Abstract
We report here that HtrA plays a role in controlling growth and competence development for genetic transformation in Streptococcus mutans. Disruption of the gene for HtrA resulted in slow growth at 37°C, reduced thermal tolerance at 42°C, and altered sucrose-dependent biofilm formation on polystyrene surfaces. The htrA mutant also displayed a significantly reduced ability to undergo genetic transformation. A direct association between HtrA and genetic competence was demonstrated by the increased expression of the htrA gene upon exposure to competence-stimulating peptide. The induction of htrA gradually reached a maximum at around 20 min, suggesting that HtrA may be involved in a late competence response. Complementation of the htrA mutation in a single copy on the chromosome of the mutant could rescue the defective growth phenotypes but not transformability, apparently because a second gene, spo0J, immediately downstream of htrA, also affects transformation. The htrA and spo0J genes were shown to be both individually transcribed and cotranscribed and probably have a functional connection in competence development. HtrA regulation appears to be finely tuned in S. mutans, since strains containing multiple copies of htrA exhibited abnormal growth phenotypes. Collectively, the results reveal HtrA to be an integral component of the regulatory network connecting cellular growth, stress tolerance, biofilm formation, and competence development and reveal a novel role for the spo0J gene in genetic transformation.
Prokaryotes are equipped with a variety of stress response mechanisms that enable them to survive under adverse environmental conditions, including a group of highly conserved proteins that help the cell to cope with the increased levels of the misfolded proteins arising from exposure to stressors. Proteins with abnormal conformations are either refolded or degraded by a sophisticated system of molecular chaperones and proteases. HtrA (high-temperature requirement A), also termed DegP or DO protease (41), is of particular interest because it can function as both a protease and a chaperone. Many of these functions are central to physiologic homeostasis, including growth, stress tolerance, transport and secretion, signal transduction, and modification of the behavior of the organisms to adapt to environmental stresses (13, 14, 48). HtrA is a heat-shock-induced, surface-associated serine protease that was first identified in Escherichia coli (26), and homologues of HtrA have been identified in a wide range of bacteria, as well as in some Eukarya, including yeast, plants, and humans (34). HtrA-like proteins characteristically possess an amino-terminal hydrophobic region, a catalytic domain containing a triad of His, Ser, and Asp residues conserved in trypsin-like serine proteases, and a PDZ domain thought to be involved in the formation of a multimeric enzyme complex (34, 38). Although many bacteria have more than one copy of HtrA (34), gram-positive cocci, including Streptococcus mutans, Streptococcus pneumoniae, and Streptococcus pyogenes, have a single htrA gene. HtrA in these organisms contains a single PDZ domain in the carboxyl-terminal portion of the protein, whereas most gram-negative proteins have two PDZ domains (34). In all cases, a primary role for HtrA is to help organisms to survive environmental insults, mainly, elevated temperature and oxidative stress (34). Interestingly, there is a wide variation in the phenotypes of htrA mutants of different bacteria. For example, htrA mutants of Salmonella enterica serovar Typhimurium, Brucella abortus, Lactococcus lactis, and S. pyogenes are sensitive to both temperature and oxidative stresses, whereas Escherichia coli and Yersinia pestis htrA mutants show sensitivity to temperature increases but not to oxidative stresses (10, 20, 26, 44, 46). The contribution of HtrA to resistance to thermal and oxidative stress appears to differ even within species. In the case of S. pneumoniae, an htrA mutant of strain D39 exhibited sensitivity to both stresses (18), but that of strain TIGR4 did not (40). The HtrA protein has also been identified as a virulence factor for several pathogenic bacteria, such as S. enterica serovar Typhimurium, Yersinia enterocolitica, B. abortus, S. pyogenes, and S. pneumoniae. In all cases, htrA null mutants were attenuated for virulence in vivo, although the specific contribution that HtrA makes to virulence is not clear. More recently, HtrA was reported to be involved in the development of competence for genetic transformation in S. pneumoniae (35, 39).
S. mutans, considered to be one of the principle causative agents of dental caries and a leading cause of infective endocarditis, depends on a biofilm lifestyle. S. mutans is known to have developed a variety of mechanisms to colonize the tooth surfaces and to tolerate the various stresses experienced during the development of dental caries, such as large fluctuations in carbohydrate availability and pH. The increase in the proportions of S. mutans in a cariogenic flora in dental plaque and the factors that make these organisms more competitive under conditions conducive to caries development hinges on the ability of these organisms to respond rapidly and efficiently to environmental fluxes. Here, we characterize the htrA-spo0J gene cluster of S. mutans. We show that HtrA plays a central role in growth, thermal tolerance, biofilm formation, and genetic transformation. It is also demonstrated that competence development is significantly influenced by the expression of both htrA and spo0J.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
E. coli strain DH10B was grown in Luria broth, and S. mutans strain UA159 and its derivatives were grown in brain heart infusion (BHI) broth (Difco). For the selection of antibiotic-resistant colonies after genetic transformation, erythromycin (300 μg/ml for E. coli or 10 μg/ml for S. mutans), kanamycin and spectinomycin (50 μg/ml for E. coli or 1,000 μg/ml for S. mutans), or ampicillin (100 μg/ml for E. coli) was added to the medium as needed. For growth rate comparisons, cultures of S. mutans were initiated with a 1:100 dilution of overnight cultures, and the optical density at 600 nm (OD600) was measured at 37°C or 42°C at routine time intervals. The cultures were removed from test tubes, and the adhesive films of cells formed on the glass bottom were observed as a growth phenotype. For biofilm formation assays, S. mutans strains were grown in the semidefined BM medium (27), supplemented with glucose or sucrose at a final concentration of 20 mM. Plasmid pDL278 (21), an E. coli-Streptococcus shuttle vector carrying a spectinomycin resistance (Spr) gene that confers resistance to spectinomycin in both organisms, was used to measure transformation efficiency.
DNA methods.
Chromosomal DNA was prepared from S. mutans UA159 and its derivatives as previously described (4), and plasmid DNA was isolated from E. coli by using columns (QIAGEN, Inc., Chatsworth, CA). Restriction endonucleases and DNA-modifying enzymes were used according to the manufacturers' instructions. PCR products were purified with QIAquick kits (QIAGEN) and used for cloning. DNA was introduced into S. mutans by natural transformation (24) and into E. coli by the calcium chloride method (37). Southern blot analysis was performed with internal fragments of the genes of interest that had been labeled with psoralen-biotin by the BrightStar labeling kit (Ambion, Inc., Austin, TX).
Construction of mutant strains.
