Abstract
Background
Millions of sepsis survivors annually face neuropsychiatric sequelae of their illness. Corticosteroids are frequently administered for sepsis, and their use improves neuropsychiatric outcomes, but the mechanisms are unknown. In light of prior work that has shown persistent inflammation in sepsis survivors, we hypothesized that short-term corticosteroid treatment during illness would reverse the long-term impact of sepsis on inflammatory gene expression in the hippocampus and rescue associated changes to affective behaviors.
Methods
Male and female mice underwent cecal ligation and puncture or a sham surgery to induce acute infection and were treated for 5 days with corticosterone or vehicle. Starting 2 weeks after the surgery, we performed functional phenotyping in the survivor mice followed by hippocampal RNA sequencing to identify underlying mechanisms.
Results
Long-term cecal ligation and puncture survivors exhibited anxiety-like behavior, increased central hypothalamic-pituitary-adrenal axis activity, and persistent systemic and neuroinflammation. Corticosterone treatment during illness did not reverse anxiety-like behavior or inflammation in survivors. Instead, corticosterone treatment impaired object memory and increased active coping behavior in females. History of corticosterone treatment influenced the expression of >10% of detectable transcripts in the dorsal and ventral hippocampus, including a coordinated downregulation of activity-dependent genes.
Conclusions
Corticosterone treatment during sepsis impaired memory formation in survivors and caused a lasting decrease in hippocampal neural activity, which could underlie its effect on memory. Future studies should focus on how this lasting effect of corticosteroid treatment on hippocampal activity and memory translates into improved neuropsychiatric outcomes in human sepsis survivors.
Keywords: Anxiety, Glucocorticoids, Illness, PTSD, Sepsis, Stress
Sepsis, a life-threatening illness due to infection, affects >15 million people each year, and approximately 10 million survive long term (1). Long-term (>30 days) survivors face high rates of persistent sequelae, most notably impairments in neuropsychiatric functioning that persist for years. Indeed, more than one-third of sepsis survivors experience cognitive decline and/or mental health problems such as anxiety, depression, and/or posttraumatic stress disorder (PTSD) (1).
Corticosteroids are commonly administered in sepsis for shock, pneumonia, and acute respiratory distress syndrome (2,3), where they decrease illness severity and in some cases mortality. Observational and randomized trials have shown that corticosteroid treatment in critically ill patients with sepsis influenced neuropsychiatric functioning in survivors, specifically decreasing the risk for PTSD (4, 5, 6, 7, 8). The mechanisms by which corticosteroid treatment during sepsis alters long-term brain function to decrease PTSD risk are unknown. Because this is the only treatment that has consistently been shown to modify neuropsychiatric risk in sepsis survivors, identifying the mechanisms is crucial for the design of better treatments for millions of affected patients.
Prior work has shown persistent inflammation in the brain of murine sepsis survivors that is correlated with prolonged anxiety-like behavior (9, 10, 11). Corticosteroids are potent immunosuppressives, suggesting that they could decrease neuroinflammation during sepsis. In addition, corticosteroid treatment immediately following psychogenic stressors has been shown to prevent delayed anxiety-like behavior (12, 13, 14, 15, 16). We hypothesized that corticosteroid treatment during sepsis would prevent delayed anxiety-like behavior in mice, at least in part via its immunosuppressive properties.
To test this hypothesis, we used the common mouse model of sepsis, cecal ligation and puncture (CLP); treated male and female mice with corticosteroids or vehicle during illness; and performed behavioral phenotyping starting 2 weeks later. We performed RNA sequencing on the hippocampus because the hippocampus is central to the neurobiology of PTSD, vulnerable to injury during infection and/or inflammation, and highly sensitive to corticosteroids (17, 18, 19, 20). We used correlated network analysis to relate biologically meaningful gene expression programs to functional outcomes.
Methods and Materials
Animals
Ten to 12-week-old C57BL/6 male and female mice (40/sex) were obtained from the Jackson Laboratory. Animals were group housed on a 14:10 light/dark cycle with free access to food and water. The experimental timeline is shown in Figure 1A.
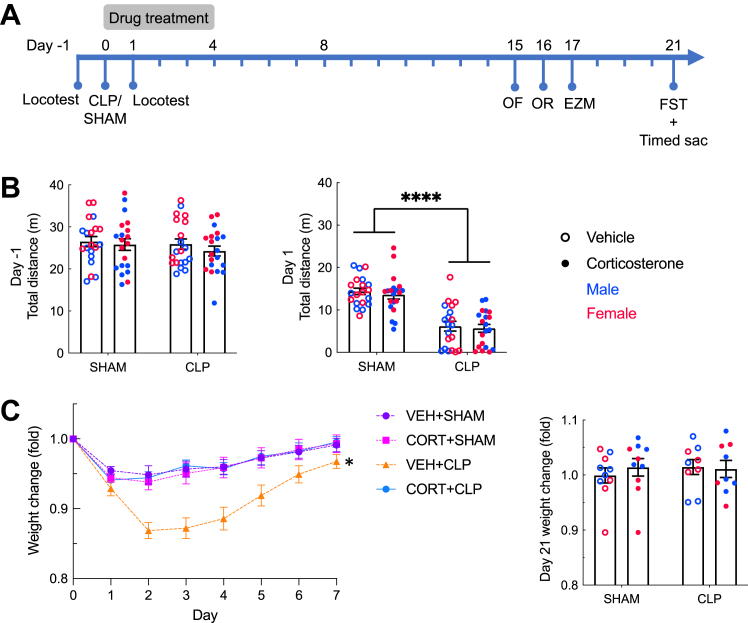
Figure 1.
