Abstract
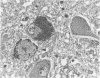
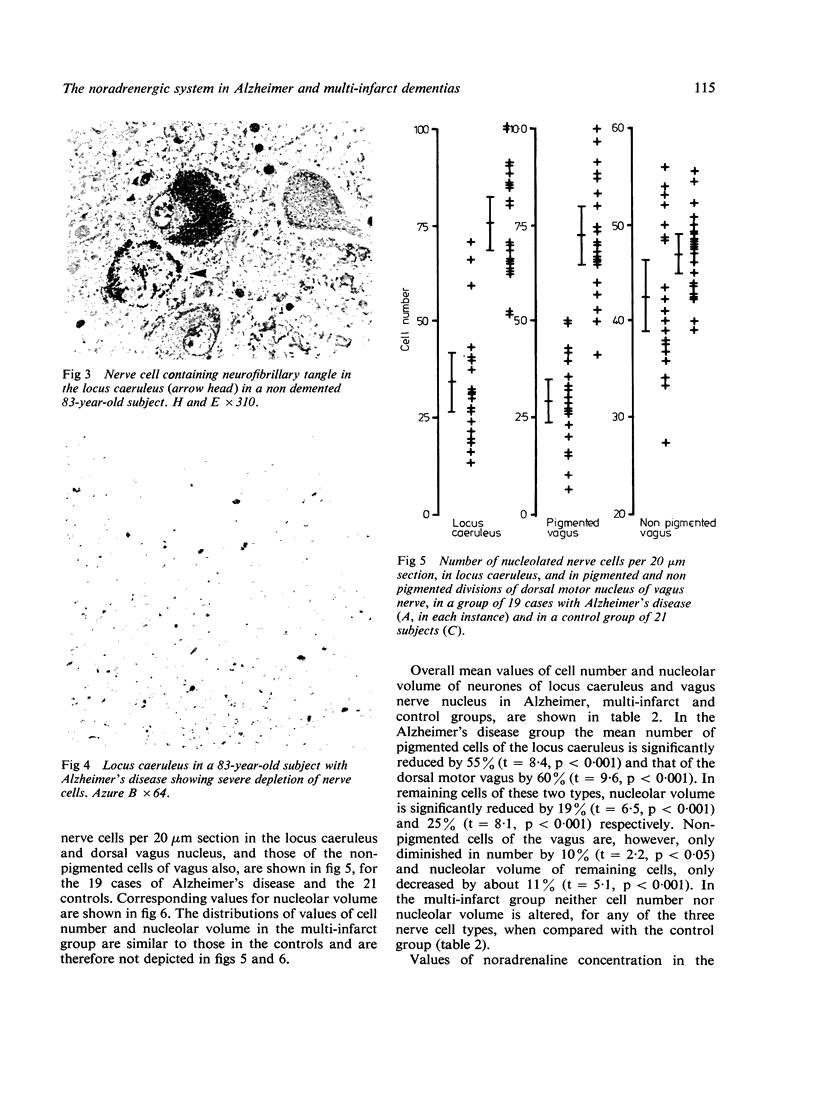
The number of melanin containing nerve cells of the locus coeruleus and vagus nucleus is reduced in Alzheimer's disease by 60% with decrease of 22% in the protein synthetic capability of remaining cells. These changes are matched by reductions in brain noradrenaline in eight regions, averaging 36%. In multi-infarct dementia, however, all three of these features are unchanged. These findings indicate that degeneration of central noradrenergic nerve cells is a specific aspect of the pathogenic process underlying Alzheimer's disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsson R., Gottfries C. G., Roos B. E., Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry. 1979 Sep;135:216–223. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- Berger B., Escourolle R., Moyne M. A. Axones catécholaminergiques du cortex cérébral humain. Observation, en histofluorescence, de biopsies cérébrales dont 2 cas de maladie d'Alzheimer. Rev Neurol (Paris) 1976 Mar;132(3):183–194. [PubMed] [Google Scholar]
- Bondareff W., Mountjoy C. Q., Roth M. Selective loss of neurones of origin of adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Lancet. 1981 Apr 4;1(8223):783–784. doi: 10.1016/s0140-6736(81)92657-x. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., Smith C. B., White P., Flack R. H., Carrasco L. H., Gedye J. L., Davison A. N. Chemical pathology of this organic dementias. II. Quantitative estimation of cellular changes in post-mortem brains. Brain. 1977 Sep;100(3):427–453. doi: 10.1093/brain/100.3.427. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., White P., Spillane J. A., Goodhardt M. J., Curzon G., Iwangoff P., Meier-Ruge W., Davison A. N. Accelerated ageing or selective neuronal loss as an important cause of dementia? Lancet. 1979 Jan 6;1(8106):11–14. doi: 10.1016/s0140-6736(79)90454-9. [DOI] [PubMed] [Google Scholar]
- Boyd W. D., Graham-White J., Blackwood G., Glen I., McQueen J. Clinical effects of choline in Alzheimer senile dementia. Lancet. 1977 Oct 1;2(8040):711–711. doi: 10.1016/s0140-6736(77)90517-7. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. A possible role of zinc in the pathology of dementia. Lancet. 1981 Jan 24;1(8213):186–188. doi: 10.1016/s0140-6736(81)90062-3. [DOI] [PubMed] [Google Scholar]
- Christie J. E., Shering A., Ferguson J., Glen A. I. Physostigmine and arecoline: effects of intravenous infusions in Alzheimer presenile dementia. Br J Psychiatry. 1981 Jan;138:46–50. doi: 10.1192/bjp.138.1.46. [DOI] [PubMed] [Google Scholar]
- Crapper D. R., Krishnan S. S., Quittkat S. Aluminium, neurofibrillary degeneration and Alzheimer's disease. Brain. 1976 Mar;99(1):67–80. doi: 10.1093/brain/99.1.67. [DOI] [PubMed] [Google Scholar]
- Cross A. J., Crow T. J., Perry E. K., Perry R. H., Blessed G., Tomlinson B. E. Reduced dopamine-beta-hydroxylase activity in Alzheimer's disease. Br Med J (Clin Res Ed) 1981 Jan 10;282(6258):93–94. doi: 10.1136/bmj.282.6258.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Maloney A. J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976 Dec 25;2(8000):1403–1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Davies P. Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res. 1979 Aug 3;171(2):319–327. doi: 10.1016/0006-8993(79)90336-6. [DOI] [PubMed] [Google Scholar]
