Abstract
Helicobacter pylori is a pathogenic bacterium associated with various gastrointestinal diseases, including chronic gastritis, peptic ulcers, mucosa-associated lymphoid tissue lymphoma, and gastric cancer. The increasing rates of H. pylori antibiotic resistance and the emergence of multidrug-resistant strains pose significant challenges to its treatment. This comprehensive review explores the mechanisms underlying the resistance of H. pylori to commonly used antibiotics and the clinical implications of antibiotic resistance. Additionally, potential strategies for overcoming antibiotic resistance are discussed. These approaches aim to improve the treatment outcomes of H. pylori infections while minimizing the development of antibiotic resistance. The continuous evolution of treatment perspectives and ongoing research in this field are crucial for effectively combating this challenging infection.
Keywords: Helicobacter pylori, Antibiotic Resistance, Multidrug Resistance, Antimicrobial Stewardship
INTRODUCTION
Helicobacter pylori is a Gram-negative bacterium that colonizes and persists in the stomach.1 It can be transmitted from an infected person to an uninfected person by direct contact via an oral-oral, fecal-oral, or both routes.2,3 Most people become infected during their childhood, and parents and siblings appear to play a significant role in pathogen transmission.3,4 Once infected, H. pylori causes lifelong chronic progressive gastric inflammation, which can lead to clinical complications in up to 10% of infected individuals.1,5 The major clinical complications include peptic ulcer disease, chronic gastritis, gastric cancer, and mucosa-associated lymphoid tissue lymphoma.1,2,5,6,7 The prevalence of H. pylori infection varies widely according to geographic area, age, and socioeconomic status.6 Although the prevalence of H. pylori is decreasing due to improving hygiene and standard of living, it remains high, particularly in the Eastern Asian countries.6,8,9,10
A combination of two to three antibiotics (from a few antibiotics such as amoxicillin, clarithromycin, metronidazole, tetracycline, levofloxacin, and rifabutin) and an acid-suppressive agent with or without bismuth are used to eradicate H. pylori.11,12,13 However, the successful eradication rate of H. pylori has decreased in the past decades, in parallel with increasing antibiotic resistance.14,15,16,17,18,19,20 Antibiotic resistance of H. pylori is particularly important because this is one of the most common causes of bacterial infections worldwide, affecting millions of people every year.1,6 Additionally, overuse or inappropriate use of antibiotics can contribute to the development of antimicrobial resistance in H. pylori and other bacteria, which can have serious public health implications.21,22,23 Recently, the World Health Organization listed H. pylori as a serious threat to human health for their resistance against most available treatment regimens.24
The molecular mechanisms underlying antibiotic resistance in H. pylori infection are diverse and complex. Several mechanisms have been proposed to drive the antibiotic resistance of H. pylori, including genetic mutations in the bacterium itself and physiological changes that can upregulate efflux pump expression in bacterial cells.25,26,27,28,29,30 Cellular adaptation associated with biofilm or coccoid formation, which protects drug penetration into bacterial cells, is another potential resistance mechanism.31,32 Many of these resistance mechanisms can work in concert to confer multidrug resistance (MDR) in H. pylori, making eradication increasingly challenging and highlighting the need for new therapeutic strategies. In this review, the mechanism of resistance of H. pylori to commonly used antibiotics and their clinical implications are explored.
MOLECULAR MECHANISMS OF ANTIBIOTIC RESISTANCE
Mechanism of single-drug resistance
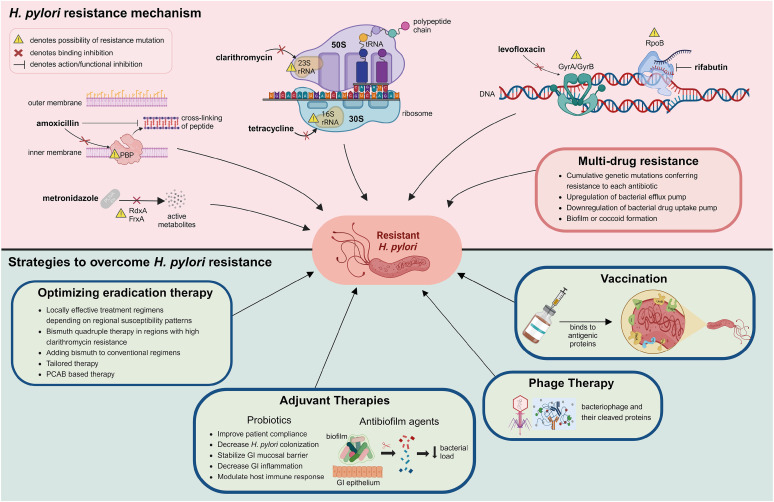
Single-drug resistance refers to a situation in which a microorganism, such as a bacterium, virus, or fungus, is resistant to only one type of drug while remaining susceptible to other drugs. Although the eradication regimen for H. pylori consists of a combination of antibiotics, resistance to a single antibiotic agent could result in eradication failure.33 Antibiotic resistance in H. pylori is mainly from de novo genetic mutations that disrupt the activity of antibiotics by either altering the drug target or inhibiting drug activation within cells (Table 1 and Fig. 1).7 With advances in next-generation sequencing (NGS), many mutations have been observed in resistant clinical isolates.25,26,34,35 However, the relative contribution of each mutation to phenotypic resistance and the effect of combinations of mutations remain unclear.36
Table 1. Mechanisms of antibiotic resistance in H. pylori .
| Antibiotics | Antibiotic resistance mechanism | Associated gene or sequence |
|---|---|---|
| Amoxicillin | Mutational change of penicillin-binding protein | pbp1A, pbp2, pbp3, pbp4 |
| Production of beta-lactamase | ||
| Outer membrane protein mutations causing decreased membrane permeability | hefC, hopC, hofH | |
| Clarithromycin | Point mutations in 23S rRNA | 23S rRNA |
| Increased antibiotic efflux mediated by efflux pump systems | rpl22, infB | |
| Levofloxacin | Point mutations in quinolone resistance determination region of DNA gyrase gene | gyrA, gyrB |
| Metronidazole | Mutations causing reduced or abolished nitroreductase activity | rdxA, frxA |
| Tetracycline | Mutations in 16S rRNA | 16S rRNA |
| Rifabutin | Point mutations in rifampicin-resistance determining region of RNA polymerase gene | rpoB |
Fig. 1. Overview of molecular mechanism of antibiotic resistance in H. pylori and strategies to overcome this resistance. This image was created with BioRender.com.
FrxA = NAD(P)H flavin oxidoreductase, GI = gastrointestinal, GyrA = DNA gyrase subunit A, GyrB = DNA gyrase subunit B, PBP = penicillin-binding protein, PCAB = potassium-competitive acid blocker, RdxA = oxygen-insensitive NAD(P)H nitroreductase, RpoB = β-subunit of DNA-dependent RNA polymerase, rRNA = ribosomal RNA, tRNA = transfer RNA.
Beta-lactams
Amoxicillin is widely used for H. pylori eradication in combination with acid-suppressive agents to improve drug stability and efficacy. After administration, amoxicillin is well absorbed into bloodstream and then released into the gastric juice.37 In a favorable environment with acid-suppressive agents, amoxicillin exerts its antimicrobial effect by binding to penicillin-binding proteins (PBPs).38,39 Binding of amoxicillin to PBPs inhibits the synthesis of peptidoglycan, a major component of bacterial cell walls, resulting in cell wall lysis in replicating bacteria.39,40 Amoxicillin generally shows a low antibiotic resistance rate.41,42,43,44 In H. pylori, amoxicillin resistance is mainly due to mutational changes in PBP1A.45,46,47,48 Mutations in other PBPs (PBP2 and PBP 3) have also reported, and multiple mutations in all three isotypes PBP1, PBP2 and PBP3 was found to confer high level of amoxicillin resistance when compared to the amoxicillin susceptible strain.49 Beta-lactamase activity, which can hydrolyze amoxicillin to attenuate the optimum concentration needed to elicit bactericidal effect, was also found in H. pylori.40,50 Furthermore, amoxicillin resistance may be contributed by mutations in hefC, hopC, and hofH, which are likely associated with changes in the composition of the outer membrane and membrane permeability of H. pylori.51,52,53
Macrolides
Clarithromycin is widely used in the frontline regimen to eradicate H. pylori. Clarithromycin has pharmacokinetic advantages over other macrolides, including increased oral bioavailability, higher plasma concentration, and longer elimination half-life.54 Concomitant administration with acid-suppressive agents also increases its stability in acidic environments.55 Clarithromycin exerts antimicrobial effects by binding to the peptidyl transferase loop of domain V in the 23S ribosomal RNA (rRNA) in the bacterial ribosomal subunit 50S. Mutations in domain V of the 23S rRNA gene of H. pylori, A2142G/C and A2143G, can result in reduced binding affinity of the antibiotic agent, making it less effective at inhibiting bacterial growth.56,57 Other point mutations are also reported in the 23S rRNA in H. pylori isolates.25,26,27,58 In addition, studies using experimentally induced resistant phenotype for clarithromycin found that mutations in rpl22 and infB genes had synergistic effects with mutations in the 23S rRNA genes, resulting in higher minimum inhibitory concentration of clarithromycin.34,35 Another relevant mechanism for clarithromycin resistance is attributed to the efflux pump system.29,59 However, the role of novel mutations and specific function of efflux pump system in the development of clarithromycin resistance in clinical isolates should be further clarified.