Sickness and recovery after CLP with and without corticosteroid treatment. (A) Experimental timeline. (B) (Left panel) Baseline locomotion. (Right panel) One day after surgery, CLP mice were less active than sham mice (n = 40 mice/group). (C) Corticosterone treatment prevented weight loss in CLP animals in the week immediately after surgery; CLP animals that were treated with vehicle lost more weight than the 4 other groups (VEH+sham: n = 20; CORT+sham: n = 17; VEH+CLP: n = 12; CORT+CLP: n = 15). On day 21, 3 weeks after CLP, there was no significant difference in weight change from baseline between groups (sham: n = 20, CLP: n = 18). Error bars show standard error of the mean. ∗p < .05 relative to all other groups based on post hoc tests, ∗∗∗∗p < .0001. CLP, cecal ligation and puncture; CORT, corticosterone; EZM, elevated zero maze; FST, forced swim test; OF, open field; OR, object recognition; VEH, vehicle.
Surgery and Drug Treatment
CLP and sham surgeries were performed as before (9,11). Animals were anesthetized with ketamine (80–120 mg/kg) and xylazine (5–10 mg/kg) and injected with 60 μL of 0.25% bupivacaine at the incision site. Under aseptic conditions, a 5-mm incision was made through the abdominal wall. The cecum was ligated 5 mm from the end with a silk suture and punctured once through-and-through with a 19-gauge needle. Sham animals underwent laparotomy, and the cecum was removed from the abdomen and replaced without puncture. The incision was closed with surgical clips removed after 8 days. After surgery, mice immediately received subcutaneous injections of 1 mL saline and 0.1 mL imipenem-cilastatin. This CLP protocol was designed to treat the mice so that most (approximately 80%) of them survive to be studied 2 to 3 weeks later. Mice received daily subcutaneous injections of 16 mg/kg corticosterone or vehicle (5% dimethyl sulfoxide in saline) for 5 days starting immediately after surgery (days 0–4).
Behavior
To measure sickness behavior, 5-minute locomotor activity was measured on day −1 and day 1 in VersaMax chambers (Omnitech Electronics). All other behavioral tests were recorded and tracked using Ethovision software (version 15). Anxiety-like behavior was assessed by measuring exploration in a novel open field (72 × 72 × 26 cm) and in an elevated zero maze (5 minutes per test at 200 lux). Novel object recognition training and testing were conducted in the open field arena under low light (30 lux), with the perimeter of the object defined as the object zone for tracking. During the 5-minute training trial, mice explored 2 identical objects for 5 minutes. During the 5-minute testing trial 90 minutes later, one of the original objects was replaced by a novel object. The forced swim test was conducted over 6 minutes in large plexiglass cylinders filled with 22 °C water.
Euthanasia and Tissue Collection
On day 21, one half of the mice underwent forced swimming followed by rapid decapitation and trunk blood collection for hormone measurements. The remaining half were euthanized by rapid decapitation, and trunk blood was collected. The dorsal and ventral hippocampus were dissected from the latter group, frozen on dry ice, and stored at −80 °C until future use. Their spleens were removed and prepared for flow cytometry.
Spleen Flow Cytometry
Splenocytes were isolated by mincing and mechanical dissociation of tissue through a 40-mm cell strainer. Cells were isolated into phosphate-buffered saline with 1% bovine serum albumin, 2 mM EDTA, and 25 mM HEPES. Red cells were removed by ammonium-chloride-potassium lysis. Cells were then stained with either a myeloid marker panel (CD45-BV421, CD11b-BV605, Ly6C-FITC, Ly6G-PECy7; BioLegend) or lymphoid marker panel (CD45-BV421, CD11b-BV605, B220-PE, CD3e-FITC; BioLegend). Cells were analyzed on an Attune cytometer and reported as the percentage of CD45 cells.
Hormone Assays
Blood samples were centrifuged immediately after collection at 1500g for 10 minutes. The supernatant was removed and stored at −20 °C. Corticosterone was measured using the Corticosterone DetectX Enzyme Immunoassay Kit (Arbor Assays), and ACTH (adrenocorticotropic hormone) was measured with the ACTH (rat, mouse) Extraction-Free Enzyme Immunoassay Kit (Phoenix Pharmaceuticals).
RNA Sequencing
Messenger RNA was isolated from dorsal and ventral hippocampi using the Qiagen RNAeasy kit in 5 batches, with groups counterbalanced across batches. RNA was assessed for quality using the TapeStation (Agilent). Samples with RNA integrity numbers of ≥8 were prepared using the KAPA messenger RNA HyperPrep Kit (KAPA/Roche #KK8581) according to the protocol using 400 ng input and 11 cycles of polymerase chain reaction. Final libraries were checked for quality and quantity by TapeStation (Agilent) and quantitative polymerase chain reaction using KAPA’s Library Quantification Kit for Illumina Sequencing platforms (catalog #KK4835; KAPA Biosystems). The samples were pooled and sequenced on 50% of an Illumina NovaSeq S4 Paired-end 50 bp according to the manufacturer’s recommended protocols.
Statistical Analysis
Weight change after surgery was analyzed with three-way repeated-measures analysis of variance (ANOVA) comparing CLP, corticosterone treatment, and time. Day 21 weight change was analyzed with a three-way ANOVA comparing CLP, treatment, and sex. The effect of treatment on survival after CLP was assessed with a two-sided Fisher’s exact test.
The effects of CLP, treatment, and sex on behavior and spleen flow cytometric data were compared using three-way ANOVA. For the forced swim test, by convention, only the final 4 minutes were used in the analysis. For the novel object recognition test, the fraction of total exploration time that was spent with the novel object was calculated (novel object fraction). The 4 treatment groups were first analyzed individually with one-sample t tests to determine whether exploration of the novel object was different from the chance value indicating object memory. Then the novel object fraction was compared between groups using three-way ANOVA. These analyses were performed using GraphPad Prism 9, with p < .05 considered significant.
