Abstract
Alzheimer’s disease (AD) is the leading cause of dementia and is characterized by a progressive decline in cognitive abilities. A pathological hallmark of AD is a region-specific accumulation of the amyloid-beta protein (Aβ). Here, we explored the association between regional Aβ deposition, sociodemographic, and local biochemical factors. We quantified the Aβ burden in postmortem cortical samples from parietal (PCx) and temporal (TCx) regions of 27 cognitively unimpaired (CU) and 15 AD donors, aged 78–100 + years. Histological images of Aβ immunohistochemistry and local concentrations of pathological and inflammatory proteins were obtained at the “Aging, Dementia and TBI Study” open database. We used the area fraction fractionator stereological methodology to quantify the Aβ burden in the gray and white matter within each cortical region. We found higher Aβ burdens in the TCx of AD octogenarians compared to CU ones. We also found higher Aβ loads in the PCx of AD nonagenarians than in AD octogenarians. Moreover, AD women exhibited increased Aβ deposition compared to CU women. Interestingly, we observed a negative correlation between education years and Aβ burden in the white matter of both cortices in CU samples. In AD brains, the Aβ40, Aβ42, and pTau181 isoforms of Aβ and Tau proteins were positively correlated with the Aβ burden. Additionally, in the TCx of AD donors, the proinflammatory cytokine TNFα showed a positive correlation with the Aβ load. These novel findings contribute to understanding the interplay between sociodemographic characteristics, local inflammatory signaling, and the development of AD-related pathology in the cerebral cortex.
Keywords: Amyloidosis, Amyloid burden, Inflammaging, Cytokines, Aging, Cognitive reserve
Introduction
Major neurocognitive disorders, also known as dementia, are the seventh leading cause of mortality among diseases [1]. Currently, dementia affects more than 55 million people worldwide, and, by 2050, about 150 million individuals may be diagnosed with these conditions [2]. Neurodegenerative dementias are characterized by a progressive decline in cognitive processes (e.g. attention, executive functions, learning, memory, language, and social cognition) and functional abilities [3]. Alzheimer’s Disease (AD) represents approximately 60–70% of the detected cases of dementia [3, 4]. AD has a strong heritable component and exists on a spectrum of genetic risk [5, 6]. At one end of the spectrum are very rare, highly penetrant mutations in the amyloid precursor protein (APP) and presenilin (PSEN1 and PSEN2) genes. These mutations cause the autosomal dominant (AD), which is a rare form of the disease that usually has an early onset [7]. At the other end of the spectrum are common alleles that have been identified in genome-wide association studies (GWAS) of late-onset sporadic AD [8, 9]. These alleles have individually small effects on the disease risk, but they can have a significant impact when they occur together [6]. Many lifestyle risk factors are also known to influence the AD aetiology, such as age, family history, sex, educational levels, environmental toxins, and head injuries [10, 11]. Yet, it is still unclear by which physiological mechanisms these factors influence deviations from healthy to pathological brain aging [12].
A major neuropathological hallmark of AD is the accumulation of amyloid-β (Aβ) oligomers in dense neuritic plaques [13]. These Aβ plaques accumulate in the extracellular space and are found in both the gray and white matter of the brain regions [13, 14]. The Aβ deposition in brain tissue follows a gradual and hierarchical sequence of affected regions [13, 14]. Typically, the accumulation starts in the basal neocortex, most often in poorly myelinated temporal areas such as the perirhinal and ectorhinal cortices [13]. Then, adjacent cortical areas and the hippocampal formation are affected [13]. Finally, deposits are found in all cortical areas, subcortical regions, brainstem, and cerebellum [13, 14].
The pathological Aβ induces shifts in glial cell phenotypes leading to the release of pro-inflammatory cytokines [15–17]. Aβ is also thought to initiate a pathophysiological cascade that leads to misfolding of the tau protein in neurofibrillary tangles, another hallmark of AD [18, 19]. The Aβ aggregates promote mitochondrial toxicity, oxidative stress, and cell membrane disruption [20–22]. Morphological changes and loss of innervation in GABAergic interneurons nearby Aβ plaques are likely associated with the initiation of synaptic inhibitory dysfunction in AD [23]. Studies employing multiple experimental approaches (e.g., microtransplantation of synaptic membranes, fluorescence deconvolution tomography, genomic, transcriptomic, and proteomic analyses), corroborate pro-excitatory shifts in the excitatory/inhibitory balance in the parietal and temporal cortices of AD individuals [24, 25]. While increased synaptic activity may trigger the release of Aβ and tau, soluble forms of Aβ also induce higher neuronal activity and glutamate release [26, 27], indicating a positive feedback loop between amyloidosis and neuronal excitability [28, 29].
Although there is a clear link between Aβ pathology and AD, Aβ aggregates can be observed decades before the onset of cognitive decline [30–33]. Up to 38% of cognitively unimpaired (CU) older individuals may carry Aβ deposits at the age of 85 years [34]. During normal aging, brain regions like the precuneus, temporal, and cingulate cortices present accelerated rates of Aβ deposition [35]. In contrast, age-related amyloid deposition seems to be less pronounced in regions such as the parietal, occipital, dorsolateral prefrontal, and orbitofrontal cortices [35]. In this perspective, histopathological post-mortem studies point to a disconnection between the amyloid load and cognitive status [36, 37]. In other words, the same brain region can be found with high or low levels of Aβ despite the AD diagnosis [37]. Thus, the Aβ deposition is likely influenced by the local concentrations of neuropathological, inflammatory, or neurotrophic factors in each brain region.
Other biological and sociodemographic factors, such as sex and cognitive engagement, also influence Aβ accumulation [38–42]. For instance, women tend to present higher global levels of neuritic plaques [39–41]. Moreover, CU individuals who engage in greater cognitively stimulating activities across life are less impacted by Aβ [38, 42]. To gain a deeper understanding of how these factors are related to region-specific vulnerability to Aβ accumulation, histological evaluations are necessary.
Therefore, our objective was to identify associations between the Aβ burden and sociodemographic and regional biochemical factors. We quantified the proportion of the area occupied by Aβ in the parietal (PCx) and temporal (TCx) cortices of older adults, either CU or diagnosed with AD. Our findings demonstrate region-specific variations in the influence of age, sex, and education on the Aβ burden. Additionally, we also discovered several connections between neuropathological and inflammatory factors and the Aβ load.
Methods
Brain samples
High-resolution digital images of postmortem human brain sections were obtained from the Aging, Dementia and Traumatic Brain Injury (TBI) Study [37], an open database compiled by the University of Washington, the Kaiser Permanente Washington Health Research Institute and the Allen Institute for Brain Science (http://aging.brain-map.org/, accessed on 17 May 2023). The database presents neuropathological, molecular, and transcriptomic data from 107 participants of the Adult Changes in Thought (ACT) study: a prospective cohort focusing on aged individuals from the Seattle—United States of America (USA) area [43]. Detailed information regarding the inclusion of participants in the ACT, collection of brain samples, neurobiological procedures, and database organization is available in the documentation provided at the database website (https://help.brain-map.org/display/aging/documentation).
Participants were chosen through a randomized selection process in the extended Seattle, USA catchment area. Individuals above 65 years, who were not residing in nursing homes and without the diagnosis of dementia, were invited to join the ACT. The cognitive functioning of these participants was measured with the cognitive abilities screening instrument (CASI) [44]. The CASI covers a range of cognitive domains, including attention, concentration, orientation, short-term memory, long-term memory, language abilities, visual construction, and verbal fluency [44]. Only individuals with a CASI score above 85, which indicates the absence of cognitive impairments, were invited to join the ACT. The participants received follow-up visits on a cycle of 2 years, in which the CASI was repeated. When a participant obtained a CASI score of 85 or lower, they underwent an additional battery of neuropsychological tests. This neuropsychological battery assessed executive functioning through measures such as the Mattis initiation, concept scales, comprehension, fluency, and clock drawing. This was evaluated together with clinical data and neuroimaging results to distinguish the dementia subtype, following the diagnostic and statistical manual of mental disorders (DSM-IV) and the NINCDS-ADRDA criteria [45, 46]. Upon enrollment at the ACT, all participants consented to the donation of brain samples at the moment of the autopsy.
Case selection for the database aimed to include all ACT subjects with a TBI and available banked frozen tissue. Starting with the youngest participants, each TBI case was matched with a control case of the same sex. Originally, 55 donors were included within the TBI cohort and 55 donors were included within the control cohort. Both groups presented 32 males and 23 females with an average of 89 years. However, the material of only 107 donors is available in the database. The database provides de-identified information about the donors such as age, sex, education years, diagnosis by the DSM-IV, and global metrics of AD pathology, such as CERAD, Braak, and NIA-Reagan scores. The database also provides histological images as well as transcriptomic and biochemical data from multiple brain regions. Major findings with this dataset were previously reported by the database organizers [37]. Recent findings with this dataset were also reported by other research groups [24, 25, 47]. All permissions for data usage in academic research and derived publications are explicit at the following links of the database: https://alleninstitute.org/legal/terms-use/ and http://aging.brain-map.org/overview/home (accessed on 17 May 2023). For referencing all datasets, we followed the citation guidelines available at https://alleninstitute.org/legal/citation-policy/ (accessed on 17 May, 2023).
From the total pool of 107 donors, 53 individuals suffered traumatic brain injuries in life and were excluded from the present study. From the remaining 54 individuals, two were diagnosed with vascular dementia, four had a multiple aetiologies diagnosis, and one was cognitively impaired due to an unspecified medical condition. For this reason, these samples were excluded from the present study. From the remaining 47 donors, PCx and TCx samples from 44 individuals were available. In some cases, neuropathological data was accessible exclusively for either the TCx or PCx, rather than for both cortical regions (Table 1). TCx samples for analysis in the present study were obtained from 27 cognitively unimpaired individuals (13 women and 14 men ranging from 78–99 years) and 15 donors diagnosed with AD (5 women and 10 men ranging from 79–over 100 years). PCx samples for analysis in the present study were obtained from 26 cognitively unimpaired individuals (14 women and 12 men ranging from 78–99 years) and 15 donors diagnosed with AD (5 women and 10 men ranging from 79–over 100 years).
Table 1.
