Abstract
Introduction
To ensure the sterility of cell products that cannot undergo conventional sterilization processes, it is imperative to establish and maintain a clean room environment, regulated through environmental monitoring, including particle counts. Nevertheless, the impact of particles generated by operators as potential contaminants remains uncertain. Thus, in this study, we conducted an accelerated test to assess the correlation between particles generated by operators and airborne bacteria, utilizing biosafety cabinets within a typical laboratory setting. These biosafety cabinets create a controlled environment with air conditioning and high-efficiency particulate air (HEPA) filters, offering fundamental data relevant to cell production.
Materials and methods
We conducted a simulation followed by real-time experiments involving human operations to explore the quantity of particles, particle sizes, and the percentage of bacteria within these particles. This investigation focused on conditions with heightened particle generation from operators within a biosafety cabinet. The experiment was conducted on operators wearing textile and non-woven dustless clothing within biosafety cabinets. It entailed tapping the upper arms for a duration of 2 min.
Results
Observations under biosafety cabinet-off conditions revealed the presence of various particles and falling bacteria in textile clothing. In contrast, no particles or falling bacteria were detected in operators wearing dustless clothing within biosafety cabinets. Notably, a correlation between 5 μm particles and colony-forming units in textile clothing was identified through this analysis. The ratio of falling bacteria to the total number of particles within the biosafety cabinet was 0.8 ± 0.5 % for textile clothing, while it was significantly lower at 0.04 ± 0.2 % for dustless clothing.
Conclusion
This study demonstrated that the number of particles and falling bacteria varied depending on the type of clothing and that quantitative data could be used to identify risks and provide basic data for operator education and evidence-based control methods in aseptic manufacturing areas. Although, this study aims to serve as an accelerated test operating under worst-case conditions, the results need to make sure the study range in general research.
Keywords: Particle, Colony-forming unit, Falling bacteria, Biosafety cabinet, Cell-product processing
Highlights
-
•
Processing of cell products poses diverse risks.
-
•
The number of particles and falling bacteria was evaluated in laboratory BSCs.
-
•
The correlation between 5-μm particles and colony forming unit were observed.
-
•
The proportion of falling bacteria was 0.8 ± 0.5 % for textile clothing.
-
•
The proportion of falling bacteria was 0.04 ± 0.2 % for non-woven dustless clothing.
1. Introduction
To ensure sterility, it is important to process cell products that cannot be sterilized by conventional means. Therefore, cell products must be protected from numerous risks, including environmental bacteria present in cell-processing facilities [[1], [2], [3], [4]] and bacteria and fungi in raw materials [5,6]. In addition, there may be residual contaminants in the biosafety cabinet, such as droplets of culture medium [[7], [8], [9]], which may be introduced into the cell products. To protect cell products from such diverse risks, a clean room environment, including a sterile production area, must be established, properly maintained, and controlled. As the use of excessive disinfectants to protect cell products places a physical burden on operators [10,11], it is important to quantify the risks and develop a strategy for maintaining and managing cell-processing facilities.
To maintain and control the clean room environment, environmental monitoring for risk management is conducted, and particle counts are measured daily. The measurement of particle counts has been operationalized as a method for environmental trend analysis [[12], [13], [14]]. Operators are considered to be the most significant sources of these particles. The particles include non-living and microbial particles that can contaminate cell products [14,15]. However, it is unclear to what extent these operator-generated particles are contaminants in biosafety cabinets used in aseptic manufacturing areas, and to what extent there is a risk of contamination of cell products. By quantifying and properly understanding these risks, it is possible to educate operators effectively and implement appropriate operations.
Particles measured in clean rooms exhibit diameters ranging from 0.5 to 20 μm. While these particles display various diameters, bacteria typically have diameters around 1 μm [16]. In clean rooms and open air, airborne bacteria often adhere to particles within the 0.5–10 μm range, which are suspended by airflow [17,18]. The aim of this study was to elucidate trends in particles, particle sizes, and the percentage of bacteria contained within these particles. However, cell processing facilities incorporate high-efficiency particulate air (HEPA) filters and stringent air conditioning controls to prevent particle generation. Analyzing the correlation between operator-generated particles and falling bacteria in such high-clean environments proves significantly challenging in statistical, as even if particles are present, they are typically in extremely small quantities. Hence, in this study, we conducted an accelerated test using biosafety cabinets in a standard laboratory environment. These biosafety cabinets, featuring HEPA filters and air conditioning, offer a controlled setting expected to yield fundamental data relevant to cell production. It is important to note that the number of culturable bacteria in particles is reported to be considerably small [14]. Therefore, simulations and experiments in this study assumed conditions involving excessive particle generation from operators, which deviates from the norm in regular manufacturing processes.
