Abstract
Introduction:
Post-COVID-19 syndrome, or Long Covid (LC) refers to symptoms persisting 12 weeks after the COVID-19 infection. LC comprises a wide range of dysautonomia symptoms, including fatigue, breathlessness, palpitations, dizziness, pain and brain fog. This study tested the feasibility and estimated the efficacy, of a Heart Rate Variability Biofeedback (HRV-B) programme via a standardised slow diaphragmatic breathing technique in individuals with LC.
Methods:
LC patients underwent a 4-week HRV-B intervention for 10 minutes twice daily for 4 weeks using the Polar H10 ECG (Electrocardiogram) chest strap and Elite HRV phone application. Outcome measures C19-YRSm (Yorkshire Rehabilitation Scale modified), Composite Autonomic Symptom Score (COMPASS-31), WHO Disability Assessment Schedule (WHODAS), EQ5D-5L (EuroQol 5 Dimensions) and Root Mean Square of Successive Differences between heartbeats (RMSSD) using a Fitbit device were recorded before and after the intervention. The study was pre-registered at clinicaltrials.gov NCT05228665.
Results:
A total of 13 participants (54% female, 46% male) completed the study with high levels of independent use of technology, data completeness and intervention adherence. There was a statistically significant improvement in C19YRS-m (P = .001), COMPASS-31 (P = .007), RMSSD (P = .047), WHODAS (P = .02) and EQ5D Global Health Score (P = .009). Qualitative feedback suggested participants could use it independently, were satisfied with the intervention and reported beneficial effects from the intervention.
Conclusion:
HRV-B using diaphragmatic breathing is a feasible intervention for LC. The small sample size limits generalisability. HRV-B in LC warrants further exploration in a larger randomised controlled study.
Keywords: Post-COVID-19 condition, post-COVID-19 syndrome, dysautonomia, autonomic dysfunction, HRV biofeedback, sympathetic, parasympathetic, rehabilitation, technology
Introduction
Post-COVID-19 syndrome or Long Covid (LC) refers to persistent symptoms 12 weeks after SARS-COV2 infection and includes symptoms of physical fatigue, cognitive fatigue or ‘brain fog’, breathlessness, pain and psychological distress.1,2 An estimated 1.9 million people are reported to be affected by LC in the UK alone. 3 A general practitioner at an average-sized practice in the UK can expect to have 65 patients with LC. 4 The condition can be highly debilitating for some, particularly middle-aged individuals who were previously functioning at a high level and in demanding vocational roles. 5 Many will experience significant disruption to employment, social and caregiving roles and participation in society.
Many LC symptoms such as palpitations, dizziness, fatigue, pain and breathlessness can be explained by dysfunction of the Autonomic Nervous System (ANS) or dysautonomia.6 -11 Estimates of prevalence of dysautonomia in LC range from 2.5 to 67%. 8,10,12 Usually the ANS can maintain a finely tuned state of homeostasis or mount an appropriate stress response where necessary. 10 However, in dysautonomia, there is episodic dysregulation in the ANS, typically with sympathetic overdrive. Dysautonomia also plays a significant role in the symptomology of many long-term conditions including multiple sclerosis, Parkinson’s disease, diabetes mellitus, fibromyalgia, chronic fatigue syndrome and migraine. 13
One way of estimating and measuring autonomic function is through heart rate variability (HRV), as cardiac rate and rhythm are controlled largely by the ANS. A low HRV is associated with sympathetic nervous system activation, also described as a state of ‘fight or flight’. Higher HRV corresponds with parasympathetic nervous system activation and is believed to reflect a state of rest and recovery. Lower HRV has been observed to be associated with fatigue and pain symptoms of chronic fatigue syndrome/myalgic encephalomyelitis (ME/CFS) and fibromyalgia,14 -16 as well as other chronic physical and mental health pathologies including asthma, anxiety and stress.14 -18
When physiological parameters such as HRV are monitored in real-time with self-regulation techniques such as breathing techniques to increase parasympathetic activity through vagus nerve activation to influence the parameters, this is known as biofeedback.19,20 To the best of our knowledge, there have not yet been any studies of HRV-B in LC. However, HRV-B using breathing techniques has been tested in other chronic conditions such as asthma, 17 depression, 21 fibromyalgia 16 and post-traumatic stress 22 and a 2021 systematic review summarises these comprehensively. 14 The optimal breathing frequency to produce maximal increase in HRV varies for each individual but on average is between 5.5 and 6 breaths per minute and is known as resonant breathing.17,23,24 Resonant breathing helps to restore autonomic balance due to increased baroreflex gain and vagal activation.17,23 -25
The aim of this study is to determine the feasibility and impact of a structured HRV-B regime incorporating diaphragmatic breathing exercise, on LC and dysautonomia symptoms.
