Abstract
Neurodegenerative diseases including Alzheimer’s disease, frontotemporal dementia, and dementia with Lewy bodies are age-related disorders and the main cause of dementia. They are characterized by the cerebral accumulation of Aβ, tau, α-synuclein, and TDP-43. Because the accumulation begins decades before disease onset, treatment should be started in the preclinical stage. Such intervention would be long-lasting, and therefore, prophylactic agents should be safe, non-invasively taken by the patients, and inexpensive. In addition, the agents should be broadly effective against etiologic proteins and capable of repairing neurons damaged by toxic oligomers. These requirements are difficult to meet with single-ingredient pharmaceuticals but may be feasible by taking proper diets composed of multiple ingredients. As a source of such diets, we focused on the Hawaiian native herb Mamaki. From its dried leaves and fruits, we made three preparations: hot water extract of the leaves, non-extracted simple crush powder of the leaves, and simple crush powder of the fruits, and examined their effects on the cognitive function and neuropathologies in four different mouse models of neurodegenerative dementia. Hot water extract of the leaves attenuated neuropathologies, restored synaptophysin levels, suppressed microglial activation, and improved memory when orally administered for 1 month. Simply crushed leaf powder showed a higher efficacy, but simply crushed fruit powder displayed the strongest effects. Moreover, the fruit powder significantly enhanced the levels of brain-derived neurotrophic factor expression and neurogenesis, indicating its ability to repair neurons. These results suggest that crushed Mamaki leaves and fruits are promising sources of dementia-preventive diets.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00950-y.
Keywords: Mamaki, Alzheimer’s disease, Frontotemporal dementia, Dementia with Lewy bodies, Brain-derived neurotrophic factor, Neurogenesis
Introduction
The number of dementia patients is rapidly growing globally with aging societies. The social costs of medical and nursing care for these patients and the economic loss due to their and family caregivers’ inability to work are becoming enormous. These are serious problems, and appropriate countermeasures are urgently needed. The main cause of dementia is neurodegenerative diseases including Alzheimer’s disease (AD), frontotemporal dementia (FTD), and dementia with Lewy bodies (DLB). These diseases are age-related [1, 2] and characterized by the cerebral accumulation of amyloidogenic proteins unique to each disorder: Aβ and tau in AD, tau or TDP-43 in FTD, and α-synuclein in DLB [3, 4]. Notably, TDP-43 also accumulates in amyotrophic lateral sclerosis (ALS), and α-synuclein also accumulates in Parkinson’s disease (PD) (3. 4); both diseases primarily impair motor function, but when the pathology extends to the cerebral cortex, they also affect cognitive function. Meanwhile, mixed pathologies of these proteins have been frequently observed in many neurodegenerative diseases, suggesting synergistic interplays between Aβ, tau, α-synuclein, and TDP-43 [3, 4]. These proteins have been shown to impair synaptic and cognitive function [5, 6] and mediate the intercellular propagation of neuropathology [7, 8] when they aggregate into soluble oligomers and/or insoluble fibrils. Since the accumulation begins decades before disease onset and clinical symptoms emerge after the neurodegeneration progresses [9, 10], treatment to remove the toxic aggregates should be started in the preclinical stage [11, 12]. Moreover, prophylactic agents for dementia taken a long time should be highly safe, non-invasively taken by the patients themselves, and inexpensive. In addition, the agents should have a broad spectrum of action against etiologic proteins and for repairing neurons damaged by toxic oligomers. These requirements are difficult to meet with single-ingredient pharmaceuticals. Furthermore, if all aged people take pharmaceuticals for dementia prevention, the medical economy would soon collapse. A feasible alternative is that aged people keep their brains in good condition with proper diets that are composed of multiple ingredients in their daily lives.
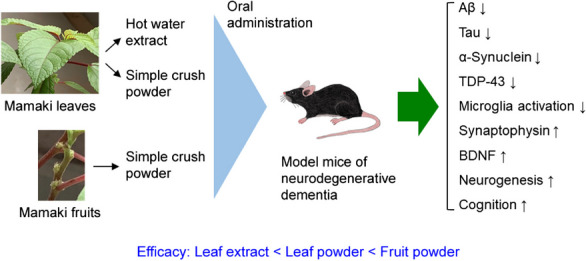
As a source of such diets, we focused on the Hawaiian native herb Mamaki (Pipturus albidus). Its leaves are used to make herbal tea that is popular in Hawaii. In Hawaii, the Mamaki plant, particularly its fruits, has a history as a natural folklore medicine that regulates blood glucose levels, blood pressure, and cholesterol levels; relieves stress and fatigue; and reduces inflammation [13]. As the major components in Mamaki leaves, three polyphenols have been identified: ( +)-catechin, chlorogenic acid, and rutin [14]. These polyphenols have multiple functions, including anti-inflammatory and antioxidant activities, and show beneficial effects against neurodegenerative diseases in model mice and humans [15–26]. These observations suggest that Mamaki should be part of an anti-dementia diet. Thus, in the present study, we made three preparations from dried Mamaki leaves and fruits and examined their effects on the cognitive function and neuropathologies in four different mouse models of neurodegenerative dementia: APP23 mice [27–29] for AD, Tau784 mice [30, 31] for FTD-tau, Huα-Syn(A53T) mice [32, 33] for DLB, and C9-500 mice [34, 35] for FTD-TDP. The hot water extract of Mamaki leaves attenuated neuropathologies, restored synaptophysin levels, suppressed microglial activation, which is an index of brain inflammation [36], and improved memory when orally administered for 1 month. Non-extracted simple crush powder of the leaves showed a higher efficacy, but simple crush powder of the fruits displayed the strongest effects. Moreover, the fruit powder significantly enhanced the levels of brain-derived neurotrophic factor (BDNF) expression and neurogenesis. BDNF and neurogenesis play an important role in repairing neurons and restoring neural function [37, 38]. We also evaluated the contribution of catechin, chlorogenic acid, and rutin on mouse cognition. A three-polyphenol mixture improved mouse memory only partially compared with Mamaki fruit powder, suggesting that Mamaki contains other functional substances besides these polyphenols. These results collectively suggest that crushed Mamaki leaves and fruits are promising components of dementia-preventive diets.
Methods
Preparation of hot water extract and non-extracted simple crush powders of Mamaki leaves and fruits
Dried Mamaki tea leaves were purchased commercially from Nakihalani Farm, LLC, Hawaii. Hot water extract of Mamaki leaves was prepared at TechnoPro L&D (Tokyo, Japan). Briefly, 250 g of Mamaki tea leaves was added to 3 L of water and soaked for 1 h. After boiling for 15 min with stirring, the mixture was allowed to stand for 24 h. After removing the tea leaves, suction filtration was performed using a filter paper with a pore size of 8 μm. The filtrate was concentrated to 500 mL using an evaporator while being heated at 40 °C. The concentrate was lyophilized to obtain 66 g of extract powder. Non-extracted simple crush powders of Mamaki leaves and fruits were prepared in our laboratory. The package of commercial Mamaki tea leaves contained a small amount of fruits and/or seeds. Tea leaves and fruits/seeds (simply referred to as fruits hereafter) were separated by hand, and each was powdered into 20–50 μm particles using a grinder (Fine Powder Mill FM-100; Labonect, Sakai, Japan). These particles were collected and used as non-extracted simple crush powders.