Fluoroquinolones
Among the fluoroquinolones, levofloxacin, moxifloxacin, and sitafloxacin have been used for H. pylori eradication therapy. Due to high resistance rate, fluoroquinolones are generally used in rescue treatment after initial eradication failure.11,60,61 Fluoroquinolones act on microbes by inhibiting bacterial topoisomerase II (DNA gyrase) and topoisomerase IV enzymes involved in bacterial synthesis of nucleic acid, a step proceeding cell division and proliferation.28 The most common mechanism of fluoroquinolone resistance in H. pylori is due to a specific mutation in one or more of gyrA and gyrB genes.62 The region where mutations arise in these genes is a short DNA sequence known as the quinolone resistance-determining region (QRDR).63,64 Fluoroquinolone resistance of H. pylori is mainly due to point mutations of codon position 87 and 91 in the QRDR of gyrA.65,66,67 These few mutations associated with most cases of phenotypic resistance indicate that molecular testing for levofloxacin can be a reliable substitute for culture and antimicrobial susceptibility testing. Mutations present outside the QRDR region of gyrA or in the QRDR region of gyrB have also been reported to be associated with levofloxacin resistance; however, the impact of these mutations requires further investigation.26,27,58,66
Nitroimidazole
Among the nitroimidazoles, metronidazole is frequently used to eradicate H. pylori infections. Metronidazole is actively released into gastric juice after oral ingestion, with the acidic condition in the stomach rarely affecting its antimicrobial activity.68 Metronidazole is a prodrug that needs to be activated by intracellular reduction of the nitro group attached to the imidazole ring.69 Reductive activation of metronidazole causes imidazole fragmentation and nitro-anion free radicals which are cytotoxic.69,70 The reduction of metronidazole is mainly mediated by oxygen-insensitive NAD(P)H nitroreductase (RdxA), NAD(P)H flavin oxidoreductase (FrxA), and ferredoxin-like enzymes (FdxB) in H. pylori. Metronidazole resistance in H. pylori is primarily due to decreased drug activation mediated by mutations in RdxA gene which encodes an oxygen-insensitive NAD(P)H nitroreductase.70,71,72,73,74,75,76 Mutations involving the FrxA gene were also reported.35,74,77 However, metronidazole resistance was observed in H. pylori isolates without the loss of functional RdxA and FrxA, suggesting that other factors are involved in metronidazole resistance.72,78,79,80,81 Other putative mechanisms of metronidazole resistance in H. pylori include mutations in FdxB, ferric uptake regulator (Fur), and enhancement of efflux pump (HefA) protein.72,82,83,84
Tetracyclines
Tetracycline is stable in gastric pH and acts as a topical agent on the surface of the gastric mucosa against H. pylori.85 At the bacterial cytoplasm, tetracycline binds to bacterial ribosomes and interacts with a highly conserved 16S rRNA target in the 30S ribosomal subunit, arresting translation and protein synthesis.86 Resistance mechanism of H. pylori against tetracycline is not widely studied because tetracycline resistance is not common in clinical isolates.14,41,44,87 Among various mechanisms of tetracycline resistance, the major resistance mechanisms are related to mutations in the 16S rRNA genes.88,89,90 However, tetracycline resistance without mutation in the 16S rRNA gene has been reported, suggesting that other mechanisms, such as efflux, are associated with tetracycline resistance.84,91
Rifamycins
Rifamycins are transcriptional inhibitors that specifically inhibit the activity of bacterial transcription by binding to RNA polymerase, mostly β-subunit encoded by the rpoB gene.92 Among the rifamycins, rifabutin has better pharmacokinetics than rifampicin and is used for H. pylori eradication. Rifabutin is chemically stable at a wide range of pH values and is not inactivated by gastric acid.93 The resistance rate of H. pylori against rifabutin is low, and most rifabutin-resistant strains were isolated after treatment failure.94,95,96 In H. pylori, the molecular mechanism driving rifabutin resistance is at least one point mutation in the rifampicin resistance-determining region of the rpoB gene.27,97,98,99,100 However, a previous study reported that rifabutin-resistant strains were successfully eradicated with rifabutin-based triple therapy.101 Correlations between rpoB gene mutation status, phenotypic resistance, and treatment outcome need further clarification.
MDR in H. pylori
The presence of H. pylori strains with MDR profiles poses a significant challenge for H. pylori eradication and complicates the management of H. pylori-related diseases. The MDR profile of H. pylori is expressed as the cumulative result of genetic mutations conferring resistance to each antibiotic agent.7 Other putative mechanisms of H. pylori MDR include physiologic changes in bacterial cells (upregulation of efflux pump systems or downregulation of drug uptake proteins in the outer membrane of bacteria) and cellular adaptation properties (biofilm or coccoid formation).59,102,103,104,105
Bacterial biofilms are complex microbiological ecosystems where adherent aggregates of microorganisms surround themselves in multidimensional extracellular polymeric substances.106,107 Biofilms are often associated with chronic infectious diseases as they protect the bacteria from unfavorable environments, antimicrobial exposure, and host immune system.31,108 Biofilm formation in H. pylori has been observed both in vitro (environmental water body) and in vivo (gastric mucosa).109,110 The presence of biofilm was shown to be associated with decreased susceptibility to antibiotics in H. pylori.31 Previous studies showed that mutations in several genes coding for flagellar protein, outer membrane protein, cytotoxin-associated gene pathogenicity island protein, or efflux pumps were responsible for biofilm formation in H. pylori, which can further potentiate antibiotic resistance.84,111,112 However, the precise mechanisms for H. pylori biofilm formation have yet to be determined. In addition, the clinical implications of biofilm formation on the development of antibiotic resistance in H. pylori and treatment outcomes should be further investigated.
An exceptional feature of H. pylori is the formation of a viable but non-culturable coccoid morphology. In H. pylori, the coccoid form becomes dominant when bacteria are exposed to environmental stress conditions, such as starvation, prolonged culture, and exposure to antibiotics.113,114 It has been reported that this dormant state of H. pylori induces ultrastructural modifications in the cell membrane and metabolic pathways that contribute to antibiotic resistance.24,107 However, the clinical relevance of coccoid formation in the development of MDR profile and treatment outcome of H. pylori is not fully understood.
Heteroresistance
Heteroresistance refers to a phenomenon where subpopulations of bacteria have different antibiotic susceptibility profiles.115 Heteroresistance in H. pylori has been reported in several studies in which both susceptible and resistant bacterial strains were isolated either from the same biopsy site (intraniche) or from different sites (interniche).87,115,116,117,118,119,120,121,122 Heteroresistance can be developed through evolutionary change in a single strain or mixed infection of multiple bacterial strains.7 Previous studies reported that heteroresistant H. pylori strains had similar fingerprinting patterns, suggesting that the presence of the same strain with mixed susceptible and resistant phenotype, rather than coinfection of different strains, is associated with the development of H. pylori heteroresistance.117,118,119,120,121 The possibility of heteroresistance should be considered during antimicrobial susceptibility testing and eradication therapy for H. pylori infection since underestimation of the presence of antibiotic-resistant strain may lead to treatment failure. To address this issue, multiple biopsies from different sites in the stomach or multiple bacterial colonies from the same sample should be obtained when evaluating the antimicrobial susceptibility of H. pylori.
Antimicrobial susceptibility testing
With increasing antibiotic resistance, it is imperative to develop individualized therapeutic approaches based on the results of antimicrobial susceptibility tests. Common methods for antimicrobial susceptibility testing include culture-based antimicrobial susceptibility testing and molecular detection. Bacterial culture is necessary for the use of conventional antimicrobial susceptibility tests, such as E-test, disk diffusion method, and agar dilution method.39,42,123 However, H. pylori culture requires specific conditions, is time-consuming, and is affected by several factors, such as transport conditions and the time interval between specimen collection and inoculation, limiting its availability in clinical practice.
Molecular methods are also used to assess antimicrobial susceptibility. Since the resistance of H. pylori to clarithromycin, fluoroquinolones, and tetracycline is mainly driven by specific point mutations in a small region of the responsible gene, molecular methods can be utilized for antimicrobial susceptibility testing. Polymerase chain reaction (PCR) was used to assess antibiotic resistance by detecting resistance-associated mutations. Similarly, PCR-restriction fragment length polymorphism, real-time PCR, multiplex PCR, and droplet digital PCR have been widely used to determine antibiotic resistance in H. pylori.124,125,126,127,128,129 Currently, several commercial kits are available to detect clarithromycin resistance, levofloxacin resistance, or both.130,131,132,133,134,135 Another diagnostic tool using loop-mediated isothermal amplification methods, combined PCR and quenching probe method, and single cell-based antimicrobial susceptibility test Ramanometry has been introduced.136,137,138,139
Compared to conventional methods, molecular detection methods have the advantages of high sensitivity, specificity, and reproducibility, and disadvantages such as high cost. Furthermore, as molecular detection methods target specific gene loci, antibiotic resistance caused by other mutations cannot be detected, leading to false-negative results.140 In addition, as metronidazole has complex resistance mechanisms and the presence of metronidazole resistance does not necessarily lead to treatment failure, the role of molecular detection of metronidazole resistance is limited.
