Abstract
Vaccination is the most feasible way of preventing rabies, an ancient zoonosis that remains a major public health concern globally. However, administration of inactivated rabies vaccination without adjuvants is always inefficient and necessitates four to five injections. In the current study, we explored the adjuvant characteristics of cordycepin, a major bioactive component of Cordyceps militaris, to boost immune responses against a commercially available rabies vaccine. We found that cordycepin could stimulate stronger phenotypic and functional maturation of dendritic cells (DCs). For animal experiments, mice were immunized 3 times with rabies vaccine in the presence or absence of cordycepin at 1-week interval. Analysis of T cell differentiation and serum antibody isotypes showed that humoral immunity was dominant with a Th2 biased immune response. These results were also supported by the raised ratio of follicular helper T cells (TFH) and germinal center B cells (GCB). Thus, titer of rabies virus neutralizing antibody (RVNAb) and rabies virus-specific memory B cells were both raised as a result. Furthermore, administration of cordycepin did not cause pathological phenomena or body weight loss. The findings indicate that cordycepin could be used as a promising adjuvant for rabies vaccines to get a higher range of protection without any side effects.
Keywords: Rabies vaccine, Cordycepin, Adjuvant
1. Introduction
Rabies is one of the oldest worldwide infectious diseases with highest mortality rate of all known diseases which cause roughly 59,000 deaths every year according to the World Health Organization [[1], [2], [3]]. This fatal disease is caused by the rabies virus (RABV), a non-segmented negative-strand RNA virus from the Rhabdoviridae family, Mononegavirales order, Lyssavirus genus, is responsible for this lethal illness [[4], [5], [6]]. Peripheral immunity is restricted during RABV infection because it is a model of a neurotropic RNA virus that causes neuronal dysfunction or injury in the central nervous system [7,8]. Vaccination is a common way to stop the spread of rabies. Each year, more than 15 million patients worldwide receive post-exposure prophylaxis (PEP) [[9], [10], [11]]. Inactivated vaccinations are still widely used because of their high level of safety and ease of storage, even though recombinant viral vectors have shown promise for research and development of novel rabies vaccines [12]. Due to the low immunogenicity, the WHO recommends 3 intramuscular administrations for pre-exposure vaccination and 5 for post-exposure vaccination with 28 days of vaccination [13]. However, the majority of rabies cases occur in developing countries, and several doses are prohibitively expensive, particularly in Asia and Africa [14]. As a result, improving vaccine immunogenicity is critical in order to build more efficient and cost-effective rabies vaccinations.
Adjuvants, substances added to vaccines, have long been known to boost immune responses to antigens and extend their longevity [15]. Adjuvants minimize the amount of antigen required for each vaccination dose and the frequency of vaccinations, while simultaneously improving antigen stability or sustained antigen release and, eventually, increasing immunogenicity [16]. The adjuvants superfamily includes a wide range of chemicals, particularly small or macromolecules capable of activating or enhancing immunological signaling or delivery mechanisms [17]. Even if adjuvants improve vaccine efficiency, there remain still restrictions. The presence of adjuvants in vaccines is sometimes blamed for potential negative effects [15]. Adjuvants must be thoroughly studied there to ensure that they do not create harmful and adverse effects when added to vaccine.
Adjuvantation of rabies vaccine was first reported in the 1960s [18,19] and Aluminum-based adjuvants were widely used to slow antigen release of rabies vaccine for over 70 years, however, insufficient induction of early antibody responses and limited cellular immunity was observed [20]. Lin et al. found that vaccines without it had a better effect and aluminium adjuvant was not permitted to be added into commercial products of rabies vaccine for human usage in China [21,22]. In addition, alum has the risk of causing aseptic abscesses, myofascitis, and eosinophilia [23]. The exploration of new types of safe adjuvants to improve the protection of rabies vaccine is under great demand.
Cordycepin (3′-deoxyadenosine), a natural bioactive component derived from Cordyceps militaris, has been demonstrated to have anti-cancer, antioxidant and antipathogenic properties [24]. Previous research has shown that cordycepin can inhibit colon cancer growth by altering the tumor immune microenvironment, which increases the activity of CD4+T, CD8+T, M1 type macrophages and NK cells [25]. Moreover, numerous studies indicate that cordycepin exerts anti-inflammatory properties against many diseases, including asthma, acute lung injury, hepatitis, Parkinson's disease and atopic dermatitis [26]. Our previous research has shown that cordycepin can accelerate HBsAg uptake by antigen presenting cells (APC) and increase HBsAg specific antibody production when paired with HBV vaccine [27]. These findings imply that cordycepin could be developed into a novel hepatitis B vaccine adjuvant.
In this study, we investigated the efficiency of cordycepin as an adjuvant for rabies vaccine. We combine cordycepin with a commercially available inactivated rabies vaccine and discovered that cordycepin could enhance the immunogenicity of rabies vaccine. Animal study demonstrated that cordycepin could improve rabies virus specific neutralizing antibodies and protect against virulent challenge.
2. Materials and methods
2.1. Mice, vaccine and antibodies
The commercially available rabies vaccine (Vero cell, S20043089) used in this study was purchased from Yangpu central hospital (Shanghai, China). 4–6 weeks female BALB/c mice were obtained from Shanghai SLAC Laboratory Animal Company and maintained under SPF conditions at Fudan University. Principles of laboratory animal care were followed and all procedures were conducted according to the guidelines established by the National Institutes of Health, and every effort was made to minimize suffering. This study was approved by the Animal Experiment Committee of Fudan University (ethical approval number 20170510). The analytic grade cordycepin was purchased from Beijing Century Bioko Bio-technology Co. (Beijing, China).
2.2. Immunization of mice and sample collection
For antibody detection, mice were divided randomly into 3 groups and each mouse was injected subcutaneously on days 0, 7 and 14 with PBS, vaccine (1 IU) or vaccine adjuvanted with cordycepin (1 IU+ 2 mg/kg). Serum samples were collected on days 0, 7, 14, 21 and 28 to determine the rabies-specific antibody titer and isotypes of the serum antibody. For the lymphocyte differentiation assay, mice were sacrificed 1 week after the last injection and splenocytes were collected for flow cytometry, ELISPOT assay or lymphocyte transformation test. For memory B cell ELISPOT, cells were collected 4 weeks after the last injection. For the RVNAb detection, mice were injected on day 0 with vaccine or vaccine adjuvanted with cordycepin. Total blood was collected on days 3, 5, 7, 9 and 14 to detect titers of RVNAb.
