Abstract
Background:
Home oxygen therapy (HOT) improves survival in patients with hypoxaemic chronic respiratory disease. Most patients evaluated for HOT are former or active smokers. Oxygen accelerates combustion and smoking may increase the risk of burn injuries and fire hazards; therefore, it is considered a contraindication for HOT in many countries. However, there is variability in the practices and policies regarding this matter. This multidisciplinary Swedish taskforce aimed to review the potential benefits and risks of smoking in relation to HOT, including medical, practical, legal and ethical considerations.
Methods:
The taskforce of the Swedish Respiratory Society comprises 15 members across respiratory medicine, nursing, medical law and ethics. HOT effectiveness and adverse risks related to smoking, as well as practical, legal and ethical considerations, were reviewed, resulting in five general questions and four PICO (population–intervention–comparator–outcome) questions. The strength of each recommendation was rated according to the GRADE (grading of recommendation assessment, development and evaluation) methodology.
Results:
General questions about the practical, legal and ethical aspects of HOT were discussed and summarised in the document. The PICO questions resulted in recommendations about assessment, management and follow-up of smoking when considering HOT, if HOT should be offered to people that meet the eligibility criteria but who continue to smoke, if a specific length of time of smoking cessation should be considered before assessing eligibility for HOT, and identification of areas for further research.
Conclusions:
Multiple factors need to be considered in the benefit/risk evaluation of HOT in active smokers. A systematic approach is suggested to guide healthcare professionals in evaluating HOT in relation to smoking.
Tweetable abstract
Smoking in people who are otherwise eligible for home oxygen therapy poses a clinical challenge, where management should include a risk–benefit evaluation and medical, practical, legal and ethical considerations. https://bit.ly/3GDzXtm
Introduction
Home oxygen therapy (HOT) is commonly prescribed to people with severe respiratory disease to correct hypoxaemia and improve health outcomes [1]. HOT is an umbrella term [2] that includes long-term oxygen therapy (LTOT) [3, 4], short-term oxygen therapy [2], ambulatory oxygen [5] and palliative oxygen therapy [2, 6]. Beneficial effects of HOT include improved survival time in people with COPD with severe resting hypoxaemia [3, 4], but not with moderate hypoxaemia [7]. Adverse events related to HOT are common, including upper airway dryness, nosebleeds, trips and falls over equipment, as well as burn injuries and fire hazards that may be related to smoking [8, 9]. HOT can increase the risk of burns and fire incidents in relation to smoking and contact with sparks or open flames. An oxygen-enriched atmosphere increases the risk of ignition at lower ambient temperatures and may cause a fierce and explosive combustion. The use of e-cigarettes, water pipes or heat-not-burn products may carry the same risk as regular cigarettes [10, 11].
Evaluation and prescription of HOT among active smokers is a difficult and controversial issue. Clinical practice guidelines recommend that patients and their informal caregivers are given adequate education on oxygen safety, including smoking cessation and fire prevention [1, 12–16]. For many years, clinical guidelines adapted the eligibility criteria from the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) trials and recommended that smoking was a contraindication for HOT [3, 4]. In recent years, some guidelines have become more permissive [1, 12, 17] and some state that smoking can be acceptable in patients with HOT if the patient and caregivers are adequately informed and understand the associated risks and that they can be managed with an acceptable risk profile (supplemental table S1). Many guidelines recommend education and precautions to be taken when prescribing oxygen to smokers and to encourage smoking cessation [12, 14, 15, 18–20], which has also been shown to slow the progression of COPD, decrease the risk of comorbidities such as cardiovascular disease and cancer, and to improve survival [21]. However, many clinical guidelines still state active smoking as an absolute contraindication for HOT and practices vary markedly between settings and countries [22].
There is a need for guidance and recommendations on how to evaluate and manage smoking in relation to HOT, given the wide variation in guidelines and practices that may influence treatment safety and patient outcomes. Recommendations should consider relevant aspects, including medical, practical, legal and ethical issues [23], and are important for valid and equal evaluation and management of HOT in relation to smoking. As HOT is one of the few treatments shown to improve survival in people with severe hypoxaemia, unnecessarily denying patients the treatment due to smoking (that may be safely managed) could lead to an earlier death, whereas smoking during HOT might increase the risk of adverse events and compromise the safety for the patient, caregivers and other people living in the same house.
The aim of this national taskforce (TF) was to review and address the complex clinical decision-making when considering the provision of HOT to smokers, incorporating medical, practical, legal and ethical considerations. A further aim was to formulate consensus recommendations regarding the factors to be taken into account in the clinical management of HOT in relation to smoking and to identify knowledge gaps for future research. The focus of this review is primarily LTOT, which is often used continuously by patients for several years and has the largest evidence base.
Methods
Design and TF panel
This was a national TF initiated by the Swedish Respiratory Society and the National Registry for Respiratory Failure (Swedevox). TF members were recruited through invitations to the Research Council of the Swedish Respiratory Society, the Swedevox registry, clinical experts (physicians and nurses) working with HOT and/or smoking-related interventions or policy, oxygen companies, university faculties for medical law, the Delegation for Medical Ethics of the Swedish Medical Society, the national patient organisation (Heart-Lung Association), and patients living with HOT.
The final TF panel consisted of a total of 15 members, namely five respiratory physicians (including one from another Nordic country), two oxygen nurses, one physician and senior clinical researcher in smoking interventions, two physicians (specialists in palliative medicine) with expertise in medical ethics, one senior lecturer in medical law, one representative from an oxygen company (AGA Linde Health Care), one patient organisation representative, and one patient representative living with LTOT.
Conflicts of interest
All potential conflicts of interest were declared and tobacco industry involvement (present or previous) was not acceptable for participation, in accordance with the policies of the Swedish and the European Respiratory Society. The oxygen company representative participated in the discussions (including related to equipment and safety issues) but not in formulating TF questions or statements.
Searches and meetings
Systematic searches relating to oxygen therapy and smoking were performed using Medline and Embase (last updated 30 August 2023) by a librarian and relevant papers were circulated to the TF. The search terms and strategies are provided as supplemental appendix 1.
A list of preliminary general and PICO (target population–intervention–comparator–outcome) questions was developed by the chair (M. Ekström) and Z. Ahmadi within the focus areas, namely the effects of HOT, practical administration of HOT, risks of smoking during HOT, smoking evaluation and management, medical legal aspects, and medical ethical aspects. The TF work comprised three online meetings:
Meeting 1 (18 November 2021): Each focus area was presented by expert(s) in the TF for this area and discussed by the whole panel. The list of questions, including four PICO questions, was finalised (table 1) through consensus based on their perceived clinical importance relating to smoking and HOT. After the meeting, the TF members were asked to draft a review of their respective focus area.
TABLE 1.
