Abstract
Background
Recent alerts have highlighted an increase in group A streptococcal (GAS) infections since 2022 in Europe and the United States. Streptococcus pyogenes can cause limited skin or mucosal disease, but can also present as severe invasive disease necessitating critical care. We performed a multicenter retrospective study of patients with GAS infections recently admitted to Belgian intensive care units (ICUs) since January 2022. We describe patient characteristics and investigate the molecular epidemiology of the S. pyogenes strains involved.
Results
Between January 2022 and May 2023, a total of 86 cases (56 adults, 30 children) with GAS disease were admitted to critical care in the university hospitals of Leuven, Antwerp and Liège. We noted a strikingly high incidence of severe community-acquired pneumonia (sCAP) (45% of adults, 77% of children) complicated with empyema in 45% and 83% of adult and pediatric cases, respectively. Two-thirds of patients with S. pyogenes pneumonia had viral co-infection, with influenza (13 adults, 5 children) predominating. Other disease presentations included necrotizing fasciitis (23% of adults), other severe skin/soft tissue infections (16% of adults, 13% of children) and ear/nose/throat infections (13% of adults, 13% of children). Cardiogenic shock was frequent (36% of adults, 20% of children). Fifty-six patients (65%) had toxic shock syndrome. Organ support requirements were high and included invasive mechanical ventilation (77% of adults, 50% of children), renal replacement therapy (29% of adults, 3% of children) and extracorporeal membrane oxygenation (20% of adults, 7% of children). Mortality was 21% in adults and 3% in children. Genomic analysis of S. pyogenes strains from 55 out of 86 patients showed a predominance of emm1 strains (73%), with a replacement of the M1global lineage by the toxigenic M1UK lineage (83% of emm1 strains were M1UK).
Conclusions
The recent rise of severe GAS infections (2022–23) is associated with introduction of the M1UK lineage in Belgium, but other factors may be at play—including intense circulation of respiratory viruses and potentially an immune debt after the COVID pandemic. Importantly, critical care physicians should include S. pyogenes as causative pathogen in the differential diagnosis of sCAP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01249-7.
Keywords: Group A streptococci, Invasive, Streptococcus pyogenes, Necrotizing fasciitis, Toxic shock syndrome, Community-acquired pneumonia, Empyema, Viral coinfection, Influenza, Critical care
Background
Streptococcus pyogenes (S. pyogenes) can cause infections of varying severity, ranging from benign skin infections or scarlet fever to severe invasive infections such as necrotizing fasciitis and toxic shock syndrome (StrepTSS) [1]. Risk factors for invasive group A streptococcal (iGAS) infection include extremes of age, diabetes, alcohol and intravenous drug use, but up to a third of cases occur in patients without comorbidities [2]. Also, circulation of specific virulent emm types in the community may impact severity and clinical presentation of iGAS infections [3, 4]. Moreover, varicella zoster and influenza A viral infections have been associated with iGAS infections [2, 5, 6]. Recent data from the Brussels Capital region (Belgium) showed an increasing incidence of iGAS infections already before the COVID pandemic, from 2.1 to 10.9/100000 inhabitants [7]. However, this rising trend has not been uniformly observed in other countries [8, 9].
Several critical care units in Belgium noted an increased burden of severe iGAS infections mainly since autumn of 2022 in parallel with a doubling (+ 122%) in mandatory notifications of the disease in the three national surveillance networks [10]. In fact, recent alerts from the CDC and WHO [11, 12] highlighted outbreaks of pediatric scarlet fever and iGAS infections, but data from critical care have been lacking. Also, the clinical phenotypes of critical iGAS infections as seen during recent months, have not been described.
We designed a multicenter, retrospective cohort study in the Belgian university hospitals of Antwerp, Liège and Leuven, to describe the patient population, clinical presentation and outcomes of this new epidemic of iGAS disease necessitating critical care. We also performed genetic subtyping of the iGAS strains involved to ascertain whether circulation of a highly virulent subtype could explain the rise in critical iGAS cases.
Methods
We retrospectively collected clinical data from patients with microbiologically documented severe infection with S. pyogenes who were consecutively admitted to the intensive care unit in the University Hospitals of Antwerp (UZA), Leuven (UZ Leuven) and Liège (CHU Liège and CHR Citadelle) between January 2022 and May 2023. Our hospitals are all tertiary referral centers that together cover a population of > 4 million inhabitants and have 211 ICU beds and 3385 hospital beds. Patients were considered to have definite iGAS infection if there was isolation of GAS from a normally sterile site (i.e., blood cultures, cerebrospinal, pleural or peritoneal fluid) as per the 1995 CDC case definition [13]; and probable iGAS infection if GAS was isolated from a non-sterile site (e.g., wound, genital, or respiratory tract) with clinical evidence of invasive infection such as necrotizing fasciitis, sepsis or toxic shock syndrome. Toxic shock syndrome was defined according to the 2010 CDC case definition [14] and requires the presence of hypotension with at least two of: renal failure, diffuse intravascular coagulation (DIC) or thrombocytopenia, liver involvement, acute respiratory distress syndrome, rash, soft tissue necrosis.