2. Materials and methods
2.1. Simulations of particle generation
For simulations of particle generation, FlowDesigner (Advanced Knowledge Laboratory Inc., Tokyo, Japan) was used to simulate 5 and 20 μm particles in a biosafety cabinet for 15 s. The simulation conditions of the biosafety cabinet were defined by inflow and outflow air velocities that met the JIS K-3800 standard. The detailed conditions of the biosafety cabinet, such as the aperture and airflow, are listed in Table 1.
Table 1.
Conditions for particle generation simulation.
| Design | Locations | Flow speed (m/s) | Size (cm) | Calculated flowrate (m3/min) | Total (m3/min) |
|---|---|---|---|---|---|
| Airflow | Roof | 0.5 | 160 (W) × 80 (D) | 38.40 | 38.40 |
| Suction | Rear | 1.5 | 160 (W) × 6 (D) | 8.64 | 37.44 |
| Suction | Front | 3 | 160 (W) × 10 (D) | 28.8 | |
| Aperture | Front | 160 (W) × 11(D) | 0.96 |
2.2. Investigation for particle count and falling bacteria
In the biosafety cabinet (SCV-1301ECⅡA, HITACHI, Tokyo, Japan) located in a standard laboratory without grade control, each upper arm underwent alternating taps for 2 min. This was done wearing particle-intensive textile clothing (a button-down shirt without gloves) and particle-less, non-woven dustless clothing (IsoClean; DuPont de Nemours, Inc., DE, USA) and surgical gloves (Toray Medical, Tokyo, Japan). The biosafety cabinet's integrity was validated using an anemometer (VELOCICALC Model 9515, TSI Inc., MN, USA) to ensure that inflow and outflow air velocities adhered to the JIS K-3800 standard. The tapping procedure was repeated five times for three operators wearing textile clothing and three times for operators in dustless clothing. Prior to each experiment, the biosafety cabinet was operated for at least 1 min to ensure that the particle counter was reset to zero, as indicated in Fig. S1. Data regarding the number of particles and falling bacteria, with the biosafety cabinet (BSC) off and in an unoperated state, are presented in Fig. S1A and S1B. Each experiment was performed with the BSC turned on and off. Four particle counters were installed in the BSC. On the left side, an instrument (ZN-PD03-S; OMRON Corporation, Kyoto, Japan) measuring 0.5 and 1 μm particles was installed, and on the right side, an instrument (ZN-PD50-S: OMRON) measuring 5 and 20 μm particles was installed. Soybean-casein digest (SCD) medium (Nissui-Seiyaku, Tokyo, Japan) was placed on each of the three plates adjacent to the 5 μm particle counter (Fig. 1A). The supplemental information displays temporal data, expressed in values per cubic meter. These values are derived from count data by using suction volume per minute values of 3.5 L for 0.5 μm particles and 7.0 L for 5 μm particles.
Fig. 1.
Simulation of particle generation in biosafety cabinets. (A) Simulation model of biosafety cabinet. (B) Trends of generated particles in simulation. (C) Time-lapse images of simulations for 5, 10, and 15 s of particle generation. Particle behavior when the biosafety cabinet is turned on (top) and turned off (bottom). Left: 5 μm and right: 20 μm.
2.3. Method to calculate the ratio of the presence of bacteria in particles
Fallen bacteria were quantified by the number of colony-forming units (CFU) in the SCD medium. The obtained results are shown in a heat map as average values according to the number of tests performed under each condition. The total number of CFUs in the three agar medias adjacent to each particle counter located in the front and back, divided by the total number of particles during the 2 min tapping of clothes, was calculated as the bacterial presence ratio in the particles.