Recent advances in high-throughput molecular detection technology, including NGS and metagenomic analysis, have enabled the detection of H. pylori infection and antibiotic resistance.25,26,27,34 While NGS provides comprehensive information, applying NGS results to clinical practice requires additional knowledge about the correlation between NGS results and phenotypic resistance as well as treatment outcomes.141 In addition, cost-effectiveness and availability should be considered for the clinical application of NGS to predict antibiotic susceptibility profiles.
CILNICAL IMPLICATION AND FUTURE DIRECTIONS
The main clinical implication of antibiotic resistance in H. pylori is significantly compromised eradication therapy efficacy. It is well-known that the eradication success rate using clarithromycin-based regimens is markedly decreased in the presence of clarithromycin resistance.33,142,143 Accordingly, clarithromycin containing standard triple therapy is recommended only in areas with a clarithromycin resistance rate of less than 15%.11 On the other hand, a lower decrease in eradication rates has been observed for metronidazole, and successful eradication was reported using bismuth quadruple therapy, especially with higher metronidazole dose, even in the presence of metronidazole resistance.33,142,143 Multiple different mechanisms leading to metronidazole resistance and diversity of identified mutations may explain the relatively poor correlation between phenotypic resistance against metronidazole and treatment outcome. The increasing number of MDR H. pylori strains has made eradication therapy more challenging. Given that the first-line treatment regimen is usually selected empirically rather than based on antimicrobial susceptibility testing, failure of initial eradication therapy is another important cause of the emergence of MDR H. pylori strains.
The development of antibiotic resistance in H. pylori has reduced available treatment options. Eradication failure necessitates additional rounds of therapy, including alternative antibiotic combinations. With the decline in the eradication rates of standard therapies, physicians face challenges in selecting effective alternatives. However, these options are not always effective, involve higher pill burdens, and can lead to more side effects. There have been some important changes to expert recommendations for H. pylori eradication: more frequent use of bismuth-containing quadruple therapy as a first-line treatment instead of clarithromycin-based triple therapy is recommended.144 The limited arsenal of effective treatments against antibiotic-resistant H. pylori strains underscores the need for novel treatment strategies, including non-antibiotic approaches, to address this growing problem (Fig. 1).
Optimizing eradication therapy
Current treatment regimens for H. pylori eradication are derived from empirical approaches developed by gastroenterologists over the past few decades. A significant decrease in the eradication rate worldwide, along with an increasing trend of H. pylori antibiotic resistances warrants more specific approach to H. pylori eradication therapy, utilizing antimicrobial stewardship.145 Given that antibiotic resistance patterns differ according to geographic regions, it is recommended to use treatment regimens that are locally effective according to regional susceptibility patterns.87,145,146 However, many regions lack reliable data on the prevalence and characteristics of antibiotic resistance in local populations to guide the selection of empirical eradication therapy. Currently, bismuth quadruple therapy is recommended in regions with high antibiotic resistance to both clarithromycin and metronidazole.11 In addition, adding bismuth to some triple regimens and prolonging the treatment duration to 14 days can increase eradication rates up to 30% or more, even in the presence of antibiotic resistance in some strains.13 Recent meta-analysis also showed that bismuth supplements as the first-line regimen showed better eradication rate compared to non-bismuth containing regimens.147
Tailored therapy
With increasing resistance rates and MDR H. pylori strains, the role of antimicrobial susceptibility testing and subsequent individualized antibiotic treatment has been emphasized.11 Tailored therapy, in which antibiotics are chosen based on the antimicrobial susceptibility profile, is an ideal therapeutic option to improve the efficacy of H. pylori eradication therapy while minimizing unnecessary prescription of antibiotics. Studies have reported better eradication success rates using tailored therapy than empiric therapy, especially when antimicrobial susceptibility testing was performed before treatment.148,149,150,151 However, the benefit of tailored therapy has not been demonstrated in clinical trials comparing tailored therapy with empirical quadruple regimens as a first-line treatment or tailored therapy after previous treatment failure.151,152,153 This suggests that the benefit of tailored therapy is not obvious when the empirical regimen is highly effective for H. pylori eradication. In addition, the limited availability of H. pylori cultures and antimicrobial susceptibility testing has made this approach difficult. More evidence is required to establish the generalized use of tailored therapy for H. pylori eradication.
Acid suppression with potassium-competitive acid blocker
To increase eradication success, antibiotic resistance patterns and patient characteristics, such as compliance and cytochrome P450 2C19 genetic polymorphisms, should be considered. Potassium-competitive acid blocker provides fast and long-lasting acid suppression and is recently used for H. pylori eradication therapy in combination with various antibiotics.154,155,156,157 Vonoprazan-based triple therapy showed higher eradication rate compared to proton pump inhibitor (PPI), especially in the subpopulation with clarithromycin resistance, potentially overcoming clarithromycin resistance.158,159 Recent meta-analysis of randomized controlled trials also showed that vonoprazan-containing regimens achieved a higher eradication success rate compared to PPI-based regimens.160 In line with this, a Japanese guideline recommends vonoprazan-based triple therapy or PPI-based triple therapy as the first-line treatment for H. pylori eradication.161 Vonoprazan-based dual therapy consisting amoxicillin may be another treatment option while minimizing unnecessary antibiotic use; however, the dosage and duration of dual therapy need to be further determined.159,162,163 Notably, most studies on vonoprazan-based regimens were conducted in Japan, and further studies are needed to confirm these promising results in different regions.164,165
Adjuvant therapies
Adjuvant therapies aim to enhance the efficacy of antibiotic treatment either by overcoming the bacterial mechanisms of antibiotic resistance or by modifying the host response. These include the use of probiotics and anti-biofilm agents. As supplementary to conventional eradication therapy, probiotics can reduce gastrointestinal adverse events and thus improve patient compliance.166,167 In a recent meta-analysis, it has been found that most probiotics added to triple therapy provided better treatment outcomes.167 The potential mechanisms of probiotic action against H. pylori include direct and indirect inhibitory effects against H. pylori colonization, stabilization of gastric mucosal protective barrier and reduction of gastric mucosal inflammation, and modulation of host immune response to the infection.168,169 Probiotic supplementation also reduced antibiotic-induced alteration of gut microbiota and helped the restoration of dysbiosis caused by eradication therapy.170,171 However, further research is needed to better understand the role and mechanism of action of probiotics in H. pylori eradication therapy, as the species and strains, dose, and duration of investigated probiotics are heterogeneous.
An alternative therapeutic approach is to target and disrupt bacterial biofilms. Previous studies have shown that the use of N-acetylcysteine (NAC) reduces bacterial load and enhances eradication rate.172,173 The effect of NAC was also found in a clinical trial, showing better clearance of H. pylori in patients treated with NAC before antibiotic treatment.174 However, the exact mechanism underlying the reported therapeutic effect of NAC in the disruption of biofilms and overcoming H. pylori antibiotic resistance has yet to be defined. In a recent study, the combination of antibiotics and rhamnolipid, a glycolipid biosurfactant capable of disrupting biofilm and potentially inhibiting bacterial adhesion, was reported to effectively inhibit biofilm formation in vitro.175 Although the results are promising, the roles of rhamnolipid and anti-biofilm compounds need further investigation in vitro and in vivo.
Phage therapy
The rise in antibiotic resistance has increased interest in studying bacteriophages, particularly lytic bacteriophages. Phage therapy has various potential advantages over antibiotics because phages and their cleaved proteins are highly specific, affecting the target strain but not the microbiome.176 Furthermore, phages exclusively replicate at the site of infection, and no secondary effects have been reported.177 Few studies have investigated the presence of phages in H. pylori strains, including prophages and lytic phages.178,179,180,181 However, the exact function and properties of phages to be used therapeutically are yet to be determined. Although phage therapy appears to be a promising approach for future treatment options for H. pylori infection, further investigations are required to improve our understanding of phage and H. pylori interactions, which are still in the exploratory stage.
Vaccination
Developing an effective vaccine against H. pylori could be a game-changer in preventing infections and reducing the reliance on antibiotic treatment. Several vaccine candidates are currently under investigation, aiming to elicit a protective immune response against H. pylori; however, few have shown a protective effect.182,183,184,185,186,187,188,189,190,191,192,193,194 In a randomized, double-blind, placebo-controlled, phase 3 clinical trial, three doses of oral recombinant H pylori vaccine were introduced in children and were followed up for the next three years.194 The vaccine was effective against H. pylori in 71.8% of subjects without any serious adverse events. However, the authors suggested a longer follow-up period to confirm protective effects against H. pylori-associated diseases. The search for an effective vaccine is in the exploratory stage and needs further investigations, considering better design of vaccine strategies, optimal combination of antigens, selection of suitable adjuvants, and proper delivery carriers. Additionally, given that H. pylori infection usually occurs in early childhood, the optimal timing of vaccination and subsequent follow-up strategies to ensure the protective effects of vaccination should be further elucidated.
CONCLUSIONS
The increasing rates of H. pylori antibiotic resistance and MDR strains pose significant challenges for eradication therapy. Substantial progress has been made in understanding the fundamental mechanisms underlying antibiotic resistance in H. pylori, including genetic mutations, efflux pump systems, and biofilm formation. To overcome antibiotic resistance of H. pylori, antibiotic stewardship and tailored therapy based on antimicrobial susceptibility testing are recommended. Strategies such as adjuvant treatment, phage therapy, and vaccine development are currently being explored. In addition to optimizing currently available treatment options, continuous monitoring of local resistance profiles, ongoing research, and the development of innovative therapies are required to effectively manage antibiotic-resistant H. pylori infections and associated gastrointestinal diseases.
