Abstract
In females, there is a continuous decline of the ovarian reserve with age, which results in menopause in women or estropause in mice. Loss of ovarian function results in metabolic alterations in mice and women. Based on this, we aimed to evaluate the effect of caloric restriction (CR) on redox status and metabolic changes in chemically induced estropause in mice. For this, mice were divided into four groups (n = 10): cyclic ad libitum (AL), cyclic 30% CR, AL estropause, and estropause 30% CR. Estropause was induced using 4-vinylcyclohexene diepoxide (VCD) for 20 consecutive days in 2-month-old females. The CR protocol started at 5 months of age and the treatments lasted for 4 months. The CR females gained less body weight than AL females (p < 0.001) and had lower glycemic curves in response to glucose tolerance test (GTT). The AL estropause females had the highest body weight and body fat, despite having lower food intake. However, the estropause females on 30% CR lost the most body weight and had the lowest amount of body fat compared to all groups. The effect of 30% CR on redox status in fat and liver tissue was similar for cyclic and estropause females. Interestingly, estropause decreased ROS in adipose tissue, while increasing it in the liver. No significant effects of CR on redox status were observed. Chemically induced estropause did not influence the response to 30% CR on glucose tolerance and redox status; however, weight loss was exarcebated compared to cyclic females.
Keywords: Menopause, ROS, Ovary, Aging
Introduction
Reproductive aging in females is characterized by a continuous and progressive decline of the ovarian reserve and fertility [1–3]. In women, as in other mammalian species, the size of the ovarian reserve is defined at birth, and gradually decreases each cycle, until complete depletion and the beginning of menopause [4, 5]. Mice also experience a progressive decline in the ovarian reserve with aging, along with increasing irregularities in estrous cycles, until cessation of cyclicity, known as estropause [5]. Estrogen levels begin to decrease at menopause in women, as also observed in ovariectomized mice [6, 7]. Ovariectomy-induced menopause in rodents also results in various metabolic alterations, such as increased adiposity, changes in lipid metabolism, hypertension, insulin resistance, and metabolic syndrome, similar to observed in women [6, 7].
Menopause creates a systemic pro-oxidant state in the body due to reduced estrogen levels, which has a well-established antioxidant role [8]. Increased reactive oxygen species (ROS) due to low estrogen levels leads to increased serum concentrations of inflammatory cytokines and pro-oxidant biomarkers [9]. These changes are linked to the development of various pathologies after menopause, such as increased frequency of vasomotor symptoms, increased risk of osteoporosis, and cardiovascular diseases [9]. The natural aging process in males and females also leads to increased inflammation and oxidative stress [9–11]; however, the extra burden of reduced estrogen in menopausal females can further increase susceptibility to a variety of age-related pathologies. Therefore, it is important to understand the role of the ovarian function and aging separately in the redox status.
Among experimental models of menopause in rodents, ovariectomy still is the most commonly used method. Despite being a functional model, it induces menopause in a more abrupt manner, unlike what naturally occurs in humans [12]. Additionally, ovarian tissue is lost, different from the observed during natural menopause. As an alternative, a chemically induced experimental model using 4-vinylcyclohexene diepoxide (VCD) has been used for menopause induction in rodents, aiming to mimic the gradual decline of hormone secretion and contribute to a better understanding of the effects of menopause [12, 13]. VCD is an ovotoxic drug that causes ovarian failure in rodents and has been used as a tool to study the neuroendocrine and behavioral changes resulting from menopause [12, 14]. Its ovotoxic activity accelerates the loss of primary and primordial follicles, resulting in an endocrine state that closely replicates the natural menopausal progression seen in humans [15, 16]. Interestingly, due to the presence of the ovarian tissue and enzymes for androgens synthesis [17], androstenedione levels were identical in VCD and control intact mice, and much higher than for ovariectomized mice [18]. Therefore, it is important to test the effects of different longevity interventions in VCD-induced estropause mouse, which may differ from the responses of ovariectomized mice, and more closely mimick the postmenopausal state.
In this perspective, calorie restriction (CR) is a widely studied dietary intervention to prevent age-associated decline [19–22]. Previous data indicated positive effects of CR on lifespan extension and preservation of the ovarian reserve in different species, associated to a better biochemical profile, reduction of visceral and subcutaneous fat deposition, and increased insulin sensitivity [19, 20]. In addition, CR has antioxidant effects and reduces the pro-inflammatory milieu during aging [21, 22]. However, the potential metabolic and oxidative effects of CR during chemically induced menopause are still poorly explored. Although CR is considered a strategy to increase lifespan [23–25], it is not understood if CR can benefit females who have already reached end of reproductive life. Based on this, we aimed to evaluate the effect of CR on body weight, glucose metabolism, and redox status in liver/adipose tissue of young mice chemically induced to estropause.
Methods
Animals and treatments
This study was approved by the Ethics Committee for Animal Experimentation from Universidade Federal de Pelotas (number 20410-2020). Female C57BL/6 mice 2-months-old were used and kept under controlled conditions of temperature, light, and humidity (22 ± 2 °C, 12-h light/12-h dark cycles, and 40–60% humidity). The chemical estropause induction protocol was initiated at 2 months of age. Females (n = 20) received i.p. injections of VCD (160 mg/kg) diluted in sesame oil for 20 consecutive days [15, 26] or placebo (n = 20) during the same period.
