Abstract
Exercise has well-characterized therapeutic benefits in the management of type 2 diabetes mellitus (T2DM). Most of the beneficial effects of exercise arise from the impact of nuclear factor erythroid 2 related factor-2 (Nrf2) activation of glucose metabolism. Nrf2 is an essential controller of cellular anti-oxidative capacity and circadian rhythms. The circadian rhythm of Nrf2 is influenced by circadian genes on its expression, where the timing of exercise effects the activation of Nrf2 and the rhythmicity of Nrf2 and signaling, such that the timing of exercise has differential physiological effects. Exercise in the evening has beneficial effects on diabetes management, such as lowering of blood glucose and weight. The mechanisms responsible for these effects have not yet been associated with the influence of exercise on the circadian rhythm of Nrf2 activity. A better understanding of exercise-induced Nrf2 activation on Nrf2 rhythm and signaling can improve our appreciation of the distinct effects of morning and evening exercise. This review hypothesizes that activation of Nrf2 by exercise in the morning, when Nrf2 level is already at high levels, leads to hyperactivation and decrease in Nrf2 signaling, while activation of Nrf2 in the evening, when Nrf2 levels are at nadir levels, improves Nrf2 signaling and lowers blood glucose levels and increases fatty acid oxidation. Exploring the effects of Nrf2 activators on rhythmic signaling could also provide valuable insights into the optimal timing of their application, while also holding promise for timed treatment of type 2 diabetes.
Abbreviations
- AMPK
AMP-activated protein kinase
- ARE
Antioxidant response element
- BMAL1
Brain and muscle Arnt-like protein-1
- CLOCK
Circadian Locomotor Output Cycles Kaput
- CREB
cAMP response element binding protein
- CRY1/2
Cryptochrome 1 and 2
- FOXo3
Forkhead box O-3
- GBE1
Glycogen branching enzyme 1
- GLP-1
Glucagon-like peptide 1
- GSIS
Glucose stimulated insulin secretion
- h
hours
- HIF-1;
Hypoxia inducible factor-1α
- IL-6
Interleukin-6
- KEAP1
Kelch-like ECH-associated protein 1
- Maf
Musculoaponeurotic fibrosarcoma
- Nrf2
Nuclear factor erythroid 2–related factor 2
- PER1
Period 1
- PER2
Period 2
- PGAM5
Phosphoglycerate mutase/protein phosphatase 5
- Phka1
Phosphorylase kinase regulatory subunit alpha 1
- PPAR-δ
Peroxisome proliferator-activated receptors-δ
- UCP-2
Uncoupling protein-2
1. Introduction
Exercise rearranges cellular metabolism in a variety of tissues, particularly in contracting skeletal muscles to increase energy metabolism.1 Lifestyle interventions that incorporate increased physical activity are important preventive approaches in the management of metabolic diseases such as obesity and type 2 diabetes mellitus (T2DM).2 Apart from the ability to increase energy expenditure, exercise also modulates glucose homeostasis to benefit diabetic patients by affecting a variety of metabolic pathways. For example, exercise decreases blood levels of visfatin, an adipokine with pro-inflammatory and insulin signaling inhibitory activities.3, 4, 5 Exercise activates Nrf2, which also modulate circadian/metabolic genes (e.g. AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor-delta (PPAR-δ), and cryptochrome 1 and 2 (CRY1/2) that leads to the regulation of metabolic processes such as increase in glucose and fatty acid metabolism.6, 7, 8
Exercise guidelines generally recommend exercise volume, frequency, and intensity that are effective in promoting health and preventing or improving metabolic diseases,9,10 but rarely suggest the optimal time of exercise, because there is insufficient evidence on the biological effects of exercise timing. The award of the 2017 Nobel Prize in Physiology or Medicine for the elucidation of the molecular mechanisms of biological clocks further stimulated research in the circadian regulation of cellular function. While sports science focuses on the type and intensity of exercise, chrono-exercise aims to maintain and improve health by considering optimal times for exercise.
The optimal timing of exercise depends on the rate and type of metabolism, which is determined by the biological clock, the organisms' natural timing devices that regulate the cycle of circadian rhythms. The biological clock is important in forming a rhythm of approximately 24 hours (h) per day, and disruption of the biological clock contributes to various metabolic abnormalities, including obesity and diabetes.11 Oxidative stress coupled with low levels of Nrf2 (which are shared components of the pathophysiology of T2DM) both exhibit diurnal variations,12, 13, 14 where exercise-induced Nrf2 activation is likely to influence the diurnal variations. Nrf2 is a transcription factor that regulates an array of detoxifying and antioxidant genes. Nrf2 is highly regulated at different levels, including by transcription and epigenetic modifications. Exercise increases the transcription of Nrf2 by brain and muscle Arnt-like protein-1 (BMAL1) gene activation. In response to various cellular stressors, Nrf2 governs a cluster of antioxidant defense genes binding to antioxidant response elements (AREs) located in their promoter regions.15 When the cell is not stressed, Nrf2 binds to the KEAP1/Cul3 ubiquitin ligase complex and is continuously ubiquitinated, leading to a short half-life.16 Exposure to cellular stress, such as moderate exercise, produces ROS which modifies disulfide bonds in the cysteine residues in KEAP1, so hindering its association with Nrf2. The free Nrf2 then translocates into the nucleus to form a heterodimer with the small protein Maf (Nrf2-Maf).17,18
Time-dependent exercise has different outcomes,19 as shown by the greater increases in endurance capacity gained by evening exercise compared to morning exercise,20 and the better glycemic control and weight control in obese and overweight individuals due to evening exercise.21, 22, 23, 24, 25 (Table 1) Moderate to vigorous exercise is more effective in the evening where it reduced insulin resistance by 25%.26 Exercise in the afternoon by patients at risk of diabetes experienced superior benefits on peripheral insulin sensitivity, fasting plasma glucose levels, exercise performance, increased fatty acid oxidation, and fat mass loss.27,28 While morning exercise leads to an increase in blood glucose concentration.21,25 In 1981, Schmidt proposed the term “dawn phenomenon” to describe the abrupt elevation of blood glucose levels that spontaneously occur in the morning.29 The rise in blood glucose following morning exercise has been linked to the extension of this phenomenon, likely caused by elevated cortisol levels. However, it is worth noting that plasma cortisol levels only experience a slight increase with morning exercise whereas they double after evening exercise, which is associated with lower blood glucose.30 These observations suggest that factors beyond cortisol alone contribute to the elevation of blood glucose following morning exercise.
Table 1.
