Abstract
Over the last few years, dramatic increases in our knowledge about diffusely adhering Escherichia coli (DAEC) pathogenesis have taken place. The typical class of DAEC includes E. coli strains harboring AfaE-I, AfaE-II, AfaE-III, AfaE-V, Dr, Dr-II, F1845, and NFA-I adhesins (Afa/Dr DAEC); these strains (i) have an identical genetic organization and (ii) allow binding to human decay-accelerating factor (DAF) (Afa/DrDAF subclass) or carcinoembryonic antigen (CEA) (Afa/DrCEA subclass). The atypical class of DAEC includes two subclasses of strains; the atypical subclass 1 includes E. coli strains that express AfaE-VII, AfaE-VIII, AAF-I, AAF-II, and AAF-III adhesins, which (i) have an identical genetic organization and (ii) do not bind to human DAF, and the atypical subclass 2 includes E. coli strains that harbor Afa/Dr adhesins or others adhesins promoting diffuse adhesion, together with pathogenicity islands such as the LEE pathogenicity island (DA-EPEC). In this review, the focus is on Afa/Dr DAEC strains that have been found to be associated with urinary tract infections and with enteric infection. The review aims to provide a broad overview and update of the virulence aspects of these intriguing pathogens. Epidemiological studies, diagnostic techniques, characteristic molecular features of Afa/Dr operons, and the respective role of Afa/Dr adhesins and invasins in pathogenesis are described. Following the recognition of membrane-bound receptors, including type IV collagen, DAF, CEACAM1, CEA, and CEACAM6, by Afa/Dr adhesins, activation of signal transduction pathways leads to structural and functional injuries at brush border and junctional domains and to proinflammatory responses in polarized intestinal cells. In addition, uropathogenic Afa/Dr DAEC strains, following recognition of β1 integrin as a receptor, enter epithelial cells by a zipper-like, raft- and microtubule-dependent mechanism. Finally, the presence of other, unknown virulence factors and the way that an Afa/Dr DAEC strain emerges from the human intestinal microbiota as a “silent pathogen” are discussed.
INTRODUCTION
Pathogenic Escherichia coli strains cause a spectrum of diseases in humans (103, 210, 219, 306, 369). Uropathogenic and diarrheagenic strains of E. coli are characterized by the expression of distinctive bacterial properties, products, or structures that are known as virulence factors because they help the organism overcome host defenses and colonize or invade the urinary or gastrointestinal tract (103, 210, 219, 306). These virulence factors allow pathogenic E. coli to interact with host molecules for colonization and usurping normal cell processes, including cytoskeletal dynamics and vesicle targeting for cellular structural and functional damage and host evasion (87, 233). In the case of uropathogenic strains of E. coli, some virulence factors specifically promote the development of pyelonephritis, whereas others promote cystitis or asymptomatic bacteremia (103, 210, 369). Consistent with the fecal-perineal-urethral hypothesis, acute pyelonephritis is recognized to be initiated by the dominance of uropathogenic strains in fecal flora (7). Moreover, although recurrent infections might occasionally be due to a persistent focus of infection (301, 302, 369), the majority have been thought to be reinfections caused by the initially infecting strain persisting in the fecal flora (7). Pathogenic enteric E. coli strains that cause human diarrhea can be divided into a least six groups based on their serotypes and the mechanism by which the disease is thought to be induced: enterotoxigenic E. coli (ETEC), attaching and effacing enteropathogenic E. coli (EPEC), enteroinvasive E. coli, enterohemorrhagic E. coli (EHEC), enteroaggregative E. coli (EAEC), and diffusely adhering E. coli (DAEC) (219, 306).
DAEC strains have been identified from their diffuse adherence (DA) pattern on cultured epithelial HEp-2 as well as HeLa cells (307, 308, 365), and they appear to form a heterogeneous group (91, 308). The first class of DAEC strains includes E. coli strains that harbor Afa/Dr adhesins (Afa/Dr DAEC) (322). These E. coli strains have been found to be associated with urinary tract infections (UTIs) (pyelonephritis, cystitis, and asymptomatic bacteriuria) and with various enteric infections (11, 92, 103, 322). The genetic determinants responsible for the adherence of Afa/Dr DAEC to human epithelial cells have been identified in recent years by genetic and molecular methods. The data indicate that all Afa/Dr DAEC adhesins act as virulence factors. In addition, searches for DNA sequences that are present in Afa/Dr strain C1845, of intestinal origin (43), but absent from a nonpathogenic K-12 strain have revealed that several C1845-specific sequences are either homologous to putative virulence genes or show no homology with known sequences (48). E. coli C1845 harbors sequences encoding several iron transport systems found in other pathotypes of E. coli, including the yersiniabactin siderophore (irp2), the aerobactin siderophore (iuc), a catechole siderophore receptor (iroN), a heme transport system (shu), and a molybdenum transport system (modD). In addition, three C1845-specific sequences (MO30, S109, and S111) are highly prevalent (77 to 80%) among Afa/Dr strains but have low prevalence (12 to 23%) among non-Afa/Dr strains. Moreover, it is interesting that the Afa/Dr strain IH11128, recovered from a patient with a UTI (316), is genetically closely related to strain C1845 of intestinal origin (43). Finally, no genes encoding factors known to subvert host cell proteins, such as the type III secretion system or effector proteins expressed by EPEC (including intimin [Eae] and its receptor [Tir]) (155, 443), have been found in strain C1845. The phylogenic analysis of EAEC and DAEC strains has revealed five large clusters of strains (91). Strain C1845 and some other DAEC strains were present in the cluster DAEC1 and appear to be phylogenetically close to the EAEC strains. Moreover, Afa/Dr DAEC strains appear generally to express several characteristics that have been associated with extraintestinal E. coli strains, including the B2 phylogenetic group (188, 341), the O75 serotype (312), the production of aerobactin (71, 130, 441), the presence of iroN (358), and the presence of sequences from PAICFT073 (168), but not including the hlyA, hlyD, hp1 to hp4, papG, or papF sequences. As an exception, the pyelonephritogenic Afa/Dr DAEC strain EC7372, which harbors the Dr-II adhesin (340), expresses a functional hemolysin that is responsible for cell death by apoptosis or necrosis (165).
The second class of DAEC strains includes E. coli strains that express an adhesin involved in diffuse adherence (AIDA-I) (26-29), which is a potential cause of infantile diarrhea. These DAEC strains are likely to contain one or more homologues of the locus of the enterocyte effacement characteristic of EPEC, which may contribute to the pathogenic potential of these DAEC strains. These diarrheagenic E. coli strains have been shown to secrete similar patterns of proteins regulated by environmental parameters, namely, the medium, temperature, pH, and iron concentration (24). Proteins homologous to the EspA, EspB, and EspD proteins, which are necessary for signal transduction events inducing attaching and effacing (A/E) lesions, have been identified that induce the accumulation of actin and tyrosine-phosphorylated proteins at sites of bacterial attachment, leading to the formation of pedestals and/or extended surface structures phenotypically similar to the A/E lesions observed with enteropathogenic and some enterohemorrhagic E. coli strains carrying the LEE pathogenicity island (454).
GENETIC ORGANIZATION
For extracellular colonization and internalization, microbial pathogens develop molecular interactions with the host cell surfaces. Bacterial pathogens, including pathogenic E. coli, have developed on their surfaces adhesins and invasins responsible for the recognition and binding of specific membrane-bound host molecules acting as receptors. In some cases, activation of complex signal transduction cascades associated with these host cell molecules follows the binding of adhesins and invasins within the active sites on these molecules. In many instances adhesins and invasins are located on the bacterial surface in extended hair-like appendages named pili or fimbriae or in amorphous outer membrane-associated structures termed afimbrial sheaths (404). The Afa/Dr family of adhesins contains representatives having fimbrial (37, 43, 90, 115, 316, 442), afimbrial (241, 243-245, 251, 455, 470), and nonfimbrial (340) architectures (Table 1).
TABLE 1.
Characteristics of Afa/Dr adhesins
| Adhesin | Type | Host | Receptors
|
||
|---|---|---|---|---|---|
| Type IV collagen | DAFa | CEACAMsb | |||
| AfaE-I | Afimbrial | Human | Negative | Positive | Negative |
| AfaE-II | Afimbrial | Human | Unknown | Positive | Unknown |
| AfaE-III | Afimbrial | Human | Negative | Positive | Positive |
| AfaE-V | Afimbrial | Human | Unknown | Positive | Unknown |
| AfaE-VII | Afimbrial | Bovine | Unknown | Negative | Unknown |
| AfaE-VIII | Afimbrial | Animal, human | Unknown | Negative | Unknown |
| Dr | Fimbrial | Human | Positive | Positive | Positive |
| Dr-II | Nonfimbrial | Human | Negative | Positive | Negative |
| F1845 | Fimbrial | Human | Negative | Positive | Positive |
| Nfa-I | Nonfimbrial | Human | Unknown | Positive | Unknown |
| AAF-I | Fimbrial | Human | Unknown | Unknown | Unknown |
| AAF-II | Fimbrial | Human | Unknown | Unknown | Unknown |
| AAF-III | Fimbrial | Human | Unknown | Unknown | Unknown |
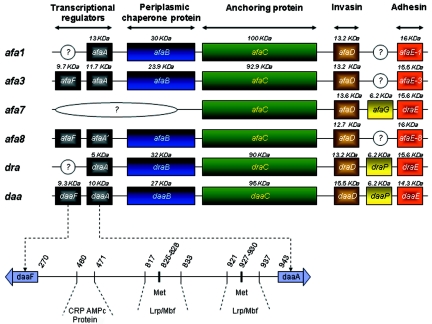
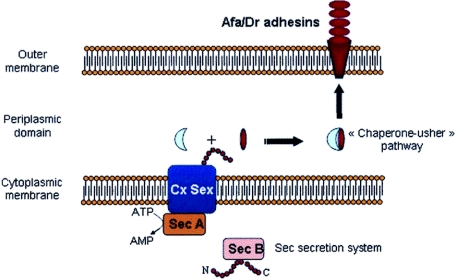
The structural assembly genes coding for Afa/Dr adhesins have a similar organization, consisting of operons of at least five genes. Genes A to D, which encode accessory proteins, are highly conserved in the different family members, whereas gene E, which encodes the adhesin molecule itself, is more divergent. On the basis of a similar genetic organization of the gene clusters involved in the biogenesis of adhesins and/or binding to the common epithelial cell receptor decay-accelerating factor (DAF, CD55) (Fig. 1), Nowicki et al. (322) have proposed that the Afa/Dr family of adhesins currently includes 13 human adhesins, i.e., AfaE-I, AfaE-II, AfaE-III, AfaE-V, Dr, Dr-II, F1845, Nfa-I, AAF-I, AAF-II, AAF-III, the bovine adhesin AfaE-VII, and the AfaE-VIII adhesin found in humans and animals (Table 1). Only the human adhesins AfaE-I, AfaE-III, Dr, Dr-II, and F1845 have been fully explored with regard to their genetic organization, receptor recognition, and involvement in Afa/Dr DAEC pathogenicity. In addition, it was noted that the EAEC adhesins AAF-I, AAF-II (90), and AAF-III (37) are probably more distantly related members of the Afa/Dr family of adhesins (91). In particular, despite similar genetic organizations of the gene clusters involved in the biogenesis of these three adhesins and Afa/Dr adhesins, it remains important to explore whether or not EAEC adhesins recognized the Afa/Dr receptors, type IV collagen, DAF (CD55), and/or carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs), which play a pivotal role in Afa/Dr DAEC pathogenesis. Finally, it has been established that Afa/Dr adhesins are assembled via the chaperone-usher pathway (Fig. 2) (360-362, 404) and that the Afa/Dr family of adhesins are members of the FGL group of the chaperone-usher class of E. coli adhesins (198).
FIG. 1.
Genetic organization of Afa/Dr operons afa1 (243), afa3 (139, 251), afa7 and afa8 (140, 244), dra (316; accession number AF329316), and daa (43, 264, 265).
FIG. 2.
Assembly of Dr adhesin via the chaperone-usher pathway.
Afa Adhesins
The afa gene clusters encode afimbrial adhesins (Afas) that are expressed by uropathogenic and diarrhea-associated E. coli strains. These gene clusters are responsible for the biosynthesis of the Afa adhesins belonging to the Afa/Dr family of adhesins and for the biosynthesis of invasins. AfaE-I, a mannose-resistant adhesin, has been isolated from the uropathogenic E. coli KS52 strain (243, 455). The genetic organization of the 6.7-kb DNA fragment encoding the AfaE-I adhesin involves five genes, afaA, afaE, afaD, afaB, and afaC (242). These five genes have been localized and shown to belong to the same transcription unit. The AfaB, AfaC, and AfaE gene products are required for mannose-resistant hemagglutination (MRHA). The afaE gene has been identified as the structural gene encoding AfaE-I adhesin. AfaE-1 adhesin has 32% identity with Dr adhesin (51 out of 160 amino acids are identical) (340).
The Afa-related operons in the A22 and A30 strains lack the Afa-I adhesin-encoding gene but do encode adhesins designated AfaE-II and AfaE-III (241). Le Bouguenec et al. (251) have reported that the cloned afa-3 gene clusters from strain A30 appeared to be carried by 9-kb plasmid regions, which displayed similar genetic organizations. The amino acid sequence of AfaE-III deduced from the nucleotide sequence of the afaE3 gene displays 98% identity to that of the Dr adhesin (157 out of 160 amino acids are identical) (340). Unlike Dr adhesin, in which receptor binding is inhibited by chloramphenicol (319), AfaE-III adhesin confers chloramphenicol-resistant adherence. The plasmid-borne afa-3 gene cluster determines the formation of an afimbrial adhesive sheath that is expressed by both uropathogenic and diarrhea-associated strains of E. coli (137). The afa-3 gene cluster has been shown to contain six open reading frames, designated afaA to afaF (139). It is organized as two divergent transcriptional units. Five of the six Afa products showed marked homologies with proteins encoded by adhesion systems that have already been described. AfaE has been identified as the structural adhesin product, whereas based on homology with the pap operon, AfaB and AfaC have been identified as periplasmic chaperone and outer membrane anchor proteins, respectively. The AfaA and AfaF products have been shown to be homologous with the PapI-PapB transcriptional regulatory proteins. Upstream of the afa-3 gene cluster, a 1.2-kb region has been found to display 96% identity with the RepFIB sequence of one of the enterotoxigenic E. coli plasmids (P307), suggesting a common plasmid ancestor. This region contains an integrase-like gene (int). Sequence analysis has revealed the presence of an IS1 element between the int gene and the afa-3 gene cluster. Two other IS1 elements have been detected and located in the vicinity of the afa-3 gene cluster by hybridization experiments. This means that the afa-3 gene cluster is flanked by two IS1 elements in a direct orientation and two in opposite orientations. The afa-3 gene cluster, flanked by two directly oriented IS1 elements, has been shown to translocate from a recombinant plasmid into the E. coli chromosome. This translocation event occurred via IS1-specific recombination mediated by a RecA-independent mechanism. The afa-3 gene cluster is closely related to the daa operon, which codes for an adhesin, fimbrial adhesin F1845 (42, 43), that is closely related to the AfaE-III adhesin (137). Chimeras constructed between the afa-3 and daa operons demonstrate that the biogenesis of a fimbrial or an afimbrial adhesin is entirely determined by the amino acid sequences of the AfaE-III and F1845 adhesins (137). Determination of the atomic resolution structure for the AfaE-III subunit reveals that the adhesin assembles by donor strand complementation and for the architecture of capped surface fibers (8).
Two other Afa-related adhesins have been identified recently. The AfaE-VII and AfaE-VIII adhesins are encoded by the afa-7 and afa-8 gene clusters, respectively, and are expressed mostly by bovine isolates (244, 245). These animal afa gene clusters are expressed by strains that produce other virulence factors, such as the CNF toxins and the F17, PAP, and CS31A adhesins. It is noteworthy that although the AfaE-VIII adhesin has been detected in human E. coli (142), it has never been detected in diarrhea-associated human isolates (252). Like the afa-3 gene cluster, both the afa-7 and afa-8 gene clusters were found to encode the afimbrial adhesin AfaE and the invasin AfaD. The afa-8 operon is carried by a 61-kb genomic region with characteristics typical of a pathogenicity island, including a size in excess of 10 kb, the presence of an integrase-encoding gene, being inserted into a tRNA locus (PheR), and the presence of a small direct repeat at each extremity (245). The location of the afa-8 gene cluster on the plasmids or chromosomes of these isolates suggests that it could be carried by a mobile element, facilitating its dissemination among bovine-pathogenic E. coli strains (244). Sequences related to the afa-8 gene cluster have been identified in E. coli strains isolated from diseased calves, pigs, humans, and poultry, whereas no sequence related to the afa-7 gene cluster has been reported (140).
The EPEC O55 serogroup includes two major electrophoretic types (ET), designated ET1 and ET5. ET5 comprises strains with different combinations of virulence genes, including those for localized adherence and DA. Interestingly, the ET5 DA strains possess an 11.6-kb chromosomal region including an operon that encodes a protein with 98% identity to AfaE-I, which is probably responsible for the DA (227).
Dr Adhesins
The uropathogenic strain E. coli IH11128 (O75:K5:H-) (442) exhibits a mannose-resistant adhesin (316). This adhesin has been variously designated O75X, Dr hemagglutinin, and Dr adhesin. For the sake of clarity, the term Dr adhesin will be used throughout this review. The genetic organization of Dr adhesin shows that a 6.6-kb DNA fragment expresses five proteins with molecular masses of 15.5, 5, 18, 90, and 32 kDa, which are encoded by the draA, draB, draC, draD, and draE genes, respectively. Four genes, draA, draC, draD, and draE, are required for the expression of full, mannose-resistant hemagglutination (324).
The Dr-II adhesin has been identified from the pyelonephritogenic strain EC7372. This adhesin has a low level of sequence identity with other members of the Afa/Dr family (17 to 20% of the 160 amino acids are identical) (340). Dr-II is 96% identical to the nonfimbrial adhesin NFA-1, an adhesin associated with a UTI whose receptor has not been identified (4). It was noted that although nonfimbrial adhesins have not previously been considered to belong to the Afa/Dr family, in fact they have a very similar genetic organization. Strain EC7372 can be viewed as a prototype of a subclass of Afa/Dr DAEC isolates that have acquired a pathogenicity island similar to that described for the pyelonephritogenic strain CFT073, which carries both hly and pap operons (370) and which, unlike other Afa/Dr DAEC strains, triggers cell death by apoptosis or necrosis (165).
Adhesin F1845
A fimbrial adhesin, designated F1845, has been shown to be responsible for the diffuse cell adherence of a diarrheal E. coli isolate. The genetic determinant of F1845 has been cloned, and the order of the genes necessary for F1845 to be produced has been determined (42, 43). Five polypeptides with apparent sizes of 10, 95, 27, 15.5, and 14.3 kDa have been shown to be encoded in that order by the daaA, daaB, daaC, daaD, and daaE F1845 determinants, respectively. The nucleotide sequence of the 14.3-kDa subunit gene was determined and was found to share extensive signal sequence homology with the gene encoding the structural subunit of the AfaE-I adhesin, but not in the region encoding the mature protein. In strain C1845, the F1845 determinants are of chromosomal origin (43). However, hybridization studies using a probe from the region encoding the 95-kDa polypeptide indicate that related sequences may be plasmid associated in some strains and chromosomal in others (43). The transcriptional organization of the gene cluster encoding the F1845 fimbrial adhesin has been investigated. Genes daaA to daaE have been shown to constitute a single transcriptional unit under the control of the daaA promoter. The nucleotide sequence of daaA and that of an upstream open reading frame encoded on the opposite strand, designated daaF, have been shown to share limited homology with the papB and papI genes of the P fimbrial adhesin, respectively (42). An open reading frame predicted to encode a 57-amino-acid polypeptide has been identified flanking the daa processing site (265). Site-directed mutagenesis introducing a limited number of mutations into the open reading frame, designated daaP, appears to show that a sequence of the DaaP peptide is important and that translation of the daaP gene is required in cis to promote processing by the endonuclease. Interestingly, whereas PapB lowered the level of expression of type 1 fimbriae, DaaA did not (192). Adhesin F1845 has 57% identity with Dr adhesin (91 amino acids out of 160 are identical) (340).
DIAGNOSIS
Phenotypic and genotypic assays have been developed for detection of E. coli harboring Afa/Dr adhesins. On the basis that pathogenic E. coli strains attach to HeLa cells in different patterns (localized, diffuse, or aggregative), an adhesion assay has been proposed for the detection of the mannose-resistant diffuse adhesiveness of DAEC strains onto cultured epithelial Hep-2 or HeLa cells (307, 308, 365). The adhesion assay is not specific for Afa/Dr DAEC detection, since other pathogenic E. coli strains, including DA EPEC strains (26-29), have been reported to develop the diffuse phenotype of adhesion without the presence of Afa/Dr adhesins.
In order to detect the DAEC strains harboring the Afa/Dr adhesins, a hemagglutination inhibition assay with human erythrocytes and with human erythrocytes preincubated with anti-DAF monoclonal antibody IH4 has been further proposed on the basis that these E. coli strains show an MRHA phenotype (241, 243) and recognize as a receptor the human DAF in its complement control protein repeat 3 (CCP-3) domain (originally known as short consensus repeats) (318). Inhibition of MRHA presents several inconveniences, including the lack of viability of fresh human erythrocytes.
A new method of detection of Afa/Dr DAEC, named the DAF clustering assay (DCA), has recently been proposed by Goluszko et al. (149). This assay associates the diffuse adhesiveness of bacteria onto cultured epithelial HeLa cells and the previously reported human DAF receptor clustering around adhering bacteria (150, 166, 213, 252). Results show a high positive correlation of DCA with the hemagglutination inhibition assay described above and with a PCR protocol conducted with primers amplifying the afaB 750-bp sequences. However, the DCA did not allow the detection of all E. coli strains expressing Afa/Dr adhesins, since Afa/Dr DAEC strains that express the AfaE-VII and AfaE-VIII adhesins do not bind to human DAF (244). This is a particular inconvenience for the detection of Afa/Dr DAEC strains expressing the AfaE-VIII adhesin present in human extraintestinal clinical isolates (142, 245, 252).
DNA probes have been constructed for colony hybridization assays. The first DNA probe, named daaC, was generated by Stapleton et al. (413) and was a 300-bp PstI fragment of plasmid pSSS1 (daa operon), coding for part of an accessory protein of F1845 adhesin expressed by the diarrheagenic Afa/Dr DAEC C1845 strain (43). This DNA probe has been used in a large majority of the epidemiological studies of the association of Afa/Dr DAEC with diarrhea and urinary tract infections (1, 5, 68, 112, 127, 130, 131, 141, 143, 152, 208, 209, 256, 257, 293, 329, 344, 364, 366, 367, 400). Another constructed DNA probe, named drb (130), was a 260-bp PstI fragment of plasmid pIL14 (afa-1 operon) coding for the AfaE-I adhesin expressed by the uropathogenic Afa/Dr DAEC KS52 strain (243). This DNA probe has been used in epidemiological studies of the association of Afa/Dr DAEC with urinary tract infection (13, 130, 131, 250, 287, 466, 467). The results show a high positive correlation of the colony hybridization assay with the adhesion assay and the hemagglutination inhibition assay described above. However, this technique requires lengthy manipulations to prepare the DNA probes and is too time-consuming for testing of individual strains.
A more practical and faster method than the colony hybridization assay uses the PCR approach. Pham et al. (339) have developed primers designed to amplify a 750-bp fragment of the afaB gene, which encodes a periplasmic chaperone protein involved in the biogenesis of Afa/Dr adhesins. Two pertinent PCR assays that allow the detection of all of the Afa/Dr adhesins have been developed by Le Bouguenec's group (250, 252). The first PCR assay used the afa1 and afa2 primers, based on the partial sequence of the afa-1 gene operon, flanking a 750-bp DNA segment overlapping the afaB and afaC genes (250). After comparison of the nucleotide sequences of the afa-3, afa-7, and afa-8 operons, Le Bouguenec et al. (252), considering that the afa1 and afa2 primers did not detect all of the afa/dr gene clusters, constructed two new primers, afa-f and afa-r, which flanked a 672-bp DNA segment internal to the afaC gene. Strains positive in afa1-afa2 PCR expressed the afaE1, afaE2, afaE3, afaE5, afaE7, afaE8, draE and daaE genes clusters. Afa/Dr DAEC strains have been found equally in control and diseased patients.
Epidemiological studies conducted by use of colony blotting with the daaC DNA probe have demonstrated an age-related incidence of Afa/Dr DAEC in diarrhea in children, which apparently begins after age 2 or 3 (112, 127, 141, 143, 152, 167, 208, 209, 256, 344, 364). Moreover, E. coli strains expressing Afa/Dr adhesins have been found with similar frequencies in patients with diarrhea and control subjects (127, 167, 356). Recently Blanc-Potard et al. (48), using representational difference analysis, have revealed that three sequences (MO30, S109, and S111) were specifically present in the wild-type, diarrhea-associated C1845 strain (43). On the basis of these sequences, it should be of interest to develop a new PCR assay with primers specific for the detection of diarrhea-associated Afa/Dr DAEC strains.
RECEPTORS FOR Afa/Dr ADHESINS
Type IV Collagen
The Dr adhesin binds specifically to the 7S domain (tetramer) of the basement membrane protein type IV collagen (321, 459, 460). Indeed, the Dr adhesin, unlike other members of the Dr family, mediates adherence that is inhibited by the presence of chloramphenicol. Moreover, when examining the ability of other members of the Afa/Dr family, such as AfaE-I, AfaE-III, and F1845, to bind to type IV collagen, Nowicki et al. (321) demonstrated that the collagen-binding phenotype was unique to the Dr adhesin. Interestingly, despite the fact that the amino acid sequence of AfaE-III deduced from the nucleotide sequence of the afaE3 gene shows 98.1% identity to that of the Dr adhesin (251, 316, 324), AfaE-III adhesin conferred chloramphenicol-resistant adherence. Swanson et al. (424) used oligonucleotide-directed, site-specific mutagenesis to construct a hybrid adhesin subunit gene containing the amino terminus of F1845 fused to the carboxy terminus of the Dr structural gene. The resulting construct confers chloramphenicol-resistant hemagglutination when introduced into an E. coli strain expressing the cloned Dr adhesin. Site-directed mutagenesis has been used to show that a negatively charged amino acid is required at position 54 of the Dr adhesin subunit to confer chloramphenicol sensitivity of binding and that mutations at positions 32, 40, 54, 90, and 113 have differing effects on type IV collagen binding and the chloramphenicol sensitivity of binding (73). In particular, replacement of a single amino acid at position 113 of the DraE subunit results in loss of type IV collagen binding. Moreover, the two conserved cysteine residues of the Afa/Dr family structural subunits form a disulfide bond, and mutations of these residues abolish both hemagglutination and binding to type IV collagen. Van Loy et al. (448) have purified the major structural subunits of Dr and F1845 fimbriae, DraE and DaaE, as fusions to maltose-binding protein and to oligohistidine tags and have examined their binding to erythrocytes, Chinese hamster ovary (CHO) cell transfectants expressing DAF, and a DAF fusion protein. The DraE and DaaE fusion proteins bind to the DAF receptor in a specific manner resembling the distinct phenotypes of the corresponding Dr and F1845 fimbriae. In contrast to results of binding studies with the DAF receptor, the DraE fusion proteins did not bind to type IV collagen. When the gene encoding the adhesive subunit, DraE, was subjected to random mutagenesis, the resulting mutants, showing amino acid changes at positions 10, 63, 65, 75, 77, 79, and 131 of the mature DraE sequence, did not display any significant reduction in the ability of the DraE adhesin to bind type IV collagen (449).
