Abstract
Helicobacter hepaticus infection in A/JCr mice results in chronic active hepatitis characterized by perivascular, periportal, and parenchymal infiltrates of mononuclear and polymorphonuclear cells. This study examined the development of hepatitis and the immune response of A/JCr mice to H. hepaticus infection. The humoral and cell-mediated T helper immune response was profiled by measuring the postinfection (p.i.) antibody response in serum, feces, and bile and by the production of cytokines and proliferative responses by splenic mononuclear cells to H. hepaticus antigens. Secretory immunoglobulin A (IgA) and systemic IgG2a antibody developed by 4 weeks p.i. and persisted through 12 months. Splenocytes from infected mice proliferated and produced more gamma interferon (IFN-γ) than interleukin-4 (IL-4) or IL-5 when cultured with H. hepaticus outer membrane proteins. The predominantly IgG2a antibody response in serum and the in vitro production of IFN-γ in excess of IL-4 or IL-5 are consistent with a Th1 immune response reported in humans and mice infected with Helicobacter pylori and Helicobacter felis, respectively. Mice infected with H. hepaticus developed progressively severe perivascular, periportal, and hepatic parenchymal lesions consisting of lymphohistiocytic and plasmacytic cellular infiltrates. In addition, transmural typhlitis was observed at 12 months p.i. The characterization of a cell-mediated Th1 immune response to H. hepaticus infection in the A/JCr mouse should prove valuable as a model for experimental regimens which manipulate the host response to Helicobacter.
Helicobacter hepaticus colonizes the cecum and colon in many strains of immunocompetent mice without evidence of causing overt clinical disease. H. hepaticus can also colonize the hepatobiliary system, particularly in male A/JCr mice, and can cause chronic active hepatitis, which may progress to hepatocellular adenoma and carcinoma (6, 7, 14, 24). Infected A/JCr mice develop numerous foci of perivascular, peribiliary, and parenchymal infiltrates of mononuclear cells. These lesions suggest that significant cell-mediated immune responses to H. hepaticus antigens develop within the hepatobiliary system (7, 30). Other than development of a titer of serum immunoglobulin G (IgG), little is known about the murine immune response to H. hepaticus. However, host susceptibility to hepatic lesions and persistent colonization of the liver vary by mouse strain (32), suggesting that genetic differences determine the character of the immune response and disease outcome.
The host response to H. hepaticus infection in A/JCr mice may have similarities to that of humans infected with H. pylori because both diseases are associated with persistent bacterial colonization and inflammatory lesions despite significant immune responses (7, 8, 30). Atrophic gastritis in humans infected with Helicobacter pylori (21) and chronic hepatitis in H. hepaticus-infected A/JCr mice (30) may, in part, be the result of autoimmune-like tissue damage. Humans infected with H. pylori may develop gastric mucosal atrophy related to serum IgG with specificity for gastric parietal cells (4). H. hepaticus-infected A/JCr mice have been shown to produce IgG with specificity for heat shock proteins that are expressed by both H. hepaticus and liver cells stressed by inflammation (30).
The role of cell-mediated immunity in protection against chronic colonization with Helicobacter or in the progression of lesions has not been well defined. In humans, mononuclear cells obtained from the blood of H. pylori-infected patients had a lower in vitro proliferative index to H. pylori antigens than similar cells isolated from control patients (12, 14). This suggested that H. pylori may suppress host cell-mediated immune responses by production of an inhibitory factor (15). Inhibition of cell-mediated immune responses was not found in Helicobacter felis-infected mice where mononuclear cell proliferation was positively correlated with infection (18). The immune response to H. pylori and H. felis have both been described as Th1-like because inflammatory cells produce gamma interferon (IFN-γ) in excess over interleukin-4 (IL-4) (3, 13, 18). Nothing is known about the cell-mediated immune response of A/JCr mice, which are unable to effectively eliminate H. hepaticus and subsequently develop chronic inflammatory lesions in the liver.
This study profiled the immune response of A/JCr mice experimentally infected with H. hepaticus by measuring postinfection (p.i.) IgG2a (Th1-like) and IgG1 (Th2-like) antibody responses in serum as well as secretory IgA in bile and feces. The proliferative responses of splenic mononuclear cells to H. hepaticus antigens were measured to determine the antigen sensitivity of systemic mononuclear cells. Antigen-stimulated production of IFN-γ (Th1-like) and IL-4 and IL-5 (both Th2-like) by splenic mononuclear cells was also evaluated.
MATERIALS AND METHODS
Animals.
Fifty-five male A/JCr mice that were free of viral antibody to specific murine viruses and H. hepaticus by culture and PCR were purchased from the National Cancer Institute, Frederick, Md. At the age of 6 to 8 weeks, half of the mice were infected with H. hepaticus and half served as uninfected controls (see “Bacterial inoculation”). The infected and control mice were housed in microisolator caging in separate areas within an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility. Replicate experiments were conducted with groups of the sizes indicated in the figures and tables.
