Abstract
Objective
Glucagon-like peptide 1 (GLP-1) receptor agonists reduce food intake, producing remarkable weight loss in overweight and obese individuals. While much of this weight loss is fat mass, there is also a loss of lean mass, similar to other approaches that induce calorie deficit. Targeting signaling pathways that regulate skeletal muscle hypertrophy is a promising avenue to preserve lean mass and modulate body composition. Myostatin and Activin A are TGFβ-like ligands that signal via the activin type II receptors (ActRII) to antagonize muscle growth. Pre-clinical and clinical studies demonstrate that ActRII blockade induces skeletal muscle hypertrophy and reduces fat mass. In this manuscript, we test the hypothesis that combined ActRII blockade and GLP-1 receptor agonism will preserve muscle mass, leading to improvements in skeletomuscular and metabolic function and enhanced fat loss.
Methods
In this study, we explore the therapeutic potential of bimagrumab, a monoclonal antibody against ActRII, to modify body composition alone and during weight loss induced by GLP-1 receptor agonist semaglutide in diet-induced obese mice. Mechanistically, we define the specific role of the anabolic kinase Akt in mediating the hypertrophic muscle effects of ActRII inhibition in vivo.
Results
Treatment of obese mice with bimagrumab induced a ∼10 % increase in lean mass while simultaneously decreasing fat mass. Daily treatment of obese mice with semaglutide potently decreased body weight; this included a significant decrease in both muscle and fat mass. Combination treatment with bimagrumab and semaglutide led to superior fat mass loss while simultaneously preserving lean mass despite reduced food intake. Treatment with both drugs was associated with improved metabolic outcomes, and increased lean mass was associated with improved exercise performance. Deletion of both Akt isoforms in skeletal muscle modestly reduced, but did not prevent, muscle hypertrophy driven by ActRII inhibition.
Conclusions
Collectively, these data demonstrate that blockade of ActRII signaling improves body composition and metabolic parameters during calorie deficit driven by GLP-1 receptor agonism and demonstrate the existence of Akt-independent pathways supporting muscle hypertrophy in the absence of ActRII signaling.
Keywords: Obesity, Glucagon-like peptide 1 (GLP-1), Activin type II receptor (ActRII), Weight loss, Akt, Body composition
Highlights
-
•
Weight loss induced by GLP-1 receptor agonism is achieved through reductions in both lean and fat mass.
-
•
ActRII blockade reduces fat mass while simultaneously driving muscle hypertrophy.
-
•
Blockade of ActRII preserves muscle mass while enhancing fat loss during GLP-1 receptor agonism.
-
•
Skeletal muscle-specific deletion of Akt modestly reduces, but does not prevent, ActRII blockade-driven muscle hypertrophy.
1. Introduction
Obesity is a chronic, multifactorial disease that is predicted to affect more than ∼25 % of the world's population by 2035 [1]. Common lifestyle interventions including diet and increased physical activity combat obesity but often fail to produce significant and sustained weight loss [2]. Until recently, surgical procedures were considered the “gold standard” for effective weight loss intervention, yielding up to 30–40 % body weight loss, as previously approved pharmacological inhibitors rarely yielded weight loss greater than 10 % [2]. Notably, individuals that achieve significant amounts of weight loss must contend with shifts in neuroendocrine and autonomic homeostatic systems that defend the body's energy stores and favor weight regain [3]. Combating weight regain due to these biological mechanisms as well as behavioral and environmental hurdles remains a major challenge.
The discovery of incretin hormones such as glucagon-like peptide 1 (GLP-1) has illuminated the neuronal and endocrine pathways that regulate energy balance and systemic metabolism [4]. The approval of semaglutide, a potent GLP-1 receptor agonist (GLP-1RA), for weight loss represents a significant breakthrough in pharmacological interventions for managing obesity, with high-dose weekly injections shown to help individuals achieve a mean weight loss of ∼14.9 % over 68 weeks [5]. Further studies evaluating the synergistic effects of combining multiple hormones led to the development of dual GLP-1 and glucose insulinotropic peptide (GIP) receptor agonists such as tirzepatide, and the GLP-1, GIP, and glucagon receptor tri-agonist retatrutide, both showing remarkable effects on lowering body in advanced clinical studies [6,7].
The efficacy of GLP-1RAs and overall positive benefits of weight loss are remarkable and undeniable, but the challenge of long-term maintenance of this weight reduced state remains unresolved. The STEP-1 extension trial investigating the use of semaglutide in adults with obesity reports that individuals regained roughly two-thirds of the weight lost over the 52-week follow-up period after cessation of treatment [8]. This is not a phenomenon unique to GLP-1RAs—weight regain is a common outcome following periods of significant weight loss [3]. The weight loss that occurs during dieting, surgery or with GLP-1 receptor agonism is driven by significant reductions in both lean and fat mass [9]. Notably, the STEP-1 clinical trial for semaglutide noted that a significant proportion of weight loss was due to lean mass (∼40 %) [5]. Skeletal muscle is an important mediator of insulin action and glucose homeostasis and contributes substantially to energy expenditure (EE) [10]. Decreased EE following weight loss is thought to promote weight regain [11], and reductions in skeletal muscle mass and increased work efficiency are reported to contribute directly to this phenomenon [12]. Moreover, low muscle mass is an independent risk factor for all-cause and cardiovascular disease-driven mortality [13]. For these reasons, a weight loss strategy that preserves lean mass could offer long-term weight maintenance as well as metabolic and functional benefits [9].