For RNA sequencing data, quality control was performed, and reads with a score of <15 were discarded (21,22). The reads were aligned to the GRCm38 genome using the STAR aligner with Ensembl annotation and quantified to gene level using featureCounts (23, 24, 25). These counts were analyzed using the R/Bioconductor framework (26). Subsequent quality control was performed using principal component analysis to identify outlier samples. During this step, it was discovered that most of the samples from one of the 5 batches were outliers, meaning that they did not cluster with the other samples by brain region (dorsal or ventral) and sex. This raised the concern that samples in this batch were mislabeled or contaminated, and so the entire batch was excluded. The analysis proceeded using the 55 samples that passed all quality control measures. Differential gene expression analysis was conducted using the limma-voom package (27). Correlation was controlled for given that we had both ventral and dorsal hippocampus samples from the same animal. Genes with a false discovery rate cutoff of .05 were considered significant (28). A gene set enrichment analysis was conducted using the biological processes pathways from the Gene Ontology resource (28, 29, 30, 31).
Clusters of genes with correlated expression and their relationship to behavior were identified using weighted gene correlation network analysis, an R package for weighted correlation network analysis (32). A Pearson correlation coefficient was used to report the association between significance of the module-level gene expression and animal behavior. The z score test for 2 proportions was used to measure enrichment of differentially expressed genes in selected modules compared with the full dataset.
For cell-type analysis, the normalized expression values were used as input to the R package BrainInABlender (33). This gave us z scores for the expression of a particular cell type in a sample relative to the other samples. In addition, t tests were used to compare the proportion of neurons between groups.
Study Approval
All experimental protocols were approved by the University of Michigan Institutional Animal Care and Use Committee.
Results
Acute Illness and Recovery
We performed CLP or sham surgery and treated mice with daily corticosterone or vehicle for 5 days during the acute illness. CLP induces systemic inflammation and illness due to endogenous intestinal flora, with an acute immune response that subsides after 48 hours using our protocol (34,35). We found that mice began to show behavioral recovery within 5 days of CLP, with complete recovery of body weight and locomotion within 14 days (11).
In humans, systemic corticosteroid treatment decreases illness severity with a modest mortality benefit in sepsis (36). We used repeated locomotion testing and weight to assess the impact of CLP and corticosterone treatment on sickness in these mice (Figure 1). Prior to surgery, there was no difference in locomotor activity between the mice destined for sham or CLP (Figure 1B) (F1,72 = 0.793, p = .376). CLP significantly decreased locomotor activity as expected: on day 1, animals that had undergone CLP traveled less than sham animals (Figure 1B) (F1,72 = 71.05, p < .0001), with no effect of corticosterone (F1,72 = 0.449, p = .505) or interaction between CLP and corticosterone (F1,72 = 0.018, p = .895) and no sex differences.
Weight during the week following surgery was affected by all 3 variables: CLP, time, and treatment (Figure 1C). Both CLP and sham animals lost weight after surgery (main effect of time: F7,420 = 53.99, p < .0001) with the nadir on day 2, but CLP animals lost more weight than sham animals (main effect of surgery: F1,60 = 6.895, p = .011). Corticosterone treatment prevented weight loss after CLP such that mice that underwent CLP and corticosterone treatment were indistinguishable from sham animals (main effect of corticosterone: F1,60 = 6.335, p = .015; interaction between corticosterone and surgery: F1,60 = 8.030, p = .006). Animals that underwent CLP and were treated with vehicle lost significantly more weight than the 3 other groups (F1,60 = 8.030, p = .0063). By day 21, all animals had returned to their starting weight (Figure 1C).
There were no deaths in the sham group. In the CLP group, fewer corticosterone- than vehicle-treated mice died (90% vs. 70% survival, respectively), but this difference was not statistically significant (p = .235).
In summary, a weight-based dose of corticosteroid similar to that used in human sepsis caused a decrease in illness severity (prevention of weight loss) and a modest (not statistically significant) mortality benefit similar to that which is seen in human sepsis (3).
Affective Behavior
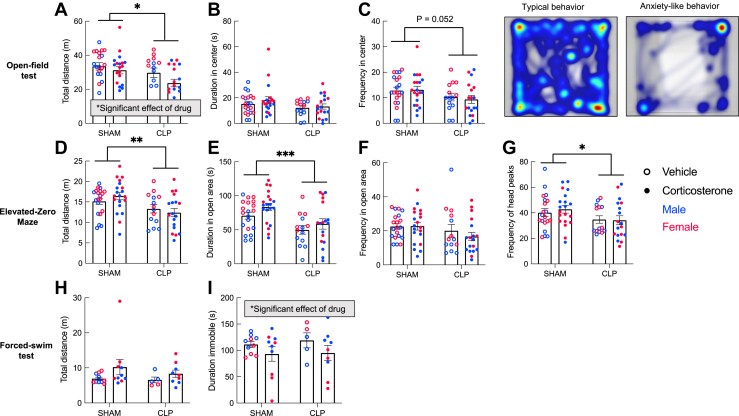
Beginning 15 days after surgery and 9 days after the completion of corticosterone or vehicle treatment, mice were tested in hippocampal-dependent tasks of affective behavior (Figure 2). In the open field, CLP survivors traveled less (Figure 2A) (F1,64 = 5.317, p = .024), with a trend toward fewer center entries (Figure 2C) (F1,64 = 3.932, p = .052). Corticosterone also decreased total distance traveled in the open field (F1,64 = 4.352, p = .041), but had no effect of corticosterone on center exploration, and corticosterone treatment did not modify the effect of CLP on anxiety-like behavior. In the elevated zero maze, CLP survivors traveled less (Figure 2D) (F1,64 = 9.473, p = .003), spent less time in the open areas (Figure 2E) (F1,64 = 14.48, p = .0003), and had fewer head peaks into the open area (Figure 2G) (F1,64 = 4.885, p = .031). Corticosterone treatment did not affect any measure in the elevated zero maze. There was no interaction between sex and either surgery or corticosterone treatment.
Figure 2.
