Abstract
Behavioral experiments have demonstrated that long-term physical exercise can be beneficial for learning and memory dysfunction caused by neuroinflammation in Alzheimer's disease (AD). However, the molecular mechanism remains poorly understood due to a lack of sufficient pertinent biochemical evidence. We investigated the potential effect of long-term physical exercise on cognition and hippocampal gene and protein expression changes in a transgenic AD mouse model. Following twenty weeks of treadmill exercise, transgenic AD mice showed improvement in cognitive functions and downregulation of Nod-like receptor protein 3 (NLRP3) (p < 0.01), interleukin-1beta (IL-1β) (p < 0.05), and amyloid-β1-42 (Aβ1-42) (p < 0.05) expression levels. In addition, we observed significant reductions of microglial activation and hippocampal neuronal damage in the exercised AD mice (p < 0.01), which might be a result of the downregulation of NLRP3-mediated signaling and neuro-inflammatory responses. As neuronal damage due to inflammation might be a likely cause of AD-associated cognitive dysfunction. Our results suggested that the anti-inflammatory effects of exercise training involved downregulating the expression of key inflammatory factors and might play an important role in protecting hippocampal neurons against damage during the course of AD.
Keywords: Exercise, NLRP3, Neuroinflammation, Hippocampus, APP/PS1 mice, Alzheimer's
Abbreviations
- AD
Alzheimer's disease
- IL-1β
interleukin-1beta
- Aβ
amyloid-β
- NFTs
neurofibrillary tangles
- CNS
central nervous system
- NLRP3
Nod-like receptor protein 3
- IL-18
interleukin-18
- APP/PS1
amyloid beta precursor protein/presenilin 1
- WT
wild-type
- MWM
Morris water maze
- RAM
radial arm maze
- PFA
paraformaldehyde
- PCR
Polymerase Chain Reaction
- RNA
ribonucleic acid
- BSA
bovine serum albumin
- Aβ1-42
amyloid-β1-42
- PBS
phosphate buffer saline
- ANOVA
analysis of variance
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder which affects wide areas of the cerebral cortex and hippocampus.1 AD is the most common cause of dementia among older adults with approximately 47 million people worldwide were reported to be living with dementia in 2015, and this number is expected to triple by 2050, causing substantial personal and societal burdens.2 AD is characterized by extracellular amyloid-β (Aβ) plaques and intra-neuronal neurofibrillary tangles (NFTs) depositions in the hippocampal formation and cerebral cortex, leading to synaptic loss and neuronal death which provokes cognitive deficits.3 In addition to amyloid plaques and NFTs, microglial activation plays a significant role in neurodegeneration, which is known as neuroinflammation. Microglia are the main immune cells in the brain and also participate in the establishment of normal neuronal connectivity and regulatory processes that are critical for central nervous system (CNS) development.4 A series of studies have shown that extracellular Aβ deposition activates innate immunity, leading to microglial activation and release of inflammatory mediators and cytokines, which promote the development of AD.5,6
Because of the multifactorial nature of AD, treatment involves a combination of lifestyle interventions targeting general brain health to combat the disease, such as exercise, diet, and cognitive training.7,8 Many experimental studies have shown that regular physical exercise training reduces the risk of chronic and age-related neurodegenerative diseases, such as Parkinson's disease or AD.9, 10, 11, 12 The neuroprotective effects of physical exercise can be mediated by multiple mechanisms. Existing evidence has shown that exercise-induced myokines, metabolic changes and positive effects on microglial status and inflammation that prevents aging and AD.12,13 Consistent physical exercise can improve immunosurveillance and immunocompetence in different tissues and exert specific biological protective effects by reducing microglial activation in the CNS.14 However, the mechanism of how exercise affects AD through neuroinflammation is still unclear.