Methods
Study design
This was a phase 2 uncontrolled open-label feasibility study of a home technology-based HRV-B in 15 individuals with LC. Participants were identified through the Leeds COVID-19 Rehabilitation Service, based at Leeds Community Healthcare NHS Trust. Five individuals with LC contacted researchers to take part in the study after seeing details on clinicaltrials.gov but they could not be included as they were not patients under the Leeds service. The intervention period was 4 weeks for each participant and the total study period was 6 weeks. Four weeks was selected as an ideal study length as this is supported by biofeedback literature.13,18 The inclusion criteria were: age ⩾18 years, confirmed LC diagnosis as per the NICE criteria for post-covid syndrome, 1 self-rating of at least ‘moderate’ or ’severe’ on dysautonomia questions of palpitations or dizziness on the C19-YRSm 26 ; and abnormal NASA Lean Test (NLT)27 -29 (HR increase of 30 or ⩾120 bpm, OR, BP decrease of 20 mmHg systolic or 10 mmHg diastolic in the first 3 minutes of standing).
NASA Lean Test is an accepted measure of cardiovascular instability and is conducted at initial assessment clinic for all LC service users in the Leeds service. 12 The patient lies down for 2 to 5 minutes prior to the test with HR and BP taken each minute to calculate average supine values. They then stand with heels 6 inches from a wall and lean back against it with HR and BP taken each minute for 10 min. Abnormal results (as described above) are demonstrated through orthostatic hypotension or tachycardia on standing which are hallmarks of dysautonomia and therefore objectively quantifiable.
Exclusion criteria were inability to use the wearable or smartphone app technology, unable to independently consent, cardiac arrhythmia, unstable respiratory disease (except asthma management). Cognitive or mental health disorders would have been apparent at the initial consultation and through the written consent process. No participants had significant cognitive or mental health disorders.
Equipment and intervention
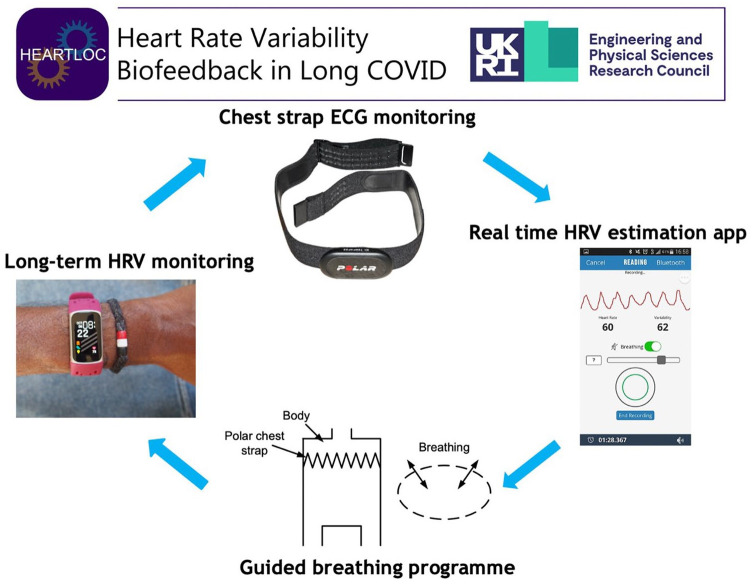
To collect medium-term HRV data, participants wore a Fitbit Charge 5 smartwatch continuously for a total of 6 weeks which collected nightly HRV data along with other measures of sleep. The HRV-B element was conducted using a Polar H10 chest strap for 10 minutes twice daily for a total of 4 weeks (Figure 1). This connected via Bluetooth to the Elite HRV smartphone app which was downloaded to participants’ phones. Researchers spent an initial induction consultation with participants to explain the technology and demonstrated the breathing technique and ensured that the participants could use the equipment independently. The diaphragmatic breathing taught was a 4-second inhale, and 6-second exhale through the nose for 10 minutes twice daily. This breathing pattern was felt to be simple to allow replication by participants at home and would produce 6 breaths per minute, a breathing frequency shown to induce HRV resonance.16,22,23 The exercises were instructed to be carried out lying down in bed upon waking in the morning and anytime in the evening before sleep. Compliance data was obtained from Elite HRV following the study to determine concordance with the intervention. Participants aimed to increase their HRV score as displayed in Elite HRV in real time using a diaphragmatic breathing technique (as explained in the protocol paper 30 ) which was the HRV-B element of the intervention and started after an initial 1 week baseline period in which HRV data was collected only by Fitbit to allow a baseline of HRV data for comparison to post-intervention. Omron M2 blood pressure monitor (endorsed by the British Hypertension Society) was used to conduct the NLT in the clinic. 31 The costs of each equipment item are approximately £130 for Fitbit Charge 5, £76.50 for Polar H10 chest strap and £40 for Omron M2 blood pressure monitor.