2.3. Culture of mouse Bone Marrow-derived DCs (BMDCs)
DCs were cultured as described previously [28]. Briefly, BM cells from BALB/c mice were separated on day 0 and cultured at a density of 2 × 106 cells/ml in complete RPMI 1640 medium in the presence of 10 ng/ml mouse rGM-CSF and 1 ng/ml rIL-4 (PeproTech, Rocky Hill, USA). On day 3, fresh medium was added and immature DCs were harvested to perform indicated experiments on day 5.
2.4. Flow cytometry
For DC maturation, DCs were collected on the 5th day and washed with PBS before stimulated with PBS, vaccine and vaccine adjuvanted with cordycepin. After 24 h culture (37 °C, 5 % CO2), 1 × 106 cells were collected and resuspended in 100 μL PBS, incubated with 2 μg FITC anti-mouse CD11c together with PE anti-mouse CD80 or PE anti-mouse CD86 antibodies (All from Biolegend, San Diego, CA, USA) for 20 min on ice and washed twice with PBS. Finally, cells were suspended in 300 μl PBS and analyzed using a Calibur flow cytometer (BD Bioscience, USA). Data was analyzed using FlowJo software (Tree Star, San Caros, CA).
For mixed lymphocyte reaction, CD4+T lymphocyte was purified using a mouse CD4+T cell enrichment kit (Miltenyi Biotec, Bergisch Gladbach, Germany) from mouse spleens and incubated with 4 μM CFSE for 10 min in the dark followed by the additional of cold RPMI 1640 medium. After washing with cold RPMI 1640 medium twice, CD4+T cells were collected and mixed with DCs at a ratio of 10:1. Before mixed with T cells, DCs were collected and stimulated with PBS, vaccine and vaccine adjuvanted with cordycepin for 24 h. After 5 days’ co-culture, cells were collected and incubated with PE anti-mouse CD4 for 20 min on ice before analyzed with Gallios flow cytometer (Beckman coulter, Florida, USA). Data was analyzed using FCS express 5 software (Denovo Software, Glendale, CA).
For lymphocyte differentiation, a total of 1 × 106 spleen cells were collected and suspended in 100 μL PBS, incubated with FITC anti-mouse CD4, PE anti-mouse CD3 for Th cells, PE anti-mouse CXCR5, APC anti-mouse PD-1, FITC anti-mouse CD4 for follicular helper T cells (TFH) and APC anti-mouse CD95, FITC anti-mouse B220, PE anti-mouse GL-7 for germinal center B cells (GCBs) (All from Biolegend, San Diego, CA, USA). After incubating for 20 min on ice, cells were collected and washed twice with PBS before flow cytometry was performed using Calibur (BD Bioscience, USA) and Gallios flow cytometer (Beckman coulter, Florida, USA). Data was analyzed using FlowJo (Tree Star, San Caros, CA) and FCS express 5 software (Denovo Software, Glendale, CA).
2.5. Measurement of cytokines in cell culture supernatant
On the 5th day, DCs were stimulated with PBS, vaccine or vaccine adjuvanted with cordycepin. Cell supernatant was collected at 12 and 24 h after stimulation. Cytokines (IL-12p70, IL-6) were measured using the LEGEND MAX™ Mouse ELISA kit (Biolegend, San Diego, CA, USA) according to the manufacturer's instructions. Briefly, diluted cytokine standards and samples were added and incubated at room temperature, then mouse IL-12p70 or IL-6 detection antibody was added. After incubation and washes, avidin-HRP solution was added followed by the substrate solution and stop solution. Absorbance was measured at 450 nm immediately on SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA).
2.6. Lymphocyte transformation test (LTT)
Splenocytes were collected and suspended at a density of 106/ml in a 96-well plate (100 μL/well). Cells were treated with concanavalin A (Con A, Sigma-Aldrich, USA) for non-specific stimulation and with rabies vaccine for specific stimulation. After incubated in a humidified 5 % CO2 incubator at 37 °C for 48 h, 10 μl CCK-8 solution (Cell Counting Kit-8, Beyotime Biotechnology, China) was added followed by 4 h's incubation. The OD450 was measured immediately on SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA).
2.7. ELISPOT assay
Percentage of IFN-γ or IL-4 secreting CD4+ T cells was analyzed with Mouse IFN-γ and IL-4 ELISPOT kits (DAKEWE, China). Briefly, splenocytes were isolated and a total of 106 cells were seeded into 96-wells plate. All cells were stimulated with according antigen followed by 48 h incubation in a humidified incubator at 37 °C and 5 % CO2. Then, plates were washed and processed according to the manufacturer's protocol, spots were scanned and enumerated by SpotReader B1 (SLT Instruments, UK).
Percentage of rabies specific memory B cells was analyzed with a B cell ELISPOT kit (U-CyTech Biosciences, Yalelaan, Netherlands). Briefly, splenocytes were suspended at a density of 5 × 106/ml and stimulated with IL-2+R848 for 2–3 days at 37 °C with 5 % CO2 in a humidified atmosphere. 100 μl stimulated cells were added into the coated wells and incubated for another 5–6 h. After washing the plate, detection antibody was added followed by the addition of streptavidin-HRP conjugate and substrate solution, spots were scanned and enumerated by SpotReader B1 (SLT Instruments, UK).
2.8. Measurement of serum antibody isotypes using ELISA methods
The rabies-specific serum antibody isotypes were measured using ELISA as described [29,30]. Briefly, 96-well plates (Grenier, Frickenhausen, Germany) were coated with 0.005 IU/well vaccine (CDBIO, Liaoning, China) overnight at 4 °C followed by blocking with 5 % bovine serum albumin in PBST. Then, 100 μl/well serially diluted mouse IgG1, IgG2a (both from Abcam, Cambridge, UK) or serum samples were added and followed by incubation with HRP conjugated goat anti-mouse IgG antibodies (Santa Cruz, CA, USA). The plates were incubated for 1 h at room temperature. After washing, 100 μl/well TMB substrate (BD Biosciences, San Diego, CA, USA) was added and the reactions were terminated by the addition of 50 μl/well 2 M H2SO4. The absorbance of 495 nm was immediately read on a microplate reader (Molecular devices, Sunnyvale, CA, USA). A standard curve of IgG1 or IgG2a was used to transform absorbance to the antibody concentration.
