Abstract
The cerebral form of severe malaria is associated with excessive intravascular sequestration of Plasmodium falciparum-infected erythrocytes (PRBC). Retention and accumulation of PRBC may lead to occlusion of brain microvessels and direct the triggering of acute pathologic changes. Here we report that by selection, cloning, and subcloning, we have identified rare P. falciparum parasites expressing a pan-adhesive phenotype linked to erythrocyte rosetting, a previously identified correlate of cerebral malaria. Rosetting PRBC not only bound uninfected erythrocytes but also formed autoagglutinates, adhered to endothelial cells, and bound to CD36, immunoglobulins, and the blood group A antigen. The linkage of rosetting, autoagglutination, and cytoadherence involved the coexpression on a single PRBC of ligands with multiple specificities and the binding to two or more receptors on erythrocytes and to at least two other cell adhesion molecules, including a new endothelial cell receptor for P. falciparum-infected erythrocytes. Limited proteolysis that differentially cleaved the rosetting ligand PfEMP1 from the PRBC surface abrogated all the binding phenotypes of these parasites, implicating the variant antigen PfEMP1 as a carrier of multiple ligand specificities. The results encourage the further study of pan-adhesion as a potentially important parasite phenotype in the pathogenesis of severe P. falciparum malaria.
Plasmodium falciparum, the parasite responsible for severe disease and mortality in human malaria (23), withdraws from the peripheral circulation during the second half of its intraerythrocytic life cycle. P. falciparum-infected erythrocytes (PRBC) carrying mature forms of the parasite adhere to the cell lining of post-capillary vessels, a phenomenon known as sequestration (22, 37). Several host receptors have been identified as potential mediators of PRBC cytoadhesion, including CD36 (3), thrombospondin (TSP) (27), intercellular adhesion molecule 1 (ICAM-1) (6), chondroitin sulfate A (CSA) (26, 29), vascular cell adhesion molecule 1 (VCAM-1), endothelial leukocyte adhesion molecule 1 (ELAM-1) (25), and, more recently, platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) (39).
Host and parasite factors are thought to participate in the etiology of severe forms of malaria disease, the most fulminant being cerebral malaria (CM). What distinguishes the parasite involved in CM is poorly understood. RBC rosetting, the binding of uninfected erythrocytes to PRBC, is a property of parasites commonly found in patients with CM but less frequently in those with milder disease (8, 30). Moreover, antibodies interfering with rosette formation are less frequent in sera from CM patients (38). It has been postulated that excessive adhesion of PRBC to the vascular endothelium of the brain (2, 20), as well as to uninfected and infected RBC (8, 30), leading to the focal accumulation of parasites at high densities, is implicated in the pathogenesis of cerebral malaria. Binding to a single receptor, CSA, on the other hand, is the hallmark of the parasite causing maternal malaria (15). Thus, augmented or selective adhesive properties of the parasite may be equivalent to virulence determinants in the causation of severe malaria.
Parasite-induced modifications of the PRBC underlie its de novo acquired adhesive and antigenic properties. The parasite exports several proteins to the membrane of the host cell (18). One is P. falciparum erythrocyte membrane protein 1 (PfEMP1), an antigenically variant protein of 200 to 350 kDa encoded by the var family of genes (4, 35). PfEMP1 has features of an adhesion molecule, binds in vitro to cytoadherence receptors (5), and mediates rosetting of uninfected RBC (12, 31).
In this paper, we report the identification of a linkage between RBC rosetting and other major adhesive phenotypes of the PRBC. Our data also indicate that PfEMP1 is a multivalent ligand on PRBC, mediating the rosetting-linked binding to several molecules on target cells, including novel receptors on RBC and endothelial cells.
MATERIALS AND METHODS
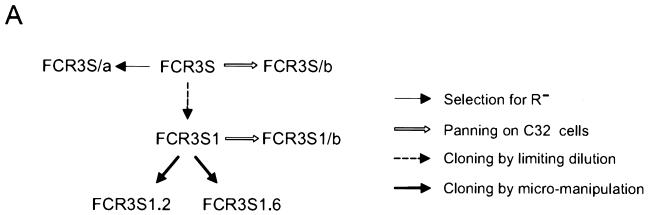
Parasites.
The P. falciparum parasite FCR3S, which originated from the FCR3 strain isolated in The Gambia, West Africa, FCR3S1, a parasite cloned by limiting dilution from FCR3S and subsequently maintained in continuous culture with periodic enrichment for the rosetting phenotype, clones FCR3S1.2 and FCR3S1.6, obtained by micromanipulation of FCR3S1 parasites with a defined R+ and R− rosetting phenotype, respectively, line FCR3S/a, derived from FCR3S by enrichment of nonrosetting parasites, and lines FCR3S/b and FCR3S1/b, generated from FCR3S and FCR3S1, respectively, by consecutive rounds of panning on C32 melanoma cells as described previously, were maintained in culture with O+ erythrocytes by standard procedures (36). FCR3S parasites and all its descendants were of the knobless (K−) phenotype, as seen by transmission electron microscopy. It should be noted that FCR3S was previously called Palo Alto (Uganda) in our publications. Molecular studies of the “Palo Alto” parasites have revealed, however, that they are identical to parasites of the FCR3 lineage (reference 14 and our own studies). The parasite R29 (K+) was cloned from ITOR, a rosetting parasite selected from the ITO strain. The Malayan Camp (MCAMP) (K+) strain of P. falciparum was first adapted to growth in spleen-intact Aotus monkeys, subsequently adapted to in vitro growth in human RBC, and later selected for the rosetting phenotype.