Recommendations for target PICO (population–intervention–comparator–outcome) questions
| PICO question | Recommendations and strength of recommendation |
| PICO 1. How should smoking be assessed before and during HOT? | In people evaluated for HOT, we recommend a thorough clinical history-taking and physical examination (strong recommendation). It is important to provide adequate information and to achieve alliance with the patient around common goals. We recommend shared decision-making to improve adherence with HOT (strong recommendation). We recommend that home visits are performed if possible (strong recommendation). We suggest analysis of COHb from ABG assessments (conditional recommendation). We recommend against the general use of other tests such as exhaled CO or U-cotinine (strong recommendation). |
| PICO 2: How should smoking be managed and followed up in people evaluated for or treated with HOT? | We recommend education and training in HOT of healthcare professionals prescribing and involved in oxygen therapy (strong recommendation). We recommend evidence-based smoking cessation interventions are offered and followed up (strong recommendation). |
| PICO 3: Should HOT be offered to people that meet the eligibility criteria but who continue to smoke (either at starting or during oxygen therapy)? | The taskforce suggests a risk-benefit evaluation when considering HOT to people that meet the eligibility criteria but who continue to smoke (conditional recommendation). Oxygen therapy should not be offered to people with impaired decision making who continue to smoke, who have increased risk of insufficient adherence or complications of the therapy and smoking (strong recommendation). Guidelines should be established on how to manage oxygen therapy in active smokers to improve quality and equality of care, including safety and risk management (strong recommendation). |
| PICO 4: Should the patient be required to have stopped smoking for a certain amount of time before being considered eligible for starting HOT? | The taskforce panel did not reach a consensus to recommend any specific length of time of smoking cessation before being considered eligible for starting HOT (conditional recommendation). Appropriate smoking cessation interventions should be offered before starting oxygen therapy (conditional recommendation). |
ABG: arterial blood gas analysis; CO: carbon monoxide; COHb: carboxyhaemoglobin; HOT: home oxygen therapy.
Meeting 2 (23 March 2022): The draft for each focus area was reviewed and discussed by the panel and a preliminary set of statements and recommendations was formulated. After the meeting, the preliminary statements and recommendations were rated separately by each TF member (except the oxygen company representative) (supplemental table S2). The results were incorporated (by Z. Ahmadi and M. Ekström) into the manuscript draft.
Meeting 3 (30 May 2023): The manuscript was reviewed and discussed in detail by the panel. The focus was to identify statements with consensus, statements without consensus, factors to consider relating to the clinical management of smoking and HOT, and knowledge gaps for future research.
Grading of evidence and recommendations
For statements and recommendations where the panel reached a consensus (defined as >70% of participants selecting the same grade), the strength of each recommendation (table 2) was graded using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology [24].
TABLE 2.
Implications of clinical guideline recommendations by stakeholder
| Stakeholder | Strong recommendation | Conditional recommendation |
| Patient | The majority of patients would want the recommended course of action in this situation and only a small number would not. | Many patients in this situation would prefer recommendation, but a substantial number may not. This is an opportunity for shared decision-making between the clinician and the patient. |
| Clinician | Most individuals should receive the course of action that is recommended. There is a low chance that additional formal decision aids are needed to help individuals make decisions consistent with their values and preferences and adherence to this recommendation could be used as a performance indicator or quality criterion. | Different choices will be applicable to different patients and additional factors will need to be considered in addition to the recommendation in order for a patient to make a decision according to their values and preferences. Decision aids may be needed to assist individuals in making their best choice. This is an opportunity for shared decision-making between the clinician and the patient. |
| Policy-maker | The recommendation can be widely adapted as policy and can be used for performance indicators. | Policy-making will require substantial additional debate and involvement of many and/or additional stakeholders. The likelihood of regional variance is also higher. Performance indicators would need to take into consideration any additional deliberation that has occurred. |
The manuscript was peer reviewed by international experts in the field (Anne Holland, Monash University, Melbourne, Australia; Yet H. Khor, Monash University, Melbourne, Australia; and Yves Lacasse, Université Laval, Quebec, Canada) and the Board and Research Council of the Swedish Respiratory Society. The TF approved the final version for publication.
Structure and role of the document
The review is divided into five general questions and four PICO questions (table 1). For each general question, a short overview and remarks from the panel are provided. The TF recommendations and suggestions are listed for each PICO question, along with background information and concluding remarks. Based on the review and statements, a suggested flow chart illustrating the decision process and the aspects involved in evaluating HOT in relation to smoking is presented. Additionally, future research requirements are identified.
This document is not an official national or international guideline; instead, it aims to provide an overview of the literature and relevant aspects to consider in the clinical management within these focus areas. The content of the document pertains to regulations and conditions in Swedish, European and similar settings. It is intended to assist health practitioners in making more evidence-based decisions during the evaluation and clinical management of HOT in relation to smoking. As available scientific evidence is often scarce and subject to local variations in terms of regulations and practicalities, the TF highlights the importance of considering individual preferences and local circumstances in clinical implementation and management.
Review
General questions
Question 1. How is HOT currently administered and followed up?
In Sweden, oxygen services are provided mainly within respiratory or internal medicine clinics and are publicly funded [14]. All 48 clinics that prescribe LTOT have agreed to comply with guidelines from the Swedevox Registry and the Swedish Respiratory Society [25], which are in line with international guidelines [1, 12]. HOT is prescribed by specialists in pulmonary medicine, internal medicine or palliative medicine after clinical assessment and evaluation of the indication for HOT. It is conducted as an inpatient in the ward or at an outpatient clinic. To assess the level of chronic hypoxaemia and HOT eligibility, arterial blood gas (ABG) analysis under steady-state conditions while breathing room air should be performed. The procedure for the use of HOT is explained to the patient, including potential benefits and risks. The decision whether to prescribe HOT involves a comprehensive review of a patient's comorbidities, clinical status, attitude towards oxygen therapy and ability to adhere to the treatment and follow-up.
The oxygen equipment is supplied to the patient by specialised oxygen nurses or sometimes by oxygen technicians at each clinical unit. The equipment is publicly funded (through taxes) and free to the patient. Oxygen nurses provide detailed information regarding oxygen therapy and its follow-up (which may include home visits) to the patient and informal caregivers and enter data into the national Swedevox registry, which has maintained a stable coverage of about 85% of patients starting LTOT in Sweden since 1987 [14].
Safety aspects should be considered before initiation of HOT. Patients are informed about the increased risk of burn injuries during any interaction with potential sources of open flames, such as cigarettes, e-cigarettes, toasters, etc., emphasising the importance of avoiding such situations. The risk of fire and explosion may vary by type of oxygen equipment. Continual oxygen therapy with oxygen concentrators (which only enrich oxygen from room air and include no oxygen storage) are considered safe. As reported to the Swedevox registry in 2021, about 86% of patients were also prescribed portable oxygen equipment, most often a portable oxygen concentrator (89%) and in some cases cylinders of compressed gas (7%) or liquid oxygen (4%) [25].