Bacterial inoculation.
H. hepaticus (type strain ATCC 51448) was grown as previously described (6). Briefly, cultures were first established under microaerobic conditions at 37°C on Trypticase soy blood agar (Remel Laboratories, Lenexa, Kans.) and then inoculated into brucella broth containing 5% fetal bovine serum. After a 48-h incubation on a rotary shaker (New Brunswick Scientific, Edison, N.J.), the culture was centrifuged at 10,000 rpm (microcentrifuge 235C; Fisher Scientific, Hampton, N.H.) for 20 min at 4°C. After examination for bacterial contaminants using Gram stain and phase microscopy, the pellet was resuspended in brucella broth containing 30% glycerol to approximately 108 organisms per ml as confirmed by spectrophotometry (8). Test mice received 0.2 ml of fresh inoculum by oral gavage every other day for three doses. Controls received medium alone on the same schedule. Both the inoculum and the medium were subcultured on blood agar to confirm the purity of the H. hepaticus inoculum and the sterility of the medium.
Reisolation of H. hepaticus from feces and cecum.
Feces and cecal tissue were cultured as previously described (28) at 4 weeks p.i. and at necropsy (6 to 12 months), respectively, to confirm H. hepaticus infection of the test mice and the H. hepaticus-negative status of the controls. Duplicate cecum samples were assessed by PCR as previously described (28) to confirm the presence or absence of H. hepaticus.
Sample preparation of serum, bile, and feces for enzyme-linked immunosorbent assay (ELISA).
Sera and feces were collected from all mice prior to dosing with H. hepaticus or medium and then monthly until necropsy. For fecal collection, all mice from each cage (approximately five) were placed in an empty cage for 1 h and a pooled fecal sample was obtained by randomly selecting four average size pellets (approximately 100 mg of freshly voided feces). The feces were then suspended in a protease inhibitor cocktail [1 μg of aprotonin per ml, 10 μM leupeptin, 3.25 μM bestatin, and 0.2 mM 4-(2-aminoethyl)-benzene sulfonylfluoride (all from Sigma, St. Louis, MO.) in 5% nonfat dry milk] as previously described (10). The fecal slurry was microcentrifuged at 10,000 rpm (microcentrifuge 235C; Fisher Scientific) for 10 min to yield supernatant for IgA measurement. Mice were fasted for 12 h prior to necropsy to increase the amount of bile in the gall bladder; the bile was aspirated into 100 μl of protease inhibitor. Serum, bile, and fecal extracts were frozen at −70°C until analyzed.
ELISA for anti-H. hepaticus IgG1 and IgG2a in serum and IgA in bile and feces.
An outer membrane antigen preparation of H. hepaticus was obtained by methods previously described for preparing H. pylori antigen (23). Briefly, H. hepaticus was cultured in Trypticase soy broth containing 5% fetal bovine serum for 48 h under microaerobic conditions as detailed above. After three washes in phosphate-buffered saline (PBS) and examination for bacterial contaminants using Gram stain and phase microscopy, the pellet was resuspended in 4 ml of 1% N-octyl-β-glucopyranoside (Sigma) for 30 min at room temperature. Insoluble material was removed by ultracentrifugation at 100,000 × g for 1 h. After dialysis against PBS for 24 h at 4°C, supernatant protein concentration was measured by the Lowry technique (Sigma).
Immulon II 96-well plates (Dynax Technologies, Chantilly, Va.) were coated with 100 μl of a 10-μg/ml concentration of H. hepaticus protein in carbonate buffer (pH 9.6) per well overnight at 4°C. Serum was diluted 1:100, and both bile and fecal extracts were assayed undiluted. Biotinylated secondary antibodies included α-chain-specific goat anti-mouse IgA (Sigma) and monoclonal rat anti-mouse antibodies produced by clones G1-6.5 and R19-15 (Pharmingen, San Diego, Calif.) for detecting IgG1 and IgG2a, respectively. Incubation with extravidin peroxidase (Sigma) was followed by incubation with a 2-2′-azino-di(3-ethyl-benzthiazoline sulfonate) (ABTS) substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for color development. Optical density (OD) development at 405 nm was recorded by an ELISA plate reader (model MR7000; Dynatech Laboratories, Inc., Chantilly, Va.). Serum IgG1 and IgG2a results are reported as OD values at a sample dilution of 1:100. Because of an unknown dilution factor inherent in sample preparation, the OD measurement of IgA specific for H. hepaticus in bile and fecal extracts was standardized against total IgA concentration of the sample. A standard curve was generated on each ELISA plate by applying known amounts of purified mouse IgA(κ) (Sigma) to wells precoated with α-chain-specific sheep anti-mouse IgA. The antibody response to H. hepaticus was considered significant if the OD exceeded a value equal to the mean OD plus 3 standard deviations measured for samples from uninfected control mice.
Splenic mononuclear cell proliferation in response to H. hepaticus antigens.