Cloning of parasites.
Limiting-dilution cloning of PRBC was performed as described elsewhere (40). Micromanipulation cloning was performed with a micromanipulator (MN-188; Narishige), sterile micropipettes with internal diameters of 3 to 5 μm, and an inverted Diaphot 300 microscope (Nikon). Rosetting PRBC binding four or more uninfected RBC and nonrosetting PRBC (binding none) were picked from a settled monolayer by aspiration and thoroughly examined for the number and stage of intracellular parasites. Rosetting PRBC were mechanically stripped from uninfected cells. Only rosetting or nonrosetting PRBC infected with a single mature trophozoite were transferred into a petri dish containing RBC at 2% hematocrit in malaria culture medium supplemented with 15% human AB+ serum. The clones were grown for 19 days before being subjected to microscopic examination.
Enrichment of rosetting and nonrosetting parasites.
A 2-ml portion of a culture at 5 to 10% parasitemia and with a rosetting rate of 20% or higher was layered over 2 ml of cold Ficoll-Isopaque (Pharmacia) and centrifuged for 10 s at the high-speed setting in a Dade Immufuge II (Baxter Diagnostics). The cells sedimenting through the Ficoll cushion were collected in a pellet, washed twice in RPMI 1640 (Gibco), and cultured as described above. To enrich for nonrosetting parasites, 2 ml of culture was layered over 60% Percoll (Pharmacia) and centrifuged at 500 × g for 20 min at room temperature (RT). The layer of cells floating at the interface was collected, washed twice in RPMI 1640, and cultured, a procedure which was repeated four times. The parasite line thus generated was named FCR3S/a.
Surface analysis of PRBC.
Surface iodination of PRBC was performed by the lactoperoxidase method. In short, 2 × 109 cells of a culture at 7 to 15% parasitemia with a majority of parasites in the trophozoite stage were gently washed in phosphate-buffered saline (PBS) and resuspended to 1 ml in PBS with 1 mM KI. Na125I (1 mCi; Amersham) and 100 μl of lactoperoxidase (2 mg/ml; Sigma) were added, and the reaction was initiated by the addition of 25 μl of 0.03% H2O2. Four subsequent additions of 25 μl of 0.03% H2O2 were made at 1-min intervals. Radioiodinated cells were washed four times with ice-cold PBS containing 5 mM KI and resuspended in 1 ml of RPMI 1640 containing 5% sorbitol. Labeling of intracellular hemoglobin accounted for less than 2% of total acid-precipitable incorporated radiolabel. To disrupt rosettes and agglutinates, 100 U of heparin (Løvens) per ml was added to the cell suspension and this was passed five times through a 23-gauge (internal diameter, 0.6 mm) needle with a 1-ml syringe. The cell suspension was overlaid on top of a four-step (40, 60, 70, and 80%) Percoll gradient in RPMI 1640–5% sorbitol and centrifuged in a JA 20 (Beckman) rotor at 10,000 rpm for 30 min at RT. The cells floating between the 40 and 60% Percoll layers (>95% mature parasite-containing RBC) were recovered and gently washed with PBS. Enriched PRBC were extracted with 1% Triton X-100 containing a cocktail of protease inhibitors. The polypeptides in the Triton-insoluble fraction were solubilized in 2% sodium dodecyl sulfate (SDS) sample buffer and separated by SDS-polyacrylamide gel electrophoresis (PAGE) (5 to 8.5% or 7.5 to 17.5% polyacrylamide) (19). The dried gels were scanned and analyzed with a PhosphorImager and ImageQuant analysis software (Molecular Dynamics).
Trypsin treatment of the PRBC surface.
Intact or radiolabeled PRBC purified on Percoll gradients were digested with trypsin (Sigma) at the indicated concentrations for 10 min at 37°C. The reaction was terminated by the addition of 1 mg of soybean trypsin inhibitor (Sigma) per ml in RPMI 1640–10% AB+ serum. The cells were washed with PBS and either resuspended in RPMI 1640–HEPES–10% AB+ serum for binding assays or extracted with Triton X-100–SDS and analyzed by SDS-PAGE as described above.
Rosetting, autoagglutination, and cytoadherence.
Rosetting of uninfected RBC and autoagglutination of parasitized erythrocytes were assessed by direct staining of cultures with acridine orange (Sigma) and examination under an epifluorescence microscope (Nikon). Rosetting rates were measured as previously described (9). Spontaneous autoagglutination of PRBC in the P. falciparum cultures was measured with a scoring system ranging from − to 5+, where − denotes lack of binding between parasite-bearing cells and 5+ indicates that >80% of the trophozoite-infected RBC in the culture are engaged in agglutinates, binding directly other infected cells. Adherence of PRBC to unfixed C32 melanoma cells, human umbilical vein endothelial cells (HUVEC), or Chinese hamster ovary (CHO) cells transfected with CD36 or ICAM-1, was performed as described previously (21). In some cytoadherence assays with HUVEC and C32 cells, rosettes were first disrupted by adding 100 U of heparin per ml to the culture and passing it five times through a 23-gauge (internal diameter, 0.6 mm) needle with a 1-ml syringe. The cell suspension was overlaid on top of a four-step (40, 60, 70, and 80%) Percoll gradient in RPMI 1640–5% sorbitol and centrifuged in a JA 20 rotor at 10,000 rpm for 30 min at RT. The cells floating between the 40 and 60% Percoll layers (>95% mature parasite-containing RBC) were recovered, washed twice in RPMI 1640, and used for the assays.