Component analysis of Mamaki preparations
Mamaki leaves are known to contain catechin, chlorogenic acid, and rutin as the major components [14]. Quantification of these components in our Mamaki preparations was outsourced to Japan Food Research Laboratories (Tokyo, Japan). Considering the properties of each component, highly versatile methanol was used for extraction. Briefly, for catechin, 0.4 g of materials was suspended in a 30-mL mixture of methanol and oxalic acid (8:2) and shaken for 10 min. After centrifugation, the supernatant was harvested, and the pellet was subjected to methanol extraction two more times. The supernatants of three extractions were combined for a total volume of 100 mL. The extracts were diluted and separated by high-performance liquid chromatography (HPLC) using a reversed-phase InertSustain C18 column (GL Sciences, Tokyo, Japan) with 0.1% acetic acid and acetonitrile mixture (89:11) as the mobile phase. Each fraction was sequentially analyzed by electrospray ionization (ESI)-mass spectrometry (MS) using a Xevo TQ MS (Waters Corporation, Milford, MA). For chlorogenic acid, 0.2 g of materials was suspended in 80 mL of methanol and 0.02 M perchloric acid mixture (1:9) and shaken for 10 min. After centrifugation, the supernatant was harvested, and the pellet was subjected to methanol extraction two more times. The supernatants of three extractions were combined for a total volume of 250 mL. The extracts were separated by HPLC using a reverse-phase CAPCELL PAK C18 ACR column (Osaka Soda, Osaka, Japan) with water, acetonitrile, and phosphoric acid mixture (920:80:2) as the mobile phase. The absorbance at 325 nm of each fraction was measured. For rutin, 0.4 g of materials was suspended in a 60-mL mixture of methanol and 2.5% acetic acid (8:2) and shaken for 10 min. After centrifugation, the supernatant was harvested, and the pellet was subjected to methanol extraction two more times. The supernatants of three extractions were combined for a total volume of 200 mL. The extracts were diluted and separated by HPLC using a reverse-phase Unison UK-C18 column (Imtakt USA, Portland, OR, USA) with water, acetonitrile, and 2-propanol mixture (200:38:2) containing 0.4% citric acid as the mobile phase. The absorbance at 360 nm of each fraction was measured. The representative HPLC and MS data are shown in the supplementary materials.
Mice
Four different mouse models of neurodegenerative dementia were used. APP23 mice are a model of AD and express human APP with the Swedish (KM670/671NL) mutation [27] and show memory impairment at 3 months [28]. We observed Aβ oligomer accumulation, synapse loss, and amyloid deposition at 15 months in these mice [29]. Tau784 mice are a model of FTD-tau that express both 3-repeat and 4-repeat human tau with the dominant expression of 4-repeat human tau at adult age by the presence of a tau intron mutation [30]. Due to the imbalanced expression of tau isoforms, the mice display tau hyperphosphorylation, tau oligomer formation, synapse loss, and memory impairment at 6 months, microglial activation at 12 months, and neurofibrillary tangle formation and neuronal loss at 15 months [31]. Huα-Syn(A53T) line G2-3 mice were originally generated as a model of PD that express human α-synuclein with A53T mutation [32]. We previously showed that the mice exhibit apparent α-synuclein oligomer accumulation, synapse loss, and cognitive impairment at 6 months, microglial activation at 7 months, and motor dysfunction at 9 months, indicating that the mice can be regarded as a model of DLB until 9 months [33]. C9-500 mice were generated as a model of C9orf72-linked FTD/ALS by introducing human full-length C9orf72 gene harboring ~ 500 repeats of the GGGGCC sequence in intron 1a [34]. Our previous study revealed that due to the presence of the hexanucleotide repeat expansion (HRE), the mice start to accumulate RNA G-quadruplexes that form RNA foci, dipeptide repeat proteins (DPRs) produced by the repeat-associated non-ATG (RAN) translation, and phosphorylated TDP-43 at 3 months and show synapse loss, neuronal loss, and microglial activation at 6 months [35]. Their cognitive function was impaired at 4.5 months, but their motor function remained normal even at 12 months, indicating that the mice can be regarded as a model of FTD-TDP until 12 months. All transgenic (Tg) mice were maintained and used as heterozygotes. All animal experiments were approved by the ethics committee of Osaka Metropolitan University (Osaka, Japan) and performed in accordance with the Guide for Animal Experimentation, Osaka Metropolitan University.
Treatment of mice
To study the effects of the hot water extract of Mamaki leaves, the extract powder was suspended in water at 3.33, 0.33, or 0.10 mg/mL by sonication. 300 L of each suspension (i.e., 1,000, 100, and 30 g powder, respectively) was orally administered using feeding needles to male and female Tau784 mice 5 days a week (Monday through Friday) for 1 month. The same volume of water was administered to age-matched Tg and non-Tg littermates as controls. For APP23, Huα-Syn(A53T), and C9-500 mice, the suspension of 0.33 mg/mL (i.e., 100 g powder/300 L) was administered to male and female mice for 1 month. To compare the effects of three Mamaki preparations: hot water extract of the leaves, simple crush powder of the leaves, and simple crush powder of the fruits, each powder was suspended in water at 0.10 mg/mL by sonication. 300 L of each suspension (i.e., 30 g powder) was administered to Tau784 mice for 1 month. To test the major components of the Mamaki leaves, catechin, chlorogenic acid, and rutin (all from Fujifilm-Wako, Osaka, Japan) were combined in water at concentrations of 0.29, 0.12, and 0.41 g/mL, respectively. 300 L of the solution (i.e., 0.087 μg catechin, 0.036 μg chlorogenic acid, and 0.123 μg rutin) was administered to Tau784 mice for 1 month. These dosages correspond to the amounts contained in 30 g simple crush powder of the fruits. Catechin alone (0.087 μg/300 L) was also administered to Tau784 mice.
Behavioral test
The spatial reference memory of the mice was assessed using the Morris water maze, as described previously [29]. Mamaki treatment was continued during the behavioral test.
Immunohistochemical analysis of neuropathologies
After the behavioral tests, the mice in each group were divided into two groups, one for histological analysis and the other for future biochemical analysis. Brain sections were prepared and stained as described previously [29, 35]. Phosphorylated tau and tau oligomers were stained with AT8 antibody to pSer202/Thr205-tau (Thermo Scientific, Waltham, MA) and T22 antibody (Sigma-Aldrich, St Louis, MO), respectively. Aβ oligomers and amyloid deposits were stained with 11A1 antibody (IBL, Fujioka, Japan) and β001 antibody [29], respectively. Phosphorylated α-synuclein and α-synuclein oligomers were stained with EP1536Y antibody to pSer129-α-synuclein (Abcam, Cambridge, UK) and Syn33 antibody (Sigma-Aldrich), respectively. C9orf72 HRE-related neuropathologies were stained with antibodies to DNA/RNA G-quadruplex (BG4; Absolute antibody, Cleveland, UK), DPRs of poly-GA and poly-GP (both from Cosmo Bio, Tokyo, Japan), phosphorylated TDP-43 (anti-pSer409/410-TDP-43; Cosmo Bio), and phosphorylated double-strand RNA-dependent protein kinase (PKR), which regulates RAN translation (anti-pThr446-PKR; MilliporeSigma, Burlington, MA). Synapse levels were measured by staining with SVP-38 antibody to synaptophysin (Sigma-Aldrich). Microglial activation was evaluated by staining with an antibody to Iba-1 (Fujifilm-Wako). The staining intensity or positive area in a constant brain region was quantified using NIH ImageJ software. Iba-1-positive cells were also counted in a constant brain region.
Histological analysis of BDNF expression and neurogenesis
BDNF expression was evaluated in Tau784 mice that received three different Mamaki preparations at 30 g/day for 1 month. Brain sections were stained with an anti-BDNF antibody (GTX132621; GeneTex, Irvine, CA). The staining intensity in a constant brain region was quantified using NIH ImageJ software. To assess neurogenesis, the suspension of simple crush powder of Mamaki fruits was administered to male and female aged Huα-Syn(A53T) mice at 30 g/day for 1 month. 5-Bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich), a thymidine analog selectively incorporated into the DNA of proliferating cells, was dissolved in Tris-buffered saline, pH 7.6 at 5 mg/mL. 300 L of BrdU solution (i.e., 1.5 mg) was intraperitoneally injected into the mice for the last 5 days of the fruit powder treatment. Brain sections were double stained with mouse monoclonal anti-BrdU (IBL) and rabbit polyclonal anti-doublecortin antibodies (Abcam). Cells positive for both BrdU and doublecortin were regarded as newly generated neurons and counted in a constant brain region.