Spleens harvested at necropsy were processed mechanically into single-cell suspensions. After lysis of erythrocytes by hypotonic shock and three washes in complete medium (RPMI 1640, 10% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 50 μM 2-mercaptoethanol, 10 mM HEPES, 2 mM l-glutamine [all from Sigma]), 2 × 105 viable cells were plated per well in 96-well flat-bottom microtiter plates and cultured in triplicate for 72 h at 37°C under 8% CO2. H. hepaticus antigen for use in cell culture was prepared as it was for ELISA but sterile filtered through a 0.2-μm-pore-size filter before protein determination. Antigen was added to achieve concentrations of 0.1, 1, or 10 μg/ml. To control for nonspecific mitogen activity, lipopolysaccharide (LPS) activity of the outer membrane preparation was measured with a commercial kit based on the Limulus amoebocyte lysate test for endotoxin (E-Toxate; Sigma). Wells precoated with 100 μl of a 10-μg/ml concentration of hamster anti-CD3 antibody (clone 145-2C11; Pharmingen) in PBS were used as positive controls for stimulation. Cells were pulse-labeled with 1 μCi of [3H]thymidine per well 6 h prior to cell harvesting (harvester 11028; Skatron, Sterling, Va.); this was followed by scintillation counting (model LS6800; Beckman, Irvine, Calif.). Mean counts of triplicate cultures were corrected for background and reported as a stimulation index (SI), which is the ratio of counts per minute measured in test wells divided by counts per minute generated by cells incubated in medium alone.
Production of IFN-γ IL-4, and IL-5 by splenic mononuclear cells.
Production of IFN-γ, IL-4, and IL-5 by splenic mononuclear cells was measured as previously reported (18). Briefly, 5 × 106 cells were cultured in 1-ml volumes under the same conditions as those described for measurement of proliferation. Supernatants for cytokine evaluation were harvested at 24 to 72 h poststimulation with H. hepaticus sterile protein. The presence of IFN-γ, IL-4, and IL-5 in supernatants was measured by a sandwich ELISA using monoclonal capture and biotinylated secondary antibodies produced by clones R4-6A2/XMG1.2, BVD4-1D11/BVD6-24G2, and TRFK5/TRFK4 (Pharmingen) for IFN-γ, IL-4, and IL-5, respectively. A standard curve was created by using recombinant standards for IFN-γ, IL-4, and IL-5 from Pharmingen.
Histologic evaluation.
Formalin-fixed liver and cecum tissue samples were embedded in paraffin, cut at a 5-μm width, and stained with hematoxylin and eosin for assessment of morphology and with Warthin-Starry stain to identify organisms with morphology consistent with H. hepaticus. Tissues were evaluated by a board-certified veterinary pathologist blinded to the sample identity. The liver lesions were scored on the basis of size and frequency of the lesions on a scale of 0 to 4 for ascending severity of inflammation (none, mild, moderate, and severe, respectively). Hepatic lesions consisted almost exclusively of parenchymal necrosis and inflammation, and inflammation centered on either portal areas or sublobular veins. Parenchymal necrosis and inflammation were scored as a single category that included sharply demarcated foci of hepatocyte necrosis and inflammatory or phagocytic cell accumulation and foci of hepatitis lacking clear borders. Portal or periportal inflammation and inflammation centered on sublobular veins were scored as independent categories. Sums of the three individual scores were used as total scores for hepatic lesion intensity. Cecal lesions among mice at 12 months p.i. were similarly assessed for mucosal hyperplasia and chronic inflammation.
Statistical analysis.
Data on serum, fecal, and bile antibody, cell proliferation, and cytokine responses are reported as a mean plus 1 standard deviation and were compared by using Student’s t test at a significance level of P < 0.05 for differences between groups. Analysis of liver and cecal lesion scores was performed by using the Mann-Whitney nonparametric test for categorical data.
RESULTS
Colonization and persistence of infection with H. hepaticus.
Feces were cultured at 4 weeks p.i. to confirm that mice that received H. hepaticus by oral gavage were colonized and that controls were uninfected. All of the pooled samples from the H. hepaticus-dosed mice were positive by fecal culture at 4 weeks p.i., and the controls were culture and PCR negative by methods previously described (28).
Serum IgG1 and IgG2a humoral responses to H. hepaticus.
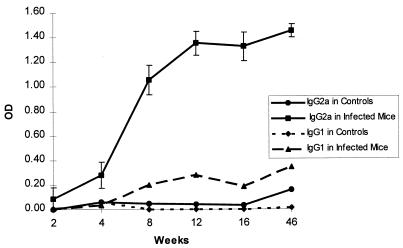
Serum IgG2a levels in infected mice significantly increased above control levels by 4 weeks p.i. (P < 0.01) (Fig. 1). The IgG2a response continued to rise through 12 weeks p.i. and was persistent at a high level up through 12 months, when the study terminated. The levels of IgG1 antibody in sera from infected mice were similar to the background levels of IgG2a or IgG1 in sera from control mice.