To construct htrA mutant strains, a DNA fragment containing the htrA gene was amplified from chromosomal DNA of S. mutans UA159 with primers HtrA-SphI-FW and HtrA-PstI-RV (Table 1) and cloned onto pGEM5 (Promega, Madison, WI) as an SphI-PstI fragment (pGEM5-htrA). To construct a polar mutant, the entire htrA structural gene was removed from pGEM5-htrA by two internal SpeI sites, blunt ended with T4 DNA polymerase, and replaced by an erythromycin resistance (Emr) gene containing a transcriptional terminator. The mutagenic plasmid for a nonpolar htrA mutant was constructed by inserting a commercial EZ::TN transposon (Epicentre, Madison, WI) including a nonpolar kanamycin resistance (Kmr) gene into the htrA gene (Fig. 1). To inactivate the spo0J gene, another EZ::TN transposon including the erythromycin resistance gene was used. The custom EZ::TN transposons were constructed by cloning a Kmr or Emr gene into the multiple cloning site (MCS) of the pMOD-2<MCS> vector and then isolating the transposon from the vector backbone by PCR amplification using pMOD<MCS> forward and reverse PCR primers that are provided with the vector. The EZ::TN transposon was incubated with pGEM5-htrA or pGEMT-spo0J, which was constructed by cloning the PCR fragment of the whole spo0J gene into pGEM-T Easy vector (Promega, Madison, WI) for 2 h at 37°C for an in vitro transposon insertion reaction. The desired mutagenic plasmids were selected by PCR amplification using vector-originated M13 primers and pMOD<MCS> forward and reverse sequencing primers that are provided with the vector and then isolated and used directly to transform S. mutans UA159. Transformants were selected on BHI agar containing the appropriate antibiotic, and double-crossover mutants of htrA or spo0J were confirmed by PCR and sequencing or by Southern blot analysis. The htrA-deficient mutants were designated SAB2 (ΔhtrA::Emr, polar mutant) and SAB2-13 (htrA::Kmr, nonpolar mutant), respectively. The spo0J-deficient mutant was named SAB3 (spo0J::Emr).
TABLE 1.
Primers used in this study
| Primer | Sequencea | Product size (bp) |
|---|---|---|
| HtrA-SphI-FW | GGACCTCCCTCTGCATGCATAAAGGCTCGA | |
| HtrA-PstI-RV | GGAATCTTATTTCTGCAGGCTAATTTTGCTG | |
| PhtrA-SacI-FW2 | TGTAAATAGCGAGCTCCATTTTCTGA | |
| htrA-SphI-RV | GATTATGGTGCATGCAGACTTATTG | |
| Spo0J-flanking-FW | GGGTTCTAAGCGCCACATTA | |
| Spo0J-flanking-RV | TGAGAAAGTTTTCCACAGGTGA | |
| HtrAspo0J-FW | CGCTGACCCTCTTTTTCA | |
| HtrAspo0J-RV | TGGGCTGTTTGATGTGGAT | |
| For real-time PCR | ||
| gtfB-FW | AGCAATGCAGCCATCTACAAAT | 98 |
| gtfB-RV | ACGAACTTTGCCGTTATTGTCA | |
| gtfC-FW | CTCAACCAACCGCCACTGTT | 98 |
| gtfC-RV | GGTTTAACGTCAAAATTAGCTGTATTAGC | |
| gtfD-FW | CACAGGCAAAAGCTGAATTAACA | 83 |
| gtfD-RV | GAATGGCCGCTAAGTCAACAG | |
| ftf-FW | CAGCTAGTACACCGGAAGTAGG | 124 |
| ftf-RV | GCAATCTTACGAGCCTGTTCTG | |
| htrA2-FW | AAGTTGTTAGACCCGCTCTTG | 101 |
| htrA2-RV | ACCGCTTGTGACATCACTTGG | |
| spo0J-FW | AATCCCTATCAACCTCGACTGC | 142 |
| spo0J-RV | GCCTTTCTCCTGCAACCAAATC |
Italics indicate restriction endonuclease sites.
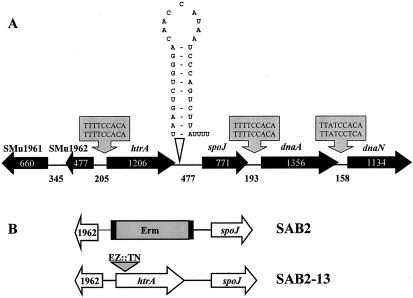
FIG. 1.
Schematic diagram of the htrA locus and construction of two htrA mutant strains. (A) Gene assignments and gene numbers above the schematic diagram are based on the genomic sequence information available for S. mutans UA159. Arrows indicate the directions of transcription. The numbers inside the schematic diagram and between open reading frames indicate the sizes of the open reading frames and intergenic regions in base pairs, respectively. The grey boxes indicate the consensus binding sites for DnaA (DnaA box). A putative transcriptional terminator is shown immediately downstream of the htrA gene. (B) The htrA gene was mutated either by allelic replacement of the whole gene encoding HtrA by a cassette containing the polar erythromycin resistance cassette (SAB2) or by in vitro insertion of a transposon (EZ::TN) containing the nonpolar kanamycin resistance gene (SAB2-13). See the text for more details.
To construct a reporter gene fusion for measuring the transcription level of the htrA gene, a 0.2-kb fragment containing the putative promoter region of htrA was cloned into the BamHI site in front of a promoterless chloramphenicol acetyltransferase (CAT) gene (cat) in pGEM3cat, where the cat gene is inserted into BamHI and SphI sites of pGEM3 (Promega), to yield plasmid pGEMcat-PhtrA. The clone containing a functional PhtrA-cat fusion was obtained on LB agar plates supplemented with chloramphenicol at 30 μg/ml, and the sequence of the promoter region was confirmed by nucleotide sequencing. The PhtrA-cat gene fusion was then excised from pGEM3cat-PhtrA, subcloned onto the S. mutans integration vector pBGK (49), and integrated into the gtfA locus of the chromosome of the wild-type strain in single copy. Double-crossover recombination of the reporter gene fusion into the S. mutans chromosome was confirmed by PCR amplification using primers internal to gtfA.
Construction of complemented strains.
To ensure that the htrA mutant phenotypes were due solely to the absence of htrA, we constructed two htrA-complemented strains with different copy numbers, SAB2MC (multicopy) and SAB2C (single copy). A wild-type copy including the entire htrA gene and its promoter region was amplified from UA159 genomic DNA by using primers PhtrA-SacI-FW2 and htrA-SphI-RV (Table 1). The amplicon was digested with SacI and SphI, ligated into the same restriction sites of plasmid pDL278 (pDL278/htrA+), and then transformed into the htrA mutant strain SAB2 (SAB2MC). To construct a complemented strain with a single copy of the htrA gene, the htrA gene fragment was excised from pDL278/htrA+, subcloned onto pBGK (49), and integrated into the gtfA locus of the chromosome of the mutant strain (SAB2C). The pBGK integration vector was also used to construct spo0J- and htrA-spo0J-complemented strains. For each construct, the entire spo0J and htrA-spo0J genes, including their promoter regions, were generated by PCR using each primer set, spo0J-flanking-FW/RV and htrAspo0J-FW/RV, respectively. These PCR fragments were subsequently cloned into pGEM-T Easy vector (Promega) and then into pBGK vector by using the proper restriction enzymes for integration into the chromosome of SAB3 (for complementation of spo0J; named SAB3C) and SAB2 (for complementation of htrA-spo0J; named SAB2Cspo0J). The integration of the genes was confirmed by PCR amplification and sequencing.
Biofilm assays.
The ability to form stable biofilms was assessed by growing the cells in 96-well, flat-bottom microtiter plates (Costar 3595; Corning, Inc., N.Y.) as previously described (23, 50). Briefly, overnight cultures of S. mutans UA159 and its derivatives were transferred to prewarmed BHI medium and grown at 37°C in a 5% CO2, aerobic atmosphere to an OD600 of approximately 0.5. The cultures were diluted 1:100 in fresh BM medium supplemented with glucose or sucrose, and then 200-μl aliquots of the cell suspension were inoculated into the wells of the microtiter plates. Wells containing uninoculated growth medium were used as negative controls. Plates were incubated at 37°C in a 5% CO2, aerobic atmosphere for 24 to 48 h. For biofilm quantification, the microtiter plates were slowly immersed in water and dumped out to remove the remaining planktonic and loosely bound cells. After this was performed twice, the plates were blotted on paper towels and air dried. The adherent bacteria were stained with 50 μl of 0.1% crystal violet for 15 min at room temperature, and then the plates were slowly immersed in water twice to rinse the wells. The bound dye was extracted from the stained cells by adding 200 μl of ethanol/acetone (8:2) mix. Biofilm formation was then quantified by measuring the optical density of the solution at 575 nm.