TGFβ-like ligands myostatin (GDF-8) and Activin A are negative regulators of skeletal muscle mass. These ligands signal via the activin type II A and B receptors (ActRII) which complex with transmembrane type I activin receptor-like kinases (ALK4 or ALK5) [14]. Engagement of these receptors leads to phosphorylation of Smad proteins downstream regulation of gene expression, and decreased IGF-1 signaling via Akt [14]. Inhibition of ActRII signaling through genetic deletion of muscle ActRIIA/B, antibody blockade of ActRIIA/B, or treatment with a soluble ActRIIB decoy receptor all lead to significant increases in muscle mass [[15], [16], [17]]. Recent studies using bimagrumab, a dual-specific human monoclonal antibody targeting both ActRIIA and B, in overweight and obese humans resulted in lowering of HbA1c, a 3.6 % increase in lean mass, and a 20.5 % decrease in fat mass, which equates to overall body weight loss of 6.5 % [18]. Notably, participants’ fat mass did not significantly change 12 weeks after completion of treatment [18]. This weight loss maintenance is not observed following cessation of semaglutide [8], indicating that ActRII inhibition not only induces weight loss targeted to fat mass, but potentially improves weight loss maintenance. Taken together, these studies highlight the potential benefits of an anti-ActRII antibody alone and in combination with a GLP-1 receptor agonist to both preserve lean mass and enhance fat loss during weight loss.
Mechanistically, ActRII inhibition is thought to induce skeletal muscle hypertrophy by reducing Smad2/3 activity and elevating Akt signaling [14,16]. The PI3K/Akt/mTOR signaling axis is well known to regulate anabolic processes, including increasing protein synthesis to support muscle hypertrophy [14]. A previous study demonstrated that global ablation of either Akt1 or Akt2 alone did not prevent skeletal muscle hypertrophy induced by treatment with a soluble ActRII decoy receptor, suggesting that neither Akt isoform is required [17]. However, recent studies utilizing skeletal muscle-specific knockouts of both Akt1 and Akt2 demonstrated that deletion of both isoforms reduces both muscle size and muscle protein synthesis, indicating that only minimal amounts of Akt in muscle are needed to transmit downstream signaling and preserve muscle mass in vivo [19,20]. Several studies point to Akt as a critical node promoting muscle hypertrophy, however, our knowledge of the specific requirement of signaling through this pro-growth pathway in mediating the hypertrophic effects of ActRII inhibition is incomplete.
GLP-1RAs induce weight loss and improve metabolic health with remarkable efficiency. To further maximize weight loss strategies, an emerging goal for new anti-obesity approaches is to preserve muscle mass and enhance loss of fat mass, which may lead to longer term weight management and additional improvements in metabolic and skeletomuscular function. In this manuscript, we explore the therapeutic potential of improving body composition through ActRII blockade alone and in combination with GLP-1 receptor agonism in a pre-clinical mouse model of obesity. Furthermore, we examine the requirement of skeletal muscle Akt signaling in mediating the hypertrophic effects of ActRII blockade on skeletal muscle in vivo.
2. Results
2.1. Blockade of ActRII enhances fat mass loss and preserves lean mass during GLP-1 receptor agonism in obese mice
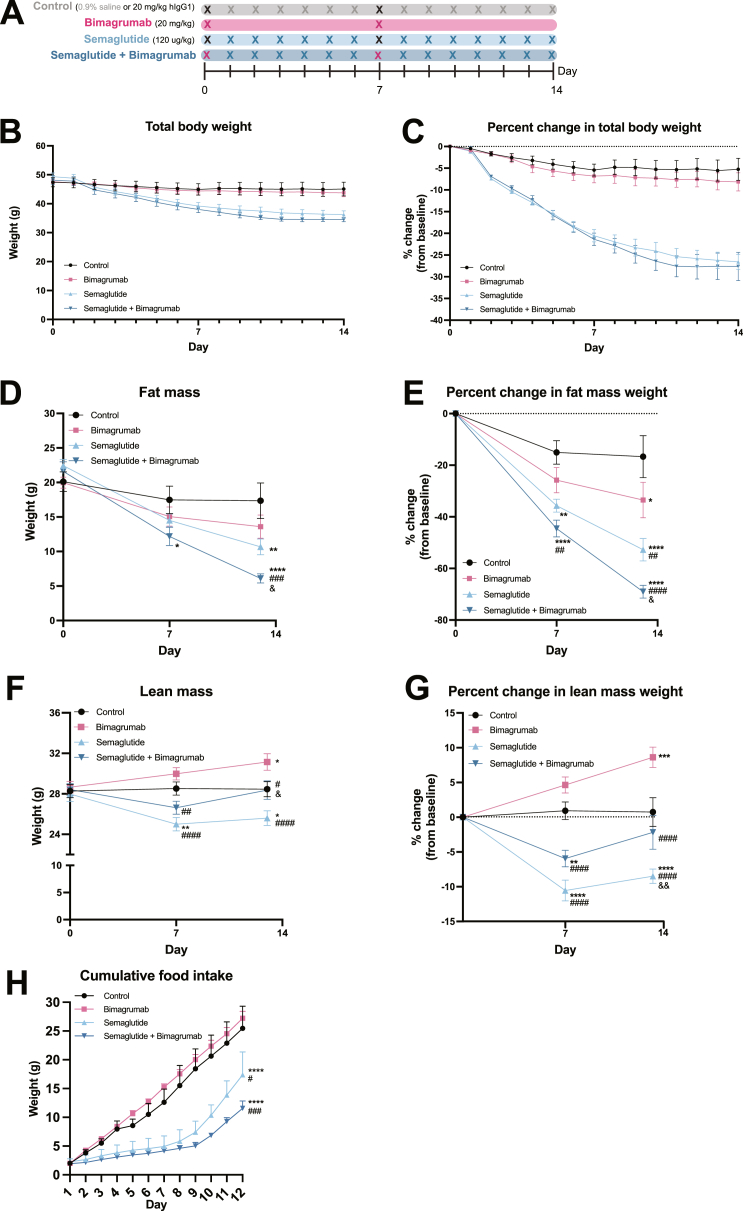