2.9. Rapid fluorescent focus inhibition test
The concentration of RVNAb was detected by the rapid fluorescent focus inhibition test (RFFIT) (Wang et al., 2008). Briefly, serum samples were heated at 56 °C for 30min to inactivate complement and diluted three fold in a 96-well plate. Titrated CVS was added and mixtures were incubated at 37 °C for 1 h, followed by the addition of 4 × 104 BHK-21 cells and another incubation for 24 h at 37 °C, fixed with 80 % acetone solution and treated with FITC conjugated anti-nucleoprotein antibody (Biorbyt, UK) for 30 min in the dark. Finally, numbers of fluorescent foci were counted under a fluorescence microscope and the RVNAb titer was calculated as compared to the titer of national reference standard serum by the Reed and Muench method and expressed as international unit per milliliter (IU/ml).
2.10. Virus challenge
For the PEP study, RABV seronegative Golden Hamster were assigned to three groups (n = 8) to evaluate the effects of cordycepin. The mice were injected i.m. with 100 μL of mouse brain suspension containing 50 LD50 of BD06. At 2 h after exposure, the animals received PBS, vaccine control or rabies vaccine adjuvanted with cordycepin. All mice were vaccinated on days 0, 3, 7, 14, 28 and monitored for 30 days for the disease development and death. Mice were euthanized by CO2 intoxication once any sickness was observed. The BD06 virus strain used was maintained at Academy of Military Medical Sciences.
2.11. Statistical analysis
Tests for normal distribution of data, a two-sided Student's t-test and one-way ANOVA for paired or unpaired data was determined using the SPSS19 software (SPSS Inc., Chicago, IL, USA). Differences between experimental and control samples with a P < 0.05 were considered statistically significant.
3. Results
3.1. Cordycepin adjuvanted rabies vaccine enhances the production of rabies specific antibody
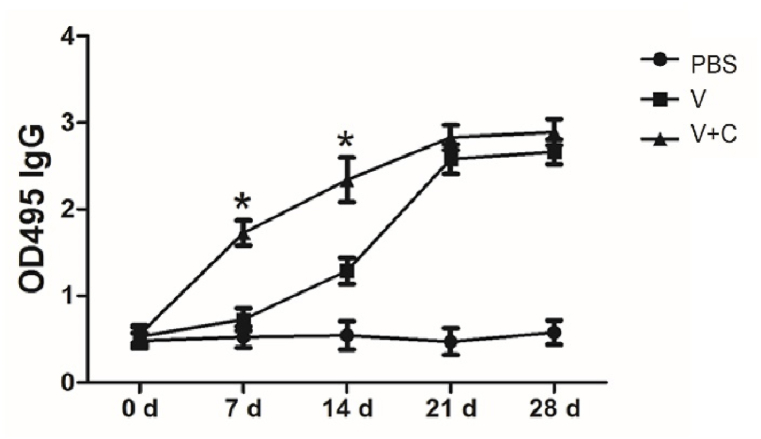
The most crucial aspect of evaluating a novel vaccine is antibody production. Following injection, rabies specific IgG was tested. Fig. 1 shows that the cordycepin adjuvanted rabies vaccine elicits significantly more IgG than vaccine group on days 7 and 14, implying that the novel vaccine may elicit earlier protection during virus defense.
Fig. 1.
Cordycepin could raise the level of rabies specific IgG. Mice (n = 5/group) were subcutaneous injected with PBS, vaccine (V) or vaccine adjuvanted with cordycepin (V + C) for 3 times with an interval of 7 days and serum samples were collected on indicated time points. Levels of rabies specific IgG were analyzed by ELISA. Results are presented as the mean ± S.D. ∗p < 0.05.
3.2. Cordycepin adjuvanted rabies vaccine elicits maturation of DCs
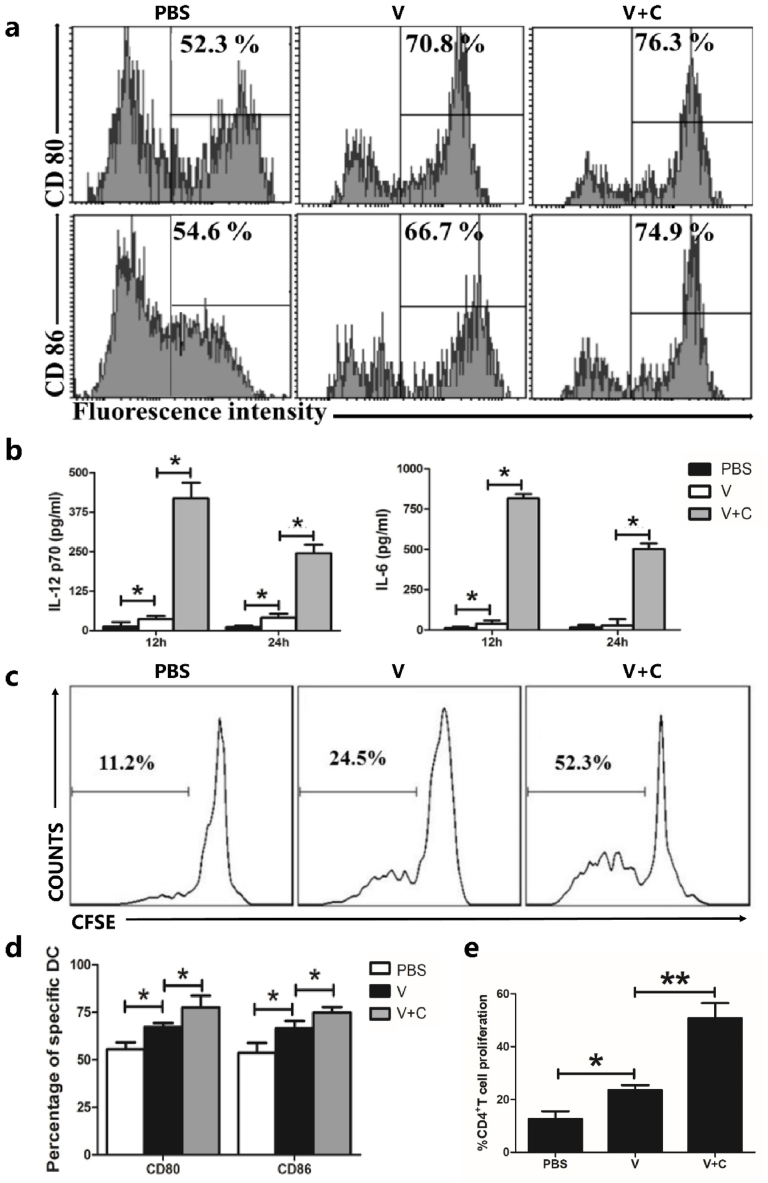
T cell activation required increased expression of costimulatory molecules during DC maturation. To test their potential to induce DC maturation, the phenotypes of DCs were analyzed by flow cytometry after treatment with PBS, vaccine alone (0.1 IU/106 cells), or cordycepin (100 ng/106 cells) adjuvanted vaccine. Surface expression of CD80 and CD86 increased following stimulation, according to Fig. 2a and d, and rabies vaccine induced lower expression rates than cordycepin adjuvanted groups.