Statistical analysis
Comparisons of means among more than two groups were performed using ANOVA or two-factor repeated measures ANOVA (for the behavioral tests), followed by Fisher’s PLSD test. Differences with a p value of < 0.05 were considered significant.
Results
Effects of hot water extracts of Mamaki leaves in four different mouse models
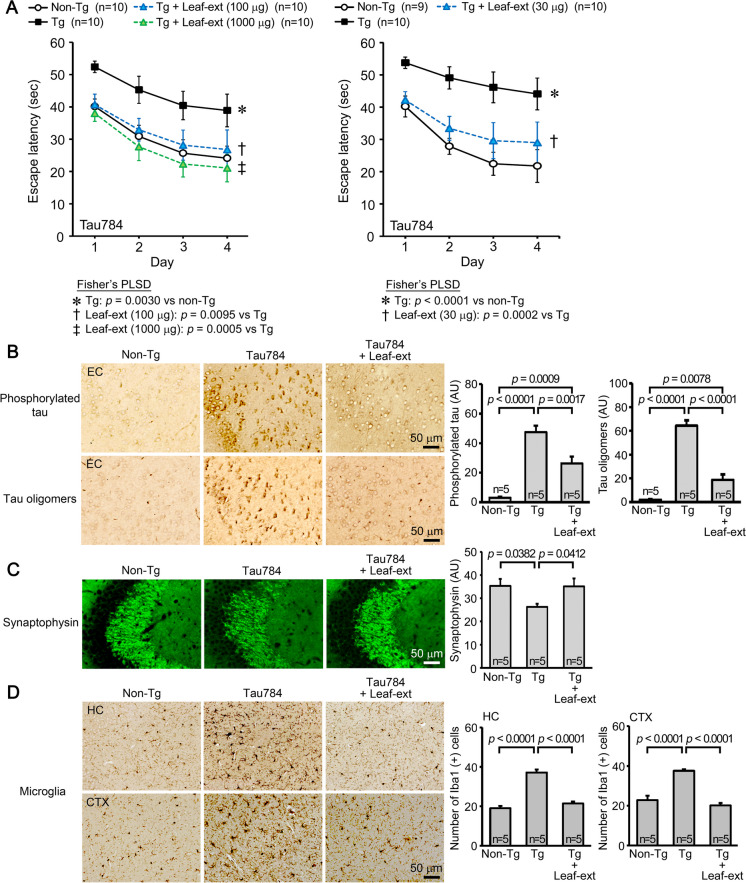
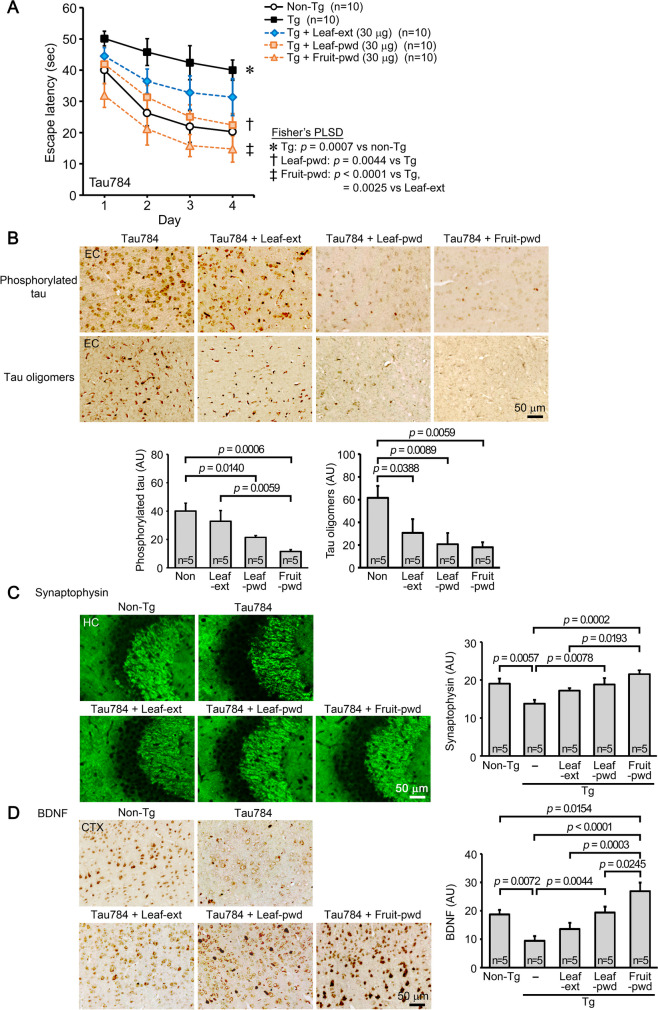
Initially, we studied the effects of hot water extract of Mamaki leaves in four different mouse models of neurodegenerative dementia. The first model, Tau784 mice, is a model of FTD-tau. The leaf extract was orally administered to 12–14-month-old mice at 1000, 100, or 30 μg/day for 1 month. Control Tg mice and age-matched non-Tg littermates received the same volume of water. In the Morris water maze test, the leaf extract improved mouse memory in a dose-dependent fashion; all doses significantly improved mouse memory but only 100 or 1000 μg/day showed an improvement to a level similar to that of non-Tg littermates (Fig. 1A). Immunostaining analysis was performed in mice treated with 100 μg leaf extract, because mouse memory was recovered almost completely at this dose. The levels of phosphorylated tau and tau oligomers in the entorhinal cortex were significantly reduced by the treatment (Fig. 1B). The levels of synaptophysin in the hippocampal CA2/3 regions were significantly recovered (Fig. 1C), and the levels of activated microglia in the hippocampus and cerebral cortex were significantly attenuated (Fig. 1D). Hereafter, the dose of Mamaki leaf extract was set at 100 μg/day to facilitate comparison of effects between model mice.
Fig. 1.