2.4. Statistical analysis
Statistical analyses of the collected data were performed using GraphPad Prism version 9 (GraphPad Software, CA, USA). Spearman's correlation analysis was performed between the particle size and total particle and CFU counts.
3. Results
3.1. Simulations of particle generation
In the simulations of particle generation, many particles behaved under biosafety cabinet-on or -off conditions (Fig. 1A and B). Under biosafety cabinet-off conditions, both particle types continued to remain in the workspace (Supplemental Videos 1 and 2). One difference was that the 5 μm particles were still floating after 15 s, whereas the 20 μm particles began to settle after 10 s. In the BSC, when both types of particles were generated, they were immediately discharged through the exhaust located at the front and rear ends, respectively. All the particles were completely exhausted within 15 s after ceasing particle generation (Fig. 1C; Supplemental Videos 3 and 4).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.reth.2024.01.001
The following are the supplementary data related to this article:
2
3
4
5
3.2. Distribution of falling bacteria caused by excessive particle generation
The number of falling bacteria was quantitatively evaluated under textile clothing and dustless clothing conditions and under excessive particle generation conditions wherein the clothing was tapped for 2 min (Fig. 2A). Numerous falling bacteria were observed on the textile clothing in the biosafety cabinet-off condition for operators a, b, and c. The highest number of bacteria was observed directly under the tapping area at the front position (Fig. 2B). In the textile clothing and BSC, falling bacteria were detected only at the front of operator a (Fig. 2B). In the dustless clothing and biosafety cabinet-off conditions, falling bacteria were detected only at the front of operator a (Fig. 2B). In the dustless clothing and BSCs, no falling bacteria were detected by any of the operators (Fig. 2B).
Fig. 2.
Distribution of falling bacteria caused by excessive particle generation. (A) Experimental conditions for excessive particle generation. Position of agar mediums and particle counters (Upper). Tapping clothes for 2 min for textile and dustless clothing (Lower). (B) Heatmap of colony-forming units (CFU) of fallen bacteria in operators a, b, and c on BSC On or Off.
3.3. Differences in particle generation by operators and clothing
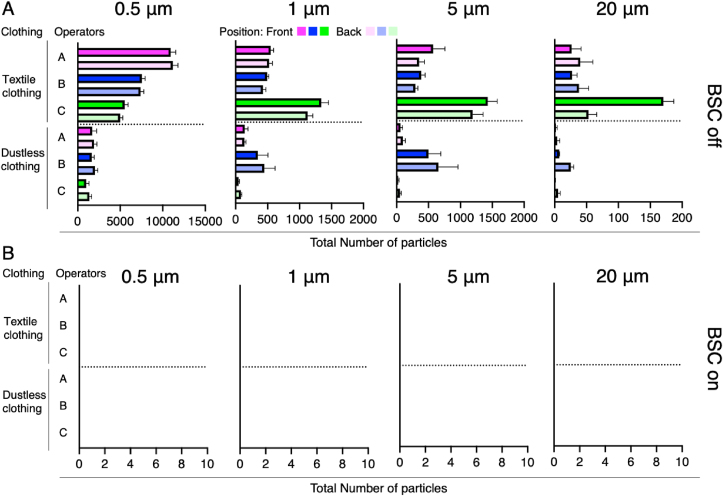
The total number of particles generated by the tapping of clothes of operators a, b, and c is shown in Fig. 3. Particles were detected under biosafety cabinet-off conditions (Fig. 3A). The number of 0.5 μm particles in textile clothing were 11,061 ± 580, 7432 ± 369, and 5265 ± 436 for operator a, b, and c, respectively. The 0.5 μm particle counts in dustless clothing were 1816 ± 402, 1840 ± 345, and 1186 ± 337 for operators a, b, and c, respectively. The 1 μm particle counts for textile clothing were 537 ± 47, 460 ± 49, and 1233 ± 147 for operators a, b, and c, respectively. The 1 μm particle counts for dustless clothing were 1339 ± 36, 398 ± 156, and 64 ± 23 for operators a, b, and c, respectively. The 5 μm particle counts for textile clothing were 462 ± 178, 339 ± 69, and 1310 ± 193 for operators a, b, c, respectively. The 5 μm particle counts for dustless clothing were 79 ± 38, 577 ± 245, and 37 ± 23 for operators a, b, and c, respectively. The 20 μm particle counts for textile clothing were 33 ± 18, 33 ± 13, and 112 ± 63 for operators a, b, and c, respectively. The 20 μm particle counts for dustless clothing were 3 ± 3, 16 ± 10, and 3 ± 3 for operator a, b, and c, respectively. Figure S2 illustrates the generation of 0.5 μm and 5 μm particles by each operator during a 2 min tapping session in the absence of BSC activation. By contrast, no particles were detected when the BSC was operational (Fig. 3B). Figure S3 presents the generation of 0.5 μm and 5 μm particles by each operator during a 2 min tapping session with the BSC in an activated state.