Effects of morning and evening exercise on metabolism.
| Study | Study Aim | Outcomes |
|
|---|---|---|---|
| Morning | Evening | ||
| Savikj et al.21 | Effect of morning and evening exercise on blood glucose levels | Morning exercise increased glucose concentrations. | Evening exercise reduced glucose concentrations |
| Moholdt et al.24 | |||
| Toghi-Eshghi et al.25 | |||
| Syeda et al.22 | |||
| Kim et al.28 | |||
| Mancilla et al.27 | Effect of exercise on weight loss and free fat mass | Weight loss is lower and free fat mass is higher | Weight loss is higher and free fat level is lower |
| Hill et al.20 | Effect of timed exercise on endurance and muscle mass | Lower muscle mass and endurance capacity | Higher endurance capacity and muscle capacity |
| Fan et al.31 | |||
While the advantages of evening exercise appear to be favorable for individuals with metabolic impairments, the exact distinction between the effects of morning and evening exercise remain unclear. Nrf2, which regulates metabolism and its circadian rhythmicity, is also activated by exercise. The effect of activated Nrf2 on the normal rhythm of Nrf2 can affect Nrf2 signaling to impact glucose metabolism especially in T2DM. Morning activation of Nrf2 by exercise is postulated to induce hyperactivation of Nrf2, which subsequently leads to a decrease in Nrf2 signaling. Conversely, exercise in the evening activates Nrf2 levels and signaling when they are typically low to enhance glucose and fatty acid metabolism.
Nrf2 modulates cellular oxidative stress by its ability to control the basal and induced expression of an array of antioxidant enzymes by binding to the antioxidant response element (ARE) in the promoter region of their genes. This greatly impacts pancreatic function. Given the association between diabetes and oxidative stress, there has been a growing interest in exploring Nrf2 activation as a potential novel approach in managing T2DM.32, 33, 34, 35, 36 Furthermore, Nrf2 can independently regulate several circadian genes such as interleukin-6 (IL-6), (which also plays a crucial role on host defenses by stimulating acute phase responses and immune reactions) as well as PPAR-δ and AMPK,37 which are major regulators of metabolism. This review discusses the circadian rhythmicity of Nrf2 and how exercise at different times of the day can modulate Nrf2 signaling and expression of metabolic genes, to modulate a differential effect of timed exercise.
1.1. Nrf2 and circadian rhythms
Biological clocks provide a constant cycle (biological rhythm) that regulates various physiological functions, including digestion, absorption, metabolism, endocrine processes,38 and pathological functions such as cell death and cardiovascular diseases.39 Biological clocks in mammals, including humans, are broadly classified into a central clock in the suprachiasmatic nucleus (SCN, which resides in the microscopic neuronal nucleus in the hypothalamus of the brain), and a peripheral clock in other brain regions and all tissues in the body (such as liver, kidney, adipose tissue, and skeletal muscle). Photic (light) stimulation is the most important stimulus for central clock synchronization, where the photic signal from the retina is transmitted to the SCN, and synchronization is initiated by promoting the transcription of Per1 and Per2 (Period1 and Period2) genes through phosphorylation of cAMP response element binding protein (CREB). Circadian regulation of peripheral organs is maintained by the central clock and a set of genes forming a transcriptional autoregulatory feedback loop that includes CLOCK, BMAL1 (stimulatory), and PER1, PER2, CRY1, and CRY2 (inhibitory). Peripheral organs are also regulated by stimuli other than the central clock (e.g., exercise and diet),40 via a negative feedback loop involving the transcription and translation of clock genes in cells.
The Nrf2/ARE pathway is regulated by the circadian clock, which also helps in the management of diurnal variations in oxidative stress.41,42 One of the main circadian genes, BMAL1, binds to the promoter region of the Nrf2 gene through an E-BOX element resulting in rhythmic activation of Nrf2. Other pathways can mediate the circadian rhythm by impacting Nrf2 levels through BMAL1 genes, such as sirtuin 1/BMAL1, HIF-1α/BMAL1 (in response to oxygen levels), FOXO3/BMAL1 (activated by insulin).43, 44, 45 The rhythmic activation of Nrf2 is confirmed by cyclic changes in ARE-regulated anti-oxidative stress proteins such that when Nrf2 levels are at a circadian nadir, the expression of antioxidants such as NQO1, GCLM, and HO1 are also at minimum levels.14,41 A negative feedback mechanism of circadian rhythm is accomplished by the binding of Nrf2 to specific enhancer regions of the core clock repressor gene CRY1 and 2, leading to increased CRY1 and 2 expression and repressed CLOCK/BMAL1-regulated E-box transcription. Together these data indicate that Nrf2 and the clock form an interlocking loop that integrates cellular redox signals into tissue-specific circadian rhythmicity. The rhythmicity of Nrf2 leads to a timing-induced differential effect of Nrf2 activators,46 as demonstrated by a greater effect of sulforaphane in preventing pulmonary fibrosis when applied at the nadir of Nrf2 levels when oxidative stress are at their highest levels.47 The redox regulation of circadian rhythms in cardiovascular health has led to proposed preventive strategies through "chrono" therapy.48
1.2. Nrf2 and metabolism
The biological clock systems collectively regulate several metabolic targets, such as insulin sensitivity, insulin secretion, cholesterol synthesis, fat synthesis, oxidation, and energy expenditure which can be rhythmically linked with Nrf2 activation throughout the 24-h daily cycle.49
Nrf2 contributes to the maintenance of glucose homeostasis in vivo by many mechanisms, one of which is the protection of pancreatic β cells from oxidative stress and improving peripheral tissue glucose utilization.50 Activation of Nrf2 in skeletal muscles enhances the transcription of GBE1 and Phka1, resulting in reduced muscle glycogen content and increased glucose uptake and leading to improved glucose tolerance.51
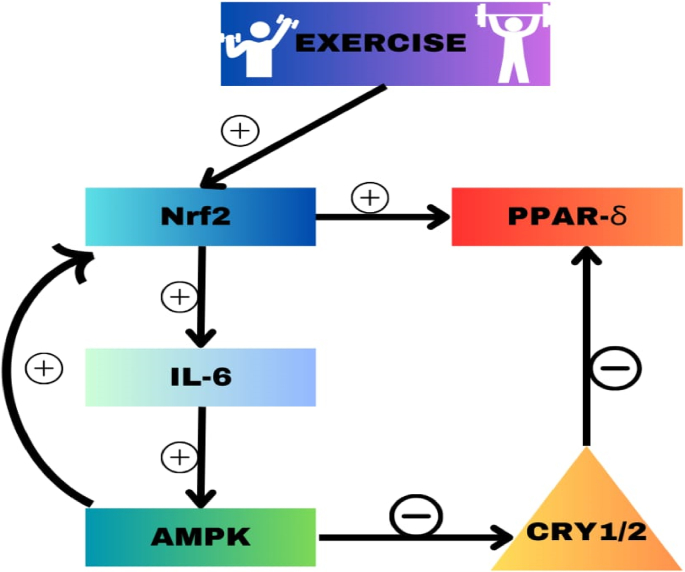
Nrf2 also improves glucose uptake in skeletal muscles by enhancing the transcription of IL-6,52 to indirectly activate AMPK via IL-6 during exercise53 (Fig. 1). Nrf2 positively regulates fatty acid oxidation by a variety of mechanisms, one of which is the stimulation of carnitine palmitoyltransferase 1 (CPT1) and CD36 to ease the passage of fatty acids into the mitochondria for oxidation.53,54 Similarly, Nrf2 influences PPARα, and PPARβ/δ which have roles in the expression of fatty acid oxidation enzymes in skeletal muscle.55, 56, 57, 58 Increased Nrf2 signaling in the mouse liver is associated with repression of lipogenesis.59 While Nrf2 activates NADPH production through the upregulation of pentose phosphate pathway, it also maintains the NADPH level by suppressing NADPH consuming processes of which lipid biosynthesis is a major one.