Effects of hot water extracts of Mamaki leaves in FTD-tau mice. Hot water extract of Mamaki leaves (Leaf-ext) was orally administered to 12–14-month-old Tau784 mice (mean body weight, 31.8 g) at 1000, 100, or 30 μg/day for 1 month. A In the water maze test, Leaf-ext improved mouse memory in a dose-dependent fashion; all doses significantly improved mouse memory but only 100 or 1000 μg/day showed an improvement to a level similar to that of non-Tg littermates. Thus, subsequent immunostaining analysis was performed in mice treated with 100 μg leaf extract. B Leaf-ext significantly reduced the levels of phosphorylated tau and tau oligomers in the entorhinal cortex (EC). AU, arbitrary unit. C The levels of synaptophysin in the hippocampal CA2/3 regions were significantly recovered. D The levels of activated microglia in the hippocampus (HC) and cerebral cortex (CTX) were significantly attenuated
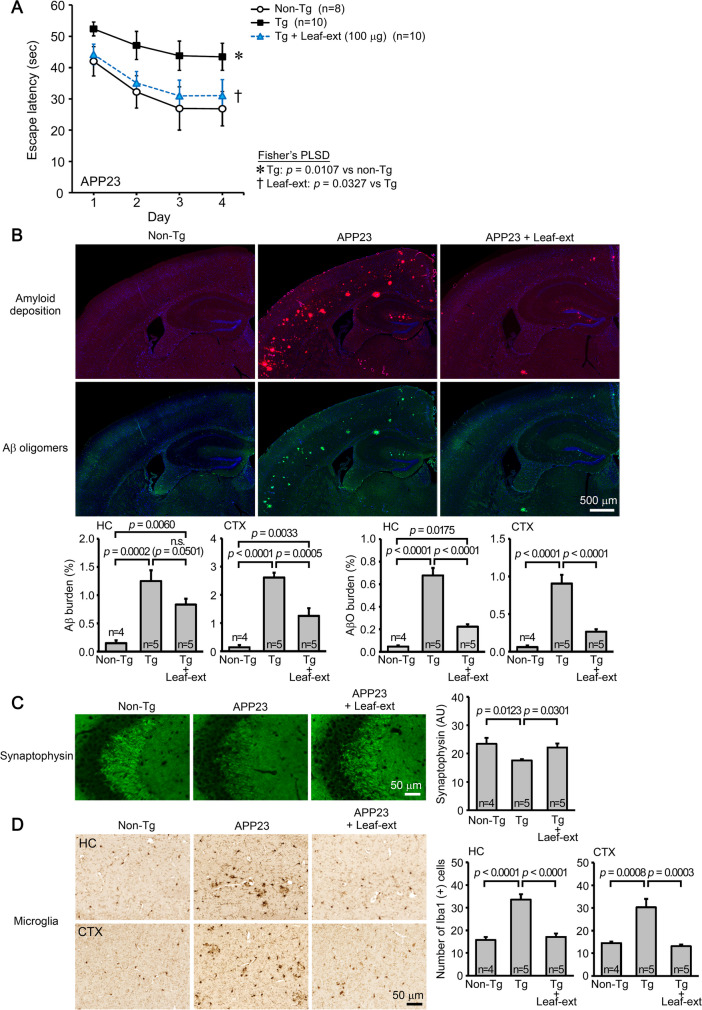
The second model, APP23, is a model of AD. The leaf extract was orally administered to 14–17-month-old mice at 100 μg/day for 1 month. Mouse memory was significantly improved to a level similar to or slightly less than that of non-Tg littermates (Fig. 2A). The levels of amyloid deposition and Aβ oligomers in the hippocampus and cerebral cortex were significantly reduced (Fig. 2B). Again, the level of synaptophysin in the hippocampal CA2/3 regions was significantly restored (Fig. 2C), and the levels of activated microglia in the hippocampus and cerebral cortex were significantly suppressed (Fig. 2D).
Fig. 2.
Effects of hot water extracts of Mamaki leaves in AD mice. Leaf extract (Leaf-ext) was orally administered to 14–17-month-old APP23 mice (mean body weight, 30.1 g) at 100 μg/day for 1 month. A Mouse memory was significantly improved to a level similar to or slightly less than that of non-Tg littermates. B The levels of amyloid deposition and Aβ oligomers in the hippocampus (HC) and cerebral cortex (CTX) were significantly reduced. C The level of synaptophysin in the hippocampal CA2/3 regions was significantly restored. D The levels of activated microglia in the hippocampus and cerebral cortex were significantly suppressed
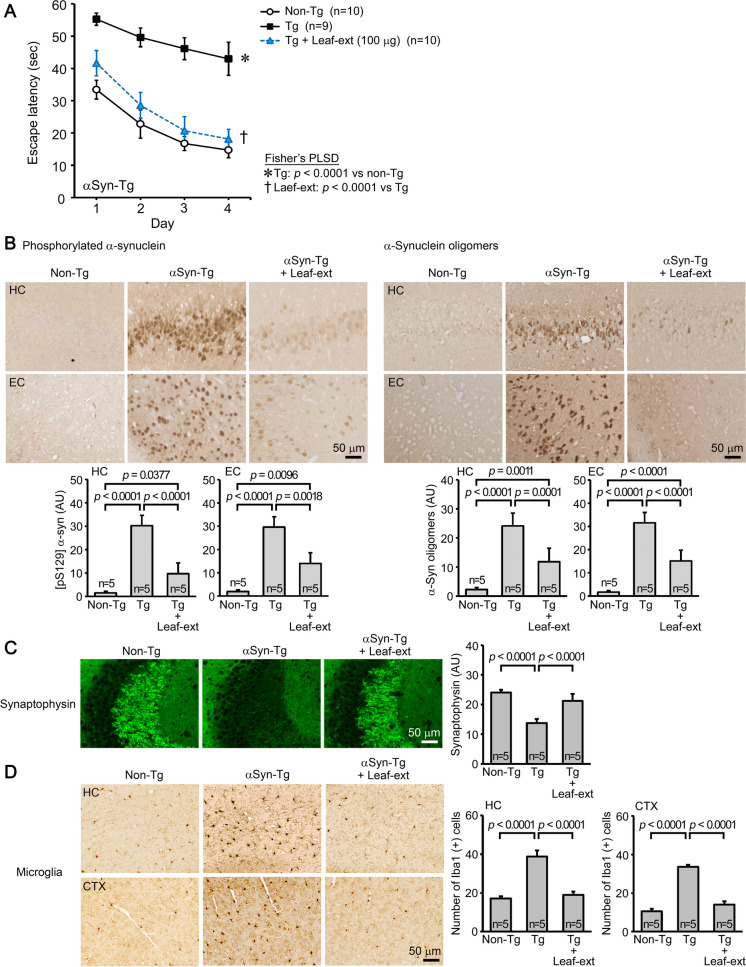
Huα-Syn(A53T) mice were used as a model of DLB. The leaf extract was orally administered to 7–8-month-old mice at 100 μg/day for 1 month, and the treatment significantly improved mouse memory to a level similar to or slightly less than that of non-Tg littermates (Fig. 3A). The levels of phosphorylated α-synuclein and α-synuclein oligomers in the hippocampus and entorhinal cortex were significantly reduced (Fig. 3B). The level of synaptophysin in the hippocampal CA2/3 regions was significantly recovered (Fig. 3C), and, as above, the levels of activated microglia in the hippocampus and cerebral cortex were significantly attenuated (Fig. 3D).
Fig. 3.