According to Swedish guidelines, contraindications for HOT in order to decrease the risk of burn injuries and fire hazards include inadequate collaboration and adherence to the therapy, severe dementia (which could lead to insufficient daily duration of HOT and increased risk of fall injuries on the oxygen tubes), active smoking (including e-cigarettes), and other contact with fire (such as gas or wood stoves and heating) [25]. Occupational therapists from the municipality can assist with home adaptions as needed.
Patients prescribed HOT should optimally be followed up within a few weeks to check adherence, eligibility (including ABG and safety issues), side-effects and the need for portable oxygen equipment and then regularly about twice a year. Treatment is ended after individual assessment if the HOT eligibility criteria are not met, at which time the oxygen equipment is returned.
Remarks
A national structured approach to the prescription, management and follow-up of HOT has facilitated standardisation of the management and adherence to the eligibility criteria for oxygen therapy in Sweden. The TF advocates for the prescription and management of HOT by healthcare professionals with adequate knowledge of oxygen therapy and that follow-up of the therapy should be carried out by specialised oxygen staff, preferably complemented by home visits to assess the patent's interaction with the treatment in their home environment. A national oxygen registry (Swedevox) effectively monitors the adherence to the eligibility criteria. The presented structure might also be suitable for implementation in other settings for improved and sustained validity of HOT prescription and management. We acknowledge that conducting adequate follow-up, particularly through home visits, may pose additional challenges in resource-limited settings, depending on the structure of healthcare systems.
Question 2: What are the potential benefits of HOT in people that meet the eligibility criteria but who continue to smoke?
LTOT is standard of care for patients with severe chronic resting room air hypoxaemia [1, 3, 4, 12]. Chronic hypoxaemia is defined as hypoxaemia that persists despite the patient being in a stable clinical condition and having optimal treatment of underlying disease [1]. The eligibility criteria for HOT vary between countries [22]. HOT assessment using pulse oximetry might misclassify hypoxaemia and repeated ABG analysis under steady-state conditions while breathing room air is recommended to confirm chronic severe hypoxaemia [26, 27]. Severe hypoxaemia was defined in the landmark LTOT trials as meeting either of the following criteria: 1) arterial carbon dioxide tension (PaO2) ≤ 55 mmHg (7.3 kPa) or 2) PaO2 56–59 mmHg (7.5–7.9 kPa) together with oedema, haematocrit ≥55% or the presence of cor pulmonale [1, 3, 4, 12]. The two landmark randomised clinical trials from the 1970s (the NOTT and MRC trials) included a total of 290 people with COPD and severe chronic hypoxaemia, defined according with the criteria above. The studies showed that LTOT decreased 2-year and 5-year mortality [3, 4].
Although the findings from the NOTT and MRC trials have been widely adopted into clinical practice guidelines [22], the generalisability of the findings for current practice is diminished by the fact that they included mostly men younger than 70 years without significant comorbidity [3, 4]. In addition, treatment for COPD, cardiovascular disease and other comorbidities has significantly improved in recent decades.
A survival benefit of HOT has not been shown in COPD patients with moderate or mild resting hypoxaemia or hypoxaemia only during exercise or at sleep [7]. In the Long-term Oxygen Therapy Trial (LOTT), 738 patients with COPD and moderate resting hypoxaemia (defined in the trial as a peripheral oxygen saturation of 89–93%), exertional hypoxaemia, or both, were randomised to LTOT or no oxygen [5]. The LOTT did not find any differences between the oxygen and no oxygen groups in the primary composite outcome of death or requirement for LTOT. There were also no differences in secondary outcomes including 6-min walk distance, lung function or health-related quality of life (HRQoL). In a recently published trial, nocturnal oxygen did not have any effect on survival or progression to HOT in COPD [28], findings that are in line with previous studies [29, 30]. Ambulatory oxygen, in the setting of isolated hypoxaemia during exertion, has shown acute beneficial effects in terms of exercise capacity and breathlessness during laboratory-based exercise tests in COPD [31] and pulmonary fibrosis [32]. However, evidence for its effect in the context of pulmonary rehabilitation or for daily life (home) treatment is less consistent [31]. Trials of palliative oxygen for relief of breathlessness or reduction of respiratory distress near death have not reported any clear benefits with oxygen compared with breathing air or no treatment [33, 34].
Trials on the efficacy of HOT in chronic conditions other than COPD, such as interstitial lung disease (ILD), are lacking. However, clinical guidelines recommend HOT for other conditions using the same criteria as in COPD [1, 16]. For people with ILD and exertional hypoxaemia, one crossover trial with a 2-week treatment period reported improvements in HRQoL, but the long-term impact on HRQoL is unknown [35]. In laboratory studies, there were small improvements in exercise capacity with ambulatory oxygen [36].
Most patients evaluated for HOT in clinical practice are former or active smokers, which poses an ethical dilemma for healthcare professionals [37]. Data from the oxygen register in Denmark, where smoking is not a strict contraindication for HOT, indicate that 20–25% of patients on HOT continue to smoke [38]. In the MRC trial, which showed a survival benefit with oxygen therapy in hypoxaemic COPD, approximately 40% of the study population were smokers at the start of the trial and smoking was not regarded as a contraindication; however, they were encouraged to quit smoking [3]. Smokers were also eligible for the NOTT. Although no mention of smoking was made in the original paper, a secondary publication indicated that 38% of participants were actively smoking at study entry [39]. The effectiveness of oxygen therapy in smokers with hypoxaemia and secondary polycythaemia was evaluated in some early studies [40, 41]. Smoking may contribute to secondary polycythaemia in hypoxic COPD patients due to the strong binding of CO to haemoglobin, which could also attenuate the effect of the supplemental oxygen [40]. However, no trial has specifically compared the benefit of HOT between smokers and nonsmokers.
The risk–benefit assessment is affected by the type of oxygen equipment used, as the risk of smoking-related harm is probably more significant in LTOT compared to ambulatory oxygen therapy. In addition, the potential benefits, including improved survival, is also likely to be greater in LTOT.
Remarks
The TF concludes that HOT is likely to improve survival in people with chronic severe hypoxaemia and may improve breathlessness and well-being in some patients. The benefit of oxygen therapy is likely to be greater in people with more severe hypoxaemia, whereas a benefit has not been shown in mild to moderate hypoxaemia. Smokers were included in the original efficacy trials. Although the benefit of HOT among smokers is uncertain due to a lack of robust evidence in the field, a positive effect among smokers cannot be precluded. Further research on the benefit of HOT among smokers is difficult to perform due to ethical issues; however, national registries could be used to improve the existing evidence base.
