848 phase II trial [98], including over 40 TMB-H (≥10mt/MB) solid tumor types, dual ICI with ipilimumab + nivolumab (ipi + nivo) produced an ORR of 35.3% (95% CI 24.1, 47.8%), mPFS of 4.1 months (95% CI 2. 8, 11.3), and mOS of 14.5 months (95% CI 7.7, NE) [48]. Accordingly, ipi + nivo can be considered for TMB-H patients in the second or later line. Further quantifying TMB with molecular profiling up-front can help predict response to immunotherapy, especially since TOPAZ-1 and KEYNOTE-966 trials have added ICI to the 1L setting. Those with a low TMB (<10 mt/MB) may not derive benefit from the addition of ICI and may be subjected to increased risk of IRAEs. Continued molecular profiling of targetable co-existing mutations along with risk stratifying TMB to assess response to ICI is needed to further evaluate the treatment landscape in eCCA.
L-type amino acid transporter (LAT1)
More recently, LAT1 has been identified as a promising target for therapy in advanced BTC. LAT1, found at low levels in non-cancer cells, is over-expressed in various cancers due to increased pathologic metabolic requirements and has been shown to be associated with poor survival [133–135]. A phase II trial [99••] compared nanvuranlat (LAT1 inhibitor) to placebo in patients with advanced, chemo-experienced BTC (N = 104; n = 15 [eCCA]). Treatment with LAT1 inhibition significantly improved PFS compared to placebo (HR 0.59 [95% CI 0.34, 0.90], p = 0.016). Notably, this was driven by patients with LAT1-high tumors (HR 0.44 [95% CI 0.23, 0.85], p = 0.013) who made up over two-thirds of the total study population (n = 65 [LAT1-high], n = 32 [LAT1-low]). These LAT-high patients also trended towards improved OS with Nanvuranlat treatment. LAT1 inhibition did not improve outcomes patients with LAT1-low BTC. The greatest PFS benefit was seen in the eCCA (HR 0.15 [95% CI 0.04, 0.52]) and GBC (HR 0.26 [95% CI 0.10, 0.82]) cohorts, likely due to Nanvuranlat’s pharmacokinetics with its active metabolites attaining a high extrahepatic biliary concentration [99••, 136]. LAT1 expression status stratified by tumor site is not available so the question as to whether eCCA and GBC tumors were more likely to be LAT1-high expressors (compared to iCCA) is not known. Overall, Nanvuranlat was well tolerated with minimal treatment-related grade 3/4 AEs, likely owing in large part to its pharmacokinetics that show limited presence of drug systemically [136]. The future of this agent will likely be in biomarker-select populations and as part of a combinatorial regimen rather than monotherapy.
Palliative considerations and supportive care
Patients with eCCA not only have symptoms associated with their cancer that may impact their quality of life (QOL) such as jaundice, pruritis, and chronic abdominal pain, but they may also experience acute and chronic treatment-related toxicities such as peripheral neuropathy with platinum-based treatment. With the addition of ICI in the 1L setting, patients may suffer from arthralgias or dermatitis, and even more severe IRAE such as pneumonitis, colitis, and hepatitis. Both chemotherapy and underlying illness can result in significant fatigue, nausea, and/or anorexia-cachexia which can all have significant impact physical/functional and psychosocial quality of life. Nutritional support from dieticians, patient and family support with psychotherapy or counseling, and early co-management with palliative care specialists to address unmet needs and conduct iterative serious-illness conversations are fundamental to providing comprehensive care for patients with eBTC.
In patients with malignant biliary obstruction, up to 26.5% can develop cholangitis prior to stenting with a 30-day mortality rate up to 30.8% [137]. Palliative endobiliary stenting during ERCP or by placing a percutaneous biliary drain may alleviate symptoms while preventing cholangitis, but also requires intermittent stent exchange [138]. The decision to pursue ERCP or percutaneous drainage should be made with the input of both interventional radiology and surgical oncologists. Alongside stenting, both photodynamic therapy (PDT) and endobiliary radiofrequency ablation (RFA) are used to alleviate obstruction or as a bridge to surgery. PDT and RFA may be performed either endoscopically or percutaneously with interventional radiology [139]. Biliary stenting with either eRFA or PDT has been associated with longer stent patency than endobiliary stenting alone with improved patient-reported QOL and may also have a dual effect of improving response to chemotherapy [140–142].