Type IV binding capacity appears to be important for urinary tract infection caused by Afa/Dr DAEC, since in the kidney the type IV collagen binding capacity of Dr adhesin leads to the formation of persistent mesangial deposits (285). Consistent with this previous observation, using an isogenic mutant in the DraE adhesin subunit that was unable to bind type IV collagen but retained binding to DAF, Selvaragan et al. (377) have shown that type IV collagen binding mediated by the DraE adhesin is a critical step for the development of persistent renal infection in a murine model of E. coli pyelonephritis. In contrast, the role of type IV collagen binding capacity in Afa/Dr DAEC-induced intestinal pathogenicity is questionable. Indeed, type IV collagen is never present at the apical domain of polarized epithelial cells, the site of Afa/Dr DAEC colonization, since it is mainly of mesenchymal origin (266). Together with fibronectin, laminin, tenascin, and heparan sulfate proteoglycans, type IV collagen is a component of the basement membrane, which is involved in complex interactions at the epithelial-mesenchymal interface. In particular, type IV collagen interacts with integrins expressed at the basal domain of polarized cells (23), to form a link between the basement membrane and epithelial cells (266). However, during inflammation, deregulated expression of membrane-bound molecules that are normally segregated in the basolateral domain of polarized intestinal cells occurs, and it is possible that in this context type IV collagen binding may contribute to the pathogenicity of Afa/Dr DAEC.
DAF (CD55)
Complement-regulating proteins (CRPs) are of vital interest in microbial pathogenicity, since functional domains and structural variations of CD46 and DAF play a pivotal role in the interaction between the pathogen and the host cells that leads to infection (14, 194, 248, 260, 295). In particular, signaling pathways associated with several CRPs are hijacked by microorganisms to promote pathogenicity. Nowicki's group was the first to report that DAF functions as a receptor for Afa/Dr adhesins (Fig. 3). The Dr adhesin (316) has been found to be able to hemagglutinate human erythrocytes that express the Dr blood group antigen but to be unable to hemagglutinate Dr-negative erythrocytes (321), a rare phenotype of the Cromer blood group system in which the Dr antigen is not expressed (272). The Dra blood group antigen is a component of the Cromer-related blood group complex, which consists of 10 antigens located on the DAF (269). Dr binding has been observed in various parts of the human digestive, urinary, genital, and respiratory tracts and in skin (316, 325).
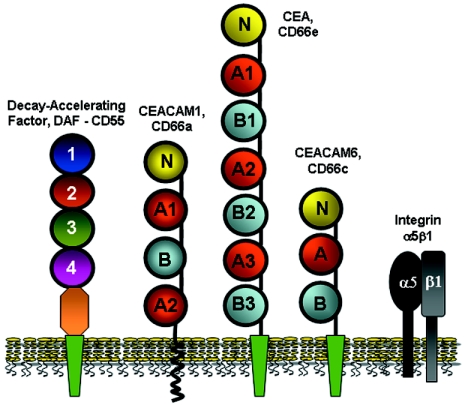
FIG. 3.
Membrane-associated receptors for Afa/Dr adhesins and invasins. DAF acts as a receptor for all of the human Afa/Dr adhesins (Afa/DrDAF) (318, 319). CEACAM1, CEA, and CEACAM6 act as receptors for AfaE-III, Dr, and F1845 adhesins (Afa/DrCEA) (33). Integrin α5β1 acts as a receptor for internalization by Dr-positive IH11128 and AfaE-III-positive A30 strains (164, 343). Integrin α5β1 recognition by AfaD-III invasin leads to cell entry (343).
Receptor for human Afa/Dr adhesins.
The biological functions on human DAF all map to a single surface of the molecule, whereas bacterial and viral pathogens recognize a variety of different sites on DAF (463, 464). All of the uropathogenic and diarrhea-associated E. coli strains expressing the fimbrial F1845, the afimbrial AfaE-I and AfaE-III adhesins, and the Dr and Dr-II adhesins of the Afa/Dr family recognized DAF as a receptor (319, 321, 325). Interestingly, despite the fact that DR-II adhesin displays only 17 to 20% amino acid identity with fimbrial F1845, afimbrial AfaE-I and AfaE-III adhesins, and Dr adhesin, it is interesting that Dr-II adhesin retains the ability to recognize the CCP-3 domain of DAF as a binding site (339), whereas AfaE-VII and AfaE-VIII adhesins do not bind DAF (244, 245).
Afa/Dr adhesins recognized the CCP-3 on DAF (318). Indeed, a single point substitution in CCP-3 (Ser165 to Leu, corresponding to the Dra-to-Drb allelic polymorphism) caused complete abolition of adhesin binding to DAF (318). Importantly, the Dr adhesin-binding and complement-regulating epitopes of DAF appear to be distinct and are approximately 20 Å apart (178). Indeed, it is residue Ser155, and not Ser165, in DAF CCP-3 that is the key amino acid that interacts with the Dr adhesin and amino acids Gly159, Tyr160, and Leu162 and also aids in binding Dr adhesin, while residues Phe123 and Phe148 at the interface of CCP-2 and CCP-3, and also Phe154 in the CCP-3 cavity, are important in complement regulation. An atomic resolution model for functions of the AfaE-III adhesin reveals the pivotal role of CCP-2 and -3 in binding of adhesin onto DAF (8). Like DraE, AfaE-III binds to CCP-2 and -3, but CCP-3 contributes most to the free energy of binding. Interestingly, the binding regions for AfaE-III and the complement pathway convertases lie in close proximity to each other on DAF. This raises the possibility, previously invoked by Nowicki et al. (325), that binding of Afa/Dr adhesins might interfere with the complement-regulatory function of DAF, leading to immunopathological lesions.
The major structural subunits of Dr adhesin (DraE) and F1845 adhesin (DaaE) bind to the DAF receptor in a specific manner resembling the distinct phenotypes of the corresponding Dr adhesin (448). Individual amino acid changes at positions 10, 63, 65, 75, 77, 79, and 131 of the mature DraE sequence significantly reduce the ability of the DraE adhesin to bind DAF. Considering that more than half of the mutants obtained had substitutions within amino acids 63 to 81, this suggests that these proximal residues may cluster to form a binding domain for DAF (449).
As described above for binding of DraE adhesin to type IV collagen, binding of DraE adhesin to DAF is sensitive to chloramphenicol, as demonstrated by the ability of chloramphenicol to inhibit the MRHA of erythrocytes (319, 321). In contrast, the MRHA produced by AfaE-I, AfaE-III, and F1845 adhesins is not sensitive to chloramphenicol (251, 319). Swanson et al. (424) reported that the domains responsible for the chloramphenicol-sensitive hemagglutination of Dr adhesin reside within the amino-terminal portion of the fimbrial subunit. Examination of the X-ray structure of a DraE-chloramphenicol complex has recently revealed the precise atomic basis for the sensitivity of DraE-DAF binding to chloramphenicol (338). The chloramphenicol-DraE complex structure reveals that chloramphenicol binds in a surface pocket between the N-terminal portion of strand B and the C-terminal portion of strand E and lies within the recently identified DAF-binding site (8). Moreover, in contrast to other chloramphenicol-proteins complexes, chloramphenicol binding to DraE is mediated via recognition of the chlorine “tail” rather than by the intercalation of the benzene rings into the hydrophobic pocket. Carnoy and Moseley demonstrated that the single Ile111Thr mutation in DraE completely abolishes chloramphenicol binding (73). The X-ray structure of a DraE-chloramphenicol complex reveals that the chloramphenicol binding site is in close proximity to two of the three sequence differences between DraE and AfaE-III (338), providing an explanation for the previously reported lack of activity of chloramphenicol against AfaE-III binding to DAF (251, 319).
Among E. coli strains isolated from gestational pyelonephritis patients and used to investigate the expression of Dr adhesin, several Dra-positive strains did not fulfill the specific criteria for Dr adhesins (339). Indeed, the binding sites of several of these E. coli strains were located within the CCP-3 domain of DAF but outside the region blocked by a monoclonal anti-CCP-3 antibody, and in other cases they were located on the CCP-4 domain. This reveals heterogeneity in the binding sites of E. coli expressing Afa/Dr adhesins that may reflect the ability of these adhesins to evolve so as to recognize alternative peptide epitopes on DAF in order to achieve efficient colonization.
As described above for Afa/Dr DAEC, DAF is hijacked by coxsackieviruses and enteroviruses as part of their pathogenicity mechanisms. DAF is a major cell attachment receptor for coxsackieviruses B-1, -3, and -5 (3, 30, 32, 277, 380, 383), but cell infection requires an association with the coxsackievirus and adenovirus receptor (CAR) (31, 75, 76, 332, 381, 382, 384). Enterovirus 70 utilizes DAF as an attachment protein (221), recognizing CCP-1 as a binding site (184, 220), but some enteroviruses that bind to DAF also bind to cells of human and murine origins in a DAF-independent manner, suggesting that they use a multiplicity of receptors to achieve infection of the host (153, 154). Finally, human DAF is the receptor that mediates attachment and infection by several echoviruses (30, 84, 153, 184, 249, 347-349). Interestingly, echovirus and coxsackie B viruses all display highly specific recognition of human DAF, since all failed to recognize rat, mouse, or pig DAF (412), like Afa/Dr DAEC (197).
DAF structure and functions.
DAF is a CRP (58, 239, 273, 294). These structurally related regulatory proteins are all encoded in the regulators of C activation gene cluster on chromosome 1q32. The regulators of C activation gene family encodes four membrane-bound proteins: C receptor 1 (CD35), C receptor 2 (CD21), membrane cofactor protein (CD46), and DAF (74, 263, 270, 271, 346, 354). Human DAF is a cell-associated protein with an Mr of 55,000 to 70,000, depending on its glycosylation level. Large quantities of membrane-associated DAF have been found on the epithelial surfaces of oral and gastrointestinal mucosae, renal tubules, ureter and bladder, and cervical and uterine mucosa (86, 199, 281).
Under physiological conditions, DAF plays a central role in preventing the amplification of the complement cascade on host cell surfaces (133, 280). DAF interacts directly with membrane-bound C3b or C4b and prevents the subsequent uptake of C2 and factor B.
The DAF domains involved in complement regulation have been characterized. Biophysical explorations of the structural biology of CRPs have shown that the five human proteins responsible for regulating the early events of complement are homologous and consist mainly of building blocks containing CCPs. The structures of the individual CCPs exhibit wide variations on a common theme, while the extent and nature of their intermodular connections are diverse. Some neighboring modules within a protein stabilize each other, and some cooperate to form specific binding surfaces (232). Molecular cloning of human DAF from HeLa cells has revealed two classes of DAF mRNA (69). The major spliced DAF mRNA (90%) encodes membrane-bound DAF, whereas the minor unspliced DAF mRNA (10%) may encode secreted DAF. The two DAF proteins have divergent C-terminal domains with differing hydrophobicities, and the deduced DAF sequence contains four repeating units homologous to a consensus repeat found in the CRP family. Membrane-bound DAF is attached to the cell surface membrane by a glycosylphosphatidylinositol (GPI) anchor (70, 95), followed by a serine-threonine-proline-rich region and by the repeating units of the CCPs, consisting of 60 to 70 amino acids arranged in tandem (74, 354). A model of the regulatory region of human DAF has revealed that the four CCPs are arranged in a helical fashion (238). Removal of CCP-1 had no effect on DAF function, but individual deletion of CCP-2, CCP-3, or CCP-4 totally abolished DAF function (59, 88). Molecular modeling of the protein has predicted that a positively charged surface area on CCP-2 and -3 and nearby exposed hydrophobic residues on CCP-3 may act as ligand-binding sites and that L147F148 in a hydrophobic area of CCP-3 is essential in the regulation of both regular and alternative pathway C3 convertase, whereas KKK125 to -127 in the positively charged pocket between CCP-2 and -3 is necessary for the regulatory activity of DAF on the alternative pathway C3 convertase but plays a lesser role in its activity on the regular pathway enzyme (58). The N-linked glycan of DAF is not involved in its regulatory function (88). Because of the increased lateral mobility due to the GPI anchor, this gives a functional advantage in contacting ligand C3b or C4b on the cell surface. However, a transmembrane (TM) version of DAF (DAF-TM) is effective in protecting CHO transfectants against cytotoxicity (268). Finally, deletion of the serine-threonine-proline-rich region totally abolished DAF function, since this region serves as a crucial but nonspecific spacer required to project the DAF functional domains above the plasma membrane (88).
CEACAMs as Receptors for Afa/Dr Adhesins
The recognition of CEACAMs as receptors by bacterial pathogens has been reported, and importantly, this recognition is followed by activation of CEACAM-associated signaling by pathogens, which triggers the cellular events that allow these pathogens to evade host defenses. Guignot et al. (166) have shown that CEA-related molecules are recruited around adhering bacteria in enterocyte-like Caco-2 cells, and an inhibition assay using an anti-CD66 antibody demonstrated that one or more CEA-related molecules function as receptors for Afa/Dr DAEC adhesins. Consistent with this, the role of CEACAMs in Afa/Dr DAEC pathogenicity has recently been documented. Berger et al. (33) have analyzed the interactions of Afa/Dr adhesins with CEACAMs by using CEACAM-expressing CHO and HeLa cells. Unlike strains expressing any of the Afa/Dr adhesins binding to DAF (318, 319, 322), only E. coli expressing a subfamily of Afa/Dr adhesins, designated Afa/Dr-I (Afa/DrCEA) and including Dr, F1845, and AfaE-III adhesins, bound to CHO cells expressing CEACAM1, CEA, or CEACAM6 (Fig. 3). Moreover, whereas all of the Afa/Dr adhesins elicited the recruitment of DAF around adhering bacteria (Afa/DrDAF) (150, 166), Afa/DrCEA was the only one to elicit the recruitment of CEACAM1, CEA, and CEACAM6. In addition, although CEACAM3 is not recognized as a receptor by all of the Afa/Dr adhesins, it is recruited around all of the adhering bacteria expressing the Afa/DrCEA adhesins. Consistent with the role of lipid rafts in Afa/Dr DAEC pathogenicity (148, 164, 218), the recruited receptors CEACAM1, CEA, and CEACAM6 are totally or partially resistant to detergent extraction, whereas the recruited nonreceptor CEACAM3 is not. Recognition of CEA and CEACAM6, but not CEACAM1, is accompanied by tight attachment of the bacterium to elongated cell surface microvillus-like extensions. This cellular response results from the activation of Rho GTPase Cdc42 and phosphorylation of ezrin/radixin/moesin (ERM).
The outer membrane protein P5, expressed by Haemophilus influenzae, a commensal of the human respiratory mucosa, recognizes CEACAM1 (189, 450). A major outer membrane protein of Moraxella catarrhalis strains, belonging to the ubiquitous surface protein family, also interacts with CEACAMs as receptors (189). CEACAM1, CEA, and CEACAM6 have been shown to bind some uncharacterized E. coli strains and some Salmonella species (253-255, 363). CEACAMs play an important role in the pathogenicity of Neisseria gonorrhoeae, since opacity (Opa) proteins mediate the adherence and signaling required to allow this bacterium to penetrate into human tissues (96, 97, 180, 182, 183, 282, 283, 310, 452). As described above for Afa/Dr adhesins, groups displaying distinct specificities of Opa interaction with CEACAMs have been identified (159). CEACAM1, CEACAM3, CEA, and CEACAM6 all act as OpaCEA receptors, whereas CEACAM4, CEACAM7, and CEACAM8 do not (44, 49-52, 79, 158, 298, 345). Opa52 binds CEACAM1, CEACAM3, CEA, and CEACAM6; Opa53 is CEACAM1 specific; Opa54 binds CEACAM1 and CEA; and Opa55 is CEA specific.
CEACAMs belong to the immunoglobulin (Ig) superfamily of adhesion molecules (163, 172, 327, 431). The members of the CEACAM gene family are clustered on chromosome 19q13.2. CEACAMs share a conserved N-terminal Ig variable (Igv)-like domain that is followed by 0 to 6 Ig constant (Igc)-like domains. The CEACAMs, consistent with the recently redefined nomenclature (22), now comprise seven members, i.e., CEACAM1 (biliary glycoprotein, CD66a), CEACAM3 (CEA gene family member 1 [CGM1], CD66d), CEACAM4 (CGM7), CEA (carcinoembryonic antigen, CD66e), CEACAM6 (nonspecific cross-reacting antigen, CD66c), CEACAM7 (CGM2), and CEACAM8 (CGM6, CD66b). CEACAM receptors are differentially expressed by various epithelial, endothelial, and hematopoietic cells in vivo (22, 163). CEACAM1, CEACAM3, and CEACAM4 are inserted into the cellular membrane via a carboxy-terminal transmembrane and cytoplasmic domain, whereas CEA, CEACAM6, CEACAM7, and CEACAM8 have a GPI anchor instead. The level of glycosylation of CEACAM receptors may vary, depending on their cell type and differentiation state, and multiple glycoforms of the same protein have been isolated. CEACAMs generally function as intercellular adhesion molecules (25). Moreover, the observation that CEACAM1, CEA, CEACAM6, and CEACAM7 are all located on the apical glycocalyx of normal colonic epithelium suggests that they could play a role in innate immunity (118).
CEACAM1 structure and functions.
CEACAM1 contains the conserved N-terminal Igv-like domain of CEACAMs, which is followed by three Igc-like domains (22, 396, 431). CEACAM1 is inserted into the cellular membrane via a carboxy-terminal transmembrane and cytoplasmic domain. Differential splicing of CEACAM1 finally yields eight transmembrane isoforms, including CEACAM1-4L, CEACAM1-3L, CEACAM1-4S, and CEACAM1-3S, with different numbers of extracellular domains, and either a long or a truncated cytoplasmic domain. CEACAM1 has been shown to be expressed on leukocytes, including granulocytes, activated T cells, B cells, and CD16− CD56+ natural killer cells (163), and has also been observed in endothelial cells, in the apical poles of enterocytes and colonic cells, and in the epithelia of esophageal and Brunner's glands, bile ducts and gallbladder, pancreatic ducts, proximal tubules of the kidney, prostate, endometrium, and mammary ducts (195, 350).
CEACAM1 functions as a cell-cell adhesion molecule that mediates homophilic cell adhesion (429, 457, 458). CEACAM1 contributes to contact inhibition of cell proliferation in confluent cells but allows proliferation when expressed at different isoform ratios (117, 129, 394). CEACAM1 expression has been reported to be generally downregulated in carcinomas of the colon and liver of human, rat, and mouse origins (235, 311, 372), and in human colon and prostate cancer downregulation is associated with the loss of cell polarity (66) and results in enhanced tumor cell growth and tumorigenicity (15). CEACAM1 directly associates with the cytoskeleton proteins actin and tropomyosin (61, 374). CEACAM1-L is located at cell-cell boundaries, and its association with the actin cytoskeleton is regulated by the Rho family of GTPases (359). Consistent with this, CEACAM1 colocalizes with paxillin at the plasma membrane, and CEACAM1-paxillin complexes have been isolated in granulocytes, the colonic cell line HT29, and human umbilical vein endothelial cells (111). In polarized Madin-Darby canine kidney (MDCK) epithelial cells, activation of Cdc42 and Rac1, or of their downstream effector PAK1, targeted CEACAM1 to sites of cell-cell contacts. The transmembrane domain of CEACAM1 was responsible for the Cdc42-induced targeting at cell-cell contacts (128).
Other cell functions mediated by CEACAM1 have also recently attracted interest. In human T and natural killer (NK) cells (291) and small intestinal intraepithelial lymphocytes (292), CEACAM1 phosphorylation undergoes a rapid increase following stimulation with the chemoattractant formyl-Met-Leu-Phe peptide (395). Ligation of CEACAM1 strongly increases adhesion to fibrinogen by Fc receptor- and β2 integrin-dependent mechanisms (419). Interestingly, coligation of CEACAM1 plus CEACAM6 and CEACAM8 has also been reported to cause increased β2 integrin-mediated adhesion and receptor clustering, whereas ligation of CEACAM6 or CEACAM8 separately did not cause neutrophil activation. CEACAM1 acts as a novel class of immunoreceptor tyrosine-based inhibition motif (ITIM)-bearing regulatory molecules on T cells that are active during the early phases of the immune response in mice (215, 216, 303). The cytoplasmic domain of CEACAM1 contains two tyrosine residues in amino acid motifs interacting with pp60c-src, which are located in ITIM consensus sequences (62). Phosphorylation of CEACAM1 tyrosine by an associated tyrosine kinase may have a functional role (395). Lyn and Hck account for much of the tyrosine kinase activity associated with CEACAM1 (395), which activates extracellular signal-regulated kinases 1 and 2 (392). The structural features surrounding the tyrosine residues in the cytoplasmic domain of CEACAM1 share similarities with the consensus sequence of the ITIM, the docking site for SHIP, SHP-1, and SHP-2 molecules. When phosphorylated, these residues associate with the protein-tyrosine phosphatases SHP-1 and SHP-2, and the C-terminal amino acids of CEACAM1 are critical for these interactions (196). The intracytoplasmic domain, which contains two ITIM-like domains, is required for activation of a fraction of T cells in the lamina propria that express CEACAM1 by interleukin-7 (IL-7) and IL-15 cytokines, indicating that CEACAM1 amplifies T-cell activation and thus could facilitate cross talk between epithelial cells and T lymphocytes in the intestinal immune response (102).
The particular role of CEACAM1 in Neisseria pathogenicity has been documented. By its functional ITIM, CEACAM1 plays pivotal role in OpaCEA-mediated signaling (283, 284). CEACAM1 functions as a microbial receptor in human granulocytes and epithelial cells, since OpaCEA proteins bind to the N-terminal domain of CEACAM1 on the nonglycosylated surface of the molecule (451, 453). Interestingly, the N-terminal domain is implicated in homophilic adhesion by CEACAM1 (458). No pathogen-directed reorganization of the actin cytoskeleton is required for invasion of the epithelial cell lines via CEACAM1 to occur (44). Neisseria infection induces the expression of CEACAM1, CEACAM1-3L, and CECAM1-4L splice variants through activation of an NF-κB heterodimer consisting of p50 and p65. Subsequently, increased Opa52-dependent binding of gonococci by these cells develops (297, 299). The ability of N. gonorrhoeae to upregulate its epithelial receptor CEACAM1 via NF-κB reveals an important pathogen-elicited mechanism that allows efficient bacterial colonization to occur during the initial infection process. In addition, the regulation of CEACAM1 expression by NF-κB also implies that this receptor plays a broader role in the general inflammatory response to infection (299). N. gonorrhoeae evades host immunity by switching off T lymphocytes (56). In N. gonorrhoeae, the Opa52 protein is able to bind the CEACAM1 expressed by primary CD4+ T lymphocytes and to suppress their activation and proliferation after the Opa gonococcal protein associates with the tyrosine phosphatases SHP-1 and SHP-2 in the ITIM of CEACAM1 (55, 80). In addition, OpaCEA interaction with CEACAM1 leads to inhibition of the activation and proliferation of Neisseria-infected CD4+ T lymphocytes (315). It remains to be determined whether or not the recognition of CEACAM1 by Afa/DrCEA adhesins is followed by the signaling events and the cellular responses observed for OpaCEA.
CEA structure and functions.
CEA is a well-established tumor-associated marker (398). CEA shares the conserved N-terminal Igv-like domain of CEACAMs, which is followed by six Igc-like domains (22, 431). CEA is expressed by M cells (19), enterocytes (34), and colonic cells (456) and is an integral component of the apical glycocalyx (173). CEA has been shown to act, in vitro at least, as a homotypic intercellular adhesion molecule. CEA is known to mediate Ca2+-independent, homotypic aggregation of cultured human colon adenocarcinoma cells. CEA is produced in excess in virtually all human colon carcinomas and in a high proportion of carcinomas at many other sites (372). The engagement of neutrophil CEA with anti-CEA Ig results in activation-associated phenomena, including shape change and activation of β2-integrin (418). CEA can also inhibit the differentiation of several other cell types and thus contributes to tumorigenesis, an activity that requires CEA-CEA interactions (78). This differentiation-blocking activity resides in its GPI anchor (375). Deregulated expression of CEA could directly contribute to colon tumorigenesis by inhibiting terminal differentiation and anoikis (201). CEA may act as a chemoattractant in colorectal cells, a function related to type IV collagen and laminin (230). In fully differentiated polarized epithelial cells, CEA is apically expressed. It has not been shown whether CEA mediates functions in normal cells. CEA is anchored in the cell membrane via a GPI anchor, and like other GPI-anchored proteins (82, 386, 387, 415, 417), CEA can signal.
The role of CEA in signaling events following its recognition as a receptor by microbial pathogens is poorly documented. OpaCEA-mediated stimulation of CEA leads to activation of the small GTPases Rac1 and Cdc42 (44) and downregulation of the tyrosine phosphatase SHP-1 (181). It remains to be analyzed what the signaling events that follow the recognition of CEA by Afa/DrCEA adhesins are. Moreover, it could be of interest to examine whether the CEA, which is a GPI-anchored protein, triggers the same signaling events observed following recognition of DAF. Finally, analyzing the cellular responses that occur after the recognition of CEA by Afa/DrCEA adhesins is of interest, considering that the functions of CEA are poorly documented.
CEACAM6 structure and functions.
CEACAM6 shares the conserved N-terminal Igv-like domain of CEACAMs, which is followed by two Igc-like domains (22, 397, 431). CEACAM6 is anchored in the cell membrane via a GPI anchor. Intriguingly, unlike GPI-anchored proteins, CEACAM6 could not be dislodged from the cell membrane by phosphatidylinositol-specific phospholipase C. CEACAM6, due to its GPI anchor, is apically expressed in polarized epithelial cells. Like CEA, the GPI-anchored CEACAM6 can signal (82, 386, 387, 415, 417). Consistent with this, CEACAM6-cross-linking increased c-Src activation and induced tyrosine phosphorylation of p125FAK focal adhesion kinase, for which caveolin-1 was required (106). Moreover, CEACAM6 cross-linking initiates c-Src-dependent cross talk between CEACAM6 and αvβ3 integrin, leading to increased extracellular matrix component adhesion (107).
