Abstract
The therapeutic potential of curcumin for many diseases are intensively investigated. However, real-world observational data documenting health and longevity effects associated with dietary curcumin in turmeric from consuming curry in food is lacking. A prospective cohort study of 4551 adults aged 55 + assessed curry consumption (never or < once/year, ≥ once/year to < once/month, ≥ once/month to < once/week, ≥ once/week to < daily, ≥ once daily), prevalent health conditions, blood biomarker indexes of atherogenicity, insulin resistance, and inflammation at baseline, and mean (SD) 11.6 (3.8) year follow up of all-cause, CVS and cancer mortality. There were linear positive associations of increasing curry consumption with waist circumference, fasting blood glucose, TyG, AIP, CRI-1, CRI-2, central obesity and diabetes prevalence, and inverse association with eGFR. There were non-linear associations with FEV1/height2 and COPD prevalence, GDS score and depression, MMSE score and cognitive impairment, comorbidity count, serum albumin and haemoglobin, being most favourable with moderate consumption. The levels of NLR, PLR and SII indices of systemic and immune inflammation decreased linearly with curry consumption. Total mortality HR adjusted for baseline co-variables, decreased across curry consumption, 0.68 (95%CI 0.56–0.82), 0.54 (95%CI 0.43–0.69), 0.70 (0.52–0.93), and 0.62 (0.41–0.95), being lowest in the middle categories. Among participants with cardio-metabolic and vascular diseases (CMVD), at least occasional curry consumption was associated with decreased mortality risk by 39%, and increased life expectancy by 1.0 years. Among those without CMVD, the associated life expectancy increase was 1.9 years. Moderate curry consumption may confer meaningful longevity benefits.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00842-1.
Keywords: Observational study, Mortality, Health, Cardio-metabolic, Cancer, Biomarkers
Introduction
Turmeric, a common spice obtained from the roots of Curcuma longa, has been in use as a traditional Indian and Chinese medicine for centuries. Curcumin isolated from turmeric is a non-toxic polyphenolic compound which has attracted great scientific interest for its therapeutic potentials. Its powerful antioxidant, anti-inflammatory, anti-neoplastic, antimicrobial, antiviral and anti-aging activities are well documented in numerous laboratory studies [1–5]. The potential of curcumin as a low-cost and well-tolerated treatment for many diseases including cancers, diabetes, obesity, cardiovascular, pulmonary, neurological and inflammatory diseases have been investigated in over 100 randomized controlled trials (RCT). Uncertainty still surround the clinical efficacy of curcumin even for those deemed most promising (in cardio-metabolic and inflammatory disorders), in which its antioxidant and anti-inflammatory actions play crucial roles. In clinical studies, curcumin reduces the levels of proinflammatory cytokines IL-1 and TNF-α [6], with dose-responses analyses indicating non-linear effects. Experimental data suggest that curcumin is notably a hormetic agent (hormetin), exhibiting biphasic dose-responses. It has stronger effects at low doses than high doses for functions such as activation of protein kinase signalling pathway), while acting at high doses for other functions such as autophagy and cell death [7].
Curcumin is most widely and naturally consumed in food through the turmeric spice commonly used to prepare curry meals among Asians. There is a paucity of studies documenting real world observations of the health and longevity effects associated with the consumption of food with curry rich in curcumin. In this population-based prospective cohort study, we investigated curry consumption and associated profiles of health and blood biomarkers of atherogenicity, insulin resistance and inflammation, and its association with 12-year mortality risk among 4,500 middle-aged and older adults in the Singapore Longitudinal Ageing Study (SLAS).
Methods
Study design and participants
We analysed data in two combined population cohorts (N = 6074), SLAS-1 and SLAS-2, of older adults aged ≥ 55 years at recruitment, excluding individuals who were unable to participate due to severe physical or mental disability. Details of the methodology have been previously described [8, 9]. Briefly, SLAS-1 recruited 2804 residents from Sep 2003–Dec 2004, and SLAS-2 recruited 3270 residents from Mar 2009–Jun 2013. Trained nurses and research assistants performed face-to-face questionnaire interviews and clinical and fasting blood sample measurements to collect a wide range of socio-demographic, lifestyle, behaviour, psychological, neurobehavioral, medical and other data at baseline. The first follow-up visits were conducted approximately 3–5 years after the initial interview. Participants were followed up over mean (SD) of 11.6 (SD 3.8) years on mortality outcome. Ethics Approval. The study was approved by National University of Singapore IRB (Ref: 04–140). All participants gave written informed consent to participate in the study. ClinicalTrials.gov Identifier: NCT03405675.
Measurement data
Baseline data of participants were available for curry consumption (N = 5226), metabolic profile (waist circumference, blood pressure and hypertension, fasting blood glucose (FBG) and diabetes, and lipid panel, N = 5533), estimated glomerular filtration rate (eGFR, N = 5433), and blood cell count (N = 5616). This resulted in a complete analytical set of data belonging to 4551 study participants. Mortality follow up was complete for all participants.
Curry consumption
Participants were asked at baseline and follow-up interviews how frequently they usually consumed curry: never or rarely (< once a year), occasionally (at least once/year to less than once/month), often (at least once/month to less than once/week, very often (at least once a week or daily). Participants were also asked whether their curry consumption had remained mostly unchanged or have changed. Strictly consistent responses at baseline and follow up interviews were used to assign participants unequivocally to the highest (daily) and lowest (never or rarely) consumption category. Participants who consistently reported ‘occasional’ or ‘often’ or ‘very often’ consumption frequencies at baseline and follow up were also assigned as such to these categories. For slightly inconsistent responses (one frequency category apart), the higher consumption response was used to assign to the nearest category. For more widely inconsistent responses, we used the average response score to assign them to the nearest approximate response category, ‘occasional’, ‘often’ or ‘very often’.
Demographic and lifestyle measures
Demographic variables included age, sex and education (0 years, 1–6 years or > 6 years). Housing characteristic (low-end 1–2 room public housing apartments, 3 rooms, or higher-end 4 + rooms or others) was used to indicate socio-economic status based on the Singapore population census data. Social isolation was assessed by marital status (single, divorced or widowed versus married) and living arrangement (live alone versus living with others). The level of instrumental social support was assessed by summed scores on three questions: having someone to confide (0 = no, 1 = yes), frequency of visits by family or friends, or phone calls by family or friends (0 = none, 1 = at least once/year, 2 = at least once/month, 3 = at least once week). Lifestyle factors included number and frequency of participation (on a 5-point Likert scale) in 16 categories of physical, social and productive activities to derive aggregate score with higher scores indicating greater participation [8].