Nod-like receptor protein 3 (NLRP3) has been implicated in several chronic inflammatory diseases as it can sense inflammatory crystals and aggregated proteins, including Aβ.15,16 NLRP3 activation causes the production of interleukin-1beta (IL-1β) and interleukin-18 (IL-18), which are involved in the development and progression of AD.17 This cascade results in the release of bioactive cytokines and induce an inflammatory form of cell death.18 Hippocampal NLRP3 is also activated in AD and may contribute to AD pathogenesis.15 Therefore, in the present study, we used an amyloid beta precursor protein/presenilin 1 (APP/PS1) transgenic AD mouse model to explore the mechanisms of the protective effect of exercise training on neuroinflammation related to the NLRP3 signaling pathway in the hippocampus.
2. Materials and methods
2.1. Animals
A total of 45 male wild-type and APP/PS1 mice (3 months old) were purchased from Nanjing Biomedical Research Institute of Nanjing University and were bred inhouse. All animals were group-housed under a normal light–dark cycle (12 h/12 h) with ad libitum access to standard laboratory food and filtered water. The 30 APP/PS1 mice were divided into the AD group (n = 15) and the AD + exercise training (AD + Ex) group (n = 15). The 15 male wild-type (WT) mice were assigned into WT group. All experimental protocols were approved by the Laboratory Animal Ethics Committee at Chengdu Sports University and were performed in accordance with the National Guidance for Animal Experiments (approval number 202113).
2.2. Long-term treadmill exercise protocol
Long-term treadmill exercise was performed as previously described.19 One six-channel treadmill apparatus (Model JD-PT, SANS Instruments, Jiangsu, China) was used (12 m/min for 10 min, then 15 m/min for 20 min, 5 days/week). After 20 weeks of treadmill exercise, the animals were subjected to behavioral testing and then sacrificed for molecular assays.
2.3. Morris Water Maze
The Morris water maze (MWM) test was used to assess spatial learning and memory functions.20 The MWM consists of a circular pool filled with water. A platform was submerged 1 cm deep in the water in the secondary quadrant. The duration of the MWM test was 7 consecutive days, including the navigation (days 1–6) and space exploration (day 7) tests. On days 1–6, each animal was released into water in different quadrants and subjected to four trials per day until reaching the platform. If the subject failed to find the platform within 120 s, it was gently guided to the platform and allowed to stay on the platform for 10 s. The escape latency was recorded within 120 s. On the 7th day, the platform was removed, and the swimming trajectory of each individual and the number of crosses over the original platform location were recorded for 90 s.
2.4. Radial Arm Maze (RAM)
Working and reference memory functions were assessed using the RAM for 7 consecutive days.21 On days 1–2, food was scattered in the center and on each arm of the maze, then animals were placed in the center of the maze and allowed to freely eat and explore for 10 min. On days 3–4, a single individual was trained and food was placed in the outer food box of all arm, and the animal was left to eat freely. The animal was then taken out when finished eating or after 10 min. On days 5–6, the four selected arms were only baited and the animal was placed in the center of the maze and allowed to move freely to find and eat the food in all four arms. If the food was not eaten within 10 min, the training session was ended. Training was performed twice per day with an at least 1 h between sessions. On day 7, the number of working memory errors (the number of times the animal re-entered the arm in which it had already eaten the food) and the number of reference memory errors (the number of times the animal entered the arm that did not have food) were recorded.
2.5. Sample harvesting of hippocampal tissues
After the corresponding behavior test, five mice selected randomly from each group were anesthetized and sacrificed by decapitation, and then perfused with 0.9% saline followed by 4% paraformaldehyde (PFA). Their brains were dissected on ice to harvest hippocampal tissues. The hippocampal tissues were immediately frozen in liquid nitrogen and then stored at −80 °C until being analyzed.
2.6. Real-time polymerase chain reaction (PCR)
Total ribonucleic acid (RNA) was extracted from three mouse hippocampal tissues using TRIzol reagent (Servicebio, G3013) according to the manufacturer's instructions. Complementary deoxyribonucleic acid was then synthesized by a reverse transcription kit (MasterMix, Abm, #G492). Real-time PCR was performed using specific primers for NLRP3 and the internal reference gene encoding β-actin. Primer sequences are shown in Table 1. Real-time PCR was performed on a fluorescent quantitative PCR cycler (SLAN-96S, Shanghai, China) under the following conditions: denaturation at 95 °C for 30 s, followed by 40 cycles of 90 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s. Quantitative analysis was performed by the 2–ΔΔCt method.
