Abstract
The female reproductive system is strongly influenced by nutrition and energy balance. It is well known that food restriction or energy depletion can induce suppression of reproductive processes, while overnutrition is associated with reproductive dysfunction. However, the intricate mechanisms through which nutritional inputs and metabolic health are integrated into the coordination of reproduction are still being defined. In this review, we describe evidence for essential contributions by hormones that are responsive to food intake or fuel stores. Key metabolic hormones—including insulin, the incretins (glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1), growth hormone, ghrelin, leptin, and adiponectin—signal throughout the hypothalamic–pituitary–gonadal axis to support or suppress reproduction. We synthesize current knowledge on how these multifaceted hormones interact with the brain, pituitary, and ovaries to regulate functioning of the female reproductive system, incorporating in vitro and in vivo data from animal models and humans. Metabolic hormones are involved in orchestrating reproductive processes in healthy states, but some also play a significant role in the pathophysiology or treatment strategies of female reproductive disorders. Further understanding of the complex interrelationships between metabolic health and female reproductive function has important implications for improving women’s health overall.
Keywords: hypothalamic-pituitary-ovarian axis, insulin, metabolic disorders, nutrient-sensing, obesity, polycystic ovary syndrome (PCOS)
Close ties between nutritional status and female reproductive function
Reproduction is an energetically expensive process, and the energetic costs are largely borne by the females of many species. The mammalian female reproductive system is responsible for producing female gametes, facilitating their fertilization with sperm, supporting embryonic-fetal growth and development, and enabling the birth and nourishment of the offspring. These complex processes require a high level of communication between various organ systems, and so reproduction is under tight control of centrally produced hormones released by the hypothalamus and pituitary, as well as signaling factors produced by the placenta, developing embryo, and tissues of the reproductive system. Considering the high energetic requirements of gamete production, gestation, and lactation, it is clear that levels of food and energy stores are additional pieces of information that must be incorporated into the control of reproductive processes. Therefore, hormones that are classically defined by their metabolic roles are also critically important for regulating reproduction, including those which relay acute changes in ingestion and nutrient levels (e.g., insulin, the incretins, growth hormone, and ghrelin), as well as hormones that communicate stored metabolic fuel levels (e.g., leptin and adiponectin).
Strong links between nutritional status and female reproductive function are evident throughout the animal kingdom. Vertebrate orexigenic and anorectic neuropeptides that are classified based on their effects on appetite also modify levels of gonadotropin hormones in the reproductive axis [1]. Many bird species breed seasonally, which restricts reproductive activity to periods when local food supply is optimal for supporting increased metabolic demands [2,3]. Food availability affects fecundity and the timing of sexual maturity in fishes, and female iteroparous fishes may skip a spawning season if nutrient levels are insufficient [4,5]. There are also insect and nematode species that can enter diapause states to reversibly suspend development and reproduction under unfavourable conditions. Entry into or recovery from diapause in these invertebrate organisms is orchestrated in part by evolutionarily conserved signaling systems that communicate nutritional status [6,7]. Mammals exhibit patterns of sexual dimorphism that are consistent with the concept that females are better suited for withstanding periods of food scarcity, which would increase chances of reproductive success in nutritionally-fluctuating environments [1]. For instance, in contrast with males, female mammals tend to favor energy storage over the capacity for rapid fuel mobilization, and are more prone to accumulate adipose mass in subcutaneous depots [8–10].
In humans, the relationship between nutrient levels and female reproductive health is most obvious when an imbalance in nutrient and energy levels pushes functioning of the reproductive system off-kilter. This is exemplified by physiological responses to food restriction and excessive energy expenditure, or conversely by the reproductive system disorders that are linked to overnutrition.
Inadequate energy and reproductive dysfunction
Energy deficiency caused by stress, low food intake, or strenuous exercise can result in a suppression of neuroendocrine signals that allow normal menstruation and ovulation. This deregulation is signified by a loss or alteration of gonadotropin hormone pulsatility, and leads to ovarian responses such as decreased estradiol production [11–13]. Low energy availability can thereby cause primary amenorrhea, a delay in menarche, or secondary amenorrhea, a temporary halt in natural menstrual cycling [14]. Studies in human populations exemplary of a negative energy balance have shed light on aberrant menstrual patterns. Ballet dancers [15,16] and athletes [17,18] engaged in high physical activity may have a delayed pubertal onset. Additionally, disordered eating, excessive exercise, or lifestyle stressors can cause menses loss in erstwhile normal-ovulatory women [19–22]. Temporary food deprivation or fasting can also induce a drop in gonadotropin levels and rise in cortisol [23–27]. Furthermore, women in poverty-stricken and/or strenuous labor-demanding societies experience increased risks of adult amenorrhea and lower birth rates [28–32].
Importantly, the ready availability of energy or metabolic fuels is more critical to reproductive fitness than adiposity per se. Food-deprived or over-exercised females can adjust food intake or activity to restore normal ovarian cyclicity and gonadotropin pulsatility before changes in adiposity or weight are evident [33–36]. Similarly, short fasting intervals halt ovarian cycles in Syrian hamsters without affecting adiposity, by reducing free fatty acid oxidation [37,38]. Maintaining glucose availability also preserves reproductive function in rodents and primates [39–43].
Amenorrhea and subfertility are not merely disorders of the reproductive system, but instead have broad physiological impacts. Just as a reduction in estrogen levels in postmenopausal women increases risks of cardiovascular disease [44], bone frailty [45], and neuropsychiatric disorders [46], a premenopausal estrogen deficiency caused by amenorrhea leads to compromised cardiovascular, skeletal, and mental health [47–51]. Thus, reproductive system responses to nutritional cues have far-reaching effects.
Excess energy and reproductive dysfunction
Undernutrition can suppress signals that allow reproduction, but chronic overnutrition is also associated with reproductive dysfunction. The recent prevalence of ultra-processed and low-satiating foods, combined with a more sedentary lifestyle, has led to a surplus of calories in everyday life [52–56]. Animal studies have shown that high-energy, high-fat diets interfere with reproductive function independent of obesity [57–60]. Additionally, obesity and a high body mass index are themselves associated with precocious menarche [61–65], menstrual cycle irregularities [66–69], infertility [70–73], miscarriage [74–76], and fetal abnormalities [77–82]. Diets that are high in refined carbohydrate also predict an earlier age of menopause [83,84].
Energy overload and reproductive dysfunction are also closely associated in the context of polycystic ovary syndrome (PCOS), the most prevalent female reproductive disorder [85,86]. The diagnostic features of PCOS include hyperandrogenism, menstrual cycle irregularities, and an accumulation of fluid-filled cysts in the ovaries [87,88]. However, PCOS has heterogeneous symptoms that often include obesity, elevated insulin, and/or a diminished capacity for glucose disposal [89,90]. These metabolic characteristics exacerbate the reproductive features of PCOS through such means as heightening testosterone levels [91–95]. Hypercaloric, high-fat diets also potentiate the traits of PCOS [96–98]. Women with PCOS tend to have difficulty conceiving, as well as a greater risk of pregnancy complications such as gestational diabetes, preeclampsia, or miscarriage [99–109]. PCOS is also associated with increased incidence of Type 2 diabetes [110–112], hypertension [110,113–117], high cholesterol [118–120], stroke [121,122], and cancer [123–126].
Weight loss [127–129], exercise [129–134], and bariatric surgery to limit food intake [135–140] are useful clinical tools for treating some aspects of the reproductive dysfunction associated with energy surplus. Lifestyle approaches such as balanced diet selections favoring whole grains, vegetables, fish, and unsaturated fats rather than saturated or trans fats are associated with increased fertility, improvements to PCOS symptoms, and beneficial impacts on other aspects of gynecologic health [141–144]. However, there is a lack of widespread awareness that diet has important implications for reproductive health (beyond effects on weight loss or perinatal health), and this is compounded by barriers preventing equal access to healthy diet options [143].
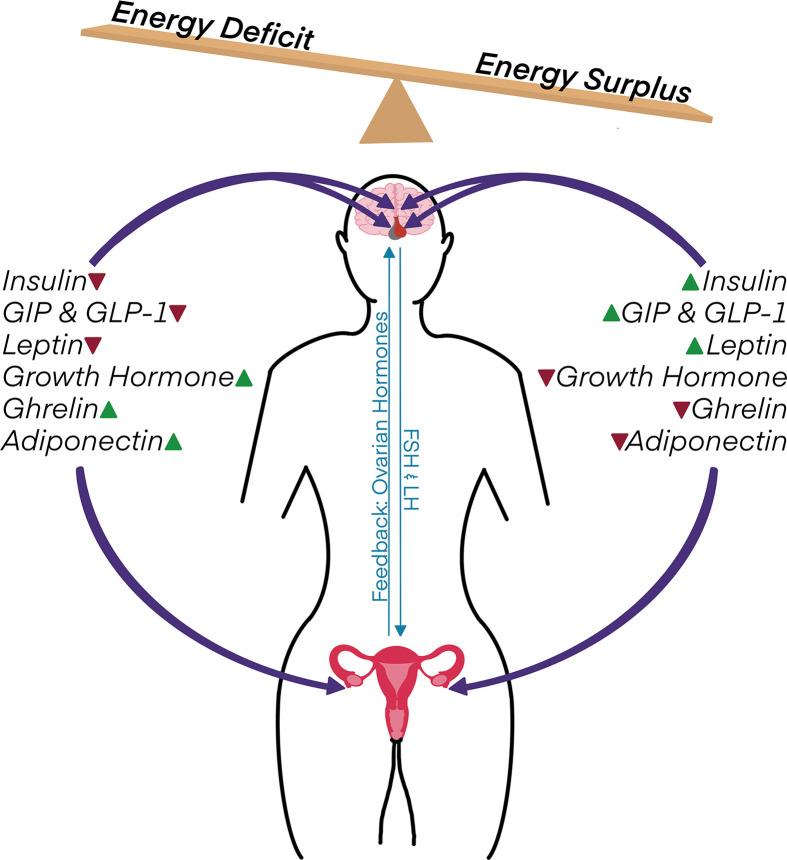
Effective management of nutritional and energy inputs is imperative for maintaining reproductive health. Nutrition and energy balance affect many aspects of reproductive health, including the menstrual cycle, fertility, pregnancy, fetal health, and age-related reproductive decline. This is due in part to the hormones that interpret food intake and fuel stores, which cooperate with cellular nutrient sensors to trigger the appropriate physiological responses for systemic energy homeostasis. These metabolic hormones engender changes across the reproductive axis, from the hypothalamus and pituitary to peripheral tissues of the female reproductive system (Figure 1).
Figure 1. Metabolic hormones act as key intermediaries in linking nutrient and energy status to female reproductive function.
Energetic deficits generally decrease levels of insulin, the incretin hormones (GIP and GLP-1), and leptin while also raising growth hormone, ghrelin, and adiponectin. Conversely, food ingestion and/or a chronic energy surplus causes the opposite shift in circulating levels of these hormones. Metabolic hormones act directly within the hypothalamus, pituitary, and ovaries to modulate reproductive processes. Their effects are thereby integrated into the reproductive axis, in which the hypothalamus and anterior pituitary communicate with the female reproductive system through the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH), while the ovaries in turn provide feedback via steroid hormones and other signaling factors.
Basic regulation of female reproduction
The hypothalamus and anterior pituitary
The hypothalamic–pituitary–gonadal (HPG) axis comprises a system wherein the hypothalamus and anterior pituitary cooperate to centrally control gonadal maturity and function. Gonadotropin-releasing hormone (GnRH) is a tropic hormone secreted by a small subset of hypothalamic neurons in response to a suite of peripheral signals and neuronal messengers, including inputs from kisspeptin (Kiss1) neurons, astrocytes, γ-aminobutyric acid (GABA) neurons and pro-opiomelanocortin (POMC) neurons [145,146]. Pulses of GnRH released into portal circulation range in frequency from pulsatile to surge mode, depending on sex, age, and menstrual cycle phase [147–150]. Although the HPG axis is first established in utero, it is largely silenced until the initiation of nocturnal GnRH pulses during the onset of puberty [151,152]. Kisspeptin signaling is a key player in reactivating the HPG axis and initiating the pulsatile hypothalamic GnRH secretion required for sexual maturity and reproductive function [153–157]. Thereafter, rhythmic changes in frequency and amplitude of GnRH pulses are integral for controlling the differential secretion pattern of the two gonadotropin hormones, together with regulatory input by other systemic and paracrine factors.
Gonadotroph cells of the anterior pituitary produce the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [158,159]. GnRH signaling induces differential expression of genes encoding LHβ and FSHβ subunits, in addition to regulating LH and FSH exocytosis [160]. In turn, FSH and LH exert controls over ovarian function, including steroidogenesis, follicular development, and ovulation. Together, forward-acting and feed-back regulatory loops facilitate a dynamic, nuanced reproductive axis that can be adjusted at multiple levels in response to internal and external conditions [161]. For instance, crucial information related to nutritional status and energy balance is incorporated into the reproductive axis via metabolic hormones exerting effects on GnRH production and release, gonadotropin secretion, and ovarian functions.
The ovaries
Oogenesis, folliculogenesis, and the production of steroid hormones and other signaling factors are tightly regulated ovarian processes that are responsive to inputs such as metabolic hormone signaling. Oogenesis begins early in embryonic development with primordial germ cells that divide by mitosis to form oogonia, which may continue to mitotically divide, undergo programmed cell death, or enter into meiosis as primary oocytes [162]. Primary oocytes do not complete meiosis, but are instead arrested and individually surrounded by a sheath of granulosa cells in structures called primordial follicles [163,164]. Female humans are born with approximately one million non-atretic primordial follicles, which constitute the initial ‘ovarian reserve’ [165].
After birth, primordial follicles are continuously recruited into a pool of growing follicles that routinely undergo atresia, or apoptosis-mediated degeneration [166–169]. Since primordial follicles lack an independent blood supply, the early stages of folliculogenesis are under limited endocrine regulation; the transition from primordial to primary follicle is largely controlled by intra-ovarian paracrine signaling [170]. Granulosa cells of a primary follicle begin to express FSH receptors, and FSH is involved in stimulating the progression into a secondary follicle [171–173]. Theca cells enveloping the follicle develop LH receptors late in the secondary follicle stage [174], and the thecal layer becomes increasingly vascularized as the antral follicle develops [175]. Preantral stages of folliculogenesis continuously generate a pool of developing follicles which almost all default to atresia [170,176]. However, with the stimulation by gonadotropins that occurs after puberty, a very small cohort of ∼10 antral follicles is recruited each month for further maturation [176].
In response to FSH and LH, granulosa cells and theca cells cooperatively participate in steroidogenesis to produce androgens, estrogens, and progesterone [177,178]. The dominant follicle heightens production of estradiol and other signaling factors that act locally as well as centrally suppressing FSH production; this ultimately pushes the non-dominant follicles to atresia, since they have fewer FSH receptors and are outcompeted for FSH, a survival factor that inhibits follicular atresia [179,180]. The oocyte resumes meiosis as the dominant antral follicle continues to mature. Eventually, sustained high estradiol levels triggers a surge in centrally produced LH and FSH, driving follicle rupture and ovulation of the oocyte surrounded by supporting granulosa cells called cumulus cells [181,182]. Meanwhile, the residual follicle somatic cells differentiate into a temporary endocrine gland called the corpus luteum [183,184]. Ovulation occurs monthly in healthy females until approximately 50–55 years of age, when the ovarian reserve is exhausted [185–188]. The resultant drop in levels of follicle-produced hormones and signaling factors ripples through the HPG feedback system, leading to fluctuations in GnRH pulses and high circulating LH and FSH before gonadotropin levels eventually decline after menopause [189,190].
Cellular nutrient sensors
Cellular nutrient sensors are expressed in the hypothalamus, pituitary and ovaries, where they interpret local quantities of metabolites or energy-carrying molecules and interact with different cellular players to govern functioning of the female reproductive system. As comprehensively described in recent review articles [191–195], these nutrient sensors exert direct and indirect effects on female reproductive function. For instance, mechanistic target of rapamycin (mTOR), a protein kinase that promotes anabolic processes in response to increased amino acids and growth signals, is involved in regulating primordial follicle activation and granulosa cell proliferation, among other metabolically important functions. Similarly, AMP-activated protein kinase (AMPK), a nutrient sensor activated by a drop in cellular energy, plays a role in maintaining organismal energy homeostasis that extends to influencing pubertal timing and oocyte maturation.
Along with their own nutrient-sensing functions, cellular nutrient sensors are integral signaling intermediaries by which metabolic hormones probably direct some of their effects on reproductive physiology. For example, adiponectin can induce mTOR inhibition and AMPK activation [196], whereas the insulin signaling cascade is capable of promoting mTOR activity and inhibiting AMPK [197,198]. However, delineating these relationships in the context of reproductive functions is complicated by tissue-specific and context-dependent interactions. For instance, the adiposity signal leptin inhibits hypothalamic AMPK but activates skeletal muscle AMPK, whereas fasting-induced ghrelin activates AMPK in the hypothalamus while inhibiting AMPK in adipose tissue and liver [199]; it is unclear how these signaling hubs interact elsewhere, such as in ovarian cells. Intricate, bidirectional cross-talk between signaling pathways of cellular nutrient sensors and metabolic hormones further exacerbates the difficulties of defining their individual roles in regulating reproductive processes.
Insulin
Insulin is best known for maintaining blood glucose levels, but it also regulates carbohydrate, lipid and protein metabolism, appetite, cell division, cell growth, and lifespan. A peptide hormone predominantly produced by β cells of the pancreatic Islets of Langerhans, insulin is secreted in response to the glucose, fatty acids, and amino acids that become elevated in circulation due to food intake. However, insulin levels are under multifactorial control, and autonomic nervous system innervation as well as other hormones (such as growth hormone and glucagon-like peptide 1) also affect insulin production and secretion; circulating levels are further controlled at the level of its clearance [200–203]. Insulin was discovered in 1921-22 with the extraction and purification of a pancreatic substance that could effectively lower blood glucose levels in patients with Type 1 diabetes [204,205]. It had a transformative impact on the treatment of diabetes, establishing its fundamental metabolic role.
Although insulin was discovered in mammals, it is now well known that insulin-like peptides and their highly conserved signaling cascades regulate metabolism, development, and aging across the animal kingdom. Ligand binding to insulin/insulin-like growth factor 1 (IGF-1) tyrosine kinase receptors leads to the activation of downstream signaling effectors, including phosphatidylinositol 3-kinase (PI3K, whose activities are counteracted by phospholipid phosphatases like PTEN) and the serine/threonine kinase AKT. The PI3K/AKT signaling pathway is associated with promoting glucose uptake and storage, suppressing hepatic glucose release, stimulating lipogenesis, and inhibiting mobilization of stored lipids [197,206]. Another major branch of insulin/IGF-1 signaling is transduced via the mitogen-activated protein kinase (MAPK)/ERK cascade, which is primarily involved in regulating growth and cell proliferation [197,206]. These signaling cascades also interact with other nutrient sensor mechanisms, through such means as activating mTOR and inactivating AMPK [197].
Lowering insulin below a critical threshold causes diabetes, but elevated insulin is also associated with detrimental changes. Insulin hypersecretion is a driving factor for insulin resistance, obesity, and other aspects of metabolic dysfunction [207]. Notably, in the ‘insulin resistant’ state that is defined by impaired insulin-induced glucose disposal, only a subset of insulin-regulated processes have diminished responses to insulin; tissues such as ovaries and the pituitary might remain mostly insulin-responsive [207–209]. The elevation in circulating insulin that often accompanies insulin resistance could thereby exacerbate insulin signaling responses in the female reproductive system.
Insulin and the female reproductive system
Insulin and the insulin signaling pathway are important regulators of reproduction, and there can be detrimental outcomes of either insufficient or excess levels. Manipulations such as brain-wide deletion of insulin receptors (InsR) suppress GnRH release in mice, leading to impaired follicle maturation and reduced fertility [210]. On the other hand, high insulin levels induced by high-fat feeding are accompanied by fewer estrous cycles, fewer preantral and antral follicles, and smaller litters; exogenous insulin also causes a reduction in murine oocyte yield and quality [198,211,212]. In humans, an infusion of insulin and lipids acutely suppresses FSH and LH levels [213]. Elevated insulin at birth and during childhood is associated with earlier puberty [214], and insulin levels in reproductive-aged women are negatively correlated with levels of anti-müllerian hormone (AMH), an ovary-produced hormone that signifies ovarian reserve [215].
Insulin receptors are expressed widely in the brain [216,217], including in GnRH neurons, astrocytes, and Kiss1 neurons [218–221]. Astrocytes are implicated in the insulin-mediated regulation of GnRH release [222], and InsR ablation in astrocytes results in altered ovarian cycling, impaired oocyte maturation, hypogonadism, and subfertility [223]. Cultured GnRH cell-lines also contain InsRs [224], and insulin stimulates GnRH expression and promotes its effects on gonadotropin secretion in vitro [225–227]. GnRH-specific InsR knockout mice are protected from obesity-associated infertility, with GnRH pulses that are comparable to lean control mice [228]. InsR knockout in the pituitary [211] or ovarian theca cells [229] also protects female mice from high-fat diet-induced infertility, showing that elevated insulin acts across multiple systems to impair reproductive function under conditions of nutrient surplus.
Insulin has direct ovarian effects on metabolism, steroidogenesis, and folliculogenesis. The InsR is expressed in oocytes, granulosa cells, and theca cells of rodent, bovine, and human ovaries [230–234]. Insulin signaling is crucial for supporting glucose uptake and glycolysis in the ovary, to provide energy for folliculogenesis [235,236]. Insulin also plays an important role in steroidogenesis, by cooperating with LH to stimulate androgen production in theca cells [91,237,238]. It promotes the primordial to primary follicle transition in a rat ovarian organ culture system [239], and supra-physiological levels of insulin stimulate bovine oocyte cleavage, maturation and meiotic progression in vitro [240,241]. In vivo, oocyte-specific InsR deletion appears to have minimal impacts on fertility in mice [242]. Similarly, InsR ablation in murine granulosa or theca cells can lead to altered steroidogenesis and gene expression changes without overt effects on gross ovarian morphology or fertility under standard dietary conditions [229,243]. However, insulin and IGF-1 are closely related and can bind to each other's receptors or hybrid insulin-IGF-1 receptors with varying affinities [244]. Notably, double knockout of InsR and IGF-1 receptors in granulosa cells causes significant infertility, by impairing oocyte development and ovulation to a greater degree than knockout of either receptor alone [243]. IGF peptides, binding proteins, and receptors are expressed in human follicles [245], and IGF-1 is itself important for regulating follicular growth and survival as well as FSH-induced processes such as estradiol production, granulosa cell differentiation, and ovulation [246–252].
Downstream insulin/IGF-1 signaling components are established players in maintaining ovarian function and balance in folliculogenesis. For instance, overexpression of PI3K in the oocytes of neonatal mice increases follicular numbers, reduces apoptosis, and triggers an anovulatory state due to an excess of overgrown follicles [253]. Similarly, oocyte-specific removal of the counter-regulatory PTEN causes premature activation and exhaustion of the quiescent follicular pool [254]. Akt is widely expressed in ovarian stromal and germ cells in humans [255] and rodents [256], and Akt-deficient mice have delayed puberty onset, reduced fertility, altered steroid hormone levels, and a predisposition for PCOS-like phenotypes [257,258]. PI3K/AKT signaling maintains the primordial follicle pool in part by phosphorylating and inactivating the FOXO3 transcription factor, which otherwise suppresses primordial follicle activation when active [259]. Mice with constitutively activated oocyte FOXO3 maintain follicle numbers, gonadotropin levels, and youthful gene expression profiles with advancing age [260].
Abnormal insulin levels—relatively common with metabolic disorders—are linked to impaired reproductive health. For instance, women with Type 1 diabetes (typically treated with exogenous insulin) are more likely to exhibit ovarian dysfunction, and those taking a higher daily dose of insulin have an increased chance of earlier menopause [261,262]. An early diagnosis of Type 2 diabetes, which is closely tied to obesity and high endogenous insulin, is also predictive of earlier menopause [261,263–265]. Elevated insulin is a cardinal feature of PCOS that aggravates its reproductive pathophysiology, by augmenting testosterone production and bioavailability as well as inhibiting follicular growth and maturation [88,89]. Therefore, while insulin signaling is essential for metabolic and reproductive functions, preventing insulin excess could have promising therapeutic potential [207].
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1)
Incretins are metabolically active gut hormones released promptly after food consumption. Seminal work in the 1960's pointed to the existence of factors that heighten insulin levels in response to ingestion [266–268]. Glucose-dependent insulinotropic polypeptide (GIP) was isolated in 1971 [269] and shown to potentiate insulin levels in response to intestinal absorption of nutrients such as glucose [270]. Glucagon-like peptide 1 (GLP-1) was later identified as another potent insulinotropic hormone [271]. GLP-1 also promotes the proliferation of β-cells [272,273] and prevents their apoptosis [274]. In addition, GLP-1 contributes towards maintaining glucose homeostasis by lowering food intake, glucagon secretion, and endogenous glucose production [275–277].
GIP and GLP-1 are produced by intestinal enteroendocrine cells. GIP is secreted by the K-cells of the small intestine in response to the ingestion and absorption of glucose, lipids, and high levels of amino acids [270,278–281]. GLP-1 is secreted by the large intestine and the L-cells of the small intestine [282,283]. GLP-1 is the post-translational cleavage product of the proglucagon gene, and is stimulated by monosaccharides such as glucose, fructose, and galactose [284,285], as well as dietary lipids [286,287] and amino acids [288–291]. Upon their release into circulation, the incretins bind to their respective G-coupled receptors (GIPR and GLP-1R), which in pancreatic β cells stimulate insulin exocytosis by inducing a rise in intracellular cAMP and calcium levels [292,293]. The insulinotropic effects of incretins are largely mediated by β cells, but incretin receptors have a broad distribution across many tissues, including in the hypothalamus [292–294]. GLP-1 produced within the brain also appears to contribute to its central effects [292,295].
Despite higher circulating levels, the insulinotropic effects of GIP dwarf in comparison to GLP-1, which is widely popularized as a therapeutic target. GLP-1 can normalize blood glucose levels in Type 2 diabetes patients [296] and promote weight loss [297]. However, GIP and GLP-1 are enzymatically inactivated after secretion, and the rapid degradation of GLP-1 limits its therapeutic potential [298]. Efforts have now shifted to using GLP-1 receptor agonists [299–301] or dual GIP- and GlP-1- receptor agonists [300,302,303]. Incretin-based therapy has expanded to target fatty liver disease [304,305], kidney disease [306], neurodegenerative diseases [307,308] and reproductive disorders such as PCOS [309,310].