FIG. 1.
Anti-H. hepaticus IgG2a and IgG1 antibody levels in serum samples from experimentally infected and control mice. The level of IgG2a against H. hepaticus antigens in serum was significantly greater than that of IgG1 antibody (P < 0.01, n = 15 infected mice and 13 controls), which is consistent with a Th1 cell-mediated immune response. Anti-H. hepaticus IgG2a and IgG1 from control mice were at background levels. Each error bar represents ±1 standard deviation of the mean. Data are representative of multiple experiments.
IgA specific for H. hepaticus in feces and bile.
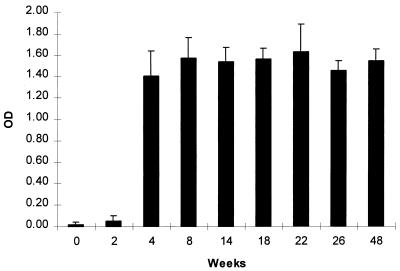
Both feces and bile collected from infected mice contained high levels of anti-H. hepaticus IgA antibody (Fig. 2). Bile from infected mice contained a mean (± standard deviation) of 3.33 ± 0.27 OD units of IgA (range, 1.15 to 3.83 OD units), whereas that from controls contained 0.89 ± 0.67 OD unit (range, 0 to 1.72). The mean level of IgA in bile samples with specificity for H. hepaticus antigens at 6 months p.i. was significantly higher than that in control samples (P < 0.02, n = 4 infected mice and 7 controls). At 2 weeks p.i. fecal IgA reactive with H. hepaticus antigens was near background levels but had clearly become significant by 4 weeks p.i. and remained elevated (P < 0.001). Feces from control mice did not have IgA reactive with H. hepaticus antigens (mean [± standard deviation] OD value of 0.02 ± 0.02). Bile IgA collected at necropsy from infected mice yielded significantly higher OD values on ELISA against H. hepaticus antigens than samples collected from controls (P < 0.002).
FIG. 2.
Anti-H. hepaticus IgA in fecal extracts from experimentally infected mice. Secretory IgA specific for H. hepaticus antigens rose rapidly by 4 weeks p.i. (P < 0.001) and remained consistently high throughout the time period studied (12 months p.i.). Values for control mice were similar to the 0 time point for infected mice (see text). Data represent assay results for five pooled fecal samples from infected mice and five pooled samples from control mice and are representative of multiple experiments. Each error bar represents 1 standard deviation of the mean.
Proliferative response of splenic mononuclear cells to H. hepaticus antigens.
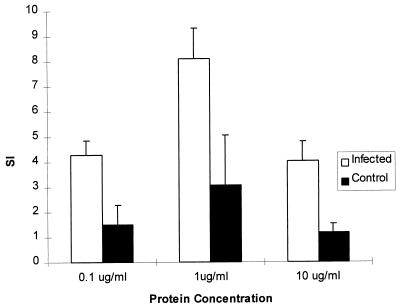
Mononuclear cells isolated from spleens of H. hepaticus-infected mice at 6 months p.i. proliferated in response to H. hepaticus antigens in vitro to a significantly greater extent than cells isolated from uninfected control mice (P < 0.001) (Fig. 3). The proliferative response of cells from infected mice was approximately threefold higher than that of control samples at each of the levels of H. hepaticus protein used to stimulate the cells in culture. The peak response to H. hepaticus protein was at a concentration of 1 μg/ml. The outer membrane preparation of antigen used to stimulate the splenocytes did not have detectable LPS activity with an assay sensitivity of 0.125 endotoxin units (EU)/ml. The positive control of plate-bound anti-CD3 antibody stimulated mononuclear cell proliferation in infected and control mice to an equivalent extent (SIs of 79 ± 18 and 85 ± 16, respectively). Proliferative responses of splenic mononuclear cells isolated from mice at 9 months p.i. were equivalent to those measured at 6 months p.i. (data not shown).
FIG. 3.
Mean SI + SD of proliferative response of splenic mononuclear cells isolated from H. hepaticus-infected and uninfected control A/JCr mice. The SI of infected mice was significantly greater than control values (P < 0.001, n = 5 infected mice and 4 controls). Data are representative of multiple experiments.
Production of IFN-γ, IL-4, and IL-5 by splenic mononuclear cells in response to H. hepaticus antigen.