Surface immunofluorescence.
The binding of human nonimmune normal Swedish serum immunoglobulins (Igs) to PRBC was assayed by direct labeling of the cells with fluorescein isothiocyanate-conjugated sheep anti-human Ig antibodies (SBL, Stockholm, Sweden), as described elsewhere (32).
Assay of serum-induced agglutination of PRBC.
Sera from a panel of individuals living in Kisumu, Kenya, who had experienced malaria were used to evaluate differences in the relative antigenic phenotype of the P. falciparum strains, derived lines, and clones. A microagglutination assay was performed and scored by a procedure modified from that of Aguiar et al. (1) by Barragan (3a).
RESULTS AND DISCUSSION
Selection for rosetting coselects for multiple linked binding phenotypes.
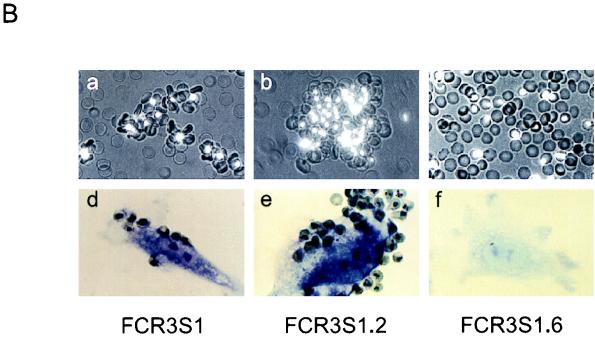
P. falciparum FCR3S1 was cloned from FCR3S by limiting dilution followed by long-term maintenance in continuous in vitro culture. Previous observations suggested that panning of FCR3S1 on some preparations of HUVEC may upregulate the rosetting phenotype (32). When FCR3S1 was repeatedly selected for increased rosetting adhesion, multiple binding phenotypes were coselected, including cytoadherence to HUVEC, adhesion to C32 melanoma and CD36-transfected CHO cells, autoagglutination of infected RBC, and binding to the blood group A sugar α-d-GalNAc(1-3)β-d-gal(1-3)α-d-fuc and to serum Igs (IgM) (Fig. 1A; Table 1). Conversely, selection of FCR3S/a parasites for decreased rosetting resulted in the concomitant loss of all these adhesive features (Table 1). These results suggested a linkage of multiple cytoadhesive phenotypes to rosetting in individual PRBC. To further examine this possibility, rosetting and nonrosetting parasites were recloned from FCR3S1 by micromanipulation and their binding phenotypes were analyzed after a minimal necessary expansion of 10 to 12 cycles. All of the six rosetting clones analyzed showed various levels of accompanying binding phenotypes. RBC harboring trophozoites of one of these clones (FCR3S1.2) adhered extensively to endothelial cells (>1,000 PRBC/100 HUVEC) and formed rosettes at high rates with blood group O RBC (80 to 90%) but used the blood group A carbohydrate as a preferred rosetting receptor if available. FCR3S1.2 PRBC bound Igs (96% of the trophozoite-infected PRBC) and adhered to the CD36 receptor (Table 1). Massive autoagglutination under regular culture conditions in the absence of immune serum was another feature of this clone. Moreover, FCR3S1.2 rosettes and autoagglutinates were commonly observed bound to fresh monolayers of endothelial cells, although infected RBC were the main cells that still adhered after processing of samples for light microscopy (Fig. 1B). Nonrosetting clones derived from FCR3S1, including FCR3S1.6 (Fig. 1; Table 1), were either devoid of binding phenotypes or showed low-level binding to the CD36 receptor (data not shown). The occurrence of the rosetting phenotype, however, was not generally predictive of accompanying multiple cytoadhesive capacity, and neither was the knob phenotype of the parasite. Rosetting parasites with a K+ phenotype such as clone R29 or strain MCAMP lacked binding phenotypes other than CD36 adhesion (Table 1), whereas an R+K+ clone recently derived from a P. falciparum clinical isolate adhered to several cell adhesion receptors and to the blood group B sugar determinant and bound normal human IgG and IgM (data not shown). These results represent the first direct evidence that rosetting, a phenotype correlated with CM (8, 30), can occur linked to all of the other major adhesive traits of intraerythrocytic P. falciparum. Previous studies have shown that a single PRBC can bind to more than one cytoadherence receptor or can simultaneously rosette and cytoadhere (11, 41). Here, by selection and cloning of rosetting parasites, we demonstrate that multiadhesive variants of P. falciparum may arise, which confer maximized binding capacities to the host cell, with regard to both the intensity of binding and the multiplicity of targets.
FIG. 1.