Fig. 2.
Influence of cordycepin adjuvanted rabies vaccine on dendritic cells maturation in vitro. (a) Costimulatory molecule expression in BMDC was upregulated by cordycepin adjuvanted vaccine. A total of 1 × 106 cells were collected and stimulated with PBS, vaccine (0.1 IU) (V) or vaccine adjuvanted with cordycepin (150 ng) (V + C) for 24 h. Cells were stained and analyzed by flow cytometry. (b) Cytokines produced by DCs after stimulation with vaccine or vaccine adjuvanted with cordycepin. DCs were stimulated with PBS, vaccine (V) or vaccine adjuvanted with cordycepin (V + C), cell culture supernatant was collected on indicated time and levels of IL-12 p70 and IL-6 were determined using ELISA methods. (c) CD4+T lymphocyte proliferation induced by cordycepin adjuvanted rabies vaccine pretreated DCs. DCs were stimulated with PBS, vaccine (V) or vaccine adjuvanted with cordycepin (V + C) for 24 h before co-cultured with CFSE-labeled CD4+T cells. After culture for 5 days, cells were collected and stained with PE anti-mouse CD4 and analyzed by flow cytometry. (d) Bar graphs of (a). (e) Bar graphs of (c). Results are presented as the mean ± S.D. ∗p < 0.05.
3.3. Cordycepin adjuvanted rabies vaccine induces innate cytokines in vitro
We stimulated immature DCs with PBS, vaccine or vaccine adjuvanted with cordycepin and measured the cytokine (IL-12 p70 and IL-6) levels in cell culture supernatant at different time points to further evaluate the capacity of cordycepin adjuvanted vaccine to increase innate immunity in vitro. The cordycepin adjuvanted rabies vaccine induced much higher cytokine levels than the other groups, as seen in Fig. 2b. Both IL-12 p70 and IL-6 peaked at 12 h and began to decline at 24 h post stimulation. These findings demonstrated that cordycepin adjuvanted rabies vaccine is capable of stimulating the secretion of type I cytokine IL-12, p70 and pro-inflammatory cytokine IL-6, which may lead to the activation of antibody secretion.
3.4. Lymphocyte proliferation activity induced by cordycepin adjuvanted rabies vaccine pretreated DCs was enhanced
DC activation was further determined using a mixed lymphocyte reaction. Before co-cultured with stimulated DCs, CD4+T cells were isolated from the spleen and stained with CFSE. According to the results (Fig. 2c and e), untreated DCs could generate 11.2 % CD4+ T cell proliferation which vaccine-treated DCs caused 24.5 % proliferation. For cordycepin adjuvanted vaccine group, this number is 52.3 %, which is twice as high as the non-adjuvanted group. These findings suggest that cordycepin adjuvanted rabies vaccine can activate DCs more effectively.
3.5. Cordycepin adjuvanted rabies vaccine promotes the lymphocyte proliferation ability and influences the differentiation of Th cells
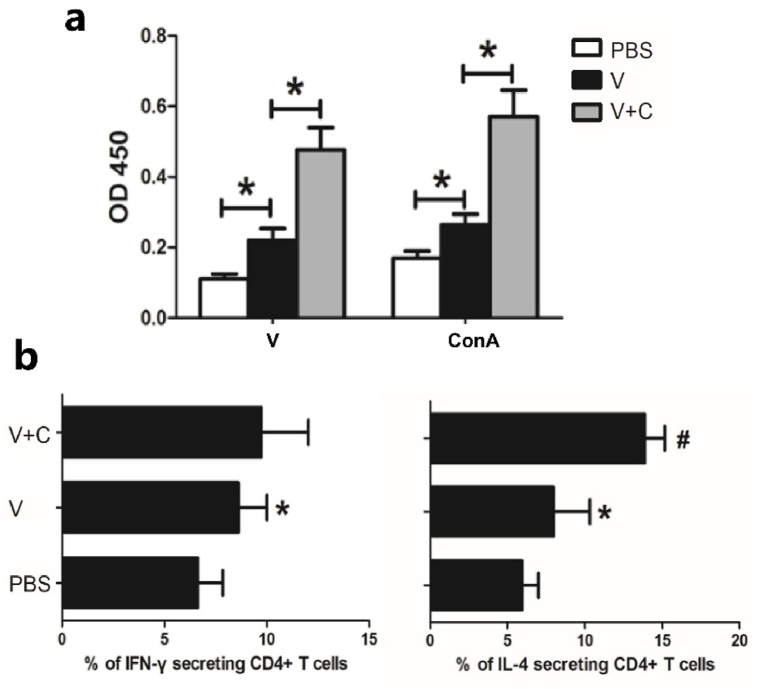
T-cell dependent responses are engaged once innate immunity is initiated, followed by B cell activation and antibody production. The CCK-8 assay was used to assess the lymphocyte proliferation ability of injected mice based on the improved immunogenicity of cordycepin adjuvanted vaccine. ELISPOT assays were also carried out to determine its ability to stimulate Th1 and Th2 responses. The lymphocyte proliferation rate was higher in the cordycepin adjuvanted group than the current vaccine (Fig. 3a). The cordycepin adjuvanted vaccine improved both Th1 (IFN-γ secreting cells) and Th2 (IL-4 secreting cells) responses, although there was no significant difference in Th1 ratio, indicating that Th2 response dominated (Fig. 3b).
Fig. 3.
Cordycepin adjuvanted vaccine enhances both Th1 and Th2 response. Mice (n = 5/group) were injected with PBS, vaccine (V) or vaccine adjuvanted with cordycepin (V + C) for 3 times with an interval of 7 days and splenocytes were harvested 14 days after the last injection. (a) Total lymphocyte proliferation activity was measured using CCK-8 methods. Nonspecific proliferation in response to ConA was used as positive control. (b) CD4+ T cells were isolated to measure the percentage of cytokine production cells using ELISAPOT assays. Results are presented as the mean ± S.D. ∗, represents p < 0.05 compared with PBS group and #, represents p < 0.05 compared with vaccine group.
