Abstract
Purpose
Photobiomodulation (PBM), encompassing low-energy laser treatment and light-emitting diode (LED) phototherapy, has demonstrated positive impacts on skin rejuvenation and wound healing. Organic light-emitting diodes (OLEDs) present a promising advancement as wearable light sources for PBM. However, the biological and biochemical substantiation of their skin rejuvenation and wound healing effects remains limited. This study aimed to ascertain the safety and efficacy of OLEDs as a next-generation PBM modality through comprehensive in vitro and in vivo investigations.
Materials and Methods
Cell viability assays and human ex vivo skin analyses were performed after exposure to OLED and LED irradiation to examine their safety. Subsequent evaluations examined expression levels and wound healing effects in human dermal fibroblasts (HDFs) using quantitative reverse transcription-polymerase chain reaction, enzyme-linked immunosorbent assay, and wound healing assays post-irradiation. Additionally, an in vivo study was conducted using a ultra violet (UV)-irradiated animal skin model to explore the impact of OLED exposure on dermal collagen density and wrinkles, employing skin replica and tissue staining techniques.
Results
OLED irradiation had no significant morphological effects on human skin tissue, but caused a considerably higher expression of collagen than the control and LED-treated groups. Moreover, OLED irradiation reduced the expression levels of matrix metalloproteinases (MMPs) more effectively than did LED on HDFs. OLED irradiation group in HDFs had significantly higher expression levels of growth factors compared to the control group, but similar to those in the LED irradiation group. In addition, OLED irradiation on photo-aged animal skin model resulted in increased collagen fiber density in the dermis while reducing ultra violet radiation-mediated skin wrinkles and roughness, as shown in the skin replica.
Conclusion
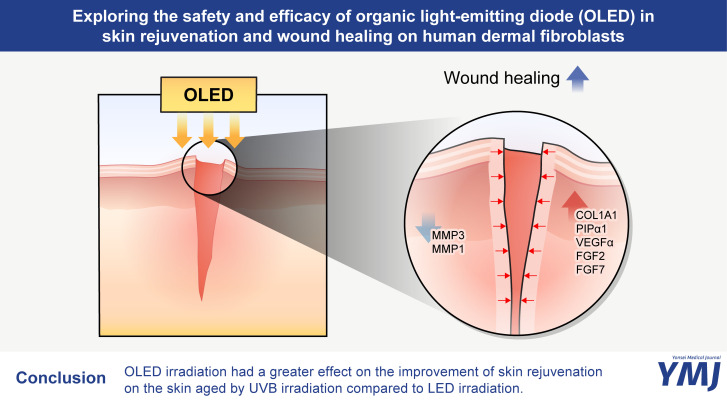
This study established comparable effectiveness between OLED and LED irradiation in upregulating collagen and growth factor expression levels while downregulating MMP levels in vitro. In the UV-irradiated animal skin model, OLED exposure post UV radiation correlated with reduced skin wrinkles and augmented dermal collagen density. Accelerated wound recovery and demonstrated safety further underscore OLEDs’ potential as a future PBM modality alongside LEDs, offering promise in the realms of skin rejuvenation and wound healing.
Keywords: Organic light-emitting diode, light-emitting diode, photobiomodulation, skin rejuvenation; wound healing
Graphical Abstract
INTRODUCTION
Photobiomodulation (PBM), which includes low-energy laser treatment and light-emitting diode (LED) phototherapy, has shown positive effects on skin rejuvenation.1 PBM acts via nonthermal or photochemical reactions, which are also referred to as photobiological or biostimulatory effects, in the irradiated cells.2,3 LED emits a narrow spectrum of noncoherent light in the near-infrared, visible, and ultraviolet ranges.4 Previous experimental models of PBM have shown the effect of LED phototherapy on mitochondria-mediated signaling pathways to preserve mitochondrial function, attenuate oxidative stress, stimulate the production of cytoprotective factors, and subsequently, prevent cell death.5 Mitochondria include not only chromophores that absorb photons emitted from PBM, but also cytochrome c oxidase that causes the various molecular activity such as calcium ions, ATP, nitric oxide, and reactive oxygen species.
A recent study described the anti-inflammatory effect of a 630 nm LED via the inhibition of reactive oxygen species and regulation of the nuclear factor-κB signaling pathway.6 In a prospective single-arm interventional study with a home-use neck LED device, a 630 nm/850 nm LED had a positive effect on skin rejuvenation after 16 weeks of daily use, which lasted for over 8 weeks of observation after the end of the device application.7 Continuous LED irradiation of visible and near-infrared wavelengths increases the synthesis of collagen and decreases the expression of matrix metalloproteinase (MMP) 1 and MMP2 in skin fibroblasts, thus producing a skin rejuvenation effect. It can also increase keratinocyte (KC) proliferation, renewing epidermal layer of the skin and thereby partially affecting skin rejuvenation and wound healing.8
However, traditional LED devices are usually relatively heavy and burdensome. In contrast, organic light-emitting diodes (OLEDs) are strong candidates to compete in the currently LED-based photorejuvenation market, as they can be constructed into desired shapes owing to their structural flexibility.9,10 Unlike LEDs, OLEDs are surface light sources that can uniformly distribute light over a wide area.11 Moreover, OLED is a useful source for PBM due to their safety, high efficiency, low power consumption, and lack of overheating. As OLED devices are flexible and lightweight, they can be easily transformed into wearable units, making it easier to utilize them as daily cosmeceutical devices compared to LED devices.
Previously, the efficacy of OLED has been investigated in several in vitro assessments. When 630–690 nm OLED was treated on KCs, cell proliferation and migration increased significantly without notable cell cytotoxicity; in the skin equivalent model, the epidermal thickness was increased by 39%, while highly inducing re-epithelialization in organ culture.12 Moreover, longer wavelengths near-infrared light in early 700 nm range emitted from OLED also demonstrated similar effect in vitro, suggesting that all wavelength regions using OLEDs produce beneficial effect on cell proliferation, with near-infrared wavelengths penetrating more deeply into the human skin to produce positive outcome.13 Thus, OLEDs enable frequent and repeated PBM treatments, thereby facilitating wound healing and skin rejuvenation.14
Although OLEDs are promising next-generation wearable light sources for PBM, biological and biochemical evidence for the skin rejuvenation and wound healing effects of OLED are lacking. A previous study on the effect of OLED irradiation on diabetic rat model for wound healing showed significantly higher percentage of wound closure on day 6 post-injury compared to the control,15 but its effect on skin aging and rejuvenation needs to be further investigated. Thus, we conducted a pilot study to screen the safety and efficacy of OLED-based PBM on skin rejuvenation and wound healing; particularly, these effects were analyzed via in vitro study through observing changes in collagen, MMP, and selected growth factor expression levels, along with wound healing assay on human dermal fibroblasts (HDFs). Furthermore, in vivo study with photo-aged skin animal model was performed to further investigate the effect of OLED on UV-exposed skin. Additional cell viability assay and ex vivo study were also performed to confirm the safety of OLED irradiation on human skin.