Affective behavior after CLP with and without corticosterone treatment. In the open field test (A–C), CLP survivors explored the entire arena less (A), spent similar amounts of time in the center (B), and exhibited a trend toward fewer entries into the center (C) (p = .052); corticosterone treatment did not prevent these anxiety-like behaviors. Heat maps show the activity of representative typical and anxiety-like behavior in the open field. In the elevated zero maze test (D–G), CLP survivors exhibited anxiety-like behavior, including decreased total exploration (D), time spent in the open area (E), and frequency of head peaks into the open area (G) without affecting the frequency in the open area (F). Again, corticosterone treatment did not prevent the effect of CLP in the elevated zero maze test (sham: n = 40, CLP: n = 32 for open field and elevated zero maze tests). In the forced swim test (H), CLP had no effect on swimming distance or time spent immobile. There was an independent effect of corticosterone treatment to decrease the time spent immobile (I) (sham: n = 20, CLP: n = 14 for forced swim test). Error bars show standard error of the mean. Statistical analysis by three-way analysis of variance for (A–I). ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. CLP, cecal ligation and puncture.
In the forced swim test, there was no effect of CLP or corticosterone treatment on swimming distance (Figure 2H). Corticosterone treatment decreased the amount of time spent immobile (Figure 2I) (F1,26 = 5.766, p = .024), and there was an interaction between corticosterone treatment and sex (F1,26 = 9.608, p = .005). Two-way ANOVAs conducted separately for male and female mice revealed that corticosterone treatment decreased the time spent immobile in female but not male mice (F1,12 = 9.179, p = .011). This apparent antidepressant-like effect of prior corticosterone treatment in female mice was independent of CLP.
In summary, male and female CLP survivors showed anxiety-like behavior which was not rescued by corticosterone during illness. Corticosterone had a CLP-independent antidepressant-like effect in the forced swim test only in females.
Object Memory
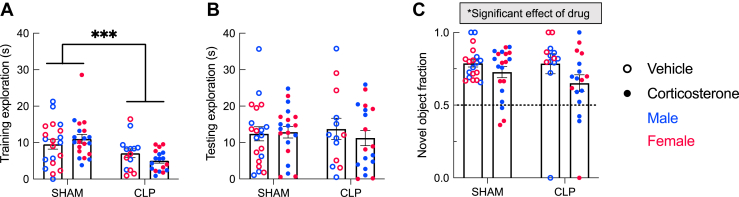
A 1-day object recognition test was used to assess formation of new object memory in CLP survivors (Figure 3). CLP survivors spent less time exploring the objects during the training session (Figure 3A) (F1,64 = 12.89, p = .0006) but not during the testing session 90 minutes later (Figure 3B). Corticosterone did not affect object exploration. During the testing trial, all 4 groups demonstrated a preference for the novel object (sham+vehicle: t18 = 11.68, p < .0001; sham+corticosterone: t19 = 5.979, p < .0001; CLP+vehicle: t12 = 4.135, p = .002; CLP+corticosterone: t16 = 2.649, p = .0175). Corticosterone-treated mice showed lower mean novel object fraction than vehicle-treated mice. Three-way ANOVA showed a main effect of corticosterone on decrease in novel object fraction, suggesting that corticosterone treatment during illness significantly impaired the ability to form a new object memory after recovery (F1,65 = 4.3, p = .042). Thus, corticosterone treatment for 5 days after CLP or sham surgery caused a lasting impairment in new object memory formation 2 weeks later.
Figure 3.
Object memory after CLP with and without corticosterone treatment. In an object recognition test in which both training and testing sessions occurred after recovery, CLP decreased total object exploration during training (A) but not testing (B). (C) Novel object fraction represents the proportion of total exploration time spent with the novel object. All groups demonstrated object memory based on one-sample t tests. There was a significant effect of corticosterone to decrease the novel object fraction toward the chance value, suggesting that corticosterone treatment impaired later object memory formation independent of CLP (sham: n = 40, CLP: n = 32). Statistical analysis by three-way analysis of variance for (A) and (B). Statistical analysis by both one-sample t tests and three-way analyses of variance for (C). ∗p < .05, ∗∗∗p < .001. Error bars show standard error of the mean. CLP, cecal ligation and puncture.
Hypothalamic-Pituitary-Adrenal Axis Activity
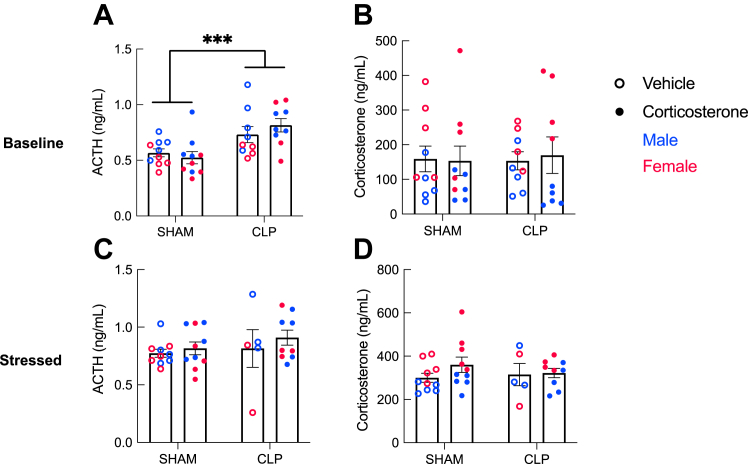
Corticosterone treatment is expected to temporarily suppress hypothalamic-pituitary-adrenal (HPA) axis activity. To assess the lasting effect of CLP and corticosterone treatment on HPA axis activity and reactivity to stress, blood corticosterone and ACTH were measured at baseline and after the forced swim stress 21 days after CLP or sham (Figure 4). CLP survivors had higher baseline ACTH (Figure 4A) (F1,30 = 18.38, p = .0002), but there was no difference in corticosterone (Figure 4B) (F1,30 = 0.292, p = .593), and there was no effect of prior corticosterone treatment on either hormone (ACTH: F1,30 = 0.278, p = .602; corticosterone: F1,30 = 0.133, p = .718). There was no effect of CLP or corticosterone on stress-induced ACTH or corticosterone after forced swimming (Figure 4C, D). While there was a statistical interaction between CLP and sex on corticosterone (F1,26 = 4.460, p = .045), post hoc two-way ANOVAs conducted separately by sex did not reveal any significant findings, likely due to the small number of animals per group. Thus, CLP survivors showed some evidence of increased central HPA axis activity with elevated baseline ACTH, but corticosterone treatment had no lasting effect on HPA axis function.