Two months after finishing the VCD treatment, acyclicity of the females was confirmed by vaginal cytology, and the CR protocol was initiated in half of the control group (n = 10) and half of the VCD group (n = 10). CR was gradually implemented, with a 10% reduction in the first week, 20% in the second week, and a 30% reduction compared to the controls, starting from the third week and maintained for 4 months. The CR was calculated based on the average consumption of the previous week in the control groups (cyclic and estropause). Body weight was evaluated every 2 weeks.
At the end of the 4-month CR treatment, mice were fasted for 4 h and euthanized by exsanguination immediately after anesthesia with isoflurane. Mice were dissected and fat, ovaries, and liver tissue collected. The amount of body fat was determined by weighing the fat tissue immediately after euthanasia, relative to body weight.
Vaginal cytology
Vaginal cytology was performed during seven consecutive days to determine if females were acyclic, according to previous protocol [27]. For sample collection, a micropipette containing 10 μL of saline was inserted into the vaginal canal. The fluid obtained was spread on a slide and stained using rapid panoptic. The slides were observed under a light microscope to determine the stage of the estrous cycle [27, 28]. Mice were considered acyclic if continuously in the diestrus stage.
Insulin tolerance test and glucose tolerance test
The insulin tolerance test (ITT) and glucose tolerance test (GTT) were performed 2 and 1 week prior to euthanasia, respectively. For the ITT, insulin (0.5 IU/kg) was injected i.p. after a 2-h fast. Blood samples to measure glucose levels were collected through a small incision at the tail tip at 0, 5, 20, and 35 min after insulin injection [29]. For the GTT, glucose (2 g/kg) was administered i.p. after a 4-h fast. Blood samples were collected at 0, 15, 30, 60, and 120 min after glucose injection [30]. A glucometer (AccuChek Activ, Roche Diagnostics®, USA) was used to measure blood glucose levels in both tests.
Follicle counting
The ovaries were removed from 10% buffered formalin, dehydrated in alcohol, cleared in xylene, embedded in paraffin, and then cut into 5 μm sections using a semiautomatic microtome (RM2245 38, Leica Biosystems Newcastle Ltd., Newcastle Upon Tyne, UK). One out of every six sections was selected and placed on standard histological slide. After drying in a 56 °C oven for 24 h, the slides were stained with hematoxylin and eosin and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, MO, USA). Images of the ovarian sections were captured using microscope (Nikon Eclipse E200, Nikon Corporation, Japan) with 4×, 10×, and 40× objectives. Follicles with clearly visible oocyte nuclei were quantified. The number of follicles counted was divided by the total number of sections for each ovary. The follicle classification protocol was based on Myers et al. [31].
Oxidative stress parameters
The liver tissue was homogenized (1/10 w/v) using a 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl. The adipose tissue was homogenized (1/10 w/v) using RIPA buffer, pH 7.5, containing 50 mM Tris-HCl, 150 mM NaCl, and 150 mM EDTA. The homogenates were centrifuged at 3500 × g using a centrifuge at 4 °C for 10 min and supernatants were collected and used for oxidative stress analysis.
Reactive oxygen species
The quantification of ROS levels was determined by the oxidation of DCFH-DA to fluorescent 2′,7′-dichlorofluorescein (DCF). Briefly, the DCF fluorescence intensity was measured at excitation wavelengths of 525 nm and 488 nm, 30 min after the addition of DCFH-DA to the homogenate. ROS formation was expressed as μmol DCF/mg of protein [32].
Total thiol content
The total thiol content was determined by the reduction of DTNB by thiols and the subsequent oxidation (disulfide) to form a yellow-colored derivative compound (TNB), which was measured at 412 nm using a spectrophotometer. The results were expressed as nmol TNB/mg of protein [33].
Thiobarbituric acid reactive substances (TBARS)
Lipid peroxidation was determined by estimating the formation of malonaldehyde (MDA). TBA and trichloroacetic acid were added to the supernatant for the reaction. TBARS levels were quantified by measuring the absorbance at 535 nm, and reported as nmol TBARS/mg of protein [34].
Nitrites
The nitrite was measured with sulfanilamide and N-1-naphthylethylenediamine dihydrochloride (NED), using the Griess reaction. The absorbance was measured at 540 nm. The results were expressed as μM nitrite/mg of protein [35].
Catalase (CAT) activity
The catalase activity was measured according to Aebi [36]. This assay is based on the decomposition of H2O2 at 240 nm. CAT activity was reported as units/mg of protein.
Superoxide dismutase (SOD) activity
Superoxide dismutase activity was measured based on the inhibition of adrenaline auto-oxidation at 480 nm. Total SOD activity is expressed as units/mg of protein [37].
Protein determination
For SOD, CAT, TBARS, and total thiol content, protein was measured using the method described by Lowry et al. [38], and for ROS and nitrite, protein was determined according to Bradford [39].