Effects of hot water extracts of Mamaki leaves in DLB mice. Leaf extract (Leaf-ext) was orally administered to 7–8-month-old Huα-Syn(A53T) mice (mean body weight, 28.4 g) at 100 μg/day for 1 month. A Mouse memory was significantly improved to a level similar to or slightly less than that of non-Tg littermates. B The levels of phosphorylated α-synuclein and α-synuclein oligomers in the hippocampus (HC) and entorhinal cortex (EC) were significantly reduced. C The level of synaptophysin in the hippocampal CA2/3 regions was significantly recovered. D The levels of activated microglia in the HC and cerebral cortex (CTX) were significantly attenuated
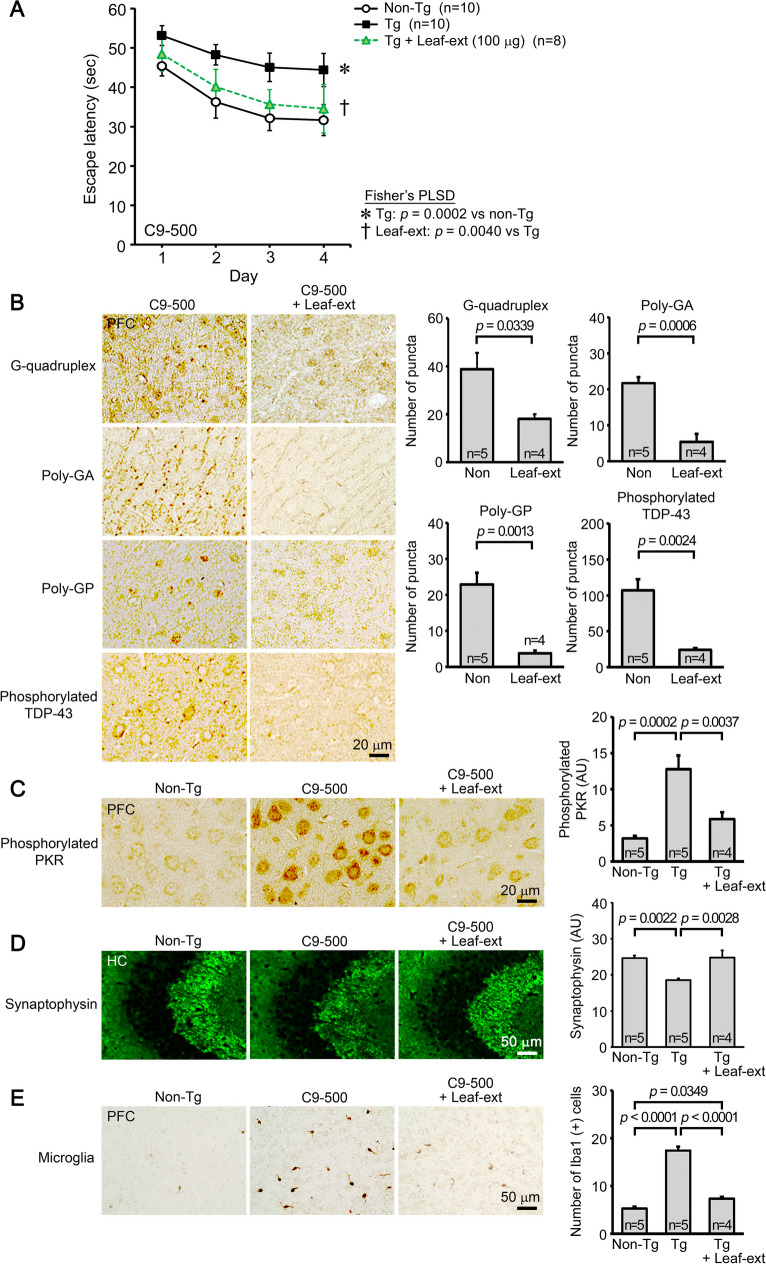
Lastly, C9-500 mice were tested as a model of FTD-TDP. The leaf extract was orally administered to 9–10-month-old mice at 100 μg/day for 1 month. Mouse memory was significantly improved to a level similar to or slightly less than that of non-Tg littermates (Fig. 4A). The treatment significantly alleviated C9orf72 HRE-associated neuropathologies, including RNA G-quadruplexes, DPRs of poly-GA and poly-GP, and phosphorylated TDP-43, in the prefrontal cortex (Fig. 4B). Furthermore, the level of phosphorylated PKR, a regulator of RAN translation, in the prefrontal cortex was significantly reduced (Fig. 4C). The level of synaptophysin in the hippocampal CA2/3 regions was significantly restored (Fig. 4D), and the level of activated microglia in the prefrontal cortex was significantly reduced (Fig. 4E).
Fig. 4.
Effects of hot water extracts of Mamaki leaves in FTD-TDP mice. Leaf extract (Leaf-ext) was orally administered to 9–10-month-old C9-500 mice (mean body weight, 29.2 g) at 100 μg/day for 1 month. A Mouse memory was significantly improved to a level similar to or slightly less than that of non-Tg littermates. B The levels of RNA G-quadruplex, poly-GA, poly-GP, and phosphorylated TDP-43 in the prefrontal cortex (PFC) were significantly reduced. C The level of phosphorylated PKR in the prefrontal cortex was also significantly reduced. D The level of synaptophysin in the hippocampal CA2/3 regions was significantly restored. E The levels of activated microglia in the in the prefrontal cortex were significantly reduced
These results suggest that hot water extract of Mamaki leaves is effective at reducing toxic oligomers and preventing neuroinflammation in various neurodegenerative diseases, thereby improving the cognitive function of these model mice.
Comparison of hot water extract and non-extracted simple crush powders of Mamaki leaves and fruits in Tau784 mice
We prepared non-extracted simple crush powders of dried Mamaki leaves and fruits and compared their effects with those of hot water extract of the leaves. To facilitate comparison of efficacy between preparations, their doses were set at 30 μg/day, below the dose showing a complete recovery of mouse cognition. The three preparations were orally administered to 7–9-month-old Tau784 mice for 1 month. The hot water extract of the leaves affected mouse memory, but its effect was not significant (Fig. 5A). This result is very similar to that in Fig. 1A except for statistical significance, demonstrating the reproducibility of the experiment. In contrast, simple crush powders of the leaves significantly improved mouse memory to a level similar to that of non-Tg littermates. Moreover, simple crush powders of the fruits markedly enhanced mouse memory to a level even higher than that of non-Tg littermates. The levels of phosphorylated tau in the entorhinal cortex were significantly decreased by simple crush powders of the leaves and fruits, but those of the fruits showed a higher effect (Fig. 5B). The hot water extract of the leaves showed only slight effects. The levels of tau oligomers in the same region were significantly reduced by all preparations, with the fruit powder showing the highest effect and the leaf extract showing the lowest (Fig. 5B). The levels of synaptophysin in the hippocampal CA2/3 regions were significantly recovered by simple crush powders of the leaves and fruits, with those of the fruits showing a higher effect (Fig. 5C). The hot water extract of the leaves restored synaptophysin levels incompletely. The result that the simple crush powder of Mamaki fruits enhanced the cognitive function of Tg mice over the age-matched wild-type littermates suggests that this preparation contains certain factors that repair damaged neurons and restore neural function that declines with age. Thus, we examined the level of BDNF expression in this model. The simple crush powder of the fruits significantly increased the level of BDNF in the cerebral cortex to a level even higher than that in non-Tg littermates (Fig. 5D). The simple crush powder of the leaves increased the level to that of non-Tg littermates, whereas hot water extract of the leaves showed only slight effects.
Fig. 5.
Comparison of hot water extract and non-extracted simple crush powders of Mamaki leaves and fruits in Tau784 mice. Hot water extract of Mamaki leaves (Leaf-ext), simple crush powder of the leaves (Leaf-pwd), and simple crush powder of the fruits (Fruit-pwd) were orally administered to 7–9-month-old Tau784 mice (mean body weight, 28.9 g) at 30 μg/day for 1 month. A Leaf-ext improved mouse memory incompletely, while Leaf-pwd significantly improved it to a level similar to that of non-Tg littermates. Fruit-pwd markedly enhanced mouse memory to a level even higher than that of non-Tg littermates. B The levels of phosphorylated tau in the entorhinal cortex (EC) were significantly decreased by Leaf-pwd and Fruit-pwd, with Fruit-pwd showing the strongest effect. Leaf-ext showed only slight effects. The levels of tau oligomers were significantly reduced by all preparations, with Fruit-pwd showing the highest effect and Leaf-ext the lowest. C The levels of synaptophysin in the hippocampal CA2/3 regions were significantly recovered by Leaf-pwd and Fruit-pwd, with Fruit-pwd showing the strongest effect. Leaf-ext restored synaptophysin levels only incompletely. D The levels of BDNF in the cerebral cortex (CTX) were significantly increased by Fruit-pwd to a level even higher than that in non-Tg littermates and also by Leaf-pwd but to a level similar to that in non-Tg littermates. Only slight effects were observed with Leaf-ext
These results suggest that certain factors that remove toxic proteins, reconstitute neural network, and enhance cognition are contained more abundantly in the simple crush powder of the leaves than the hot water extract and are most concentrated in the fruits.