Figure 1.
Heart Rate Variability Biofeedback (HRV-B) using a breathing technique and chest strap for real time HRV monitoring. Polar H10 picture from Wikimedia commons, reprinted under CC BY-SA 3.0 license. EliteHRV screenshot from Wikimedia commons, reprinted under CC BY-SA 4.0 license.
Measures
The primary outcome measure was the COVID-19 Yorkshire Rehabilitation Scale modified version (C19YRSm). The C19YRS is the literature’s first validated condition-specific patient-recorded outcome measure in LC.32,33 The modified version of the scale comprises a symptom severity score (out of 30), functional disability score (out of 15), other symptoms score (out of 25) and overall health score (out of 10). 26
Secondary outcome measures
Fitbit data measures
These included root mean square of successive differences between heartbeats (RMSSD), sleeping resting heart rate, non-rapid eye movement sleep heart rate (NREM HR), overall sleep score (based on heart rate, time spent awake and sleep stages), composition score (based on sleep stages), revitalisation score (based on breathing disturbances and heart rate during sleep compared to awake), sleep duration score (length of time asleep compared to a user’s average bedtime and wake times) and deep sleep score (length of time in deep sleep and REM sleep)
Patient reported outcome measures
COMPASS (Composite Autonomic Symptom Score): The COMPASS 31 was completed by the participant at the initial visit and again 6 weeks later at the end of the study. Autonomic symptoms were scored for different domains including orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder and pupillomotor. Scores range from 0 to 100 representing severity of autonomic symptoms. A higher score represents greater severity. 34
World Health Organisation Disability Assessment Schedule (WHODAS): This is a 36-item scale captures 6 domains of life (cognition, mobility, self-care, getting along, life activities and participation) with a summary score ranging from 0 (no disability) to 100 (full disability)35,36
EQ-5D-5L: The EQ-5D-5L is a widely used quality of life measure, consists of 5 items covering: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. 37 The
A summary of the schedule for the completion of outcome measures is shown in Table 1.
Table 1.
Outcome measures summary schedule.
| Initial assessment clinic | Pre HRV-B phase (1 week) | HRV-B phase (4 weeks) | Post HRV-B phase (1 week) | |
|---|---|---|---|---|
| Autonomic screening (NASA Lean Test) | √ | √ | ||
| Autonomic function (COMPASS 31) | √ | √ | ||
| Fitbit wrist strap HRV, sleep data | √ | √ daily | √ | |
| LC specific PROM C19-YRSm | √ | √ weekly | √ | |
| WHODAS | √ | √ | ||
| Quality of life (EQ5D-5L) | √ | √ |
At the last visit, participants were informally asked what they felt about the technology and its effect on their LC symptoms. Their main comments were recorded in free text. We were not aiming to use structured interviews or undertake formal qualitative analysis of the comments made.