CEACAM6 is coexpressed with CEA in normal colorectal epithelia and is deregulated in colorectal cancers, where it could play a role in tumorigenesis (201). CEACAM6 revealed a broader expression zone in proliferating cells in hyperplasic polyps and adenomas than in the normal mucosa. Anoikis is the apoptotic response induced in normal intestinal cells by inadequate or inappropriate adhesion to substrate. Deregulated overexpression of CEA/CEACAM6 inhibits anoikis (330). Furthermore, increased CEACAM6 expression and CEACAM6 cross-linking both induced a significant increase in cellular resistance to anoikis, and CEACAM6 gene silencing reversed this acquired resistance (108). It has been observed that Akt, which is known to mediate cell survival, is activated in colonic T84 cells expressing CEA and CEACAM6 and infected with Afa/Dr DAEC (F. Betis, A. L. Servin, and P. Hofman, unpublished data).
CEACAM6 is involved in the invasion of epithelial cell lines by Neisseria. As for CEACAM1, no pathogen-directed reorganization of the actin cytoskeleton is required for OpaCEA-expressing bacteria (44). Moreover, the CEACAM6-mediated uptake of Neisseria is not blocked by dominant-negative versions of the small GTPase Rac (371). This mechanism of cell entry resembles the mechanism by which Afa/Dr DAEC is internalized following recognition of CEA and/or CEACAM6, which does not require mobilization of the actin cytoskeleton and which is not inhibited by substances that block the signaling molecules involved in F-actin rearrangements (218). It remains to be determined whether Neisseria uses lipid rafts, like Afa/Dr DAEC (148, 164, 218), to invade the cells following recognition of the GPI-anchored CEACAM.
MECHANISMS OF PATHOGENICITY
UTIs
Epidemiological studies show that DAEC strains that express adhesins of the Afa/Dr family are involved in 25 to 50% of cases of cystitis in children and 30% of cases of pyelonephritis in pregnant women (11, 92, 103, 322). Moreover, E. coli expressing Dr adhesin has been shown to be associated with a twofold increase in the risk of a second UTI, suggesting its possible association with recurrent or chronic UTI (131). Forestier et al. (127) found that daaC-positive strains were significantly associated with a past record of urinary tract infections. Zhang et al. (470) screened UTI and fecal E. coli isolates for the presence of Dr sequences (drb) and found that among the drb-positive strains examined, 18% were afaE1 positive, 1.3% were afaE2 positive, 1.3% were afaE3 positive, 12% were draE positive, and 1.3% were daaE positive, whereas 12% were draE-afaE3 hybrid. It is noteworthy that daaC-positive E. coli isolates from human patients with disease have been found that express other virulence factors, including aerobactin (130, 441), the CS31A antigen reported for septicemic and bovine ETEC strains (207), cytotoxic necrotizing factor (130, 131, 212, 441), and hemolysin (92, 93, 130, 131, 165, 176, 209, 312, 441). Recent data indicate that AfaE-1, AfaE-III, and F1845 adhesins are found in isolates from both human diarrhea and UTI (252).
DAF has been shown to regulate complement activation on glomerular epithelial cells (351), and expression of DAF was increased on the glomerulus of patients with diffuse proliferative glomerulonephritis (12). Experimental studies have shown that urinary complement components have a role in mediating tubulointerstitial damage, which is known to be closely correlated with the progression of chronic renal diseases. Both GPI-anchored and transmembrane-anchored DAF proteins, each of which can be derived from two different genes (Daf1 and Daf2), are produced in mice, and nephrotoxic serum nephritis develops in both wild-type mice and Daf1 gene-floxed mice (259). Increased susceptibility to antiglomerular basement membrane glomerulonephritis has been reported in DAF-deficient mice (401). The possible role of virulence factors of Dr-positive E. coli in the persistence of bacteria in renal tissue and in the pathogenesis of chronic pyelonephritis has been investigated. Goluszsko et al. (146) examined the hypothesis that E. coli renal interstitial binding mediated by the Dr adhesin is important for the development of chronic ascending pyelonephritis in mice. Dr+ E. coli colonized the renal interstitium, since a substantial amount of fimbrial antigen was detected in the injured parenchymal regions, and significant histological changes corresponding to tubulointerstitial nephritis, including interstitial inflammation, fibrosis, and tubular atrophy, were found in the kidney tissue of Dr+-infected mice but not in that of Dr−-infected mice. Considering that the Dr adhesin mediates interaction with DAF and type IV collagen (321, 459, 460), whether these phenotypes are necessary for the development of tubulointerstitial nephritis in mice has been investigated. Meittinen et al. (285) observed that in kidney, the type IV collagen binding capacity of Dr adhesin results in the formation of mesangial deposits that persist but does not induce histological damage, indicating that additional factors provided by the bacteria and/or the host are needed for glomerular damage to occur. Selvarangan et al. (377) recently demonstrated that the type IV, collagen-binding phenotype is crucial for E. coli virulence in the mouse model of chronic pyelonephritis. Indeed, an isogenic DraE adhesin subunit mutant that was unable to bind type IV collagen but retained binding to DAF was eliminated from the mouse renal tissues, while the parent strain caused persistent renal infection. In addition, transcomplementation with the intact Dr operon restored type IV collagen-binding activity, basement membrane interstitial tropism, and the ability to cause persistent renal infection. The role of DAF in the development of chronic ascending pyelonephritis in Dr-positive E. coli-infected mice is currently not established, and a recent report is not in favor of a role of mouse DAF. Indeed, Hudault et al. (197) showed that like the echovirus and coxsackie B viruses, which bind specifically to human DAF but fail to recognize rat and mouse DAF (412), Dr and F1845 adhesins fail to recognize mouse, rat, or pig DAF. This could result because although mouse DAF contains four CCPs similar to those found in human and guinea pig DAF (174), the base sequences of mouse and human DAF show 63.7% identity and the deduced degree of amino acid sequence identity between mouse and human DAF is only about 47% (134, 410). In addition, considering that in rodents Crry is a membrane-associated complement-regulating protein (126, 258, 290, 352) expressed on glomerular mesangial, endothelial, and epithelial cells and that like DAF, Crry protects against complement injury (231, 289, 304, 313, 314, 368), Hudault et al. (197), examining the role of Crry in Afa/Dr binding, showed that Crry does not act as a receptor for human Afa/Dr adhesins. In conclusion, from these reports, it is probable that the recognition of DAF by Dr adhesin is not necessary for the induction of tubulointerstitial nephritis in Dr-positive E. coli-infected mice, whereas the recognition of type IV collagen is a critical step for the development of persistent renal infection in mice.
UTIs are associated with approximately 27% of premature births. E. coli Dr family adhesins have been found to be frequently expressed in strains associated with pyelonephritis in pregnant females (176, 320, 339, 340). Within the uterus, DAF has been found in the endometrial glands, spiral arterioles, and myometrial arteries protecting tissues against complement-induced damage, and the DAF density in the endometrium may affect sensitivity to complement activation (222, 223). The presence of DAF in the endometrium and interindividual differences in DAF density in the endometrium may affect sensitivity to the attachment of Dr-bearing E. coli (223, 225). Moreover, DAF has been found to be overexpressed in endometrial biopsies from patients without malignancy at the proliferative phase (326).
Using the experimental model of chronic pyelonephritis developed with E. coli bearing Dr adhesin, Kaul et al. (224) observed that nearly 90% of pregnant mice infected with Dr-positive E. coli delivered preterm, compared to 10% of mice infected with Dr-negative E. coli. Urogenital tract colonization by Dr-positive E. coli is accompanied by a defense mechanism involving nitric oxide (NO), which is known to induce antimicrobial activity (120). NO is generated in the uterus, and one of its functions is to inhibit uterine contractility (465). Current data support the suggestion that gestation, parturition, steroid hormones, and prostaglandins all modulate both the generation and the effects of NO on the uterus (439). Moreover, the NO synthases (NOS) that produce NO are induced by lipopolysaccharide (LPS) and/or cytokines. One theory is that NO plays a role in uterine quiescence during pregnancy and that any change in this system at term or preterm could play a role in inhibiting labor and delivery (309). An increase in rat uterine NOS activity has been found in pregnancy and declines at term, suggesting that NO functions in an autocrine and/or paracrine manner (355). Indeed, two isoforms of NOS have been found, an endothelial constitutive form located in vascular endothelium and an inducible form that is expressed in the myometrium of the pregnant rat uterus but not in that of the virgin rat and the expression of which declines at term when labor occurs. A localized increase in type II NOS expression and NO production occurs in response to intrauterine infection (121, 122). Moreover, the invasion of human endometrial adenocarcinoma Ishikawa cells by Dr-positive E. coli was reduced by elevated NO production and increased by NO inhibition. In addition, elevated NO production significantly reduced DAF protein and mRNA expression in Ishikawa cells in a time- and dose-dependent manner (123). In addition, changes in NO and LPS responsiveness were significantly associated with the increased sensitivity of C3H/HeJ mice to experimental Dr-induced pyelonephritis. Infection of LPS responder (C3H/HeN) and nonresponder (C3H/HeJ) mice with E. coli strain O75 (bearing Dr fimbriae) and an O75 strain (bearing P fimbriae) has shown that the E. coli infection rate in Dr-infected C3H/HeN mice treated with the inhibitor of nitric oxide, L-NAME, was approximately 100-fold greater than that in the P-infected group (323). E. coli infection is followed by complications in pregnancy, and death occurs in pregnant mice within 24 to 48 h following infection. This death rate was increased twofold by treatment with the NO blocker L-NAME. In contrast, no deaths occurred in nonpregnant animals with or without L-NAME treatment, suggesting that infectious complications of pregnancy may be related to gestation-dependent sensitivity to the pathogenic microorganism and to the host's NO status (317). Overall, these findings add to our understanding of the NO-dependent mechanism of defense and have provided reliable insights into how this system works as an epithelial defense against urogenital tract infection. As already discussed for Dr-induced pyelonephritis, the role of mouse CRPs in Dr-induced urogenital tract infection is intriguing, since mouse DAF and Cryy do not act as receptors for human Afa/Dr adhesins (197).
One study indicates that CEACAM1 could be implicated in the human implantation process (16, 17). Data from immunohistochemistry studies and flow cytometry and Western blotting of isolated trophoblast populations show that CEACAM1 is present in epithelial cells of the pregnant endometrium as well as in small endometrial vessels, whereas it is absent from decidual cells. In the fetus, CEACAM1 is strongly expressed by the extravillous, intermediate trophoblast at the implantation site, as well as by extravillous trophoblastic cells. Expression is also observed in placental villous core vessels but is absent from both villous cyto- and syncytiotrophoblasts throughout pregnancy. A subfamily of Afa/Dr adhesins, including Dr, AfaE-III, and F1845, bind to CEACAM1, CEA, or CEACAM6 (33). Human CEACAM1, CEA, and CEACAM6 are not expressed in mice. However, it has been reported that murine CEACAM1 and CEACAM2 can serve as receptors for mouse hepatitis virus, a murine coronavirus (234, 288, 392, 428, 432, 438). The possibility that murine CEACAM1 acts as a receptor for human Dr adhesin in the infectious mouse model has been investigated by using BHK cells transfected with mouse CEACAM1a, and the results show that it does not act as a receptor (S. Hudault and A. L. Servin, unpublished data).
Internalization
Uropathogenic E. coli strains can invade and replicate within uroepithelial cells, which gives them a survival advantage, as it enhances the ability of these microbes to resist detection and clearance by both innate and adaptive immune defense mechanisms (301, 302, 369). Afa/Dr DAEC strains enter epithelial cells by a zipper-like mechanism (213, 218), but to a lesser extent than is achieved by invasive bacteria such as Salmonella. The bacterial factor(s) involved in Afa/Dr DAEC internalization has not been clearly identified, and both DraE and AfaD proteins seems to be involved in the internalization process.
According to Nowicki's group, the DraE adhesin harbored by Dr-positive strains and encoded by the draE gene is sufficient to promote internalization, even though this strain expresses a DraD invasin (469). Indeed, purified Dr fimbriae applied to polystyrene beads were capable of triggering receptor clustering and the accumulation of actin at the adhesion sites on cells where beads were engulfed and ultimately internalized by the cells (150). In addition, the internalization of Dr-positive E. coli was inhibited by anti-Dr fimbria IgG and anti-CCP-3 of DAF, and the draE, draC, and draB insertional mutants and adherent draD mutant were unable to enter epithelial cells, whereas complementation of the dra mutation restored their invasiveness (147). Consistent with a role of DraE adhesin in internalization, Selvaragan et al. (376) have demonstrated the role of extracellular domains and the GPI anchor of DAF in the internalization process of Dr-positive E. coli. Binding to the CCP-3 domain and replacement of the GPI anchor of DAF were critical for internalization to occur. Internalization of Dr-positive E. coli is associated with the recruitment of α5β1 integrin around the adhering bacteria (164, 218). Interestingly, it has been reported that β1 integrin plays a critical role in echovirus-1 binding preceding the DAF-dependent entry (99, 421). Dr-mediated internalization is inhibited by nocodazole (148, 164), indicating that the microtubules play a role in the entry process, as has been observed for a few pathogens, including Campylobacter jejuni (328). In enterocyte-like epithelial cells, an Afa/Dr diffusely adhering E. coli strain bearing the Dr adhesin entered basolaterally but not apically (164). In addition, it has been observed that surviving Dr-positive bacteria residing within the host cell have no effect on the functional differentiation of these cells (164).
According to Le Bouguenec's group, the AfaD protein harbored by an Afa-III-positive strain and encoded by the afaD gene acts as an invasin (137). The AfaD invasin is structurally and functionally conserved among Afa-expressing human strains, independently of the AfaE subtype and clinical origin of the E. coli isolate (138). The AfaD protein, like the AfaE protein, was exposed at the bacterial cell surface, but unlike AfaE, it was able to detach itself from the surface of bacterium to become internalized (137, 138, 157). Moreover, recent data suggest that the AfaE-III adhesin assembles into a flexible fiber that provides the link between the bacterial membrane usher and the invasin at the tip (8). Recombinant E. coli producing the AfaD or AfaE-III protein demonstrated that AfaE-III allows the E. coli to bind to cells and that AfaD mediates the internalization of the adherent bacteria (213). Moreover, colloidal gold tagging of AfaE-III and AfaD proteins has shown that AfaE-III-gold complexes are simply bound to the cell surface, whereas AfaD-gold complexes actually enter the cells. The role of AfaD in cell entry has been confirmed by the observation that coating of polycarbonate beads with AfaD protein enables the beads to enter the cell (137). As observed for Dr-positive bacteria (164), the entry of recombinant AfaD-coated beads into both cervical HeLa and undifferentiated intestinal Caco-2 cells was dependent on the accessibility of β1 integrins (343) (Fig. 3). It has been suggested that AfaD could be the prototype of a family of invasins encoded by adhesion-associated operons in pathogenic E. coli (138). This hypothesis is based on two observations. First, the AggB protein from enteroaggregative E. coli has also been found to be an AfaD-related invasin (138). Second, despite their differences, the recombinant AfaD-III and AfaD-VIII proteins both bind to β1 integrins (343).
To reconcile the two mechanisms proposed for Afa/Dr DAEC internalization, it could be of interest to consider the mechanism of internalization by coxsackieviruses, which also recognizes DAF as a receptor (32, 379), as well as that of Afa/Dr DAEC. Two individual components of the CAR complex have been identified as DAF and the CAR protein (31, 76, 384). Interestingly, in the lytic action of the coxsackievirus, DAF acts as a virus sequestration site, enhancing the presentation of the virus to the functional CAR protein. In light of this mechanism, it is tempting to propose that a prerequisite for the zipper-like internalization of Afa/Dr DAEC to occur is the attachment of Afa/Dr DAEC to DAF, followed by the interaction of the Afa/Dr invasins with α5β1 integrin.
Pathogens entering host cells engage molecular mechanisms that vary widely from one pathogen to another (87, 233). A zipper-like internalization process is utilized by Dr- and AfaE-III-positive bacteria to internalize into host cells (213, 218). The initial engulfment of Neisseria (44), Listeria (203), Helicobacter (240), EPEC (135), and Streptococcus (100) occurs via a zipper-like endocytosis mechanism. The prototype of zipper-like bacterial internalization is that of Yersinia, which involves the subversion of the α5β1 integrin in a receptor-mediated mechanism that promotes the microfilament cytoskeleton-dependent advance of the pseudopod and involves receptor-ligand affinity, receptor clustering, signaling through focal adhesion kinase, and stimulation of cytoskeletal rearrangements by small GTP-binding proteins (204, 205). It is important to note that the zipper-like internalization of Dr-positive bacteria is independent of the events related to the microfilament cytoskeleton that accompany Afa/Dr DAEC cell infection (218). The internalization of Afa/Dr DAEC resembles the uptake of Neisseria mediated by GPI-anchored CEA and CEACAM6, which is a zipper-like mechanism, independent of activated tyrosine kinases and F-actin microfilaments (45, 279). The mechanism of cell entry used by Dr-positive E. coli is different from those used by the uropathogenic FimH-positive strain and the EAIC LF82 E. coli strain. Indeed, although FimH-positive E. coli strains use a raft-dependent internalization mechanism (18, 389, 390), the entry of bacteria into epithelial cells results from massive cell membrane reorganization characteristic of a macropinocytic mechanism, involving activation of a cell signaling pathway involving protein tyrosine phosphorylation and two Rho-GTPase family members, namely, Cdc42 and Rac1, that control an F-actin-dependent process (275, 276). In LF82 E. coli, type 1 pili alone are not sufficient to trigger bacterial internalization, but type 1 pilus-mediated adherence is involved in disrupting host cell signaling, leading to membrane elongations that closely resemble Salmonella- or Shigella-induced macropinocytosis (53, 54).
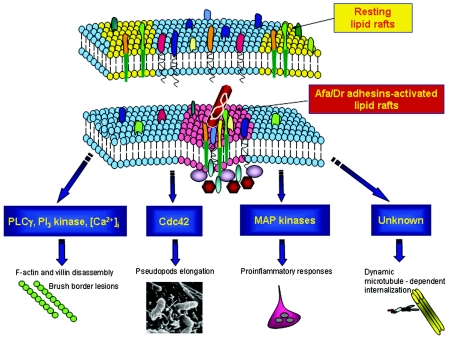
Caveolae and lipid rafts are being increasingly recognized as significant portals of entry into host cells for a wide variety of pathogenic microorganisms and bacterial toxins (104, 274, 337, 357, 446). For example, pathogenic bacteria, including Shigella flexneri, Chlamydia trachomatis, uropathogenic FimH-positive E. coli, and Mycobacterium kansasii, use lipid rafts to enter the host cells. It has been shown that Dr-positive E. coli is one of the pathogenic bacteria that uses lipid rafts for internalization (148, 164). By investigating the initial steps in the infection process that depend on Dr adhesin, it has been recently demonstrated that adhering bacteria recruit the lipid raft-associated molecules ganglioside GM1 and VIP21/caveolin (218) (Fig. 4 and 5). Interestingly, like that of Afa/Dr DAEC, the DAF-dependent echovirus 11 cell entry (421) is dependent upon the presence of cholesterol and an intact actin cytoskeleton and microtubule network (420). Moreover, as it has been demonstrated for Dr-positive bacteria (148, 164, 218), the zipper-like mechanism of internalization used by Listeria monocytogenes involves the mobilization of raft-associated molecules such as ganglioside GM1 and organized lipid rafts (378). Unlike clathrin-mediated endocytosis, internalization of pathogenic microorganisms via lipid rafts or caveolae is a triggered event that involves complex signaling, consistent with the function of lipid rafts as platforms for signaling molecules (200, 391, 399). In view of the facts that two of the receptors for Afa/Dr adhesins are the signaling DAF and CEA GPI-anchored proteins and that GPI-anchored proteins in lipid rafts function as signaling molecules, the role of lipid rafts in Afa/Dr DAEC has been investigated (Fig. 4). Increased receptor ligand density occurs at the site of internalization (148, 164). Consistent with the fact that signaling through GPI-anchored proteins requires lipid raft integrity (342), it has been observed that dissociation of lipid rafts completely prevents the internalization of Dr-positive E. coli (148, 164).
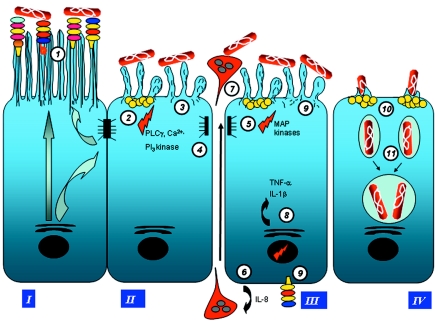
FIG. 4.
Roles of lipid rafts and signaling pathways in Afa/Dr DAEC pathogenicity. Afa/DrDAF and Afa/DrCEA adhesins recognize as receptors the GPI-anchored proteins DAF, CEA, and CEACAM6, which are known to be associated with lipid rafts that act as a platform for signaling molecules (200, 391, 399). Afa/Dr DAEC bacteria adhere to epithelial cells, mobilize raft-associated molecules ganglioside GM1 and VIP21/caveolin (218) and GPI-anchored proteins DAF, CEA, and CEACAM6, which act as receptors (33, 150, 166, 213), and recruit the α5β1 integrin as a receptor for Afa/Dr invasins (164, 218, 343). Signaling involving protein tyrosine kinase(s), phospholipase Cγ, phosphatidylinositol 3-kinase, protein kinase C, and an increase in [Ca2+]i leads to structural rearrangements of the cytoskeleton (335, 336). Signaling involving the Rho GTPase Cdc42 leads to pseudopod elongation (33, 85). Signaling involving MAPKs leads to proinflammatory responses, including IL-8 production and PMNL transmigration (39, 40). The dynamic microtubule-dependent internalization of Dr-positive bacteria is a lipid raft-dependent phenomenon (148, 164, 218).
FIG. 5.
Virulence mechanisms of Afa/Dr DAEC. (I) In epithelial cells, Afa/Dr DAEC interacts with membrane-bound receptors (circle 1) including the recognition of DAF by Afa/DrDAF adhesins (318, 319) and of CEACAM1, CEA, and CEACAM6 by Afa/DrCEA adhesins (33, 166). In the polarized epithelial cells forming epithelium, receptor recognition develops at the apical domain expressing the brush border (arrows indicate the transport pathways of functional proteins in polarized epithelial cells) (36, 335). (II) The loss of microvilli results from a signaling pathway involving protein tyrosine kinase(s), phospholipase Cγ, phosphatidylinositol 3-kinase, protein kinase C, and an increase in [Ca2+]i (circle 2) (336), which controls the rearrangements of brush border-associated F-actin and villin cytoskeletal proteins (circle 3) (36, 335). The elongation of the microvilli (36, 85) also involves the activation of Rho GTPase Cdc42 (33). Structural lesions in the brush border are accompanied by a decrease in the expression and enzyme activities of functional brush border-associated proteins, including SI, DPP IV, glucose transporter SGLT1, and fructose transporter GLUT5 (circle 3) (333, 335). The increased paracellular permeability is associated with the reorganization of TJ-associated proteins, ZO-1 and occludin, but does not affect the TER (circle 4) (334). (III) A MAPK-dependent signaling mechanism (circle 5) leads to the production of the proinflammatory cytokine IL-8, which triggers the transepithelial migration of PMNLs (circle 6) (39). Afa/Dr DAEC bacteria interact with PMNLs (circle 7) (57, 211). In turn, PMNL transmigration promotes the production of proinflammatory cytokines TNF-α and IL-1β (circle 8) (40). The proinflammatory response induces the upregulation of DAF and MICA and abnormal expression of DAF at the basolateral domain (circle 9) (40, 433). (IV) Internalization occurs in nonpolarized epithelial cells via a mechanism involving lipid rafts and dynamic microtubules (circle 10) (137, 148, 164, 213, 218). Recognition of α5β1 integrin by Afa/Dr invasins plays a pivotal role in bacterial internalization (164, 343). Internalized Afa/Dr DAEC bacteria survive within a large, late vacuole (circle 11), which seems to result from the fusion of the early vacuoles, containing one bacterium, that are formed during the initial step of internalization (148, 164, 213, 343).
Internalized Afa/Dr DAEC bacteria do not significantly multiply in the HeLa cell line and survive within a large, late vacuole which seems to result from the fusion of the early vacuoles containing one bacterium formed during the initial step of internalization (148, 164, 213). It is known that intracellular pathogens, such as Salmonella enterica serovar Typhimurium, residing within a single cytoplasmic organelle (41, 156, 414) control the organization of the vacuolar membrane by acquiring functional cellular molecules that allow the intravacuolar bacteria to survive. The molecules associated with both the early and late vacuole-containing Afa/Dr DAEC bacteria are unknown, as is the mechanism by which the Afa/Dr DAEC bacteria survive within the late vacuole.
Concomitantly with internalization, other cellular events contribute to the recurrence of infection by Afa/Dr DAEC. For example, Dr adhesin mediates the adherence of Afa/Dr DAEC to polymorphonuclear leukocyte (PMNLs), resulting in minimal bacterial killing (211). Moreover, the Afa/Dr DAEC strain C1845 induces F-actin-dependent long, thin membrane processes that extend from the cell surface (85). These projections promote gentamicin protection, indicating that they may play a role in enabling the bacterium to survive host defenses. Finally, a significant increase in ampicillin resistance among gestational pyelonephritis E. coli has been found to be associated with the dra gene cluster (175).
Cell Signaling
GPI-anchored CRPs localize in the plasma membranes of cells in patches and microdomains that are resistant to detergent extraction. These membrane microdomains, or lipid rafts, contain high levels of glycosphingolipids and cholesterol and have been implicated in cell processes such as membrane sorting and signal transduction (60, 237). GPI-anchored proteins present in microdomains in the cell membrane are implicated in processes such as sorting in polarized cells and signal transduction when a clustered rearrangement of GPI-anchored proteins has been promoted (132, 200, 391, 399). Despite lacking transmembrane or intracellular domains, GPI-anchored proteins can modulate intracellular signaling events, in many cases by aggregating within membrane lipid raft microdomains. Recombinant strains of E. coli that express the Afa/Dr family of adhesins (Dr, Dr-II, F1845, AfaE-I, and AfaE-III) promote a major rearrangement of DAF at the adherence sites of recombinant strains expressing Dr, Dr-II, and F1845 adhesins, which occurs to a significantly lesser extent on cells infected with E. coli bearing AfaE-I or AfaE-III afimbrial adhesins (150, 165, 166) (Fig. 4). Mapping of the DAF epitopes involved in DAF clustering by using DAF deletion mutants expressed in CHO cells has shown that a deletion in the CCP-1 domain abolished the induced DAF clustering, whereas a deletion in the CCP-4 domain did not (166). Using structural draE gene mutants (73), it has been demonstrated that a mutant in which cysteine replaces aspartic acid at position 54 displays conserved binding capacity but fails to induce DAF clustering (166). CEA and CEACAM6, the GPI-anchored receptors for the Afa/DrCEA adhesins, are recruited around Dr-positive bacteria (33). Consistent with the fact that non-lipid raft-associated molecules are recruited into activated rafts, it has been reported that the other receptor for Afa/DrCEA adhesins, the transmembrane receptor CEACAM1, is recruited into lipid rafts in infected cells, whereas the recruited transmembrane nonreceptor CEACAM3 is not (33).