Health measures
The presence of chronic diseases was determined by a structured questionnaire listing diagnosis and treatment of 17 named and other medical conditions, corroborated by drug names on medication packages, or report of diagnostic procedures, or clinical or blood measurements. Fasted blood samples were analysed for total blood cell counts, haemoglobin, serum albumin, fasting blood glucose (FBG), lipid profiles: total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), triglyceride (TG), and eGFR.
Cardio-metabolic and vascular diseases (CMVD)
Prevalent cardiovascular disease was assessed by self-reported history of myocardial infarct, heart failure, atrial fibrillation, peripheral vascular disease or stroke. Prediabetes or diabetes was determined by FBG ≥ 5.6 mmol/L or self-reported history of diabetes diagnosis and anti-diabetic treatment. Hypertension was determined by self-reported history of hypertension diagnosis and anti-hypertensive treatment, or measurements of systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg. Central obesity was defined by waist circumference ≥ 80 cm in women and ≥ 90 cm in men. Dyslipidemia was defined by TG ≥ 1.7 mmol/L or HDL-c < 1 mmol/L in men and < 1.3 mmol/L in women, or use of lipid lowering drugs, based on clinical practice guideline recommendations.
Other health measures
Pulmonary function including forced expiratory volume in 1 s (FEV1), and forced vital capacity (FVC), and the presence of chronic obstructive pulmonary disease (FEV1/FVC < 0.70) was assessed by pre-bronchodilatation spirometry (the ndd EasyOne™ Spirometer) [10], in accordance with the American Thoracic Society standardization of spirometric tests. Depression was assessed by summed scores of the Geriatric Depression Scale 15-items (GDS-15) score (0–15), and the presence of depressive symptoms was defined by GDS ≥ 5) [11]. Prevalent depression was defined by GDS ≥ 5 or history of depression in the previous year or the use of anti-depressant medications. Global cognitive function was assessed by scores on the validated translated and modified version of the Mini-Mental State Examination (MMSE) [12], and cognitive impairment was defined by MMSE ≤ 23. Serum creatinine was measured by the Jaffe’s kinetic method and eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration equation in the Chinese population [13]. The presence of chronic kidney disease (CKD) was defined by eGFR below 60 mL/min/1.73 m2.
Metabolic indices
Fasting blood measurements of TC, LDLc, HDLc, TG and FBG in mmol/L concentrations were measured using standard laboratory methods. They were used to calculate atherogenic indexes including Atherogenic Index of Plasma (AIP) = log10 (TG/HDLc), Castelli Risk Index-1 (CRI-1 = TC/HDLc) and Castelli Risk Index-2 (CRI-II = HDLc/LDLc), which have been documented to be more robust predictors of atherosclerosis and cardiovascular events [14, 15] than individual lipid abnormalities. (The Atherogenic Coefficient (AC = TC –HDLc / HDLc) was also calculated, but given its singularity with the CRI-1, was omitted in the analysis). Triglyceride-glucose index (TyG), calculated using mg/dl concentrations of fasting TG and FBG by Ln(TG × FBG/2), is a validated surrogate index of insulin resistance and diagnostic marker of metabolic syndrome [16].
Blood inflammatory markers
Simple routine blood counts of neutrophils (N), lymphocytes (L) and platelets (P) were used to calculate neutrophil–lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), and systemic and immune inflammation (SII) index = P x N/L), which have been shown in recent years to be accurate and reliable measures of systemic and immune inflammation, and useful for clinical prognostic evaluation of patients with cancers, cardio-metabolic syndromes, and COPD, and others [17–21].
Mortality outcome
Date and cause of death from baseline up to 31 Dec 2020 was determined using the participant’s unique National Registration Identity Card number for computerized record linkage with the National Death Registry through the National Disease Registry Office of the Ministry of Health.
Statistical analysis
Differences in prevalent health biomarkers between curry exposure groups were examined using ANOVA for continuous variables, and Chi-square for categorical variables, using general linear models and logistic regression to control for confounding by risk factor. The association of curry consumption with mortality was evaluated using Kaplan–Meier survival analysis and Cox proportional hazards regression analysis. Hazard ratio (HR) and 95% confidence intervals (95%CI) were estimated in hierarchical models controlling for age, sex and ethnicity (Model 1), and additionally for socio-demographic and behavioural factors (Model 2), health and blood biomarker indices (Model 3), and inflammatory indices (Model 4). The joint exposure of CMVDs with curcumin consumption was assessed by a four-category variable: (1) CMD non-consumer: CMDs with never/rarely curry consumption, (2) CMD consumer: CMDs with at least occasional to daily curry consumption, (3) CMD-free non-consumer: CMD-free with never/rarely curry consumption, (4) CMD-free consumer: CMD-free with at least occasional to daily curry consumption. All statistical analyses were performed using IBM SPSS Statistics version 27.
Results
Socio-demographic and behavioural profile
The mean (SD) age of the study participants (N = 4551) was 66.1 (7.6) years, and 63.4% were women. Among them, 18% never or rarely consumed curry, and 82% consumed curry at least occasionally (once a year); 11% consumed curry at least once/week but not daily, and 3% consumed curry daily.
Curry consumption and baseline characteristics (Table 1)
Table 1.