Table 1.
The primer sequences of the gene NLR family pyrin domain-containing 3 (NLRP3) and β-actin.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| NLRP3 | 5′-ATCTTGGCGATCTGTGCGTG-3′ | 5′-GAGCGCTTCTAAGGCACGTT-3′ |
| β-actin | 5′-GTGCTATGTTGCTCTAGACTTCG-3′ | 5′-ATGCCACAGGATTCCATACC-3′ |
2.7. Western blotting
Tissue lysates were extracted from mouse hippocampus samples with lysis buffer (70-WB020, LiankeBio, Hangzhou, China) containing protease inhibitors. After quantification using a bicinchoninic acid kit (70-PQ0012, LiankeBio, Hangzhou, China), 30 μg of total protein was separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis at 75–120 V for 90 min and transferred to a polyvinylidene fluoride membrane at 300 mA for 30 min. After being blocked in 5% bovine serum albumin (BSA) for 1 h, the membrane was incubated overnight at 4 °C with primary antibodies against NLRP3 (1:1 000, ab270449, Abcam), IL-1β (1:1 000, ab9722, Abcam), IL-18 (1:1 000, A1115, Abclonal), amyloid-β1-42 (Aβ1-42) (1:1 000, YT5773, Immunoway), and β-actin (1:5 000, AF7018, Affinity). The membrane was then washed three times for 10 min each and incubated in the secondary antibody for 2 h at room temperature. Protein bands were visualized using an imaging system (ChemiScope 6100, ClinX, China). The integrated gray values of each band were measured using ImageJ (National Institutes of Health, Bethesda, MD, USA) to determine the relative protein expressions.
2.8. Nissl staining
Hippocampal tissues were sectioned and deparaffinized, then they were placed in dimethylbenzene I for 20 min, dimethylbenzene II for 20 min, absolute ethanol I for 5 min, anhydrous ethanol II for 5 min, and 75% ethanol for 5 min, and then placed in the dye solution for 5 min. The slices were sealed with neutral balsam and images were captured under a microscope (Eclipse E100, Nikon, Japan) to assess hippocampal morphology.
2.9. Immunofluorescence staining
Mice were euthanized with chloral hydrate and perfused with 4% PFA. The brain was sectioned into 4 μm slices using a sliding microtome (Cryotome E, Thermo, USA). After being washed three times with phosphate buffer saline (PBS) and blocked with BSA for 30 min, brain slices were incubated with Iba1 primary antibodies (1:100, ET1705-78, Huabio) at 4 °C overnight, followed by incubation with secondary antibodies (1:300, GB21303, Servicebio). The slices were then washed in PBS, DAPI solution was then added tissues were incubated for 10 min. Images were captured with an upright fluorescence microscope (Nikon Eclipse C1, Japan), and the fluorescence intensity was analyzed using ImageJ.