3.6. Cordycepin adjuvanted vaccine enhances the differentiation of TFH and GCB cells
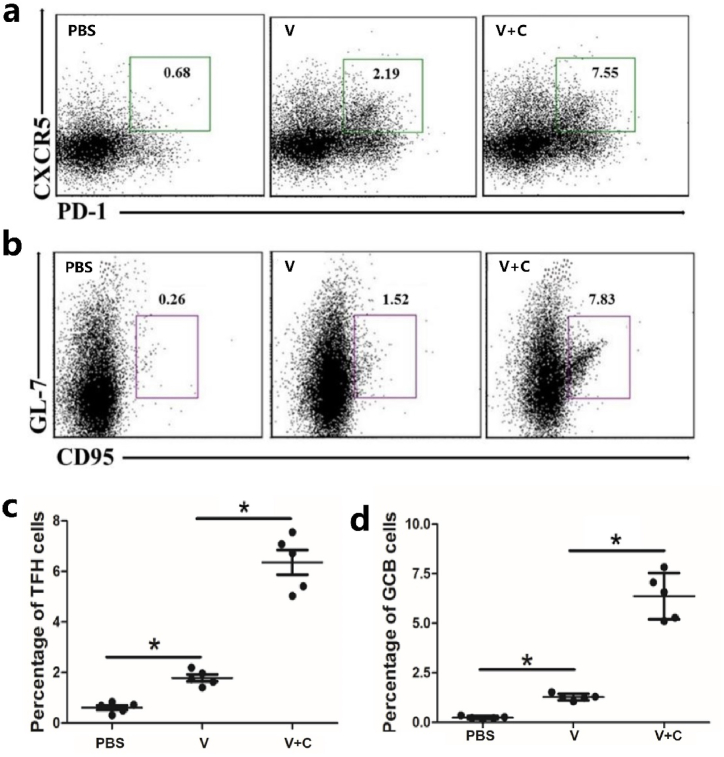
T follicular helper (TFH) cells are essential for B cell development. They can help B cells in germinal centers (GCs) become antibody-secreting cells or memory B cells. Flow cytometry was used to assess the percentages of TFH and GCB cells in different immune groups, and the results show that a higher percentage of TFH and GCB cells were induced by the cordycepin adjuvanted vaccine (Fig. 4a–d), indicating the new vaccine's enhanced antibody production activity.
Fig. 4.
Cordycepin adjuvanted vaccine enhances the differentiation of (a) TFH and (b) GCB cells. Mice (n = 5/group) were injected with PBS, vaccine (V) or vaccine adjuvanted with cordycepin (V + C) for 2 times with an interval of 7 days and splenocytes were harvested 3 days after the last injection, cells were incubated with labeled antibodies and analyzed by flow cytometry. (c) Each point represents a mouse of (a). (d) Each point represents a mouse of (b). ∗p < 0.05.
3.7. Cordycepin enhances the antibody production and memory B cell formation of rabies vaccine
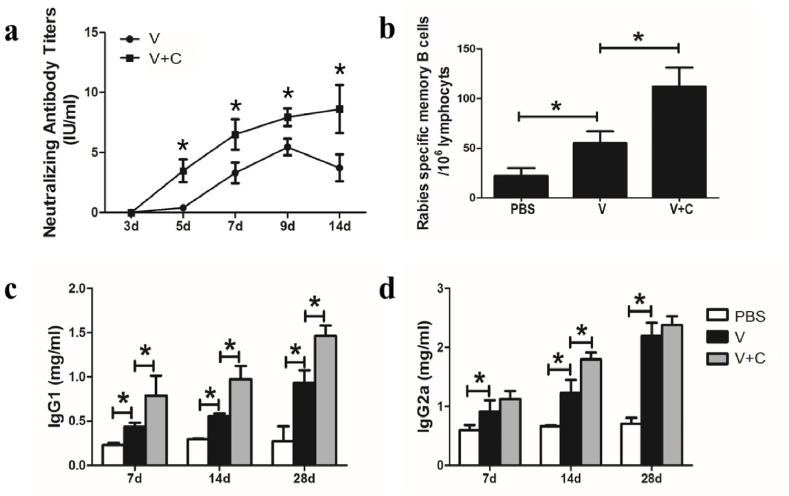
The serum RVNAb titer, which is connected to the outcomes of protection, is the most significant marker for the effectiveness of the rabies vaccine. We hypothesized that increased antibody production and memory B cell development would result from the increased ratio of GCB cells. As a result, RFFIT was used to assess RVNAb titers, and ELISPOT was used to determine the percentage of memory B cells specific to rabies. The results showed that compared to the vaccine group, the cordycepin adjuvanted rabies vaccine induced greater RVNAb production from days 5–14 (Fig. 5a). According to Fig. 5b, cordycepin also encourages the development of memory cells since memory B cells in the cordycepin adjuvanted group were considerably greater than those in the vaccine group.
Fig. 5.
Humoral response is induced by adjuvanted and non-adjuvanted rabies vaccines. (a) Mice were injected with a vaccine (V) or vaccine adjuvanted with cordycepin (V + C) on day 0 and RVNAb titers were evaluated by RFFIT method. Mice were immunized with three injections of vaccine (V), vaccine adjuvanted with cordycepin (V + C) at 1-week intervals. (b) Rabies specific memory B cells were evaluated by ELISPOT. (c) IgG1 and (d) IgG2a isotypes were evaluated by ELISA. Results are presented as the mean ± S.D. ∗p < 0.05.
3.8. Cordycepin adjuvanted vaccine induces different isotypes of serum IgG
The cordycepin adjuvanted vaccination increased the total blood IgG level. In order to investigate its function in adaptive immunity, IgG subclass distribution was assessed using ELISA techniques. In contrast to the vaccine group, the cordycepin adjuvanted rabies vaccine increased the blood levels of both IgG1 and IgG2a (Fig. 5c/5 d). It is possible that the cordycepin adjuvanted vaccine induces a Th2 biased immunoreaction to the rabies virus because IgG1 level was predominate in the cordycepin adjuvanted group while IgG2a only significantly differed on day 14 compared to the non-adjuvanted group.