Question 3: What are the potential risks of adverse effects of HOT in people who otherwise meet the eligibility criteria but continue to smoke?
HOT provides oxygen-enriched air within the home setting, which, together with smoking or another heat source, provides the necessary elements to ignite and magnify fires and cause burn injuries and fire hazards [42]. Tobacco smoking is the main cause of burn injuries during HOT [43–46]. The incidence of burn injuries during LTOT is reported at 85/100 000 person-years in Sweden (where smoking is a strong contraindication) compared with 170/100 000 person-years in Denmark (where smoking is allowed in some patients during LTOT) [47]. Burn injuries include head and neck burns, and inhalation injuries. Compared to other burns, burn injuries in relation to HOT are associated with a higher risk of inhalation injury and mortality [45]. The use of e-cigarettes and other vaping devices carries the same risk as regular cigarettes [11]. The risk of burns during HOT also extends to persons surrounding the patient, such as family, home care providers, informal caregivers, as well as neighbours [43, 44]. The risk of fire and explosion likely differs by type of HOT equipment. Cylinders of compressed gas or liquid oxygen can explode after about 20 min of fire. In contrast, oxygen concentrators do not explode and pose fewer safety and health risks. Furthermore, smoking can contaminate and decrease the life span of oxygen concentrators. Although studies are lacking, it is known from clinical practice that smoking (by the patient or others) near the concentrator can lead to the requirement for filters to be cleaned and replaced more frequently; in addition, the concentrator will have a shorter operating time. Therefore, patients and their families must be informed of the risks of burn injuries and fire and how to avoid them. The prescribing physician and the oxygen supplier must assess on case-to-case basis whether HOT can be used safely.
Remarks
The TF concludes that smoking during HOT is associated with several potential adverse effects for patients and caregivers, who need to be adequately informed about the associated risks and safety precautions. Oxygen can increase the risk of fire and burn injuries for the patient and others. The risk of fire accidents depends on the type of oxygen equipment used, where cylinders of compressed oxygen may be associated with a higher risk compared with liquid oxygen and oxygen concentrators (lowest risks). Tobacco smoke exposure reduces the life span of oxygen equipment, thereby increasing HOT-related costs.
Question 4: Which legal aspects (laws and regulations) are relevant when considering HOT among active smokers?
For the discussion, see supplementary material S4.
Remarks
Several key legal aspects need to be considered in the evaluation of HOT in active smokers. Health professionals are obliged to offer patients good healthcare based on the best available evidence and clinical expertise. Limiting the care and treatment that a patient needs requires that there is underpinning empirical evidence or reduced efficacy and/or increased risks. The patient and relevant persons such as informal caregivers need to receive information tailored to their needs. There is no law or regulation that unequivocally prohibits the administration of HOT to active smokers or states that HOT must be offered to active smokers who otherwise meet the eligibility criteria. An individual evaluation of the benefits and risks needs to be conducted, involving all relevant aspects including underlying ethical principles.
Question 5: what ethical considerations are relevant when considering HOT among active smokers?
For the discussion, see supplementary material S5.
Remarks
Healthcare professionals are obligated to provide treatment to all individuals and adhere to the ethical principle that everyone has the right to access healthcare and available evidence-based treatments, irrespective of their age, medical condition or personal practices. According to the principle of beneficence, treatments with a net benefit should be offered to patients. Although the net benefit of HOT among active smokers is unknown, we need to consider that smoking most likely reduces the benefits associated with oxygen therapy. Regarding the potential safety issues, the combination of HOT and smoking might also be dangerous due to the risk of fires and burn injuries. In agreement with the principle of nonmaleficence, the risk of fire and burn injuries associated with HOT to the patient, carers as well as to third parties needs to be carefully assessed in each individual case.
PICO questions
PICO 1: How should smoking be assessed before and during HOT?
Recommendation
In people evaluated for HOT, we recommend a thorough clinical history-taking and physical examination (strong recommendation).
It is important to provide adequate information and to achieve alliance with the patient around common goals. We recommend shared decision-making to improve adherence to HOT (strong recommendation).
We recommend that home visits should be performed if possible (strong recommendation).
We suggest analysis of carboxyhaemoglobin (COHb) from ABG assessments (conditional recommendation).
We recommend against the general use of other tests such as exhaled carbon monoxide (CO) or U-cotinine (strong recommendation).
Background
Given the potential hazards of smoking during HOT, assessing a patient's smoking habits is important at every visit. Objective measurement can easily be done by measuring the concentration of CO in exhaled air [48]. A battery-powered handheld device can identify, within 15 s, someone who has smoked within the past 3–4 h based on their exhaled air [49]. Several blood biomarkers have been identified to indicate smoking status, including cotinine levels in the plasma or urine. Cotinine, a metabolite of nicotine, can be detected 2–3 days (15–40 h half-life) after nicotine consumption. Cotinine can be analysed in saliva, urine, serum and hair. Quantitative methods are expensive, but newer and cheaper semi-quantitative ones may also be considered.
Remarks
The TF emphasises the importance of shared decision-making to improve adherence to oxygen therapy. Some patients may not be truthful about their smoking history if they perceive that their honesty might potentially disqualify them of life-saving treatment. The patient must feel comfortable in giving relevant medical information to healthcare professionals, including lifestyle information such as smoking habits that might be important in determining treatment choice and success rate. Passive smoking exposure, including from caregivers, needs to be discussed as part of the overall risk assessment and safety education. The aim is to encourage and motivate smokers to quit and to not penalise them for their behaviours.
There was agreement in the TF that home visits are valuable and an opportunity to assess patient adherence to therapy. Home visits follow a standardised routine, involving interviews with the patient and their family, along with equipment checks and, if needed, appropriate adjustments. If a patient has relapsed to smoking, he or she will be advised to stop, and offered support with smoking cessation. If multiple attempts to quit smoking prove unsuccessful, it is essential to reiterate the necessary precautions for preventing fires. However, if the risk of fire or other accidents is deemed unacceptable, then HOT must be discontinued. However, most clinics do not have resources to perform home visits routinely or at all. We acknowledge that home-visit practices might not be possible in resource-limited settings depending on the structure of healthcare systems.
The TF agreed upon a conditional recommendation for analysis of COHb from ABG assessment (when obtained), but other tests such as exhaled CO or U-cotinine were not recommended as diagnostic tools due to a lack of evidence and the high costs associated with the instruments. However, costs may vary depending on the healthcare system. There is a need to identify cost-effective and reliable ways of objectively assessing active smoking when considering HOT.
PICO 2: How should smoking be managed and followed up in people evaluated for or treated with HOT?
Recommendation
We recommend education and training in HOT of healthcare professionals prescribing and managing oxygen therapy (strong recommendation).
