Abstract
Lipid droplet formation is a defining histological feature in clear-cell renal cell carcinoma (ccRCC) but the underlying mechanisms and importance of this biological behaviour have remained enigmatic. De novo fatty acid (FA) synthesis, uptake and suppression of FA oxidation have all been shown to contribute to lipid storage, which is a necessary tumour adaptation rather than a bystander effect. Clinical studies and mechanistic investigations into the roles of different enzymes in FA metabolism pathways have revealed new metabolic vulnerabilities that hold promise for clinical effect. Several metabolic alterations are associated with worse clinical outcomes in patients with ccRCC, as lipogenic genes drive tumorigenesis. Enzymes involved in the intrinsic FA metabolism pathway include FA synthase, acetyl-CoA carboxylase, ATP citrate lyase, stearoyl-CoA desaturase 1, cluster of differentiation 36, carnitine palmitoyltransferase 1A and the perilipin family, and each might be potential therapeutic targets in ccRCC owing to the link between lipid deposition and ccRCC risk. Adipokines and lipid species are potential biomarkers for diagnosis and treatment monitoring in patients with ccRCC. FA metabolism could potentially be targeted for therapeutic intervention in ccRCC as small-molecule inhibitors targeting the pathway have shown promising results in preclinical models.
Renal cell carcinoma (RCC) has been described as a metabolic disease as metabolic alterations underlie otherwise disparate cancer aetiologies1. Dysregulations in oxygen, nutrients, iron and energy metabolism are common in RCC1, suggesting that understanding tumour metabolism could be a key to the development of novel therapeutic strategies. For, example, ccRCC (which is the most common subtype of RCC, and accounts for ~80% of renal malignancies2) has a distinct histological phenotype characterized by cytoplasmic lipid deposits that underlie the pathological nomenclature (FIG. 1). The lipid droplets contain different lipid species (fatty acids (FAs), triglycerides and cholesterol esters) and a non-lipid component, glycogen3. Efforts to understand the mechanisms that drive lipid storage in ccRCC have repeatedly revealed a tumour dependency that could potentially be exploited.
Fig. 1 |. H&E staining of ccrCC and normal adjacent kidney tissues.
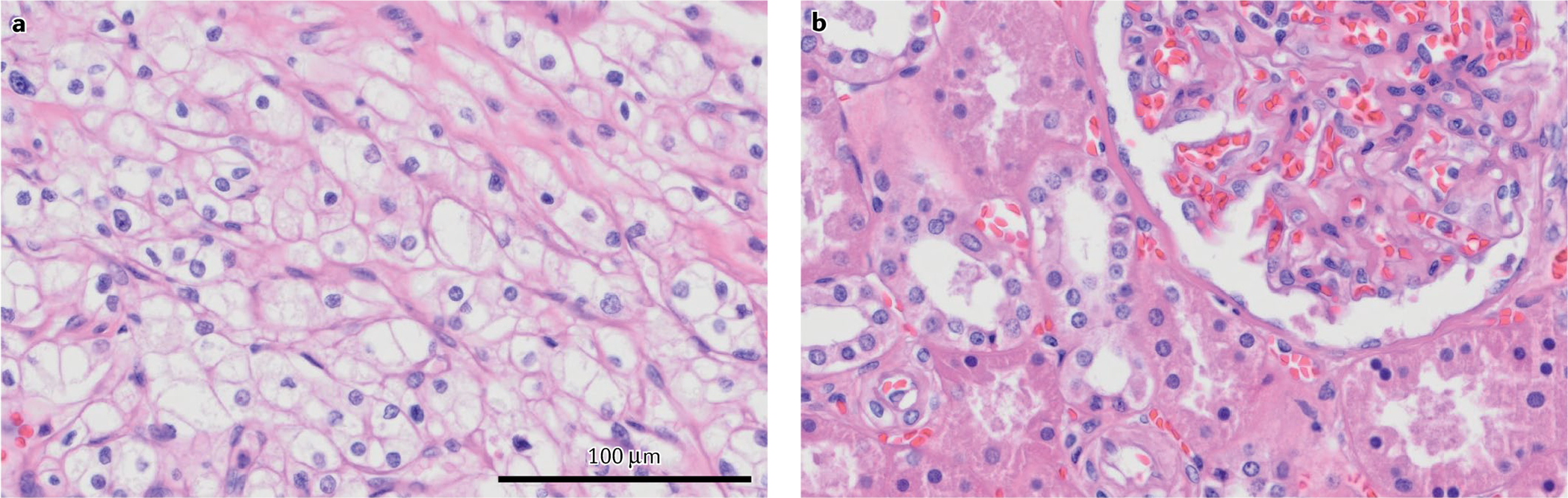
a | The ‘clear’ cytoplasm seen in clear-cell renal cell carcinoma (ccRCC) tissue sections compared with b | non-malignant adjacent tissue shows the washout of lipid and glycogen content during histological preparation, which also gives the name to this disease.
Genetically, ccRCC commonly displays inactivation of the von Hippel–Lindau (VHL) tumour suppressor through a near universal loss of chromosome 3p, together with either mutation or promoter hypermethylation of the remaining VHL allele, showing an important initiating mutation at the convergence of both familial and sporadic tumours1. VHL is a component of an E3 ubiquitin ligase complex that regulates the stability of the hypoxia-inducible factors (HIFs)4. In normoxia, prolyl hydroxylase (PHD) enzymes hydroxylate two key proline residues in the oxygen-dependent degradation domains of the HIFs subunits HIF1α and HIF2α, enabling recognition by the VHL complex for subsequent ubiquitin-dependent degradation. Under oxygen deprivation, or after loss of VHL, the HIFα proteins are stabilized and translocate to the nucleus, where they partner with a constitutive HIF1β to form heterodimeric transcription factors5 (FIG. 2). HIF proteins regulate numerous pathways, including cell survival, energy production, and angiogenesis, which are often exploited by cancer cells6. Metabolic adaptations in ccRCC are a result of HIF-mediated increases in the production of GLUT1 (SLC2A1), hexokinase 2 (HK2), lactate dehydrogenase A (LDHA) and pyruvate dehydrogenase kinase 1 (PDK1), which increase glycolysis and suppress pyruvate entry into the tricarboxylic acid (TCA) cycle7 (FIG. 2). To maintain crucial TCA cycle intermediates, ccRCC cells use an anaplerotic process via elevated uptake of glutamine that is converted to glutamate by the HIF-dependent expression of glutaminase 1 (GLS1)8,9. Glutamate is then converted into α-ketoglutarate, which, among other outcomes, leads to the increased citrate that fuels lipid synthesis. Concurrently, HIF-driven suppression of carnitine palmitoyltransferase 1 A (CPT1A) prevents FA oxidation and results in accumulation of cytoplasmic lipids10 (FIG. 2).
Fig. 2 |. VHl and HIF pathway regulation in ccrCC.
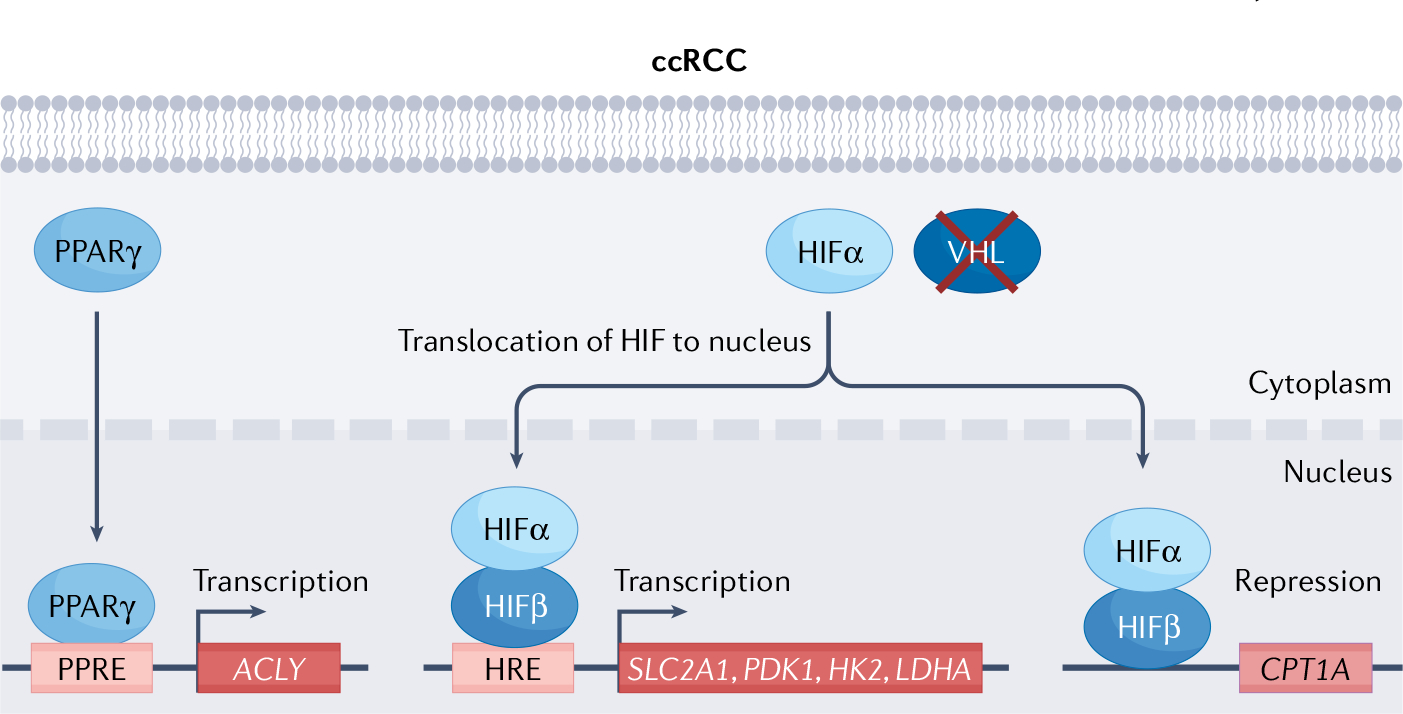
In clear-cell renal cell carcinoma (ccRCC), von Hippel–Lindau (VHL) inactivation leads to hypoxia-inducible factor (HIF)1α and HIF2α stabilization irrespective of oxygenation status (although HIF1α protein lost in roughly 30% of ccRCC, and HIF2α has been argued to be the driving oncogenic subunit211–213). Stabilized HIFα translocates to the nucleus, dimerizes with HIFβ and functions as a transcription factor through binding to conserved hypoxia response elements (HREs) present in hypoxia-responsive genes, such as SLC2A1, PDK1, HK2 and LDHA, HIFα translocation and dimerization with HIFβ also results in repression of CPT1A transcription. VHL inactivation also results in stabilization of PPARγ, which translocases to nucleus to bind to PPRE, activating transcription of lipogenic gene ACLY. CPT1A, carnitine palmitoyltransferase 1A; SLC2A1, glucose transporter 1; HK2, hexokinase 2; LDHA, lactate dehydrogenase A; PDK1, pyruvate dehydrogenase kinase 1; PPARγ, peroxisome proliferator-activated receptor-γ; PPRE, PPAR response elements.