Stress response and CAT assays.
Acid and H2O2 tolerance experiments were performed as previously described (23). To measure the induction of htrA under various stress conditions, CAT activity expressed from the htrA promoter was measured by a spectrophotometric method (42) using the colorimetric substrate 5,5′-dinitro-bis-nitrobenzoic acid (Boehringer Mannheim, Indianapolis, Ind.). One unit of CAT activity was defined as the amount of enzyme needed to acetylate 1 nmol of chloramphenicol min−1. Protein concentrations were determined by the bicinchoninic acid assay (Sigma).
RNA methods.
To compare the levels of expression of gtfB, gtfC, gtfD, and ftf genes and to examine the transcriptional profile of the htrA-spo0J locus, S. mutans UA159 and SAB2 were grown in 50 ml of BHI to mid-exponential phase (OD600 ≈ 0.5). To measure the expression of htrA after competence-stimulating peptide (CSP) treatment, the wild-type strain was grown in 50 ml of BHI to early exponential phase (OD600 ≈ 0.2) and 1 μM CSP was added. Samples (10 ml) were removed at 0, 5, 10, 20, and 30 min after inoculation and transferred into 15-ml Falcon tubes (Becton Dickinson Labware, Franklin Lakes, NJ) containing rifampin at a final concentration of 150 μg ml−1. Total RNA was extracted by using a previously described protocol (5), except the extraction with hot phenol (60 to 65°C) and chilling on ice were repeated twice instead of using cold phenol. For quantitative real-time PCR analysis, after preliminary purification with phenol and chloroform and precipitation with isopropanol, the crude RNA was treated with DNaseI (Ambion, Inc., Austin, TX) and then further purified using the RNeasy mini kit (QIAGEN, Inc., Chatsworth, CA), including on-column DNase digestion with the RNase-free DNase set (QIAGEN).
For Northern blot analysis, RNA was separated on a 1% formaldehyde gel as described elsewhere (37). RNAs were UV cross-linked to nylon membranes, and membranes were probed with internal fragments of the desired genes that had been labeled with psoralen-biotin by the BrightStar labeling kit (Ambion). Signals obtained on autoradiographs were detected with an IS1000 digital imaging system (Alpha Innotech Corp., San Leandro, CA). For reverse transcription (RT)-PCR and real-time PCR, cDNA templates were created from 1 μg of RNA using the SuperScript first-strand synthesis system (Invitrogen Corp., Carlsbad, CA) according to the recommended procedure. The primers used for RT-PCR and real-time PCR are shown in Table 1.
Real-time PCR.
Real-time PCRs were carried out in an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) using iQSYBR green supermix (Bio-Rad) and primers designed using DNA mfold (http://www.bioinfo.rpi.edu/applications/mfold/old/dna/) and Beacon Designer 2.0 (PREMIER Biosoft International, Palo Alto, CA) to generate PCR products that were 85 to 150 bp in length. SYBR green signal measurements were collected for experimental samples in triplicate, and all experiments were performed at least twice. A standard curve was prepared using eight 10-fold serial dilutions of the PCR products to determine the starting amount for each cDNA template, based on its threshold cycle. The concentrations of purified PCR products were estimated at OD260, and the numbers of copies/ml for standard curves were calculated according to the following formula (53): copies/ml = (6.023 × 1023 × C × OD260)/MWt, where C is 5 × 10−5 g/ml for DNA and MWt is the molecular weight of PCR product (base pairs × 6.58 × 102 g). Standards were made to concentrations of 108 copies/μl.
The gradient thermocycling program was set for 40 cycles of 95°C for 10 s and 60°C for 45 s, with an initial cycle at 95°C for 30 s. During each cycle, the accumulation of PCR products was detected by monitoring the increase in fluorescence of the reporter dye from double-stranded-DNA-binding SYBR green. Melt curves were run immediately after the last PCR cycle. Melt curves were constructed by plotting the fluorescence intensities against temperatures as the set point temperatures (60°C) were increased by 0.4°C for 10 s (100 cycles). Data were collected and analyzed using the software and graphics programs provided with the iCycler iQ. For the confirmation of amplicon presence and purity, the real-time PCR products were run on a 1.0% Tris-acetate-EDTA gel and stained with ethidium bromide.
Transformation of S. mutans.
Overnight cultures of S. mutans strains were diluted 1:20 in BHI medium containing horse serum (10% vol/vol). A 0.2-ml aliquot of the cultures was incubated at 37°C for 100 min in a 96-well microtiter plate (Costar 3595; Corning) and then treated with or without 5 μl of synthetic CSP solution (1 nmol/μl). CSP consisted of a 21-amino-acid peptide (SGSLSTFFRLFNRSFTQALGK) (25) that was synthesized at the ICBR Protein Chemistry and Biomarkers Core Facility of University of Florida. The purity of the synthetic CSP was confirmed by high-performance liquid chromatography and mass spectrometry profiles, and the material was freeze-dried and stored at −20°C for further studies. After incubation for 20 min to allow the induction of competence, the cultures were exposed to 500 ng of plasmid pDL278. After 150 min at 37°C, cultures were chilled on ice and transformants and total CFU were enumerated by plating cells on BHI agar plates with or without 1 mg/ml spectinomycin, respectively. Transformation efficiency was determined after 48 h of incubation and was expressed as the percentage of transformants among the total viable recipient cells.
RESULTS
The htrA-spo0J locus of S. mutans.
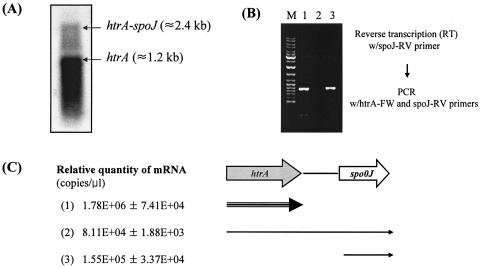
The htrA gene was identified in the complete genome of S. mutans UA159 from nucleotides 2028472 through 2029677 (1). The gene encodes a putative 402-amino-acid protein that showed high levels of similarity with HtrA proteins of S. pneumoniae (50% identity), Lactococcus lactis (49% identity), and E. coli (37% identity). The organization of open reading frames in the region surrounding htrA appeared similar to those in other streptococcal strains, such as S. pneumoniae (47) and S. pyogenes (11), but a gene that is most similar to spo0J of Bacillus subtilis is located 477 bp downstream of the stop codon of htrA (Fig. 1A). Consensus binding sites for DnaA (DnaA box), which forms initiation complexes for chromosome replication, are found in nontranslated regions upstream of htrA and upstream and downstream of dnaA (Fig. 1A). The 477-bp intergenic region between htrA and the gene immediately downstream is much longer than those of other bacteria, including S. pneumoniae (57 bp) and S. pyogenes (58 bp), suggesting that the S. mutans htrA gene is monocistronic, as implied in other studies (8, 34). A putative transcriptional terminator was found downstream of the stop codon of htrA (Fig. 1A). Interestingly, our analysis revealed that htrA and spo0J appear to be both independently transcribed and cotranscribed (Fig. 2). Northern blot analysis with RNA isolated from the wild-type strain, which was probed with a portion of htrA, detected two transcripts corresponding to an individual transcript of htrA and an htrA-spo0J transcript (Fig. 2A). No signal was detected in the htrA-deficient mutant (SAB2) (data not shown). The ability of htrA and spo0J transcripts to be cotranscribed was confirmed by RT-PCR in which a reverse transcript formed by a primer internal to spo0J (spo0J-RV) could be amplified with an internal forward primer in htrA (htrA2-FW) and an internal reverse primer in spo0J (spo0J-RV) (Fig. 2B).