Effects of simple crush powder of Mamaki fruits on neurogenesis in Huα-Syn(A53T) mice
We further explored the activity of Mamaki fruits against brain aging and disorder. We speculated that Mamaki fruits may promote brain rejuvenation and thereby reinforce the memory of dementia-suffering mice over their healthy controls. Thus, we examined the effect of the fruits on neurogenesis. The simple crush powder of the fruits was administered to 10–11-month-old Huα-Syn(A53T) mice at 30 μg/day for 1 month, and the levels of neurogenesis were assessed in the dentate gyrus and substantia nigra. The latter brain region is particularly vulnerable to α-synuclein-induced neurodegeneration, leading to motor dysfunction in PD. Compared to non-Tg littermates, neurogenesis in Tg mice tended to decrease in both brain regions (Fig. 6). The crushed fruit powder significantly increased the number of newly generated neurons to levels higher than those in non-Tg littermates in both regions.
Fig. 6.
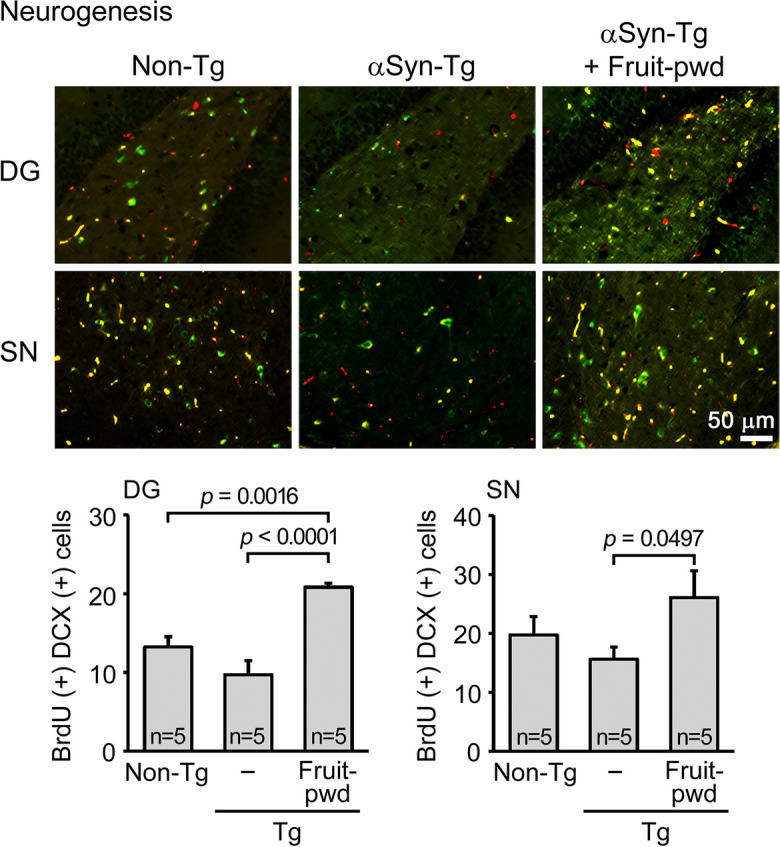
Effects of simple crush powder of Mamaki fruits on neurogenesis in Huα-Syn(A53T) mice. Crushed fruit powder (Fruit-pwd) was orally administered to 10–11-month-old Huα-Syn(A53T) mice (mean body weight, 28.9 g) at 30 μg/day for 1 month. Neurogenesis was evaluated by immunofluorescence for BrdU (red) and doublecortin (DCX, green), where double-positive cells (yellow) were regarded as newly generated neurons. The number of newly generated neurons in the dentate gyrus (DG) and substantia nigra (SN) was significantly increased by the treatment
Analysis of the three components of Mamaki preparations and their effects in Tau784 mice
The differences in efficacy among the three Mamaki preparations may be attributable to the differences in their polyphenol contents. Thus, we analyzed the amounts of three major polyphenols: catechin, chlorogenic acid, and rutin, in those preparations. As shown in Table 1, only catechin content matched the therapeutic efficacy of the preparations. We also tried to measure epigallocatechin-3-gallate, which is a strong antioxidant and neuroprotective catechin derivative and abundantly contained in green tea leaves [15, 16], in the hot water extract of Mamaki leaves; however, its level was under the detection limit (< 0.5 mg/100 g).
Table 1.
The content of three polyphenols in 100 g of each Mamaki preparation
| Polyphenols | Leaf-ext | Leaf-pwd | Fruit-pwd |
|---|---|---|---|
| Catechin | 0.031 g | 0.15 g | 0.29 g |
| Chlorogenic acid | 0.44 g | 0.72 g | 0.12 g |
| Rutin | 0.83 g | 1.3 g | 0.41 g |
Leaf-ext, hot water extract of the leaves; Leaf-pwd, simple crush powder of the leaves; Fruit-pwd, simple crush powder of the fruits
To evaluate the contribution of catechin, chlorogenic acid, and rutin on mouse cognition, we tested catechin alone and the mixture of these three polyphenols at doses corresponding to those contained in 30 μg simple crush powder of Mamaki fruits: 0.087 μg catechin, 0.036 μg chlorogenic acid, and 0.123 μg rutin. Catechin alone or the mixture was orally administered to 8–10-month-old Tau784 mice for 1 month. The three-polyphenol mixture improved mouse memory, but its effect was incomplete (Fig. 7) and weaker than that of 30 μg Mamaki fruit powder (Fig. 5A). Catechin alone had an even smaller effect on mouse memory. These results suggest that the three polyphenols represent the nootropic ingredients of Mamaki preparations only partially and that Mamaki has other functional substances that are particularly abundant in the fruits.
Fig. 7.
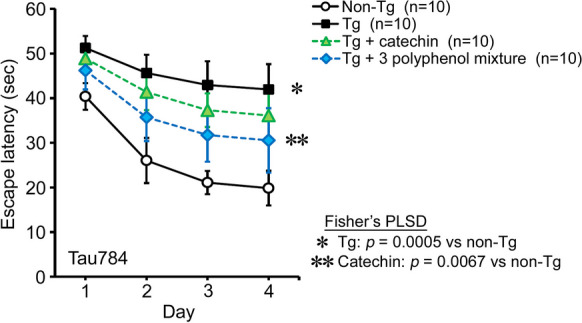
Effects of three polyphenols in Tau784 mice. A mixture of catechin, chlorogenic acid, and rutin or catechin alone was orally administered to 8–10-month-old Tau784 mice (mean body weight, 32.0 g) for 1 month. The doses corresponded to those contained in 30 μg simple crush powder of Mamaki fruits: 0.087 μg catechin, 0.036 μg chlorogenic acid, and 0.123 μg rutin. The three-polyphenol mixture improved mouse memory incompletely, and catechin alone had an even weaker effect
Discussion
Prevention is an important issue for eradicating dementia. We propose five requirements for dementia-prophylactic agents: they should be (1) highly safe, (2) non-invasively taken by patients themselves, (3) inexpensive, (4) broadly effective against etiologic proteins, and (5) capable of repairing neurons damaged by toxic oligomers. From the viewpoint of medical economics and the limited actions of single-ingredient pharmaceuticals, diets composed of multiple ingredients are a feasible way to meet these requirements. As a source of such diets, we selected the Hawaiian native herb Mamaki. In Hawaii, Mamaki tea has long been consumed safely and inexpensively, and the fruits have been taken as home remedies [13]. In the present study, hot water extract of Mamaki leaves reduced the levels of Aβ, tau, α-synuclein, and TDP-43 pathologies, attenuated brain inflammation, and improved memory in four different mouse models of neurodegenerative dementia. Moreover, the simple crush powder of Mamaki fruits enhanced BDNF expression and neurogenesis, indicating its ability to repair neurons. Thus, Mamaki meets the requirements for dementia-preventive diets.