The lipid droplet formation process is crucial in ccRCC tumorigenesis. Storage of excess FAs is necessary for maintaining endoplasmic reticulum (ER) integrity11, and suppressing lipid reactive oxygen species to prevent lipotoxicity12. Elevated lipid storage levels might also benefit renal tumours, as elevated phosphatidylcholine can maintain cell membrane fluidity, which enhances metastatic capability13, as has been found in other malignancies such as lung carcinoma and glioma14,15. By contrast, the coincident accumulation of glycogen granules in ccRCC, which is also HIF dependent, is currently thought to be dispensable for tumorigenesis suggesting that the lipid component is more important than the other non-lipid component16. Altered FA metabolism has also been reported in multiple non-renal malignancies, including prostate, breast and lung17–19, and results of several studies have enabled identification of potential therapeutic targets20,21, with a select few potential therapeutic targets progressing to clinical trials22. For example, in breast cancer, a crosstalk between fatty acid synthase (FASN) and the oestrogen receptor exists whereby FASN inhibition suppresses the tumorigenic effects of oestrogen23. In prostate cancer, FASN expression is regulated by androgens, but becomes androgen independent in castration-resistant disease20. Thus, as therapeutic strategies for ccRCC continue to evolve, investigating untapped tumour-dependent mechanisms will open up new opportunities for clinical success.
In this Perspective, we discuss the enzymes involved in the intrinsic FA metabolism pathway and the current advances in the development of therapeutic strategies against these targets. We also highlight the potential use of circulating adipokines and lipid species as serum biomarkers for ccRCC diagnosis and treatment monitoring.
Targets in the fatty acid pathway in ccRCC
FAs are important constituents of cell membranes, energy sources, and signalling molecules; they can either be imported from exogenous supply or synthesized de novo from cytosolic citrate or acetate9,24–27. ccRCC cells increase both the import and the synthesis of FAs to facilitate metabolic plasticity28, unlike kidney proximal tubule cells, which have been shown to rely more heavily on lipid uptake29–33. ccRCC cells make use of the metabolic advantage of not relying on a single pathway to sustain their rapid proliferation rate, similar to the Warburg elevation of glycolysis even in the presence of abundant oxygen34. However, notably, not all lipid species follow the same paradigm of lipid production in cancer: for example, biosynthesis of cholesterol has been shown to be suppressed in ccRCC tumour cells, causing full reliance on cholesterol uptake35. These seemingly contradicting lipid metabolism pathways demonstrate the complexity of how cancer cells use lipids for tumorigenesis and the need to fully understand them in order to improve development of new treatment strategies. Expression of specialized transporters (such as cluster of differentiation 36 (CD36)) are upregulated to maximize FA uptake across the plasma membrane, and de novo lipogenesis enzymes (such as FASN) are overexpressed in cancer cells, including ccRCC36. In addition to synthesis and uptake, the FA pathway includes modification, storage and degradation37. Stearoyl-CoA desaturase 1 (SCD1) expression is upregulated in ccRCC, resulting in increased endogenous FA desaturation, which is required for proliferation when exogenous sources of monounsaturated FAs (MUFAs) are limited38,39. Many FA enzyme alterations occur in ccRCC40, and some correlate with clinical outcomes (TABLE 1). For example, upregulation of FASN36 and SCD1 (REF.39) are associated with worse clinical outcomes in patients with ccRCC.
Table 1 |.
Fatty acid pathway enzymes implicated in renal cell carcinoma-related alterations and their prognostic values
| Fatty acid pathway enzyme | Mechanism of action | Clinical significance | Small-molecule targeting agents | Refs. |
|---|---|---|---|---|
| FASN | Condenses seven malonyl-CoA and one acetyl-CoA to produce palmitate | Positive correlation with T stage (pT3–4), grade, lymph node involvement, distant metastasis and microvascular invasion Reduced overall survival Negative correlation with cancer-specific survival |
CD97 Orlistat Triclosan G28UCM GSK837149A |
35,49,76 |
| ACC | Carboxylates acetyl-CoA to produce malonyl-CoA | Reduced overall survival | Metformin TOFA |
49 |
| ACLY | Produces acetyl-CoA from citrate | Positive correlation with T stage and Fuhrman grade Improved overall survival |
None | 48,49 |
| SCD1 | Desaturates palmitate to form monounsaturated fatty acids | Reduced overall survival | A939572 | 38 |
| CD36 | Transport fatty acids across membrane | Positive correlation with TNM stage and visceral adiposity Reduced progression-free survival and overall survival |
None | 117 |
| CPT1A | Transports long-chain fatty acids across mitochondrial membrane for fatty acid oxidation | Improved overall survival | None | 10,49 |
| PLIN3 | Lipid droplet protein | Positive correlation with TNM stage and tumour grade Reduced disease-free survival and overall survival |
None | 145 |
| PLIN2 | Lipid droplet protein | Negative correlation with prognosis | None | 144 |
ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; CD36, cluster of differentiation 36; CPT1A, carnitine palmitoyltransferase 1 A; FASN, fatty acid synthase; PLIN, perilipin.
Key intrinsic fatty acid metabolism enzymes such as ATP citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), FASN, SCD1, CD36, carnitine palmitoyltransferase 1 A, and perilipin (PLIN) proteins have proposed roles in ccRCC and provide potential clinical targets (FIG. 3). HIF2α inhibitors, such as belzutifan, have the potential to impair the wide array of HIF-regulated metabolic genes, but an approach that directly targets FA metabolism enzymes might offer improved specificity. Improved understanding of the pathway enzymes can, therefore, be expected to accelerate the development of additional treatment strategies.
Fig. 3 |. Fatty acid metabolism pathways in clear-cell renal cell carcinoma and potential therapeutic targets.
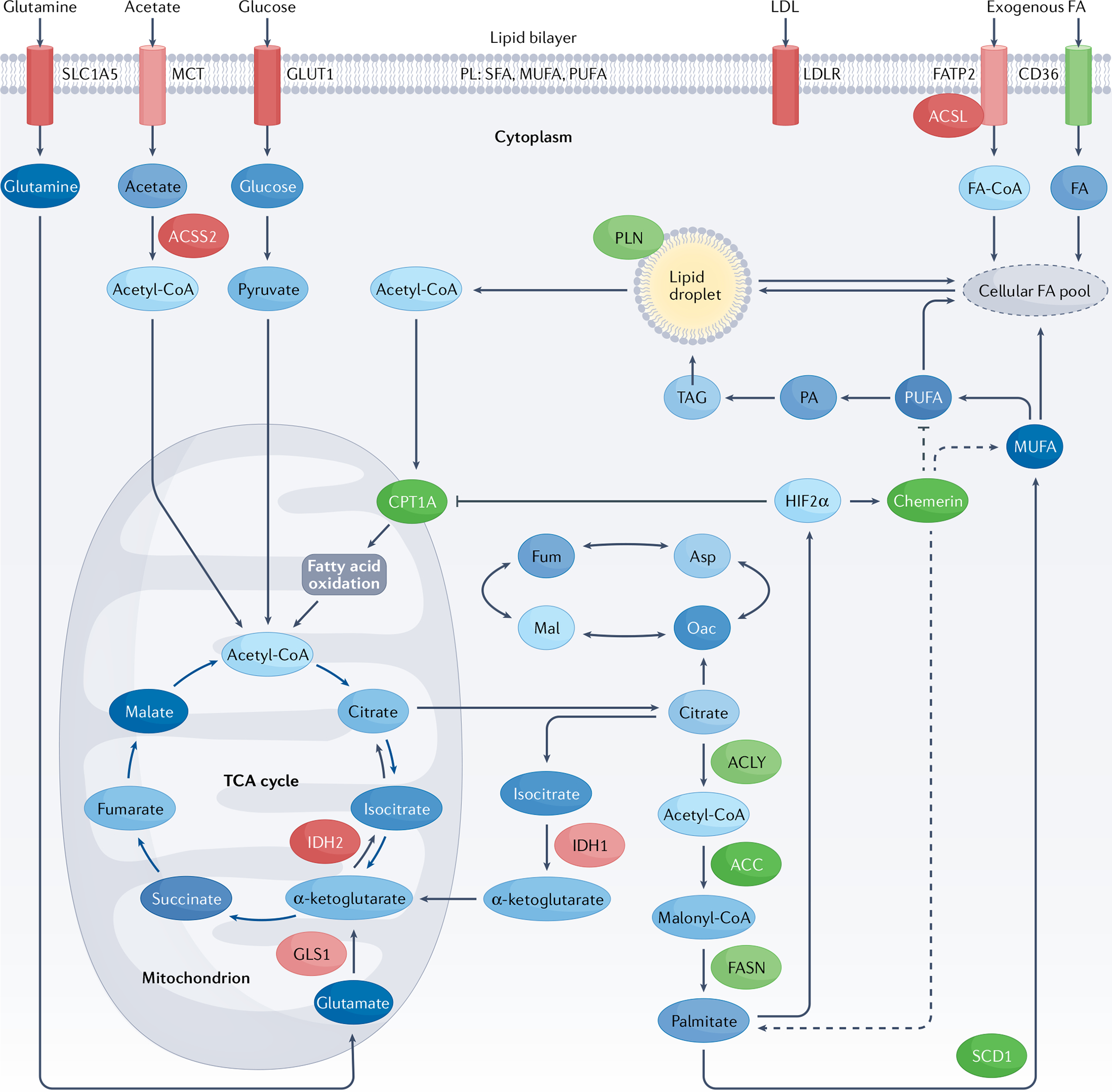
Fatty acids (FAs) are the products of de novo lipogenesis, are carboxylic acid molecules with an unbranched aliphatic chain, and can be either saturated or unsaturated29. In de novo lipogenesis, cells synthesize citrate from glucose, acetate and glutamine. Palmitate (16:0 FA) is produced via a multistep process: through the action of ATP-citrate lyase (ACLY), citrate is cleaved into oxaloacetate and acetyl-coenzyme A (acetyl-CoA). In the cytosol, acetyl-CoA is irreversibly carboxylated to form malonyl-CoA via acetyl-CoA carboxylase (ACC), which is the rate-limiting step. FA synthase (FASN) later elongates the malonyl-CoA into long-chain FAs. Palmitate can then be further elongated by FA elongases (such as ELOVL6) or desaturated by stearoyl-CoA desaturase (SCD). Exogeneous FAs can be transported into cells by CD36 located on the plasma membrane. Both exogenously and endogenously sourced FAs can be stored as triacylglycerol (TAG) in lipid droplets. FAs can alternatively be transported into mitochondria via carnitine palmitoyltransferase 1 A (CPT1A) for mitochondrial β-oxidation, providing an energy source through the production of ATP. The highly relevant targets that are described in this Review are highlighted in green. ACC, acetyl-CoA carboxylase; ACLY, ATP-citrate lyase; ACSL, long-chain acyl-CoA synthetase; ACSS2, acyl Co-A synthethase-2; Asp, aspartate; FASN, FA synthase; FATP2, fatty acid transport protein 2; Fum, fumarate; GLS, glutaminase; GLUT1, glucose transporter 1; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; Mal, malate; MCT, monocarboxylate transporter; MUFA, monounsaturated FA; Oac, oxaloacetate; PA, phosphatidic acid; PL, phospholipid; PUFA, polyunsaturated FA; SFA, saturated FA; TCA, tricyclic acid cycle.