Fig. 3.
Differences in particle generation by operators and clothing. (A) Total number of particles at each particle size during 2 min of particle generation under BSC-Off conditions. (B) Total number of particles at each particle size during 2 min of particle generation under BSC-On conditions.
3.4. Correlations between particle size and general or dustless clothing under BSC-Off conditions
The correlation between each particle type under biosafety cabinet-off conditions was then analyzed (Fig. 4). Correlations were found between 1 μm/20 μm (r = 0.625, p < 0.05) and 5 μm/20 μm (r = 0.664, p < 0.01) particles in textile clothing, along with a high correlation between 1 μm/5 μm (r = 0.859, p < 0.001) particles (Fig. 4A). In dustless clothing, correlations were observed between 0.5 μm/1 μm (r = 0.612, p < 0.01) and 1 μm/20 μm (r = 0.674, p < 0.01) particles, with higher correlations between 1 μm/5 μm (r = 0.930, p < 0.001) and 5 μm/20 μm (r = 0.815, p < 0.001) particles (Fig. 4B).
Fig. 4.
Correlations between particle size during general or dustless clothing and BSC-Off conditions. (A) Correlations between particle size under BSC-Off with textile clothing conditions. (B) Correlations between particle size under BSC-Off with dustless clothing conditions.
3.5. Correlations between particle size and colony-forming unit for general or dustless clothing under BSC-Off
The correlation between each particle and the total number of CFUs on the SCD agar medium under biosafety cabinet-off conditions was analyzed (Fig. 5). The results showed a correlation between 5 μm particles and CFU for textile clothing (r = 0.614, p < 0.001) (Fig. 5A). No valid results were obtained for dustless clothing because the number of falling bacteria was exceedingly low (Fig. 5B). In the calculation of the percentage of bacteria present in the total particle count, the correlated results for 5 μm particles in textile clothing were valid: 1.0 ± 0.5 % for operator a, 0.8 ± 0.5 % for operator b, and 0.6 ± 0.3 % for operator c. The mean for all operators was 0.8 ± 0.5 % for textile clothing and 0.04 ± 0.2 % for dustless clothing (Fig. 5C).
Fig. 5.
Correlations between particle size and colony-forming unit (CFU) during general or dustless clothing and BSC-Off conditions. (A) Correlations between particle size and CFU with textile clothing under BSC-Off conditions. (B) Correlations between particle size and CFU with dustless clothing under BSC-Off conditions. (C) Number of CFU per particles at each particle size.
4. Discussion
4.1. Discussion
The processing of cell products, which cannot undergo sterilization, necessitates the careful maintenance of cleanliness in the manufacturing environment to ensure crucial sterility during the process. While many manufacturing operations are currently performed manually [10], humans are one of the major sources of generating particles, which causes contamination. However, the specific risk these human-generated particles pose to cell products remains unclear. Therefore, in this study, we analyzed the correlation between particles and falling bacteria in BSCs, which has not yet been quantitatively evaluated. In this investigation, we analyzed data derived from excessive particles originating from clothing in general cell cultures conducted under typical laboratory conditions. This analysis serves as fundamental data with practical utility for cell production processes.
