Abstract
Hypoxia is the key pathobiological trigger of tubular oxidative stress and cell death that drives the transition of acute kidney injury (AKI) to chronic kidney disease (CKD). The mitochondrial-rich proximal tubular epithelial cells (PTEC) are uniquely sensitive to hypoxia and thus, are pivotal in propagating the sustained tubular loss of AKI-to-CKD transition. Here, we examined the role of PTEC-derived small extracellular vesicles (sEV) in propagating the ‘wave of tubular death’.
Ex vivo patient-derived PTEC were cultured under normoxia (21 % O2) and hypoxia (1 % O2) on Transwell inserts for isolation and analysis of sEV secreted from apical versus basolateral PTEC surfaces. Increased numbers of sEV were secreted from the apical surface of hypoxic PTEC compared with normoxic PTEC. No differences in basolateral sEV numbers were observed between culture conditions. Biological pathway analysis of hypoxic-apical sEV cargo identified distinct miRNAs linked with cellular injury pathways. In functional assays, hypoxic-apical sEV selectively induced ferroptotic cell death (↓glutathione peroxidase-4, ↑lipid peroxidation) in autologous PTEC compared with normoxic-apical sEV. The addition of ferroptosis inhibitors, ferrostatin-1 and baicalein, attenuated PTEC ferroptosis. RNAse A pretreatment of hypoxic-apical sEV also abrogated PTEC ferroptosis, demonstrating a role for sEV RNA in ferroptotic ‘wave of death’ signalling. In line with these in vitro findings, in situ immunolabelling of diagnostic kidney biopsies from AKI patients with clinical progression to CKD (AKI-to-CKD transition) showed evidence of ferroptosis propagation (increased numbers of ACSL4+ PTEC), while urine-derived sEV (usEV) from these ‘AKI-to-CKD transition’ patients triggered PTEC ferroptosis (↑lipid peroxidation) in functional studies.
Our data establish PTEC-derived apical sEV and their intravesicular RNA as mediators of tubular lipid peroxidation and ferroptosis in hypoxic kidney injury. This concept of how tubular pathology is propagated from the initiating insult into a ‘wave of death’ provides novel therapeutic check-points for targeting AKI-to-CKD transition.
Keywords: Hypoxia, Proximal tubular epithelial cells, Small extracellular vesicles, Lipid peroxidation, Ferroptosis, AKI-to-CKD transition
Graphical abstract
Highlights
-
•
Elevated extracellular vesicle signalling by hypoxic kidney tubular cells.
-
•
Extracellular vesicles possess a unique ‘pro-injurious’ miRNA signature.
-
•
Extracellular vesicles trigger tubular lipid peroxidation and ferroptotic death.
-
•
Extracellular vesicle signalling drives loss of kidney tissue in AKI-to-CKD transition.
1. Introduction
Acute kidney injury (AKI) is a devastating public health issue, with an annual global incidence in excess of 13 million people [1]. AKI is a risk factor for chronic kidney disease (CKD) development and progression, with AKI survivors at an 8.8-fold increased risk of CKD compared to hospital patients with no AKI [2]. Hypoxia of the tubulointerstitial compartment is a hallmark of AKI-to-CKD transition [3,4]. Peritubular capillary rarefaction and reduced blood flow following severe AKI generate localized tubulointerstitial hypoxic microenvironments that trigger tubular epithelial cell (TEC) oxidative stress and death pathways, culminating in tubulointerstitial fibrosis and progression to CKD [5]. Elucidating the complex molecular signalling mechanisms propagating this hypoxic tubular cell depletion is essential for developing precision therapeutics that target the transition of AKI to CKD.
Proximal tubular epithelial cells (PTEC) play a central role in driving AKI-to-CKD transition [6,7]. The high energy demands of PTEC are primarily supplied by mitochondrial fatty acid oxidation, an oxygen intensive process [8]. Thus, PTEC are particularly vulnerable to hypoxic injury after severe AKI [9]. Human PTEC in hypoxic conditions undergo oxidative stress and ferroptotic cell death [10], an iron-dependent form of necrosis triggered by: [i] ferroptosis activator and gold-standard biomarker acyl-CoA synthetase long-chain family member 4 (ACSL4) [11]; [ii] the reduced expression of lipid repair enzyme glutathione peroxidase 4 (GPX4); and [iii] the accumulation of toxic lipid peroxides (end-product 4-hydroxynonenal; 4-HNE) [12]. The Linkermann group reported that hypoxic AKI injury is transmitted along mouse proximal tubules via a mechanism of synchronized ferroptosis, triggering ongoing tubular cell death in adjacent cells [13]. However, the identity of the PTEC-derived death signals propagating this ferroptotic ‘wave of tubular death’ remain to be defined.
Small extracellular vesicles (sEV) are fundamental components of intercellular signalling [14]. They are defined based on their size distribution (50–150 nm diameter) and expression of canonical sEV proteins - tetraspanins (i.e., CD9), components of the ESCRT (endosomal sorting complex required for transport) system (i.e., tumour susceptibility gene 101; TSG101) and heat shock proteins (i.e., HSP70, HSP84) [14,15]. sEV signal between cells by transfer of their incorporated molecular cargo (i.e., microRNA; miRNA) from parental to recipient cells [16]. We previously showed that human hypoxic PTEC secrete increased numbers of sEV (termed exosomes in our earlier publication) with a distinct molecular (miRNA, protein) cell death signalling signature [17]. However, PTEC are polarized epithelial cells and the extracellular environment at their apical membranes differs from that at their basolateral membranes (i.e., tubular lumen/urinary space versus tubulointerstitium). Studies have characterized the polarity-dependent response of PTEC to injurious stimuli (i.e., apical versus basal membrane expression of surface molecules and cytokine production) [18,19]. We have also demonstrated that the release of sEV by human PTEC under inflammatory diseased conditions is polarized [20].
The aim of this study was to examine the polarity-dependent differences in numbers and cargo of sEV released by ex vivo patient-derived PTEC under hypoxic conditions of AKI-to-CKD transition. We demonstrate that: [i] sEV production from the apical PTEC membrane, but not basolateral membrane, is significantly increased in hypoxic conditions; and [ii] hypoxic-apical sEV have a unique ‘pro-injurious’ miRNA signature and trigger lipid peroxidation and a ferroptotic ‘wave of tubular death’ in autologous PTEC. Moreover, we provide first in situ evidence of this concept in AKI-to-CKD transition, with urine-derived sEV (usEV) from AKI patients with clinical transition to CKD inducing PTEC lipid peroxidation in functional studies.
2. Materials and methods
2.1. Isolation and culture of human primary PTEC
Kidney cortical tissue was obtained with informed patient consent from the macroscopically/microscopically healthy portion of tumour nephrectomies performed at the Royal Brisbane and Women’s Hospital (RBWH) (Table S1), following approval by the RBWH Human Research Ethics Committee (2002/011). Tissue specimens were classified as ‘healthy’ based on histopathological assessment of the non-tumour kidney parenchyma by a renal histopathologist blinded to the experimental procedure. Human primary PTEC were purified from kidney cortical tissue following the method of Glynne and Evans [21] and cultured in Defined Medium (DM) as previously described [22]. All PTEC were used in experiments at passage 4.
2.2. Hypoxic treatment of human primary PTEC on permeable membranes
Apical versus basolateral sEV production by PTEC was examined using Transwell® plates (Corning, Cambridge, MA, USA). Human primary PTEC were seeded onto 6-well transparent polyester Transwell inserts (0.4 μm pore size, 24 mm diameter, 4.67 cm2 surface area) at a concentration of 1.2 × 105 cells/cm2 in DM (2.5 ml volume in the upper compartment). DM alone (3.1 ml) was added to the lower compartment. PTEC were grown to confluence as confirmed in permeability studies (detailed protocol provided in Supplementary Methods).
Once monolayer integrity was confirmed, the DM in the upper and lower compartments were exchanged with: (1) fresh DM followed by further culture for 72 h for normoxic control PTEC; or (2) hypoxia pre-conditioned fresh DM followed by 72 h culture in an InvivO2 1000 Hypoxia Workstation (Ruskinn, Laftec, Bayswater North, Victoria, Australia) for hypoxic PTEC (1% O2). PTEC culture medium was subsequently harvested from the upper (apical) compartment (2.5 ml for each Transwell) and lower (basolateral) compartment (3.1 ml for each Transwell). Individual collections of apical and basolateral media for each culture condition from each PTEC donor were pooled for downstream sEV isolation.
2.3. Immunofluorescent studies of PTEC monolayer permeability and polarity
Cellular distribution and maintenance of PTEC polarity were examined by immunofluorescent (IF) staining (detailed protocol provided in Supplementary Methods).