Supernatants for cytokine evaluation were harvested at 24 to 72 h poststimulation with H. hepaticus outer membrane protein. Production of IFN-γ by splenic mononuclear cells isolated from mice 6 months p.i. peaked at the 72-h time point and to a protein concentration of 10 μg/ml (data not shown). IFN-γ production by cells isolated from infected mice in response to H. hepaticus protein under these optimal conditions was equivalent to that from stimulation with the positive control stimulus, concanavalin A (ConA) (434 ± 97 and 384 ± 137 pg/ml, respectively). Splenic mononuclear cells isolated from control mice released a low level of IFN-γ in comparison to a sevenfold higher level of γ-IFN released from cells obtained from infected mice (Table 1). Production of IL-4 and IL-5 was either undetectable or similar to background levels. Splenocyte cultures from control and infected mice that were stimulated with ConA as a positive control released equivalent levels of IL-5 (239 ± 112 and 303 ± 164 pg/ml, respectively). IL-4 was detectable by ELISA only when samples were artificially spiked with recombinant IL-4 to validate the assay.
TABLE 1.
Production of IFN-γ, IL-4, and IL-5 from splenic mononuclear cells stimulated with H. hepaticus antigens in culturea
| Culture | Cytokine production (pg/ml [mean ± SD])
|
||
|---|---|---|---|
| IFN-γ | IL-4 | IL-5 | |
| Medium alone | 23 ± 31 | NDb | 12 ± 16 |
| Spleen cells | |||
| Infected | 434 ± 97 | ND | 34 ± 42 |
| Control | 61 ± 51 | ND | 7 ± 9 |
Splenic mononuclear cells were isolated from mice at 9 months p.i. and then stimulated in culture for 72 h with outer membrane proteins from H. hepaticus at 10 μg/ml. IFN-γ production was significantly higher from cells from infected mice than from control cells (P < 0.006, n = 5 infected mice and 9 controls), approximately a sevenfold difference. Both IL-4 and IL-5 were essentially undetectable except for cells stimulated by ConA as a positive control for the assay (see text). Data are representative of multiple experiments.
ND, not detectable.
Histologic evaluation.
The total scores for liver lesions at 6, 9, and 12 months p.i. were significantly higher for infected mice than for controls (Table 2). An increasing trend in inflammation surrounding the sublobular veins with increased duration of infection was observed. The median scores for sublobular phlebitis in the infected mice were higher than those of controls at 9 and 12 months p.i., with significant differences at 12 months p.i. (P < 0.05). Inflammation of the sublobular veins progressed from subendothelial infiltration by mononuclear inflammatory cells to intramural and adventitial infiltration (Fig. 4). Multifocal aggregates of mixed mononuclear cells, primarily lymphocytes, formed in the vessel walls and surrounding stroma and extended into the adjacent hepatic parenchyma.
TABLE 2.
Hepatic and cecal lesion scores in control and H. hepaticus-infected micea
| Mouse group and MO p.i. | n | Median lesion score (range)
|
|||||
|---|---|---|---|---|---|---|---|
| Liver
|
Cecum
|
||||||
| Parenchymal necrosis and inflammation | Portal inflammation | Sublobular phlebitis | Total score | Mucosal hyperplasia | Inflammation | ||
| Control | |||||||
| 6 | 8 | 1 (0–1) | 0 (0) | 0 (0–2) | 1 (0–3) | NDb | ND |
| 9 | 6 | 0.5 (0–2) | 0.5 (0–1) | 0 (0–2) | 1 (0–5) | ND | ND |
| 12 | 6 | 1.5 (1–2) | 1 (0–1) | 0 (0–1) | 2.5 (1–4) | 2 (0–3) | 2 (0–2) |
| Infected mice | |||||||
| 6 | 5 | 2c (1–2) | 0 (0–1) | 0 (0–2) | 2c (1–5) | ND | ND |
| 9 | 6 | 1.5 (0–3) | 1.5c (0–3) | 2 (0–3) | 5c (2–8) | ND | ND |
| 12 | 6 | 2 (1–3) | 1 (0–4) | 2c (0–3) | 4.5c (2–10) | 2.5 (0–3) | 3d (2–4) |
Median scores (see “Histologic evaluation” in Materials and Methods) for hepatic lesions were significantly higher in infected mice than in controls at 6, 9, and 12 months p.i. The scores for chronic cecal inflammation were significantly higher (P < 0.01) in the infected mice than in the controls at 12 months p.i.
ND, not done.
P < 0.05, compared to value for control mice (by Mann-Whitney test).
P < 0.01, compared to value for control mice (by Mann-Whitney test).
FIG. 4.
Liver tissue from an A/JCr mouse at 9 months p.i. Focal infiltration is visible in the wall of a sublobular vein associated with H. hepaticus infection. The inflammatory mononuclear cell infiltrate is predominantly lymphocytic. Stain, hematoxylin and eosin. Bar, 50 μm.