First, the behavior of the particles in a BSC was analyzed using an in silico simulation. Although the particles typically measured in clean rooms are small, such as 0.5 and 5 μm particles, the terminal settling velocity of these small particles is known to be less than 1 mm/s. In other words, the gravitational and drag forces due to the air are balanced and suspended. In an environment without wind, these particles remain in the air for a long time and become sources of contamination. Meanwhile, the terminal settling velocity becomes 1 mm/s or more when the particles are 10 μm or 20 μm in size. As particles of these sizes are light, they are exhausted by the airflow in clean rooms. In this analysis, we confirmed the previously known behavior of the particles in the simulation. However, in actual manufacturing sites, multiple factors, such as the turbulence and static electricity associated with the nature of the particles generated, cannot be assumed. Therefore, it is necessary to verify what happens during actual human operations.
Experimental conditions for actual human operations are necessary for verification. Because the number of culturable airborne bacteria contained in particles is speculated to be very small [14], in the current study, we conducted experiments under the condition of excessive particle generation, which does not occur in normal manufacturing processes. The experiment was conducted under the condition that the three operators tap their clothes for 2 min. The experimental results were generally stable among the operators, despite the difference in the force applied by each operator. In addition, falling bacteria were observed even on agar plates placed just below the fall point under conditions of no downward flow, suggesting that falling bacteria were suspended by the airflow generated by arm tapping. In concordance with the simulation, a greater number of 5-μm particles were registered in the particle counter situated at the front of the biosafety cabinet—directly below the tapping zone—compared to the counter located at the back of the cabinet. Notably, the detection of these particles ceased when the BSC was operational, implying that the clean environment may be compromised during periods of abnormal airflow or when airflow is impeded by an abundance of culture materials. Therefore, routine airflow assessments coupled with adherence to proper culture protocols by operators stand as a straightforward and dependable approach for ensuring the secure production of cell products.
Textile clothing with excessive particle generation under biosafety cabinet-off conditions exhibited numerous particles, along with a significant presence of falling bacteria. This observation may be attributed not only to the clothing quality but also to the release of indigenous skin bacteria from the cuffs [19]. Interestingly, in the case of operator A, who experienced a substantial occurrence of falling bacteria in textile clothing, these bacteria were detected even under biosafety cabinet-on conditions, though no particles could be measured. This suggests that particle measurement may not consistently be effective in detecting falling bacteria. Conversely, even in the dustless clothing under the generation of excess particles on the biosafety cabinet-off conditions, equal or more particles were observed in operator b than in the textile clothing, but no falling bacteria were detected in operator b with dustless clothing.
These data are consistent with previous reports that suggested that sterilized clothing releases fewer microbiological contaminants than non-sterilized clothing [20]. Most importantly, when the BSC was on, no particles or falling bacteria were observed in the dustless clothing. This suggests that a clean environment can be maintained during the manufacturing of cell products if proper facility maintenance and operation are managed, followed by proper clothing and operational practices.
The measurement of particles is not used to infer a relationship with microorganisms but rather to evaluate whether cleanliness in the environment is maintained, such as whether the HEPA filters are functioning. The HEPA filters are defined in ISO 29463-1 or JIS Z-8122 as filters with a particle collection efficiency of 99.97 % or higher for 0.3 μm particles [21]. Therefore, the significance of measuring 0.5 μm particles is that it is a real-time means of confirming that there are no leaks in the HEPA and that cleanliness is maintained. Furthermore, the data are supported by the fact that 0.5 μm particle size is smaller than the typical bacterial diameter and is the smallest reliable particle size that can be measured [16,22]. In our analysis, 0.5 μm particles in textile clothing showed no correlation with other particle diameters, while in dustless clothing, 0.5 μm particles showed a correlation with 1 μm particles. This suggests that the tendency of particles to be emitted differs depending on the clothing material. In addition, ISO 14644-1 and JIS B-9920 specify correlative reference values for 0.5 and 5 μm particles for each class [22]; however, no correlation was observed in this study. In other words, it was suggested that each measurement of 0.5 and 5 μm particle size has a certain significance. By contrast, unlike industrial clean rooms where semiconductors are manufactured, biological clean rooms where cell products are manufactured are made of biological materials, and the raw materials themselves may be contaminated with microorganisms such as bacteria [5,6,23]. Therefore, managing clean rooms using the same standards can be challenging. Furthermore, 5 μm particles are immediately captured under biosafety cabinets-on conditions; at the same time, antibiotics are often used in cell culture media [24,25]. Thus, whether 5 μm control is necessary would still need to be debated. Scientific validation of the relationship between antibiotics, contamination risk, and the risk of microbial contamination of cell products is essential to advance this debate.