Figure 4.
Hypothalamic-pituitary-adrenal axis activity. Three weeks after surgery, CLP survivor mice demonstrated elevated baseline ACTH (A) but no change in baseline corticosterone (B) (sham: n = 20, CLP: n = 18). CLP did not affect stress-induced ACTH (C) or corticosterone (D). A history of corticosterone treatment had no effect on basal or stress-induced ACTH or corticosterone. Error bars show standard error of the mean. Statistical analysis by three-way analysis of variance for (A–D). ∗∗∗p < .001. ACTH, adrenocorticotropic hormone; CLP, cecal ligation and puncture.
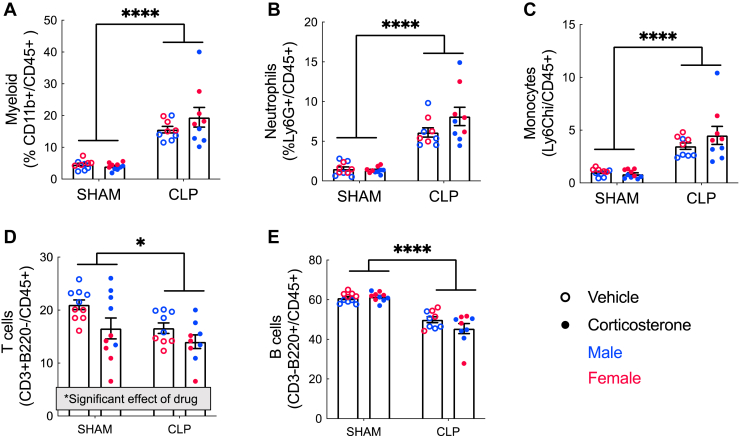
Systemic Inflammation
Corticosteroids are potent immunosuppressives, but whether their use in sepsis has a lasting effect on immune function is not known. To measure ongoing systemic inflammation 21 days after CLP/sham, we performed flow cytometry on dissociated splenocyte populations (Figure 5). CLP survivor mice showed evidence of ongoing systemic myeloid inflammation, with an increased proportion of myeloid cells (F1,34 = 70.73, p < .0001), neutrophils (F1,34 = 82.01, p < .0001), and monocytes (F1,34 = 49.19, p < .0001) and a decreased proportion of B cells (F1,34 = 81.82, p < .0001) and T cells (F1,34 = 6.3, p = .02). Corticosterone decreased the T cell population independently of CLP (F1,34 = 6.5, p = .02), but had no effect on the other cell populations.
Figure 5.
Splenic cell counts. CLP survivor mice showed evidence of ongoing systemic inflammation, with an increased proportion of myeloid cells (A), neutrophils (B), and monocytes (C) and a decreased proportion of B and T cells (D–E). Corticosterone also decreased the T cell population independently of CLP (D) but had no effect on the other cell populations. n = 10 for sham+vehicle and sham+corticosterone, 9 for CLP+vehicle and CLP+corticosterone. Error bars show standard error of the mean. ∗p < .05, ∗∗∗∗p < .0001. CLP, cecal ligation and puncture.
RNA Sequencing
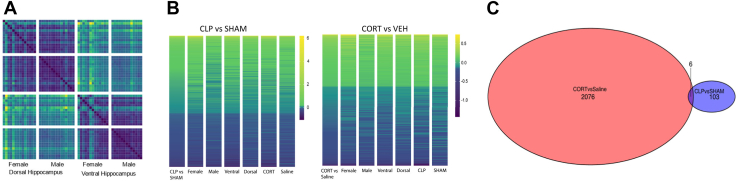
To understand the hippocampal gene expression programs underlying the effects of illness and corticosterone treatment on behavior, we performed RNA sequencing from the dorsal and ventral hippocampus of male and female mice 21 days after CLP/sham (Figure 6) (GSE225868). The dorsal and ventral hippocampus have different functions related to cognition (dorsal) and emotion (ventral), in addition to large differences in baseline gene expression and function (37). We previously found a greater effect of CLP on ventral hippocampal activity in survivors (11). For these reasons, the dorsal and ventral hippocampus from each animal were included separately for sequencing. Poisson distance for all samples demonstrated that dorsal versus ventral region and sex explained the greatest amount of differential gene expression (Figure 6A). In the CLP versus sham comparison, 109 genes were differentially expressed, the vast majority of which are upregulated by CLP (only 9 downregulated genes). Corticosterone affected the expression of many more genes than CLP, with 2084 genes differentially expressed more than 2 weeks after the last corticosterone dose (more than 10% of detectable transcripts). The effect of CLP was qualitatively similar across sexes, brain regions, and treatment groups (Figure 6B). However, the quantitative effect of CLP on gene expression (degree of change) was greater in females than in males and in the ventral hippocampus than in the dorsal hippocampus (Figure 6B). Corticosterone had the same effect across sexes and brain regions irrespective of CLP or sham surgery (Figure 6B). CLP and corticosterone altered the expression of almost completely different sets of genes, with only 6 overlapping genes (Figure 6C). In summary, CLP and corticosterone both had significant and largely independent effects on hippocampal gene expression 3 weeks later.
Figure 6.
(A) Poisson distance heatmap for all samples showing clear separation between samples from the dorsal and ventral hippocampus and then between samples from male and female mice, demonstrating that these factors explain most of the variation between samples. (B) Heatmap of the log-fold change top 500 genes (by p value) in the CLP vs. sham and CORT vs. VEH comparisons ordered based on log-fold change. (C) Venn diagram showing the number of significant genes (at a false discovery rate–corrected p value of .05) in CORT vs. VEH and CLP vs. sham comparisons. We saw 2082 genes that were significant in the former and 109 genes that were significant in the latter, and only 6 genes were significantly changed in both comparisons. n = 7 sham-CORT-male, n = 8 sham-CORT-female, n = 8 sham-VEH-male, n = 7 sham-VEH-female, n = 8 CLP-CORT-male, n = 6 CLP-CORT-female, n = 5 CLP-VEH-male, n = 6 CLP-VEH-female. CLP, cecal ligation and puncture; CORT, corticosterone; VEH, vehicle.