Footnotes
Funding: This research was supported by National Research Foundation (NRF) grant funded by the Korean government (MIST) (NRF-2021R1C1C1010631) and Hallym University Research Fund.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Gong EJ.
- Funding acquisition: Gong EJ.
- Investigation: Hasanuzzaman M, Bang CS.
- Methodology: Gong EJ.
- Project administration: Gong EJ.
- Supervision: Gong EJ.
- Writing - original draft: Hasanuzzaman M.
- Writing - review & editing: Bang CS, Gong EJ.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayali S, Manfredi M, Gaiani F, Bianchi L, Bizzarri B, Leandro G, et al. Helicobacter pylori, transmission routes and recurrence of infection: state of the art. Acta Biomed. 2018;89(8-S):72–76. doi: 10.23750/abm.v89i8-S.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokota S, Konno M, Fujiwara S, Toita N, Takahashi M, Yamamoto S, et al. Intrafamilial, preferentially mother-to-child and intraspousal, Helicobacter pylori infection in Japan determined by mutilocus sequence typing and random amplified polymorphic DNA fingerprinting. Helicobacter. 2015;20(5):334–342. doi: 10.1111/hel.12217. [DOI] [PubMed] [Google Scholar]
- 5.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 6.Hooi JK, Lai WY, Ng WK, Suen MM, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18(9):613–629. doi: 10.1038/s41575-021-00449-x. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Choi KD, Jung HY, Baik GH, Park JK, Kim SS, et al. Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter. 2018;23(2):e12463. doi: 10.1111/hel.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(6):553–564. doi: 10.1016/S2468-1253(23)00070-5. [DOI] [PubMed] [Google Scholar]
- 10.Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8(1):8. doi: 10.1186/s13099-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71(9):1724–1762. [Google Scholar]
- 12.Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver. 2021;15(2):168–195. doi: 10.5009/gnl20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65(5):870–878. doi: 10.1136/gutjnl-2015-311019. [DOI] [PubMed] [Google Scholar]
- 14.Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155(5):1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JW, Kim N, Choi SI, Jang JY, Song CH, Nam RH, et al. Prevalence and trends of multiple antimicrobial resistance of Helicobacter pylori in one tertiary hospital for 20 years in Korea. Helicobacter. 2023;28(1):e12939. doi: 10.1111/hel.12939. [DOI] [PubMed] [Google Scholar]
- 17.Ho JJ, Navarro M, Sawyer K, Elfanagely Y, Moss SF. Helicobacter pylori antibiotic resistance in the united states between 2011 and 2021: a systematic review and meta-analysis. Am J Gastroenterol. 2022;117(8):1221–1230. doi: 10.14309/ajg.0000000000001828. [DOI] [PubMed] [Google Scholar]
- 18.Kim SE, Park MI, Park SJ, Moon W, Choi YJ, Cheon JH, et al. Trends in Helicobacter pylori eradication rates by first-line triple therapy and related factors in eradication therapy. Korean J Intern Med. 2015;30(6):801–807. doi: 10.3904/kjim.2015.30.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong EJ, Yun SC, Jung HY, Lim H, Choi KS, Ahn JY, et al. Meta-analysis of first-line triple therapy for Helicobacter pylori eradication in Korea: is it time to change? J Korean Med Sci. 2014;29(5):704–713. doi: 10.3346/jkms.2014.29.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choe Y. Antibiotic resistance and Helicobacter pylori eradication therapy. Korean J Helicobacter Up Gastrointest Res. 2023;23(3):218–221. [Google Scholar]
- 21.Megraud F, Bruyndonckx R, Coenen S, Wittkop L, Huang TD, Hoebeke M, et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021;70(10):1815–1822. doi: 10.1136/gutjnl-2021-324032. [DOI] [PubMed] [Google Scholar]
- 22.Shin WG, Lee SW, Baik GH, Huh KC, Lee SI, Chung JW, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016;21(4):266–278. doi: 10.1111/hel.12279. [DOI] [PubMed] [Google Scholar]
- 23.Boltin D, Levi Z, Gingold-Belfer R, Gabay H, Shochat T, Niv Y, et al. Impact of previous exposure to macrolide antibiotics on Helicobacter pylori infection treatment outcomes. Am J Gastroenterol. 2019;114(6):900–906. doi: 10.14309/ajg.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 24.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 25.Gong EJ, Ahn JY, Kim JM, Lee SM, Na HK, Lee JH, et al. Genotypic and phenotypic resistance to clarithromycin in Helicobacter pylori strains. J Clin Med. 2020;9(6):1930. doi: 10.3390/jcm9061930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuan VP, Narith D, Tshibangu-Kabamba E, Dung HD, Viet PT, Sokomoth S, et al. A next-generation sequencing-based approach to identify genetic determinants of antibiotic resistance in Cambodian Helicobacter pylori clinical isolates. J Clin Med. 2019;8(6):858. doi: 10.3390/jcm8060858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauener FN, Imkamp F, Lehours P, Buissonnière A, Benejat L, Zbinden R, et al. Genetic determinants and prediction of antibiotic resistance phenotypes in Helicobacter pylori . J Clin Med. 2019;8(1):53. doi: 10.3390/jcm8010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53(10):1565–1574. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Liu ZQ, Zheng PY, Tang FA, Yang PC. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori . World J Gastroenterol. 2010;16(10):1279–1284. doi: 10.3748/wjg.v16.i10.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonezawa H, Osaki T, Kamiya S. Biofilm formation by Helicobacter pylori and its involvement for antibiotic resistance. BioMed Res Int. 2015;2015:914791. doi: 10.1155/2015/914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadkhodaei S, Siavoshi F, Akbari Noghabi K. Mucoid and coccoid Helicobacter pylori with fast growth and antibiotic resistance. Helicobacter. 2020;25(2):e12678. doi: 10.1111/hel.12678. [DOI] [PubMed] [Google Scholar]
- 33.Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000;45(1):68–76. doi: 10.1023/a:1005457226341. [DOI] [PubMed] [Google Scholar]
- 34.Binh TT, Shiota S, Suzuki R, Matsuda M, Trang TT, Kwon DH, et al. Discovery of novel mutations for clarithromycin resistance in Helicobacter pylori by using next-generation sequencing. J Antimicrob Chemother. 2014;69(7):1796–1803. doi: 10.1093/jac/dku050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tshibangu-Kabamba E, Ngoma-Kisoko PJ, Tuan VP, Matsumoto T, Akada J, Kido Y, et al. Next-generation sequencing of the whole bacterial genome for tracking molecular insight into the broad-spectrum antimicrobial resistance of Helicobacter pylori clinical isolates from the Democratic Republic of Congo. Microorganisms. 2020;8(6):8. doi: 10.3390/microorganisms8060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domanovich-Asor T, Motro Y, Khalfin B, Craddock HA, Peretz A, Moran-Gilad J. Genomic analysis of antimicrobial resistance genotype-to-phenotype agreement in Helicobacter pylori . Microorganisms. 2020;9(1):2. doi: 10.3390/microorganisms9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura M, Spiller RC, Barrett DA, Wibawa JI, Kumagai N, Tsuchimoto K, et al. Gastric juice, gastric tissue and blood antibiotic concentrations following omeprazole, amoxicillin and clarithromycin triple therapy. Helicobacter. 2003;8(4):294–299. doi: 10.1046/j.1523-5378.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 38.Zullo A. The current role of dual therapy for treatment of Helicobacter pylori: back to the future? Eur J Gastroenterol Hepatol. 2020;32(5):555–556. doi: 10.1097/MEG.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 39.Gerrits MM, van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6(11):699–709. doi: 10.1016/S1473-3099(06)70627-2. [DOI] [PubMed] [Google Scholar]
- 40.Wilke MS, Lovering AL, Strynadka NC. Beta-lactam antibiotic resistance: a current structural perspective. Curr Opin Microbiol. 2005;8(5):525–533. doi: 10.1016/j.mib.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Kuo YT, Liou JM, El-Omar EM, Wu JY, Leow AH, Goh KL, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- 42.Gong EJ, Ahn JY. Antimicrobial resistance of Helicobacter pylori isolates in Korea. Korean J Helicobacter Up Gastrointest Res. 2018;18(2):82–88. [Google Scholar]
- 43.Cosme A, Torrente Iranzo S, Montes Ros M, Fernández-Reyes Silvestre M, Alonso Galán H, Lizasoain J, et al. Helicobacter pylori antimicrobial resistance during a 5-year period (2013-2017) in northern Spain and its relationship with the eradication therapies. Helicobacter. 2019;24(1):e12557. doi: 10.1111/hel.12557. [DOI] [PubMed] [Google Scholar]
