Abstract
Transcription of the Escherichia coli osmB gene is induced by several stress conditions. osmB is expressed from two promoters, osmBp1 and osmBp2. The downstream promoter, osmBp2, is induced after osmotic shock or upon entry into stationary phase in a σS-dependent manner. The upstream promoter, osmBp1, is independent of σS and is activated by RcsB, the response regulator of the His-Asp phosphorelay signal transduction system RcsCDB. RcsB is responsible for the induction of osmBp1 following treatment with chlorpromazine. Activation of osmBp1 by RcsB requires a sequence upstream of its −35 element similar to the RcsB binding site consensus, suggesting a direct regulatory role. osmB appears as another example of a multistress-responsive gene whose transcription involves both a σS-dependent promoter and a second one independent of σS but controlled by stress-specific transcription factors.
To adapt to adverse conditions, bacterial cells induce specific families of genes that promote growth or survival in stressful environments. Many such genes can be induced by a variety of stresses through several transcriptional regulators acting on one or several stress-inducible promoters. In Escherichia coli, osmB is an example of a multistress-responsive gene, and we are investigating its regulation with the aim of understanding better the complex interplay of regulators resulting in multistress response. osmB encodes an outer membrane lipoprotein of an as yet unknown function (21). First identified as an osmotically inducible gene, osmB is also induced upon entry into stationary phase in a σS-dependent manner (13, 18, 20). RNA mapping experiments suggested that osmB is expressed under the control of two promoters. The downstream promoter, named osmBp2, was unambiguously characterized by deletion mapping and RNA analysis (20). In contrast, the proposed upstream promoter, osmBp1, contained no sequences similar to the canonical −10 and −35 elements upstream from the mRNA 5′ end determined by RNase protection experiments, and its identification remained uncertain (20).
Recently, transcriptome analysis revealed that osmB is a target of the RcsCDB His-Asp phosphorelay system (7, 16; our unpublished results). Initially found to regulate the synthesis of the capsular polysaccharide in E. coli (11), the response regulator RcsB was also shown to activate osmCp1, a σS-independent promoter of the multistress-responsive gene osmC. This activation occurs through the binding of RcsB to a site upstream from the −35 box of osmCp1 (5, 25, 26) and is responsible for the induction of osmCp1 upon exposure to the cationic amphipathic molecule chlorpromazine (3).
We investigated here the organization of the osmB promoter region and the mechanism of activation of osmB transcription by RcsCDB. We demonstrate that osmB is transcribed under the control of two independent promoters. The downstream promoter, osmBp2, is σS dependent and responsible for the response to the phase of growth and to osmotic shock, whereas the upstream promoter, osmBp1, is σS independent and the target of the response regulator RcsB.
Transcription of osmB is activated by RcsB but not by its cofactor, RcsA.
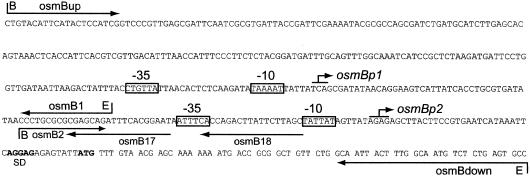
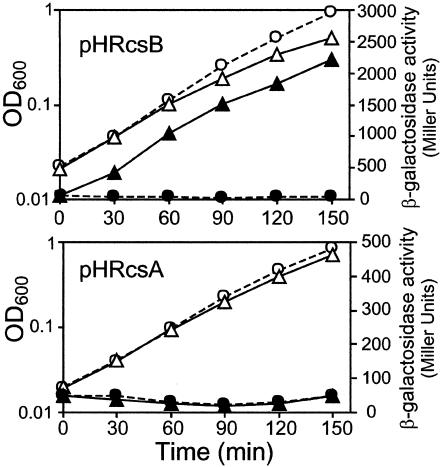
To investigate the regulation of osmB transcription, we constructed a set of osmB-lac transcriptional fusions. DNA fragments amplified from chromosomal DNA with various oligonucleotide pairs (Fig. 1) were cloned in the vector pRS550, recombined on bacteriophage λRS45, and installed in single copy in the chromosome of the wild-type strain CF6343 (9) as described by Simons et al. (24). In strain SK1755, the osmB-lac fusion is driven by a 465-bp DNA fragment amplified with the oligonucleotides osmBup and osmBdown (Fig. 1). SK1755 was transformed with the plasmid pHRcsB (2) or pHRcsA (5), expressing rcsB or rcsA, respectively, under the control of lacp, and the effect of overproduction of RcsB or RcsA on osmB promoter activity was tested by monitoring β-galactosidase activity (22) in the absence or presence of IPTG (isopropyl-β-d-thiogalactopyranoside) (Fig. 2). In agreement with previous data from transcriptome analysis (7, 16), these experiments demonstrated that the overproduction of RcsB strongly induced transcription from the osmB promoter region. In contrast, the overproduction of RcsA, a cofactor of RcsB necessary for the full activation of the capsule synthesis cps genes (11, 27), had no effect on transcription from osmBp. Since it has been shown previously that in identical experimental conditions, RcsA accumulation is sufficient to repress transcription from the flhD promoter (9), we conclude that osmB belongs to the RcsA-independent subfamily of RcsB targets.