3.9. Cordycepin adjuvanted rabies vaccine in defending virus challenge
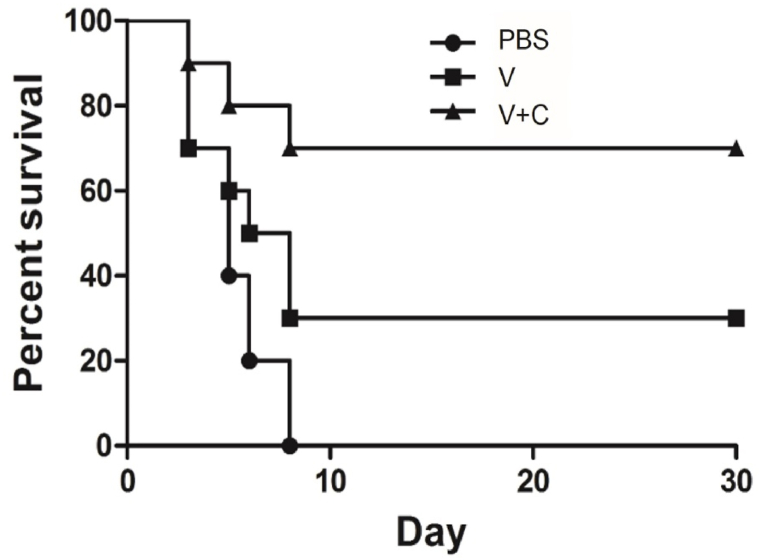
To determine whether cordycepin adjuvanted vaccine could induce enough protection against virus infection, post-exposure test was performed. All mice were infected with 50 LD50 rabies virus BD06, and by the third day, typical clinic signs were observed on these animals. The survival rate demonstrated in Fig. 6 revealed that all of the mice immunized with PBS died within 8 days. Meanwhile, 70 % of mice died in vaccine group, and the cordycepin adjuvanted group provided a 70 % survival rate respectively. Two weeks post challenge, there was still significant difference in the protective effect between the traditional vaccine group (30 %) and the adjuvanted group (70 %). These results revealed that the immune responses stimulated by cordycepin adjuvanted vaccine can provide protection against high dose of rabies virus challenge.
Fig. 6.
Protection assay induced by adjuvanted and non-adjuvated rabies vaccines. RABV seronegative Golden Hamster were challenged with 50 LD50 of wild rabies virus through intramuscular injection, and followed by immunization with PBS, rabies vaccine control or vaccine plus cordycepin on day 0, 3, 7, 14, 28. The survival of mice was observed for 30 days, and data were analyzed using GraphPad Prism 5.
3.10. Cordycepin did not cause immune toxicity
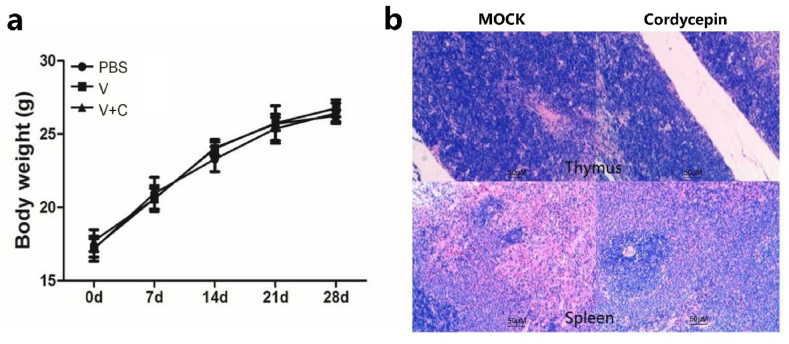
The body weight of each mouse was measured during experiments to evaluate the potential adverse effects of cordycepin, and a high dose of cordycepin was injected to detect the tissue pathological change. As demonstrated in Fig. 7a and b, no harmful effects were observed in our studies, indicating that cordycepin is safe to use as an adjuvant.
Fig. 7.
Cordycepin did not cause immune toxicity. (a) Mice were injected with different reagents at 7-day intervals for 3 times and body weight of each mouse was measured at different time points. (b) Mice were injected with a high dose of cordycepin for 3 times at 7-day intervals and sacrificed 7 days after the final injection. Representative photomicrographs of thymus and spleen are shown with original magnification: × 200 (HE staining).
4. Discussion
Unlike most pre-exposure vaccinations, the rabies vaccine is often administered following rabies virus exposure [31]. As a result, it is critical to ensure that the rabies vaccine induces an earlier and higher virus-neutralizing antibody titer. On account of the limited immunogenicity of existing inactivated rabies vaccines, a complete PEP schedule needed at least 4 injections, making it unaffordable for residents in developing counties [20]. Given these considerations, adjuvants capable of eliciting a quick antibody response and promoting early anti-viral activity are optimal for inactivated vaccines. In a prior investigation, we discovered that cordycepin can boost the immunogenicity of a hepatitis B vaccination. In this investigation, we discovered that cordycepin increased antibody production when paired with a commercially available rabies vaccine. Then, its adjuvanticity was evaluated through various parameters.
Antigen presenting cells (APCs) are essential for the activation of the host immune system. Many vaccines are considering stimulation of dendritic cells (DCs) because of their ability to link innate and adaptive immunity as the most effective APCs [[32], [33], [34]]. The expression of costimulatory molecules increases and cytokines are secreted during DC activation [35]. According to our results, the addition of cordycepin to rabies vaccine boosted the expression of CD80 and CD86, indicating the maturation of DCs. Cytokines also play a significant role in the immune process, with IL-6 being required for the development of plasma cells and IL-12 p70 acting as a bridge between innate and adaptive immunity [29,36]. The cordycepin adjuvanted rabies vaccine stimulated the synthesis of IL-6 and IL-12 p70, which are crucial for the establishment of immunologic synapse [37]. Immature DCs dwell in non-lymphoid tissues before migrating to regional lymph nodes to initiate a T cell response [38,39]. DCs primed with cordycepin adjuvanted rabies vaccine could stimulate the proliferation of CD4+ T cells more efficiently. This could be due to increased expression of the costimulatory molecules.
Th cell differentiation is critical for the development of immunological responses [40]. Th1 and Th2 lymphocyte balance is required for immunological homeostasis [41], with Th1 cells producing IFN-γ to promote cellular immune response and Th2 cells producing IL-4 to promote humoral immunity [42]. Because cordycepin adjuvanted rabies vaccine increased total lymphocyte proliferation activity, we investigated whether cordycepin may impact the Th1/Th2 balance. The results reveal that rabies vaccine adjuvanted with cordycepin generates a Th2-biased immune response. Th2 secreted IL-4 is known as the “B cell growth factor” and is associated with antibody production. Follicular helper T cells (TFH) are another group of CD4+ T helper cells that play a key role in the formation and maintenance of germinal center B cells (GCBs). It is required for GCB cell development into high-affinity long-lived plasma cells and memory B cells [[43], [44], [45]]. In mice, IL-6 produced by DCs can activate the transcriptional repressor Bcl-6, which is required for TFH cell differentiation [46]. Thus, the raised ratio in TFH and GCB could be linked to the high level of IL-6 induced by cordycepin adjuvanted rabies vaccine.