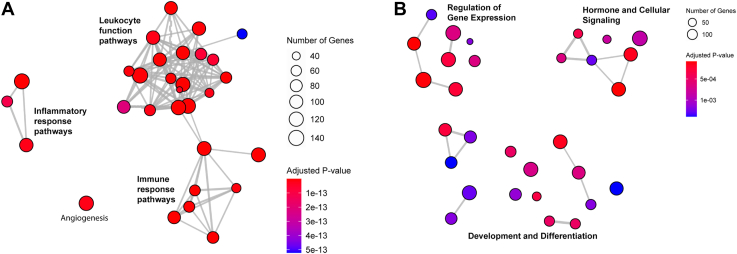
We used gene set enrichment analysis to identify the major biological pathways altered in the hippocampus by CLP and corticosterone treatment (Figure 7). This analysis confirmed that CLP and corticosterone altered gene expression in biologically distinct pathways. CLP largely caused upregulation of genes in interconnected pathways regulating inflammation, leukocyte function, and the immune response, consistent with persistent inflammation in the brain 3 weeks after the systemic infection (Figure 7A; Figure S1). In contrast, a history of corticosterone treatment altered a broader array of less interconnected pathways regulating biological processes distinct from inflammation and immunity, including genes involved in hormone and cellular signaling, development and differentiation, and the regulation of gene expression (Figure 7B; Figure S2).
Figure 7.
Top 30 pathways from a gene set enrichment analysis of the CLP vs. sham (A) and CORT vs. VEH (B) comparisons. The analysis was conducted using the Gene Ontology pathways, specifically the pathways related to biological processes. The pathways are connected if there is a >30% overlap between them. CLP caused upregulation to pathways regulating leukocyte function and inflammation, while the primary pathways altered by corticosterone included many nonoverlapping pathways in the areas of regulation of gene expression, hormone and cellular signaling, and development and differentiation. n = 7 sham-CORT-male, n = 8 sham-CORT-female, n = 8 sham-VEH-male, n = 7 sham-VEH-female, n = 8 CLP-CORT-male, n = 6 CLP-CORT-female, n = 5 CLP-VEH-male, n = 6 CLP-VEH-female. CLP, cecal ligation and puncture; CORT, corticosterone; VEH, vehicle.
To relate changes in specific biological processes to behavior in individual mice, we used weighted gene correlation network analysis. The analysis identified 17 modules (gene sets) with correlated expression, each arbitrarily assigned a color name (GSE225868). We singled out 2 different modules, pink and tan, for further study based on their enrichment for differential expressed genes by CLP or CORT treatment. Pink was a 136-gene module enriched for CLP-regulated genes (z = 52.8, p < .00001). Most (59%) of the pink genes were differentially expressed in the CLP versus sham comparison. Because CLP survivors exhibited anxiety-like behavior, we measured the relationship between pink gene expression and these behaviors. Pink expression was not significantly correlated with exploration of bright open areas in the open field or elevated zero maze (center time: R = 0.19, p = .1; center entries: R = 0.16, p = .2; open area time: R = 0.14, p = .2; open area entries: R = 0.16, p = .2).
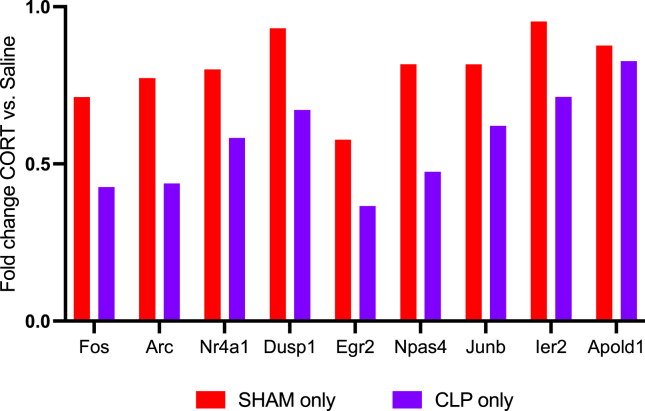
Tan was a module of 9 gene markers of neural activity, and these were all significantly downregulated by corticosterone to a greater degree in CLP than in sham (Figure 8). This suggests that corticosterone treatment led to a prolonged decrease in hippocampal activity long after treatment cessation. To determine whether the decrease in activity-dependent gene expression after corticosterone treatment reflected a decreased number of neurons, we performed a cell-type analysis (33) (GSE225868). This analysis showed no difference in the proportion of neurons based on corticosterone treatment (t27 = 0.029, p = .98). Because corticosterone influenced performance in the object recognition test, we examined the association between tan expression and novel object fraction. Tan expression was not significantly correlated with novel object fraction (R = 0.17, p = .1).
Figure 8.
Correlation of activity-dependent gene expression and behavior. Fold change of the activity-dependent genes in the tan module in the corticosterone vs. saline comparison, shown separately for the sham and CLP groups, shows downregulation of gene expression by corticosterone. CLP, cecal ligation and puncture; CORT, corticosterone.
Discussion
In this study, we sought to determine the lasting impact of 5 days of corticosteroid treatment for acute infectious illness on hippocampal function in 2- to 3-week survivors using a combination of behavioral, neuroendocrine, and gene expression phenotypes in the same mice. We found that corticosterone did not reverse persistent neuroinflammatory gene expression or anxiety-like behavior after CLP. Instead, it caused distinct and independent effects on hippocampal function and memory in survivors.