Conclusion
Extrahepatic BTCs are diverse cancers with distinct molecular features but share an overall poor prognosis. With the addition of ICI and growing identification of actionable mutations paired with targeted therapy development, patients with eCCA may have more treatment options beyond standard chemotherapy. However, patients with eCCA are less likely to receive upfront molecular profiling (50.5% [eCCA] vs 64.3% [iCCA]; p < 0.001) than patients with iCCA, which may be in part due to difficulty obtaining enough tissue on diagnosis (especially if this is done with ERCP) [25•]. Fortunately, with the development of blood-based circulating tumor DNA testing, targetable mutations can be evaluated upfront even without tumor tissue. Given the high mortality rate for patients with advanced eCCA, patients with refractory disease may not be appropriate candidates for continued therapy with targeted agents even if they had identified mutations. Reportedly, only 15–40% of patients have a performance status after 1L therapy appropriate for 2L therapy [143]. Therefore, more prospective data is needed to see if there is benefit to incorporating targeted agents earlier in the treatment course or in combination with chemotherapy or ICI for additive/synergistic/complimentary effect. Continued upfront molecular testing, early clinical trial enrollment, and integration of multidisciplinary management are critical factors in improving survival and optimize quality of life for patients with eCCA.
Declarations
Author contribution
Conceptualization: MW and TH; methodology: MW and TH; software: MW; validation: MW, TH, LG, RA, NL, CP; data curation: MW and TH; writing — original draft preparation: MW; writing — review and editing: TH, RA, LG, NL, CP; visualization: MW. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge funding NIH K12 CA090625-17 (TH).
Disclaimer
These entities had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Compliance with Ethical Standards
Conflict of Interest
TH serves as a medical advisor for Eisai, consults for Primum, and has received an honorarium from Total Health. LG serves as a medical advisor or consultant for QED Therapeutics, Genentech, Merck, AstraZeneca, Exelixis, Boehringer Ingelheim, Cardinal Health, Athenum Consulting, and Relay Therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Yang Y, Zhang X. An overview of extrahepatic cholangiocarcinoma: from here to where? Front Oncol. 2023;13:1171098. doi: 10.3389/fonc.2023.1171098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Biliary tract cancers (version 2.2023) [Available from: https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf. Accessed 5/1/23 [DOI] [PubMed]
- 3.Folseraas T, Boberg KM. Cancer risk and surveillance in primary sclerosing cholangitis. Clin Liver Dis. 2016;20(1):79–98. doi: 10.1016/j.cld.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77(6):1690–8. doi: 10.1016/j.jhep.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based study in SEER-Medicare. PLoS One. 2017;12(10):e0186643. doi: 10.1371/journal.pone.0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farha N, Dima D, Ullah F, Kamath S. Precision oncology targets in biliary tract cancer. Cancers (Basel). 2023;15(7):2105. 10.3390/cancers15072105. [DOI] [PMC free article] [PubMed]
- 7.Jiang Y, Jiang L, Li F, Li Q, Yuan S, Huang S, et al. The epidemiological trends of biliary tract cancers in the United States of America. BMC Gastroenterol. 2022;22(1):546. doi: 10.1186/s12876-022-02637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56(2):69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 9.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–44. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 10.Ali H, Zweigle J, Patel P, Tedder B, Khan R, Agrawal S. Survival analysis of extrahepatic cholangiocarcinoma based on surveillance, epidemiology, and end results database. Ann Hepatobiliary Pancreat Surg. 2023;27(2):151–7. doi: 10.14701/ahbps.22-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Society AC. Survival rates for bile duct cancer 2018 [Available from: https://www.cancer.org/content/dam/CRC/PDF/Public/8554.00.pdf. Accessed 5/1/23
- 12.Ethun CG, Le N, Lopez-Aguiar AG, Pawlik TM, Poultsides G, Tran T, et al. Pathologic and prognostic implications of incidental versus nonincidental gallbladder cancer: a 10-institution study from the United States extrahepatic biliary malignancy consortium. Am Surg. 2017;83(7):679–86. doi: 10.1177/000313481708300721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol. 2013;3(1):541–65. doi: 10.1002/cphy.c120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gkountakos A, Martelli FM, Silvestris N, Bevere M, De Bellis M, Alaimo L, Sapuppo E, Masetto F, Mombello A, Simbolo M, Bariani E, Milella M, Fassan M, Scarpa A, Luchini C. Extrahepatic distal cholangiocarcinoma vs. pancreatic ductal adenocarcinoma: Histology and molecular profiling for differential diagnosis and treatment. Cancers (Basel). 2023;15(5):1454. 10.3390/cancers15051454 [DOI] [PMC free article] [PubMed]