MATERIALS AND METHODS
Light source and irradiation protocol
The LED device, which emitted a wavelength of 628 nm, was provided by Samsung Display Co., Ltd. (Yongin, Korea). The spectral characteristics of the LED device were as follows: peak wavelength of 628±3 nm and full width at half maximum of approximately 15 nm. For comparison, OLED lighting devices with the same spectral characteristics were prepared. The spectral characteristics of the OLED device, which was also provided by Samsung Display Co., Ltd., were as follows: peak wavelength of 625–630 nm and full width at half maximum of approximately 35 nm. The radiant energy on the cell plates were adjusted to 0, 1.5, 3, 6, 9, 10, and 20 J/cm2. The adjusted effective irradiance values were calculated as follows: {[irradiance at target (mW/cm2)×exposure time (sec)]/1000 (mJ/J)=Radiant energy (J/cm2)}. As default parameters, the irradiance at target and the distance from the panel were pre-set for each light source by the manufacturer. For OLED, the irradiance at target was 4.6 mW/cm2 when the distance from the OLED panel and the cell plates were 2 cm away; for LED, the irradiance at target was 5 mW/cm2 when the distance from the LED panel and the cell plates were 4 cm away (Supplementary Fig. 1, only online). Detailed information of LED and OLED irradiation are shown in Table 1. Cells that were not irradiated by any light sources were used as controls.
Table 1. Detailed Information of LED and OLED Devices.
| Parameter | LED | OLED |
|---|---|---|
| Irradiance at target (mW/cm2) | 5 | 4.6 |
| Radiant energy (J/cm2) | 1.5, 3, 6, 9, 10, 20 | 1.5, 3, 6, 9, 10, 20 |
| Exposure time (sec) | 300, 600, 1200, 1800, 2000, 4000 | 326, 652, 1304, 1956, 2173, 4347 |
| Panel size (cm) | 14×20 | 8×16 |
LED, light-emitting diode; OLED, organic light-emitting diode.
Cell culture
HDF and human epidermal KC cells were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The HDF cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Lonza, Walkersville, MD, USA) containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and 1% penicillin–streptomycin (Gibco). KC cells were cultured in KBM Gold Basal Medium (Lonza) with KGM Gold Single Quots supplements (Lonza). All cells were incubated in a humidified atmosphere containing 5% CO2 at 37℃. When the seeded HDF cells (1×105 cells) reached approximately 80% confluence, the cells were irradiated with OLED or LED (625–630 nm) light, or untreated, and incubated for 24 h.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
To extract the total RNA, cells were irradiated and harvested after 24 h. Total RNA was extracted using an RNA extraction kit (RNeasy Mini Kit, Qiagen, Hilden, Germany), and cDNA was synthesized using an RNA-to-cDNA EcoDry Premix (Oligo dT; Clontech, Berkley, CA, USA). For qRT-PCR, TaqMan Gene Expression Master Mix (Applied Biosystems, Waltham, MA, USA) and TaqMan primers for each target (COL1A1, Hs00164004_m1; MMP1, Hs00899658_m1; VEGFα, Hs00900055_m1; MMP3, Hs00968305_m1; FGF2, Hs00266645_m1; FGF7, Hs00940253_m1; Applied Biosystems) were used. GAPDH (Hs02786624_g1, Applied Biosystems) was used as a housekeeping gene for the normalization of expression levels. The gene expression levels were calculated via the 2-ΔΔCt method. The experiment was independently repeated three times (n=3).
Enzyme-linked immunosorbent assay (ELISA)
HDF cells (1×104 cells/well) were seeded in a 96-well plate and incubated for 24 h. When they reached approximately 80% confluence, the cells were irradiated with OLED or LED (625–630 nm) light, or untreated, and incubated. After 24 h, to detect pro-collagen type I peptide α1 (PIP1 α1) and MMP1 concentrations, human PIP1 α1 SimpleStep ELISA Kit (ab210966, Abcam, Cambridge, UK) and MMP1 Human ELISA Kit (EHMMP1, Invitrogen, Waltham, MA, USA) were used according to the manufacturers’ instructions. The experiment was independently repeated three times (n=3).
Cytotoxicity assay
To measure the cell viability after OLED and LED light irradiation, the HDF and KC cells were seeded into 96-well black plates (HDF, 1×104 cells/well; KC, 5×103 cells/well) and incubated at 37℃. When approximately 80% confluence was reached, the plates were irradiated using OLED or LED (625–630 nm) light at five energy irradiation points. After 24 h, using the CCK-8 (CK04, Dojindo, Mashiki, Japan) reagent and microplate reader (VARIOSKAN LUX, Thermo Fisher Scientific), cell viability was observed at 450 nm absorbance and calculated. The experiment was independently repeated three times (n=3).
Wound healing assay
The wound healing assay was performed using the IBIDI Culture-Inserts (Ibidi GmbH, Gewerbehof Gräfelfing, Germany), which were used according to the manufacturer’s instructions. HDF cells were seeded into the IBIDI Culture-Insert chamber composed of two reservoirs separated by two 500±100 µm plastic membranes. After 24 h, wound areas were formed by removing the plastic membranes, OLED or LED (625–630 nm) light irradiation was performed according to the experimental conditions, and the cells were incubated in a humidified atmosphere containing 5% CO2 at 37℃ for 24 h. Afterward, the cells were fixed using 4% paraformaldehyde (BPP-9004, T&I), stained with crystal violet (V5265, Sigma Aldrich, Saint Louis, MO, USA), and observed under a microscope (CKX53, Olympus, Shinjuku, Japan). The experiment was independently repeated three times (n=3).
Animal study with photo-aged skin mouse model
Female SKH-1 hairless mice, 5 weeks old, were purchased from OrientBio (Seongnam, Korea) and acclimated for 1 week. The experimental animals were housed under controlled conditions with a temperature of 24℃±0.5℃, humidity of 55%–65%, and a 12-hour light-dark cycle. They were provided with ad libitum access to experimental animal feed. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Yonsei University (Approval No. 2022-0073) and conducted in compliance with ethical regulations in the SPF facility. After the acclimation period, mice were weighed and categorized into groups with six mice each. Finally, the changes in collagen fiber density and skin roughness were measured after OLED irradiation on the UV-irradiated mouse skin.