Fig. 1.
Exercise activates nuclear factor erythroid 2-related factor 2 (Nrf2) in skeletal muscles and binds to the antioxidant response element (ARE) region in the promoter region of the interleukin 6 (IL-6) gene, leading to increased transcription and increased IL-6 protein which in turn causes the activation of AMP-activated protein kinase (AMPK). AMPK is also directly activated by exercise and activates Nrf2 through a feedback loop. AMPK negatively regulates phosphoglycerate mutase/protein phosphatase 5 (PGAM5) which increases NRF2 signaling.
In summary Nrf2 activation by exercise is a robust zeitgeber (external regulator),60,61 that modulates glucose metabolism and fatty acid oxidation and fat synthesis to improve the management of T2DM (Table 2).
Table 2.
Metabolic effect of circadian genes.
| Gene | Activity |
|---|---|
| IL-6 |
|
| Nrf-2 |
|
| CRY 1/2 |
|
| PPAR- δ |
|
| AMPK |
|
Abbreviation: IL-6 = interleukin-6, Nrf-2 = Nuclear factor E2 related factor-2, CRY1/2 = Cryptochrome 1and 2, PPAR-δ = Peroxisome proliferator-activated receptors-δ, AMPK = AMP-activated protein kinase.
1.3. Exercise and Nrf2 signaling in skeletal muscle
Exercise in humans is affected by the time of day in which it is performed, typically falling into morning or evening chronotype.62,63 Tissue sensitivity and response to exercise vary according to the time of day, indicating that the expression of purported molecular clock genes may be related to the time of exercise.64,65 Exercise performed at different times of day influences skeletal muscle metabolic pathways and endurance capacity. Thus, the timing of exercise may favor a close alignment between tissue clocks and promote coherent and efficient temporal gating of metabolic processes.
One of the main outcomes of contraction on skeletal muscle is the activation of Nrf2, which stimulates the transcription of IL-6 and regulates glucose and fatty acid metabolism.66 (Fig. 1) IL-6 is the most abundant myokine produced and released by skeletal muscle fibers in response to muscle contraction.67 IL-6 promotes pancreatic α-cell expansion and improves insulin secretion and hyperglycemia by stimulating glucagon-like peptide 1 (GLP-1) secretion from intestinal L cells and pancreatic α cells.68,69
IL-6 also activates AMPK (Fig. 1) to stimulate glucose uptake and lipid oxidation to produce energy,70 while turning off energy-consuming processes including glucose and lipid production to restore energy balance. AMPK controls whole-body glucose homeostasis by regulating metabolism in multiple peripheral tissues, such as skeletal muscle, liver, adipose tissues, and pancreatic β cells.71 AMPK also activates the Nrf2/HO-1 pathway, revealing a strong interaction between cellular redox and energy/metabolism. Genetic ablation and pharmacological inhibition of AMPK downgrades Nrf2-dependent HO-1 expression.72 In addition to IL-6 induced activation of AMPK, AICAR monophosphate (ZMP), which is also exercise induced also activates AMPK in a time-dependent manner with peak activation in the later part of the day. The summation of these effects is higher levels of AMPK with evening exercise compared to morning exercise (Table 4).73 Since the metabolic consequences of AMPK activation are stimulation of glycolysis and inhibition of lipid synthesis as well as activation of fatty acid oxidation,74 variations in AMPK level results in high blood glucose levels after morning exercise and lower level of glucose with evening exercise.
Table 4.
Summary of the impact of morning and afternoon exercise on metabolism.
| Parameters | Morning exercise | Afternoon exercise |
|---|---|---|
| Nrf2 signaling | Low | High |
| Glucose metabolism | Low | High |
| Fatty acid metabolism | Low | High |
| Fat synthesis | High | Low |
Abbreviation: Nrf2= Nuclear factor E2 related factor-2.
AMPK also influences fatty acid metabolism by activating peroxisome proliferator-activated receptor-delta (PPAR-δ) by AMPK-dependent phosphorylation of PGC1α,75 and degradation of CRY1/2 which is responsible for metabolizing PPAR-δ (Fig. 2). Activation of PPAR-δ regulates fatty acid uptake, transport, and β-oxidation as well as insulin secretion and sensitivity.76 Ligand activation of PPAR-δ in muscle cells switches energy production from glycolysis to fatty acid oxidation as an alternative energy source to enhance muscle endurance.31 Activation of PPAR-δ in skeletal muscle cells increases fatty acid uptake and catabolism via β-oxidation.77 PPAR-δ is expressed by pancreatic islet cells to enhance the secretion of insulin,78, 79, 80 and PPAR-δ reduces insulin resistance by regulating hepatic and peripheral energy substrate utilization.81,82 (Table 2) Evening exercise serving as a non-pharmacological activator of Nrf2 and PPAR- δ, reduces oxidative stress to enhances pancreatic secretion of insulin.83
Fig. 2.
Circadian gene expression for metabolism in the pancreas and skeletal muscle. Morning exercise lowers Nrf2 signaling due to low AMP-activated protein kinase (AMPK) and high cyptochrome 1/2 (CRY1/2) levels, causing low levels of activation of peroxisome proliferator-activated receptors-δ
(PPAR-δ). Evening exercise leads to high nuclear factor erythroid 2–related factor 2 (Nrf2) signaling and high AMPK and low CRY1/2 levels and increases in the activation of PPAR-δ. AMPK activates NRF2 through a feedback loop.
1.4. Morning vs. evening exercise and Nrf2 rhythmicity and signaling
Nrf2 enhances glucose and fatty acid oxidation and improves glucose tolerance through several mechanisms. However, it is surprising that morning exercise which activates Nrf2 also increases blood glucose levels and lowers fatty acid oxidation compared to evening exercise (Table 2).