FIG. 1.
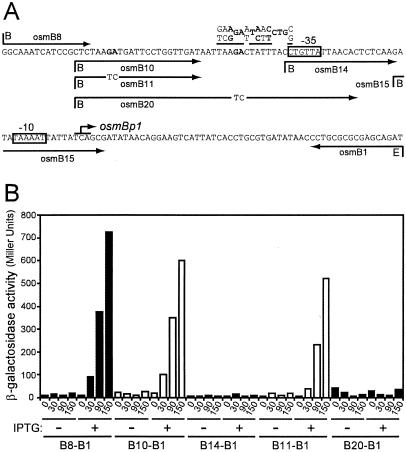
Sequence of the osmB promoter region. Arrows indicate the oligonucleotides used for mRNA 5′-end mapping or to amplify the DNA fragments used to construct transcriptional lac fusions. B and E, BamHI and EcoRI restriction sites. The −35 and −10 elements of osmBp1 and osmBp2 are boxed. Broken arrows indicate the transcription starts. The Shine-Dalgarno sequence (SD) and translation start of osmB are shown in bold letters.
FIG. 2.
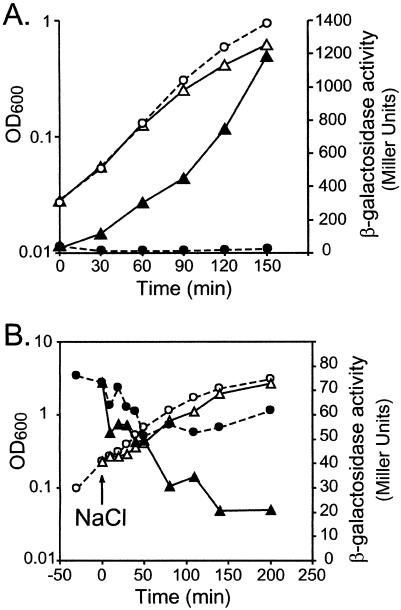
osmB transcription is stimulated by RcsB but not by its cofactor, RcsA. Strain SK1755 (Φ[osmBp1-osmBp2-lac]), transformed with plasmid pHRcsB or pHRcsA, as indicated, was grown in LB broth, and the optical density at 600 nm (OD600) (open symbols) and β-galactosidase activity (filled symbols) were followed during growth. At time zero, half of each culture was kept in LB broth (circles, dashed lines) and the other half (triangles, full lines) was treated with IPTG (0.5 mM) to induce the production of RcsB or RcsA.
Expression of the proximal promoter osmBp2 is controlled by σS but not by RcsB.