Statistical analyses
Statistical analyses were performed using GraphPad Prism 8.4. Repeated measures ANOVA was used for the analysis of body weight, ITT, and GTT. Two-way ANOVA was used for the analysis of other variables (body weight gain, follicle count, glucose, and oxidative stress parameters). P values lower or equal than 0.05 were considered statistically significant.
Results
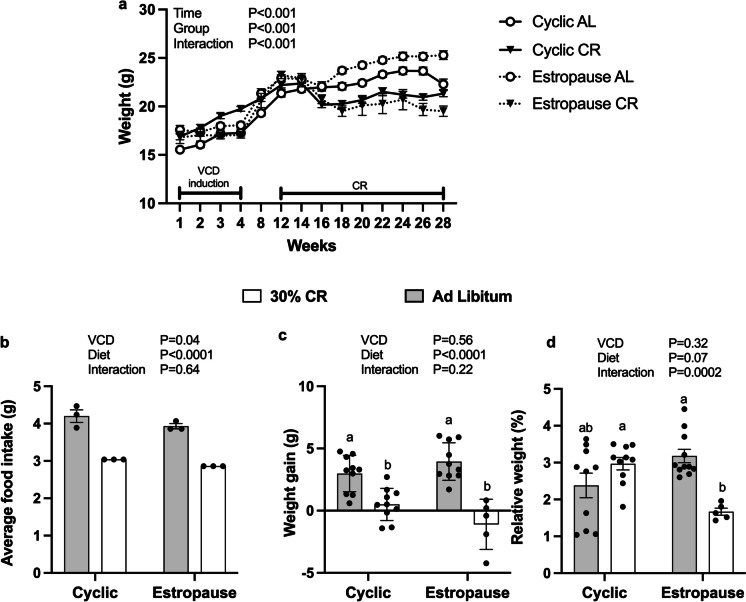
The AL estropause females had the highest body weight. In general, the CR groups had lower body weight compared to AL groups, and CR estropause females had the lowest body weight (Fig. 1a). However, when analyzing body weight gain only after the start of CR, no significant difference was found between the cyclic and estropause groups. A clear reduction in body weight gain was evident in both groups subjected to CR, as expected (Fig. 1b). Despite increased body weight gain, the estropause females had lower food intake than cyclic controls (Fig. 1c). Additionally, the CR estropause females had the lowest percentage of body fat (Fig. 1d), in agreement with the more severe reduction in body weight in this group.
Fig. 1.
a Body weight of females from the start of VCD injection to the end of the experimental period; b body weight gain during the period of calorie restriction from 5 to 9 months of age. c Food intake/mice/day during the period of calorie restriction from 5 to 9 months of age. d Relative amount of body fat at the time of euthanasia
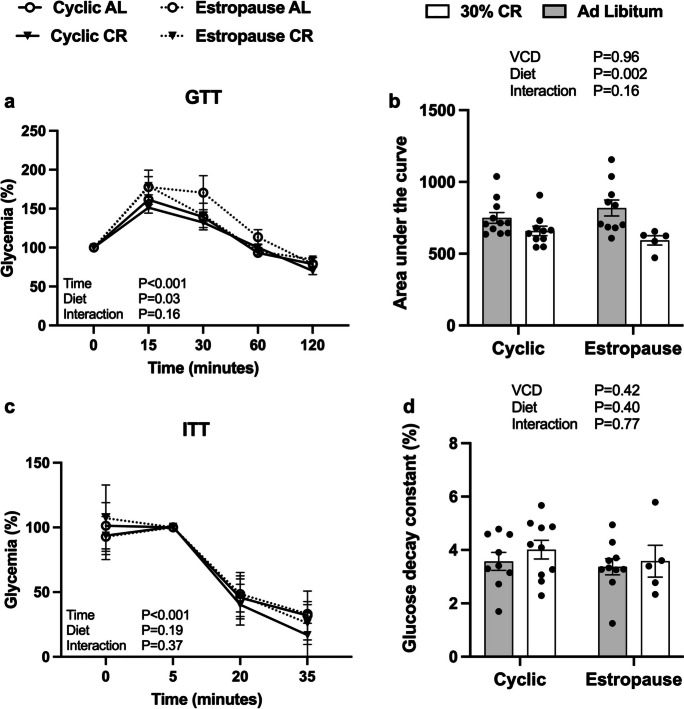
In the GTT, AL estropause females had a higher glycemic index compared to the other groups (Fig. 2a). Overall, the glycemic curves of CR females were lower than AL females as expected (Fig. 2b). There was no difference in insulin tolerance (Fig. 2c) and the rate of glucose decay in response to insulin (Fig. 2d).