We found increased expression of proinflammatory genes in the hippocampus of CLP survivors and increased myeloid cell counts in the spleen, which when taken together suggests persistence of the innate immune response 3 weeks after CLP and is consistent with prior work (9). While this response is necessary acutely for pathogen clearance (38), inflammation can also damage the structural integrity of the brain including the hippocampus (18,19,39) and has been suggested as one cause of neuropsychiatric sequelae (40,41). Corticosteroids have potent immunosuppressive properties and did cause a lasting decrease in the splenic T cell fraction but otherwise did not attenuate the persistent expression of inflammatory genes in the brain or the expansion of splenic myeloid cell populations in CLP survivors despite improving illness severity (3). Corticosterone treatment also had no lasting effect on HPA axis function. Increased ACTH with normal corticosterone in the CLP group may be interpreted as recovery from adrenal atrophy, which is seen during critical illness (42).
CLP survivor mice showed anxiety-like behavior, as measured by avoidance of bright open areas in the open field and elevated zero maze. Decreased locomotion in these novel environments, as well as decreased object exploration during training, can also be interpreted as anxiety-like behavior because we found no effect of CLP on locomotion in a familiar arena at 14 days (11). Corticosterone did not modify the anxiety-like phenotype seen in CLP survivors. This is in contrast to other preclinical studies in which a single injection of similar weight-based doses of corticosterone prevented the development of delayed anxiety in rats following a psychogenic stressor, restraint, or predator odor (12,14). On the other hand, corticosterone-treated females showed more active coping (less immobility) in the forced swim test, which is often interpreted as an antidepressant-like effect. The possibility of a sex-dependent effect of corticosteroid treatment in improving affective outcomes is important given the increased prevalence of psychiatric morbidity in female critical illness survivors (4). The gene expression analysis did not reveal sex-dependent gene expression patterns that would explain this sex difference in behavior. This behavioral finding should be replicated in additional studies that are explicitly powered to uncover sex differences in the effect of corticosteroid treatment on brain outcomes.
Corticosterone-treated mice showed object memory impairment. This finding is consistent with one prior study in which a single corticosterone injection given to rats after predator odor stress caused long-term impairment in object recognition (14). Corticosterone treatment also decreased activity-dependent gene expression in the dorsal and ventral hippocampus in survivors, suggesting a lasting decrease in hippocampal activity. Expression of these genes was not correlated with novel object fraction on the single-animal level. Nevertheless, decreased hippocampal activity remains a plausible mechanism of impaired new memory formation in mice with a history of corticosterone treatment. How might short-term corticosteroid treatment during illness influence hippocampal activity in survivors? Corticosteroids are known to cause hippocampal dendritic atrophy (43), which may take time to reverse. Furthermore, corticosteroids can exacerbate neuronal injury in other acute physiological stressors such as hypoxia-ischemia, excitotoxicity, and hypoglycemia (44). We did not see evidence of a decreased number of neurons in the hippocampus of corticosterone-treated mice based on cell-type analysis of the RNA sequencing data. Furthermore, using the same CLP model, we have not seen histological evidence of neuron loss (9). Taken together, the data suggest that short-term corticosterone treatment during illness has a lasting impact on the activity of existing neurons.
In patients, corticosteroid treatment during sepsis is associated with decreased risk of PTSD in survivors. Both traumatic and nontraumatic memories are important for PTSD pathology (20). In humans, the effect of corticosteroids seems to be independent of traumatic memories (8,45,46). Previously, we found that corticosterone treatment during CLP-induced illness improved memory of an object seen during illness/treatment without influencing fear memory retention (47). In total, our findings suggest that corticosteroid treatment during illness influences cognitive outcomes in survivors, enhancing nonfear memories that are formed during illness/treatment at the expense of lasting impairment in new memory formation. This could indicate a decrease in cognitive flexibility during recovery that should be studied in relation to its role in PTSD vulnerability. Importantly, we saw similar effects of corticosteroid treatment on hippocampal gene expression and behavior in sham and CLP mice, suggesting that corticosteroids may act via similar mechanisms to influence neuropsychiatric risk after multiple types of trauma.
Conclusions
In conclusion, we showed that corticosteroid treatment during sepsis caused distinct changes to memory in survivors with parallel changes to hippocampal activity–dependent gene expression. The importance of these durable effects of corticosteroid treatment for neuropsychiatric outcomes in survivors should be tested in future preclinical and clinical studies.
Acknowledgments and Disclosures
This work was supported by the Hope for Depression Research Foundation, the National Institutes of Health (Grant Nos. U01DA043098, T32K007245, and MH116267), and the Brain and Behavior Research Foundation.
AH and JLS-S conducted experiments, analyzed data, and wrote the manuscript. HK and SG analyzed data. KL conducted experiments. SK-W, CJ, and BHS conducted experiments and analyzed data.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.08.001.