Analysis
Data from questionnaires were scored using standardised procedures for each measure. Data from the Fitbit was downloaded securely, cleaned and analysed using Python statistical platform. 2 participants were excluded from analysis, therefore a total of 13 individual datasets were analysed. Participant 6 could not complete the full 6 week study period due to unrelated health issues. Participant 12 was unwell during the final assessment with an acute respiratory infection. This was a cause extraneous to the study process and encompassed by our exclusion criteria therefore this participant was removed from data analysis. To explore the impact of the intervention, pre and post measures were compared on a within-participant basis using Wilcoxon signed rank tests. The pre-HRV phase was the baseline pre intervention period. The post-HRV-B phase data was used for post-intervention effect. Fitbit data and Elite HRV data for HRV were analysed during the HRV-B intervention phase and compared to pre-HRV-B data. A formal sample size calculation was not required for this feasibility study as it did not mimic a definitive randomised trial, and the aim was not to measure effect size. 38
Results
15 participants were enrolled in the study, out of which 13 completed the study and their data was included for the final analysis (Table 2). Table 3 shows the results of the NLT for the 13 participants. There was a 92% compliance rate with the breathing intervention and a total of 720 sessions were completed over the 4-week period. 92% compliance refers to 12 participants (out of 13) completing the 10-minute session twice daily.
Table 2.
Participant demographics.
| Patient demographics | Population, n = 13 |
|---|---|
| Age/years | 41.7 (11.8) |
| Mean (standard deviation, SD) | |
| Average time since initial COVID positivity/months | 14.7 (5.8) |
| Mean (standard deviation, SD) | |
| Gender | |
| Female n (%) | 7 (53.8) |
| Male n (%) | 6 (46.2) |
| Ethnicity | |
| White n (%) | 12 (92.3) |
| Mixed race n (%) | 1 (7.7) |
| Symptoms | |
| Fatigue n (%) | 10 (76.9) |
| Breathlessness n (%) | 8 (61.5) |
| Medication history | |
| Tricyclic antidepressants n (%) | 2 (15.9) |
| SSRI/ n (%) | 2 (15.9) |
| Codene/ibuprofen n (%) | 3 (23.1) |
| Ivabridine/bisoprolol n (%) | 3 (23.1) |
| Past medical history | |
| Anxiety/depression n (%) | 3 (23.1) |
| Atopy n (%) | 4 (30.8) |
Table 3.
Results of baseline NASA Lean test.
| Percentage of participants with significant HR changes | 92.3 % |
| Mean HR difference (SD) | +37 (7.4) bpm |
| Percentage of participants with significant BP changes | 40% |
| Average systolic BP Change (SD) | −10.2 (18.4) mm Hg |
| Average diastolic BP Change (SD) | 2.3 (14.0) mm Hg |
Statistically significant improvements were noted in Long Covid specific outcome measure C19YRS-m for symptom severity, functional ability, overall health score as well as total ‘other’ symptoms, COMPASS31, WHODAS and EQ5D-5L (Table 4). The effect size for C19-YRSm was large.
Table 4.
Change and effect size for the patient-reported outcome measure scores.
| Mean (SD) at baseline pre HRV-B phase | Mean (SD) at post HRV-B phase | Change (compared to baseline) | P value for paired pre/port difference | Effect size (Cohen’s d) | |
|---|---|---|---|---|---|
| C19YRS-m symptom severity | 19.5 (4.4) | 13.5(6.5) | −6 | 0.001 | 1.09 |
| C19YRS-m functional ability | 7.6 (3.4) | 5.2 (4.9) | −2.4 | 0.001 | 0.57 |
| C19YRS-m global health score | 3.9 (1.5) | 5.0 (1.8) | +1.1 | 0.014 | 0.66 |
| C19YRS-m total ‘other’ LC symptoms | 6.5 (4.5) | 4.0 (4.7) | −2.5 | 0.006 | 0.54 |
| COMPASS-31 | 38.5 (18.1) | 32.5 (19.6) | −6 | 0.008 | 0.32 |
| WHODAS disability scale | 36.3 (18.4) | 29.4 (20.2) | −6.9 | 0.021 | 0.36 |
| EQ5D-5L global health score | 46.3 (14.1) | 56.7 (19.9) | +10.4 | 0.008 | 0.60 |
C19YRS symptoms severity – range from 0 to 30, higher score = greater severity. C19YRS functional disability – range from 0 to 15, higher score = greater disability. C19yrs Global Health Score – range from 0 to 10, higher score = greater total health perception. C19YRS Total ‘other’ symptoms – total of 40 other miscellaneous symptoms possible. COMPASS-31 – range from 1 to 100, greater score = higher autonomic symptom burden. EQ5D-L Global Health Score – greater score = higher global health perception. WHODAS Disability Score – range from 0 to 100, higher score = greater disability.