DAF is known to have signal transduction capacity (388). The mechanism by which DAF is able to transduce signals has been characterized in part. Lipid rafts containing DAF also contain protein tyrosine kinases (82, 415, 417). In human T cells, in which DAF expression rapidly increases after T-cell activation by mitogens, DAF transmits signals for T-cell activation after DAF antibodies have been cross-linked with a secondary antibody (94). Cross-linking of DAF is followed by tyrosine phosphorylation on proteins with molecular masses of 45, 72, 78, and approximately 100 kDa (236), and this is sufficient to induce the phosphorylation of tyrosine residues on p56lck, both the T-cell receptor zeta chain and ZAP-70, followed by IL-2 secretion, which demonstrates that DAF-mediated T-cell activation depends on the expression of this chain within the CD3-T-cell receptor complex (435). Moreover, DAF immunoprecipitates with the Src family protein tyrosine kinases p56lck and p59fyn, leading to the phosphorylation of proteins (262, 387, 415, 417), whereas DAF-TM does not (387). For instance, it has been reported that an association between DAF and the Src-like protein tyrosine kinases p56lck and p59fyn occurs only when both palmitylation of the amino-terminal cysteine residue(s) and myristylation of the amino-terminal glycine residue have occurred (386). Cross-linking of DAF leads to capping and is associated with cytoskeletal reorganization (214), and receptor aggregation following Dr+ E. coli infection is associated with the redistribution of cytoskeleton-associated proteins such as actin, α-actinin, ezrin, and occasionally tropomyosin (36, 150, 335, 336).
Structural and Functional Lesions in the Intestinal Barrier
The involvement of Afa/Dr DAEC in diarrhea is controversial. Indeed, among adult volunteers who received diffusely adherent E. coli strain C1845, only one patient developed symptoms of diarrhea, although all duodenal string cultures and stools were positive for E. coli C1845 (425). During an investigation of the possible role of diffusely adhering E. coli strains in causing diarrhea in infants in Sao Paulo, Brazil, the role of these strains was still uncertain (151). Analysis of fecal specimens obtained from Mexican children during the first 2 years of life showed that the presence of strains with diffuse adherence was not related to the type or duration of diarrhea (89). In contrast, a community-based case-control study conducted in a southern Mexican Mayan village during the peak diarrhea period prospectively identified DAEC strains associated with childhood diarrheal disease (143). Analysis of E. coli isolated from diarrheal stool specimens from infants, children, and adults hospitalized in Clermont-Ferrand, France, showed that 38.2% of the strains from the group with diarrhea exhibited a DA to HEp-2 cells, versus only 8.9% from the control group. Only 33% of them hybridized with the daaC DNA probe, and only 2% hybridized with the AIDA-I DNA probe. In another study, many of the E. coli strains isolated from sporadic cases of watery diarrhea in patients hospitalized during 1991 and 1992 belonged to the DAEC group, since 15.2% of them hybridized with the daaC DNA probe and 3.9% hybridized with the AIDA-I DNA probe. By way of comparison, the other pathogenic E. coli groups were only weakly represented: 0.6% of ETEC, 0.6% of EHEC, and 3.9% of EAEC. Neither EPEC or enteroinvasive E. coli was isolated during the study period (208, 209). Importantly, an age-related incidence of Afa/Dr DAEC in diarrhea has been demonstrated. In Thailand a study has shown that an E. coli strain that had hybridized with F1845 was not associated with infantile diarrhea, whereas EAEC O44:H18 (400), which adhered to HeLa cells in a DA pattern and hybridized with the F1845 DNA probe, was the predominant E. coli strain found in a 5-month-old girl with diarrhea (112). Analysis of E. coli isolated from fecal samples from Australian aboriginal children revealed that age stratification of children of ≥18 months showed a significant association of Afa/Dr DAEC with diarrhea (167). The incidence of diarrhea due to E. coli determined in two pediatric cohorts from a low-socioeconomic-level community in Santiago, Chile, showed that in all cohorts ETEC strains were important pathogens, EPEC strains were present during the first year of life in the newborn cohort, and DAEC strains increased with age in the age-cross-sectional cohort (256). A retrospective case-control study has shown that among children suffering from gastroenteritis in whom DAEC was present, those who were daaC positive spent significantly longer in the hospital than those who were daaC negative (344). A 1-year prospective study of hospitalized children in France, including 220 patients with diarrhea and 211 matched controls, showed that Afa/Dr DAEC was the predominant pathotype, since 30.7% were detected by their adherence pattern and 13.7% were detected with the daaC probe (127). The clinical significance of E. coli in children with diarrhea in New Caledonia has been investigated (141). The difference in the rate of isolation between age-matched patients and control children 2 to 6 years old was significant only when afa or daa sequences were detected. In E. coli isolates from 1- to 4-year-old children with and without diarrhea in Sao Paulo, Brazil, isolates presenting DA adherence or which hybridized with the related daaC probe, or both, were by far the most frequent (152). In a prospective study carried out in two urban centers in northeastern Brazil, daaC-positive E. coli stratification for children over 12 months of age revealed a significant correlation between bacterial infection and diarrhea (364). Taken together, these studies indicate that Afa/Dr DAEC isolates should be considered to be potential pathogens and show an association with age-dependent diarrhea. It remains to be determined why a window of susceptibility occurs, which apparently begins after age 2 or 3, due to unknown factors, and closes later when individuals are exposed and become immune. A hypothesis, which does not exclude others is that an age-dependent variation of the expression of membrane-bound receptors for Afa/Dr adhesins in human intestinal epithelium develops, which in turn modulates the intestinal colonization by Afa/Dr DAEC and the adhesin-dependent structural and functional injuries in intestinal cells.
Recombinant E. coli strains bearing the Dr adhesin and the afimbrial adhesin AfaE-I harbored by uropathogenic E. coli adhere to cultured human colonic intestinal cells expressing the structural and functional characteristics of both fluid-transporting and mucus-secreting cells (229) expressing DAF at the brush border (34). Moreover, using freshly isolated ileal or colonic enterocytes, Adlerberth et al. (2) have observed that E. coli strains expressing the Dr adhesin adhere preferentially to the brush borders and slightly better to colonic than to ileal enterocytes. Adhering E. coli C1845 bacteria bearing the F1845 adhesin strikingly bind diffusely in the apical domains of human colon carcinoma HT-29 and Caco-2 cells, in a fashion dependent on cell differentiation (228). This finding corroborates the intestinal colonization by uropathogenic E. coli of the Afa/Dr family, which is related to the fecal-perineal-urethral hypothesis of the etiology of urinary tract infection. Following adhesion, the wild-type C1845 strain and the recombinant E. coli strain HB101(pSSS1) expressing the F1845 adhesin induced injuries in microvilli that were characterized by elongation and nucleation of the microvilli (36) (Fig. 4 and 5). The Afa/Dr DAEC C1845, IH11128, and EC7372 strains all promote the disassembly of F-actin, villin, and fimbrin, which play pivotal roles in brush border assembly (36, 165, 335). Using draE mutants, it has been shown that a mutant in which cysteine replaces aspartic acid at position 54 conserves DAF binding capacity but fails to induce F-actin disassembly. In human embryonic, nondifferentiated intestinal INT407 cells and fully differentiated intestinal Caco-2 cells expressing DAF, infection by wild-type strain C1845 and E. coli recombinants carrying plasmids encoding the fimbrial adhesin F1845 or the Dr adhesin provokes dramatic F-actin rearrangements following clustering of phosphotyrosines and activation of a cascade of signaling molecules, including protein tyrosine kinase, phospholipase Cγ, phosphatidylinositol 3-kinase, and protein kinase C, and an increase in [Ca2+]i (335, 336). Although the brush border injuries by Afa/Dr DAEC result in the disappearance of the microvilli, as was observed following EPEC infection of intestinal cells, it is important to note that the mechanism controlling the lesions is very different from those of EPEC (155, 443). Indeed, EPEC is the prototype for a family of A/E lesion-causing bacteria. EPEC remains extracellular and transmits signals through the host cell plasma membrane via direct injection of virulence factors via a “molecular syringe,” the bacterial type III secretion system. Several of these factors are translocated directly into the infected cell, including the bacterium's own receptor (Tir), which is linked directly to extracellular EPEC through the epithelial membrane and firmly anchors it to the host cell actin cytoskeleton, thereby initiating pedestal formation. The translocated EPEC proteins also activate signaling pathways that lead to tight-junction (TJ) disruption, inhibition of phagocytosis, altered ion secretion, and immune responses. None of the virulence factors expressed by EPEC that are necessary to induce these cell injuries have been found in Afa/Dr DAEC strains (48).
Elongations of the microvilli in Afa/Dr DAEC-infected enterocyte-like cells (36) resemble the elongations of microvillus-like extensions observed by Cookson et al. (85) in C1845-infected Hep-2 cells. Dr-induced elongation of microvillus-like extensions is visible in HeLa cells constitutively expressing DAF (33). Elongation of microvillus-like extensions occurs in CHO cells expressing the GPI-anchored receptors for Afa/DrCEA adhesins CEA and CEACAM6, whereas this phenomenon is absent in CHO cells expressing the other receptor for Afa/DrCEA adhesins, the transmembrane receptor CEACAM1. The Afa/DrCEA-induced microvillus-like extensions are microfilament dependent and result from the activation of the Rho GTPase Cdc42, accompanied by the phosphorylation of ERM (Fig. 4). The involvement of Cdc42 is consistent with the established role of the Rho family of GTPases in the regulation of cytoskeletal reorganization and, in particular in the case of Cdc42, in the elongation of the cell membrane (46). The observation that phosphorylation of ERM accompanied the Afa/DrCEA-induced microvillus-like extensions is consistent with the reciprocal regulation of Rho GTPases and ERM in the remodeling of the actin cytoskeleton that mediates cell shape change (206). In particular, Rho GTPases can activate ERM and so lead to the formation of microvillus-like structures (331, 427, 468).
Accompanying the brush border injuries, the distribution of brush border-associated functional intestinal proteins such as sucrase-isomaltase (SI), dipeptidylpeptidase IV (DPP IV), glucose transporter SGLT1, and fructose transporter GLUT5 was dramatically altered (335) (Fig. 5). SI and DPPIV enzyme activities decreased simultaneously. No changes in the mRNA levels of SI and DPP IV occurred, and the enzyme stabilities of SI and DPP IV were not altered, whereas enzyme biosynthesis decreased dramatically (333). It is noteworthy that in cells infected with recombinant E. coli strains expressing adhesin homologous to that of the wild-type strain, no decrease in sucrase or DPP IV enzyme activities and no inhibition of enzyme biosynthesis were observed. This revealed that another pathogenic factor(s), distinct from the Afa/Dr adhesins, may play a crucial role in the pathogenicity mechanism of Afa/Dr DAEC strain C1845.
As pathogenic enteric bacteria (124, 190), Afa/Dr DAEC strains produce lesions in the intestinal epithelial barrier (Fig. 5). Infection of a monolayer of fully differentiated Caco-2 cells by the wild-type C1845 strain is followed by an increase in the paracellular permeability and alterations in the distribution of TJ-associated occludin and ZO-1 protein (334). However, unlike the case for other enteric pathogens, the increase in the paracellular permeability induced by C1845 develops without any decrease in the transepithelial resistance of the monolayers. Moreover, it is important to note that the C1845-induced lesions in TJs are not mimicked by the recombinant E. coli strain HB101(pSSS1) expressing the F1845 fimbrial adhesin, indicating once again that a pathogenic factor(s) other than F1845 adhesin may be operating in Afa/Dr DAEC strain C1845.
The brush border structural lesions (36, 39, 335) and the increase in monolayer permeability (334) following Afa/Dr DAEC infection of cultured human intestinal Caco-2 cells resemble the changes observed after intestinal cells have been exposed to hydrogen peroxide (H2O2) (353). In intestinal cells, the DUOX2 enzyme responsible for H2O2 production is brush border associated (246). H2O2 production via the DUOX2 enzyme has been investigated in Afa/Dr DAEC C1845-infected Caco-2 cells, and the absence of production demonstrates that the Afa/Dr adhesin-induced lesions did not result from the deleterious effect of H2O2 (C. Rougeot and A. L. Servin, unpublished data).
The CEACAM receptors for the Afa/DrCEA adhesins CEACAM1, CEA, and CEACAM6 are expressed by the apical glycocalix/microvillus domain in enterocytes (172). Blebbing of the microvillus membrane to form vesicles containing CEACAMs has been recently described. Hammarstrom and Baranov (173) have suggested that when this phenomenon occurs following bacterial intestinal infection, it may be a mechanism of the host's defense intended to remove adhering infectious agents from the intraluminal surface of the gut. Interestingly, it has been reported that the infection of enterocyte-like cells by Afa/Dr DAEC is followed by intense bacteriolytic activity against the adhering E. coli, characterized by dramatic alterations in the bacterial cell, suggesting lysis, and bacterial death (35). In the future it would be interesting to try to find out whether the recognition of DAF, CEACAM1, CEA, and/or CEACAM6 by Afa/DrCEA adhesins is followed by host cell defense mechanisms, including microvillus blebbing and the production of antimicrobial molecules.
Inflammatory Responses
The pathogeneses of inflammatory bowel disease (IBD), ulcerative colitis (UC), and Crohn's disease (CD) remain elusive (98). The current general opinion is that the appearance and chronicity of IBD involve an extremely complex chain of events that includes the physiology and genetic characteristics of the host. Moreover, in inflammatory states, interleukins regulate the intensity of the intestinal immune response either directly or via the production of additional effector molecules. Although it is controversial, a possible causal link between microbial pathogens and IBD, UC, and CD has been suggested, based on the identification of retroviruses and enterovirulent bacteria (67, 77). These pathogens include Salmonella spp., Yersinia enterocolitica spp., Shigella spp., and L. monocytogenes (63, 81). In addition, E. coli strains present in the colon, some of which express mannose-resistant adhesion (64, 65, 177, 247, 373, 385, 434), may play a crucial role in the pathogenesis of IBD. E. coli immunoreactivity has been located in ulcers, within the lamina propria, and along fissures that may contribute to the onset of CD (77). More importantly, recent studies have demonstrated the presence of a pathogenic adherent-invasive E. coli strain (LF82) in patients with CD and UC (20, 21, 53, 54, 93, 145, 278). Moreover, the observation that overexpression at the mRNA and protein levels of human α-defensins 5 and 6, β-defensins 1 and 2, and lysozyme, which are involved in the first line of defense against microbial pathogens (136), is observed in the epithelial cells of patients with UC and when compared to controls with no history of IBD (119) indicates that the pathogens may play a role in the onset and/or chronicity of these inflammatory diseases of the intestine.
The proinflammatory effects of Afa/Dr DAEC strains have been recently demonstrated in polarized monolayers of intestinal T84 cells (39) (Fig. 4 and 5). Infection with the wild-type Afa/Dr DAEC strain C1845, harboring the fimbrial F1845 adhesin, and strain IH11128, harboring the Dr adhesin, with E. coli laboratory strain HB101, expressing the F1845 adhesin, is followed by transmigration across the epithelial monolayers of PMNLs, which are important cellular mediators in IBD (160). PMNL migrations have been shown to be correlated with a basolateral secretion of IL-8 by T84 cells and were abolished after incubation of the epithelial cells with the monoclonal anti-DAF antibody 1H4, which recognizes the short consensus repeat 3 domain of DAF. Moreover, Afa/Dr DAEC strains induced tyrosine phosphorylation of several T84 proteins and activated the mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases 1 and 2, p38, and stress-activated protein kinase/c-Jun NH2-terminal kinases.
After infection by a pathogen, eukaryotic cells can undergo programmed cell death as an ultimate response (471). The balance between PMNL apoptosis and necrosis in inflamed tissues is an important factor in determining the degree of tissue injury, and deregulation of PMNL apoptosis may lead to the development of chronic inflammatory disease. The behavior of human PMNLs in the presence of pathogens and/or their products is variable, since some pathogens delay PMNL apoptosis, whereas others kill PMNLs by inducing or accelerating apoptosis. The pathogenic adherent-invasive E. coli LF82 strain isolated from patients with CD (93) survives and replicates within macrophages but does not induce host cell death (145), and conversely, cyto-detaching EAEC strains induce macrophage cell death (125). Afa/Dr DAEC strains expressing Dr or F1845 adhesins are able to accelerate the apoptosis of PMNLs dramatically after procaspase 3 has been cleaved, and caspase activity was dramatically induced in infected PMNLs (57). This indicates that the Afa/Dr DAEC-PMNL interaction could overcome the microbiocidal weapon of PMNL death, which is detrimental to the host cells. Importantly, in a Dr-positive strain, the induction of PMNL cell death is independent of recognition of DAF, CEACAM1, and CEA. An increased rate of apoptosis was linked to the agglutination process developed by Dr adhesin, a phenomenon also observed by Johnson et al. (211). Moreover, PMNLs that had transmigrated showed increased phagocytic activity towards E. coli (191). In contrast, PMNLs that had transmigrated after Afa/Dr DAEC infection showed phagocytic activity similar to that of nontransmigrated PMNLs, suggesting that Afa/Dr DAEC strains have the ability to block the phagocytic activity of PMNLs (57).
Afa/Dr DAEC-induced PMNL transmigration initiates epithelial synthesis of tumor necrosis factor alpha (TNF-α) and IL-1β, which in turn promote the upregulation of DAF, increasing the adhesion of Afa/Dr DAEC bacteria (40) (Fig. 5). DAF has been found to be upregulated in the intestine in the context of inflammatory processes, ulcerative colitis, and autoimmune diseases (202, 226, 403, 440), and uncontrolled complement activation may be of immunopathological importance in inflammatory diseases of the gastrointestinal tract (38). Upregulation of the other inflammation-associated molecule, MICA, has been found to be markedly increased in intestinal Caco-2 cells infected by AfaE-III-positive bacteria, an effect mediated by the specific interaction between bacterial adhesin and DAF (433). MICA is a distant homologue of major histocompatibility complex class I molecules that is expressed in the normal intestinal epithelium and has been found to be increased at the surface of epithelial cells in colonic biopsies from CD-affected patients compared to controls (144). Proinflammatory cytokines are known to modulate DAF expression in various human cells and UC lesions. The expression of DAF was enhanced by TNF-α, transforming growth factor β, IL-1β, and IL-4, whereas IL-6, IL-8, and IL-10 had no effect (9, 10, 47, 296, 305, 411).
Afa/Dr DAEC-induced PMNL transmigration in turn induces the abnormal expression of DAF at the basolateral domain of polarized epithelial intestinal cells (40) (Fig. 5). An activation-induced antigen, known as CD97, is expressed on leukocytes and belongs to a new group of seven-span transmembrane (7-TM) molecules, designated the EGF-TM7 family (447). This antigen acts as a receptor for DAF. CD97 expression has been found on activated lymphocytes, monocytes, macrophages, granulocytes, and numerous hematopoietic and nonhematopoietic cell lines, and abundant expression of CD97 has been detected on all types of macrophages and dendritic cells, other than microglia (113, 114). Adhesion of CD97 to the NH2-terminal CCPs of DAF has been reported (171) and requires the interaction of at least three tandemly linked EGF domains of CD97 (170). The significance of the CD97-DAF interaction is little understood, but interestingly, CD97 is upregulated on leukocytes during inflammatory activation (114).
DAF is a part of the multimeric LPS receptor complex (116, 185, 186). It has been established that LPS elicits several immediate proinflammatory responses via a pathway including CD14, Toll-like receptors (TLRs), serine-threonine kinases, and the NF-κB transcription factor. The activation of immunocompetent cells by LPS occurs during severe gram-negative infections. Receptor molecules that are implicated in LPS-induced cellular activation include heat shock proteins 70 and 90, chemokine receptor 4, growth differentiation factor 5, and TLR4. These molecules are recruited at the site of CD14-LPS ligation, within the lipid rafts (436, 437). When examining the activated cell signaling that accompanies Afa/Dr DAEC infection in T84 cells, Betis et al. (39) observed that LPS does not mimic the Dr adhesin-induced proinflammatory signaling. This is consistent with the facts that CD14 mRNA and protein expression is not detectable in fully differentiated T84 and Caco-2 cells and that these cells are unresponsive to LPS stimulation (72, 423). However, by expressing TLR2, TLR3, and TLR4 mRNAs, Caco-2 cells respond to LPS stimulation in a way that results in the activation of stress-activated protein kinase/c-Jun NH2-terminal kinases and p38 MAPK (72). Since p38 MAPK is activated in response to Dr adhesin-induced signaling (39), it remains to be determined whether Afa/Dr DAEC cells use TLR-associated cell signaling to promote proinflammatory responses.
The roles of CEACAM1, CEA, and CEACAM6, which act as receptors for Dr, AfaE-III, and F1845 adhesins in proinflammatory responses, will be an interesting topic for future exploration. Indeed, CEACAM1, CEA, and CEACAM6 have been found to be overexpressed in inflammatory situations. CEA and CEACAM6 are strongly upregulated in inflammatory colon diseases, in the early stages of colon tumor, and in uterine and kidney carcinomas (162). Induction of CEACAM1 expression develops after stimulation of human umbilical vein endothelial cells with the proinflammatory cytokine TNF-α (298). In the colon carcinoma cell line HT-29, IFN-γ, but not IL-1β, live bacteria, or LPS, induces marked upregulation of CEACAM1, CEA, and CEACAM6 mRNAs and also induces increased cell surface expression of CEACAM1, CEA, and CEACAM6 (118). N. gonorrhoeae upregulates CEACAM1 through an NF-κB-dependent mechanism (396). CEACAM1, CEA, and CEACAM6 are apically expressed in colonic T84 cells (456), in which Afa/Dr DAEC promotes proinflammatory responses (39, 40), but it remains to be determined whether CEACAM1, CEA, and/or CEACAM6 is upregulated after Afa/Dr DAEC infection, as are DAF (40) and MICA (433).
CONCLUDING REMARKS
It is tempting to now propose a classification of DAEC consisting of two classes of strains, the typical DAEC strains and the atypical DAEC strains, with each subdivided into two subclasses of strains. The typical class of DAEC includes E. coli strains harboring Afa/Dr adhesins (i) having an identical genetic organization, (ii) allowing binding onto human DAF, and (iii) promoting DAF clustering. Currently, the typical class of DAEC (Afa/DrDAF) includes strains that express the AfaE-I (243, 455), AfaE-II (251), AfaE-III (251), AfaE-V (470), Dr (316, 442), Dr-II (340), F1845 (43), and NFA-I (4) adhesins. This class includes two subclasses of strains, the typical subclass 1, including the AfaE-III, Dr, and F1845 adhesins that bind to human CEA (Afa/DrCEA), and the typical subclass 2, including AfaE-I and Dr-II adhesins that do not bind to human CEA (33). The atypical class of DAEC includes two subclasses of strains. The atypical subclass 1 includes E. coli strains harboring Afa/Dr adhesins or others adhesins (i) having an identical genetic organization and (ii) not binding to human DAF. Currently, the atypical subclass 1 of DAEC includes strains that express the AfaE-VII (244, 245), AfaE-VIII (244, 245), AAF-I (90, 115), AAF-II (90, 115), and AAF-III (37) adhesins. The atypical subclass 2 includes E. coli strains that harbor Afa/Dr adhesins or others adhesins promoting diffuse adhesion, together with pathogenicity islands known to be expressed by the others classes of enterovirulent E. coli (219, 306). Currently, the atypical subclass 2 of DAEC includes DA-EPEC strains (having the AIDA-I adhesin and the LEE pathogenicity island) (26-29) and ET5 DA strains (having AfaE-I and the LEE pathogenicity island) (227).
The importance of human Afa/Dr DAEC in UTIs has already been demonstrated. In contrast, the role of human Afa/Dr DAEC as a cause of diarrhea still remains controversial. On the basis of the in vitro observation discussed above, one question remains: how does an Afa/Dr DAEC strain emerge as a pathogen in the intestinal tract? Experimental data obtained with cultured human intestinal cells have consistently revealed mechanisms by which human Afa/Dr DAEC strains induce structural and functional lesions in the intestinal brush border, impairment of the epithelial barrier, and proinflammatory responses in cultured human intestinal cells that express the structural and functional characteristics of enterocytes of the small intestine or colonic cells (Fig. 5). It is necessary to examine whether these lesions in intestinal barrier develop in vivo by using appropriate rodent models. In view of the recently demonstrated high specificity of human Afa/Dr adhesins for human DAF (197) and that rodent CEACAM1, CEA, and CEACAM6 were not homologous to human CEACAM1, CEA, and CEACAM6, transgenic mice expressing functional human DAF (300, 402, 444, 445, 461) or CEA (83, 109, 110, 193, 430, 462) had to be used.
Several of the cellular effects observed in cultured human intestinal cells are not Afa/Dr adhesin dependent (333, 334). This suggests that another, unknown virulence factor(s) must be active in Afa/Dr DAEC. Interestingly, it has recently been reported that mannose-resistant DAEC strains isolated from stools of children with and without diarrhea and with UTI in Brazil, although uncharacterized for daaC presence, were 64 and 21%, positive, respectively, for sat, which encodes the secreted autotransporter toxin, Sat (426). More interestingly, this report indicates that Afa/Dr DAEC strain C1845 is positive for sat. This autotransporter protein, which is present in uropathogenic E. coli and in some categories of enterovirulent E. coli, belongs to the family of high-molecular-weight serine protease autotransporters of Enterobacteriaceae (187). Serine protease autotransporters of Enterobacteriaceae produced by E. coli include EspC, EspP, Pet, Sat, Tsh, Pic, AIDA-I, TibA, and Ag23. Despite homologies, these autotransporter proteins have differing pathogenic functions that are only partially dependent on their substrate specificities (105). Since the Sat autotransporter protein is a vacuolating cytotoxin (169), it is important to find out whether Sat is involved in Afa/Dr DAEC pathogenicity.