To determine the effects of antibody ActRII blockade (bimagrumab) and GLP-1 receptor agonism (semaglutide) on body composition, 25-week-old diet-induced obese mice (DIO) were obtained and maintained on a high fat diet (60 % kcal from fat). Mice received subcutaneous injection of either 120 ug/kg semaglutide or equal volume of vehicle as a control, 20 mg/kg bimagrumab or human recombinant IgG1 as a control, or a combination of semaglutide and bimagrumab over the course of 14 days (Figure 1A) Body weight was monitored daily, and body composition was assessed by EchoMRI at days 0, 7, and 13. Over 14 days of treatment, mice treated with semaglutide or semaglutide plus bimagrumab lost greater than 25 % of their body weight (Figure 1B,C). Body composition analysis revealed a ∼30 % decrease in fat mass with bimagrumab and a ∼50 % decrease with semaglutide (Figure 1D,E). Co-administration of bimagrumab and semaglutide led to 70 % fat mass loss, superior to bimagrumab or semaglutide alone (Figure 1D,E). Treatment with semaglutide alone decreased lean mass by ∼10 % compared to control animals, while bimagrumab alone induced an ∼8 % increase (Figure 1F,G). Co-treatment of bimagrumab and semaglutide preserved lean mass—the proportion of lean mass was indistinguishable between the co-treatment and control groups at day 13 (Figure 1F,G). Cumulative food intake over 12 days was assessed in a separate cohort of DIO mice using the same treatment strategy (Figure 1H). Mice treated with semaglutide and semaglutide plus bimagrumab consumed significantly less food than control and bimagrumab-treated counterparts over a 12 day period. Food consumption in the semaglutide and semaglutide plus bimagrumab increased around day 9, which is consistent with previous animal studies [21]. Overall, these data suggest that ActRII inhibition can increase lean mass and decrease fat mass alone and in combination with GLP-1 receptor agonism.
Figure 1.
ActRII inhibition enhances fat mass loss and preserves lean mass during weight loss induced by GLP-1 receptor agonism in obese mice.
A) Experimental schematic.
B) Change in total body weight (g) over 14 days.
C) Percent change in total body weight from baseline over 14 days.
D) Change in fat mass (g) determined by MRI at day 0, 7, and 13.
E) Percent change in fat mass from baseline at day 0, 7, and 13.
F) Change in lean mass (g) determined by MRI at day 0, 7, and 13.
G) Percent change in lean mass from baseline at day 0, 7, and 13.
H) Cumulative food intake over 12 days (n = 3 mice per group for days 1–4; n = 2 for control group (one mouse removed due to animal welfare complications) and n = 3 for semaglutide, bimagrumab, and semaglutide plus bimagrumab groups for days 5–12).
n = 8 mice for control and semaglutide + bimagrumab groups, n = 9 mice for semaglutide and bimagrumab groups unless otherwise indicated.∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0005, ∗∗∗∗p < 0.0001 for control vs. bimagrumab, semaglutide, and semaglutide + bimagrumab; #p < 0.05, ##p < 0.001, ###p < 0.0005, ####p < 0.0001 for bimagrumab vs. semaglutide and semaglutide + bimagrumab; & p < 0.05, && p < 0.001 for semaglutide vs. semaglutide + bimagrumab.
2.2. ActRII inhibition plus GLP-1 receptor agonism further decreases adipose tissue mass during weight loss
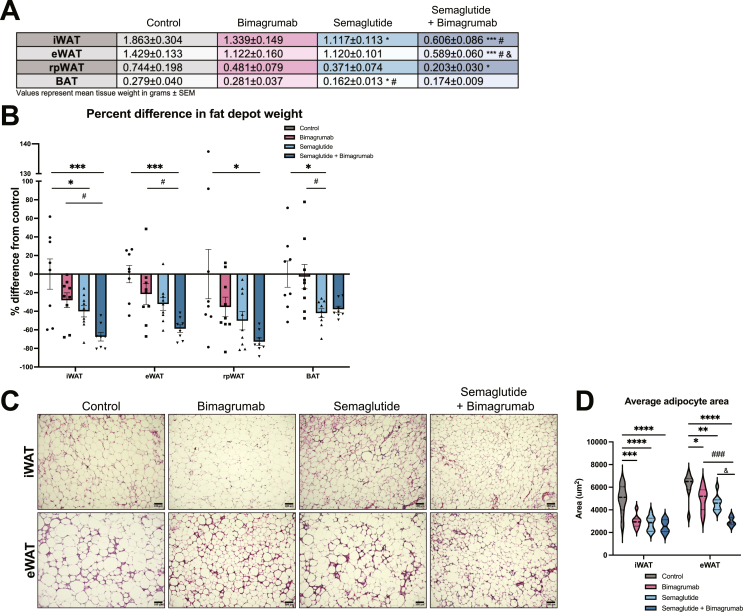
To evaluate the effect of bimagrumab and semaglutide treatment on white adipose tissue (WAT) depots, we isolated and weighed inguinal (iWAT), epididymal (eWAT), retroperitoneal (rpWAT), and brown adipose tissue (BAT) depots at 14 days. Semaglutide plus bimagrumab produced the largest reductions in eWAT, iWAT, and rpWAT size, with greater than 60 % reductions in tissue weights relative to control animals (Figure 2A,B). Similar reductions in BAT weight were observed with semaglutide and semaglutide plus bimagrumab (Figure 2A,B). Adipocyte size was quantified from hematoxylin and eosin (H&E) stained sections from eWAT and iWAT depots. Across all three treatment groups, average adipocyte size was significantly decreased compared to control animals (Figure 2C,D). Consistent with trends in overall fat mass and fat depot weights, adipocyte size was smallest in animals co-treated with semaglutide and bimagrumab (Figure 2C,D). These data demonstrate that the combination of ActRII blockade and GLP-1 receptor agonism leads to profound reductions in both adipose tissue mass and adipocyte size.