Baseline characteristics of SLAS study participants (N = 4551) by curry consumption levels at baseline
| Never or rarely | Occasional | Often | Very Often | Daily | Crude | Adjusted* | |
|---|---|---|---|---|---|---|---|
| Never or < once/year | > once/year, < once/month | > once/month, < once/week | > once/week, not daily | ≥ once daily | p | P | |
| N of participants | 828 | 2060 | 1006 | 514 | 143 | ||
| Sex: Women | 68.5 (567) | 66.1 (1361) | 60.4 (608) | 52.5 (270) | 52.5 (81) | < 0.001c | |
| Age, years | 68.1 ± 8.2 | 65.9 ± 7.3 | 65.6 ± 7.3 | 64.9 ± 7.2 | 66.8 ± 7.6 | 0.001b | |
| Ethnicity: Chinese | 97.0 (803) | 94.4 (1945) | 90.3 (908) | 72.9 (375) | 63.6 (91) | ||
| Malay, Indian and Other | 3.0 (25) | 5.6 (115) | 9.8 (98) | 27.1 (139) | 36.4 (52) | < 0.001c | |
| Education: 0 years | 28.1 (233) | 16.3 (336) | 13.8 (139) | 13.4 (69) | 23.8 (34) | < 0.001 | |
| 1–6 years | 41.2 (341) | 38.8 (800) | 34.3 (345) | 33.7 (173) | 41.3 (59) | ||
| > 6 years | 30.7 (254) | 44.9 (924) | 51.9 (522) | 52.9 (272) | 35.0 (50) | ||
| Housing: 1–2 room | 17.3 (143) | 14.3 (294) | 10.4 (105) | 19.1(98) | 19.6 (28) | < 0.001 | |
| 3-room | 30.0 (248) | 26.4 (543) | 25.0 (252) | 24.3 (125) | 30.8 (44) | ||
| 4 + room and private | 52.8 (437) | 59.4 (1223) | 64.5 (649) | 56.6 (291) | 49.7 (71) | ||
| Single/divorced/widowed | 36.8 (305) | 30.0 (617) | 27.0 (272) | 33.1 (170) | 31.5 (45) | < 0.001 | |
| Instrumental support (0–13) | 29.7 (246) | 23.8 (490) | 21.0 (211) | 24.9 (128) | 25.2 (36) | < 0.001 | |
| (14–15) | 36.2 (300) | 37.5 (772) | 35.2 (354) | 35.6 (183) | 36.4 (52) | ||
| (16–17) | 34.1 (282) | 38.7 (798) | 43.8 (441) | 39.5 (203) | 38.5 (55) | ||
| Smoking: Past smoker | 11.7 (97) | 8.8 (181) | 11.5 (116) | 13.2 (68) | 11.2 (16) | < 0.001 | |
| Current smoker | 7.2 (60) | 7.6 (156) | 6.3 (63) | 11.9 (61) | 9.8 (14) | ||
| Alcohol > = once /week | 4.5 (37) | 3.6 (74) | 5.3 (53) | 6.4 (33) | 5.6 (8) | 0.024 | |
| Physical activity score | 2.18 ± 1.75 | 2.35 ± 1.68 | 2.32 ± 1.79 | 2.35 ± 1.80 | 1.92 ± 1.61 | 0.004 | |
| Social activity score | 2.85 ± 2.51 | 3.17 ± 2.46 | 3.27 ± 2.48 | 3.11 ± 2.49 | 2.48 ± 2.07 | 0.001 | |
| Productive activity score | 3.72 ± 1.92 | 3.87 ± 1.82 | 4.12 ± 1.90 | 3.98 ± 1.91 | 3.59 ± 1.77 | 0.001 | |
| Medical comorbidities count | 2.45 ± 1.58 | 2.33 ± 1.47 | 2.37 ± 1.47 | 2.36 ± 1.52 | 2.62 ± 1.47 | 0.018b | 0.349a |
| Cardiovascular diseases | 13.0 (108) | 11.0 (226) | 10.7 (108) | 10.7 (55) | 12.6 (18) | 0.483 | 0.830 |
| Hypertension | 64.1 (531) | 59.7 (1229) | 62.8 (632) | 64.0 (329) | 65.0 (93) | 0.090 | 0.568 |
| Central obesity | 47.9 (397) | 52.6 (1083) | 53.3 (536) | 56.2 (289) | 55.9 (80) | 0.003c | 0.026 |
| Diabetes | 16.2 (134) | 14.7 (302) | 14.9 (150) | 15.8 (81) | 26.6 (38) | 0.001c | 0.316 |
| Metabolic syndrome | 43.0 (356) | 47.5 (978) | 46.3 (466) | 47.9 (246) | 53.1 (76) | 0.045 | 0.022 |
| COPD (FEV1/FVC < 0.70) | 22.9 (190) | 17.8 (366) | 18.3 (184) | 14.6 (75) | 15.4 (22) | < 0.001c | 0.305 |
| Depression (GDS ≥ 5, treatment) | 9.8 (41) | 5.8 (60) | 3.6 (29) | 7.6 (30) | 7.7 (21) | < 0.001 | 0.030 |
| Cognitive impairment (MMSE < 23) | 13.0 (108) | 6.0 (123) | 6.2 (62) | 9.3 (48) | 15.4 (22) | < 0.001 | 0.008 |
| Chronic kidney disease | 5.6 (46) | 4.1 (84) | 5.2 (52) | 5.1 (26) | 5.6 (8) | 0.410 | 0.905 |
| Cancer | 2.1 (17) | 2.7 (56) | 2.6 (26) | 1.6 (8) | 0.7 (1) | 0.305 | 0.417 |
| Gastro-intestinal conditions | 5.9 (49) | 6.9 (142) | 6.3 (63) | 5.4 (28) | 7.0 (10) | 0.727 | 0.661 |
| Musculoskeletal conditions | 19.2 (159) | 16.1 (331) | 17.7 (178) | 17.2 (88) | 15.4 (22) | 0.330 | 0.302 |
| GDS score | 1.44 ± 2.33 | 1.00 ± 1.82 | 1.07 ± 2.04 | 1.14 ± 2.16 | 1.75 ± 2.81 | < 0.001b | 0.001b |
| MMSE score | 26.9 ± 3.5 | 27.9 ± 2.6 | 27.9 ± 2.6 | 27.8 ± 3.0 | 26.6 ± 3.4 | < 0.001b | 0.001b |
| FEV1/Ht, L/m | 1.05 ± 0.30 | 1.15 ± 0.30 | 1.15 ± 0.31 | 1.18 ± 0.33 | 1.05 ± 0.33 | < 0.001b | 0.002b |
| Albumin | 42.1 ± 3.0 | 42.4 ± 2.7 | 42.5 ± 2.6 | 42.5 ± 2.4 | 42.1 ± 3.2 | 0.001b | 0.095b |
| Haemoglobin | 13.3 ± 1.3 | 13.4 ± 1.4 | 13.5 ± 1.3 | 13.7 ± 1.5 | 13.4 ± 1.5 | < 0.001b | 0.178b |
| eGFR | 119.3 ± 40.5 | 113.0 ± 37.9 | 115.7 ± 41.5 | 113.1 ± 40.7 | 109.7 ± 38.0 | 0.001a | 0.009b |
| Waist circumference (men) | 86.9 ± 9.8 | 88.1 ± .5 | 88.4 ± 8.4 | 88.9 ± 10.2 | 91.4 ± 11.5 | 0.011a | 0.029a |
| Waist circumference (women) | 80.2 ± 10.1 | 81.7 ± 9.9 | 81.9 ± 9.7 | 83.9 ± 10.6 | 82.8 ± 12.0 | < 0.001a | 0.016b |
| Sitting SBP | 131.0 ± 16.7 | 129.2 ± 15.5 | 131.1 ± 16.2 | 130.8 ± 16.8 | 130.7 ± 14.9 | 0.006b | 0.022b |
| Sitting DBP | 80.1 ± 10.1 | 80.6 ± 9.6 | 81.6 ± 9.5 | 82.3 ± 8.9 | 81.0 ± 10.3 | 0.001b | 0.068b |
| Fasting blood glucose, mmol/L | 5.31 ± 1.58 | 5.35 ± 1.51 | 5.40 ± 1.51 | 5.48 ± 1.61 | 5.83 ± 2.37 | 0.002a | 0.050b |
| TyG | 9.19 ± 0.54 | 9.23 ± 0.53 | 9.25 ± 0.53 | 9.25 ± 0.55 | 9.28 ± 0.61 | 0.009a | 0.122b |
| AIP = Log (TG/HDLc) | –0.08 ± 0.27 | –0.06 ± 0.27 | –0.05 ± 0.26 | –0.04 ± 0.27 | –0.05 ± 0.29 | 0.018a | 0.044b |
| CRI-I = TChol/HDL-Chol | 3.84 ± 1.06 | 3.85 ± 1.00 | 3.94 ± 1.00 | 3.96 ± 1.03 | 3.92 ± 1.25 | 0.006a | 0.289b |
| CRI-II = LDLc/HDLc | 2.37 ± 0.87 | 2.37 ± 0.80 | 2.45 ± 0.82 | 2.46 ± 0.82 | 2.42 ± 0.97 | 0.011a | 0.205b |
| LMR | 5.21 ± 2.45 | 5.33 ± 2.63 | 5.30 ± 1.88 | 5.11 ± 1.75 | 5.46 ± 1.91 | 0.242a | 0.304b |