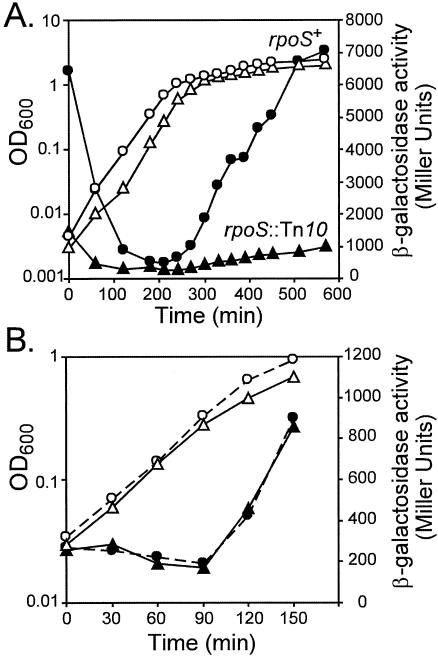
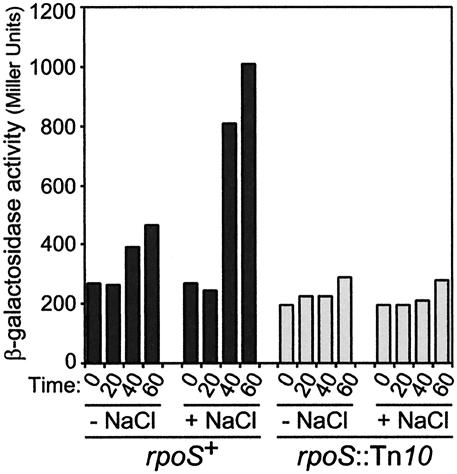
Strain CLG250 contains an osmB-lac transcriptional fusion driven by a 176-bp DNA fragment amplified with the oligonucleotides osmB2 and osmBdown (Fig. 1). This DNA fragment carries the previously characterized osmBp2 promoter (20). The introduction of an rpoS::Tn10 mutation in this strain yielded strain CLG254. As shown in Fig. 3A, the pattern of β-galactosidase-specific activity was typical of promoters poorly expressed during exponential phase and induced upon entry into stationary phase in a σS-dependent manner (Fig. 3A). The overproduction of RcsB in strain CLG250 had no effect on the production of β-galactosidase, modifying neither the level of expression nor the stimulation at the end of exponential phase (Fig. 3B). Therefore, osmBp2 appears to be an RcsB-independent promoter. We also tested the response of this promoter after an osmotic shock (Fig. 4). During exponential growth in a medium of low osmolarity (LB broth without NaCl [LB0N]), the addition of NaCl to a 0.4 M final concentration stimulated transcription from osmBp2 in strain CLG250. In contrast, NaCl had no effect in the rpoS mutant strain CLG254, demonstrating that the osmotic shock induction of osmBp2 is entirely dependent on σS. This behavior is in agreement with previous work that demonstrated that σS accumulates in the cells after an osmotic shock (23), leading to the osmotic induction of a number of its target genes (17, 19). It is also consistent with data obtained in vitro showing that transcription of osmBp2 by EσS is stimulated in the presence of high concentrations of K+ glutamate, a compound known to accumulate in osmotically stressed cells (6).
FIG. 3.
osmBp2 is induced upon entry into stationary phase in a σS-dependent manner but is not stimulated by overexpression of RcsB. (A) Strains CLG250 (Φ[osmBp2-lac]) (circles) and CLG254 (Φ[osmBp2-lac] rpoS::Tn10) (squares) were grown in LB0N, and OD600 (open symbols) and β-galactosidase activity (filled symbols) were monitored during growth. (B) CLG250 transformed with plasmid pHRcsB was grown in LB broth, and the OD600 (open symbols) and β-galactosidase activity (filled symbols) were monitored during growth. At time zero, half of the culture was kept in LB broth (circles, dashed lines) and the other half was treated with IPTG (0.5 mM) to induce the production of RcsB (triangles, full lines).
FIG. 4.
σS is responsible for osmotic shock induction of osmBp2. Strains CLG250 (Φ[osmBp2-lac]) (dark gray bars) and CLG254 (Φ[osmBp2-lac] rpoS::Tn10) (light gray bars) were grown in LB0 until early exponential phase. At time zero, half of the culture was kept in LB0 (− NaCl) and the other half was treated with 0.4 M NaCl (+ NaCl), and β-galactosidase activity was monitored at 0, 20, 40, and 60 min.
Expression of the upstream promoter osmBp1 is controlled by RcsB and independent of σS.