FIG. 2.
Transcriptional analysis in the htrA-spo0J locus of S. mutans UA159. (A) Northern blot analysis. Total RNA (10 μg) from UA159 strain was separated in a 0.9% formaldehyde gel, transferred to a nylon membrane, and hybridized to a probe specific for the htrA gene. (B) Following reverse transcription with reverse primer spo0J-RV, PCR amplification was performed with a primer set of htrA2-FW and spo0J-RV. The PCR products were run on Tris-acetate-EDTA gel. Lane M, size marker; lane 1, RT-PCR product; lane 2, negative control of RT; lane 3, positive control of PCR from chromosomal DNA of UA159. (C) Real-time PCR. For measuring the htrA (1) and htrA-spo0J (2) mRNAs, total RNA from UA159 was used for RT with htrA2-RV and spo0J-RV, respectively. For measuring the spo0J mRNA, total RNA from SAB2 was used for RT with the spo0J-RV primer. See the text for more details. Data represent means ± standard deviations which were from two separate experiments. Arrows indicate the relative amounts of mRNA.
To quantify the relative amounts of the htrA and spo0J transcripts, the mRNAs for htrA, spo0J, and the cotranscript were quantified by real-time PCR (Fig. 2C). To examine spo0J mRNA arising from transcription immediately upstream of spo0J and not as a result of cotranscription with htrA, a polar htrA mutant described below was used to obtain RNA. The htrA-spo0J mRNA was about 20-fold less abundant than the htrA mRNA, indicating that transcription through the putative transcription terminator is not completely efficient. Notably, the spoJ transcript alone was present in about twofold-greater quantity than the cotranscript. Thus, both genes appear to carry at least one functional promoter, and the ability of the genes to be cotranscribed suggests the possibility of a functional relationship between the htrA and spo0J.
Construction of htrA mutants of S. mutans UA159.
To phenotypically characterize the role of HtrA in S. mutans, the htrA gene was disrupted by polar and nonpolar insertions so as to also evaluate effects on transcription of the spo0J gene (Fig. 1B). The polar mutant (SAB2) was generated by replacing the entire htrA gene with a polar erythromycin resistance cassette. In the nonpolar mutant (SAB2-13), a commercial EZ::TN transposon containing a nonpolar kanamycin resistance gene was integrated into the 5′ portion of the htrA structural gene. In addition, a polar spo0J mutant (SAB3) was constructed by inserting the EZ::TN transposon containing a polar erythromycin resistance cassette into the middle of the spo0J gene of the wild type (details not shown).
Growth phenotypes and stress tolerance by htrA mutants.
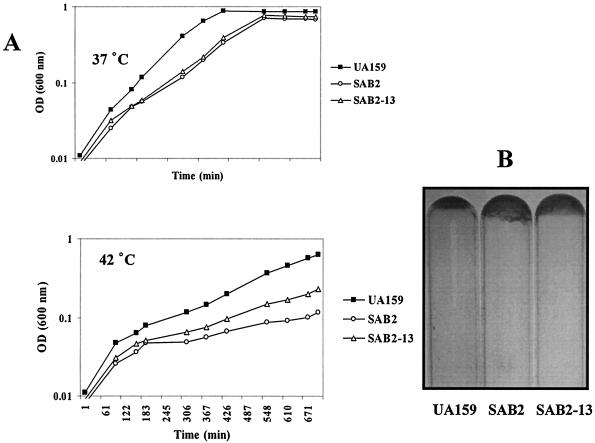
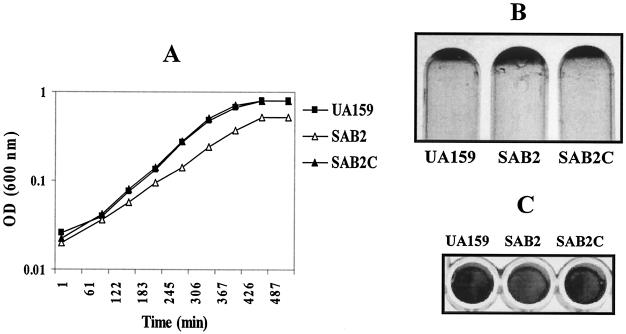
When grown at 37°C in liquid BHI medium, strains SAB2 and SAB2-13 had generation times about 10 to 20% longer than those of the wild-type strain and reached a lower final OD600 than the wild-type strain (Fig. 3A). The mutant strains formed clumps during the mid-exponential phase of growth and settled to the bottom of the tube to form adhesive films (Fig. 3B), probably due to altered surface properties. Alterations of chain lengths and differences in colony morphology on agar medium were not observed for the mutant strains. At 42°C, both the polar and nonpolar htrA mutants SAB2 and SAB2-13 grew about 20% slower than the wild-type strain (Fig. 3A), indicating that disruption of htrA resulted in reduced thermal tolerance.
FIG. 3.
Growth phenotypes of S. mutans UA159 (wild type) and two htrA-disrupted mutants, SAB2 (polar) and SAB2-13 (nonpolar). (A) Growth curves grown in BHI broth at 37°C (top) and 42°C (bottom). The data shown are from a single experiment representative of three independent experiments. (B) Adhesive films formed on the bottom of the glass test tubes. After being grown in BHI at 37°C for 1 day, the culture broths were removed.
Strain SAB2 was also tested for its response to oxidative, acid, ethanol, and salt stresses. Acid and H2O2 killing experiments performed by acidification at pH 2.8 and treatment with 0.2% H2O2, respectively, did not reveal significant differences in survival rates of mid-exponential-phase cells between SAB2 and the parental strain (data not shown). In CAT assays to measure the transcription levels of htrA, the addition of NaCl (4.5%, wt/vol) and ethanol (10%, vol/vol) to mid-exponential-phase liquid culture had no effect on htrA transcription (data not shown), and growth of the wild-type and mutant strains was the same as assessed by counting of viable cells. These data indicate that the htrA gene of S. mutans UA159 is not significantly involved in these stress responses and that coping with damage induced by the tested stressors, except for heat, may not be a primary role of S. mutans HtrA.
Effects of htrA deficiency on biofilm formation.