| NLR | 1.83 ± 0.85 | 1.80 ± 0.84 | 1.75 ± 0.87 | 1.79 ± 0.76 | 1.65 ± 0.73 | 0.018a | 0.091a |
| PLR | 137.5 ± 55.1 | 139.9 ± 56.3 | 135.6 ± 53.4 | 133.4 ± 53.0 | 118.4 ± 40.0 | < 0.001a | 0.012b |
| SII index | 460.4 ± 269.4 | 458.9 ± 276.9 | 442.6 ± 263.5 | 456.3 ± 228.5 | 404.5 ± 199.8 | 0.022a | 0091b |
Figures shown are percentage (number) or mean ± SD
COPD: self-report or FEV1/FVC < 0.70; Chronic kidney disease; self-report or eGFR < 60 ml/min/1.73 m; Low HDL-Chol: HDL-Chol < 1.03 (male) or < 1.29 (female)
ANOVA: alinear-p; bquadratic-p; Chi-squared: clinear
*Adjusted for age, sex and ethnicity, education, housing, marital status, social support, smoking, alcohol, physical, social and productive activity
GDS: Geriatric Depression Scale; MMSE: Mini Mental State Examination; eGFR: estimated glomerular filtration rate;
TyG: Triglyceride-glucose index (TyG) = Ln(TG × FBG/2) calculated using mg/dl concentrations of fasting TG and FBG
AIP: Atherogenic Index of Plasma = log10 (TG/HDLc), CRI-1: Castelli Risk Index-1 = TC/HDLc; CRI-II: Castelli Risk Index-2 = HDLc/LDLc;
NLR: neutrophil–lymphocyte ratio, LMR: lymphocyte-monocyte ratio, PLR: platelet-lymphocyte ratio, SII index: systemic and immune inflammation index
There were significant linear trends across curry consumption groups of fewer women or Chinese, but non-linear associations were found for other sociodemographic factors. Participants who never/rarely or daily consumed curry were more likely to be older, less educated, housed in lower-end apartments, single/divorced/widowed, and had less instrumental social support. They were more likely to be smokers, alcohol drinkers, and less physically, socially and vocationally active.
Cardio-metabolic and vascular disease
Waist circumference and prevalent central obesity, FBG, TyG and prevalent diabetes increased linearly with increasing curry consumption, and were highest in those who consumed curry at least once/week or daily. Higher levels of AIP, CRI-1 and CRI-2 indices were also linearly associated with increasing curry consumption. Systolic BP and prevalent hypertension showed no discernible trends across curry consumption level, but diastolic BP showed an inverse U-shaped trend. eGFR decreased linearly across increasing curry consumption. The prevalence cardiovascular disease was not significantly different across curry consumption levels.
Other clinical and blood measurement profiles
There were non-linear trends of association of curry consumption with FEV1/height2 and COPD prevalence, GDS score and depression, MMSE score and cognitive impairment, as well as comorbidity count, being most favourable in those with moderate curry consumption, between ≥ once year and ≥ once/month to < daily. Serum albumin and haemoglobin also showed significant non-linear relationships, and were most favourable in the middle curry consumption categories. There were no significant differences in the prevalence of cancer, musculoskeletal and gastro-intestinal conditions.
Inflammation biomarkers
The levels of NLR, PLR and SII indices of systemic and immune inflammation showed significantly decreasing linear trends with increasing curry consumption.
Mortality
A total of 831 deaths occurred over 52,958 person-years of observation Table 2. All-cause mortality rate was highest among participants who never/rarely consumed curry, and decreased to the lowest among those who consumed curry often, thereafter to a higher level among daily consumers. Adjusted for age, sex and ethnicity (base model), the HR referenced to never/rarely (HR = 1) were 0.64, 0.47, 0.66 and 0.59.
Table 2.
All-cause mortality rates by curry consumption (N = 4552)
| Sample | Curry consumption | N | Person-years (py) | Deaths | Mortality rate per 1000 py | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% | CI | ||||||
| Whole | Never or rarely | (Never or < once/year) | 828 | 9568.6 | 250 | 26.1 | 23.0 | 29.5 |
| Occasionally | (> once/year, < once/month | 2060 | 23522.9 | 317 | 13.5 | 12.0, | 15.0 | |
| Often | (> once/month, < once/week) | 1006 | 12201.1 | 140 | 11.5 | 9.7 | 13.5 | |
| Very often | (> once/week, not daily) | 514 | 5891.3 | 90 | 15.3 | 12.4 | 18.7 | |
| Daily | (≥ once daily) | 143 | 1774.5 | 34 | 19.2 | 13.5 | 26.5 | |
| CMVD: Yes | Never or rarely | (Never or < once/year) | 400 | 4435.2 | 153 | 34.5 | 29.3 | 40.3 |
| CMVD: Yes | Occasional to daily | (> once/year to daily) | 1919 | 21902.2 | 360 | 16.4 | 14.8 | 18.2 |
| CMVD: No | Never or rarely | (Never or < once/year) | 428 | 5133.3 | 97 | 18.9 | 15.4 | 22.9 |
| CMVD: No | Occasional to daily | (> once/year to daily) | 1804 | 21487.5 | 221 | 10.3 | 8.8 | 11.5 |
Additional co-variables in the model altered HRs toward the null, but the adjusted HRs remained significantly decreased, by 32% in those with occasional curry consumption, maximally at 46% in those who often consumed curry, but with no greater decrease in all-cause mortality (30% and 38%) at higher consumption levels (Table 3, Fig. 1). There were similar results for CVS and cancer mortality. In Kaplan–Meier survival analysis, the mean survival time was 14.4 ± SE 0.17 years, 15.7 years ± SE 0.09 years, 16.0 ± SE 0.12 years, 15.5 ± SE 0.20 years, and 15.0 ± 0.40 years respectively in participants who never or rarely, occasionally, often, very often and daily consumed curry, suggesting possible increased life expectancy by 0.6 to 1.6 years.