Figure 2.
ActRII inhibition plus GLP-1 receptor agonism further decreases adipose tissue mass during weight loss.
A) Weights (g) of iWAT, eWAT, rpWAT, and BAT depots isolated from HFD-fed mice treated for 14 days.
B) Percent difference in fat depot weights compared to control group.
C) Representative iWAT and eWAT sections stained with H&E (10x magnification, scale bar = 100 um).
D) Quantification of average adipocyte size in iWAT and eWAT sections (n = 8: 2 images analyzed per animal, 4 animals per group).
n = 8 mice for control and semaglutide + bimagrumab groups, n = 9 mice for semaglutide and bimagrumab groups unless otherwise indicated.∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0005, ∗∗∗∗p < 0.0001 for control vs. bimagrumab, semaglutide, and semaglutide + bimagrumab; #p < 0.05, ##p < 0.001, ###p < 0.0005, ####p < 0.0001 for bimagrumab vs. semaglutide and semaglutide + bimagrumab; & p < 0.05, && p < 0.001 for semaglutide vs. semaglutide + bimagrumab.
2.3. ActRII inhibition drives skeletal muscle hypertrophy and preserves muscle mass during weight loss induced by GLP-1 receptor agonism
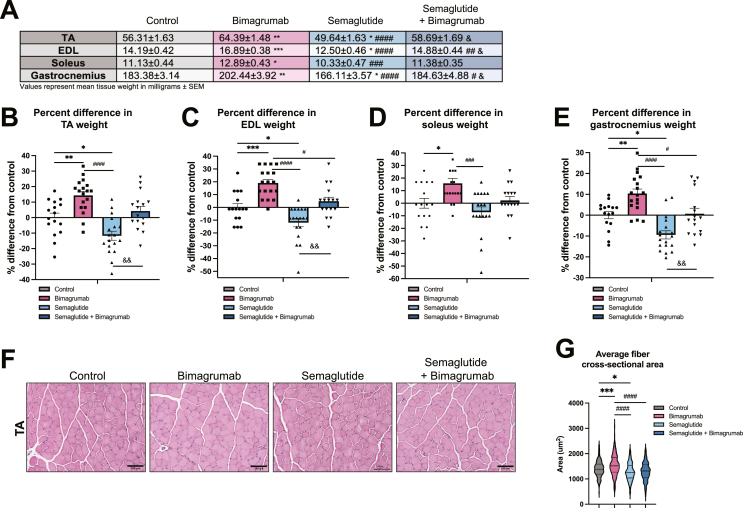
In the same cohort of mice, we evaluated the effect of GLP-1 receptor agonism and ActRII blockade on individual tibialis anterior (TA), soleus, extensor digitorum longus (EDL), and gastrocnemius muscles. Bimagrumab induced increases in skeletal muscle size (∼10–20 %) across all four muscles (Figure 3A–E). On the contrary, semaglutide treatment reduced TA, EDL, and gastrocnemius muscle weights and a trend towards decreased soleus weights compared to control animals (Figure 3A–E). Strikingly, ActRII blockade preserved skeletal muscle weights despite significant body weight loss, as there were no significant differences in muscle sizes between control and semaglutide plus bimagrumab animals (Figure 3A–E). Muscle fiber cross-sectional area (CSA) was quantified from H&E stained sections of TA muscles, revealing that muscle fibers of bimagrumab-treated animals were significantly larger than those of control animals, and average CSA of both control and bimagrumab-treated animals were larger than those of animals treated with semaglutide (Figure 3F,G). The average CSA of fibers of mice treated with semaglutide plus bimagrumab were not different than muscle fibers from control mice (Figure 3F,G). Overall, these data demonstrate that ActRII blockade can stimulate skeletal muscle hypertrophy and preserve muscle size during weight loss stimulated by GLP-1 receptor agonism.
Figure 3.
ActRII inhibition drives skeletal muscle hypertrophy and preserves muscle mass during weight loss induced by GLP-1 receptor agonism.
A) Weights (mg) of TA, EDL, soleus, and gastrocnemius muscles isolated from HFD-fed mice treated for 14 days.
B) Percent difference in weight of TA muscles compared to control group.
C) Percent difference in weight of EDL muscles compared to control group.
D) Percent difference in weight of soleus muscles compared to control group.
E) Percent difference in weight of gastrocnemius muscles compared to control group.
F) Representative TA sections stained with H&E (20x magnification, scale bar = 100 um).
G) Quantification of average muscle fiber cross-sectional area in TA sections (n = 4: 2 images analyzed per animal, 2 animals per group).
n = 8 mice for control and semaglutide + bimagrumab groups, n = 9 mice for semaglutide and bimagrumab groups unless otherwise indicated.∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0005, ∗∗∗∗p < 0.0001 for control vs. bimagrumab, semaglutide, and semaglutide + bimagrumab; #p < 0.05, ##p < 0.001, ###p < 0.0005, ####p < 0.0001 for bimagrumab vs. semaglutide and semaglutide + bimagrumab; & p < 0.05, && p < 0.001 for semaglutide vs. semaglutide + bimagrumab.