Summary of donor characteristics
| Donor ID* | DSM-IV diagnosis | Age (years) | Sex | Education years | ApoE4 genotype (yes/no) | CERAD score | Braak stage | NIA-Reagan | Analyzed region (PCx/TCx) |
|---|---|---|---|---|---|---|---|---|---|
| H14.09.024 | ND | 78 | M | 21 | N | 1 | 3 | 1 | Both |
| H14.09.028 | ND | 78 | M | 16 | N | 2 | 3 | 2 | Both |
| H14.09.094 | ND | 78 | M | 18 | N | 1 | 2 | 1 | Both |
| H14.09.076 | ND | 78 | F | 14 | N | 1 | 2 | 1 | Both |
| H14.09.016 | ND | 81 | M | 21 | Y | 2 | 3 | 2 | Both |
| H14.09.074 | ND | 82 | F | 16 | N | 1 | 3 | 1 | Both |
| H14.09.096 | ND | 84 | M | 12 | N | 2 | 3 | 2 | Both |
| H14.09.060 | ND | 86 | F | 12 | N/A | 2 | 4 | 2 | Both |
| H15.09.106 | ND | 86 | M | 14 | N | 0 | 2 | 1 | TCx |
| H14.09.078 | ND | 87 | M | 16 | N | 0 | 1 | 1 | Both |
| H14.09.080 | ND | 87 | M | 21 | N | 0 | 0 | 0 | Both |
| H14.09.058 | ND | 88 | M | 14 | N | 1 | 1 | 1 | Both |
| H14.09.062 | ND | 89 | F | 16 | Y | 1 | 3 | 1 | Both |
| H14.09.072 | ND | 89 | F | 15 | N | 1 | 4 | 1 | Both |
| H14.09.090 | ND | 89 | F | 12 | N | 1 | 5 | 2 | PCx |
| H14.09.004 | ND | 90–94 | M | 14 | N | 1 | 3 | 1 | Both |
| H14.09.014 | ND | 90–94 | M | 10 | N | 1 | 4 | 1 | Both |
| H14.09.006 | ND | 90–94 | F | 14 | N | 0 | 3 | 1 | Both |
| H14.09.030 | ND | 90–94 | F | 15 | Y | 3 | 3 | 2 | Both |
| H14.09.032 | ND | 90–94 | F | 15 | N | 1 | 4 | 1 | Both |
| H14.09.052 | ND | 90–94 | F | 14 | N | 1 | 2 | 1 | Both |
| H15.09.104 | ND | 90–94 | F | 12 | N | 2 | 5 | 2 | Both |
| H15.09.108 | ND | 90–94 | F | 12 | N | 3 | 4 | 2 | Both |
| H14.09.046 | ND | 95–99 | M | 13 | N | 2 | 1 | 2 | Both |
| H14.09.050 | ND | 95–99 | M | 8 | N | 3 | 3 | 2 | Both |
| H14.09.070 | ND | 95–99 | M | 16 | N | 0 | 2 | 1 | Both |
| H14.09.020 | ND | 95–99 | F | 18 | N | 2 | 3 | 2 | Both |
| H14.09.102 | ND | 95–99 | F | 16 | N | 0 | 1 | 1 | Both |
| H14.09.018 | AD | 79 | M | 18 | N | 0 | 2 | 1 | Both |
| H15.09.110 | AD | 82 | F | 14 | N | 2 | 2 | 1 | Both |
| H14.09.040 | AD | 85 | M | 12 | Y | 1 | 6 | 2 | Both |
| H14.09.068 | AD | 85 | M | 16 | Y | 3 | 5 | 3 | Both |
| H14.09.098 | AD | 86 | M | 9 | N | 3 | 6 | 3 | Both |
| H14.09.044 | AD | 87 | F | 14 | N/A | 2 | 6 | 2 | TCx |
| H14.09.022 | AD | 88 | M | 12 | N | 0 | 1 | 1 | Both |
| H14.09.034 | AD | 88 | M | 16 | Y | 3 | 5 | 3 | Both |
| H14.09.054 | AD | 89 | M | 9 | N | 1 | 3 | 1 | Both |
| H14.09.002 | AD | 90–94 | M | 16 | Y | 0 | 1 | 1 | Both |
| H14.09.082 | AD | 95–99 | M | 15 | N | 0 | 4 | 1 | Both |
| H14.09.042 | AD | 95–99 | F | 11 | N/A | 3 | 5 | 3 | PCx |
| H14.09.086 | AD | 95–99 | F | 14 | N | 3 | 6 | 3 | Both |
| H14.09.010 | AD | 100 + | M | 16 | Y | 3 | 4 | 2 | Both |
| H14.09.056 | AD | 100 + | F | 17 | N/A | 3 | 5 | 3 | Both |
| H14.09.088 | AD | 100 + | F | 12 | N | 3 | 5 | 3 | Both |
*Histological images from the donors were obtained at the public database aging, dementia, and TBI study (http://aging.brain-map.org/overview/home). Donors are listed here with the same ID as coded at the online database
DSM-IV diagnostic and statistical manual of mental disorders, CERAD Consortium to Establish a Registry for Alzheimer’s Disease, NIA-Reagan National Institute on Aging and Ronald and Nancy Reagan Institute of the Alzheimer’s Association criteria, PCx parietal cortex, TCx temporal cortex, ND no dementia, AD Alzheimer’s disease
Tissue processing
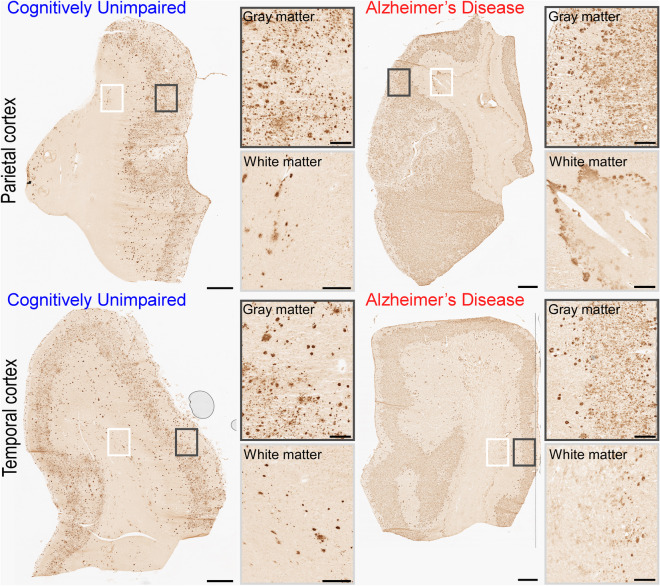
The brain samples obtained from the donors were processed through immunohistochemistry (IHC) for Aβ detection (Fig. 1). Biochemical assays of gas chromatography-mass spectrometry (GC/MS) and Luminex were also performed to establish local F2-isoprostane (F2-IP) and neuropathological/inflammatory protein concentrations in these samples. These procedures are described in detail at the following link: https://help.brain-map.org/display/aging/Documentation (accessed on 17 May, 2023). For each donor, IHC images and isoprostane/protein quantification data can be freely downloaded at http://aging.brain-map.org/donors/summary (accessed on 17 May 2023). We briefly summarized here these procedures, which were performed at the Allen Institute and the University of Washington.
Fig. 1.
Amyloid-beta (Aβ) immunoreactive profiles in the parietal (PCx, top) and temporal (TCx, bottom) cortices. The top-left chart illustrates a PCx section from a cognitively unimpaired (CU) donor (H14.09.030). The top-right chart illustrates a PCx section from a donor (H14.09.010) diagnosed with Alzheimer’s Disease (AD) through the DSM-IV. The bottom-left chart illustrates a TCx section from a CU donor (H14.09.030). The bottom-right chart illustrates a TCx section from an AD donor (H14.09.010). Panels placed at the right side of the sections illustrate the high-resolution gray (top) and white (bottom) matter squares traced in the charts. Scale bars: 1 mm (topographic view of sections) and 200 µm (high-resolution gray and white matter panels). Images were obtained from the aging, dementia, and TBI study database and are publicly available at: http://aging.brain-map.org/ (accessed on 17 May, 2023). Anatomical charts were produced by the authors of the present work
Brain samples were obtained using a rapid autopsy protocol with a post-mortem interval of less than 8 h. This included the collection of ventricular cerebrospinal fluid and mid-sagittal hemisections from 60 brain tissue samples, which were flash-frozen in liquid nitrogen and stored at − 80 °C. The remaining tissue was immersion-fixed in 10% normal buffered formalin for 2–3 weeks. Routine diagnostic stains, such as hematoxylin–eosin and luxol fast blue, were used to evaluate the neuropathological tissue viability. Fresh frozen samples of the parietal and temporal cortex were sliced into 25 µm thick coronal sections using a Leica CM3050S cryostat (Leica Biosystems). Brain sections were collected systematically and at uniform intervals to ensure stereological sampling. Each cortical block of tissue was sliced into 62 sections. For each sample, the 5th section and every other section within a 20-interval were collected for Aβ detection. The last sections of each tissue block were submitted to microdissection for quantification of neuropathological, inflammatory, and neurotrophic protein concentrations (in ng/mg or pg/mg) by the GC/MS and Luminex methods (Table 2).
Table 2.
Pathological, oxidative stress, inflammatory, and neurotrophic proteins quantified by gas chromatography–mass spectrometry and luminex
| Name | Full name | Description | Measured in |
|---|---|---|---|
| Aβ40 | Amyloid-beta 1–40 | Amyloid-beta isoform with 40 amino acids | pg/mg |
| Aβ42 | Amyloid-beta 1–42 | Amyloid-beta isoform with 42 amino acids, considered a major component of amyloid plaques | pg/mg |
| Tau | Total Tau protein | Microtubule binding protein expressed in neurons | ng/mg |
| pTau-181 | Tau protein phosphorylated at threonine 181 | Abnormal alteration of the Tau protein, considered a highly specific marker of the Alzheimer’s disease | ng/mg |
| αSNCA | Alpha synuclein | Major component of Lewy bodies | pg/mg |
| F2-IP | F2-isoprostanes | Mediators of oxidative stress | pg/mg |
| RANTES | Regulated on activation, normal T cell expressed and secreted (CCL5) | Pro-inflammatory chemokine | pg/mg |
| MIP-1α | Macrophage inflammatory protein-1 alpha (CCL3) | Pro-inflammatory chemokine | pg/mg |
| MCP-1 | Monocyte chemotactic protein 1 (CCL2) | Pro-inflammatory chemokine | pg/mg |
| IL-6 | Interleukin-6 | Pro-inflammatory cytokine | pg/mg |
| IL-7 | Interleukin-7 | Hematopoietic factor | pg/mg |
| IL-10 | Interleukin-10 | Anti-inflammatory cytokine | pg/mg |
| IFNγ | Interferon-gamma | Pro-inflammatory cytokine | pg/mg |
| VEGF | Vascular endothelial growth factor | Vasculogenesis growth factor | pg/mg |
| BDNF | Brain-derived neurotrophic factor | Neuronal differentiation growth factor | pg/mg |
Protein quantifications were obtained at the public database aging, dementia, and TBI study (http://aging.brain-map.org/overview/home)
For the IHC procedure, samples were removed from storage at − 80 °C, allowed to acclimate to room temperature, and fixed with 100% acetone at − 20 °C. Sections were then rehydrated in 1 × phosphate-buffered saline (PBS), pH 7.4. To retrieve the antigen, sections were incubated in 10 mM sodium citrate for 10 min at 98 °C. The tissue was washed in PBS-Tween 20 (0.05%) prior to starting the staining protocol. To block the activity of endogenous peroxidases, sections were treated with 3% hydrogen peroxide in PBS. Subsequently, sections were washed in PBS-Tween 20 before incubating in a blocking solution containing 4% horse serum and 0.3% Triton-X in PBS. Next, sections were incubated overnight with the Ab6E10 primary antibody obtained from mice (Biolegend, #SIG-39320) at a 1:2000 dilution. Following this, the samples were washed in PBS-Tween 20 before incubation with a biotinylated anti-mouse secondary antibody (Vector Laboratories, #BA2000) at a 1:500 dilution. After rinsing, the sections were immersed for 30 min in ABC (Vectastain, Vector Laboratories). The reaction product was visualized with 0.05% 3,3′-Diaminobenzidine (DAB) (Sigma Aldrich) and 0.01% hydrogen peroxide. The specimens were then washed in PBS, dehydrated using a graded series of alcohol, and cleared in Formula 83, allowing for the production of histological slides. The slices were scanned using the Leica ScanScope scanner (Leica Biosystems) with a 20 × objective (0.75 NA Pan Apo). Images were captured at cellular resolution (1 µm/pixel) and underwent quality control before being indexed on the web database.