2.10. Statistical analysis
All data are shown as the mean ± standard deviation (mean ± SD). Statistical analyses were performed using one-way analysis of variance (ANOVA). For time-dependent analysis, two-way ANOVA was used in conjunction with Bonferroni's post-hoc comparison. GraphPad Prism version 8.0 package (La Jolla, CA, USA) was used for data analysis and plotting. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Exercise attenuates cognitive dysfunction in AD mice given physical exercise
We assessed the potential neuroprotective effects of aerobic exercise using the MWM test to evaluate spatial learning and memory capacity (Fig. 1 A–C) and the RAM test to evaluate working and reference memory (Fig. 1 D–F) in an APP/PS1 transgenic mouse model of AD. In the MWM test, there was no difference between the groups on days 1–2. From days 3–6, the escape latency in the AD group was significantly longer (p < 0.001, Fig. 1A) than the wild-type (WT) and AD + Ex groups. There was no significance difference between WT and AD + Ex mice on days 1–6 of MWM training (p > 0.05, Fig. 1A). On day 7, the number of platform crossings in the AD group significantly decreased, indicating deficits in learning and memory abilities (p < 0.001, Fig. 1B). Moreover, the AD + Ex group had markedly increased numbers of target platform crossings compared with those in the AD group (p < 0.01, Fig. 1D and E), and showed significant difference compared to the WT group (p > 0.05, Fig. 1D and E). In the RAM test, the number of working and reference memory errors in the AD group significantly increased, indicating deficits in learning and memory (p < 0.001, Fig. 1D–F). Similar to the results of the MWM test, the AD + Ex mice showed fewer errors than the AD mice (p < 0.001) and no significant difference with the WT group (p > 0.05, Fig. 1D–F). These results indicate that the APP/PS1 transgenic mice that did not receive exercise training suffered decreased cognitive functions and that long-term aerobic exercise could significantly improve learning and memory capacities and attenuate dysfunctional cognition associated with AD.
Fig. 1.
Exercise attenuated learning and memory dysfunction in Alzheimer's disease (AD) mice. (A) Escape latency of the mice to reach the platform on days 1–6 in the Morris Water Maze (MWM). (B) The number of crossings target platform on day 7 of MWM training. (C) Swimming track in the MWM. (D) The number of working memory errors in the Radial Arm Maze (RAM) test on day 7. (E) The number of reference memory errors in the RAM test on day 7. (F) Motion trajectory in the RAM test. WT, wild-type mice; AD, Alzheimer's disease model amyloid beta precursor protein/presenilin 1 (APP/PS1) transgenic mice; AD + Ex, APP/PS1 transgenic mice given exercise regimen. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.2. Exercise inhibits the expression of NLRP3 and neuroinflammation in the hippocampus of AD mice