- 15.Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabro R, Pinyol R, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73(2):315–27. doi: 10.1016/j.jhep.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Ren J, Ten Dijke P. Targeting TGFbeta signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):8. doi: 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73(7):2259–70. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–61. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzo A, Tavolari S, Ricci AD, Frega G, Palloni A, Relli V, Salati M, Fenocchio E, Massa A, Aglietta M, Brandi G. Molecular features and targeted therapies in extrahepatic cholangiocarcinoma: Promises and failures. Cancers (Basel). 2020;12(11):3256. 10.3390/cancers12113256. [DOI] [PMC free article] [PubMed]
- 20.Javle M, Bekaii-Saab T, Jain A, Wang Y, Kelley RK, Wang K, et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016;122(24):3838–47. doi: 10.1002/cncr.30254. [DOI] [PubMed] [Google Scholar]
- 21.Malats N, Porta M, Piñol JL, Corominas JM, Rifà J, Real FX. Ki-ras mutations as a prognostic factor in extrahepatic bile system cancer. PANK-ras I Project Investigators. J Clin Oncol. 1995;13(7):1679–86. doi: 10.1200/JCO.1995.13.7.1679. [DOI] [PubMed] [Google Scholar]
- 22.Mondaca S, Nervi B, Pinto M, Abou-Alfa GK. Biliary tract cancer prognostic and predictive genomics. Chin Clin Oncol. 2019;8(4):42. doi: 10.21037/cco.2019.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobi O, Ross JS, Goshen-Lago T, Haddad R, Moore A, Sulkes A, et al. ERBB2 pathway in biliary tract carcinoma: clinical implications of a targetable pathway. Oncol Res Treat. 2021;44(1–2):20–7. doi: 10.1159/000511919. [DOI] [PubMed] [Google Scholar]
- 24.Moffat GT, Hu ZI, Armas ADD, Ross JS, Javle MM, Knox JJ. Characterizing KRAS allele variants within biliary tract cancers. J Clin Oncol. 2023;41(16_suppl):4088-.
- 25.• Spencer K, Pappas L, Baiev I, Maurer J, Bocobo AG, Zhang K, et al. Molecular profiling and treatment pattern differences between intrahepatic and extrahepatic cholangiocarcinoma. J Natl Cancer Inst. 2023. This is an important reference for discussing key molecular features and genomic profiles that distinguish eCCA from iCCA. [DOI] [PMC free article] [PubMed]
- 26.Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol. 2019;10(4):751–65. doi: 10.21037/jgo.2019.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyal L, Kongpetch S, Crolley VE, Bridgewater J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021;95:102170. doi: 10.1016/j.ctrv.2021.102170. [DOI] [PubMed] [Google Scholar]
- 28.de Bitter TJJ, de Reuver PR, de Savornin Lohman EAJ, Kroeze LI, Vink-Borger ME, van Vliet S, et al. Comprehensive clinicopathological and genomic profiling of gallbladder cancer reveals actionable targets in half of patients. NPJ Precis Oncol. 2022;6(1):83. doi: 10.1038/s41698-022-00327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilyas SI, Affo S, Goyal L, Lamarca A, Sapisochin G, Yang JD, et al. Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat Rev Clin Oncol. 2023;20(7):470–86. doi: 10.1038/s41571-023-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondaca S, Schultz N, Roa JC, Walch HS, Sepulveda S, Harding JJ, et al. Clinical and genomic characterization of ERBB2-altered gallbladder cancer. J Clin Oncol. 2022;40(16_suppl):4114-. [DOI] [PMC free article] [PubMed]
- 31.Giraldo NA, Drill E, Satravada BA, Dika IE, Brannon AR, Dermawan J, et al. Comprehensive molecular characterization of gallbladder carcinoma and potential targets for intervention. Clin Cancer Res. 2022;28(24):5359–67. doi: 10.1158/1078-0432.CCR-22-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder RA. Survival benefit of neoadjuvant therapy for extrahepatic cholangiocarcinoma: real or artifact? Ann Surg Oncol. 2023;30(1):15–7. doi: 10.1245/s10434-022-12352-z. [DOI] [PubMed] [Google Scholar]
- 33.De Moura DTH, Moura EGH, Bernardo WM, De Moura ETH, Baraca FI, Kondo A, et al. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: systematic review and meta-analysis. Endosc Ultrasound. 2018;7(1):10–9. doi: 10.4103/2303-9027.193597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks C, Gausman V, Kokoy-Mondragon C, Munot K, Amin SP, Desai A, et al. Role of fluorescent in situ hybridization, cholangioscopic biopsies, and EUS-FNA in the evaluation of biliary strictures. Dig Dis Sci. 2018;63(3):636–44. doi: 10.1007/s10620-018-4906-x. [DOI] [PubMed] [Google Scholar]
- 35.Juratli MA, Hofmann K, Balaban U, El Youzouri H, Pession U, Heise M, et al. Introduction of an interdisciplinary tumor board leads to improvement of treatment outcome of cholangiocarcinoma (bile duct cancer) Chirurg. 2020;91(8):650–61. doi: 10.1007/s00104-019-01100-x. [DOI] [PubMed] [Google Scholar]