Data were collected from the patient electronic health records and entered into an anonymized database. We documented demographics, comorbidities, viral coinfections, clinical phenotype, laboratory characteristics and scores (ISTH score for DIC [15], APACHE II [16] and SOFA [17] as scores for severity of illness and organ failure, respectively), need for respiratory, cardiovascular or renal support, antibiotic therapy, use of intravenous immunoglobulins, length of stay and mortality. The Ethics committees of the participating hospitals waived the requirement for informed consent for this retrospective data collection.
Streptococcus pyogenes isolates from invasive infections are routinely sent, although not mandatory, to the Belgian Reference Laboratory at the Antwerp University Hospital for emm gene typing of all isolates (according to the CDC emm typing protocol [18]) as GAS strains are classified by genetic differences in the surface M protein encoded by the emm gene [19]. Whole genome sequencing (Illumina MiSeq) is performed on a selection of isolates in search of pathogen-specific factors such as superantigens and hypervirulent clones, like the toxigenic M1UK variant [20, 21].
Data are reported as mean ± standard deviation, median (interquartile range), numbers or proportions, as appropriate. For comparison between groups, Mann–Whitney U and Chi square tests were used. We used Prism 9 (version 9.5.1, GraphPad Software) for these analyses. Adult and pediatric patient data are presented separately.
Results
Patient characteristics
During the study period, a total of 86 critically ill patients (of which 56 adults) with iGAS infection were identified. Three quarters of patients were referred from other hospitals, after a median of 1 day (IQR 0–3). Clinical characteristics of patients can be found in Table 1.
Table 1.
Clinical data from 86 critically ill patients with invasive group A streptococcal infections
| Adult cases (n = 56) | Pediatric cases (n = 30) | |
|---|---|---|
| Demographics | ||
| Age (years) | 48 ± 16 | 2 (1–5) |
| Male gender (n, %) | 34 (61%) | 17 (57%) |
| Weight (kg) | 80 ± 19 | 14 (10–19) |
| BMI (kg/m2) | 26 (23–28) | NA |
| Clinical presentation | ||
| Duration of symptoms prior to hospital admission (days) | 2 (1–4) | 3 (2–5) |
| Pneumonia (n, %) | 25 (45%) | 23 (77%) |
| With empyema (n) | 12 | 19 |
| Necrotizing fasciitis (n, %) | 13 (23%) | 0 |
| Other SSTI (n, %) | 9 (16%) | 4 (13%) |
| ENT infection (n, %) | 7 (13%) | 4 (13%) |
| Puerperal sepsis (n, %) | 2 (4%) | NA |
| Toxic shock syndrome (n, %) | 43 (77%) | 13 (43%) |
| Viral co-infection (n, %) | 18 (32%) | 21 (70%) |
| Influenza | 14 | 7 |
| HMPV | 3 | 4 |
| RSV | 1 | 3 |
| SARS CoV-2 | 0 | 3 |
| Varicella | 0 | 3 |
| Severity of illness and organ support | ||
| APACHE II score | 22 ± 9 | 21 ± 9 |
| SOFA score at ICU admission | 12 ± 5 | NA |
| Highest SOFA score in ICU | 13 ± 5 | NA |
| Invasive mechanical ventilation at ICU admission | 35 (63%) | 9 (30%) |
| Invasive mechanic ventilation (n, %) | 43 (77%) | 15 (50%) |
| Ventilator days | 12 (3–24) | 4 (2–12) |
| RRT at ICU admission | 13 (23%) | 0 |
| RRT (n, %) | 16 (29%) | 1 (3%) |
| Days on RRT | 16 ± 13 | 15 |
| Requiring vasoactive drugs at ICU admission | 45 (80%) | 9 (30%) |
| Septic shock (n, %) | 49 (88%) | 9 (30%) |
| Cardiogenic shock (n, %) | 20 (36%) | 6 (20%) |
| ECMO (n, %) | 11 (20%) | 2 (7%) |
| Days on ECMO | 15 (10–18) | 9 (8–10) |
| Outcomes | ||
| ICU length-of-stay (days) | 16 (6–40) | 7 (4–17) |
| Hospital length-of-stay (days) | 29 (20–65) | 18 (16–30) |
| 28 day mortality (n, %) | 11 (20%) | 1 (3%) |
| ICU mortality (n, %) | 12 (21%) | 1 (3%) |
| In-hospital mortality (n, %) | 12 (21%) | 1 (3%) |
BMI: body mass index; n number of patients; NA: not applicable; ENT: ear, nose and throat; SSTI: skin or soft tissue infection; HMPV: human metapneumovirus; RSV: Respiratory syncytial virus; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; APACHE II: acute physiology, age and chronic health evaluation; SOFA: sequential organ failure assessment; RRT: renal replacement therapy; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit
Chronic airway disease (n = 8 patients), malignancies (n = 7), arterial hypertension (n = 7), immune suppression (n = 5), psychiatric disorders (n = 6), alcoholism (n = 5) and intravenous drug use (n = 4) were the most frequent comorbidities. Adults were often overweight or obese (median BMI of 26 kg/m2). About half of patients (21/56 adults and 24/30 children) had no underlying medical conditions. A large proportion of cases (45% of adults, 77% of children) presented with severe community-acquired pneumonia (sCAP) and many (77% of adults, 43% of children) had toxic shock syndrome. Indeed, lab results on admission to ICU showed thrombocytopenia (< 100*109/L) in 40% of cases (34/86), overt DIC in 23% (20/86), acute liver injury in 34% (29/86) and acute kidney injury in 44% (38/86). In 37 adults and 15 children, S. pyogenes was cultured from blood cultures. Viral coinfection was documented in one out of three adults, and two out of three children, with influenza and HMPV predominating.