The number of CFUs found on the agar medium had the highest correlation with 5 μm particles. These data are important, as they directly demonstrate the significance of 5-μm particle measurements in environmental monitoring. However, among the 5 μm particles, extremely small amounts of bacteria were present, approximately 0.8 % in textile clothing and 0.04 % in dustless clothing. In other words, particles are trapped by the airflow during normal BSC operation, and the possibility of bacteria affecting cell products in the culture is considered extremely low. The cell products fabricated in properly controlled facilities are often contaminated by raw human tissue, contamination during harvesting from humans, and contamination from aseptic testing environment [[4], [5], [6],[26], [27], [28]]. The data from our study suggest that the risk of environmental contamination is low. However, this does not imply that the 5-μm measurement is not significant. For example, autologous and allogeneic cell products have different risks of contamination. Autologous cell products are at a 1:1 risk, whereas allogeneic cell products can be at a 1:100–1000 risk. This implies that unique control methods should be considered through risk assessment based on the characteristics of each individual product.
In the current situation, where various costs, such as electricity bills, are increasing, it is necessary to consider appropriate management methods based on the characteristics of cell products for facility-related costs, which dominate 30%–50 % of the cost structure of cell products [29,30]. To adopt new management practices, it is necessary to dispel concerns by adopting a scientific approach and developing new technologies from the current stage.
In our current study, we identified a limitation in the method employed for calculating the percentage of bacteria, which did not involve simultaneous particle measurements. Certain systems, for instance, irradiate airborne particulates with a laser and utilize scattered light to identify microorganisms [14]. However, it is not certain whether such systems pose a risk to actual cell products because some bacteria cannot be cultured in an agar medium. Additionally, there might be bacteria that do not thrive on the SCD agar medium utilized in this study but flourish in a liquid medium containing fetal bovine serum or various growth factors used in cell product production. Consequently, future verification of results with non-cultured microorganisms using alternative analytical methods is imperative. Another limitation is ensuring that the results obtained in this study range are applicable more broadly. While this research aims to present novel, previously unreported data, the experiments deliberately took place in a general laboratory environment to quantify a worst-case scenario for cell product contamination. The objective is to extrapolate from these maximum risk factors to assess risk levels within a clean room setting. For example, our study estimated the risk of environmental bacterial contamination from dust-free clothing during biosafety cabinet (BSC) operation to be 0.04 ± 0.2 %. If extrapolated to a clean room setting, this risk logically decreases further. However, it is crucial not to entirely dismiss the potential for contamination in actual manufacturing facilities. Thus, this study serves as an accelerated test under worst-case conditions. The quantified risks identified in this study are expected to provide foundational data for risk assessments across various facilities.
5. Conclusion
Our study revealed that the number of particles and falling bacteria under conditions of excessive particle generation varied greatly depending on the type of clothing. Furthermore, we were able to quantitatively show the ratio of falling bacteria to the number of particles in the BSC. Such quantitative data can be used to correctly identify risks and provide basic information for the education of operators manufacturing cell products, which can facilitate the development of evidence-based control methods for aseptic manufacturing.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ichiro Sekiya reports financial support was provided by Shimizu Corporation.
Funding
This research was funded by a joint research grant from the SHIMIZU CORPORATION. One of the authors (MM) of this paper is employed by the Japan Agency for Medical Research and Development (AMED) under grant number JP22bk0304003, which was provided to IS.
Authors' contributions
MM, KA, and TK: Data acquisition, analysis, and interpretation. MM: Drafting the manuscript. HH and NK: Proposals and debates questioning the validity and interpretation of the data. MM, KA, TK, HH, NK and IS: Manuscript revision for important intellectual content. All the authors have read and approved the final manuscript.