2.4. sEV isolation and quantitation
sEV protocols are reported as per Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018) guidelines [15]. PTEC culture medium was centrifuged at 300×g for 10 min at 4 °C and filtered through a 0.22 μm filter (Merck Millipore, Bayswater, Victoria, Australia) to remove contaminating apoptotic bodies and cell debris. The clarified medium was transferred to an Amicon® Ultra-15 100,000 Da device (Merck Millipore) and concentrated to a 500 μl volume by centrifugation at 3,500×g at 4 °C. The concentrated supernatant was loaded onto a qEV size exclusion column (Izon Science Ltd, Christchurch, New Zealand), with sEV isolation performed as per the manufacturer’s instructions. Briefly, the concentrated supernatant was overlaid on the qEV exclusion column followed by elution with PBS (0.22 μm filtered). The flow-through was eluted in 500 μl fractions, with sEV fractions 5–11 collected and pooled. sEV were then ultracentrifuged at 100,000×g in a Hitachi CS150NX fixed rotor centrifuge for 1.5 h at 4 °C to concentrate samples. The resulting pellets were resuspended in 100 μl PBS (0.22 μm filtered). The total number and size distribution of sEV was analyzed with tuneable resistive pulse sensing (TRPS) (qNano, Izon Science Ltd) following the method of Wang et al. [17] Briefly, total particles/Transwell were calculated as: total number of sEV/volume of the initial pooled PTEC culture medium x Transwell chamber volume (TCV); where TCV is 2.5 ml for apical (upper chamber) samples and 3.1 ml for basolateral (lower chamber) samples. Total particles/Transwell were then normalized to total particles from equivalent 1 cm2 area of confluent Transwell monolayer (total particles/cm2), based on a surface area of 6-well Transwell inserts of 4.67 cm2.
2.5. Electron microscopy
Purified sEV were applied on a Formvar-coated and carbon stabilized copper grid and stained with 2 % aqueous uranyl acetate. Samples were examined using a JEM 1011 transmission electron microscope operated at 80 kV and equipped with a digital camera.
2.6. Proteomic analysis
Purified sEV were subjected to single phase solvent extraction using 100 % v/v methanol containing 50 μg/ml butylated hydroxytoluene (BHT). Briefly, ten volumes of cold methanol (+50 μg/ml BHT) were added to each sample, followed by incubation at -20°C for 24 h. Samples were then centrifuged at 16,000×g for 15 min at 4 °C, with protein recovered in the pellets. sEV proteins were reduced, alkylated and digested for tandem liquid chromatography-mass spectrometry (LC-MS/MS) and proteomic analysis (detailed protocol provided in Supplementary Methods).
2.7. miRNA analysis
Total RNA isolation, miRNA-seq library construction, sequencing and analysis were performed at Beijing Genomics Institute (BGI) (Shenzhen, China) (detailed protocol provided in Supplementary Methods).
2.8. Pathway analysis
Annotations for significantly dysregulated miRNA were obtained using the Ingenuity Pathway Analysis (IPA) program (Qiagen, Melbourne, Victoria, Australia) following the core analysis workflow using standard parameters: stringent filter for molecules and relationship and no protein fold change cut-off applied. Readouts included the “Disease and Biological Function”, “Tox” clustering and “Networks Function” pathway analyses.
2.9. sEV co-culture with human primary PTEC
Human primary PTEC were seeded (100,000 cells/well in DM) in 24-well flat-bottom plates to allow overnight adherence, then cultured in fresh DM for 72 h in the presence of normoxic- or hypoxic-apical sEV derived from autologous PTEC (cell:sEV ratio of 1:10,000; 1 ml final volume).
PTEC were harvested by trypsin treatment and assessed for viability using the Annexin-V Detection kit I (BD Biosciences, San Jose, CA, USA). Briefly, harvested PTEC were incubated with Annexin-V FITC and propidium iodide (PI) in binding buffer for 15 min at room temperature. The percentage of apoptotic cells (Annexin-V+ PI−) and necrotic cells (Annexin-V+ PI+) was determined by flow cytometry performed on a LSR Fortessa (BD Biosciences), with data analyzed using FlowJo software (TreeStar, Ashland, OR, USA). Harvested PTEC were also examined for protein expression by Western blotting (detailed protocol provided in Supplementary Methods).
2.10. Immunofluorescent (IF) staining of co-cultured PTEC
Human primary PTEC were seeded (10,000 cells/well in DM) into CellCarrier-96 Ultra Microplates (Thermo Fisher Scientific) to allow overnight adherence, then cultured in fresh DM for 72 h in the presence of normoxic- or hypoxic-apical sEV derived from autologous PTEC (cell:sEV ratio of 1:10,000; 100 μl final volume). Following the co-culture period, the cells were analyzed for DNA damage marker γ-H2AX and lipid peroxidation end-product 4-HNE by IF staining (detailed protocol provided in Supplementary Methods).
2.11. Image-iT™ lipid peroxidation assay of PTEC co-cultured with sEV
Human primary PTEC were seeded (20,000 cells/well in DM) in 96-well black/clear bottom plates to allow overnight adherence, then cultured in fresh DM for 48 h in the presence of normoxic- or hypoxic-apical sEV derived from autologous PTEC (cell:sEV ratio of 1:10,000; 200 μl final volume). For inhibitor studies, 10 μM ferrostatin-1 (Sigma-Aldrich, St Louis, MO, USA), 1 μM baicalein (Sigma-Aldrich) or 0.03 % dimethyl sulfoxide (DMSO) vehicle control were added to PTEC-sEV co-cultures for the 48 h treatment period. In selected experiments, hypoxic-apical sEV were pre-treated with 20 μg/ml RNase A (Thermo Fisher Scientific, Waltham, MA, USA) in the absence or presence of 0.1 mg/ml saponin (Sigma-Aldrich) for 20 min at 37 °C. The reaction was stopped by adding 1 IU RNaseOUT (Thermo Fisher Scientific) per 5 ng RNase A used. Removal of residual RNase A/RNase OUT reagents was performed using an Exosome Spin Column (MW3000; Life Technologies, Grand Island, NY, USA) according to the manufacturer’s instructions.
Following the PTEC-sEV co-culture period, the cells were incubated with 10 μM of BODIPY 581/591 C11 reagent (Image-iT™ lipid peroxidation kit, Thermo Fisher Scientific) for 30 min at 37 °C. Wells were washed three times with PBS, before 200 μL Live Cell Imaging Solution (Thermo Fisher Scientific) was applied to each well. The wells were visualized on a Zeiss 780 NLO confocal microscope (Carl Zeiss, Hamburg, Germany). Images of both FITC and Texas Red® channels were captured from each well, with median channel intensity from three randomly selected areas for each well quantified using QuPath (v0.3.2, University of Edinburgh, United Kingdom) [23]. The ratio of oxidized 510 nm (green)/reduced 590 nm (red) emission fluorescence intensities representing lipid peroxidation was determined for each well.
2.12. AKI clinical samples
Clinical specimens (urine and/or kidney tissue) were obtained from AKI patients at the time of diagnostic kidney biopsy (n = 10; 6 females/4 males; mean age 50 ± 19; mean estimated glomerular filtration rate (eGFR) at specimen collection 23 ± 15 ml/min/1.73 m2) (Table S2). AKI urine was collected 1–2 h prior to the kidney biopsy, while biopsy tissue (excess to diagnostic requirements) was immediately frozen in Tissue-Tek OCT compound (Sakura, Torrance, CA, USA) for IF analysis. Control kidney tissue was obtained from the histologically healthy portion (nil pathology; no interstitial fibrosis/tubular atrophy) of tumour nephrectomies (n = 4; 3 females/1 male; mean age 53 ± 14; mean eGFR at specimen collection 84 ± 7 ml/min/1.73 m2). Control urine was obtained from age/sex-matched healthy donors (n = 4; 2 females/2 males; mean age 48 ± 16). Informed written consent was obtained from all participants, with study approval from the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (2002/011 and 2006/072).
AKI was confirmed as per: [i] Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines [24]; and [ii] a histopathological diagnosis of acute tubular injury (ATI) (i.e., tubular luminal dilatation, epithelial flattening, brush border loss) [25]. AKI progression to CKD was defined as eGFR <60 ml/min/1.73 m2 for ≥3 months post-AKI episode. AKI patients were stratified into two cohorts based on this criterion: AKI recovery or non-progression to CKD (AKI-CKD; n = 4; 2 females/2 males; mean age 37 ± 17; mean eGFR at specimen collection 22 ± 19 ml/min/1.73 m2) or AKI progression/transition to CKD (AKI + CKD; n = 6; 4 females/2 males; mean age 59 ± 16; mean eGFR at specimen collection 23 ± 14 ml/min/1.73 m2).
2.13. IF staining of kidney tissue
Frozen 7 μm tissue sections were fixed with 75 % acetone: 25 % ethanol for 5 min at room temperature, followed by a protein block with 10 % Donkey Serum for 30 min at room temperature. Sections were subsequently probed with primary antibodies against ferroptosis biomarker ACSL4 (1:400; Rabbit monoclonal IgG; Clone EPR8640; Cat. No. ab155282; Abcam, Cambridge, MA, USA) and PTEC marker aquaporin-1 (AQP-1; 1:400; Mouse monoclonal IgG; Clone B-11; Cat. No. sc-25287; Santa Cruz, Dallas, TX, USA) overnight at 4 °C in a humidified chamber. Fluorescent detection was obtained by secondary incubation with Alexa Fluor™ Plus 555 anti-rabbit IgG and Alexa Fluor™ Plus 647 anti-mouse IgG (1:500 dilutions; all from Thermo Fisher) for 30 min at room temperature. Nuclei were stained with DAPI (1:10,000; Sigma-Aldrich). Slides were coverslipped in fluorescence mounting medium (Agilent Technologies, Santa Clara, CA, USA) and visualized using a Zeiss 780 NLO confocal microscope (Carl Zeiss).