There was a significant difference in RMSSD, a HRV measure reflecting parasympathetic activity, 14 between preHRV-B and post HRV-B phases (Table 5).
Table 5.
HRV data from Fitbit device.
| Mean (SD) at baseline pre HRV-B phase | Mean SD at post HRV-B phase | P value for paired difference between post HRV-B and baseline | Effect Size (Cohen’s d) | |
|---|---|---|---|---|
| Fitbit RMSSD summaries | 34.2 (19.6) | 40.9 (29.4) | 0.048 | 0.27 |
| Fitbit sleeping resting HR | 67.8 (6.5) | 67.7 (5.9) | 0.898 | 0.02 |
| Fitbit NREM HR | 66.6 (7.6) | 66.1 (7.1) | 0.542 | 0.07 |
| Fitbit overall sleep score | 76.8 (4.7) | 76.3 (5.1) | 0.848 | 0.10 |
| Fitbit composition score | 20.1 (1.2) | 19.7 (1.0) | 0.414 | 0.37 |
| Fitbit revitalisation score | 19.2 (1.9) | 18.7 (2.5) | 0.339 | 0.22 |
| Fitbit sleep duration score | 37.5 (3.2) | 37.9 (2.9) | 0.685 | 0.13 |
| Fitbit deep sleep score | 72.1 (22.1) | 69.2 (16.6) | 0.191 | 0.15 |
Abbreviations: HR, heart rate; NREM, non-rapid eye movement; RMSSD, Root Mean Square of Successive Differences between heartbeats.
The patient quotes, when asked about how they felt about the technology and its effect on their symptoms, are summarised in Table 6.
Table 6.
Participant feedback upon study completion.
| Participant No. | Qualitative Feedback Highlights |
|---|---|
| 1 | ‘Sleep is improved’ |
| ‘I’m Enjoying the exercise, it’s easier and more instinctive over time’ | |
| 2 | ‘Would continue doing the breathing exercises’ |
| ‘Feel more relaxed’ | |
| ‘Helped me get to sleep at night’ | |
| ‘Would do the exercises at work when my heart was going’ | |
| ‘Helps to control my panic’ | |
| 3 | ‘Technology was user friendly’ |
| ‘Improves sleep’ | |
| 4 | ‘Huge improvement in fatigue levels, not got the same levels of fatigue anymore’ |
| ‘No longer crashing’ | |
| ‘Able to start trying to go to work’ | |
| ‘The breathing exercises make me feel like I can cope with my situation and give me confidence in addressing my fatigue’ | |
| 5 | ‘Helps when I get palpitations or if I’m struggling to sleep’ |
| ‘I do the breathing exercises when I’m getting stressed’ | |
| ‘I don’t wake up stressed as much anymore’ | |
| ‘I will carry on doing the breathing exercises, even if I get better, I will always do them’ | |
| 7 | ‘Equipment was straightforward to use’ |
| ‘I will keep doing the breathing exercises afterwards’ | |
| ‘I found it hard to work it into my morning routine. That’s because I don’t have a very leisurely morning routine’ | |
| 8 | ‘I feel a lot better, my fatigue is better, I’m not falling asleep as much, my sleeping patterns has improved’ |
| ‘I’m feeling a lot more positive, I’m able to enjoy work and do my job again’ | |
| ‘I feel like I'm back to what I was pre covid’ | |
| 10 | ‘I feel massively better, it helps me manage my trigger of stress’ |
| ‘Physically a lot better now, I can think about doing exercise again’ | |
| 11 | ‘I do have some more energy’ |
| ‘When I do activity, I am able to do it for a longer period of time’ | |
| ‘I found the breathing quite difficult’ | |
| 13 | ‘It did help me get to sleep quicker at night’ |
| ‘I found the actual breathing exercises really hard compared to what I’ve done normally’ | |
| 14 | ‘Equipment was easy to use’ |
| ‘It definitely helps at nighttime, helped me sleep’ |
Discussion
The HRV-B intervention using diaphragmatic breathing, smartphone HRV application and an ECG chest strap appears feasible for participants with LC in a home-based setting. This study represents the first use of HRV-B with diaphragmatic breathing modulation in a LC population. The study adds to the notion that dysautonomia plays a critical role in LC symptom generation and interventions to target this may prove beneficial. It should be noted that unlike many potential LC interventions, diaphragmatic breathing is a non-pharmacological treatment, well tolerated and scalable with app-based technologies. The results of this study support a further controlled trial in LC to explore its actual efficacy on symptoms, particularly dysautonomia.