Table 3.
Associations of curry consumption with all-cause, CVS and cancer mortality risks
| All-cause mortality | CVS mortality | Cancer mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Curry consumption | HR | 95% | CI | HR | 95% | CI | HR | 95% | CI | ||||
| Model 1 | Never or rarely | 1 | 1 | 1 | |||||||||
| Occasionally | 0.64 | 0.54 | 0.76 | *** | 0.63 | 0.46 | 0.86 | ** | 0.62 | 0.47 | 0.84 | ** | |
| Often | 0.47 | 0.38 | 0.57 | *** | 0.48 | 0.33 | 0.70 | *** | 0.48 | 0.33 | 0.69 | ** | |
| Very often | 0.66 | 0.51 | 0.85 | ** | 0.57 | 0.36 | 0.91 | * | 0.65 | 0.42 | 1.02 | ||
| Daily | 0.59 | 0.41 | 0.86 | ** | 0.48 | 0.24 | 0.95 | * | 0.69 | 0.36 | 1.33 | ||
| Model 2 | Never or rarely | 1 | 1 | 1 | |||||||||
| Occasionally | 0.68 | 0.57 | 0.81 | *** | 0.70 | 0.52 | 0.95 | * | 0.62 | 0.46 | 0.83 | ** | |
| Often | 0.50 | 0.41 | 0.62 | *** | 0.54 | 0.37 | 0.79 | ** | 0.47 | 0.33 | 0.69 | *** | |
| Very often | 0.72 | 0.56 | 0.93 | * | 0.68 | 0.43 | 1.09 | 0.63 | 0.40 | 0.98 | * | ||
| Daily | 0.60 | 0.41 | 0.86 | ** | 0.49 | 0.24 | 0.97 | * | 0.67 | 0.35 | 1.29 | ||
| Model 3 | Never or rarely | 1 | 1 | 1 | |||||||||
| Occasionally | 0.68 | 0.57 | 0.83 | *** | 0.71 | 0.50 | 0.99 | * | 0.59 | 0.43 | 0.82 | ** | |
| Often | 0.54 | 0.43 | 0.69 | *** | 0.57 | 0.37 | 0.87 | ** | 0.45 | 0.30 | 0.68 | *** | |
| Very often | 0.69 | 0.52 | 0.92 | * | 0.74 | 0.44 | 1.23 | 0.49 | 0.29 | 0.83 | ** | ||
| Daily | 0.61 | 0.40 | 0.93 | * | 0.54 | 0.25 | 1.17 | 0.78 | 0.39 | 1.55 | |||
| Model 4 | Never or rarely | 1 | 1 | 1 | |||||||||
| Occasionally | 0.68 | 0.56 | 0.82 | *** | 0.71 | 0.50 | 1.01 | 0.59 | 0.42 | 0.81 | *** | ||
| Often | 0.54 | 0.43 | 0.69 | *** | 0.58 | 0.38 | 0.88 | * | 0.45 | 0.30 | 0.67 | *** | |
| Very often | 0.70 | 0.52 | 0.93 | * | 0.75 | 0.44 | 1.25 | 0.49 | 0.29 | 0.83 | ** | ||
| Daily | 0.62 | 0.41 | 0.95 | * | 0.54 | 0.25 | 1.17 | 0.80 | 0.40 | 1.60 | |||
Model 1: age, sex, ethnicity
Model 2: Model 1 + housing type, education, marital status, living alone, instrumental social support score, smoking, alcohol, physical activity, social activity, productive activity
Model 3: Model 2 + comorbidity count, metabolic syndrome, waist circumference, SBP, DBP, fasting blood glucose, eGFR, GDS score, MMSE score, FEV1/Ht, albumin, haemoglobin,TyG, AIP, CRI-1, CRI-2,
Model 4: Model 3 + blood inflammatory indices
*p < 0.05, **p < 0.01, ***p < 0.001
Fig. 1.
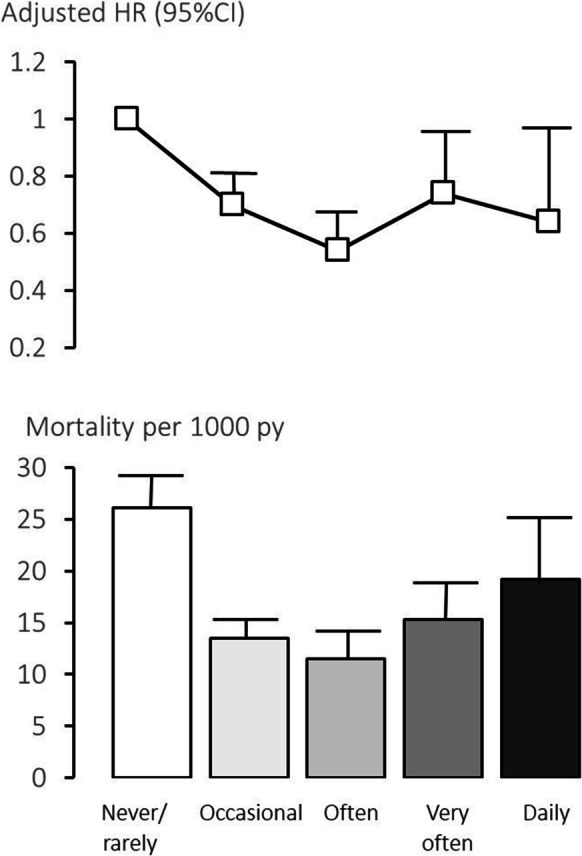
12-year mortality rate and adjusted hazard ratio (HR) associated with curry consumption (Singapore Longitudinal Ageing Study). Footnote: Usual curry consumption frequency. ‘Never or rarely’: < once a year), ‘occasionally’: at least once/year to less than once/month, ‘often’: at least once/month to less than once/week, ‘very often’: at least once a week or daily. Hazard ratio (HR): Adjusted for age, sex, ethnicity, housing type, education, marital status, living alone, instrumental social support score, smoking, alcohol, physical activity, social activity, productive activity, comorbidity count, metabolic syndrome, waist circumference, SBP, DBP, fasting blood glucose, eGFR, GDS score, MMSE score, FEV1/Ht, albumin, haemoglobin, TyG, AIP, CRI-1, CRI-2, and blood inflammatory indices. Reference exposure category: ‘never or rarely’
Joint effect analysis
Referent to those with CMDs who rarely or never consumed curry, the multivariable-adjusted mortality HR suggested that curry consumption reduced the CMD-related mortality risk by approximately 39% (Table 4). In Kaplan–Meier survival analysis, the mean survival duration was 15.2 ± SE 0.22 years in participants with CMDs who never or rarely consumed curry, and 16.2 years ± SE 0.09 years in those with CMVDs who occasionally to daily consumed curry. The mean survival duration was 13.5 ± SE 0.27 years in those who were CMVD-free and never or rarely consumed curry, and 15.4 ± 0.10 years in those who were CMVD-free and occasionally to daily consumed curry, suggesting increased life expectancy by 1.0 years among those with CMVDs and by 1.9 years among those without CMVD (Fig. 2).