- 44.Bujanda L, Nyssen OP, Vaira D, Saracino IM, Fiorini G, Lerang F, et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013-2020: results of the European Registry on H. pylori Management (Hp-EuReg) Antibiotics (Basel) 2021;10(9):1058. doi: 10.3390/antibiotics10091058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerrits MM, Godoy AP, Kuipers EJ, Ribeiro ML, Stoof J, Mendonça S, et al. Multiple mutations in or adjacent to the conserved penicillin-binding protein motifs of the penicillin-binding protein 1A confer amoxicillin resistance to Helicobacter pylori . Helicobacter. 2006;11(3):181–187. doi: 10.1111/j.1523-5378.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 46.Kwon YH, Kim JY, Kim N, Park JH, Nam RH, Lee SM, et al. Specific mutations of penicillin-binding protein 1A in 77 clinically acquired amoxicillin-resistant Helicobacter pylori strains in comparison with 77 amoxicillin-susceptible strains. Helicobacter. 2017;22(6):e12437. doi: 10.1111/hel.12437. [DOI] [PubMed] [Google Scholar]
- 47.Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006;28(1):6–13. doi: 10.1016/j.ijantimicag.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Kim BJ, Kim JG. Substitutions in penicillin-binding protein 1 in amoxicillin-resistant Helicobacter pylori strains isolated from Korean patients. Gut Liver. 2013;7(6):655–660. doi: 10.5009/gnl.2013.7.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rimbara E, Noguchi N, Kawai T, Sasatsu M. Mutations in penicillin-binding proteins 1, 2 and 3 are responsible for amoxicillin resistance in Helicobacter pylori . J Antimicrob Chemother. 2008;61(5):995–998. doi: 10.1093/jac/dkn051. [DOI] [PubMed] [Google Scholar]
- 50.Tseng YS, Wu DC, Chang CY, Kuo CH, Yang YC, Jan CM, et al. Amoxicillin resistance with beta-lactamase production in Helicobacter pylori . Eur J Clin Invest. 2009;39(9):807–812. doi: 10.1111/j.1365-2362.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 51.Co EM, Schiller NL. Resistance mechanisms in an in vitro-selected amoxicillin-resistant strain of Helicobacter pylori . Antimicrob Agents Chemother. 2006;50(12):4174–4176. doi: 10.1128/AAC.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qureshi NN, Gallaher B, Schiller NL. Evolution of amoxicillin resistance of Helicobacter pylori in vitro: characterization of resistance mechanisms. Microb Drug Resist. 2014;20(6):509–516. doi: 10.1089/mdr.2014.0019. [DOI] [PubMed] [Google Scholar]
- 53.Park SY, Lee EH, Kim D, Song YG, Jeong SJ. Novel mutations conferring amoxicillin resistance in Helicobacter pylori in South Korea. Antibiotics (Basel) 2023;12(4):748. doi: 10.3390/antibiotics12040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodvold KA. Clinical pharmacokinetics of clarithromycin. Clin Pharmacokinet. 1999;37(5):385–398. doi: 10.2165/00003088-199937050-00003. [DOI] [PubMed] [Google Scholar]
- 55.Erah PO, Goddard AF, Barrett DA, Shaw PN, Spiller RC. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother. 1997;39(1):5–12. doi: 10.1093/jac/39.1.5. [DOI] [PubMed] [Google Scholar]
- 56.Versalovic J, Shortridge D, Kibler K, Griffy MV, Beyer J, Flamm RK, et al. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori . Antimicrob Agents Chemother. 1996;40(2):477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41(12):2621–2628. doi: 10.1128/aac.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyu T, Cheung KS, Deng Z, Ni L, Chen C, Wu J, et al. Whole genome sequencing reveals novel genetic mutations of Helicobacter pylori associating with resistance to clarithromycin and levofloxacin. Helicobacter. 2023;28(4):e12972. doi: 10.1111/hel.12972. [DOI] [PubMed] [Google Scholar]
- 59.Hirata K, Suzuki H, Nishizawa T, Tsugawa H, Muraoka H, Saito Y, et al. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori . J Gastroenterol Hepatol. 2010;25(Suppl 1):S75–S79. doi: 10.1111/j.1440-1746.2009.06220.x. [DOI] [PubMed] [Google Scholar]
- 60.Choi JH, Yang YJ, Bang CS, Lee JJ, Baik GH. Current status of the third-line Helicobacter pylori eradication. Gastroenterol Res Pract. 2018;2018:6523653. doi: 10.1155/2018/6523653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liou JM, Lee YC, Wu MS Taiwan Gastrointestinal Disease and Helicobacter Consortium. Treatment of refractory Helicobacter pylori infection-tailored or empirical therapy. Gut Liver. 2022;16(1):8–18. doi: 10.5009/gnl20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22(8):438–445. doi: 10.1016/j.tim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli . Antimicrob Agents Chemother. 1990;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida H, Bogaki M, Nakamura M, Yamanaka LM, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli . Antimicrob Agents Chemother. 1991;35(8):1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JW, Kim N, Nam RH, Park JH, Kim JM, Jung HC, et al. Mutations of Helicobacter pylori associated with fluoroquinolone resistance in Korea. Helicobacter. 2011;16(4):301–310. doi: 10.1111/j.1523-5378.2011.00840.x. [DOI] [PubMed] [Google Scholar]
- 66.Rimbara E, Noguchi N, Kawai T, Sasatsu M. Fluoroquinolone resistance in Helicobacter pylori: role of mutations at position 87 and 91 of GyrA on the level of resistance and identification of a resistance conferring mutation in GyrB. Helicobacter. 2012;17(1):36–42. doi: 10.1111/j.1523-5378.2011.00912.x. [DOI] [PubMed] [Google Scholar]
- 67.Miyachi H, Miki I, Aoyama N, Shirasaka D, Matsumoto Y, Toyoda M, et al. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter. 2006;11(4):243–249. doi: 10.1111/j.1523-5378.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 68.Debets-Ossenkopp YJ, Namavar F, MacLaren DM. Effect of an acidic environment on the susceptibility of Helicobacter pylori to trospectomycin and other antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1995;14(4):353–355. doi: 10.1007/BF02116532. [DOI] [PubMed] [Google Scholar]
- 69.Dingsdag SA, Hunter N. Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms. J Antimicrob Chemother. 2018;73(2):265–279. doi: 10.1093/jac/dkx351. [DOI] [PubMed] [Google Scholar]
- 70.Martínez-Júlvez M, Rojas AL, Olekhnovich I, Espinosa Angarica V, Hoffman PS, Sancho J. Structure of RdxA--an oxygen-insensitive nitroreductase essential for metronidazole activation in Helicobacter pylori. FEBS J. 2012;279(23):4306–4317. doi: 10.1111/febs.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong Y, Zhai K, Sun L, He L, Wang H, Guo Y, et al. RdxA diversity and mutations associated with metronidazole resistance of Helicobacter pylori . Microbiol Spectr. 2023;11(2):e0390322. doi: 10.1128/spectrum.03903-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SM, Kim N, Kwon YH, Nam RH, Kim JM, Park JY, et al. rdxA, frxA, and efflux pump in metronidazole-resistant Helicobacter pylori: their relation to clinical outcomes. J Gastroenterol Hepatol. 2018;33(3):681–688. doi: 10.1111/jgh.13906. [DOI] [PubMed] [Google Scholar]
- 73.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten SJ, Berg DE, Hoffman PS. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28(2):383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 74.Jeong JY, Mukhopadhyay AK, Dailidiene D, Wang Y, Velapatiño B, Gilman RH, et al. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori . J Bacteriol. 2000;182(18):5082–5090. doi: 10.1128/jb.182.18.5082-5090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binh TT, Suzuki R, Trang TT, Kwon DH, Yamaoka Y. Search for novel candidate mutations for metronidazole resistance in Helicobacter pylori using next-generation sequencing. Antimicrob Agents Chemother. 2015;59(4):2343–2348. doi: 10.1128/AAC.04852-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Albert TJ, Dailidiene D, Dailide G, Norton JE, Kalia A, Richmond TA, et al. Mutation discovery in bacterial genomes: metronidazole resistance in Helicobacter pylori . Nat Methods. 2005;2(12):951–953. doi: 10.1038/nmeth805. [DOI] [PubMed] [Google Scholar]
- 77.Kwon DH, Kato M, El-Zaatari FA, Osato MS, Graham DY. Frame-shift mutations in NAD(P)H flavin oxidoreductase encoding gene (frxA) from metronidazole resistant Helicobacter pylori ATCC43504 and its involvement in metronidazole resistance. FEMS Microbiol Lett. 2000;188(2):197–202. doi: 10.1111/j.1574-6968.2000.tb09193.x. [DOI] [PubMed] [Google Scholar]
- 78.Kim SY, Joo YM, Lee HS, Chung IS, Yoo YJ, Merrell DS, et al. Genetic analysis of Helicobacter pylori clinical isolates suggests resistance to metronidazole can occur without the loss of functional rdxA. J Antibiot (Tokyo) 2009;62(1):43–50. doi: 10.1038/ja.2008.6. [DOI] [PubMed] [Google Scholar]
- 79.Zhang S, Wang X, Wise MJ, He Y, Chen H, Liu A, et al. Mutations of Helicobacter pylori RdxA are mainly related to the phylogenetic origin of the strain and not to metronidazole resistance. J Antimicrob Chemother. 2020;75(11):3152–3155. doi: 10.1093/jac/dkaa302. [DOI] [PubMed] [Google Scholar]
- 80.An B, Moon BS, Lim HC, Lee YC, Kim H, Lee G, et al. Analysis of gene mutations associated with antibiotic resistance in Helicobacter pylori strains isolated from Korean patients. Korean J Helicobacter Up Gastrointest Res. 2014;14(2):95–102. [Google Scholar]
- 81.Lee SY, Lee YC, Pyo JH, Kwon DH, Rhee JC, Kim JJ. Quasispecies of antibacterial heteroresistant Helicobacter pylori . Korean J Helicobacter Up Gastrointest Res. 2004;4(1):7–14. [Google Scholar]