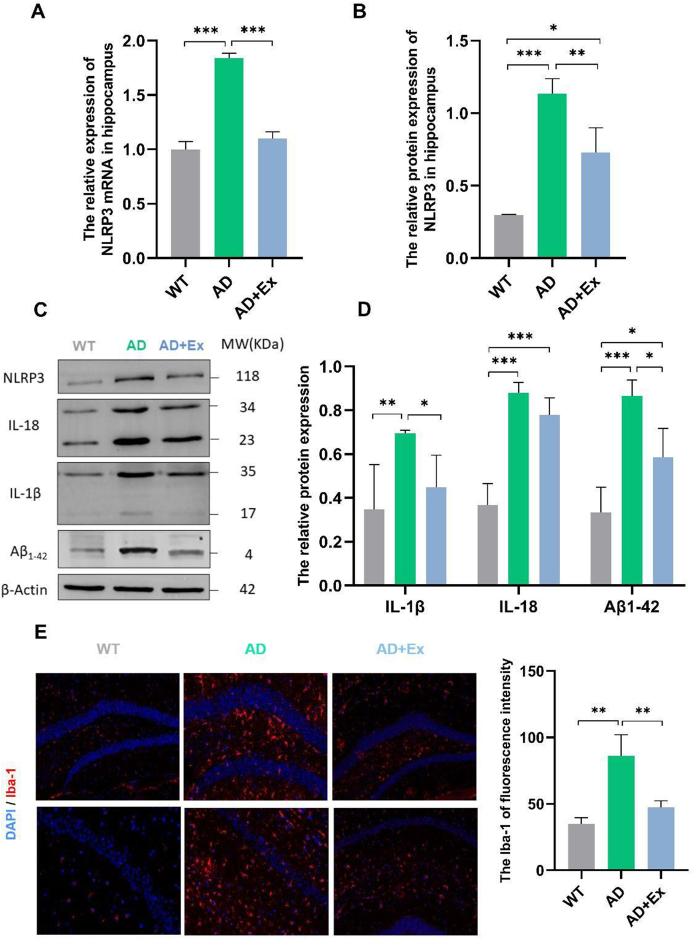
We next investigated the molecular changes in AD mice. NLRP3 gene and protein expressions were upregulated in the hippocampal tissue of AD mice (p < 0.001, Fig. 2A–C), while the expression levels were significantly suppressed in mice received treadmill exercise (p < 0.01, Fig. 2A–C). We measured the expression of Aβ1−42 in mice as an indicator of neurotoxicity and found that it was significantly increased in the AD group (p < 0.001, Fig. 2C and D). Following the same pattern as the behavioral tests, we found that treadmill exercise significantly decreased the expression of Aβ1−42 in the AD + Ex group compared to the AD group (p < 0.05, Fig. 2C and D). We next examined expressions of the cytokines IL-1β and IL-18, which are downstream of NLRP3, in the hippocampus. The data suggested that the levels of these inflammatory cytokines are significantly increased in hippocampal tissue of the AD group (p < 0.01), but were significantly decreased with treadmill exercise (p < 0.05, Fig. 2C and D). The protein expression of IL-18 showed no significant difference between the AD and AD + Ex groups (p > 0.05, Fig. 2C and D). We next assessed whether neuroinflammation was changed in AD mice after treadmill exercise with immunofluorescent staining. Staining for the microglial marker ionized calcium binding adapter molecule-1 (Iba-1) indicated significantly higher microglial density in the hippocampus of the AD group than in the WT group, and exercise resulted in reduced microglial density, indicating reduced neuroinflammation (p < 0.01, Fig. 2E).
Fig. 2.
Long-term treadmill exercise suppresses NLRP3 in the hippocampus of AD mice. (A) The relative expression of NLRP3 mRNA in the hippocampus (B) The relative protein expression of NLRP3 in the hippocampus. (C) Representative western blots. MW, molecular weight. (D) The relative protein expressions of interleukin-1beta (IL-1β), interleukin-18 (IL-18), and amyloid-β1-42 (Aβ1-42) in the hippocampus. (E) Immunostaining and quantification of ionized calcium binding adapter molecule-1 (Iba-1). WT, wild-type mice; AD, Alzheimer's disease model APP/PS1 transgenic mice; AD + Ex, APP/PS1 transgenic mice given exercise regimen. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.3. Exercise mitigates the damage to hippocampal neurons in AD mice
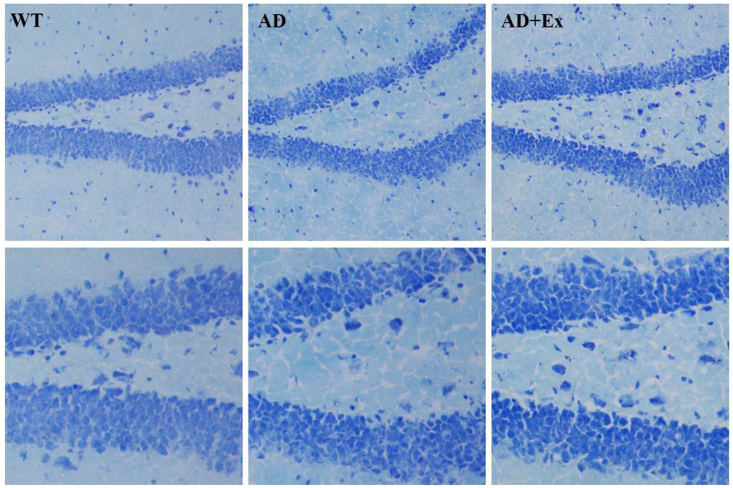
We next examined histopathological changes by Nissl staining to evaluate the damage to hippocampal neurons. Neurons in the hippocampal dentate gyrus regions in the AD group showed fewer neurons and disorderly arrangement compared to WT mice. However, compared with those in the AD group, the number of neurons in the hippocampal structures of the AD + Ex group were increased and neatly arranged, suggesting that neuronal damage or loss caused by AD was minimized (Fig. 3).
Fig. 3.