Exercise is a zeitgeber that activates Nrf2 in skeletal muscles and other tissues,84, 85, 86 but the effect of exercise timing on Nrf2 activation and signaling is poorly described. Morning exercise appears to result in diminished Nrf2 signaling in comparison to evening exercise, as indicated by the evidence of genes under Nrf2 control. The expression level of BMAL1 serves as a direct indicator of Nrf2 levels,87 as BMAL1 typically binds to the Nrf2/ARE promoter through the E-box element to directly regulate the transcription of Nrf2.88(Fig. 3 and 4) The impact on the rhythm of clock gene expression was assessed by measuring BMAL1 gene expression following a single session of moderate-intensity endurance exercise using a bicycle ergometer. The exercise was conducted at two different time points: 7:00 a.m. (morning exercise) and 4:00 p.m. (evening exercise). The findings were then compared to individuals who did not engage in any exercise.89 Individuals who did not engage in exercise demonstrated a peak expression of BMAL1 at 3:00 p.m. In contrast, those who performed morning exercise exhibited a peak expression at 9:00 a.m., which remained at a steady plateau for 6 h before undergoing a rapid decline. (Fig. 3). The sustained activation of the Nrf2 pathway with morning exercise can result in reduced Nrf2 signaling and disrupt the balance of redox reactions, leading to the initiation of detrimental oxidative stress.90,91 Others studies also demonstrates that the response to repetitive Nrf2 signaling was diminished, suggesting an adaptive process taking place.92 Following activation, Nrf2 exhibits oscillations between the cytoplasm and nucleus, with the cytoplasmic refresh rate of Nrf2 playing a crucial role in modulating the transcriptional response, particularly during persistent activation.93 The mitochondrial phosphatase PGAM5 play a role in reducing the refresh rate of Nrf2 by interacting with the KEAP-Nrf2 complex. This interaction leads to the sequestration of Nrf2 within the mitochondria, thereby repressing Nrf2-dependent signaling.94 Inhibition or silencing of PGAM5 leads to a robust activation of Nrf2. Intriguingly, AMP-activated protein kinase (AMPK) can negatively regulate PGAM5 by reducing its expression. This regulation by AMPK contributes to the modulation of Nrf2 activity.95 Hence, engaging in morning exercise, which is associated with decreased activation of AMPK, may lead to an elevation in PGAM5 levels, resulting in reduced Nrf2 signaling. Conversely, evening exercise, characterized by increased AMPK activation could decrease PGAM5 levels and enhance Nrf2 signaling (as illustrated in Fig. 1). The impact of Nrf2 signaling can be confirmed by examining the levels of substances whose transcription is facilitated by Nrf2 as IL-6 and antioxidants. Following morning exercise, the effects of Nrf2 on these agents may be diminished due to persistent activation, leading to increased oxidative stress, as indicated by elevated levels of malondialdehydes.90 To further verify the reduced Nrf2 signaling following exercise, studies have shown that high-intensity exercise which depicts persistent activation of Nrf2 leads to decrease levels of superoxide dismutase (SOD) after exercise because SOD levels are regulated by Nrf2 transcription.96,97
Fig. 3.
Illustration of the effect of morning exercise on brain and muscle Arnt-like protein-1 (BMAL1) gene expression adapted from Tanaka et al.89Morning exercise at 7:00 a.m. shifts the peak expression to 9:00 a.m. from 3:00 p.m. followed by a plateau for 6 h before declining rapidly.
Following evening exercise, there was a gradual and consistent rise in BMAL1 expression, reaching the highest point of the experiment around 6:00 p.m., followed by a decline to its lowest level at 6:00 a.m. the following morning (as depicted in Fig. 4). The elevated levels of IL-6 observed after evening exercise, compared to morning exercise are likely attributed to the increased Nrf2 signaling in the evening.28 Nrf2 serves as a transcription factor by binding to the promoter region known as the antioxidant response element (ARE).98 The IL-6 gene consists of an ARE (ARE 5′-TGACXXXGC-3′) in the promoter region. Findings from Nrf2 knock-out mice demonstrate that Nrf2 is a potent activator of IL-6 gene transcription in vivo. 98Exercise triggers NRF2 activation, resulting in a significant elevation of plasma IL-6 levels, up to 100-fold as well as an increase in glucose consumption.
Fig. 4.
Illustration of the effect of evening exercise on brain and muscle Arnt-like protein-1 (BMAL1) expression adapted from Tanaka et al.89 Evening at exercise at 4:00 p.m. extends the peak to 6:00 p.m. before declining.
Following morning exercise, AMPK levels are typically lower, which can be attributed to the diminished stimulatory effect of IL-6 on AMPK activation. However exercise itself can directly activate AMPK due to the energy-depleted state induced.99,100 AMPK in turn, activates Nrf2 through phosphorylation (Fig. 1), highlighting the interconnected and cooperative nature of AMPK and Nrf2 signaling in restoring cellular homeostasis.101,102 Consequently, the disruption of Nrf2 signaling after morning exercise leads to reduced levels of IL-6 and AMPK (Table 3).
Table 3.
Exercise timing and exerkine/myokine level.
| Exerkine/myokine | Level of exerkine after morning exercise | Level of exerkine after evening exercise |
|---|---|---|
| Nrf2 signaling | Low | High |
| IL-6 | Low | High |
| AICAR | Low | High |
| AMPK | Low | High |
| CRY1/2 | High | Low |
| PPAR-δ | Low | High |
| PGAM5 | High | Low |
Abbreviation: Nrf2= Nuclear factor E2 related factor-2, IL-6 = interleukin-6, AICAR = 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, AMPK = AMP-activated protein kinase, CRY1/2 = Cryptochrome 1/2 = PPAR-δ = Peroxisome proliferator-activated receptors-δ, PGAM5 = mitochondrial serine/threonine-protein phosphatase.
1.5. Benefits of evening exercise on pancreatic function
The initial evidence of circadian rhythms in glucose metabolism was documented during the 1960s.103 Studies indicated that glucose tolerance in humans was generally lower in the evening compared to the morning.104,105 The diurnal variation in glucose tolerance is remarkably significant, with adults who exhibit normal glucose in the morning potentially displaying metabolic profiles similar to those of individuals with prediabetes in the evening.106,107 Oral glucose tolerance studies conducted on individuals with prediabetes reveal that blood glucose levels are approximately 40 mg/dL higher in the evening compared to the morning in non-diabetic patients. These elevated evening levels of blood glucose are comparable to those observed in individuals with pre-diabetes and early-stage diabetes during dinner time. Insulin sensitivity and β-cell reactivity to glucose also exhibit diurnal variations, leading to variations in glucose levels.108 The rhythmicity of blood glucose levels is related to fluctuations in Nrf2 levels, which are higher in the morning at the onset of the fed/active phase compared to the evening.109 Oxidative stress is also higher in the evening due to the low levels of Nrf2,110,111 as several antioxidant genes regulated by Nrf2 via ARE promoter regions also display a circadian rhythm.112,113
Pancreatic β cells are particularly susceptible to oxidative stress due to their high endogenous production of reactive oxygen species (ROS) and their low antioxidant capacity, suggesting that oxidative stress plays an important role in β cell activity and failure.114 Nrf2 limits oxidative damage to pancreatic β-cells by repressing apoptosis and enhancing proliferation,50 decreasing inflammation, increasing insulin secretion, and preserving β-cell mass.115 Insulin secretion follows a circadian rhythm, with higher level of glucose stimulated secretion (GSIS) in the morning compared to the evening.116 Melatonin activates the phospholipase C/IP3 and Nrf2 pathways which mobilizes Ca2+ from organelles and consequently increases insulin secretion.117,118
Levels of Nrf2 rapidly decline during the inactive phase, causing simultaneous increases in oxidative stress that eventually peaks in the early evening,13 leading to the activation of uncoupling protein-2 (UCP-2) (Fig. 5) and the uncoupling of the electron transport chain function (to reduce ATP production and insulin secretion). However, engaging in evening exercise gradually raises the level of BMAL1 (Fig. 4). This increase in BMAL1 peaks around 6:00 p.m. and then gradually decrease throughout the night.89 Consequently, Nrf2 activation increases, leading to the transcription of antioxidants and subsequently reducing oxidative stress. This reduction in oxidative stress promotes an increase in glucose stimulated insulin secretion (GSIS).