For pneumonia specifically, 16/25 adults and 17/23 children had evidence of viral coinfection. Conversely, of all patients with combined viral and severe GAS infection, the clinical presentation was pneumonia in 16/18 adults and 17/21 children. For pneumonia cases, the majority was multilobar (31/48 or 65%) and many were complicated with empyema. Eight patients with pneumonia even developed lung parenchymal necrosis and/or cavitation. The median pO2/FiO2 ratio at ICU admission was 140 (IQR 88–250). The highest number of ICU admissions occurred during winter, coinciding with the peak of the influenza epidemic in Belgium [22] (Additional file 1: Fig. S1).
Patients were severely ill, more than half required invasive ventilation, a third had cardiogenic shock and one in five required kidney support. Eleven adults and 2 children required extracorporeal membrane oxygenation, including 4 who required veno-arterial or veno-arteriovenous ECMO.
Patients were treated with appropriate antibiotics, including adjunctive clindamycin (in 50/56 adults and 25/30 children). Beta-lactam antibiotics for S. pyogenes infections were given for median 15 days (IQR 11–22) as some patients received prolonged courses for presentations such as empyema, arthritis, spondylodiscitis, mastoiditis, infective endocarditis. Clindamycin was given for 6 (IQR 4–8) days. Only 1 adult and 1 child were receiving antibiotics prior to hospital presentation. In three quarters of referred patients (48 of 64 referred cases), antibiotic therapy was started in the first hospital. For necrotizing fasciitis cases, surgery was performed either the day of admission, or the day thereafter, patients required a median of 4 (1.5–5) surgeries. Intravenous immunoglobulins were used less frequently in the total population (in 24/56 adults and 6/30 children) and in 27/56 patients with toxic shock syndrome.
One in five adults with critical iGAS infection did not survive their admission. In-hospital mortality from critical GAS pneumonia was 16% (4/25) in adults and 4% (1/23) in children; for necrotizing fasciitis case fatality rate was higher at 38% (5/13). Toxic shock syndrome was associated with a mortality of 26% (11/43) in adults and 8% (1/13) in children. Seventy-seven percent (10/13) of all patients who temporarily required ECMO survived. Co-isolation of viruses was not associated with a higher mortality rate (4/39 patients with viral co-infection, as compared to 9/47 patients without evidence for viral coinfection, died in hospital). Factors significantly associated with mortality in univariate analysis for the adult patients were severity of illness and organ failure (APACHE II score, SOFA score and receipt of invasive mechanical ventilation or renal replacement therapy at admission, as well as DIC and lactate level) (Additional file 2: Table S1).
We looked up the number of iGAS infections requiring ICU admission in a similar pre-pandemic period (Jan 2018-May 2019) in our three centers, and found a total of 44 admitted to ICU for iGAS disease. This corroborates a higher ICU admission rate with critical GAS infections and supports the assumption of an increased incidence of critical GAS infections in the population. Also, data from the reference laboratory show an increase in invasive isolates of group A Streptococcus [10].