Incretin hormones and the female reproductive system
The incretin hormones might elicit indirect, insulin-mediated impacts on female reproductive function due to their insulinotropic nature, but they also directly affect reproduction. Mice deficient in either GLP-1R or GIPR exhibit disrupted estrous cycling, reduced fertility, and smaller litter sizes [311], and GLP-1R knockout additionally leads to delayed puberty in female mice [312]. GLP-1 may exert many of these reproductive effects through central actions. Both hypothalamic GLP-1r expression and plasma GLP-1 concentrations vary across estrous phases in rats, and either central or peripheral administration of GLP-1 increases the preovulatory LH surge [313,314]. Intracerebral GLP-1 also synchronizes the onset of puberty, and improves implantation rates, birthing rates, and mature follicle numbers [314]. Changes in gonadotropin secretion appear to be due in part to GLP-1 positively regulating GnRH release. Early evidence in a rat hypothalamic cell-line pointed to GLP-1 promoting GnRH release via intracellular cAMP signaling [315], and subsequent work implicated the involvement of Kiss1 neurons [314,316,317] and GABAergic signals [318] in bolstering the direct effects of GLP-1 on GnRH neurons. While there is less known about the role of GIP, GIPR is expressed in the murine hypothalamus [319] and pituitary [311], and intracerebroventricular GIP administration decreases plasma FSH levels in rats [320]. Receptors for both GLP-1 and GIP are also expressed in the rodent ovary [311,321], and both of these incretins suppress progesterone synthesis in the presence of FSH [321]. Incretins have also been detected in human follicular fluid, and tend to be higher in the follicular fluid of obese women (particularly GLP-1) [322].
There has been some investigation into the therapeutic effects of incretin receptor agonists for female reproductive disorders. For instance, GLP-1 receptor analogues can mitigate the ovarian inflammation, fibrosis, oxidative stress, and AMH reduction that is induced in a rat model of diabetes [323]. In animal models of PCOS, incretin receptor agonists improve ovarian morphology and gonadotropin levels [324,325]. Incretin analogues are also being applied in clinical practice with PCOS patients. They can effectively alter steroid hormone levels [326–329], decrease body weight and enhance metabolic health [330–333], regularize menstrual cycling [329,331,334], and improve pregnancy rates and outcomes [331,333]. However, in general there is a paucity of information on the details and mechanisms by which anti-obesity pharmaceuticals such as incretin receptor agonists affect the female reproductive system [309,335].
Growth hormone (GH)
First isolated in the 1940s, growth hormone (GH) was defined by impacts on longitudinal growth largely driven by a promotion of bone growth in children and adolescents [336,337]. However, it is now known that GH also has a broader reach in regulating energy balance, including effects on puberty timing, reproductive function, insulin resistance, metabolic fuel selection, lipolysis, hepatic glucose production, protein synthesis, muscle building, and immune function [337–339]. In general, GH is anabolic in nature. It stimulates an increase in lean body mass under energy-replete conditions, and preserves lean body mass and carbohydrate stores during fasting by promoting lipid usage [337]. GH elicits its physiological outcomes through a combination of indirect, IGF-1-mediated effects and direct intracellular signaling via the widely expressed GH receptor, which activates Janus kinase 2 (JAK2)-signal transducers and activators of transcription (STAT) as well as other signaling cascades [339]. Since GH promotes a rise in circulating and locally produced levels of the insulin-like growth factor IGF-1, it can be difficult to mechanistically distinguish between direct GH effects and ancillary effects carried out through IGF-1 signaling [337].
GH is secreted in a pulsatile manner by somatotroph cells in the anterior pituitary gland, with levels and patterns that depend on age, sex, and energy balance [340–342]. Hypothalamic-produced GH-releasing hormone stimulates its secretion while somatostatin inhibits it [340,343]. Additionally, ghrelin potently stimulates GH secretion, and both estrogens and androgens promote GH release [340,343–348]. Outside of the brain, GH mRNA is also expressed in peripheral tissues, including in the uterus, mammary glands, and ovaries; locally produced GH likely has local autocrine and/or paracrine effects, rather than traveling through circulation [349]. In general, circulating GH is higher in females than males, and levels rise in response to puberty, sleep, exercise, and fasting, whereas GH is decreased in response to elevated blood glucose, glucocorticoids, and aging [340,348]. Circulating GH levels peak at puberty and decline steadily afterwards, with only residual levels detectable at age 50 [350–354].
Growth hormone and the female reproductive system
Having sufficient GH is important for multiple facets of female reproductive competency. Women with GH deficiency have delayed menarche, fewer children, reduced uterine volume, low prolactin levels, and higher FSH [355,356]. Similarly, GH-deficient rodents have a later onset of puberty, smaller litter sizes, delayed parturition, irregular estrous cycles, and fewer corpora lutea and follicles [357–360]. GH replacement therapy has therapeutic potential in GH-deficient infertile women [361], and also improves ovulation rates and embryo implantation rates in women undergoing IVF when combined with gonadotropin treatment [362,363]. Genetically engineered GH-overexpressing animals have increased ovarian weights as well as higher ovulation rates and implantation sites, but also exhibit lower mating rates and reduced offspring survival [364,365].
In addition to regulating pubertal growth, GH is implicated in controlling the timing of puberty. Puberty is delayed in mammals with GH deficiency, although the fact that they can reach sexual maturation indicates that GH is not a requirement [366,367]. GH transgene expression expedites puberty in mice [365], and GH treatment in GH-deficient children stimulates an earlier age of puberty [368]. GH affects GnRH release [369,370], and GH and/or IGF-1 signaling in GnRH neurons or Kiss1 neurons could play a role in the activation of pulsatile hypothalamic GnRH secretion linked to the onset of puberty (reviewed in [366]). However, the start of ovarian steroidogenesis and consequential rise in steroid hormones is instrumental in promoting the steep elevation in GH levels during puberty, making it difficult to tease apart these causal relationships [366,367]. Ultimately, it is most likely that interactions between GH and the HPG axis are bidirectional during the complex endocrine shifts of puberty, and GH may be one of the integrated endocrine signals that conveys whether nutrient levels are sufficient for puberty to proceed [366,367].
At the level of the anterior pituitary, it is noteworthy that there are interactions between GH-producing somatotroph cells and gonadotropin-producing gonadotroph cells (reviewed in [367]). Therefore, GH likely exerts some of its reproductive effects within the pituitary itself, through such means as influencing the secretion of LH and FSH [367]. However, GH plays a more apparent role in regulating reproductive function through its ovarian actions.
In the ovary, GH is involved in governing gametogenesis, gonadotropin sensitivity, follicle survival, and the preservation of tissue health [371]. GH receptors are present in the oocytes and granulosa cells of antral follicles [372], and levels are significantly decreased in lower-quality oocytes of aging women [373]. In vitro studies of goat oocytes reveal that GH treatment stimulates early antral follicle development, promotes fertilization, development of healthy oocyte-cumulus complexes and growth of a healthy embryo [374]. Similarly, in canine oocytes GH acts alongside FSH to promote antrum formation, resulting in improved follicular viability [375]. Other studies have suggested that GH prevents follicular apoptosis via IGF-1 and the PI3K/AKT signaling pathway [376,377]. In vivo work has shown that GH may improve ovulation rates by increasing the number of superovulated oocytes reaching meiosis II [378]. In vitro studies point to a similar trend of GH supplementation leading to increased meiotic progression rates [374], and improved nuclear maturation in rodent [379], dog [380], sheep [381], bovine [382], equine [383], and human [384] oocytes. These effects may be partially mediated by cumulus cells. GH stimulates proliferation and inhibits apoptosis of cumulus cells [385,386], and regulates the expression of the gap junction proteins that allow oocytes to exchange nutrients with surrounding cumulus cells [385,387]. Murine oocytes cultured with GH form thecal layers that are rich in mitochondria and rough ER, implicating an additional role in theca cell proliferation [388].
The clinical potential for GH also stems from its role in improving uterine receptivity to incoming embryos. GH-stimulated upregulation of IGF-1 mediates estrogen-related improvements in endometrial receptivity and increase uterine thickness across several species [389–393]. Although more contentious, human studies also suggest benefits of GH therapy among female IVF and embryo transfer patients, especially if they have endocrine disorders or are overweight/obese [394,395]. GH concentrations are higher in the follicular fluid of oocytes that result in successful pregnancy [396,397], and GH supplementation increases oocyte yield as well as rates of pregnancies and live births with IVF [398,399]. Thus, GH is becoming an increasingly important compound-of-interest in assisted reproductive technologies [363,400–405].
Ghrelin
Identified in 1999 as the endogenous ligand of the growth hormone secretagogue receptor (GHSR) [406], acetylated ghrelin is a gastric hormone involved in sensing nutrient availability and coordinating meal anticipation, which complements its stimulation of growth hormone secretion and other metabolic effects [407]. Dubbed the ‘hunger hormone’ for its appetite-boosting effects [408], acetylated ghrelin is in fact a multi-faceted hormone that also stimulates gastric acid secretion and gut motility, promotes adiposity, decreases insulin sensitivity, and modulates glucose and lipid metabolism [407,409]. Ghrelin and its receptor are both widely expressed in human tissues, including in reproductive and endocrine organs [410], but most circulating ghrelin originates from enteroendocrine cells of the stomach [411]. Levels rise before meals and during fasting, largely due to neural regulation of gastric ghrelin secretion; conversely, there is a postprandial drop in ghrelin in response to nutrients and bitter compounds in the gastrointestinal system as well as input by hormones such as insulin and leptin [408,409,411,412]. Circulating ghrelin exists in two distinct forms due to its enzyme-catalyzed acetylation. Less than 10% is acetylated and capable of binding to the GHSR, while des-acylated ghrelin functionally antagonizes acetylated ghrelin and may also have independent effects [407,409].
Ghrelin and the female reproductive system
As an orexigenic hormone that signals nutrient insufficiency, ghrelin is generally a negative modifier of female reproduction. Women with amenorrhea associated with intense exercise or anorexia have higher levels of ghrelin [413–415]. Ghrelin levels decline during childhood and into puberty [416], and pubertal onset in female rats is delayed by high doses of ghrelin [417–419]. High ghrelin or ghrelin analog treatment reduces rates of ovulation, pregnancy, fertilization, and embryo implantation in mice, and suppresses ovine embryo development [420–423]. Interestingly, ghrelin levels increase with age and over the menopausal transition [412,424,425], which may contribute to postmenopausal shift in metabolic health [426].
Central in vivo effects of ghrelin include decreasing GnRH secretion and pulsatility, as well as lowering LH and/or FSH [415,418,427–431]. The GHSR is expressed in regions of the hypothalamus [432,433], Kiss1 neurons [434], and pituitary gonadotrophs [435]. Although GnRH neurons themselves do not express this receptor, ghrelin might act via upstream neuronal regulators to suppress GnRH release [221]. In contrast, ghrelin can stimulate LH secretion by pituitary tissue in vitro, pointing to an opposing, tissue-specific mode of action that might depend on such factors as age, sex, and interacting gonadal inputs [417,418,436].
Ghrelin also exerts direct ovarian effects. Ghrelin expression has been documented in the ovaries of species ranging from chicken to human [437–443], including in oocytes, corpus lutea, and stromal cells [437,439,440]. Ghrelin injections lower estrogen and progesterone levels in female rats [444], and it acts directly through GHSR of corpus luteal cells to reduce progesterone secretion [445,446], pointing to a role in regulating steroidogenesis. Ghrelin may also contribute toward repressing follicle maturation: ghrelin administration leads to greater numbers of small follicles coupled with fewer corpus lutea in rat ovaries [447], whereas mice lacking endogenous acetylated ghrelin have a decrease in small follicles [448]. Generally, elevated ghrelin suppresses female reproductive functions both centrally and peripherally, which is consistent with a message of energy depletion.
Leptin
Leptin, an indicator of stored fuel levels in adipose tissue, is involved in regulating long-term energy balance by influencing parameters such as energy expenditure, appetite, and reproductive function. Leptin is a peptide hormone principally secreted by adipocytes in white adipose tissue, though it can also be produced by other tissues such as the placenta, stomach, and skeletal muscle [449–453]. Serum leptin and adipocyte expression of the leptin gene (LEP or OB) are proportional to adipose tissue mass, with levels that generally rise with obesity and fall with weight loss [454]. LEP expression and circulating leptin are also affected by short-term energy imbalances, cytokines, the sympathetic nervous system, and other hormones such as insulin, glucocorticoids, and gonadal steroids [455]. In both humans and rodents, leptin levels are higher in females than in adiposity-matched males (particularly for premenopausal women); this is likely due to the regulation of leptin production by estrogens and testosterone, as well as sexual dimorphism in adipose tissue distribution [456–460]. Although leptin is not alone in controlling energy balance, its crucial role is evidenced by the excessive obesity, hyperphagia, and infertility of the leptin-deficient ob/ob mouse [461–463]. Similarly, rare mutations causing congenital leptin deficiency or leptin resistance in humans are associated with rapid weight gain, severe obesity, low gonadotropin levels, and delayed or absent puberty [464–467].
In response to elevated leptin—which indicates that energy balance has tipped towards abundant metabolic fuel stores—the hypothalamus induces a series of physiological processes that boost satiety and energy expenditure [467,468]. However, obesity is often coupled with both increased leptin levels and leptin resistance, which diminishes its effectiveness in promoting weight loss [467]. Low leptin levels correlate with reduced adiposity, which is interpreted by the hypothalamus as an energy deficit that requires neurological and physiological changes to promote food intake while reducing energy expenditure to restore energy balance [467].
The canonical leptin signaling pathway involves activation of JAK2 and phosphorylation of the transcription factor STAT3. However, due to pathway cross-talk leptin also activates other signal transduction cascades, such as the PI3K and MAPK pathways [468]. Only the full-length, long-form isoform of the leptin receptor has the intracellular domains required for signal transduction [469,470]. Although particularly abundant in the hypothalamus, there is nearly universal tissue distribution of leptin receptors, including expression of the long-form receptor in many brain regions and in the uterus and ovaries [469–472]. This highlights the fact that leptin signaling has a wide breadth of effects. In addition to its trademark impacts on appetite and energy expenditure, leptin is also involved in controlling lipolysis, immune function, angiogenesis, bone formation, and reproduction [468].
Leptin and the female reproductive system
Leptin is a fundamental regulator of female reproductive function that affects processes ranging from steroidogenesis and ovulation to puberty and pregnancy. Negative energy balance leads to decreased leptin, amenorrhea, and subfertility [473,474], and leptin administration in women with hypothalamic amenorrhea is sufficient to restore their menses and fertility, raise serum estradiol, and increase the number of dominant follicles [475–477]. Exogenous leptin also restores the fertility of ob/ob mice independent of body weight effects, by restoring HPG axis functioning [478,479]. Similarly, daily leptin injections in leptin-deficient children correct pubertal timing and gonadotropin pulsatility [480]. In rodents, leptin aids in pubertal activation of the HPG axis [481,482], although it alone cannot trigger puberty alone [478,483–486]. Low doses of leptin increase LH and FSH levels in mice [487], induce ovulation in an LH-dependent manner [488], and stimulate meiotic progression of bovine oocytes [489].
Leptin exerts some of these effects through indirect modulation of GnRH neurons and the pituitary. Leptin receptors are undetectable in murine GnRH neurons, and GnRH neuron-specific leptin receptor deletion does not affect fertility or puberty onset. However, mice lacking leptin receptors in all forebrain neurons have delayed puberty, severe infertility, and a suppressed estradiol-stimulated LH surge [490]. Leptin likely impacts GnRH release via upstream neuronal inputs [221], such as Kiss1 neurons [490–493]. It may also elicit direct effects on the pituitary [494], since cultured pituitary tissue dose-dependently releases LH, FSH and prolactin in response to leptin exposure [495].
There is also a relationship between leptin and the ovarian steroid hormones. LEP is expressed by granulosa, cumulus, and oocyte cells, with leptin protein detectable in mature follicles and follicular fluid [496,497]. Human and rat ovaries express leptin receptors on their theca, granulosa and interstitial cells [471,496–499], thus acting as target sites for leptin to regulate steroidogenesis. In vitro studies of human [499,500], bovine [501], and rat [502–504] cells or tissues have demonstrated that leptin attenuates steroid hormone production. Interestingly, there are reports of circulating leptin changing across the menstrual cycle, with a rise from menses into the luteal phase and a mid-cycle peak corresponding with the LH surge; leptin therefore shows some synchronicity with estradiol, progesterone, testosterone, and LH [475,505–507]. Some of these rhythmic changes may be due to leptin production by ovarian structures such as the corpus luteum [508,509]. However, leptin cyclicity during the menstrual cycle is controversial, with other studies reporting no differences [510–512].
During pregnancy, there is a two-fold increase in circulating leptin levels [513,514], due to both increased maternal adiposity and leptin secretion by the placenta [452]. As pregnancy progresses, the rise in leptin induces central resistance to its appetite-suppressing effects, and leptin takes on modified roles that include support of blastocyst formation, implantation, placentation, and human chorionic gonadotropin production [515–518]. Supraphysiological levels of leptin are associated with pregnancy disorders such as preeclampsia [519–524] and gestational diabetes [525]. Thus, leptin is involved in optimising and maintaining many aspects of female reproductive health and function.
Adiponectin
In addition to leptin, adipocytes also secrete another signaling molecule in large quantities: adiponectin. First discovered and characterized in 1995–1996, adiponectin is a 244-amino acid protein [526–529] with insulin-sensitizing [530,531], anti-inflammatory [532], anti-atherogenic [532–535], and cardioprotective [536,537] properties. Adiponectin is primarily secreted by adipocytes, but has been detected in other tissues, including the brain [538,539], gonads [540,541], and placenta [542]. In contrast with leptin, adiponectin levels are inverse to adiposity; adiponectin is lower among obese individuals [543–545], and restricting caloric intake can increase circulating adiponectin [546]. Testosterone also reduces adiponectin secretion [547], which may contribute toward the lower levels in men compared with women [543,544]. There are no apparent repercussions of menopause, estrogen therapy or ovary removal for adiponectin levels [548–550].
Adiponectin signals through binding to adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2), which are found abundantly among several tissues, but especially in the skeletal muscle and liver [551,552]. Ligand binding leads to a number of downstream signaling responses, including AMPK activation, mTOR inhibition, stimulation and cross-talk with the insulin/IGF-1 signaling pathways, and interactions with other signal transduction adaptor proteins [196]. These adiponectin-induced signaling cascades in central and peripheral tissues induce metabolically important responses. Exogenous adiponectin administration increases blood insulin levels in vivo [553], and promotes insulin gene expression and secretion in vitro [554]. Adiponectin-deficient mice develop hepatic insulin resistance and hyperglycemia, and are more sensitive to diet-induced metabolic dysfunction [530]. In addition, adiponectin-deficient female mice are subfertile, with altered menstrual cycles, altered gonadotropin profiles, reduced ovulation and a greater number of atretic follicles [555]. This points to a role in reproductive regulation.
Adiponectin and the female reproductive system
Adiponectin elicits both central and peripheral reproductive effects. Adiponectin receptors are found throughout the hypothalamus in a variety of species [556–558], and adiponectin is present in cerebrospinal fluid [557,559–562], which suggests a potential route of entry into the brain. Consistent with a function in promoting energy preservation, adiponectin suppresses GnRH secretion and inhibits Kiss1 gene expression by activating AMPK in a hypothalamic cell line [563,564], and it attenuates activity of a subpopulation of mouse GnRH neurons via AMPK activation [565]. Humans also express adiponectin and its receptors on pituitary cells, including gonadotrophs [566]. Adiponectin can reduce basal and GnRH-stimulated LH levels and GnRH receptor expression in cultured rodent pituitary cells [538,567], but reported effects are not consistent for pigs [568] or non-human primates [569].
In the ovary, adiponectin signaling influences oocyte maturation as well as the production and release of steroid hormones. Adiponectin and adiponectin receptors are expressed in ovarian theca cells, granulosa cells, oocytes, and corpus lutea [541,570–572], and levels appear somewhat responsive to gonadotropins [573–575]. Adiponectin regulates the expression of genes encoding steroidogenic enzymes and gonadotropin receptors, augments the IGF-1-stimulated release of progesterone and estradiol, and decreases androgen levels [541,570,576–579]. Adiponectin is also implicated in promoting oocyte meiotic maturation and early embryo development [572,580–583]. The mechanisms by which adiponectin exerts these ovarian effects have not been fully defined, but may involve interactions with the insulin/IGF-1 MAPK/ERK signaling pathway [541,570,576,579,581,582].
Low adiponectin (which can signify overabundant energy stores) is linked to reduced female reproductive health. Adiponectin levels are positively correlated with levels of the ovarian reserve biomarker AMH [215,584], and obesity corresponds with both low adiponectin and low AMH, among other endocrine changes [585]. Women with PCOS also have significantly lower adiponectin levels that correlate with lower metabolic health, compared with individuals matched for body mass index [586,587]. In addition, a decreased proportion of theca cells express adiponectin receptors in polycystic ovaries [588]. Low serum adiponectin or a low ratio of follicular fluid:serum adiponectin has been associated with unsuccessful IVF outcomes [589], higher rates of implantation failures [590], and low oocyte retrieval [591]. Therefore, adiponectin is yet another metabolic hormone at the nexus of metabolic health and reproductive function.
Conclusions and future perspectives
Research related to female reproductive health is disproportionately underfunded [592–595], with consequential impacts on the well-being of half the global population. Moreover, despite the existence of sex differences in the prevalence, pathophysiology, and responses to treatment of metabolic disorders such as Type 2 diabetes [9,10,596–598], a significant underrepresentation of female participants and female animals in metabolic health studies has persisted in recent decades [599–604]. As a result, each of these fields alone holds substantial knowledge gaps—to say nothing of the gaps in knowledge that exist at the interface of metabolic health and female reproductive health.
We believe that there are many critical questions at the junction of metabolism and reproduction. For instance:
While this review highlights effects of metabolic hormones on reproductive function, lines of communication between metabolic tissues and the reproductive system are bidirectional, and merit further study. For example, FSH was recently shown to regulate insulin secretion via FSH receptors expressed in pancreatic islets [605], and gonadal steroid hormones contribute towards sexual dimorphism in energy partitioning and metabolic homeostasis [8–10,606].
Exogenous hormonal contraceptives cause metabolic changes [607–609], and conversely, the presence of diabetes, obesity, and/or other metabolic disorders has implications for the systemic impacts of hormonal contraceptives [610,611]. Research into these intertwining effects is complicated by the heterogeneous nature of disorders such as Type 2 diabetes or polycystic ovary syndrome, in addition to wide variety in hormonal contraceptive formulations, and interplay of factors such as age, ethnicity, genetics, environment, and duration of contraceptive use [608,612].
Similar complications affect investigations of interactions between metabolic health and hormone replacement therapy, or between metabolic health and menstrual cycle characteristics, but these challenges should not preclude exploring such fundamental biomedical and biological topics.
Puberty, pregnancy, and perimenopause are defined by changes to the female reproductive system, and all three life stages also feature marked metabolic changes. For instance, insulin resistance and β-cell mass are transiently elevated during puberty [613–616] and during pregnancy [617–620], and the incidence or severity of metabolic syndrome increases significantly during perimenopause [621,622]. Therefore, it seems especially pertinent to understand the relationships between nutritional status, metabolic health, and female reproductive function during these transitional periods.
The high energetic requirements of supporting reproduction mean that the signaling systems communicating food intake, metabolic fuel stores, and energy levels play an integral role in regulating reproductive function. Consequently, impaired metabolic health has repercussions for female reproductive health that extend beyond fertility or fetal effects. Changes to nutritional status induce a suite of responses, and it is necessary to consider the context of a broad landscape of nutrient- and energy-responsive signaling systems instead of focusing on isolated hormones under specific conditions. For instance, food ingestion or a surplus of energy stores generally leads to increased levels of insulin, GIP, GLP-1, and leptin, together with suppression of growth hormone, ghrelin, and adiponectin; energy deficits tend to cause the opposite endocrine shifts (Figure 1). Each of these hormones can cause their own effects within the reproductive axis, in addition to generating signaling pathway cross-talk and interplay with other hormones. Moreover, the precise effects of each hormone might vary depending on nutritional conditions and interacting signaling factors. Delineating the complexities of these mechanistic relationships is essential for understanding how metabolic disorders or energy imbalance deregulates female reproductive health.
Abbreviations
- AdipoR
adiponectin receptor
- AdipoR1 and AdipoR2
adiponectin receptors 1 and 2
- AMH
anti-müllerian hormone
- AMPK
AMP- activated protein kinase
- FSH
follicle-stimulating hormone
- GH
growth hormone
- GHSR
growth hormone secretagogue receptor
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide 1
- GnRH
Gonadotropin-releasing hormone
- HPG
hypothalamic-pituitary-gonadal
- IGF-1
insulin-like growth factor 1
- InsR
insulin receptor
- JAK2
janus kinase 2
- Kiss1
kisspeptin
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- mTOR
mechanistic target of rapamycin
- PCOS
polycystic ovary syndrome
- PI3K
phosphatidylinositol 3-kinase
- STAT
signal transducers and activators of transcription
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The Templeman laboratory is supported by funding from the Canadian Institutes of Health Research [grant number PJT-183618] and the Natural Sciences Engineering Research Council of Canada [grant number RGPIN-2022-05149]. N.M.T. is a Tier 2 Canada Research Chair in Cell Biology and a Michael Smith Health Research BC Scholar, and this work was undertaken in part thanks to funding from the Canada Research Chairs Program and Michael Smith Health Research BC.
CRediT Author Contribution
Faria Athar: Writing—original draft. Muskan Karmani: Visualization, Writing—original draft. Nicole M. Templeman: Writing—original draft, Writing—review & editing.