Other studies of HRV-B as an adjunct in chronic disease management have found similar positive results amongst other conditions. 14 In fibromyalgia, Hassett et al demonstrated significant improvement in fibromyalgia physical functioning scores which was evident at 3 months follow up (p 0.002). 15 As demonstrated by the qualitative feedback, participants were satisfied with the HRV-B practice which is comparable to other studies. 14 There are many indices for capturing HRV and most significant improvement was the RMSSD metric, reflecting greater parasympathetic activity. 14 This was also demonstrated by Shumann et al albeit in a different population of veterans with post-traumatic stress disorder, although no p-values were provided for comparison. 22
All participants reported that they appreciated the concept of the technology and found it easy to use and empowering. Challenges came with replacing the battery to the Polar H10 strap, and occasional issues with data not ‘syncing’ to the Elite HRV database. A researcher would call the participants weekly to troubleshoot technology issues which were most often fixed over the telephone. HRV data collected in this study was diverse and rich. The Elite HRV data was collected only during the 10 minute breathing intervention. The Fitbit collected data during sleep offered a reasonable longer-term measure of HRV. There was a significant improvement in RMSSD from the Fitbit data, however the effect size was not as pronounced as clinical outcome measures. The reason for this may be due to the fact that HRV is impacted by many factors including nutrition, sleep, exercise and menstrual periods in females.39 -41 We did not control for these factors and future trials should be large in size and may consider propensity score matching in an attempt to control for them to truly quantify effect of HRV-B on LC and dysautonomia symptoms whilst also being representative of a broader population.
The study had several limitations. It was a small open-label uncontrolled feasibility study, and we need to bear caution regarding the generalisability of study findings. The ideal study design would have been to include a control (standard care) group, but that will be done in future pilot and RCT studies. Our study group was homogenous with little ethnic diversity. We did not collect detailed information on educational background or income that affects access to the intervention and ease of use. The participants in this study are more likely to be confident in the use of a technological intervention and therefore need not be representative of the wider population of people with Long Covid. The study has however showed the technology was used in a home setting independently by individuals with LC. The changes in physiological parameters did not match the clinical outcome measures in terms of significance values and effect sizes. This can be explained by the World Health Organisation’s International Classification of Functioning Disability and Health (ICF) framework which suggests non-linear changes in different domains of the health condition (body function, functional activities and quality of life). The intervention duration could be argued to be short to have a reasonable effect on autonomic imbalance and future research could explore the relationship between dose (duration) and response. Finally, we did not undertake a long follow-up after stopping the use of technology. This needs to be explored in future larger-scale studies.
In summary, a diaphragmatic breathing technique using HRV-B is feasible to be used in a home setting by individuals with LC and the intervention seems to have a potential effect on improving LC symptoms, particularly those related to dysautonomia.
Acknowledgments
The authors would like to thank all study participants and the Leeds LC Advisory Group (PAG) for their involvement in all stages of this study.