Nissl staining showing hippocampal neurons in the dentate gyrus. Representative photomicrographs showing histopathological changes in hippocampal tissues with Nissl staining (top and bottom are 100 × and 200 × , respectively). WT, wild-type mice; AD, Alzheimer's disease model APP/PS1 transgenic mice; AD + Ex, APP/PS1 transgenic mice given exercise regimen.
4. Discussion
Many studies have used the Aβ injection-induced AD model in the hippocampus.22, 23, 24 Although this model can simulate some of the characteristic behavioral changes of AD in humans, it cannot reveal the progressive pathological changes of the disease.25 The APP/PS1 transgenic mouse model of AD, however, expresses a human/mouse chimeric amyloid precursor protein and human Presenilin-1, leading to chronic Aβ deposition, neuroinflammation, and cognitive impairment, simulating the main pathological changes in the brain in AD.15 Previous studies have indicated that chronic Aβ deposition stimulates the persistent activation of microglial cells in AD.26 Increased expression of inflammatory factors suggest that activation of the innate immune system may contribute to AD pathogenesis.27 Indeed, microglial activation may elicit AD progression, and several therapeutic strategies have been developed to treat this disease by targeting this.17 The benefits of exercise, physical activity, and environmental enrichment on improvement of neurodegenerative and aging disorders have been well-documented.28,29 Exercise can exert anti-inflammatory and inhibitory effects on microglial activation. In our study, we assessed the protective effects of exercise on neuroinflammation and cognitive ability in APP/PS1 transgenic AD mice. It is well-established that Aβ is a pathogenetic peptide in AD.8,30 Here, we measured the expression of Aβ1−42 because it has amyloidogenic characteristics (is more prone to aggregation).1,31,32 Consistent with previous studies, our data show that the Aβ1−42 levels were significantly higher in the AD model than WT mice, but this was reduced by physical exercise. The activation of Aβ stimulates NLRP3 to produce inflammatory factors and causes an inflammatory response in the brain tissue. Previous studies have reported that NLRP3 expression is upregulated in AD patients.33 Therefore, we investigated whether exercise could reduce neuroinflammation and improve cognitive deficits that may be mediated by inhibiting NLRP3 signaling. Reducing NLRP3 signaling would decrease the release of IL-1β to prevent or weaken the inflammatory response. Interestingly, the expression of the cytokine IL-18 showed no significant difference between different treatment groups. The mechanisms underlying microglial activation may be related to the formation of the NLRP3 inflammasome, a multiprotein complex that is composed of an NLRP3 sensor, an apoptosis-associated speck-like protein containing a caspase-recruitment domain adaptor, and a Caspase-1 enzyme,34 but require further studies. Activated microglia cause the release of cytokines, chemokines, reactive oxygen species, and neurotoxic products which are involved in neuronal and synaptic damage.35 The anti-inflammatory effects of exercise training may be related to indirectly protecting hippocampal neurons from damage by decreasing the expression of NLRP3. Our data demonstrate that neuronal damage occurs concomitantly with neuroinflammation in the hippocampus in AD mice, but can be mitigated by exercise training (Fig. 4). Neuroinflammation and neuronal damage can cause cognitive impairment. Hippocampus-dependent spatial learning/memory and working memory functions were evaluated by the MWM and RAM tests, which are important assessment tools for rodents.20,21 The results show cognitive deficits caused by the AD model, which were improved in mice given physical exercise, which suggest that physical exercise can positively affect learning and memory traits (Fig. 1). There is evidence to suggest that changes in the expression of inflammatory factors are specifically associated with microglial activation and advanced cognitive decline.36,37 Our results indicate that the deficits in learning and memory abilities in AD mice could effectively be rescued by long-term exercise, which is could be mediated, at least in part, by the suppression of the mechanistic target of the NLRP3 pathway.
Fig. 4.
Diagram of the proposed mechanism.
5. Conclusions
In summary, our study demonstrates that long-term exercise training can attenuate neuroinflammation and improve cognitive deficits, possibly by inhibiting NLRP3 signaling to indirectly protected hippocampal neurons from damage in transgenic APP/PS1 mice. This suggests that exercise training could be beneficial for people diagnosed with or at risk of developing AD.