A UV cross-linker (BLX 312; Vilber Lourmat, Baden-Württemberg, Germany) emitting 312 nm UV-B light was used as the light source for UV irradiation. After anesthesia, except for the control group, the mice were irradiated on the dorsal side with an initial dose of 50 mJ/cm2 for 1 week, followed by a weekly increase of 50 mJ/cm2 for up to 7 weeks, and a fixed dose of 100 mJ/cm2 for the 8th week with three sessions per week. For the experimental groups, after UV-B irradiation, OLED devices were applied to the irradiated area with doses of 6 J/cm2 and 10 J/cm2 for five sessions. At the end of each 4-week and 8-week experimental period, mice were euthanized with CO2 anesthesia, and skin tissues were extracted for hematoxylin and eosin (H&E) and Masson’s trichrome (M-T) staining. Epidermis and papillary dermis of the fixed animal tissues were observed at 400× magnification using an optical microscope (BX43F; Olympus, Shinjuku, Japan). Images of the tissue sections were captured, and image analysis software (Zen image analysis; Zeiss, Oberkochen, Germany) was utilized to measure the total area of the papillary dermis and the area occupied by collagen fibers (stained in blue in M-T staining). The ratio of collagen fiber area to the total tissue area was expressed as collagen fiber density (%), where higher collagen fiber density indicates a greater presence of collagen fibers within the tissue section. Collagen fiber density (%) was calculated as follows: Collagen fiber density (%)=(Collagen fiber area/Papillary dermis area)×100.
In addition to the measurement of collagen fiber density after the OLED irradiation, the average roughness of the skin with or without OLED irradiation was measured to reveal the effect of OLED on UV-irradiated skin. For the objective evaluation of wrinkle severity, silicone polymer (Replica kit, Biobridge, Waltham, MA, USA) was applied to the dorsal area of the mice after sacrifice, using a spatula, to create replicas. The replicas were allowed to set for approximately 5 minutes. The created replicas were observed using Visioscan (VC98, CK electronic GmbH, Koeln, Germany) to visualize the wrinkle patterns, and the parameter R3, representing the roughness of the skin, was measured. R3 (average roughness) represents the mean difference between the highest and lowest grayscale values in five equal parts of the selected area. This value was calculated by obtaining the difference between the values of the highest and lowest points of each wrinkle. The average of these values, after removing artifacts, provided the R3 value, which indicates the degree of skin roughness. A smaller R3 value indicates smoother and less pronounced wrinkles on the skin.
Tissue analyses
Human skin tissues for tissue analyses were obtained after obtaining approval from the Institutional Review Board of Severance Hospital, Yonsei University (IRB No. 4-2021-1036). The subcutaneous fat on the human skin specimen was cut and discarded, and the remaining human skin was washed with phosphate-buffered saline until the remaining impurities were eliminated. The specimen was cut into 1×1 cm pieces and placed on 6-well plates filled with semi-solid DMEM. The specimen was irradiated using OLED or LED lights with selected energies and incubated in a humidified atmosphere with 5% CO2 at 37℃. After 24 h, the specimens were fixed in 10% formalin and embedded in paraffin blocks. Sections (4-µm thick) from human skin tissue, as well as the animal tissues, were prepared and used for H&E and M-T staining. After the slides were deparaffinized, H&E (hematoxylin: 104302, Merck, NJ, USA; eosin: 230251, Sigma Aldrich) or M-T (Biebrich scarlet-acid fuchsin solution: B6008, phosphomolybdic acid hydrate: 221856, phosphotungstic acid hydrate: 79690, Sigma Aldrich; aniline blue: Showa, Tokyo, Japan) staining was performed using a standard experimental protocol. All stained slides were visualized under a light microscope (BX43F, Olympus).
Statistics
All data are indicated as percentages or mean values±standard deviations, and were analyzed using SPSS statistics version 25.0 software (IBM Corp., Armonk, NY, USA). Data were analyzed using Student’s t-test for the assessment of statistical significance. The animal study at each group were compared through the analysis of variance. At p<0.05, p<0.01, and p<0.005, the mean differences were significant. All experiments in the present study were independently repeated three times (n=3).
RESULTS
Cell viability assay and human ex vivo skin analyses
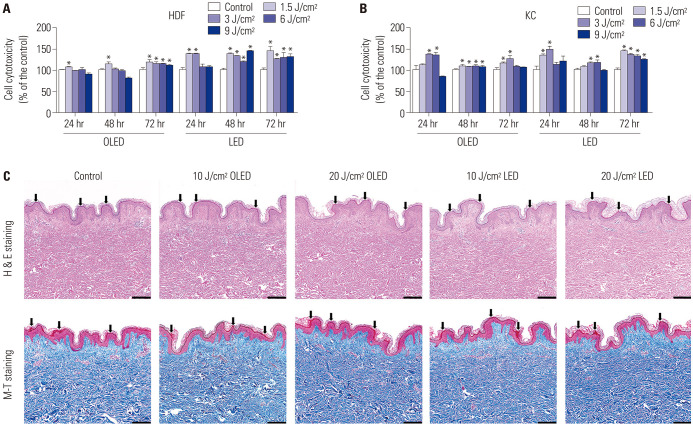
The results of the viability of HDF cells before and after irradiation with OLED or LED light are shown in Fig. 1A. When HDF cells were incubated for 24 and 48 h after OLED irradiation, the cell viability in the 1.5 J/cm2 OLED-irradiated group was significantly higher than that in the control group (control: 100.00±1.63; 106.43±1.77%, independent samples t-test, p<0.05). When incubated for 72 h, the cell viabilities in all OLED-irradiated groups were significantly higher than those in the control group (control: 100.00±5.62; 1.5 J/cm2: 117.73±7.43; 3 J/cm2: 115.18±4.23; 6 J/cm2: 115.12±0.72; 9 J/cm2: 110.60±3.04, independent samples t-test, p<0.05). No significant differences in cell viability were observed between the OLED- and LED-irradiated groups. In Fig. 1B, the results of the cell viability assay of KC cells are indicated. Cell viabilities after 3 and 6 J/cm2 OLED light irradiation were significantly higher than those in the control group when KC cells were incubated for 24 h (control: 100.00±10.62; 3 J/cm2: 137.17±0.93; 6 J/cm2: 134.47±7.09, independent samples t-test, p<0.05). After 48 h of incubation, the cell viability in all OLED-irradiated groups was significantly higher than that in the control group (control: 100.00±1.72; 1.5 J/cm2: 108.72±3.53; 3 J/cm2: 107.48±1.29; 6 J/cm2: 108.08±3.95; 9 J/cm2: 106.44±3.23, independent samples t-test, p<0.05). The cell viability after 1.5 and 3 J/cm2 OLED light irradiation in the 72-h incubation group was significantly higher than that in the control group (control: 100.00±3.67; 1.5 J/cm2: 115.59±2.56; 3 J/cm2: 125.16±8.55, independent samples t-test, p<0.05). To confirm the safety of OLED and LED device applications on human skin tissue, H&E and M-T staining of human skin tissues were performed after 10 and 20 J/cm2 OLED or LED irradiation. After irradiation, the results of H&E and M-T staining showed that there were no vacuolization and thinning of the basal layer of the epidermis occurred by harmful influence (marked black arrows) (Fig. 1C), and the separation between the epidermis and dermis, induced by severe heat, was also not observed. Additionally, for the result of the M-T staining, we confirmed no effects of homogenization of collagen and dermal vascular damage. Therefore, the results of H&E and M-T staining suggested that OLED or LED irradiation is safe, as there were no significant histological changes in the skin.