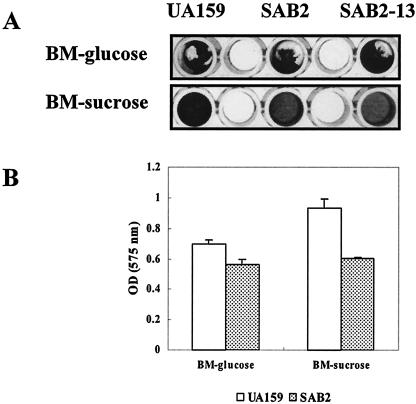
Since the htrA mutants showed a distinctive growth phenotype by forming clumps and an adhesive film in BHI liquid medium (Fig. 3B), the capacity of the htrA mutants for forming biofilms was compared with that of the wild-type strain. The biofilm assay was performed with BM medium supplemented with glucose or sucrose at a final concentration of 20 mM (27). On polystyrene surfaces with BM-sucrose medium, both strains formed relatively stable biofilms, although less biofilm mass was formed by the mutant strains, SAB2 and SAB2-13 (Fig. 4). Consistent with the biofilm data, the biofilms formed by the mutant in BM-sucrose medium had reduced numbers of cells compared to the wild type, confirming the results of the crystal violet staining of the biofilms (data not shown). In BM-glucose medium, the amounts of biofilm formed by strain SAB2 and by the parent were not significantly different (Fig. 4A).
FIG. 4.
Biofilm formation of S. mutans UA159 (wild type) and SAB2 in BM medium supplemented with glucose (BM-glucose) or sucrose (BM-sucrose) at a final concentration of 20 mM. Biofilm was assayed on polystyrene microtiter plates, stained by using crystal violet (A) and quantified by adding ethanol/acetone mix (B). See the text for more details. Data are representative of no fewer than three separate experiments. The error bars represent standard deviations.
Sucrose-dependent biofilm formation in S. mutans is mediated primarily by the production of glucan polymers from sucrose by glucosyltransferase enzymes (Gtfs) and binding mediated through the Gtfs and other glucan binding proteins (52). S. mutans also produces fructan exopolymers from sucrose via a single secreted fructosyltransferase enzyme. To determine if the reduction in biofilm formation by the mutant was due to aberrant expression of genes encoding the exopolysaccharide synthesis machinery of S. mutans, expression levels of the gft and ftf genes in UA159 and SAB2 were compared using real-time PCR. The data showed differential expression of gtfB and gtfC genes in the mutant and wild-type strains. The GtfBC enzymes are primarily responsible for production of the α1,3-linked adhesive glucans, suggesting that the reduction of biofilm formation in SAB2 may, at least in part, be due to the reduced expression of these two genes (Table 2). There was no significant difference between the levels of expression of the gtfD and ftf genes.
TABLE 2.
Real-time PCR-based expression files of gtfB, gtfC, gtfD, and ftf genes
| Gene | Relative quantity of mRNA (copies/μl)a
|
Ratiob | |
|---|---|---|---|
| UA159 | SAB2 | ||
| gtfB | 1.03E+05 ± 3.00E+04 | 4.70E+04 ± 1.00E+04 | 1:0.45 |
| gtfC | 3.47E+06 ± 9.00E+05 | 2.18E+06 ± 8.00E+05 | 1:0.63 |
| gtfD | 7.52E+05 ± 4.00E+05 | 1.19E+06 ± 9.00E+05 | N/D |
| ftf | 4.61E+05 ± 2.00E+05 | 7.52E+05 ± 4.00E+05 | N/D |
Following reverse transcription from 1 μg of total RNA from S. mutans strains UA159 and SAB2, the amounts of gtf and ftf gene cDNA were determined by real-time PCR using SYBR green. The data represent means±standard deviations which were obtained from three different RNA preparation and reverse-transcription reactions. See the text for experimental procedures in detail.
Ratio of UA159 mRNA to SAB2 mRNA. N/D indicates that there was statistically no difference (P < 0.01; Student's t test).
Complementation of the htrA mutation.
To evaluate the contribution of HtrA in the different phenotypes observed in the htrA mutants, we integrated the htrA gene in a single copy, driven by its own promoter, into the chromosome of the mutant SAB2. In this case, the number of relative copies of htrA mRNA in SAB2C (1.40 × 105 ± 1.21 × 104) was similar to that in the wild type (1.27 × 105 ± 2.40 × 104), indicating that the levels of htrA mRNA in the htrA-complemented strain (SAB2C) were restored to those seen in the wild-type strain. Complementation with htrA restored the normal growth phenotypes and stress tolerance characteristics, as well as the capacity for forming biofilms (Fig. 5). Interestingly, when the intact htrA gene was provided in trans on a plasmid (pDL278/htrA+), the transformants showed severe colony morphology changes and aberrant growth characteristics (irregular and tiny) (data not shown). S. mutans strains that were transformed with an empty vector (pDL278) behaved identically like the parental strains (data not shown).
FIG. 5.
Effects of complementation with htrA on growth phenotypes. The complemented strain (SAB2C) was constructed by integrating the entire htrA gene into the genome of the mutant strain (SAB2) in a single copy. SAB2C behaved almost identically like the parent strain in terms of growth rate (A), the formation of adhesive films on the bottom of glass test tubes (B), and biofilm formation on polystyrene microtiter plates in BM medium supplemented with sucrose (C). The data shown were obtained from at least three independent experiments. See the text for more details.
HtrA and genetic competence.
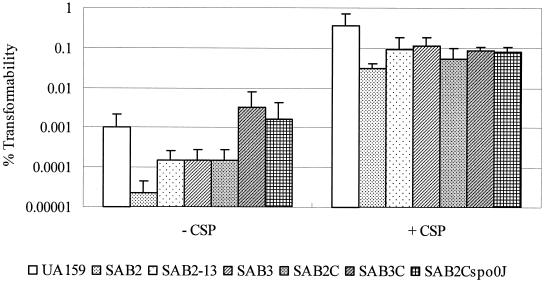
The possibility that HtrA is involved in genetic competence has recently emerged through microarray studies with competent S. pneumoniae (39). To assess the contribution of the S. mutans HtrA protein in competence, the efficiencies of transformation of the htrA mutant strains were evaluated by transformation with plasmid pDL278, which contains a Spr marker (Fig. 6). The htrA polar mutant (SAB2) showed a significant reduction in the number of cells that could develop competence in the absence of treatment with exogenous CSP. Interestingly, the decrease in transformation efficiency in the htrA polar mutant appeared to be due to effects elicited both by the loss of HtrA and decreases in the expression of spo0J. Specifically, both the nonpolar htrA mutant and the spo0J mutant caused significant reductions in transformation efficiency, and the complementation of only htrA in strain SAB2 (htrA polar mutant) could not completely restore transformability. However, complementation of spo0J in SAB3 (spo0J polar mutant) and the complete htrA-spo0J locus in SAB2 resulted in the complete restoration of transformation in the absence of CSP. In all cases, the impairment in transformation of each of the mutants could be significantly reversed by the addition of CSP to the growth medium, suggesting that HtrA and Spo0J may participate in CSP processing. Alternatively, the provision of excess CSP may somehow allow the cells to compensate for the deficiency of HtrA and Spo0J, perhaps through overexpression of CSP-regulated pathways.
FIG. 6.
Transformability of htrA mutants compared to UA159. The S. mutans strains used were UA159 (wild type), SAB2(polar htrA mutant), SAB2-13 (nonpolar htrA mutant), SAB3 (polar spo0J mutant), SAB2C (htrA-complemented strain of SAB2), SAB3C (spo0J-complemented strain), and SAB2Cspo0J (htrA-spo0J-complemented strain of SAB2). Percent transformability was determined by the ratio of the number of transformants and that of the total viable recipients, multiplied by 100. The data shown are means ± standard deviations (error bars) of at least three independent experiments. The transformation was performed in 200-μl culture with or without CSP (5 nmol). See the text for more details.