ATP citrate lyase
ACLY forms a physiological link between carbohydrate metabolism and FA biogenesis by converting the TCA intermediate citrate into acetyl-CoA41,42. ccRCC cells divert glucose into the lactic acid pool through the HIF-dependent activation of PDK1 and, therefore, rely on the reductive carboxylation of glutamine-derived α-ketoglutarate for citrate production9 (FIG. 3). ACLY then acts on the citrate product to generate acetyl-CoA to provide a substrate for the subsequent commitment step in FA synthesis43. VHL deficiency increases ACLY through a HIF-independent mechanism, because the PPARγ transcription factor that controls ACLY expression is another VHL degradation target44 (FIG. 2). The PI3K–AKT pathway, which is hyperactive in ccRCC45, also contributes to FA synthesis by phosphorylating and activating ACLY41,46. ACLY controls acetyl-CoA synthesis; thus, dysregulated epigenetics, including histone acetylation patterns that are characteristic of ccRCC could be caused by ACLY activity47. For example, in pancreatic ductal adenocarcinoma, ACLY modulates cellular acetyl-CoA abundance, which in turn regulates histone acetylation marks (AcH4 and H3K27Ac) in genetic loci that promote tumour progression48.
Little is known about the clinical significance of ACLY alterations in ccRCC. ACLY is expressed at higher levels in ccRCC than surrounding non-malignant kidney tissue, based on the results of a small institutional cohort of 33 paired tumour and normal adjacent tissue samples49. Tumour mRNA and protein levels of ACLY were higher than in adjacent non-malignant tissues, and correlated positively with T stage and Fuhrman grade49. However, increased ACLY expression was associated with favourable outcomes such as overall survival (OS) in patients with ccRCC in an Oncomine query, (HR 0.43, 95% CI: 0.29–0.62)50. These seemingly contradictory findings suggest that the role of ACLY in ccRCC needs additional study. The biproduct of ACLY, oxaloacetic acid, was speculated to indirectly inhibit FA synthesis and might provide an explanation for the contradiction51. Importantly, when ACLY was inhibited in vitro, ccRCC cells showed decreased proliferation and increased apoptosis, demonstrating its potential to be used as a therapeutic target49. Two ACLY inhibitors, ETC-1002 and hydroxycitrate, have been studied in clinical trials to reduce low-density lipoprotein-cholesterol in patients with cardiovascular disease and type 2 diabetes, respectively, and have demonstrated favourable toxic effect profiles52,53. In both in vitro and in vivo studies using human hepatoma cells and miniature pigs with atherosclerosis, respectively, the use of these ACLY inhibitors upregulated the expression and activity of hepatic LDL receptor, which ultimately reduced the plasma LDL-cholesterol level and, therefore, could potentially reduce the lipid level in cancer cells54,55. This observation suggests that existing ACLY inhibitors could be repurposed for ccRCC treatment if results of further preclinical studies provide the justification.
Acetyl-CoA carboxylase
ACC is a rate-limiting enzyme in FA synthesis56 ACC carboxylates acetyl-CoA to form malonyl-CoA56,57 (FIG. 3). Results of a gene expression analysis study showed that ACC mRNA is upregulated in ccRCC compared with non-malignant kidney tissue, and the overexpression of ACC was associated with poor OS in patients with ccRCC (HR 1.43, 95% CI 1.03–1.99, P = 0.033)50. In a large multi-omic study of over 400 primary ccRCC tumours, increased ACC and decreased AMP-activated kinase (AMPK) were found to be the top protein correlates of worse survival58. AMPK, thought to be a master regulator of cellular energy balance59, directly inhibits ACC and, therefore, the altered ACC:AMPK balance promotes FA synthesis in ccRCC60.
An ACC-targeted inhibitor as well as indirect inhibition via AMPK activation have been investigated in preclinical studies. 5-tetradecyloxy-2-furoic acid (TOFA), an allosteric ACC inhibitor, reduces cell growth in both ccRCC and papillary RCC cell lines by inhibiting the mTOR pathway and causing cell-cycle arrest in the G2/M phase61. To indirectly target ACC, metformin, an oral diabetes medication that activates AMPK29 and reduces lipid accumulation in a kidney fibrosis model62, has been used. Metformin was found to similarly inhibit mTOR, induce cell-cycle arrest, inhibit proliferation and cause apoptosis in multiple ccRCC cell lines in several studies63–66. Lipid accumulation might be ACC dependent, but whether the effects of metformin rest solely on the inhibition of ACC is unclear. Metformin also inhibits mitochondrial complex I67, which could increase FA oxidation and explain the observed lipid reduction. The interest in the role of metformin in cancer is high, in part owing to the wealth of retrospective data from patients with diabetes. Studies examining the effect of metformin on ccRCC outcomes have been unfortunately limited by selection bias and difficulty controlling important confounding factors68. Results of a meta-analysis of 8 retrospective studies demonstrated that metformin improved OS (HR 0.643, 95% CI 0.520–0.795, P < 0.001) and cancer-specific survival (CSS) (HR 0.618, 95% CI 0.446–0.858) in patients with RCC and diabetes, but the conclusions are limited by a high level of heterogeneity amongst the studies69. Additionally, benefits were more uniformly observed in patients with localized disease than in those with metastatic disease for unknown reasons. Thus, the role of metformin in ccRCC treatment remains to be studied, as the observations justify a prospective study in which proper biological correlates such as ACC phosphorylation could be performed.
Fatty acid synthase
FASN is the final enzyme in de novo FA synthesis, and its expression is altered in ccRCC in addition to a wide range of malignancies70,71. FASN condenses seven malonyl-CoA and one acetyl-CoA to produce palmitate72–74 (FIG. 3). With multifaceted roles in the anabolic FA pathway and oncogenic metabolism (such as suppression of the oestrogen receptor in breast cancer when FASN is inhibited75), FASN has justifiably received the most widespread interest in cancer therapeutics. A role for FASN in RCC was first explored in 2008 (REF.76): in a cohort of 120 patients with RCC (93% of whom had ccRCC) who underwent nephrectomy, immunohistochemistry (IHC) staining for FASN showed that positive expression correlated positively with tumour stage (P = 0.0009), lymph node involvement (P = 0.0429) and distant metastasis (P = 0.0042)76. Increased FASN expression was associated with reduced CSS (HR 3.736, 95% CI 1.087–12.837, P = 0.036)76. In a cohort of 126 patients from The Cancer Genome Atlas (TCGA) with localized or metastatic ccRCC, FASN upregulation was associated with increased cancer-specific mortality (P < 0.001)77. Results of a study including a cohort from the International Metastatic Renal Cell Carcinoma Database (IMDC) showed that patients with FASN-positive disease on IHC staining had significantly shorter OS than those with FASN-negative disease (14.5 months versus 27.5 months; HR 1.71, 95% CI 1.17–2.51, P = 0.005)78. Thus, increased FASN expression is prevalent in ccRCC and has considerable prognostic implications.
The oncogenic functions of FASN have been studied in numerous malignancies79,80 FASN is involved in producing phospholipids that participate in detergent-resistant membrane microdomains, which have been implicated in promoting signal transduction of the tyrosine kinase receptors EGFR and HER2 (REF.81). This implication is important as tyrosine kinase inhibitors are a mainstay of RCC treatment. Results of another study suggested that FASN confers a metabolic advantage to otherwise nutritionally deprived colorectal cancer cells82. However, overall, the mechanism by which FASN contributes to cancer remains ambiguous. Nonetheless, FASN is a potential therapeutic target and several pharmacological agents against this enzyme are being investigated. The most well-studied small molecule inhibitor is C75. In multiple ccRCC cell lines, C75 reduced cell viability and growth via cell-cycle arrest, reduced cell migration and increased apoptosis83. In human breast cancer xenograft models in mice, C75 decreased tumour volumes compared with control animals after 28 days84. Several mechanisms for the effects were proposed, such as reduced mitogenic signalling of membrane receptors83. Another posited mechanism from the breast cancer studies is that FASN inhibition causes malonyl-CoA accumulation, which has been shown to be cytotoxic to cancer cells;20,84 this mechanism could also be important in renal cancer. Other molecules, including orlistat, triclosan, G28UCM and GSK837149A, have been tested and found to be effective in inhibiting the proliferation of ovarian and prostate cancer cells85,86 and, therefore, might also be useful in ccRCC. Clinically, old-generation FASN inhibitors have not been successful owing primarily to unselective cytotoxicity and unpleasant off-target effects, including weight loss and anorexia, because the inhibitors act centrally in the hypothalamus87. However, a new generation of inhibitors with increased specificity holds the potential to realize clinical efficacy; for example, the FASN inhibitor, TVB-2640, has been tested in a phase I trial in patients with advanced solid tumours and displayed a favourable toxic effect profile22.
Stearoyl-CoA desaturase 1
SCD1 is highly expressed in multiple tumour types88–91 and appears to participate in the biology of ccRCC as well39. SCD1 produces MUFAs from saturated FAs (SFAs, such as palmitate) to be incorporated into glycerophospholipids and sphingolipids for cell membranes, or serve as signalling molecules92 (FIG. 3). SCD1 is expressed in ccRCC tumour specimens across all stages but is not detected in patient-matched benign tissue39. In 359 patients with ccRCC who underwent primary nephrectomy, SCD1 expression by IHC was significantly higher in malignant than in adjacent non-malignant tissue in 61.6% of the samples (P < 0.001). Increased SCD1 expression was also an independent prognostic indicator of reduced OS (HR 2.94, 95% CI 1.44–6.03, P = 0.003)93.
The complex balance between the levels of SFAs and MUFAs, and the roles of FAs in cell metabolism underlie the importance of SCD1 in kidney cells. Chronic kidney disease secondary to obesity is associated with lipid metabolism dysfunction, in which excess SFAs induce ER stress, inflammation and apoptosis94. A particularly susceptible cell is the proximal tubular epithelial cell, the presumed cell of origin for ccRCC95, owing to its high mitochondrial content. SCD1 has been shown to protect proximal tubule cells from lipotoxicity-mediated apoptosis96 and, therefore, might have the same role in ccRCC cells. Additionally, unsaturated FAs might have a role in promoting cancer cell stemness, as shown in ovarian cancer cells97, which is broadly associated with therapeutic resistance that is often seen in RCC98.