- Etienne P., Gauthier S., Dastoor D., Collier B., Ratner J. Lecithin in Alzheimer's disease. Lancet. 1978 Dec 2;2(8101):1206–1206. doi: 10.1016/s0140-6736(78)92191-8. [DOI] [PubMed] [Google Scholar]
- Etienne P., Gauthier S., Johnson G., Collier B., Mendis T., Dastoor D., Cole M., Muller H. F. Clinical effects of choline in Alzheimer's disease. Lancet. 1978 Mar 4;1(8062):508–509. doi: 10.1016/s0140-6736(78)90180-0. [DOI] [PubMed] [Google Scholar]
- Jellinger K., Flament H., Riederer P., Schmid H., Ambrozi L. Levodopa in the treatment of (pre) senile dementia. Mech Ageing Dev. 1980 Sep-Oct;14(1-2):253–264. doi: 10.1016/0047-6374(80)90125-6. [DOI] [PubMed] [Google Scholar]
- Johnson K., Presly A. S., Ballinger B. R. Levodopa in senile dementia. Br Med J. 1978 Jun 17;1(6127):1625–1625. doi: 10.1136/bmj.1.6127.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C., Ballinger B. R., Presly A. S. Trial of levodopa in senile dementia. Br Med J. 1978 Mar 4;1(6112):550–550. doi: 10.1136/bmj.1.6112.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. M., Lincoln J., Yates P. O., Brennan C. M. Monoamine metabolism in Down syndrome. Lancet. 1980 Dec 20;2(8208-8209):1366–1367. doi: 10.1016/s0140-6736(80)92427-7. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Lincoln J., Yates P. O., Stamp J. E., Toper S. Changes in the monoamine containing neurones of the human CNS in senile dementia. Br J Psychiatry. 1980 Jun;136:533–541. doi: 10.1192/bjp.136.6.533. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Neary D., Yates P. O., Lincoln J., Snowden J. S., Stanworth P. Alterations in protein synthetic capability of nerve cells in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1981 Feb;44(2):97–102. doi: 10.1136/jnnp.44.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Bansal D. V., Marshall D. J. Hypothalamus and dementia. Lancet. 1981 Feb 14;1(8216):393–394. doi: 10.1016/s0140-6736(81)91721-9. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Barton C. M. Cytophotometric mapping of neuronal changes in senile dementia. J Neurol Neurosurg Psychiatry. 1977 Mar;40(3):299–302. doi: 10.1136/jnnp.40.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Stamp J. E. The relationship between lipofuscin pigment and ageing in the human nervous system. J Neurol Sci. 1978 Jun;37(1-2):83–93. doi: 10.1016/0022-510x(78)90229-0. [DOI] [PubMed] [Google Scholar]
- Muramoto O., Sugishita M., Sugita H., Toyokura Y. Effect of physostigmine on constructional and memory tasks in Alzheimer's disease. Arch Neurol. 1979 Aug;36(8):501–503. doi: 10.1001/archneur.1979.00500440071014. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Gibson P. H., Blessed G., Perry R. H., Tomlinson B. E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J Neurol Sci. 1977 Nov;34(2):247–265. doi: 10.1016/0022-510x(77)90073-9. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Perry R. H., Blessed G., Tomlinson B. E. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978 Jul-Aug;4(4):273–277. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Tomlinson B. E., Blessed G., Perry R. H., Cross A. J., Crow T. T. Noradrenergic and cholinergic systems in senile dementia of Alzheimer type. Lancet. 1981 Jul 18;2(8238):149–149. doi: 10.1016/s0140-6736(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Hartman B. K., Eichling J. O., Sharpe L. G. Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3726–3730. doi: 10.1073/pnas.72.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T. D., Yamamura H. I., Bird E. D., Spokes E., Enna S. J. Pre- and postsynaptic neurochemical alterations in Alzheimer's disease. Brain Res. 1978 Dec 29;159(2):477–481. doi: 10.1016/0006-8993(78)90562-0. [DOI] [PubMed] [Google Scholar]