S. pyogenes characteristics
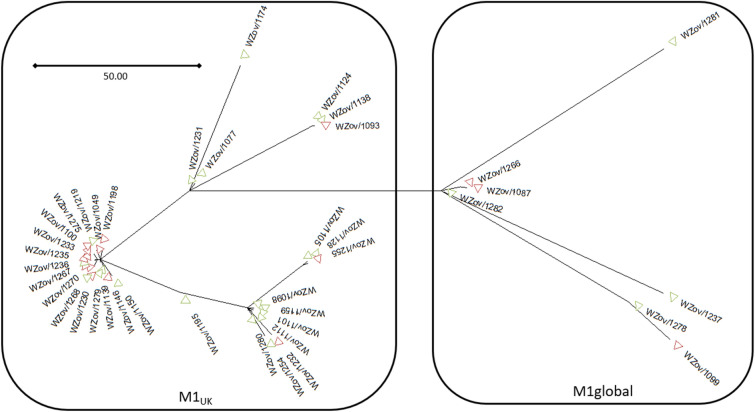
For 55 out of the 86 patients (36/56 adult and 19/30 pediatric patients) the S. pyogenes strain was sent to the Belgian Reference Centre at the Antwerp University Hospital for typing. Emm typing showed a predominance of emm1 (40/55, 73%) among emm4 and emm75 (both 2/55, 4%) and other emm-types occurring only once (emm12, emm22, emm65, emm76, emm87, emm89, emm94). Whole genome sequencing of the emm1 strains revealed that most of them (33/40, 83%) belonged to the toxigenic M1UK lineage while the M1global lineage was underrepresented (7/40, 17%) (Fig. 1). Pneumonia and viral coinfection were more frequent in patients infected with emm1 strains. We did not find significant differences in severity of illness (APACHE II), length of stay or mortality between patients infected with emm1 S. pyogenes strains as compared to patients with other emm-types (Additional file 3: Table S2).
Fig. 1.
Maximum likelihood phylogenetic tree constructed from core SNPs of the 40 emm1 S. pyogenes isolates, with MGAS5005 as reference, differentiating between the M1global and M1UK lineage. Green: adult (n = 24), red: pediatric (n = 16) strains
Discussion
We report our recent observation of a high number of admissions to Belgian tertiary critical care units of patients with severe invasive S. pyogenes infections, with a striking predominance of cases with pneumonia and empyema. These patients, both adults and children, were severely ill, as reflected by the high need for mechanical ventilation, cardiovascular support and even extracorporeal membrane oxygenation. They required prolonged ICU and hospital stays. Mortality was 21% in adults and 3% in children.
This rise in critically ill patients with iGAS infections parallels the increase in hospital admissions for iGAS that has been noted in different countries worldwide in 2022–23 [11, 12, 23–26], although the clinical presentations have not been clearly described before in a large cohort, and these health alerts have mainly stressed pediatric cases.
Our data show that critical iGAS infections affect both adults and children and are associated with considerable morbidity and mortality. Case fatality rates from iGAS infections (not necessarily requiring critical care) quoted in literature are high, up to 29–45% [9], and even in young children with iGAS during 2022, 21% did not survive [23]. In adults, mortality from S. pyogenes pneumonia of > 20–30% has been described [27, 28]. About one in five patients with iGAS infections are admitted to ICU [29]. We found a low mortality in critically ill children and a high mortality of 21% in adults. Of note, patients with empyema and necrotizing fasciitis, as well as patients on extracorporeal support, often required multiple interventions for source control and management of bleeding complications. Finally, the length of ICU and hospital stay in our cohort also reflect the impact these severe infections have on patients and the healthcare system.
Unexpectedly, our critically ill patients had a predominance of pneumonia (56%) as clinical presentation and more than half of patients had toxic shock syndrome, which is different from previous findings of 29–39% pneumonia and 10–16% toxic shock syndrome in iGAS infections in critical care [29, 30] and from the Belgian data mentioned earlier (4% pneumonia, 10% toxic shock) [7]. Despite the fact that pneumonia was not mentioned as a common clinical presentation of iGAS infections in a recent review [31], and that S. pyogenes was only a rare cause (< 1%) of CAP in the CDC EPIC study 10 years ago [32, 33], we believe that intensivists and clinicians today should not discard the possibility of severe S. pyogenes CAP, especially after viral respiratory tract infections in the winter season [34]. It has been hypothesized that widespread pneumococcal vaccination may play a role in an increasing incidence of S. pyogenes CAP in children [35].
While influenza and other viral infections are often found in patients with severe or critical GAS pneumonia [28, 36, 37] and GAS incidence is higher during winter months [5], many cases of iGAS infections occur in the absence of documented viral coinfection, so other pathogen- and/or host-specific factors are likely at play.
Streptococcus pyogenes produces many surface-bound and extracellular virulence factors that contribute to the pathogenesis of iGAS infection [38]. The predominance (73%) of emm1 S. pyogenes strains in our study is striking as this type, although the most prevalent among the different emm-types in Belgium, usually represents around 20% of Belgian iGAS strains like in other high income countries [19]. All the emm1 strains carried streptococcal pyrogenic exotoxins (Spe) and streptococcal mitogenic exotoxin (Sme) virulence genes speA, speG, speJ and smeZ, superantigen genes associated with invasive disease and severity of presentation [39]. Furthermore, the dominance of the toxigenic M1UK variant among emm1 isolates suggests replacement of circulating emm1 strains in Belgium.