Multiple SCD1 inhibitors exist in the preclinical setting in RCC91,99. Inhibiting SCD1 with either a short-hairpin RNA or the small molecule A939572 in ccRCC cell lines decreased ccRCC proliferation and induced apoptosis39. Adding back the MUFA oleic acid, the principal product of SCD1, rescued the proliferation defect, confirming that MUFA levels, or the balance of unsaturated FAs and SFA, affect tumour cell behaviour39. In addition, high levels of unsaturated FAs might alter responses to anti-angiogenic agents by forming lipid mediators such as cyclooxygenase-derived prostaglandins100 and lipoxygenase-derived 4-hydroxy-docosahexaenoic acid101, which regulate inflammation and angiogenesis102. A combination of A939572 and the mTOR inhibitor temsirolimus used in ccRCC treatment displayed synergistic effects in reducing ccRCC growth in vitro and in vivo39. Furthermore, SCD1 and HIF2α synergistically enhance ccRCC growth, suggesting that the combination of SCD1 and HIF2α inhibitors might enhance effectiveness over HIF2α inhibition alone103. Overall, the mechanistic relationship between FA regulation and synergistic antitumour growth remains to be elucidated, but early studies suggest that combinatorial drug approaches including the targeting of FA metabolism might have value. Owing to the role of SCD1 in sebum production, many SCD1 inhibitors are associated with considerable adverse effects in animal models99. However, two SCD1 inhibitors have been tested in vivo with good tolerance: SSI-4 reduced ccRCC cell proliferation in vitro by inducing ER stress and showed minimal toxic effects in vivo104; T-3764518 inhibited xenograft renal cancer growth in mice without severe adverse effects105. Currently, SCD1 inhibitors with acceptable toxic effects are in clinical trials for type 2 diabetes and non-alcoholic steatohepatitis106. Aramchol inhibits SCD1 in hepatic stellate cells and reduces fibrosis in patients with non-alcoholic steatohepatitis107. No SCD1 inhibitors have been tested for cancer in the clinical setting, but, as interest in fat metabolism pathways continues to increase, more drugs will probably enter clinical trials.
CD36
CD36, which is also known as FA translocase, is a multifunctional transmembrane protein integral to FA uptake108,109. Acting as a translocase, CD36 can transfer extracellular FAs to cytosolic FA-binding proteins for cell metabolism in a variety of tissues110,111 (FIG. 3). Increased CD36 expression has been observed in numerous malignancies, including prostate, breast, brain and gastric cancers and ccRCC112–115. Beyond FA metabolism, CD36 has also been implicated in angiogenesis through binding of thrombospondin-1, inflammation through interaction with Toll-like receptors and metastasis through regulation of integrins and the cytoskeleton108.
The association between CD36 and ccRCC has been investigated. Results of one investigation showed that CD36 was expressed at higher levels in the cell membranes of 74 ccRCC samples than in 80 non-malignant kidney samples116. Results of another study, involving a cohort of 367 patients with ccRCC undergoing nephrectomy (10% of patients were M+), showed that high CD36 expression was associated with increased TNM stage and visceral adiposity117. On multivariate Cox regression analysis, patients with high CD36 expression had significantly reduced progression-free survival (PFS) (median PFS of 44 months for medium expression versus 29 months for high expression, HR 3.24, 95% CI 2.15–4.89, P < 0.001) and OS (median OS of 80 months for medium versus 52 months for high expression, HR 2.49, 95% CI 1.55–4.00, P < 0.001) when controlling for stage and body mass index (BMI)117. Thus, CD36 has potentially meaningful involvement in ccRCC.
Preclinical mechanistic studies of CD36 in ccRCC are notably lacking. Insights from other models suggest that CD36 is regulated by the HIF pathway118, which would explain its elevation in ccRCC. In a cervical cancer xenograft model, CD36 was shown to mediate tumour growth enhancement caused by a high oleic acid diet, which could have obvious ramifications for the obesity-dependent increase in ccRCC tumour incidence119. Oleic acid was found to promote tumour cell proliferation by activating Src kinase and the downstream ERK1–ERK2 pathway in a CD36-dependent manner119. In gastric cancer, palmitic acid uptake via CD36 has been found to induce AKT phosphorylation120, which is also elevated in ccRCC121. Collectively, the data suggest that CD36 is highly likely to have a functional role in ccRCC that is yet to be documented.
CD36 inhibitory strategies are currently under development. A number of peptide strategies have been used in preclinical studies, taking advantage of known binding partners such as TSP-1 and apolipoprotein122–124, but have suffered in early-phase trials from toxic effects or lack of efficacy122,123. Inhibiting CD36 using a monoclonal antibody has demonstrated high efficacy in reducing tumour metastasis in oral squamous cell carcinoma models, with no described adverse effects, suggesting possible promising outcomes in other highly metastatic cancers and ccRCC125,126.
Carnitine palmitoyltransferase 1 A
CPT1A is a mitochondrial outer membrane protein that has an essential role in the transport of long-chain FAs across the membrane for β-oxidation in the mitochondrial matrix127. CPT1A is the rate-limiting enzyme in FA transport, which involves transfer of a long-chain acyl group from coenzyme A to carnitine, transport into the inner membrane space in exchange for a free carnitine, and finally reversion to long-chain acyl-CoA by CPT2 on the inner matrix128 (FIG. 3). Deficiencies in CPT1A result in hypoketotic hypoglycaemia, hepatomegaly and elevated levels of free FAs with fat accumulation in skeletal muscles129.
In ccRCC, CPT1A levels are reduced compared with non-malignant kidney tissue, in concordance with reduced FA oxidation in favour of storage10,130. IHC staining of CPT1A in 50 ccRCC samples and 50 non-malignant kidney samples showed a reduction of CPT1A protein expression in cancer tissue130. Mechanistically, CPT1A expression was found to correlate positively with VHL status, and elevated HIF transcriptionally represses CPT1A mRNA synthesis10. Functionally, CPT1A repression is important because exogenously added CPT1A reduced lipid deposition and tumour formation in preclinical ccRCC models both in vitro and in vivo10. Furthermore, survival analysis using TCGA data showed a correlation between reduced CPT1A expression with reduced OS in patients with ccRCC (P = 2.58 × 10−6)10.
Therapeutically, CPT1A is an atypical target in ccRCC. The preclinical and prognostic data suggest that agents that could elevate expression or activity of CPT1A would be beneficial in patients with ccRCC, which precludes the use of CPT1A inhibitors such as etomoxir131. However, currently, no direct approaches to upregulating or relieving the repression of CPT1A exist. Inducing FA oxidation to treat ccRCC would align with the demonstration that suppression of FA oxidation is a necessary means for cancer cells to escape from the iron accumulation-induced and lipid peroxidation-induced form of cell death known as ferroptosis132. Redox balance in cancer cells is an essential facet of all of the hallmarks of cancer133, and cancer cells invest heavily in regulating antioxidant enzymes to survive high reactive oxygen species production134. Glutathione peroxidase 4 (GPX4), an antioxidant enzyme that detoxifies lipid reactive oxygen species, has been shown to be necessary for ccRCC cells to evade ferroptosis and importantly highlights the idea that targeting glutathione biology is an alternative strategy to achieve the same outcome as inducing CPT1A activity135,136.
Perilipin proteins
The PLIN proteins comprise a family of five proteins (PLIN1–PLIN5) that are associated with lipid droplets and are involved in lipid droplet function and formation in various tissue types137,138 (FIG. 3). Post-translational modifications of PLINs are key to their roles in controlling lipid homeostasis, and differential expression of the five members suggest divergence of function: PLIN1 and PLIN4 are exclusively present in professional lipid-storing cells such as adipocytes139,140; PLIN2 is expressed in immature adipocytes141; PLIN3 is ubiquitously expressed in many tissue types and is present in cell cytosol and lipid droplets142; and PLIN5 is exclusively expressed in cells with high energy demand, such as cardiomyocytes143. PLIN2 and PLIN3 have been studied in the context of ccRCC. Investigation of the lipid phenotype in tumour cell lines first showed elevated expression of PLIN2 in ccRCC, in which expression was found to be specifically dependent on HIF2α12. In 80 matched tumour–non-malignant tissue pairs, PLIN2 and PLIN3 were found to have higher expression in ccRCC tissue than in non-malignant tissue144,145. However, PLIN2 and PLIN3 expression correlate with disease severity and survival differently: PLIN2 expression decreases with increasing T stage and grade144, but PLIN3 expression increases145. In TCGA database, when stratifying by PLIN2 expression, high PLIN2 expression correlated with improved survival (HR 0.586, 95% CI 0.429–0.803, P = 0.001)144, whereas high PLIN3 (REFS.144,145) was associated with worse disease-free survival (77.9 months, 95% CI 69.2–86.6 versus 98.4 months, 95% CI 89.4–107.5, P = 0.0005)145. The high-PLIN3 expression group also had worse OS than the group with low-PLIN3 expression (79.5 months, 95% CI 71.5–86.6 versus 102.5 months, 95% CI 92.4–112.6, P = 0.0006)145.
Structural proteins might not seem to be ideal drug targets, but preclinical studies have shown that PLIN2 depletion in ccRCC cells results in reduced lipid droplets and cell death owing to ER stress, supporting the theory that lipid storage is an essential activity of ccRCC12. PLINs are heavily post-translationally modified and control of their expression is key to their function146; thus, targeting upstream regulators might be a possible approach. The natural compound sulforaphane is an isothiocyanate derived from broccoli with noted protective effects against cancer147. Sulforaphane targets PLIN2 and PLIN5 through a PPARγ-dependent mechanism to reduce hepatic lipid droplet formation in a model of non-alcoholic fatty liver disease148. Clearly, inhibitors of master regulators such as PPARγ would be expected to have multiple downstream effects, but the concept is still valid and currently untested in ccRCC.
In summary, multiple enzymes in the FA metabolism pathway are altered in RCC. Some, such as FASN and SCD1, are better studied as potential targets than others and have inhibitors that are being studied in the clinical setting. Others are being further studied in the preclinical setting. Together, the pathway as a whole offers promise for developing novel cancer therapeutics.
Lipid metabolism-related biomarkers
Lipid metabolism alterations in ccRCC compared with renal epithelia provide the potential for the discovery of biomarkers of the disease, and high-throughput ‘-omic’ technologies, including lipidomic analysis, have been increasingly used to identify biochemical or molecular signatures149. However, currently, the use of biomarkers as a whole is limited in RCC. In the metastatic setting, the only validated biomarker is the IMDC risk model, which is a composite model of six clinical factors150. Other biomarkers, including lipid biomarkers, are considered investigational in both localized and metastatic settings. Nonetheless, a prognostic or predictive biomarker could be beneficial in several situations, from active surveillance of small renal masses to consideration of adjuvant therapy after excision of locally advanced tumours.
Adipokines
Adipokines are cytokines released by adipocytes151. Many adipokines have been examined in the context of ccRCC, and three well-studied adipokines have a potential role as serum biomarkers. Adiponectin and chemerin were chosen owing to their established connections with RCC, and leptin owing to its implications in multiple malignancies.
Adiponectin.
Adiponectin is one of the most physiologically active molecules secreted by adipose tissue and stimulates glucose uptake in skeletal muscle, increases FA oxidation in muscle and liver, and has anti-atherosclerotic effects152. Serum adiponectin levels are low in patients with metabolic syndrome, type 2 diabetes mellitus153 and cardiovascular disease152. Weight loss has been associated with increased adiponectin as well as improved insulin sensitivity152,154. Adiponectin has been studied in detail in the context of cancer owing to the relationship between obesity and several malignancies, such as colorectal and breast cancers155–157. Adiponectin has also been studied in RCC because of its anti-angiogenic properties158.