Quantitative image analysis of kidney tissue was performed using QuPath (v0.4.3) [23]. Briefly, ACSL4+ and AQP-1+ cells were enumerated in 2–3 randomly selected areas for each tissue section. Each area was assessed using the Watershed Cell Detection algorithm [23,26], with staining intensity threshold-based cell classification identifying ACSL4+ cells or AQP-1+ cells for quantitative analysis.
2.14. Urine-derived sEV (usEV) isolation, quantitation and functional characterisation
Fresh urine samples (50–100 ml) were collected and immediately centrifuged at 650×g for 10 min at room temperature to remove contaminating cells. 50 mL aliquots of urinary supernatant were then mixed with one tablet of protease inhibitor cocktail dissolved in 2 ml H2O (Roche Diagnostics, Mannheim, Germany) and stored at -80°C until required for usEV isolation.
For isolation of usEV, frozen urine samples were thawed, centrifuged at 2000×g for 20 min at 4 °C to remove cellular debris, followed by a filtering step using a 0.22 μm filter (Merck Millipore) and concentrated to a 500 μl volume using an Amicon® Ultra-15 100,000 Da device (Merck Millipore). The concentrated urinary supernatant was subsequently loaded onto a qEV size exclusion column (Izon Science Ltd) for usEV isolation and downstream analysis (qNano, electron microscopy) in the same manner as PTEC culture medium-derived sEV. All usEV counts were normalized to urine creatinine (μmol/L). In functional studies, human primary PTEC were co-cultured with usEV (cell:usEV ratio of 1:10,000; 200 μl final volume) and assessed for lipid peroxidation as described for PTEC culture medium-derived sEV (Image-iT™ lipid peroxidation kit, Thermo Fisher Scientific).
2.15. Statistics
Sample sizes were selected based on previous publications from our laboratory with similar experimental design [20]. Statistical tests for sEV enumeration, functional co-culture assays and IF microscopy were performed using Prism 7.0 analysis software (GraphPad Software, La Jolla, CA, USA). Comparisons between paired groups were performed using a Wilcoxon matched pairs signed rank test. Statistical comparisons between unpaired groups were performed using a Mann-Whitney test. P values ≤ 0.05 were considered statistically significant.
3. Results
3.1. Human primary PTEC maintain monolayer impermeability and polarity under hypoxic conditions
Human primary PTEC were cultured to confluency on 0.4 μm Transwell inserts to form an impermeable cell monolayer, thus enabling the characterisation of discrete sEV produced from the apical plasma membrane into the upper compartment and basolateral plasma membrane into the lower compartment (Fig. 1a). The impermeability of confluent monolayers at this time-point (time = 0 h) was confirmed by assessing the diffusion of FITC-Dextran from the upper (apical) to the lower (basolateral) chamber. The confluent PTEC monolayer was shown to effectively prevent diffusion from the apical to the basolateral compartment, with a percent permeability of 0.55 ± 0.14 % measured at time = 0 h (Table 1).
Fig. 1.
Human primary PTEC cultured on Transwell inserts maintain impermeability and polarity under hypoxic conditions. (a) Human primary PTEC were seeded onto Transwell inserts (0.4 μm pore size) and grown to confluence. Human PTEC monolayers were subsequently cultured under normoxic control (21 % O2) or hypoxic (1 % O2) conditions for 72 h. PTEC culture medium was then harvested from the upper (apical) compartment and lower (basolateral) compartment for downstream sEV isolation. (b) IF microscopy of a hypoxic PTEC monolayer on Transwell insert (lateral projection) stained for β-tubulin (green), multidrug resistance-associated protein (MRP)-4 (red) and DAPI (blue). Scale bar represents 2 μm. One representative of three individual donor PTEC experiments. Equivalent staining profile was observed for normoxic PTEC monolayers. (c) IF staining of PTEC monolayers on Transwell inserts for ZO1 (green), E-Cadherin (red) and DAPI (blue) at time = 0 h and following 72 h culture under normoxic and hypoxic conditions. Scale bars represents 20 μm. One representative of three individual donor PTEC experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Apical-to-basolateral diffusion (expressed as percent permeability; % permeability) for Transwells with confluent PTEC monolayers at time = 0 h and following culture under normoxic (control) or hypoxic conditions (time = 72 h). Results represent mean ± SEM of eight individual PTEC donor experiments.
| Condition | % Permeability (n = 8) |
|---|---|
| Time = 0 h | |
| Confluent monolayer | 0.55 ± 0.14 |
| Time = 72 h | |
| Normoxia | 0.75 ± 0.05 |
| Hypoxia | 0.70 ± 0.13 |
Human PTEC monolayers were subsequently cultured under normoxic control (21 % O2) or hypoxic (1 % O2) conditions for 72 h. The PTEC monolayer remained impermeable under both culture conditions, with the percent permeability measured at 0.75 ± 0.05 % under normoxia and 0.70 ± 0.13 % under hypoxia (Table 1). The formation of a restrictive PTEC monolayer was further confirmed by the maintained expression of tight junction protein Zonula occludens-1 (ZO1) and intercellular junction marker E-cadherin at cell-cell contact points in PTEC monolayers at time = 0 h and following 72-h culture under normoxic and hypoxic conditions (Fig. 1c). IF staining of confluent monolayers cultured under normoxia and hypoxia also showed expression of PTEC apical protein MRP-4 (red) to be retained on the upper surface and facing the top compartment, indicating a maintenance of cellular polarity (Fig. 1b). These data establish the Transwell model as suitable for interrogating the polarized secretion of sEV by hypoxic human primary PTEC.
3.2. Significantly elevated apical sEV production by human primary PTEC under hypoxic conditions
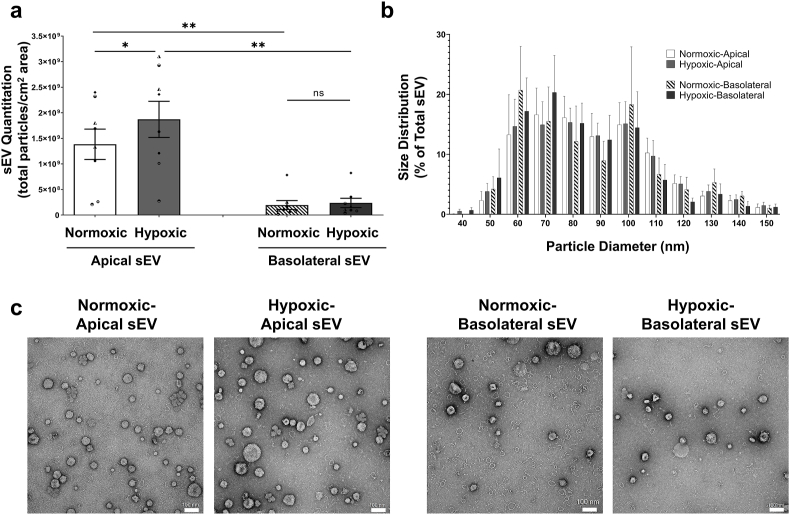
PTEC culture media were collected from the apical and basolateral compartments for both normoxic and hypoxic conditions. The individual collections of apical and basolateral media for each culture condition from each PTEC donor were pooled for sEV isolation and quantitiative analysis. Numbers of sEV produced from the apical membrane (normalized to total particles from equivalent 1 cm2 area of confluent Transwell monolayer) were significantly increased compared with the basolateral membrane for both normoxia and hypoxia (Fig. 2a). Of note, numbers of apical sEV produced under hypoxia were significantly elevated compared with apical sEV from normoxic conditions (Fig. 2a). No differences in basolateral sEV numbers were observed between culture conditions. There were no significant differences in sEV size distributions, with all populations exhibiting equivalent sEV size profiles of 50–150 nm (Fig. 2b). Electron microscopy analysis of purified sEV further confirmed the equivalent size profiles of all sEV populations (Fig. 2c).
Fig. 2.
Significantly elevated apical sEV production by human primary PTEC under hypoxic conditions. (a) Apical and basolateral sEV numbers (normalized to total particles from equivalent 1 cm2 area of confluent Transwell monolayer) produced by human primary PTEC under normoxic and hypoxic culture conditions. Results represent mean ± SEM; symbols represent individual donor PTEC; n = 8. *P < 0.05, **P < 0.01, Wilcoxon matched-pairs signed-rank test. (b) Equivalent size distribution of apical and basolateral sEV derived from primary human PTEC under normoxia and hypoxia; analyzed with tunable resistive pulse sensing (TRPS) using a NP100 nanopore at a 45 mm stretch. Size distribution data represent the proportion of total sEV particles for each condition, with the mean ± SEM of eight individual PTEC donor experiments presented. (c) Electron microscopy images of apical and basolateral sEV purified from primary human PTEC under normoxia and hypoxia. Scale bars represent 100 nm. One representative of four PTEC donor experiments.