Fig. 5.
Theoretical diurnal variations in nuclear factor erythroid 2-related factor 2 (Nrf2) and oxidative stress in the pancreas. Oxidative stress activates uncoupling protein 2 (UCP2) and reduces ATP synthesis and insulin secretion at the nadir of NRF2 activation leading to low glucose stimulated insulin secretion (GSIS).
2. Future directions
To comprehend the connection between T2DM and oxidative stress more effectively, it is crucial to gain a deeper understanding of the Nrf2/KEAP/ARE pathway and its involvement in the circadian regulation of physiological functions. This pathway serves as a master antioxidant system, and any dysfunction within it is associated with the pathophysiology of diabetes and numerous complications associated with the condition. By investigating the role of the Nrf2/KEAP/ARE pathway in circadian rhythm, we can shed light on its impact on T2DM and potentially develop a timed therapeutic approaches for managing T2DM and its related complications.119
The activation of Nrf2 presents a unique and innovative strategy for managing diabetes and its associated complications.34 Exercise induces the activation of Nrf2, and its effects vary depending on whether it is performed in the morning or evening, implying that there might be a specific optimal timeframe to activate Nrf2 in patients with T2DM. Insulin secretion follows a circadian rhythm, with higher level of GSIS in the morning compared to the evening.116 The combination of exercise and Nrf2 activators leads to greater activation of Nrf2 than either exercise or Nrf2 activators alone. A combination of Nrf2 activators, exercise, and metformin reduced diabetic complications (e.g., gain weight, water, calorie intake, blood glucose, insulin, and GLUT4 content) more efficiently than each treatment.120, 121, 122 The combinations exhibited a greater impact on enhancing oxidative stress homeostasis by effectively activating Nrf2 signaling pathway and reducing the KEAP1 protein to a greater extent, but without considering the timing of Nrf2 activation.123 The safety of the long-term effects of the combination therapy that includes Nrf2 activators is complicated by the role of Nrf2 in cancer onset and treatment.124
The activation of Nrf2 has demonstrated effectiveness in modulating glucose metabolism through exercise, pharmacological means, or the circadian rhythm. However, limited attention has been given to the timed activation of Nrf2. By attributing the variation in Nrf2 level and signaling between morning and evening exercise, it becomes evident that there might be an optimal time for Nrf2 activation. Although the effects have been observed with exercise, it remain uncertain whether Nrf2 activators, with lower activation potential will exhibit similar differential effect when applied. Further studies will be necessary in the future to investigate this aspect. Additionally, considering that blood sugar levels are notably higher in the evening it may be crucial to determine the most suitable time for administering antidiabetic medication. Furthermore, additional research should be conducted to confirm the oxidative state during morning and evening exercise to establish Nrf2 signaling status.
3. Conclusion
Despite previous reports that evening exercise reduces blood glucose levels and leads to weight loss, the mechanisms of these effects are poorly understood. Comprehending the role of Nrf2 in glucose metabolism, its rhythmicity, and the impact of chrono exercise on Nrf2 signaling helps in establishing an optimal exercise time for individuals with type 2 diabetes. The hypothesis proposed in this review suggests that Nrf2 signaling is diminished after morning exercise compared to evening exercise, leading to decreased blood glucose levels and heightened fatty acid oxidation with afternoon/evening exercise. This observation sheds light on the observed benefits of evening exercise for type 2 diabetics and instills confidence in recommending exercise during that time. Furthermore, this understanding can aid in determining the ideal timing for administering Nrf2 activators which holds promise as a future treatment for diabetes.125 Chronotherapy is effective in several instances, such as timely administration of aspirin to prevent myocardial infarction.126 Lastly the study may also contribute to identifying the most effective timing for administering antidiabetic drugs to achieve maximum efficacy in individuals with type 2 diabetes.
Submission statement
All authors have read and agree with manuscript content. While this manuscript is being reviewed for this journal, the manuscript will not be submitted elsewhere for review and publication.
Authors’ contribution statement
Babatunde Fasipe provided concept and writing.
Ismail Laher participated in review and supervision.
Conflict of interest
Ismail Laher is is an editorial board member for Sports Medicine and Health Science and was not in the editorial review or the decision to publish this article. Otherwise the authors have no conflicts of interest to declare.
References
- 1.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic Biol Med. 2015;88(Pt B):147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Hawley J.A., Hargreaves M., Joyner M.J., Zierath J.R. Integrative biology of exercise. Cell. 2014;159(4):738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Heo Y.J., Choi S.E., Jeon J.Y., et al. Visfatin induces inflammation and insulin resistance via the NF-κB and STAT3 signaling pathways in hepatocytes. J Diabetes Res. 2019;2019 doi: 10.1155/2019/4021623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dakroub A., A Nasser S., Younis N., et al. Visfatin: a possible role in cardiovasculo-metabolic disorders. Cells. 2020;9(11):2444. doi: 10.3390/cells9112444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haider D.G., Pleiner J., Francesconi M., Wiesinger G.F., Müller M., Wolzt M. Exercise training lowers plasma visfatin concentrations in patients with type 1 diabetes. J Clin Endocrinol Metab. 2006;91(11):4702–4704. doi: 10.1210/jc.2006-1013. [DOI] [PubMed] [Google Scholar]
- 6.He F., Antonucci L., Karin M. Nrf2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41(4):405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteras N., Blacker T.S., Zherebtsov E.A., et al. Nrf2 regulates glucose uptake and metabolism in neurons and astrocytes. Redox Biol. 2023;62 doi: 10.1016/j.redox.2023.102672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter E.A., Ruderman N.B. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskell W.L., Lee I.M., Pate R.R., et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 10.Jakicic J.M., Otto A.D. Treatment and prevention of obesity: what is the role of exercise? Nutr Rev. 2006;64(2 Pt 2):S57–S61. doi: 10.1111/j.1753-4887.2006.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 11.Doi M. Circadian clock-deficient mice as a tool for exploring disease etiology. Biol Pharm Bull. 2012;35(9):1385–1391. doi: 10.1248/bpb.b12-00364. [DOI] [PubMed] [Google Scholar]