Frozen cortical tissue, immediately adjacent to those submitted for IHC, was used for F2-IP and Luminex quantifications. GC/MS was used to quantify F2-IP, which indicates free radical injury in the parietal and temporal cortices [48, 49]. Firstly, a modified Folch procedure was performed for lipid extraction and release of esterified prostanes. Then, isoprostanes were isolated using reversed-phase and normal-phase solid-phase extraction. GC/MS analysis was performed using a 6890N Agilent gas chromatograph coupled to a 5973-quadrupole-mass spectrometer in the negative-ion mode. The multiplex Luminex assay was used to determine the local concentrations of proteins involved in neuropathological, inflammatory, and neurotrophic cascades. Cortical tissue was sequentially homogenized and centrifuged in a reassembly buffer (RAB), followed by 5 M guanidine-HCL or a radio immunoprecipitation assay (RIPA) buffer. This yielded supernatants labeled RAB extracts, G extracts, or RIPA extracts. These extracts were incubated with antibodies and the resulting fluorescence was measured to determine the sample concentration by comparison to a standard curve. This was performed in a LiquiChip Workstation (Qiagen). RAB extracts were utilized to quantify brain-derived neurotrophic factor (BDNF) and 11-plex proteins, G extracts were used to quantify Amyloid-beta (Aβ) 40 and 42 isoforms, the Tau protein, and Tau isoform abnormally phosphorylated at threonine 181 (pTau-181). RIPA extracts were used to quantify alpha-synuclein.
Image analysis
The aforementioned procedures (such as case selection, brain sectioning and sampling, Aβ immunohistochemistry, the GC/MS, and the luminex methods) were carried out by the database organizers. In the present study, we undertook the extraction of morphological data from the histological images available in the database. Firstly, we downloaded full-resolution images of fresh-frozen brain slices processed for Aβ IHC from the database. All samples were blind-coded therefore the donors could not be identified in morphometric evaluations. Before morphometric analyses, we delimited the gray and white matter of the PCx and TCx images. The borders between these two sub-regions were evident in the histological material (Fig. 1). We estimated the percentage of the area (area fraction) occupied by the Aβ IHC using the area fraction fractionator stereological methodology [50]. This method was performed with a built-in function of the software StereoInvestigator v11.0 (MBF Bioscience).
After region delimitation, the software superimposed a Cavalieri point-counting grid on the blind-coded IHC image. Grids sampled the delimited cortical region at an interval of 2500 × 3000 µm (XY). Each grid matrix for point counting was defined with a height and width of 500 × 500 µm, and the spacing between two adjacent points was set at 25 µm. Two independent observers checked every delimited region for hit points superimposed on IHC signals. The hit points at the histological stain were correlated with the area occupied by the IHC marker, whereas the total number of points indicated the total regional area. Thus, the percentage of hit points per total point indicated the area fraction occupied by Aβ aggregates. In addition to the regional estimations, we also used a single delimitation encompassing the entire cortex to estimate the Aβ area fraction for the total cortex. With this design, we were able to retrieve three sub-regionally distinct morphometric parameters for evaluation: Aβ load in the gray, white, and total cortical areas.
Statistics
We verified the normality of group distributions after Kolmogorov–Smirnov tests and evaluation of kurtosis and skewness. Data from F2-IP and Luminex assays identified as highly skewed were transformed into a log curve (Y = Log (Y + 1)) and normalized. Initially, we conducted a two-way analysis of variance (ANOVA) to examine differences in Aβ area fractions across regions (PCx or TCx) and sub-regions (gray matter, white matter, and total cortex) among different age and sex groups. In this analysis, one factor was sociodemographic characteristics (age or sex), while the other factor was the DSM-IV diagnosis (CU or AD). We also investigated the interaction between these two factors.
To determine specific group differences, we performed multiple comparisons using the Sidak post hoc test. Due to the age categorization in the database, which groups individuals over 90 years into age intervals of 90–94, 95–99, or above 100 years, we were unable to directly evaluate Pearson’s correlation between age and the Aβ load. Therefore, we classified the donors into age groups to assess changes within the octogenarian to nonagenarian age range. Since there were only five donors aged 78–79 years, we included them in the 78–89-year-old group. Similarly, as there were only three donors above 100 years, we included them in the 90–100-year-old group. Consequently, the age groups were divided as follows: 78–89 years (PCx: n = 13 CU and n = 8 AD; TCx: n = 14 CU and n = 9 AD) and 90–100 years (PCx: n = 13 CU and n = 7 AD; TCx: n = 13 CU and n = 6 AD).
The education years were presented as a continuous variable in the database. Hence, we performed Pearson’s correlation and simple linear regression analysis to explore the relationship between the Aβ area fraction with education years in each region and sub-region of interest. To account for potential influences of age and sex, multiple regression modeling was performed to adjust these correlations.
We conducted Pearson’s correlation and linear regression analysis to examine the relation between the Aβ area fraction and various measurements, including F2-IP, neuropathological, and inflammatory protein quantifications obtained through GC/MS and Luminex. To account for potential confounding factors, such as age, sex, and education years, multiple regression modeling was performed. Since these biochemical estimates were available only for the total cortex and lacked sub-region specificity, we could only analyze the total cortical Aβ area fraction. Interleukin 1 beta, one of the listed biochemicals in the database, was not detected in the TCx and PCx and was excluded from the present analysis. Additionally, data for alpha-synuclein and BDNF were not available for the TCx. To present all the associations between the measured Aβ burden and local protein concentrations, we constructed a Pearson correlation matrix.
Descriptive results are reported as mean ± standard deviation, and p-values were set with α < 0.05. All statistical analyses were performed using GraphPad Prism version 7.0 software (GraphPad). The Pearson correlation matrix was generated using Rstudio software (Rstudio team).
Results
Effects of age, biological sex, and education years on the cortical amyloid-beta load
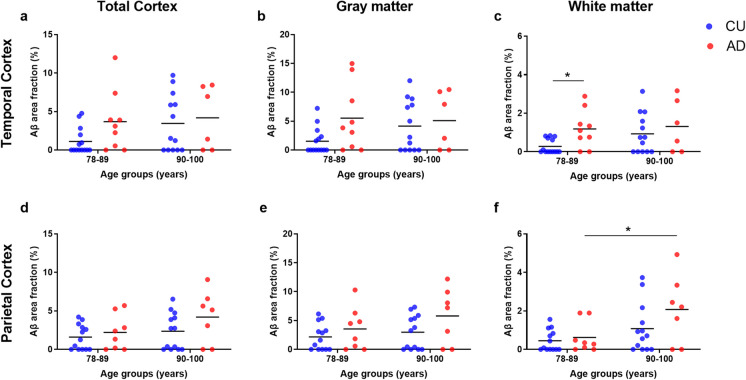
In the TCx, we observed a significant main effect of the diagnosis group on the white matter Aβ load (F(1, 38) = 4.72, p = 0.036). However, we did not find any significant effects of age or the interaction between age and diagnosis on this region. Similarly, no significant effects were observed for any comparisons involving other sub-regions of the TCx. The Sidak test for multiple comparisons revealed a higher Aβ load in the TCx of the 78–89 AD group (1.18 ± 0.98) compared to the 78–89 CU group (0.28 ± 0.37, p = 0.049). In the white matter of the PCx, on the other hand, we observed a significant main effect of age on the Aβ load (F(1, 37) = 8.16, p = 0.007), but we did not find any significant effects of the diagnosis group or the interaction between age and diagnosis. Multiple comparisons indicated a higher Aβ load in the white matter of the 90–100 AD group (2.07 ± 1.77) compared to the 78–89 AD group (0.62 ± 0.80, p = 0.033). No significant effects were observed in the other sub-regions of the PCx (Fig. 2).
Fig. 2.
Age-related differences in the amyloid-beta (Aβ) area fractions of the temporal (TCx, a–c) and parietal (PCx, d–f) cortices. This analysis was performed in cognitively unimpaired (CU; blue scatter plot) and in Alzheimer’s disease (AD; red scatter plot) donors. Donors were divided into 78–89- or 90–100-year-old groups. At each cortical region, the Aβ percentage was estimated in the total cortex (a and d), in the gray matter (b and e), and in the white matter (c and f). Two-way ANOVA and Sidak multiple comparison tests were performed to establish differences between the age and diagnosis groups. *P-values less than 0.05
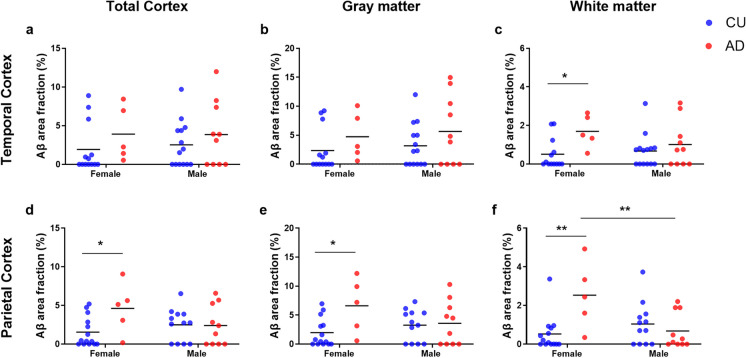
In the TCx, we found a significant main effect of the diagnosis group on the white matter Aβ load (F(1, 38) = 6.03, p = 0.019). However, no significant effects of sex or the interaction between sex and diagnosis were observed in this region or in any other sub-regions of the TCx. The Sidak test for multiple comparisons revealed that women with AD had a higher Aβ load of the TCx (1.69 ± 0.85) compared to CU women (0.50 ± 0.79, p = 0.039). In the PCx, we observed an effect of the interaction between sex and diagnosis group on the Aβ load in the total cortex (F(1, 37) = 4.04, p = 0.05), the gray matter (F(1, 37) = 4.04, p = 0.049) and white matter (F(1, 37) = 10.4, p = 0.003). Additionally, we found a main effect of the diagnosis group in the gray matter (F(1, 37) = 5.24, p = 0.028) and white matter (F(1, 37) = 5.06, p = 0.030). However, no significant effects of sex as an isolated factor were observed in any sub-regions of the PCx. Multiple comparison analyses revealed a higher Aβ load in AD women when compared to CU women in the total cortical region (AD: 4.62 ± 3.28; CU: 1.54 ± 1.93, p = 0.031), in the gray matter (AD: 6.60 ± 4.77; CU: 1.99 ± 2.47, p = 0.017), and white matter (AD: 2.53 ± 1.73; CU: 0.53 ± 0.90, p = 0.002). Furthermore, in the PCx white matter, AD women presented higher Aβ percentages comparison to AD men (0.68 ± 0.92, p = 0.007, see Fig. 3).
Fig. 3.