Table 4.
Associations of curry consumption with all-cause mortality risks by cardio-metabolic and vascular disease
| Cardio-metabolic and vascular disease | Curry consumption | HR | 95% | CI | ||
|---|---|---|---|---|---|---|
| Adjusted for age, sex, ethnicity | Yes | Never or rarely | ||||
| Yes | At once/year to daily | 0.54 | 0.45 | 0.66 | *** | |
| No | Never or rarely | 0.70 | 0.55 | 0.91 | ** | |
| No | At once/year to daily | 0.45 | 0.36 | 0.55 | *** | |
| Adjusted for age, sex, ethnicity, social, economic, health behaviour, other medical morbidities and blood biomarkers †¶ | Yes | Never or rarely | 1 | |||
| Yes | At once/year to daily | 0.61 | 0.47 | 0.76 | *** | |
| No | Never or rarely | 0.93 | 0.69 | 1.24 | ||
| No | At once/year to daily | 0.61 | 0.47 | 0.78 | *** |
†Age, sex, ethnicity, housing type, education, marital status, living alone, instrumental social support score, smoking, alcohol, physical, social and productive activity scores, comorbidity count, cancer, depression, cognitive impairment, eGFR, FEV1/Ht, Hb, Alb
¶Statistical interaction (CMVD x curry), p = 0.006
*p < 0.05, **p < 0.01, ***p < 0.001
Fig. 2.
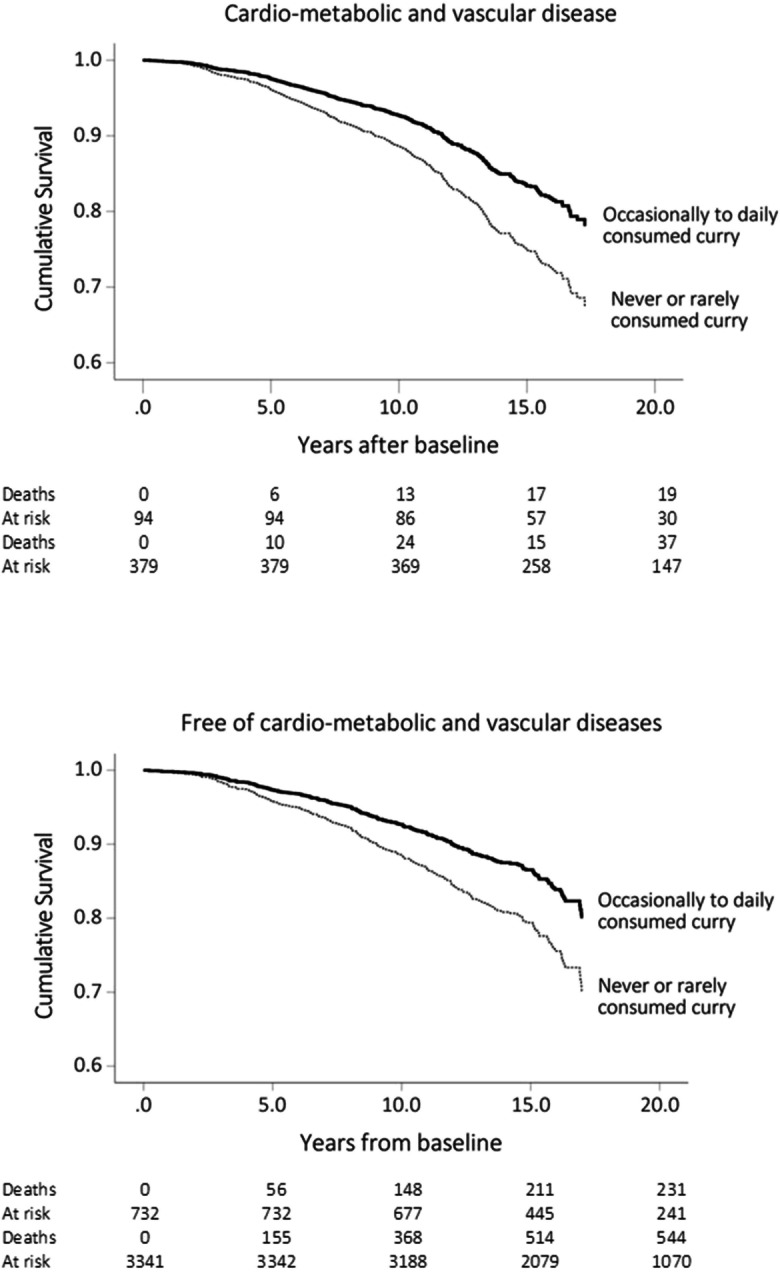
Kaplan–Meier cumulative survivals by curry consumption at least one a year to daily versus never or rarely (less than once a year) among participants with cardio-metabolic and vascular diseases (top panel) and those without (bottom panel)
Discussion
Cardio-metabolic health effects
Laboratory studies suggest curcumin improves glucose homeostasis, lipid metabolism, endothelial function and insulin signalling [2], and decreases cardiac hypertrophy and chronic heart failure [4], but clinical evidence of cardio-metabolic health benefits remain limited or contradictory [22, 23]. Among a few naturalistic studies, a randomized, controlled crossover study has demonstrated that a single curry meal consumption significantly improved postprandial endothelial function in healthy Japanese men [24]. Only one population-based observational study has reported significantly lower blood glucose and triglyceride levels in Koreans with moderate (2–4 times month) curry consumption versus no curry consumption [25]. However, in our observational study, participants with greater curry consumption were more likely to show an adverse cardio-metabolic profile. This was not unexpected in this study population. Curry meals are typically prepared with meat or vegetables together with other ingredients such as coconut milk or dairy milk, sugar, and cooking oils with higher content of saturated fatty acids (ghee, coconut oil, palm oil, corn oil). It is thus probable that the dietary consumption of turmeric is associated with higher calorie and saturated fats intake in the Singapore population.