- 36.Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(12):1015–27. doi: 10.1200/JCO.18.02178. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: a comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354. doi: 10.1016/j.ctarc.2021.100354. [DOI] [PubMed] [Google Scholar]
- 38.Radtke A, Konigsrainer A. Surgical therapy of cholangiocarcinoma. Visc Med. 2016;32(6):422–6. doi: 10.1159/000452921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18(3):651–8. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- 40.Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klumpen HJ, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015;15:564. doi: 10.1186/s12885-015-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019;37(8):658–67. doi: 10.1200/JCO.18.00050. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto R, Sugiura T, Ashida R, Ohgi K, Yamada M, Otsuka S, et al. Prognostic value of carbohydrate antigen 19–9 and the surgical margin in extrahepatic cholangiocarcinoma. Ann Gastroenterol Surg. 2022;6(2):307–15. doi: 10.1002/ags3.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izquierdo-Sanchez L, Lamarca A, La Casta A, Buettner S, Utpatel K, Klumpen HJ, et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. 2022;76(5):1109–21. doi: 10.1016/j.jhep.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10(3):427–32. doi: 10.3748/wjg.v10.i3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bridgewater J, Fletcher P, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Long-term outcomes and exploratory analyses of the randomized phase III BILCAP study. J Clin Oncol. 2022;40(18):2048–57. doi: 10.1200/JCO.21.02568. [DOI] [PubMed] [Google Scholar]
- 46.Jeong H, Kim KP, Jeong JH, Hwang DW, Lee JH, Kim KH, et al. Adjuvant gemcitabine plus cisplatin versus capecitabine in node-positive extrahepatic cholangiocarcinoma: the STAMP randomized trial. Hepatology. 2023;77(5):1540–9. doi: 10.1097/HEP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 47.Adjuvant Chemotherapy With Gemcitabine and Cisplatin Compared to Standard of Care After Curative Intent Resection of Biliary Tract Cancer (ACTICCA-1) [Internet]. 2023 [cited April]. Available from: https://clinicaltrials.gov/show/NCT02170090
- 48.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–73. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: a phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015;33(24):2617–22. doi: 10.1200/JCO.2014.60.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 51.Shroff RT, Guthrie KA, Scott AJ, Borad MJ, Goff LW, Matin K, et al. SWOG 1815: a phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. Journal of Clinical Oncology. 2023;41(4_suppl):LBA490-LBA.
- 52.Andre T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99(6):862–7. doi: 10.1038/sj.bjc.6604628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23(10):2332–8. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 54.Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, et al. Modified FOLFIRINOX versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): a randomized phase II study. J Clin Oncol. 2022;40(3):262–71. doi: 10.1200/JCO.21.00679. [DOI] [PubMed] [Google Scholar]
- 55.Sharma A, Kalyan Mohanti B, Pal Chaudhary S, Sreenivas V, Kumar Sahoo R, Kumar Shukla N, et al. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: results of a phase III randomised controlled trial. Eur J Cancer. 2019;123:162–70. doi: 10.1016/j.ejca.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 56.•• Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence. 2022;1(8):EVIDoa2200015. This paradigm changing study (oustanding importance) was the first to demonstrate the additative survival benefit of adding immune checkpoint inhibition to standard of care chemotherapy. While the benefit can be argued as being modest, it nonetheless has changed practice and also is important for the future of immunotherapy in BTC (both optimizing its first-line effect as well as designing trials in the 2nd line for patients who are no longer immunotherapy-naïve). [DOI] [PubMed]
- 57.•• Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023. Together with TOPAZ-1, KEYNOTE-966 cemented the use of immune checkpoint inhibition with Gem-Cis in the first-line setting. It also improtantly maintained a significant benefit compared to SOC-treated patients who continued on with maintenance gemcitabine. [DOI] [PubMed]
- 58.El-Khoueiry AB, Ren Z, Chon H, Park JO, Kim JW, Pressiani T, et al. IMbrave151: a phase 2, randomized, double-blind, placebo-controlled study of atezolizumab with or without bevacizumab in combination with cisplatin plus gemcitabine in patients with untreated, advanced biliary tract cancer. J Clin Oncol. 2023;41(4_suppl):491-.
- 59.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22(11):1560–72. doi: 10.1016/S1470-2045(21)00486-1. [DOI] [PubMed] [Google Scholar]
- 61.Vogel A, Wenzel P, Folprecht G, Schütt P, Wege H, Kretzschmar A, et al. 53MO Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabine-based therapies (NALIRICC – AIO-HEP-0116). Ann Oncol. 2022;33:S563–S4.