Strain CLG249 contains an osmB-lac transcriptional fusion driven by a 306-bp DNA fragment amplified with the oligonucleotides osmBup and osmB1 (Fig. 1). During growth in LB broth, CLG249 expressed β-galactosidase at a low basal level, and this activity was strongly induced by the overproduction of RcsB from plasmid pHRcsB (Fig. 5A). In contrast, this activity was repressed after osmotic shock and showed no stimulation upon entry into stationary phase (Fig. 5B and data not shown). In addition, introduction of an rpoS::Tn10 mutation in strain CLG249 had no effect on β-galactosidase production. Altogether, these data demonstrate that the 306-bp osmBup/osmB1 DNA fragment carries a σS-independent promoter that is stimulated by RcsB. This promoter must be responsible for the increase in osmB transcription following activation of the RcsCDB phosphorelay (Fig. 2) (7, 16).
FIG. 5.
osmBp1 is stimulated by overexpression of RcsB but neither upon entry into stationary phase nor by osmotic shock. (A) Strain CLG249 (Φ[osmBp1-lac]) transformed with plasmid pHRcsB was grown in LB broth, and the OD600 (open symbols) and β-galactosidase activity (filled symbols) were monitored during growth. At time zero, half of the culture was kept in LB (circles, dashed lines) and the other half was treated with IPTG (0.5 mM) to induce the production of RcsB (triangles, full lines). (B) CLG249 was grown in LB0N, and the OD600 (open symbols) and β-galactosidase activity (filled symbols) were monitored during growth. At time zero, half of the culture was kept in LB0N (circles, dashed lines) and the other half (triangles, full lines) was treated with NaCl (0.4 M).
The physiologically relevant signal sensed by the RcsCDB phosphorelay is complex and, as yet, not totally clear (10, 16). However, it has been shown that RcsB can induce transcription of some of its targets after treatment with the amphipathic molecule chlorpromazine (3). A culture of strain CLG249 was treated with 0.1 mM chlorpromazine during exponential growth in LB0N, and this resulted in 4.5-fold induction of β-galactosidase activity (from 57 to 257 units after 100 min of treatment). This stimulation was totally abolished in the rcsB mutant strain CLG258 (from 54 to 45 units after 100 min of treatment). Therefore, osmBp1 is inducible by treatment with chlorpromazine and RcsB is necessary for this induction.
Identification of osmBp1.
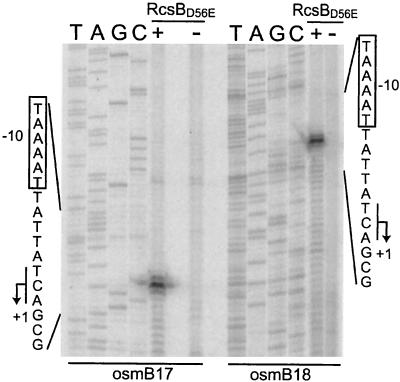
We performed primer extension experiments to investigate the osmBp1 mRNA 5′ end. Strain SK1158 [MG1655 ΔlacIZ(MluI) rcsB::tet] (9) was transformed with the expression vector pIM10 (4) or its derivative, pHRcsBD56E, which can overproduce RcsBD56E, a constitutive form of RcsB (2, 12). The two resulting strains were grown in LB broth until early exponential phase, IPTG (0.5 mM) was added, and total RNAs were extracted after 10 min by the hot-phenol method. We chose to use the RcsBD56E variant because its overproduction resulted in a higher and earlier induction of a number of targets of the RcsCDB system than that of wild-type RcsB (our unpublished data). Ten micrograms of the total RNA was hybridized with 1 pmol of oligonucleotide osmB17 or osmB18, radiolabeled with [γ-32P]dATP (3,000 Ci/mmol; Amersham) and T4 polynucleotide kinase (New England Biolabs), and primer extension experiments were performed as described previously (15), except that 25 units of AMV reverse transcriptase (FINNZYMES) was used for each reaction. The two oligonucleotide probes, osmB17 and osmB18, are complementary of the osmB mRNA specie starting from osmBp1 but not of the mRNA starting from osmBp2 (Fig. 1). As shown in Fig. 6, the experiments with both probes identified a single 5′-end region, centered 148 nucleotides upstream from the translation start of osmB. The amount of this RNA was strongly dependent on the overproduction of RcsBD56E. Just upstream from this 5′ end, a putative promoter that is composed of an almost-consensus −10 element and a more divergent −35 element (Fig. 1) separated by a 17-nucleotide spacer is found. We confirmed this location of osmBp1 by deletion mapping. A DNA fragment amplified with the oligonucleotide pair osmB14/osmB1 (Fig. 7A) showed promoter activity similar to the basal activity exhibited by longer DNA fragments, whereas the DNA fragment obtained with osmB15/osmB1 (Fig. 7A) exhibited no promoter activity at all (Fig. 7B and data not shown). The osmBp1 mRNA 5′ end identified here by primer extension analysis is located approximately 45-bp 5′ of that reported previously (20). This discrepancy may result from an artifact of the RNase protection experiments, and, because the nature of osmBp1 as identified here is confirmed by both biochemical and genetic data, we propose to reassess this promoter.