References
- 1.Schneider J., Klingerman C. and Abdulhay A. (2012) Sense and nonsense in metabolic control of reproduction. Front Endocrinol. 3, 26 10.3389/fendo.2012.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball G.F. and Ketterson E.D. (2007) Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Philos. Trans. R. Soc. B Biol. Sci. 363, 231–246 10.1098/rstb.2007.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas D.W., Blondel J., Perret P., Lambrechts M.M. and Speakman J.R. (2001) Energetic and Fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600 10.1126/science.1057487 [DOI] [PubMed] [Google Scholar]
- 4.Luquet P. and Watanabe T. (1986) Interaction “nutrition-reproduction” in fish. Fish Physiol. Biochem. 2, 121–129 10.1007/BF02264080 [DOI] [PubMed] [Google Scholar]
- 5.Rideout R.M., Rose G.A. and Burton M.P.M. (2005) Skipped spawning in female iteroparous fishes. Fish Fish 6, 50–72 10.1111/j.1467-2679.2005.00174.x [DOI] [Google Scholar]
- 6.Karp X. (2021) Hormonal regulation of diapause and development in nematodes, insects, and fishes. Front Ecol. Evol. 9, 735924 10.3389/fevo.2021.735924 [DOI] [Google Scholar]
- 7.Short C.A. and Hahn D.A. (2023) Fat enough for the winter? Does nutritional status affect diapause? J. Insect Physiol. 145, 104488 10.1016/j.jinsphys.2023.104488 [DOI] [PubMed] [Google Scholar]
- 8.Bond S.T., Calkin A.C. and Drew B.G. (2021) Sex differences in white adipose tissue expansion: emerging molecular mechanisms. Clin. Sci. 135, 2691–2708 10.1042/CS20210086 [DOI] [PubMed] [Google Scholar]
- 9.Mauvais-Jarvis F. (2015) Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 6, 14 10.1186/s13293-015-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tramunt B., Smati S., Grandgeorge N., Lenfant F., Arnal J.-F., Montagner A.et al. (2020) Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63, 453–461 10.1007/s00125-019-05040-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meczekalski B., Tonetti A., Monteleone P., Bernardi F., Luisi S., Stomati M.et al. (2000) Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropin-releasing hormone test. Eur. J. Endocrinol. 142, 280–285 10.1530/eje.0.1420280 [DOI] [PubMed] [Google Scholar]
- 12.Bomba M., Gambera A., Bonini L., Peroni M., Neri F., Scagliola P.et al. (2007) Endocrine profiles and neuropsychologic correlates of functional hypothalamic amenorrhea in adolescents. Fertil. Steril. 87, 876–885 10.1016/j.fertnstert.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 13.Perkins R.B., Hall J.E. and Martin K.A. (2001) Aetiology, previous menstrual function and patterns of neuro-endocrine disturbance as prognostic indicators in hypothalamic amenorrhoea. Hum. Reprod. 16, 2198–2205 10.1093/humrep/16.10.2198 [DOI] [PubMed] [Google Scholar]
- 14.Golden N.H. and Carlson J.L. (2008) The pathophysiology of amenorrhea in the adolescent. Ann. N. Y. Acad. Sci. 1135, 163–178 10.1196/annals.1429.014 [DOI] [PubMed] [Google Scholar]
- 15.Frisch R.E., Wyshak G. and Vincent L. (1980) Delayed menarche and amenorrhea in ballet dancers. N. Engl. J. Med. 303, 17–19 10.1056/NEJM198007033030105 [DOI] [PubMed] [Google Scholar]
- 16.Warren M.P. (1980) The effects of exercise on pubertal progression and reproductive function in girls. J. Clin. Endocrinol. Metab. 51, 1150–1157 10.1210/jcem-51-5-1150 [DOI] [PubMed] [Google Scholar]
- 17.Frisch R.E., Gotz-Welbergen A.V., McArthur J.W., Albright T., Witschi J., Bullen B.et al. (1981) Delayed menarche and amenorrhea of college athletes in relation to age of onset of training. JAMA 246, 1559–1563 10.1001/jama.1981.03320140047029 [DOI] [PubMed] [Google Scholar]
- 18.Ravi S., Valtonen M., Ihalainen J.K., Holopainen E., Kosola S., Heinonen S.et al. (2023) Eating behaviours, menstrual history and the athletic career: a retrospective survey from adolescence to adulthood in female endurance athletes. BMJ Open Sport Exerc. Med. 9, e001489 10.1136/bmjsem-2022-001489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden N.H. and Shenker I.R. (1994) Amenorrhea in anorexia nervosa. Neuroendocrine control of hypothalamic dysfunction. Int. J. Eat. Disord. 16, 53–60 [DOI] [PubMed] [Google Scholar]
- 20.Warren M.P. (2011) Endocrine manifestations of eating disorders. J. Clin. Endocrinol. Metab. 96, 333–343 10.1210/jc.2009-2304 [DOI] [PubMed] [Google Scholar]
- 21.Hetland M.L., Haarbo J., Christiansen C. and Larsen T. (1993) Running induces menstrual disturbances but bone mass is unaffected, except in amenorrheic women. Am. J. Med. 95, 53–60 10.1016/0002-9343(93)90232-E [DOI] [PubMed] [Google Scholar]
- 22.Morrison A.E., Fleming S. and Levy M.J. (2021) A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin. Endocrinol. (Oxf) 95, 229–238 10.1111/cen.14399 [DOI] [PubMed] [Google Scholar]
- 23.Højlund K., Wildner-Christensen M., Eshøj O., Skjærbæk C., Holst J.J., Koldkjær O.et al. (2001) Reference intervals for glucose, β-cell polypeptides, and counterregulatory factors during prolonged fasting. Am. J. Physiol.-Endocrinol. Metab. 280, E50–E58 10.1152/ajpendo.2001.280.1.E50 [DOI] [PubMed] [Google Scholar]
- 24.Fahrenholtz I.L., Sjödin A., Benardot D., Tornberg Å.B., Skouby S., Faber J.et al. (2018) Within-day energy deficiency and reproductive function in female endurance athletes. Scand. J. Med. Sci. Sports 28, 1139–1146 10.1111/sms.13030 [DOI] [PubMed] [Google Scholar]
- 25.Kumar S. and Kaur G. (2013) Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo-hypophysial-gonadal axis. PLoS ONE 8, e52416 10.1371/journal.pone.0052416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoshdel A., Kheiri S., Hashemi-Dehkordi E., Nasiri J., Shabanian-Borujeni S. and Saedi E. (2014) The effect of Ramadan fasting on LH, FSH, oestrogen, progesterone and leptin in pregnant women. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 34, 634–638 10.3109/01443615.2014.920791 [DOI] [PubMed] [Google Scholar]
- 27.Cameron J.L. and Nosbisch C. (1991) Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey. (Macaca mulatta). Endocrinology 128, 1532–1540 10.1210/endo-128-3-1532 [DOI] [PubMed] [Google Scholar]
- 28.Gopalan C. and Nadamuni Naidu A. (1972) Nutrition and fertility. Lancet North Am. Ed. 300, 1077–1079 10.1016/S0140-6736(72)92355-0 [DOI] [PubMed] [Google Scholar]
- 29.Amegah A.K., Damptey O.K., Sarpong G.A., Duah E., Vervoorn D.J. and Jaakkola J.J.K. (2013) Malaria infection, poor nutrition and indoor air pollution mediate socioeconomic differences in adverse pregnancy outcomes in Cape Coast, Ghana. PLoS One 8, e69181 10.1371/journal.pone.0069181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamshed S., Khan F.-, Begum A., Barkat Ali B., Akram Z. and Ariff M. (2020) Frequency of low birth weight and its relationship with maternal nutritional and dietary factors: a cross-sectional study. Cureus 12, e8731 10.7759/cureus.8731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girsen A.I., Mayo J.A., Carmichael S.L., Phibbs C.S., Shachar B.Z., Stevenson D.K.et al. (2016) Women's prepregnancy underweight as a risk factor for preterm birth: a retrospective study. BJOG Int. J. Obstet. Gynaecol. 123, 2001–2007 10.1111/1471-0528.14027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cates J.E., Unger H.W., Briand V., Fievet N., Valea I., Tinto H.et al. (2017) Malaria, malnutrition, and birthweight: A meta-analysis using individual participant data. PLoS Med. 14, e1002373 10.1371/journal.pmed.1002373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams N.I., Helmreich D.L., Parfitt D.B., Caston-Balderrama A. and Cameron J.L. (2001) Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J. Clin. Endocrinol. Metab. 86, 5184–5193 10.1210/jcem.86.11.8024 [DOI] [PubMed] [Google Scholar]
- 34.Szymanski L.A., Schneider J.E., Friedman M.I., Ji H., Kurose Y., Blache D.et al. (2007) Changes in insulin, glucose and ketone bodies, but not leptin or body fat content precede restoration of luteinising hormone secretion in ewes. J. Neuroendocrinol. 19, 449–460 10.1111/j.1365-2826.2007.01551.x [DOI] [PubMed] [Google Scholar]
- 35.Jones J.E. and Lubbers L.S. (2001) Suppression and recovery of estrous behavior in Syrian hamsters after changes in metabolic fuel availability. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 280, R1393–R1398 10.1152/ajpregu.2001.280.5.R1393 [DOI] [PubMed] [Google Scholar]
- 36.Schneider J.E., Blum R.M. and Wade G.N. (2000) Metabolic control of food intake and estrous cycles in Syrian hamsters. I. Plasma insulin and leptin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R476–R485 10.1152/ajpregu.2000.278.2.R476 [DOI] [PubMed] [Google Scholar]
- 37.Schneider J.E. and Wade G.N. (1990) Decreased availability of metabolic fuels induces anestrus in golden hamsters. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 258, R750–R755 10.1152/ajpregu.1990.258.3.R750 [DOI] [PubMed] [Google Scholar]
- 38.Schneider J.E. and Wade G.N. (1989) Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science 244, 1326–1328 10.1126/science.2734610 [DOI] [PubMed] [Google Scholar]
- 39.Lado-Abeal J., Clapper J.A., Chen Zhu B., Hough C.M., Syapin P.J. and Norman R.L. (2002) Hypoglycemia-induced suppression of luteinizing hormone. (LH) secretion in intact female rhesus macaques: role of vasopressin and endogenous opioids. Stress Amst. Neth. 5, 113–119 10.1080/10253890290027886 [DOI] [PubMed] [Google Scholar]
- 40.Mircea C.N., Lujan M.E. and Pierson R.A. (2007) Metabolic Fuel and Clinical Implications for Female Reproduction. J. Obstet. Gynaecol. Can. 29, 887–902 10.1016/S1701-2163(16)32661-5 [DOI] [PubMed] [Google Scholar]
- 41.Bucholtz D.C., Vidwans N.M., Herbosa C.G., Schillo K.K. and Foster D.L. (1996) Metabolic interfaces between growth and reproduction. V. Pulsatile luteinizing hormone secretion is dependent on glucose availability. Endocrinology 137, 601–607 10.1210/endo.137.2.8593808 [DOI] [PubMed] [Google Scholar]
- 42.Roland A.V. and Moenter S.M. (2011) Regulation of gonadotropin-releasing hormone neurons by glucose. Trends Endocrinol. Metab. TEM 22, 443–449 10.1016/j.tem.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinoshita M., Moriyama R., Tsukamura H. and Maeda K.-I. (2003) A rat model for the energetic regulation of gonadotropin secretion: role of the glucose-sensing mechanism in the brain. Domest. Anim. Endocrinol. 25, 109–120 10.1016/S0739-7240(03)00050-X [DOI] [PubMed] [Google Scholar]
- 44.Nappi R.E., Chedraui P., Lambrinoudaki I. and Simoncini T. (2022) Menopause: a cardiometabolic transition. Lancet Diab. Endocrinol. 10, 442–456 10.1016/S2213-8587(22)00076-6 [DOI] [PubMed] [Google Scholar]
- 45.Cheng C.-H., Chen L.-R. and Chen K.-H. (2022) Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 23, 1376 10.3390/ijms23031376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogervorst E., Craig J. and O'Donnell E. (2022) Cognition and mental health in menopause: a review. Best Pract. Res. Clin. Obstet. Gynaecol. 81, 69–84 10.1016/j.bpobgyn.2021.10.009 [DOI] [PubMed] [Google Scholar]
- 47.Baker L., Meldrum K.K., Wang M., Sankula R., Vanam R., Raiesdana A.et al. (2003) The role of estrogen in cardiovascular disease. J. Surg. Res. 115, 325–344 10.1016/S0022-4804(03)00215-4 [DOI] [PubMed] [Google Scholar]
- 48.Lanyon L.E. (1996) Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone 18, S37–S43 10.1016/8756-3282(95)00378-9 [DOI] [PubMed] [Google Scholar]
- 49.Kalervo Väänänen H. and Härkönen P.L. (1996) Estrogen and bone metabolism. Maturitas 23, S65–S69 10.1016/0378-5122(96)01015-8 [DOI] [PubMed] [Google Scholar]
- 50.Schiessl H., Frost H.M. and Jee W.S.S. (1998) Estrogen and bone-muscle strength and mass relationships. Bone 22, 1–6 10.1016/S8756-3282(97)00223-8 [DOI] [PubMed] [Google Scholar]
- 51.Shufelt C.L., Torbati T. and Dutra E. (2017) Hypothalamic amenorrhea and the long-term health consequences. Semin. Reprod. Med. 35, 256–262 10.1055/s-0037-1603581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kearney J. (2010) Food consumption trends and drivers. Philos. Trans. R. Soc. B. Biol. Sci. 365, 2793–2807 10.1098/rstb.2010.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nardocci M., Leclerc B.-S., Louzada M.-L., Monteiro C.A., Batal M. and Moubarac J.-C. (2018) Consumption of ultra-processed foods and obesity in Canada. Can J. Public Health Rev. Can Santé Publique 110, 4–14 10.17269/s41997-018-0130-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abarca-Gómez L., Abdeen Z.A., Hamid Z.A., Abu-Rmeileh N.M., Acosta-Cazares B., Acuin C.et al. (2017) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet North Am. Ed. 390, 2627–2642 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speakman J.R., de Jong J.M.A., Sinha S., Westerterp K.R., Yamada Y., Sagayama H.et al. (2023) Total daily energy expenditure has declined over the past three decades due to declining basal expenditure, not reduced activity expenditure. Nat. Metab. 5, 579–588 10.1038/s42255-023-00782-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swinburn B.A., Sacks G., Hall K.D., McPherson K., Finegood D.T., Moodie M.L.et al. (2011) The global obesity pandemic: shaped by global drivers and local environments. Lancet North Am. Ed. 378, 804–814 10.1016/S0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- 57.Hohos N.M. and Skaznik-Wikiel M.E. (2017) High-fat diet and female fertility. Endocrinology 158, 2407–2419 10.1210/en.2017-00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skaznik-Wikiel M.E., Swindle D.C., Allshouse A.A., Polotsky A.J. and McManaman J.L. (2016) High-fat diet causes subfertility and compromised ovarian function independent of obesity in mice. Biol. Reprod. 94, 108 10.1095/biolreprod.115.137414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hohos N.M., Cho K.J., Swindle D.C. and Skaznik-Wikiel M.E. (2018) High-fat diet exposure, regardless of induction of obesity, is associated with altered expression of genes critical to normal ovulatory function. Mol. Cell. Endocrinol. 470, 199–207 10.1016/j.mce.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 60.Gonnella F., Konstantinidou F., Di Berardino C., Capacchietti G., Peserico A., Russo V.et al. (2022) A systematic review of the effects of high-fat diet exposure on oocyte and follicular quality: a molecular point of view. Int. J. Mol. Sci. 23, 8890 10.3390/ijms23168890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee J.M., Appugliese D., Kaciroti N., Corwyn R.F., Bradley R.H. and Lumeng J.C. (2007) Weight status in young girls and the onset of puberty. Pediatrics 119, e624–e630 10.1542/peds.2006-2188 [DOI] [PubMed] [Google Scholar]
- 62.Davison K.K., Susman E.J. and Birch L.L. (2003) Percent body fat at age 5 predicts earlier pubertal development among girls at age 9. Pediatrics 111, 815–821 10.1542/peds.111.4.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biro F.M., Pajak A., Wolff M.S., Pinney S.M., Windham G.C., Galvez M.P.et al. (2018) Age of menarche in a longitudinal US cohort. J. Pediatr. Adolesc. Gynecol. 31, 339–345 10.1016/j.jpag.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bralić I., Tahirović H., Matanić D., Vrdoljak O., Stojanović-Spehar S., Kovacić V.et al. (2012) Association of early menarche age and overweight/obesity. J. Pediatr. Endocrinol. Metab. JPEM 25, 57–62 10.1515/jpem-2011-0277 [DOI] [PubMed] [Google Scholar]
- 65.Barros B de S., Kuschnir M.C.M.C., Bloch K.V. and da Silva T.L.N. (2019) ERICA: age at menarche and its association with nutritional status. J. Pediatr. (Rio. J) 95, 106–111 10.1016/j.jped.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 66.Hillman J.B., Miller R.J. and Inge T.H. (2011) Menstrual concerns and intrauterine contraception among adolescent bariatric surgery patients. J. Womens Health 2002 20, 533–538 10.1089/jwh.2010.2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ko K.M., Han K., Chung Y.J., Yoon K.H., Park Y.G. and Lee S.H. (2017) Association between body weight changes and menstrual irregularity: the Korea National Health and Nutrition Examination Survey 2010 to 2012. Endocrinol. Metab. Seoul Korea 32, 248–256 10.3803/EnM.2017.32.2.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei S., Schmidt M.D., Dwyer T., Norman R.J. and Venn A.J. (2009) Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obes. Silver Spring Md. 17, 1070–1076 10.1038/oby.2008.641 [DOI] [PubMed] [Google Scholar]
- 69.He Y., Tian J., Blizzard L., Oddy W.H., Dwyer T., Bazzano L.A.et al. (2020) Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: the Childhood Determinants of Adult Health Study and the Bogalusa Heart Study. Hum Reprod. Oxf. Engl. 35, 1185–1198 10.1093/humrep/deaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silvestris E., de Pergola G., Rosania R. and Loverro G. (2018) Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. RBE 16, 22 10.1186/s12958-018-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasquali R., Patton L. and Gambineri A. (2007) Obesity and infertility. Curr. Opin. Endocrinol. Diab. Obes. 14, 482 10.1097/MED.0b013e3282f1d6cb [DOI] [PubMed] [Google Scholar]
- 72.Dağ Z.Ö. and Dilbaz B. (2015) Impact of obesity on infertility in women. J. Turk. Ger. Gynecol. Assoc. 16, 111–117 10.5152/jtgga.2015.15232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Talmor A. and Dunphy B. (2015) Female Obesity and Infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 29, 498–506 10.1016/j.bpobgyn.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 74.Lashen H., Fear K. and Sturdee D.W. (2004) Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum. Reprod. Oxf. Engl. 19, 1644–1646 10.1093/humrep/deh277 [DOI] [PubMed] [Google Scholar]
- 75.Fedorcsák P., Dale P.O., Storeng R., Ertzeid G., Bjercke S., Oldereid N.et al. (2004) Impact of overweight and underweight on assisted reproduction treatment. Hum. Reprod. Oxf. Engl. 19, 2523–2528 10.1093/humrep/deh485 [DOI] [PubMed] [Google Scholar]
- 76.Bellver J., Rossal L.P., Bosch E., Zúñiga A., Corona J.T., Meléndez F.et al. (2003) Obesity and the risk of spontaneous abortion after oocyte donation. Fertil. Steril. 79, 1136–1140 10.1016/S0015-0282(03)00176-6 [DOI] [PubMed] [Google Scholar]
- 77.Leddy M.A., Power M.L. and Schulkin J. (2008) The impact of maternal obesity on maternal and fetal health. Rev. Obstet. Gynecol. 1, 170–178 [PMC free article] [PubMed] [Google Scholar]
- 78.Persson M., Cnattingius S., Villamor E., Söderling J., Pasternak B., Stephansson O.et al. (2017) Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ 357, j2563 10.1136/bmj.j2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castro L.C. and Avina R.L. (2002) Maternal obesity and pregnancy outcomes. Curr. Opin. Obstet. Gynecol. 14, 601 10.1097/00001703-200212000-00005 [DOI] [PubMed] [Google Scholar]
- 80.Ray J.G., Wyatt P.R., Vermeulen M.J., Meier C. and Cole D.E.C. (2005) Greater maternal weight and the ongoing risk of neural tube defects after folic acid flour fortification. Obstet. Gynecol. 105, 261–265 10.1097/01.AOG.0000151988.84346.3e [DOI] [PubMed] [Google Scholar]
- 81.Watkins M.L., Rasmussen S.A., Honein M.A., Botto L.D. and Moore C.A. (2003) Maternal obesity and risk for birth defects. Pediatrics 111, 1152–1158 10.1542/peds.111.S1.1152 [DOI] [PubMed] [Google Scholar]
- 82.Cedergren M.I. and Källén B.A.J. (2003) Maternal obesity and infant heart defects. Obes. Res. 11, 1065–1071 10.1038/oby.2003.146 [DOI] [PubMed] [Google Scholar]
- 83.Nagel G., Altenburg H.P., Nieters A., Boffetta P. and Linseisen J. (2005) Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas 52, 337–347 10.1016/j.maturitas.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 84.Dunneram Y., Greenwood D.C., Burley V.J. and Cade J.E. (2018) Dietary intake and age at natural menopause: results from the UK Women's Cohort Study. J. Epidemiol. Community Health 72, 733–740 10.1136/jech-2017-209887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gambineri A., Pelusi C., Vicennati V., Pagotto U. and Pasquali R. (2002) Obesity and the polycystic ovary syndrome. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 26, 883–896 10.1038/sj.ijo.0801994 [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez Paris V., Solon-Biet S.M., Senior A.M., Edwards M.C., Desai R., Tedla N.et al. (2020) Defining the impact of dietary macronutrient balance on PCOS traits. Nat. Commun. 11, 5262 10.1038/s41467-020-19003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witchel S.F., Oberfield S.E. and Peña A.S. (2019) Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J. Endocr. Soc. 3, 1545–1573 10.1210/js.2019-00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teede H., Deeks A. and Moran L. (2010) Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 8, 41 10.1186/1741-7015-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diamanti-Kandarakis E. and Dunaif A. (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33, 981–1030 10.1210/er.2011-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Melo A.S., Dias S.V., de Carvalho Cavalli R., Cardoso V.C., Bettiol H., Barbieri M.A.et al. (2015) Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reprod. Camb. Engl. 150, R11–R24 10.1530/REP-14-0499 [DOI] [PubMed] [Google Scholar]
- 91.Poretsky L., Cataldo N.A., Rosenwaks Z. and Giudice L.C. (1999) The insulin-related ovarian regulatory system in health and disease. Endocr. Rev. 20, 535–582 10.1210/edrv.20.4.0374 [DOI] [PubMed] [Google Scholar]
- 92.Rosenfield R.L. and Ehrmann D.A. (2016) The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520 10.1210/er.2015-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Villa J. and Pratley R.E. (2011) Adipose tissue dysfunction in polycystic ovary syndrome. Curr. Diab. Rep. 11, 179–184 10.1007/s11892-011-0189-8 [DOI] [PubMed] [Google Scholar]
- 94.Delitala A.P., Capobianco G., Delitala G., Cherchi P.L. and Dessole S. (2017) Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch. Gynecol. Obstet. 296, 405–419 10.1007/s00404-017-4429-2 [DOI] [PubMed] [Google Scholar]
- 95.Kershaw E.E. and Flier J.S. (2004) Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 96.Guo W., Wang Y., Ma Y., Cui Z., Zhang L., Nie L.et al. (2021) Contribution of high-fat diet-induced PCSK9 upregulation to a mouse model of PCOS is mediated partly by SREBP2. Reproduction 162, 397–410 10.1530/REP-21-0164 [DOI] [PubMed] [Google Scholar]
- 97.Zheng Y.-H., Xu Y., Ma H.-X., Liang C.-J. and Yang T. (2021) Effect of high-fat diet on the intestinal flora in letrozole-induced polycystic ovary syndrome rats. Evid. Based Complement Alternat. Med. 2021, e6674965 10.1155/2021/6674965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H., Yi M., Zhang Y., Jin H., Zhang W., Yang J.et al. (2016) High-fat diets exaggerate endocrine and metabolic phenotypes in a rat model of DHEA-induced PCOS. Reprod. Camb. Engl. 151, 431–441 10.1530/REP-15-0542 [DOI] [PubMed] [Google Scholar]
- 99.Boomsma C.M., Eijkemans M.J.C., Hughes E.G., Visser G.H.A., Fauser B.C.J.M. and Macklon N.S. (2006) A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 12, 673–683 10.1093/humupd/dml036 [DOI] [PubMed] [Google Scholar]
- 100.Lo J.C., Feigenbaum S.L., Escobar G.J., Yang J., Crites Y.M. and Ferrara A. (2006) Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study. Diabetes Care 29, 1915–1917 10.2337/dc06-0877 [DOI] [PubMed] [Google Scholar]
- 101.Legro R.S. (2007) Pregnancy considerations in women with polycystic ovary syndrome. Clin. Obstet. Gynecol. 50, 295–304 10.1097/GRF.0b013e31803057ed [DOI] [PubMed] [Google Scholar]
- 102.Sawada M., Masuyama H., Hayata K., Kamada Y., Nakamura K. and Hiramatsu Y. (2015) Pregnancy complications and glucose intolerance in women with polycystic ovary syndrome. Endocr. J. 62, 1017–1023 10.1507/endocrj.EJ15-0364 [DOI] [PubMed] [Google Scholar]
- 103.Manoharan V. and Wong V.W. (2020) Impact of comorbid polycystic ovarian syndrome and gestational diabetes mellitus on pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth 20, 484 10.1186/s12884-020-03175-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roos N., Kieler H., Sahlin L., Ekman-Ordeberg G., Falconer H. and Stephansson O. (2011) Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 343, d6309 10.1136/bmj.d6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Vries M.J., Dekker G.A. and Schoemaker J. (1998) Higher risk of preeclampsia in the polycystic ovary syndrome: A case control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 76, 91–95 10.1016/S0301-2115(97)00164-4 [DOI] [PubMed] [Google Scholar]
- 106.Rees D.A., Jenkins-Jones S. and Morgan C.L. (2016) Contemporary reproductive outcomes for patients with polycystic ovary syndrome: a retrospective observational study. J. Clin. Endocrinol. Metab. 101, 1664–1672 10.1210/jc.2015-2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ashrafi M., Sheikhan F., Arabipoor A., Hosseini R., Nourbakhsh F. and Zolfaghari Z. (2014) Gestational diabetes mellitus risk factors in women with polycystic ovary syndrome. (PCOS). Eur. J. Obstet. Gynecol. Reprod. Biol. 181, 195–199 10.1016/j.ejogrb.2014.07.043 [DOI] [PubMed] [Google Scholar]