The observation that human Afa/Dr DAEC promotes proinflammatory responses by interaction of Afa/Dr adhesins with membrane-bound receptors suggests that an Afa/Dr DAEC interaction could play a role in the pathogenesis of IBD. It is tempting to propose that Afa/Dr DAEC is a “silent pathogen” that can emerge from the human intestinal microbiota. Indeed, it is intriguing that epidemiological studies have revealed that Afa/Dr DAEC strains may be regarded as resident colonic strains, since daaC-positive E. coli strains have been isolated with similar frequencies from patients and control subjects (127, 167, 356). The resident luminal bacteria seem to be an important factor in the development and chronicity of inflammatory bowel diseases, and an aggressive immunological response may be triggered by these bacteria rather than as a result of a change in the normal flora (261). However, the mechanism(s) by which Afa/Dr DAEC emerges as a proinflammatory pathogen remains to be identified. One possible mechanism would be that the emergence of pathogenic Afa/Dr DAEC is regulated by a quorum-sensing-dependent mechanism and/or by its environment. It has been recently established that pathogenic bacteria are able to sense both the cell density and the metabolic potential of their environment and to secrete small organic molecules that are involved in intercellular communication (286). These quorum-sensing molecules play a role in pathogenesis (101, 422) and are countered by the mammalian cells via a hitherto-unknown mechanism of defense (179). In E. coli, quorum sensing involves a transcription regulator (LuxR homologue) and an autoinducer, AI-2 or AI-3, depending on the function encoded by the luxS gene. For example, recent studies have demonstrated that for EHEC and EPEC, quorum-sensing molecules encoded by the luxS gene influence transcription from four of the LEE operon promoters that cause a characteristic histopathology in intestinal cells known as attaching and effacing lesions (6, 161, 217, 405-407, 409). Despite the fact that the Dr-positive IH11128 strain produces high levels of AI-2 at the end of the exponential phase of growth, recent results (O. Bouvet, S. Diard, and A. L. Servin, unpublished data) have indicated that expression of Dr is not influenced by AI-2 production. Moreover, other unpublished results (Bouvet et al., unpublished data) show that the expression and the secretion of Dr adhesin are controlled by norepinephrine, which is consistent with the hormone-dependent bacterium-host communication language described recently by Sperandio et al. (408).
Acknowledgments
I express my sincere thanks to Marie-Françoise Bernet-Camard, Cedric Berger, Julie Guignot, Sylvie Hudault, Imad Kansau, Sophie Kerneis, Isabelle Peiffer, and all of the members, past and present, of INSERM Unit 510, Pathogènes et Fonctions des Cellules Epithéliales Polarisées, as well as to Bogdan Nowicki, Steve Moseley, Paul Hofman, Chantal Le Bouguenec, Doug Lublin, Oliver Billker, and Brad O. Spiller for their outstanding contributions to the understanding of the mechanisms of pathogenicity of Afa/Dr DAEC.
REFERENCES
- 1.Abduch Fabrega, V. L., A. J. Piantino Ferreira, F. Reis da Silva Patricio, C. Brinkley, and I. C. Affonso Scaletsky. 2002. Cell-detaching Escherichia coli (CDEC) strains from children with diarrhea: identification of a protein with toxigenic activity. FEMS Microbiol. Lett. 217:191-197. [DOI] [PubMed] [Google Scholar]
- 2.Adlerberth, I., L. A. Hanson, C. Svanborg, A. M. Svennerholm, S. Nordgren, and A. E. Wold. 1995. Adhesins of Escherichia coli associated with extra-intestinal pathogenicity confer binding to colonic epithelial cells. Microb. Pathog. 18:373-385. [DOI] [PubMed] [Google Scholar]
- 3.Agrez, M. V., D. R. Shafren, X. Gu, K. Cox, D. Sheppard, and R. D. Barry. 1997. Integrin alpha v beta 6 enhances coxsackievirus B1 lytic infection of human colon cancer cells. Virology 239:71-77. [DOI] [PubMed] [Google Scholar]
- 4.Ahrens, R., M. Ott, A. Ritter, H. Hoschutzky, T. Buhler, F. Lottspeich, G. J. Boulnois, K. Jann, and J. Hacker. 1993. Genetic analysis of the gene cluster encoding nonfimbrial adhesin I from an Escherichia coli uropathogen. Infect. Immun. 61:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert, M. J., A. S. Faruque, S. M. Faruque, R. B. Sack, and D. Mahalanabis. 1999. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 37:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand, S. K., and M. W. Griffiths. 2003. Quorum sensing and expression of virulence in Escherichia coli O157:H7. Int. J. Food Microbiol. 85:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, G. G., K. W. Dodson, T. M. Hooton, and S. J. Hultgren. 2004. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 12:424-430. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, K. L., J. Billington, D. Pettigrew, E. Cota, P. Simpson, P. Roversi, H. A. Chen, P. Urvil, L. Du Merle, P. N. Barlow, M. E. Medof, R. A. Smith, B. Nowicki, C. Le Bouguenec, S. M. Lea, and S. Matthews. 2004. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol. Cell 15:647-657. [DOI] [PubMed] [Google Scholar]
- 9.Andoh, A., Y. Fujiyama, K. Sumiyoshi, H. Sakumoto, and T. Bamba. 1996. Interleukin 4 acts as an inducer of decay-accelerating factor gene expression in human intestinal epithelial cells. Gastroenterology 111:911-918. [DOI] [PubMed] [Google Scholar]
- 10.Andoh, A., Y. Fujiyama, K. Sumiyoshi, H. Sakumoto, H. Okabe, and T. Bamba. 1997. Tumour necrosis factor-alpha up-regulates decay-accelerating factor gene expression in human intestinal epithelial cells. Immunology 90:358-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archambaud, M., P. Courcoux, and A. Labigne-Roussel. 1988. Detection by molecular hybridization of pap, afa, and sfa adherence systems in Escherichia coli strains associated with urinary and enteral infections. Ann. Inst. Pasteur Microbiol. 139:575-588. [DOI] [PubMed] [Google Scholar]
- 12.Arora, M., R. Arora, S. C. Tiwari, N. Das, and L. M. Srivastava. 2000. Expression of complement regulatory proteins in diffuse proliferative glomerulonephritis. Lupus 9:127-131. [DOI] [PubMed] [Google Scholar]
- 13.Arthur, M., C. E. Johnson, R. H. Rubin, R. D. Arbeit, C. Campanelli, C. Kim, S. Steinbach, M. Agarwal, R. Wilkinson, and R. Goldstein. 1989. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect. Immun. 57:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson, J. P., M. Krych, M. Nickells, D. Birmingham, V. B. Subramanian, L. Clemenza, J. Alvarez, and K. Liszewski. 1994. Complement receptors and regulatory proteins: immune adherence revisited and abuse by microorganisms. Clin. Exp. Immunol. 97(Suppl. 2):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bamberger, A. M., H. Kappes, C. Methner, G. Rieck, J. Brummer, C. Wagener, T. Loning, and K. Milde-Langosch. 2002. Expression of the adhesion molecule CEACAM1 (CD66a, BGP, C-CAM) in breast cancer is associated with the expression of the tumor-suppressor genes Rb, Rb2, and p27. Virchows Arch. 440:139-144. [DOI] [PubMed] [Google Scholar]
- 16.Bamberger, A. M., S. Sudahl, T. Loning, C. Wagener, C. M. Bamberger, P. Drakakis, C. Coutifaris, and A. Makrigiannakis. 2000. The adhesion molecule CEACAM1 (CD66a, C-CAM, BGP) is specifically expressed by the extravillous intermediate trophoblast. Am. J. Pathol. 156:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamberger, A. M., S. Sudahl, C. Wagener, and T. Loning. 2001. Expression pattern of the adhesion molecule CEACAM1 (C-CAM, CD66a, BGP) in gestational trophoblastic lesions. Int. J. Gynecol Pathol. 20:160-165. [DOI] [PubMed] [Google Scholar]
- 18.Baorto, D. M., Z. Gao, R. Malaviya, M. L. Dustin, A. van der Merwe, D. M. Lublin, and S. N. Abraham. 1997. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636-639. [DOI] [PubMed] [Google Scholar]
- 19.Baranov, V., and S. Hammarstrom. 2004. Carcinoembryonic antigen (CEA) and CEA-related cell adhesion molecule 1 (CEACAM1), apically expressed on human colonic M cells, are potential receptors for microbial adhesion. Histochem. Cell Biol. 121:83-89. [DOI] [PubMed] [Google Scholar]
- 20.Barnich, N., J. Boudeau, L. Claret, and A. Darfeuille-Michaud. 2003. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol. Microbiol. 48:781-794. [DOI] [PubMed] [Google Scholar]
- 21.Barnich, N., M. A. Bringer, L. Claret, and A. Darfeuille-Michaud. 2004. Involvement of lipoprotein NlpI in the virulence of adherent invasive Escherichia coli strain LF82 isolated from a patient with Crohn's disease. Infect. Immun. 72:2484-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beauchemin, N., P. Draber, G. Dveksler, P. Gold, S. Gray-Owen, F. Grunert, S. Hammarstrom, K. V. Holmes, A. Karlsson, M. Kuroki, S. H. Lin, L. Lucka, S. M. Najjar, M. Neumaier, B. Obrink, J. E. Shively, K. M. Skubitz, C. P. Stanners, P. Thomas, J. A. Thompson, M. Virji, S. von Kleist, C. Wagener, S. Watt, and W. Zimmermann. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243-249. [DOI] [PubMed] [Google Scholar]
- 23.Beaulieu, J. F. 1999. Integrins and human intestinal cell functions. Front. Biosci. 4:D310-D321. [DOI] [PubMed] [Google Scholar]
- 24.Beinke, C., S. Laarmann, C. Wachter, H. Karch, L. Greune, and M. A. Schmidt. 1998. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect. Immun. 66:528-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benchimol, S., A. Fuks, S. Jothy, N. Beauchemin, K. Shirota, and C. P. Stanners. 1989. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 57:327-334. [DOI] [PubMed] [Google Scholar]
- 26.Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. [DOI] [PubMed] [Google Scholar]
- 27.Benz, I., and M. A. Schmidt. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benz, I., and M. A. Schmidt. 1993. Diffuse adherence of enteropathogenic Escherichia coli strains—processing of AIDA-I. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 278:197-208. [DOI] [PubMed] [Google Scholar]
- 29.Benz, I., and M. A. Schmidt. 1992. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27). Infect. Immun. 60:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 32.Bergelson, J. M., J. G. Mohanty, R. L. Crowell, N. F. St John, D. M. Lublin, and R. W. Finberg. 1995. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J. Virol. 69:1903-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger, C. N., O. Billker, T. F. Meyer, A. L. Servin, and I. Kansau. 2004. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC). Mol. Microbiol. 52:963-983. [DOI] [PubMed] [Google Scholar]
- 34.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut 38:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Differentiation-associated antimicrobial functions in human colon adenocarcinoma cell lines. Exp. Cell Res. 226:80-89. [DOI] [PubMed] [Google Scholar]
- 36.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect. Immun. 64:1918-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernier, C., P. Gounon, and C. Le Bouguenec. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 70:4302-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berstad, A. E., and P. Brandtzaeg. 1998. Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract. Gut 42:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Betis, F., P. Brest, V. Hofman, J. Guignot, M. F. Bernet-Camard, B. Rossi, A. Servin, and P. Hofman. 2003. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect. Immun. 71:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betis, F., P. Brest, V. Hofman, J. Guignot, I. Kansau, B. Rossi, A. Servin, and P. Hofman. 2003. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infect. Immun. 71:1774-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beuzon, C. R., S. Meresse, K. E. Unsworth, J. Ruiz-Albert, S. Garvis, S. R. Waterman, T. A. Ryder, E. Boucrot, and D. W. Holden. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilge, S. S., J. M. Apostol, Jr., K. J. Fullner, and S. L. Moseley. 1993. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol. Microbiol. 7:993-1006. [DOI] [PubMed] [Google Scholar]
- 43.Bilge, S. S., C. R. Clausen, W. Lau, and S. L. Moseley. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billker, O., A. Popp, V. Brinkmann, G. Wenig, J. Schneider, E. Caron, and T. F. Meyer. 2002. Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42-dependent and -independent pathways. EMBO J. 21:560-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billker, O., A. Popp, S. D. Gray-Owen, and T. F. Meyer. 2000. The structural basis of CEACAM-receptor targeting by neisserial Opa proteins. Trends Microbiol. 8:258-260. [DOI] [PubMed] [Google Scholar]
- 46.Bishop, A. L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 348:241-255. [PMC free article] [PubMed] [Google Scholar]
- 47.Bjorge, L., T. S. Jensen, and R. Matre. 1996. Characterisation of the complement-regulatory proteins decay-accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) on a human colonic adenocarcinoma cell line. Cancer Immunol. Immunother. 42:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanc-Potard, A. B., C. Tinsley, I. Scaletsky, C. Le Bouguenec, J. Guignot, A. L. Servin, X. Nassif, and M. F. Bernet-Camard. 2002. Representational difference analysis between Afa/Dr diffusely adhering Escherichia coli and nonpathogenic E. coli K-12. Infect. Immun. 70:5503-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bos, M. P., F. Grunert, and R. J. Belland. 1997. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect. Immun. 65:2353-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bos, M. P., D. Hogan, and R. J. Belland. 1999. Homologue scanning mutagenesis reveals CD66 receptor residues required for neisserial Opa protein binding. J. Exp. Med. 190:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bos, M. P., D. Kao, D. M. Hogan, C. C. Grant, and R. J. Belland. 2002. Carcinoembryonic antigen family receptor recognition by gonococcal Opa proteins requires distinct combinations of hypervariable Opa protein domains. Infect. Immun. 70:1715-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bos, M. P., M. Kuroki, A. Krop-Watorek, D. Hogan, and R. J. Belland. 1998. CD66 receptor specificity exhibited by neisserial Opa variants is controlled by protein determinants in CD66 N-domains. Proc. Natl. Acad. Sci. USA 95:9584-9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boudeau, J., N. Barnich, and A. Darfeuille-Michaud. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol. Microbiol. 39:1272-1284. [DOI] [PubMed] [Google Scholar]
- 54.Boudeau, J., A. L. Glasser, E. Masseret, B. Joly, and A. Darfeuille-Michaud. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 67:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulton, I. C., and S. D. Gray-Owen. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229-236. [DOI] [PubMed] [Google Scholar]
- 56.Bradbury, J. 2002. Neisseria gonorrhoeae evades host immunity by switching off T lymphocytes. Lancet 359:681. [DOI] [PubMed] [Google Scholar]
- 57.Brest, P., F. Bétis, N. Cuburu, E. Selva, M. Herrant, P. Auberger, A. Servin, and P. Hofman. 2004. Increased rate of apoptosis and diminished phagocytic ability of human neutrophils infected with Afa/Dr diffusely adheing Escherichia coli strains. Infect. Immun. 72:5741-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brodbeck, W. G., L. Kuttner-Kondo, C. Mold, and M. E. Medof. 2000. Structure/function studies of human decay-accelerating factor. Immunology 101:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brodbeck, W. G., D. Liu, J. Sperry, C. Mold, and M. E. Medof. 1996. Localization of classical and alternative pathway regulatory activity within the decay-accelerating factor. J. Immunol. 156:2528-2533. [PubMed] [Google Scholar]
- 60.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 61.Brummer, J., A. Ebrahimnejad, R. Flayeh, U. Schumacher, T. Loning, A. M. Bamberger, and C. Wagener. 2001. Cis interaction of the cell adhesion molecule CEACAM1 with integrin beta3. Am. J. Pathol. 159:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brummer, J., M. Neumaier, C. Gopfert, and C. Wagener. 1995. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene 11:1649-1655. [PubMed] [Google Scholar]
- 63.Bulois, P., P. Desreumaux, C. Neut, A. Darfeuille-Michaud, A. Cortot, and J. F. Colombel. 1999. Infectious agents and Crohn's disease. Clin. Microbiol. Infect. 5:601-604. [DOI] [PubMed] [Google Scholar]
- 64.Burke, D. A., and A. T. Axon. 1988. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. Br. Med. J. 297:102-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burke, D. A., and A. T. Axon. 1988. Hydrophobic adhesin of E coli in ulcerative colitis. Gut 29:41-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Busch, C., T. A. Hanssen, C. Wagener, and B. O'Brink. 2002. Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum. Pathol. 33:290-298. [DOI] [PubMed] [Google Scholar]
- 67.Campieri, M., and P. Gionchetti. 2001. Bacteria as the cause of ulcerative colitis. Gut 48:132-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campos, L. C., M. A. M. Vieira, L. R. Trabulsi, L. A. da Silva, V. Monteiro-Neto, and T. A. T. Gomes. 1999. Diffusely adhering Escherichia coli (DAEC) strains of fecal origin rarely express F1845 adhesin. Microbiol. Immunol. 43:167-170. [DOI] [PubMed] [Google Scholar]
- 69.Caras, I. W., M. A. Davitz, L. Rhee, G. Weddell, D. W. Martin, Jr., and V. Nussenzweig. 1987. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature 325:545-549. [DOI] [PubMed] [Google Scholar]
- 70.Caras, I. W., G. N. Weddell, M. A. Davitz, V. Nussenzweig, and D. W. Martin, Jr. 1987. Signal for attachment of a phospholipid membrane anchor in decay accelerating factor. Science 238:1280-1283. [DOI] [PubMed] [Google Scholar]
- 71.Carbonetti, N. H., S. Boonchai, S. H. Parry, V. Vaisanen-Rhen, T. K. Korhonen, and P. H. Williams. 1986. Aerobactin-mediated iron uptake by Escherichia coli isolates from human extraintestinal infections. Infect. Immun. 51:966-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 73.Carnoy, C., and S. L. Moseley. 1997. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol. Microbiol. 23:365-379. [DOI] [PubMed] [Google Scholar]
- 74.Carroll, M. C., E. M. Alicot, P. J. Katzman, L. B. Klickstein, J. A. Smith, and D. T. Fearon. 1988. Organization of the genes encoding complement receptors type 1 and 2, decay-accelerating factor, and C4-binding protein in the RCA locus on human chromosome 1. J. Exp. Med. 167:1271-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carson, S. D. 2001. Receptor for the group B coxsackieviruses and adenoviruses: CAR. Rev. Med. Virol. 11:219-226. [DOI] [PubMed] [Google Scholar]
- 76.Carson, S. D., N. N. Chapman, and S. M. Tracy. 1997. Purification of the putative coxsackievirus B receptor from HeLa cells. Biochem. Biophys. Res. Commun. 233:325-328. [DOI] [PubMed] [Google Scholar]
- 77.Cartun, R. W., H. J. Van Kruiningen, C. A. Pedersen, and M. M. Berman. 1993. An immunocytochemical search for infectious agents in Crohn's disease. Mod. Pathol. 6:212-219. [PubMed] [Google Scholar]
- 78.Charbonneau, J., and C. P. Stanners. 1999. Role of carbohydrate structures in CEA-mediated intercellular adhesion. Cell. Adhes. Commun. 7:233-244. [DOI] [PubMed] [Google Scholar]
- 79.Chen, T., F. Grunert, A. Medina-Marino, and E. C. Gotschlich. 1997. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 185:1557-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen, T., W. Zimmermann, J. Parker, I. Chen, A. Maeda, and S. Bolland. 2001. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J. Leukoc. Biol. 70:335-340. [PubMed] [Google Scholar]
- 81.Chiba, M., T. Fukushima, S. Inoue, Y. Horie, M. Iizuka, and O. Masamune. 1998. Listeria monocytogenes in Crohn's disease. Scand. J. Gastroenterol. 33:430-434. [DOI] [PubMed] [Google Scholar]
- 82.Cinek, T., and V. Horejsi. 1992. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J. Immunol. 149:2262-2270. [PubMed] [Google Scholar]
- 83.Clarke, P., J. Mann, J. F. Simpson, K. Rickard-Dickson, and F. J. Primus. 1998. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res. 58:1469-1477. [PubMed] [Google Scholar]
- 84.Clarkson, N. A., R. Kaufman, D. M. Lublin, T. Ward, P. A. Pipkin, P. D. Minor, D. J. Evans, and J. W. Almond. 1995. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J. Virol. 69:5497-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cookson, S. T., and J. P. Nataro. 1996. Characterization of HEp-2 cell projection formation induced by diffusely adherent Escherichia coli. Microb. Pathog. 21:421-434. [DOI] [PubMed] [Google Scholar]
- 86.Cosio, F. G., D. D. Sedmak, J. D. Mahan, and N. S. Nahman, Jr. 1989. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 36:100-107. [DOI] [PubMed] [Google Scholar]
- 87.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. [DOI] [PubMed] [Google Scholar]
- 88.Coyne, K. E., S. E. Hall, S. Thompson, M. A. Arce, T. Kinoshita, T. Fujita, D. J. Anstee, W. Rosse, and D. M. Lublin. 1992. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J. Immunol. 149:2906-2913. [PubMed] [Google Scholar]
- 89.Cravioto, A., A. Tello, A. Navarro, J. Ruiz, H. Villafan, F. Uribe, and C. Eslava. 1991. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet 337:262-264. [DOI] [PubMed] [Google Scholar]
- 90.Czeczulin, J. R., S. Balepur, S. Hicks, A. Phillips, R. Hall, M. H. Kothary, F. Navarro-Garcia, and J. P. Nataro. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 65:4135-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Czeczulin, J. R., T. S. Whittam, I. R. Henderson, F. Navarro-Garcia, and J. P. Nataro. 1999. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun. 67:2692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daigle, F., J. Harel, J. M. Fairbrother, and P. Lebel. 1994. Expression and detection of pap-, sfa-, and afa-encoded fimbrial adhesin systems among uropathogenic Escherichia coli. Can. J. Microbiol. 40:286-291. [DOI] [PubMed] [Google Scholar]
- 93.Darfeuille-Michaud, A., C. Neut, N. Barnich, E. Lederman, P. Di Martino, P. Desreumaux, L. Gambiez, B. Joly, A. Cortot, and J. F. Colombel. 1998. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115:1405-1413. [DOI] [PubMed] [Google Scholar]
- 94.Davis, L. S., S. S. Patel, J. P. Atkinson, and P. E. Lipsky. 1988. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J. Immunol. 141:2246-2252. [PubMed] [Google Scholar]
- 95.Davitz, M. A., M. G. Low, and V. Nussenzweig. 1986. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J. Exp. Med. 163:1150-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 2000. Host cell invasion by pathogenic Neisseriae. Subcell. Biochem. 33:61-96. [DOI] [PubMed] [Google Scholar]
- 97.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6:489-495. [DOI] [PubMed] [Google Scholar]
- 98.Desreumaux, P., and J. F. Colombel. 2003. Intestinal flora and Crohn's disease. Ann. Pharm. Fr. 61:276-281. [PubMed] [Google Scholar]
- 99.Dickeson, S. K., N. L. Mathis, M. Rahman, J. M. Bergelson, and S. A. Santoro. 1999. Determinants of ligand binding specificity of the alpha1beta1 and alpha2beta1 integrins. J. Biol. Chem. 274:32182-32191. [DOI] [PubMed] [Google Scholar]
- 100.Dombek, P. E., D. Cue, J. Sedgewick, H. Lam, S. Ruschkowski, B. B. Finlay, and P. P. Cleary. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31:859-870. [DOI] [PubMed] [Google Scholar]
- 101.Donabedian, H. 2003. Quorum sensing and its relevance to infectious diseases. J. Infect. 46:207-214. [DOI] [PubMed] [Google Scholar]
- 102.Donda, A., L. Mori, A. Shamshiev, I. Carena, C. Mottet, M. H. Heim, C. Beglinger, F. Grunert, C. Rochlitz, L. Terracciano, P. Jantscheff, and G. De Libero. 2000. Locally inducible CD66a (CEACAM1) as an amplifier of the human intestinal T cell response. Eur. J. Immunol. 30:2593-2603. [DOI] [PubMed] [Google Scholar]
- 103.D'Orazio, S. E., and C. M. Collins. 1998. Molecular pathogenesis of urinary tract infections. Curr. Top. Microbiol. Immunol. 225:137-164. [DOI] [PubMed] [Google Scholar]
- 104.Duncan, M. J., J. S. Shin, and S. N. Abraham. 2002. Microbial entry through caveolae: variations on a theme. Cell. Microbiol. 4:783-791. [DOI] [PubMed] [Google Scholar]
- 105.Dutta, P. R., R. Cappello, F. Navarro-Garcia, and J. P. Nataro. 2002. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect. Immun. 70:7105-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duxbury, M. S., H. Ito, S. W. Ashley, and E. E. Whang. 2004. CEACAM6 crosslinking induces caveolin-1-dependent, Src-mediated FAK phosphorylation in BxPC3 pancreatic adenocarcinoma cells. J. Biol. Chem. 279:23176-23182. [DOI] [PubMed] [Google Scholar]
- 107.Duxbury, M. S., H. Ito, S. W. Ashley, and E. E. Whang. 2004. c-Src-dependent cross-talk between CEACAM6 and alphavbeta3 integrin enhances pancreatic adenocarcinoma cell adhesion to extracellular matrix components. Biochem. Biophys. Res. Commun. 317:133-141. [DOI] [PubMed] [Google Scholar]
- 108.Duxbury, M. S., H. Ito, M. J. Zinner, S. W. Ashley, and E. E. Whang. 2004. CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene. 23:465-473. [DOI] [PubMed] [Google Scholar]
- 109.Eades-Perner, A. M., H. van der Putten, A. Hirth, J. Thompson, M. Neumaier, S. von Kleist, and W. Zimmermann. 1994. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 54:4169-4176. [PubMed] [Google Scholar]
- 110.Eades-Perner, A. M., and W. Zimmermann. 1995. Carcinoembryonic antigen-transgenic mice: a model for tumor immunotherapy. Tumour Biol. 16:56-61. [DOI] [PubMed] [Google Scholar]
- 111.Ebrahimnejad, A., R. Flayeh, G. Unteregger, C. Wagener, and J. Brummer. 2000. Cell adhesion molecule CEACAM1 associates with paxillin in granulocytes and epithelial and endothelial cells. Exp. Cell Res. 260:365-373. [DOI] [PubMed] [Google Scholar]