3.3. Molecular profile of apical sEV produced by human primary PTEC under hypoxic conditions is associated with injury/disease pathways
Mass spectrometry proteomic analysis of purified sEV from three individual donor PTEC experiments identified a total of 614 proteins, of which 377 proteins were subjected to normalization/differential analysis after filtering out low quality data points (see Supplementary Methods). Of these 377 proteins, established sEV markers, CD9, TSG101 and HSP84, were identified in similar abundance across all samples (Fig. S1a), confirming the sEV identity of specimens as per MISEV2018 guidelines [15]. Proteomics data was supported by Western blot analysis, with all sEV populations expressing CD9 (Fig. S1b). In contrast, the endoplasmic reticulum protein Calnexin was detected only in control PTEC lysate and not in sEV samples, further validating the purity and lack of cellular contamination in sEV preparations (Fig. S1b).
Strikingly, quantitative proteomic analysis identified only a single protein, hypoxia-inducible Carbonic Anhydrase 12 (log2FC >6.1449; FDR adjusted P-value <0.0324), to be significantly differentially expressed between hypoxic and normoxic sEV from the apical membrane. There were no significantly differentially expressed proteins between hypoxic and normoxic sEV from the basolateral membrane.
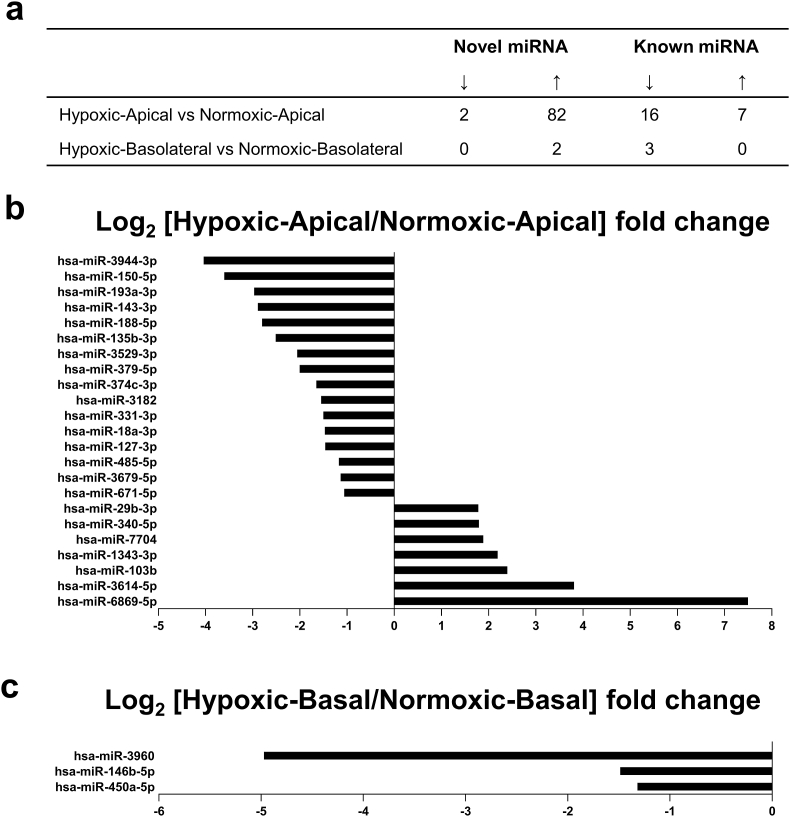
sEV from four individual donor PTEC experiments were next examined for miRNA content – both undefined/novel miRNA predicted using miRDeep2 [27] and defined/known miRNA (i.e., those registered and annotated in the public miRNA database – miRBase: http://www.mirbase.org). High-stringency quantitative analysis identified 84 novel miRNA as significantly differentially expressed (FDR adjusted P-value ≤0.001) between hypoxic and normoxic sEV from the apical membrane (two down-regulated; 82 up-regulated) and two to be significantly differentially expressed between hypoxic and normoxic sEV from the basolateral membrane (both up-regulated) (Fig. 3a and Table S3). Of known miRNA identified, 23 were significantly differentially expressed between hypoxic and normoxic sEV from the apical membrane (16 down-regulated; seven up-regulated) (Fig. 3a and b and Table S4), with three molecules significantly differentially expressed between hypoxic and normoxic sEV from the basolateral membrane (three down-regulated) (Fig. 3c and Table S4).
Fig. 3.
Differential miRNA profile of apical and basolateral sEV produced by human primary PTEC under hypoxic conditions. (a) Numbers of significantly differentially expressed [upregulated (↑) and downregulated (↓)] sEV novel and defined/known miRNA. (b) Differentially expressed miRNA between hypoxic and normoxic sEV produced from the apical membrane. Log2 fold changes for miRNA with FDR adjusted P-value ≤0.001 are shown. (c) Differentially expressed miRNA between hypoxic and normoxic sEV produced from the basolateral membrane. Log2 fold changes for miRNA with FDR adjusted P-value ≤0.001 are shown.
The functional associations of significantly differentially expressed miRNA were interrogated using the “Networks Function” of the Ingenuity Pathway Analysis (IPA) program, with a stringent P-value cut-off of <1e-4. Mapping of the significantly differentially expressed hypoxic-apical sEV miRNA associated 11 of the 23 known miRNA with a top-ranked pathway of ‘Organismal Injury’ (Fig. 4). Additional downstream effects analysis of these hypoxic-apical sEV miRNA within the “Diseases and Biological Functions” feature of IPA identified a ‘fibrosis’ biological function (overlap P-value 1.14e-07). Equivalent mapping of the significantly differentially expressed known miRNA of hypoxic-basolateral sEV failed to identify shared pathways. These findings suggest that PTEC-derived apical sEV play a specialised pathobiological function in the hypoxic tubulointerstitial micro-environment.
Fig. 4.
Eleven out of the 23 significantly differentially expressed defined/known miRNA between hypoxic-apical sEV versus normoxic-apical sEV associate with an ‘organismal injury’ pathway, as identified by the “Networks Function” of IPA. Molecules highlighted in bold within the data table are the eleven significantly differentially hypoxic-apical sEV miRNA.
3.4. Hypoxic-apical sEV induce ferroptotic cell death of autologous human primary PTEC
To define the pathobiological function of hypoxic-apical sEV, we established a model of tubular cell signalling where human primary PTEC were co-cultured with apical sEV derived from autologous PTEC. We showed significantly elevated expression of DNA damage marker γ-H2AX in PTEC co-cultured with hypoxic-apical sEV compared with normoxic-apical sEV and PTEC alone (-sEV) (Fig. 5a). Subsequent cell viability assays showed significantly increased PTEC necrosis (% Annexin-V+ PI+ cells), but not PTEC apoptosis (% Annexin-V+ PI− cells), in response to hypoxic-apical sEV compared with normoxic-apical sEV and PTEC alone (Fig. 5b).
Fig. 5.
Hypoxic-apical sEV induce DNA damage and cellular necrosis in autologous PTEC. (a) Top panel: Fold changes (relative to PTEC culture alone; -sEV) in γ-H2AX levels (measured as % cells containing >10 γ-H2AX foci) for PTEC alone (-sEV) and co-cultured with normoxic-apical sEV or hypoxic-apical sEV. Bar graphs represent mean ± SEM. Symbols represent individual donor PTEC; n = 6. *P < 0.05, Wilcoxon matched-pairs signed-rank test. Bottom panel: IF labelling of a representative PTEC donor stained for γ-H2AX (magenta) and DAPI (blue). Scale bars represent 20 μm. (b) Top panels: Fold changes (relative to PTEC culture alone; -sEV) in cellular apoptosis (measured as % Annexin-V+ PI− cells) and cellular necrosis (measured as % Annexin-V+ PI+ cells) for PTEC alone (-sEV) and co-cultured with normoxic-apical sEV or hypoxic-apical sEV. Bar graphs represent mean ± SEM. Symbols represent individual donor PTEC; n = 6. ns - not significant. *P < 0.05, Wilcoxon matched-pairs signed-rank test. Bottom panels: Annexin-V/PI dot plots from a representative PTEC donor. The percentage of Annexin-V+ PI− apoptotic cells and Annexin-V+ PI+ necrotic cells for each dot plot are presented. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
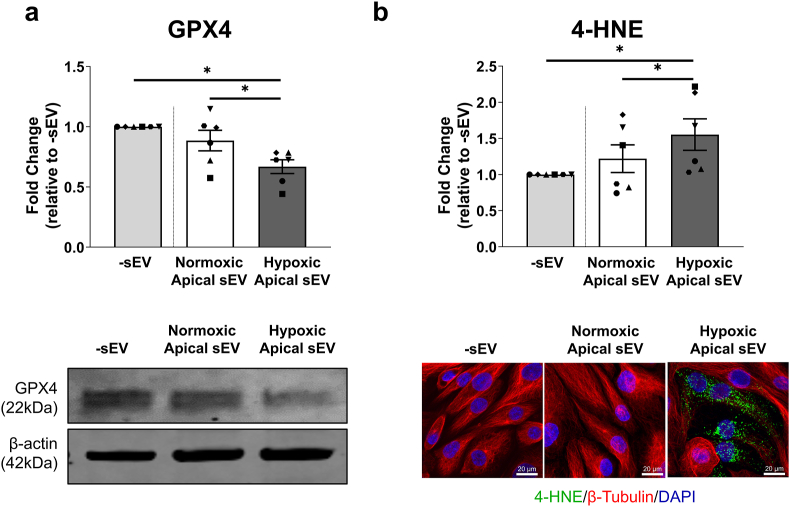
The molecular pathways of discrete cell death modes were next assessed. Apoptosis marker cleaved caspase-3 and necroptosis marker phosphorylated MLKL (pMLKL) were not detectable in PTEC-sEV co-cultures (data not shown). In contrast, lipid repair enzyme GPX4 was significantly decreased (Fig. 6a) and lipid peroxidation end-product 4-HNE (Fig. 6b) was significantly increased in PTEC co-cultured with hypoxic-apical sEV – both hallmarks of ferroptotic cell death [12].