Mamaki leaves are usually brewed to make herbal tea, but hot water extraction may break down heat-sensitive ingredients and lose volatile substances by evaporation. In addition, some functional ingredients may remain in the residues of leaves after decoction. In Japan, in addition to brewed green tea, beverages of powdered green tea are also popular. Powdered green tea is made by grinding dried tea leaves that have been steamed immediately after being picked. Compared to brewed tea, powdered tea contains all ingredients in the leaves such as catechins and dietary fibers, which presumably makes the latter more functional and better for our health. As for Mamaki, the non-extracted simple crush powder of the leaves showed a higher efficacy than the hot water extract. Mamaki plant, particularly its fruits, is used in home remedies by native Hawaiians [13]. This use suggests that certain functional ingredients are concentrated in the fruits more than in the leaves. Our results clearly indicate that the simple crush powder of Mamaki fruits has stronger effects than the leaves to alleviate brain disorders. Furthermore, the fruits improved the cognitive function of disease-suffering mice compared with healthy controls, suggesting that the fruits promote brain rejuvenation, at least in part, by enhancing BDNF expression and neurogenesis.
While our findings indicate the advantage of multi-ingredient diets in dementia prevention, the identification of the active ingredients would facilitate the development of anti-dementia pharmaceuticals. As the major components in Mamaki leaves, three polyphenols have been identified: catechin, chlorogenic acid, and rutin [14]. These polyphenols have been shown to possess beneficial effects against neurodegenerative diseases in model mice and humans. Catechins, which include catechin and its derivatives and comprise a class of flavonoids, have the greatest abundance in tea leaves. They show anti-inflammatory and antioxidant effects by blocking cytokine production and inflammatory pathways as well as chelating metal ions and scavenging free radicals [15, 16]. Catechins, in particular epigallocatechin-3-gallate, have been shown to inhibit Aβ production, tau phosphorylation, and amyloidogenic protein aggregation and thereby prevent cognitive decline in AD and PD [15, 16]. Furthermore, they increase BDNF expression and neurogenesis when orally administered to mice [17, 18]. Chlorogenic acid, also called 5-O-caffeoylquinic acid, is abundantly contained in coffee beans. It exerts multiple health benefits through anti-inflammatory, antioxidant, neuroprotective, hepatoprotective, cardioprotective, chemo-preventive, antidiabetic, and anti-obesity activities, and its regular intake is suggested to reduce the risk of neurodegenerative diseases and improve cognition [19]. In model mice of AD and PD, the oral administration of chlorogenic acid improved mouse memory and motor function [20, 21]. It also promotes the expression of BDNF and nerve growth factor in a rat model of cerebral ischemia/reperfusion [22]. Rutin, a type of flavonol and subtype of flavonoids, is contained in various plants. It too has anti-inflammatory, antioxidant, and neuroprotective properties [23]. Its oral administration reduces Aβ and tau oligomers and improves cognition in model mice of AD [24, 25]. Moreover, rutin increases BDNF expression in Aβ-injected rats when administered intraperitoneally [26]. In these studies, polyphenols were administered to animals at 25–100 mg/kg per day to obtain significant effects. In contrast, in the present study, Mamaki fruit powder was sufficiently effective at 30 μg/head (i.e., approximately 1 mg/kg) per day, unlike a mixture of the three polyphenols. These results suggest that Mamaki contains other nootropic substances besides catechins, chlorogenic acid, and rutin and that these unidentified substances are abundant in Mamaki fruits. Recent evidence suggests that the gut microbiome affects brain function [39–41]. Plant-derived soluble and insoluble dietary fibers are known to make the intestinal environment favorable to gut microbes [42, 43], and dried Mamaki leaves contain higher amounts of fibers compared to other commercial teas (44). Thus, it is likely that simple crush powders of Mamaki leaves and fruits, which may be rich in dietary fibers, have stronger nootropic activities than hot water extract by exerting a greater influence on gut microbiota.
Mamaki has a long dietary history in Hawaii, and the crushed leaf powder of green tea has been safely taken in Japan. Nevertheless, the safety of the long-term intake of crushed powders of Mamaki leaves and fruits as dementia-preventing diets should be confirmed. Clinical trials are also needed to evaluate the true efficacy in humans. Our results show that Mamaki has a broad spectrum against neurodegenerative dementia and reduces α-synuclein and TDP-43 pathologies as well as Aβ and tau pathologies. However, it remains to be studied whether Mamaki is also effective at preventing PD and ALS, which primarily impair motor function. Despite such challenges, non-extracted simple crush powders of Mamaki leaves and fruits are promising sources of dementia-preventive diets.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ayumi Yokota, Yu Masumoto, and Yuki Kinjo for technical assistance, and Peter Karagiannis for reading the manuscript.
Author contribution
T.U. performed the experiments, analyzed the data, and prepared the figures. K.S. and R.U. carried out the experiments. K.M. prepared the materials. T.T. designed the study, performed the experiments, and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by funding from Cerebro Pharma Inc., Osaka, Japan.
Data availability
Datasets used for analysis and materials are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
All animal experiments were approved by the ethics committee of Osaka Metropolitan University and performed in accordance with the Guide for Animal Experimentation, Osaka Metropolitan University.
Competing interests
T.T. and K.M. are the founders of Cerebro Pharma Inc., and T.U. is a member of that company. The company funded this study and applied together with Osaka Metropolitan University for a patent on Mamaki. The other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 3.Forrest SL, Kovacs GG. Current concepts of mixed pathologies in neurodegenerative diseases. Can J Neurol Sci. 2022;31:1–17. doi: 10.1017/cjn.2022.34. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta U, Kayed R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog Neurobiol. 2022;214:102270. doi: 10.1016/j.pneurobio.2022.102270. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Selkoe DJ. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J Neurochem. 2020;154(6):583–597. doi: 10.1111/jnc.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez BA, Limon A. Synaptic disruption by soluble oligomers in patients with Alzheimer’s and Parkinson’s disease. Biomedicines. 2022;10(7):1743. doi: 10.3390/biomedicines10071743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaunmuktane Z, Brandner S. Invited review: The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol Appl Neurobiol. 2020;46(6):522–545. doi: 10.1111/nan.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umeda T, Uekado R, Shigemori K, Eguchi H, Tomiyama T. Nasal rifampicin halts the progression of tauopathy by inhibiting Tau oligomer propagation in Alzheimer brain extract-injected mice. Biomedicines. 2022;10(2):297. doi: 10.3390/biomedicines10020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossenkoppele R, PichetBinette A, Groot C, Smith R, Strandberg O, Palmqvist S, Stomrud E, Tideman P, Ohlsson T, Jögi J, Johnson K, Sperling R, Dore V, Masters CL, Rowe C, Visser D, van Berckel BNM, van der Flier WM, Baker S, Jagust WJ, Wiste HJ, Petersen RC, Jack CR, Jr, Hansson O. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med. 2022;28(11):2381–2387. doi: 10.1038/s41591-022-02049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Howard RS, Schneider LS. The current landscape of prevention trials in dementia. Neurotherapeutics. 2022;19(1):228–247. doi: 10.1007/s13311-022-01236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafii MS, Sperling RA, Donohue MC, Zhou J, Roberts C, Irizarry MC, Dhadda S, Sethuraman G, Kramer LD, Swanson CJ, Li D, Krause S, Rissman RA, Walter S, Raman R, Johnson KA, Aisen PS. The AHEAD 3–45 study: Design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. 2022. 10.1002/alz.12748 [DOI] [PMC free article] [PubMed]
- 13.Chun MN. Mamaki. In: Native Hawaiian medicines. Honolulu, HI: First People’s Productions; 1994; 216–217.