Longevity effect
Remarkably, despite their adverse cardio-metabolic profile, participants with occasional to daily curry consumption had 12-year all-cause and CVS-related mortality risks that were between 30 and 46% lower than those who never or rarely consumed curry. Curry consumption also appeared to lower cardio-metabolic-related mortality by 39%. This striking paradox may be explained by the decreasing levels of NLR, PLR and SII indices of systemic and immune inflammation associated with increasing curry consumption, suggesting the protective anti-inflammatory effects of curcumin.
Hormetic dose–response
Laboratory studies indicate a dose dependent effect of curcumin for some biological functions which are greater at lower doses [9, 10]. Our results showed discernible U-shaped trends of relationships with mortality risks, as well as for prevalent GDS/depression, MMSE/cognitive impairment, and levels of serum albumin and haemoglobin, most favourable in the low to moderate range of curry consumption. On the other hand, the results suggest a linear relationship between increasing curry consumption and lower COPD prevalence.
Biological mechanisms
On its own, curcumin as a pure chemical compound has low water solubility and bioavailability and is rapidly metabolised [26]. For this reason, lipid-based therapeutic formulations of curcumin in the forms of liposomes, polymeric micelles, phospholipid complexes, and micro-emulsions or nanoparticles are used in clinical trials. The observable effects of dietary intake of curcumin may be made possible by different routes to systemic bioavailability and efficacy. Dietary curcumin is ingested via turmeric, which contains structural analogues of essential oils (turmeric oil) such as zingiberene, α-Turmerone and β-Turmerone [27]. Spices such as chilli, pepper, cumin, coriander, and plant-based cooking oils, are commonly blended with turmeric in the preparation of curry meals. Turmeric oil, together with spice oils, as well as the actions of intestinal bile salts, may act as a unique lipid-based oral delivery system for enhanced solubility and bioavailability. Moreover, the combination of piperine in black pepper with curcumin in turmeric has been shown to increase the bioavailability of curcumin by 2000%, as well as inhibiting the intestinal and hepatic glucuronidation of curcumin [28]. High concentrations of curcumin remain in the intestines after oral ingestion. Curcumin could exert direct regulatory effects on physiological systems primarily in the gastrointestinal tract. Bacterial enzymatic actions on curcumin may form pharmacologically more active metabolites than curcumin. Studies show that by enhancing the diversity of the gut microbiota, curcumin and its metabolites restore gut microbial dysbiosis, decreases gut permeability and inflammation, and metabolic endotoxemia associated with a high fat diet, obesity, metabolic syndrome, diabetes, CVD and neurodegenerative diseases [29].
Turmeric is often used in curry meals together with other culinary herbs and spices such as cardamom, cinnamon, coriander seeds, fennel, and others that have also been investigated for their therapeutic potentials as anti-tumour, ant-microbial, ant-inflammatory and anti-oxidative agents with broad spectrum benefits across multiple diseases [30–32]. The health and longevity benefits observed in this study may arguably be attributed not only to turmeric and curcumin, but to other spices as well. However, curcumin is the most prominent and commonly used spice among them, and, it has the most extensive body of scientific data in support of its potential health and longevity benefits, far more than any other spice. This should be explicated with more studies.
Strengths and limitations
Potential exposure misclassification of curry consumption was minimized by repeated baseline and follow up measurements, and mortality ascertainment was complete. Some observed associations between curry consumption and baseline health and biomarker profiles may be subject to prevalence bias, hence calling for caution in interpretation. Curry consumption is associated with many socio-demographic and health behavioural factors in this population. Although their confounding influences were controlled in multivariable analyses, the possibility of residual confounding cannot be completely excluded. As curry consumption is especially related to non-Chinese ethnicities and associated socio-economic factors, we performed a sensitivity analysis by repeating the Cox regression modelling using only data of participants of Chinese ethnicity. The results shown in the online Supplementary Table S1 reveal very similar findings. Finally, turmeric consumption in this study may be typical of populations in South and South East Asia, where turmeric is commonly used in cooking, but more studies should be conducted in a diversity of other populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the following voluntary welfare organizations for their support: Geylang East Home for the Aged, Presbyterian Community Services, St Luke’s Eldercare Services, Thye Hua Kwan Moral Society (Moral Neighbourhood Links), Yuhua Neighbourhood Link, Henderson Senior Citizens’ Home, NTUC Eldercare Co-op Ltd, Thong Kheng Seniors Activity Centre (Queenstown Centre) and Redhill Moral Seniors Activity Centre.
Abbreviations
- AIP
Atherogenic Index of Plasma (AIP)
- CRI-1
Castelli Risk Index-1
- CRI-2
Castelli Risk Index-2
- TyG
Triglyceride-glucose index
- NLR
Neutrophil-lymphocyte ratio
- LMR
Lymphocyte-monocyte ratio
- PLR
Platelet-lymphocyte ratio
- SIII
Systemic and immune inflammation index
- GDS
Geriatric Depression Scale
- MMSE
Mini Mental State Examination
- eGFR
Estimated glomerular filtration rate
- FEV1
Forced expiratory volume in one second
- COPD
Chronic obstructive pulmonary disease
Authors’ contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Tze Pin Ng, Shwe Zin Nyunt, Qi Gao, Xinyi Gwee, Denise Qian Ling Chua, and Keng Bee Yap. Data analysis was performed by Tze Pin Ng. The first draft of the manuscript was written by Tze Pin Ng, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by research grants from the Agency for Science Technology and Research (A*STAR) Biomedical Research Council (grant number BMRC/08/1/21/19/567) and the National Medical Research Council (grant numbers NMRC/1108/2007, NMRC/CIRG/1409/2014.
Data availability
Data is available upon request from the corresponding authors.
Declarations
Conflict of interest
The authors declare no conflict of interest, including relevant financial interests, activities, relationships or affiliations.
Role of the funding source
The study sponsor had no role in the study design, collection, analysis, and interpretation of data, the writing of the report and in the decision to submit the paper for publication.