2.4. Combined ActRII inhibition and GLP-1 receptor agonism improves circulating markers of adipose inflammation and function
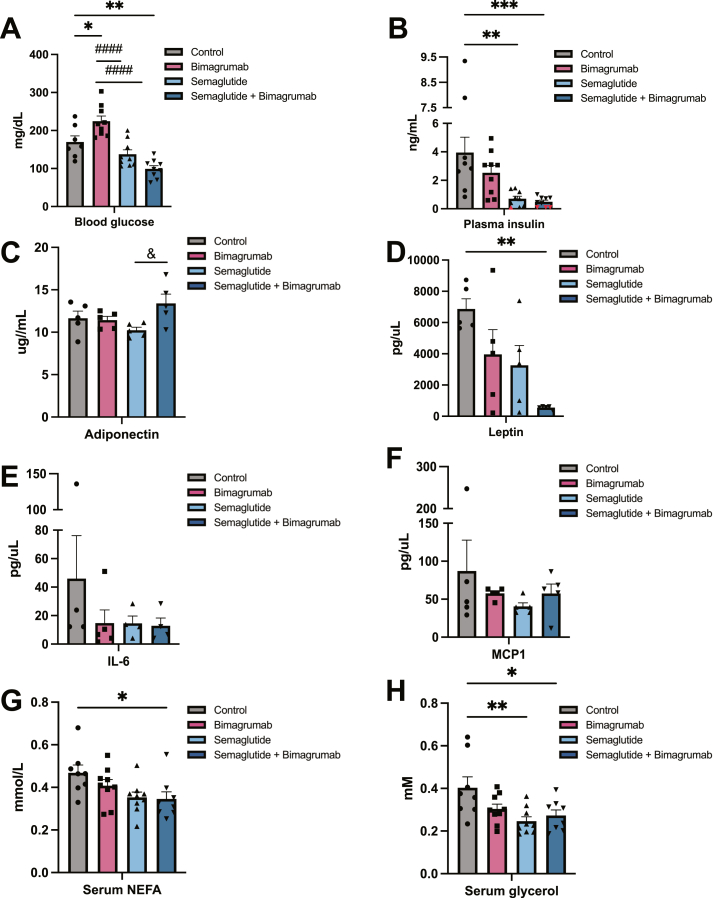
Next, we determined if the changes in body composition induced by ActRII inhibition were associated with improved metabolic parameters, including blood glucose, insulin, and circulating adipokines and cytokines. Treatment with semaglutide or semaglutide plus bimagrumab led to significantly lower fed blood glucose (Figure 4A). Interestingly, mice treated with bimagrumab alone displayed modestly elevated blood glucose levels (Figure 4A). Both semaglutide and semaglutide plus bimagrumab treatment reduced plasma insulin levels, while bimagrumab treatment trended toward a decrease compared to control animals (Figure 4B). Levels of adiponectin, an adipokine with anti-inflammatory properties, were comparable across control, bimagrumab, and semaglutide treatment groups, while semaglutide plus bimagrumab increased adiponectin levels compared to semaglutide alone (Figure 4C). Semaglutide plus bimagrumab treatment dramatically decreased levels of circulating leptin compared to control, while leptin levels in the individual treatment groups trended towards a decrease (Figure 4D). Circulating levels of inflammatory cytokines interleukin 6 (IL-6) and monocyte chemoattractant protein 1 (MCP1) trended lower in all treatment groups compared to control, although differences did not reach statistical significance (Figure 4E,F). Circulating levels of glycerol and free fatty acids were reduced across treatment groups, most significantly in animals treated with semaglutide or semaglutide plus bimagrumab (Figure 4G,H). These data indicate that ActRII blockade alone may offer metabolic benefits, while semaglutide plus bimagrumab have additional effects on improving metrics of metabolic health, including markers of adipose tissue function.
Figure 4.
Combined ActRII blockade plus GLP-1 receptor agonism improves circulating markers of adipose inflammation and function.
A) Ad libitum blood glucose levels from HFD-fed mice.
B) Ad libitum plasma insulin levels from HFD-fed mice. Values below the limit of detection were given the concentration value of 0.1 ng/mL and marked in red.
C) Serum adiponectin levels from HFD-fed mice (n = 5).
D) Serum leptin levels from HFD-fed mice (n = 5).
E) Serum IL-6 levels from HFD-fed mice (n = 5).
F) Serum MCP1 levels from HFD-fed mice (n = 5).
G) Serum glycerol levels from HFD-fed mice.
H) Serum NEFA levels from HFD-fed mice.
n = 8 mice for control and semaglutide + bimagrumab groups, n = 9 mice for semaglutide and bimagrumab groups unless otherwise indicated. ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0005, ∗∗∗∗p < 0.0001 for control vs. bimagrumab, semaglutide, and semaglutide + bimagrumab; #p < 0.05, ##p < 0.001, ###p < 0.0005, ####p < 0.0001 for bimagrumab vs. semaglutide and semaglutide + bimagrumab; & p < 0.05, && p < 0.001 for semaglutide vs. semaglutide + bimagrumab.