- 108.Mills G., Badeghiesh A., Suarthana E., Baghlaf H. and Dahan M.H. (2020) Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: a population-based study on 9.1 million pregnancies. Hum. Reprod. Oxf. Engl. 35, 1666–1674 10.1093/humrep/deaa099 [DOI] [PubMed] [Google Scholar]
- 109.Toulis K.A., Goulis D.G., Kolibianakis E.M., Venetis C.A., Tarlatzis B.C. and Papadimas I. (2009) Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil. Steril. 92, 667–677 10.1016/j.fertnstert.2008.06.045 [DOI] [PubMed] [Google Scholar]
- 110.Wild S., Pierpoint T., Jacobs H. and McKeigue P. (2000) Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil. Camb. Engl. 3, 101–105 10.1080/1464727002000198781 [DOI] [PubMed] [Google Scholar]
- 111.Pierpoint T., McKeigue P.M., Isaacs A.J., Wild S.H. and Jacobs H.S. (1998) Mortality of women with polycystic ovary syndrome at long-term follow-up. J. Clin. Epidemiol. 51, 581–586 10.1016/S0895-4356(98)00035-3 [DOI] [PubMed] [Google Scholar]
- 112.Legro R.S., Kunselman A.R., Dodson W.C. and Dunaif A. (1999) Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J. Clin. Endocrinol. Metab. 84, 165–169 [DOI] [PubMed] [Google Scholar]
- 113.Cheang K.I., Nestler J.E. and Futterweit W. (2008) Risk of cardiovascular events in mothers of women with polycystic ovary syndrome. Endocr. Pract. Off. J. Am. Coll Endocrinol. Am. Assoc. Clin. Endocrinol. 14, 1084–1094 10.4158/EP.14.9.1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joham A.E., Boyle J.A., Zoungas S. and Teede H.J. (2015) Hypertension in reproductive-aged women with polycystic ovary syndrome and association with obesity. Am. J. Hypertens. 28, 847–851 10.1093/ajh/hpu251 [DOI] [PubMed] [Google Scholar]
- 115.Ding D.-C., Tsai I.-J., Wang J.-H., Lin S.-Z. and Sung F.-C. (2018) Coronary artery disease risk in young women with polycystic ovary syndrome. Oncotarget 9, 8756–8764 10.18632/oncotarget.23985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glintborg D., Hass Rubin K., Nybo M., Abrahamsen B. and Andersen M. (2015) Morbidity and medicine prescriptions in a nationwide Danish population of patients diagnosed with polycystic ovary syndrome. Eur. J. Endocrinol. 172, 627–638 10.1530/EJE-14-1108 [DOI] [PubMed] [Google Scholar]
- 117.Sirmans S.M., Parish R.C., Blake S. and Wang X. (2014) Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J. Investig Med. Off. Publ. Am. Fed. Clin. Res. 62, 868–874 10.1097/01.JIM.0000446834.90599.5d [DOI] [PubMed] [Google Scholar]
- 118.Kim J.J. and Choi Y.M. (2013) Dyslipidemia in women with polycystic ovary syndrome. Obstet. Gynecol. Sci. 56, 137–142 10.5468/ogs.2013.56.3.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Legro R.S., Kunselman A.R. and Dunaif A. (2001) Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am. J. Med. 111, 607–613 10.1016/S0002-9343(01)00948-2 [DOI] [PubMed] [Google Scholar]
- 120.Roe A., Hillman J., Butts S., Smith M., Rader D., Playford M.et al. (2014) Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J. Clin. Endocrinol. Metab. 99, E841–E847 10.1210/jc.2013-3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Y., Wang X., Jiang Y., Ma H., Chen L., Lai C.et al. (2017) Association between polycystic ovary syndrome and the risk of stroke and all-cause mortality: insights from a meta-analysis. Gynecol. Endocrinol. 33, 904–910 10.1080/09513590.2017.1347779 [DOI] [PubMed] [Google Scholar]
- 122.Okoroh E.M., Boulet S.L., George M.G. and Craig Hooper W. (2015) Assessing the intersection of cardiovascular disease, venous thromboembolism, and polycystic ovary syndrome. Thromb. Res. 136, 1165–1168 10.1016/j.thromres.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Indhavivadhana S., Rattanachaiyanont M., Wongwananuruk T., Techatraisak K., Rayasawath N. and Dangrat C. (2018) Endometrial neoplasia in reproductive-aged Thai women with polycystic ovary syndrome. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 142, 170–175 10.1002/ijgo.12522 [DOI] [PubMed] [Google Scholar]
- 124.Fearnley E.J., Marquart L., Spurdle A.B., Weinstein P., Webb P.M., Australian Ovarian Cancer Study Group et al. (2010) Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control CCC 21, 2303–2308 10.1007/s10552-010-9658-7 [DOI] [PubMed] [Google Scholar]
- 125.Schildkraut J.M., Schwingl P.J., Bastos E., Evanoff A. and Hughes C. (1996) Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet. Gynecol. 88, 554–559 10.1016/0029-7844(96)00226-8 [DOI] [PubMed] [Google Scholar]
- 126.Yin W., Falconer H., Yin L., Xu L. and Ye W. (2019) Association Between Polycystic Ovary Syndrome and Cancer Risk. JAMA Oncol. 5, 106–107 10.1001/jamaoncol.2018.5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kiddy D.S., Hamilton-Fairley D., Bush A., Short F., Anyaoku V., Reed M.J.et al. (1992) Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. (Oxf) 36, 105–111 10.1111/j.1365-2265.1992.tb02909.x [DOI] [PubMed] [Google Scholar]
- 128.Hollmann M., Runnebaum B. and Gerhard I. (1996) Effects of weight loss on the hormonal profile in obese, infertile women. Hum Reprod. Oxf. Engl. 11, 1884–1891 10.1093/oxfordjournals.humrep.a019512 [DOI] [PubMed] [Google Scholar]
- 129.Huber-Buchholz M.M., Carey D.G. and Norman R.J. (1999) Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J. Clin. Endocrinol. Metab. 84, 1470–1474 10.1210/jc.84.4.1470 [DOI] [PubMed] [Google Scholar]
- 130.Guzick D.S., Wing R., Smith D., Berga S.L. and Winters S.J. (1994) Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertil. Steril. 61, 598–604 10.1016/S0015-0282(16)56632-1 [DOI] [PubMed] [Google Scholar]
- 131.Sim K.A., Dezarnaulds G.M., Denyer G.S., Skilton M.R. and Caterson I.D. (2014) Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial. Clin. Obes. 4, 61–68 10.1111/cob.12048 [DOI] [PubMed] [Google Scholar]
- 132.Palomba S., Falbo A., Giallauria F., Russo T., Rocca M., Tolino A.et al. (2010) Six weeks of structured exercise training and hypocaloric diet increases the probability of ovulation after clomiphene citrate in overweight and obese patients with polycystic ovary syndrome: a randomized controlled trial. Hum Reprod. Oxf. Engl. 25, 2783–2791 10.1093/humrep/deq254 [DOI] [PubMed] [Google Scholar]
- 133.Moran L., Tsagareli V., Norman R. and Noakes M. (2011) Diet and IVF pilot study: short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. Aust. N. Z. J. Obstet. Gynaecol. 51, 455–459 10.1111/j.1479-828X.2011.01343.x [DOI] [PubMed] [Google Scholar]
- 134.Legro R.S., Dodson W.C., Kris-Etherton P.M., Kunselman A.R., Stetter C.M., Williams N.I.et al. (2015) Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 100, 4048–4058 10.1210/jc.2015-2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goldman R.H., Missmer S.A., Robinson M.K., Farland L.V. and Ginsburg E.S. (2016) Reproductive outcomes differ following Roux-en-Y gastric bypass and adjustable gastric band compared with those of an obese non-surgical group. Obes. Surg. 26, 2581–2589 10.1007/s11695-016-2158-4 [DOI] [PubMed] [Google Scholar]
- 136.Edison E., Whyte M., van Vlymen J., Jones S., Gatenby P., de Lusignan S.et al. (2016) Bariatric surgery in obese women of reproductive age improves conditions that underlie fertility and pregnancy outcomes: retrospective cohort study of UK National Bariatric Surgery Registry. (NBSR). Obes. Surg. 26, 2837–2842 10.1007/s11695-016-2202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee R., Mathew C.J., Jose M.T., Elshaikh A.O., Shah L., Cancarevic I.et al. (2020) A review of the impact of bariatric surgery in women with polycystic ovary syndrome. Cureus 12, e10811 10.7759/cureus.10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jamal M., Gunay Y., Capper A., Eid A., Heitshusen D. and Samuel I. (2012) Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: a 9-year analysis. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr Surg. 8, 440–444 10.1016/j.soard.2011.09.022 [DOI] [PubMed] [Google Scholar]
- 139.Khazraei H., Hosseini S.V., Amini M., Bananzadeh A., Najibpour N., Ganji F.et al. (2017) Effect of weight loss after laparoscopic sleeve gastrectomy on infertility of women in Shiraz. J. Gynecol. Surg. 33, 43–46 10.1089/gyn.2016.0064 [DOI] [Google Scholar]
- 140.Legro R.S., Dodson W.C., Gnatuk C.L., Estes S.J., Kunselman A.R., Meadows J.W.et al. (2012) Effects of gastric bypass surgery on female reproductive function. J. Clin. Endocrinol. Metab. 97, 4540–4548 10.1210/jc.2012-2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cowan S., Lim S., Alycia C., Pirotta S., Thomson R., Gibson-Helm M.et al. (2023) Lifestyle management in polycystic ovary syndrome - beyond diet and physical activity. BMC Endocr. Disord. 23, 14 10.1186/s12902-022-01208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gaskins A.J. and Chavarro J.E. (2018) Diet and fertility: a review. Am. J. Obstet. Gynecol. 218, 379–389 10.1016/j.ajog.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kudesia R., Alexander M., Gulati M., Kennard A. and Tollefson M. (2021) Dietary approaches to women's sexual and reproductive health. Am. J. Lifestyle Med. 15, 414–424 10.1177/15598276211007113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Panth N., Gavarkovs A., Tamez M. and Mattei J. (2018) The influence of diet on fertility and the implications for public health nutrition in the United States. Front Public Health 6, 211 10.3389/fpubh.2018.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ojeda S.R., Lomniczi A. and Sandau U.S. (2008) Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J. Neuroendocrinol. 20, 732–742 10.1111/j.1365-2826.2008.01712.x [DOI] [PubMed] [Google Scholar]
- 146.Ruiz-Cruz M., Torres-Granados C., Tena-Sempere M. and Roa J. (2023) Central and peripheral mechanisms involved in the control of GnRH neuronal function by metabolic factors. Curr. Opin. Pharmacol. 71, 102382 10.1016/j.coph.2023.102382 [DOI] [PubMed] [Google Scholar]
- 147.Marshall J.C., Dalkin A.C., Haisenleder D.J., Griffin M.L. and Kelch R.P. (1993) GnRH pulses–the regulators of human reproduction. Trans. Am. Clin. Climatol. Assoc. 104, 31–46 [PMC free article] [PubMed] [Google Scholar]
- 148.Okamura H., Tsukamura H., Ohkura S., Uenoyama Y., Wakabayashi Y. and Maeda K. (2013) Kisspeptin and GnRH pulse generation. In Kisspeptin Signaling in Reproductive Biology(Kauffman A.S. and Smith J.T., eds), pp. 297–323, Springer, New York, NY: 10.1007/978-1-4614-6199-9_14 [DOI] [PubMed] [Google Scholar]
- 149.Maeda K., Ohkura S., Uenoyama Y., Wakabayashi Y., Oka Y., Tsukamura H.et al. (2010) Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 1364, 103–115 10.1016/j.brainres.2010.10.026 [DOI] [PubMed] [Google Scholar]
- 150.Lehman M.N. (2022) Origins of the ‘KNDy hypothesis’ of GnRH pulse generation. Nat. Rev. Endocrinol. 18, 521–521 10.1038/s41574-022-00703-5 [DOI] [PubMed] [Google Scholar]
- 151.Schwanzel-Fukuda M., Crossin K.L., Pfaff D.W., Bouloux P.M., Hardelin J.P. and Petit C. (1996) Migration of luteinizing hormone-releasing hormone. (LHRH) neurons in early human embryos. J. Comp. Neurol. 366, 547–557 [DOI] [PubMed] [Google Scholar]
- 152.Bizzarri C. and Cappa M. (2020) Ontogeny of hypothalamus-pituitary gonadal axis and minipuberty: an ongoing debate? Front Endocrinol. 11, 187 10.3389/fendo.2020.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Clarkson J., Han S.-K., Liu X., Lee K. and Herbison A.E. (2010) Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone. (GnRH) neurons at puberty. Mol. Cell. Endocrinol. 324, 45–50 10.1016/j.mce.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 154.Spaziani M., Tarantino C., Tahani N., Gianfrilli D., Sbardella E., Lenzi A.et al. (2021) Hypothalamo-pituitary axis and puberty. Mol. Cell. Endocrinol. 520, 111094 10.1016/j.mce.2020.111094 [DOI] [PubMed] [Google Scholar]
- 155.de Roux N., Genin E., Carel J.-C., Matsuda F., Chaussain J.-L. and Milgrom E. (2003) Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U. S. A. 100, 10972–10976 10.1073/pnas.1834399100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seminara S.B., Messager S., Chatzidaki E.E., Thresher R.R., Acierno J.S., Shagoury J.K.et al. (2003) The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349, 1614–1627 10.1056/NEJMoa035322 [DOI] [PubMed] [Google Scholar]
- 157.Navarro V.M., Castellano J.M., Fernández-Fernández R., Barreiro M.L., Roa J., Sanchez-Criado J.E.et al. (2004) Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145, 4565–4574 10.1210/en.2004-0413 [DOI] [PubMed] [Google Scholar]
- 158.Schally A.V., Arimura A., Kastin A.J., Matsuo H., Baba Y., Redding T.W.et al. (1971) Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 173, 1036–1038 10.1126/science.173.4001.1036 [DOI] [PubMed] [Google Scholar]
- 159.Schally A.V., Arimura A., Baba Y., Nair R.M., Matsuo H., Redding T.W.et al. (1971) Isolation and properties of the FSH and LH-releasing hormone. Biochem. Biophys. Res. Commun. 43, 393–399 10.1016/0006-291X(71)90766-2 [DOI] [PubMed] [Google Scholar]
- 160.McNeilly A.S., Crawford J.L., Taragnat C., Nicol L. and McNeilly J.R. (2003) The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod. Camb. Engl. Suppl. 61, 463–476 [PubMed] [Google Scholar]
- 161.Plant T.M. (2015) The hypothalamo-pituitary-gonadal axis. J. Endocrinol. 226, T41–T54 10.1530/JOE-15-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pinkerton J.H., McKay D.G., Adams E.C. and Hertig A.T. (1961) Development of the human ovary–a study using histochemical technics. Obstet. Gynecol. 18, 152–181 [PubMed] [Google Scholar]
- 163.Pepling M.E. (2012) Follicular assembly: mechanisms of action. Reprod. Camb. Engl. 143, 139–149 10.1530/REP-11-0299 [DOI] [PubMed] [Google Scholar]
- 164.O'Connell J.M. and Pepling M.E. (2021) Primordial follicle formation - some assembly required. Curr. Opin. Endocr. Metab. Res. 18, 118–127 10.1016/j.coemr.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baker T.G. (1963) A quantitative and cytological study of germ cells in human ovaries. Proc. R. Soc. Lond. B Biol. Sci. 158, 417–433 10.1098/rspb.1963.0055 [DOI] [PubMed] [Google Scholar]
- 166.Findlay J.K., Hutt K.J., Hickey M. and Anderson R.A. (2015) How is the number of primordial follicles in the ovarian reserve established? Biol. Reprod. 93, 111, 1-7 10.1095/biolreprod.115.133652 [DOI] [PubMed] [Google Scholar]
- 167.Hansen K.R., Hodnett G.M., Knowlton N. and Craig L.B. (2011) Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil. Steril. 95, 170–175 10.1016/j.fertnstert.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 168.Wallace W.H.B. and Kelsey T.W. (2010) Human ovarian reserve from conception to the menopause. PLoS ONE 5, e8772 10.1371/journal.pone.0008772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kelsey T.W., Anderson R.A., Wright P., Nelson S.M. and Wallace W.H.B. (2012) Data-driven assessment of the human ovarian reserve. Mol. Hum. Reprod. 18, 79–87 10.1093/molehr/gar059 [DOI] [PubMed] [Google Scholar]
- 170.Williams C.J. and Erickson G.F. (2000) Morphology and physiology of the ovary. In Endotext(Feingold K.R., Anawalt B. and Blackman M.R.et al., eds), MDText.com, Inc., South Dartmouth. (MA) [Google Scholar]
- 171.Allan C.M., Wang Y., Jimenez M., Marshan B., Spaliviero J., Illingworth P.et al. (2006) Follicle-stimulating hormone increases primordial follicle reserve in mature female hypogonadal mice. J. Endocrinol. 188, 549–557 10.1677/joe.1.06614 [DOI] [PubMed] [Google Scholar]
- 172.McGee E.A., Perlas E., LaPolt P.S.et al. (1997) Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol. Reprod. 57, 990–998 10.1095/biolreprod57.5.990 [DOI] [PubMed] [Google Scholar]
- 173.Oktay K., Newton H., Mullan J. and Gosden R.G. (1998) Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. Oxf. Engl. 13, 1133–1138 10.1093/humrep/13.5.1133 [DOI] [PubMed] [Google Scholar]
- 174.Kishi H., Kitahara Y., Imai F., Nakao K. and Suwa H. (2018) Expression of the gonadotropin receptors during follicular development. Reprod. Med. Biol. 17, 11–19 10.1002/rmb2.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rodgers R.J. and Irving-Rodgers H.F. (2010) Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 82, 1021–1029 10.1095/biolreprod.109.082941 [DOI] [PubMed] [Google Scholar]
- 176.McGee E.A. and Hsueh A.J. (2000) Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 21, 200–214 [DOI] [PubMed] [Google Scholar]
- 177.Franks S. (2021) Androgen production and action in the ovary. Curr. Opin. Endocr. Metab. Res. 18, 48–53 10.1016/j.coemr.2021.02.002 [DOI] [Google Scholar]
- 178.Hillier S.G., Whitelaw P.F. and Smyth C.D. (1994) Follicular oestrogen synthesis: the “two-cell, two-gonadotrophin” model revisited. Mol. Cell. Endocrinol. 100, 51–54 10.1016/0303-7207(94)90278-X [DOI] [PubMed] [Google Scholar]
- 179.Zeleznik A.J. (2004) The physiology of follicle selection. Reprod. Biol. Endocrinol. RBE 2, 31 10.1186/1477-7827-2-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeleznik A.J. (1981) Premature elevation of systemic estradiol reduces serum levels of follicle-stimulating hormone and lengthens the follicular phase of the menstrual cycle in rhesus monkeys. Endocrinology 109, 352–355 10.1210/endo-109-2-352 [DOI] [PubMed] [Google Scholar]
- 181.Strauss J.F. and Williams C.J. (2019) Chapter 8 - Ovarian Life Cycle. In Yen and Jaffe's Reproductive Endocrinology Eighth Edition(Strauss J.F. and Barbieri R.L., eds), pp. 167.e9–205.e9, Elsevier, Philadelphia [Google Scholar]
- 182.Turathum B., Gao E.-M. and Chian R.-C. (2021) The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 10, 2292 10.3390/cells10092292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reynolds L.P. and Redmer D.A. (1999) Growth and development of the corpus luteum. J. Reprod. Fertil. Suppl. 54, 181–191 [PubMed] [Google Scholar]
- 184.Mlyczyńska E., Kieżun M., Kurowska P., Dawid M., Pich K., Respekta N.et al. (2022) New aspects of corpus luteum regulation in physiological and pathological conditions: involvement of adipokines and neuropeptides. Cells 11, 957 10.3390/cells11060957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stolk L., Perry J.R.B., Chasman D.I., He C., Mangino M., Sulem P.et al. (2012) Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat. Genet. 44, 260–268 10.1038/ng.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pelosi E., Simonsick E., Forabosco A., Garcia-Ortiz J.E. and Schlessinger D. (2015) Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biol. Reprod. 92, 130, 1-9 10.1095/biolreprod.114.127381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Appiah D., Nwabuo C.C., Ebong I.A., Wellons M.F. and Winters S.J. (2021) Trends in age at natural menopause and reproductive life span among US women, 1959-2018. JAMA 325, 1328–1330 10.1001/jama.2021.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gold E.B. (2011) The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. North Am. 38, 425–440 10.1016/j.ogc.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hoyt L.T. and Falconi A. (2015) Puberty and perimenopause: reproductive transitions and their implications for women's health. Soc. Sci. Med. 1982 132, 103 10.1016/j.socscimed.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Atwood C.S., Meethal S.V., Liu T., Wilson A.C., Gallego M., Smith M.A.et al. (2005) Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J. Neuropathol. Exp. Neurol. 64, 93–103 10.1093/jnen/64.2.93 [DOI] [PubMed] [Google Scholar]
- 191.Estienne A., Bongrani A., Ramé C., Kurowska P., Błaszczyk K., Rak A.et al. (2021) Energy sensors and reproductive hypothalamo-pituitary ovarian axis. (HPO) in female mammals: Role of mTOR. (mammalian target of rapamycin), AMPK. (AMP-activated protein kinase) and SIRT1. (Sirtuin 1). Mol. Cell. Endocrinol. 521, 111113 10.1016/j.mce.2020.111113 [DOI] [PubMed] [Google Scholar]
- 192.Guo Z. and Yu Q. (2019) Role of mTOR signaling in female reproduction. Front Endocrinol. 10, 1–13 10.3389/fendo.2019.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Correia B., Sousa M.I. and Ramalho-Santos J. (2020) The mTOR pathway in reproduction: from gonadal function to developmental coordination. Reprod. Camb. Engl. 159, R173–R188 10.1530/REP-19-0057 [DOI] [PubMed] [Google Scholar]
- 194.Yang W., Wang L., Wang F. and Yuan S. (2020) Roles of AMP-activated protein kinase (AMPK) in mammalian reproduction. Front Cell Dev. Biol. 8, 593005 10.3389/fcell.2020.593005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nguyen T.M.D. (2019) Role of AMPK in mammals reproduction: specific controls and whole-body energy sensing. C. R. Biol. 342, 1–6 10.1016/j.crvi.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 196.Ruan H. and Dong L.Q. (2016) Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 8, 101–109 10.1093/jmcb/mjw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saltiel A.R. (2021) Insulin signaling in health and disease. J. Clin. Invest. 131, e142241 10.1172/JCI142241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang Q.-L., Wang Y., Liu J.-S. and Du Y.-Z. (2022) Effects of hypercaloric diet-induced hyperinsulinemia and hyperlipidemia on the ovarian follicular development in mice. J. Reprod. Dev. 68, 173–180 10.1262/jrd.2021-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jeon S.-M. (2016) Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 48, e245–e245 10.1038/emm.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Faber C.L., Deem J.D., Campos C.A., Taborsky G.J. and Morton G.J. (2020) CNS control of the endocrine pancreas. Diabetologia 63, 2086–2094 10.1007/s00125-020-05204-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fu Z., Gilbert E.R. and Liu D. (2013) Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diab. Rev. 9, 25–53 10.2174/157339913804143225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Melloul D., Marshak S. and Cerasi E. (2002) Regulation of insulin gene transcription. Diabetologia 45, 309–326 10.1007/s00125-001-0728-y [DOI] [PubMed] [Google Scholar]
- 203.Thomas D.D., Corkey B.E., Istfan N.W. and Apovian C.M. (2019) Hyperinsulinemia: an early indicator of metabolic dysfunction. J. Endocr. Soc. 3, 1727–1747 10.1210/js.2019-00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rostène W. and De Meyts P. (2021) Insulin: a 100-year-old discovery with a fascinating history. Endocr. Rev. 42, 503–527 10.1210/endrev/bnab020 [DOI] [PubMed] [Google Scholar]
- 205.Banting F. and Best C. (1922) The internal secretion of the pancreas. J. Lab. Clin. Med. 7, 251–266 [PubMed] [Google Scholar]
- 206.Cheatham B. and Kahn C.R. (1995) Insulin action and the insulin signaling network. Endocr. Rev. 16, 117–142 [DOI] [PubMed] [Google Scholar]
- 207.Nolan C.J. and Prentki M. (2019) Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab. Vasc. Dis. Res. 16, 118–127 10.1177/1479164119827611 [DOI] [PubMed] [Google Scholar]
- 208.Biddinger S.B. and Kahn C.R. (2006) From mice to men: Insights into the insulin resistance syndromes. Annu. Rev. Physiol. 68, 123–158 10.1146/annurev.physiol.68.040104.124723 [DOI] [PubMed] [Google Scholar]
- 209.Lei C., Wang J., Li X., Mao Y.-Y. and Yan J.-Q. (2023) Changes of insulin receptors in high fat and high glucose diet mice with insulin resistance. Adipocyte 12, 2264444 10.1080/21623945.2023.2264444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brüning J.C., Gautam D., Burks D.J., Gillette J., Schubert M., Orban P.C.et al. (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 10.1126/science.289.5487.2122 [DOI] [PubMed] [Google Scholar]
- 211.Brothers K.J., Wu S., DiVall S.A., Messmer M.R., Kahn C.R., Miller R.S.et al. (2010) Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 12, 295–305 10.1016/j.cmet.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ou X.-H., Li S., Wang Z.-B., Li M., Quan S., Xing F.et al. (2012) Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod. Oxf. Engl. 27, 2130–2145 10.1093/humrep/des137 [DOI] [PubMed] [Google Scholar]
- 213.Chosich J., Bradford A.P., Allshouse A.A., Reusch J.E.B., Santoro N. and Schauer I.E. (2017) Acute recapitulation of the hyperinsulinemia and hyperlipidemia characteristic of metabolic syndrome suppresses gonadotropins. Obes. Silver Spring Md. 25, 553–560 10.1002/oby.21754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang G., Radovick S., Buckley J.P., Hauser R., Williams P.L., Hong X.et al. (2023) Plasma insulin concentration in newborns and children and age at menarche. Diabetes Care. 46, 1231–1238 10.2337/dc22-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Park H.T., Cho G.J., Ahn K.H., Shin J.H., Kim Y.T., Hur J.Y.et al. (2010) Association of insulin resistance with anti‐Mullerian hormone levels in women without polycystic ovary syndrome. (PCOS). Clin. Endocrinol. (Oxf) 72, 26–31 10.1111/j.1365-2265.2009.03614.x [DOI] [PubMed] [Google Scholar]