A linkage between competence development and HtrA was also revealed by demonstrating that exposure to CSP induced htrA expression, which reached a maximum after 20 min and then declined (Table 3). Given the kinetics of induction, HtrA could be involved in later steps of the transformation process. Notably, HtrA does not appear to influence the transcription of comAB, comDE, or comX as examined by real-time PCR of mRNA from the wild-type and SAB2 strains (data not shown).
TABLE 3.
Induction of the htrA gene by CSP treatment over time by using real-time PCRa
| Parameter | Parameter by time after adding CSP (min)
|
||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 30 | |
| Relative quantity of htrA mRNA (× 105 copies/μl) | 2.03 ± 0.26 | 2.04 ± 0.82 | 2.99 ± 0.36 | 7.09 ± 0.44 | 4.05 ± 0.11 |
| n-fold induction | 1.0 | 1.5 | 3.5 | 2.0 | |
Early-exponential-phase culture of S. mutans UA159 was supplemented with CSP and then removed over time. Following reverse transcription from 1 μg of total RNA, the amount of htrA gene cDNA was determined by real-time PCR using SYBR green. These are typical results representative of two independent experiments. The data represent means ± standard deviations. See the text for more details.
DISCUSSION
As was shown to be the case for htrA of S. pneumoniae (12, 47) and S. pyogenes (11, 28), htrA of S. mutans is near the apparent origin of chromosome replication. Immediately downstream of htrA, genes homologous to spo0J and dnaA were identified. DnaA boxes, which are bound by DnaA proteins to form the initiation complex for chromosome replication were identified in this region. Similar to those of S. pneumoniae (12) and S. pyogenes (28), the DnaA boxes of S. mutans are localized to untranslated regions upstream of htrA and upstream and downstream of dnaA (Fig. 1). The spo0J gene was found to encode a polypeptide of 257 amino acid residues with a high degree of similarity to a chromosome partitioning factor, which is also required for the initiation of sporulation, in B. subtilis (3, 19, 22, 32). In B. subtilis, spo0J is downstream and cotranscribed with the soj gene. Based on sequence similarities with the ParA/ParB families of proteins (16), it is suggested that Spo0J is a DNA binding protein and Soj is an ATPase and that the two proteins interact (15), possibly participating in chromosome partitioning. Since S. mutans is a nonsporulating bacterium, the role of the spo0J gene product is not clear, although our results indicate that its activity may be linked to the functions of HtrA.
Our transcriptional analysis of the htrA-spo0J locus revealed that the two genes are able to be individually transcribed and cotranscribed. At first, we thought that the htrA-spo0J cotranscript was due to incomplete termination of the htrA transcription, since a putative transcription terminator is found immediately behind the stop codon of htrA and there is 477 bp of noncoding DNA between htrA and spo0J. Also, from an analysis of the polar htrA mutant, spo0J appears to have its own promoter. However, the level of cotranscription of htrA and spo0J is similar to that of the spo0J transcript alone, suggesting that transcription of spo0J from the htrA promoter contributes substantively to spo0J expression, further supporting a functional connection between htrA and spo0J.
Disruption of htrA and spo0J resulted in significant decreases in transformation efficiency. In the absence of treatment with exogenous CSP, both the spo0J mutant (SAB3) and the htrA nonpolar mutant (SAB2-13) had significant reductions in transformability. The results with HtrA are similar to those reported for S. pneumoniae, for which it was shown that inactivation of htrA decreased transformation and complementation with htrA restored competence to wild-type levels (18). The deficiency of transformability in SAB2 (htrA polar mutant) of S. mutans could be completely restored only by complementation with both the htrA and spo0J genes, suggesting that the cotranscription of htrA-spo0J is required for full development of the transformation process and that these two gene products may be functionally connected, at least in terms of competence development. However, with the addition of exogenous CSP, the mutant strains were not as efficiently transformed as the parent strain, even after complementation (Fig. 6). We postulate that this observation is due to the fact that the complemented strains carry htrA-spo0J at a locus far from the origin and there may be additional effects on htrA-spo0J expression when linked to the origin of replication. More recently, the effects of HtrA on competence were shown to be exerted through the ciaHR two-component system (17). The spo0J gene is not cotranscribed with htrA in the pneumococcus, so effects of spo0J on competence have not been explored. However, S. pneumoniae spo0J has recently been identified as a delayed CSP-induced gene that showed the same level of induction as htrA after treatment with CSP, implying a role for Spo0J in competence development (35). Collectively, these findings are consistent with results showing that Spo0J of B. subtilis is part of a regulatory network controlling the initiation of sporulation and the development of genetic competence in B. subtilis (15).
Treatment of S. mutans with CSP dramatically increases induction of competence (Fig. 6). Interestingly, after treatment with CSP, transformation efficiency of both the htrA and spo0J mutants was dramatically enhanced, albeit not to the same levels as UA159. These results are consistent with the hypothesis that HtrA and Spo0J may participate in the maturation of CSP but also point to the involvement of these genes either in the detection and signaling of CSP or in downstream events in the competence cascade. Consistent with this idea is our finding that htrA induction after exposure to competence peptide peaks at around 20 min, indicating that it may also have a role in late competence processes. Our results also indicate that the inactivation of htrA does not impact the expression of the early competence genes, such as comAB, comDE, and comX, as assessed by real-time PCR. Unfortunately, the expression of comC could not be precisely measured, since we could not obtain good primers for real-time PCR from this very small gene. Supporting a role for protease chaperones in the latter phases of competence development, a recent microarray analysis of S. pneumoniae (35) revealed that a diverse set of genes were induced by CSP treatment. Among them were genes encoding chaperones or proteases, such as DnaK, GroEL/GroES, ClpL, and HtrA, which were identified as delayed induced genes because transcription of these genes gradually increased during the first 15 to 17 min of CSP treatment. The roles of these chaperones or proteases in competence development are not clear at this point, but it has been speculated that they may be involved in processing or modulating the stability of proteins required for competence development. Alternatively, competence in S. pneumoniae was reported to trigger growth arrest (7), probably resulting from shutdown of most of the protein synthesis except for competence-associated proteins during differentiation to competence. During competence development in Bacillus subtilis, DNA replication and the synthesis of stable RNA are known to be arrested (9, 31, 33). Consequently, the severe physiological changes in cells due to the blockage of essential cell functions during competence development can possibly create a stress or can generate stress signals that induce stress response genes (7, 35).
The ciaRH two-component signaling system has been shown to regulate htrA expression and to impact competence, suggesting that the regulation of competence by HtrA may be exerted via the CiaRH regulation system (7, 29, 39). Recently, it was proposed that the CiaRH regulon is required to cope with the competence-induced physiological changes of cells and for normal exit from competence (7). Also, increasing expression of HtrA in a ciaR mutant of S. pneumoniae could restore genetic competence. However, the mutation of ciaH resulted in up-regulation of htrA expression by about 2 logs in our real-time PCR assay (unpublished data), suggesting that the expression and the role of htrA may be different for S. mutans. Consequently, effects on the later stages of competence of HtrA may be related to the function of the ciaRH system. However, for S. mutans, ciaRH was shown to affect biofilm formation and stress tolerance in ways that we did not observe with HtrA-deficient strains (36). It seems, then, that although there is some degree of cross talk between HtrA and CiaRH, the two systems can function independently of one another.