Acknowledgements
We thank Hisako Katano and Sayaka Komura for managing laboratory work.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2024.01.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mizuno M., Endo K., Katano H., Tsuji A., Kojima N., Watanabe K., et al. The environmental risk assessment of cell-processing facilities for cell therapy in a Japanese academic institution. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martín P.G., González M.B., Martínez A.R., Lara V.G., Naveros B.C. Isolation and characterization of the environmental bacterial and fungi contamination in a pharmaceutical unit of mesenchymal stem cell for clinical use. Biologicals. 2012;40:330–337. doi: 10.1016/j.biologicals.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Raval J.S., Koch E., Donnenberg A.D. Real-time monitoring of non-viable airborne particles correlates with airborne colonies and represents an acceptable surrogate for daily assessment of cell-processing cleanroom performance. Cytotherapy. 2012;14:1144–1150. doi: 10.3109/14653249.2012.698728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negre H., Pinte L., Manduke R., Cunningham A., Anderson H., Richard S., et al. Personnel environmental monitoring during manufacture of manipulated cell therapy products. Cytotherapy. 2018;20(5):S 68. doi: 10.1016/j.jcyt.2018.02.192. [DOI] [Google Scholar]
- 5.Mizutani M., Samejima H., Terunuma H., Kino-Oka M. Experience of contamination during autologous cell manufacturing in cell processing facility under the Japanese Medical Practitioners Act and the Medical Care Act. Regen Ther. 2016;5:25–30. doi: 10.1016/j.reth.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi R., Kobayashi S., Yamato M., Owaki T., Kasai Y., Hosoi T., et al. How to prevent contamination with Candida albicans during the fabrication of transplantable oral mucosal epithelial cell sheets. Regen Ther. 2015;1:1–4. doi: 10.1016/j.reth.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa Y., Mizutani M., Okamoto R., Kitajima H., Ezoe S., Kino-Oka M. Understanding the formation and behaviors of droplets toward consideration of changeover during cell manufacturing. Regen Ther. 2019;12:36–42. doi: 10.1016/j.reth.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno M., Matsuda J., Watanabe K., Shimizu N., Sekiya I. Effect of disinfectants and manual wiping for processing the cell product changeover in a biosafety cabinet. Regen Ther. 2023;22:169–175. doi: 10.1016/j.reth.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuno M., Yori K., Takeuchi T., Yamaguchi T., Watanabe K., Tomaru Y., et al. Cross-contamination risk and decontamination during changeover after cell-product processing. Regen Ther. 2023;22:30–38. doi: 10.1016/j.reth.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno M., Sugahara Y., Iwayama D., Miyashita N., Katano H., Sekiya I. Stress and motivation of cell processing operators: a pilot study of an online questionnaire survey. Regen Ther. 2022;21:547–552. doi: 10.1016/j.reth.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno M., Abe K., Kakimoto T., Yano K., Ota Y., Tomita K., et al. Volatile organic compounds and ionic substances contamination in cell processing facilities during rest period; a preliminary assessment of exposure to cell processing operators. Regen Ther. 2023;24:211–218. doi: 10.1016/j.reth.2023.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandle T., Leavy C., Jindal H., Rhodes R. Application of rapid microbiological methods for the risk assessment of controlled biopharmaceutical environments. J Appl Microbiol. 2014;116:1495–1505. doi: 10.1111/jam.12487. [DOI] [PubMed] [Google Scholar]
- 13.Tham K.W., Zuraimi M.S. Size relationship between airborne viable bacteria and particles in a controlled indoor environment study. Indoor Air. 2005;15(9):48–57. doi: 10.1111/j.1600-0668.2005.00303.x. supplement 9. [DOI] [PubMed] [Google Scholar]
- 14.Kawai M., Ichijo T., Takahashi Y., Noguchi M., Katayama H., Cho O., et al. Culture independent approach reveals domination of human-oriented microbes in a pharmaceutical manufacturing facility. Eur J Pharmaceut Sci. 2019;137 doi: 10.1016/j.ejps.2019.104973. [DOI] [PubMed] [Google Scholar]