Antibody production is the most considerable part in the development of rabies vaccine to combat the virus's rapid spread. We discovered that mice received rabies vaccine with cordycepin as an adjuvant has significantly higher and earlier RVNAb levels, suggesting that they may have formed effective immune protection prior to the viral epidemic. Furthermore, both IgG1 and IgG2a production is accelerated, and IgG1-biased Th2 response dominates, which is consistent with prior findings. A Th2 dominant response indicates that humoral immunity will play a dominant part in defending against the rabies virus. Furthermore, the cordycepin adjuvanted vaccine boosted rabies virus-specific memory B cells as a result of the raised ratio of GCB cells.
The present study indicated that vaccine combined with cordycepin provided an enhanced protection against rabies virus in an animal model, which might be mediated by enhanced T cell differentiation and antibody production. Although cordycepin improved the immunogenicity of rabies vaccine, there were limitations in the present study. During our study, we did not measure the antigen-specific cellular immune responses induced by cordycepin adjuvanted rabies vaccine. According to prior research, Th1-biased immune responses have critical role in preventing rabies infection [31,47] and it is believed that Th1 type cytokines are critical for early anti-viral activities [48]. Thus, the influence of cordycepin on Th1 activation should be evaluated further. In conclusion, our findings highlighted the potential utility of cordycepin in developing a more effective and affordable rabies vaccine adjuvant which may be beneficial to the control of rabies.
Patient consent for publication
Not applicable.
CRediT authorship contribution statement
Xin Chen: Methodology, Formal analysis, Data curation. Boyu Liao: Software, Methodology, Investigation, Formal analysis, Data curation. Tianci Ren: Software, Investigation. Zhipeng Liao: Validation, Methodology. Zijie Huang: Software. Yujuan Lin: Methodology. Shouhao Zhong: Investigation. Jiaying Li: Methodology. Shun Wen: Investigation. Yingyan Li: Methodology. Xiaohan Lin: Software. Xingchen Du: Software. Yuhui Yang: Formal analysis, Data curation. Jiubiao Guo: Supervision, Software. Xiaohui Zhu: Supervision, Methodology, Data curation. Haishu Lin: Supervision, Project administration. Rui Liu: Visualization, Supervision, Formal analysis, Data curation, Conceptualization. Jingbo Wang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Youth Innovative Talents Project of Guangdong Education Department (No. 2021KQNCX082) and the Science and Technology Project of Shenzhen (NO. JCYJ20180228164407689).
Contributor Information
Rui Liu, Email: liur1@shrcb.com.
Jingbo Wang, Email: wangjingbo@sztu.edu.cn.
References
- 1.Gilbert A., et al. Antibody response of cattle to vaccination with commercial modified live rabies vaccines in Guatemala. Prev. Vet. Med. 2015;118(1):36–44. doi: 10.1016/j.prevetmed.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Koraka P., et al. A recombinant rabies vaccine expressing the trimeric form of the glycoprotein confers enhanced immunogenicity and protection in outbred mice. Vaccine. 2014;32(36):4644–4650. doi: 10.1016/j.vaccine.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 3.Luo J., et al. Artesunate enhances the immune response of rabies vaccine as an adjuvant. Vaccine. 2019;37(51):7478–7481. doi: 10.1016/j.vaccine.2019.09.077. [DOI] [PubMed] [Google Scholar]
- 4.Dastkhosh M., et al. Cell culture extraction and purification of rabies virus nucleoprotein. Jundishapur J. Microbiol. 2014;7(9) doi: 10.5812/jjm.11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., et al. A CpG oligodeoxynucleotide acts as a potent adjuvant for inactivated rabies virus vaccine. Vaccine. 2008;26(15):1893–1901. doi: 10.1016/j.vaccine.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Callaway H.M., et al. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci. Adv. 2022;8(24) doi: 10.1126/sciadv.abp9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C.T., et al. Enhancement of blood-brain barrier permeability is required for intravenously administered virus neutralizing antibodies to clear an established rabies virus infection from the brain and prevent the development of rabies in mice. Antivir. Res. 2014;110:132–141. doi: 10.1016/j.antiviral.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur M., et al. Rabies vaccines: where do we stand, where are we heading? Expert Rev. Vaccines. 2015;14(3):369–381. doi: 10.1586/14760584.2015.973403. [DOI] [PubMed] [Google Scholar]
- 9.Kang H., et al. Chimeric rabies virus-like Particles containing membrane-Anchored GM-CSF enhances the immune response against rabies virus. Viruses. 2015;7(3):1134–1152. doi: 10.3390/v7031134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shwiff S., Hampson K., Anderson A. Potential economic benefits of eliminating canine rabies. Antivir. Res. 2013;98(2):352–356. doi: 10.1016/j.antiviral.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C., et al. Immunogenicity after pre- and post-exposure rabies vaccination: a systematic review and dose-response meta-analysis. Vaccine. 2021;39(7):1044–1050. doi: 10.1016/j.vaccine.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Chen C., et al. Monophosphoryl-lipid A (MPLA) is an Efficacious adjuvant for inactivated rabies vaccines. Viruses-Basel. 2019;11(12) doi: 10.3390/v11121118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwaki Y., et al. Enhancement of antibody production against rabies virus by uridine 5'-triphosphate in mice. Microb. Infect. 2014;16(3):196–202. doi: 10.1016/j.micinf.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Fisher C.R., Schnell M.J. New developments in rabies vaccination. Revue Scientifique Et Technique-Office International Des Epizooties. 2018;37(2):657–672. doi: 10.20506/rst.37.2.2831. [DOI] [PubMed] [Google Scholar]
- 15.Brai A., et al. Progress towards adjuvant development: focus on Antiviral Therapy. Int. J. Mol. Sci. 2023;24(11) doi: 10.3390/ijms24119225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negm I.I., Ragab Y.M., Mohamed A.F. Outer membrane proteins of as an adjuvant in rabies vaccine. Clinical and Experimental Vaccine Research. 2021;10(2):132–+. doi: 10.7774/cevr.2021.10.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morel S., et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29(13):2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 18.DiStefano D., et al. Immunogenicity of a reduced-dose whole killed rabies vaccine is significantly enhanced by ISCOMATRIX™ adjuvant, Merck amorphous aluminum hydroxylphosphate sulfate (MAA) or a synthetic TLR9 agonist in rhesus macaques. Vaccine. 2013;31(42):4888–4893. doi: 10.1016/j.vaccine.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 19.McKee A.S., Munks M.W., Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W.J., et al. Adjuvant activity of PCP-II, a polysaccharide from Poria cocos, on a whole killed rabies vaccine. Virus Res. 2019:270. doi: 10.1016/j.virusres.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Lin H., Perrin P. [Influence of aluminum adjuvant to experimental rabies vaccine] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1999;13(2):133–135. [PubMed] [Google Scholar]