Sex-specific differences in the amyloid-beta (Aβ) area fractions of the temporal (TCx, a–c) and parietal (PCx, d–f) cortices. This analysis was performed in cognitively unimpaired (CU; blue scatter plot) and in Alzheimer’s disease (AD; red scatter plot) donors. Donors were divided into male or female groups. At each cortical region, the Aβ percentage was estimated in the total cortex (a and d), in the gray matter (b and e), and in the white matter (c and f). Two-way ANOVA and Tukey’s multiple comparison test were performed to establish differences between the sex and diagnosis groups. *P-values less than 0.05
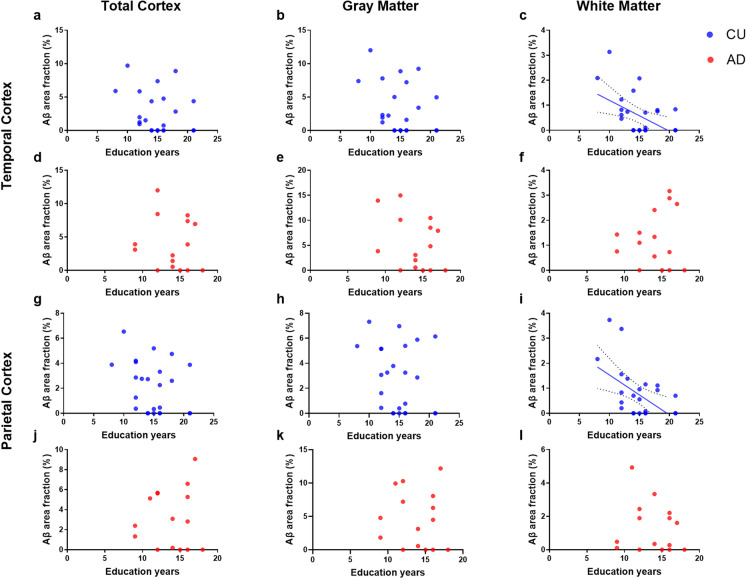
Finally, the regression analysis revealed that a higher number of education years were associated with a decrease in the Aβ burden in the white matter of both TCx (r (27) = − 0.47, R2 = 0.22, p adj. = 0.048) and PCx (r (26) = − 0.50, R2 = 0.25, p adj. = 0.012) among CU donors. These effects remained significant after controlling for age and sex (see Table 3). However, no significant associations between education and the Aβ load were observed in any other cortical sub-region among either CU or AD donors (see Fig. 4).
Table 3.
Relation between the cortical amyloid burden and education years in normal cognition and Alzheimer’s disease
| Region of interest | Cognitively unimpaired | Alzheimer’s disease | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted model | Unadjusted model | Adjusted model | |||||
| β (SE) | p value | β (SE) | p value | β (SE) | p value | β (SE) | p value | |
| TCx total | − 0.22 (0.19) | 0.254 | − 0.08 (0.20) | 0.689 | − 0.15 (0.39) | 0.699 | − 0.20 (0.44) | 0.655 |
| TCx GM | − 0.31 (0.22) | 0.177 | − 0.17 (0.24) | 0.486 | − 0.65 (0.50) | 0.219 | − 0.69 (0.57) | 0.249 |
| TCx WM | − 0.12 (0.04) | 0.013 | − 0.09 (0.04) | 0.048 | 0.05 (0.11) | 0.651 | 0.05 (0.12) | 0.665 |
| PCx total | − 0.17 (0.12) | 0.169 | − 0.17 (0.13) | 0.207 | 0.08 (0.29) | 0.775 | 0.05 (0.29) | 0.868 |
| PCx GM | − 0.18 (0.16) | 0.288 | − 0.18 (0.17) | 0.319 | − 0.09 (0.41) | 0.834 | − 0.12 (0.43) | 0.785 |
| PCx WM | − 0.16 (0.05) | 0.008 | − 0.15 (0.06) | 0.012 | − 0.08 (0.14) | 0.593 | − 0.10 (0.12) | 0.400 |
Multiple regression modeling was used to adjust for potential contributions of age and sex
TCx temporal cortex, PCx parietal cortex, GM gray matter, WM white matter, β standardized β coefficient, SE standard error
Significant p values (< 0.05) are highlighted in bold
Fig. 4.
Relation between education years and the amyloid-beta (Aβ) burden in the temporal (TCx, a–f) and parietal (PCx, g–i) cortices. This analysis was performed in cognitively unimpaired (CU; blue scatter plot) and in Alzheimer’s disease (AD; red scatter plot) donors. At each cortical region, the Aβ percentage was estimated in the total cortex (left), in the gray matter (middle) and in the white matter (right). Regression lines are shown with the 95% confidence interval for the significant (P-values less than 0.05) Pearson correlations between Aβ burden and the number of education years. Results remained significant after correction for age and sex
Biochemical factors underlying the cortical amyloid-beta load
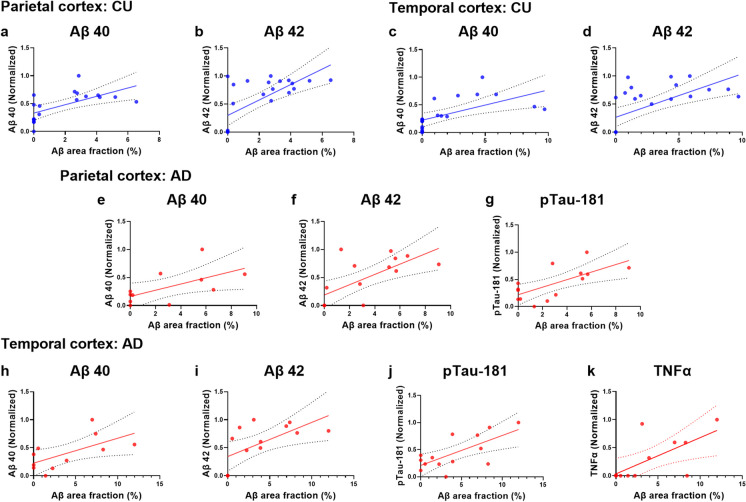
The concentration of local proteins involved in oxidative stress, pathological, and inflammatory processes was analyzed for possible associations with the Aβ load in the TCx (see Fig. 5 and Table 4) and PCx (see Fig. 6 and Table 5). Among CU donors, we observed positive correlations between the Aβ percentage and the amyloid isoforms Aβ40 and Aβ42 in both the TCx (Aβ40: r (20) = 0.61, R2 = 0.38, p adj. = 0.005; Aβ42: r (24) = 0.63, R2 = 0.002, p adj. = 0.011) and PCx (Aβ40: r (19) = 0.63, R2 = 0.39, p adj. = 0.034; Aβ42: r (23) = 0.69, R2 = 0.47, p adj. = 0.001). Similarly, among AD donors, we observed positive correlations between the Aβ percentage and the local concentrations of the amyloid isoforms Aβ40 and Aβ42 in both the TCx (Aβ40: r (11) = 0.62, R2 = 0.39, p adj. = 0.049; Aβ42: r (13) = 0.63, R2 = 0.40, p adj. = 0.011) and PCx (Aβ40: r (11) = 0.61, R2 = 0.37, p adj. = 0.012; Aβ42: r (15) = 0.68, R2 = 0.47, p adj. = 0.001). These associations remained significant after adjusting for age, sex and education years (see Table 4).
Fig. 5.
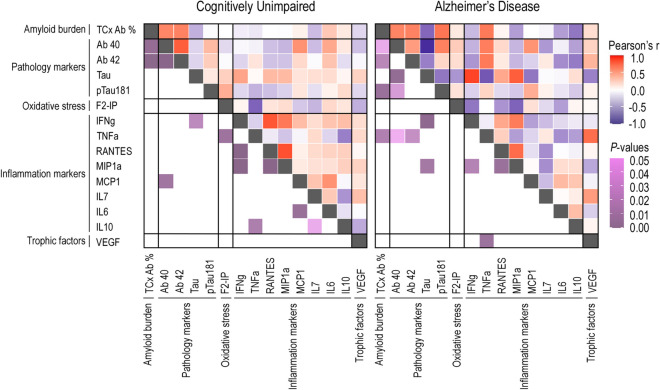
Correlation matrix for associations between the Aβ area fractions, F2-isoprostane and Luminex concentrations in the total temporal (TCx) cortex. Heat maps for P-values and Pearson’s r are found in the lower and upper triangles, respectively, of the matrix. This analysis was performed in cognitively unimpaired (CU; left) and in Alzheimer’s disease (AD; right) donors
Table 4.
Relation between the amyloid burden of the temporal cortex and pathological, oxidative stress, inflammatory, and neurotrophic protein concentrations
| Protein marker | Cognitively unimpaired | Alzheimer’s disease | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted model | Unadjusted model | Adjusted model | |||||
| β (SE) | p value | β (SE) | p value | β (SE) | p value | β (SE) | p value | |
| Aβ40 | 6.89 (2.09) | 0.004 | 6.79(2.01) | 0.005 | 8.69 (3.65) | 0.041 | 9.79 (3.99) | 0.049 |
| Aβ42 | 5.09(1.35) | 0.001 | 4.79 (1.33) | 0.002 | 6.53 (2.42) | 0.021 | 8.86 (2.70) | 0.011 |
| F2-IP | − 0.03 (3.11) | 0.993 | 1.88 (3.48) | 0.594 | 1.46 (5.62) | 0.804 | 2.34 (10.89) | 0.844 |
| Tau | − 3.58 (2.52) | 0.171 | − 2.74 (2.46) | 0.279 | − 5.13 (2.32) | 0.055 | − 5.67 (3.08) | 0.115 |
| pTau-181 | − 0.21 (2.62) | 0.938 | − 0.06 (2.46) | 0.981 | 8.52 (2.6) | 0.007 | 9.05 (2.85) | 0.011 |
| IFNg | − 0.74 (2.18) | 0.737 | 0.97 (2.18) | 0.661 | − 3.29 (3.75) | 0.409 | − 6.12 (4.84) | 0.246 |
| TNFα | − 0.82 (1.81) | 0.653 | − 3.09 (1.65) | 0.075 | 6.98 (2.43) | 0.017 | 8.41 (2.96) | 0.025 |
| RANTES | − 0.04 (1.98) | 0.983 | 0.85 (1.92) | 0.661 | 0.68 (4.61) | 0.885 | − 1.86 (8.17) | 0.826 |
| MIP1α | − 0.13 (2.14) | 0.951 | 0.39 (2.07) | 0.853 | − 5.26 (4.11) | 0.230 | − 8.87 (6.04) | 0.185 |
| MCP-1 | 0.89 (2.53) | 0.728 | 1.38 (2.54) | 0.593 | 0.03 (4.31) | 0.995 | 2.12 (8.43) | 0.809 |
| IL-7 | − 2.89 (2.27) | 0.216 | − 4.80 (2.39) | 0.058 | − 0.57 (4.81) | 0.907 | − 3.29 (6.66) | 0.634 |
| IL-6 | 3.69 (2.68) | 0.181 | 2.88 (2.92) | 0.334 | − 4.94 (3.73) | 0.212 | − 5.34 (4.88) | 0.306 |
| IL-10 | 0.98 (1.87) | 0.605 | 2.72 (1.85) | 0.158 | − 6.75 (2.84) | 0.037 | − 8.10 (3.74) | 0.061 |
| VEGF | − 1.85 (2.51) | 0.469 | − 2.01 (2.38) | 0.407 | 2.54 (4.07) | 0.546 | 1.54 (5.81) | 0.797 |
Multiple regression modeling was used to adjust for potential contributions of age, sex, and education years
β standardized β coefficient; SE standard error
Significant p values (< 0.05) are highlighted in bold
Fig. 6.