- 216.Havrankova J., Roth J. and Brownstein M. (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272, 827–829 10.1038/272827a0 [DOI] [PubMed] [Google Scholar]
- 217.Pomytkin I., Costa-Nunes J.P., Kasatkin V., Veniaminova E., Demchenko A., Lyundup A.et al. (2018) Insulin receptor in the brain: mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 24, 763–774 10.1111/cns.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Qiu X., Dao H., Wang M., Heston A., Garcia K.M., Sangal A.et al. (2015) Insulin and leptin signaling interact in the mouse Kiss1 neuron during the peripubertal period. PLoS ONE 10, e0121974 10.1371/journal.pone.0121974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Evans M.C., Rizwan M., Mayer C., Boehm U. and Anderson G.M. (2014) Evidence that insulin signalling in gonadotrophin-releasing hormone and kisspeptin neurones does not play an essential role in metabolic regulation of fertility in mice. J. Neuroendocrinol. 26, 468–479 10.1111/jne.12166 [DOI] [PubMed] [Google Scholar]
- 220.Qiu X., Dowling A.R., Marino J.S., Faulkner L.D., Bryant B., Brüning J.C.et al. (2013) Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology 154, 1337–1348 10.1210/en.2012-2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Navarro V.M. and Kaiser U.B. (2013) Metabolic influences on neuroendocrine regulation of reproduction. Curr. Opin. Endocrinol. Diab. Obes. 20, 335 10.1097/MED.0b013e32836318ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Evans M.C., Hill J.W. and Anderson G.M. (2021) Role of insulin in the neuroendocrine control of reproduction. J. Neuroendocrinol. 33, e12930 10.1111/jne.12930 [DOI] [PubMed] [Google Scholar]
- 223.Manaserh I.H., Chikkamenahalli L., Ravi S., Dube P.R., Park J.J. and Hill J.W. (2019) Ablating astrocyte insulin receptors leads to delayed puberty and hypogonadism in mice. PLoS Biol. 17, e3000189 10.1371/journal.pbio.3000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kim H.H., DiVall S.A., Deneau R.M. and Wolfe A. (2005) Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Mol. Cell. Endocrinol. 242, 42–49 10.1016/j.mce.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 225.Adashi E.Y., Hsueh A.J. and Yen S.S. (1981) Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology 108, 1441–1449 10.1210/endo-108-4-1441 [DOI] [PubMed] [Google Scholar]
- 226.Buggs C., Weinberg F., Kim E., Wolfe A., Radovick S. and Wondisford F. (2006) Insulin augments GnRH-stimulated LHβ gene expression by Egr-1. Mol. Cell. Endocrinol. 249, 99–106 10.1016/j.mce.2006.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Salvi R., Castillo E., Voirol M.-J., Glauser M., Rey J.-P., Gaillard R.C.et al. (2006) Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology 147, 816–826 10.1210/en.2005-0728 [DOI] [PubMed] [Google Scholar]
- 228.DiVall S.A., Herrera D., Sklar B., Wu S., Wondisford F., Radovick S.et al. (2015) Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS ONE 10, e0119995 10.1371/journal.pone.0119995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu S., Divall S., Nwaopara A., Radovick S., Wondisford F., Ko C.et al. (2014) Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes 63, 1270–1282 10.2337/db13-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.el-Roeiy A Chen X., Roberts V.J., Shimasakai S., Ling N., LeRoith D.et al. (1994) Expression of the genes encoding the insulin-like growth factors. (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1-6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J. Clin. Endocrinol. Metab. 78, 1488–1496 [DOI] [PubMed] [Google Scholar]
- 231.Lighten A.D., Hardy K., Winston R.M. and Moore G.E. (1997) Expression of mRNA for the insulin-like growth factors and their receptors in human preimplantation embryos. Mol. Reprod. Dev 47, 134–139 [DOI] [PubMed] [Google Scholar]
- 232.Samoto T., Maruo T., Ladines-Llave C.A., Matsuo H., Deguchi J., Barnea E.R.et al. (1993) Insulin receptor expression in follicular and stromal compartments of the human ovary over the course of follicular growth, regression and atresia. Endocr. J. 40, 715–726 10.1507/endocrj.40.715 [DOI] [PubMed] [Google Scholar]
- 233.Schultz G.A., Hogan A., Watson A.J., Smith R.M. and Heyner S. (1992) Insulin, insulin-like growth factors and glucose transporters: temporal patterns of gene expression in early murine and bovine embryos. Reprod. Fertil. Dev. 4, 361–371 10.1071/RD9920361 [DOI] [PubMed] [Google Scholar]
- 234.Acevedo N., Ding J. and Smith G.D. (2007) Insulin signaling in mouse oocytes. Biol. Reprod. 77, 872–879 10.1095/biolreprod.107.060152 [DOI] [PubMed] [Google Scholar]
- 235.Xu S., Wu X., Dong Y., Xu M., Li Z., Chen S.et al. (2020) Glucose activates the primordial follicle through the AMPK/mTOR signaling pathway. Clin. Transl. Med. 10, e122 10.1002/ctm2.122 [DOI] [Google Scholar]
- 236.Zhou J., Bievre M. and Bondy C.A. (2000) Reduced GLUT1 expression in Igf1-/- null oocytes and follicles. Growth Horm. IGF Res. 10, 111–117 10.1054/ghir.2000.0147 [DOI] [PubMed] [Google Scholar]
- 237.Diamanti-Kandarakis E., Argyrakopoulou G., Economou F., Kandaraki E. and Koutsilieris M. (2008) Defects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome. (PCOS). J. Steroid Biochem. Mol. Biol. 109, 242–246 10.1016/j.jsbmb.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 238.Dupont J. and Scaramuzzi R.J. (2016) Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem. J. 473, 1483–1501 10.1042/BCJ20160124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kezele P.R., Nilsson E.E. and Skinner M.K. (2002) Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol. Cell. Endocrinol. 192, 37–43 10.1016/S0303-7207(02)00114-4 [DOI] [PubMed] [Google Scholar]
- 240.Sakaguchi M., Dominko T., Yamauchi N., Leibfried-Rutledge M.L., Nagai T. and First N.L. (2002) Possible mechanism for acceleration of meiotic progression of bovine follicular oocytes by growth factors in vitro. Reprod Camb. Engl. 123, 135–142 10.1530/rep.0.1230135 [DOI] [PubMed] [Google Scholar]
- 241.Stefanello J.R., Barreta M.H., Porciuncula P.M., Arruda J.N., Oliveira J.F., Oliveira M.A.et al. (2006) Effect of angiotensin II with follicle cells and insulin-like growth factor-I or insulin on bovine oocyte maturation and embryo development. Theriogenology 66, 2068–2076 10.1016/j.theriogenology.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 242.Pitetti J.L., Torre D., Conne B., Papaioannou M.D., Cederroth C.R., Xuan S.et al. (2009) Insulin receptor and IGF1R are not required for oocyte growth, differentiation, and maturation in mice. Sex. Dev Genet. Mol. Biol. Evol. Endocrinol. Embryol. Pathol. Sex Determ Differ 3, 264–272 10.1159/000252813 [DOI] [PubMed] [Google Scholar]
- 243.Sekulovski N., Whorton A.E., Shi M., Hayashi K. and MacLean J.A. (2020) Periovulatory insulin signaling is essential for ovulation, granulosa cell differentiation, and female fertility. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 34, 2376–2391 10.1096/fj.201901791R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Belfiore A., Frasca F., Pandini G., Sciacca L. and Vigneri R. (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 30, 586–623 10.1210/er.2008-0047 [DOI] [PubMed] [Google Scholar]
- 245.Bøtkjær J.A., Pors S.E., Petersen T.S., Kristensen S.G., Jeppesen J.V., Oxvig C.et al. (2019) Transcription profile of the insulin-like growth factor signaling pathway during human ovarian follicular development. J. Assist. Reprod. Genet. 36, 889–903 10.1007/s10815-019-01432-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Baumgarten S.C., Convissar S.M., Fierro M.A., Winston N.J., Scoccia B. and Stocco C. (2014) IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells. J. Clin. Endocrinol. Metab. 99, 2995–3004 10.1210/jc.2014-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhao J., Taverne M.A.M., Van Der Weijden G.C., Bevers M.M. and Van Den Hurk R. (2001) Insulin-like growth factor-I. (IGF-I) stimulates the development of cultured rat pre-antral follicles. Mol. Reprod. Dev 58, 287–296 [DOI] [PubMed] [Google Scholar]
- 248.Guthrie H.D., Garrett W.M. and Cooper B.S. (1998) Follicle-stimulating hormone and insulin-like growth factor-I attenuate apoptosis in cultured porcine granulosa cells. Biol. Reprod. 58, 390–396 10.1095/biolreprod58.2.390 [DOI] [PubMed] [Google Scholar]
- 249.Zhou P., Baumgarten S.C., Wu Y., Bennett J., Winston N., Hirshfeld-Cytron J.et al. (2013) IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 27, 511–523 10.1210/me.2012-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Walters K.A., Binnie J.P., Campbell B.K., Armstrong D.G. and Telfer E.E. (2006) The effects of IGF-I on bovine follicle development and IGFBP-2 expression are dose and stage dependent. Reproduction 131, 515–523 10.1530/rep.1.00682 [DOI] [PubMed] [Google Scholar]
- 251.Magalhães-Padilha D.M., Duarte A.B.G., Araújo V.R., Saraiva M.V.A., Almeida A.P., Rodrigues G.Q.et al. (2012) Steady-state level of insulin-like growth factor-I. (IGF-I) receptor mRNA and the effect of IGF-I on the in vitro culture of caprine preantral follicles. Theriogenology 77, 206–213 10.1016/j.theriogenology.2011.07.036 [DOI] [PubMed] [Google Scholar]
- 252.Baker J., Hardy M.P., Zhou J., Bondy C., Lupu F., Bellvé A.R.et al. (1996) Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 10, 903–918 [DOI] [PubMed] [Google Scholar]
- 253.Kim S.-Y., Ebbert K., Cordeiro M.H., Romero M., Zhu J., Serna V.A.et al. (2015) Cell autonomous phosphoinositide 3-kinase activation in oocytes disrupts normal ovarian function through promoting survival and overgrowth of ovarian follicles. Endocrinology 156, 1464–1476 10.1210/en.2014-1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Reddy P., Liu L., Adhikari D., Jagarlamudi K., Rajareddy S., Shen Y.et al. (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319, 611–613 10.1126/science.1152257 [DOI] [PubMed] [Google Scholar]
- 255.Goto M., Iwase A., Ando H., Kurotsuchi S., Harata T. and Kikkawa F. (2007) PTEN and Akt expression during growth of human ovarian follicles. J. Assist. Reprod. Genet. 24, 541–546 10.1007/s10815-007-9156-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Reddy P., Adhikari D., Zheng W., Liang S., Hämäläinen T., Tohonen V.et al. (2009) PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 18, 2813–2824 10.1093/hmg/ddp217 [DOI] [PubMed] [Google Scholar]
- 257.Brown C., LaRocca J., Pietruska J., Ota M., Anderson L., Smith S.D.et al. (2010) Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKB alpha/Akt1. Biol. Reprod. 82, 246–256 10.1095/biolreprod.109.077925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Restuccia D.F., Hynx D. and Hemmings B.A. (2012) Loss of PKBβ/Akt2 predisposes mice to ovarian cyst formation and increases the severity of polycystic ovary formation in vivo. Dis. Model Mech. 5, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.John G.B., Gallardo T.D., Shirley L.J. and Castrillon D.H. (2008) Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev. Biol. 321, 197–204 10.1016/j.ydbio.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pelosi E., Omari S., Michel M., Ding J., Amano T., Forabosco A.et al. (2013) Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat. Commun. 4, 1843 10.1038/ncomms2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wellons M.F., Matthews J.J. and Kim C. (2017) Ovarian aging in women with diabetes: an overview. Maturitas 96, 109–113 10.1016/j.maturitas.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yi Y., El Khoudary S.R., Buchanich J.M., Miller R.G., Rubinstein D., Orchard T.J.et al. (2021) Predictors of the age at which natural menopause occurs in women with type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications (EDC) study. Menopause N. Y. N. 28, 735–740 10.1097/GME.0000000000001772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Brand J.S., Onland-Moret N.C., Eijkemans M.J.C., Tjønneland A., Roswall N., Overvad K.et al. (2015) Diabetes and onset of natural menopause: results from the European Prospective Investigation into Cancer and Nutrition. Hum. Reprod. 30, 1491–1498 10.1093/humrep/dev054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sekhar T.V.D.S., Medarametla S., Rahman A. and Adapa S.S. (2015) Early menopause in type 2 diabetes - a study from a South Indian Tertiary Care Centre. J. Clin. Diagn. Res. 9, OC08–OC10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mehra V.M., Costanian C., McCague H., Riddell M.C. and Tamim H. (2023) The association between diabetes type, age of onset, and age at natural menopause: a retrospective cohort study using the Canadian Longitudinal Study on Aging. Menopause N. Y. N. 30, 37–44 10.1097/GME.0000000000002085 [DOI] [PubMed] [Google Scholar]
- 266.Elrick H., Stimmler L., Hlad C.J. and Arai Y. (1964) Plasma insulin response to oral and intravenous glucose administration. J. Clin. Endocrinol. Metab. 24, 1076–1082 10.1210/jcem-24-10-1076 [DOI] [PubMed] [Google Scholar]
- 267.Mcintyre N., Holdsworth C.D. and Turner D.S. (1964) New interpretation of oral glucose tolerance. Lancet Lond. Engl. 2, 20–21 10.1016/S0140-6736(64)90011-X [DOI] [PubMed] [Google Scholar]
- 268.Perley M.J. and Kipnis D.M. (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J. Clin. Invest. 46, 1954–1962 10.1172/JCI105685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Brown J.C. and Dryburgh J.R. (1971) A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can. J. Biochem. 49, 867–872 10.1139/o71-122 [DOI] [PubMed] [Google Scholar]
- 270.Dupre J., Ross S.A., Watson D. and Brown J.C. (1973) Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J. Clin. Endocrinol. Metab. 37, 826–828 10.1210/jcem-37-5-826 [DOI] [PubMed] [Google Scholar]
- 271.Schmidt W.E., Siegel E.G. and Creutzfeldt W. (1985) Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia 28, 704–707 10.1007/BF00291980 [DOI] [PubMed] [Google Scholar]
- 272.Perfetti R., Zhou J., Doyle M.E. and Egan J.M. (2000) Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141, 4600–4605 10.1210/endo.141.12.7806 [DOI] [PubMed] [Google Scholar]
- 273.Stoffers D.A., Kieffer T.J., Hussain M.A., Drucker D.J., Bonner-Weir S., Habener J.F.et al. (2000) Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49, 741–748 10.2337/diabetes.49.5.741 [DOI] [PubMed] [Google Scholar]
- 274.Farilla L., Hui H., Bertolotto C., Kang E., Bulotta A., Di Mario U.et al. (2002) Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143, 4397–4408 10.1210/en.2002-220405 [DOI] [PubMed] [Google Scholar]
- 275.Prigeon R.L., Quddusi S., Paty B. and D'Alessio D.A. (2003) Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am. J. Physiol. Endocrinol. Metab. 285, E701–E707 10.1152/ajpendo.00024.2003 [DOI] [PubMed] [Google Scholar]
- 276.Willms B., Werner J., Holst J.J., Orskov C., Creutzfeldt W. and Nauck M.A. (1996) Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1. (GLP-1)-(7-36) amide in type 2. (noninsulin-dependent) diabetic patients. J. Clin. Endocrinol. Metab. 81, 327–332 [DOI] [PubMed] [Google Scholar]
- 277.Komatsu R., Matsuyama T., Namba M., Watanabe N., Itoh H., Kono N.et al. (1989) Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7-36)-amide. Diabetes 38, 902–905 10.2337/diab.38.7.902 [DOI] [PubMed] [Google Scholar]
- 278.Elliott R.M., Morgan L.M., Tredger J.A., Deacon S., Wright J. and Marks V. (1993) Glucagon-like peptide-1. (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J. Endocrinol. 138, 159–166 10.1677/joe.0.1380159 [DOI] [PubMed] [Google Scholar]
- 279.Fehmann H.C., Göke R. and Göke B. (1995) Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr. Rev. 16, 390–410 10.1210/edrv-16-3-390 [DOI] [PubMed] [Google Scholar]
- 280.Kim W. and Egan J.M. (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 60, 470–512 10.1124/pr.108.000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pederson R.A., Schubert H.E. and Brown J.C. (1975) Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes 24, 1050–1056 10.2337/diab.24.12.1050 [DOI] [PubMed] [Google Scholar]
- 282.Doyle M.E. and Egan J.M. (2007) Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 113, 546–593 10.1016/j.pharmthera.2006.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jang H.-J., Kokrashvili Z., Theodorakis M.J., Carlson O.D., Kim B.-J., Zhou J.et al. (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. U.S.A. 104, 15069–15074 10.1073/pnas.0706890104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gribble F.M., Williams L., Simpson A.K. and Reimann F. (2003) A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52, 1147–1154 10.2337/diabetes.52.5.1147 [DOI] [PubMed] [Google Scholar]
- 285.Herrmann C., Göke R., Richter G., Fehmann H.C., Arnold R. and Göke B. (1995) Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56, 117–126 10.1159/000201231 [DOI] [PubMed] [Google Scholar]
- 286.Ekberg J.H., Hauge M., Kristensen L.V., Madsen A.N., Engelstoft M.S., Husted A.-S.et al. (2016) GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology 157, 4561–4569 10.1210/en.2016-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Husted A.S., Trauelsen M., Rudenko O., Hjorth S.A. and Schwartz T.W. (2017) GPCR-mediated signaling of metabolites. Cell Metab. 25, 777–796 10.1016/j.cmet.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 288.Greenfield J.R., Farooqi I.S., Keogh J.M., Henning E., Habib A.M., Blackwood A.et al. (2009) Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am. J. Clin. Nutr. 89, 106–113 10.3945/ajcn.2008.26362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hira T., Mochida T., Miyashita K. and Hara H. (2009) GLP-1 secretion is enhanced directly in the ileum but indirectly in the duodenum by a newly identified potent stimulator, zein hydrolysate, in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G663–G671 10.1152/ajpgi.90635.2008 [DOI] [PubMed] [Google Scholar]
- 290.Lejeune M.P.G.M., Westerterp K.R., Adam T.C.M., Luscombe-Marsh N.D. and Westerterp-Plantenga M.S. (2006) Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 83, 89–94 10.1093/ajcn/83.1.89 [DOI] [PubMed] [Google Scholar]
- 291.Tolhurst G., Zheng Y., Parker H.E., Habib A.M., Reimann F. and Gribble F.M. (2011) Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 152, 405–413 10.1210/en.2010-0956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Campbell J.E. and Drucker D.J. (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17, 819–837 10.1016/j.cmet.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 293.Mayo K.E., Miller L.J., Bataille D., Dalle S., Göke B., Thorens B.et al. (2003) International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 55, 167–194 10.1124/pr.55.1.6 [DOI] [PubMed] [Google Scholar]
- 294.Singh I., Wang L., Xia B., Liu J., Tahiri A., El Ouaamari A.et al. (2022) Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci. 12, 178 10.1186/s13578-022-00914-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Daniels D. and Mietlicki-Baase E.G. (2019) Glucagon-like peptide 1 in the brain: where is it coming from, where is it going? Diabetes 68, 15–17 10.2337/dbi18-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Nauck M.A., Kleine N., Orskov C., Holst J.J., Willms B. and Creutzfeldt W. (1993) Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1. (7-36 amide) in type 2. (non-insulin-dependent) diabetic patients. Diabetologia 36, 741–744 10.1007/BF00401145 [DOI] [PubMed] [Google Scholar]
- 297.Flint A., Raben A., Astrup A. and Holst J.J. (1998) Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 101, 515–520 10.1172/JCI990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kuhre R.E., Wewer Albrechtsen N.J., Hartmann B., Deacon C.F. and Holst J.J. (2015) Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J. Diabetes Complications 29, 445–450 10.1016/j.jdiacomp.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 299.Brunton S.A. and Wysham C.H. (2020) GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. Postgrad. Med. 132, 3–14 10.1080/00325481.2020.1798099 [DOI] [PubMed] [Google Scholar]
- 300.Gallwitz B. (2019) Clinical use of DPP-4 inhibitors. Front. Endocrinol. 10, 389 10.3389/fendo.2019.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nauck M.A., Quast D.R., Wefers J. and Meier J.J. (2021) GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol. Metab. 46, 101102 10.1016/j.molmet.2020.101102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Holst J.J. and Rosenkilde M.M. (2020) GIP as a therapeutic target in diabetes and obesity: insight from incretin co-agonists. J. Clin. Endocrinol. Metab. 105, e2710–e2716 10.1210/clinem/dgaa327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nauck M.A., Bartels E., Orskov C., Ebert R. and Creutzfeldt W. (1993) Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 76, 912–917 [DOI] [PubMed] [Google Scholar]
- 304.Andreasen C.R., Andersen A. and Vilsbøll T. (2023) The future of incretins in the treatment of obesity and non-alcoholic fatty liver disease. Diabetologia 66, 1846–1858 10.1007/s00125-023-05966-9 [DOI] [PubMed] [Google Scholar]
- 305.Targher G., Mantovani A. and Byrne C.D. (2023) Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 8, 179–191 10.1016/S2468-1253(22)00338-7 [DOI] [PubMed] [Google Scholar]
- 306.Alicic R.Z., Cox E.J., Neumiller J.J. and Tuttle K.R. (2021) Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 17, 227–244 10.1038/s41581-020-00367-2 [DOI] [PubMed] [Google Scholar]
- 307.Girges C., Vijiaratnam N., Athauda D., Auld G., Gandhi S. and Foltynie T. (2021) The future of incretin-based approaches for neurodegenerative diseases in older adults: which to choose? a review of their potential efficacy and suitability Drugs Aging 38, 355–373 10.1007/s40266-021-00853-7 [DOI] [PubMed] [Google Scholar]
- 308.Nowell J., Blunt E. and Edison P. (2023) Incretin and insulin signaling as novel therapeutic targets for Alzheimer's and Parkinson's disease. Mol. Psychiatry 28, 217–229 10.1038/s41380-022-01792-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Abdalla M.A., Deshmukh H., Atkin S. and Sathyapalan T. (2021) The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther. Adv. Endocrinol. Metab. 12, 2042018821989238 10.1177/2042018821989238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Glendining K.A. and Campbell R.E. (2023) Recent advances in emerging PCOS therapies. Curr. Opin. Pharmacol. 68, 102345 10.1016/j.coph.2022.102345 [DOI] [PubMed] [Google Scholar]
- 311.Khan D., Ojo O.O., Woodward O.R., Lewis J.E., Sridhar A., Gribble F.M.et al. (2022) Evidence for involvement of GIP and GLP-1 receptors and the gut-gonadal axis in regulating female reproductive function in mice. Biomolecules 12, 1736 10.3390/biom12121736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.MacLusky N.J., Cook S., Scrocchi L., Shin J., Kim J., Vaccarino F.et al. (2000) Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 141, 752–762 10.1210/endo.141.2.7326 [DOI] [PubMed] [Google Scholar]
- 313.Johnson M.L., Saffrey M.J. and Taylor V.J. (2017) Glucagon-like peptide-1 (GLP-1) increases in plasma and colon tissue prior to estrus and circulating levels change with increasing age in reproductively competent Wistar rats. Peptides 90, 55–62 10.1016/j.peptides.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 314.Outeiriño-Iglesias V., Romaní-Pérez M., González-Matías L.C., Vigo E. and Mallo F. (2015) GLP-1 increases preovulatory LH source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology 156, 4226–4237 10.1210/en.2014-1978 [DOI] [PubMed] [Google Scholar]
- 315.Beak S.A., Heath M.M., Small C.J., Morgan D.G., Ghatei M.A., Taylor A.D.et al. (1998) Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J. Clin. Invest. 101, 1334–1341 10.1172/JCI610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Arbabi L., Li Q., Henry B.A. and Clarke I.J. (2021) Glucagon-like peptide-1 control of GnRH secretion in female sheep. J. Endocrinol. 248, 325–335 10.1530/JOE-20-0335 [DOI] [PubMed] [Google Scholar]
- 317.Heppner K.M., Baquero A.F., Bennett C.M., Lindsley S.R., Kirigiti M.A., Bennett B.et al. (2017) GLP-1R signaling directly activates arcuate nucleus kisspeptin action in brain slices but does not rescue luteinizing hormone inhibition in ovariectomized mice during negative energy balance. eNeuro 4, ENEURO.0198-16.2016 10.1523/ENEURO.0198-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Farkas I., Vastagh C., Farkas E., Bálint F., Skrapits K., Hrabovszky E.et al. (2016) Glucagon-Like Peptide-1 Excites Firing and Increases GABAergic Miniature Postsynaptic Currents. (mPSCs) in Gonadotropin-Releasing Hormone. (GnRH) Neurons of the Male Mice via Activation of Nitric Oxide. (NO) and Suppression of Endocannabinoid Signaling Pathways. Front Cell Neurosci. 10, 214 10.3389/fncel.2016.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Adriaenssens A.E., Biggs E.K., Darwish T., Tadross J., Sukthankar T., Girish M.et al. (2019) Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 30, 987.e6–996.e6 10.1016/j.cmet.2019.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ottlecz A., Samson W.K. and McCann S.M. (1985) The effects of gastric inhibitory polypeptide (GIP) on the release of anterior pituitary hormones. Peptides 6, 115–119 10.1016/0196-9781(85)90086-5 [DOI] [PubMed] [Google Scholar]