Fig. 1. Safety assessment of OLED and LED light irradiation of human skin cells and human skin tissue. Safety assessments of OLED and LED light irradiation on human skin cells were performed by confirmation of cell viability (A and B). Except for the 9 J/cm2 OLED light irradiation after 48 h of incubation on HDF cells and 9 J/cm2 LED light irradiation after 24 h of incubation on KC cells, all results of light irradiation had a positive effect. There were no histological variations in the human skin tissue after OLED or LED light irradiation (C). *p<0.05, independent samples t-test. Scale bar: 200 µm. HDF, human dermal fibroblast; H&E, hematoxylin and eosin; KC, keratinocyte; LED, light-emitting diode; M-T, Masson’s trichrome; OLED, organic light-emitting diode.
Expression levels of collagen and MMPs after OLED and LED irradiation on HDFs
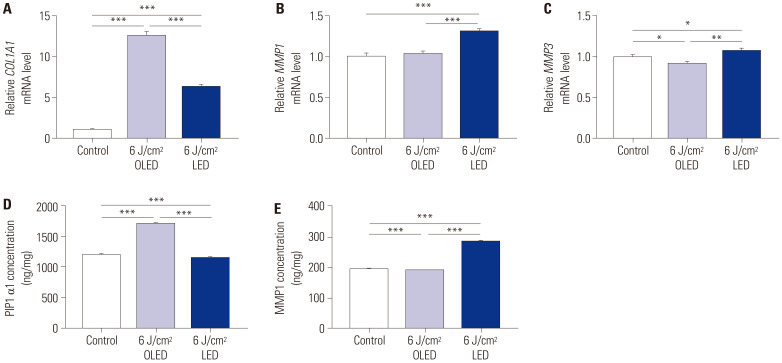
To explore the effect of OLED compared with that of LED on collagen synthesis, we performed qRT-PCR and ELISA on HDFs. The COL1A1 mRNA expression levels in the control, 6 J/cm2 OLED, and 6 J/cm2 LED irradiation groups were 1.000±0.023, 12.543±0.592, and 6.319±0.262, respectively (Fig. 2A). The increase in COL1A1 mRNA expression after OLED irradiation was significantly higher than that after LED irradiation (independent samples t-test, p<0.005). Furthermore, there was no significant difference in MMP1 mRNA expression between the control group and 6 J/cm2 OLED-irradiated group, whereas the 6 J/cm2 LED-irradiated group showed significantly higher MMP1 gene expression (control: 1.000±0.044; 6 J/cm2 OLED: 1.031±0.034; 6 J/cm2 LED: 1.307±0.035, independent samples t-test, p<0.005) (Fig. 2B). In contrast, the MMP3 expression level after 6 J/cm2 OLED light irradiation was significantly lower than that in the control group, whereas its expression level after 6 J/cm2 LED light irradiation was significantly higher than that in both the control and OLED-irradiated groups (control: 1.000±0.030; 6 J/cm2 OLED: 0.918±0.023; 6 J/cm2 LED: 1.078±0.030, independent samples t-test, p<0.05, p<0.01) (Fig. 2C). We further investigated the production of collagen and MMP1 using ELISA (Fig. 2D and E). The PIP1 α1 concentrations in the control, 6 J/cm2 OLED, and 6 J/cm2 LED light irradiation groups were 1196.533±13.322 ng/mg, 1710.483±12.987 ng/mg, and 1152.100±2.257 ng/mg, respectively. The PIP1 α1 concentration after 6 J/cm2 OLED irradiation was significantly higher than that in both the control and LED-irradiated groups (independent samples t-test, p<0.005). MMP1 production after 6 J/cm2 OLED light irradiation was significantly lower than that in the control group, whereas its production after LED irradiation was notably higher than that in the control group (control: 194.856±0.291 ng/mg; 6 J/cm2 OLED: 189.911±0.084; 6 J/cm2 LED: 286.722±0.234 ng/mg, independent samples t-test, p<0.005).
Fig. 2. Comparison of concentrations of PIP1 α1 and MMP1 and mRNA expression levels of COL1A1, MMP1, and MMP3 after OLED and LED light irradiation. The 6 J/cm2 OLED light irradiation significantly induced COL1A1 mRNA expression (A) and PIP1 α1 production (D). The 6 J/cm2 LED light irradiation significantly induced MMP1 (B) and MMP3 (C) mRNA expression and MMP1 production (E), whereas the 6 J/cm2 OLED light irradiation significantly reduced MMP3 mRNA expression and MMP1 production. *p<0.05, **p<0.01, ***p<0.005, independent samples t-test. PIP1 α1, pro-collagen I α1; MMP, matrix metalloproteinase; OLED, organic light-emitting diode; LED, light-emitting diode; HDF, human dermal fibroblast.