Coselection of adhesive phenotypes on RBC infected with P. falciparum. (A) Derivation of lines and clones from the FCR3S strain. FCR3S1 was cloned by limiting dilution, subjected to long-term culture, and repeatedly enriched for the rosetting phenotype. Clones FCR3S1.2 and FCR3S1.6 were derived by micromanipulation from rosetting and nonrosetting parasites, respectively. The line FCR3S/a was obtained by enrichment of nonrosetting parasites. The lines FCR3S/b and FCR3S1/b were generated by five consecutive rounds of panning on C32 melanoma cells. (B) Binding phenotypes of parasites derived from FCR3S. (a to c) Rosetting of uninfected RBC and autoagglutination of parasitized RBC. PRBC are stained with acridine orange and visualized by UV microscopy. (d to f) Cytoadherence to unfixed HUVEC. Cells were stained with Giemsa and examined by light microscopy. All magnifications, ×400.
TABLE 1.
Adhesive capacity of FCR3S lines and clones and other P. falciparum parasites
| Parasitea | Rosetting
|
Autoaggluti- natione | Cytoadherencef to:
|
|||||
|---|---|---|---|---|---|---|---|---|
| RRb | Ig (%)c | Blood group Ad | HUVEC | Melanoma cells | CHO-CD36 | CHO-ICAM-1 | ||
| FCR3S | 15–20 | 24 | ± | − | 50–100 | 50–100 | 25–50 | — |
| FCR3S1 | 40–70 | 57 | + | 1+ | 300–500 | 100–200 | 50–100 | — |
| FCR3S1.2 | 80–90 | 96 | + | 5+ | 1,200–1,600 | 400–500 | 200–300 | — |
| FCR3S1.6 | <10 | − | − | <10 | <10 | — | — | |
| FCR3S/a | <5 | − | − | 10–20 | <10 | — | — | |
| FCR3S/b | <5 | − | − | <10 | 500–700 | 300–400 | 25–50 | |
| FCR3S1/b | <5 | − | − | <10 | 800–1,000 | 300–400 | <5 | |
| R29 | 40–60 | 4 | NDg | 1+ | <5 | 400–500 | 200–300 | — |
| MCAMP | 50–70 | ND | − | <5 | 400–600 | 300–500 | — | |
The FCR3S1 parasite was cloned by limiting dilution from FCR3S. FCR3S1.2 and FCR3S1.6 were cloned by micromanipulation from FCR3S1. FCR3S/a was derived from FCR3S by enrichment of non-rosetting parasites, and FCR3S/b and FCR3S1/b were generated from FCR3S and FCR3S1, respectively, by panning on C32 melanoma cells as described in Materials and Methods. R29 was cloned from ITOR, a rosetting parasite selected from the ITO strain. The MCAMP strain was adapted to growth in Aotus monkeys, subsequently grown in human RBC, and later selected for the rosetting phenotype.
Rosetting rates (RR) are expressed as the range of percent rosetting.
The binding of serum IgM to the PRBC surface (Ig) is expressed as the mean percentage of total mature parasite-infected RBC binding Ig.
The binding of blood group A RBC by PRBC is indicated relative to the binding of blood group O RBC.
The autoagglutination of PRBC was scored 1+ to 5+ as described in Materials and Methods.
The cytoadherence results are expressed as the range of the number of PRBC bound per 1,000 target cells. —, lack of detectable adhesion. Results are from three experiments.
ND, not done.
Several ligands and receptors are involved in the linkage of rosetting with multiple adhesive phenotypes.
FCR3S1 parasites, propagated for more than 300 generations in blood group O erythrocytes with periodic enrichment for the rosetting phenotype, retained their preference for binding to RBC of blood group A, as assayed in competition experiments, where single-rosetting PRBC bound uninfected RBC of blood groups A and O at an approximate ratio of 3:1. This result indicates that parasites of the FCR3S lineage express a single ligand or genetically linked ligands on the PRBC surface that account for binding to the A substance, which is a receptor for rosetting on RBC carrying this blood group antigen (10), and to another receptor(s) on RBC. None of the parasites studied here bound significantly to either ICAM-1, VCAM-1, ELAM-1, or CSA (Table 1 and data not shown).
Autoagglutination of mature infected RBC in serum from donors never exposed to malaria, a phenotype first described as rosetting unrelated (28), indeed seems to be closely associated with the rosetting capacity of the parasite. All autoagglutinating laboratory strains and derived lines and clones examined so far did form rosettes (Table 1 and our unpublished observations). Spontaneous autoagglutination has also been observed in highly rosetting parasites freshly recovered from P. falciparum malaria patients, but not in nonrosetting isolates (3b, 8). Autoagglutination could be an upregulated form of rosetting, leading to local high concentrations of parasitized RBC. The significance of this phenotype in the pathogenesis of severe malaria remains to be studied.
Thus, the linkage of rosetting, autoagglutination, and cytoadhesion in FCR3S entails the binding of a single PRBC to two or more receptors on RBC and at least two other cell adhesion receptors, one presumably being CD36 but the second being distinct from this receptor since the HUVEC used in this study lacked the expression of CD36.
Rosetting and endothelial-cell binding of FCR3S mediated by distinct receptors.