Fig. 2.
a Glucose levels during the glucose tolerance test (GTT); b area under the curve (AUC) for the GTT; c glucose levels during the insulin tolerance test (ITT); d glucose decay constant (KITT) during the first 15 min of the ITT for the AL cyclic, 30% CR cyclic, AL estropause and 30% CR estropause
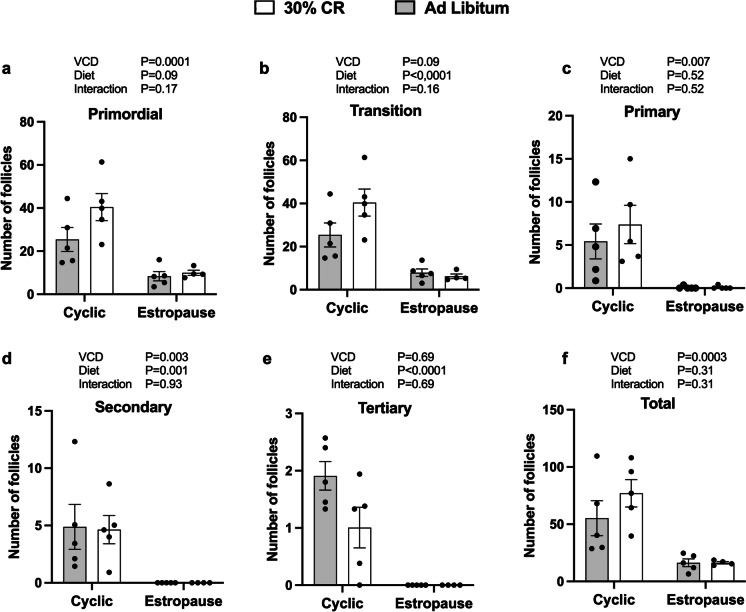
Regarding ovarian activity, we observed a reduction in the number of follicles at all stages of development in estropause females compared with cyclic controls (Fig. 3a–f). This supports the effectiveness of estropause induction with VCD in these groups. Additionally, all VCD injected females were acyclic before the start of CR, as confirmed by vaginal citology. CR cyclic female tended to have more primordial and transition follicles (Fig. 3a and b).
Fig. 3.
Number of follicles a primordial, b transition, c primary, d secondary, e tertiary, and f total, relative to the ovarian area
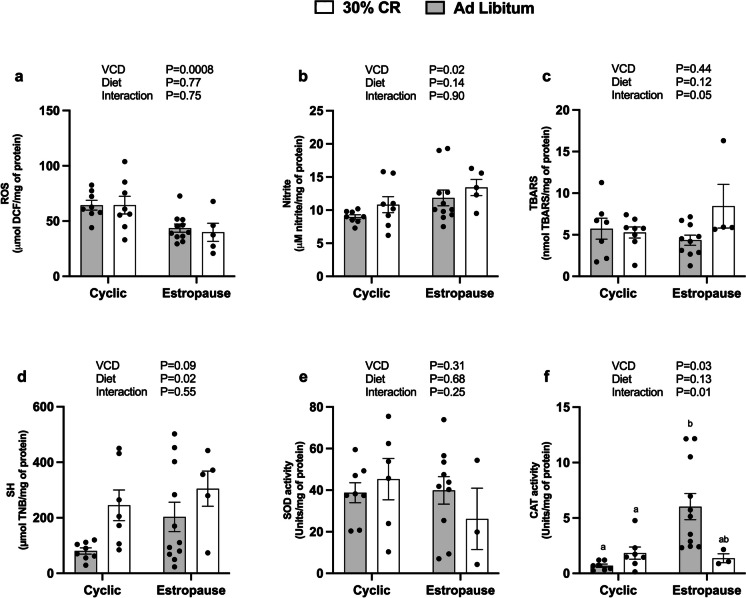
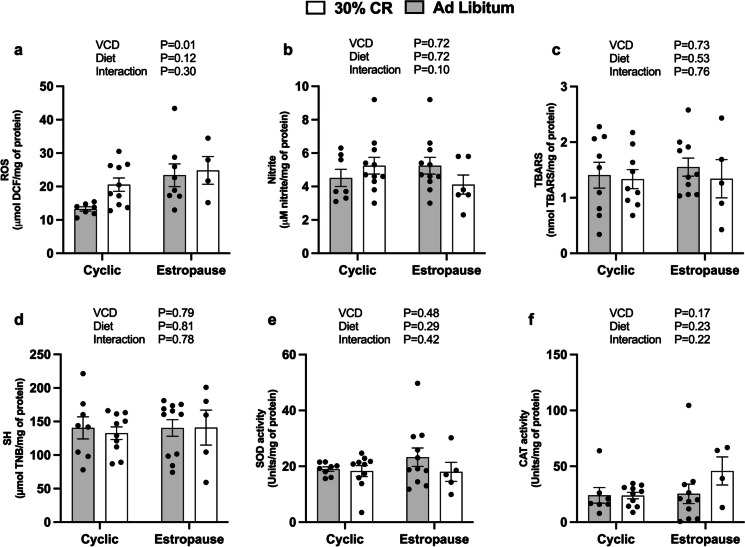
Regarding oxidative stress parameters, we observed that estropause females had a decrease in ROS levels (Fig. 4a) and an increase in nitrite levels (Fig. 4b) in adipose tissue. Females subjected to CR had an increase in sulfhydryl levels in adipose tissue (Fig. 4d). Interestingly, CR estropause females had reduction in CAT activity in adipose tissue compared to AL estropause females (Fig. 4f). There were no differences in TBARS levels (Fig. 4c) or SOD activity in adipose tissue (Fig. 4e). In the liver, only a slight increase in ROS levels was observed for estropause females (Fig. 5a). No differences were found for nitrite (Fig. 5b), TBARS (Fig. 5c), sulfhydryl (Fig. 5d), SOD (Fig. 5e), and CAT activity (Fig. 5f).