The M1UK variant was first described in 2019 [20], has been associated with iGAS meningitis cases in the Netherlands [4], and seems to have become dominant in Belgium, as recently reported in the Netherlands and the UK [28]. This specific variant is characterized by 27 single nucleotide polymorphisms of which some have shown to increase the expression of exotoxin SpeA explaining its more toxigenic nature. It is associated with increase in scarlet fever and might explain the observed severity. Indeed, a recent Danish study showed that patients infected with emm1 variants more often required intensive care treatment although mortality rate and length of hospital stay did not differ [40], in keeping with our findings. Further clinical studies are required to assess whether iGAS infections caused by the M1UK variant are more severe and to discriminate the overlapping association between invasive disease, S. pyogenes types and clones and superantigen carriage although unraveled to some extent for specific emm types [41].
The relaxing of nonpharmacological infection control measures such as hand washing, face masks and social distancing could also contribute to the observed rise in iGAS [42], and potentially other invasive infections. Interestingly, the national reference laboratory for Streptococcus pneumoniae also saw an increase in notifications during December 2022 and January 2023, compared to the average of the years 2015–2019 [43].
Although our study describes extensively the patient and pathogen characteristics of iGAS infections requiring intensive care treatment, some limitations are of note. Since our hospitals are all tertiary care centers and the patients involved were often referred from peripheral hospitals, the characterized patient population is rather specific and might not reflect the general iGAS patient population, both in terms of disease and treatment characteristics as well as pathogen specifications. Even though the study combines data from four hospitals during a time frame of over a year, the sample size is rather limited and there is no comparison with an observation during the same time period in the pre-COVID-19 era. Both adult and pediatric iGAS infections are well represented in this study although some iGAS patients most probably were missed as S. pyogenes had to be isolated to be included. In case antibiotic treatment was already initiated before sample taking, this might have hampered the chances of S. pyogenes growth especially since molecular assays for S. pyogenes detection in clinical samples other than throat swabs are not routinely used [44]. Even if S. pyogenes was cultured, in only 64% of the cases the strains were preserved and available for further typing at the reference laboratory. Also extensive screening for viral (respiratory) co-infections is not systematically performed in clinical practice which complicates assessing their possible association with iGAS.
Faced with this new reality of severe GAS infections, a multifaceted approach seems warranted. Heightened awareness of the diverse disease manifestations and potential severe clinical course of S. pyogenes infections is required, as early treatment, antibiotics, source control, clindamycin and IVIG could improve outcome [2]. Indeed, there seems to be room for improvement in the use of adjunctive clindamycin and IVIG, as also seen in recent data from Australasia [45]. In our series, immunoglobulins as well as clindamycin were administered very early in the majority of the patients (certainly when presenting with necrotizing fasciitis or severe toxic shock). Moreover, beta-lactam antibiotics were started early too in the course of iGAS infection, though with a median duration somewhat longer than recommended [2] which can be explained by a high incidence of persistent abscess formation at different sites (including empyema, arthritis, spondylodiscitis or mastoiditis).
We speculate that reinforcement of face mask use in healthcare professionals [46] and in the community for people with coryzal symptoms or pharyngitis, could prevent cases of GAS disease, as it is a human host-restricted pathogen. A similar effect of nonpharmacological measures has been shown for pneumococcal disease even though it did not prevent pharyngeal carriage [42]. Also, prompt antibiotic treatment of streptococcal pharyngitis and scarlet fever could prevent household or school clusters of infection [25], and maybe even avoid invasive disease. Finally, vaccines that reduce invasive GAS infections without immunological adverse effects, have been eagerly awaited for years [47].
Conclusions
This multicenter, retrospective study describes patient and pathogen characteristics of increased iGAS infections in Belgian tertiary care hospitals from January 2022 until May 2023. The large proportion of patients presenting with severe CAP especially in combination with a viral co-infection, mostly influenza virus is remarkable, highlighting the importance of considering GAS as a causative pathogen in severe CAP. Although the explanation for the observed epidemiological pattern of increased iGAS cases is still unclear, the introduction and replacement of the M1global S. pyogenes clone by the toxigenic M1UK strain may play a role. Reduced GAS transmission and exposure during COVID-19-related restrictions (social distancing and/or masking) and possibly an immune debt may have enhanced the rapid expansion of individual lineages. The high circulating rates of respiratory viruses during the 2022–2023 winter season, including influenza and COVID-19, may have predisposed to subsequent iGAS infection.
Supplementary Information
Additional file 1: Fig. S1. Seasonal distribution of critical S. pyogenes infections in four Belgian centers.