In vitro scratch wound healing assay
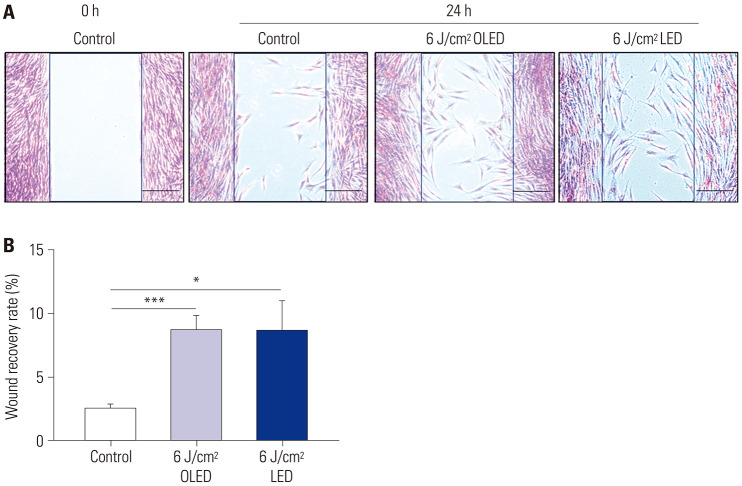
In HDF cells, a wound healing assay was performed to investigate the wound recovery effect (Fig. 3). The wound recovery rates after 6 J/cm2 OLED and 6 J/cm2 LED light irradiation were significantly higher than those in the control group (control: 2.491±0.374%; 6 J/cm2 OLED: 8.632±1.184%; 6 J/cm2 LED: 8.649±2.374%, independent samples t-test, p<0.05, p<0.005).
Fig. 3. Wound recovery effect of OLED and LED light irradiation on the HDF cells. In comparison with the control group, wound recovery was induced via 6 J/cm2 OLED or LED light irradiation on HDF cells (A and B). *p<0.05, ***p<0.005, independent samples t-test. Scale bar: 200 µm. OLED, organic light-emitting diode; LED, light-emitting diode; HDF, human dermal fibroblast.
Expression levels of growth factors after OLED and LED irradiation on HDFs
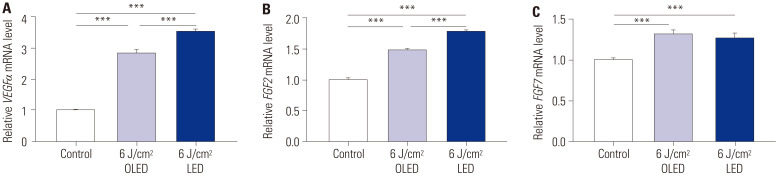
To explore the effects of 6 J/cm2 OLED and 6 J/cm2 LED light irradiation on the expression of growth factor genes, such as VEGFα, FGF2, and FGF7, qRT-PCR, was performed on HDF cells. The expression levels of VEGFα and FGF2 were significantly higher in both the 6 J/cm2 OLED- and 6 J/cm2 LED-irradiated groups than in the control group, and LED irradiation resulted in a higher expression of both genes [VEGFα (control: 1.000±0.025; 6 J/cm2 OLED: 2.834±0.107; 6 J/cm2 LED: 3.557±0.062), FGF2 (control: 1.000±0.030; 6 J/cm2 OLED: 1.474±0.017; 6 J/cm2 LED: 1.773±0.022), independent samples t-test, p<0.005] (Fig. 4A and B). FGF7 mRNA expression levels in the control group and after 6 J/cm2 OLED or 6 J/cm2 LED irradiation were 1.000±0.025, 1.306±0.051, and 1.256±0.064, respectively (Fig. 4C). The 6 J/cm2 OLED- and LED-irradiated groups showed significantly higher FGF7 expression compared to the control group (independent samples t-test, p<0.005).
Fig. 4. Relative mRNA expression levels confirm growth factor gene expressions, such as VEGFα, FGF2, and FGF7, in the HDF cells via quantitative reverse transcription polymerase chain reaction. The VEGFα and FGF2 mRNA expressions were significantly induced by 6 J/cm2 OLED or LED light irradiation on HDF cells (A and B). The 6 J/cm2 OLED light irradiation induced more FGF7 mRNA expression than 6 J/cm2 LED light irradiation (C). ***p<0.005, independent samples t-test. HDF, human dermal fibroblast; LED, light-emitting diode; OLED, organic light-emitting diode.
Collagen fiber density and skin roughness measurement after OLED irradiation in the animal study
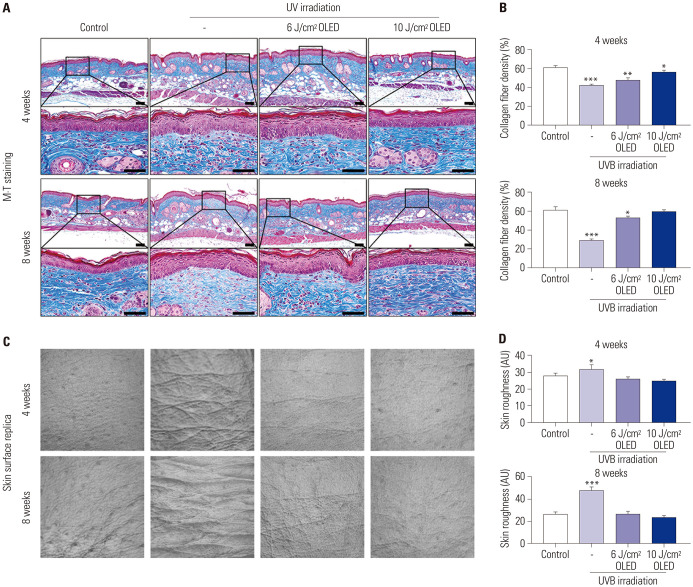
To explore the effect of OLED in dermal collagen, the collagen fiber density (%) of the skin tissue from SKH-1 hairless mice was evaluated through M-T staining after 4 weeks and 8 weeks of OLED treatment on chronic UV-irradiated mice [8 weeks of ultraviolet B (UVB) irradiation as written in the method section]. When the irradiation of 6 J/cm2 and 10 J/cm2 OLED on the photo-aged mouse skin, the collagen fiber density was increased more than the only UVB irradiation in both 4 weeks and 8 weeks (4 weeks: control; 61.677±1.344, UVB; 42.147±0.977, 6 J/cm2 OLED; 48.208±1.553, 10 J/cm2 OLED; 56.020±1.946, 8 weeks: control; 61.446±2.508, UVB; 28.493±1.492, 6 J/cm2 OLED; 52.733±1.883, 10 J/cm2 OLED; 59.579±1.049) (Fig. 5A and B). For the 10 J/cm2 OLED result in 8 weeks of irradiation, the collagen fiber density was not significantly different compared to the control group. The skin surface roughness of control, UVB irradiation, 6 J/cm2, and 10 J/cm2 OLED irradiation groups at 4 weeks was 27.833±1.772, 31.833±2.478, 26.167±1.067, and 24.933±0.745 AU, respectively (Fig. 5C and D). Additionally, for 8 weeks, the skin surface roughness was 26.500±1.803, 47.500±2.872, 26.500±2.217, and 23.500±0.957 AU, respectively. Likewise, in the skin surface results of all OLED irradiation groups, the skin roughness was not significantly different compared with the results of the control group. The results suggest that OLED irradiation affected the improvement of collagen fiber density and the reduction of skin roughness regarding the rejuvenation of photo-aged skin.