Fig. 4.
Redox status in adipose tissue. a Reactive oxygen species (ROS); b nitrites. c Thiobarbituric acid reactive substances (TBARS); d sulfhydryls (SH); e superoxide dismutase (SOD) activity; f catalase (CAT) activity for the AL cyclic, 30% CR cyclic, AL estropause and 30% CR estropause
Fig. 5.
Redox status in the liver. a Reactive oxygen species (ROS); b nitrites. c Thiobarbituric acid reactive substances (TBARS); d sulfhydryls (SH); e superoxide dismutase (SOD) activity; f catalase (CAT) activity for the AL cyclic, 30% CR cyclic, AL estropause and 30% CR estropause
Discussion
We observed that VCD induced an estropause state in females, as evidenced by the severe reduction in preantral follicles and absence of tertiary follicles. Previous studies in rodents using VCD had shown depletion of small preantral follicles [12, 40, 41]. The VCD-induced follicular depletion in rodents is due to accelerated atresia of primordial and primary follicles [12, 41, 42]. We also observed through vaginal cytology that the estropause state was established 2 months after the end of VCD treatment, when females exhibited a pattern of acyclicity and remained in diestrus. This is in line with previous reports suggesting estropause initiates between 2 and 3 months after the end of VCD injections [12, 15, 41]. Therefore, the CR intervention was started only after estropause was fully established.
Females in estropause had increased body weight gain over time compared to cyclic females. This increased body weight during estropause is consistent with previous studies in mice receiving standard chow [14, 43, 44]. Previous studies also observed increased body weight gain in ovariectomized mice [43]. Despite increased body weight gain, females in estropause had lower food intake compared to cyclic females. It was previously observed that loss of ovarian function led to decreased 24-h energy expenditure without changes in the energy intake pattern [43]. Therefore, our data furter reinforces that loss of ovarian activity can indeed reduce energy expenditure, since even with lower food intake, estropause females were heavier than their cyclic controls. This suggests metabolic adaptations that could hinder the response to diets such as CR, hence our hypothesis for this study.
Our results revealed a more intense weight loss in estropause compared to cyclic females. Levels of 25 to 40% of CR have been pointed out to increase longevity in several species [23, 24]. Although the mechanisms related to lifespan extension mediated by CR are not fully elucidated, there is a strong relationship with changes in energy metabolism, oxidative stress, insulin sensitivity, and inflammation [45, 46]. The effects of CR seem to be also partly mediated by increased cellular repair, stress resistance, and protection from oxidative damage [24]. However, there is no data regarding the effects of CR in chemically induced estropause in rodents, only ovariectomy. An intense CR (50%) in rats starting at weaning mitigated the effects of ovariectomy in body weight gain [19]. However, ovariectomization occurred at 90 days of age, after the beginning of CR, suggesting that CR has beneficial effects when initiated before ovariectomy. This is unlike our study, where CR initiated after the establishment of the estropause state. In another study CR was initiated after ovariectomization in rats. As expected, ovariectomized rats exhibited the highest body weight, while 50% CR reduced body weight compared to ovariectomized controls [47]. Ovariectomized females display increased rate of body weight change compared with intact females [48], which seems to be due to increased feed efficiency, as rats gain weight even without an increase in food intake [49]. This is similar to our observations, and raises an interesting question regarding the effects of CR on estropause females, as it had the most pronounced reduction in body weight compared to its AL estroupause couterparts. Despite increase feed efficiency, these females have increase response to CR, suggesting a different metabolic adaptation to food shortage and fasting promoted by this strategy. Further studies are needed to clarify these questions and point out a potential role of ovarian tissue in aging, independent of cyclicity.
Estroupause females showed the most significant reduction in body fat in response to CR. A previous study observed that both VCD-treated and cyclic mice receiving a high-fat diet exhibited a significant increase in body fat compared to mice on a standard diet [14]. However, estropause females accumulated less body fat than cyclic females on a high-fat diet [14]. This is similar to our current data, which shows that estropause females did not had increased body fat. Nevertheless, others observed that ovariectomized females had a higher volume of both subcutaneous and visceral fat compared to intact females [50]. Transdermal estradiol replacement was able to reduce both subcutaneous and visceral body fat in ovariectomized females [50], as was the transplantation of young ovarian tissue in naturally estropausal females, even without increased estradiol levels [51]. Therefore, further studies are needed to confirm the role of CR on fat accumulation in response to reduced estradiol levels in the absensce or presence of the ovarian tissue.
The glucose tolerance tests indicated lower tolerance in the AL estropause females. Similarly, reduced glucose tolerance was observed in VCD-treated mice on a standard diet [21]. This lower glucose tolerance may be associated with the reduction of estrogen levels in menopause, both in rodents and humans [14, 51], and the protective effect of estrogen replacement in postmenopause women [14, 52–54]. On the other hand, CR females had higher glucose tolerance regardless of estropause. This is consistent with previous studies suggesting improved glucose tolerance and insulin sensitivity in ovariectomized females undergoing 40% CR for 30 days [55]. It is worth noting that insulin sensitivity appears to decrease with age in mice, as shown by several studies. However, as our study used young mice to minimize the confouding effects of age, this may differ from other CR studies using much older mice. Older estropausal-aged mice that received young ovarian tissue transplants displayed improved glucose tolerance compared with age-matched controls [56], further confirming the role of young ovaries in glucose tolerance. It is possible that age may have a more significant effect than cyclicity in females regarding insulin sensitivity.