Footnotes
Author Contributions: MS and AC conceptualised the study. MS, AC and RJOC were awarded EPSRC IAA pump-priming grant for the feasibility study with MS as the Principal Investigator. All authors contributed to the study design and obtained ethical approval. JC and NI performed the initial statistical analysis independently and verified the results. JC wrote an initial draft of the paper by adapting the grant proposal, the ethics protocol and the protocol paper. All authors approved the final manuscript. MS is the corresponding author and guarantor.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Manoj Sivan is an advisor to the World Health Organisation (WHO) for the long COVID policy in Europe. Manoj Sivan serves on the Editorial Board of the journal.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by IAA EPSRC grant [Ref 112538] with the University of Leeds as the sponsor organisation and the Leeds Community Healthcare NHS Trust Covid Rehabilitation service as the research site organisation.
Ethics and Dissemination: The study received ethical approval from Health Research Authority (HRA) Leicester South Research Ethics Committee (21/EM/0271).
Patient and Public Involvement: Members of the PAG provided input to analyse and develop the research aims, objectives and questions, ensuring these align with the key research priorities of those with LC and dysautonomia. All advisory group members have lived experience of LC. The PAG met quarterly to review progress, ensure the research continues to answer relevant issues and that findings can inform LC care.
References
- 1. National Institute for Health and Care Excellence (NICE) Scottish Intercollegiate Guidelines Network (SIGN) and Royal College of General Practitioners (RCGP). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. NICE; 2022. [Google Scholar]
- 2. Crook H, Raza S, Nowell J, et al. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 3. Office of National Statistics. Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK: 1 June 2022. ONS; 2022. [Google Scholar]
- 4. Greenhalgh T, Sivan M, Delaney B, et al. Long covid-an update for primary care. BMJ. 2022;378:e072117. [DOI] [PubMed] [Google Scholar]
- 5. Office for National Statistics. Coronavirus and the social impacts of ‘long COVID’ on people’s lives in Great Britain: 7 April to 13 June 2021. 2021. Accessed February 5, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronavirusandthesocialimpactsoflongcovidonpeopleslivesingreatbritain/7aprilto13june2021
- 6. Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med. 2021;21:e63-e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31(3):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larsen NW, Stiles LE, Shaik R, et al. Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults. Front Neurol. 2022;13:1012668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iftekhar N, Sivan M. Venous insufficiency and acrocyanosis in long COVID: dysautonomia. Lancet. 2023;402:e9. [DOI] [PubMed] [Google Scholar]
- 10. Carmona-Torre F, Minguez-Olaondo A, Lopez-Bravo A, et al. Dysautonomia in COVID-19 patients: a narrative review on clinical course, diagnostic and therapeutic strategies. Front Neurol. 2022;13:886609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sivan M, McKeever V, Natt M, et al. A global need for more awareness of dysautonomia in postviral syndromes. J Med Virol. 2023;95:e29048. [DOI] [PubMed] [Google Scholar]
- 12. Isaac RO, Corrado J, Sivan M. Detecting Orthostatic Intolerance in Long COVID in a Clinic Setting. Int J Environ Res Public Health. 2023;20:5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zalewski P, Slomko J, Zawadka-Kunikowska M. Autonomic dysfunction and chronic disease. Br Med Bull. 2018;128:61-74. [DOI] [PubMed] [Google Scholar]
- 14. Fournie C, Chouchou F, Dalleau G, et al. Heart rate variability biofeedback in chronic disease management: A systematic review. Complement Ther Med. 2021;60:102750. [DOI] [PubMed] [Google Scholar]
- 15. Escorihuela RM, Capdevila L, Castro JR, et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2020;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hassett AL, Radvanski DC, Vaschillo EG, et al. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl Psychophysiol Biofeedback. 2007;32:1-10. [DOI] [PubMed] [Google Scholar]
- 17. Lehrer PM, Vaschillo E, Vaschillo B, et al. Biofeedback treatment for asthma. Chest. 2004;126(2):352-361. [DOI] [PubMed] [Google Scholar]