- 62.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–40. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 63.Ren B, Guo Q, Yang Y, Liu L, Wei S, Chen W, et al. A meta-analysis of the efficacy of postoperative adjuvant radiotherapy versus no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma. Radiat Oncol. 2020;15(1):15. doi: 10.1186/s13014-020-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shridhar R, Blinn P, Huston J, Meredith KL. Outcomes of adjuvant radiation therapy after chemotherapy in resected extrahepatic cholangiocarcinoma: a National Cancer Database analysis. Cancer. 2023;129(6):890–900. doi: 10.1002/cncr.34625. [DOI] [PubMed] [Google Scholar]
- 65.Toyoda J, Sahara K, Takahashi T, Miyake K, Yabushita Y, Sawada Y, Homma Y, Matsuyama R, Endo I, Pawlik TM. Neoadjuvant therapy for extrahepatic biliary tract cancer: A propensity score-matched survival analysis. J Clin Med. 2023;12(7):2654. 10.3390/jcm12072654. [DOI] [PMC free article] [PubMed]
- 66.Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - a systematic review. Eur J Surg Oncol. 2019;45(2):83–91. doi: 10.1016/j.ejso.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 67.Ioka T, Shindo Y, Ueno M, Nagano H. Current progress in perioperative chemotherapy for biliary tract cancer. Ann Gastroenterol Surg. 2023;7(4):565–71. doi: 10.1002/ags3.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adam MA, Glencer A, AlMasri S, Winters S, Bahary N, Singhi A, et al. Neoadjuvant therapy versus upfront resection for nonpancreatic periampullary adenocarcinoma. Ann Surg Oncol. 2023;30(1):165–74. doi: 10.1245/s10434-022-12257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson JW, Ghafoori AP, Willett CG, Tyler DS, Pappas TN, Clary BM, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73(1):148–53. doi: 10.1016/j.ijrobp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krankenhaus N, German Research F. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC. 2024. [DOI] [PMC free article] [PubMed]
- 71.Goetze TO, Bechstein WO, Bankstahl US, Keck T, Königsrainer A, Lang SA, Pauligk C, Piso P, Vogel A, Al-Batran SE. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC (ICC/ECC) - a phase III study of the German registry of incidental gallbladder carcinoma platform (GR)- the AIO/ CALGP/ ACO- GAIN-trial. BMC Cancer. 2020;20(1):122. 10.1186/s12885-020-6610-4. [DOI] [PMC free article] [PubMed]
- 72.Cristina D, Ramón C. Liver transplantation for perihilar cholangiocarcinoma. Do we need to move forward? Hepatoma Res. 2023;9:10.
- 73.Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B, Jr, McCashland T, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2(8):774–9. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 74.Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24(2):201–7. doi: 10.1055/s-2004-828896. [DOI] [PubMed] [Google Scholar]
- 75.Breuer E, Mueller M, Doyle MB, Yang L, Darwish Murad S, Anwar IJ, et al. Liver transplantation as a new standard of care in patients with perihilar cholangiocarcinoma? Results from an international benchmark study. Ann Surg. 2022;276(5):846–53. doi: 10.1097/SLA.0000000000005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6(3):309–16. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 77.• Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824–30. This is an important study that, despite being a negative study, has some notable takeaways: (1) the doublet of Gem-Cis remains the chemo base of frontline therapy for BTC as a whole; (2) there was a notable trend in increased efficacy of the study triplet observed in gallbladder cancer patients. This is hypothesis-generating and may support testing this regimen prospectively in a gallbladder cancer-specific patient population in the future. [DOI] [PMC free article] [PubMed]
- 78.Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28(30):4581–6. doi: 10.1200/JCO.2010.29.3605. [DOI] [PubMed] [Google Scholar]
- 79.Kang S, El-Rayes BF, Akce M. Evolving role of immunotherapy in advanced biliary tract cancers. Cancers (Basel). 2022;14(7):1748. 10.3390/cancers14071748. [DOI] [PMC free article] [PubMed]
- 80.Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6(6):888–94. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. 2020;6(9):1405–9. doi: 10.1001/jamaoncol.2020.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ioka T, Ueno M, Oh D-Y, Fujiwara Y, Chen J-S, Doki Y, et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). Journal of Clinical Oncology. 2019;37(4_suppl):387-.