The following observations prompted us to consider the possibility of an identical ligand-receptor pair mediating rosetting and adhesion to HUVEC: (i) the increase in rosetting rates observed when FCR3S1-infected RBC were panned on some preparations of HUVEC; (ii) the binding of parasites of the FCR3S lineage to an unknown receptor on HUVEC comodulated with induced changes in rosetting; and (iii) the unprecedentedly high levels of these two phenotypes displayed by the newly cloned FCR3S1.2. Our data supported neither a common parasite ligand domain structure for rosetting and HUVEC adhesion (see below) nor identical receptor usage on RBC and HUVEC. We have recently found that while heparan sulfate or a heparan sulfate-like receptor on RBC mediates rosetting due to FCR3S and its offspring (12), these parasites adhered to HUVEC via a previously undescribed endothelial receptor for malaria parasites, PECAM-1/CD31 (39). CD31, with a broad distribution on hematopoietic and endothelial cells, is not expressed on human RBC.
Selection of rosetting parasites for cytoadherence on the CD36 receptor.
To investigate the relationship between CD36 adhesion and the linkage of adhesive phenotypes in rosetting parasites, we studied whether selection of parasites on cells expressing this receptor would reciprocally coselect for rosetting, as did binding to HUVEC. By panning FCR3S and FCR3S1 parasites on C32 melanoma cells, the lines FCR3S/b and FCR3S1/b were generated, both showing greatly increased adhesion (5- to 10-fold) to C32 and CD36-transfected CHO cells (Table 1). These two independently selected parasites were devoid of rosetting, autoagglutinating, or endothelial cell-binding capacity. By using the microagglutination assay, it was determined that FCR3S/b and FCR3S1/b were of distinct agglutination serotypes, different from the one common to the multiadhesive rosetting parasites of the FCR3S lineage and, in all likelihood, were two different antigenic variants (Table 2). The results underline the uniqueness of the rosetting-driven comodulation of adhesive phenotypes, including adherence to melanoma and CD36-transfected CHO cells. They also point to CD36 adhesion as a relatively conserved and constitutive property of the intraerythrocytic parasite, which may or may not occur in conjunction with other binding specificities and occur independently from changes in surface variant antigens. Numerous observations indicate that most parasites isolated in the field or laboratory adhere to CD36 (15, 24, 28), suggesting that this may be a characteristic of a majority of P. falciparum variant antigenic types. It should be noted that the parasites selected on melanoma cells displayed minor levels of adhesion to ICAM-1-transfected CHO cells (Table 1). This new binding specificity, not detected in the parent lines, could presumably have arisen by antigenic variation and residually coselected on melanoma cells expressing a restricted number of ICAM-1 receptors (17).
TABLE 2.
Agglutination of FCR3S lines and clones with serum from asymptomatic individuals with repeated exposures to P. falciparum
| Parasite | Agglutinationa for patient:
|
||||
|---|---|---|---|---|---|
| 011 | 039 | 080 | 118 | 209 | |
| FCR3S | 3+ | 3+ | 2+ | 2+ | − |
| FCR3S1 | 3+ | 1+ | 3+ | − | − |
| FCR3S1.2 | 2+ | − | 2+ | − | − |
| FCR3S1.6 | − | − | − | − | − |
| FCR3S/a | − | − | 2+ | 2+ | − |
| FCR3S/b | − | − | − | 2+ | − |
| FCR3S1/b | 1+ | − | − | − | − |
Scored 1+ to 5+ as described in Materials and Methods.
Modulation of binding phenotypes and antigen expression on the PRBC surface.
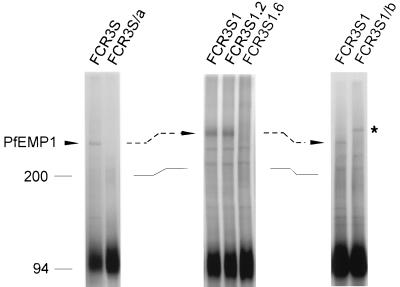
A radioiodination analysis of intact PRBC was used to monitor the expression of polypeptides on the infected RBC surface upon selection or cloning of FCR3S parasites with different adhesive phenotypes. Antigens with the characteristics of PfEMP1 were modulated together with the adhesion phenotype of the cell (Fig. 2). A trypsin-sensitive and Triton X-100-insoluble polypeptide with an Mr of 285,000 was readily 125I labeled on RBC infected with mature FCR3S parasites but not on ring-infected or uninfected RBC. The expression of the Mr 285,000 PfEMP1 was stable and enhanced on RBC infected with the cloned FCR3S1 parasites enriched for rosetting binding or the highly adhesive subclone FCR3S1.2. In contrast, the Mr 285,000 PfEMP1 was not detected either in FCR3S/a parasites, derived from FCR3S by selection for decreased rosetting, or in clones devoid of rosetting or other binding phenotypes, e.g., FCR3S1.6, or in parasite lines FCR3S/b and FCR3S1/b, which lost most of their binding phenotypes, including rosetting, but expressed new PfEMP1 antigens (Mr 320,000 to 350,000) after selection for adherence on melanoma cells (Fig. 2 and data not shown). In general, PfEMP1 polypeptides were not readily 125I labeled on nonbinding parasites. Additional labeled polypeptides with low molecular weights (Mr 31,000 to 38,000) were detected on RBC carrying adhesive parasites of the FCR3S lineage but generally not on nonbinding PRBC. Subsequent analysis indicated that these small polypeptides were not further associated with rosetting or linked adhesive phenotypes of FCR3S parasites (data not shown). Taken together, the results suggested the following. (i) The Mr 285,000 PfEMP1 was likely to have a functional relationship with rosetting and/or linked adhesive phenotypes of the FCR3S lineage. (ii) Selected or cloned nonbinding parasites did not express PfEMP1 or did so at low copy number. This is in keeping with the microagglutination data, showing a narrower antigenic display on FCR3S/a and FCR3S1.6 PRBC (Table 2). (iii) The appearance of phenotypic variants, e.g., exclusive CD36 binders, and new forms of PfEMP1, occurred simultaneously.