- 112.Echeverria, P., O. Serichantalerg, S. Changchawalit, B. Baudry, M. M. Levine, F. Orskov, and I. Orskov. 1992. Tissue culture-adherent Escherichia coli in infantile diarrhea. J. Infect. Dis. 165:141-143. [DOI] [PubMed] [Google Scholar]
- 113.Eichler, W. 2000. CD97 isoform expression in leukocytes. J. Leukoc. Biol. 68:561-567. [PubMed] [Google Scholar]
- 114.Eichler, W., J. Hamann, and G. Aust. 1997. Expression characteristics of the human CD97 antigen. Tissue Antigens 50:429-438. [DOI] [PubMed] [Google Scholar]
- 115.Elias, W. P., S. Suzart, L. R. Trabulsi, J. P. Nataro, and T. A. Gomes. 1999. Distribution of aggA and aafA gene sequences among Escherichia coli isolates with genotypic or phenotypic characteristics, or both, of enteroaggregative E. coli. J. Med. Microbiol. 48:597-599. [DOI] [PubMed] [Google Scholar]
- 116.El-Samalouti, V. T., J. Schletter, I. Chyla, A. Lentschat, U. Mamat, L. Brade, H. D. Flad, A. J. Ulmer, and L. Hamann. 1999. Identification of the 80-kDa LPS-binding protein (LMP80) as decay-accelerating factor (DAF, CD55). FEMS. Immunol. Med. Microbiol. 23:259-269. [DOI] [PubMed] [Google Scholar]
- 117.Estrera, V. T., D. T. Chen, W. Luo, D. C. Hixson, and S. H. Lin. 2001. Signal transduction by the CEACAM1 tumor suppressor. Phosphorylation of serine 503 is required for growth-inhibitory activity. J. Biol. Chem. 276:15547-15553. [DOI] [PubMed] [Google Scholar]
- 118.Fahlgren, A., V. Baranov, L. Frangsmyr, F. Zoubir, M. L. Hammarstrom, and S. Hammarstrom. 2003. Interferon-gamma tempers the expression of carcinoembryonic antigen family molecules in human colon cells: a possible role in innate mucosal defence. Scand. J. Immunol. 58:628-641. [DOI] [PubMed] [Google Scholar]
- 119.Fahlgren, A., S. Hammarstrom, A. Danielsson, and M. L. Hammarstrom. 2003. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin. Exp. Immunol. 131:90-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fang, F. C. 1997. Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fang, L., B. Nowicki, and C. Yallampalli. 2001. Differential expression of uterine NO in pregnant and nonpregnant rats with intrauterine bacterial infection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R1356-R1363. [DOI] [PubMed] [Google Scholar]
- 122.Fang, L., B. J. Nowicki, Y. L. Dong, and C. Yallampalli. 1999. Localized increase in nitric oxide production and the expression of nitric oxide synthase isoforms in rat uterus with experimental intrauterine infection. Am. J. Obstet. Gynecol. 181:601-609. [DOI] [PubMed] [Google Scholar]
- 123.Fang, L., B. J. Nowicki, P. Urvil, P. Goluszko, S. Nowicki, S. L. Young, and C. Yallampalli. 2004. Epithelial invasion by Escherichia coli bearing Dr fimbriae is controlled by nitric oxide-regulated expression of CD55. Infect. Immun. 72:2907-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fasano, A., and J. P. Nataro. 2004. Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins. Adv. Drug Deliv. Rev. 56:795-807. [DOI] [PubMed] [Google Scholar]
- 125.Fernandez-Prada, C., B. D. Tall, S. E. Elliott, D. L. Hoover, J. P. Nataro, and M. M. Venkatesan. 1998. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect. Immun. 66:3918-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Foley, S., B. Li, M. Dehoff, H. Molina, and V. M. Holers. 1993. Mouse Crry/p65 is a regulator of the alternative pathway of complement activation. Eur. J. Immunol. 23:1381-1384. [DOI] [PubMed] [Google Scholar]
- 127.Forestier, C., M. Meyer, S. Favre-Bonte, C. Rich, G. Malpuech, C. Le Bouguenec, J. Sirot, B. Joly, and C. De Champs. 1996. Enteroadherent Escherichia coli and diarrhea in children: a prospective case-control study. J. Clin. Microbiol. 34:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fournes, B., J. Farrah, M. Olson, N. Lamarche-Vane, and N. Beauchemin. 2003. Distinct Rho GTPase activities regulate epithelial cell localization of the adhesion molecule CEACAM1: involvement of the CEACAM1 transmembrane domain. Mol. Cell. Biol. 23:7291-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fournes, B., S. Sadekova, C. Turbide, S. Letourneau, and N. Beauchemin. 2001. The CEACAM1-L Ser503 residue is crucial for inhibition of colon cancer cell tumorigenicity. Oncogene 20:219-230. [DOI] [PubMed] [Google Scholar]
- 130.Foxman, B., L. Zhang, K. Palin, P. Tallman, and C. F. Marrs. 1995. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J. Infect. Dis. 171:1514-1521. [DOI] [PubMed] [Google Scholar]
- 131.Foxman, B., L. Zhang, P. Tallman, K. Palin, C. Rode, C. Bloch, B. Gillespie, and C. F. Marrs. 1995. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J. Infect. Dis. 172:1536-1541. [DOI] [PubMed] [Google Scholar]
- 132.Friedrichson, T., and T. V. Kurzchalia. 1998. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 394:802-805. [DOI] [PubMed] [Google Scholar]
- 133.Fujita, T., T. Inoue, K. Ogawa, K. Iida, and N. Tamura. 1987. The mechanism of action of decay-accelerating factor (DAF). DAF inhibits the assembly of C3 convertases by dissociating C2a and Bb. J. Exp. Med. 166:1221-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fukuoka, Y., A. Yasui, N. Okada, and H. Okada. 1996. Molecular cloning of murine decay accelerating factor by immunoscreening. Int. Immunol. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 135.Gabastou, J. M., S. Kerneis, M. F. Bernet-Camard, A. Barbat, M. H. Coconnier, J. B. Kaper, and A. L. Servin. 1995. Two stages of enteropathogenic Escherichia coli intestinal pathogenicity are up and down-regulated by the epithelial cell differentiation. Differentiation 59:127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 137.Garcia, M. I., P. Gounon, P. Courcoux, A. Labigne, and C. Le Bouguenec. 1996. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol. Microbiol. 19:683-693. [DOI] [PubMed] [Google Scholar]
- 138.Garcia, M. I., M. Jouve, J. P. Nataro, P. Gounon, and C. Le Bouguenec. 2000. Characterization of the AfaD-like family of invasins encoded by pathogenic Escherichia coli associated with intestinal and extra-intestinal infections. FEBS Lett. 479:111-117. [DOI] [PubMed] [Google Scholar]
- 139.Garcia, M. I., A. Labigne, and C. Le Bouguenec. 1994. Nucleotide sequence of the afimbrial-adhesin-encoding afa-3 gene cluster and its translocation via flanking IS1 insertion sequences. J. Bacteriol. 176:7601-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gerardin, J., L. Lalioui, E. Jacquemin, C. Le Bouguenec, and J. G. Mainil. 2000. The afa-related gene cluster in necrotoxigenic and other Escherichia coli from animals belongs to the afa-8 variant. Vet. Microbiol. 76:175-184. [DOI] [PubMed] [Google Scholar]
- 141.Germani, Y., E. Begaud, P. Duval, and C. Le Bouguenec. 1996. Prevalence of enteropathogenic, enteroaggregative, and diffusely adherent Escherichia coli among isolates from children with diarrhea in New Caledonia. J. Infect. Dis. 174:1124-1126. [DOI] [PubMed] [Google Scholar]
- 142.Girardeau, J. P., L. Lalioui, A. M. Said, C. De Champs, and C. Le Bouguenec. 2003. Extended virulence genotype of pathogenic Escherichia coli isolates carrying the afa-8 operon: evidence of similarities between isolates from humans and animals with extraintestinal infections. J. Clin. Microbiol. 41:218-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Giron, J. A., T. Jones, F. Millan-Velasco, E. Castro-Munoz, L. Zarate, J. Fry, G. Frankel, S. L. Moseley, B. Baudry, J. B. Kaper, et al. 1991. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J. Infect. Dis. 163:507-513. [DOI] [PubMed] [Google Scholar]
- 144.Glas, J., K. Martin, G. Brunnler, R. Kopp, C. Folwaczny, E. H. Weiss, and E. D. Albert. 2001. MICA, MICB and C1_4_1 polymorphism in Crohn's disease and ulcerative colitis. Tissue Antigens 58:243-249. [DOI] [PubMed] [Google Scholar]
- 145.Glasser, A. L., J. Boudeau, N. Barnich, M. H. Perruchot, J. F. Colombel, and A. Darfeuille-Michaud. 2001. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect. Immun. 69:5529-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goluszko, P., D. Niesel, B. Nowicki, R. Selvarangan, S. Nowicki, A. Hart, E. Pawelczyk, M. Das, P. Urvil, and R. Hasan. 2001. Dr operon-associated invasiveness of Escherichia coli from pregnant patients with pyelonephritis. Infect. Immun. 69:4678-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goluszko, P., V. Popov, R. Selvarangan, S. Nowicki, T. Pham, and B. J. Nowicki. 1997. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J. Infect. Dis. 176:158-167. [DOI] [PubMed] [Google Scholar]
- 149.Goluszko, P., R. Selvarangan, B. J. Nowicki, S. Nowicki, A. Hart, E. Pawelczyk, and K. Nguyen. 2001. Rapid receptor-clustering assay to detect uropathogenic and diarrheal Escherichia coli isolates bearing adhesins of the Dr family. J. Clin. Microbiol. 39:2317-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goluszko, P., R. Selvarangan, V. Popov, T. Pham, J. W. Wen, and J. Singhal. 1999. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect. Immun. 67:3989-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gomes, T. A., P. A. Blake, and L. R. Trabulsi. 1989. Prevalence of Escherichia coli strains with localized, diffuse, and aggregative adherence to HeLa cells in infants with diarrhea and matched controls. J. Clin. Microbiol. 27:266-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gomes, T. A. T., M. A. M. Vieira, C. M. Abe, D. Rodrigues, P. M. Griffin, and S. R. T. S. Ramos. 1998. Adherence patterns and adherence-related DNA sequences in Escherichia coli isolates from children with and without diarrhea in Sao Paulo City, Brazil. J. Clin. Microbiol. 36:3609-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goodfellow, I. G., R. M. Powell, T. Ward, O. B. Spiller, J. W. Almond, and D. J. Evans. 2000. Echovirus infection of rhabdomyosarcoma cells is inhibited by antiserum to the complement control protein CD59. J. Gen. Virol. 81:1393-1401. [DOI] [PubMed] [Google Scholar]
- 154.Goodfellow, I. G., A. B. Sioofy, R. M. Powell, and D. J. Evans. 2001. Echoviruses bind heparan sulfate at the cell surface. J. Virol. 75:4918-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goosney, D. L., S. Gruenheid, and B. B. Finlay. 2000. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 16:173-189. [DOI] [PubMed] [Google Scholar]
- 156.Gorvel, J. P., and S. Meresse. 2001. Maturation steps of the Salmonella-containing vacuole. Microbes Infect. 3:1299-1303. [DOI] [PubMed] [Google Scholar]
- 157.Gounon, P., M. Jouve, and C. Le Bouguenec. 2000. Immunocytochemistry of the AfaE adhesin and AfaD invasin produced by pathogenic Escherichia coli strains during interaction of the bacteria with HeLa cells by high-resolution scanning electron microscopy. Microbes Infect. 2:359-365. [DOI] [PubMed] [Google Scholar]
- 158.Gray-Owen, S. D., C. Dehio, A. Haude, F. Grunert, and T. F. Meyer. 1997. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 16:3435-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gray-Owen, S. D., D. R. Lorenzen, A. Haude, T. F. Meyer, and C. Dehio. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26:971-980. [DOI] [PubMed] [Google Scholar]
- 160.Grimm, M. C., S. K. Elsbury, P. Pavli, and W. F. Doe. 1996. Interleukin 8: cells of origin in inflammatory bowel disease. Gut 38:90-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gruenheid, S., and B. B. Finlay. 2000. Crowd control: quorum sensing in pathogenic E. coli. Trends Microbiol. 8:442-443. [DOI] [PubMed] [Google Scholar]
- 162.Grunert, F., S. Daniel, G. Nagel, S. von Kleist, and P. Jantscheff. 1995. CD66b, CD66c and carcinoembryonic antigen (CEA) are independently regulated markers in sera of tumor patients. Int. J. Cancer 63:349-355. [DOI] [PubMed] [Google Scholar]
- 163.Grunert, F., M. Kuroki, and, S. S. C. 1998. CEA family members expressed on hematopoeitic cells and their possible role in cell adhesion ans signaling, p. 99-120. In C. P. Stanners (ed.), Cell adhesion and communication mediated by the CEA family—basic and clinical perspective. Harwood Academic Publisher, Amsterdam, The Netherlands.10626907
- 164.Guignot, J., M. F. Bernet-Camard, C. Pous, L. Plancon, C. Le Bouguenec, and A. L. Servin. 2001. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for a5b1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 69:1856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guignot, J., J. Breard, M. F. Bernet-Camard, I. Peiffer, B. J. Nowicki, A. L. Servin, and A. B. Blanc-Potard. 2000. Pyelonephritogenic diffusely adhering Escherichia coli EC7372 harboring Dr-II adhesin carries classical uropathogenic virulence genes and promotes cell lysis and apoptosis in polarized epithelial Caco-2/TC7 cells. Infect. Immun. 68:7018-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guignot, J., I. Peiffer, M. F. Bernet-Camard, D. M. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely-adhering family of Escherichia coli infecting the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 68:3554-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gunzburg, S. T., B. J. Chang, S. J. Elliott, V. Burke, and M. Gracey. 1993. Diffuse and enteroaggregative patterns of adherence of enteric Escherichia coli isolated from aboriginal children from the Kimberley region of Western Australia. J. Infect. Dis. 167:755-758. [DOI] [PubMed] [Google Scholar]
- 168.Guyer, D. M., J. S. Kao, and H. L. Mobley. 1998. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect. Immun. 66:4411-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guyer, D. M., S. Radulovic, F. E. Jones, and H. L. Mobley. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70:4539-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hamann, J., C. Stortelers, E. Kiss-Toth, B. Vogel, W. Eichler, and R. A. van Lier. 1998. Characterization of the CD55 (DAF)-binding site on the seven-span transmembrane receptor CD97. Eur. J. Immunol. 28:1701-1707. [DOI] [PubMed] [Google Scholar]
- 171.Hamann, J., B. Vogel, G. M. van Schijndel, and R. A. van Lier. 1996. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 184:1185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hammarstrom, S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9:67-81. [DOI] [PubMed] [Google Scholar]
- 173.Hammarstrom, S., and V. Baranov. 2001. Is there a role for CEA in innate immunity in the colon? Trends Microbiol. 9:119-125. [DOI] [PubMed] [Google Scholar]
- 174.Harris, C. L., N. K. Rushmere, and B. P. Morgan. 1999. Molecular and functional analysis of mouse decay accelerating factor (CD55). Biochem. J. 341:821-829. [PMC free article] [PubMed] [Google Scholar]
- 175.Hart, A., B. J. Nowicki, B. Reisner, E. Pawelczyk, P. Goluszko, P. Urvil, G. Anderson, and S. Nowicki. 2001. Ampicillin-resistant Escherichia coli in gestational pyelonephritis: increased occurrence and association with the colonization factor Dr adhesin. J. Infect. Dis. 183:1526-1529. [DOI] [PubMed] [Google Scholar]
- 176.Hart, A., T. Pham, S. Nowicki, E. B. Whorton, Jr., M. G. Martens, G. D. Anderson, and B. J. Nowicki. 1996. Gestational pyelonephritis-associated Escherichia coli isolates represent a nonrandom, closely related population. Am. J. Obstet. Gynecol. 174:983-989. [DOI] [PubMed] [Google Scholar]
- 177.Hartley, M. G., M. J. Hudson, E. T. Swarbrick, A. E. Gent, M. D. Hellier, and R. H. Grace. 1993. Adhesive and hydrophobic properties of Escherichia coli from the rectal mucosa of patients with ulcerative colitis. Gut 34:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hasan, R. J., E. Pawelczyk, P. T. Urvil, M. S. Venkatarajan, P. Goluszko, J. Kur, R. Selvarangan, S. Nowicki, W. A. Braun, and B. J. Nowicki. 2002. Structure-function analysis of decay-accelerating factor: identification of residues important for binding of the Escherichia coli Dr adhesin and complement regulation. Infect. Immun. 70:4485-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hastings, J. W. 2004. Bacterial quorum-sensing signals are inactivated by mammalian cells. Proc. Natl. Acad. Sci. USA 101:3993-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hauck, C. R., H. Grassme, J. Bock, V. Jendrossek, K. Ferlinz, T. F. Meyer, and E. Gulbins. 2000. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of Neisseria gonorrhoeae. FEBS Lett. 478:260-266. [DOI] [PubMed] [Google Scholar]
- 181.Hauck, C. R., E. Gulbins, F. Lang, and T. F. Meyer. 1999. Tyrosine phosphatase SHP-1 is involved in CD66-mediated phagocytosis of Opa52-expressing Neisseria gonorrhoeae. Infect. Immun. 67:5490-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hauck, C. R., and T. F. Meyer. 2003. ‘Small’ talk: Opa proteins as mediators of Neisseria-host-cell communication. Curr. Opin. Microbiol. 6:43-49. [DOI] [PubMed] [Google Scholar]
- 183.Hauck, C. R., T. F. Meyer, F. Lang, and E. Gulbins. 1998. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 17:443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.He, Y., F. Lin, P. R. Chipman, C. M. Bator, T. S. Baker, M. Shoham, R. J. Kuhn, M. E. Medof, and M. G. Rossmann. 2002. Structure of decay-accelerating factor bound to echovirus 7: a virus-receptor complex. Proc. Natl. Acad. Sci. USA 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Heine, H., V. T. El-Samalouti, C. Notzel, A. Pfeiffer, A. Lentschat, S. Kusumoto, G. Schmitz, L. Hamann, and A. J. Ulmer. 2003. CD55/decay accelerating factor is part of the lipopolysaccharide-induced receptor complex. Eur. J. Immunol. 33:1399-1408. [DOI] [PubMed] [Google Scholar]
- 186.Heine, H., A. J. Ulmer, V. T. El-Samalouti, A. Lentschat, and L. Hamann. 2001. Decay-accelerating factor (DAF/CD55) is a functional active element of the LPS receptor complex. J. Endotoxin Res. 7:227-231. [PubMed] [Google Scholar]
- 187.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Herzer, P. J., S. Inouye, M. Inouye, and T. S. Whittam. 1990. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J. Bacteriol. 172:6175-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hill, D. J., M. A. Toleman, D. J. Evans, S. Villullas, L. Van Alphen, and M. Virji. 2001. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol. Microbiol. 39:850-862. [DOI] [PubMed] [Google Scholar]
- 190.Hofman, P. 2003. Pathological interactions of bacteria and toxins with the gastrointestinal epithelial tight junctions and/or the zonula adherens: an update. Cell Mol. Biol. 49:65-75. [PubMed] [Google Scholar]
- 191.Hofman, P., M. Piche, D. F. Far, G. Le Negrate, E. Selva, L. Landraud, A. Alliana-Schmid, P. Boquet, and B. Rossi. 2000. Increased Escherichia coli phagocytosis in neutrophils that have transmigrated across a cultured intestinal epithelium. Infect. Immun. 68:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Holden, N., C. Cotterill, and D. Gally. 2000. Examination of regulatory cross-talk between the decay accelerating factor-binding fimbrial/afimbrial adhesins and type I fimbriae. Adv. Exp. Med. Biol. 485:143-150. [DOI] [PubMed] [Google Scholar]
- 193.Horig, H., A. Wainstein, L. Long, D. Kahn, S. Soni, A. Marcus, W. Edelmann, R. Kucherlapati, and H. L. Kaufman. 2001. A new mouse model for evaluating the immunotherapy of human colorectal cancer. Cancer Res. 61:8520-8526. [PubMed] [Google Scholar]
- 194.Hourcade, D., M. K. Liszewski, M. Krych-Goldberg, and J. P. Atkinson. 2000. Functional domains, structural variations and pathogen interactions of MCP, DAF and CR1. Immunopharmacology 49:103-116. [DOI] [PubMed] [Google Scholar]
- 195.Huang, J., J. D. Hardy, Y. Sun, and J. E. Shively. 1999. Essential role of biliary glycoprotein (CD66a) in morphogenesis of the human mammary epithelial cell line MCF10F. J. Cell Sci. 112:4193-4205. [DOI] [PubMed] [Google Scholar]
- 196.Huber, M., L. Izzi, P. Grondin, C. Houde, T. Kunath, A. Veillette, and N. Beauchemin. 1999. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J. Biol. Chem. 274:335-344. [DOI] [PubMed] [Google Scholar]
- 197.Hudault, S., O. B. Spiller, B. P. Morgan, and A. L. Servin. 2004. Human diffusely adhering Escherichia coli expressing Afa/Dr adhesins that use human CD55 (decay-accelerating factor) as a receptor does not bind the rodent and pig analogues of CD55. Infect. Immun. 72:4859-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hung, D. L., S. D. Knight, R. M. Woods, J. S. Pinkner, and S. J. Hultgren. 1996. Molecular basis of two subfamilies of immunoglobulin-like chaperones. EMBO J. 15:3792-3805. [PMC free article] [PubMed] [Google Scholar]
- 199.Ichida, S., Y. Yuzawa, H. Okada, K. Yoshioka, and S. Matsuo. 1994. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 46:89-96. [DOI] [PubMed] [Google Scholar]
- 200.Ilangumaran, S., H. T. He, and D. C. Hoessli. 2000. Microdomains in lymphocyte signalling: beyond GPI-anchored proteins. Immunol. Today 21:2-7. [DOI] [PubMed] [Google Scholar]
- 201.Ilantzis, C., L. DeMarte, R. A. Screaton, and C. P. Stanners. 2002. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia 4:151-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Inaba, T., M. Mizuno, S. Ohya, M. Kawada, T. Uesu, J. Nasu, K. Takeuchi, M. Nakagawa, H. Okada, T. Fujita, and T. Tsuji. 1998. Decay-accelerating factor (DAF) in stool specimens as a marker of disease activity in patients with ulcerative colitis (UC). Clin. Exp. Immunol. 112:237-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 204.Isberg, R. R., and P. Barnes. 2001. Subversion of integrins by enteropathogenic Yersinia. J. Cell Sci. 114:21-28. [DOI] [PubMed] [Google Scholar]
- 205.Isberg, R. R., Z. Hamburger, and P. Dersch. 2000. Signaling and invasin-promoted uptake via integrin receptors. Microbes Infect. 2:793-801. [DOI] [PubMed] [Google Scholar]
- 206.Ivetic, A., and A. J. Ridley. 2004. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 112:165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jallat, C., A. Darfeuille-Michaud, J. P. Girardeau, C. Rich, and B. Joly. 1994. Self-transmissible R plasmids encoding CS31A among human Escherichia coli strains isolated from diarrheal stools. Infect. Immun. 62:2865-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jallat, C., A. Darfeuille-Michaud, C. Rich, and B. Joly. 1994. Survey of clinical isolates of diarrhoeogenic Escherichia coli: diffusely adhering E. coli strains with multiple adhesive factors. Res. Microbiol. 145:621-632. [DOI] [PubMed] [Google Scholar]
- 209.Jallat, C., V. Livrelli, A. Darfeuille-Michaud, C. Rich, and B. Joly. 1993. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J. Clin. Microbiol. 31:2031-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Johnson, J. R., K. M. Skubitz, B. J. Nowicki, K. Jacques-Palaz, and R. M. Rakita. 1995. Nonlethal adherence to human neutrophils mediated by Dr antigen-specific adhesins of Escherichia coli. Infect. Immun. 63:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 213.Jouve, M., M. I. Garcia, P. Courcoux, A. Labigne, P. Gounon, and C. Le Bouguenec. 1997. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect. Immun. 65:4082-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kammer, G. M., E. I. Walter, and M. E. Medof. 1988. Association of cytoskeletal re-organization with capping of the complement decay-accelerating factor on T lymphocytes. J. Immunol. 141:2924-2928. [PubMed] [Google Scholar]
- 215.Kammerer, R., S. Hahn, B. B. Singer, J. S. Luo, and S. von Kleist. 1998. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur. J. Immunol. 28:3664-3674. [DOI] [PubMed] [Google Scholar]
- 216.Kammerer, R., D. Stober, B. B. Singer, B. Obrink, and J. Reimann. 2001. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J. Immunol. 166:6537-6544. [DOI] [PubMed] [Google Scholar]
- 217.Kanamaru, K., I. Tatsuno, T. Tobe, and C. Sasakawa. 2000. SdiA, an Escherichia coli homologue of quorum-sensing regulators, controls the expression of virulence factors in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 38:805-816. [DOI] [PubMed] [Google Scholar]
- 218.Kansau, I., C. Berger, M. Hospital, R. Amsellem, V. Nicolas, A. L. Servin, and M. F. Bernet-Camard. 2004. Zipper-like internalization of Dr-positive Escherichia coli by epithelial cells is preceded by an adhesin-induced mobilization of raft-associated molecules in the initial step of adhesion. Infect. Immun. 72:3733-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kaper, J. B., J. P. Nataro, and H. L. T. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123140. [DOI] [PubMed] [Google Scholar]
- 220.Karnauchow, T. M., S. Dawe, D. M. Lublin, and K. Dimock. 1998. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J. Virol. 72:9380-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kaul, A., M. Nagamani, and B. Nowicki. 1995. Decreased expression of endometrial decay accelerating factor (DAF), a complement regulatory protein, in patients with luteal phase defect. Am. J. Reprod. Immunol. 34:236-240. [DOI] [PubMed] [Google Scholar]
- 223.Kaul, A., B. J. Nowicki, M. G. Martens, P. Goluszko, A. Hart, M. Nagamani, D. Kumar, T. Q. Pham, and S. Nowicki. 1994. Decay-accelerating factor is expressed in the human endometrium and may serve as the attachment ligand for Dr pili of Escherichia coli. Am. J. Reprod. Immunol. 32:194-199. [DOI] [PubMed] [Google Scholar]
- 224.Kaul, A. K., S. Khan, M. G. Martens, J. T. Crosson, V. R. Lupo, and R. Kaul. 1999. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect. Immun. 67:5958-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kaul, A. K., D. Kumar, M. Nagamani, P. Goluszko, S. Nowicki, and B. J. Nowicki. 1996. Rapid cyclic changes in density and accessibility of endometrial ligands for Escherichia coli Dr fimbriae. Infect. Immun. 64:611-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kawano, M. 2000. Complement regulatory proteins and autoimmunity. Arch. Immunol. Ther. Exp. 48:367-372. [PubMed] [Google Scholar]