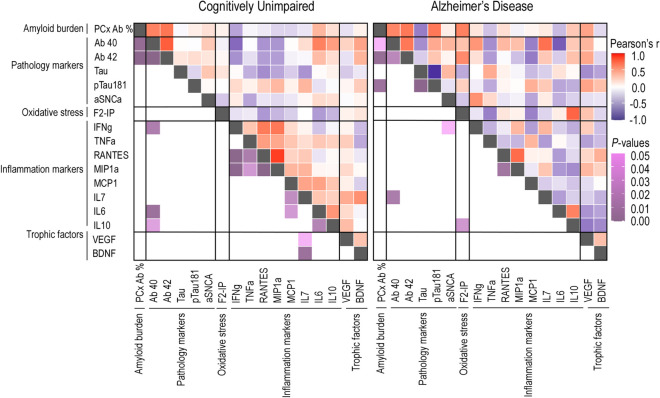
Correlation matrix for associations between the Aβ area fractions, F2-isoprostane, and Luminex concentrations in the total parietal (PCx) cortex. Heat maps for P-values and Pearson’s r are found in the lower and upper triangles, respectively, of the matrix. This analysis was performed in cognitively unimpaired (CU; left) and in Alzheimer’s disease (AD; right) donors
Table 5.
Relation between the amyloid burden of the parietal cortex and pathological, oxidative stress, inflammatory, and neurotrophic protein concentrations
| Protein marker | Cognitively unimpaired | Alzheimer’s disease | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted model | Unadjusted model | Adjusted model | |||||
| β (SE) | p value | β (SE) | p value | β (SE) | p value | β (SE) | p value | |
| Aβ40 | 5.24 (1.57) | 0.004 | 3.90 (1.66) | 0.034 | 6.65 (2.90) | 0.047 | 8.59 (2.43) | 0.012 |
| Aβ42 | 3.43 (0.79) | 0.0003 | 2.86 (0.76) | 0.001 | 5.09 (1.50) | 0.005 | 5.90 (1.31) | 0.001 |
| F2-IP | 0.56 (1.72) | 0.749 | 0.66 (1.61) | 0.686 | 6.77 (3.09) | 0.065 | 8.32 (3.77) | 0.092 |
| Tau | − 0.22 (1.82) | 0.906 | − 0.92 (1.35) | 0.506 | − 6.41 (3.06) | 0.056 | − 6.08 (3.25) | 0.091 |
| pTau-181 | 0.80 (1.90) | 0.678 | 0.36 (1.71) | 0.837 | 6.55 (2.06) | 0.008 | 6.29 (2.40) | 0.028 |
| αSNC | 0.98 (1.37) | 0.482 | 0.59 (1.47) | 0.691 | 0.84 (2.93) | 0.779 | − 0.15 (3.45) | 0.966 |
| IFNg | − 2.25 (1.38) | 0.119 | − 1.12 (1.41) | 0.438 | 1.48 (3.20) | 0.651 | 1.67 (3.42) | 0.634 |
| TNFα | − 0.26 (1.42) | 0.854 | − 0.43 (1.27) | 0.739 | − 1.64 (2.03) | 0.436 | − 4.48 (2.41) | 0.096 |
| RANTES | − 0.57 (1.50) | 0.705 | 0.12 (1.35) | 0.923 | 1.73 (3.13) | 0.592 | 3.13 (3.88) | 0.446 |
| MIP1α | − 1.07 (1.48) | 0.477 | − 0.08 (1.37) | 0.951 | − 0.47 (2.68) | 0.865 | − 0.49 (3.35) | 0.887 |
| MCP-1 | − 1.39 (1.82) | 0.454 | − 0.49 (1.74) | 0.783 | 0.72 (3.47) | 0.837 | − 2.63 (4.43) | 0.569 |
| IL-7 | − 0.79 (1.65) | 0.637 | − 0.84 (1.47) | 0.572 | 1.05 (2.82) | 0.716 | 2.30 (3.51) | 0.529 |
| IL-6 | 2.56 (1.47) | 0.098 | 1.89 (1.53) | 0.237 | − 2.29 (2.67) | 0.411 | − 1.97 (3.30) | 0.569 |
| IL-10 | 1.26 (1.60) | 0.441 | 0.44 (1.47) | 0.767 | 2.67 (2.95) | 0.388 | 3.90 (3.13) | 0.259 |
| VEGF | − 0.65 (1.27) | 0.614 | − 0.86 (1.09) | 0.436 | 4.71 (2.82) | 0.126 | 4.78 (3.03) | 0.159 |
| BDNF | 0.55 (1.71) | 0.748 | 1.38 (1.56) | 0.387 | − 1.39 (3.17) | 0.670 | − 1.27 (3.51) | 0.729 |
Multiple regression modeling was used to adjust for potential contributions of age, sex, and education years
β standardized β coefficient, SE standard error
Significant p values (< 0.05) are highlighted in bold
Moreover, in both cortices of AD donors, we found positive correlations between the Aβ burden and the isoform pTau-181 of the Tau protein (TCx: r(14) = 0.69, R2 = 0.47,p adj. = 0.011; PCx: r(14) = 0.67, R2 = 0.45, p adj. = 0.028). Additionally, for AD donors, a negative correlation between the Aβ load and the anti-inflammatory protein interleukin 10 was observed in the TCx, but this correlation did not remain significant after adjustment (p adj. = 0.061). Finally, we identified a region-specific correlation between the Aβ load and the concentration of the proinflammatory cytokine tumor necrosis factor alpha (TNFα) in the TCx of AD donors (r (13) = 0.67, R2 = 0.45, p adj. = 0.025; Fig. 7).
Fig. 7.
Relation between pathological and inflammatory factors and the amyloid-beta (Aβ) burden of the temporal (TCx) and parietal (PCx) cortices. This analysis was performed in cognitively unimpaired (CU; blue scatter plots, a–d) and in Alzheimer’s disease (AD; red scatter plots, e–n) donors. Protein concentrations were measured in ng/mg or pg/mg and normalized. Significance was determined for p-values less than 0.05. Regression lines are shown with the 95% confidence interval for the significant relation between Aβ burden and protein concentrations. Results remained significant after correction for age, sex and education years
Discussion
This study investigated sociodemographic and biochemical factors associated with Aβ accumulation in the cerebral cortex of CU and AD individuals within the octogenarian to nonagenarian age range. We found regionally distinct age-specific effects in the white matter Aβ burden among AD individuals. Additionally, we observed sex-specific differences. In all cortical sub-regions of the PCx, AD women presented higher Aβ loads than CU women. Conversely, AD women exhibited a higher percentage of Aβ only in the white matter of the TCx. No differences were found between CU and AD male samples. Moreover, an antagonistic relationship between education years and the white matter Aβ burden was observed in both TCx and PCx of CU donors.
Notably, certain age-, sex-, and education-related effects on the Aβ load were only evident in the white matter, being hidden in the analysis of the entire cortical region without a sub-regional distinction. Moreover, distinct regional association patterns were found between the Aβ burden and soluble isoforms of Aβ, Tau, and inflammatory proteins. In both cortices of CU and AD individuals, Aβ load exhibited positive correlations with Aβ40 and Aβ42 concentrations. Among AD donors, Aβ percentage was also positively correlated with the isoform pTau181 of the tau protein in both cortical regions. Lastly, the Aβ load in the TCx of AD donors showed a positive correlation with the pro-inflammatory cytokine TNFα.
Age-related differences in the cortical Aβ deposition
Chronological age is the greatest risk factor for AD and other dementias [51]. The prevalence rates of dementia increase acutely with age [52]. Previous PET neuroimaging studies have also indicated a positive association between advanced age and increased Aβ deposition in the cerebral cortex of CU individuals aged 30–89 years [35, 53]. However, when focusing on individuals above 60 years, this relationship was reduced to a non-significant trend [35]. Furthermore, in CU adults ranging from 70 to 89 years, no significant age-related effects on cortical Aβ deposition were observed [53]. A neuropathological study investigating CU individuals aged 60 to 104 years also did not find any age-related changes in the neocortical plaque burden [54]. Similarly, Miller et al. reported no correlations between Aβ immunoreactivity and age in the total PCx of CU donors within the octogenarian-nonagenarian range [37]. Consistent with these findings, our study found no significant differences in Aβ accumulation between age groups in any of the analyzed regions or sub-regions in CU individuals.
In AD individuals, the prevalence of Aβ abnormalities in the cerebrospinal fluid (CSF) and positron emission tomography (PET), after adjustment for CSF-cutoffs, exhibited a trend of age-related decline which was not statistically significant [34]. While previous studies have reported an age-related decline in Aβ plaques in regions such as the hippocampus and visual and motor cortices, the PCx and TCx remained unaffected [55]. Similarly, no age-related effects were found in the Aβ load of the PCx among older adults diagnosed with dementia [37]. Our data for the total TCx and PCx from AD donors support these previous studies, as we observed no differences in the percentage of Aβ deposition between octogenarians and nonagenarians.
It is important to note that previous research on this topic has primarily focused on global or regional Aβ levels, without considering sub-regional differences in the gray and white matter [34, 35, 37, 53–55]. However, by accounting for such distinctions, we discovered a higher Aβ percentage deposition in the TCx white matter of AD individuals aged 78–89, compared to CU individuals of the same age. Additionally, within the PCx white matter, the 90–100-year-old AD group exhibited greater Aβ deposition compared to AD individuals aged 78–89. These effects, however, did not manifest in the overall cortical Aβ burden. Collectively, these findings suggest that while changes in Aβ deposition may be less detectable at old ages, they can still be identified in the cortical white matter.
Sex-specific differences in the cortical Aβ deposition
The prevalence of AD is higher in women than men, even after accounting for women’s longer lifespans [52, 56, 57]. This observation has led to interest in investigating sex differences in the neuropathological hallmarks of AD. In a genome-wide association study, a strong positive correlation was found between serine protease inhibitor genes (SERPINB1, SERPINB6, and SERPINB9) and amyloidosis in the prefrontal cortex of women, but not in men [58]. Previous studies using Aβ PET imaging in CU individuals have reported either no sex differences [41, 59, 60] or higher Aβ levels in women [39, 61]. At the neuropathological level, brain samples from both CU and AD women have demonstrated higher levels of overall AD pathology, primarily driven by tau tangle densities [40, 55, 62, 63]. Animal models of Aβ overexpression have shown accelerated rates of hippocampal Aβ deposition in females compared to males [64, 65]. Consistent with these findings, our data also revealed no significant differences in Aβ load when comparing women and men in total cortical regions. However, when analyzing individual sub-regions, we observed a significant difference in the white matter of the PCx in AD individuals, where females exhibited higher percentages of Aβ deposition.