While both htrA and spo0J are required for full development of competence, the distinctive growth phenotypes appear to result from the loss of only HtrA, since htrA polar (SAB2) and nonpolar (SAB2-13) mutants showed the same growth characteristics and the htrA-complemented strain (SAB2C) completely restored the growth and stress resistance to wild-type levels. Also, no significant effects on the growth of the spo0J mutant (SAB3) were observed (data not shown). The S. mutans htrA mutant exhibited slower growth and tended to aggregate and form biofilms on glass surfaces in BHI medium, perhaps indicating that alterations in the expression or maturation of surface proteins or other components of the cell envelope occur in the mutant. The potential role of HtrA in the processing of surface proteins has been previously implicated in S. mutans (8) and in the proper presentation of the SpeB protease of S. pyogenes. Preliminary two-dimensional-gel comparisons of surface-associated proteins prepared from SAB2 and UA159 showed differences in the amounts of a variety of gene products (data not shown). A possible role of S. mutans HtrA, as a membrane-associated chaperone/protease, may be to influence the biogenesis of surface proteins, although the behavior of the mutant may also be a function of alterations in metabolic processes.
The htrA mutant exhibited reduced biofilm formation in BM medium supplemented with sucrose, but not with glucose, compared to UA159. This finding alone is surprising, since most studies with S. mutans to date reveal that mutations that affect biofilm formation in genes other than the gtf or gbp generally affect biofilm maturation in glucose to a much greater degree than in sucrose. Our results demonstrated that, at least in part, the reduction in biofilms in sucrose-containing media could be attributed to lower expression levels of the gtfBC gene. This decrease was seen at the transcriptional level, suggesting that S. mutans HtrA impacts sucrose-dependent biofilm formation mediated by water-insoluble glucans synthesized by glucosyltransferases GtfBC, which are primarily responsible for establishing the extracellular polysaccharide matrix, and in the adhesion and accumulation of the organisms on surfaces (24). The levels of GtfBC enzyme were not measured in this study, and thus, it is not clear if the reduced sucrose-dependent biofilm formation of the htrA mutant is due solely to decreased transcription of the gtfBC genes. In previous work by Diaz-Torres and Russell (8), no significant differences were observed between S. mutans LT11 and its htrA mutant in measurements of extracellular proteins, including Gtf and Ftf, by using Western immunoblotting (8). There were no statistically significant differences in the expression of gtfD, which encodes an enzyme that catalyzes the formation of a water-soluble glucan, or ftf, which encodes a fructosyltransferase that catalyses the synthesis of fructans from sucrose (43). In addition, we did not observe a significant difference between the levels of expression of genes that encode extracellular proteins that may be involved in biofilm formation, such as brpA (50), spaP (2, 6), and gbpB (30, 45) (data not shown). However, the possibility that posttranscriptional effects of the loss of HtrA on one or all of these gene products contribute to the behavior of the mutants cannot be excluded.
Another alteration of growth phenotypes by the htrA mutation is the reduced thermal tolerance at 42°C. Notably, unlike other molecular chaperones and proteases in S. mutans, htrA expression was not transcriptionally regulated by heat shock, as determined by using a transcriptional cat fusion of the htrA promoter region (data not shown). Other stress responses of the S. mutans htrA mutant were also strikingly different from those of many gram-negative and gram-positive htrA null mutants, since the S. mutans htrA mutant did not exhibit sensitivity to H2O2, low pH, NaCl, and ethanol stresses. This is in contrast to what was reported by Diaz-Torres and Russell (8), for whom the htrA mutant derived from strain LT11 showed a restricted pH range of growth and also exhibited sensitivity to various H2O2 concentrations. One probable reason for this is due to fact that their htrA mutant was constructed by single-crossover homologous recombination, which may have an impact on the phenotype because of the insertion of a large block of foreign DNA in a region needed for DNA replication. This idea is supported by the fact that the LT11 htrA mutant had greater growth defects, such as a slower growth rate, the formation of small and irregular colonies, and a very restricted temperature range. The mutants constructed in this study by the replacement of htrA with a similarly sized antibiotic resistance determinant would be less likely to have growth defects associated with disruption of the spacing of DnaA boxes. Thus, we propose that the stress-sensitive responses of the LT11 htrA mutant may be related to a general defect in growth. We also recognize that strain LT11 may harbor other genetic or physiologic differences from UA159, which, in turn, could affect the behavior of HtrA-deficient derivatives.
For complementation of the htrA mutant, the intact htrA gene was provided in trans in a single copy on the chromosome of the mutant, restoring growth, biofilm formation, and stress tolerance to wild-type levels. However, when htrA was provided on a plasmid, the transformants displayed abnormal growth phenotypes, suggesting that the overexpression of HtrA in S. mutans could affect normal cellular homeostatic mechanisms. This is clearly an interesting phenomenon, since the htrA mutation of other species, including S. pneumoniae (18) and Listeria monocytogenes (51), could be fully complemented by provision of the gene on a plasmid. The effect on S. mutans of htrA gene dosage provides additional evidence that HtrA in this organism is regulated differently and may play distinctly different roles.
In summary, we demonstrated that the mutation of the S. mutans htrA results in multiple phenotypes, such as a slower growth, reduced thermal tolerance, altered biofilm formation, and significantly diminished transformation efficiency. The htrA and spo0J gene products appear to work both cooperatively and independently to govern homeostatic mechanisms, to coordinate competence and stress tolerance, and to modulate the expression of known virulence determinants. A more detailed analysis of the functions and interactions of HtrA and Spo0J in regulatory networks connecting the growth, stress tolerance, biofilm formation, and the competence regulon is under way.
Acknowledgments
We thank Z. T. Wen and J. Abranches for technical assistance in biofilm and real-time PCR experiments, respectively, Y. M. Chen for helpful discussions on experiments, and our colleagues for support and advice.