FIG. 2.
Surface radioiodination analysis of RBC infected with FCR3S lines and clones. Parasite cultures were radiolabeled with Na125I, fractionated on Percoll gradients, detergent solubilized, and separated by SDS-PAGE in 5.0 to 8.5% acrylamide gradient gels as described in Materials and Methods. Labeled polypeptides were detected by phosphorimaging. The Mr 285,000 PfEMP1 of FCR3S, FCR3S1, and FCR3S1.2 is indicated by the arrows. The variant PfEMP1 of FCR3S1/b is marked by the asterisk. RBC α/β spectrin (bands between the 200-kDa molecular mass marker and PfEMP1) and two or three polypeptides with Mrs of 120,000 to 150,000 were faintly labelled in some experiments. Molecular sizes are indicated in kilodaltons.
Considerable evidence demonstrates that PfEMP1 is a variant antigen that mediates the adhesion of PRBC to host receptors (4, 35). The expression of variant-specific PfEMP1 proteins or its gene, var, correlates with the capacity of the PRBC to bind to various cell adhesion receptors, including CD36 and ICAM-1 (7, 21, 34). Fragments of PfEMP1 can bind in vitro to soluble CD36, TSP, or ICAM-1 (5). PfEMP1 has been identified as the parasite ligand in a rosetting clone (31). In our laboratory, a var gene, FCR3S1.2 var 1, has been cloned which codes for a PfEMP1 polypeptide with an estimated molecular mass of 260 kDa. This PfEMP1 is exported and expressed on the outer membrane of PRBC infected with mature stages of the multiadhesive clone FCR3S1.2, and it mediates rosetting through binding to heparan sulfate or a heparan sulfate-like receptor (12).
The data presented here, showing a comodulation of adhesive phenotypes linked to rosetting and the expression of PfEMP1 variants on the PRBC surface in the FCR3S lineage, raised the following question: is the Mr 285,000 PfEMP1 the parasite ligand responsible for all the binding specificities of the multiadhesive FCR3S parasites?
Trypsin sensitivity of PfEMP1 and binding phenotypes.
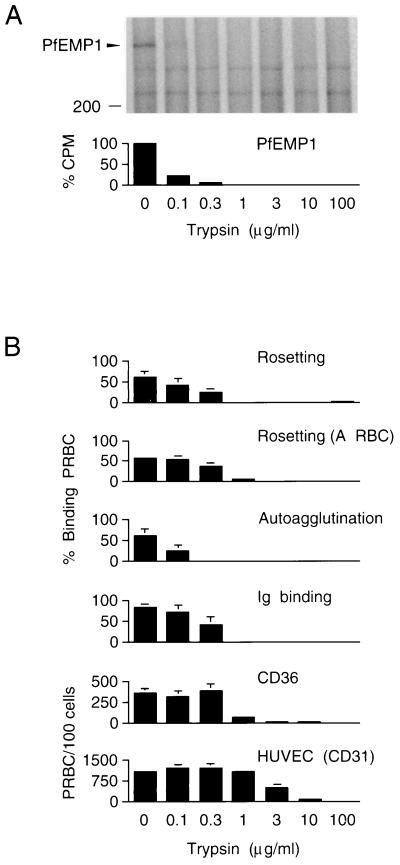
To investigate the role of PfEMP1 in rosetting-linked multibinding, we compared the effect of proteolytic treatment on the Mr 285,000 polypeptide and on each one of the adhesive phenotypes of FCR3S1 parasites (Fig. 3). When intact 125I-labeled PRBC were digested with serial dilutions of trypsin, the Mr 285,000 PfEMP1 band was no longer evident at 1 μg of the protease per ml (Fig. 3A). The sensitivity of the Mr 285,000 band correlated with a loss in the capacity of the digested PRBC to form rosettes, to spontaneously autoagglutinate, to bind Igs, and, to a large extent, to adhere to the CD36 receptor (Fig. 3B). Cytoadherence to HUVEC was, in contrast, a relatively less trypsin-sensitive phenotype of FCR3S1 than were the other binding features of this parasite. Similar results were obtained in experiments where chymotrypsin was used for digestion of PRBC (data not shown).
FIG. 3.