2.5. Muscle hypertrophy driven by ActRII blockade is associated with improved exercise performance
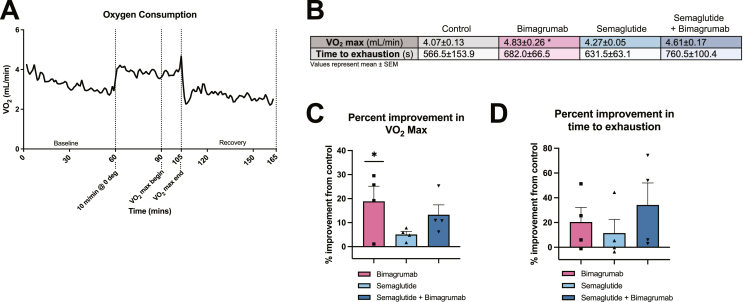
To assess whether ActRII blockade improves muscle function, we performed an exercise performance protocol to measure VO2 max and time to exhausation in a separate cohort of animals treated as in Figure 1A. Mice were acclimated in metabolic treadmills to obtain baseline oxygen consumption measurements followed by 30 min of pre-running (10 m/min) before initiating the VO2 max protocol (Figure 5A). Mice treated with bimagrumab had significantly greater VO2 max values than control mice, with an average of an 18 % improvement (Figure 5B,C). Animals treated with semaglutide displayed a modest improvement in excercise capacity (∼5 % over control) (Figure 5B,C). VO2 max values of animals in the semaglutide plus bimagrumab group trended to be greater than control (13 % average improvement and ∼2 fold higher than animals treated with semaglutide alone) (Figure 5B,C). The time to exhaustion of animals in the three treatment groups trended to be greater than those of control animals with the largest effect in animals co-treated with bimagrumab and semaglutide (Figure 5B,D). ANCOVA analysis using lean mass as a covariate eliminates the bimagrumab-driven increases, indicating that changes in VO2 max are lean mass-dependent (data not shown). These data support the hypothesis that increased lean mass due to ActRII inhbition alone and during GLP-1 receptor agonism improves functional outcomes.
Figure 5.
Muscle hypertrophy driven by ActRII blockade is associated with improved exercise performance.
A) Example oxygen consumption trace from control animals during exercise performance protocol.
B) VO2 max (mL/min) and time to exhausation (s) during VO2 protocol.
C) Percent improvement in VO2 max over the control group.
D) Percent improvement in time to exhaustion over the control group.
n = 4 mice for control, bimagrumab, semaglutide, semaglutide + bimagrumab groups. ∗p < 0.05 for control vs. bimagrumab, semaglutide, and semaglutide + bimagrumab.
2.6. Akt is not required for muscle hypertrophy driven by ActRII blockade
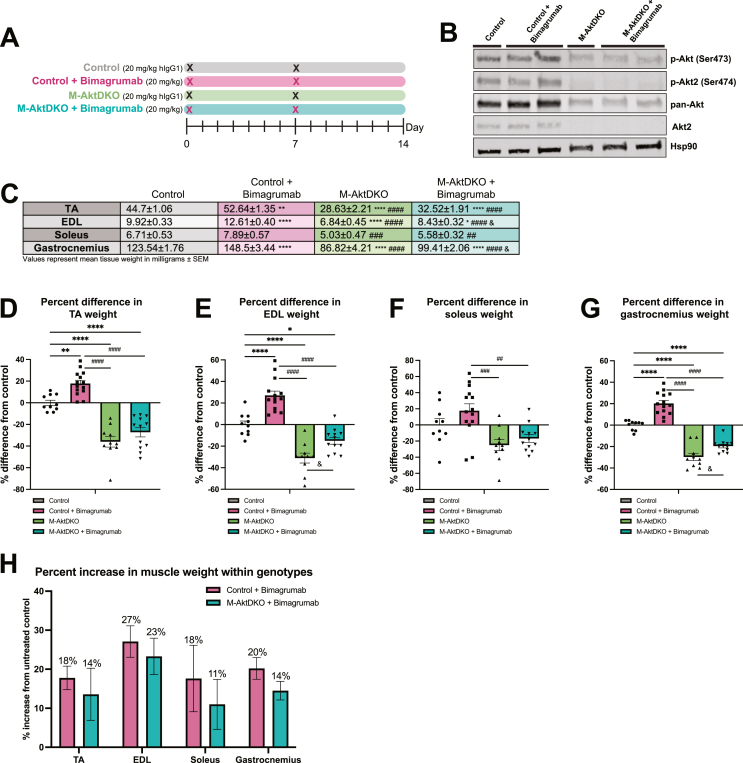
Skeletal muscle Akt signaling is both required and sufficient for muscle hypertrophy, as genetic loss of Akt isoforms in the muscle leads to decreased muscle size in adult mice [19,20,22]. Mechanistically, bimagrumab treatment in human primary myotubes prevents myostatin-induced Smad activation and increases Akt signaling [16]. To determine if Akt signaling is required for the hypertrophic effects of ActRII blockade using bimagrumab, skeletal muscle-specific Akt1 and Akt2 knockout (M-AktDKO) mice were generated. As described in prior studies, these mice display significant deficits in muscle growth and protein synthesis, consistent with a major anabolic role for Akt in muscle [19,20]. Control and M-AktDKO mice were administered bimagrumab or human recombinant IgG1 over 14 days as indicated (Figure 6A). Western blot analysis of gastrocnemius muscle from control and M-AktDKO mice confirmed knockout of Akt2 as well as a significant reduction in expression of all Akt isoforms (detected by the pan-Akt antibody) in M-AktDKO mice (Figure 6B). Residual Akt1 expression in whole muscle tissue is due to the expression of Akt1 in non-muscle cells [19]. Bimagrumab treatment increased skeletal muscle weights in control animals, with greater than ∼15–20 % increases in size observed in all muscle groups (Figure 6C–G). Muscles isolated from M-AktDKO mice were smaller than those of control animals, consistent with previous work [19,20]. Surprisingly, EDL and gastrocnemius muscles from M-AktDKO mice treated with bimagrumab were significantly larger than those of control M-AktDKO mice, and TA and soleus muscles trended towards being larger (Figure 6C–G). The percent increase in muscle weights induced by bimagrumab were similar between control and M-AktDKO animals (17–27 % vs. 11–23 %) (Figure 6H). Collectively, these data demonstrate that ActRII inhibition can drive muscle growth via an Akt-independent pathway.