Fig. 6.
Hypoxic-apical sEV induce ferroptotic cell death in autologous PTEC. (a) Top panel: Fold changes (relative to PTEC culture alone; -sEV) in GPX4 protein levels (as a ratio of loading control β-actin) for PTEC alone (-sEV) and co-cultured with normoxic-apical sEV or hypoxic-apical sEV. Bar graphs represent mean ± SEM. Symbols represent individual donor PTEC; n = 6. *P < 0.05, Wilcoxon matched-pairs signed-rank test. Bottom panel: GPX4 Western blot from a representative PTEC donor (10 μg total protein per lane). (b) Top panel: Fold changes (relative to PTEC culture alone; -sEV) in 4-HNE levels (measured as mean corrected total cellular fluorescence (CTCF) of >70 cells per condition) for PTEC alone (-sEV) and co-cultured with normoxic-apical sEV or hypoxic-apical sEV. Bar graphs represent mean ± SEM. Symbols represent individual donor PTEC; n = 6. *P < 0.05, Wilcoxon matched-pairs signed-rank test. Bottom panel: IF labelling of a representative PTEC donor stained for 4-HNE (green), β-tubulin (red) and DAPI (blue). Scale bars represent 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
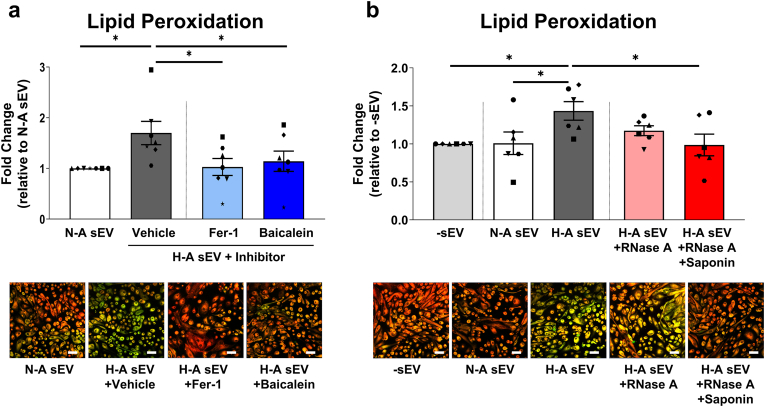
We confirmed this ferroptotic PTEC death using increased lipid peroxidation (BODIPY 581/591 C11 staining) as a readout. Significantly increased lipid peroxidation was detected in PTEC co-cultured with hypoxic-apical sEV compared with normoxic-apical sEV (Fig. 7a). The addition of ferroptosis inhibitors, ferrostatin-1 [28] and baicalein [29], significantly attenuated this elevated lipid peroxidation in PTEC co-cultured with hypoxic-apical sEV (Fig. 7a). To examine the functional contribution of sEV miRNA cargo in driving PTEC ferroptosis, we pre-treated hypoxic-apical sEV with both saponin (for active permeabilization) and RNase A prior to co-culture. Saponin-assisted RNase A pre-treatment significantly attenuated ferroptosis induction by hypoxic-apical sEV (Fig. 7b). In contrast, pre-treatment of hypoxic-apical sEV with RNase A alone failed to mitigate PTEC ferroptosis (Fig. 7b). These data establish the intravesicular RNA cargo of PTEC-derived apical sEV as key mediators of the ferroptotic ‘wave of tubular death’ in hypoxic kidney injury.
Fig. 7.
RNA digestion of hypoxic-apical sEV inhibits PTEC ferroptosis. (a) Top panel: Fold changes (relative to normoxia-apical sEV) in lipid peroxidation (measured as a ratio of oxidized 510 nm (green)/reduced 590 nm (red) fluorescence) for PTEC cultured with normoxic-apical sEV (N-A sEV) or hypoxic-apical sEV (H-A sEV) in the presence of a vehicle control (DMSO), ferrostatin-1 (Fer-1) or baicalein. Bar graphs represent mean ± SEM. Symbols represent individual donor PTEC; n = 7. *P < 0.05, Wilcoxon matched-pairs signed-rank test. Bottom panel: IF labelling of a representative PTEC donor stained with Image-iT™ Lipid Peroxidation Sensor. Lipid peroxidation is highlighted by the shift in fluorescence from red to green. Scale bars represent 50 μm. (b) Top panel: Fold changes (relative to PTEC culture alone; -sEV) in lipid peroxidation for PTEC alone (-sEV) and co-cultured with normoxic-apical sEV or hypoxic-apical sEV. Experiments included pre-treatment of hypoxic-apical sEV with RNase A alone or both saponin and RNase A prior to co-culture. Bar graphs represent mean ± SEM. Symbols represent individual donor PTEC; n = 6. *P < 0.05, Wilcoxon matched-pairs signed-rank test. Bottom panel: IF labelling of a representative PTEC donor stained with Image-iT™ Lipid Peroxidation Sensor. Scale bars represent 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Urine-derived sEV from AKI patients with clinical progression to CKD induce PTEC lipid peroxidation
We extended these findings to examine whether sEV mediate ferroptosis propagation in AKI-to-CKD transition. Urine and kidney tissue were collected from AKI patients at the time of kidney biopsy, with patients stratified into two cohorts based on eGFR ≥3 months post-AKI episode: AKI recovery or non-progression to CKD (AKI-CKD; eGFR ≥60 ml/min/1.73 m2) or AKI progression/transition to CKD (AKI + CKD; eGFR <60 ml/min/1.73 m2).
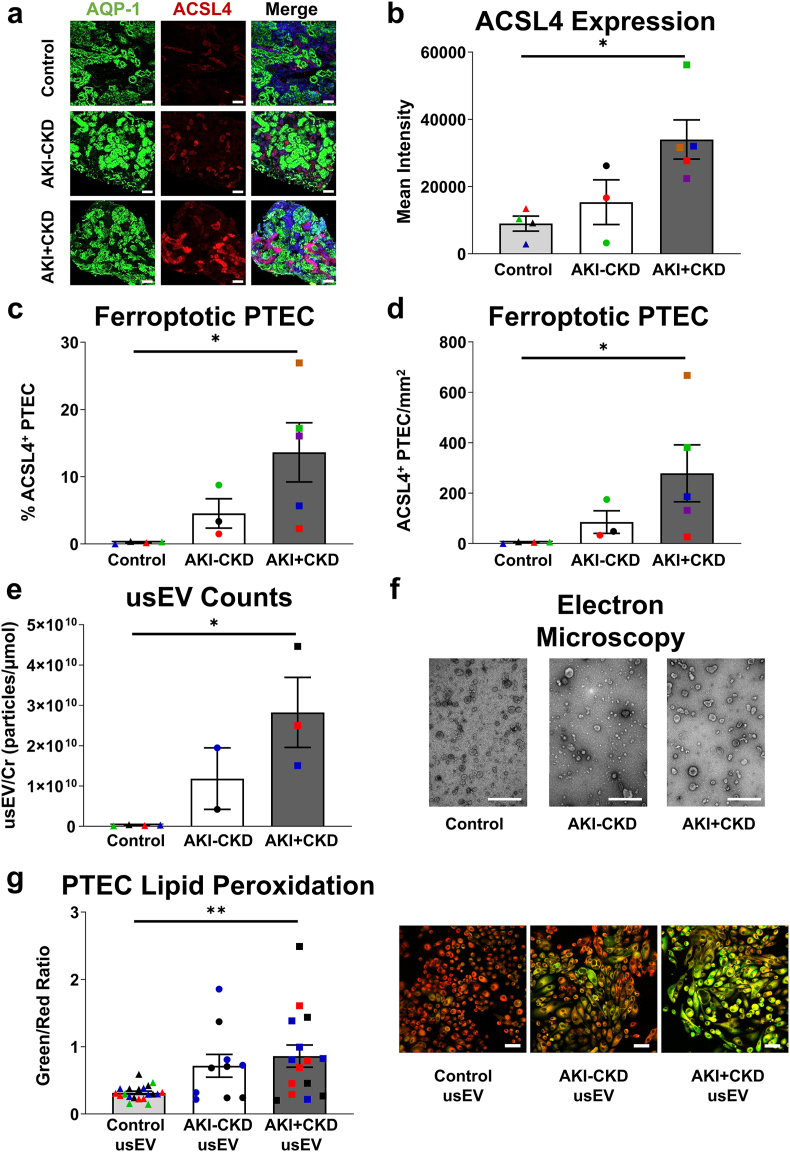
IF staining revealed widespread ferroptosis (ACSL4 expression) in AKI + CKD biopsies compared with AKI-CKD biopsies and control kidney tissue (Fig. 8a). Quantitative analysis was performed, showing significantly increased ACSL4 expression intensity (Fig. 8b), % ACSL4+ PTEC (Fig. 8c) and absolute numbers of ACSL4+ PTEC (Fig. 8d) in AKI + CKD biopsies.