- 22.Yao S.Y., et al. Staphylococcal enterotoxin C2 as an adjuvant for rabies vaccine induces specific immune responses in mice. Pathogens and Disease. 2018;76(5) doi: 10.1093/femspd/fty049. [DOI] [PubMed] [Google Scholar]
- 23.Singh D., et al. Adjuvant activity of ethanol extract of leaves with inactivated rabies virus antigen. Pharmaceut. Biol. 2017;56(1):25–31. doi: 10.1080/13880209.2017.1413662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei P.J., et al. Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury. J. Neuroinflammation. 2021;18(1) doi: 10.1186/s12974-021-02188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Q.F., et al. Cordycepin enhances anti-tumor immunity in colon cancer by inhibiting phagocytosis immune checkpoint CD47 expression. Int. Immunopharm. 2022;107 doi: 10.1016/j.intimp.2022.108695. [DOI] [PubMed] [Google Scholar]
- 26.Tan L., et al. Anti-inflammatory effects of cordycepin: a review. Phytother Res. 2021;35(3):1284–1297. doi: 10.1002/ptr.6890. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., et al. Systems Pharmacology-based strategy to screen new adjuvant for hepatitis B vaccine from Traditional Chinese Medicine Ophiocordyceps sinensis. Sci. Rep. 2017;7 doi: 10.1038/srep44788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y.C., Liu S.J. A TLR9 agonist enhances the anti-tumor immunity of peptide and lipopeptide vaccines via different mechanisms. Sci. Rep. 2015;5 doi: 10.1038/srep12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaur G., et al. rIL-22 as an adjuvant enhances the immunogenicity of rGroEL in mice and its protective efficacy against S. Typhi and S. Typhimurium. Cell. Mol. Immunol. 2015;12(1):96–106. doi: 10.1038/cmi.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeisy-Scott V., et al. Increased MDSC accumulation and Th2 biased response to influenza A virus infection in the absence of TLR7 in mice. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R., et al. Rabies virus lipopeptide conjugated to a TLR7 agonist improves the magnitude and quality of the Thi-biased humoral immune response in mice. Virology. 2016;497:102–110. doi: 10.1016/j.virol.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Yashiro T., et al. PU.1 Suppresses Th2 cytokine expression via silencing of GATA3 transcription in dendritic cells. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahoor M.A., et al. Genome-wide transcriptional profiling reveals that HIV-1 Vpr differentially regulates interferon-stimulated genes in human monocyte-derived dendritic cells. Virus Res. 2015;208:156–163. doi: 10.1016/j.virusres.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Calderon-Gonzalez R., et al. Identification and characterisation of T-cell epitopes for incorporation into dendritic cell-delivered Listeria vaccines. J. Immunol. Methods. 2015;424:111–119. doi: 10.1016/j.jim.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boks M.A., et al. MPLA incorporation into DC-targeting glycoliposomes favours anti-tumour T cell responses. J Control Release. 2015;216:37–46. doi: 10.1016/j.jconrel.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 36.Gao Q., et al. Effects of porcine epidemic diarrhea virus on porcine monocyte-derived dendritic cells and intestinal dendritic cells. Vet. Microbiol. 2015;179(3–4):131–141. doi: 10.1016/j.vetmic.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Jendrysik M.A., et al. NADPH oxidase-2 derived ROS dictates murine DC cytokine-mediated cell fate decisions during CD4 T helper-cell commitment. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsui K., Mori A., Ikeda R. Langerhans cell-like dendritic cells stimulated with an adjuvant direct the development of Th1 and Th2 cells in vivo. Clin. Exp. Immunol. 2015;182(1):101–107. doi: 10.1111/cei.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q.L., et al. Role of growth hormone in maturation and activation of dendritic cells via miR-200a and the Keap1/Nrf2 pathway. Cell Prolif. 2015;48(5):573–581. doi: 10.1111/cpr.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X., et al. Arctigenin exerts anti-colitis efficacy through inhibiting the differentiation of Th1 and Th17 cells via an mTORC1-dependent pathway. Biochem. Pharmacol. 2015;96(4):323–336. doi: 10.1016/j.bcp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Hochreiter R. TH1-promoting DNA immunization against allergens modulates the ratio of IgG1/IgG2a but does not affect the anaphylactic potential of IgG1 antibodies No evidence for the synthesis of nonanaphylactic IgG1. J. Allergy Clin. Immunol. 2003;112(3):579–584. doi: 10.1016/s0091-6749(03)01623-3. [DOI] [PubMed] [Google Scholar]
- 42.Maassen C.B.M., et al. Growth phase of orally administered Lactobacillus strains differentially affects IgG1/IgG2a ratio for soluble antigens: implications for vaccine development. Vaccine. 2003;21(21–22):2751–2757. doi: 10.1016/s0264-410x(03)00220-2. [DOI] [PubMed] [Google Scholar]
- 43.Choi Y.S., et al. LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 2015;16(9):980–990. doi: 10.1038/ni.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butler N.S., Kulu D.I. The regulation of T follicular helper responses during infection. Curr. Opin. Immunol. 2015;34:68–74. doi: 10.1016/j.coi.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allam A., et al. TFH cells accumulate in mucosal tissues of humanized-DRAG mice and are highly permissive to HIV-1. Sci. Rep. 2015;5 doi: 10.1038/srep10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L., et al. The transcription factor TCF-1 initiates the differentiation of TFH cells during acute viral infection. Nat. Immunol. 2015;16(9):991–999. doi: 10.1038/ni.3229. [DOI] [PubMed] [Google Scholar]
- 47.Liu R., et al. A novel rabies virus lipopeptide provides a better protection by improving the magnitude of DCs activation and T cell responses. Virus Res. 2016;221:66–73. doi: 10.1016/j.virusres.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Nair P.K.R., et al. Mechanism of macrophage activation by (1,4)-α-D-glucan isolated from. Int. Immunopharm. 2006;6(12):1815–1824. doi: 10.1016/j.intimp.2006.07.028. [DOI] [PubMed] [Google Scholar]