- 321.Nishiyama Y., Hasegawa T., Fujita S., Iwata N., Nagao S., Hosoya T.et al. (2018) Incretins modulate progesterone biosynthesis by regulating bone morphogenetic protein activity in rat granulosa cells. J. Steroid Biochem. Mol. Biol. 178, 82–88 10.1016/j.jsbmb.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 322.Bou Nemer L., Shi H., Carr B.R., Word R.A. and Bukulmez O. (2019) Effect of body weight on metabolic hormones and fatty acid metabolism in follicular fluid of women undergoing in vitro fertilization: a pilot study. Reprod. Sci. Thousand Oaks. Calif. 26, 404–411 10.1177/1933719118776787 [DOI] [PubMed] [Google Scholar]
- 323.Artunc-Ulkumen B., Pala H.G., Pala E.E., Yavasoglu A., Yigitturk G. and Erbas O. (2015) Exenatide improves ovarian and endometrial injury and preserves ovarian reserve in streptozocin induced diabetic rats. Gynecol. Endocrinol. 31, 196–201 10.3109/09513590.2014.975686 [DOI] [PubMed] [Google Scholar]
- 324.Kabel A.M., Al-Shehri A.H., Al-Talhi R.A. and Abd Elmaaboud M.A. (2017) The promising effect of linagliptin and/or indole-3-carbinol on experimentally-induced polycystic ovarian syndrome. Chem. Biol. Interact. 273, 190–199 10.1016/j.cbi.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 325.Tao X., Zhang X., Ge S.-Q., Zhang E.-H. and Zhang B. (2015) Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int. J. Clin. Exp. Pathol. 8, 8276–8283 [PMC free article] [PubMed] [Google Scholar]
- 326.Elkind-Hirsch K., Marrioneaux O., Bhushan M., Vernor D. and Bhushan R. (2008) Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 2670–2678 10.1210/jc.2008-0115 [DOI] [PubMed] [Google Scholar]
- 327.Jensterle M., Salamun V., Kocjan T., Vrtacnik Bokal E. and Janez A. (2015) Short term monotherapy with GLP-1 receptor agonist liraglutide or PDE 4 inhibitor roflumilast is superior to metformin in weight loss in obese PCOS women: a pilot randomized study. J. Ovarian Res. 8, 32 10.1186/s13048-015-0161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Jensterle M., Kravos N.A., Pfeifer M., Kocjan T. and Janez A. (2015) A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome. Hormones 14, 81–90 10.1007/BF03401383 [DOI] [PubMed] [Google Scholar]
- 329.Nylander M., Frøssing S., Clausen H.V., Kistorp C., Faber J. and Skouby S.O. (2017) Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod. Biomed. Online 35, 121–127 10.1016/j.rbmo.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 330.Kahal H., Aburima A., Ungvari T., Rigby A.S., Coady A.M., Vince R.V.et al. (2015) The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocr. Disord. 15, 14 10.1186/s12902-015-0005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu X., Zhang Y., Zheng S., Lin R., Xie Y., Chen H.et al. (2017) Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin. Endocrinol. (Oxf) 87, 767–774 10.1111/cen.13454 [DOI] [PubMed] [Google Scholar]
- 332.Rasmussen C.B. and Lindenberg S. (2014) The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Front Endocrinol. 5, 140 10.3389/fendo.2014.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Salamun V., Jensterle M., Janez A. and Vrtacnik Bokal E. (2018) Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur. J. Endocrinol. 179, 1–11 10.1530/EJE-18-0175 [DOI] [PubMed] [Google Scholar]
- 334.Elkind-Hirsch K.E., Chappell N., Shaler D., Storment J. and Bellanger D. (2022) Liraglutide 3 mg on weight, body composition, and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil. Steril. 118, 371–381 10.1016/j.fertnstert.2022.04.027 [DOI] [PubMed] [Google Scholar]
- 335.Nuako A., Tu L., Campoverde Reyes K.J., Chhabria S.M. and Stanford F.C. (2023) Pharmacologic treatment of obesity in reproductive aged women. Curr. Obstet. Gynecol. Rep. 12, 138–146 10.1007/s13669-023-00350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Li C.H. and Evans H.M. (1944) The isolation of pituitary growth hormone. Science 99, 183–184 10.1126/science.99.2566.183 [DOI] [PubMed] [Google Scholar]
- 337.Tidblad A. (2022) The history, physiology and treatment safety of growth hormone. Acta. Paediatr. Oslo. Nor. 1992 111, 215–224 10.1111/apa.15948 [DOI] [PubMed] [Google Scholar]
- 338.Aguiar-Oliveira M.H. and Bartke A. (2019) Growth hormone deficiency: health and longevity. Endocr. Rev. 40, 575–601 10.1210/er.2018-00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kopchick J.J., Berryman D.E., Puri V., Lee K.Y. and Jorgensen J.O.L. (2020) The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat. Rev. Endocrinol. 16, 135–146 10.1038/s41574-019-0280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Olarescu N.C., Gunawardane K., Hansen T.K., Møller N. and Jørgensen J.O.L. (2019) Normal physiology of growth hormone in adults. In Endotext(Feingold K.R., Anawalt B. and Blackman M.R.et al., eds), MDText.com, Inc., South Dartmouth. (MA) [Google Scholar]
- 341.Rose S.R., Municchi G., Barnes K.M., Kamp G.A., Uriarte M.M., Ross J.L.et al. (1991) Spontaneous growth hormone secretion increases during puberty in normal girls and boys. J. Clin. Endocrinol. Metab. 73, 428–435 10.1210/jcem-73-2-428 [DOI] [PubMed] [Google Scholar]
- 342.van den Berg G., Veldhuis J.D., Frölich M. and Roelfsema F. (1996) An amplitude-specific divergence in the pulsatile mode of growth hormone. (GH) secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J. Clin. Endocrinol. Metab. 81, 2460–2467 [DOI] [PubMed] [Google Scholar]
- 343.Devesa J., Lima L. and Tresguerres J.A.F. (1992) Neuroendocrine control of growth hormone secretion in humans. Trends Endocrinol. Metab. 3, 175–183 10.1016/1043-2760(92)90168-Z [DOI] [PubMed] [Google Scholar]
- 344.Birzniece V. and Ho K.K.Y. (2017) Sex steroids and the GH axis: Implications for the management of hypopituitarism. Best Pract. Res. Clin. Endocrinol. Metab. 31, 59–69 10.1016/j.beem.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 345.Hassan H.A., Enright W.J., Tucker H.A. and Merkel R.A. (2001) Estrogen and androgen elicit growth hormone release via dissimilar patterns of hypothalamic neuropeptide secretion. Steroids 66, 71–80 10.1016/S0039-128X(00)00168-9 [DOI] [PubMed] [Google Scholar]
- 346.Marin G., Domené H.M., Barnes K.M., Blackwell B.J., Cassorla F.G. and Cutler G.B. Jr (1994) The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. J. Clin. Endocrinol. Metab. 79, 537–541 [DOI] [PubMed] [Google Scholar]
- 347.Mauras N., Rogol A.D. and Veldhuis J.D. (1990) Increased hGH production rate after low-dose estrogen therapy in prepubertal girls with Turner's syndrome. Pediatr. Res. 28, 626–630 10.1203/00006450-199012000-00018 [DOI] [PubMed] [Google Scholar]
- 348.Wideman L., Weltman J.Y., Shah N., Story S., Veldhuis J.D. and Weltman A. (1999) Effects of gender on exercise-induced growth hormone release. J. Appl. Physiol. Bethesda Md. 1985 87, 1154–1162 [DOI] [PubMed] [Google Scholar]
- 349.Harvey S. (2010) Extrapituitary growth hormone. Endocrine 38, 335–359 10.1007/s12020-010-9403-8 [DOI] [PubMed] [Google Scholar]
- 350.Veldhuis J.D. and Bowers C.Y. (2003) Human GH pulsatility: an ensemble property regulated by age and gender. J. Endocrinol. Invest. 26, 799–813 10.1007/BF03345229 [DOI] [PubMed] [Google Scholar]
- 351.Finkelstein J.W., Roffwarg H.P., Boyar R.M., Kream J. and Hellman L. (1972) Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J. Clin. Endocrinol. Metab. 35, 665–670 10.1210/jcem-35-5-665 [DOI] [PubMed] [Google Scholar]
- 352.Zadik Z., Chalew A., McCarter R.J.J.R., Meistas M. and Kowarski A.A. (1985) The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J. Clin. Endocrinol. Metab. 60, 513–516 10.1210/jcem-60-3-513 [DOI] [PubMed] [Google Scholar]
- 353.Bartke A., Sun L.Y. and Longo V. (2013) Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 93, 571–598 10.1152/physrev.00006.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Giustina A. and Veldhuis J.D. (1998) Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 19, 717–797 [DOI] [PubMed] [Google Scholar]
- 355.Fassnacht M., Tsagarakis S., Terzolo M., Tabarin A., Sahdev A., Newell-Price J.et al. (2023) European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 189, G1–G42 10.1093/ejendo/lvad066 [DOI] [PubMed] [Google Scholar]
- 356.Menezes M., Salvatori R., Oliveira C.R.P., Pereira R.M.C., Souza A.H.O., Nobrega L.M.A.et al. (2008) Climacteric in untreated isolated growth hormone deficiency. Menopause N. Y. N. 15, 743 10.1097/gme.0b013e31815b97d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Bachelot A., Monget P., Imbert-Bolloré P., Coshigano K., Kopchick J.J., Kelly P.A.et al. (2002) Growth hormone is required for ovarian follicular growth. Endocrinology 143, 4104–4112 10.1210/en.2002-220087 [DOI] [PubMed] [Google Scholar]
- 358.Danilovich N., Wernsing D., Coschigano K.T., Kopchick J.J. and Bartke A. (1999) Deficits in female reproductive function in GH-R-KO mice; role of IGF-I. Endocrinology 140, 2637–2640 10.1210/endo.140.6.6992 [DOI] [PubMed] [Google Scholar]
- 359.Slot K.A., Kastelijn J., Bachelot A., Kelly P.A., Binart N. and Teerds K.J. (2006) Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction 131, 525–532 10.1530/rep.1.00946 [DOI] [PubMed] [Google Scholar]
- 360.Sonntag W.E., Carter C.S., Ikeno Y., Ekenstedt K., Carlson C.S., Loeser R.F.et al. (2005) Adult-onset growth hormone and insulin-like growth factor i deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology 146, 2920–2932 10.1210/en.2005-0058 [DOI] [PubMed] [Google Scholar]
- 361.Giampietro A., Milardi D., Bianchi A., Fusco A., Cimino V., Valle D.et al. (2009) The effect of treatment with growth hormone on fertility outcome in eugonadal women with growth hormone deficiency: report of four cases and review of the literature. Fertil. Steril. 91, 930.e7–1011.e7 10.1016/j.fertnstert.2008.09.065 [DOI] [PubMed] [Google Scholar]
- 362.Homburg R., West C., Torresani T. and Jacobs H.S. (1990) Cotreatment with human growth hormone and gonadotropins for induction of ovulation: a controlled clinical trial. Fertil. Steril. 53, 254–260 10.1016/S0015-0282(16)53277-4 [DOI] [PubMed] [Google Scholar]
- 363.Yovich J.L. and Stanger J.D. (2010) Growth hormone supplementation improves implantation and pregnancy productivity rates for poor-prognosis patients undertaking IVF. Reprod. Biomed. Online 21, 37–49 10.1016/j.rbmo.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 364.Adams N.R. and Briegel J.R. (2005) Multiple effects of an additional growth hormone gene in adult sheep1. J. Anim. Sci. 83, 1868–1874 10.2527/2005.8381868x [DOI] [PubMed] [Google Scholar]
- 365.Cecim M., Kerr J. and Bartke A. (1995) Effects of bovine growth hormone (bGH) transgene expression or bGH treatment on reproductive functions in female mice. Biol. Reprod. 52, 1144–1148 10.1095/biolreprod52.5.1144 [DOI] [PubMed] [Google Scholar]
- 366.Devesa J. and Caicedo D. (2019) The role of growth hormone on ovarian functioning and ovarian angiogenesis. Front Endocrinol. 10, 450 10.3389/fendo.2019.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Hull K.L. and Harvey S. (2014) Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int. J. Endocrinol. 2014, 234014 10.1155/2014/234014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Olwi D., Day F. and Ong K. (2023) Effect of growth hormone therapy on pubertal timing: systematic review and meta-analysis. Horm. Res. Paediatr. 1–10 10.1159/000530578 [DOI] [PubMed] [Google Scholar]
- 369.Bhattarai J.P., Kim S.H., Han S.K. and Park M.J. (2010) Effects of human growth hormone on gonadotropin-releasing hormone neurons in mice. Korean J. Pediatr. 53, 845–851 10.3345/kjp.2010.53.9.845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Martínez-Moreno C.G., Calderón-Vallejo D., Harvey S., Arámburo C. and Quintanar J.L. (2018) Growth hormone (GH) and gonadotropin-releasing hormone (GnRH) in the central nervous system: a potential neurological combinatory therapy. Int. J. Mol. Sci. 19, 375 10.3390/ijms19020375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Yigiter M., Halici Z., Odabasoglu F., Keles O.N., Atalay F., Unal B.et al. (2011) Growth hormone reduces tissue damage in rat ovaries subjected to torsion and detorsion: biochemical and histopathologic evaluation. Eur. J. Obstet. Gynecol. Reprod. Biol. 157, 94–100 10.1016/j.ejogrb.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 372.Martins F.S., Saraiva M.V.A., Magalhães-Padilha D.M., Almeida A.P., Celestino J.J.H., Padilha R.T.et al. (2014) Presence of growth hormone receptor. (GH-R) mRNA and protein in goat ovarian follicles and improvement of in vitro preantral follicle survival and development with GH. Theriogenology 82, 27–35 10.1016/j.theriogenology.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 373.Weall B.M., Al-Samerria S., Conceicao J., Yovich J.L. and Almahbobi G. (2015) A direct action for GH in improvement of oocyte quality in poor-responder patients. Reprod. Camb. Engl. 149, 147–154 10.1530/REP-14-0494 [DOI] [PubMed] [Google Scholar]
- 374.Magalhães D.M., Duarte A.B.G., Araújo V.R., Brito I.R., Soares T.G., Lima I.M.T.et al. (2011) In vitro production of a caprine embryo from a preantral follicle cultured in media supplemented with growth hormone. Theriogenology 75, 182–188 10.1016/j.theriogenology.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 375.Serafim M.K.B., Duarte A.B.G., Silva G.M., Souza C.E.A., Magalhães-Padilha D.M., Moura A.A.A.et al. (2015) Impact of growth hormone. (GH) and follicle stimulating hormone. (FSH) on in vitro canine preantral follicle development and estradiol production. Growth Horm. IGF Res. 25, 85–89 10.1016/j.ghir.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 376.Costoya J.A., Finidori J., Moutoussamy S., Señaris R., Devesa J. and Arce V.M. (1999) Activation of growth hormone receptor delivers an antiapoptotic signal: evidence for a role of Akt in this pathway. Endocrinology 140, 5937–5943 10.1210/endo.140.12.7209 [DOI] [PubMed] [Google Scholar]
- 377.Eisenhauer K.M., Chun S.-Y., Billig H. and Hsueh A.J.W. (1995) Growth hormone suppression of apoptosis in preovulatory rat follicles and partial neutralization by insulin-like growth factor binding protein. Biol. Reprod. 53, 13–20 10.1095/biolreprod53.1.13 [DOI] [PubMed] [Google Scholar]
- 378.Semiz O. and Evirgen O. (2009) The effect of growth hormone on ovarian follicular response and oocyte nuclear maturation in young and aged mice. Acta Histochem. 111, 104–111 10.1016/j.acthis.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 379.Kiapekou E., Loutradis D., Drakakis P., Zapanti E., Mastorakos G. and Antsaklis A. (2005) Effects of GH and IGF-I on the in vitro maturation of mouse oocytes. Horm. Athens Greece 4, 155–160 10.14310/horm.2002.11153 [DOI] [PubMed] [Google Scholar]
- 380.Chigioni S., Secchi C., Borromeo V., Modina S., Beretta M.S. and Luvoni G.C. (2008) Effects of growth hormone on oocyte in vitro maturation and its localization in the canine cumulus-oocyte complexes. Vet. Res. Commun. 32, 131–134 10.1007/s11259-008-9098-y [DOI] [PubMed] [Google Scholar]
- 381.Shirazi A., Shams-Esfandabadi N., Ahmadi E. and Heidari B. (2010) Effects of growth hormone on nuclear maturation of ovine oocytes and subsequent embryo development. Reprod. Domest. Anim. 45, 530–536 10.1111/j.1439-0531.2008.01290.x [DOI] [PubMed] [Google Scholar]
- 382.Mtango N.R., Varisanga M.D., Dong Y.J., Rajamahendran R. and Suzuki T. (2003) Growth factors and growth hormone enhance in vitro embryo production and post-thaw survival of vitrified bovine blastocysts. Theriogenology 59, 1393–1402 10.1016/S0093-691X(02)01163-9 [DOI] [PubMed] [Google Scholar]
- 383.Pereira G.R., Lorenzo P.L., Carneiro G.F.et al. (2012) The effect of growth hormone (GH) and insulin-like growth factor-I (IGF-I) on in vitro maturation of equine oocytes. Zygote 20, 353–360 10.1017/S0967199411000335 [DOI] [PubMed] [Google Scholar]
- 384.Li Y., Liu H., Yu Q., Liu H., Huang T., Zhao S.et al. (2019) Growth hormone promotes in vitro maturation of human oocytes. Front Endocrinol. 10, 485 10.3389/fendo.2019.00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kölle S., Stojkovic M., Boie G., Wolf E. and Sinowatz F. (2003) Growth hormone-related effects on apoptosis, mitosis, and expression of connexin 43 in bovine in vitro maturation cumulus-oocyte complexes. Biol. Reprod. 68, 1584–1589 10.1095/biolreprod.102.010264 [DOI] [PubMed] [Google Scholar]
- 386.Songsasen N., Yu I. and Leibo S.P. (2002) Nuclear maturation of canine oocytes cultured in protein-free media. Mol. Reprod. Dev 62, 407–415 10.1002/mrd.10130 [DOI] [PubMed] [Google Scholar]
- 387.Kaiser G.G., Kölle S., Boie G., Sinowatz F., Palma G.A. and Alberio R.H. (2006) In vivo effect of growth hormone on the expression of connexin-43 in bovine ovarian follicles. Mol. Reprod. Dev 73, 600–606 10.1002/mrd.20438 [DOI] [PubMed] [Google Scholar]
- 388.Kobayashi J., Mizunuma H., Kikuchi N., Liu X., Andoh K., Abe Y.et al. (2000) Morphological assessment of the effect of growth hormone on preantral follicles from 11-day-old mice in an in vitro culture system. Biochem. Biophys. Res. Commun. 268, 36–41 10.1006/bbrc.1999.2072 [DOI] [PubMed] [Google Scholar]
- 389.Bezecný I., Bártová J. and Skarda J. (1992) Growth hormone treatment increases oestrogen receptor concentration in the guinea-pig uterus. J. Endocrinol. 134, 5–9 10.1677/joe.0.1340005 [DOI] [PubMed] [Google Scholar]
- 390.Chilton B.S. and Daniel J.C. (1987) Differences in the rabbit uterine response to progesterone as influenced by growth hormone or prolactin. J. Reprod. Fertil. 79, 581–587 10.1530/jrf.0.0790581 [DOI] [PubMed] [Google Scholar]
- 391.Guzeloglu A., Bilby T.R., Meikle A., Kamimura S., Kowalski A., Michel F.et al. (2004) Pregnancy and bovine somatotropin in nonlactating dairy cows: II. Endometrial gene expression related to maintenance of pregnancy. J. Dairy Sci. 87, 3268–3279 10.3168/jds.S0022-0302(04)73463-3 [DOI] [PubMed] [Google Scholar]
- 392.Liu F.-T., Wu Z., Yan J., Norman R.J. and Li R. (2020) The potential role of growth hormone on the endometrium in assisted reproductive technology. Front Endocrinol. 11, 49 10.3389/fendo.2020.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Santos J.E.P., Juchem S.O., Cerri R.L.A., Galvão K.N., Chebel R.C., Thatcher W.W.et al. (2004) Effect of bST and reproductive management on reproductive performance of Holstein dairy cows. J. Dairy Sci. 87, 868–881 10.3168/jds.S0022-0302(04)73231-2 [DOI] [PubMed] [Google Scholar]
- 394.Drakopoulos P., Pluchino N., Bischof P., Cantero P., Meyer P. and Chardonnens D. (2016) Effect of growth hormone on endometrial thickness and fertility outcome in the treatment of women with panhypopituitarism: a case report. J. Reprod. Med. 61, 78–82 [PubMed] [Google Scholar]
- 395.Du X.-F., Yang X.-H., Li J., Hao M. and Guo Y.-H. (2016) Growth hormone co-treatment within a GnRH agonist long protocol improves implantation and pregnancy rates in patients undergoing IVF-ET. Arch. Gynecol. Obstet. 294, 877–883 10.1007/s00404-016-4163-1 [DOI] [PubMed] [Google Scholar]
- 396.Mendoza C., Ruiz-Requena E., Ortega E., Cremades N., Martinez F., Bernabeu R.et al. (2002) Follicular fluid markers of oocyte developmental potential. Hum. Reprod. 17, 1017–1022 10.1093/humrep/17.4.1017 [DOI] [PubMed] [Google Scholar]
- 397.Mendoza C., Cremades N., Ruiz-Requena E., Martinez F., Ortega E., Bernabeu S.et al. (1999) Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum. Reprod. 14, 628–635 10.1093/humrep/14.3.628 [DOI] [PubMed] [Google Scholar]
- 398.Kolibianakis E.M., Venetis C.A., Diedrich K., Tarlatzis B.C. and Griesinger G. (2009) Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in-vitro fertilization: a systematic review and meta-analysis. Hum. Reprod. Update 15, 613–622 10.1093/humupd/dmp026 [DOI] [PubMed] [Google Scholar]
- 399.Yu X., Ruan J., He L.-P., Hu W., Xu Q., Tang J.et al. (2015) Efficacy of growth hormone supplementation with gonadotrophins in vitro fertilization for poor ovarian responders: an updated meta-analysis. Int. J. Clin. Exp. Med. 8, 4954–4967 [PMC free article] [PubMed] [Google Scholar]
- 400.Cai M., Gao L., Liang X., Fang C., Wu Y. and Yang X. (2019) The Effect of Growth Hormone on the Clinical Outcomes of Poor Ovarian Reserve Patients Undergoing in vitro Fertilization/Intracytoplasmic Sperm Injection Treatment: A Retrospective Study Based on POSEIDON Criteria. Front Endocrinol. 10, 775 10.3389/fendo.2019.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Chang C.-W., Sung Y.-W., Hsueh Y.-W., Chen Y.-Y., Ho M., Hsu H.-C.et al. (2022) Growth hormone in fertility and infertility: Mechanisms of action and clinical applications. Front Endocrinol. 13, 1040503 10.3389/fendo.2022.1040503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Keane K.N., Hinchliffe P.M., Rowlands P.K., Borude G., Srinivasan S., Dhaliwal S.S.et al. (2018) DHEA Supplementation confers no additional benefit to that of growth hormone on pregnancy and live birth rates in ivf patients categorized as poor prognosis. Front Endocrinol. 9, 14 10.3389/fendo.2018.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Keane K.N., Yovich J.L., Hamidi A., Hinchliffe P.M. and Dhaliwal S.S. (2017) Single-centre retrospective analysis of growth hormone supplementation in IVF patients classified as poor-prognosis. BMJ Open 7, e018107 10.1136/bmjopen-2017-018107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Li J., Chen Q., Wang J., Huang G. and Ye H. (2020) Does growth hormone supplementation improve oocyte competence and IVF outcomes in patients with poor embryonic development? A randomized controlled trial BMC Pregnancy Childbirth 20, 310 10.1186/s12884-020-03004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Wang N., Huang Y., Wen J., Su Q., Huang Y., Cai L.et al. (2019) Early life exposure to famine and reproductive aging among Chinese women. Menopause 26, 463–468 10.1097/GME.0000000000001259 [DOI] [PubMed] [Google Scholar]
- 406.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H. and Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 407.Deschaine S.L. and Leggio L. (2022) From “Hunger Hormone” to “It's Complicated”: Ghrelin Beyond Feeding Control. Physiol. Bethesda Md. 37, 5–15 10.1152/physiol.00024.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Tschöp M., Smiley D.L. and Heiman M.L. (2000) Ghrelin induces adiposity in rodents. Nature 407, 908–913 10.1038/35038090 [DOI] [PubMed] [Google Scholar]
- 409.Nunez-Salces M., Li H., Feinle-Bisset C., Young R.L. and Page A.J. (2021) The regulation of gastric ghrelin secretion. Acta Physiol. Oxf. Engl. 231, e13588 10.1111/apha.13588 [DOI] [PubMed] [Google Scholar]
- 410.Ueberberg B., Unger N., Saeger W., Mann K. and Petersenn S. (2009) Expression of ghrelin and its receptor in human tissues. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 41, 814–821 10.1055/s-0029-1233462 [DOI] [PubMed] [Google Scholar]
- 411.Ariyasu H., Takaya K., Tagami T., Ogawa Y., Hosoda K., Akamizu T.et al. (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 86, 4753–4758 10.1210/jcem.86.10.7885 [DOI] [PubMed] [Google Scholar]
- 412.Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E. and Weigle D.S. (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 10.2337/diabetes.50.8.1714 [DOI] [PubMed] [Google Scholar]
- 413.De Souza M.J., Leidy H.J., O'Donnell E., Lasley B. and Williams N.I. (2004) Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J. Clin. Endocrinol. Metab. 89, 3536–3542 10.1210/jc.2003-032007 [DOI] [PubMed] [Google Scholar]
- 414.Tolle V., Kadem M., Bluet-Pajot M.-T., Frere D., Foulon C., Bossu C.et al. (2003) Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J. Clin. Endocrinol. Metab. 88, 109–116 10.1210/jc.2002-020645 [DOI] [PubMed] [Google Scholar]
- 415.Schneider L.F. and Warren M.P. (2006) Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil. Steril. 86, 1744–1749 10.1016/j.fertnstert.2006.05.051 [DOI] [PubMed] [Google Scholar]
- 416.Soriano-Guillén L., Barrios V., Chowen J.A., Sánchez I., Vila S., Quero J.et al. (2004) Ghrelin levels from fetal life through early adulthood: relationship with endocrine and metabolic and anthropometric measures. J. Pediatr. 144, 30–35 10.1016/j.jpeds.2003.08.050 [DOI] [PubMed] [Google Scholar]