This work was supported by grants DE13239 and DE12236 from the National Institute for Dental and Craniofacial Research.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaker, R. P., S. V. Seddon, C. Tredwin, and E. Lynch. 1998. Detection of Streptococcus mutans by PCR amplification of the spaP gene in teeth rendered caries free. J. Dent. 26:443-445. [DOI] [PubMed] [Google Scholar]
- 3.Autret, S., R. Nair, and J. Errington. 2001. Genetic analysis of the chromosome segregation protein Spo0J of Bacillus subtilis: evidence for separate domains involved in DNA binding and interactions with Soj protein. Mol. Microbiol. 41:743-755. [DOI] [PubMed] [Google Scholar]
- 4.Burne, R. A., K. Schilling, W. H. Bowen, and R. E. Yasbin. 1987. Expression, purification, and characterization of an exo-β-d-fructosidase of Streptococcus mutans. J. Bacteriol. 169:4507-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y. Y., C. A. Weaver, D. R. Mendelsohn, and R. A. Burne. 1998. Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J. Bacteriol. 180:5769-5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowley, P. J., L. J. Brady, S. M. Michalek, and A. S. Bleiweis. 1999. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect. Immun. 67:1201-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagkessamanskaia, A., M. Moscoso, V. Henard, S. Guiral, K. Overweg, M. Reuter, B. Martin, J. Wells, and J. P. Claverys. 2004. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol. Microbiol. 51:1071-1086. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Torres, M. L., and R. R. Russell. 2001. HtrA protease and processing of extracellular proteins of Streptococcus mutans. FEMS Microbiol. Lett. 204:23-28. [DOI] [PubMed] [Google Scholar]
- 9.Dooley, D. C., C. T. Hadden, and E. W. Nester. 1971. Macromolecular synthesis in Bacillus subtilis during development of the competent state. J. Bacteriol. 108:668-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elzer, P. H., R. W. Phillips, G. T. Robertson, and R. M. Roop II. 1996. The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect. Immun. 64:4838-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasc, A. M., P. Giammarinaro, S. Richter, and M. Sicard. 1998. Organization around the dnaA gene of Streptococcus pneumoniae. Microbiology 144:433-439. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman, S., S. Wickner, and M. R. Maurizi. 1997. Protein quality control: triage by chaperones and proteases. Genes Dev. 11:815-823. [DOI] [PubMed] [Google Scholar]
- 15.Grossman, A. D. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu. Rev. Genet. 29:477-508. [DOI] [PubMed] [Google Scholar]
- 16.Hiraga, S. 1992. Chromosome and plasmid partition in Escherichia coli. Annu. Rev. Biochem. 61:283-306. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Control of virulence by the two-component system CiaR/H is mediated via HtrA, a major virulence factor of Streptococcus pneumoniae. J. Bacteriol. 186:5258-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect. Immun. 72:3584-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ireton, K., N. W. Gunther IV, and A. D. Grossman. 1994. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 176:5320-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, K., I. Charles, G. Dougan, D. Pickard, P. O'Gaora, G. Costa, T. Ali, I. Miller, and C. Hormaeche. 1991. The role of a stress-response protein in Salmonella typhimurium virulence. Mol. Microbiol. 5:401-407. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc, D. J., L. N. Lee, and A. Abu-Al-Jaibat. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130-145. [DOI] [PubMed] [Google Scholar]
- 22.Lee, P. S., D. C. Lin, S. Moriya, and A. D. Grossman. 2003. Effects of the chromosome partitioning protein Spo0J (ParB) on oriC positioning and replication initiation in Bacillus subtilis. J. Bacteriol. 185:1326-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemos, J. A., T. A. Brown, Jr., and R. A. Burne. 2004. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect. Immun. 72:1431-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Y., and R. A. Burne. 2001. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology 147:2841-2848. [DOI] [PubMed] [Google Scholar]
- 25.Li, Y. H., P. C. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipinska, B., O. Fayet, L. Baird, and C. Georgopoulos. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J. Bacteriol. 171:1574-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyon, W. R., and M. G. Caparon. 2004. Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect. Immun. 72:1618-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascher, T., D. Zahner, M. Merai, N. Balmelle, A. B. de Saizieu, and R. Hakenbeck. 2003. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J. Bacteriol. 185:60-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattos-Graner, R. O., S. Jin, W. F. King, T. Chen, D. J. Smith, and M. J. Duncan. 2001. Cloning of the Streptococcus mutans gene encoding glucan binding protein B and analysis of genetic diversity and protein production in clinical isolates. Infect. Immun. 69:6931-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy, C., and E. W. Nester. 1967. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J. Bacteriol. 94:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mysliwiec, T. H., J. Errington, A. B. Vaidya, and M. G. Bramucci. 1991. The Bacillus subtilis spo0J gene: evidence for involvement in catabolite repression of sporulation. J. Bacteriol. 173:1911-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nester, E. W., and B. A. Stocker. 1963. Biosynthetic latency in early stages of deoxyribonucleic acid transformation in Bacillus subtilis. J. Bacteriol. 86:785-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26:209-221. [DOI] [PubMed] [Google Scholar]
- 35.Peterson, S. N., C. K. Sung, R. Cline, B. V. Desai, E. C. Snesrud, P. Luo, J. Walling, H. Li, M. Mintz, G. Tsegaye, P. C. Burr, Y. Do, S. Ahn, J. Gilbert, R. D. Fleischmann, and D. A. Morrison. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51:1051-1070. [DOI] [PubMed] [Google Scholar]
- 36.Qi, F., J. Merritt, R. Lux, and W. Shi. 2004. Inactivation of the ciaH gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 72:4895-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sassoon, N., J. P. Arie, and J. M. Betton. 1999. PDZ domains determine the native oligomeric structure of the DegP (HtrA) protease. Mol. Microbiol. 33:583-589. [DOI] [PubMed] [Google Scholar]
- 39.Sebert, M. E., L. M. Palmer, M. Rosenberg, and J. N. Weiser. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect. Immun. 70:4059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sebert, M. E., K. P. Patel, and J. N. Weiser. 2004. Pneumococcal htrA impacts competence for genetic transformation, p. 239, D-265. Abstr. 104th Gen. Meet. Am. Soc. Microbiol. ASM Press, Washington, D.C.
- 41.Seol, J. H., S. K. Woo, E. M. Jung, S. J. Yoo, C. S. Lee, K. J. Kim, K. Tanaka, A. Ichihara, D. B. Ha, and C. H. Chung. 1991. Protease Do is essential for survival of Escherichia coli at high temperatures: its identity with the htrA gene product. Biochem. Biophys. Res. Commun. 176:730-736. [DOI] [PubMed] [Google Scholar]
- 42.Shaw, W. V. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737-755. [DOI] [PubMed] [Google Scholar]
- 43.Shiroza, T., and H. K. Kuramitsu. 1988. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J. Bacteriol. 170:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skorko-Glonek, J., A. Wawrzynow, K. Krzewski, K. Kurpierz, and B. Lipinska. 1995. Site-directed mutagenesis of the HtrA (DegP) serine protease, whose proteolytic activity is indispensable for Escherichia coli survival at elevated temperatures. Gene 163:47-52. [DOI] [PubMed] [Google Scholar]
- 45.Smith, D. J., W. F. King, L. A. Barnes, Z. Peacock, and M. A. Taubman. 2003. Immunogenicity and protective immunity induced by synthetic peptides associated with putative immunodominant regions of Streptococcus mutans glucan-binding protein B. Infect. Immun. 71:1179-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatum, F. M., N. F. Cheville, and D. Morfitt. 1994. Cloning, characterization and construction of htrA and htrA-like mutants of Brucella abortus and their survival in BALB/c mice. Microb. Pathog. 17:23-36. [DOI] [PubMed] [Google Scholar]
- 47.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 48.Visick, J. E., and S. Clarke. 1995. Repair, refold, recycle: how bacteria can deal with spontaneous and environmental damage to proteins. Mol. Microbiol. 16:835-845. [DOI] [PubMed] [Google Scholar]
- 49.Wen, Z. T., and R. A. Burne. 2001. Construction of a new integration vector for use in Streptococcus mutans. Plasmid 45:31-36. [DOI] [PubMed] [Google Scholar]
- 50.Wen, Z. T., and R. A. Burne. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 68:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wonderling, L. D., B. J. Wilkinson, and D. O. Bayles. 2004. The htrA (degP) gene of Listeria monocytogenes 10403S is essential for optimal growth under stress conditions. Appl. Environ. Microbiol. 70:1935-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita, Y., W. H. Bowen, R. A. Burne, and H. K. Kuramitsu. 1993. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 61:3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin, J. L., N. A. Shackel, A. Zekry, P. H. McGuinness, C. Richards, K. V. Putten, G. W. McCaughan, J. M. Eris, and G. A. Bishop. 2001. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR green I. Immunol. Cell Biol. 79:213-221. [DOI] [PubMed] [Google Scholar]