- 227.Keller, R., J. G. Ordonez, R. R. de Oliveira, L. R. Trabulsi, T. J. Baldwin, and S. Knutton. 2002. Afa, a diffuse adherence fibrillar adhesin associated with enteropathogenic Escherichia coli. Infect. Immun. 70:2681-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kerneis, S., S. S. Bilge, V. Fourel, G. Chauviere, M. H. Coconnier, and A. L. Servin. 1991. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect. Immun. 59:4013-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kerneis, S., J. M. Gabastou, M. F. Bernet-Camard, M. H. Coconnier, B. J. Nowicki, and A. L. Servin. 1994. Human cultured intestinal cells express attachment sites for uropathogenic Escherichia coli bearing adhesins of the Dr adhesin family. FEMS Microbiol. Lett. 119:27-32. [DOI] [PubMed] [Google Scholar]
- 230.Kim, J. C., K. H. Koo, B. S. Kim, K. C. Park, D. C. Bicknell, and W. F. Bodmer. 1999. Carcino-embryonic antigen may function as a chemo-attractant in colorectal-carcinoma cell lines. Int. J. Cancer 82:880-885. [DOI] [PubMed] [Google Scholar]
- 231.Kim, Y. U., T. Kinoshita, H. Molina, D. Hourcade, T. Seya, L. M. Wagner, and V. M. Holers. 1995. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J. Exp. Med. 181:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kirkitadze, M. D., and P. N. Barlow. 2001. Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol. Rev. 180:146-161. [DOI] [PubMed] [Google Scholar]
- 233.Knodler, L. A., J. Celli, and B. B. Finlay. 2001. Pathogenic trickery: deception of host cell processes. Nat. Rev. Mol. Cell. Biol. 2:578-588. [DOI] [PubMed] [Google Scholar]
- 234.Krueger, D. K., S. M. Kelly, D. N. Lewicki, R. Ruffolo, and T. M. Gallagher. 2001. Variations in disparate regions of the murine coronavirus spike protein impact the initiation of membrane fusion. J. Virol. 75:2792-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kunath, T., C. Ordonez-Garcia, C. Turbide, and N. Beauchemin. 1995. Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene 11:2375-2382. [PubMed] [Google Scholar]
- 236.Kuraya, M., and T. Fujita. 1998. Signal transduction via a protein associated with a glycosylphosphatidylinositol-anchored protein, decay-accelerating factor (DAF/CD55). Int. Immunol. 10:473-480. [DOI] [PubMed] [Google Scholar]
- 237.Kurzchalia, T. V., and R. G. Parton. 1999. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11:424-431. [DOI] [PubMed] [Google Scholar]
- 238.Kuttner-Kondo, L., M. E. Medof, W. Brodbeck, and M. Shoham. 1996. Molecular modeling and mechanism of action of human decay-accelerating factor. Protein Eng. 9:1143-1149. [DOI] [PubMed] [Google Scholar]
- 239.Kuttner-Kondo, L. A., L. Mitchell, D. E. Hourcade, and M. E. Medof. 2001. Characterization of the active sites in decay-accelerating factor. J. Immunol. 167:2164-2171. [DOI] [PubMed] [Google Scholar]
- 240.Kwok, T., S. Backert, H. Schwarz, J. Berger, and T. F. Meyer. 2002. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect. Immun. 70:2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Labigne-Roussel, A., and S. Falkow. 1988. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect. Immun. 56:640-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Labigne-Roussel, A., M. A. Schmidt, W. Walz, and S. Falkow. 1985. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J. Bacteriol. 162:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Labigne-Roussel, A. F., D. Lark, G. Schoolnik, and S. Falkow. 1984. Cloning and expression of an afimbrial adhesin (Afa-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect. Immun. 46:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lalioui, L., M. Jouve, P. Gounon, and C. Le Bouguenec. 1999. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia coli strains associated with diarrhea or septicemia in calves. Infect. Immun. 67:5048-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lalioui, L., and C. Le Bouguenec. 2001. afa-8 gene cluster is carried by a pathogenicity island inserted into the tRNA(Phe) of human and bovine pathogenic Escherichia coli isolates. Infect. Immun. 69:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lambeth, J. D. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4:181-189. [DOI] [PubMed] [Google Scholar]
- 247.Langman, M. J., and R. N. Allan. 1999. Escherichia coli for ulcerative colitis. Lancet 354:2000-2001. [DOI] [PubMed] [Google Scholar]
- 248.Lea, S. 2002. Interactions of CD55 with non-complement ligands. Biochem. Soc. Trans. 30:1014-1019. [DOI] [PubMed] [Google Scholar]
- 249.Lea, S. M., R. M. Powell, T. McKee, D. J. Evans, D. Brown, D. I. Stuart, and P. A. van der Merwe. 1998. Determination of the affinity and kinetic constants for the interaction between the human virus echovirus 11 and its cellular receptor, CD55. J. Biol. Chem. 273:30443-30447. [DOI] [PubMed] [Google Scholar]
- 250.Le Bouguenec, C., M. Archambaud, and A. Labigne. 1992. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 30:1189-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Le Bouguenec, C., M. I. Garcia, V. Ouin, J. M. Desperrier, P. Gounon, and A. Labigne. 1993. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect. Immun. 61:5106-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Le Bouguenec, C., L. Lalioui, L. du Merle, M. Jouve, P. Courcoux, S. Bouzari, R. Selvarangan, B. J. Nowicki, Y. Germani, A. Andremont, P. Gounon, and M. I. Garcia. 2001. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J. Clin. Microbiol. 39:1738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Leusch, H. G., Z. Drzeniek, S. A. Hefta, Z. Markos-Pusztai, and C. Wagener. 1991. The putative role of members of the CEA-gene family (CEA, NCA and BGP) as ligands for the bacterial colonization of different human epithelial tissues. Zentralbl. Bakteriol. 275:118-122. [DOI] [PubMed] [Google Scholar]
- 254.Leusch, H. G., Z. Drzeniek, Z. Markos-Pusztai, and C. Wagener. 1991. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect. Immun. 59:2051-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Leusch, H. G., S. A. Hefta, Z. Drzeniek, K. Hummel, Z. Markos-Pusztai, and C. Wagener. 1990. Escherichia coli of human origin binds to carcinoembryonic antigen (CEA) and non-specific crossreacting antigen (NCA). FEBS Lett. 261:405-409. [DOI] [PubMed] [Google Scholar]
- 256.Levine, M. M., C. Ferreccio, V. Prado, M. Cayazzo, P. Abrego, J. Martinez, L. Maggi, M. M. Baldini, W. Martin, D. Maneval, et al. 1993. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am. J. Epidemiol. 138:849-869. [DOI] [PubMed] [Google Scholar]
- 257.Levine, M. M., V. Prado, R. Robins-Browne, H. Lior, J. B. Kaper, S. L. Moseley, K. Gicquelais, J. P. Nataro, P. Vial, and B. Tall. 1988. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J. Infect. Dis. 158:224-228. [DOI] [PubMed] [Google Scholar]
- 258.Li, B., C. Sallee, M. Dehoff, S. Foley, H. Molina, and V. M. Holers. 1993. Mouse Crry/p65. Characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J. Immunol. 151:4295-4305. [PubMed] [Google Scholar]
- 259.Lin, F., S. N. Emancipator, D. J. Salant, and M. E. Medof. 2002. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab. Investig. 82:563-569. [DOI] [PubMed] [Google Scholar]
- 260.Lindahl, G., U. Sjobring, and E. Johnsson. 2000. Human complement regulators: a major target for pathogenic microorganisms. Curr. Opin. Immunol. 12:44-51. [DOI] [PubMed] [Google Scholar]
- 261.Linskens, R. K., X. W. Huijsdens, P. H. Savelkoul, C. M. Vandenbroucke-Grauls, and S. G. Meuwissen. 2001. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand. J. Gastroenterol. Suppl. 234:29-40. [DOI] [PubMed] [Google Scholar]
- 262.Lisanti, M. P., M. Sargiacomo, and P. E. Scherer. 1999. Purification of caveolae-derived membrane microdomains containing lipid-anchored signaling molecules, such as GPI-anchored proteins, H-Ras, Src-family tyrosine kinases, eNOS, and G-protein alpha-, beta-, and gamma-subunits. Methods Mol. Biol. 116:51-60. [DOI] [PubMed] [Google Scholar]
- 263.Liszewski, M. K., T. C. Farries, D. M. Lublin, I. A. Rooney, and J. P. Atkinson. 1996. Control of the complement system. Adv. Immunol. 61:201-283. [DOI] [PubMed] [Google Scholar]
- 264.Loomis, W. P., J. T. Koo, T. P. Cheung, and S. L. Moseley. 2001. A tripeptide sequence within the nascent DaaP protein is required for mRNA processing of a fimbrial operon in Escherichia coli. Mol. Microbiol. 39:693-707. [DOI] [PubMed] [Google Scholar]
- 265.Loomis, W. P., and S. L. Moseley. 1998. Translational control of mRNA processing in the F1845 fimbrial operon of Escherichia coli. Mol. Microbiol. 30:843-853. [DOI] [PubMed] [Google Scholar]
- 266.Louvard, D., M. Kedinger, and H. P. Hauri. 1992. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu. Rev. Cell Biol. 8:157-195. [DOI] [PubMed] [Google Scholar]
- 267.Reference deleted.
- 268.Lublin, D. M., and K. E. Coyne. 1991. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J. Exp. Med. 174:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lublin, D. M., S. Kompelli, J. R. Storry, and M. E. Reid. 2000. Molecular basis of Cromer blood group antigens. Transfusion 40:208-213. [DOI] [PubMed] [Google Scholar]
- 270.Lublin, D. M., R. S. Lemons, M. M. Le Beau, V. M. Holers, M. L. Tykocinski, M. E. Medof, and J. P. Atkinson. 1987. The gene encoding decay-accelerating factor (DAF) is located in the complement-regulatory locus on the long arm of chromosome 1. J. Exp. Med. 165:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lublin, D. M., M. K. Liszewski, T. W. Post, M. A. Arce, M. M. Le Beau, M. B. Rebentisch, L. S. Lemons, T. Seya, and J. P. Atkinson. 1988. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J. Exp. Med. 168:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lublin, D. M., E. S. Thompson, A. M. Green, C. Levene, and M. J. Telen. 1991. Dr(a-) polymorphism of decay accelerating factor. Biochemical, functional, and molecular characterization and production of allele-specific transfectants. J. Clin. Investig. 87:1945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lukacik, P., P. Roversi, J. White, D. Esser, G. P. Smith, J. Billington, P. A. Williams, P. M. Rudd, M. R. Wormald, D. J. Harvey, M. D. Crispin, C. M. Radcliffe, R. A. Dwek, D. J. Evans, B. P. Morgan, R. A. Smith, and S. M. Lea. 2004. Complement regulation at the molecular level: the structure of decay-accelerating factor. Proc. Natl. Acad. Sci. USA 101:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Manes, S., G. del Real, and A. C. Martinez. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557-568. [DOI] [PubMed] [Google Scholar]
- 275.Martinez, J. J., and S. J. Hultgren. 2002. Requirement of Rho-family GTPases in the invasion of type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 4:19-28. [DOI] [PubMed] [Google Scholar]
- 276.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Martino, T. A., M. Petric, M. Brown, K. Aitken, C. J. Gauntt, C. D. Richardson, L. H. Chow, and P. P. Liu. 1998. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology 244:302-314. [DOI] [PubMed] [Google Scholar]
- 278.Masseret, E., J. Boudeau, J. F. Colombel, C. Neut, P. Desreumaux, B. Joly, A. Cortot, and A. Darfeuille-Michaud. 2001. Genetically related Escherichia coli strains associated with Crohn's disease. Gut 48:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.McCaw, S. E., E. H. Liao, and S. D. Gray-Owen. 2004. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect. Immun. 72:2742-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Medof, M. E., T. Kinoshita, and V. Nussenzweig. 1984. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J. Exp. Med. 160:1558-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Medof, M. E., E. I. Walter, J. L. Rutgers, D. M. Knowles, and V. Nussenzweig. 1987. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J. Exp. Med. 165:848-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Merz, A. J., and M. So. 2000. Interactions of pathogenic Neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 283.Meyer, T. F. 1999. Pathogenic Neisseriae: complexity of pathogen-host cell interplay. Clin. Infect. Dis. 28:433-441. [DOI] [PubMed] [Google Scholar]
- 284.Meyer, T. F. 1998. Pathogenic Neisseria—interplay between pro- and eukaryotic worlds. Folia Microbiol. 43:311-319. [DOI] [PubMed] [Google Scholar]
- 285.Miettinen, A., B. Westerlund, A. M. Tarkkanen, T. Tornroth, P. Ljungberg, O. V. Renkonen, and T. K. Korhonen. 1993. Binding of bacterial adhesins to rat glomerular mesangium in vivo. Kidney Int. 43:592-600. [DOI] [PubMed] [Google Scholar]
- 286.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 287.Mitsumori, K., A. Terai, S. Yamamoto, and O. Yoshida. 1997. Virulence characteristics and DNA fingerprints of Escherichia coli isolated from women with acute uncomplicated pyelonephritis. J. Urol. 158:2329-2332. [DOI] [PubMed] [Google Scholar]
- 288.Miura, H. S., K. Nakagaki, and F. Taguchi. 2004. N-terminal domain of the murine coronavirus receptor CEACAM1 is responsible for fusogenic activation and conformational changes of the spike protein. J. Virol. 78:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Miwa, T., L. Zhou, B. Hilliard, H. Molina, and W. C. Song. 2002. Crry, but not CD59 and DAF, is indispensable for murine erythrocyte protection in vivo from spontaneous complement attack. Blood 99:3707-3716. [DOI] [PubMed] [Google Scholar]
- 290.Molina, H. 2002. The murine complement regulator Crry: new insights into the immunobiology of complement regulation. Cell Mol. Life Sci. 59:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Moller, M. J., R. Kammerer, F. Grunert, and S. von Kleist. 1996. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int. J. Cancer 65:740-745. [DOI] [PubMed] [Google Scholar]
- 292.Morales, V. M., A. Christ, S. M. Watt, H. S. Kim, K. W. Johnson, N. Utku, A. M. Texieira, A. Mizoguchi, E. Mizoguchi, G. J. Russell, S. E. Russell, A. K. Bhan, G. J. Freeman, and R. S. Blumberg. 1999. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J. Immunol. 163:1363-1370. [PubMed] [Google Scholar]
- 293.Morelli, R., L. Baldassarri, V. Falbo, G. Donelli, and A. Caprioli. 1994. Detection of enteroadherent Escherichia coli associated with diarrhoea in Italy. J. Med. Microbiol. 41:399-404. [DOI] [PubMed] [Google Scholar]
- 294.Morgan, B. P., M. Daha, S. Meri, and A. Nicholson-Weller. 2000. Into the third century of complement research. Immunol. Today 21:603-605. [DOI] [PubMed] [Google Scholar]
- 295.Moulds, J. M., S. Nowicki, J. J. Moulds, and B. J. Nowicki. 1996. Human blood groups: incidental receptors for viruses and bacteria. Transfusion 36:362-374. [DOI] [PubMed] [Google Scholar]
- 296.Moutabarrik, A., I. Nakanishi, M. Namiki, T. Hara, M. Matsumoto, M. Ishibashi, A. Okuyama, D. Zaid, and T. Seya. 1993. Cytokine-mediated regulation of the surface expression of complement regulatory proteins, CD46 (MCP), CD55 (DAF), and CD59 on human vascular endothelial cells. Lymphokine Cytokine Res. 12:167-172. [PubMed] [Google Scholar]
- 297.Muenzner, P., O. Billker, T. F. Meyer, and M. Naumann. 2002. Nuclear factor-kappa B directs carcinoembryonic antigen-related cellular adhesion molecule 1 receptor expression in Neisseria gonorrhoeae-infected epithelial cells. J. Biol. Chem. 277:7438-7446. [DOI] [PubMed] [Google Scholar]
- 298.Muenzner, P., C. Dehio, T. Fujiwara, M. Achtman, T. F. Meyer, and S. D. Gray-Owen. 2000. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun. 68:3601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Muenzner, P., M. Naumann, T. F. Meyer, and S. D. Gray-Owen. 2001. Pathogenic Neisseria trigger expression of their carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1; previously CD66a) receptor on primary endothelial cells by activating the immediate early response transcription factor, nuclear factor-kappaB. J. Biol. Chem. 276:24331-24340. [DOI] [PubMed] [Google Scholar]
- 300.Mulder, L. C., M. Mora, E. Ciccopiedi, C. Melli, S. Nuti, G. Marinucci, P. Bruzzone, M. Lazzeri, R. Lorenzini, D. Alfani, et al. 1995. Mice transgenic for human CD46 and CD55 are protected from human complement attack. Transplant. Proc. 27:333-335. [PubMed] [Google Scholar]
- 301.Mulvey, M. A. 2002. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 4:257-271. [DOI] [PubMed] [Google Scholar]
- 302.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nakajima, A., H. Iijima, M. F. Neurath, T. Nagaishi, E. E. Nieuwenhuis, R. Raychowdhury, J. Glickman, D. M. Blau, S. Russell, K. V. Holmes, and R. S. Blumberg. 2002. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J. Immunol. 168:1028-1035. [DOI] [PubMed] [Google Scholar]
- 304.Nangaku, M., R. J. Quigg, S. J. Shankland, N. Okada, R. J. Johnson, and W. G. Couser. 1997. Overexpression of Crry protects mesangial cells from complement-mediated injury. J. Am. Soc. Nephrol. 8:223-233. [DOI] [PubMed] [Google Scholar]
- 305.Nasu, J., M. Mizuno, T. Uesu, K. Takeuchi, T. Inaba, S. Ohya, M. Kawada, K. Shimo, H. Okada, T. Fujita, and T. Tsuji. 1998. Cytokine-stimulated release of decay-accelerating factor (DAF;CD55) from HT-29 human intestinal epithelial cells. Clin. Exp. Immunol. 113:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nataro, J. P., J. B. Kaper, R. Robins-Browne, V. Prado, P. Vial, and M. M. Levine. 1987. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr. Infect. Dis. J. 6:829-831. [DOI] [PubMed] [Google Scholar]
- 308.Nataro, J. P., I. C. Scaletsky, J. B. Kaper, M. M. Levine, and L. R. Trabulsi. 1985. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect. Immun. 48:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Natuzzi, E. S., P. C. Ursell, M. Harrison, C. Buscher, and R. K. Riemer. 1993. Nitric oxide synthase activity in the pregnant uterus decreases at parturition. Biochem. Biophys. Res. Commun. 194:1-8. [DOI] [PubMed] [Google Scholar]
- 310.Naumann, M., T. Rudel, and T. F. Meyer. 1999. Host cell interactions and signalling with Neisseria gonorrhoeae. Curr. Opin. Microbiol. 2:62-70. [DOI] [PubMed] [Google Scholar]
- 311.Neumaier, M., S. Paululat, A. Chan, P. Matthaes, and C. Wagener. 1993. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc. Natl. Acad. Sci. USA 90:10744-10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Nimmich, W., W. Voigt, and G. Seltmann. 1997. Characterization of urinary Escherichia coli O75 strains. J. Clin. Microbiol. 35:1112-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nishikage, H., L. Baranyi, H. Okada, N. Okada, K. Isobe, A. Nomura, F. Yoshida, and S. Matsuo. 1995. The role of a complement regulatory protein in rat mesangial glomerulonephritis. J. Am. Soc. Nephrol. 6:234-241. [DOI] [PubMed] [Google Scholar]
- 314.Nomura, A., K. Nishikawa, Y. Yuzawa, H. Okada, N. Okada, B. P. Morgan, S. J. Piddlesden, M. Nadai, T. Hasegawa, and S. Matsuo. 1995. Tubulointerstitial injury induced in rats by a monoclonal antibody that inhibits function of a membrane inhibitor of complement. J. Clin. Investig. 96:2348-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Normark, S., B. Albiger, and A. B. Jonsson. 2002. Gonococci cause immunosuppression by engaging a coinhibitory receptor on T lymphocytes. Nat. Immunol. 3:210-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Nowicki, B., J. P. Barrish, T. Korhonen, R. A. Hull, and S. I. Hull. 1987. Molecular cloning of the Escherichia coli O75X adhesin. Infect. Immun. 55:3168-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nowicki, B., L. Fang, J. Singhal, S. Nowicki, and C. Yallampalli. 1997. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death rate with inhibition of nitric oxide. Am. J. Reprod. Immunol. 38:309-312. [DOI] [PubMed] [Google Scholar]
- 318.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Nowicki, B., A. Labigne, S. Moseley, R. Hull, S. Hull, and J. Moulds. 1990. The Dr hemagglutinin, afimbrial adhesins Afa-I and Afa-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect. Immun. 58:279-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nowicki, B., M. Martens, A. Hart, and S. Nowicki. 1994. Gestational age-dependent distribution of Escherichia coli fimbriae in pregnant patients with pyelonephritis. Ann. N. Y. Acad. Sci. 730:290-291. [DOI] [PubMed] [Google Scholar]
- 321.Nowicki, B., J. Moulds, R. Hull, and S. Hull. 1988. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect. Immun. 56:1057-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Nowicki, B., R. Selvarangan, and S. Nowicki. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl. 1):S24-S27. [DOI] [PubMed] [Google Scholar]
- 323.Nowicki, B., J. Singhal, L. Fang, S. Nowicki, and C. Yallampalli. 1999. Inverse relationship between severity of experimental pyelonephritis and nitric oxide production in C3H/HeJ mice. Infect. Immun. 67:2421-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nowicki, B., C. Svanborg-Eden, R. Hull, and S. Hull. 1989. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect. Immun. 57:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Nowicki, B., L. Truong, J. Moulds, and R. Hull. 1988. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am. J. Pathol. 133:1-4. [PMC free article] [PubMed] [Google Scholar]
- 326.Nowicki, S., B. Nowicki, T. Pham, R. Hasan, and M. Nagamani. 2001. Expression of decay accelerating factor in endometrial adenocarcinoma is inversely related to the stage of tumor. Am. J. Reprod. Immunol. 46:144-148. [DOI] [PubMed] [Google Scholar]
- 327.Öbrink, B. 1997. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr. Opin. Cell Biol. 9:616-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Oelschlaeger, T. A., and D. J. Kopecko. 2000. Microtubule dependent invasion pathways of bacteria. Subcell. Biochem. 33:3-19. [DOI] [PubMed] [Google Scholar]
- 329.Okeke, I. N., A. Lamikanra, H. Steinruck, and J. B. Kaper. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 38:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ordonez, C., R. A. Screaton, C. Ilantzis, and C. P. Stanners. 2000. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 60:3419-3424. [PubMed] [Google Scholar]
- 331.Oshiro, N., Y. Fukata, and K. Kaibuchi. 1998. Phosphorylation of moesin by Rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J. Biol. Chem. 273:34663-34666. [DOI] [PubMed] [Google Scholar]
- 332.Pasch, A., J. H. Kupper, A. Wolde, R. Kandolf, and H. C. Selinka. 1999. Comparative analysis of virus-host cell interactions of haemagglutinating and non-haemagglutinating strains of coxsackievirus B3. J. Gen. Virol. 80:3153-3158. [DOI] [PubMed] [Google Scholar]
- 333.Peiffer, I., M. F. Bernet-Camard, M. Rousset, and A. L. Servin. 2001. Impairments in enzyme activity and biosynthesis of brush border-associated hydrolases in human intestinal Caco-2/TC7 cells infected by members of the Afa/Dr family of diffusely adhering Escherichia coli. Cell. Microbiol. 3:341-357. [DOI] [PubMed] [Google Scholar]
- 334.Peiffer, I., A. B. Blanc-Potard, M. F. Bernet-Camard, J. Guignot, A. Barbat, and A. L. Servin. 2000. Afa/Dr diffusely adhering Escherichia coli C1845 infection promotes selective injuries in the junctional domain of polarized human intestinal Caco-2/TC7 cells. Infect. Immun. 68:3431-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Peiffer, I., J. Guignot, A. Barbat, C. Carnoy, S. L. Moseley, B. J. Nowicki, A. L. Servin, and M. F. Bernet-Camard. 2000. Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect. Immun. 68:5979-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Peiffer, I., A. L. Servin, and M. F. Bernet-Camard. 1998. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect. Immun. 66:4036-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Pelkmans, L., and A. Helenius. 2002. Endocytosis via caveolae. Traffic 3:311-320. [DOI] [PubMed] [Google Scholar]
- 338.Pettigrew, D., K. L. Anderson, J. Billington, E. Cota, P. Simpson, P. Urvil, F. Rabuzin, P. Roversi, B. Nowicki, L. Du Merle, C. Le Bouguenc, S. Matthews, and S. M. Lea. 2004. High resolution studies of the Afa/Dr adhesin DraE and its interaction with chloramphenicol. J. Biol. Chem. 279:46851-46857. [DOI] [PubMed] [Google Scholar]
- 339.Pham, T., A. Kaul, A. Hart, P. Goluszko, J. Moulds, S. Nowicki, D. M. Lublin, and B. J. Nowicki. 1995. Dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect. Immun. 63:1663-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Pham, T. Q., P. Goluszko, V. Popov, S. Nowicki, and B. J. Nowicki. 1997. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect. Immun. 65:4309-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Picard, B., J. S. Garcia, S. Gouriou, P. Duriez, N. Brahimi, E. Bingen, J. Elion, and E. Denamur. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pizzo, P., E. Giurisato, M. Tassi, A. Benedetti, T. Pozzan, and A. Viola. 2002. Lipid rafts and T cell receptor signaling: a critical re-evaluation. Eur. J. Immunol. 32:3082-3091. [DOI] [PubMed] [Google Scholar]
- 343.Plancon, L., L. Du Merle, S. Le Friec, P. Gounon, M. Jouve, J. Guignot, A. Servin, and C. Le Bouguenec. 2003. Recognition of the cellular beta1-chain integrin by the bacterial AfaD invasin is implicated in the internalization of afa-expressing pathogenic Escherichia coli strains. Cell. Microbiol. 5:681-693. [DOI] [PubMed] [Google Scholar]
- 344.Poitrineau, P., C. Forestier, M. Meyer, C. Jallat, C. Rich, G. Malpuech, and C. De Champs. 1995. Retrospective case-control study of diffusely adhering Escherichia coli and clinical features in children with diarrhea. J. Clin. Microbiol. 33:1961-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Popp, A., C. Dehio, F. Grunert, T. F. Meyer, and S. D. Gray-Owen. 1999. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell. Microbiol. 1:169-181. [DOI] [PubMed] [Google Scholar]
- 346.Post, T. W., M. A. Arce, M. K. Liszewski, E. S. Thompson, J. P. Atkinson, and D. M. Lublin. 1990. Structure of the gene for human complement protein decay accelerating factor. J. Immunol. 144:740-744. [PubMed] [Google Scholar]