Fig. 5. Improvement effects of OLED irradiation regarding the collagen fiber density (A and B) and the skin surface roughness (C and D) on the mice’s skin. The OLED irradiation on photo-aged mouse skin affected the improvement of collagen fiber density and the reduction of skin roughness depending on the energy of OLED irradiation. *p<0.05, **p<0.01, ***p<0.005, independent samples t-test compared with the control group. Scale bar indicates 50 µm. Control, non-treatment; UVB, only UVB irradiation; 6 J/cm2 OLED group, 6 J/cm2 OLED treatment after UVB irradiation; 10 J/cm2 OLED group, 10 J J/cm2 OLED treatment after UVB irradiation; OLED, organic light-emitting diode; M-T, Masson’s trichromel; UVB, ultraviolet B.
DISCUSSION
In this study, we demonstrated the potential role of OLEDs as a next-generation PBM modality in the dermatological fields of skin rejuvenation and wound healing. OLED irradiation promoted the expression levels of collagens and growth factors, while reducing the expression levels of MMPs in HDFs. The in vitro wound healing assay also revealed an increased rate of wound recovery after OLED irradiation. Furthermore, we extended our investigation to an in vivo mouse model with 8 weeks of chronic UV exposure on the dorsal skin. The results showed that OLED irradiation on the chronic UV-exposed skin increased collagen fiber density and led to a reduction in skin wrinkles and roughness in the replica study. These findings suggest that OLED irradiation in photoaged skin may contribute to skin rejuvenation and improvement of skin wrinkles.
PBM occurs as a result of low-intensity photochemical reactions in the intended cells, in contrast to thermal photoablation produced by high-intensity lasers.5 Phototherapy based on OLED or LED is relatively safe, noninvasive, and can be easily combined with other treatment options, such as laser treatments.4 In particular, red and near-infrared LEDs are widely used as skin biostimulators to decelerate fibroblast aging by exerting both anti-oxidative and collagen-enhancing effects via absorption by chromophores located in the skin.16,17 Our comparative analysis of OLED and LED irradiations on HDF and KC cells showed similar positive effect on cell viability. Ex vivo analysis of human skin tissue also revealed no adverse effects, such as epidermal shrinkage or heat-induced dermal damage, from both OLED and LED irradiation. These results indicate that these devices are safe to be used for irradiation at cellular and tissue levels.
The 6 J/cm2 OLED irradiation led to significantly higher expression levels of collagen in HDF cells, whereas MMP1 protein and MMP3 gene expression levels were notably reduced compared to those in the control group. Both OLED and LED irradiation resulted in an increased collagen expression; however, the expression of collagen was significantly higher after OLED irradiation than after LED irradiation. MMP1 is a zinc-containing endopeptidase that degrades dermal collagen and other extracellular matrix molecules.18 Repetitive ultraviolet radiation exposure, which can produce one of the most powerful extrinsic aging effects, alters collagen homeostasis by the UVR-induced hyperactivity of MMPs, including MMP1, MMP3, and MMP9.19 Hence, the increased collagen production and decreased MMP expression after OLED irradiation highlights its potential as an advanced PBM device for skin anti-aging.
Skin aging is associated with changes that include skin atrophy, wrinkles, and pigmentation, as well as a decreased ability for wound healing.4 Wound healing is a complex process that involves a series of molecular and cellular events.20 This process depends on the interplay among extracellular matrix molecules, various chemokines, skin resident cells, and the underlying vascular system. Previous studies on the wound healing effect of LED have demonstrated that LED stimulates the proliferation and migration of various types of cells while promoting angiogenesis, the expression of growth factors, and the production of collagen, thereby assisting the process of wound healing.11,21,22,23,24 Recent studies have also applied OLEDs and observed improved cellular function and wound healing in vivo.11,15 We performed cell scratch wound healing assay in vitro and observed that the migration ability of HDFs was significantly increased after exposure to 6 J/cm2 OLED and LED, similar to the previous report on the positive effect of OLED on the fibroblast proliferation.25 Additionally, our group performed in vivo study with animal mouse model to investigate skin rejuvenation effect of OLEDs on UV-exposed skin. As a result, the OLED irradiation on chronically UV-exposed animal skin showed increased collagen fiber density and improved skin surface roughness. These results suggest that OLED treatment can clinically improve the signs of skin photoaging, such as skin elasticity and wrinkles.
Growth factors are important therapeutic options to avoid aging, since they are responsible for cell differentiation, survival, proliferation, and growth and are directly correlated with the deceleration of aging.26,27 VEGF is one of the most important regulators of angiogenesis, which binds to the tyrosine kinase receptors VEGFR-1 and VEGFR-2, thereby initiating a downstream signaling cascade that promotes angiogenesis.28 Angiogenesis is essential for accelerating wound repair as it enhances blood flow and extracellular matrix microenvironment formation.29,30 In our study, we observed a significant increase in VEGFα gene expression in both OLED- and LED-irradiated groups, suggesting a positive effect on angiogenesis and wound healing.
Fibroblast growth factors (FGFs) induce the synthesis of type 1 collagen and, therefore, play a relevant role in the process of skin aging.31 FGF family members not only increase the proliferation and activation of fibroblasts but also stimulate endothelial cell division. They also promote angiogenesis, which plays an important role in cell repair.27,32 We observed increased expressions of FGF-2 and FGF-7 in HDF cells upon exposure to either LED or OLED irradiation. In particular, OLED irradiation resulted in a higher production of FGF-7 compared to LED irradiation. FGF-2 reduces skin aging by stimulating the proliferation of mesodermal, ectodermal, and endodermal cells, including fibroblasts and KCs.31 Moreover, FGF-7, which is also considered a KC growth factor, plays an important role in the regulation of epithelial proliferation.33 Additionally, FGF-7 plays a pivotal role in inducing hair follicle proliferation and progenitor cell differentiation, thereby promoting epithelialization and wound healing.34 LED therapy promotes better tissue organization, with larger granulation tissue areas, the presence of hair follicles, and well-developed dermal papillae.35 Although we did not fully investigate the effect of OLED on hair growth, our preliminary laboratory study on human follicle dermal papilla cells (DPCs) showed significantly increased mRNA expressions of 5a-reductase-1, VEGFa, FGF-7, and FGF-10 after OLED irradiation (Supplementary Fig. 2, only online). Detailed study method and materials are in Supplementary Material (only online). The treatment also increased the survival of human follicle DPCs, suggesting its possible cosmeceutical ability to enhance hair growth (Supplementary Fig. 3, only online). Further studies on the safety and efficacy of OLED irradiation for hair growth are warranted.