Additional file 2: Table S1. Clinical data from 56 adult critically ill patients with invasive group A streptococcal infections, with univariate analysis of factors associated with mortality. Significant p-values are highlighted in boldface.
Additional file 3: Table S2. Clinical data from 55 critically ill patients with invasive group A streptococcal infections, for whom strain subtyping was performed. Comparison was made between patients infected with an emm1 S. pyogenes strain, as compared to patients infected with other strains.
Acknowledgements
We would like to thank all Belgian clinical laboratories for sending invasive β-hemolytic streptococcal isolates to the Belgian Reference Centre, and for sharing basic patient clinical information; and the "Agence pour une Vie de Qualité" (AVIQ) of Wallonia and the "Afdeling Preventief Gezondheidsbeleid" of the Department of Care of Flanders, for sharing information on contact tracing.
Abbreviations
- APACHE II
Acute physiology, age and chronic health evaluation
- BMI
Body mass index
- CAP
Community acquired pneumonia
- CDC
Centers for Disease Control
- COVID
Coronavirus disease
- DIC
Diffuse intravascular coagulation
- ECMO
Extracorporeal membrane oxygenation
- ENT
Ear, nose and throat
- GAS
Group A Streptococcus/streptococcal
- HMPV
Human metapneumovirus
- iGAS
Invasive group A Streptococcus/Streptococcal
- ICU
Intensive care unit
- NA
Not applicable
- RRT
Renal replacement therapy
- RSV
Respiratory Syncytial Virus
- S. pyogenes
Streptococcus pyogenes
- sCAP
Severe community-acquired pneumonia
- SOFA
Sequential organ failure assessment
- SSTI
Skin or soft tissue infection
- TSS
Toxic shock syndrome
- StrepTSS
Streptococcal toxic shock syndrome
- WHO
World Health Organisation
Author contributions
MP, JW, PGJ, VM and NL conceptualized the study, MP, FM, FDR, ST, EP and NL acquired the clinical data. MP analyzed the clinical data and drafted the manuscript, VM analyzed the microbiological data, with the help of DG, VM performed subtyping and drafted the manuscript. All authors assisted in interpretation of the data, performed substantive revisions of the manuscript and approved the final submitted version. All authors vow for the accuracy and integrity of the work.
Funding
The Belgian Reference Centre for invasive β-haemolytic Streptococci is supported by the Belgian Ministry of Social Affairs through a fund within the Health Insurance System. The funding body was not involved in the design of this study, data collection, analysis, interpretation nor in writing of the manuscript.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The ethical committee of the university hospitals of Leuven (EC Research, S-67532), Liège and Antwerp waived the need for informed consent for this retrospective, anonymized data collection from the patient data management system.
Consent for publication
Not applicable.
Competing interests
JW received investigator-initiated grants from Pfizer, Gilead and MSD and speakers’ and travel fees from Pfizer, Gilead and MSD, and declares participation in advisory boards of Pfizer and Gilead, all outside the scope of this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marijke Peetermans and Veerle Matheeussen contributed equally.
Nathalie Layios, Joost Wauters and Philippe G. Jorens contributed equally.
References
- 1.Brouwer S, Rivera-Hernandez T, Curren BF, Harbison-Price N, De Oliveira DMP, Jespersen MG, et al. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat Rev Microbiol. 2023;21(7):431–447. doi: 10.1038/s41579-023-00865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz M, Roux X, Huttner B, Pugin J. Streptococcal toxic shock syndrome in the intensive care unit. Ann Intensive Care. 2018;8(1):88. doi: 10.1186/s13613-018-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiska DL, Thiede B, Caracciolo J, Jordan M, Johnson D, Kaplan EL, et al. Invasive group A streptococcal infections in North Carolina: epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J Infect Dis. 1997;176(4):992–1000. doi: 10.1086/516540. [DOI] [PubMed] [Google Scholar]
- 4.van der Putten BCL, Vlaminckx BJM, de Gier B, Freudenburg-de Graaf W, van Sorge NM. Group A Streptococcal meningitis with the M1UK variant in the Netherlands. JAMA. 2023;329(20):1791–1792. doi: 10.1001/jama.2023.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Gier B, Vlaminckx BJM, Woudt SHS, van Sorge NM, van Asten L. Associations between common respiratory viruses and invasive group A streptococcal infection: a time-series analysis. Influenza Other Respir Viruses. 2019;13(5):453–458. doi: 10.1111/irv.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachariadou L, Stathi A, Tassios PT, Pangalis A, Legakis NJ, Papaparaskevas J, et al. Differences in the epidemiology between paediatric and adult invasive Streptococcus pyogenes infections. Epidemiol Infect. 2014;142(3):512–519. doi: 10.1017/S0950268813001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zangarini L, Martiny D, Miendje Deyi VY, Hites M, Maillart E, Hainaut M, et al. Incidence and clinical and microbiological features of invasive and probable invasive streptococcal group A infections in children and adults in the Brussels-Capital Region, 2005–2020. Eur J Clin Microbiol Infect Dis. 2023;42(5):555–567. doi: 10.1007/s10096-023-04568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couture-Cossette A, Carignan A, Mercier A, Desruisseaux C, Valiquette L, Pepin J. Secular trends in incidence of invasive beta-hemolytic streptococci and efficacy of adjunctive therapy in Quebec, Canada, 1996–2016. PLoS ONE. 2018;13(10):e0206289. doi: 10.1371/journal.pone.0206289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson GE, Pondo T, Toews KA, Farley MM, Lindegren ML, Lynfield R, et al. Epidemiology of invasive Group A Streptococcal Infections in the United States, 2005–2012. Clin Infect Dis. 2016;63(4):478–486. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risk Assessment Group. Increasing number of invasive infections with group A streptococci (iGAS). https://www.sciensano.be/sites/default/files/rag_advice_igas_170123.pdf. Accessed 6 Sep 2023.