Adiponectin levels are reduced in patients with RCC. In one case–control study, patients with RCC (74% ccRCC) had lower serum adiponectin levels than matched healthy individuals after controlling for BMI159. Similar inverse associations between RCC incidence and adiponectin levels have been reported in two other studies160,161. One of the studies was a prospective case–control study including 273 patients with incidentally diagnosed RCC and BMI-matched healthy individuals, and the results suggested that high adiponectin levels might be protective against future RCC risk160. Some evidence also suggests that adiponectin levels correlate inversely with measures of tumour severity, such as tumour size and metastatic disease162. Results of a meta-analysis of 10 studies showed that adiponectin is lower in patients with RCC than in healthy individuals, but serum adiponectin levels did not correlate with stage, grade or subtype160,163.
In terms of long-term clinical outcomes, the association between adiponectin and survival has been examined. Measurement of adiponectin before nephrectomy in 131 patients with various stages of cancer showed that each 1-μg/ml increase in preoperative adiponectin resulted in an 8% decrease in hazard of death (0.92, 95% CI 0.86–0.98, P = 0.007) after controlling for tumour characteristics but not body composition variables164. Notably, in another study involving 129 patients undergoing nephrectomy mostly for localized ccRCC, low serum adiponectin was associated with reduced OS (P = 0.035) but not CSS (P = 0.3)165. This observation is interesting because adiponectin is inversely associated with obesity and insulin resistance; thus, the association with OS but not CSS suggests that adiponectin levels might be predictive of mortality from metabolic disorders rather than RCC165. Moreover, this inference is consistent with the short follow-up duration (30 months) in a cohort of patients with mostly localized RCC, which has relatively low mortality165. In the metastatic setting, the adiponectin receptor 1 (AdipoR1) was investigated in a study involving 127 patients with RCC (71% ccRCC) receiving sunitinib. Low receptor expression correlated significantly with disease progression compared with patients with high expression over a 64-month follow-up period (40.0% versus 11.1%, P = 0.02; median PFS 19.5 months versus 37.8 months, P = 0.001). On Cox regression analysis, after controlling for cancer-specific features including IMDC score, low adiponectin levels remained a significant predictor of PFS (HR 0.55, 95% CI 0.31–0.98, P = 0.042)166. Overall, adiponectin levels are reduced in patients with RCC and might be associated with survival, but no threshold has been suggested, limiting its use as a biomarker.
Leptin.
The connection of leptin with adiposity is well-established. Leptin suppresses appetite and promotes metabolic activity when it binds to receptors in the brain as a ‘satiety’ hormone167,168. Patients with congenital leptin deficiency have symptoms such as severe obesity and insulin resistance169. Leptin is elevated in the serum of patients with BMI ≥ 25 kg/m2 compared with those with BMI < 25 kg/m2 and has a contrasting biological function to adiponectin167,170.
Leptin has tumorigenic effects that have been demonstrated in several malignancies171. In breast cancer, chronic leptin treatment reduces reactive oxygen species levels and apoptosis in MCF-7 cells172. Leptin also mediates tumour–stromal interactions between breast cancer cells and tumour-associated macrophages via stimulation of IL-8 and IL-18 synthesis in order to promote metastasis172. However, the role of leptin in ccRCC is not well studied, and most evidence showed no association between leptin and RCC173. Serum leptin levels were shown to inversely correlate with RCC risk in one study174, but in other studies and meta-analyses no association between serum leptin levels and RCC was found161,164,173,175. Thus, serum leptin does not seem to be a good candidate as a serum biomarker for ccRCC, despite its well-studied role in adiposity.
Chemerin.
Chemerin is a multifunctional adipokine that both promotes adipocyte differentiation and increases fat deposition in mature adipocytes176. Systemically, chemerin has been linked to obesity and metabolic syndrome176. Chemerin is also involved in inflammation, promoting chemotaxis of macrophages and dendritic cells177. Serum chemerin correlates positively with levels of many pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α, IL-6 and C reactive protein (CRP)178. Inflammation is associated with many cancers, but the combination of lipid regulation and inflammation makes chemerin an ideal candidate to have a role in ccRCC. Chemerin expression has been shown to be elevated in ccRCC tumour tissue, in which it promotes lipid droplet formation, resulting in increased expression of HIF1α and HIF2α mRNA, which are responsive to SFAs such as palmitate12,179. The mechanism yields a positive feedback loop because HIF2α then suppresses FA oxidation via downregulation of CPT1A and further induces chemerin12 (FIG. 3). A similar effect was also observed in hepatocellular carcinoma cells, in which HIF1A mRNA level was upregulated transiently when treated with palmitate179. Thus, chemerin depletion results in drastic lipid reprogramming in ccRCC, leading to excess FA catabolism that induces mitochondrial dysfunction and ferroptosis180.
Increased chemerin expression in ccRCC correlates significantly with poor patient outcomes according to TCGA data when patients are stratified according to upper-third versus lower-third of chemerin mRNA expression (P = 9.02e-07); furthermore, plasma chemerin is also elevated in patients with ccRCC compared with healthy individuals, suggesting a potential use of chemerin as a circulating biomarker12. Chemerin might, therefore, be part of the protumorigenic effects of obesity in renal cancer. Chemerin has attractive therapeutic potential as a circulating target, because a monoclonal antibody or a small molecule approach can affect tumour inhibition without the need to enter into tumour cells. Chemerin antagonism could also affect the immune-suppressive tumour microenvironment, which is thought to decrease the effectiveness of current checkpoint therapies181.
Serum lipid species as biomarkers
The overt lipid metabolic reprogramming in ccRCC raises the prospect of using serum lipids themselves as biomarkers. However, few studies to date have explored this concept, and most of the results are inconsistent. In a discovery-based lipid profiling study including 112 patients with ccRCC of various stages and 52 healthy individuals, a 16-lipid panel from serum enabled patients with ccRCC to be distiguished, and a 26-lipid panel distinguished early-stage (I and II) from late-stage (III and IV) disease with 77–95% accuracy182. The lipid components included were lyso-glycerophospholipids, phospholipids and SFAs. However, further validation is necessary to determine prognostic potential. Results of another exploratory study of a broad portfolio of serum metabolites (amino acids, bile acids, vitamins, FAs and lipids) in a cohort of 28 patients, the majority of whom had RCC (89%), receiving immune checkpoint inhibitor therapy for metastatic disease showed that 9 of 10 metabolites associated with response to therapy were very-long-chain FAs (VLCFAs)183. VLCFAs are carboxylic acids with at least 22 carbons and have been proposed to be involved in regulated cell death184,185. The observational nature of this study means that potential mechanisms for the association between VLCFA levels and treatment response were not explored, but a connection to peroxisome signalling in immune T cells was suggested. Overall, the paucity of data currently limits the use of serum lipids as biomarkers.
Some evidence exists for an association between tissue FA levels and prognosis. Certain classes of FAs seem to be more concentrated in tumour tissue than in normal tissue186. In one study, 47 ccRCC samples were compared with paired non-malignant renal cortex and 8 tissue lipid biomarkers with high signal intensity in tumours were identified185. Among the candidate biomarkers, low maximum intensity ratio values of oleic acid correlated with shorter PFS than high maximum ratio values185. However, the study was limited by the small cohort.
In summary, tumour or circulating lipid biomarkers have shown some correlation with clinical outcomes in RCC, but currently published studies are too small to draw firm clinical conclusions. Larger, prospective studies will be necessary to validate any useful prognostic or predictive tools in the clinical setting in ccRCC.
The obesity paradox
Obesity is associated with increased cancer incidence187–189 and poor outcomes in several malignancies190, and is one of the few known risk factors for RCC187,191–193. The causal relationship of obesity and cancer is not fully defined, but one theory is that increased body size leads to increased oxidative stress194 and increased inflammation, which are both associated with carcinogenesis195. Numerous adipocyte-linked molecules such as adipokines, insulin and insulin-like growth factor, interleukins, and gastric inhibitory polypeptides could also promote tumorigenesis196. The effect is probably multifactorial, rather than dependent on a single mechanism.
Paradoxically, ccRCC disease incidence is increased in individuals with obesity, but patients with obesity and ccRCC tend to have more favourable outcomes than patients with a BMI within the ‘normal’ range197. This ‘obesity paradox’ has been demonstrated in multiple disease states including localized and metastatic settings198–200. For example, in localized disease, patients with obesity who underwent nephrectomy in a single-institution retrospective study had improved median 5-year recurrence-free survival (90.7% versus 84.9%, P < 0.001)198. Consistent with the paradox, recurrence-free survival was also improved with increasing BMI on multivariate analysis (HR 0.93, 95% CI 0.89–0.97, P = 0.002)198. This phenomenon might be driven by the fact that patients with obesity tend to present with a low-stage and low-grade disease77, although results of other studies involving patients with localized disease show a persistent, independent positive effect of BMI on improved OS and CSS after correcting for stage and grade199. A considerable difference in survival was observed in the metastatic setting as well: for patients receiving first-line targeted therapy in the IMDC, the median OS was 25.6 months for patients with BMI ≥25 kg/m2, compared with 17.1 months among patients with BMI <25 kg/m2 (adjusted HR 0.84, 95% CI 0.73–0.95)78. In the immunotherapy era, the phenomenon has been borne out as well. Patients in the IMDC receiving PD1–PDL1 immune checkpoint inhibitors have a 1-year OS of 79% in the high-BMI group compared with 66% in the low-BMI group (adjusted HR 0.75, 95% CI 0.57–0.97, P = 0.03)200.
FA molecular changes that might underlie a potential mechanism of the obesity paradox have been studied. The first of these is FASN-mediated; FASN is generally reduced in individuals with obesity and might independently mediate survival77. Results of a study including 126 patients with ccRCC who underwent nephrectomy in a single institution showed that patients with obesity had a decreased cancer-specific mortality (P < 0.05)77. Genomic analysis showed that patients with obesity (BMI ≥30) had lower tumour FASN expression than those with a BMI within the ‘normal’ range, and was associated with improved CSS in TCGA (P < 0.001)77. In another study that included 145 patients with localized or metastatic ccRCC from a single institution, improved cancer survival independent of BMI was observed in the low-FASN cohort with a 3-year follow-up duration based on logrank analysis (logrank = 14.9, P < 0.001)36. Cox regression analysis controlling for clinical characteristics including tumour grade, stage, size and clinical metastasis showed an HR of 1.88 (95% CI 1.021–3.460, P = 0.043)36. In the same study, the LinkedOmics and The Cancer Protein Atlas were analysed and FASN expression was shown to be negatively correlated with OS in both databases. Thus, reduced tumour FASN expression as a BMI-dependent variable could be one mechanistic driver of the improved clinical outcomes shown by patients with obesity.