- 347.Powell, R. M., V. Schmitt, T. Ward, I. Goodfellow, D. J. Evans, and J. W. Almond. 1998. Characterization of echoviruses that bind decay accelerating factor (CD55): evidence that some haemagglutinating strains use more than one cellular receptor. J. Gen. Virol. 79:1707-1713. [DOI] [PubMed] [Google Scholar]
- 348.Powell, R. M., T. Ward, D. J. Evans, and J. W. Almond. 1997. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J. Virol. 71:9306-9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Powell, R. M., T. Ward, I. Goodfellow, J. W. Almond, and D. J. Evans. 1999. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J. Gen. Virol. 80:3145-3152. [DOI] [PubMed] [Google Scholar]
- 350.Prall, F., P. Nollau, M. Neumaier, H. D. Haubeck, Z. Drzeniek, U. Helmchen, T. Loning, and C. Wagener. 1996. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J. Histochem. Cytochem. 44:35-41. [DOI] [PubMed] [Google Scholar]
- 351.Quigg, R. J., A. Nicholson-Weller, A. V. Cybulsky, J. Badalamenti, and D. J. Salant. 1989. Decay accelerating factor regulates complement activation on glomerular epithelial cells. J. Immunol. 142:877-882. [PubMed] [Google Scholar]
- 352.Quigg, R. J., and A. E. Sneed. 1994. Molecular characterization of rat glomerular epithelial cell complement receptors. J. Am. Soc. Nephrol. 4:1912-1919. [DOI] [PubMed] [Google Scholar]
- 353.Rao, R. K., R. D. Baker, S. S. Baker, A. Gupta, and M. Holycross. 1997. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am. J. Physiol. 273:G812-G823. [DOI] [PubMed] [Google Scholar]
- 354.Rey-Campos, J., P. Rubinstein, and S. Rodriguez de Cordoba. 1988. A physical map of the human regulator of complement activation gene cluster linking the complement genes CR1, CR2, DAF, and C4BP. J. Exp. Med. 167:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Riemer, R. K., C. Buscher, R. K. Bansal, S. M. Black, Y. He, and E. S. Natuzzi. 1997. Increased expression of nitric oxide synthase in the myometrium of the pregnant rat uterus. Am. J. Physiol. 272:E1008-E1015. [DOI] [PubMed] [Google Scholar]
- 356.Rosa, A. C., A. T. Mariano, A. M. Pereira, A. Tibana, T. A. Gomes, and J. R. Andrade. 1998. Enteropathogenicity markers in Escherichia coli isolated from infants with acute diarrhoea and healthy controls in Rio de Janeiro, Brazil. J. Med. Microbiol. 47:781-790. [DOI] [PubMed] [Google Scholar]
- 357.Rosenberger, C. M., J. H. Brumell, and B. B. Finlay. 2000. Microbial pathogenesis: lipid rafts as pathogen portals. Curr. Biol. 10:R823-R825. [DOI] [PubMed] [Google Scholar]
- 358.Russo, T. A., U. B. Carlino, A. Mong, and S. T. Jodush. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sadekova, S., N. Lamarche-Vane, X. Li, and N. Beauchemin. 2000. The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell-cell contact through activation of Rho-like GTPases. Mol. Biol. Cell 11:65-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sauer, F. G., M. Barnhart, D. Choudhury, S. D. Knight, G. Waksman, and S. J. Hultgren. 2000. Chaperone-assisted pilus assembly and bacterial attachment. Curr. Opin. Struct. Biol. 10:548-556. [DOI] [PubMed] [Google Scholar]
- 361.Sauer, F. G., K. Futterer, J. S. Pinkner, K. W. Dodson, S. J. Hultgren, and G. Waksman. 1999. Structural basis of chaperone function and pilus biogenesis. Science 285:1058-1061. [DOI] [PubMed] [Google Scholar]
- 362.Sauer, F. G., M. A. Mulvey, J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bacterial pili: molecular mechanisms of pathogenesis. Curr. Opin. Microbiol. 3:65-72. [DOI] [PubMed] [Google Scholar]
- 363.Sauter, S. L., S. M. Rutherfurd, C. Wagener, J. E. Shively, and S. A. Hefta. 1991. Binding of nonspecific cross-reacting antigen, a granulocyte membrane glycoprotein, to Escherichia coli expressing type 1 fimbriae. Infect. Immun. 59:2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Scaletsky, I. C., S. H. Fabbricotti, R. L. Carvalho, C. R. Nunes, H. S. Maranhao, M. B. Morais, and U. Fagundes-Neto. 2002. Diffusely adherent Escherichia coli as a cause of acute diarrhea in young children in Northeast Brazil: a case-control study. J. Clin. Microbiol. 40:645-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Scaletsky, I. C., M. L. Silva, and L. R. Trabulsi. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45:534-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Scaletsky, I. C. A., M. Z. Pedroso, C. A. G. Oliva, R. L. B. Carvalho, M. B. Morais, and U. Fagundes-Neto. 1999. A localized adherence-like pattern as a second pattern of adherence of classic enteropathogenic Escherichia coli to HEp-2 cells that is associated with infantile diarrhea. Infect. Immun. 67:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Scaletsky, I. C. A., M. Z. Pedroso, and R. M. Silva. 1999. Phenotypic and genetic features of Escherichia coli strains showing simultaneous expression of localized and diffuse adherence. FEMS. Immunol. Med. Microbiol. 23:181-188. [DOI] [PubMed] [Google Scholar]
- 368.Schiller, B., P. N. Cunningham, J. J. Alexander, L. Bao, V. M. Holers, and R. J. Quigg. 2001. Expression of a soluble complement inhibitor protects transgenic mice from antibody-induced acute renal failure. J. Am. Soc. Nephrol. 12:71-79. [DOI] [PubMed] [Google Scholar]
- 369.Schilling, J. D., M. A. Mulvey, and S. J. Hultgren. 2001. Dynamic interactions between host and pathogen during acute urinary tract infections. Urology 57:56-61. [DOI] [PubMed] [Google Scholar]
- 370.Schmidt, H., and M. Hensel. 2004. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 17:14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Schmitter, T., F. Agerer, L. Peterson, P. Munzner, and C. R. Hauck. 2004. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J. Exp. Med. 199:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Scholzel, S., W. Zimmermann, G. Schwarzkopf, F. Grunert, B. Rogaczewski, and J. Thompson. 2000. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am. J. Pathol. 156:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Schultsz, C., M. Moussa, R. van Ketel, G. N. Tytgat, and J. Dankert. 1997. Frequency of pathogenic and enteroadherent Escherichia coli in patients with inflammatory bowel disease and controls. J. Clin. Pathol. 50:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Schumann, D., C. J. Chen, B. Kaplan, and J. E. Shively. 2001. Carcinoembryonic antigen cell adhesion molecule 1 directly associates with cytoskeleton proteins actin and tropomyosin. J. Biol. Chem. 276:47421-47433. [DOI] [PubMed] [Google Scholar]
- 375.Screaton, R. A., L. DeMarte, P. Draber, and C. P. Stanners. 2000. The specificity for the differentiation blocking activity of carcinoembryonic antigen resides in its glycophosphatidyl-inositol anchor. J. Cell Biol. 150:613-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Selvarangan, R., P. Goluszko, V. Popov, J. Singhal, T. Pham, D. M. Lublin, S. Nowicki, and B. Nowicki. 2000. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect. Immun. 68:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Selvarangan, R., P. Goluszko, J. Singhal, C. Carnoy, S. Moseley, B. Hudson, S. Nowicki, and B. Nowicki. 2004. Interaction of Dr adhesin with collagen type IV is a critical step in Escherichia coli renal persistence. Infect. Immun. 72:4827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Seveau, S., H. Bierne, S. Giroux, M. C. Prevost, and P. Cossart. 2004. Role of lipid rafts in E-cadherin- and HGF-R/Met-mediated entry of Listeria monocytogenes into host cells. J. Cell Biol. 166:743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Shafren, D. R. 1998. Viral cell entry induced by cross-linked decay-accelerating factor. J. Virol. 72:9407-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay-accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Shafren, D. R., D. J. Dorahy, R. F. Thorne, and R. D. Barry. 2000. Cytoplasmic interactions between decay-accelerating factor and intercellular adhesion molecule-1 are not required for coxsackievirus A21 cell infection. J. Gen. Virol. 81:889-894. [DOI] [PubMed] [Google Scholar]
- 383.Shafren, D. R., D. J. Dorahy, R. F. Thorne, T. Kinoshita, R. D. Barry, and G. F. Burns. 1998. Antibody binding to individual short consensus repeats of decay-accelerating factor enhances enterovirus cell attachment and infectivity. J. Immunol. 160:2318-2323. [PubMed] [Google Scholar]
- 384.Shafren, D. R., D. T. Williams, and R. D. Barry. 1997. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J. Virol. 71:9844-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Shen, W., H. Steinruck, and A. Ljungh. 1995. Expression of binding of plasminogen, thrombospondin, vitronectin, and fibrinogen, and adhesive properties by Escherichia coli strains isolated from patients with colonic diseases. Gut 36:401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Shenoy-Scaria, A. M., L. K. Gauen, J. Kwong, A. S. Shaw, and D. M. Lublin. 1993. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol. Cell. Biol. 13:6385-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Shenoy-Scaria, A. M., J. Kwong, T. Fujita, M. W. Olszowy, A. S. Shaw, and D. M. Lublin. 1992. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J. Immunol. 149:3535-3541. [PubMed] [Google Scholar]
- 388.Shibuya, K., T. Abe, and T. Fujita. 1992. Decay-accelerating factor functions as a signal transducing molecule for human monocytes. J. Immunol. 149:1758-1762. [PubMed] [Google Scholar]
- 389.Shin, J. S., and S. N. Abraham. 2001. Glycosylphosphatidylinositol-anchored receptor-mediated bacterial endocytosis. FEMS Microbiol. Lett. 197:131-138. [DOI] [PubMed] [Google Scholar]
- 390.Shin, J. S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 391.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 392.Singer, B. B., I. Scheffrahn, R. Heymann, K. Sigmundsson, R. Kammerer, and B. Obrink. 2002. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J. Immunol. 168:5139-5146. [DOI] [PubMed] [Google Scholar]
- 393.Reference deleted.
- 394.Singer, B. B., I. Scheffrahn, and B. Obrink. 2000. The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res. 60:1236-1244. [PubMed] [Google Scholar]
- 395.Skubitz, K. M., K. D. Campbell, K. Ahmed, and A. P. Skubitz. 1995. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J. Immunol. 155:5382-5390. [PubMed] [Google Scholar]
- 396.Skubitz, K. M., M. Kuroki, P. Jantscheff, A. P. Skubitz, and F. Grunert. 1999. CD66a. J. Biol. Regul. Homeost. Agents 13:240-241. [PubMed] [Google Scholar]
- 397.Skubitz, K. M., M. Kuroki, P. Jantscheff, A. P. Skubitz, and F. Grunert. 1999. CD66c. J. Biol. Regul. Homeost. Agents 13:244-245. [PubMed] [Google Scholar]
- 398.Skubitz, K. M., M. Kuroki, P. Jantscheff, A. P. Skubitz, and F. Grunert. 1999. CD66e. J. Biol. Regul. Homeost. Agents 13:248-249. [PubMed] [Google Scholar]
- 399.Smart, E. J., G. A. Graf, M. A. McNiven, W. C. Sessa, J. A. Engelman, P. E. Scherer, T. Okamoto, and M. P. Lisanti. 1999. Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19:7289-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Smith, H. R., S. M. Scotland, G. A. Willshaw, B. Rowe, A. Cravioto, and C. Eslava. 1994. Isolates of Escherichia coli O44:H18 of diverse origin are enteroaggregative. J. Infect. Dis. 170:1610-1613. [DOI] [PubMed] [Google Scholar]
- 401.Sogabe, H., M. Nangaku, Y. Ishibashi, T. Wada, T. Fujita, X. Sun, T. Miwa, M. P. Madaio, and W. C. Song. 2001. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J. Immunol. 167:2791-2797. [DOI] [PubMed] [Google Scholar]
- 402.Somerville, C., B. van Denderen, B. Adam, A. Aminian, J. Allison, M. Pearse, and A. d'Apice. 1995. Expression and function of human CD59 and human CD55 in transgenic mice. Transplant. Proc. 27:3565-3566. [PubMed] [Google Scholar]
- 403.Song, W. C. 2004. Membrane complement regulatory proteins in autoimmune and inflammatory tissue injury. Curr. Dir. Autoimmun. 7:181-199. [DOI] [PubMed] [Google Scholar]
- 404.Soto, G. E., and S. J. Hultgren. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sperandio, V., C. C. Li, and J. B. Kaper. 2002. Quorum-sensing Escherichia coli regulator A: a regulator of the LysR family involved in the regulation of the locus of enterocyte effacement pathogenicity island in enterohemorrhagic E. coli. Infect. Immun. 70:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 183:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 410.Spicer, A. P., M. F. Seldin, and S. J. Gendler. 1995. Molecular cloning and chromosomal localization of the mouse decay-accelerating factor genes. Duplicated genes encode glycosylphosphatidylinositol-anchored and transmembrane forms. J. Immunol. 155:3079-3091. [PubMed] [Google Scholar]
- 411.Spiller, O. B., O. Criado-Garcia, S. Rodriguez De Cordoba, and B. P. Morgan. 2000. Cytokine-mediated up-regulation of CD55 and CD59 protects human hepatoma cells from complement attack. Clin. Exp. Immunol. 121:234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Spiller, O. B., I. G. Goodfellow, D. J. Evans, J. W. Almond, and B. P. Morgan. 2000. Echoviruses and coxsackie B viruses that use human decay-accelerating factor (DAF) as a receptor do not bind the rodent analogues of DAF. J. Infect. Dis. 181:340-343. [DOI] [PubMed] [Google Scholar]
- 413.Stapleton, A., S. Moseley, and W. E. Stamm. 1991. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J. Infect. Dis. 163:773-779. [DOI] [PubMed] [Google Scholar]
- 414.Steele-Mortimer, O., S. Meresse, J. P. Gorvel, B. H. Toh, and B. B. Finlay. 1999. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell. Microbiol. 1:33-49. [DOI] [PubMed] [Google Scholar]
- 415.Stefanova, I., and V. Horejsi. 1991. Association of the CD59 and CD55 cell surface glycoproteins with other membrane molecules. J. Immunol. 147:1587-1592. [PubMed] [Google Scholar]
- 416.Reference deleted.
- 417.Stefanova, I., V. Horejsi, I. J. Ansotegui, W. Knapp, and H. Stockinger. 1991. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254:1016-1019. [DOI] [PubMed] [Google Scholar]
- 418.Stocks, S. C., M. A. Kerr, C. Haslett, and I. Dransfield. 1995. CD66-dependent neutrophil activation: a possible mechanism for vascular selectin-mediated regulation of neutrophil adhesion. J. Leukoc. Biol. 58:40-48. [DOI] [PubMed] [Google Scholar]
- 419.Stocks, S. C., M. H. Ruchaud-Sparagano, M. A. Kerr, F. Grunert, C. Haslett, and I. Dransfield. 1996. CD66: role in the regulation of neutrophil effector function. Eur. J. Immunol. 26:2924-2932. [DOI] [PubMed] [Google Scholar]
- 420.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Stuart, A. D., T. A. McKee, P. A. Williams, C. Harley, S. Shen, D. I. Stuart, T. D. Brown, and S. M. Lea. 2002. Determination of the structure of a decay-accelerating factor-binding clinical isolate of echovirus 11 allows mapping of mutants with altered receptor requirements for infection. J. Virol. 76:7694-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Suzuki, M., T. Hisamatsu, and D. K. Podolsky. 2003. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect. Immun. 71:3503-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Swanson, T. N., S. S. Bilge, B. Nowicki, and S. L. Moseley. 1991. Molecular structure of the Dr adhesin: nucleotide sequence and mapping of receptor-binding domain by use of fusion constructs. Infect. Immun. 59:261-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Tacket, C. O., S. L. Moseley, B. Kay, G. Losonsky, and M. M. Levine. 1990. Challenge studies in volunteers using Escherichia coli strains with diffuse adherence to HEp-2 cells. J. Infect. Dis. 162:550-552. [DOI] [PubMed] [Google Scholar]
- 426.Taddei, C. R., A. C. Moreno, A. Fernandes Filho, L. P. Montemor, and M. B. Martinez. 2003. Prevalence of secreted autotransporter toxin gene among diffusely adhering Escherichia coli isolated from stools of children. FEMS Microbiol. Lett. 227:249-253. [DOI] [PubMed] [Google Scholar]
- 427.Takeuchi, K., N. Sato, H. Kasahara, N. Funayama, A. Nagafuchi, S. Yonemura, and S. Tsukita. 1994. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J. Cell Biol. 125:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Tan, K., B. D. Zelus, R. Meijers, J. H. Liu, J. M. Bergelson, N. Duke, R. Zhang, A. Joachimiak, K. V. Holmes, and J. H. Wang. 2002. Crystal structure of murine sCEACAM1a[1,4]: a coronavirus receptor in the CEA family. EMBO J. 21:2076-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Teixeira, A. M., J. Fawcett, D. L. Simmons, and S. M. Watt. 1994. The N-domain of the biliary glycoprotein (BGP) adhesion molecule mediates homotypic binding: domain interactions and epitope analysis of BGPc. Blood 84:211-219. [PubMed] [Google Scholar]
- 430.Thompson, J. A., A. M. Eades-Perner, M. Ditter, W. J. Muller, and W. Zimmermann. 1997. Expression of transgenic carcinoembryonic antigen (CEA) in tumor-prone mice: an animal model for CEA-directed tumor immunotherapy. Int. J. Cancer 72:197-202. [DOI] [PubMed] [Google Scholar]
- 431.Thompson, J. A., F. Grunert, and W. Zimmerman. 1991. Carcinoembrionic antigen family: molecular biology and clinical perspective. J. Clin. Lab Anal. 5:344-366. [DOI] [PubMed] [Google Scholar]
- 432.Thorp, E. B., and T. M. Gallagher. 2004. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 78:2682-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Tieng, V., C. Le Bouguenec, L. du Merle, P. Bertheau, P. Desreumaux, A. Janin, D. Charron, and A. Toubert. 2002. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc. Natl. Acad. Sci. USA 99:2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Tiveljung, A., J. D. Soderholm, G. Olaison, J. Jonasson, and H. J. Monstein. 1999. Presence of eubacteria in biopsies from Crohn's disease inflammatory lesions as determined by 16S rRNA gene-based PCR. J. Med. Microbiol. 48:263-268. [DOI] [PubMed] [Google Scholar]
- 435.Tosello, A. C., F. Mary, M. Amiot, A. Bernard, and D. Mary. 1998. Activation of T cells via CD55: recruitment of early components of the CD3-TCR pathway is required for IL-2 secretion. J. Inflamm. 48:13-27. [PubMed] [Google Scholar]
- 436.Triantafilou, M., K. Miyake, D. T. Golenbock, and K. Triantafilou. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603-2611. [DOI] [PubMed] [Google Scholar]
- 437.Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 23:301-304. [DOI] [PubMed] [Google Scholar]
- 438.Tsai, J. C., B. D. Zelus, K. V. Holmes, and S. R. Weiss. 2003. The N-terminal domain of the murine coronavirus spike glycoprotein determines the CEACAM1 receptor specificity of the virus strain. J. Virol. 77:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Tschugguel, W., C. Schneeberger, G. Unfried, K. Czerwenka, W. Weninger, M. Mildner, J. R. Bishop, and J. C. Huber. 1998. Induction of inducible nitric oxide synthase expression in human secretory endometrium. Hum. Reprod. 13:436-444. [DOI] [PubMed] [Google Scholar]
- 440.Uesu, T., M. Mizuno, H. Inoue, J. Tomoda, and T. Tsuji. 1995. Enhanced expression of decay accelerating factor and CD59/homologous restriction factor 20 on the colonic epithelium of ulcerative colitis. Lab. Investig. 72:587-591. [PubMed] [Google Scholar]
- 441.Usein, C. R., M. Damian, D. Tatu-Chitoiu, C. Capusa, R. Fagaras, D. Tudorache, M. Nica, and C. Le Bouguenec. 2001. Prevalence of virulence genes in Escherichia coli strains isolated from Romanian adult urinary tract infection cases. J. Cell Mol. Med. 5:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Vaisanen-Rhen, V. 1984. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect. Immun. 46:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.van Denderen, B., C. Somerville, M. Pearse, M. Nottle, Z. T. Du, T. Shinkel, and A. d'Apice. 1995. Expression of functional decay-accelerating factor in transgenic mice. Transplant. Proc. 27:3567-3568. [PubMed] [Google Scholar]
- 445.van Denderen, B. J., M. J. Pearse, M. Katerelos, M. B. Nottle, Z. T. Du, A. Aminian, W. R. Adam, A. Shenoy-Scaria, D. M. Lublin, T. A. Shinkel, and A. J. d'Apice. 1996. Expression of functional decay-accelerating factor (CD55) in transgenic mice protects against human complement-mediated attack. Transplantation 61:582-588. [DOI] [PubMed] [Google Scholar]
- 446.van der Goot, F. G., and T. Harder. 2001. Raft membrane domains: from a liquid-ordered membrane phase to a site of pathogen attack. Semin. Immunol. 13:89-97. [DOI] [PubMed] [Google Scholar]
- 447.van Lier, R. A., W. Eichler, and J. Hamann. 1996. Sevenspan transmembrane molecules: novel receptors involved in leukocyte adhesion. Immunol. Lett. 54:185-187. [DOI] [PubMed] [Google Scholar]
- 448.Van Loy, C. P., E. V. Sokurenko, and S. L. Moseley. 2002. The major structural subunits of Dr and F1845 fimbriae are adhesins. Infect. Immun. 70:1694-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Van Loy, C. P., E. V. Sokurenko, R. Samudrala, and S. L. Moseley. 2002. Identification of amino acids in the Dr adhesin required for binding to decay-accelerating factor. Mol. Microbiol. 45:439-452. [DOI] [PubMed] [Google Scholar]
- 450.Virji, M., D. Evans, J. Griffith, D. Hill, L. Serino, A. Hadfield, and S. M. Watt. 2000. Carcinoembryonic antigens are targeted by diverse strains of typable and non-typable Haemophilus influenzae. Mol. Microbiol. 36:784-795. [DOI] [PubMed] [Google Scholar]
- 451.Virji, M., D. Evans, A. Hadfield, F. Grunert, A. M. Teixeira, and S. M. Watt. 1999. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol. Microbiol. 34:538-551. [DOI] [PubMed] [Google Scholar]
- 452.Virji, M., K. Makepeace, D. J. Ferguson, and S. M. Watt. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic Neisseriae. Mol. Microbiol. 22:941-950. [DOI] [PubMed] [Google Scholar]
- 453.Virji, M., S. M. Watt, S. Barker, K. Makepeace, and R. Doyonnas. 1996. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol. Microbiol. 22:929-939. [DOI] [PubMed] [Google Scholar]
- 454.Wachter, C., C. Beinke, M. Mattes, and M. A. Schmidt. 1999. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 31:1695-1707. [DOI] [PubMed] [Google Scholar]
- 455.Walz, W., M. A. Schmidt, A. F. Labigne-Roussel, S. Falkow, and G. Schoolnik. 1985. Afa-I, a cloned afimbrial X-type adhesin from a human pyelonephritic Escherichia coli strain. Purification and chemical, functional and serologic characterization. Eur. J. Biochem. 152:315-321. [DOI] [PubMed] [Google Scholar]
- 456.Wang, J., S. D. Gray-Owen, A. Knorre, T. F. Meyer, and C. Dehio. 1998. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol. Microbiol. 30:657-671. [DOI] [PubMed] [Google Scholar]
- 457.Watt, S. M., J. Fawcett, S. J. Murdoch, A. M. Teixeira, S. E. Gschmeissner, N. M. Hajibagheri, and D. L. Simmons. 1994. CD66 identifies the biliary glycoprotein (BGP) adhesion molecule: cloning, expression, and adhesion functions of the BGPc splice variant. Blood 84:200-210. [PubMed] [Google Scholar]
- 458.Watt, S. M., A. M. Teixeira, G. Q. Zhou, R. Doyonnas, Y. Zhang, F. Grunert, R. S. Blumberg, M. Kuroki, K. M. Skubitz, and P. A. Bates. 2001. Homophilic adhesion of human CEACAM1 involves N-terminal domain interactions: structural analysis of the binding site. Blood 98:1469-1479. [DOI] [PubMed] [Google Scholar]
- 459.Westerlund, B., and T. K. Korhonen. 1993. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9:687-694. [DOI] [PubMed] [Google Scholar]
- 460.Westerlund, B., P. Kuusela, J. Risteli, L. Risteli, T. Vartio, H. Rauvala, R. Virkola, and T. K. Korhonen. 1989. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol. Microbiol. 3:329-337. [DOI] [PubMed] [Google Scholar]
- 461.White, D. J., T. Oglesby, M. K. Liszewski, I. Tedja, D. Hourcade, M. W. Wang, L. Wright, J. Wallwork, and J. P. Atkinson. 1992. Expression of human decay accelerating factor or membrane cofactor protein genes on mouse cells inhibits lysis by human complement. Transplant. Proc. 24:474-476. [PubMed] [Google Scholar]
- 462.Wilkinson, R. W., E. L. Ross, D. Ellison, W. Zimmermann, D. Snary, and S. J. Mather. 2002. Evaluation of a transgenic mouse model for anti-human CEA radioimmunotherapeutics. J. Nucl. Med. 43:1368-1376. [PubMed] [Google Scholar]
- 463.Williams, D. T., Y. Chaudhry, I. G. Goodfellow, S. Lea, and D. J. Evans. 2004. Interactions of decay-accelerating factor (DAF) with haemagglutinating human enteroviruses: utilizing variation in primate DAF to map virus binding sites. J. Gen. Virol. 85:731-738. [DOI] [PubMed] [Google Scholar]
- 464.Williams, P., Y. Chaudhry, I. G. Goodfellow, J. Billington, R. Powell, O. B. Spiller, D. J. Evans, and S. Lea. 2003. Mapping CD55 function. The structure of two pathogen-binding domains at 1.7 A. J. Biol. Chem. 278:10691-10696. [DOI] [PubMed] [Google Scholar]
- 465.Yallampalli, C., Y. L. Dong, P. R. Gangula, and L. Fang. 1998. Role and regulation of nitric oxide in the uterus during pregnancy and parturition. J. Soc. Gynecol. Investig. 5:58-67. [DOI] [PubMed] [Google Scholar]
- 466.Yamamoto, S., A. Terai, K. Yuri, H. Kurazono, Y. Takeda, and O. Yoshida. 1995. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 12:85-90. [DOI] [PubMed] [Google Scholar]
- 467.Yamamoto, S., T. Tsukamoto, A. Terai, H. Kurazono, Y. Takeda, and O. Yoshida. 1997. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J. Urol. 157:1127-1129. [PubMed] [Google Scholar]
- 468.Yonemura, S., and S. Tsukita. 1999. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J. Cell Biol. 145:1497-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Zalewska, B., R. Piatek, H. Cieslinski, B. Nowicki, and J. Kur. 2001. Cloning, expression, and purification of the uropathogenic Escherichia coli invasin DraD. Protein Expr. Purif. 23:476-482. [DOI] [PubMed] [Google Scholar]
- 470.Zhang, L., B. Foxman, P. Tallman, E. Cladera, C. Le Bouguenec, and C. F. Marrs. 1997. Distribution of drb genes coding for Dr binding adhesins among uropathogenic and fecal Escherichia coli isolates and identification of new subtypes. Infect. Immun. 65:2011-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]