We recommend that evidence-based smoking cessation interventions are offered and followed up (strong recommendation).
Background
The beneficial effects of HOT in terms of survival depend on prescribing the treatment to the right patients (according to evidence-based eligibility criteria), providing adequate information on associated risks with the treatment and improving adherence to treatment [1, 12, 14]. A recent American Thoracic Society workshop concluded that many healthcare providers that prescribe oxygen lack knowledge about the eligibility criteria and lack the necessary resources to prescribe HOT delivery systems and devices correctly and efficiently to meet the specific needs of their patients [50]. Clinicians need better access to educational programmes [50].
Achieving smoking cessation and sustained abstinence from smoking is a complex, personal and often lengthy process that typically requires multiple quit attempts, often including both assisted and unassisted efforts [51]. Expert advice and support plus pharmacological treatment with follow-up is an effective strategy [52]. Guidelines recommend a combination of behavioural support and pharmacologic therapy to achieve higher smoking quit rates than either type of treatment alone due to complementary or potentially additive effects [53]. First-line pharmacotherapies for smoking cessation include nicotine replacement therapy, bupropion hydrochloride or varenicline tartrate, which aim to reduce symptoms of nicotine withdrawal and increase smoking abstinence [53]. The choice of pharmacotherapy is typically based on patient preference after shared clinical decision-making and considering contraindications and comorbidities. Pharmacotherapy for smoking cessation is recommended for at least 3 months. A referral to a tobacco cessation clinic or “quit line” is recommended. The prevalence of post-quit smoking relapses is high, which necessitates long-term follow-up and encouragement and support. As a last resort for individuals with heavy nicotine dependence, noncombustible nicotine products could be an alternative.
Remarks
HOT is an established treatment with the capacity to improve survival and should be offered when indicated according to guidelines. Smoking cessation is an important intervention aiming at improving the prognosis of these frail patients. Discussing and supporting smoking cessation when considering HOT might be an important window of opportunity to improve health outcomes for the patient. We recommend that healthcare professionals in hospital and primary care settings address the topic of smoking with their patients in all suitable situations, not only when considering HOT but also in situations where the patient is dependent upon help from healthcare. The TF do not have any specific recommendations regarding which clinical setting has the ultimate responsibility for provision of smoking-cessation services. It depends on variations in available resources and the structure of a particular health system.
PICO 3: Should HOT be offered to people that meet the eligibility criteria but who continue to smoke (either at starting or during oxygen therapy)?
Recommendation
The TF suggests that an individual risk–benefit evaluation should be performed when considering the administration of HOT to people that meet the eligibility criteria but who continue to smoke (conditional recommendation).
Oxygen therapy should not be offered to people with impaired decision-making who continue to smoke, who have increased risk for insufficient adherence to and adverse events related to the therapy (strong recommendation).
Guidelines should be established on how to manage oxygen therapy in active smokers to improve quality and equality of care, including safety and risk management (strong recommendation).
Background
When considering the provision of HOT to active smokers, there is a need for initial and continuous assessment of the benefits and risks associated with the treatment. HOT is an effective treatment that improves survival among people with chronic severe hypoxaemia, but its efficacy among smokers specifically is unknown. When prescribed to active smokers, HOT may be associated with adverse effects such as increased risk burn injuries and fire accidents involving the patient and third parties [43–45, 47]. Tobacco smoke exposure may reduce the life span of the oxygen equipment and thus increase healthcare costs.
Guidelines to date have based the eligibility criteria for LTOT on two landmark studies showing efficacy [3, 4]. In addition, several guidelines state smoking as an absolute contraindication for HOT [13–15, 19, 20]. In recent years, some guidelines have become more nuanced and state that smoking can be acceptable in patients with HOT if the patient is adequately informed about the risks and that treatment can be managed with an acceptable risk profile [12, 14, 18].
Remarks
The TF panel did not reach a consensus on whether HOT should be offered to people that meet the eligibility criteria but who continue to smoke. We want to emphasise the importance of providing adequate information to the patient on the potential benefits and risks associated with the treatment, ensuring that they have really understood the given information and can make an informed decision. In order to minimize the fire hazard, smoking and oxygen treatment should be separated. This has not been a subject in any guidelines. The oxygen concentrator and any cylinders need to be positioned with sufficient ventilation and at a safe distance from any naked flame, cooking equipment or heating appliance. We recommend that smoking does not take place in the same room where the oxygen equipment is located and preferably takes place outside. Patients with beards should wash their face before smoking to avoid high oxygen concentration near the face. With knowledge of the identified safety risks, it is essential to establish the acceptable level of risk for each individual item from a benefit–risk perspective when contemplating the prescription of HOT.
Being a smoker should not as a rule preclude HOT, but continued smoking might be considered a contraindication in the individual based on insufficient treatment effectiveness and/or high risk of complications and fire accidents. In some cases, it may be relevant to require behavioural changes from the patient, such as smoking cessation or specified safety precautions for the treatment, to be meaningful and appropriate. A prerequisite is then that the patient is treated professionally by the healthcare providers and is offered relevant help such as smoking cessation support to be able to achieve and sustain the needed change in lifestyle.
PICO 4: Should the patient be required to have stopped smoking for a certain amount of time before being considered eligible for starting HOT?
Recommendation
The TF panel did not reach a consensus to recommend any specific length of time of smoking cessation before being considered eligible for starting HOT. Appropriate smoking cessation interventions should be offered before starting oxygen therapy (conditional recommendation).
Background
Current guidelines do not specify a specific length of time of smoking cessation before starting HOT [1, 12, 14] and the TF is not aware of any studies evaluating the utility of such time periods. Potential advantages of specifying a required period of time of not smoking before prescribing LTOT are to define a goal for both the patient and the healthcare team to strive towards and to decrease the risk of smoking relapse during HOT. The role of the team will be to support the patient and to restore autonomy and return control by providing smoking cessation support and counselling. A potential risk is that postponing HOT could have detrimental effects on the individual and even increase the risk of mortality, especially in people with more severe hypoxaemia, comorbidities and frailty.
Smoking cessation is not a simple process for many of those strongly addicted to smoking, with high rates of relapse. The rate of relapse is greatest in the first few weeks and decreases rapidly over time [54]. This might be of importance when HOT is initiated during hospitalisation for, e.g., COPD exacerbation to facilitate early hospital discharge [2]. To reduce the risk of early relapse, it is important to arrange a follow-up visit after hospitalisation and make sure that people are given adequate time for recovery.
A parallel could be drawn with organ transplant surgery, which might be denied to active smokers with the justification of an increased risk of post-operative morbidity and mortality among active smokers [55]. While not categorically denying liver transplantation to people with alcoholic cirrhosis, it is common in US liver transplant programmes to use the “6-month rule” as a listing criterion and require patients to be alcohol-free for 6 months to be accepted for an organ transplant [56].