Tumour and adipose microenvironments have also been investigated in the context of the protective effect of adiposity in patients with RCC. Using the cohort from the COMPARZ trial (patients with metastatic RCC receiving pazopanib or sunitinib), immune cell infiltration in tumour tissues and peritumoural fat was examined using gene expression analyses201. No significant difference in the degree of immune cell infiltration was found between patients with obesity and those with a BMI within the ‘normal’ range, but the tumours from patients with obesity expressed lower levels of immune checkpoint molecules and had decreased PDL1 expression. This mechanism might explain the improved survival seen in patients with obesity receiving ICIs200, but the data are immature. Furthermore, tumours from those with obesity had higher angiogenic scores than those with a BMI within the ‘normal’ range in a separate cohort receiving nephrectomy for localized RCC201. One hypothesis from the data would be that improved vascularization in tumours leads to improved drug delivery and/or immune access. However, no direct mechanistic studies comparing tumours from individuals with different BMIs have been conducted to establish whether fundamental biological differences exist.
In contrast to the relationship between altered FA metabolism with the obesity paradox, other lipid species have less clear associations with obesity and outcome. Elevated LDL cholesterol (LDL-C) and HDL cholesterol (HDL-C) are both associated with a reduced risk of developing ccRCC202, whereas individuals with obesity tend to have high LDL-C but low HDL-C203. Thus, how cholesterol mediates RCC risk in unclear. Furthermore, increased preoperative serum HDL-C correlated with improved OS in a series of 308 patients with ccRCC eligible for surgery (HR 0.32, 95% CI 0.13–0.78, P = 0.013204. Thus, in summary, the contribution of obesity in ccRCC outcome is still far from clear and warrants further investigation.
Future perspectives
Altered metabolism is well recognized as a hallmark of cancer. FA metabolism is grossly disrupted in ccRCC205, which has a lipid phenotype, affecting tumour growth and therapeutic resistance, and is obviously connected with the histological definition of clear-cell tumours. Understanding the mechanisms of FA metabolism in ccRCC oncogenesis might lead to novel personalized treatment strategies. Historically, therapeutic approaches in ccRCC have focused on tyrosine kinase inhibition using sunitinib, pazopanib or cabozantinib, with moderate but limited success206,207. The FDA approval of a HIF2α small molecule inhibitor (belzutifan) has great promise for further improving patient outcomes, but HIF2α inhibitors also cause anaemia and fatigue, which might be a result of the broad roles of HIF targets208,209; thus, developing additional treatment modalities for ccRCC is imperative. Currently, no actively targeted lipid metabolic pathways in ccRCC clinical management exist, but as possibly one of the most appropriate tumours for a metabolic approach, future investigations should be aimed at this tumour-specific characteristic209. Moreover, the different cell types that comprise the tumour microenvironment have been shown to have different metabolic characteristics, adding complexity but also revealing potentially targetable pathways210.
Conclusions
Multiple lipid metabolism pathway alterations that drive lipid deposition are associated with ccRCC. Historically, the value of excess lipids in ccRCC has been confined to its use in a histological definition. However, FA metabolic rewiring has now been established as a necessary adaptation of ccRCC tumour cells, which reveals a possible metabolic vulnerability. The fact that lipid storage is a dramatic biological difference between tumour and non-malignant tissue provides an important therapeutic window if appropriate inhibitors with acceptable toxic effect profiles (in the kidney or elsewhere) can be developed. Currently, numerous attractive targets have been discovered, including enzymes, structural proteins and secreted molecules, each with their own advantages and disadvantages. Preclinical studies improve understanding of the mechanisms that define ccRCC, meaning new pharmacological approaches are bound to emerge.
Acknowledgements
This work was supported in part by a grant from the National Institutes of Health R01CA254409 to S.M.W.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Urology thanks Kimryn Rathmell, who co-reviewed with Dakim Gaines, Barrie Peck and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Linehan WM et al. The metabolic basis of kidney cancer. Cancer Discov. 9, 1006–1021 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Cairns P Renal cell carcinoma. Cancer Biomark. 9, 461–473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tugnoli V, Trinchero A & Tosi MR Evaluation of the lipid composition of human healthy and neoplastic renal tissues. Ital. J. Biochem. 53, 169–182 (2004). [PubMed] [Google Scholar]
- 4.Kaelin WG Jr The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin. Cancer Res. 13, 680s–684s (2007). [DOI] [PubMed] [Google Scholar]
- 5.Kaelin WG Jr & Ratcliffe PJ Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Schito L & Semenza GL Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2, 758–770 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Gameiro PA et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 17, 372–385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metallo CM et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du W et al. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat. Commun. 8, 1769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu B et al. HIF2α-dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma. Cancer Discov. 5, 652–667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan SK et al. Obesity-dependent adipokine chemerin suppresses fatty acid oxidation to confer ferroptosis resistance. Cancer Discov. 10.1158/2159-8290.Cd-20-1453 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y et al. Lysophosphatidylcholine acyltransferase 1 upregulation and concomitant phospholipid alterations in clear cell renal cell carcinoma. J. Exp. Clin. Cancer Res. 36, 66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sok M, Šentjurc M, Schara M, Stare J & Rott T Cell membrane fluidity and prognosis of lung cancer. Ann. Thorac. Surg. 73, 1567–1571 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Campanella R Membrane lipids modifications in human gliomas of different degree of malignancy. J. Neurosurg. Sci. 36, 11–25 (1992). [PubMed] [Google Scholar]
- 16.Xie H et al. Glycogen metabolism is dispensable for tumour progression in clear cell renal cell carcinoma. Nat. Metab. 3, 327–336 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H et al. Fatty acid metabolism reprogramming in advanced prostate cancer. Metabolites 10.3390/metabo11110765 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monaco ME Fatty acid metabolism in breast cancer subtypes. Oncotarget 8, 29487–29500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson RU & Shaw RJ Lipid synthesis is a metabolic liability of non-small cell lung cancer. Cold Spring Harb. Symp. Quant. Biol. 81, 93–103 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Pizer ES et al. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate 47, 102–110 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Davis AL & Kridel SJ Trimming the fat in non-small cell lung cancer: a new small molecule inhibitor of acetyl-CoA carboxylase to target fatty acid synthesis. Transl. Cancer Res. 5(S7), S1449–S1452 (2016). [Google Scholar]
- 22.Falchook G et al. First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. EClinicalMedicine 34, 100797 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menendez JA & Lupu R Fatty acid synthase regulates estrogen receptor-α signaling in breast cancer cells. Oncogenesis 6, e299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan SK & Welford SM Lipid in renal carcinoma: queen bee to target. Trends Cancer 10.1016/j.trecan.2020.02.017 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Koundouros N & Poulogiannis G Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 122, 4–22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C & Thompson CB ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24, 6314–6322 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Schug ZT et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ookhtens M, Kannan R, Lyon I & Baker N Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am. J. Physiol. 247, R146–R153 (1984). [DOI] [PubMed] [Google Scholar]
- 29.Pinthus JH, Whelan KF, Gallino D, Lu JP & Rothschild N Metabolic features of clear-cell renal cell carcinoma: mechanisms and clinical implications. Can. Urol. Assoc. J. 5, 274–282 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medes G, Thomas A & Weinhouse S Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 13, 27–29 (1953). [PubMed] [Google Scholar]
- 31.Butler LM et al. Lipids and cancer: emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 159, 245–293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan S et al. Kidney proximal tubule lipoapoptosis is regulated by fatty acid transporter-2 (FATP2). J. Am. Soc. Nephrol. 29, 81–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhlén M et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 34.DeBerardinis RJ & Chandel NS Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riscal R et al. Cholesterol auxotrophy as a targetable vulnerability in clear cell renal cell carcinoma. Cancer Discov. 11, 3106–3125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y et al. Expression and prognostic significance of fatty acid synthase in clear cell renal cell carcinoma. Pathol. Res. Pract. 216, 153227 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Fujita Y, Matsuoka H & Hirooka K Regulation of fatty acid metabolism in bacteria. Mol. Microbiol. 66, 829–839 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Peck B et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 4, 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Roemeling CA et al. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin. Cancer Res. 19, 2368–2380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain IH et al. Genetic screen for cell fitness in high or low oxygen highlights mitochondrial and lipid metabolism. Cell 181, 716–727 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaidi N, Swinnen JV & Smans K ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 72, 3709 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Watson JA, Fang M & Lowenstein JM Tricarballylate and hydroxycitrate: substrate and inhibitor of ATP: citrate oxaloacetate lyase. Arch. Biochem. Biophys. 135, 209–217 (1969). [DOI] [PubMed] [Google Scholar]
- 43.Sun T, Hayakawa K, Bateman KS & Fraser ME Identification of the citrate-binding site of human ATP-citrate lyase using X-ray crystallography. J. Biol. Chem. 285, 27418–27428 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noh KH et al. Ubiquitination of PPAR-gamma by pVHL inhibits ACLY expression and lipid metabolism, is implicated in tumor progression. Metabolism 110, 154302 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Guo H et al. The PI3K/AKT pathway and renal cell carcinoma. J. Genet. Genomics 42, 343–353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migita T et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 68, 8547–8554 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Wang S et al. Novel molecular subtypes and related score based on histone acetylation modification in renal clear cell carcinoma. Front. Cell Dev. Biol. 9, 668810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrer A et al. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov. 9, 416–435 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teng L et al. Overexpression of ATP citrate lyase in renal cell carcinoma tissues and its effect on the human renal carcinoma cells in vitro. Oncol. Lett. 15, 6967–6974 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Z et al. The mRNA expression signature and prognostic analysis of multiple fatty acid metabolic enzymes in clear cell renal cell carcinoma. J. Cancer 10, 6599–6607 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi X, Li Q, Che X, Wang Q & Wu G The uniqueness of clear cell renal cell carcinoma: summary of the process and abnormality of glucose metabolism and lipid metabolism in ccRCC. Front. Oncol. 10.3389/fonc.2021.727778 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Luo S, Gan X, He C & Huang R Safety and efficacy of ETC-1002 in hypercholesterolaemic patients: a meta-analysis of randomised controlled trials. Kardiol. Pol. 77, 207–216 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Chuah LO, Yeap SK, Ho WY, Beh BK & Alitheen NB In vitro and in vivo toxicity of garcinia or hydroxycitric acid: a review. Evid. Based Complement. Altern. Med. 2012, 197920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berkhout TA, Havekes LM, Pearce NJ & Groot PH The effect of (−)-hydroxycitrate on the activity of the low-density-lipoprotein receptor and 3-hydroxy-3-methylglutaryl-CoA reductase levels in the human hepatoma cell line Hep G2. Biochem. J. 272, 181–186 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burke AC et al. Bempedoic acid lowers low-density lipoprotein cholesterol and attenuates atherosclerosis in low-density lipoprotein receptor-deficient (LDLR+/− and LDLR−/−) Yucatan Miniature Pigs. Arterioscler. Thromb. Vasc. Biol. 38, 1178–1190 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Wang C, Rajput S, Watabe K, Liao DF & Cao D Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Front. Biosci. 2, 515–526 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Bianchi A et al. Identification of an isozymic form of acetyl-CoA carboxylase. J. Biol. Chem. 265, 1502–1509 (1990). [PubMed] [Google Scholar]