Oxidative stress is associated with the aging process and age-related diseases such as cancer [57, 58], neurodegeneration [59, 60], cardiovascular diseases [61, 62], and diabetes [63], and CR is considered a protective factor against oxidative stress-related diseases [55]. Despite this, we observed that CR had very minor effects in liver and adipose tissue redox status. We even observed that CR increased sulfhydryl levels and reduced CAT activity in adipose tissue of estropausal females, indicating a negative effect on the redox balance. This is surprising, as studies indicate that redox status in adipose tissue is related to the presence of excess adipose tissue, which is a source of pro-inflammatory cytokines and chronic inflammation [63, 64]. No effects of CR on liver redox status were observed. However, estropause overall reduced ROS levels and increase nitrite in adipose tissue. In the liver, estropause slightly increased ROS levels. Ovariectomized rats have lower CAT activity than their intact couterparts in adipose tissue, suggesting reduced antioxidant defense [65]. This is in contrast with our findings, since we observed a reduction in ROS levels in adipose tissue of estropause females. However, we did observe increased nitrite levels and a tendency for increased sulfidryl levels in estropause mice, suggesting impaired redox state in this tissue. However, in our study, females retained ovarian tissue, which closely associated to what is observed in women than ovariectomy. The presence of the ovarian tissue, even depleted of follicles, may have beneficial effects on redox status [66]. In the liver, we observed only a modest increase in ROS levels in estropause females. Increased ROS production is associated with aging and the estrogen reduction resulting from menopause can further reduce antioxidant defenses [9]. Others also indicate a reduction in liver antioxidant defenses after estropause in mice, suggesting an effect more related to reproductive function than age itself [66]. In line with these findings, another study did not observe changes in TBARS and CAT levels in liver of young ovariectomized rats [67]. Reduction in TBARS content was observed in the liver of ovariectomized mice under 40% CR, suggesting a reduction in oxidative stress [49]. It is noteworthy that we observed opposite behavior regarding ROS levels between adipose and hepatic tissue in response to estropause, suggesting tissue-specific adapatations.
Although some studies suggest that menopause creates a pro-oxidant systemic state due to decreased estrogen production, we did not observe that chemically induced estropause potentiated oxidative damage in liver and adipose tissue significantly. We did not observe that CR had different effects on cyclic or estropausal females, except for greater weight loss and reduction in body fat. Glucose tolerance was impaired in estropausal females but was not differentially affected by CR. There is a clear need for further investigation into the effects of CR and other longevity-promoting interventions in estropause-induced female mice retaining the ovarian tissue.
Funding
Research reported in this publication was supported by CAPES, CNPq, and FAPERGS.
Data availability
The data presented in the work are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;145(1-2):67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 2.Neves MM, AD MARQUESJR. Senescência reprodutiva feminina em mamíferos. Rev Bras Reprod Anim. 2008;32(2):133–140. [Google Scholar]
- 3.Cavalcante MB, Sampaio OGM, Câmara FEA, Schneider A, de Ávila BM, Prosczek J, Masternak MM, Campos AR. Ovarian aging in humans: potential strategies for extending reproductive lifespan. Geroscience. 2023; 10.1007/s11357-023-00768-8. [DOI] [PMC free article] [PubMed]
- 4.Ferreira VN, Chinelato RS, Castro MR, Ferreira ME. Menopausa: marco biopsicossocial do envelhecimento feminino. Psicol Soc. 2013;25(2):410–419. doi: 10.1590/S0102-71822013000200018. [DOI] [Google Scholar]
- 5.Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol. 2014;142:132–141. doi: 10.1016/j.jsbmb.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Medeiros SF, Maitelli A, Nince APB. Efeitos da terapia hormonal na menopausa sobre o sistema imune. Rev Bras Ginecol Obstet. 2007;29(11):593–601. doi: 10.1590/S0100-72032007001100008. [DOI] [Google Scholar]
- 7.Bitto A, Altavilla D, Bonaiuto A, Polito F, Minutoli L, Di Stefano V, Giuliani D, Guarini S, Arcoraci V, Squadrito F. Effects of aglycone genistein in a rat experimental model of postmenopausal metabolic syndrome. J Endocrinol. 2009;200(3):367–376. doi: 10.1677/JOE-08-0206. [DOI] [PubMed] [Google Scholar]