FIG. 6.
Determination of osmBp1 mRNA 5′ end. Total RNAs were extracted from a strain that was (+) or was not (−) overproducing RcsBD56E, hybridized with 32P-labeled osmB17 or osmB18 oligonucleotides, and treated with AMV reverse transcriptase before analysis on a denaturing polyacrylamide gel and autoradiography. The sequence ladders (lanes T, A, G, and C) were obtained by sequencing the osmBp region by using the same primers and the CircumVent sequencing kit (Biolabs) as described by the manufacturer.
FIG. 7.
Activation of osmBp1 by RcsB necessitates a site upstream of the −35 element. (A) Arrows indicate the oligonucleotides used for the construction of Φ[osmBp1-lac] transcriptional fusions. osmB11 and osmB20 carry the GA-to-TC double mutations indicated. B and E, BamHI and EcoRI restriction sites. The consensus sequence of RcsB binding sites is shown above the osmB sequence, with the most-conserved positions in bold letters. (B) Strains carrying the indicated transcriptional fusions were transformed with plasmid pHRcsB and grown in LB broth. At time zero (OD600 of 0.1), cultures were not treated or were treated with IPTG (0.5 mM) to induce the production of RcsB, and β-galactosidase activity was assayed over time.
Activation by RcsB requires a site upstream from the −35 element of osmBp1.
To identify the sequence required for stimulation by RcsB, we constructed a set of osmBp1-lac fusion strains in which osmBp1 was carried on DNA fragments with different 5′ ends upstream of the promoter (Fig. 7A). These strains were transformed with pHRcsB, and β-galactosidase activities were monitored with or without the induction of rcsB expression. The results of this deletion analysis (Fig. 7B) indicated that the 145-bp osmB8-osmB1 and 130-bp osmB10-osmB1 DNA fragments exhibited similar promoter activities and were sufficient to support activation after the overproduction of RscB. In contrast, the 95-bp osmB14-osmB1 DNA fragment carried a basal promoter activity that was insensitive to RcsB overproduction. A consensus has been proposed for the RcsB box (5), and several putative RcsB boxes could be recognized upstream from the −35 element of osmBp1. We then constructed osmBp1-lac transcriptional fusions expressed under the control of 130-bp DNA fragments similar to osmB10-osmB1 but carrying the GA-to-TC double mutations shown in Fig. 7A. The osmB11-osmB1 fragment remained fully responsive to RcsB overproduction. In contrast, the mutations introduced on fragment osmB20-osmB1 abolished the RcsB-dependent stimulation (Fig. 7B), demonstrating that the putative RcsB box located closest to the osmBp1 −35 element is necessary for this stimulation. Altogether, these observations suggest a direct binding of RcsB at the identified box. However, the most-conserved positions in the RcsB boxes identified so far are GAnnnnnC (2, 9, 28), and we note that the putative RcsB box of osmBp1 is lacking the C in its right half (Fig. 7). A search in the National Center for Biotechnology Information microbial genomes databases (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi) revealed that OsmB is present and identical in all the E. coli, Shigella flexneri and Salmonella strains sequenced so far. Alignment of the promoter regions upstream of the osmB gene in some of these enterobacteria (8; EnteriX [http://globin.cse.psu.edu/enterix/]) shows a strong conservation of the sequence around the −10 element of osmBp1 and downstream. In addition, in spite of a high degree of divergence within the spacer of osmBp1, a region upstream from the −35 element and encompassing the putative RcsB box is also strongly conserved. Therefore, it is likely that osmBp1 is functional and regulated similarly by the same regulators in the different enterobacterial species.