- 58.Creighton CJ et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo J et al. Acetyl-CoA carboxylase rewires cancer metabolism to allow cancer cells to survive inhibition of the Warburg effect by cetuximab. Cancer Lett. 384, 39–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu YY et al. Inactivation of the AMPK-GATA3-ECHS1 pathway induces fatty acid synthesis that promotes clear cell renal cell carcinoma growth. Cancer Res. 80, 319–333 (2020). [DOI] [PubMed] [Google Scholar]
- 61.He D, Sun X, Yang H, Li X & Yang D TOFA induces cell cycle arrest and apoptosis in ACHN and 786-O cells through inhibiting PI3K/Akt/mTOR pathway. J. Cancer 9, 2734–2742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee M et al. Phosphorylation of acetyl-CoA carboxylase by AMPK reduces renal fibrosis and is essential for the anti-fibrotic effect of metformin. J. Am. Soc. Nephrol. 29, 2326–2336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song A, Zhang C & Meng X Mechanism and application of metformin in kidney diseases: an update. Biomed. Pharmacother. 138, 111454 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Liu J et al. Metformin inhibits renal cell carcinoma in vitro and in vivo xenograft. Urol. Oncol. 31, 264–270 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Fang Z, Xu X, Zhou Z, Xu Z & Liu Z Effect of metformin on apoptosis, cell cycle arrest migration and invasion of A498 cells. Mol. Med. Rep. 9, 2251–2256 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Li J, Song M, Qi G & Meng L High-concentration metformin reduces oxidative stress injury and inhibits the growth and migration of clear cell renal cell carcinoma. Comput. Math. Methods Med. 2022, 1466991 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Mir MY et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000). [DOI] [PubMed] [Google Scholar]
- 68.Psutka SP et al. The association between metformin use and oncologic outcomes among surgically treated diabetic patients with localized renal cell carcinoma. Urol. Oncol. 33, e15–e23 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Hu L, Xia Q, Yuan Y & Mi Y The impact of metformin use on survival in kidney cancer patients with diabetes: a meta-analysis. Int. Urol. Nephrol. 49, 975–981 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahiro T, Shinichi K & Toshimitsu S Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas. Clin. Cancer Res. 9, 2204–2212 (2003). [PubMed] [Google Scholar]
- 71.Notarnicola M et al. Serum levels of fatty acid synthase in colorectal cancer patients are associated with tumor stage. J. Gastrointest. Cancer 43, 508–511 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Menendez JA & Lupu R Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 7, 763–777 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Maier T, Jenni S & Ban N Architecture of mammalian fatty acid synthase at 4.5 A resolution. Science 311, 1258–1262 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Asturias FJ et al. Structure and molecular organization of mammalian fatty acid synthase. Nat. Struct. Mol. Biol. 12, 225–232 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Menendez JA et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc. Natl Acad. Sci. USA 101, 10715–10720 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horiguchi A et al. Fatty acid synthase over expression is an indicator of tumor aggressiveness and poor prognosis in renal cell carcinoma. J. Urol. 180, 1137–1140 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Hakimi AA et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J. Natl Cancer Inst. 105, 1862–1870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albiges L et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J. Clin. Oncol. 34, 3655–3663 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang L et al. Inhibition of FASN suppresses the malignant biological behavior of non-small cell lung cancer cells via deregulating glucose metabolism and AKT/ERK pathway. Lipids Health Dis. 18, 118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schroeder B et al. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis. 12, 977 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swinnen JV et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem. Biophys. Res. Commun. 302, 898–903 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Kuchiba A et al. Body mass index and risk of colorectal cancer according to fatty acid synthase expression in the nurses’ health study. J. Natl Cancer Inst. 104, 415–420 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horiguchi A et al. Pharmacological inhibitor of fatty acid synthase suppresses growth and invasiveness of renal cancer cells. J. Urol. 180, 729–736 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Pizer ES et al. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 60, 213–218 (2000). [PubMed] [Google Scholar]
- 85.Sadowski MC et al. The fatty acid synthase inhibitor triclosan: repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget 5, 9362–9381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Mijn JC, Panka DJ, Geissler AK, Verheul HM & Mier JW Novel drugs that target the metabolic reprogramming in renal cell cancer. Cancer Metab. 4, 14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimokawa T, Kumar MV & Lane MD Effect of a fatty acid synthase inhibitor on food intake and expression of hypothalamic neuropeptides. Proc. Natl Acad. Sci. USA 99, 66–71 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vargas T et al. ColoLipidGene: signature of lipid metabolism-related genes to predict prognosis in stage-II colon cancer patients. Oncotarget 6, 7348–7363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi H et al. Macrophage migration inhibitory factor and stearoyl-CoA desaturase 1: potential prognostic markers for soft tissue sarcomas based on bioinformatics analyses. PLoS One 8, e78250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holder AM et al. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res. Treat. 137, 319–327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang J et al. SCD1 is associated with tumor promotion, late stage and poor survival in lung adenocarcinoma. Oncotarget 7, 39970–39979 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeffords E et al. Y-box binding protein 1 acts as a negative regulator of stearoyl CoA desaturase 1 in clear cell renal cell carcinoma. Oncol. Lett. 20, 165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J et al. High expression of stearoyl-CoA desaturase 1 predicts poor prognosis in patients with clear-cell renal cell carcinoma. PLoS One 11, e0166231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castro BBA, Foresto-Neto O, Saraiva-Camara NO & Sanders-Pinheiro H Renal lipotoxicity: insights from experimental models. Clin. Exp. Pharmacol. Physiol. 48, 1579–1588 (2021). [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc. Natl Acad. Sci. USA 10.1073/pnas.2103240118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iwai T et al. Stearoyl-CoA desaturase-1 protects cells against lipotoxicity-mediated apoptosis in proximal tubular cells. Int. J. Mol. Sci. 10.3390/ijms17111868 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell 20, 303–314 e305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malta TM et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 173, 338–354.e315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tracz-Gaszewska Z & Dobrzyn P Stearoyl-CoA desaturase 1 as a therapeutic target for the treatment of cancer. Cancers 10.3390/cancers11070948 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang P et al. Formation and antiproliferative effect of prostaglandin E3 from eicosapentaenoic acid in human lung cancer cells. J. Lipid Res. 45, 1030–1039 (2004). [DOI] [PubMed] [Google Scholar]
- 101.Sapieha P et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω−3 polyunsaturated fatty acids. Sci. Transl. Med. 3, 69ra12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iwamoto H et al. Cancer lipid metabolism confers antiangiogenic drug resistance. Cell Metab. 28, 104–117.e105 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y, Wang H, Zhang J, Lv J & Huang Y Positive feedback loop and synergistic effects between hypoxia-inducible factor-2α and stearoyl-CoA desaturase-1 promote tumorigenesis in clear cell renal cell carcinoma. Cancer Sci. 104, 416–422 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.von Roemeling CA et al. Accelerated bottom-up drug design platform enables the discovery of novel stearoyl-CoA desaturase 1 inhibitors for cancer therapy. Oncotarget 9, 3–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Imamura K et al. Discovery of novel and potent stearoyl coenzyme A desaturase 1 (SCD1) inhibitors as anticancer agents. Bioorg. Med. Chem. 25, 3768–3779 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Oballa RM et al. Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia. J. Med. Chem. 54, 5082–5096 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Bhattacharya D et al. Aramchol downregulates stearoyl CoA-desaturase 1 in hepatic stellate cells to attenuate cellular fibrogenesis. JHEP Rep. 3, 100237–100237 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J & Li Y CD36 tango in cancer: signaling pathways and functions. Theranostics 9, 4893–4908 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E & Grimaldi PA Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268, 17665–17668 (1993). [PubMed] [Google Scholar]
- 110.Pepino MY, Kuda O, Samovski D & Abumrad NA Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 34, 281–303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coburn CT et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529 (2000). [DOI] [PubMed] [Google Scholar]
- 112.Zaoui M et al. Breast-associated adipocytes secretome induce fatty acid uptake and invasiveness in breast cancer cells via CD36 independently of body mass index, menopausal status and mammary density. Cancers 10.3390/cancers11122012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Watt MJ et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 10.1126/scitranslmed.aau5758 (2019). [DOI] [PubMed] [Google Scholar]
- 114.Jiang M et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics 9, 5359–5373 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hale JS et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cell 32, 1746–1758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim YS et al. High membranous expression of fatty acid transport protein 4 is associated with tumorigenesis and tumor progression in clear cell renal cell carcinoma. Dis. Markers 2019, 5702026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu WH et al. Elevated CD36 expression correlates with increased visceral adipose tissue and predicts poor prognosis in ccRCC patients. J. Cancer 10, 4522–4531 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mwaikambo BR, Yang C, Chemtob S & Hardy P Hypoxia up-regulates CD36 expression and function via hypoxia-inducible factor-1- and phosphatidylinositol 3-kinase-dependent mechanisms. J. Biol. Chem. 284, 26695–26707 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang P et al. Dietary oleic acid-induced CD36 promotes cervical cancer cell growth and metastasis via up-regulation Src/ERK pathway. Cancer Lett. 438, 76–85 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Pan J et al. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3beta/beta-catenin pathway. J. Exp. Clin. Cancer Res. 38, 52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li J et al. TCPA: a resource for cancer functional proteomics data. Nat. Methods 10, 1046–1047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bocharov AV et al. Synthetic amphipathic helical peptides targeting CD36 attenuate lipopolysaccharide-induced inflammation and acute lung injury. J. Immunol. 197, 611–619 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Wang S et al. Development of a prosaposin-derived therapeutic cyclic peptide that targets ovarian cancer via the tumor microenvironment. Sci. Transl. Med. 8, 329ra334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coronella J et al. Selective activity against proliferating tumor endothelial cells by CVX-22, a thrombospondin-1 mimetic CovX-Body. Anticancer. Res. 29, 2243–2252 (2009). [PubMed] [Google Scholar]
- 125.Pascual G et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Pascual G et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 599, 485–490 (2021). [DOI] [PubMed] [Google Scholar]
- 127.Lee K, Kerner J & Hoppel CL Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J. Biol. Chem. 286, 25655–25662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McGarry JD, Mannaerts GP & Foster DW A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Investig. 60, 265–270 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rasmussen BB et al. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J. Clin. Invest. 110, 1687–1693 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu Y et al. Identification of CPT1A as a prognostic biomarker and potential therapeutic target for kidney renal clear cell carcinoma and establishment of a risk signature of CPT1A-related genes. Int. J. Genomics 2020, 9493256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lopaschuk GD, Wall SR, Olley PM & Davies NJ Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ. Res. 63, 1036–1043 (1988). [DOI] [PubMed] [Google Scholar]