Supplementary Material
References
- 1.Prescott H.C., Angus D.C. Enhancing recovery from sepsis: A review. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annane D., Pastores S.M., Rochwerg B., Arlt W., Balk R.A., Beishuizen A., et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017;45:2078–2088. doi: 10.1097/CCM.0000000000002737. [DOI] [PubMed] [Google Scholar]
- 3.Rochwerg B., Oczkowski S.J., Siemieniuk R.A.C., Agoritsas T., Belley-Cote E., D’Aragon F., et al. Corticosteroids in sepsis: An updated systematic review and meta-analysis. Crit Care Med. 2018;46:1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 4.Spencer-Segal J.L., Hyzy R.C., Iwashyna T.J., Standiford T.J. Psychiatric symptoms in survivors of the acute respiratory distress syndrome: Effects of age, sex, and immune modulation. Ann Am Thorac Soc. 2017;14:960–967. doi: 10.1513/AnnalsATS.201606-468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienvenu O.J., Gellar J., Althouse B.M., Colantuoni E., Sricharoenchai T., Mendez-Tellez P.A., et al. Post-traumatic stress disorder symptoms after acute lung injury: A 2-year prospective longitudinal study. Psychol Med. 2013;43:2657–2671. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amos T., Stein D.J., Ipser J.C. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2014;7:CD006239. doi: 10.1002/14651858.CD006239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schelling G., Stoll C., Kapfhammer H.P., Rothenhäusler H.B., Krauseneck T., Durst K., et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Crit Care Med. 1999;27:2678–2683. doi: 10.1097/00003246-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Schelling G., Briegel J., Roozendaal B., Stoll C., Rothenhäusler H.B., Kapfhammer H.P. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 9.Singer B.H., Newstead M.W., Zeng X., Cooke C.L., Thompson R.C., Singer K., et al. Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denstaedt S.J., Spencer-Segal J.L., Newstead M., Laborc K., Zeng X., Standiford T.J., Singer B.H. Persistent neuroinflammation and brain-specific immune priming in a novel survival model of murine pneumosepsis. Shock. 2020;54:78–86. doi: 10.1097/SHK.0000000000001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer-Segal J.L., Singer B.H., Laborc K., Somayaji K., Watson S.J., Standiford T.J., Akil H. Sepsis survivor mice exhibit a behavioral endocrine syndrome with ventral hippocampal dysfunction. Psychoneuroendocrinology. 2020;117 doi: 10.1016/j.psyneuen.2020.104679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao R.P., Anilkumar S., McEwen B.S., Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry. 2012;72:466–475. doi: 10.1016/j.biopsych.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen H., Zohar J., Gidron Y., Matar M.A., Belkind D., Loewenthal U., et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Cohen H., Matar M.A., Buskila D., Kaplan Z., Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Kozlovsky N., Zohar J., Zeev K., Cohen H. High dose corticosterone immediately after stress exposure prevents hippocampal cytoarchitecture and neuronal plasticity damage in an animal model of PTSD. Eur J Psychotraumatol. 2012;3:796–809. [Google Scholar]
- 16.Zohar J., Yahalom H., Kozlovsky N., Cwikel-Hamzany S., Matar M.A., Kaplan Z., et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Yuan M., Yan D.Y., Xu F.S., Zhao Y.D., Zhou Y., Pan L.F. Effects of sepsis on hippocampal volume and memory function. World J Emerg Med. 2020;11:223–230. doi: 10.5847/wjem.j.1920-8642.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavan S.S., Huerta P.T., Robbiati S., Valdes-Ferrer S.I., Ochani M., Dancho M., et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpe B.T., Berlin R.A., Frankfurt M. The brain at risk: The sepsis syndrome and lessons from preclinical experiments. Immunol Res. 2015;63:70–74. doi: 10.1007/s12026-015-8704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberzon I., Abelson J.L. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewels P., Magnusson M., Lundin S., Käller M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S., Zhou Y., Chen Y., Gu J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Y., Smyth G.K., Shi W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham F., Achuthan P., Akanni W., Allen J., Amode M.R., Armean I.M., et al. Ensembl 2019. Nucleic Acids Res. 2019;47:D745–D751. doi: 10.1093/nar/gky1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber W., Carey V.J., Gentleman R., Anders S., Carlson M., Carvalho B.S., et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12:115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 29.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., et al. Gene Ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G., Wang L.G., Yan G.R., He Q.Y. DOSE: An R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics. 2015;31:608–609. doi: 10.1093/bioinformatics/btu684. [DOI] [PubMed] [Google Scholar]
- 31.Yu G. Enrichplot: Visualization of functional enrichment result. R package version 1.12.3. 2021. https://yulab-smu.top/biomedical-knowledge-mining-book/enrichplot.html Available at: Accessed January 1, 2021.
- 32.Langfelder P., Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birt I.A., Hagenauer M.H., Clinton S.M., Aydin C., Blandino P., Stead J.D.H., et al. Genetic liability for internalizing versus externalizing behavior manifests in the developing and adult hippocampus: Insight from a meta-analysis of transcriptional profiling studies in a selectively bred rat model. Biol Psychiatry. 2021;89:339–355. doi: 10.1016/j.biopsych.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walley K.R., Lukacs N.W., Standiford T.J., Strieter R.M., Kunkel S.L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buras J.A., Holzmann B., Sitkovsky M. Animal models of sepsis: Setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 36.Fang F., Zhang Y., Tang J., Lunsford L.D., Li T., Tang R., et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: A systematic review and meta-analysis. JAMA Intern Med. 2019;179:213–223. doi: 10.1001/jamainternmed.2018.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanselow M.S., Dong H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer B.H., Dickson R.P., Denstaedt S.J., Newstead M.W., Kim K., Falkowski N.R., et al. Bacterial dissemination to the brain in sepsis. Am J Respir Crit Care Med. 2018;197:747–756. doi: 10.1164/rccm.201708-1559OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huerta P.T., Robbiati S., Huerta T.S., Sabharwal A., Berlin R.A., Frankfurt M., Volpe B.T. Preclinical models of overwhelming sepsis implicate the neural system that encodes contextual fear memory. Mol Med. 2016;22:789–799. doi: 10.2119/molmed.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hori H., Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. 2019;73:143–153. doi: 10.1111/pcn.12820. [DOI] [PubMed] [Google Scholar]
- 41.Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., et al. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 42.Boonen E., Langouche L., Janssens T., Meersseman P., Vervenne H., De Samblanx E.D., et al. Impact of duration of critical illness on the adrenal glands of human intensive care patients. J Clin Endocrinol Metab. 2014;99:4214–4222. doi: 10.1210/jc.2014-2429. [DOI] [PubMed] [Google Scholar]
- 43.Woolley C.S., Gould E., McEwen B.S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 44.Sapolsky R.M. Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- 45.Weis F., Kilger E., Roozendaal B., de Quervain D.J.-F., Lamm P., Schmidt M., et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: A randomized study. J Thorac Cardiovasc Surg. 2006;131:277–282. doi: 10.1016/j.jtcvs.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 46.Schelling G., Kilger E., Roozendaal B., de Quervain D.J.-F., Briegel J., Dagge A., et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: A randomized study. Biol Psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Hill A., Johnston C., Agranoff I., Gavade S., Spencer-Segal J. Corticosterone enhances formation of non-fear but not fear memory during infectious illness. Front Behav Neurosci. 2023;17 doi: 10.3389/fnbeh.2023.1144173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.