Recent literature suggests that loss of neuroprotective sex hormones after menopause may influence a higher susceptibility of the female brain to neurodegeneration and AD (reviewed by Demetrius et al. [66]). Animal studies have shown that depleting ovarian hormones through ovariectomy exacerbates Aβ expression in the hippocampus [64, 67]. Additionally, treatment with 17β-estradiol has been shown to reduce Aβ plaque density in the cortex and hippocampus [68]. We observed a higher Aβ load in AD women compared to CU women, not only in the total PCx but also in both the gray and white matter of the PCx, as well as in the white matter of the TCx. Therefore, the decline in gonadal hormones after menopause may contribute to the increased Aβ deposition found in AD women.
Antagonistic association between education and the white matter Aβ burden
Education level can be either a risk or a protection factor for AD and other dementias [64, 65]. Increased years of education have been associated with a 7% reduction in the risk of developing dementia per year [65]. In older adults, higher educational attainment has been positively linked to neuronal activity in the inferior temporal lobe during memory tasks [66]. Moreover, greater education has been identified as a neuroprotective factor against hippocampal atrophy and loss of inhibitory GABAergic neurons [69, 70].
These findings can be understood in light of the cognitive reserve (CR) hypothesis, which posits that lifelong engagement in mentally stimulating activities influences individual tolerance to cerebral pathology [71, 72]. Educational attainment is a proxy for CR, along with occupational engagement and cognitively stimulating activities throughout the lifespan [72]. CR has been associated with the preservation of gray matter volume, increased metabolic activity in the temporal area, robust functional connectivity, slower decline in memory, and a reduced risk of dementia [73–77]. Additionally, CU individuals who engage in higher levels of mentally stimulating activities throughout their lives have been found to exhibit lower levels of cerebral Aβ deposition [38].
It is proposed that the CR facilitates either the efficient utilization of existing neural networks or the capacity to activate alternative networks in response to disruptions in the primary network [72, 78]. A previous cohort study reported that some non-demented centenarians maintain cognitive performance despite having elevated Aβ levels [78]. This resilience is partially attributed to a genetically defined tolerance for AD-associated neuropathology [78, 79]. However, the authors also acknowledge that the centenarians in this cohort had high levels of educational attainment, which may have contributed to their individual resilience [78, 80, 81]. Several lines of evidence also indicate that the negative association between Aβ load and episodic memory is attenuated in CU individuals with higher education [42, 82, 83].
Previous studies have yielded divergent findings regarding the relationship between educational attainment and Aβ pathology. Some studies have reported a negative association between the Aβ PET signal and education in CU individuals [84, 85], while other studies found no significant associations [86]. Similarly, in AD individuals, some studies did not find a link between education and Aβ deposition [85–88], while one study observed higher Aβ levels in the frontal, but not in parietal or temporal, regions of higher educated individuals [89]. The discrepancy in these findings may be attributed, at least in part, to the evaluation of global Aβ levels without considering region-specific differences [85].
In this study, CU, but not AD, individuals with higher education years presented lower Aβ burden in the temporal and parietal cortical regions. This relation was observed only in the white matter. The gray and white matter undergo distinct morphological alterations during the lifespan [90, 91]. The gray matter volume tends to increase until around middle childhood (approximately 6 years), after which it starts to decline from young adulthood onwards. In contrast, the white matter achieves its maximum volume in adulthood (between 20 and 40 years), stabilizing and subsequently decreasing in late adulthood. Both gray and white matter exhibit accelerated atrophy during the late stages of adulthood [91–94].
While traditional research on amyloidosis has primarily focused on the gray matter, recent studies have recognized the significance of Aβ alterations in the white matter during both normal aging and AD [95, 96]. Elevated levels of soluble Aβ isoforms (Aβ40 and Aβ42) have been found in the white matter of AD individuals in comparison with CU ones [95]. White matter hyperintensities, a radiological marker of white matter abnormalities, are increased in AD and associated with higher Aβ levels in CU individuals [96–100]. Additionally, cerebrovascular dysfunctions driven by Aβ deposition frequently involve white matter lesions [101]. Patients with cerebral amyloid angiopathy (CAA), which is characterized by the loss of vessel integrity due to Aβ deposition in the arteries, arterioles, and capillaries, often present microbleeds at the border between the gray matter and white matter of the parietal and occipital lobes [102]. Cerebral microbleeds and CAA also have been characterized by the presence of amyloid-associated white matter hyperintensities [97, 103, 104].
Our findings here suggest that education years, and by extension the CR, might promote neuroprotection against the Aβ load in the white matter of CU individuals. However, the pathophysiological mechanisms that underlie this relationship remain to be determined. This highlights the importance of future investigations to explore the potential associations between the CR, white matter alterations, and region-specific amyloid deposition.
Pathological and inflammatory proteins and the cortical Aβ burden
The cleavage cascade of the APP originates Aβ soluble isoforms ranging in length from 37 to 43 amino acids [105], in which Aβ40 predominates [106, 107]. Although the Aβ42 product is released in fewer amounts, these peptides have a longer C-terminus and are more hydrophobic, being thought to be the most contributing factor for neuritic plaque formation [105–108]. Indeed, in a postmortem study, it is described that neuritic plaques contain primarily Aβ42 and occasionally Aβ40 [109]. For this reason, we were expecting positive correlations between the Aβ burden and either only with Aβ42 or with both isoforms. We confirmed the latter possibility. These data reinforce the participation of both isoforms in neuritic plaque formation in normal aging and AD.
During the progression of AD, the build-up of Aβ plaques is viewed as an initial occurrence, while the development of tau neurofibrillary tangles occurs temporally later and is in a closer association with subsequent neuronal dysfunction. Accordingly, patients with autosomal dominant AD present early Aβ deposition, which precedes increased levels of total tau and pTau-181 [110]. In this context, it was proposed that either Aβ and Tau pathologies are two unrelated pathologies that happen to coincide during AD progression or these proteins act synergistically to potentiate neural damage and the onset of cognitive decline [111]. A significant body of evidence supports the latter proposition [17–19, 112].
In a previous study, the injection of human AD-brain-derived pathological tau into Aβ plaque-bearing mouse models enhanced the formation of tau pathologies [113]. Another study combined a mice lineage that overexpresses Aβ with a lineage for tau overexpression and observed a threefold increase in tau seeding activity [111]. Using a HEK cell biosensor array for tau isolated from humans, these authors also found that cases with Aβ plaques had an enhanced propensity to promote tau aggregation in comparison with cases without plaques [111]. Here, we found that AD donors, but not CU ones, presented a positive correlation between the Aβ load and the concentration of pTau-181 in both cortical regions. We did not find any correlations with total Tau. The pTau-181 is a highly specific pathological marker for AD being able to differentiate it from other neurodegenerative diseases [114]. Thus, we corroborate the view that Aβ contributes to a cellular microenvironment prone to the development of AD-related tau pathologies.
During normal aging and AD, the brain undergoes structural and functional changes that vary across different regions [115–120]. This region-specific vulnerability to brain changes is influenced by distinct local neuroinflammatory responses [121, 122]. Higher levels of proinflammatory cytokines, such as TNFα, are detectable in individuals with mild cognitive impairment and are correlated with a greater likelihood of advancing to severe AD [123]. In a mouse model for amyloidosis, TNFα levels were found to be elevated in comparison with control ones and were positively correlated with soluble and insoluble Aβ40 and Aβ42 isoforms [124]. Similarly, TNFα induced Aβ production from astrocytes in APP transgenic mice [125]. Chronic TNFα exposition also induced substantial production of Aβ aggregates in human AD neurons but not in healthy control ones [126]. In accordance, we found a positive correlation between the Aβ load and TNFα concentration in the TCx from AD individuals but not in CU ones. This effect seems highly region-specific, since it was not observed in the PCx.
Our findings are particularly relevant since therapies based on TNFα inhibitors have been shown to reduce Aβ plaque deposition in mice [127, 128] and improve cognitive function in mice and humans [127–129]. Thus, the identification of regions in which the association between TNFα and Aβ is more evident may facilitate the validation of these therapies for humans. In other words, region-specific findings can guide future research to areas where local neuroinflammation is more likely to occur and should be targeted. In our adjusted models, no other neuroinflammatory protein concentration was associated with the Aβ load in the PCx and TCx of AD and CU donors. It would be interesting to investigate whether similar associations are present in other brain regions.
Limitations
This study has certain limitations inherent to the use of autopsy-based data available in online databases. Detailed information regarding the exact postmortem interval (PMI) and cause of death of the donors was not provided in the database. However, it is stated that a rapid autopsy protocol was implemented to ensure a PMI of less than 8 h, thereby minimizing potential postmortem tissue reactions that could impact the immunohistochemistry signal.
The histochemical procedures performed in the database used only 3 tissue samples per histological marker for each region of interest. Ideally, it would be better for morphometric quantifications if each region was represented in more images. However, histological sampling was performed through a fractionator scheme which is a rigorous method to standardize the collection of tissue in a manner that each individual has representative sections of the regions of interest as matched as possible. In this scheme, sections were collected in a systematic interval at 1 in every 20th for each marker. Also, the area fraction fractionator ensures the morphometric evaluation in an assumption-free manner, which has advantages in relation to other manual methods of counting (which might introduce biases when placing counting grids) or automatic counting methods (which might introduce biases when standardizing the background of the images to apply a pixel threshold).
Since the age range of the subjects included in the cohort was from 78 to over 100 years, all our conclusions are limited to age-related changes within the octogenarian—nonagenarian interval. We had no means to evaluate the factors underlying the Aβ build-up between young, middle-aged, and old brains. Moreover, the age of the donors in the database was categorized into intervals for those within the 90–100-year-old range, which restricted our analysis to treating age as a categorical variable. While the participants of the ACT cohort study underwent evaluation of cognitive activity, this information was not available for correlation with the presented findings. Our final AD sample was uneven in gender representation, with 10 men and only 5 women. However, our regression models were adjusted for sex, as well as age and education, to account for any potential influence of gender or other confounding factors. It should be noted that information regarding the ApoE genotype was missing for several donors, preventing us from including this variable in our models.
Conclusion
Our study provides insights into the factors influencing region-specific Aβ accumulation in the cerebral cortex during normal aging and AD. We found higher Aβ burdens in the TCx of AD octogenarians compared to CU ones. We also found higher Aβ loads in the PCx of AD nonagenarians than in AD octogenarians. Furthermore, AD women exhibited a greater Aβ burden compared to CU women. Our findings also suggest that educational attainment may have a protective effect against Aβ deposition in the white matter of CU individuals. Moreover, we observed positive associations between the Aβ burden and Aβ40 and Aβ42 isoforms in both cortical regions of CU and AD individuals. In contrast, the pTau-181 isoform was found to be positively associated with the Aβ burden only in AD individuals. Finally, we found a positive association between the Aβ load and the proinflammatory cytokine TNFα exclusively in the TCx of AD individuals. These findings shed light on region-specific vulnerabilities for Aβ deposition in the brain, which could guide targeted therapeutic interventions in the future.
Acknowledgements
The authors express their gratitude to the University of Washington, the Kaiser Permanente Washington Health Research Institute, and the Allen Institute for Brain Sciences for organizing the Aging Dementia and TBI study and generously providing case studies to the neuroscience community.