- 8.Bourgonje AR, Abdulle AE, Al-Rawas AM, Al-Maqbali M, Al-Saleh M, Enriquez MB, Al-Siyabi S, Al-Hashmi K, Al-Lawati I, Bulthuis MLC, Mulder DJ, Gordijn SJ, van Goor H, Saleh J. Systemic oxidative stress is increased in postmenopausal women and independently associates with homocysteine levels. Int J Mol Sci. 2020;21(1):314. doi: 10.3390/ijms21010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi SB, Agarwal A. The role of oxidative stress in menopause. J Midlife Health. 2013;4(3):140–146. doi: 10.4103/0976-7800.118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Signorelli SS, Neri S, Sciacchitano S, Pino LD, Costa MP, Marchese G, Celotta G, Cassibba N, Pennisi G, Caschetto S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53(1):77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 11.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7(4):134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Perez JN, Constantopoulos E, McKee L, Regan J, Hoyer PB, Brooks HL, Konhilas J. A method to study the impact of chemically-induced ovarian failure on exercise capacity and cardiac adaptation in mice. J Vis Exp. 2014;86:51083. doi: 10.3791/51083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das M, Ellies LG, Kumar D, Sauceda C, Oberg A, Gross E, Mandt T, Newton IG, Kaur M, Sears DD, Webster NJG. Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nat Commun. 2021;12(1):565. doi: 10.1038/s41467-020-20743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Aleshire MJ, Diamond-Stanic MK, Hasty AH, Hoyer PB, Brooks HL. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R587–R592. doi: 10.1152/ajpregu.90762.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda). 2016;31(4):250–257. doi: 10.1152/physiol.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–187. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera Z, Christian PJ, Marion SL, Brooks HL, Hoyer PB. Steroidogenic capacity of residual ovarian tissue in 4-vinylcyclohexene diepoxide-treated mice. Biol Reprod. 2009;80(2):328–336. doi: 10.1095/biolreprod.108.070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150(9):4248–4259. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues CM, Domingues TE, de Sousa SC, Costa-Pereira LV, Mendes BF, Dos Santos JM, Costa KB, Silva G, Cantuária VL, Rocha-Vieira E, Dias-Peixoto MF, Honorato-Sampaio K. Cardioprotective effects of severe calorie restriction from birth in adult ovariectomized rats. Life Sci. 2021;275:119411. doi: 10.1016/j.lfs.2021.119411. [DOI] [PubMed] [Google Scholar]
- 20.Normandin E, Sénéchal M, Prud’homme D, Rabasa-Lhoret R, Brochu M. Effects of caloric restriction with or without resistance training in dynapenic-overweight and obese menopausal women: a MONET study. J Frailty Aging. 2015;4(3):155–162. doi: 10.14283/jfa.2015.54. [DOI] [PubMed] [Google Scholar]
- 21.Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy. Cell Signal. 2009;21(9):1356–1360. doi: 10.1016/j.cellsig.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Silva WJM da, Ferrari CKB. Metabolismo mitocondrial, radicais livres e envelhecimento. Rev Bras Geriatr Gerontol. 2011;14(3):441–451. 10.1590/S1809-98232011000300005
- 23.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 24.de Genaro PS, Sarkis KS, Martini LA. O efeito da restrição calórica na longevidade. Arq Bras Endocrinol Metab. 2009;53(5):667–672. doi: 10.1590/S0004-27302009000500019. [DOI] [PubMed] [Google Scholar]
- 25.de Magalhães JP. Open-minded scepticism: inferring the causal mechanisms of human ageing from genetic perturbations. Ageing Res Rev. 2005;4(1):1–22. doi: 10.1016/j.arr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp Med. 2005;55(6):523–527. [PubMed] [Google Scholar]
- 27.McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012;67:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koebele SV, Bimonte-Nelson HA. The endocrine-brain-aging triad where many paths meet: female reproductive hormone changes at midlife and their influence on circuits important for learning and memory. Exp Gerontol. 2017;94:14–23. doi: 10.1016/j.exger.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, Wang F, Javors MA, Chen J, Sun LY, Bartke A. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17(3):456–462. doi: 10.1016/j.cmet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39(1):51–59. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 32.Ali SF, LeBel CP, Bondy SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13(3):637–648. [PubMed] [Google Scholar]
- 33.Aksenov MY, Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett. 2001;302(2-3):141–145. doi: 10.1016/s0304-3940(01)01636-6. [DOI] [PubMed] [Google Scholar]
- 34.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 35.Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 37.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- 38.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 39.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 40.Kao SW, Sipes IG, Hoyer PB. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 1999;13(1):67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 41.Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16(6):775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 42.Borman SM, VanDePol BJ, Kao S, Thompson KE, Sipes IG, Hoyer PB. A single dose of the ovotoxicant 4-vinylcyclohexene diepoxide is protective in rat primary ovarian follicles. Toxicol Appl Pharmacol. 1999;158(3):244–252. doi: 10.1006/taap.1999.8702. [DOI] [PubMed] [Google Scholar]
- 43.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vasconcellos LS, Sabino KR, Petroianu A. Relação entre ooforectomia e peso em modelo experimental. Rev Col Bras Cir. 2005;32(3):132–135. doi: 10.1590/S0100-69912005000300006. [DOI] [Google Scholar]