Trypsin sensitivity of PfEMP1 and binding phenotypes. (A) 125I-labeled PRBC containing FCR3S1 parasites at the trophozoite stage were enzyme digested for 10 min at 37°C, and the Triton X-100-insoluble fraction was analyzed by SDS-PAGE in 5.0 to 8.5% acrylamide gradient gels. The radioactivity associated with trypsin-sensitive polypeptides comodulating with adhesion was normalized to values in trypsin-resistant bands and assessed by phosphorimaging. (B) Treated cells were washed in PBS, resuspended in culture medium supplemented with 10% AB+ serum, and either mixed with uninfected RBC of blood group O at a final hematocrit of 5% (except in experiments performed with group A RBC), and allowed to re-form rosettes and autoagglutinates for 45 min or incubated for 3 h on ice to bind serum Igs or assayed for cytoadherence on monolayers of CHO-CD36 transfectants or HUVEC as described in Materials and Methods. Assessment of autoagglutinate re-formation was carried out with clone FCR3S1.2. Results represent the mean ± standard deviation for three experiments.
The results provide evidence that the variant antigen, PfEMP1, mediates the various adhesive features linked to rosetting in the FCR3S lineage and are consistent with a multivalent molecule carrying binding sites of distinct specificity, as well as variant antigenic epitopes. Binding to the rosetting receptors on O and A RBCs and the related autoagglutinating phenotype displayed the highest sensitivity to trypsinization, in line with previous data, mapping the rosetting ligand to the distal first Duffy binding-like domain (DBL-1) (12, 31), with the highest concentration of potential trypsin cleavage sites in the FCR3S1.2 PfEMP1 (data not shown) and, presumably, the most exposed part of the molecule. Interestingly, binding of serum Igs to the PRBC surface was at least as sensitive to trypsin as rosetting was. The adhesion by trophozoite-infected erythrocytes of Igs in sera from individuals never exposed to malaria is a trait expressed by most but not all rosetting parasites as well as by some nonrosetting strains and isolates (33). By electron microscopy, it has been shown that bound Ig localizes to electron-dense complexes on the surface of K+ or K− PRBC in the same way as PfEMP1 does (4, 32). Deposition of Igs on PRBC of some rosetting strains and, in particular, IgM binding by PRBC of rosetting FCR3S lines and clones has a positive effect on the rosette formation capacity of the parasite (32). Considering the data together, we propose the existence of an Ig binding domain(s) in the PfEMP1 molecule. This putative semiconserved region would typically be located in the relative vicinity of the rosetting ligand domain and would be subjected to primary structure variability. However, although band 3 was not altered by trypsinization (not shown), a role in Ig binding for a parasite-modified, perhaps senescent form of band 3, or another undefined molecule, remains a possibility.
Cytoadherence to the CD36 receptor is presumably the most highly conserved adhesive phenotype of intraerythrocytic P. falciparum. This could make an appealing case for a conserved ligand (13), rather than a variant molecule. Our data, in agreement with the work by others (5, 16), suggest that a binding site(s) in PfEMP1 mediates most, if not all, of the binding to this receptor. Adhesion to the CD31 receptor on HUVEC was, on the other hand, distinctly affected by limited trypsinization of PRBC. At present we cannot conclude whether a CD31 binding ligand, located proximally between the rosetting domain and the PRBC membrane, exists in PfEMP1 or whether another molecule, e.g., the Mr 35,000 rosettin, accounts for cytoadherence to HUVEC in FCR3S and its progeny. The first alternative presupposes that the incorporation of label during radioiodination occurs N-terminal relative to the putative CD31 ligand domain and that a trypsin cleavage site of reduced sensitivity exists C-terminal to the proposed ligand. We favor this interpretation for two reasons. First, a majority of the tyrosine residues present in the FCR3S1.2 var/PfEMP1 are clustered in the N-terminal portion of the molecule (12). Second, analysis of the deduced amino acid sequence of this gene predicts a second DBL domain mapping to the suggested location for the CD31 ligand.
Our data show that through cloning of FCR3S, followed by selection for the rosetting phenotype and subcloning of rosetting PRBC, a singular parasite has been isolated, displaying multiple binding phenotypes at unusually high rates, including rosetting. We argue that this rosetting-linked P. falciparum pan-adhesion is an exceptional event rather than a general phenomenon, originated by the appearance of a member of the var/PfEMP1 family carrying a particular set of ligand specificities. Whether additional parasite factors can enhance adhesion, by a direct involvement or through modifications of the RBC surface environment, is unknown.
In clinical studies, the virulence of P. falciparum correlates with a propensity of parasitized cells to bind uninfected erythrocytes. It has been a matter of conjecture whether rosetting represents a surrogate marker for another adhesive interaction or itself contributes to the pathologic changes by exacerbating microvascular obstruction. Although the data presented here does not support either alternative, it demonstrates the occurrence of a cluster of adhesive phenotypes in rosetting parasites that express a unique PfEMP1 variant carrying an array of binding sites for host molecules. A parasite endowing its host cell with concurrent sticky features such as binding to the postcapillary endothelium and tight aggregation with infected and uninfected RBCs is likely to increase the chances of being retained and accumulated in the cerebral microvasculature. The results encourage the further study of pan-adhesion as a potentially important parasite phenotype in the pathogenesis of the severe form of malaria associated with the ability of P. falciparum to form rosettes.
ACKNOWLEDGMENTS
We thank A. Barragan for the gift of sera, R. J. Howard for CHO transfectants, and J. Carlson and P. Perlmann for helpful comments on the manuscript.
These studies were supported by grants from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR), the Swedish Medical Research Council, and the Swedish International Development Authority (SIDA). V.F. was supported in part by the Swedish Society for Medical Research, and C.J.T. was supported in part by the Clas Groschinsky and the Sigurd and Elsa Golje Foundations.