Author contribution
Conceptualization: Sayonara P. da Silva and Felipe P. Fiuza; methodology: Sayonara P. da Silva; formal analysis and investigation: Sayonara P. da Silva, Carla C. M. de Castro, and Livia N. Rabelo; data curation: Sayonara P. da Silva; writing—original draft preparation: Sayonara P. da Silva, Bernardino Fernández-Calvo, Rovena C. G. J. Engelberth, and Felipe P. Fiuza; writing—review and editing: Sayonara P. da Silva, Bernardino Fernández-Calvo, Rovena C. G. J. Engelberth, and Felipe P. Fiuza; supervision: Felipe P. Fiuza. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Brazilian Ministry of Education (MEC) and Brazilian funding agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). BF-C holds a Senior Distinguished Researcher position (Beatriz Galindo Programme) in the Department of Psychology at the University of Córdoba (ref. BEA-GAL18/00006).
Data availability
Please contact the author for data requests.
Declarations
Ethics approval and consent to participate
Ethical approval was waived for this study due to the fact that this work did not involve any human or animal model experimentation, nor clinical trials or direct usage of brain tissues. Instead, the present work is an in silico analysis of digital images previously obtained and compiled by the “Aging, Dementia and TBI study” database organizers (Allen Institute for Brain Sciences, University of Washington and the Kaiser Permanente Washington Health Research Institute). Permission for usage in academic research of the downloadable content, including these images, is explicit and encouraged at https://alleninstitute.org/legal/terms-use/ (accessed on 17 May, 2023). As it is stated in the Allen Institute legal terms, no further approval is requested for the usage of these data. Informed consent was obtained from all subjects involved in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaborators G 2019. Global mortality from dementia: application of a new method and results from the Global Burden Of Disease Study 2019. Alzheimer’s Dement: Transl Res Clin Interv 2021. 10.1002/trc2.12200. [DOI] [PMC free article] [PubMed]
- 2.GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022. 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Washington: American Psychiatric Publishing, Inc.; 2013. [Google Scholar]
- 4.Mecocci P, Boccardi V. The impact of aging in dementia: it is time to refocus attention on the main risk factor of dementia. Ageing Res Rev. 2020 doi: 10.1016/j.arr.2020.101210. [DOI] [PubMed] [Google Scholar]
- 5.Golde TE. Alzheimer’s disease – the journey of a healthy brain into organ failure. Mol Neurodegener. 2022 doi: 10.1186/s13024-022-00523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews SJ, Renton AE, Fulton-Howard B, et al. The complex genetic architecture of Alzheimer’s disease: novel insights and future directions. EBioMedicine. 2023 doi: 10.1016/j.ebiom.2023.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanoiselée H-M, Nicolas G, Wallon D, et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLoS Med. 2017 doi: 10.1371/journal.pmed.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wightman DP, Jansen IE, Savage JE, et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat Genet. 2021 doi: 10.1038/s41588-021-00921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellenguez C, Küçükali F, Jansen IE, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022 doi: 10.1038/s41588-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, Srivastav S, Yadav AK, Srikrishna S, Perry G. Overview of Alzheimer's disease and some therapeutic approaches targeting Aβ by using several synthetic and herbal compounds. Hindawi Publishing Corporation; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geraets AFJ, Leist AK. Sex/gender and socioeconomic differences in modifiable risk factors for dementia. Sci Rep. 2023 doi: 10.1038/s41598-022-27368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decourt B, D’Souza GX, Shi J, et al. The cause of Alzheimer’s disease: the theory of multipathology convergence to chronic neuronal stress. Aging Dis. 2022. 10.14336/AD.2021.0529. [DOI] [PMC free article] [PubMed]
- 13.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997 doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 14.Thal DR, Rüb U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002 doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 15.Wang W-Y, Tan M-S, Yu J-T, et al. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015 doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.d’Errico P, Ziegler-Waldkirch S, Aires V, et al. Microglia contribute to the propagation of Aβ into unaffected brain tissue. Nat Neurosci. 2022 doi: 10.1038/s41593-021-00951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DW, Tu KJ, Wei A, et al. Amyloid-beta and tau pathologies act synergistically to induce novel disease stage-specific microglia subtypes. Mol Neurodegener. 2022 doi: 10.1186/s13024-022-00589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripathi T, Kalita P. Synergistic effect of amyloid-β and tau disrupts neural circuits. ACS Chem Neurosci. 2019 doi: 10.1021/acschemneuro.9b00037. [DOI] [PubMed] [Google Scholar]
- 19.Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci. 2020 doi: 10.1038/s41593-020-0687-6. [DOI] [PubMed] [Google Scholar]
- 20.Tillement L, Lecanu L, Papadopoulos V. Alzheimer’s disease: effects of β-amyloid on mitochondria. Mitochondrion. 2011 doi: 10.1016/j.mito.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Park J, Jang M, Chang S. Deleterious effects of soluble amyloid-β oligomers on multiple steps of synaptic vesicle trafficking. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Reiss AB, Arain HA, Stecker MM, et al. Amyloid toxicity in Alzheimer’s disease. Rev Neurosci. 2018 doi: 10.1515/revneuro-2017-0063. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Marin V, Blazquez-Llorca L, Rodriguez J, et al. Diminished perisomatic GABAergic terminals on cortical neurons adjacent to amyloid plaques. Front Neuroanat. 2009 doi: 10.3389/neuro.05.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauterborn JC, Scaduto P, Cox CD, et al. Increased excitatory to inhibitory synaptic ratio in parietal cortex samples from individuals with Alzheimer’s disease. Nat Commun. 2021 doi: 10.1038/s41467-021-22742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaduto P, Lauterborn JC, Cox CD, et al. Functional excitatory to inhibitory synaptic imbalance and loss of cognitive performance in people with Alzheimer’s disease neuropathologic change. Acta Neuropathol. 2023 doi: 10.1007/s00401-022-02526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busche MA, Chen X, Henning HA, et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2012.10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed]
- 27.Hascup KN, Findley CA, Sime LN, et al. Hippocampal alterations in glutamatergic signaling during amyloid progression in AβPP/PS1 mice. Sci Rep. 2020 doi: 10.1038/s41598-020-71587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zott B, Simon MM, Hong W, et al. A vicious cycle of β amyloid–dependent neuronal hyperactivation. Science. 2019 doi: 10.1126/science.aay0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maestú F, de Haan W, Busche MA, et al. Neuronal excitation/inhibition imbalance: core element of a translational perspective on Alzheimer pathophysiology. Ageing Res Rev. 2021 doi: 10.1016/j.arr.2021.101372. [DOI] [PubMed] [Google Scholar]
- 30.Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968 doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- 31.Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988 doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 32.Verfaillie SCJ, Tijms B, Versteeg A, et al. Thinner temporal and parietal cortex is related to incident clinical progression to dementia in patients with subjective cognitive decline. Alzheimers Dement (Amst) 2016 doi: 10.1016/j.dadm.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michalowska MM, Herholz K, Hinz R, et al. Evaluation of in vivo staging of amyloid deposition in cognitively unimpaired elderly aged 78–94. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen WJ, Janssen O, Tijms BM, et al. Prevalence estimates of amyloid abnormality across the Alzheimer disease clinical spectrum. JAMA Neurol. 2022 doi: 10.1001/jamaneurol.2021.5216. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigue KM, Kennedy KM, Devous MD, et al. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012 doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnen JA, Santa Cruz K, Hemmy LS, et al. Ecology of the aging human brain. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JA, Guillozet-Bongaarts A, Gibbons LE, et al. Neuropathological and transcriptomic characteristics of the aged brain. eLife. 2017 doi: 10.7554/eLife.31126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landau SM, Marks SM, Mormino EC, et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology. 2017 doi: 10.1212/WNL.0000000000004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oveisgharan S, Arvanitakis Z, Yu L, et al. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 2018 doi: 10.1007/s00401-018-1920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2018.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joannette M, Bocti C, Dupont PS, et al. Education as a moderator of the relationship between episodic memory and amyloid load in normal Aging. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002 doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 44.Teng EL, Hasegawa K, Homma A, et al. The cognitive abilities screening instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994 doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 45.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association . Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association, Inc; 1994. p. 886. [Google Scholar]
- 47.Amro Z, Ryan M, Collins-Praino LE, et al. Unexpected classes of aquaporin channels detected by transcriptomic analysis in human brain are associated with both patient age and Alzheimer’s disease status. Biomedicines. 2023 doi: 10.3390/biomedicines11030770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montine TJ, Montine KS, McMahan W, et al. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid redox signal. 2005 doi: 10.1089/ars.2005.7.269. [DOI] [PubMed] [Google Scholar]
- 49.Milatovic D, VanRollins M, Li K, et al. Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by alpha- or gamma-tocopherol. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 doi: 10.1016/j.jchromb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 50.Howard V, Reed N. Unbiased stereology: three-dimensional measurement in microscopy. 2. Oxon: Garland Science; 2005. [Google Scholar]
- 51.Stephan Y, Sutin AR, Luchetti M, et al. Subjective age and risk of incident dementia: evidence from the national health and aging trends survey. J Psychiatr Res. 2018 doi: 10.1016/j.jpsychires.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alzheimer’s Association. Alzheimer’s disease facts and figures. 2022. 10.1002/alz.12638
- 53.Bischof GN, Rodrigue KM, Kennedy KM, et al. Amyloid deposition in younger adults is linked to episodic memory performance. Neurology. 2016 doi: 10.1212/WNL.0000000000003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liesinger AM, Graff-Radford NR, Duara R, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. 2018 doi: 10.1007/s00401-018-1908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beam CR, Kaneshiro C, Jang JY, et al. Differences between women and men in incidence rates of dementia and Alzheimer’s disease. J Alzheimers Dis. 2018 doi: 10.3233/JAD-180141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vina J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;S520:S527–S533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 58.Deming Y, Dumitrescu L, Barnes LL, et al. Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta Neuropathol. 2018 doi: 10.1007/s00401-018-1881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012 doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimer’s Dement. 2018 doi: 10.1016/j.jalz.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luchsinger JA, Palta P, Rippon B, et al. Sex differences in in-vivo Alzheimer’s disease neuropathology in late middle-aged Hispanics. J Alzheimers Dis. 2020 doi: 10.3233/JAD-191183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnes LL, Wilson RS, Bienias JL, et al. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005 doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 63.Hu Y-T, Boonstra J, McGurran H, et al. Sex differences in the neuropathological hallmarks of Alzheimer’s disease: focus on cognitively intact elderly individuals. Neuropathol Appl Neurobiol. 2021 doi: 10.1111/nan.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carroll JC, Rosario ER, Chang L, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007 doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu Y-T, Chen X-L, Zhang Y-N, et al. Sex differences in hippocampal β-amyloid accumulation in the triple-transgenic mouse model of Alzheimer’s disease and the potential role of local estrogens. Front Neurosci. 2023 doi: 10.3389/fnins.2023.1117584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demetrius LA, Eckert A, Grimm A. Sex differences in Alzheimer’s disease: metabolic reprogramming and therapeutic intervention. Trends Endocrinol Metab. 2021 doi: 10.1016/j.tem.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Yao J, Irwin R, Chen S, et al. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amtul Z, Wang L, Westaway D, et al. Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer’s disease. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Belleville S, Mellah S, Cloutier S, et al. Neural correlates of resilience to the effects of hippocampal atrophy on memory. NeuroImage: Clinical 2021. 10.1016/j.nicl.2020.102526. [DOI] [PMC free article] [PubMed]