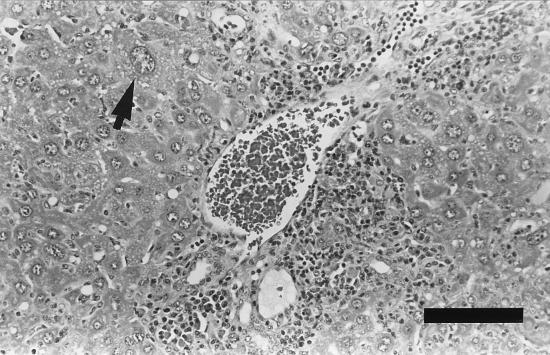
The median scores for hepatic parenchymal necrosis and inflammation were higher in the infected mice than in the controls at 6, 9, and 12 months p.i., with significant differences at 6 months p.i. (P < 0.01). Marked infiltrative inflammation and parenchymal necrosis along with severe periportal inflammation were present in infected mice at both 9 and 12 months p.i. (Fig. 5). Periportal inflammation was significantly increased among infected mice at 9 months p.i. (P < 0.05) and was comprised of mixed mononuclear cell infiltrates. The proportions of macrophages and plasma cells were higher than those of the inflammatory foci oriented around the sublobular vein. Small numbers of argyrophilic bacteria consistent with H. hepaticus were observed in two infected mice at 9 and 12 months p.i. Both mice had moderate parenchymal necrosis and inflammation, moderate to severe portal inflammation, and mild to moderate sublobular phlebitis.
FIG. 5.
Liver tissue from an A/JCr mouse at 9 months p.i. Periportal inflammation encloses the portal structures and extends into the surrounding hepatic parenchyma. The mixed mononuclear cell infiltrate is comprised of macrophages, lymphocytes, and plasma cells. Individual hepatocytes with karyomegaly (arrow) are present in mice with marked chronic hepatitis. Stain, hematoxylin and eosin. Bar, 100 μm.
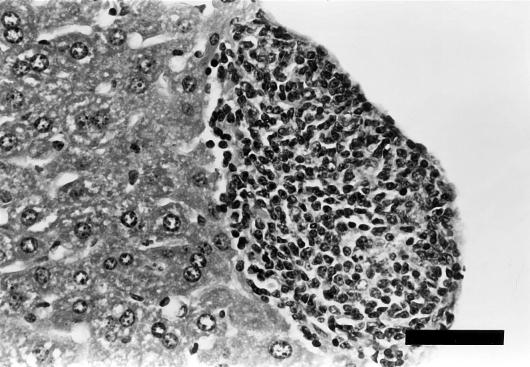
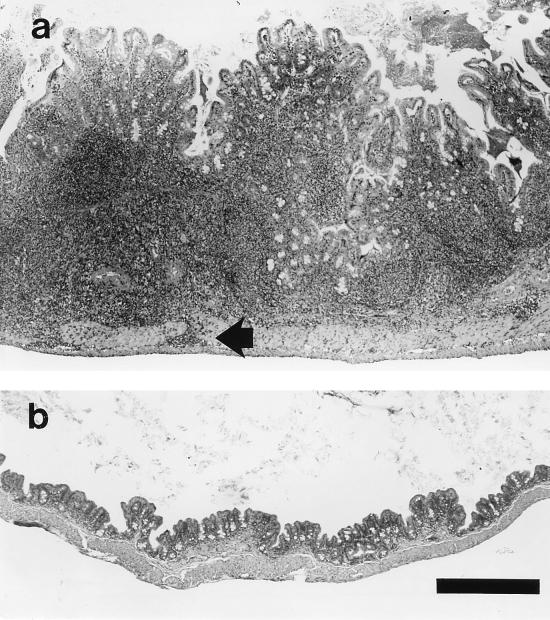
Chronic typhlitis was significantly higher (P < 0.01) in the infected mice than in the controls at 12 months p.i. (Table 2). Although the median score for cecal mucosal hyperplasia was higher for infected mice, the difference between infected and control groups was not statistically significant. One infected mouse at 12 months p.i. had marked typhlitis associated with suppuration, transmural inflammation (Fig. 6), and focal ulceration of the mucosa (data not shown).
FIG. 6.
Cecal tissue from an A/JCr mouse at 12 months p.i. (a) and from a control mouse (b). (a) Marked, multifocal thickening and inflammation of the mucosa and submucosa were observed in association with H. hepaticus infection. Multifocal extension through the tunica muscularis (arrow) and mucosal ulceration (not shown) were also observed in this case. (b) The cecal tissue from the control mouse has no significant alterations. Stain, hematoxylin and eosin. Bar, 500 μm.