- 132.Galluzzi L et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on cell death 2018. Cell death Differ. 25, 486–541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hornsveld M & Dansen TB The hallmarks of cancer from a redox perspective. Antioxid. Redox Signal. 25, 300–325 (2016). [DOI] [PubMed] [Google Scholar]
- 134.Gius D & Spitz DR Redox signaling in cancer biology. Antioxid. Redox Signal. 8, 1249–1252 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Zou Y et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 10, 1617 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miess H et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 37, 5435–5450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Itabe H, Yamaguchi T, Nimura S & Sasabe N Perilipins: a diversity of intracellular lipid droplet proteins. Lipids Health Dis. 16, 83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brasaemle DL et al. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 275, 38486–38493 (2000). [DOI] [PubMed] [Google Scholar]
- 139.Blanchette-Mackie EJ et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J. Lipid Res. 36, 1211–1226 (1995). [PubMed] [Google Scholar]
- 140.Wolins NE et al. Adipocyte protein S3–12 coats nascent lipid droplets. J. Biol. Chem. 278, 37713–37721 (2003). [DOI] [PubMed] [Google Scholar]
- 141.Brasaemle DL et al. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38, 2249–2263 (1997). [PubMed] [Google Scholar]
- 142.Lu X et al. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm. Genome 12, 741–749 (2001). [DOI] [PubMed] [Google Scholar]
- 143.Zheng P et al. Plin5 alleviates myocardial ischaemia/reperfusion injury by reducing oxidative stress through inhibiting the lipolysis of lipid droplets. Sci. Rep. 7, 42574 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cao Q et al. Overexpression of PLIN2 is a prognostic marker and attenuates tumor progression in clear cell renal cell carcinoma. Int. J. Oncol. 53, 137–147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang K et al. PLIN3 is up-regulated and correlates with poor prognosis in clear cell renal cell carcinoma. Urol. Oncol. 36, 343.e349–343.e319 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Xu G et al. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J. Biol. Chem. 280, 42841–42847 (2005). [DOI] [PubMed] [Google Scholar]
- 147.Bayat Mokhtari R et al. The role of Sulforaphane in cancer chemoprevention and health benefits: a mini-review. J. Cell Commun. Signal. 12, 91–101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tian S et al. Targeting PLIN2/PLIN5-PPARγ: sulforaphane disturbs the maturation of lipid droplets. Mol. Nutr. Food Res. 63, e1900183 (2019). [DOI] [PubMed] [Google Scholar]
- 149.Xiao Y, Bi M, Guo H & Li M Multi-omics approaches for biomarker discovery in early ovarian cancer diagnosis. EBioMedicine 79, 104001 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Graham J et al. Cytoreductive nephrectomy in metastatic papillary renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). J. Clin. Oncol. 36, 581–581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ouchi N, Parker JL, Lugus JJ & Walsh K Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arita Y et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 (1999). [DOI] [PubMed] [Google Scholar]
- 153.Hotta K et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 20, 1595–1599 (2000). [DOI] [PubMed] [Google Scholar]
- 154.Matsuzawa Y, Funahashi T, Kihara S & Shimomura I Adiponectin and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 24, 29–33 (2004). [DOI] [PubMed] [Google Scholar]
- 155.Miyoshi Y et al. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 9, 5699–5704 (2003). [PubMed] [Google Scholar]
- 156.Wei EK, Giovannucci E, Fuchs CS, Willett WC & Mantzoros CS Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J. Natl Cancer Inst. 97, 1688–1694 (2005). [DOI] [PubMed] [Google Scholar]
- 157.Goktas S et al. Prostate cancer and adiponectin. Urology 65, 1168–1172 (2005). [DOI] [PubMed] [Google Scholar]
- 158.Bråkenhielm E et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl Acad. Sci. USA 101, 2476–2481 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spyridopoulos TN et al. Low adiponectin levels are associated with renal cell carcinoma: a case-control study. Int. J. Cancer 120, 1573–1578 (2007). [DOI] [PubMed] [Google Scholar]
- 160.Liao LM et al. Prediagnostic circulating adipokine concentrations and risk of renal cell carcinoma in male smokers. Carcinogenesis 34, 109–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choi SH, Chun SY, Kim TH & Kwon TG Identifying the emerging role of adipokine as a diagnostic and prognostic biomarker of renal cell carcinoma. Urol. Oncol. 34, 259.e215–259 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Pinthus JH et al. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur. Urol. 54, 866–873 (2008). [DOI] [PubMed] [Google Scholar]
- 163.Yap NY, Yap FN, Perumal K & Rajandram R Circulating adiponectin as a biomarker in renal cell carcinoma: a systematic review and meta-analysis. Biomarkers 24, 607–614 (2019). [DOI] [PubMed] [Google Scholar]
- 164.de Martino M et al. Serum adiponectin predicts cancer-specific survival of patients with renal cell carcinoma. Eur. Urol. Focus. 2, 197–203 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Ito R et al. The impact of obesity and adiponectin signaling in patients with renal cell carcinoma: a potential mechanism for the “obesity paradox”. PLoS One 12, e0171615 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun G et al. The adiponectin-AdipoR1 axis mediates tumor progression and tyrosine kinase inhibitor resistance in metastatic renal cell carcinoma. Neoplasia 21, 921–931 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rajandram R, Perumal K & Yap NY Prognostic biomarkers in renal cell carcinoma: is there a relationship with obesity. Transl. Androl. Urol. 8, S138–S146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Y et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). [DOI] [PubMed] [Google Scholar]
- 169.Farooqi IS et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumar R et al. Association of leptin with obesity and insulin resistance. Cureus 12, e12178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Housa D, Housova J, Vernerova Z & Haluzik M Adipocytokines and cancer. Physiol. Res. 55, 233–244 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L & Sánchez-Margalet V Obesity and breast cancer: role of leptin. Front. Oncol. 10.3389/fonc.2019.00596 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perumal K et al. Role of leptin as a biomarker for early detection of renal cell carcinoma? No evidence from a systematic review and meta-analysis. Med. Hypotheses 129, 109239 (2019). [DOI] [PubMed] [Google Scholar]
- 174.Spyridopoulos TN et al. Inverse association of leptin levels with renal cell carcinoma: results from a case-control study. Hormones 8, 39–46 (2009). [DOI] [PubMed] [Google Scholar]
- 175.Zhu H, Li W, Mao S & Wang L Association between leptin level and renal cell carcinoma susceptibility and progression: a meta-analysis. J. Cancer Res. Ther. 14, 873–880 (2018). [DOI] [PubMed] [Google Scholar]
- 176.Goralski KB et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 282, 28175–28188 (2007). [DOI] [PubMed] [Google Scholar]
- 177.Wittamer V et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 198, 977–985 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lehrke M et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur. J. Endocrinol. 161, 339–344 (2009). [DOI] [PubMed] [Google Scholar]
- 179.Yoo W et al. HIF-1α expression as a protective strategy of HepG2 cells against fatty acid-induced toxicity. J. Cell Biochem. 115, 1147–1158 (2014). [DOI] [PubMed] [Google Scholar]
- 180.Stockwell BR et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin E et al. Roles of the dynamic tumor immune microenvironment in the individualized treatment of advanced clear cell renal cell carcinoma. Front. Immunol. 12, 653358 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Manzi M et al. Coupled mass-spectrometry-based lipidomics machine learning approach for early detection of clear cell renal cell carcinoma. J. Proteome Res. 20, 841–857 (2021). [DOI] [PubMed] [Google Scholar]
- 183.Mock A et al. Serum very long-chain fatty acid-containing lipids predict response to immune checkpoint inhibitors in urological cancers. Cancer Immunol. Immunother. 68, 2005–2014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Parisi LR, Li N & Atilla-Gokcumen GE Very long chain fatty acids are functionally involved in necroptosis. Cell Chem. Biol. 24, 1445–1454.e1448 (2017). [DOI] [PubMed] [Google Scholar]
- 185.Tamura K, Horikawa M, Sato S, Miyake H & Setou M Discovery of lipid biomarkers correlated with disease progression in clear cell renal cell carcinoma using desorption electrospray ionization imaging mass spectrometry. Oncotarget 10, 1688–1703 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lucarelli G et al. Integration of lipidomics and transcriptomics reveals reprogramming of the lipid metabolism and composition in clear cell renal cell carcinoma. Metabolites 10.3390/metabo10120509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Renehan AG, Tyson M, Egger M, Heller RF & Zwahlen M Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008). [DOI] [PubMed] [Google Scholar]
- 188.Peck B & Schulze A Lipid metabolism at the nexus of diet and tumor microenvironment. Trends Cancer 5, 693–703 (2019). [DOI] [PubMed] [Google Scholar]
- 189.Lien EC & Vander Heiden MG A framework for examining how diet impacts tumour metabolism. Nat. Rev. Cancer 19, 651–661 (2019). [DOI] [PubMed] [Google Scholar]
- 190.Petrelli F et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw. Open 4, e213520 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pischon T et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and nutrition (EPIC). Int. J. Cancer 118, 728–738 (2006). [DOI] [PubMed] [Google Scholar]
- 192.Chow WH, Dong LM & Devesa SS Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 7, 245–257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chow WH, Gridley G, Fraumeni JF Jr & Jarvholm B Obesity, hypertension, and the risk of kidney cancer in men. N. Engl. J. Med. 343, 1305–1311 (2000). [DOI] [PubMed] [Google Scholar]
- 194.Keaney JF Jr et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 23, 434–439 (2003). [DOI] [PubMed] [Google Scholar]
- 195.Wang Q et al. Circulating obesity-driven biomarkers are associated with risk of clear cell renal cell carcinoma: a two-stage, case-control study. Carcinogenesis 40, 1191–1197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bertrand LA et al. Obesity as defined by waist circumference but not body mass index is associated with higher renal mass complexity. Urol. Oncol. 35, 661.e661–661.e666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gan CL & Heng DYC New insights into the obesity paradox in renal cell carcinoma. Nat. Rev. Nephrol. 16, 253–254 (2020). [DOI] [PubMed] [Google Scholar]
- 198.Seon DY, Kwak C, Kim HH, Ku JH & Kim HS Prognostic implication of body mass index on survival outcomes in surgically treated nonmetastatic renal cell carcinoma: a single-institutional retrospective analysis of a large cohort. Ann. Surg. Oncol. 27, 2459–2467 (2020). [DOI] [PubMed] [Google Scholar]
- 199.Choi Y et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int. J. Cancer 132, 625–634 (2013). [DOI] [PubMed] [Google Scholar]
- 200.Lalani A-KA et al. Assessment of immune checkpoint inhibitors and genomic alterations by body mass index in advanced renal cell carcinoma. JAMA Oncol. 7, 773–775 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sanchez A et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 21, 283–293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang C et al. Association of dyslipidemia with renal cell carcinoma: a 1∶2 matched case-control study. PLoS One 8, e59796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang H & Peng DQ New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 10, 176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hao B et al. Preoperative serum high-density lipoprotein cholesterol as a predictor of poor survival in patients with clear cell renal cell cancer. Int. J. Biol. Markers 34, 168–175 (2019). [DOI] [PubMed] [Google Scholar]
- 205.van der Mijn JC et al. Combined metabolomics and genome-wide transcriptomics analyses show multiple HIF1α-induced changes in lipid metabolism in early stage clear cell renal cell carcinoma. Transl. Oncol. 13, 177–185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Motzer RJ et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 24, 16–24 (2006). [DOI] [PubMed] [Google Scholar]
- 207.Choueiri TK et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1814–1823 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jonasch E et al. Belzutifan for renal cell carcinoma in von Hippel–Lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen W et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Reinfeld BI et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gordan JD, Bertout JA, Hu C-J, Diehl JA & Simon MC HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11, 335–347 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Raval RR et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell Biol. 25, 5675–5686 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gordan JD et al. HIF-α effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell 14, 435–446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