Architecture of multistress-responsive promoters.
It is interesting to compare the organization of the osmB promoter region with that of other multistress-responsive genes. In particular, work on the osmC gene demonstrated that it is also a growth-phase and osmotically regulated gene transcribed under the control of two promoters: a downstream σS-dependent one and an upstream promoter independent of σS but regulated by auxiliary transcription factors (1, 5, 14, 25, 26). Thus, osmB and osmC respond to common stresses and exhibit promoter regions built on similar schemes. However, in spite of this great similarity, these two promoter regions use different ways to achieve the same type of regulatory response. For instance, the osmotic shock induction of osmC results from activation of its σS-independent osmCp1 promoter by the NhaR activator, whereas its σS-dependent osmCp2 promoter is not stimulated by osmotic shock (25, 26). In contrast, the osmotic induction of osmB occurs in a σS-dependent manner at the osmBp2 promoter (Fig. 4), whereas the σS-independent osmBp1 promoter is not activated by osmotic shock. At present, the molecular details explaining why the two σS-dependent promoters osmBp2 and osmCp2 respond differently to osmotic shock still await more investigations.
Finally, a general picture emerges from the comparison of genes such as osmB and osmC. Multistress-responsive promoters of this family are built by combining a patchwork of elements, based either on the general stress response sigma factor σS or on auxiliary regulators, each specific for a particular stress, that activate promoters recognized probably by the housekeeping sigma factor σ70. These different elements cooperate to finely tune the transcription of each multistress-responsive gene, resulting in a precise level of expression in the different stress conditions.
Acknowledgments
This work was supported in part by a grant from the microbiology program of the Génopôle of Toulouse. A.F.-C. was the recipient of a fellowship from the Fondation pour la Recherche Médicale.
REFERENCES
- 1.Bouvier, J., S. Gordia, G. Kampmann, R. Lange, R. Hengge-Aronis, and C. Gutierrez. 1998. Interplay between global regulators of Escherichia coli: effect of RpoS, Lrp and H-NS on transcription of the gene osmC. Mol. Microbiol. 28:971-980. [DOI] [PubMed] [Google Scholar]
- 2.Carballes, F., C. Bertrand, J. P. Bouche, and K. Cam. 1999. Regulation of Escherichia coli cell division genes ftsA and ftsZ by the two-component system rcsC-rcsB. Mol. Microbiol. 34:442-450. [DOI] [PubMed] [Google Scholar]
- 3.Conter, A., R. Sturny, C. Gutierrez, and K. Cam. 2002. The RcsCB His-Asp phosphorelay system is essential to overcome chlorpromazine-induced stress in Escherichia coli. J. Bacteriol. 184:2850-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornet, F., I. Mortier, J. Patte, and J. M. Louarn. 1994. Plasmid pSC101 harbors a recombination site, psi, which is able to resolve plasmid multimers and to substitute for the analogous chromosomal Escherichia coli site dif. J. Bacteriol. 176:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davalos-Garcia, M., A. Conter, I. Toesca, C. Gutierrez, and K. Cam. 2001. Regulation of osmC gene expression by the two-component system rcsB-rcsC in Escherichia coli. J. Bacteriol. 183:5870-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding, Q., S. Kusano, M. Villarejo, and A. Ishihama. 1995. Promoter selectivity control of Escherichia coli RNA polymerase by ionic strength: differential recognition of osmoregulated promoters by EσD and EσS holoenzymes. Mol. Microbiol. 16:649-656. [DOI] [PubMed] [Google Scholar]
- 7.Ferrieres, L., and D. J. Clarke. 2003. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol. Microbiol. 50:1665-1682. [DOI] [PubMed] [Google Scholar]