Authors’ contributions
Xue Li: Writing - Review & Editing, Conceptualization; Yu Jin: Writing - Original Draft, Visualization; Xianyi Ding: Data Curation; Tongyang Zhu: Completion of the experiment; Changling Wei: Modification; Li Yao: Methodology.
Funding
This work is supported by the Sports Medicine Key Laboratory of Sichuan Province/Sports Medicine Key Laboratory of State Sport General Administration (Grant No. 2023-A015), the Innovative Project of Sports Medicine and Health Institute/Zheng Huaixian Bone and Trauma Research Institute (Grant No. CX21A02), the "14th Five Year Plan" Scientific Research and Innovation Team of Chengdu Sport University (Grant No. 23CXTD02).
Submission statement
All authors have read and agree with manuscript content. The manuscript is only communicated to the Journal, the manuscript will not be submitted elsewhere for review and publication.
Ethical approval statement for animal use
All experimental protocols about animals were approved by the Laboratory Animal Ethics Committee at Chengdu Sports University and were performed in accordance with the National Guidance for Animal Experiments (approval number 202113).
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships related to this work.
Acknowledge Statement
The authors have no acknowledgments to report.
References
- 1.Masters C.L., Bateman R., Blennow K., Rowe C.C., Sperling R.A., Cummings J.L. Alzheimer's disease. Nat Rev Dis Prim. 2015;1 doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart M., Snyder H.M., Carrillo M.C., Fazio S., Kim H., Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Calsolaro V., Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 2016;12(6):719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Pierre W.C., Smith P.L.P., Londono I., Chemtob S., Mallard C., Lodygensky G.A. Neonatal microglia: the cornerstone of brain fate. Brain Behav Immun. 2017;59:333–345. doi: 10.1016/j.bbi.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Sarlus H., Heneka M.T. Microglia in Alzheimer's disease. J Clin Invest. 2017;127(9):3240–3249. doi: 10.1172/JCI90606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M.M., Miao D., Cao X.P., Tan L., Tan L. Innate immune activation in Alzheimer's disease. Ann Transl Med. 2018;6(10):177. doi: 10.21037/atm.2018.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngandu T., Lehtisalo J., Solomon A., et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 8.Scheltens P., Blennow K., Breteler M.M., et al. Alzheimer's disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 9.Petzinger G.M., Fisher B.E., McEwen S., Beeler J.A., Walsh J.P., Jakowec M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. 2013;12(7):716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lourenco M.V., Frozza R.L., de Freitas G.B., et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med. 2019;25(1):165–175. doi: 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horowitz A.M., Fan X., Bieri G., et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369(6500):167–173. doi: 10.1126/science.aaw2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenzuela P.L., Castillo-García A., Morales J.S., et al. Exercise benefits on Alzheimer's disease: State-of-the-science. Ageing Res Rev. 2020;62 doi: 10.1016/j.arr.2020.101108. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Wang L., Zhang S., Hu X., Yang H., Xi L. Timing-dependent protection of swimming exercise against d-galactose-induced aging-like impairments in spatial learning/memory in rats. Brain Sci. 2019;9(9):236. doi: 10.3390/brainsci9090236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheffer D.D.L., Latini A. Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta, Mol Basis Dis. 2020;1866(10) doi: 10.1016/j.bbadis.2020.165823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heneka M.T., Kummer M.P., Stutz A., et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halle A., Hornung V., Petzold G.C., et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y.S., Tan Z.X., Wu L.Y., Dong F., Zhang F. The involvement of NLRP3 inflammasome in the treatment of Alzheimer's disease. Ageing Res Rev. 2020;64 doi: 10.1016/j.arr.2020.101192. [DOI] [PubMed] [Google Scholar]