- 417.Fernández-Fernández R., Navarro V.M., Barreiro M.L.et al. (2005) Effects of chronic hyperghrelinemia on puberty onset and pregnancy outcome in the rat. Endocrinology 146, 3018–3025 10.1210/en.2004-1622 [DOI] [PubMed] [Google Scholar]
- 418.Fernández-Fernández R., Tena-Sempere M., Navarro V.M., Barreiro M.L., Castellano J.M., Aguilar E.et al. (2006) Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology 82, 245–255 10.1159/000092753 [DOI] [PubMed] [Google Scholar]
- 419.Tena-Sempere M. (2008) Ghrelin as a pleotrophic modulator of gonadal function and reproduction. Nat. Clin. Pract. Endocrinol. Metab. 4, 666–674 10.1038/ncpendmet1003 [DOI] [PubMed] [Google Scholar]
- 420.Kawamura K., Sato N., Fukuda J., Kodama H., Kumagai J., Tanikawa H.et al. (2003) Ghrelin inhibits the development of mouse preimplantation embryos in vitro. Endocrinology 144, 2623–2633 10.1210/en.2003-0033 [DOI] [PubMed] [Google Scholar]
- 421.Luque E.M., Torres P.J., de Loredo N.et al. (2015) Role of ghrelin in fertilization, early embryo development, and implantation periods. Reproduction 148, 159–167 10.1530/REP-14-0129 [DOI] [PubMed] [Google Scholar]
- 422.Wang Z., Lin P. and Yu S. (2013) Effects of ghrelin on developmental competence and gene expression of in vitro fertilized ovine embryos. Theriogenology 79, 695–701 10.1016/j.theriogenology.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 423.Puechagut P.B., Martini A.C., Stutz G., Santillán M.E., Luque E.M., Fiol de Cuneo M.et al. (2012) Reproductive performance and fertility in male and female adult mice chronically treated with hexarelin. Reprod. Fertil. Dev. 24, 451–460 10.1071/RD11009 [DOI] [PubMed] [Google Scholar]
- 424.Sowers M.R., Wildman R.P., Mancuso P., Eyvazzadeh A.D., Karvonen-Gutierrez C.A., Rillamas-Sun E.et al. (2008) Change in adipocytokines and ghrelin with menopause. Maturitas 59, 149–157 10.1016/j.maturitas.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Wildman R.P., Mancuso P., Wang C., Kim M., Scherer P.E. and Sowers M.R. (2008) Adipocytokine and ghrelin levels in relation to cardiovascular disease risk factors in women at midlife: longitudinal associations. Int. J. Obes. 2005 32, 740–748 10.1038/sj.ijo.0803782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Abdalla M.M.I. and Jegasothy R. (2020) Role of Ghrelin in Postmenopausal obesity. Int. J. Womens Health Reprod Sci. 8, 119–124 10.15296/ijwhr.2020.19 [DOI] [Google Scholar]
- 427.Furuta M., Funabashi T. and Kimura F. (2001) Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem. Biophys. Res. Commun. 288, 780–785 10.1006/bbrc.2001.5854 [DOI] [PubMed] [Google Scholar]
- 428.Lebrethon M.C., Aganina A., Fournier M., Gérard A., Parent A.S. and Bourguignon J.P. (2007) Effects of in vivo and in vitro administration of ghrelin, leptin and neuropeptide mediators on pulsatile gonadotrophin-releasing hormone secretion from male rat hypothalamus before and after puberty. J. Neuroendocrinol. 19, 181–188 10.1111/j.1365-2826.2006.01518.x [DOI] [PubMed] [Google Scholar]
- 429.Vulliémoz N.R., Xiao E., Xia-Zhang L.et al. (2004) Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J. Clin. Endocrinol. Metab. 89, 5718–5723 10.1210/jc.2004-1244 [DOI] [PubMed] [Google Scholar]
- 430.Iqbal J., Kurose Y., Canny B. and Clarke I.J. (2006) Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology 147, 510–519 10.1210/en.2005-1048 [DOI] [PubMed] [Google Scholar]
- 431.Forbes S., Li X.F., Kinsey-Jones J. and O'Byrne K. (2009) Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci. Lett. 460, 143–147 10.1016/j.neulet.2009.05.060 [DOI] [PubMed] [Google Scholar]
- 432.Burdyga G., Varro A., Dimaline R., Thompson D.G. and Dockray G.J. (2006) Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am. J. Physiol.-Gastrointest Liver Physiol. 290, G1289–G1297 10.1152/ajpgi.00543.2005 [DOI] [PubMed] [Google Scholar]
- 433.Zigman J.M., Jones J.E., Lee C.E., Saper C.B. and Elmquist J.K. (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 494, 528–548 10.1002/cne.20823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Yasrebi A., Hsieh A., Mamounis K.J., Krumm E.A., Yang J.A., Magby J.et al. (2016) Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17β-estradiol. Mol. Cell. Endocrinol. 422, 42–56 10.1016/j.mce.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Reichenbach A., Steyn F.J., Sleeman M.W. and Andrews Z.B. (2012) Ghrelin receptor expression and colocalization with anterior pituitary hormones using a GHSR-GFP mouse line. Endocrinology 153, 5452–5466 10.1210/en.2012-1622 [DOI] [PubMed] [Google Scholar]
- 436.Fernández-Fernández R., Tena-Sempere M., Roa J., Castellano J.M., Navarro V.M., Aguilar E.et al. (2007) Direct stimulatory effect of ghrelin on pituitary release of LH through a nitric oxide-dependent mechanism that is modulated by estrogen. Reprod. Camb. Engl. 133, 1223–1232 10.1530/REP-06-0227 [DOI] [PubMed] [Google Scholar]
- 437.Caminos J.E., Tena-Sempere M., Gaytán F., Sanchez-Criado J.E., Barreiro M.L., Nogueiras R.et al. (2003) Expression of ghrelin in the cyclic and pregnant rat ovary. Endocrinology 144, 1594–1602 10.1210/en.2002-221058 [DOI] [PubMed] [Google Scholar]
- 438.Gaytan F., Barreiro M.L., Chopin L.K.et al. (2003) Immunolocalization of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in the cyclic human ovary. J. Clin. Endocrinol. Metab. 88, 879–887 10.1210/jc.2002-021196 [DOI] [PubMed] [Google Scholar]
- 439.Du C., Li H., Cao G., Xilingaowa ., Wang C. and Li C. (2010) Expression of the orexigenic peptide ghrelin and the type 1a growth hormone secretagogue receptor in sheep oocytes and pre-implantation embryos produced in vitro. Reprod. Domest. Anim. Zuchthyg 45, 92–98 10.1111/j.1439-0531.2008.01259.x [DOI] [PubMed] [Google Scholar]
- 440.Komarowska H., Waśko R., Iwanik K., Majewski P., Rafińska L., Warenik-Szymankiewicz A.et al. (2006) Ghrelin ovarian cell expression in patients with polycystic ovary syndrome: an immunohistochemical evaluation. Horm. Metab. Res. 38, 783–788 10.1055/s-2006-956506 [DOI] [PubMed] [Google Scholar]
- 441.Sirotkin A.V., Grossmann R., María-Peon M.T., Roa J., Tena-Sempere M. and Klein S. (2006) Novel expression and functional role of ghrelin in chicken ovary. Mol. Cell. Endocrinol. 257-258, 15–25 10.1016/j.mce.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 442.Miller D.W., Harrison J.L., Brown Y.A., Doyle U., Lindsay A., Adam C.L.et al. (2005) Immunohistochemical evidence for an endocrine/paracrine role for ghrelin in the reproductive tissues of sheep. Reprod. Biol. Endocrinol. RBE 3, 60 10.1186/1477-7827-3-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Zhang W., Lei Z., Su J. and Chen S. (2008) Expression of ghrelin in the porcine hypothalamo-pituitary-ovary axis during the estrous cycle. Anim. Reprod. Sci. 109, 356–367 10.1016/j.anireprosci.2007.12.020 [DOI] [PubMed] [Google Scholar]
- 444.Fang F., Wang L., Zhang Y., Li Y., Su S. and Zhang X. (2012) Role of ghrelin on estrogen and progesterone secretion in the adult rat ovary during estrous cycle. Syst. Biol. Reprod. Med. 58, 116–119 10.3109/19396368.2011.637607 [DOI] [PubMed] [Google Scholar]
- 445.Tropea A., Tiberi F., Minici F., Orlando M., Gangale M.F., Romani F.et al. (2007) Ghrelin affects the release of luteolytic and luteotropic factors in human luteal cells. J. Clin. Endocrinol. Metab. 92, 3239–3245 10.1210/jc.2007-0180 [DOI] [PubMed] [Google Scholar]
- 446.Viani I., Vottero A., Tassi F., Cremonini G., Sartori C., Bernasconi S.et al. (2008) Ghrelin inhibits steroid biosynthesis by cultured granulosa-lutein cells. J. Clin. Endocrinol. Metab. 93, 1476–1481 10.1210/jc.2007-2063 [DOI] [PubMed] [Google Scholar]
- 447.Kheradmand A., Roshangar L., Taati M. and Sirotkin A.V. (2009) Morphometrical and intracellular changes in rat ovaries following chronic administration of ghrelin. Tissue Cell 41, 311–317 10.1016/j.tice.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 448.Sominsky L., Goularte J.F., Andrews Z.B. and Spencer S.J. (2018) Acylated ghrelin supports the ovarian transcriptome and follicles in the mouse: implications for fertility. Front Endocrinol. 9, 815 10.3389/fendo.2018.00815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Bado A., Levasseur S., Attoub S., Kermorgant S., Laigneau J.P., Bortoluzzi M.N.et al. (1998) The stomach is a source of leptin. Nature 394, 790–793 10.1038/29547 [DOI] [PubMed] [Google Scholar]
- 450.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D.et al. (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 10.1126/science.7624777 [DOI] [PubMed] [Google Scholar]
- 451.Maffei M., Fei H., Lee G.H., Dani C., Leroy P., Zhang Y.et al. (1995) Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc. Natl. Acad. Sci. U.S.A. 92, 6957–6960 10.1073/pnas.92.15.6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Masuzaki H., Ogawa Y., Sagawa N., Hosoda K., Matsumoto T., Mise H.et al. (1997) Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 3, 1029–1033 10.1038/nm0997-1029 [DOI] [PubMed] [Google Scholar]
- 453.Wang J., Liu R., Hawkins M., Barzilai N. and Rossetti L. (1998) A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393, 684–688 10.1038/31474 [DOI] [PubMed] [Google Scholar]
- 454.Maffei M., Halaas J., Ravussin E., Pratley R.E., Lee G.H., Zhang Y.et al. (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1, 1155–1161 10.1038/nm1195-1155 [DOI] [PubMed] [Google Scholar]
- 455.Rayner D.V. and Trayhurn P. (2001) Regulation of leptin production: sympathetic nervous system interactions. J. Mol. Med. Berl. Ger. 79, 8–20 10.1007/s001090100198 [DOI] [PubMed] [Google Scholar]
- 456.Hellström L., Wahrenberg H., Hruska K., Reynisdottir S. and Arner P. (2000) Mechanisms behind gender differences in circulating leptin levels. J. Intern. Med. 247, 457–462 10.1046/j.1365-2796.2000.00678.x [DOI] [PubMed] [Google Scholar]
- 457.Hickey M.S., Israel R.G., Gardiner S.N., Considine R.V., McCammon M.R., Tyndall G.L.et al. (1996) Gender differences in serum leptin levels in humans. Biochem. Mol. Med. 59, 1–6 10.1006/bmme.1996.0056 [DOI] [PubMed] [Google Scholar]
- 458.Shimizu H., Shimomura Y., Nakanishi Y., Futawatari T., Ohtani K., Sato N.et al. (1997) Estrogen increases in vivo leptin production in rats and human subjects. J. Endocrinol. 154, 285–292 10.1677/joe.0.1540285 [DOI] [PubMed] [Google Scholar]
- 459.Van Harmelen V., Reynisdottir S., Eriksson P., Thörne A., Hoffstedt J., Lönnqvist F.et al. (1998) Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 47, 913–917 10.2337/diabetes.47.6.913 [DOI] [PubMed] [Google Scholar]
- 460.Wabitsch M., Blum W.F., Muche R., Braun M., Hube F., Rascher W.et al. (1997) Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J. Clin. Invest. 100, 808–813 10.1172/JCI119595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Coleman D.L. (1973) Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 9, 294–298 10.1007/BF01221857 [DOI] [PubMed] [Google Scholar]
- 462.Ingalls A.M., Dickie M.M. and Snell G.D. (1950) Obese, a new mutation in the house mouse. J. Hered. 41, 317–318 10.1093/oxfordjournals.jhered.a106073 [DOI] [PubMed] [Google Scholar]
- 463.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L. and Friedman J.M. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 464.Farooqi I.S. (2002) Leptin and the onset of puberty: insights from rodent and human genetics. Semin. Reprod. Med. 20, 139–144 10.1055/s-2002-32505 [DOI] [PubMed] [Google Scholar]
- 465.Ozata M., Ozdemir I.C. and Licinio J. (1999) Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 84, 3686–3695 10.1210/jcem.84.10.5999 [DOI] [PubMed] [Google Scholar]
- 466.Strobel A., Issad T., Camoin L., Ozata M. and Strosberg A.D. (1998) A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 18, 213–215 10.1038/ng0398-213 [DOI] [PubMed] [Google Scholar]
- 467.Obradovic M., Sudar-Milovanovic E., Soskic S., Essack M., Arya S., Stewart A.J.et al. (2021) Leptin and obesity: role and clinical implication. Front Endocrinol. 12, 585887 10.3389/fendo.2021.585887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Frühbeck G. (2006) Intracellular signalling pathways activated by leptin. Biochem. J. 393, 7–20 10.1042/BJ20051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Ghilardi N., Ziegler S., Wiestner A., Stoffel R., Heim M.H. and Skoda R.C. (1996) Defective STAT signaling by the leptin receptor in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 93, 6231–6235 10.1073/pnas.93.13.6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Tartaglia L.A. (1997) The leptin receptor. J. Biol. Chem. 272, 6093–6096 10.1074/jbc.272.10.6093 [DOI] [PubMed] [Google Scholar]
- 471.Karlsson C., Lindell K., Svensson E., Bergh C., Lind P., Billig H.et al. (1997) Expression of functional leptin receptors in the human ovary. J. Clin. Endocrinol. Metab. 82, 4144–4148 10.1210/jc.82.12.4144 [DOI] [PubMed] [Google Scholar]
- 472.Mercer J.G., Hoggard N., Williams L.M., Lawrence C.B., Hannah L.T. and Trayhurn P. (1996) Localization of leptin receptor mRNA and the long form splice variant. (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 387, 113–116 10.1016/0014-5793(96)00473-5 [DOI] [PubMed] [Google Scholar]
- 473.Park H.-K. and Ahima R.S. (2015) Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 64, 24–34 10.1016/j.metabol.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Paz-Filho G., Mastronardi C.A. and Licinio J. (2015) Leptin treatment: facts and expectations. Metabolism 64, 146–156 10.1016/j.metabol.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 475.Licinio J., Negrão A.B., Mantzoros C., Kaklamani V., Wong M.L., Bongiorno P.B.et al. (1998) Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc. Natl. Acad. Sci. U. S. A. 95, 2541–2546 10.1073/pnas.95.5.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Welt C.K., Chan J.L., Bullen J., Murphy R., Smith P., DePaoli A.M.et al. (2004) Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 351, 987–997 10.1056/NEJMoa040388 [DOI] [PubMed] [Google Scholar]
- 477.Chou S.H., Chamberland J.P., Liu X., Matarese G., Gao C., Stefanakis R.et al. (2011) Leptin is an effective treatment for hypothalamic amenorrhea. Proc. Natl. Acad. Sci. 108, 6585–6590 10.1073/pnas.1015674108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Barash I.A., Cheung C.C., Weigle D.S., Ren H., Kabigting E.B., Kuijper J.L.et al. (1996) Leptin is a metabolic signal to the reproductive system. Endocrinology 137, 3144–3147 10.1210/endo.137.7.8770941 [DOI] [PubMed] [Google Scholar]
- 479.Chehab F.F., Lim M.E. and Lu R. (1996) Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 12, 318–320 10.1038/ng0396-318 [DOI] [PubMed] [Google Scholar]
- 480.Farooqi I.S., Matarese G., Lord G.M., Keogh J.M., Lawrence E., Agwu C.et al. (2002) Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 10.1172/JCI0215693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Ahima R.S., Dushay J., Flier S.N., Prabakaran D. and Flier J.S. (1997) Leptin accelerates the onset of puberty in normal female mice. J. Clin. Invest. 99, 391–395 10.1172/JCI119172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Chehab F.F., Mounzih K., Lu R. and Lim M.E. (1997) Early onset of reproductive function in normal female mice treated with leptin. Science 275, 88–90 10.1126/science.275.5296.88 [DOI] [PubMed] [Google Scholar]
- 483.Castellano J.M., Roa J., Luque R.M., Dieguez C., Aguilar E., Pinilla L.et al. (2009) KiSS-1/kisspeptins and the metabolic control of reproduction: Physiologic roles and putative physiopathological implications. Peptides 30, 139–145 10.1016/j.peptides.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 484.Cheung C.C., Thornton J.E., Kuijper J.L., Weigle D.S., Clifton D.K. and Steiner R.A. (1997) Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology 138, 855–858 10.1210/endo.138.2.5054 [DOI] [PubMed] [Google Scholar]
- 485.Roa J., García-Galiano D., Castellano J.M., Gaytan F., Pinilla L. and Tena-Sempere M. (2010) Metabolic control of puberty onset: new players, new mechanisms. Mol. Cell. Endocrinol. 324, 87–94 10.1016/j.mce.2009.12.018 [DOI] [PubMed] [Google Scholar]
- 486.True C., Kirigiti M.A., Kievit P., Grove K.L. and Smith M.S. (2011) Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J. Neuroendocrinol. 23, 1099–1112 10.1111/j.1365-2826.2011.02144.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Swain J.E., Dunn R.L., McConnell D., Gonzalez-Martinez J. and Smith G.D. (2004) Direct effects of leptin on mouse reproductive function: regulation of follicular, oocyte, and embryo development. Biol. Reprod. 71, 1446–1452 10.1095/biolreprod.104.033035 [DOI] [PubMed] [Google Scholar]
- 488.Barkan D., Hurgin V., Dekel N., Amsterdam A. and Rubinstein M. (2005) Leptin induces ovulation in GnRH-deficient mice. FASEB J. 19, 133–135 10.1096/fj.04-2271fje [DOI] [PubMed] [Google Scholar]
- 489.Paula-Lopes F.F., Boelhauve M., Habermann F.A., Sinowatz F. and Wolf E. (2007) Leptin promotes meiotic progression and developmental capacity of bovine oocytes via cumulus cell-independent and -dependent mechanisms. Biol. Reprod. 76, 532–541 10.1095/biolreprod.106.054551 [DOI] [PubMed] [Google Scholar]
- 490.Quennell J.H., Mulligan A.C., Tups A., Liu X., Phipps S.J., Kemp C.J.et al. (2009) Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150, 2805–2812 10.1210/en.2008-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Backholer K., Smith J.T., Rao A., Pereira A., Iqbal J., Ogawa S.et al. (2010) Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 151, 2233–2243 10.1210/en.2009-1190 [DOI] [PubMed] [Google Scholar]
- 492.Louis G.W., Greenwald-Yarnell M., Phillips R., Coolen L.M., Lehman M.N. and Myers M.G. Jr (2011) Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology 152, 2302–2310 10.1210/en.2011-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Morelli A., Marini M., Mancina R., Luconi M., Vignozzi L., Fibbi B.et al. (2008) Sex Steroids and Leptin Regulate the “First Kiss”. (KiSS 1/G-Protein-Coupled Receptor 54 System) in Human Gonadotropin-Releasing-Hormone-Secreting Neuroblasts. J. Sex Med. 5, 1097–1113 10.1111/j.1743-6109.2008.00782.x [DOI] [PubMed] [Google Scholar]
- 494.Jin L., Zhang S., Burguera B.G., Couce M.E., Osamura R.Y., Kulig E.et al. (2000) Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology 141, 333–339 10.1210/endo.141.1.7260 [DOI] [PubMed] [Google Scholar]
- 495.Yu W.H., Walczewska A., Karanth S. and McCann S.M. (1997) Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology 138, 5055–5058 10.1210/endo.138.11.5649 [DOI] [PubMed] [Google Scholar]
- 496.Archanco M., Muruzábal F.J., Llopiz D., Garayoa M., Gómez-Ambrosi J., Frühbeck G.et al. (2003) Leptin expression in the rat ovary depends on estrous cycle. J. Histochem. Cytochem. 51, 1269–1277 10.1177/002215540305101003 [DOI] [PubMed] [Google Scholar]
- 497.Cioffi J.A., Van Blerkom J., Antczak M., Shafer A., Wittmer S. and Snodgrass H.R. (1997) The expression of leptin and its receptors in pre-ovulatory human follicles. Mol. Hum. Reprod. 3, 467–472 10.1093/molehr/3.6.467 [DOI] [PubMed] [Google Scholar]
- 498.Duggal P.S., Weitsman S.R., Magoffin D.A. and Norman R.J. (2002) Expression of the long (OB-RB) and short (OB-RA) forms of the leptin receptor throughout the oestrous cycle in the mature rat ovary. Reproduction 123, 899–905 10.1530/rep.0.1230899 [DOI] [PubMed] [Google Scholar]
- 499.Agarwal S.K., Vogel K., Weitsman S.R. and Magoffin D.A. (1999) Leptin antagonizes the insulin-like growth factor-i augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J. Clin. Endocrinol. Metab. 84, 1072–1076 10.1210/jc.84.3.1072 [DOI] [PubMed] [Google Scholar]
- 500.Brannian J.D., Zhao Y. and McElroy M. (1999) Leptin inhibits gonadotrophin-stimulated granulosa cell progesterone production by antagonizing insulin action. Hum. Reprod. 14, 1445–1448 10.1093/humrep/14.6.1445 [DOI] [PubMed] [Google Scholar]
- 501.Spicer L.J. and Francisco C.C. (1998) Adipose obese gene product, leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol. Reprod. 58, 207–212 10.1095/biolreprod58.1.207 [DOI] [PubMed] [Google Scholar]
- 502.Zachow R.J., Weitsman S.R. and Magoffin D.A. (1999) Leptin impairs the synergistic stimulation by transforming growth factor-β of follicle-stimulating hormone-dependent aromatase activity and messenger ribonucleic acid expression in rat ovarian granulosa cells. Biol. Reprod. 61, 1104–1109 10.1095/biolreprod61.4.1104 [DOI] [PubMed] [Google Scholar]
- 503.Zachow R.J. and Magoffin D.A. (1997) Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17β production by rat ovarian granulosa cells. Endocrinology 138, 847–850 10.1210/endo.138.2.5035 [DOI] [PubMed] [Google Scholar]
- 504.Duggal P.S., Van der Hoek K.H., Milner C.R., Ryan N.K., Armstrong D.T., Magoffin D.A.et al. (2000) The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology 141, 1971–1976 10.1210/endo.141.6.7509 [DOI] [PubMed] [Google Scholar]
- 505.Ahrens K., Mumford S.L., Schliep K.C., Kissell K.A., Perkins N.J., Wactawski-Wende J.et al. (2014) Serum leptin levels and reproductive function during the menstrual cycle. Am. J. Obstet. Gynecol. 210, 248.e1–248.e9 10.1016/j.ajog.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Sir-Petermann T., Piwonka V., Pérez F., Maliqueo M., Recabarren S.E. and Wildt L. (1999) Are circulating leptin and luteinizing hormone synchronized in patients with polycystic ovary syndrome? Hum. Reprod. 14, 1435–1439 10.1093/humrep/14.6.1435 [DOI] [PubMed] [Google Scholar]
- 507.Riad-Gabriel M.G., Jinagouda S.D., Sharma A., Boyadjian R. and Saad M.F. (1998) Changes in plasma leptin during the menstrual cycle. Eur. J. Endocrinol. 139, 528–531 10.1530/eje.0.1390528 [DOI] [PubMed] [Google Scholar]
- 508.Sarkar M., Schilffarth S., Schams D., Meyer H.H.D. and Berisha B. (2010) The expression of leptin and its receptor during different physiological stages in the bovine ovary. Mol. Reprod. Dev 77, 174–181 10.1002/mrd.21129 [DOI] [PubMed] [Google Scholar]
- 509.Zendron C., Gonçalves H.F., Cavalcante F.S., Pereira T.R., Evangelista A., Ramos C.F.et al. (2014) Increased expression of the leptin receptor in human ovaries affected by endometrioma and detection of high levels of leptin in the ovarian endometriomal fluid. J. Ovarian Res. 7, 2 10.1186/1757-2215-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Šrámková M., Dušková M., Vítků J., Včelák J., Matucha P., Bradnová O.et al. (2015) Levels of adipokines and some steroids during the menstrual cycle. Physiol. Res. 64, S147–S154 10.33549/physiolres.933116 [DOI] [PubMed] [Google Scholar]
- 511.Teirmaa T., Luukkaa V., Rouru J., Koulu M. and Huupponen R. (1998) Correlation between circulating leptin and luteinizing hormone during the menstrual cycle in normal-weight women. Eur. J. Endocrinol. 139, 190–194 10.1530/eje.0.1390190 [DOI] [PubMed] [Google Scholar]
- 512.Maruyama S., Minami S., Kaseki H., Ishihara K., Araki T. and Suzue R. (2001) A Comparison of Serum Leptin Concentrations in Obese and Normal Weight Japanese Women with Regular Menstrual Cycle. J. Nutr. Sci. Vitaminol. (Tokyo) 47, 87–89 10.3177/jnsv.47.87 [DOI] [PubMed] [Google Scholar]
- 513.Anim-Nyame N., Sooranna S.R., Steer P.J. and Johnson M.R. (2000) Longitudinal analysis of maternal plasma leptin concentrations during normal pregnancy and pre-eclampsia. Hum Reprod. Oxf. Engl. 15, 2033–2036 10.1093/humrep/15.9.2033 [DOI] [PubMed] [Google Scholar]
- 514.Khant Aung Z., Grattan D.R. and Ladyman S.R. (2020) Pregnancy-induced adaptation of central sensitivity to leptin and insulin. Mol. Cell. Endocrinol. 516, 110933 10.1016/j.mce.2020.110933 [DOI] [PubMed] [Google Scholar]
- 515.Schanton M., Maymó J.L., Pérez-Pérez A., Sánchez-Margalet V. and Varone C.L. (2018) Involvement of leptin in the molecular physiology of the placenta. Reproduction 155, R1–R12 10.1530/REP-17-0512 [DOI] [PubMed] [Google Scholar]
- 516.D'Ippolito S., Tersigni C., Scambia G. and Di Simone N. (2012) Adipokines, an adipose tissue and placental product with biological functions during pregnancy. Biofactors 38, 14–23 10.1002/biof.201 [DOI] [PubMed] [Google Scholar]
- 517.Barrientos G., Toro A., Moschansky P., Cohen M., Garcia M.G., Rose M.et al. (2015) Leptin promotes HLA-G expression on placental trophoblasts via the MEK/Erk and PI3K signaling pathways. Placenta 36, 419–426 10.1016/j.placenta.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 518.Lappas M., Yee K., Permezel M. and Rice G.E. (2005) Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J. Endocrinol. 186, 457–465 10.1677/joe.1.06227 [DOI] [PubMed] [Google Scholar]
- 519.Masuyama H., Segawa T., Sumida Y., Masumoto A., Inoue S., Akahori Y.et al. (2010) Different profiles of circulating angiogenic factors and adipocytokines between early- and late-onset pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 117, 314–320 10.1111/j.1471-0528.2009.02453.x [DOI] [PubMed] [Google Scholar]