- 82.Kwon DH, El-Zaatari FA, Kato M, Osato MS, Reddy R, Yamaoka Y, et al. Analysis of rdxA and involvement of additional genes encoding NAD(P)H flavin oxidoreductase (FrxA) and ferredoxin-like protein (FdxB) in metronidazole resistance of Helicobacter pylori . Antimicrob Agents Chemother. 2000;44(8):2133–2142. doi: 10.1128/aac.44.8.2133-2142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsugawa H, Suzuki H, Satoh K, Hirata K, Matsuzaki J, Saito Y, et al. Two amino acids mutation of ferric uptake regulator determines Helicobacter pylori resistance to metronidazole. Antioxid Redox Signal. 2011;14(1):15–23. doi: 10.1089/ars.2010.3146. [DOI] [PubMed] [Google Scholar]
- 84.Attaran B, Falsafi T, Ghorbanmehr N. Effect of biofilm formation by clinical isolates of Helicobacter pylori on the efflux-mediated resistance to commonly used antibiotics. World J Gastroenterol. 2017;23(7):1163–1170. doi: 10.3748/wjg.v23.i7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115(5):1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 86.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JH, Ahn JY, Choi KD, Jung HY, Kim JM, Baik GH, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter. 2019;24(4):e12592. doi: 10.1111/hel.12592. [DOI] [PubMed] [Google Scholar]
- 88.Dailidiene D, Bertoli MT, Miciuleviciene J, Mukhopadhyay AK, Dailide G, Pascasio MA, et al. Emergence of tetracycline resistance in Helicobacter pylori: multiple mutational changes in 16S ribosomal DNA and other genetic loci. Antimicrob Agents Chemother. 2002;46(12):3940–3946. doi: 10.1128/AAC.46.12.3940-3946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gerrits MM, de Zoete MR, Arents NL, Kuipers EJ, Kusters JG. 16S rRNA mutation-mediated tetracycline resistance in Helicobacter pylori . Antimicrob Agents Chemother. 2002;46(9):2996–3000. doi: 10.1128/AAC.46.9.2996-3000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trieber CA, Taylor DE. Mutations in the 16S rRNA genes of Helicobacter pylori mediate resistance to tetracycline. J Bacteriol. 2002;184(8):2131–2140. doi: 10.1128/JB.184.8.2131-2140.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu JY, Kim JJ, Reddy R, Wang WM, Graham DY, Kwon DH. Tetracycline-resistant clinical Helicobacter pylori isolates with and without mutations in 16S rRNA-encoding genes. Antimicrob Agents Chemother. 2005;49(2):578–583. doi: 10.1128/AAC.49.2.578-583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rothstein DM. Rifamycins, alone and in combination. Cold Spring Harb Perspect Med. 2016;6(7):a027011. doi: 10.1101/cshperspect.a027011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crabol Y, Catherinot E, Veziris N, Jullien V, Lortholary O. Rifabutin: where do we stand in 2016? J Antimicrob Chemother. 2016;71(7):1759–1771. doi: 10.1093/jac/dkw024. [DOI] [PubMed] [Google Scholar]
- 94.Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35(2):209–221. doi: 10.1111/j.1365-2036.2011.04937.x. [DOI] [PubMed] [Google Scholar]
- 95.Hays C, Burucoa C, Lehours P, Tran CT, Leleu A, Raymond J. Molecular characterization of Helicobacter pylori resistance to rifamycins. Helicobacter. 2018;23(1):e12451. doi: 10.1111/hel.12451. [DOI] [PubMed] [Google Scholar]
- 96.Choi YI, Jeong SH, Chung JW, Park DK, Kim KO, Kwon KA, et al. Rifabutin and furazolidone could be the candidates of the rescue regimen for antibiotic-resistant H. pylori in Korea. Can J Infect Dis Med Microbiol. 2019;2019:9351801. doi: 10.1155/2019/9351801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heep M, Beck D, Bayerdörffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori . Antimicrob Agents Chemother. 1999;43(6):1497–1499. doi: 10.1128/aac.43.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishizawa T, Suzuki H, Matsuzaki J, Muraoka H, Tsugawa H, Hirata K, et al. Helicobacter pylori resistance to rifabutin in the last 7 years. Antimicrob Agents Chemother. 2011;55(11):5374–5375. doi: 10.1128/AAC.05437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heep M, Lehn N, Brandstätter B, Rieger U, Senzenberger S, Wehrl W. Detection of rifabutin resistance and association of rpoB mutations with resistance to four rifamycin derivatives in Helicobacter pylori . Eur J Clin Microbiol Infect Dis. 2002;21(2):143–145. doi: 10.1007/s10096-001-0672-2. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki S, Suzuki H, Nishizawa T, Kaneko F, Ootani S, Muraoka H, et al. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori . Digestion. 2009;79(1):1–4. doi: 10.1159/000191204. [DOI] [PubMed] [Google Scholar]
- 101.Mori H, Suzuki H, Matsuzaki J, Tsugawa H, Fukuhara S, Miyoshi S, et al. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: a pilot study. United European Gastroenterol J. 2016;4(3):380–387. doi: 10.1177/2050640615618043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsugawa H, Suzuki H, Muraoka H, Ikeda F, Hirata K, Matsuzaki J, et al. Enhanced bacterial efflux system is the first step to the development of metronidazole resistance in Helicobacter pylori . Biochem Biophys Res Commun. 2011;404(2):656–660. doi: 10.1016/j.bbrc.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 103.Ge X, Cai Y, Chen Z, Gao S, Geng X, Li Y, et al. Bifunctional enzyme SpoT Is involved in biofilm formation of Helicobacter pylori with multidrug resistance by upregulating efflux pump Hp1174 (gluP) Antimicrob Agents Chemother. 2018;62(11):e00957-18. doi: 10.1128/AAC.00957-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu ZQ, Zheng PY, Yang PC. Efflux pump gene hefA of Helicobacter pylori plays an important role in multidrug resistance. World J Gastroenterol. 2008;14(33):5217–5222. doi: 10.3748/wjg.14.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai Y, Wang C, Chen Z, Xu Z, Li H, Li W, et al. Transporters HP0939, HP0497, and HP0471 participate in intrinsic multidrug resistance and biofilm formation in Helicobacter pylori by enhancing drug efflux. Helicobacter. 2020;25(4):e12715. doi: 10.1111/hel.12715. [DOI] [PubMed] [Google Scholar]
- 106.Singh S, Singh SK, Chowdhury I, Singh R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J. 2017;11(1):53–62. doi: 10.2174/1874285801711010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 108.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15(12):740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yonezawa H, Osaki T, Kurata S, Zaman C, Hanawa T, Kamiya S. Assessment of in vitro biofilm formation by Helicobacter pylori . J Gastroenterol Hepatol. 2010;25(Suppl 1):S90–S94. doi: 10.1111/j.1440-1746.2009.06213.x. [DOI] [PubMed] [Google Scholar]
- 110.Carron MA, Tran VR, Sugawa C, Coticchia JM. Identification of Helicobacter pylori biofilms in human gastric mucosa. J Gastrointest Surg. 2006;10(5):712–717. doi: 10.1016/j.gassur.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 111.Cole SP, Harwood J, Lee R, She R, Guiney DG. Characterization of monospecies biofilm formation by Helicobacter pylori . J Bacteriol. 2004;186(10):3124–3132. doi: 10.1128/JB.186.10.3124-3132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong EH, Ng CG, Chua EG, Tay AC, Peters F, Marshall BJ, et al. Comparative genomics revealed multiple Helicobacter pylori genes associated with biofilm formation in vitro. PLoS One. 2016;11(11):e0166835. doi: 10.1371/journal.pone.0166835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bode G, Mauch F, Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect. 1993;111(3):483–490. doi: 10.1017/s0950268800057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kusters JG, Gerrits MM, Van Strijp JA, Vandenbroucke-Grauls CM. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65(9):3672–3679. doi: 10.1128/iai.65.9.3672-3679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Halfawy OM, Valvano MA. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev. 2015;28(1):191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kocsmár É, Kocsmár I, Buzás GM, Szirtes I, Wacha J, Takáts A, et al. Helicobacter pylori heteroresistance to clarithromycin in adults-new data by in situ detection and improved concept. Helicobacter. 2020;25(1):e12670. doi: 10.1111/hel.12670. [DOI] [PubMed] [Google Scholar]
- 117.Kim JJ, Kim JG, Kwon DH. Mixed-infection of antibiotic susceptible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter. 2003;8(3):202–206. doi: 10.1046/j.1523-5378.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 118.Lee SY, Kim JJ, Kwon DH. Synchronous infection of heteroresistant Helicobacter pylori . Korean J Helicobacter Res Pract. 2003;3(2):110–116. [Google Scholar]