- 11.World Health Organization. Increased incidence of scarlet fever and invasive Group A Streptococcus infection - multi-country 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429. Accessed on 6 Sep 2023.
- 12.Centers for Disease Control and Prevention. Increase in Pediatric Invasive Group A Streptococcal Infections 2022. https://emergency.cdc.gov/han/2022/han00484.asp. Accessed 6 Sep 2023.
- 13.Centers for Disease Control and Prevention. Streptococcus Disease, Invasive, Group A (GAS) (Streptococcus pyogenes) 1995 Case Definition. https://ndc.services.cdc.gov/case-definitions/streptococcus-disease-invasive-group-a-1995. Accessed 6 Sep 2023.
- 14.Centers for Disease Control and Prevention. Streptococcal Toxic Shock Syndrome (STSS) (Streptococcus pyogenes) 2010 Case Definition. Available from: https://ndc.services.cdc.gov/case-definitions/streptococcal-toxic-shock-syndrome-2010. Accessed 6 Sep 2023.
- 15.Taylor FB, Jr, Toh CH, Hoots WK, et al. Scientific Subcommittee on Disseminated Intravascular Coagulation of the ISTH. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. doi: 10.1055/s-0037-1616068. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. emm Typing Protocol [updated July 23, 2021]. https://www.cdc.gov/streplab/groupa-strep/resources.html#typing-protocol. Accessed 6 Sep 2023.
- 19.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9(10):611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 20.Lynskey NN, Jauneikaite E, Li HK, Zhi X, Turner CE, Mosavie M, et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis. 2019;19(11):1209–1218. doi: 10.1016/S1473-3099(19)30446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 Group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192(5):771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 22.Bossuyt N, Vermeulen M, Denayer S, Barbezange C, De Schreye R, Moreels S, et al. Weekly bulletin acute respiratory infections - week 34. Sciensano 2023. https://www.sciensano.be/nl/gezondheidsonderwerpen/influenza/cijfers. Accessed 6 Sep 2023.
- 23.de Gier B, Marchal N, de Beer-Schuurman I, Te Wierik M, Hooiveld M, Group I-AS et al. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Euro Surveill. 2023;28(1):2200941. doi: 10.2807/1560-7917.ES.2023.28.1.2200941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lassoued Y, Assad Z, Ouldali N, Caseris M, Mariani P, Birgy A, et al. Unexpected increase in invasive group A Streptococcal infections in children after respiratory viruses outbreak in France: a 15-year time-series analysis. Open Forum Infect Dis. 2023;10(5):ofad188. doi: 10.1093/ofid/ofad188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain N, Lansiaux E, Reinis A. Group A streptococcal (GAS) infections amongst children in Europe: Taming the rising tide. New Microbes New Infect. 2023;51:101071. doi: 10.1016/j.nmni.2022.101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagcchi S. Surge of invasive Group A streptococcus disease. Lancet Infect Dis. 2023;23(3):284. doi: 10.1016/S1473-3099(23)00043-9. [DOI] [PubMed] [Google Scholar]
- 27.Tamayo E, Montes M, Vicente D, Perez-Trallero E. Streptococcus pyogenes pneumonia in adults: clinical presentation and molecular characterization of isolates 2006–2015. PLoS ONE. 2016;11(3):e0152640. doi: 10.1371/journal.pone.0152640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies PJB, Russell CD, Morgan AR, Taori SK, Lindsay D, Ure R, et al. Increase of severe pulmonary infections in adults caused by M1(UK) Streptococcus pyogenes, Central Scotland, UK. Emerg Infect Dis. 2023;29(8):1638. doi: 10.3201/eid2908.230569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stockmann C, Ampofo K, Hersh AL, Blaschke AJ, Kendall BA, Korgenski K, et al. Evolving epidemiologic characteristics of invasive group a streptococcal disease in Utah, 2002–2010. Clin Infect Dis. 2012;55(4):479–487. doi: 10.1093/cid/cis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeddha NP, Atkins L, de Groot R, Driessen G, Hazelzet J, Zenz W, et al. Group A streptococcal disease in paediatric inpatients: a European perspective. Eur J Pediatr. 2023;182(2):697–706. doi: 10.1007/s00431-022-04718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev. 2014;27(2):264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson PA, Varadhan H. Severe community-acquired pneumonia due to Streptococcus pyogenes in the Newcastle area. Commun Dis Intell. 2018;2020:44. doi: 10.33321/cdi.2020.44.82. [DOI] [PubMed] [Google Scholar]