DISCUSSION
The results reported here demonstrate that A/JCr mice respond to H. hepaticus with a Th1-like immune response which is consistent with observations others have made on the immune response to gastric helicobacters (3, 13, 18). The serum IgG that was reactive with H. hepaticus antigens was predominantly IgG2a in isotype, which functionally categorizes the response as Th1-like (25–27). Secretory IgA and systemic IgG2a antibodies developed rapidly in the infected mice, with high levels persisting during chronic infection. Both serum IgG2a and secretory IgA in feces from infected mice significantly increased above control levels by 4 weeks p.i. and hence were discriminatory between infected and control mice. Sera from control mice had low levels of H. hepaticus-reactive IgG1 and IgG2a that did not change in magnitude during the first 16 weeks p.i. There was an insignificant trend in elevated serum IgG2a levels in controls between the 16- and 46-week time points that may represent cross-reactivity of antibody with bacterial antigens such as urease and heat shock proteins expressed by other gut flora (18). In addition, significant levels of H. hepaticus-specific IgA were present in bile at 6 months p.i. and was most likely secreted by IgA+ cells that accumulate around the bile ducts of H. hepaticus-infected mice (16). Antigenic stimulation of these cells may have resulted from enterohepatic circulation of H. hepaticus antigens through the portal circulation or by local colonization of H. hepaticus in the biliary system. This observation suggests that the mouse liver should be included as an important component of GALT, the gut-associated lymphoid system. In some strains of mice, colonization of H. hepaticus appears to be restricted to the lower bowel and may have a commensal relationship with the host. The ability of H. hepaticus to colonize the liver in A/JCr mice may explain why the expected immune hyporesponsiveness to nonpathogenic gut flora (29) is abrogated in infected A/JCr mice and is further evidence that H. hepaticus is a true pathogen for at least select strains of mice.
Development of a predominant Th1 or Th2 cell-mediated immune response is known to be influenced by the balance of antagonistic cytokines in the local tissue environment being colonized by a given pathogen (20, 22, 27). IFN-γ, IL-4, and IL-5 have all been shown to influence B-cell isotype switching and potentiation of immunoglobulin secretion (27). Differentiation of B cells into cells committed to IgG2a secretion is associated with early cellular activation events that result in elevated levels of IFN-γ in the microenvironment of first antigen contact (25). Production of IgG1 class antibody, which was very low in the sera from infected and control mice, is regulated at least in part by IL-4 (34), which was not detectable in supernatants from A/JCr mouse splenocytes stimulated with H. hepaticus protein.
The most important evidence that A/JCr mice responded to H. hepaticus infection with a bias towards a Th1 response was the large amount of IFN-γ produced by splenocytes isolated from infected mice. The sevenfold increase in IFN-γ production suggests that antigen recognition contributed to the cellular activation events resulting in cytokine release. The excess of IFN-γ with essentially undetectable production of IL-4 and IL-5 by cell cultures established from infected mice is consistent with the mouse model of gastric colonization with H. felis (18). Natural killer and Th1 lymphocytes produce IFN-γ, which is proinflammatory and promotes production of IL-12 that further commits naive T cells to the Th1 phenotype (11, 33). IFN-γ plays an important role in inflammation and the immune response by modulating macrophage effector functions, isotype switching, and secretion of immunoglobulins by B cells, along with upregulating expression of class I and II major histocompatibility complex antigens, Fc receptors, and leukocyte adhesion molecules (19).
Splenocytes from infected mice had a significant proliferative response to H. hepaticus antigens, and these results are similar to data obtained in the H. felis model (18). As with H. felis, cells from control mice had a low proliferative response that was higher than the background level measured for cells stimulated with medium alone. The low proliferative response is most likely explained by the outer membrane preparation containing antigens shared by many bacteria. LPS in the H. hepaticus antigen preparation was negligible so that the control responses may have been to other putative mitogens such as urease. Interestingly, the results reported here and for the H. felis mouse model contrast with the suppressed proliferative response to H. pylori antigens by peripheral blood mononuclear cells isolated from H. pylori-infected humans relative to that in H. pylori-free subjects (12). The different results between the mouse and human data may relate to species, tissue source of lymphoid cells, variability in dominant antigens contained in the preparations used to stimulate the cells in vitro, or other undefined conditions of cell culture.
Median scores for hepatic lesions were significantly higher in infected mice than in controls at 6, 9, and 12 months p.i. Also, the severity of lesions progressed as the infection persisted through 12 months. Foci of parenchymal necrosis and inflammation were the most notable lesions at 6 months p.i., with progression of hepatitis to include periportal and perivascular mononuclear cell infiltrates by 9 months p.i. Typhlitis, a feature of inflammatory bowel disease, noted in the group necropsied at 12 months p.i. in this study, has been associated with H. hepaticus infection in defined-flora scid mice (1) and was described previously in older A/JCr mice naturally infected with H. hepaticus (8, 31).
Helicobacter virulence factors are antigenic (2, 5, 9, 17), and thus lesions result at least in part from the host immune response and associated inflammation. The immune response to H. hepaticus does not appear to provide significant protection against chronic colonization or disease. The proinflammatory Th1 cell-mediated response to H. hepaticus may be detrimental to the host, as suggested by others (14, 18, 21). Thus, there is significant interest in developing immunization strategies to divert the cell-mediated immune response towards a Th2 response, which may be more protective either by inhibiting colonization or by attenuation of lesion progression.
ACKNOWLEDGMENTS
We thank Zeli Shen and Shilu Xu for technical assistance.
This research was supported in part by NIH grants RO1 CA 67529, RO1 DK 52413, and RR 07036 from the National Center for Research Resources.