- 12.Wilking M., Ndiaye M., Mukhtar H., Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxidants Redox Signal. 2013;19(2):192–208. doi: 10.1089/ars.2012.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanabrocki E.L., Murray D., Hermida R.C., et al. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol Int. 2002;19(2):423–439. doi: 10.1081/cbi-120002914. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y.Q., Zhang D., Jin T., et al. Diurnal variation of hepatic antioxidant gene expression in mice. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0044237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He F., Ru X., Wen T. Nrf2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21(13):4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao S., Liu P., Luo G., et al. p97 negatively regulates Nrf2 by extracting ubiquitylated Nrf2 from the KEAP1-CUL3 E3 complex. Mol Cell Biol. 2017;37(8) doi: 10.1128/MCB.00660-16. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxidants Redox Signal. 2018;29(17):1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo D.Y., Lee S., Kim N., et al. Morning and evening exercise. Integr Med Res. 2013;2(4):139–144. doi: 10.1016/j.imr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill D.W., Leiferman J.A., Lynch N.A., Dangelmaier B.S., Burt S.E. Temporal specificity in adaptations to high-intensity exercise training. Med Sci Sports Exerc. 1998;30(3):450–455. doi: 10.1097/00005768-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Savikj M., Gabriel B.M., Alm P.S., et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019;62(2):233–237. doi: 10.1007/s00125-018-4767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syeda U.A., Battillo D., Visaria A., Malin S.K. The importance of exercise for glycemic control in type 2 diabetes. Am J Med Open. 2023;9 doi: 10.1016/j.ajmo.2023.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creasy S.A., Wayland L., Panter S.L., et al. Effect of morning and evening exercise on energy balance: a pilot study. Nutrients. 2022;14(4):816. doi: 10.3390/nu14040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moholdt T., Parr E.B., Devlin B.L., Debik J., Giskeødegård G., Hawley J.A. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: a randomised trial. Diabetologia. 2021;64(9):2061–2076. doi: 10.1007/s00125-021-05477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toghi-Eshghi S.R., Yardley J.E. Morning (Fasting) vs afternoon resistance exercise in individuals with type 1 diabetes: a randomized crossover study. J Clin Endocrinol Metab. 2019;104(11):5217–5224. doi: 10.1210/jc.2018-02384. [DOI] [PubMed] [Google Scholar]
- 26.van der Velde J.H.P.M., Boone S.C., Winters-van Eekelen E., et al. Timing of physical activity in relation to liver fat content and insulin resistance. Diabetologia. 2023;66(3):461–471. doi: 10.1007/s00125-022-05813-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancilla R., Brouwers B., Schrauwen-Hinderling V.B., Hesselink M.K.C., Hoeks J., Schrauwen P. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol Rep. 2021;8(24) doi: 10.14814/phy2.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H.K., Furuhashi S., Takahashi M., et al. Late-afternoon endurance exercise is more effective than morning endurance exercise at improving 24-h glucose and blood lipid levels. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.957239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt M.I., Hadji-Georgopoulos A., Rendell M., Margolis S., Kowarski A. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care. 1981;4(6):579–585. doi: 10.2337/diacare.4.6.579. [DOI] [PubMed] [Google Scholar]
- 30.Kanaley J.A., Weltman J.Y., Pieper K.S., Weltman A., Hartman M.L. Cortisol and growth hormone responses to exercise at different times of day. J Clin Endocrinol Metab. 2001;86(6):2881–2889. doi: 10.1210/jcem.86.6.7566. [DOI] [PubMed] [Google Scholar]
- 31.Fan W., Waizenegger W., Lin C.S., et al. PPARδ promotes running endurance by preserving glucose. Cell Metabol. 2017;25(5):1186–1193.e4. doi: 10.1016/j.cmet.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson R.P. Nrf2 and antioxidant response in animal models of type 2 diabetes. Int J Mol Sci. 2023;24(4):3082. doi: 10.3390/ijms24043082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David J.A., Rifkin W.J., Rabbani P.S., Ceradini D.J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res. 2017;2017 doi: 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Haan J.B. Nrf2 activators as attractive therapeutics for diabetic nephropathy. Diabetes. 2011;60(11):2683–2684. doi: 10.2337/db11-1072. 10.2337/db11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiménez-Osorio A.S., González-Reyes S., Pedraza-Chaverri J. Natural Nrf2 activators in diabetes. Clin Chim Acta. 2015;448:182–192. doi: 10.1016/j.cca.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Crunkhorn S. Deal watch: abbott boosts investment in Nrf2 activators for reducing oxidative stress. Nat Rev Drug Discov. 2012;11(2):96. doi: 10.1038/nrd3655. [DOI] [PubMed] [Google Scholar]
- 37.Torrente L., DeNicola G.M. Targeting Nrf2 and its downstream processes: opportunities and challenges. Annu Rev Pharmacol Toxicol. 2022;62:279–300. doi: 10.1146/annurev-pharmtox-052220-104025. [DOI] [PubMed] [Google Scholar]
- 38.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabinovich-Nikitin I., Lieberman B., Martino T.A., Kirshenbaum L.A. Circadian-regulated cell death in cardiovascular diseases. Circulation. 2019;139(7):965–980. doi: 10.1161/CIRCULATIONAHA.118.036550. [DOI] [PubMed] [Google Scholar]
- 40.Tahara Y., Aoyama S., Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci. 2017;67(1):1–10. doi: 10.1007/s12576-016-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Q., Zeng C., Du L., Dong C. Mechanism of circadian regulation of the Nrf2/ARE pathway in renal ischemia-reperfusion. Exp Ther Med. 2021;21(3):190. doi: 10.3892/etm.2021.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bevinakoppamath S., Ramachandra S.C., Yadav A.K., Basavaraj V., Vishwanath P., Prashant A. Understanding the emerging link between circadian rhythm, Nrf2 pathway, and breast cancer to overcome drug resistance. Front Pharmacol. 2022;12 doi: 10.3389/fphar.2021.719631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaves I., van der Horst G.T., Schellevis R., et al. Insulin-FOXO3 signaling modulates circadian rhythms via regulation of clock transcription. Curr Biol. 2014;24(11):1248–1255. doi: 10.1016/j.cub.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Jo Early, Menon D., Wyse C.A., et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via Nrf2. Proc Natl Acad Sci U S A. 2018;115(36):E8460–E8468. doi: 10.1073/pnas.1800431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asher G., Gatfield D., Stratmann M., et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 46.Kitakaze T., Makiyama A., Yamashita Y., Ashida H. Low dose of luteolin activates Nrf2 in the liver of mice at start of the active phase but not that of the inactive phase. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pekovic-Vaughan V., Gibbs J., Yoshitane H., et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28(6):548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daiber A., Frenis K., Kuntic M., et al. Redox regulatory changes of circadian rhythm by the environmental risk factors traffic noise and air pollution. Antioxidants Redox Signal. 2022;37(10-12):679–703. doi: 10.1089/ars.2021.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li M.D., Li C.M., Wang Z. The role of circadian clocks in metabolic disease. Yale J Biol Med. 2012;85(3):387–401. [PMC free article] [PubMed] [Google Scholar]
- 50.Yagishita Y., Fukutomi T., Sugawara A., et al. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes. 2014;63(2):605–618. doi: 10.2337/db13-0909. [DOI] [PubMed] [Google Scholar]
- 51.Uruno A., Yagishita Y., Katsuoka F., et al. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol Cell Biol. 2016;36(11):1655–1672. doi: 10.1128/MCB.01095-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glund S., Deshmukh A., Long Y.C., et al. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes. 2007;56(6):1630–1637. doi: 10.2337/db06-1733. [DOI] [PubMed] [Google Scholar]