Fig. 8.
Urine-derived sEV from AKI patients with progression to CKD (AKI + CKD) induce PTEC ferroptosis. (a) Representative immunofluorescent labelling of control, AKI-CKD and AKI + CKD kidney tissue stained for PTEC marker aquaporin-1 (AQP-1; green), ferroptosis marker ACSL4 (red) and DAPI (blue). Scale bars represent 100 μm. (b–d) Quantitative analysis of mean ACSL4 fluorescence intensity (b), % ACSL4+ PTEC (proportion of AQP-1+ cells (i.e. PTEC) expressing ACSL4) (c) and number (mean cells/mm2) of ACSL4+ PTEC (d) in control, AKI-CKD and AKI + CKD kidney tissue. Symbols represent values for individual donor tissue. Results represent mean ± SEM of values from 2 to 3 randomly selected areas for each tissue sample. *P < 0.05, **P < 0.01, Mann-Whitney test. (e) Numbers of sEV normalized to urine creatinine (particles per μmol urine creatinine (Cr)) isolated from control, AKI-CKD and AKI + CKD urine. Symbols represent values for individual donor urine specimens. Results represent mean ± SEM. *P < 0.05, Mann-Whitney test. (f) Electron microscopy images of representative usEV. Scale bars represent 500 nm. (g) Left panel: Lipid peroxidation (measured as a ratio of oxidized 510 nm (green)/reduced 590 nm (red) fluorescence) in human primary PTEC co-cultured with control, AKI-CKD or AKI + CKD usEV. Bar graphs represent mean ± SEM. Symbols represent values for each donor usEV in biological replicate co-culture experiments against five different human primary PTEC. **P < 0.01, Mann-Whitney test. Right panel: IF labelling of a representative PTEC experiment stained with Image-iT™ Lipid Peroxidation Sensor. Lipid peroxidation is highlighted by the shift in fluorescence from red to green. Scale bars represent 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We next examined urinary-derived sEV (usEV) from AKI patients as a clinical representation of tubular sEV released into the apical lumen/urinary space. usEV were isolated from AKI urine at the time of biopsy, with numbers of usEV (particles per μmol urine creatinine) significantly increased in AKI + CKD urine compared with the control urine (Fig. 8e). There were no significant differences in usEV mean diameter or size distributions between AKI + CKD, AKI-CKD and control cohorts (Fig. S2), with characteristic cup-shaped sEV morphology identified in all populations by electron microscopy (Fig. 8f). In functional co-culture studies, only AKI + CKD usEV induced significantly increased PTEC ferroptosis (↑lipid peroxidation) compared with control usEV (Fig. 8g). Collectively, these findings identify a novel sEV-mediated mechanism of ferroptosis propagation in AKI-to-CKD transition.
4. Discussion
The lack of tools for early AKI detection and intervention is a key factor in the associated rising healthcare burden of CKD, with global prevalence of this chronic disease increasing by ∼25 % in the decade 2007–2017 [30]. Compounding the rising prevalence of AKI is the paucity of therapeutic agents to effectively block disease progression in the 20–50 % of AKI patients that transition to CKD [31]. Current suboptimal clinical outcomes highlight our limited understanding of how AKI progresses to CKD. This study addresses our knowledge gap of the pathobiology of kidney disease progression, with a focus on hypoxia, a central driver of tubulointerstitial injury in AKI-to-CKD transition [32]. We demonstrate that hypoxic-apical sEV trigger a process of synchronized tubular lipid peroxidation and ferroptosis in human ex vivo PTEC, identifying a key intercellular signalling mechanism driving the ferroptotic ‘wave of tubular death’ of hypoxic kidney disease progression [6,13,33]. Moreover, we confirm these in vitro findings in clinical biospecimens from AKI patients who subsequently progressed to CKD. Our novel data have the potential to expand therapeutic paradigms in AKI beyond haemodynamic management and fluid therapy to precision blockade of irreversible tubular loss - a major advance in improving the long-term outcomes of AKI survivors.
sEV biogenesis and release is shaped by the extracellular environment of the parent cell [34]. Increased sEV release is reported in cancer cells [[35], [36], [37]], mesenchymal stem cells [38] and adipocytes [39] under hypoxic conditions. We previously reported that total production of sEV/exosomes by human primary PTEC is significantly elevated in hypoxic conditions [17]. Here, we examined the effect of PTEC polarity on sEV production. We show that more sEV are secreted from the apical membrane of PTEC than the basolateral membrane under normoxic conditions. The number of sEV secreted from the apical membrane increases under hypoxic conditions. In contrast, there is no change in the number of sEV secreted from the basolateral membrane of hypoxic PTEC. This finding is consistent with previous reports of asymmetric sEV/exosome release by human epithelial cells of breast, retinal pigment, intestinal and hepatic origin [[40], [41], [42], [43]] and may reflect independent intracellular trafficking pathways of apical versus basolateral sEV release by epithelial cells - i.e., the ALIX–Syntenin1–Syndecan1 machinery (apical membrane) as opposed to the sphingomyelinase-dependent ceramide production machinery (basolateral membrane) [44].
We also show that the molecular repertoire of sEV is dependent on epithelial polarity. miRNA are a class of non-coding RNA that regulate gene expression by either repressing translation or directing sequence-specific degradation of their target messenger RNAs [45] and are key functional elements of the hypoxic response [46]. miRNA dysregulation in hypoxic PTEC is associated with the development of epithelial-to-mesenchymal transition (EMT) and kidney fibrosis [[47], [48], [49], [50]]. In line with this concept, biological effects analysis of the defined/known miRNA repertoire of hypoxic PTEC-derived apical sEV identify a ‘fibrosis’ biological function. These 23 significantly differentially expressed miRNA include upregulated molecules previously shown to amplify injury in hypoxic models of myocardial ischemia-reperfusion (miR-29b-3p) [51] and pulmonary artery hypertension (miR-340-5p) [52] and, now in this study, in a hypoxic model of PTEC sEV signalling.
Further network mapping of these known miRNA (Fig. 4) associate hypoxic-apical sEV with a process of ‘organismal injury’. Indeed, of the 23 known miRNA, three out of seven upregulated miRNA (miR-29b-3p, miR-103b, miR-6869-5p) and seven out of 16 downregulated miRNA (miR-127-3p, miR-18a-3p, miR-3182, miR-379-5p, miR-135b-3p, miR-143-3p, miR-150-5p) have been implicated in pathways of kidney injury [[53], [54], [55], [56], [57], [58], [59], [60], [61], [62]]. In our model of tubulointerstitial hypoxia, we establish this injurious pathway to be lipid peroxidation and ferroptotic cell death, mediated via the intravesicular RNA cargo of hypoxic-apical sEV. The miRNA signature of our hypoxic-apical sEV (i.e., ↓ miR-193a-3p, ↑ miR-340-5p, ↑ miR-1343-5p) is consistent with a functional role in ferroptosis induction. The link between reduced miR-193a-3p and ferroptosis has been reported in human congenital heart disease biosamples [63], while elevated levels of miR-340-5p and miR-1343-5p are associated with ferroptotic cell death in hepatocellular carcinoma and chronic obstructive pulmonary disease respectively [64,65]. In particular, the respective target genes of miR-340-5p and miR-1343-5p are nuclear factor E2-related factor 2 (NRF2) and aldo-keto reductase family 1 member C3 (AKR1C3), both inhibitors of lipid peroxidation and ferroptosis [[66], [67], [68]].
Ferroptosis is biologically distinct from other patterns of regulated cell death (i.e., apoptosis, necroptosis, pyroptosis) as it lacks a terminal executioner protein [69] and spreads through cell populations in a non-random, wave-like manner [13,[70], [71], [72]]. This synchronized transmission of ferroptotic cell death was first reported in mouse tubular cells following hypoxic AKI damage (ischemia-reperfusion injury; IRI) [13]. Recent publications propose that this ferroptotic ‘wave of tubular death’ may be propagated to neighbouring cells via: [i] cell-cell contacts [73]; [ii] an osmotic mechanism independently of cell rupture [71]; or [iii] a redox imbalance in the local micro-environment [33,74]. Here, we identify PTEC-derived sEV as a key ferroptosis-triggering signal in the hypoxic tubulointerstitium and extend these findings to urinary-derived sEV from patients with AKI-to-CKD transition. This study provides the proof of concept for repurposing established inhibitors of sEV trafficking/release (e.g. calpeptin, manumycin A [75]) for therapeutic targeting of the hypoxic-initiated tubular cell loss observed in AKI-to-CKD transition. Notably, the calpain inhibitor calpeptin has been recently shown to alleviate tubular pathological damage in an IRI-induced AKI mouse model [76]. Our findings identify PTEC sEV as credible targets of calpeptin in this mouse model.