REFERENCES
- 1.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Censini S, Lange C, Xiang Z Y, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delios M M, Manghetti M, Decarli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Delprete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 4.Faller G, Steininger H, Kranzlein J, Maul H, Kerkau T, Hensen J, Hahn E G, Kirchner T. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut. 1997;41:619–623. doi: 10.1136/gut.41.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of Helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham D Y, Genta R M, Graham D P, Crabtree J E. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: lack of correlation of IgG antibody in patients with peptic ulcer or asymptomatic Helicobacter pylori gastritis. J Clin Pathol. 1996;49:829–832. doi: 10.1136/jcp.49.10.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayglass Y, Li, Rempel J D, Wang M D, Simons F E R. Exogenous IL-12 and directed induction of human and murine Th1-associated responses. Int Arch Allergy Immunol. 1997;113:281–283. doi: 10.1159/000237573. [DOI] [PubMed] [Google Scholar]
- 12.Karttunen R. Blood lymphocyte proliferation, cytokine secretion and appearance of T cells with activation surface markers in cultures with Helicobacter pylori. Comparison of the responses with and without antibodies to H. pylori. Clin Exp Immunol. 1991;83:396–400. doi: 10.1111/j.1365-2249.1991.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karttunen R, Karttunen T, Ekre H P, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knipp U, Birkholz S, Kaup W, Opferkuch W. Immune suppressive effects of Helicobacter pylori on human peripheral blood mononuclear cells. Med Microbiol Immunol. 1993;182:63–76. doi: 10.1007/BF00189374. [DOI] [PubMed] [Google Scholar]
- 15.Knipp U, Birkholz S, Kaup W, Opferkuch W. Partial characterization of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect Immun. 1996;64:3491–3496. doi: 10.1128/iai.64.9.3491-3496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, X., M. Whary, Z. Zhao, and J. G. Fox. 1997. Unpublished data.
- 17.Mobley H L T, Island M D, Hausinger R P. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 19.Momosaki S, Yano H, Ogasawara S, Higaki K, Hisaka T, Kojiro M. Expression of intercellular adhesion molecule 1 in human hepatocellular carcinoma. Hepatology. 1995;22:1708–1713. [PubMed] [Google Scholar]
- 20.Nakamura T, Lee R K, Nam S Y, Podack E R, Bottomly K, Flavell R A. Roles of IL-4 and IFN-gamma in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol. 1997;158:2648–2653. [PubMed] [Google Scholar]
- 21.Negrini R, Savio A, Poiesi C, Appelmelk B J, Buffoli F, Paterlini A, Cesari P, Graffeo M, Vaira D, Franzin G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111:655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- 22.Pearce E J, Reiner S L. Induction of Th2 responses in infectious diseases. Curr Opin Immunol. 1995;7:497–504. doi: 10.1016/0952-7915(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 23.Pronovost A D, Rose S L, Pawlak J W, Robin H, Schneider R. Evaluation of a new immunodiagnostic assay for Helicobacter pylori antibody detection: correlation with histopathological and microbiological results. J Clin Microbiol. 1994;32:46–50. doi: 10.1128/jcm.32.1.46-50.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice J. Helicobacter hepaticus, a recently recognized bacterial pathogen, associated with chronic hepatitis and hepatocellular neoplasia in laboratory mice. Emerg Infect Dis. 1995;1:129–131. doi: 10.3201/eid0104.950404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 26.Secord E A, Rizzo L V, Barroso E W S, Umetsu D T, Thorbecke G J, Dekruyff R H. Reconstitution of germinal center formation in nude mice with Th1 and Th2 clones. Cell Immunol. 1996;174:173–179. doi: 10.1006/cimm.1996.0307. [DOI] [PubMed] [Google Scholar]
- 27.Sedlik C. Th1 and Th2 subsets of T lymphocytes: characteristics, physiological role and regulation. Bull Inst Pasteur. 1996;94:173–200. [Google Scholar]
- 28.Shames B, Fox J G, Dewhurst F, Yan L L, Shen Z L, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shroff K E, Meslin K, Cebra J J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward J M, Benveniste R E, Fox C H, Battles J K, Gonda M A, Tully J G. Autoimmunity in chronic active Helicobacter hepatitis of mice. Serum antibodies and expression of heat shock protein 70 in liver. Am J Pathol. 1996;148:509–517. [PMC free article] [PubMed] [Google Scholar]
- 31.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 32.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 33.Xiong H, Ohya S, Tanabe Y, Mitsuyama M. Persistent production of interferon-gamma (IFN-gamma) and IL-12 is essential for the generation of protective immunity against Listeria monocytogenes. Clin Exp Immunol. 1997;108:456–462. doi: 10.1046/j.1365-2249.1997.4101301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshino S. Effect of an anti-interleukin-4 monoclonal antibody on Th1 and Th2 cell-dependent antibody production in mice. Med Sci Res. 1997;25:301–302. [Google Scholar]