- 83.Hack SP, Verret W, Mulla S, Liu B, Wang Y, Macarulla T, et al. IMbrave 151: a randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer. Ther Adv Med Oncol. 2021;13:17588359211036544. doi: 10.1177/17588359211036544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hyung J, Kim I, Kim KP, Ryoo BY, Jeong JH, Kang MJ, et al. Treatment with liposomal irinotecan plus fluorouracil and leucovorin for patients with previously treated metastatic biliary tract cancer: the phase 2b NIFTY randomized clinical trial. JAMA Oncol. 2023;9(5):692–9. doi: 10.1001/jamaoncol.2023.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellis H, Raghavan S, Wolpin BM, Cleary JM. How we treat advanced biliary tract cancers in the second-line setting. Clin Adv Hematol Oncol. 2023;21(1):35–42. [PubMed] [Google Scholar]
- 86.Choi IS, Kim KH, Lee JH, Suh KJ, Kim JW, Park JH, et al. A randomised phase II study of oxaliplatin/5-FU (mFOLFOX) versus irinotecan/5-FU (mFOLFIRI) chemotherapy in locally advanced or metastatic biliary tract cancer refractory to first-line gemcitabine/cisplatin chemotherapy. Eur J Cancer. 2021;154:288–95. doi: 10.1016/j.ejca.2021.06.019. [DOI] [PubMed] [Google Scholar]
- 87.Harding JJ, Piha-Paul SA, Shah RH, Cleary JM, Quinn DI, Brana I, et al. Targeting HER2 mutation–positive advanced biliary tract cancers with neratinib: final results from the phase 2 SUMMIT basket trial. J Clin Oncol. 2022;40(16_suppl):4079-.
- 88.Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22(9):1290–300. doi: 10.1016/S1470-2045(21)00336-3. [DOI] [PubMed] [Google Scholar]
- 89.Ohba A, Morizane C, Kawamoto Y, Komatsu Y, Ueno M, Kobayashi S, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): an investigator-initiated multicenter phase 2 study (HERB trial). J Clin Oncol. 2022;40(16_suppl):4006-.
- 90.Meric-Bernstam F, Makker V, Oaknin A, Oh D-Y, Banerjee SN, Martin AG, et al. Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results. J Clin Oncol. 2023;41(17_suppl):LBA3000-LBA. [DOI] [PMC free article] [PubMed]
- 91.•• Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW, Chang HM, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023. This is an important study for a couple of reasons: (1) it, along with the references surrounding this, shows a potent anti-tumor efficacy of HER2-targeted therapies; (2) zanidatamab in particular also has a unique mechanism of action: a bispecific antibody that binds to two distinct extracellular domains of HER2. From mAbs, TKIs, ADC, and bispecfics, there is wealth HER2-targeted potential for, currently, the most druggable molecular alteration in eBTC. [DOI] [PubMed]
- 92.Nakamura Y, Mizuno N, Sunakawa Y, Hamilton EP, Hayashi H, Kim ST, et al. Tucatinib and trastuzumab for previously treated HER2-positive metastatic biliary tract cancer (SGNTUC-019): A phase 2 basket study. J Clin Oncol. 2023;41(16_suppl):4007-. [DOI] [PMC free article] [PubMed]
- 93.Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med. 2023;29(5):1103–12. doi: 10.1038/s41591-023-02321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bekaii-Saab TS, Yaeger R, Spira AI, Pelster MS, Sabari JK, Hafez N, et al. Adagrasib in advanced solid tumors harboring a KRAS(G12C) mutation. J Clin Oncol. 2023:101200JCO2300434. [DOI] [PMC free article] [PubMed]
- 95.Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardiere C, Boucher E, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15(8):819–28. doi: 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13(2):181–8. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 97.Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33(9):929–38. doi: 10.1016/j.annonc.2022.05.519. [DOI] [PubMed] [Google Scholar]
- 98.Schenker M, Burotto M, Richardet M, Ciuleanu T, Goncalves A, Steeghs N, et al. Abstract CT022: CheckMate 848: a randomized, open-label, phase 2 study of nivolumab in combination with ipilimumab or nivolumab monotherapy in patients with advanced or metastatic solid tumors of high tumor mutational burden. Cancer Res. 2022;82(12_Supplement):CT022–CT. [DOI] [PMC free article] [PubMed]
- 99.•• Furuse J, Ikeda M, Ueno M, Furukawa M, Morizane C, Takehara T, et al. Nanvuranlat, an L-type amino acid transporter (LAT1) inhibitor for patients with pretreated advanced refractory biliary tract cancer (BTC): primary endpoint results of a randomized, double-blind, placebo-controlled phase 2 study. J Clin Oncol. 2023;41(4_suppl):494-. This is an important study because it highlights a new, seemingly ubiqituous target for therapy in BTC of inhibiting LAT1 in LAT1-high expressors. Limited as monotherapy but may be valuable adjunct treatment in future clinical trials and has unique pharmakokinetics that, particularly in eBTC, could be exploited for therapuetic gain.
- 100.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023;388(3):228–39. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 102.Loriot Y, Schuler MH, Iyer G, Witt O, Doi T, Qin S, et al. Tumor agnostic efficacy and safety of erdafitinib in patients (pts) with advanced solid tumors with prespecified fibroblast growth factor receptor alterations (FGFRalt) in RAGNAR: interim analysis (IA) results. J Clin Oncol. 2022;40(16_suppl):3007-.