Ferroptotic tubular cell death has been reported in experimental mouse models [13,[77], [78], [79], [80], [81]], in vitro primary PTEC studies [10,82] and clinical biopsy specimens [10,83,84]. Ferrostatin-1, a potent small-molecule compound that blocks lipid peroxidation [85], has been the gold-standard ferroptosis inhibitor in the majority of pre-clinical studies. However, the clinical translatability of ferrostatin-1 is compromised by its poor in vivo metabolic stability [86]. We show that the plant-derived flavonoid, baicalein, currently under investigation in a Phase II influenza clinical trial (NCT03830684), is equally efficacious in inhibiting ferroptosis. Baicalein, or this class of biologics, are promising agents in the treatment of ferroptosis-associated nephropathies.
Collectively, our results provide the first comprehensive molecular and functional characterisation of polarity-dependent sEV secretion by hypoxic human primary PTEC. We also offer unique insights into the sEV-mediated mechanisms by which human PTEC propagate synchronized ferroptosis along the tubular lumen in AKI-to-CKD transition. Our findings open up novel fields of therapeutics with clinical specificity for targeting the pathways of tubular lipid peroxidation/loss in AKI-to-CKD transition (i.e., inhibiting sEV signalling and ferroptosis). Moreover, sEV and their molecular repertoire have been intensely investigated for their diagnostic utility as non-invasive, urinary biomarkers of AKI and CKD [87]. We propose that the miRNA cargo of apical sEV released into the tubular lumen and detected in patient urine also represent promising biomarkers for future diagnostic and prognostic studies of hypoxic kidney disease progression.
Author contributions
Each author has participated sufficiently in the work to take public responsibility for the content. X.W., R.W., J.U., H.H. and A.J.K. conceived and designed the study; X.W., C.S.K., B.C.A., M.M.H., A.K.S., A.M., M.D., M.S.N and A.J.K. carried out experiments and analyzed/interpreted the data; X.W., H.H. and A.J.K. drafted the paper. All authors read and approved the final version of the manuscript.
Funding details
The work was funded in part by Pathology Queensland, a Royal Brisbane and Women’s Hospital Research Grant, the Kidney Research Foundation, an Avant Early Career Research Program Grant (2022/000125) and National Health and Medical Research Council Project Grant (GNT1161319).
Data availability statement
The data supporting the findings of this study are openly available at Mendeley Data (https://data.mendeley.com/datasets/z76d5m8hbx/2). We have also submitted all relevant data of our experiments to the EV-TRACK knowledgebase (EV-TRACK ID: EV230957) (Van Deun J et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nature Methods. 2017; 14(3):228-32).
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgements
We would like to express our profound thanks to our surgical colleagues and especially our patients for tissue donation. We gratefully acknowledge The University of Queensland Centre for Clinical Research (UQCCR) mass spectrometry facility for assistance with proteomics data acquisition. The authors also acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103042.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Lewington A.J., Cerda J., Mehta R.L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ow C.P.C., Ngo J.P., Ullah M.M., et al. Renal hypoxia in kidney disease: cause or consequence? Acta Physiol. 2018;222 doi: 10.1111/apha.12999. [DOI] [PubMed] [Google Scholar]
- 4.Ullah M.M., Basile D.P. Role of renal hypoxia in the progression from acute kidney injury to chronic kidney disease. Semin. Nephrol. 2019;39:567–580. doi: 10.1016/j.semnephrol.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka S., Tanaka T., Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Ren. Physiol. 2014;307:F1187–F1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 6.Ide S., Kobayashi Y., Ide K., et al. Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. Elife. 2021;10 doi: 10.7554/eLife.68603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J., Kumar S., Dolzhenko E., et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang H.M., Ahn S.H., Choi P., et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turman M.A., Bates C.M. Susceptibility of human proximal tubular cells to hypoxia: effect of hypoxic preconditioning and comparison to glomerular cells. Ren. Fail. 1997;19:47–60. doi: 10.3109/08860229709026259. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani K.T.K., Grivei A., Nag P., et al. Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c(+) dendritic cells. Cell Death Dis. 2022;13:739. doi: 10.1038/s41419-022-05191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan H., Li X., Zhang X., et al. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 12.Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linkermann A., Skouta R., Himmerkus N., et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thery C., Witwer K.W., Aikawa E., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grange C., Bussolati B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022;18:499–513. doi: 10.1038/s41581-022-00586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Wilkinson R., Kildey K., et al. Unique molecular profile of exosomes derived from primary human proximal tubular epithelial cells under diseased conditions. J. Extracell. Vesicles. 2017;6 doi: 10.1080/20013078.2017.1314073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton C.J., Combe C., Walls J., et al. Fibronectin production by human tubular cells: the effect of apical protein. Kidney Int. 1996;50:760–767. doi: 10.1038/ki.1996.374. [DOI] [PubMed] [Google Scholar]
- 19.Lai K.N., Leung J.C., Chan L.Y., et al. Interaction between proximal tubular epithelial cells and infiltrating monocytes/T cells in the proteinuric state. Kidney Int. 2007;71:526–538. doi: 10.1038/sj.ki.5002091. [DOI] [PubMed] [Google Scholar]
- 20.Wang X., Wilkinson R., Kildey K., et al. Molecular and functional profiling of apical versus basolateral small extracellular vesicles derived from primary human proximal tubular epithelial cells under inflammatory conditions. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glynne P.A., Evans T.J. Inflammatory cytokines induce apoptotic and necrotic cell shedding from human proximal tubular epithelial cell monolayers. Kidney Int. 1999;55:2573–2597. doi: 10.1046/j.1523-1755.2002.t01-1-00456.x. [DOI] [PubMed] [Google Scholar]
- 22.Kassianos A.J., Sampangi S., Wang X., et al. Human proximal tubule epithelial cells modulate autologous dendritic cell function. Nephrol. Dial. Transplant. 2013;28:303–312. doi: 10.1093/ndt/gfs136. [DOI] [PubMed] [Google Scholar]
- 23.Bankhead P., Loughrey M.B., Fernandez J.A., et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group K.A.W. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2:1–138. [Google Scholar]
- 25.Fogo A.B., Lusco M.A., Najafian B., et al. AJKD atlas of renal pathology: ischemic acute tubular injury. Am. J. Kidney Dis. 2016;67:e25. doi: 10.1053/j.ajkd.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Roerdink J.B.T.M., Meijster A. The watershed transform: definitions, algorithms and parallelization strategies. Fundam. Inf. 2000;41:187–228. [Google Scholar]
- 27.Friedlander M.R., Chen W., Adamidi C., et al. Discovering microRNAs from deep sequencing data using miRDeep. Nat. Biotechnol. 2008;26:407–415. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- 28.Miotto G., Rossetto M., Di Paolo M.L., et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Y., Song X., Sun X., et al. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016;473:775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 30.GdaIIaP Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein S.L., Jaber B.L., Faubel S., et al. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin. J. Am. Soc. Nephrol. 2013;8:476–483. doi: 10.2215/CJN.12101112. [DOI] [PubMed] [Google Scholar]
- 32.Heyman S.N., Khamaisi M., Rosen S., et al. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am. J. Nephrol. 2008;28:998–1006. doi: 10.1159/000146075. [DOI] [PubMed] [Google Scholar]
- 33.Belavgeni A., Meyer C., Stumpf J., et al. Ferroptosis and necroptosis in the kidney. Cell Chem. Biol. 2020;27:448–462. doi: 10.1016/j.chembiol.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Bister N., Pistono C., Huremagic B., et al. Hypoxia and extracellular vesicles: a review on methods, vesicular cargo and functions. J. Extracell. Vesicles. 2020;10 doi: 10.1002/jev2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrmann A., Rice M., Lévy R., et al. Cellular memory of hypoxia elicits neuroblastoma metastasis and enables invasion by non-aggressive neighbouring cells. Oncogenesis. 2015;4:e138. doi: 10.1038/oncsis.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zonneveld M.I., Keulers T.G.H., Rouschop K.M.A. Extracellular vesicles as transmitters of hypoxia tolerance in solid cancers. Cancers. 2019;11 doi: 10.3390/cancers11020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T., Gilkes D.M., Takano N., et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-King H., García N.A., Ontoria-Oviedo I., et al. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cell. 2017;35:1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 39.Mleczko J., Ortega F.J., Falcon-Perez J.M., et al. Extracellular vesicles from hypoxic adipocytes and obese subjects reduce insulin-stimulated glucose uptake. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreekumar P.G., Kannan R., Kitamura M., et al. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bu H.F., Wang X., Tang Y., et al. Toll-like receptor 2-mediated peptidoglycan uptake by immature intestinal epithelial cells from apical side and exosome-associated transcellular transcytosis. J. Cell. Physiol. 2010;222:658–668. doi: 10.1002/jcp.21985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies B.A., Morton L.O., Jefferson J.R., et al. Polarized human cholangiocytes release distinct populations of apical and basolateral small extracellular vesicles. Mol. Biol. Cell. 2020;31:2463–2474. doi: 10.1091/mbc.E19-03-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin A.R., Yan W., Cao M., et al. Polarized secretion of extracellular vesicles by mammary epithelia. J. Mammary Gland Biol. Neoplasia. 2018;23:165–176. doi: 10.1007/s10911-018-9402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui T., Osaki F., Hiragi S., et al. ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep. 2021;22 doi: 10.15252/embr.202051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng Y., Yi R., Cullen B.R. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nallamshetty S., Chan S.Y., Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic. Biol. Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zell S., Schmitt R., Witting S., et al. Hypoxia induces mesenchymal gene expression in renal tubular epithelial cells: an in vitro model of kidney transplant fibrosis. Nephron Extra. 2013;3:50–58. doi: 10.1159/000351046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du R., Sun W., Xia L., et al. Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie S., Chen H., Li F., et al. Hypoxia-induced microRNA-155 promotes fibrosis in proximal tubule cells. Mol. Med. Rep. 2015;11:4555–4560. doi: 10.3892/mmr.2015.3327. [DOI] [PubMed] [Google Scholar]