- 119.Kao CY, Lee AY, Huang AH, Song PY, Yang YJ, Sheu SM, et al. Heteroresistance of Helicobacter pylori from the same patient prior to antibiotic treatment. Infect Genet Evol. 2014;23:196–202. doi: 10.1016/j.meegid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 120.Arévalo-Jaimes BV, Rojas-Rengifo DF, Jaramillo CA, de Molano BM, Vera-Chamorro JF, Del Pilar Delgado M. Genotypic determination of resistance and heteroresistance to clarithromycin in Helicobacter pylori isolates from antrum and corpus of Colombian symptomatic patients. BMC Infect Dis. 2019;19(1):546. doi: 10.1186/s12879-019-4178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van der Ende A, van Doorn LJ, Rooijakkers S, Feller M, Tytgat GN, Dankert J. Clarithromycin-susceptible and -resistant Helicobacter pylori isolates with identical randomly amplified polymorphic DNA-PCR genotypes cultured from single gastric biopsy specimens prior to antibiotic therapy. J Clin Microbiol. 2001;39(7):2648–2651. doi: 10.1128/JCM.39.7.2648-2651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keikha M, Karbalaei M. Prevalence of antibiotic heteroresistance associated with Helicobacter pylori infection: a systematic review and meta-analysis. Microb Pathog. 2022;170:105720. doi: 10.1016/j.micpath.2022.105720. [DOI] [PubMed] [Google Scholar]
- 123.Li H, Shen Y, Song X, Tang X, Hu R, Marshall BJ, et al. Need for standardization and harmonization of Helicobacter pylori antimicrobial susceptibility testing. Helicobacter. 2022;27(2):e12873. doi: 10.1111/hel.12873. [DOI] [PubMed] [Google Scholar]
- 124.Kim YJ, Chung WC. Eradication therapy for Helicobacter pylori with diagnostic test for clarithromycin resistance. Korean J Helicobacter Up Gastrointest Res. 2019;19(4):225–230. [Google Scholar]
- 125.Woo HY, Park DI, Park H, Kim MK, Kim DH, Kim IS, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 2009;14(1):22–28. doi: 10.1111/j.1523-5378.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 126.Redondo JJ, Keller PM, Zbinden R, Wagner K. A novel RT-PCR for the detection of Helicobacter pylori and identification of clarithromycin resistance mediated by mutations in the 23S rRNA gene. Diagn Microbiol Infect Dis. 2018;90(1):1–6. doi: 10.1016/j.diagmicrobio.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 127.Binmaeil H, Hanafiah A, Mohamed Rose I, Raja Ali RA. Development and validation of multiplex quantitative PCR assay for detection of Helicobacter pylori and mutations conferring resistance to clarithromycin and levofloxacin in gastric biopsy. Infect Drug Resist. 2021;14:4129–4145. doi: 10.2147/IDR.S325056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lehours P, Siffré E, Mégraud F. DPO multiplex PCR as an alternative to culture and susceptibility testing to detect Helicobacter pylori and its resistance to clarithromycin. BMC Gastroenterol. 2011;11(1):112. doi: 10.1186/1471-230X-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nahm JH, Kim WK, Kwon Y, Kim H. Detection of Helicobacter pylori with clarithromycin resistance-associated mutations using peptide nucleic acid probe-based melting point analysis. Helicobacter. 2019;24(5):e12634. doi: 10.1111/hel.12634. [DOI] [PubMed] [Google Scholar]
- 130.Cambau E, Allerheiligen V, Coulon C, Corbel C, Lascols C, Deforges L, et al. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori . J Clin Microbiol. 2009;47(11):3600–3607. doi: 10.1128/JCM.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee JW, Kim N, Nam RH, Park JH, Choi YJ, Kim JM, et al. GenoType HelicoDR test in the determination of antimicrobial resistance of Helicobacter pylori in Korea. Scand J Gastroenterol. 2014;49(9):1058–1067. doi: 10.3109/00365521.2014.894117. [DOI] [PubMed] [Google Scholar]
- 132.Schabereiter-Gurtner C, Hirschl AM, Dragosics B, Hufnagl P, Puz S, Kovách Z, et al. Novel real-time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing of stool and biopsy specimens. J Clin Microbiol. 2004;42(10):4512–4518. doi: 10.1128/JCM.42.10.4512-4518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jehanne Q, Bénéjat L, Mégraud F, Bessède E, Lehours P. Evaluation of the Allplex™ H pylori and ClariR PCR Assay for Helicobacter pylori detection on gastric biopsies. Helicobacter. 2020;25(4):e12702. doi: 10.1111/hel.12702. [DOI] [PubMed] [Google Scholar]
- 134.Kim I, Maeng LS, Kim JS, Kim BW, Cheung DY, Kim JI, et al. Quantitative multiplex real-time polymerase chain reaction assay for the detection of Helicobacter pylori and clarithromycin resistance. BMC Microbiol. 2023;23(1):155. doi: 10.1186/s12866-023-02868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van den Poel B, Gils S, Micalessi I, Carton S, Christiaens P, Cuyle PJ, et al. Molecular detection of Helicobacter pylori and clarithromycin resistance in gastric biopsies: a prospective evaluation of RIDA®GENE Helicobacter pylori assay. Acta Clin Belg. 2021;76(3):177–183. doi: 10.1080/17843286.2019.1685741. [DOI] [PubMed] [Google Scholar]
- 136.Park CG, Kim S, Jeon HS, Han S. Validation of loop-mediated isothermal amplification to detect Helicobacter pylori and 23S rRNA mutations: a prospective, observational clinical cohort study. J Clin Lab Anal. 2021;35(1):e23563. doi: 10.1002/jcla.23563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yari F, Abiri R, Aryan E, Ahmadi Jouybari T, Navabi J, Alvandi A. Loop-mediated isothermal amplification as a fast noninvasive method of Helicobacter pylori diagnosis. J Clin Lab Anal. 2016;30(5):464–470. doi: 10.1002/jcla.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kakiuchi T, Okuda M, Matsuo M, Fujimoto K. Smart Gene™ as an effective non-invasive point-of-care test to detect Helicobacter pylori clarithromycin-resistant mutation. J Gastroenterol Hepatol. 2022;37(9):1719–1725. doi: 10.1111/jgh.15887. [DOI] [PubMed] [Google Scholar]
- 139.Liu M, Zhu P, Zhang L, Gong Y, Wang C, Sun L, et al. Single-cell identification, drug susceptibility test, and whole-genome sequencing of Helicobacter pylori directly from gastric biopsy by clinical antimicrobial susceptibility test Ramanometry. Clin Chem. 2022;68(8):1064–1074. doi: 10.1093/clinchem/hvac082. [DOI] [PubMed] [Google Scholar]
- 140.Saruuljavkhlan B, Yamaoka Y. Benefits of a molecular-based method for the detection of clarithromycin-resistant Helicobacter pylori . Gut Liver. 2021;15(4):487–489. doi: 10.5009/gnl210278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hulten KG, Genta RM, Kalfus IN, Zhou Y, Zhang H, Graham DY. Comparison of culture with antibiogram to next-generation sequencing using bacterial isolates and formalin-fixed, paraffin-embedded gastric biopsies. Gastroenterology. 2021;161(5):1433–1442.e2. doi: 10.1053/j.gastro.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148(4):719–731.e3. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zou Y, Qian X, Liu X, Song Y, Song C, Wu S, et al. The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: a systematic review and meta-analysis. Helicobacter. 2020;25(4):e12714. doi: 10.1111/hel.12714. [DOI] [PubMed] [Google Scholar]
- 144.Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019;157(1):44–53. doi: 10.1053/j.gastro.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 145.Shiotani A, Roy P, Lu H, Graham DY. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Therap Adv Gastroenterol. 2021;14:17562848211064080. doi: 10.1177/17562848211064080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim N, Kim JM, Kim CH, Park YS, Lee DH, Kim JS, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006;40(8):683–687. doi: 10.1097/00004836-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 147.Ko SW, Kim YJ, Chung WC, Lee SJ. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: systemic review and meta-analysis. Helicobacter. 2019;24(2):e12565. doi: 10.1111/hel.12565. [DOI] [PubMed] [Google Scholar]
- 148.Wenzhen Y, Yumin L, Quanlin G, Kehu Y, Lei J, Donghai W, et al. Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med. 2010;49(12):1103–1109. doi: 10.2169/internalmedicine.49.3031. [DOI] [PubMed] [Google Scholar]
- 149.Bae JH, Jo HH, Kwon JG, Kim EY. Efficacy of 7-day tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance. Korean J Gastroenterol. 2023;82(1):10–17. doi: 10.4166/kjg.2023.039. [DOI] [PubMed] [Google Scholar]
- 150.Cho SH, Park MS, Park SY, Kim DH, You HS, Kim HS. Effectiveness of 7-day triple therapy with half-dose clarithromycin for the eradication of Helicobacter pylori without the A2143G and A2142G point mutations of the 23S rRNA gene in a high clarithromycin resistance area. Front Med (Lausanne) 2023;10:1150396. doi: 10.3389/fmed.2023.1150396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nyssen OP, Espada M, Gisbert JP. Empirical vs. susceptibility-guided treatment of Helicobacter pylori infection: a systematic review and meta-analysis. Front Microbiol. 2022;13:913436. doi: 10.3389/fmicb.2022.913436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.López-Góngora S, Puig I, Calvet X, Villoria A, Baylina M, Muñoz N, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70(9):2447–2455. doi: 10.1093/jac/dkv155. [DOI] [PubMed] [Google Scholar]
- 153.Gingold-Belfer R, Niv Y, Schmilovitz-Weiss H, Levi Z, Boltin D. Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36(10):2649–2658. doi: 10.1111/jgh.15575. [DOI] [PubMed] [Google Scholar]
- 154.Du RC, Ouyang YB, Lu NH, Hu Y. Research trends on vonoprazan-based therapy for Helicobacter pylori eradication: a bibliometric analysis from 2015 to 2023. Helicobacter. 2023;28(5):e13012. doi: 10.1111/hel.13012. [DOI] [PubMed] [Google Scholar]