- 8.Florea, L., C. Riemer, S. Schwartz, Z. Zhang, N. Stojanovic, W. Miller, and M. McClelland. 2000. Web-based visualization tools for bacterial genome alignments. Nucleic Acids Res. 28:3486-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francez-Charlot, A., B. Laugel, A. Van Gemert, N. Dubarry, F. Wiorowski, M.-P. Castanie-Cornet, C. Gutierrez, and K. Cam. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49:823-832. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman, S. 1995. Regulation of capsule synthesis: modification of the two-component paradigm by an accessory unstable regulator, p. 253-262. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 11.Gottesman, S., P. Trisler, and A. Torres-Cabassa. 1985. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J. Bacteriol. 162:1111-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupte, G., C. Woodward, and V. Stout. 1997. Isolation and characterization of rcsB mutations that affect colanic acid capsule synthesis in Escherichia coli K-12. J. Bacteriol. 179:4328-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez, C., J. Barondess, C. Manoil, and J. Beckwith. 1987. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. Analysis of osmotically regulated genes in Escherichia coli. J. Mol. Biol. 195:289-297. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez, C., and J. C. Devedjian. 1991. Osmotic induction of gene osmC expression in Escherichia coli K12. J. Mol. Biol. 220:959-973. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez, C., S. Gordia, and S. Bonnassie. 1995. Characterization of the osmotically inducible gene osmE of Escherichia coli K-12. Mol. Microbiol. 16:553-563. [DOI] [PubMed] [Google Scholar]
- 16.Hagiwara, D., M. Sugiura, T. Oshima, H. Mori, H. Aiba, T. Yamashino, and T. Mizuno. 2003. Genome-wide analyses revealing a signaling network of the RcsC-YojN-RcsB phosphorelay system in Escherichia coli. J. Bacteriol. 185:5735-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengge-Aronis, R. 1996. Back to log phase: σS as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol. Microbiol. 21:887-893. [DOI] [PubMed] [Google Scholar]
- 18.Hengge-Aronis, R., W. Klein, R. Lange, M. Rimmele, and W. Boos. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173:7918-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengge-Aronis, R., R. Lange, N. Henneberg, and D. Fischer. 1993. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J. Bacteriol. 175:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung, J. U., C. Gutierrez, F. Martin, M. Ardourel, and M. Villarejo. 1990. Transcription of osmB, a gene encoding an Escherichia coli lipoprotein, is regulated by dual signals. Osmotic stress and stationary phase. J. Biol. Chem. 265:10574-10581. [PubMed] [Google Scholar]
- 21.Jung, J. U., C. Gutierrez, and M. R. Villarejo. 1989. Sequence of an osmotically inducible lipoprotein gene. J. Bacteriol. 171:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1992. A short course in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Muffler, A., D. D. Traulsen, R. Lange, and R. Hengge-Aronis. 1996. Posttranscriptional osmotic regulation of the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 178:1607-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 25.Sturny, R., K. Cam, C. Gutierrez, and A. Conter. 2003. NhaR and RcsB independently regulate the osmCp1 promoter of Escherichia coli at overlapping regulatory sites. J. Bacteriol. 185:4298-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toesca, I., C. Perard, J. Bouvier, C. Gutierrez, and A. Conter. 2001. The transcriptional activator NhaR is responsible for the osmotic induction of osmCp1, a promoter of the stress-inducible gene osmC in Escherichia coli. Microbiology 147:2795-2803. [DOI] [PubMed] [Google Scholar]
- 27.Torres-Cabassa, A. S., and S. Gottesman. 1987. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J. Bacteriol. 169:981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehland, M., C. Kiecker, D. L. Coplin, O. Kelm, W. Saenger, and F. Bernhard. 1999. Identification of an RcsA/RcsB recognition motif in the promoters of exopolysaccharide biosynthetic operons from Erwinia amylovora and Pantoea stewartii subspecies stewartii. J. Biol. Chem. 274:3300-3307. [DOI] [PubMed] [Google Scholar]