Remarks
The TF panel did not reach a consensus to recommend any specific length of time of smoking cessation before being considered eligible for starting HOT.
As smoking cessation is associated with high rates of relapse, adequate behavioural support and pharmacologic therapy for smoking cessation needs to be provided and followed up. Sometimes patients are discharged to short-term care facilities after a hospitalisation and HOT can be provided in these facilities. These short-term care facilities could provide a window of 1–2 weeks to avoid early relapse to smoking, which might be common when patients come home and fall back into their usual routines and habits. However, stressful life events and chronic stressors may play a role in relapse, making relapse a possibility at any future point.
Thus, it might be adequate to recommend smoking cessation prior to prescribing HOT, but it might not be ethically correct to require it for a specific amount of time. Taken together, decision-making should involve a risk–benefit evaluation that considers the multiple psychosocial, ethical, practical and medical factors (including the patient's severity of hypoxaemia, comorbidities and frailty) rather than applying a rigid rule.
Suggested decision process and factors to consider
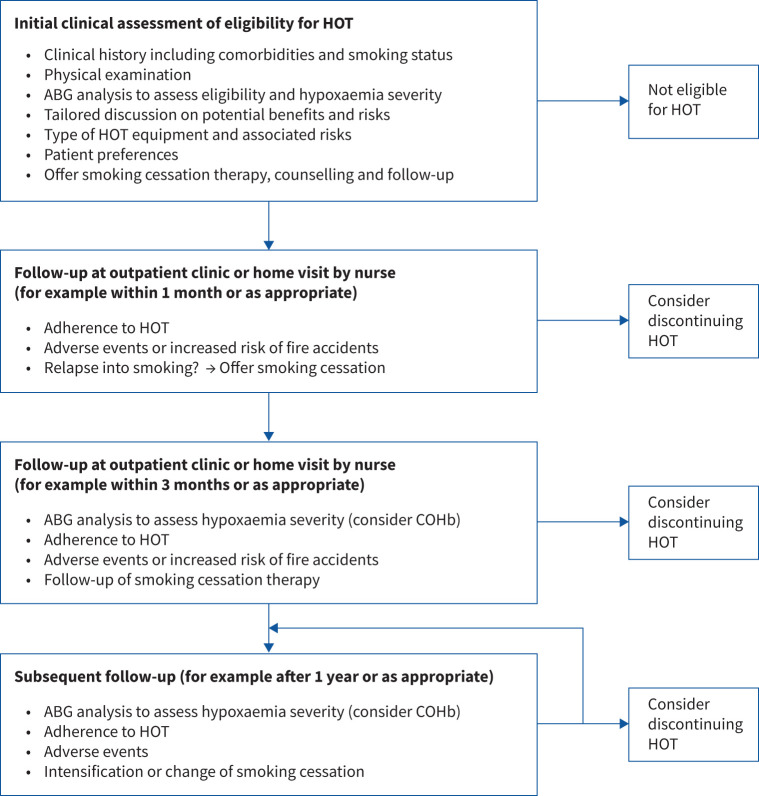
Based on the review, the panel formulated a suggested decision process (figure 1) when evaluating HOT in relation to smoking. Aspects that may need to be considered in this process are listed in table 3. The clinical management needs to be tailored to local circumstances and the needs of the individual patient.
FIGURE 1.
Illustration of the decision process of evaluating home oxygen therapy (HOT) in active smokers. The decision process is best suited for long-term oxygen therapy and might need to be adapted when considering oxygen therapy in the palliative and end of life setting. It is important to have shared decision-making in alliance with the patient and informal caregivers. ABG: arterial blood gas; COHb: carboxyhaemoglobin.
TABLE 3.
Factors to consider in the benefit/risk evaluation of home oxygen therapy (HOT) in people in relation to smoking
| Patient-related factors | Healthcare professional related factors |
| Patient characteristics such as decision-making capacity (consider use of a cognitive-assessment tool) | Provide treatment based on best available evidence and clinical expertise regarding relevant legislations and ethical principles |
| Expected benefit of correction of hypoxaemia | Health services must be provided without prejudice |
| Adherence to oxygen therapy | Education and knowledge of professionals involved in HOT |
| Smoking habits (type and frequency) | Provide smoking cessation counselling and pharmacotherapy |
| Risk of adverse events | Provide adequate information on associated risks with HOT and smoking and provide safety education |
| Adherence to smoking cessation programme and other interventions | Analysis of COHb in ABG |
| Patient preference and shared decision making in alliance with patient and informal caregivers | Home visits are valuable and an opportunity to follow patient adherence to therapy |
ABG: arterial blood gas analysis; COHb: carboxyhaemoglobin.
Knowledge gaps and questions for future research
Based on the present review, the TF identified several research needs relating to HOT in active smokers (table 4).
TABLE 4.
Knowledge gaps and future research requirements identified from the review on home oxygen therapy (HOT) in relation to smoking
| • Data on current HOT practices and procedures across settings and countries |
| • Effects of HOT in conditions other than COPD |
| • Clinical outcomes including the safety profile of HOT in relation to smoking, such as longitudinal studies using health administrative and clinical data in settings where smoking is not an absolute contraindication to HOT |
| • How smoking and oxygen therapy interact in people with hypoxaemia |
| • Implementation research on effectiveness of individualised information and safety precautions in relation to HOT in active smokers |
Conclusions
This national TF has identified several important aspects that affect the evaluation of HOT in smokers, including medical, practical, legal and ethical considerations. HOT is a well-established treatment for adults with chronic lung disease and should be prescribed according to evidence-based clinical practice guidelines. HOT may have positive effects on survival and well-being in people with chronic severe hypoxaemia, but it is also associated with an increased risk of adverse effects such as burn injuries and home fires among smokers. The risk of fire accidents is greater with cylinders of compressed gas or with liquid oxygen. There is no law or regulation that unequivocally forbids HOT being given to active smokers or that HOT must be offered to active smokers who otherwise meet the eligibility criteria. An individual risk–benefit evaluation should be performed in all patients, involving relevant aspects including underlying ethical principles. Smoking cessation is important and needs to be repeatedly discussed with the patient and adequate support and follow-up provided. Educating patients and carers of the associated risks of burn injuries and home fires is vital and every effort should be made to improve patient safety and reduce the risks involved. This multidisciplinary document is not an official guideline, but may inform clinicians and stakeholders to make rational, evidence-based decisions about smoking and HOT.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0194-2023.SUPPLEMENT (368.5KB, pdf)
Acknowledgements
The authors thank Anne Holland (Monash University, Melbourne, Australia), Jerry Krischnan (University of Illinois, Chicago, IL, USA) and Yves Lacasse (Laval University, Quebec, Canada) for their constructive review of a previous version of this article. We would like to thank Susanne Andersson, Leif Oscarsson, representative from AGA Linde oxygen company, Peter Edfelt, representative for the national Heart–Lung Association and Per Svenningsson, patient representative, for their valuable participation in the task force. The authors thank Mats Reenbom (research librarian in Karlskrona, Blekinge) for help with the search strategy for this review.