- 155.Sugimoto M, Yamaoka Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Front Pharmacol. 2019;9:1560. doi: 10.3389/fphar.2018.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee JW, Kim N, Nam RH, Yu JE, Son JH, Lee SM, et al. Efficacy of tegoprazan for improving the susceptibility of antimicrobial agents against antibiotic-resistant Helicobacter pylori . Gut Liver. 2021;15(1):53–60. doi: 10.5009/gnl20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yeom DH, Kim YS. History and pharmacological mechanism of gastric acid-suppressive drugs. Korean J Helicobacter Up Gastrointest Res. 2023;23(3):159–166. [Google Scholar]
- 158.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65(9):1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: randomized clinical trial. Gastroenterology. 2022;163(3):608–619. doi: 10.1053/j.gastro.2022.05.055. [DOI] [PubMed] [Google Scholar]
- 160.Yang C, Li S, Huang T, Lin H, Jiang Z, He Y, et al. Effectiveness and safety of vonoprazan-based regimen for Helicobacter pylori eradication: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2022;47(7):897–904. doi: 10.1111/jcpt.13637. [DOI] [PubMed] [Google Scholar]
- 161.Kato M, Ota H, Okuda M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24(4):e12597. doi: 10.1111/hel.12597. [DOI] [PubMed] [Google Scholar]
- 162.Ouyang Y, Wang M, Xu YL, Zhu Y, Lu NH, Hu Y. Amoxicillin-vonoprazan dual therapy for Helicobacter pylori eradication: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(9):1666–1672. doi: 10.1111/jgh.15917. [DOI] [PubMed] [Google Scholar]
- 163.Suzuki S, Kusano C, Horii T, Ichijima R, Ikehara H. The ideal Helicobacter pylori treatment for the present and the future. Digestion. 2022;103(1):62–68. doi: 10.1159/000519413. [DOI] [PubMed] [Google Scholar]
- 164.Graham DY. Why the vonoprazan Helicobacter pylori therapies in the US-European trial produced unacceptable cure rates. Dig Dis Sci. 2023;68(5):1691–1697. doi: 10.1007/s10620-023-07886-5. [DOI] [PubMed] [Google Scholar]
- 165.Sue S, Maeda S. Is a potassium-competitive acid blocker truly superior to proton pump inhibitors in terms of Helicobacter pylori eradication? Gut Liver. 2021;15(6):799–810. doi: 10.5009/gnl20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mohtasham M, Joukar F, Maroufizadeh S, Mojtahedi K, Asgharnezhad M, Mansour-Ghanaei F. Lactobacillus ruteri compared with placebo as an adjuvant in quadruple therapy for Helicobacter pylori eradication: a randomized, double-blind, controlled trial. Arab J Gastroenterol. 2023;24(1):40–44. doi: 10.1016/j.ajg.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 167.Wang Y, Wang X, Cao XY, Zhu HL, Miao L. Comparative effectiveness of different probiotics supplements for triple Helicobacter pylori eradication: a network meta-analysis. Front Cell Infect Microbiol. 2023;13:1120789. doi: 10.3389/fcimb.2023.1120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hsieh PS, Tsai YC, Chen YC, Teh SF, Ou CM, King VA. Eradication of Helicobacter pylori infection by the probiotic strains Lactobacillus johnsonii MH-68 and L. salivarius ssp. salicinius AP-32. Helicobacter. 2012;17(6):466–477. doi: 10.1111/j.1523-5378.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- 169.Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther. 2006;23(8):1077–1086. doi: 10.1111/j.1365-2036.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- 170.Oh B, Kim BS, Kim JW, Kim JS, Koh SJ, Kim BG, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter. 2016;21(3):165–174. doi: 10.1111/hel.12270. [DOI] [PubMed] [Google Scholar]
- 171.Yuan Z, Xiao S, Li S, Suo B, Wang Y, Meng L, et al. The impact of Helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter. 2021;26(6):e12848. doi: 10.1111/hel.12848. [DOI] [PubMed] [Google Scholar]
- 172.Huynh HQ, Couper RT, Tran CD, Moore L, Kelso R, Butler RN. N-acetylcysteine, a novel treatment for Helicobacter pylori infection. Dig Dis Sci. 2004;49(11-12):1853–1861. doi: 10.1007/s10620-004-9583-2. [DOI] [PubMed] [Google Scholar]
- 173.Gurbuz AK, Ozel AM, Ozturk R, Yildirim S, Yazgan Y, Demirturk L. Effect of N-acetyl cysteine on Helicobacter pylori . South Med J. 2005;98(11):1095–1097. doi: 10.1097/01.smj.0000182486.39913.da. [DOI] [PubMed] [Google Scholar]
- 174.Cammarota G, Branca G, Ardito F, Sanguinetti M, Ianiro G, Cianci R, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clin Gastroenterol Hepatol. 2010;8(9):817–820.e3. doi: 10.1016/j.cgh.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 175.Chen X, Li P, Shen Y, Zou Y, Yuan G, Hu H. Rhamnolipid-involved antibiotics combinations improve the eradication of Helicobacter pylori biofilm in vitro: a comparison with conventional triple therapy. Microb Pathog. 2019;131:112–119. doi: 10.1016/j.micpath.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 176.Brüssow H. Infection therapy: the problem of drug resistance - and possible solutions. Microb Biotechnol. 2017;10(5):1041–1046. doi: 10.1111/1751-7915.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1(2):111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vale FF, Nunes A, Oleastro M, Gomes JP, Sampaio DA, Rocha R, et al. Genomic structure and insertion sites of Helicobacter pylori prophages from various geographical origins. Sci Rep. 2017;7(1):42471. doi: 10.1038/srep42471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lehours P, Vale FF, Bjursell MK, Melefors O, Advani R, Glavas S, et al. Genome sequencing reveals a phage in Helicobacter pylori . mBio. 2011;2(6):e00239-11. doi: 10.1128/mBio.00239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Abdel-Haliem ME, Askora A. Isolation and characterization of bacteriophages of Helicobacter pylori isolated from Egypt. Future Virol. 2013;8(8):821–826. [Google Scholar]
- 181.Cuomo P, Papaianni M, Fulgione A, Guerra F, Capparelli R, Medaglia C. An innovative approach to control H. pylori-induced persistent inflammation and colonization. Microorganisms. 2020;8(8):1214. doi: 10.3390/microorganisms8081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, et al. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116(4):804–812. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 183.Kreiss C, Buclin T, Cosma M, Corthésy-Theulaz I, Michetti P. Safety of oral immunisation with recombinant urease in patients with Helicobacter pylori infection. Lancet. 1996;347(9015):1630–1631. doi: 10.1016/s0140-6736(96)91119-8. [DOI] [PubMed] [Google Scholar]
- 184.DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine. 1999;18(5-6):449–459. doi: 10.1016/s0264-410x(99)00246-7. [DOI] [PubMed] [Google Scholar]
- 185.Angelakopoulos H, Hohmann EL. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun. 2000;68(4):2135–2141. doi: 10.1128/iai.68.4.2135-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69(6):3581–3590. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bumann D, Metzger WG, Mansouri E, Palme O, Wendland M, Hurwitz R, et al. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2001;20(5-6):845–852. doi: 10.1016/s0264-410x(01)00391-7. [DOI] [PubMed] [Google Scholar]
- 188.Sougioultzis S, Lee CK, Alsahli M, Banerjee S, Cadoz M, Schrader R, et al. Safety and efficacy of E. coli enterotoxin adjuvant for urease-based rectal immunization against Helicobacter pylori . Vaccine. 2002;21(3-4):194–201. doi: 10.1016/s0264-410x(02)00467-x. [DOI] [PubMed] [Google Scholar]
- 189.Banerjee S, Medina-Fatimi A, Nichols R, Tendler D, Michetti M, Simon J, et al. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut. 2002;51(5):634–640. doi: 10.1136/gut.51.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Metzger WG, Mansouri E, Kronawitter M, Diescher S, Soerensen M, Hurwitz R, et al. Impact of vector-priming on the immunogenicity of a live recombinant Salmonella enterica serovar typhi Ty21a vaccine expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2004;22(17-18):2273–2277. doi: 10.1016/j.vaccine.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 191.Aebischer T, Bumann D, Epple HJ, Metzger W, Schneider T, Cherepnev G, et al. Correlation of T cell response and bacterial clearance in human volunteers challenged with Helicobacter pylori revealed by randomised controlled vaccination with Ty21a-based Salmonella vaccines. Gut. 2008;57(8):1065–1072. doi: 10.1136/gut.2007.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Malfertheiner P, Selgrad M, Wex T, Romi B, Borgogni E, Spensieri F, et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: a randomised, placebo-controlled phase 1/2 study. Lancet Gastroenterol Hepatol. 2018;3(10):698–707. doi: 10.1016/S2468-1253(18)30125-0. [DOI] [PubMed] [Google Scholar]
- 193.Malfertheiner P, Schultze V, Rosenkranz B, Kaufmann SH, Ulrichs T, Novicki D, et al. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008;135(3):787–795. doi: 10.1053/j.gastro.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 194.Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(10002):1457–1464. doi: 10.1016/S0140-6736(15)60310-5. [DOI] [PubMed] [Google Scholar]