- Rossor M. N., Iversen L. L., Mountjoy C. Q., Roth M., Hawthorn J., Ang V. Y., Jenkins J. S. Arginine vasopressin and choline acetyltransferase in brains of patients with Alzheimer type senile dementia. Lancet. 1980 Dec 20;2(8208-8209):1367–1368. doi: 10.1016/s0140-6736(80)92428-9. [DOI] [PubMed] [Google Scholar]
- Rossor M., Fahrenkrug J., Emson P., Mountjoy C., Iversen L., Roth M. Reduced cortical choline acetyltransferase activity in senile dementia of Alzheimer type is not accompanied by changes in vasoactive intestinal polypeptide. Brain Res. 1980 Nov 10;201(1):249–253. doi: 10.1016/0006-8993(80)90795-7. [DOI] [PubMed] [Google Scholar]
- Shea J. R., Jr A method for in situ cytophotometric estimation of absolute amount of ribonucleic acid using azure B1. J Histochem Cytochem. 1970 Feb;18(2):143–152. doi: 10.1177/18.2.143. [DOI] [PubMed] [Google Scholar]
- Signoret J. L., Whiteley A., Lhermitte F. Influence of choline on amnesia in early Alzheimer's disease. Lancet. 1978 Oct 14;2(8094):837–837. doi: 10.1016/s0140-6736(78)92612-0. [DOI] [PubMed] [Google Scholar]
- Sims N. R., Bowen D. M., Smith C. C., Flack R. H., Davison A. N., Snowden J. S., Neary D. Glucose metabolism and acetylcholine synthesis in relation to neuronal activity in Alzheimer's disease. Lancet. 1980 Feb 16;1(8164):333–336. doi: 10.1016/s0140-6736(80)90884-3. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Swash M., Exton-Smith A. N., Phillips M. J., Overstall P. W., Piper M. E., Bailey M. R. Choline therapy in Alzheimer's disease. Lancet. 1978 Aug 5;2(8084):318–318. doi: 10.1016/s0140-6736(78)91721-x. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Swash M. Physostigmine in Alzheimer's disease. Lancet. 1979 Jan 6;1(8106):42–42. doi: 10.1016/s0140-6736(79)90479-3. [DOI] [PubMed] [Google Scholar]
- Spillane J. A., White P., Goodhardt W. J., Flack R. H., Bowen D. M., Davison A. N. Selective vulnerability of neurones in organic dementia. Nature. 1977 Apr 7;266(5602):558–559. doi: 10.1038/266558a0. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Hartman B. K. Biochemical specificity in central pathways related to peripheral and intracerebral homeostatic functions. Neurosci Lett. 1980 Jan;16(1):55–60. doi: 10.1016/0304-3940(80)90100-7. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E., Irving D., Blessed G. Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J Neurol Sci. 1981 Mar;49(3):419–428. doi: 10.1016/0022-510x(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Vijayashankar N., Brody H. A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol. 1979 Sep;38(5):490–497. doi: 10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- Yates C. M., Allison Y., Simpson J., Maloney A. F., Gordon A. Dopamine in Alzheimer's disease and senile dementia. Lancet. 1979 Oct 20;2(8147):851–852. doi: 10.1016/s0140-6736(79)92202-5. [DOI] [PubMed] [Google Scholar]
- Yates C. M., Ritchie I. M., Simpson J., Maloney A. F., Gordon A. Noradrenaline in Alzheimer-type dementia and Down syndrome. Lancet. 1981 Jul 4;2(8236):39–40. doi: 10.1016/s0140-6736(81)90269-5. [DOI] [PubMed] [Google Scholar]