Provenance: Submitted article, peer reviewed.
Author contributions: M. Ekström takes responsibility for the contents of the manuscript. Z. Ahmadi drafted the manuscript together with all authors who provided important insights. All authors contributed significantly to manuscript writing and critical revisions for intellectually important content. All authors have read and approved the final version of the manuscript.
Conflict of interest: The authors have no conflict of interest relevant for this study to declare.
Support statement: The task force was funded by an unrestricted grant from the Swedish Heart Lung Foundation (20200023). Z. Ahmadi was supported by Swedish Heart–Lung foundation (ID: 20200295). M. Ekström was supported by an unrestricted grant from the Swedish Research Council (2019-02081). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Jacobs SS, Kishnan JA, Lederer DJ, et al. . Home oxygen therapy for adults with chronic lung disease. An Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e121–e141. doi: 10.1164/rccm.202009-3608ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacasse Y, Tan A-YM, Maltais F, et al. . Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1254–1264. doi: 10.1164/rccm.201802-0382CI [DOI] [PubMed] [Google Scholar]
- 3.Medical Research Council Working Party . Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1: 681–686. [PubMed] [Google Scholar]
- 4.Nocturnal Oxygen Therapy Trial Group . Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980; 93: 391–398. doi: 10.7326/0003-4819-93-3-391 [DOI] [PubMed] [Google Scholar]
- 5.Long-Term Oxygen Treatment Trial Research Group . A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016; 375: 1617–1627. doi: 10.1056/NEJMoa1604344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochovska S, Ferreira DH, Garcia MV, et al. . Perspectives on palliative oxygen for breathlessness: systematic review and meta-synthesis. Eur Respir J 2021; 58: 2004613. doi: 10.1183/13993003.04613-2020 [DOI] [PubMed] [Google Scholar]
- 7.Emtner M, Porszasz J, Burns M, et al. . Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med 2003; 168: 1034–1042. doi: 10.1164/rccm.200212-1525OC [DOI] [PubMed] [Google Scholar]
- 8.Tanash HA, Huss F, Ekstrom M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis 2015; 10: 2479–2484. doi: 10.2147/COPD.S91508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorklund F, Ekstrom M. Adverse effects, smoking, alcohol consumption, and quality of life during long-term oxygen therapy: a nationwide study. Ann Am Thorac Soc 2022; 19: 1677–1686. doi: 10.1513/AnnalsATS.202110-1174OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacasse Y, Legare M, Maltais F. E-cigarette use in patients receiving home oxygen therapy. Can Respir J 2015; 22: 83–85. doi: 10.1155/2015/215932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montoya A, Ozhathil D, Hollowed K, et al. . Burn injury from smoking electronic cigarettes while on supplemental oxygen. J Burn Care Res 2023; 44: 249–253. doi: 10.1093/jbcr/irac087 [DOI] [PubMed] [Google Scholar]
- 12.Hardinge M, Annandale J, Bourne S, et al. . British Thoracic Society guidelines for home oxygen use in adults. Thorax 2015; 70: Suppl. 1, i1–i43. doi: 10.1136/thoraxjnl-2015-206865 [DOI] [PubMed] [Google Scholar]
- 13.Strom K, Boe J. A national register for long-term oxygen therapy in chronic hypoxia: preliminary results. Eur Respir J 1988; 1: 952–958. doi: 10.1183/09031936.93.01100952 [DOI] [PubMed] [Google Scholar]
- 14.Ekstrom M, Ahmadi Z, Larsson H, et al. . A nationwide structure for valid long-term oxygen therapy: 29-year prospective data in Sweden. Int J Chron Obstruct Pulmon Dis 2017; 12: 3159–3169. doi: 10.2147/COPD.S140264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young IH, Crockett AJ, McDonald CF. Adult domiciliary oxygen therapy. Position statement of the Thoracic Society of Australia and New Zealand. Med J Aust 1998; 168: 21–25. doi: 10.5694/j.1326-5377.1998.tb123340.x [DOI] [PubMed] [Google Scholar]
- 16.Raghu G, Rochwerg B, Zhang Y, et al. . An Official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015; 192: e3–e19. doi: 10.1164/rccm.201506-1063ST [DOI] [PubMed] [Google Scholar]
- 17.Magnet FS, Schwarz SB, Callegari J, et al. . Long-term oxygen therapy: comparison of the German and British guidelines. Respiration 2017; 93: 253–263. doi: 10.1159/000455879 [DOI] [PubMed] [Google Scholar]
- 18.McDonald CF, Whyte K, Jenkins S, et al. . Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology 2016; 21: 76–78. doi: 10.1111/resp.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax 1997; 52: Suppl. 5, S1–S28. doi: 10.1136/thx.52.2008.S1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siafakas NM, Vermeire P, Pride NB, et al. . Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J 1995; 8: 1398–1420. doi: 10.1183/09031936.95.08081398 [DOI] [PubMed] [Google Scholar]
- 21.Anthonisen NR, Skeans MA, Wise RA, et al. . The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005; 142: 233–239. doi: 10.7326/0003-4819-142-4-200502150-00005 [DOI] [PubMed] [Google Scholar]
- 22.Saleem F, Hur SA, Cahalan M, et al. . International guideline recommendations and eligibility criteria for home oxygen therapy. Lancet Respir Med 2023; 11: 402–405. doi: 10.1016/S2213-2600(23)00100-5 [DOI] [PubMed] [Google Scholar]
- 23.Khoo K, Yoon J, Hultman C, et al. . Ethical considerations to prevent burns in patients who smoke while receiving home oxygen therapy. J Burn Care Res 2022; 53: Suppl. 1, S152. doi: 10.1093/jbcr/irac012.249 [DOI] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swedevox . Annual report. Riktlinjer för syrgasbehandling i hemmet. Date last accessed: 27 September 2023. Date last updated: 19 September 2023. www.ucr.uu.se/swedevox/ [Google Scholar]
- 26.Garnet B, Diaz-Lankenau R, Jean E, et al. . Accuracy of pulse oximetry for long-term oxygen therapy assessment in COPD. Ann Am Thorac Soc 2023; 20: 1587–1594. doi: 10.1513/AnnalsATS.202209-837OC [DOI] [PubMed] [Google Scholar]
- 27.Sjodahl Matsson V, Ekstrom M. Peripheral oxygen saturation misclassifies hypoxemia. Ann Am Thorac Soc 2023; 20: 1070–1073. doi: 10.1513/AnnalsATS.202208-702RL [DOI] [PubMed] [Google Scholar]