- 18. Goessl VC, Curtiss JE, Hofmann SG. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol Med. 2017;47:2578-2586. [DOI] [PubMed] [Google Scholar]
- 19. Lehrer P, Vaschillo B, Zucker T, et al. Protocol for heart rate variability biofeedback training. Biofeedback. 2013;41:98-109. [Google Scholar]
- 20. Gevirtz R. The promise of heart rate variability biofeedback: evidence-based application. Biofeedback. 2013;41:110-120. [Google Scholar]
- 21. Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback. 2007;32:19-30. [DOI] [PubMed] [Google Scholar]
- 22. Schuman DL, Killian MO. Pilot study of a single session heart rate variability biofeedback intervention on veterans’ posttraumatic stress symptoms. Appl Psychophysiol Biofeedback. 2019;44:9-20. [DOI] [PubMed] [Google Scholar]
- 23. Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Front Psychol. 2014;5:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaschillo EG, Vaschillo B, Lehrer PM. Characteristics of resonance in heart rate variability stimulated by biofeedback. Appl Psychophysiol Biofeedback. 2006;31:129-142. [DOI] [PubMed] [Google Scholar]
- 25. Pagaduan JC, Chen YS, Fell JW, et al. Can heart rate variability biofeedback improve athletic performance? A systematic review. J Hum Kinet. 2020;73:103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sivan M, Preston N, Parkin A, et al. The modified COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm) patient-reported outcome measure for Long Covid or Post-COVID-19 syndrome. J Med Virol. 2022;94:4253-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bungo MW, Charles JB, Johnson PC., Jr. Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med. 1985;56:985-990. [PubMed] [Google Scholar]
- 28. Lee J, Vernon SD, Jeys P, et al. Hemodynamics during the 10-minute NASA Lean Test: evidence of circulatory decompensation in a subset of ME/CFS patients. J Transl Med. 2020;18:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hyatt KH, Jacobson LB, Schneider VS. Comparison of 70 degrees tilt, LBNP, and passive standing as measrues of orthostatic tolerance. Aviat Space Environ Med. 1975;46:801-808. [PubMed] [Google Scholar]
- 30. Corrado J, Halpin S, Preston N, et al. HEART rate variability biofeedback for long COVID symptoms (HEARTLOC): protocol for a feasibility study. BMJ Open. 2022;12:e066044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sivan M, Corrado J, Mathias C. The adapted autonomic profile (aAP) home-based test for the evaluation of neuro-cardiovascular autonomic dysfunction. Adv Clin Neurosci Rehabil. 2022;21:10-13. [Google Scholar]
- 32. Sivan M, Halpin S, Gee J, et al. The self-report version and digital format of the COVID-19 Yorkshire Rehabilitation Scale (C19-YRS) for Long Covid or Post-COVID syndrome assessment and monitoring. Adv Clin Neurosci Rehabil. 2021;20:2-5. [Google Scholar]
- 33. O’Connor RJ, Preston N, Parkin A, et al. The COVID-19 Yorkshire Rehabilitation Scale (C19-YRS): application and psychometric analysis in a post-COVID-19 syndrome cohort. J Med Virol. 2022;94:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sletten DM, Suarez GA, Low PA, et al. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87(12):1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garin O, Ayuso-Mateos JL, Almansa J, et al. Validation of the “World Health Organization Disability Assessment Schedule, WHODAS-2” in patients with chronic diseases. Health Qual Life Outcomes. 2010;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Üstün TB, Chatterji S, Kostanjsek N, et al. Developing the World Health Organization disability assessment schedule 2.0. Bull World Health Organ. 2010;88:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitehead AL, Sully BG, Campbell MJ. Pilot and feasibility studies: is there a difference from each other and from a randomised controlled trial? Contemp Clin Trials. 2014;38:130-133. [DOI] [PubMed] [Google Scholar]
- 39. Young HA, Benton D. Heart-rate variability: a biomarker to study the influence of nutrition on physiological and psychological health? Behav Pharmacol. 2018;29:140-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sajjadieh A, Shahsavari A, Safaei A, et al. The association of sleep duration and quality with heart rate variability and blood pressure. Tanaffos. 2020;19:135-143. [PMC free article] [PubMed] [Google Scholar]
- 41. Sandercock GR, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433-439. [DOI] [PubMed] [Google Scholar]