Figure 6.
ActRII inhibition drives skeletal muscle hypertrophy in the absence of Akt.
A) Experimental schematic.
B) Immunoblot analysis for indicated proteins in control and M-AktDKO mice refed for 1 h following 16 h of fasting.
C) Weights (mg) of TA, EDL, soleus, and gastrocnemius muscles isolated from control and M-AktDKO mice treated for 14 days.
D) Percent difference in weight of TA muscles compared to control group.
E) Percent difference in weight of EDL muscles compared to control group.
F) Percent difference in weight of soleus muscles compared to control group.
G) Percent difference in weight of gastrocnemius muscles compared to control group.
H) Percent increase in muscle weights induced by bimagrumab within genotypes.
n = 5 mice for control and M-AktDKO groups, n = 6 mice for M-AktDKO + bimagrumab group, and n = 7 for control + bimagrumab group.∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0005, ∗∗∗∗p < 0.0001 for control vs. Control + bimagrumab, M-AktDKO, and M-AktDKO + bimagrumab; #p < 0.05, ##p < 0.001, ###p < 0.0005, ####p < 0.0001 for control + bimagrumab vs. M-AktDKO and M-AktDKO + bimagrumab; & p < 0.05 for M-AktDKO vs. M-AktDKO + bimagrumab.
3. Discussion
The approval of the GLP-1RA semaglutide for the treatment of obesity is a landmark achievement for pharmacological approaches to treat obesity and its comorbidities [8]. As such, approaches to capitalize and improve on changes in body composition achieved with GLP-1 receptor agonism are of significant interest. Recent evidence demonstrates that targeting the activin type II receptor can effectively reduce fat mass while building lean mass in obese individuals, thereby improving body composition and lowering HbA1c [18]. In this manuscript, we explored the therapeutic potential of combining bimagrumab, a monoclonal antibody against ActRII, with GLP-1RA semaglutide to improve body composition, optimize fat loss, and improve metabolic health. Strikingly, treatment with bimagrumab preserves lean mass while enhancing fat mass loss during treatment with semaglutide, and this preservation of lean mass was associated with both improved metabolic and functional outcomes. Mechanistically, we show that ActRII inhibition drives skeletal muscle hypertrophy even in the absence of Akt, suggesting that there are ActRII-dependent, Akt-independent anabolic pathways that control skeletal muscle growth.
The weight loss observed in a clinical trial of semaglutide is attributable to losses of both fat and lean mass, with ∼40 % of weight loss reported to be fat-free mass [5]. In this manuscript, we report that obese mice given daily injections of high-dose semaglutide lose an average of ∼10 % of their lean mass (accounting for ∼20 % of the total weight loss) over 14 days of treatment (Figure 1). The weight loss attributable to lean mass reported here is less than what is reported in humans, and this could be due to a number of factors including: differences in starting body composition in a human vs mouse, species specific differences in metabolic rates, drug concentrations, and dose scheduling. Nevertheless, using this model we recapitulate lean mass loss as observed in overweight and obese humans treated with GLP-1 receptor agonists. Some degree of lean mass loss is a common phenomenon with acute weight loss induced by diet restrictions or bariatric surgery, with 5–25 % reductions in muscle mass being reported across various studies [23]. Weight loss through dietary or surgical approaches can lead to significant functional and metabolic benefits, and for many individuals these positive outcomes outweigh any concerns of loss of lean mass. This manuscript does not seek to dispute these clear positive outcomes, but rather to understand how manipulating body composition can further improve metabolic and functional outcomes. Along these lines, data presented herein indicate preserved lean mass is associated with improved exercise performance and markers of adipose inflammation (Figure 5). This finding is consistent with a previous study of exercise performance and weight loss, where obese adults who exercised during weight loss exhibited VO2 max levels greater than those who dieted alone [24]. These data indicate that maintaining lean mass through exercise or pharmacological intervention can improve functional outcomes. Furthermore, because of skeletal muscle's contribution to EE [10,12], future studies will be aimed at understanding whether preserved muscle mass during weight loss has the potential to prolong weight loss maintenance.
It is well appreciated that ActRII ligands such as myostatin and activin A negatively regulate muscle size, and ActRII blockade increases muscle size as well as reduces fat mass [15,18]; however, the molecular mechanisms mediating this effect on fat are ill-defined. Multiple mechanisms are proposed, including through direct action on adipocytes or indirect effects via the muscle, potentially altered myokine secretion or changes in systemic energy balance [25]. Adipocyte-specific expression of a dominant negative form of Acvr2b did not induce any changes in body composition, suggesting that changes in fat mass are not directly due to loss of ActRII signaling in adipose tissue [26]. However, this work utilized an aP2/Fabp4 promoter to drive adipocyte-specific Cre expression, which is known to have problems with mosaicism and also drive expression in skeletal muscle and brain, highlighting the pitfalls of this model for adipose-specific targeting [27]. Global deletion of Smad3 protects mice from weight gain on HFD, elevates Akt signaling in WAT, and induces a browning phenotype in WAT [28,29], highlighting the relevance of this signaling pathway in fat. However, this genetic approach does not eliminate the possibility that these effects could be secondary to changes outside of adipose tissue. Indeed, deletion of Acvr2a and Acvr2b in muscle cells protects against weight gain in response to high-fat diet feeding, demonstrating that loss of ActRII signaling in myofibers can indirectly influence fat accumulation [30]. However, this protection from weight gain with muscle Acvr2a/b deletion is not as great as that seen in Mstn−/− mice, making it plausible that signaling through this pathway could still directly affect adipose tissue [30]. Lastly, Activin E (INHBE), a liver-derived member of the TGFβ superfamily which signals through ACVR1C to activate Smad proteins, was shown to suppress adipose tissue lipolysis and result in decreased fat mass in INHBE knockout mice [31], demonstrating an adipose-specific effect of signaling via TGFβ-family ligands. This body of work highlights the ongoing need for both adipose- and muscle-specific manipulation of this signaling pathway to detangle direct versus indirect effects of ActRII blockade on alterations to body composition.