- 18.Haneklaus M., O'Neill L.A. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 19.Baker E.J., Gleeson T.T. The effects of intensity on the energetics of brief locomotor activity. J Exp Biol. 1999;202(Pt 22):3081–3087. doi: 10.1242/jeb.202.22.3081. [DOI] [PubMed] [Google Scholar]
- 20.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei J., Kohler J., Winter Y., et al. Automated radial 8-arm maze: a voluntary and stress-free behavior test to assess spatial learning and memory in mice. Behav Brain Res. 2020;381 doi: 10.1016/j.bbr.2019.112352. [DOI] [PubMed] [Google Scholar]
- 22.Baerends E., Soud K., Folke J., et al. Modeling the early stages of Alzheimer's disease by administering intracerebroventricular injections of human native Aβ oligomers to rats. Acta Neuropathol Commun. 2022;10(1):113. doi: 10.1186/s40478-022-01417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faucher P., Mons N., Micheau J., Louis C., Beracochea D.J. Hippocampal injections of oligomeric amyloid β-peptide (1-42) induce selective working memory deficits and long-lasting alterations of ERK signaling pathway. Front Aging Neurosci. 2016;7:245. doi: 10.3389/fnagi.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo-Flores Guzmán B., Elizabeth Chaffey T., Hansika Palpagama T., et al. The interplay between beta-amyloid 1-42 (Aβ1-42)-Induced hippocampal inflammatory response, p-tau, vascular pathology, and their synergistic contributions to neuronal death and behavioral deficits. Front Mol Neurosci. 2020;13 doi: 10.3389/fnmol.2020.552073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H.Y., Lee D.K., Chung B.R., Kim H.V., Kim Y. Intracerebroventricular injection of amyloid-β peptides in normal mice to acutely induce alzheimer-like cognitive deficits. J Vis Exp. 2016;109 doi: 10.3791/53308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prinz M., Priller J., Sisodia S.S., Ransohoff R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14(10):1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 27.Heneka M.T., Golenbock D.T., Latz E. Innate immunity in Alzheimer's disease. Nat Immunol. 2015;16(3):229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 28.Meng Q., Lin M.S., Tzeng I.S. Relationship between exercise and Alzheimer's disease: a narrative literature review. Front Neurosci. 2020;14:131. doi: 10.3389/fnins.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W.W., Zhang X., Huang W.J. Role of physical exercise in Alzheimer's disease. Biomed Rep. 2016;4(4):403–407. doi: 10.3892/br.2016.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S.H., Lee D.K., Shin J., et al. Nec-1 alleviates cognitive impairment with reduction of Aβ and tau abnormalities in APP/PS1 mice. EMBO Mol Med. 2017;9(1):61–77. doi: 10.15252/emmm.201606566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finch M.S., Bagit A., Marko D.M. Amyloid beta 42 oligomers induce neuronal and synaptic receptor dysfunctions. J Physiol. 2020;598(17):3545–3546. doi: 10.1113/JP280038. [DOI] [PubMed] [Google Scholar]
- 32.Nirmalraj P.N., List J., Battacharya S., et al. Complete aggregation pathway of amyloid β (1-40) and (1-42) resolved on an atomically clean interface. Sci Adv. 2020;6(15) doi: 10.1126/sciadv.aaz6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saresella M., La Rosa F., Piancone F., et al. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer's disease. Mol Neurodegener. 2016;11:23. doi: 10.1186/s13024-016-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y., Sheng H., Bao Q., Wang Y., Lu J., Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175–186. doi: 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Birch A.M., Katsouri L., Sastre M. Modulation of inflammation in transgenic models of Alzheimer's disease. J Neuroinflammation. 2014;11:25. doi: 10.1186/1742-2094-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Miguel Z., Khoury N., Betley M.J., et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 2021;600(7889):494–499. doi: 10.1038/s41586-021-04183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sominsky L., De Luca S., Spencer S.J. Microglia: key players in neurodevelopment and neuronal plasticity. Int J Biochem Cell Biol. 2018;94:56–60. doi: 10.1016/j.biocel.2017.11.012. [DOI] [PubMed] [Google Scholar]