- 53.Herzig S., Shaw R.A.M.P.K. Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka Y., Ikeda T., Yamamoto K., Ogawa H., Kamisako T. Dysregulated expression of fatty acid oxidation enzymes and iron-regulatory genes in livers of Nrf2-null mice. J Gastroenterol Hepatol. 2012;27(11):1711–1717. doi: 10.1111/j.1440-1746.2012.07180.x. [DOI] [PubMed] [Google Scholar]
- 55.Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812(8):1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 57.Ito K., Carracedo A., Weiss D., et al. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18(9):1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka T., Yamamoto J., Iwasaki S., et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100(26):15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slocum S.L., Skoko J.J., Wakabayashi N., et al. Keap1/Nrf2 pathway activation leads to a repressed hepatic gluconeogenic and lipogenic program in mice on a high-fat diet. Arch Biochem Biophys. 2016;591:57–65. doi: 10.1016/j.abb.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva B.S.A., Uzeloto J.S., Lira F.S., et al. Exercise as a peripheral circadian clock resynchronizer in vascular and skeletal muscle aging. Int J Environ Res Publ Health. 2021;18(24) doi: 10.3390/ijerph182412949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gabriel B.M., Zierath J.R. Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol. 2019;15(4):197–206. doi: 10.1038/s41574-018-0150-x. [DOI] [PubMed] [Google Scholar]
- 62.Aoyama S., Shibata S. Time-of-day-dependent physiological responses to meal and exercise. Front Nutr. 2020;7:18. doi: 10.3389/fnut.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blazer H.J., Jordan C.L., Pederson J.A., et al. Effects of time-of-day training preference on resistance-exercise performance. Res Q Exerc Sport. 2021;92(3):492–499. doi: 10.1080/02701367.2020.1751032. [DOI] [PubMed] [Google Scholar]
- 64.Martin R.A., Esser K.A. Time for exercise? Exercise and its influence on the skeletal muscle clock. J Biol Rhythm. 2022;37(6):579–592. doi: 10.1177/07487304221122662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chow L.S., Gerszten R.E., Taylor J.M., et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25(22):5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffman N.J., Parker B.L., Chaudhuri R., et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metabol. 2015;22(5):922–935. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellingsgaard H., Hauselmann I., Schuler B., et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellingsgaard H., Ehses J.A., Hammar E.B., et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A. 2008;105(35):13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daval M., Foufelle F., Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574(Pt 1):55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long Y.C., Zierath J.R. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116(7):1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmermann K., Baldinger J., Mayerhofer B., Atanasov A.G., Dirsch V.M., Heiss E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis--A role for the unfolded protein response. Free Radic Biol Med. 2015;88(Pt B):417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ezagouri S., Zwighaft Z., Sobel J., et al. Physiological and molecular dissection of daily variance in exercise capacity. Cell Metabol. 2019;30(1):78–91.e4. doi: 10.1016/j.cmet.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Srivastava R.A., Pinkosky S.L., Filippov S., Hanselman J.C., Cramer C.T., Newton R.S. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res. 2012;53(12):2490–2514. doi: 10.1194/jlr.R025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jäger S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y., Colby J.K., Zuo X., Jaoude J., Wei D., Shureiqi I. The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J Mol Sci. 2018;19(11):3339. doi: 10.3390/ijms19113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holst D., Luquet S., Nogueira V., Kristiansen K., Leverve X., Grimaldi P.A. Nutritional regulation and role of peroxisome proliferator-activated receptor delta in fatty acid catabolism in skeletal muscle. Biochim Biophys Acta. 2003;1633(1):43–50. doi: 10.1016/s1388-1981(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 78.Iglesias J., Barg S., Vallois D., et al. PPARβ/δ affects pancreatic β cell mass and insulin secretion in mice. J Clin Invest. 2012;122(11):4105–4117. doi: 10.1172/JCI42127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang T., Abbott M.J., Ahmadian M., Lopes A.B., Wang Y., Sul H.S. Desnutrin/ATGL activates PPARδ to promote mitochondrial function for insulin secretion in islet β cells. Cell Metabol. 2013;18(6):883–895. doi: 10.1016/j.cmet.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li L., Li T., Zhang Y., et al. Peroxisome proliferator-activated receptorβ/δ activation is essential for modulating p-Foxo1/Foxo1 status in functional insulin-positive cell differentiation. Cell Death Dis. 2015;6(4) doi: 10.1038/cddis.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee C.H., Olson P., Hevener A., et al. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006;103(9):3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peeters A., Baes M. Role of PPARα in hepatic carbohydrate metabolism. PPAR Res. 2010;2010 doi: 10.1155/2010/572405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravnskjaer K., Frigerio F., Boergesen M., Nielsen T., Maechler P., Mandrup S. PPARdelta is a fatty acid sensor that enhances mitochondrial oxidation in insulin-secreting cells and protects against fatty acid-induced dysfunction. J Lipid Res. 2010;51(6):1370–1379. doi: 10.1194/jlr.M001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fasipe B., Li S., Laher I. Harnessing the cardiovascular benefits of exercise: are Nrf2 activators useful? Sports Med Health Sci. 2021;3(2):70–79. doi: 10.1016/j.smhs.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Done A.J., Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitaoka Y. The role of Nrf2 in skeletal muscle on exercise capacity. Antioxidants. 2021;10(11):1712. doi: 10.3390/antiox10111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J., Moulik M., Fang Z., et al. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol Cell Biol. 2013;33(11):2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chhunchha B., Kubo E., Singh D.P. Clock Protein Bmal1 and Nrf2 cooperatively control aging or oxidative response and redox homeostasis by regulating rhythmic expression of Prdx6. Cells. 2020;9(8):1861. doi: 10.3390/cells9081861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tanaka Y., Ogata H., Kayaba M., et al. Effect of a single bout of exercise on clock gene expression in human leukocyte. J Appl Physiol. (1985) 2020;128(4):847–854. doi: 10.1152/japplphysiol.00891.2019. [DOI] [PubMed] [Google Scholar]
- 90.Lian C.Y., Chu B.X., Xia W.H., Wang Z.Y., Fan R.F., Wang L. Persistent activation of Nrf2 in a p62-dependent non-canonical manner aggravates lead-induced kidney injury by promoting apoptosis and inhibiting autophagy. J Adv Res. 2023;46:87–100. doi: 10.1016/j.jare.2022.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan R.F., Tang K.K., Wang Z.Y., Wang L. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology. 2021;464 doi: 10.1016/j.tox.2021.152999. [DOI] [PubMed] [Google Scholar]
- 92.Bischoff L.J.M., Kuijper I.A., Schimming J.P., et al. A systematic analysis of Nrf2 pathway activation dynamics during repeated xenobiotic exposure. Arch Toxicol. 2019;93(2):435–451. doi: 10.1007/s00204-018-2353-2. [DOI] [PubMed] [Google Scholar]