- 50.Kuo M.C., Chang W.A., Wu L.Y., et al. Hypoxia-induced epithelial-to-mesenchymal transition in proximal tubular epithelial cells through miR-545-3p-TNFSF10. Biomolecules. 2021:11. doi: 10.3390/biom11071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He D., Yan L. MiR-29b-3p aggravates cardiac hypoxia/reoxygenation injury via targeting PTX3. Cytotechnology. 2021;73:91–100. doi: 10.1007/s10616-020-00446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C., So J., Davis-Dusenbery B.N., et al. Hypoxia potentiates MicroRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol. Cell Biol. 2011;31:4760–4774. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai X., Geng J., Zhou Z., et al. MicroRNA-130b improves renal tubulointerstitial fibrosis via repression of Snail-induced epithelial-mesenchymal transition in diabetic nephropathy. Sci. Rep. 2016;6 doi: 10.1038/srep20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beltrami C., Simpson K., Jesky M., et al. Association of elevated urinary miR-126, miR-155, and miR-29b with diabetic kidney disease. Am. J. Pathol. 2018;188:1982–1992. doi: 10.1016/j.ajpath.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Liu H.-H., Li X.-Q., Liu J.-F., et al. miR-6869-5p transported by plasma extracellular vesicles mediates renal tubule injury and renin-angiotensin system activation in obesity. Frontiers in Medicine [Original Research. 2021;8 doi: 10.3389/fmed.2021.725598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu L., Han X., Jiang X., et al. Downregulation of renal hsa-miR-127-3p contributes to the overactivation of type I interferon signaling pathway in the kidney of lupus nephritis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.747616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng J., Tan W., Luo Q., et al. Long non-coding RNA MEG3 promotes renal tubular epithelial cell pyroptosis by regulating the miR-18a-3p/GSDMD pathway in lipopolysaccharide-induced acute kidney injury. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.663216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker M.A., Davis S.J., Liu P., et al. Tissue-specific MicroRNA expression patterns in four types of kidney disease. J. Am. Soc. Nephrol. 2017;28:2985–2992. doi: 10.1681/ASN.2016121280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li N., Wang L.J., Xu W.L., et al. MicroRNA-379-5p suppresses renal fibrosis by regulating the LIN28/let-7 axis in diabetic nephropathy. Int. J. Mol. Med. 2019;44:1619–1628. doi: 10.3892/ijmm.2019.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weng L., Wu X., Gao H., et al. MicroRNA profiling of clear cell renal cell carcinoma by whole-genome small RNA deep sequencing of paired frozen and formalin-fixed, paraffin-embedded tissue specimens. J. Pathol. 2010;222:41–51. doi: 10.1002/path.2736. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Z., Dong Y., Zhou H., et al. MiR-143-3p directly targets GLUT9 to reduce uric acid reabsorption and inflammatory response of renal tubular epithelial cells. Biochem. Biophys. Res. Commun. 2019;517:413–420. doi: 10.1016/j.bbrc.2019.07.114. [DOI] [PubMed] [Google Scholar]
- 62.Pawluczyk I.Z.A., Didangelos A., Barbour S.J., et al. Differential expression of microRNA miR-150-5p in IgA nephropathy as a potential mediator and marker of disease progression. Kidney Int. 2021;99:1127–1139. doi: 10.1016/j.kint.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 63.Zhong L., Yang H., Zhu B., et al. The TBX1/miR-193a-3p/TGF-β2 Axis mediates CHD by promoting ferroptosis. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/5130546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo Y., Huang Q., He B., et al. Regulation of ferroptosis by non-coding RNAs in the development and treatment of cancer. Oncol. Rep. 2021;45:29–48. doi: 10.3892/or.2020.7836. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin Z., Xu Y., Guan L., et al. Seven ferroptosis-specific expressed genes are considered as potential biomarkers for the diagnosis and treatment of cigarette smoke-induced chronic obstructive pulmonary disease. Ann. Transl. Med. 2022;10:331. doi: 10.21037/atm-22-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu L.L., Cai W.P., Lei X., et al. NRAL mediates cisplatin resistance in hepatocellular carcinoma via miR-340-5p/Nrf2 axis. J Cell Commun Signal. 2019;13:99–112. doi: 10.1007/s12079-018-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anandhan A., Dodson M., Schmidlin C.J., et al. Breakdown of an ironclad defense system: the critical role of NRF2 in mediating ferroptosis. Cell Chem. Biol. 2020;27:436–447. doi: 10.1016/j.chembiol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dixon S.J., Patel D.N., Welsch M., et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3 doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galluzzi L., Vitale I., Aaronson S.A., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim S.E., Zhang L., Ma K., et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riegman M., Sagie L., Galed C., et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 2020;22:1042–1048. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katikaneni A., Jelcic M., Gerlach G.F., et al. Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat. Cell Biol. 2020;22:1049–1055. doi: 10.1038/s41556-020-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roeck B.F., Vorndran M.R.H., Garcia-Saez A.J. Ferroptosis propagates to neighboring cells via cell-cell contacts. bioRxiv. 2023;2023 2003.2024. [Google Scholar]
- 74.Maremonti F., Meyer C., Linkermann A. Mechanisms and models of kidney tubular necrosis and nephron loss. J. Am. Soc. Nephrol. 2022;33:472–486. doi: 10.1681/ASN.2021101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Catalano M., O'Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles. 2020;9 doi: 10.1080/20013078.2019.1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y., Yang H., Cheng M., et al. Calpain inhibitor calpeptin alleviates ischemia/reperfusion-induced acute kidney injury via suppressing AIM2 inflammasome and upregulating klotho protein. Front. Med. 2022;9 doi: 10.3389/fmed.2022.811980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin-Sanchez D., Ruiz-Andres O., Poveda J., et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 2017;28:218–229. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su L., Jiang X., Yang C., et al. Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J. Biol. Chem. 2019;294:19395–19404. doi: 10.1074/jbc.RA119.010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng F., Sharma I., Dai Y., et al. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Invest. 2019;129:5033–5049. doi: 10.1172/JCI129903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guerrero-Hue M., Garcia-Caballero C., Palomino-Antolin A., et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. Faseb. J. 2019;33:8961–8975. doi: 10.1096/fj.201900077R. [DOI] [PubMed] [Google Scholar]
- 81.Li X., Peng X., Zhou X., et al. Small extracellular vesicles delivering lncRNA WAC-AS1 aggravate renal allograft ischemia‒reperfusion injury by inducing ferroptosis propagation. Cell Death Differ. 2023;30:2167–2186. doi: 10.1038/s41418-023-01198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan M.A., Nag P., Grivei A., et al. Adenine overload induces ferroptosis in human primary proximal tubular epithelial cells. Cell Death Dis. 2022;13:104. doi: 10.1038/s41419-022-04527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Z., Wu J., Xu H., et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis. 2020;11:629. doi: 10.1038/s41419-020-02871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S., Kang S.W., Joo J., et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12:160. doi: 10.1038/s41419-021-03452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skouta R., Dixon S.J., Wang J., et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devisscher L., Van Coillie S., Hofmans S., et al. Discovery of novel, drug-like ferroptosis inhibitors with in vivo efficacy. J. Med. Chem. 2018;61:10126–10140. doi: 10.1021/acs.jmedchem.8b01299. [DOI] [PubMed] [Google Scholar]
- 87.Lv L.L., Feng Y., Tang T.T., et al. New insight into the role of extracellular vesicles in kidney disease. J. Cell Mol. Med. 2019;23:731–739. doi: 10.1111/jcmm.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are openly available at Mendeley Data (https://data.mendeley.com/datasets/z76d5m8hbx/2). We have also submitted all relevant data of our experiments to the EV-TRACK knowledgebase (EV-TRACK ID: EV230957) (Van Deun J et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nature Methods. 2017; 14(3):228-32).