- 103.Hollebecque A, Borad M, Goyal L, Schram A, Park JO, Cassier PA, et al. LBA12 Efficacy of RLY-4008, a highly selective FGFR2 inhibitor in patients (pts) with an FGFR2-fusion or rearrangement (f/r), FGFR inhibitor (FGFRi)-naïve cholangiocarcinoma (CCA): ReFocus trial. Ann Oncol. 2022;33:S1381. doi: 10.1016/j.annonc.2022.08.006. [DOI] [Google Scholar]
- 104.Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7(11):1669–77. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krzakowski MJ, Lu S, Cousin S, Smit EF, Springfeld C, Goto K, et al. Updated analysis of the efficacy and safety of entrectinib in patients (pts) with locally advanced/metastatic NTRK fusion-positive (NTRK-fp) solid tumors. J Clin Oncol. 2022;40(16_suppl):3099-.
- 107.Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–40. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 2022;23(10):1261–73. doi: 10.1016/S1470-2045(22)00541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Subbiah V, Cassier PA, Siena S, Garralda E, Paz-Ares L, Garrido P, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022;28(8):1640–5. doi: 10.1038/s41591-022-01931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943–62. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harding JJ, Cleary JM, Quinn DI, Braña I, Moreno V, Borad MJ, et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: results from the phase II SUMMIT ‘basket’ trial. J Clin Oncol. 2021;39(3_suppl):320-.
- 112.Du J, Lv X, Zhang Z, Huang Z, Zhang E. Revisiting targeted therapy and immunotherapy for advanced cholangiocarcinoma. Front Immunol. 2023;14:1142690. doi: 10.3389/fimmu.2023.1142690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Subbiah V, Lassen U, Elez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21(9):1234–43. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 114.Jeong SY, Hong JY, Park JO, Park YS, Lim HY, Jang JY, et al. The efficacy of immune checkpoint inhibitors in biliary tract cancer with KRAS mutation. Therap Adv Gastroenterol. 2023;16:17562848231170484. doi: 10.1177/17562848231170484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Study of MRTX1133 in patients with advanced solid tumors harboring a KRAS G12D mutation [Internet]. 2026 [cited August 30]. Available from: https://classic.clinicaltrials.gov/show/NCT05737706.
- 116.Kim D, Herdeis L, Rudolph D, Zhao Y, Bottcher J, Vides A, et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature. 2023;619(7968):160–6. doi: 10.1038/s41586-023-06123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.LaPelusa M, Heumann T, Goff L, Agarwal R. Targeted therapies in advanced biliary tract cancers-a narrative review. Chin Clin Oncol. 2023;12(2):14. 10.21037/cco-22-93. [DOI] [PMC free article] [PubMed]
- 118.Chen JS, Hsu C, Chiang NJ, Tsai CS, Tsou HH, Huang SF, et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol. 2015;26(5):943–9. doi: 10.1093/annonc/mdv035. [DOI] [PubMed] [Google Scholar]
- 119.Leone F, Marino D, Cereda S, Filippi R, Belli C, Spadi R, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: a randomized phase 2 trial (Vecti-BIL study) Cancer. 2016;122(4):574–81. doi: 10.1002/cncr.29778. [DOI] [PubMed] [Google Scholar]
- 120.Vogel A, Kasper S, Bitzer M, Block A, Sinn M, Schulze-Bergkamen H, et al. PICCA study: panitumumab in combination with cisplatin/gemcitabine chemotherapy in KRAS wild-type patients with biliary cancer-a randomised biomarker-driven clinical phase II AIO study. Eur J Cancer. 2018;92:11–9. doi: 10.1016/j.ejca.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 121.Spizzo G, Puccini A, Xiu J, Goldberg RM, Grothey A, Shields AF, et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open. 2020;5(3):e000682. doi: 10.1136/esmoopen-2020-000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olaparib in treating patients with metastatic biliary tract cancer with aberrant DNA repair gene mutations [Internet]. 2023 [cited September 30]. Available from: https://clinicaltrials.gov/show/NCT04042831.
- 123.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–27. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li XY, Chen JQ, Aisa A, Ding YW, Zhang D, Yuan Y. Targeting BRCA-mutant biliary tract cancer: current evidence and future perspectives. J Dig Dis. 2023;24(2):85–97. doi: 10.1111/1751-2980.13168. [DOI] [PubMed] [Google Scholar]
- 125.University of Michigan Rogel Cancer C, Dana-Farber Cancer I, Vanderbilt University Medical C. Rucaparib in combination with nivolumab in patients with advanced or metastatic biliary tract cancer following platinum therapy. 2023.