- 28.Lacasse Y, Sériès F, Corbeil F, et al. . Randomized trial of nocturnal oxygen in chronic obstructive pulmonary disease. N Engl J Med 2020; 383: 1129–1138. doi: 10.1056/NEJMoa2013219 [DOI] [PubMed] [Google Scholar]
- 29.Chaouat A, Weitzenblum E, Kessler R, et al. . A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J 1999; 14: 1002–1008. doi: 10.1183/09031936.99.14510029 [DOI] [PubMed] [Google Scholar]
- 30.Fletcher EC, Luckett RA, Goodnight-White S, et al. . A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis 1992; 145: 1070–1076. doi: 10.1164/ajrccm/145.5.1070 [DOI] [PubMed] [Google Scholar]
- 31.Ekström M, Ahmadi Z, Bornefalk-Hermansson A, et al. . Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev 2016; 11: CD006429. doi: 10.1002/14651858.CD006429.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaeffer MR, Ryerson CJ, Ramsook AH, et al. . Effects of hyperoxia on dyspnoea and exercise endurance in fibrotic interstitial lung disease. Eur Respir J 2017; 49: 1602494. doi: 10.1183/13993003.02494-2016 [DOI] [PubMed] [Google Scholar]
- 33.Abernethy AP, McDonald CF, Frith PA, et al. . Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376: 784–793. doi: 10.1016/S0140-6736(10)61115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell ML, Yarandi H, Dove-Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage 2013; 45: 517–523. doi: 10.1016/j.jpainsymman.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 35.Visca D, Mori L, Tsipouri V, et al. . Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med 2018; 6: 759–770. doi: 10.1016/S2213-2600(18)30289-3 [DOI] [PubMed] [Google Scholar]
- 36.Sharp C, Adamali H, Millar AB. Ambulatory and short-burst oxygen for interstitial lung disease. Cochrane Database Syst Rev 2016; 7: CD011716. 10.1002/14651858.CD011716.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadi Z, Bornefalk-Hermansson A, Fanklin K, et al. . Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective study. Respir Res 2014; 15: 30. doi: 10.1186/1465-9921-15-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ringbaek TJ, Lange P. The impact of the Danish Oxygen Register on adherence to guidelines for long-term oxygen therapy in COPD patients. Respir Med 2006; 100: 218–225. doi: 10.1016/j.rmed.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 39.McSweeny AJ, Grant I, Heaton RK, et al. . Life quality of patients with chronic obstructive pulmonary disease. Arch Intern Med 1982; 142: 473–478. doi: 10.1001/archinte.1982.00340160057014 [DOI] [PubMed] [Google Scholar]
- 40.Calverley PM, Leggett RJ, McElderry L, et al. . Cigarette smoking and secondary polycythemia in hypoxic cor pulmonale. Am Rev Respir Dis 1982; 125: 507–510. doi: 10.1164/arrd.1982.125.5.507 [DOI] [PubMed] [Google Scholar]
- 41.Kambam JR, Chen LH, Hyman SA. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg 1986; 65: 1186–1188. doi: 10.1213/00000539-198611000-00015 [DOI] [PubMed] [Google Scholar]
- 42.Jonsson A. Dödsbränder i Sverige: en analys av datakvalitet, orsaker och riskmönster (PhD thesis). Karlstad, Karlstad University, 2018. [Google Scholar]
- 43.Al Kassis S, Savetamal A, Assi R, et al. . Characteristics of patients with injury secondary to smoking on home oxygen therapy transferred intubated to a burn center. J Am Coll Surg 2014; 218: 1182–1186. doi: 10.1016/j.jamcollsurg.2013.12.055 [DOI] [PubMed] [Google Scholar]
- 44.Cooper BG. Home oxygen and domestic fires. Breathe 2015; 11: 4–12. doi: 10.1183/20734735.000815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assimacopoulos EM, Liao J, Heard JP, et al. . The national incidence and resource utilization of burn injuries sustained while smoking on home oxygen therapy. J Burn Care Res 2016; 37: 25–31. doi: 10.1097/BCR.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 46.Yoon JS, Khoo KH, Puthumana JS, et al. . Outcomes of patients with burns associated with home oxygen therapy: an institutional retrospective review. J Burn Care Res 2022; 43: 1024–1031. doi: 10.1093/jbcr/irac090 [DOI] [PubMed] [Google Scholar]
- 47.Tanash HA, Ringbaek T, Huss F, et al. . Burn injury during long-term oxygen therapy in Denmark and Sweden: the potential role of smoking. Int J Chron Obstruct Pulmon Dis 2017; 12: 193–197. doi: 10.2147/COPD.S119949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandberg A, Skold CM, Grunewald J, et al. . Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS One 2011; 6: e28864. doi: 10.1371/journal.pone.0028864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chatkin G, Chatkin JM, Aued G, et al. . Evaluation of the exhaled carbon monoxide levels in smokers with COPD. J Bras Pneumol 2010; 36: 332–338. doi: 10.1590/S1806-37132010000300011 [DOI] [PubMed] [Google Scholar]
- 50.Jacobs SS, Lederer DJ, Garvey CM, et al. . Optimizing home oxygen therapy. An official American Thoracic Society workshop report. Ann Am Thorac Soc 2018; 15: 1369–1381. doi: 10.1513/AnnalsATS.201809-627WS [DOI] [PubMed] [Google Scholar]
- 51.Smith AL, Carter SM, Dunlop SM, et al. . Revealing the complexity of quitting smoking: a qualitative grounded theory study of the natural history of quitting in Australian ex-smokers. Tob Control 2018; 27: 568–576. doi: 10.1136/tobaccocontrol-2017-053919 [DOI] [PubMed] [Google Scholar]
- 52.National Board of Health and Welfare . National guidelines for prevention and treatment of unhealthy lifestyles. Date last updated: 24 June 2018. www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2018-6-24.pdf
- 53.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff . A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008; 35: 158–176. doi: 10.1016/j.amepre.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99: 29–38. doi: 10.1111/j.1360-0443.2004.00540.x [DOI] [PubMed] [Google Scholar]
- 55.Corbett C, Armstrong MJ, Neuberger J. Tobacco smoking and solid organ transplantation. Transplantation 2012; 94: 979–987. doi: 10.1097/TP.0b013e318263ad5b [DOI] [PubMed] [Google Scholar]
- 56.Bramstedt KA, Jabbour N. When alcohol abstinence criteria create ethical dilemmas for the liver transplant team. J Med Ethics 2006; 32: 263–265. doi: 10.1136/jme.2005.012856 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0194-2023.SUPPLEMENT (368.5KB, pdf)