- 70.Castro CCM, Silva SP, Rabelo LN, et al. Age, education years, and biochemical factors are associated with selective neuronal changes in the elderly hippocampus. Cells. 2022 doi: 10.3390/cells11244033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- 72.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020 doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ersoezlue E, Rauchmann B-S, Schneider-Axmann T, et al. Lifelong experiences as a proxy of cognitive reserve moderate the association between connectivity and cognition in Alzheimer’s disease. Neurobiol Aging. 2023 doi: 10.1016/j.neurobiolaging.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Liesto S, Sipilä R, Hietanen M, et al. Cognitive function is well preserved in a cohort of breast cancer survivors: roles of cognitive reserve, resilience, and general health. The Breast. 2022 doi: 10.1016/j.breast.2022.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez M, Knížková K, Keřková B, et al. The relationships between cognitive reserve, cognitive functioning and quality of life in first-episode schizophrenia spectrum disorders. Psychiatry Res. 2022 doi: 10.1016/j.psychres.2022.114479. [DOI] [PubMed] [Google Scholar]
- 76.Brenner EK, Thomas KR, Weigand AJ, et al. Cognitive reserve moderates the association between cerebral blood flow and language performance in older adults with mild cognitive impairment. Neurobiol Aging. 2023 doi: 10.1016/j.neurobiolaging.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Contador I, Alzola P, Stern Y, et al. Is cognitive reserve associated with the prevention of cognitive decline after stroke? A systematic review and meta-analysis. Ageing Res Rev. 2023 doi: 10.1016/j.arr.2022.101814. [DOI] [PubMed] [Google Scholar]
- 78.Zhang M, Ganz AB, Rohde S, et al. Resilience and resistance to the accumulation of amyloid plaques and neurofibrillary tangles in centenarians: an age-continuous perspective. Alzheimers Dement. 2023 doi: 10.1002/alz.12899. [DOI] [PubMed] [Google Scholar]
- 79.Tesi N, van der Lee SJ, Hulsman M, et al. Centenarian controls increase variant effect sizes by an average twofold in an extreme case–extreme control analysis of Alzheimer’s disease. Eur J Hum Genet. 2019 doi: 10.1038/s41431-018-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holstege H, Beker N, Dijkstra T, et al. The 100-plus study of cognitively healthy centenarians: rationale, design and cohort description. Eur J Epidemiol. 2018 doi: 10.1007/s10654-018-0451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beker N, Ganz A, Hulsman M, et al. Association of cognitive function trajectories in centenarians with postmortem neuropathology, physical health, and other risk factors for cognitive decline. JAMA Netw Open. 2021 doi: 10.1001/jamanetworkopen.2020.31654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010 doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jansen WJ, Ossenkoppele R, Tijms BM, et al. Association of cerebral amyloid-β aggregation with cognitive functioning in persons without dementia. JAMA Psychiat. 2018 doi: 10.1001/jamapsychiatry.2017.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yasuno F, Kazui H, Morita N, et al. Low amyloid-β deposition correlates with high education in cognitively normal older adults: a pilot study. Int J Geriatr Psychiatry. 2015 doi: 10.1002/gps.4235. [DOI] [PubMed] [Google Scholar]
- 85.Arenaza-Urquijo EM, Bejanin A, Gonneaud J, et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging. 2017 doi: 10.1016/j.neurobiolaging.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 86.Wada M, Noda Y, Shinagawa S, et al. Effect of education on Alzheimer’s disease-related neuroimaging biomarkers in healthy controls, and participants with mild cognitive impairment and Alzheimer’s disease: a cross-sectional study. J Alzheimers Dis. 2018 doi: 10.3233/JAD-171168. [DOI] [PubMed] [Google Scholar]
- 87.Serrano-Pozo A, Qian J, Monsell SE, et al. Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the national Alzheimer coordinating center. J Neuropathol Exp Neurol. 2013 doi: 10.1097/NEN.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoenig M, Caprioglio CC, Collij L, et al. Inverse relationship between education and amyloid burden in individuals with subjective cognitive decline plus and mild cognitive impairment. J Nucl Med. 2022;63(supplement 2):2609–2609. [Google Scholar]
- 89.Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008 doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- 90.Schilling KG, Archer D, Yeh F-C, et al. Aging and white matter microstructure and macrostructure: a longitudinal multi-site diffusion MRI study of 1218 participants. Brain struct funct. 2022 doi: 10.1007/s00429-022-02503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schilling KG, Archer D, Rheault F, et al. Superficial white matter across development, young adulthood, and aging: volume, thickness, and relationship with cortical features. Brain struct funct. 2023 doi: 10.1007/s00429-023-02642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hedman AM, van Haren NEM, Schnack HG, et al. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum brain mapp. 2012 doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lebel C, Gee M, Camicioli R, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 94.Bethlehem RaI, Seidlitz J, White SR, et al. Brain charts for the human lifespan. Nature. 2022. 10.1038/s41586-022-04554-y. [DOI] [PMC free article] [PubMed]
- 95.Collins-Praino LE, Francis YI, Griffith EY, et al. Soluble amyloid beta levels are elevated in the white matter of Alzheimer’s patients, independent of cortical plaque severity. Acta Neuropathol Commun. 2014 doi: 10.1186/s40478-014-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caballero MÁA, Song Z, Rubinski A, et al. Age-dependent amyloid deposition is associated with white matter alterations in cognitively normal adults during the adult life span. Alzheimers Dement. 2020 doi: 10.1002/alz.12062. [DOI] [PubMed] [Google Scholar]
- 97.Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain. 2019 doi: 10.1093/brain/awz162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dupont PS, Bocti C, Joannette M, et al. Amyloid burden and white matter hyperintensities mediate age-related cognitive differences. Neurobiol Aging. 2020 doi: 10.1016/j.neurobiolaging.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 99.Walker KA, Silverstein N, Zhou Y, et al. Brain white matter structure and amyloid deposition in black and white older adults: the ARIC-PET study. J Am Heart Assoc. 2021 doi: 10.1161/JAHA.121.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alban SL, Lynch KM, Ringman JM, et al. The association between white matter hyperintensities and amyloid and tau deposition. Neuroimage Clin. 2023 doi: 10.1016/j.nicl.2023.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inoue Y, Shue F, Bu G, et al. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol Neurodegener. 2023 doi: 10.1186/s13024-023-00640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke. 1983 doi: 10.1161/01.str.14.6.915. [DOI] [PubMed] [Google Scholar]
- 103.Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, et al. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology. 2014 doi: 10.1212/WNL.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng H, Yuan Y, Zhang Z, et al. Analysis of risk factors for cerebral microbleeds and the relationship between cerebral microbleeds and cognitive impairment. Brain Sci. 2022 doi: 10.3390/brainsci12111445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chai AB, Lam HHJ, Kockx M, et al. Apolipoprotein E isoform-dependent effects on the processing of Alzheimer’s amyloid-β. Biochim Biophys Acta (BBA) – Mol Cell Biol Lipids. 2021. 10.1016/j.bbalip.2021.158980. [DOI] [PubMed]
- 106.Murphy MP, LeVine H. Alzheimer’s disease and the amyloid-β peptide. Lovell MA. ed. JAD. 2010.10.3233/JAD-2010-1221.
- 107.Steiner H, Fukumori A, Tagami S, et al. Making the final cut: pathogenic amyloid-β peptide generation by γ-secretase. CST. 2018. 10.15698/cst2018.11.162. [DOI] [PMC free article] [PubMed]
- 108.Iwatsubo T, Odaka A, Suzuki N, et al. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43) Neuron. 1994 doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 109.Cummings BJ, Satou T, Head E, et al. Diffuse plaques contain C-terminal a beta 42 and not a beta 40: evidence from cats and dogs. Neurobiol Aging. 1996 doi: 10.1016/0197-4580(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 110.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bennett RE, DeVos SL, Dujardin S, et al. Enhanced tau aggregation in the presence of amyloid β. Am J Pathol. 2017 doi: 10.1016/j.ajpath.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014 doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 113.He Z, Guo JL, McBride JD, et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med. 2018 doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 115.Fiuza FP, Silva KDA, Pessoa RA, et al. Age-related changes in neurochemical components and retinal projections of rat intergeniculate leaflet. Age (Dordr) 2016 doi: 10.1007/s11357-015-9867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fiuza FP, Aquino ACQ, Câmara DA, et al. Region-specific glial hyperplasia and neuronal stability of rat lateral geniculate nucleus during aging. Exp Gerontol. 2017 doi: 10.1016/j.exger.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 117.Engelberth RCGJ, Silva KD de A, Fiuza FP, et al. Retinal, NPY- and 5ht- inputs to the aged suprachiasmatic nucleus in common marmosets (Callithrix jacchus). Neurosci Res. 2017;121:54–59. 10.1016/j.neures.2017.03.005. [DOI] [PubMed]
- 118.Feng X, Guo J, Sigmon HC, et al. Brain regions vulnerable and resistant to aging without Alzheimer’s disease. PLoS One. 2020 doi: 10.1371/journal.pone.0234255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bader AS, Gnädig M-U, Fricke M, et al. Brain region-specific differences in amyloid-β plaque composition in 5XFAD mice. Life (Basel) 2023 doi: 10.3390/life13041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Das S, Ramanan N. Region-specific heterogeneity in neuronal nuclear morphology in young, aged and in Alzheimer’s disease mouse brains. Front Cell Dev Biol. 2023 doi: 10.3389/fcell.2023.1032504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGeer PL, McGeer EG. Local neuroinflammation and the progression of Alzheimer’s disease. J Neurovirol. 2002 doi: 10.1080/13550280290100969. [DOI] [PubMed] [Google Scholar]
- 122.Andronie-Cioara FL, Ardelean AI, Nistor-Cseppento CD, et al. Molecular mechanisms of neuroinflammation in aging and Alzheimer’s disease progression. Int J Mol Sci. 2023 doi: 10.3390/ijms24031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bermejo P, Martín-Aragón S, Benedí J, et al. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunol Lett. 2008 doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Patel NS, Paris D, Mathura V, et al. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J Neuroinflammation. 2005 doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamamoto M, Kiyota T, Horiba M, et al. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007 doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Whiten DR, Brownjohn PW, Moore S, et al. Tumour necrosis factor induces increased production of extracellular amyloid-β- and α-synuclein-containing aggregates by human Alzheimer’s disease neurons. Brain Commun. 2020 doi: 10.1093/braincomms/fcaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi J-Q, Shen W, Chen J, et al. Anti-TNF-α reduces amyloid plaques and tau phosphorylation and induces CD11c-positive dendritic-like cell in the APP/PS1 transgenic mouse brains. Brain Res. 2011 doi: 10.1016/j.brainres.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 128.Park J, Lee S-Y, Shon J, et al. Adalimumab improves cognitive impairment, exerts neuroprotective effects and attenuates neuroinflammation in an Aβ1-40-injected mouse model of Alzheimer’s disease. Cytotherapy. 2019 doi: 10.1016/j.jcyt.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 129.Tobinick E, Gross H, Weinberger A, et al. TNF-alpha modulation for treatment of Alzheimer’s disease: a 6-month pilot study. MedGenMed. 2006;8(2):25. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the author for data requests.