- 520.Hendler I., Blackwell S.C., Mehta S.H., Whitty J.E., Russell E., Sorokin Y.et al. (2005) The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am. J. Obstet. Gynecol. 193, 979–983 10.1016/j.ajog.2005.06.041 [DOI] [PubMed] [Google Scholar]
- 521.Laivuori H., Gallaher M.J., Collura L., Crombleholme W.R., Markovic N., Rajakumar A.et al. (2006) Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol. Hum. Reprod. 12, 551–556 10.1093/molehr/gal064 [DOI] [PubMed] [Google Scholar]
- 522.El shahat A.M., Ahmed A.B., Ahmed M.R. and Mohamed H.S. (2013) Maternal serum leptin as a marker of preeclampsia. Arch. Gynecol. Obstet. 288, 1317–1322 10.1007/s00404-013-2915-8 [DOI] [PubMed] [Google Scholar]
- 523.Salimi S., Farajian-Mashhadi F., Naghavi A., Mokhtari M., Shahrakipour M., Saravani M.et al. (2014) Different profile of serum leptin between early onset and late onset preeclampsia. Dis. Markers 2014, e628476 10.1155/2014/628476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Song Y., Gao J., Qu Y., Wang S., Wang X. and Liu J. (2016) Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin. Chim. Acta 458, 133–137 10.1016/j.cca.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 525.Kautzky-Willer A., Pacini G., Tura A., Bieglmayer C., Schneider B., Ludvik B.et al. (2001) Increased plasma leptin in gestational diabetes. Diabetologia 44, 164–172 10.1007/s001250051595 [DOI] [PubMed] [Google Scholar]
- 526.Hu E., Liang P. and Spiegelman B.M. (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 10.1074/jbc.271.18.10697 [DOI] [PubMed] [Google Scholar]
- 527.Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y. and Matsubara K. (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1. (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 221, 286–289 10.1006/bbrc.1996.0587 [DOI] [PubMed] [Google Scholar]
- 528.Nakano Y., Tobe T., Choi-Miura N.H., Mazda T. and Tomita M. (1996) Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. (Tokyo) 120, 803–812 10.1093/oxfordjournals.jbchem.a021483 [DOI] [PubMed] [Google Scholar]
- 529.Scherer P.E., Williams S., Fogliano M., Baldini G. and Lodish H.F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 10.1074/jbc.270.45.26746 [DOI] [PubMed] [Google Scholar]
- 530.Nawrocki A.R., Rajala M.W., Tomas E., Pajvani U.B., Saha A.K., Trumbauer M.E.et al. (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 281, 2654–2660 10.1074/jbc.M505311200 [DOI] [PubMed] [Google Scholar]
- 531.Semple R.K., Cochran E.K., Soos M.A., Burling K.A., Savage D.B., Gorden P.et al. (2008) Plasma adiponectin as a marker of insulin receptor dysfunction: clinical utility in severe insulin resistance. Diabetes Care. 31, 977–979 10.2337/dc07-2194 [DOI] [PubMed] [Google Scholar]
- 532.Delaigle A.M., Jonas J.-C., Bauche I.B., Cornu O. and Brichard S.M. (2004) Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 145, 5589–5597 10.1210/en.2004-0503 [DOI] [PubMed] [Google Scholar]
- 533.Pischon T., Girman C.J., Hotamisligil G.S., Rifai N., Hu F.B. and Rimm E.B. (2004) Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291, 1730–1737 10.1001/jama.291.14.1730 [DOI] [PubMed] [Google Scholar]
- 534.Schulze M.B., Shai I., Rimm E.B., Li T., Rifai N. and Hu F.B. (2005) Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes 54, 534–539 10.2337/diabetes.54.2.534 [DOI] [PubMed] [Google Scholar]
- 535.Takemoto F., Katori H., Sawa N., Hoshino J., Suwabe T., Nakanishi S.et al. (2008) Plasma adiponectin: a predictor of coronary heart disease in hemodialysis patients - a Japanese prospective eight-year study. Nephron Clin. Pract. 111, c12–c20 10.1159/000178818 [DOI] [PubMed] [Google Scholar]
- 536.Peng J., Chen Q. and Wu C. (2023) The role of adiponectin in cardiovascular disease. Cardiovasc. Pathol. 64, 107514 10.1016/j.carpath.2022.107514 [DOI] [PubMed] [Google Scholar]
- 537.Tu W.-J., Qiu H.-C., Liu Y.-K., Liu Q., Zeng X. and Zhao J. (2020) Elevated levels of adiponectin associated with major adverse cardiovascular and cerebrovascular events and mortality risk in ischemic stroke. Cardiovasc. Diabetol. 19, 125 10.1186/s12933-020-01096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Rodriguez-Pacheco F., Martinez-Fuentes A.J., Tovar S., Pinilla L., Tena-Sempere M., Dieguez C.et al. (2007) Regulation of pituitary cell function by adiponectin. Endocrinology 148, 401–410 10.1210/en.2006-1019 [DOI] [PubMed] [Google Scholar]
- 539.Thundyil J., Pavlovski D., Sobey C.G. and Arumugam T.V. (2012) Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 165, 313–327 10.1111/j.1476-5381.2011.01560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Caminos J.E., Nogueiras R., Gaytán F., Pineda R., González C.R., Barreiro M.L.et al. (2008) Novel expression and direct effects of adiponectin in the rat testis. Endocrinology 149, 3390–3402 10.1210/en.2007-1582 [DOI] [PubMed] [Google Scholar]
- 541.Chabrolle C., Tosca L. and Dupont J. (2007) Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reprod. Camb. Engl. 133, 719–731 10.1530/REP-06-0244 [DOI] [PubMed] [Google Scholar]
- 542.Caminos J.E., Nogueiras R., Gallego R., Bravo S., Tovar S., García-Caballero T.et al. (2005) Expression and regulation of adiponectin and receptor in human and rat placenta. J. Clin. Endocrinol. Metab. 90, 4276–4286 10.1210/jc.2004-0930 [DOI] [PubMed] [Google Scholar]
- 543.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J.et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- 544.Cnop M., Havel P.J., Utzschneider K.M., Carr D.B., Sinha M.K., Boyko E.J.et al. (2003) Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46, 459–469 10.1007/s00125-003-1074-z [DOI] [PubMed] [Google Scholar]
- 545.Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R.E.et al. (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 86, 1930–1935 10.1210/jcem.86.5.7463 [DOI] [PubMed] [Google Scholar]
- 546.Qiao L., Lee B., Kinney B., sun Yoo H. and Shao J. (2011) Energy intake and adiponectin gene expression. Am. J. Physiol. - Endocrinol. Metab. 300, E809–E816 10.1152/ajpendo.00004.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Nishizawa H., Shimomura I., Kishida K., Maeda N., Kuriyama H., Nagaretani H.et al. (2002) Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51, 2734–2741 10.2337/diabetes.51.9.2734 [DOI] [PubMed] [Google Scholar]
- 548.Combs T.P., Berg A.H., Rajala M.W., Klebanov S., Iyengar P., Jimenez-Chillaron J.C.et al. (2003) Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52, 268–276 10.2337/diabetes.52.2.268 [DOI] [PubMed] [Google Scholar]
- 549.Gui Y., Silha J.V. and Murphy L.J. (2012) Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes. Res. 12, 1481–1491 10.1038/oby.2004.185 [DOI] [PubMed] [Google Scholar]
- 550.Yu H., Chhabra K.H., Thompson Z., Jones G.L., Kiran S., Shangguan G.et al. (2020) Hypothalamic POMC deficiency increases circulating adiponectin despite obesity. Mol. Metab. 35, 100957 10.1016/j.molmet.2020.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Yamauchi T., Iwabu M., Okada-Iwabu M. and Kadowaki T. (2014) Adiponectin receptors: a review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 28, 15–23 10.1016/j.beem.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 552.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S.et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- 553.Okamoto M., Ohara-Imaizumi M., Kubota N., Hashimoto S., Eto K., Kanno T.et al. (2008) Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia 51, 827–835 10.1007/s00125-008-0944-9 [DOI] [PubMed] [Google Scholar]
- 554.Wijesekara N., Krishnamurthy M., Bhattacharjee A., Suhail A., Sweeney G. and Wheeler M.B. (2010) Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J. Biol. Chem. 285, 33623–33631 10.1074/jbc.M109.085084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Cheng L., Shi H., Jin Y., Li X., Pan J., Lai Y.et al. (2016) Adiponectin deficiency leads to female subfertility and ovarian dysfunctions in mice. Endocrinology 157, 4875–4887 10.1210/en.2015-2080 [DOI] [PubMed] [Google Scholar]
- 556.Kaminski T., Smolinska N., Maleszka A., Kiezun M., Dobrzyn K., Czerwinska J.et al. (2014) Expression of adiponectin and its receptors in the porcine hypothalamus during the oestrous cycle. Reprod. Domest. Anim. 49, 378–386 10.1111/rda.12282 [DOI] [PubMed] [Google Scholar]
- 557.Kusminski C.M., McTernan P.G., Schraw T., Kos K., O'Hare J.P., Ahima R.et al. (2007) Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 50, 634–642 10.1007/s00125-006-0577-9 [DOI] [PubMed] [Google Scholar]
- 558.Kubota N., Yano W., Kubota T., Yamauchi T., Itoh S., Kumagai H.et al. (2007) Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 6, 55–68 10.1016/j.cmet.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 559.Caja S., Torrente M., Martínez I., Abelenda M. and Puerta M. (2005) Adiponectin values are unchanged during pregnancy in rats. J. Endocrinol. Invest. 28, 609–615 10.1007/BF03347259 [DOI] [PubMed] [Google Scholar]
- 560.Kos K., Harte A.L., da Silva N.F., Tonchev A., Chaldakov G., James S.et al. (2007) Adiponectin and Resistin in Human Cerebrospinal Fluid and Expression of Adiponectin Receptors in the Human Hypothalamus. J. Clin. Endocrinol. Metab. 92, 1129–1136 10.1210/jc.2006-1841 [DOI] [PubMed] [Google Scholar]
- 561.Neumeier M., Weigert J., Buettner R., Wanninger J., Schäffler A., Müller A.M.et al. (2007) Detection of adiponectin in cerebrospinal fluid in humans. Am. J. Physiol.-Endocrinol. Metab. 293, E965–E969 10.1152/ajpendo.00119.2007 [DOI] [PubMed] [Google Scholar]
- 562.Qi Y., Takahashi N., Hileman S.M., Patel H.R., Berg A.H., Pajvani U.B.et al. (2004) Adiponectin acts in the brain to decrease body weight. Nat. Med. 10, 524–529 10.1038/nm1029 [DOI] [PubMed] [Google Scholar]
- 563.Cheng X.-B., Wen J.-P., Yang J., Yang Y., Ning G., Li X.-Y.et al. (2011) GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine 39, 6–12 10.1007/s12020-010-9375-8 [DOI] [PubMed] [Google Scholar]
- 564.Wen J.-P., Liu C., Bi W.-K., Hu Y.-T., Chen Q., Huang H.et al. (2012) Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1-7 neurons. J. Endocrinol. 214, 177–189 10.1530/JOE-12-0054 [DOI] [PubMed] [Google Scholar]
- 565.Klenke U., Taylor-Burds C. and Wray S. (2014) Metabolic influences on reproduction: adiponectin attenuates GnRH neuronal activity in female mice. Endocrinology 155, 1851–1863 10.1210/en.2013-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Psilopanagioti A., Papadaki H., Kranioti E.F., Alexandrides T.K. and Varakis J.N. (2009) Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology 89, 38–47 10.1159/000151396 [DOI] [PubMed] [Google Scholar]
- 567.Lu M., Tang Q., Olefsky J.M., Mellon P.L. and Webster N.J.G. (2008) Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in lβt2 gonadotropes. Mol. Endocrinol. 22, 760–771 10.1210/me.2007-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Kiezun M., Smolinska N., Maleszka A., Dobrzyn K., Szeszko K. and Kaminski T. (2014) Adiponectin expression in the porcine pituitary during the estrous cycle and its effect on LH and FSH secretion. Am. J. Physiol.-Endocrinol. Metab. 307, E1038–E1046 10.1152/ajpendo.00299.2014 [DOI] [PubMed] [Google Scholar]
- 569.Sarmento-Cabral A., Peinado J.R., Halliday L.C., Malagon M.M., Castaño J.P., Kineman R.D.et al. (2017) Adipokines. (Leptin, Adiponectin, Resistin) Differentially Regulate All Hormonal Cell Types in Primary Anterior Pituitary Cell Cultures from Two Primate Species. Sci. Rep. 7, 43537 10.1038/srep43537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Maillard V., Uzbekova S., Guignot F., Perreau C., Ramé C., Coyral-Castel S.et al. (2010) Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod. Biol. Endocrinol. 8, 23 10.1186/1477-7827-8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Rak A., Mellouk N., Froment P. and Dupont J. (2017) Adiponectin and resistin: potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction 153, R215–R226 10.1530/REP-17-0002 [DOI] [PubMed] [Google Scholar]
- 572.Richards J.S., Liu Z., Kawai T., Tabata K., Watanabe H., Suresh D.et al. (2012) Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil. Steril. 98, 471.e1–479.e1 10.1016/j.fertnstert.2012.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Gutman G., Barak V., Maslovitz S., Amit A., Lessing J.B. and Geva E. (2009) Recombinant luteinizing hormone induces increased production of ovarian follicular adiponectin in vivo: implications for enhanced insulin sensitivity. Fertil. Steril. 91, 1837–1841 10.1016/j.fertnstert.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 574.Lagaly D.V., Aad P.Y., Grado-Ahuir J.A., Hulsey L.B. and Spicer L.J. (2008) Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol. Cell. Endocrinol. 284, 38–45 10.1016/j.mce.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 575.Wickham E.P., Tao T., Nestler J.E. and McGee E.A. (2013) Activation of the LH receptor up regulates the type 2 adiponectin receptor in human granulosa cells. J. Assist. Reprod. Genet. 30, 963–968 10.1007/s10815-013-0012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Chabrolle C., Tosca L., Ramé C., Lecomte P., Royère D. and Dupont J. (2009) Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil. Steril. 92, 1988–1996 10.1016/j.fertnstert.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 577.Comim F.V., Gutierrez K., Bridi A., Bochi G., Chemeris R., Rigo M.L.et al. (2016) Effects of adiponectin including reduction of androstenedione secretion and ovarian oxidative stress parameters in vivo. PLoS ONE 11, e0154453 10.1371/journal.pone.0154453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Maleszka A., Smolinska N., Nitkiewicz A., Kiezun M., Chojnowska K., Dobrzyn K.et al. (2014) Adiponectin Expression in the Porcine Ovary during the Oestrous Cycle and Its Effect on Ovarian Steroidogenesis. Int. J. Endocrinol. 2014, e957076 10.1155/2014/957076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Pierre P., Froment P., Nègre D., Ramé C., Barateau V., Chabrolle C.et al. (2009) Role of adiponectin receptors, AdipoR1 and AdipoR2, in the steroidogenesis of the human granulosa tumor cell line, KGN. Hum. Reprod. 24, 2890–2901 10.1093/humrep/dep292 [DOI] [PubMed] [Google Scholar]
- 580.Chappaz E., Albornoz M.S., Campos D., Che L., Palin M.-F., Murphy B.D.et al. (2008) Adiponectin enhances in vitro development of swine embryos. Domest. Anim. Endocrinol. 35, 198–207 10.1016/j.domaniend.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 581.Gomes E.T., Costa J.A.S., Silva D.M.F., Al Shebli W., Azevedo M.L., Monteiro P.L.J.et al. (2018) Effects of adiponectin during in vitro maturation of goat oocytes: MEK 1/2 pathway and gene expression pattern. Reprod. Domest. Anim Zuchthyg 53, 1323–1329 10.1111/rda.13251 [DOI] [PubMed] [Google Scholar]
- 582.Ledoux S., Campos D.B., Lopes F.L., Dobias-Goff M., Palin M.-F. and Murphy B.D. (2006) Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 147, 5178–5186 10.1210/en.2006-0679 [DOI] [PubMed] [Google Scholar]
- 583.Oliveira B.S.P., Costa J.A.S., Gomes E.T., Silva D.M.F., Torres S.M., Monteiro P.L.J. Jret al. (2017) Expression of adiponectin and its receptors. (AdipoR1 and AdipoR2) in goat ovary and its effect on oocyte nuclear maturation in vitro. Theriogenology 104, 127–133 10.1016/j.theriogenology.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 584.Merhi Z., Bazzi A.A., Bonney E.A. and Buyuk E. (2019) Role of adiponectin in ovarian follicular development and ovarian reserve. Biomed. Rep. 10, 337–342 10.3892/br.2019.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Durmanova A.K., Otarbayev N.K., Kaiyrlykyzy A., Zhangazieva K.K., Ibrayeva Z.N., Donenbayeva G.B.et al. (2016) Ovarian reserve and adipokine levels in reproductive-aged obese women. Ter. Arkh. 88, 46–50 [DOI] [PubMed] [Google Scholar]
- 586.Sepilian V. and Nagamani M. (2005) Adiponectin Levels in Women With Polycystic Ovary Syndrome and Severe Insulin Resistance. J. Soc. Gynecol. Investig. 12, 129–134 10.1016/j.jsgi.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 587.Toulis K.A., Goulis D.G., Farmakiotis D., Georgopoulos N.A., Katsikis I., Tarlatzis B.C.et al. (2009) Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum. Reprod. Update 15, 297–307 10.1093/humupd/dmp006 [DOI] [PubMed] [Google Scholar]
- 588.Comim F.V., Hardy K. and Franks S. (2013) Adiponectin and its receptors in the ovary: further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PloS ONE 8, e80416 10.1371/journal.pone.0080416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Qin L., Sitticharoon C., Petyim S., Keadkraichaiwat I., Sririwhitchai R., Maikeaw P.et al. (2021) The Effects of Adiponectin on Infertile Women Undergoing IVF/ICSI Treatment and on Human Granulosa Cells. J. Endocr. Soc. 5, A772 10.1210/jendso/bvab048.1570 [DOI] [Google Scholar]
- 590.Bersinger N.A., Birkhäuser M.H. and Wunder D.M. (2006) Adiponectin as a marker of success in intracytoplasmic sperm injection/embryo transfer cycles. Gynecol. Endocrinol. 22, 479–483 10.1080/09537100600931316 [DOI] [PubMed] [Google Scholar]
- 591.Liu Y.-H., Tsai E.-M., Wu L.-C., Chen S.-Y., Chang Y.-H., Jong S.-B.et al. (2005) Higher Basal Adiponectin Levels Are Associated with Better Ovarian Response to Gonadotropin Stimulation during in vitro Fertilization. Gynecol. Obstet. Invest. 60, 167–170 10.1159/000086633 [DOI] [PubMed] [Google Scholar]
- 592.Galea L.A. and Parekh R.S. (2023) Ending the neglect of women's health in research. BMJ 381, p1303 10.1136/bmj.p1303 [DOI] [PubMed] [Google Scholar]
- 593.Mercuri N.D. and Cox B.J. (2022) The need for more research into reproductive health and disease. eLife 11, e75061 10.7554/eLife.75061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Mirin A.A. (2021) Gender Disparity in the Funding of Diseases by the U.S. National Institutes of Health. J. Womens Health 30, 956–963 10.1089/jwh.2020.8682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Rice L.W., Cedars M.I., Sadovsky Y., Siddiqui N.Y., Teal S.B., Wright J.D.et al. (2020) Increasing NIH funding for academic departments of obstetrics and gynecology: a call to action. Am. J. Obstet. Gynecol. 223, 79.e1–79.e8 10.1016/j.ajog.2020.03.022 [DOI] [PubMed] [Google Scholar]
- 596.Huebschmann A.G., Huxley R.R., Kohrt W.M., Zeitler P., Regensteiner J.G. and Reusch J.E.B. (2019) Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 62, 1761–1772 10.1007/s00125-019-4939-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Kautzky-Willer A., Harreiter J. and Pacini G. (2016) Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 37, 278–316 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Mauvais-Jarvis F., Bairey Merz N., Barnes P.J., Brinton R.D., Carrero J.-J., DeMeo D.L.et al. (2020) Sex and gender: modifiers of health, disease, and medicine. Lancet North Am. Ed. 396, 565–582 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Beery A.K. and Zucker I. (2011) Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Cherian C., Reeves H., Silva D.D., Tsao S., Marshall K.E. and Rideout E.J. (2023) Consideration of sex as a biological variable in diabetes research across twenty years. bioRxiv 2023.06.13.544882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Day S., Wu W., Mason R. and Rochon P.A. (2019) Measuring the data gap: inclusion of sex and gender reporting in diabetes research. Res. Integr. Peer Rev. 4, 9 10.1186/s41073-019-0068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Flórez-Vargas O., Brass A., Karystianis G., Bramhall M., Stevens R., Cruickshank S.et al. (2016) Bias in the reporting of sex and age in biomedical research on mouse models. eLife 5, e13615 10.7554/eLife.13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Herskind A.E.J. and Nørgaard B. (2023) Gender representation in drug development studies for diabetes mellitus. A systematic review. Dia. Metab. Syndr. Clin. Res. Rev. 17, 102815 10.1016/j.dsx.2023.102815 [DOI] [PubMed] [Google Scholar]
- 604.Khan M.S., Shahid I., Siddiqi T.J., Khan S.U., Warraich H.J., Greene S.J.et al. (2020) Ten‐Year Trends in Enrollment of Women and Minorities in Pivotal Trials Supporting Recent US Food and Drug Administration Approval of Novel Cardiometabolic Drugs. J. Am. Heart Assoc. 9, e015594 10.1161/JAHA.119.015594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Cheng Y., Zhu H., Ren J., Wu H.-Y., Yu J.-E., Jin L.et al. (2023) Follicle-stimulating hormone orchestrates glucose-stimulated insulin secretion of pancreatic islets. Nat. Commun. 14, 6991 10.1038/s41467-023-42801-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Mauvais-Jarvis F., Clegg D.J. and Hevener A.L. (2013) The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 34, 309–338 10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Amiri M., Ramezani Tehrani F., Nahidi F., Kabir A., Azizi F. and Carmina E. (2017) Effects of oral contraceptives on metabolic profile in women with polycystic ovary syndrome: A meta-analysis comparing products containing cyproterone acetate with third generation progestins. Metabolism 73, 22–35 10.1016/j.metabol.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 608.Nader S. and Diamanti-Kandarakis E. (2007) Polycystic ovary syndrome, oral contraceptives and metabolic issues: new perspectives and a unifying hypothesis. Hum. Reprod. 22, 317–322 10.1093/humrep/del407 [DOI] [PubMed] [Google Scholar]
- 609.Wang Q., Würtz P., Auro K., Morin-Papunen L., Kangas A.J., Soininen P.et al. (2016) Effects of hormonal contraception on systemic metabolism: cross-sectional and longitudinal evidence. Int. J. Epidemiol. 45, 1445–1457 10.1093/ije/dyw147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Preumont V. (2022) Contraception and diabetes: Which modalities should we consider in 2021? Ann. Endocrinol. 10.1016/j.ando.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 611.Rocha A.L.L., Campos R.R., Miranda M.M.S., Raspante L.B.P., Carneiro M.M., Vieira C.S.et al. (2017) Safety of hormonal contraception for obese women. Expert Opin. Drug Saf. 16, 1387–1393 10.1080/14740338.2018.1389893 [DOI] [PubMed] [Google Scholar]
- 612.Taylor C.M., Pritschet L. and Jacobs E.G. (2021) The scientific body of knowledge - Whose body does it serve? A spotlight on oral contraceptives and women's health factors in neuroimaging Front. Neuroendocrinol. 60, 100874 10.1016/j.yfrne.2020.100874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Castell A.-L., Goubault C., Ethier M., Fergusson G., Tremblay C., Baltz M.et al. (2022) β Cell mass expansion during puberty involves serotonin signaling and determines glucose homeostasis in adulthood. JCI Insight 7, e160854 10.1172/jci.insight.160854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Jeffery S.C., Hosking J., Jeffery A.N., Murphy M.J., Voss L.D., Wilkin T.J.et al. (2018) Insulin resistance is higher in prepubertal girls but switches to become higher in boys at age 16: A Cohort Study. (EarlyBird 57). Pediatr. Diabetes 19, 223–230 10.1111/pedi.12571 [DOI] [PubMed] [Google Scholar]
- 615.Kelly L.A., Lane C.J., Weigensberg M.J., Toledo-Corral C.M. and Goran M.I. (2011) Pubertal Changes of Insulin Sensitivity, Acute Insulin Response, and β-Cell Function in Overweight Latino Youth. J. Pediatr. 158, 442–446 10.1016/j.jpeds.2010.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Moran A., Jacobs D.R. Jr, Steinberger J., Hong C.P., Prineas R., Luepker R.et al. (1999) Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 48, 2039–2044 10.2337/diabetes.48.10.2039 [DOI] [PubMed] [Google Scholar]
- 617.Baeyens L., Hindi S., Sorenson R.L. and German M.S. (2016) β-Cell Adaptation in Pregnancy. Diabetes Obes. Metab. 18, 63–70 10.1111/dom.12716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Butler A.E., Cao-Minh L., Galasso R., Rizza R.A., Corradin A., Cobelli C.et al. (2010) Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 53, 2167–2176 10.1007/s00125-010-1809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Rieck S. and Kaestner K.H. (2010) Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab. 21, 151–158 10.1016/j.tem.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Van Assche F.A., Aerts L. and Prins F.D. (1978) A morphological study of the endocrine pancreas in human pregnancy. BJOG Int. J. Obstet. Gynaecol. 85, 818–820 10.1111/j.1471-0528.1978.tb15835.x [DOI] [PubMed] [Google Scholar]
- 621.Gurka M.J., Vishnu A., Santen R.J. and DeBoer M.D. (2016) Progression of metabolic syndrome severity during the menopausal transition. J. Am. Heart Assoc. 5, e003609 10.1161/JAHA.116.003609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Janssen I., Powell L.H., Crawford S., Lasley B. and Sutton-Tyrrell K. (2008) Menopause and the metabolic syndrome: the study of women's health across the nation. Arch. Intern. Med. 168, 1568–1575 10.1001/archinte.168.14.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]