- 14.Kartika H, Li QX, Wall MM, Nakamoto ST, Iwaoka WT. Major phenolic acids and total antioxidant activity in Mamaki leaves. Pipturus albidus J Food Sci. 2007;72(9):S696–701. doi: 10.1111/j.1750-3841.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 15.Afzal O, Dalhat MH, Altamimi ASA, Rasool R, Alzarea SI, Almalki WH, Murtaza BN, Iftikhar S, Nadeem S, Nadeem MS, Kazmi I. Green tea catechins attenuate neurodegenerative diseases and cognitive deficits. Molecules. 2022;27(21):7604. doi: 10.3390/molecules27217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payne A, Nahashon S, Taka E, Adinew GM, Soliman KFA. Epigallocatechin-3-gallate (EGCG): New therapeutic perspectives for neuroprotection, aging, and neuroinflammation for the modern age. Biomolecules. 2022;12(3):371. doi: 10.3390/biom12030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Cai MY, Li Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159(4):1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Yoo KY, Choi JH, Hwang IK, Lee CH, Lee SO, Han SM, Shin HC, Kang IJ, Won MH. (-)-Epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice. Phytother Res. 2010;24(7):1065–1070. doi: 10.1002/ptr.3083. [DOI] [PubMed] [Google Scholar]
- 19.Socała K, Szopa A, Serefko A, Poleszak E, Wlaź P. Neuroprotective effects of coffee bioactive compounds: A review. Int J Mol Sci. 2020;22(1):107. doi: 10.3390/ijms22010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L, Li X, Meng S, Ma T, Wan L, Xu S. Chlorogenic acid alleviates Aβ25–35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des Devel Ther. 2020;14:1705–1716. doi: 10.2147/DDDT.S235969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Dilnashin H, Singh R, Singh SP. Neuroprotective effect of chlorogenic acid on mitochondrial dysfunction-mediated apoptotic death of DA neurons in a Parkinsonian mouse model. Oxid Med Cell Longev. 2020;2020:6571484. doi: 10.1155/2020/6571484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Wang H, Zhang Y, Zhang Z. Protective effects of chlorogenic acid on cerebral ischemia/reperfusion injury rats by regulating oxidative stress-related Nrf2 pathway. Drug Des Devel Ther. 2020;14:51–60. doi: 10.2147/DDDT.S228751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Tahir MS, Almezgagi M, Zhang Y, Bashir A, Abdullah HM, Gamah M, Wang X, Zhu Q, Shen X, Ma Q, Ali M, Solangi ZA, Malik WS, Zhang W. Mechanistic new insights of flavonols on neurodegenerative diseases. Biomed Pharmacother. 2021;137:111253. doi: 10.1016/j.biopha.2021.111253. [DOI] [PubMed] [Google Scholar]
- 24.Xu PX, Wang SW, Yu XL, Su YJ, Wang T, Zhou WW, Zhang H, Wang YJ, Liu RT. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res. 2014;1(264):173–180. doi: 10.1016/j.bbr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Sun XY, Li LJ, Dong QX, Zhu J, Huang YR, Hou SJ, Yu XL, Liu RT. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J Neuroinflammation. 2021;18(1):131. doi: 10.1186/s12974-021-02182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghbelinejad S, Nassiri-Asl M, Farivar TN, Abbasi E, Sheikhi M, Taghiloo M, Farsad F, Samimi A, Hajiali F. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol Lett. 2014;224(1):108–113. doi: 10.1016/j.toxlet.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94(24):13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Dam D, D'Hooge R, Staufenbiel M, Van Ginneken C, Van Meir F, De Deyn PP. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur J Neurosci. 2003;17(2):388–96. doi: 10.1046/j.1460-9568.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 29.Umeda T, Sakai A, Shigemori K, Yokota A, Kumagai T, Tomiyama T. Oligomer-targeting prevention of neurodegenerative dementia by intranasal rifampicin and resveratrol combination - A preclinical study in model mice. Front Neurosci. 2021;13(15):763476. doi: 10.3389/fnins.2021.763476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umeda T, Yamashita T, Kimura T, Ohnishi K, Takuma H, Ozeki T, Takashima A, Tomiyama T, Mori H. Neurodegenerative disorder FTDP-17-related tau intron 10 +16C → T mutation increases tau exon 10 splicing and causes tauopathy in transgenic mice. Am J Pathol. 2013;183(1):211–225. doi: 10.1016/j.ajpath.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Umeda T, Eguchi H, Kunori Y, Matsumoto Y, Taniguchi T, Mori H, Tomiyama T. Passive immunotherapy of tauopathy targeting pSer413-tau: A pilot study in mice. Ann Clin Transl Neurol. 2015;2(3):241–255. doi: 10.1002/acn3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 –> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99(13):8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umeda T, Hatanaka Y, Sakai A, Tomiyama T. Nasal rifampicin improves cognition in a mouse model of dementia with Lewy bodies by reducing α-Synuclein oligomers. Int J Mol Sci. 2021;22:8453. doi: 10.3390/ijms22168453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Pattamatta A, Zu T, Reid T, Bardhi O, Borchelt DR, Yachnis AT, Ranum LP. C9orf72 BAC mouse model with motor deficits and neurodegenerative features of ALS/FTD. Neuron. 2016;90(3):521–534. doi: 10.1016/j.neuron.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Hatanaka Y, Umeda T, Shigemori K, Takeuchi T, Nagai Y, Tomiyama T. C9orf72 hexanucleotide repeat expansion-related neuropathology is attenuated by nasal rifampicin in mice. Biomedicines. 2022;10(5):1080. doi: 10.3390/biomedicines10051080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muzio L, Viotti A, Martino G. Microglia in neuroinflammation and neurodegeneration: From understanding to therapy. Front Neurosci. 2021;15:742065. doi: 10.3389/fnins.2021.742065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colucci-D'Amato L, Speranza L, Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci. 2020;21(20):7777. doi: 10.3390/ijms21207777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horgusluoglu E, Nudelman K, Nho K, Saykin AJ. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am J Med Genet B Neuropsychiatr Genet. 2017;174(1):93–112. doi: 10.1002/ajmg.b.32429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Annu Rev Neurosci. 2017;25(40):21–49. doi: 10.1146/annurev-neuro-072116-031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nandwana V, Nandwana NK, Das Y, Saito M, Panda T, Das S, Almaguel F, Hosmane NS, Das BC. The role of microbiome in brain development and neurodegenerative diseases. Molecules. 2022;27(11):3402. doi: 10.3390/molecules27113402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Góralczyk-Bińkowska A, Szmajda-Krygier D, Kozłowska E. The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci. 2022;23(19):11245. doi: 10.3390/ijms231911245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puhlmann ML, de Vos WM. Intrinsic dietary fibers and the gut microbiome: Rediscovering the benefits of the plant cell matrix for human health. Front Immunol. 2022;13:954845. doi: 10.3389/fimmu.2022.954845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuervo-Zanatta D, Syeda T, Sánchez-Valle V, Irene-Fierro M, Torres-Aguilar P, Torres-Ramos MA, Shibayama-Salas M, Silva-Olivares A, Noriega LG, Torres N, Tovar AR, Ruminot I, Barros LF, García-Mena J, Perez-Cruz C. Dietary fiber modulates the release of gut bacterial products preventing cognitive decline in an Alzheimer’s mouse model. Cell Mol Neurobiol. 2022: 10.1007/s10571-022-01268-7 [DOI] [PMC free article] [PubMed]
- 44.Kartika H, Shido J, Nakamoto ST, Li QX, Iwaoka WT. Nutrient and mineral composition of dried Mamaki leaves (Pipturus albidus) and infusions. J Food Composition Anal. 2011;24(1):44–48. doi: 10.1016/j.jfca.2010.03.027. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets used for analysis and materials are available from the corresponding author upon reasonable request.