REFERENCES
- 1.Aguiar J C, Albrecht G R, Cegielski P, Greenwood B M, Jensen J B, Lallinger G, Martinez A, McGregor I A, Minjas J N, Neequaye J, et al. Agglutination of Plasmodium falciparum-infected erythrocytes from east and west African isolates by human sera from distant geographic regions. Am J Trop Med Hyg. 1992;47:621–632. doi: 10.4269/ajtmh.1992.47.621. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988;39:3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- 3.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Barragan, A. Unpublished data.
- 3b.Barragan, A. Submitted for publication.
- 4.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 5.Baruch D I, Gormley J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 7.Biggs B-A, Anders R F, Dillon H E, Davern K M, Martin M, Petersen C, Brown G V. Adherence of infected erythrocytes to venular endothelium selects for antigenic variants of Plasmodium falciparum. J Immunol. 1992;149:2047–2054. [PubMed] [Google Scholar]
- 8.Carlson J, Helmby H, Hill A V S, Brewster D, Greenwood B M, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 9.Carlson J, Holmquist G, Taylor D W, Perlmann P, Wahlgren M. Antibodies to a histidine-rich protein (PfHRP1) disrupt spontaneously formed Plasmodium falciparum erythrocyte rosettes. Proc Natl Acad Sci USA. 1990;87:2511–2515. doi: 10.1073/pnas.87.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson J, Wahlgren M. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaiyaroj S C, Coppel R L, Novakovic S, Brown G V. Multiple ligands for cytoadherence can be present simultaneously on the surface of Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci USA. 1994;91:10805–10808. doi: 10.1073/pnas.91.23.10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Barragan A, Fernandez V, Sundström A, Schlichtherle M, Sahlen A, Carlson J, Datta S, Wahlgren M. Identification of PfEMP1 as the rosetting ligand of the malaria parasite Plasmodium falciparum. J Exp Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crandall I, Collins W E, Gysin J, Sherman I W. Synthetic peptides based on motifs present in human band 3 protein inhibit cytoadherence/sequestration of the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1993;90:4703–4707. doi: 10.1073/pnas.90.10.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fandeur T, Bonnefoy S, Mercereau-Puijalon O. In vivo and in vitro derived Palo Alto lines of Plasmodium falciparum are genetically unrelated. Mol Biochem Parasitol. 1991;47:167–178. doi: 10.1016/0166-6851(91)90176-7. [DOI] [PubMed] [Google Scholar]
- 15.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 16.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasler T, Albrecht G R, van Schravendijk M R, Aguiar J C, Morehead K E, Pasloske B L, Ma C, Barnwell J W, Greenwood B M, Howard R J. An improved microassay for Plasmodium falciparum cytoadherence using stable transformants of Chinese hamster ovary cells expressing CD36 or intercellular adhesion molecule-1. Am J Trop Med Hyg. 1993;48:332–347. doi: 10.4269/ajtmh.1993.48.332. [DOI] [PubMed] [Google Scholar]
- 18.Howard R J. Malarial proteins at the membrane of Plasmodium falciparum-infected erythrocytes and their involvement in cytoadherence to endothelial cells. Prog Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 21.Magowan C, Wollish W, Anderson L, Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988;168:1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller L H. Distribution of mature trophozoites and schizonts of Plasmodium falciparum in the organs of Aotus trivirgatus, the night monkey. Am J Trop Med Hyg. 1969;18:860–865. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- 23.Miller L H, Good M F, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 24.Ockenhouse C F, Ho M, Tandon N N, Van Seventer G A, Shaw S, White N J, Jamieson G A, Chulay J D, Webster H K. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis. 1991;164:163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- 25.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert C, Pouvelle B, Meyer P, Muanza K, Fujioka H, Aikawa M, Scherf A, Gysin J. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-erythrocyte adherence on brain microvascular endothelial cells. Res Immunol. 1995;146:383–393. doi: 10.1016/0923-2494(96)81042-x. [DOI] [PubMed] [Google Scholar]
- 27.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 28.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G V. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe A, Obeiro J, Newbold C I, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe A, Moulds J M, Newbold C I, Miller L H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 32.Scholander C, Treutiger C J, Hultenby K, Wahlgren M. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat Med. 1996;2:204–208. doi: 10.1038/nm0296-204. [DOI] [PubMed] [Google Scholar]
- 33.Scholander C, Carlson J, Kremsner P G, Wahlgren M. Extensive immunoglobulin binding of Plasmodium falciparum-infected erythrocytes in a group of children with moderate anemia. Infect Immun. 1998;66:361–363. doi: 10.1128/iai.66.1.361-363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su X, Heatwole V M, Werttheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 36.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 37.Trager W, Rudzinska M A, Bradbury P C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull W H O. 1966;35:883–885. [PMC free article] [PubMed] [Google Scholar]
- 38.Treutiger C J, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood B M, Wahlgren M. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992;46:503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- 39.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 40.Udomsangpetch R, Wahlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Udomsangpetch R, Webster H K, Pattanapanyasat K, Pitchayangkul S, Thaithong S. Cytoadherence characteristics of rosette-forming Plasmodium falciparum. Infect Immun. 1992;60:4483–4490. doi: 10.1128/iai.60.11.4483-4490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]