- 35.Liese JG, Schoen C, van der Linden M, Lehmann L, Goettler D, Keller S, et al. Changes in the incidence and bacterial aetiology of paediatric parapneumonic pleural effusions/empyema in Germany, 2010–2017: a nationwide surveillance study. Clin Microbiol Infect. 2019;25(7):857–864. doi: 10.1016/j.cmi.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Herrera AL, Huber VC, Chaussee MS. The association between invasive group A Streptococcal diseases and viral respiratory tract infections. Front Microbiol. 2016;7:342. doi: 10.3389/fmicb.2016.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto S, Kawabata S, Nakagawa I, Okuno Y, Goto T, Sano K, et al. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J Virol. 2003;77(7):4104–4112. doi: 10.1128/JVI.77.7.4104-4112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens DL, Kaplan EL. Streptococcal infections: clinical aspects, microbiology and molecular pathogenesis. ISBN 9780195099218 (2000).
- 39.Alcolea-Medina A, Snell LB, Alder C, Charalampous T, Williams TGS, Synnovis Microbiology Laboratory Group et al. The ongoing Streptococcus pyogenes (Group A Streptococcus) outbreak in London, United Kingdom, in December 2022: a molecular epidemiology study. Clin Microbiol Infect. 2023;29(7):887–890. doi: 10.1016/j.cmi.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johannesen TB, Munkstrup C, Edslev SM, Baig S, Nielsen S, Funk T, et al. Increase in invasive group A streptococcal infections and emergence of novel, rapidly expanding sub-lineage of the virulent Streptococcus pyogenes M1 clone, Denmark, 2023. Euro Surveill. 2023;28(26):2300291. doi: 10.2807/1560-7917.ES.2023.28.26.2300291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekelund K, Darenberg J, Norrby-Teglund A, Hoffmann S, Bang D, Skinhoj P, et al. Variations in emm type among group A streptococcal isolates causing invasive or noninvasive infections in a nationwide study. J Clin Microbiol. 2005;43(7):3101–3109. doi: 10.1128/JCM.43.7.3101-3109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen R, Ashman M, Taha MK, Varon E, Angoulvant F, Levy C, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51(5):418–423. doi: 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Reference Centre for invasive S. pneumonia. Report National Reference Centre S. pneumoniae 2022. https://www.sciensano.be/sites/default/files/report_nrc_srpn_2022_final_0.pdf. Accessed 6 Sep 2023.
- 44.Spellerberg B BC. Laboratory Diagnosis of Streptococcus pyogenes (group A streptococci) 2016. In: Streptococcus pyogenes : Basic Biology to Clinical Manifestations. University of Oklahoma Health Sciences Center. https://www.ncbi.nlm.nih.gov/books/NBK343617/. Accessed 6 Sep 2023.
- 45.Dotel R, Bowen AC, Xie O, Gibney KB, Carapetis JR, Davis JS, Tong YC. Is it time for clinical trials of invasive group A and groups C and G Streptococcus infections? Clin Microbiol Infect. 2023;29(9):1205–1207. doi: 10.1016/j.cmi.2023.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Klompas M, Baker MA, Rhee C, Baden LR. Strategic masking to protect patients from all respiratory viral infections. N Engl J Med. 2023;389:4–6. doi: 10.1056/NEJMp2306223. [DOI] [PubMed] [Google Scholar]
- 47.Andrejko K, Whittles LK, Lewnard JA. Health-economic value of vaccination against group A Streptococcus in the United States. Clin Infect Dis. 2022;74(6):983–992. doi: 10.1093/cid/ciab597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Seasonal distribution of critical S. pyogenes infections in four Belgian centers.
Additional file 2: Table S1. Clinical data from 56 adult critically ill patients with invasive group A streptococcal infections, with univariate analysis of factors associated with mortality. Significant p-values are highlighted in boldface.
Additional file 3: Table S2. Clinical data from 55 critically ill patients with invasive group A streptococcal infections, for whom strain subtyping was performed. Comparison was made between patients infected with an emm1 S. pyogenes strain, as compared to patients infected with other strains.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.