In conclusion, this is the first pilot study to investigate in vitro and in vivo the safety and efficacy of OLED irradiation in altering the expression levels of collagens, MMPs, and growth factors, while accelerating the cellular wound recovery rate; the additional tissue analysis after OLED irradiation on human skin suggests that OLED is a safe source for PBM, thereby demonstrating its potential role in skin rejuvenation and wound healing. Our pilot study has a few limitations; in particular, the in vitro study did not include UV-irradiated fibroblasts as the positive control. The addition of UV-irradiation before OLED or LED exposures similar to our in vivo study would have shown more consistent and definite results with the expressions levels of MMPs. Further laboratory and randomized controlled clinical studies are needed to fully understand the effect of OLED irradiation on anti-aging and wound healing of the skin.
ACKNOWLEDGEMENTS
This research was supported by Samsung Display Co., Ltd. This study was supported by faculty research grant of Yonsei University College of Medicine (6-2022-0089) and partially by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2022R1I1A1A01073097).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Young In Lee, Sang Gyu Lee, Inhee Jung, and Jangmi Suk.
- Data curation: Young In Lee, Sang Gyu Lee, Inhee Jung, and Jangmi Suk.
- Formal analysis: Young In Lee, Sang Gyu Lee, Seoyoon Ham, Inhee Jung, and Jangmi Suk.
- Funding acquisition: Inhee Jung, Jangmi Suk, and Ju Hee Lee.
- Investigation: Young In Lee, Sang Gyu Lee, Seoyoon Ham, Inhee Jung, and Jangmi Suk.
- Methodology: Seoyoon Ham, Inhee Jung, and Jangmi Suk.
- Project administration: Inhee Jung, Jangmi Suk, and Ju Hee Lee.
- Resources: Inhee Jung and Jangmi Suk.
- Software: Sang Gyu Lee and Inhee Jung.
- Supervision: Ju Hee Lee.
- Validation: Young In Lee and Sang Gyu Lee.
- Visualization: Young In Lee and Sang Gyu Lee.
- Writing—original draft: Young In Lee and Sang Gyu Lee.
- Writing—review & editing: Young In Lee, Sang Gyu Lee, and Ju Hee Lee.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Laboratory setting for light source irradiation (A and B). The distance from OLED panel to the cell plate was 2 cm, whereas the distance from LED panel to the cell plate was 4 cm, as suggested by the manufacturer. A pre-designed laboratory fan was applied on the top of the LED source during the irradiation period to reduce the possible effect by the heat generated from the light source. LED, light-emitting diode; OLED, organic light-emitting diode.
Relative mRNA expression levels confirming growth factor gene expressions, such as 5α-reductase-1 (A), VEGFα (B), FGF7 (C), and FGF10 (D), in the HFDPCs via qRT-PCR. *p<0.05, **p<0.01, ***p<0.005, independent samples t-test. LED, light-emitting diode; OLED, organic light-emitting diode; HFDPCs, hair follicle dermal papilla cells; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
Safety assessment of OLED and LED light irradiation on HFDPCs were performed via confirmation of cell viability. *p<0.05, independent samples t-test. HFDPCs, hair follicle dermal papilla cells; LED, light-emitting diode; OLED, organic light-emitting diode.
References
- 1.Houreld NN. The use of lasers and light sources in skin rejuvenation. Clin Dermatol. 2019;37:358–364. doi: 10.1016/j.clindermatol.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Caruso-Davis MK, Guillot TS, Podichetty VK, Mashtalir N, Dhurandhar NV, Dubuisson O, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21:722–729. doi: 10.1007/s11695-010-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arany PR. Craniofacial wound healing with photobiomodulation therapy: new insights and current challenges. J Dent Res. 2016;95:977–984. doi: 10.1177/0022034516648939. [DOI] [PubMed] [Google Scholar]
- 4.Huang A, Nguyen JK, Ho D, Jagdeo J. Light emitting diode phototherapy for skin aging. J Drugs Dermatol. 2020;19:359–364. doi: 10.36849/JDD.2020.4711. [DOI] [PubMed] [Google Scholar]
- 5.Nonarath HJ, Hall AE, SenthilKumar G, Abroe B, Eells JT, Liedhegner ES. 670nm photobiomodulation modulates bioenergetics and oxidative stress, in rat Müller cells challenged with high glucose. PLoS One. 2021;16:e0260968. doi: 10.1371/journal.pone.0260968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Wei S, Zhang K, Fang Y, Liu H, Jin Z, et al. The inflammation and reactive oxygen species regulated by Nrf2 and NF-κB signaling pathways in 630-nm light-emitting diode irradiation treated THP-1 monocytes/macrophages. Lasers Med Sci. 2021;36:1411–1419. doi: 10.1007/s10103-020-03172-2. [DOI] [PubMed] [Google Scholar]
- 7.Lee YI, Lee E, Nam KH, Shin DY, Kim J, Suk J, et al. The use of a light-emitting diode device for neck rejuvenation and its safety on thyroid glands. J Clin Med. 2021;10:1774. doi: 10.3390/jcm10081774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HS, Park WS, Baek JI, Lee BS, Yoo DS, Park SJ. Continuous irradiation with a 633-nm light-emitting diode exerts an anti-aging effect on human skin cells. Int J Mol Med. 2015;35:383–390. doi: 10.3892/ijmm.2014.2030. [DOI] [PubMed] [Google Scholar]
- 9.Song J, Lee H, Jeong EG, Choi KC, Yoo S. Organic light-emitting diodes: pushing toward the limits and beyond. Adv Mater. 2020;32:e1907539. doi: 10.1002/adma.201907539. [DOI] [PubMed] [Google Scholar]
- 10.Jeon Y, Choi HR, Lim M, Choi S, Kim H, Kwon JH, et al. A wearable photobiomodulation patch using a flexible red-wavelength OLED and its in vitro differential cell proliferation effects. Adv Mater Technol. 2018;3:1700391 [Google Scholar]