- 45.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14(2):275–287. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia DN, Saccon TD, Pradiee J, Rincón JAA, Andrade KRS, Rovani MT, Mondadori RG, Cruz LAX, Barros CC, Masternak MM, Bartke A, Mason JB, Schneider A. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience. 2019;41(4):395–408. doi: 10.1007/s11357-019-00087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pósa A, Szabó R, Kupai K, Csonka A, Szalai Z, Veszelka M, Török S, Daruka L, Varga C. Exercise training and calorie restriction influence the metabolic parameters in ovariectomized female rats. Oxid Med Cell Longev. 2015;2015:787063. doi: 10.1155/2015/787063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason JB, Cargill SL, Anderson GB, Carey JR. Ovarian status influenced the rate of body-weight change but not the total amount of body-weight gained or lost in female CBA/J mice. Exp Gerontol. 2010;45(6):435–441. doi: 10.1016/j.exger.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boldarine VT, Pedroso AP, Brandão-Teles C, LoTurco EG, Nascimento CMO, Oyama LM, Bueno AA, Martins-de-Souza D, Ribeiro EB. Ovariectomy modifies lipid metabolism of retroperitoneal white fat in rats: a proteomic approach. Am J Physiol Endocrinol Metab. 2020;319(2):E427–E437. doi: 10.1152/ajpendo.00094.2020. [DOI] [PubMed] [Google Scholar]
- 50.Nishio E, Hayashi T, Nakatani M, Aida N, Suda R, Fujii T, Wakatsuki T, Honda S, Harada N, Shimono Y. Lack of association of ovariectomy-induced obesity with overeating and the reduction of physical activities. Biochem Biophys Rep. 2019;20:100671. doi: 10.1016/j.bbrep.2019.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Habermehl TL, Mason JB. Decreased sarcopenia in aged females with young ovary transplants was preserved in mice that received germ cell-depleted young ovaries. J Clin Med. 2019;8(1):40. doi: 10.3390/jcm8010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J. Howard BV; Women’s Health Initiative Investigators. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 53.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E, Heart and Estrogen/Progestin Replacement Study Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 54.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 55.Sapatini LRL, Calsa B, Marim LJ, Helaehil JV, Chiarotto GB, do Corezola Amaral ME. Caloric restriction prevents inflammation and insulin dysfunction in middle-aged ovariectomized mice. Mol Biol Rep. 2023;50(7):5675–5685. doi: 10.1007/s11033-023-08508-z. [DOI] [PubMed] [Google Scholar]
- 56.Tyler KA, Habermehl TL, Mason JB. Manipulation of ovarian function influenced glucose metabolism in CBA/J mice. Exp Gerontol. 2019;126:110686. doi: 10.1016/j.exger.2019.110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longo VD, Anderson RM. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. 2022;185(9):1455–1470. doi: 10.1016/j.cell.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer AK, Jensen MD. Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Invest. 2022;132(16):e158451. doi: 10.1172/JCI158451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Souza Nunes Faria MS, Pimentel VE, Helaehil JV, Bertolo MC, NTH S, da Silva-Neto PV, Thomazini BF, de Oliveira CA, do Amaral MEV. Caloric restriction overcomes pre-diabetes and hypertension induced by a high fat diet and renal artery stenosis. Mol Biol Rep. 2022;49(7):5883–5895. doi: 10.1007/s11033-022-07370-9. [DOI] [PubMed] [Google Scholar]
- 60.Wu QJ, Zhang TN, Chen HH, Yu XF, Lv JL, Liu YY, Liu YS, Zheng G, Zhao JQ, Wei YF, Guo JY, Liu FH, Chang Q, Zhang YX, Liu CG, Zhao YH. The sirtuin family in health and disease. Signal Transduct Target Ther. 2022;7(1):402. doi: 10.1038/s41392-022-01257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JY, Mondaca-Ruff D, Singh S, Wang Y. SIRT1 and autophagy: implications in endocrine disorders. Front Endocrinol (Lausanne). 2022;13:930919. doi: 10.3389/fendo.2022.930919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tozzi R, Cipriani F, Masi D, Basciani S, Watanabe M, Lubrano C, Gnessi L, Mariani S. Ketone bodies and SIRT1, synergic epigenetic regulators for metabolic health: a narrative review. Nutrients. 2022;14(15):3145. doi: 10.3390/nu14153145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh ME, Shi Y, Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med. 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lissarassa YPS, Vincensi CF, Costa-Beber LC, Dos Santos AB, Goettems-Fiorin PB, Dos Santos JB, Donato YH, Wildner G, de Bittencourt H, Júnior PI, Frizzo MN, Heck TG, Ludwig MS. Chronic heat treatment positively impacts metabolic profile of ovariectomized rats: association with heat shock response pathways. Cell Stress Chaperones. 2020;25(3):467–479. doi: 10.1007/s12192-020-01087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.King TL, Underwood KB, Hansen KK, Kinter MT, Schneider A, Masternak MM, Mason JB. Chronological and reproductive aging-associated changes in resistance to oxidative stress in post-reproductive female mice. Geroscience. 2023; 10.1007/s11357-023-00865-8. [DOI] [PMC free article] [PubMed]
- 67.Vuković R, Blažetić S, Oršolić I, Heffer M, Vari SG, Gajdoš M, Krivošíková Z, Kramárová P, Kebis A, Has-Schön E. Impact of ovariectomy, high fat diet, and lifestyle modifications on oxidative/antioxidative status in the rat liver. Croat Med J. 2014;55(3):218–227. doi: 10.3325/cmj.2014.55.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in the work are available from the corresponding author upon request.