- 126.Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers (Basel). 2021;13(3):558. 10.3390/cancers13030558. [DOI] [PMC free article] [PubMed]
- 127.Silva VW, Askan G, Daniel TD, Lowery M, Klimstra DS, Abou-Alfa GK, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016;5(5):62. doi: 10.21037/cco.2016.10.04. [DOI] [PubMed] [Google Scholar]
- 128.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Riviere P, Goodman AM, Okamura R, Barkauskas DA, Whitchurch TJ, Lee S, et al. High tumor mutational burden correlates with longer survival in immunotherapy-naive patients with diverse cancers. Mol Cancer Ther. 2020;19(10):2139–45. doi: 10.1158/1535-7163.MCT-20-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–1. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jung J, Heo YJ, Park S. High tumor mutational burden predicts favorable response to anti-PD-(L)1 therapy in patients with solid tumor: A real-world pan-tumor analysis. J Immunother Cancer. 2023;11(4):e006454. 10.1136/jitc-2022-006454. [DOI] [PMC free article] [PubMed]
- 132.Jain A, Shroff RT, Zuo M, Weatherly J, Meric-Bernstam F, Isaacs R, et al. Tumor mutational burden (TMB) and co-existing actionable mutations in biliary tract cancers (BTC). J Clin Oncol. 2017;35(15_suppl):4086-.
- 133.Zhang C, Xu J, Xue S, Ye J. Prognostic value of L-type amino acid transporter 1 (LAT1) in various cancers: a meta-analysis. Mol Diagn Ther. 2020;24(5):523–36. doi: 10.1007/s40291-020-00470-x. [DOI] [PubMed] [Google Scholar]
- 134.Nishikubo K, Ohgaki R, Okanishi H, Okuda S, Xu M, Endou H, et al. Pharmacologic inhibition of LAT1 predominantly suppresses transport of large neutral amino acids and downregulates global translation in cancer cells. J Cell Mol Med. 2022;26(20):5246–56. doi: 10.1111/jcmm.17553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Achmad A, Lestari S, Holik HA, Rahayu D, Bashari MH, Faried A, et al. Highly specific L-type amino acid transporter 1 inhibition by JPH203 as a potential pan-cancer treatment. Processes. 2021;9(7):1170. doi: 10.3390/pr9071170. [DOI] [Google Scholar]
- 136.Wempe MF, Rice PJ, Lightner JW, Jutabha P, Hayashi M, Anzai N, et al. Metabolism and pharmacokinetic studies of JPH203, an L-amino acid transporter 1 (LAT1) selective compound. Drug Metab Pharmacokinet. 2012;27(1):155–61. doi: 10.2133/dmpk.DMPK-11-RG-091. [DOI] [PubMed] [Google Scholar]
- 137.Niemela J, Kallio R, Ohtonen P, Saarnio J, Syrjala H. Impact of cholangitis on survival of patients with malignant biliary obstruction treated with percutaneous transhepatic biliary drainage. BMC Gastroenterol. 2023;23(1):91. doi: 10.1186/s12876-023-02704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hunter LA, Soares HP. Quality of life and symptom management in advanced biliary tract cancers. Cancers. 2021;13(20):5074. [DOI] [PMC free article] [PubMed]
- 139.Mohan BP, Chandan S, Khan SR, Kassab LL, Ponnada S, Artifon ELA, et al. Photodynamic therapy (PDT), radiofrequency ablation (RFA) with biliary stents in palliative treatment of unresectable extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Clin Gastroenterol. 2022;56(2):e153–e60. doi: 10.1097/MCG.0000000000001524. [DOI] [PubMed] [Google Scholar]
- 140.Wu TT, Li WM, Li HC, Ao GK, Zheng F, Lin H. Percutaneous intraductal radiofrequency ablation for extrahepatic distal cholangiocarcinoma: a method for prolonging stent patency and achieving better functional status and quality of life. Cardiovasc Intervent Radiol. 2017;40(2):260–9. doi: 10.1007/s00270-016-1483-2. [DOI] [PubMed] [Google Scholar]
- 141.Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125(5):1355–63. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez-Carmona MA, Mohring C, Mahn R, Zhou T, Bartels A, Sadeghlar F, et al. Impact of regular additional endobiliary radiofrequency ablation on survival of patients with advanced extrahepatic cholangiocarcinoma under systemic chemotherapy. Sci Rep. 2022;12(1):1011. doi: 10.1038/s41598-021-04297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vienot A, Neuzillet C. Cholangiocarcinoma: the quest for a second-line systemic treatment. Transl Cancer Res. 2019;8(Suppl 3):S275–S88. doi: 10.21037/tcr.2018.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]