- 11.Kim YJ, Jeon HR, Kim SW, Kim YH, Im GB, Im J, et al. Lightwave-reinforced stem cells with enhanced wound healing efficacy. J Tissue Eng. 2021;12:20417314211067004. doi: 10.1177/20417314211067004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon Y, Choi HR, Kwon JH, Choi S, Nam KM, Park KC, et al. Sandwich-structure transferable free-form OLEDs for wearable and disposable skin wound photomedicine. Light Sci Appl. 2019;8:114. doi: 10.1038/s41377-019-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park Y, Choi HR, Jeon Y, Kim H, Shin JW, Huh CH, et al. Cell proliferation effect of deep-penetrating microcavity tandem NIR OLEDs with therapeutic trend analysis. Sci Rep. 2022;12:10935. doi: 10.1038/s41598-022-15197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo S, Chung PS, Ahn JC. 630 nm-OLED accelerates wound healing in mice via regulation of cytokine release and genes expression of growth factors. Curr Opt Photonics. 2019;3:485–495. [Google Scholar]
- 15.Wu X, Alberico S, Saidu E, Rahman Khan S, Zheng S, Romero R, et al. Organic light emitting diode improves diabetic cutaneous wound healing in rats. Wound Repair Regen. 2015;23:104–114. doi: 10.1111/wrr.12258. [DOI] [PubMed] [Google Scholar]
- 16.Maldaner DR, Azzolin VF, Barbisan F, Mastela MH, Teixeira CF, Dihel A, et al. In vitro effect of low-level laser therapy on the proliferative, apoptosis modulation, and oxi-inflammatory markers of premature-senescent hydrogen peroxide-induced dermal fibroblasts. Lasers Med Sci. 2019;34:1333–1343. doi: 10.1007/s10103-019-02728-1. [DOI] [PubMed] [Google Scholar]
- 17.Sorbellini E, Rucco M, Rinaldi F. Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: an update. Lasers Med Sci. 2018;33:1431–1439. doi: 10.1007/s10103-018-2584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17:868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krutmann J, Schikowski T, Morita A, Berneburg M. Environmentally-induced (extrinsic) skin aging: exposomal factors and underlying mechanisms. J Invest Dermatol. 2021;141(4S):1096–1103. doi: 10.1016/j.jid.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Liu Y, Zhang X, Zhu D, Qi X, Cao X, et al. Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics. 2018;8:5348–5361. doi: 10.7150/thno.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- 22.Barolet D. Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg. 2008;27:227–238. doi: 10.1016/j.sder.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22:7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang Y, Liu B, Liu L, Ye X, Bi X, Zhang Y, et al. The 800-nm diode laser irradiation induces skin collagen synthesis by stimulating TGF-β/Smad signaling pathway. Lasers Med Sci. 2011;26:837–843. doi: 10.1007/s10103-011-0985-z. [DOI] [PubMed] [Google Scholar]
- 25.Park Y, Choi HR, Jeon Y, Kim H, Shin JW, Huh CH, et al. Cell proliferation effect of deep-penetrating microcavity tandem NIR OLEDs with therapeutic trend analysis. Sci Rep. 2022;12:10935. doi: 10.1038/s41598-022-15197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maddaluno L, Urwyler C, Werner S. Fibroblast growth factors: key players in regeneration and tissue repair. Development. 2017;144:4047–4060. doi: 10.1242/dev.152587. [DOI] [PubMed] [Google Scholar]
- 27.Mine S, Fortunel NO, Pageon H, Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS One. 2008;3:e4066. doi: 10.1371/journal.pone.0004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 29.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 30.Guerra A, Belinha J, Jorge RN. Modelling skin wound healing angiogenesis: a review. J Theor Biol. 2018;459:1–17. doi: 10.1016/j.jtbi.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 31.de Araújo R, Lôbo M, Trindade K, Silva DF, Pereira N. Fibroblast growth factors: a controlling mechanism of skin aging. Skin Pharmacol Physiol. 2019;32:275–282. doi: 10.1159/000501145. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Zhang D, Wu H, Xie S, Zhang M, Zhang B, et al. Basic fibroblast growth factor influences epidermal homeostasis of living skin equivalents through affecting fibroblast phenotypes and functions. Skin Pharmacol Physiol. 2018;31:229–237. doi: 10.1159/000488992. [DOI] [PubMed] [Google Scholar]
- 33.An JJ, Eum WS, Kwon HS, Koh JS, Lee SY, Baek JH, et al. Protective effects of skin permeable epidermal and fibroblast growth factor against ultraviolet-induced skin damage and human skin wrinkles. J Cosmet Dermatol. 2013;12:287–295. doi: 10.1111/jocd.12067. [DOI] [PubMed] [Google Scholar]
- 34.Taghiabadi E, Nilforoushzadeh MA, Aghdami N. Maintaining hair inductivity in human dermal papilla cells: a review of effective methods. Skin Pharmacol Physiol. 2020;33:280–292. doi: 10.1159/000510152. [DOI] [PubMed] [Google Scholar]
- 35.Simões TMS, de Alencar Fernandes Neto J, Nonaka CFW, de Vasconcelos Catão MHC. Effects of photobiomodulation therapy with red LED on inflammatory cells during the healing of skin burns. Lasers Med Sci. 2022;37:2817–2822. doi: 10.1007/s10103-022-03537-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Laboratory setting for light source irradiation (A and B). The distance from OLED panel to the cell plate was 2 cm, whereas the distance from LED panel to the cell plate was 4 cm, as suggested by the manufacturer. A pre-designed laboratory fan was applied on the top of the LED source during the irradiation period to reduce the possible effect by the heat generated from the light source. LED, light-emitting diode; OLED, organic light-emitting diode.
Relative mRNA expression levels confirming growth factor gene expressions, such as 5α-reductase-1 (A), VEGFα (B), FGF7 (C), and FGF10 (D), in the HFDPCs via qRT-PCR. *p<0.05, **p<0.01, ***p<0.005, independent samples t-test. LED, light-emitting diode; OLED, organic light-emitting diode; HFDPCs, hair follicle dermal papilla cells; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
Safety assessment of OLED and LED light irradiation on HFDPCs were performed via confirmation of cell viability. *p<0.05, independent samples t-test. HFDPCs, hair follicle dermal papilla cells; LED, light-emitting diode; OLED, organic light-emitting diode.