Footnotes
Trial registration. ClinicalTrials.gov: NCT03405675.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pagano E, Romano B, Izzo AA, Borrelli F. The clinical efficacy of curcumin-containing nutraceuticals: An overview of systematic reviews. Pharmacol Res. 2018;134:79–91. doi: 10.1016/j.phrs.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y, Clifton P. Curcumin, Cardiometabolic health and dementia. Int J Environ Res Public Health. 2018;15(10):2093. doi: 10.3390/ijerph15102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed Pharmacother. 2021;134:111119. doi: 10.1016/j.biopha.2020.111119. [DOI] [PubMed] [Google Scholar]
- 4.Xu XY, Meng X, Li S, Gan RY, Li Y, Li HB. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. 2018;10(10):1553. doi: 10.3390/nu10101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salehi B, Stojanović-Radić Z, Matejić J, et al. The therapeutic potential of curcumin: A review of clinical trials. Eur J Med Chem. 2019;1(163):527–545. doi: 10.1016/j.ejmech.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Gorabi AM, Razi B, Aslani S, et al. Effect of curcumin on proinflammatory cytokines: A meta-analysis of randomized controlled trials. Cytokine. 2021;143:155541. doi: 10.1016/j.cyto.2021.155541. [DOI] [PubMed] [Google Scholar]
- 7.Moghaddam NSA, Oskouie MN, Butler AE, Petit PX, Barreto GE, Sahebkar A. Hormetic effects of curcumin: What is the evidence? J Cell Physiol. 2019;234(7):10060–10071. doi: 10.1002/jcp.27880. [DOI] [PubMed] [Google Scholar]
- 8.Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon4 genotype in Chinese older adults. Int Psychogeriatr. 2008;11:1–15. doi: 10.1017/S1041610207006655. [DOI] [PubMed] [Google Scholar]
- 9.Wei K, Nyunt MSZ, Gao Q, Wee SL, Ng TP. Frailty and malnutrition: related and distinct syndrome prevalence and association among community-dwelling older adults: Singapore longitudinal ageing studies. J Am Med Dir Assoc. 2017;18(12):1019–1028. doi: 10.1016/j.jamda.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. (2020 Report). [cited 2021 Dec 5]. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf. Accessed 2 Dec 2021
- 11.Nyunt MSZ, Fones C, Niti M, Ng TP. Criterion-based validity and reliability of the Geriatric Depression Screening Scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Heal. 2009;13:376–382. doi: 10.1080/13607860902861027. [DOI] [PubMed] [Google Scholar]
- 12.Feng L, Sian Chong M, Shiong Lim W, Pin NT. The Modified Mini-Mental State Examination test: normative data for Singapore Chinese older adults and its performance in detecting early cognitive impairment. Singapore Med J. 2012;53:458–462. [PubMed] [Google Scholar]
- 13.Kong X, Ma Y, Chen J, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant. 2012;28:641–651. doi: 10.1093/ndt/gfs491. [DOI] [PubMed] [Google Scholar]
- 14.Zhou K, Qin Z, Tian J, Cui K, Yan Y, Lyu S. The atherogenic index of plasma: A powerful and reliable predictor for coronary artery disease in patients with type 2 diabetes. Angiology. 2021;72(10):934–941. doi: 10.1177/00033197211012129. [DOI] [PubMed] [Google Scholar]
- 15.Caselli C, De Caterina R, Smit JM, et al. Triglycerides and low HDL cholesterol predict coronary heart disease risk in patients with stable angina. Sci Rep. 2021;11(1):20714. doi: 10.1038/s41598-021-00020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;5(10):74. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-020-79431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardash Y, Olson C, Herman W, et al. Platelet-lymphocyte ratio as a predictor of prognosis in head and neck cancer: a systematic review and meta-analysis. Oncol Res Treat. 2019;42(12):665–677. doi: 10.1159/000502750. [DOI] [PubMed] [Google Scholar]
- 19.Fan W, Zhang Y, Gao X, Liu Y, Shi F, Liu J, Sun L. The prognostic value of a derived neutrophil-lymphocyte ratio in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost. 2021;27:10760296211034579. doi: 10.1177/10760296211034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Zhang Q, Wang R, Ji H, Chen Y, Quan X, Zhang C. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;18(25):9690–9701. doi: 10.12659/MSM.919802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paliogiannis P, Fois AG, Sotgia S, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27(147):170113. doi: 10.1183/16000617.0113-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azhdari M, Karandish M, Mansoori A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2019;33(5):1289–1301. doi: 10.1002/ptr.6323. [DOI] [PubMed] [Google Scholar]
- 23.Saberi-Karimian M, Parizadeh SMR, Ghayour-Mobarhan M, et al. Evaluation of the effects of curcumin in patients with metabolic syndrome. Comp Clin Pathol. 2018;27(3):555–563. doi: 10.1007/s00580-017-2624-y. [DOI] [Google Scholar]
- 24.Nakayama H, Tsuge N, Sawada H, Masamura N, Yamada S, Satomi S, Higashi Y. A single consumption of curry improved postprandial endothelial function in healthy male subjects: a randomized, controlled crossover trial. Nutr J. 2014;28(13):67. doi: 10.1186/1475-2891-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon Y. Association of curry consumption with blood lipids and glucose levels. Nutr Res Pract. 2016;10(2):212–220. doi: 10.4162/nrp.2016.10.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopresti AL. The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv Nutr. 2018;9(1):41–50. doi: 10.1093/advances/nmx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin NY, Yang FQ, Wang YT, Li SP. Quantitative determination of eight components in rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using pressurized liquid extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2007;43:486–492. doi: 10.1016/j.jpba.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 29.Scazzocchio B, Minghetti L, D'Archivio M. Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients. 2020;12(9):2499. doi: 10.3390/nu12092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satheeshkumar N, Vijayan RS, Lingesh A, Santhikumar S, Vishnuvardhan Ch. Spices: potential therapeutics for Alzheimer's disease. Adv Neurobiol. 2016;12:57–78. doi: 10.1007/978-3-319-28383-8_4. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad R, Khan MA, Srivastava AN, Gupta A, Srivastava A, Jafri TR, Siddiqui Z, Chaubey S, Khan T, Srivastava AK. Anticancer potential of dietary natural products: a comprehensive review. Anticancer Agents Med Chem. 2020;20(2):122–236. doi: 10.2174/1871520619666191015103712. [DOI] [PubMed] [Google Scholar]
- 32.Rakhi NK, Tuwani R, Mukherjee J, Bagler G. Data-driven analysis of biomedical literature suggests broad-spectrum benefits of culinary herbs and spices. PLoS ONE. 2018;13(5):e0198030. doi: 10.1371/journal.pone.0198030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request from the corresponding authors.