- 93.Xue M., Momiji H., Rabbani N., et al. Frequency modulated translocational oscillations of Nrf2 mediate the antioxidant response element cytoprotective transcriptional response. Antioxidants Redox Signal. 2015;23(7):613–629. doi: 10.1089/ars.2014.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang M.Z., Ke T.L., Chen L. Mitochondrial protein PGAM5 emerges as a new regulator in neurological diseases. Front Mol Neurosci. 2021;14 doi: 10.3389/fnmol.2021.730604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y.S., Yu P., Wang Y., et al. AMP-activated protein kinase protects against necroptosis via regulation of Keap1-PGAM5 complex. Int J Cardiol. 2018;259:153–162. doi: 10.1016/j.ijcard.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parker L., Trewin A., Levinger I., Shaw C.S., Stepto N.K. Exercise-intensity dependent alterations in plasma redox status do not reflect skeletal muscle redox-sensitive protein signaling. J Sci Med Sport. 2018;21(4):416–421. doi: 10.1016/j.jsams.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 97.Souza A.V., Giolo J.S., Teixeira R.R., et al. Salivary and plasmatic antioxidant profile following continuous, resistance, and high-Intensity interval exercise: preliminary study. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/5425021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wruck C.J., Streetz K., Pavic G., et al. Nrf2 induces interleukin-6 (IL-6) expression via an antioxidant response element within the IL-6 promoter. J Biol Chem. 2011;286(6):4493–4499. doi: 10.1074/jbc.M110.162008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jansen T., Kvandová M., Schmal I., et al. Lack of Endothelial α1AMPK reverses the vascular protective effects of exercise by causing eNOS uncoupling. Antioxidants. 2021;10(12) doi: 10.3390/antiox10121974. 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Neill H.M. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J. 2013;37(1):1–21. doi: 10.4093/dmj.2013.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matzinger M., Fischhuber K., Pölöske D., Mechtler K., Heiss E.H. AMPK leads to phosphorylation of the transcription factor Nrf2, tuning transactivation of selected target genes. Redox Biol. 2020;29 doi: 10.1016/j.redox.2019.101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petsouki E., Cabrera S.N.S., Heiss E.H. AMPK and Nrf2: interactive players in the same team for cellular homeostasis? Free Radic Biol Med. 2022;190:75–93. doi: 10.1016/j.freeradbiomed.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 103.Jarrett R.J., Keen H. Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br Med J. 1969;2(5653):341–344. doi: 10.1136/bmj.2.5653.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poggiogalle E., Jamshed H., Peterson C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11–27. doi: 10.1016/j.metabol.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qian J., Dalla Man C., Morris C.J., Cobelli C., Scheer F.A.J.L. Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metabol. 2018;20(10):2481–2485. doi: 10.1111/dom.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jarrett R.J., Baker I.A., Keen H., Oakley N.W. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. Br Med J. 1972;1(5794):199–201. doi: 10.1136/bmj.1.5794.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carroll K.F., Nestel P.J. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22(5):333–348. doi: 10.2337/diab.22.5.333. [DOI] [PubMed] [Google Scholar]
- 108.Van Cauter E., Polonsky K.S., Scheen A.J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18(5):716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 109.Seshadri N., Doucette C.A. Circadian regulation of the pancreatic beta cell. Endocrinology. 2021;162(9):bqab089. doi: 10.1210/endocr/bqab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rigas A.N., Bittles A.H., Hadden D.R., Montgomery D.A. Circadian variation of glucose, insulin, and free fatty acids during long-term use of oral hypoglycaemic agents in diabetes mellitus. Br Med J. 1968;4(5622):25–28. [PMC free article] [PubMed] [Google Scholar]
- 111.Malherbe C., De Gasparo M., De Hertogh R., Hoet J.J. Circadian variations of blood sugar and plasma insulin levels in man. Diabetologia. 1969;5(6):397–404. doi: 10.1007/BF00427978. [DOI] [PubMed] [Google Scholar]
- 112.Panda S., Antoch M.P., Miller B.H., et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 113.Xu Y.Q., Zhang D., Jin T., et al. Diurnal variation of hepatic antioxidant gene expression in mice. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0044237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gurgul-Convey E., Mehmeti I., Plötz T., Jörns A., Lenzen S. Sensitivity profile of the human EndoC-βH1 beta cell line to proinflammatory cytokines. Diabetologia. 2016;59(10):2125–2133. doi: 10.1007/s00125-016-4060-y. [DOI] [PubMed] [Google Scholar]
- 115.Baumel-Alterzon S., Katz L.S., Brill G., Garcia-Ocaña A., Scott D.K. Nrf2: the master and captain of beta cell fate. Trends Endocrinol Metab. 2021;32(1):7–19. doi: 10.1016/j.tem.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boden G., Ruiz J., Urbain J.L., Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271(2 Pt 1):E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 117.Bona S., Fernandes S.A., Moreira A.C.J., et al. Melatonin restores zinc levels, activates the Keap1/Nrf2 pathway, and modulates endoplasmic reticular stress and HSP in rats with chronic hepatotoxicity. World J Gastrointest Pharmacol Therapeut. 2022;13(2):11–22. doi: 10.4292/wjgpt.v13.i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sharma S., Singh H., Ahmad N., Mishra P., Tiwari A. The role of melatonin in diabetes: therapeutic implications. Arch Endocrinol Metab. 2015;59(5):391–399. doi: 10.1590/2359-3997000000098. [DOI] [PubMed] [Google Scholar]
- 119.Mohan T., Narasimhan K.K.S., Ravi D.B., et al. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: therapeutic prospect of epigallocatechin-3-gallate. Free Radic Biol Med. 2020;160:227–238. doi: 10.1016/j.freeradbiomed.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 120.Wang X., Chen Y., Abdelkader D., Hassan W., Sun H., Liu J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J Diabetes Res. 2015;2015 doi: 10.1155/2015/973287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neerati P., Devde R., Gangi A.K. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother Res. 2014;28(12):1796–1800. doi: 10.1002/ptr.5201. [DOI] [PubMed] [Google Scholar]
- 122.Kumar R., Negi P.S., Singh B., Ilavazhagan G., Bhargava K., Sethy N.K. Cordyceps sinensis promotes exercise endurance capacity of rats by activating skeletal muscle metabolic regulators. J Ethnopharmacol. 2011;136(1):260–266. doi: 10.1016/j.jep.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 123.Rahimi G., Heydari S., Rahimi B., et al. A combination of herbal compound (SPTC) along with exercise or metformin more efficiently alleviated diabetic complications through down-regulation of stress oxidative pathway upon activating Nrf2-Keap1 axis in AGE rich diet-induced type 2 diabetic mice. Nutr Metab. 2021;18(1):14. doi: 10.1186/s12986-021-00543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bai X., Chen Y., Hou X., Huang M., Jin J. Emerging role of Nrf2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab Rev. 2016;48(4):541–567. doi: 10.1080/03602532.2016.1197239. [DOI] [PubMed] [Google Scholar]
- 125.Matzinger M., Fischhuber K., Heiss E.H. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol Adv. 2018;36(6):1738–1767. doi: 10.1016/j.biotechadv.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bonten T.N., Saris A., van Oostrom M.J., et al. Effect of aspirin intake at bedtime versus on awakening on circadian rhythm of platelet reactivity. A randomised cross-over trial. Thromb Haemost. 2014;112(6):1209–1218. doi: 10.1160/TH14-05-0453. [DOI] [PubMed] [Google Scholar]