- 15.Rappé M.S., Giovannoni S.J. The uncultured microbial majority. Annu Rev Microbiol. 2003;57:369–394. doi: 10.1146/annurev.micro.57.030502.090759. [DOI] [PubMed] [Google Scholar]
- 16.Choi J., Kang M., Jung J.H. Integrated micro-optofluidic platform for real-time detection of airborne microorganisms. Sci Rep. 2015;5 doi: 10.1038/srep15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orsini D.A., Rhoads K., McElhoney K., Schick E., Koehler D., Hogrefe O. A water cyclone to preserve insoluble aerosols in liquid flow—an interface to flow cytometry to detect airborne nucleic acid. Aerosol Sci Technol. 2008;42:343–356. doi: 10.1080/02786820802072881. [DOI] [Google Scholar]
- 18.Brągoszewska E., Pastuszka J.S. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland) Aerobiologia. 2018;34:241–255. doi: 10.1007/s10453-018-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markel T.A., Gormley T., Greeley D., Ostojic J., Wagner J. Wearing long sleeves while prepping a patient in the operating room decreases airborne contaminants. Am J Infect Control. 2018;46:369–374. doi: 10.1016/j.ajic.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Romano F., Milani S., Joppolo C.M. Airborne particle and microbiological human emission rate investigation for cleanroom clothing combinations. Build Environ. 2020;180 doi: 10.1016/j.buildenv.2020.106967. [DOI] [Google Scholar]
- 21.Ma D., Li S., IEC/ISO . In: Handbook of indoor air quality. Zhang Y., Hopke P.K., Mandin C., editors. Nature Publishing; Singapore, Singapore: 2022. Standards of air cleaners; pp. 1547–1564. springer. [Google Scholar]
- 22.Xu Z. Springer; Berlin Heidelberg, Berlin, Heidelberg: 2014. Classification of air cleanliness, fundamentals of air cleaning technology and its application in cleanrooms; pp. 339–376. [Google Scholar]
- 23.Watanabe K., Otabe K., Shimizu N., Komori K., Mizuno M., Katano H., et al. High-sensitivity virus and mycoplasma screening test reveals high prevalence of parvovirus B19 infection in human synovial tissues and bone marrow. Stem Cell Res Ther. 2018;9:80. doi: 10.1186/s13287-018-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno M., Endo K., Katano H., Amano N., Nomura M., Hasegawa Y., et al. Transplantation of human autologous synovial mesenchymal stem cells with trisomy 7 into the knee joint and 5 years of follow-up. Stem Cells Transl Med. 2021;10:1530–1543. doi: 10.1002/sctm.20-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekiya I., Koga H., Otabe K., Nakagawa Y., Katano H., Ozeki N., et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: a case report. Cell Transplant. 2019;28:1445–1454. doi: 10.1177/0963689719863793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panch S.R., Bikkani T., Vargas V., Procter J., Atkins J.W., Guptill V., et al. Prospective evaluation of a practical guideline for managing positive sterility test results in cell therapy products. Biol Blood Marrow Transplant. 2019;25:172–178. doi: 10.1016/j.bbmt.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirji Z., Saragosa R., Dedier H., Crump M., Franke N., Burrows L., et al. Contamination of bone marrow products with an actinomycete resembling Microbacterium species and reinfusion into autologous stem cell and bone marrow transplant recipients. Clin Infect Dis. 2003;36:e115–e121. doi: 10.1086/374051. [DOI] [PubMed] [Google Scholar]
- 28.Schwella N., Rick O., Heuft H.G., Miksits K., Zimmermann R., Zingsem J., et al. Bacterial contamination of autologous bone marrow: reinfusion of culture-positive grafts does not result in clinical sequelae during the posttransplantation course. Vox Sang. 1998;74:88–94. doi: 10.1046/j.1423-0410.1998.7420088.x. [DOI] [PubMed] [Google Scholar]
- 29.Ten Ham R.M.T., Hövels A.M., Hoekman J., Frederix G.W.J., Leufkens H.G.M., Klungel O.H., et al. What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings. Cytotherapy. 2020;22:388–397. doi: 10.1016/j.jcyt.2020.03.432. [DOI] [PubMed] [Google Scholar]
- 30.Ten Ham R.M.T., Nievaart J.C., Hoekman J., Cooper R.S., Frederix G.W.J., Leufkens H.G.M., et al. Estimation of manufacturing development costs of cell-based therapies: a feasibility study. Cytotherapy. 2021;23:730–739. doi: 10.1016/j.jcyt.2020.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2
3
4
5