Beyond storage of lipids, adipose tissue acts as an endocrine organ that secretes adipokines and chemokines which modulate a wide array of metabolic function [32]. These include several molecules that correlate directly with adipose tissue mass and can act as biological markers of inflammation and metabolic dysfunction [[32], [33], [34], [35]]. ActRII inhibition in combination with GLP-1 receptor agonism further reduces blood glucose levels and improves adipokine secretion while reducing serum markers of inflammation and circulating glycerol similar in a manner similar to semaglutide (Figure 4). Interestingly, obese mice treated with bimagrumab exhibited significantly elevated blood glucose and lowered insulin compared to control animals, suggesting an impairment in beta cell function. Notably, administration of a soluble ActRIIB fragment impaired glucose tolerance in lean mice, which was correlated with increased spleen and pancreas weights [36]. However, this effect on glucose tolerance was not observed in mice treated with antibodies specific to two ActRII ligands, GDF-8 and activin A, suggesting the potential involvement of additional ActRII ligands in the regulation of glucose homeostasis [36]. In contrast to these rodent studies, treatment with an anti-ActRII antibody for 48 weeks in a phase 2 clinical trial induced significant improvements in HbA1c and insulin sensitivity [18]. Collectively, these data suggest that length of treatment time could play a role in this phenomenon, or there are inherent species-specific differences related to glucose homeostasis with systemic inhibition of ActRII.
Skeletal muscle hypertrophy can occur through increases in protein synthesis resulting in individual myofiber hypertrophy, or through the differentiation of progenitor satellite cells and their fusion with mature myofibers [10]. Administration of bimagrumab (BYM338) in SCID mice induced significant increases in individual weights and fiber diameters in TA, EDL, soleus, and gastrocnemius muscles [16]. Furthermore, no changes were observed in fiber type composition of muscle treated with BYM338, indicating that hypertrophy occurred regardless of fiber type [37]. Whole body Akt1 or Akt2 knockout mice administered a soluble ActRIIB fragment revealed a similar increase in muscle mass across multiple muscle depots in response to ActRIIB inhibition [17]. Since both Akt isoforms are required for maintaining muscle mass in vivo, it is plausible that compensation between isoforms was responsible for muscle growth following ActRIIB inhibition in this model. However, data presented herein demonstrate muscle hypertrophy was largely maintained in mice lacking skeletal muscle Akt when administered bimagrumab (Figure 6). These data suggest the existence of Akt-independent pathways that contribute significantly to hypertrophy downstream of ActRII inhibition. Notably, changes in muscle mass are mediated by rates of protein synthesis and degradation which result in muscle growth or atrophy, respectively [10]. Food intake, physical exercise, and amino acid availability are factors that influence skeletal muscle protein turnover and nitrogen balance [38,39]. Therefore, it is of significant interest to understand ActRII-dependent effects on protein accretion, amino acid and fat metabolism and subsequent muscle hypertrophy. Moreover, studies exploring the ability of resistance exercise and amino acid supplementation to potentiate the effects of ActRII blockade on body composition and muscle function are warranted.
In conclusion, data presented herein indicate that combined ActRII blockade with bimagrumab and GLP-1 receptor agonism with semaglutide leads to improved body composition during weight loss and is associated with improved serum markers of metabolic health. Furthermore, preservation of lean mass through ActRII inhibition improved exercise performance. Interestingly, bimagrumab appears to drive muscle hypertrophy in an Akt-independent manner, suggesting additional anabolic pathways are engaged to drive muscle growth. Notably, a phase 2b clinical study has recently completed enrollment to assess the efficacy of this combination therapy over 72 weeks plus continued monitoring through 102 weeks to assess weight loss maintenance, with an estimated completion date in 2025 [40]. Future studies will be aimed at determining the underlying mechanisms by which ActRII inhibition alters body composition and how these changes alter adipose, muscle and systemic metabolism during normal conditions, calorie deficit, and following cessation of GLP-1 receptor agonism.
4. Materials and methods
4.1. Mice
All mouse experiments were reviewed and approved by the University of Pennsylvania IACUC in accordance with NIH guidelines. Muscle-specific AktDKO (M-AktDKO) mice were generated by crossing Akt1 loxp/loxp;Akt2 loxp/loxp floxed mice with mice carrying Cre recombinase driven by skeletal muscle actin promoter ACTA1–Cre (human skeletal actin (HSA)–Cre) (Jackson Laboratory #006149) as previously described [19]. M-AktDKO mice were males between 4 and 6 months of age. Male, diet-induced obese mice on a C57Bl/6J background (Jackson Laboratory #380050, 24–25 weeks) were maintained on high fat diet (60 % kcal from fat, Research Diets D12492) for the duration of experiments. Body composition was assessed using an EchoMRI body composition analyzer (EchoMRI, Houston TX). Mice were euthanized in a fed state unless otherwise indicated.
4.2. Food intake
Male, diet-induced obese mice on a C57Bl/6J background (Jackson Laboratory #380050, 12 weeks) were single-housed and maintained on high fat diet (60 % kcal from fat, Research Diets D12492). Cumulative food intake was determined by manually weighing food from single cages every 24 h over 12 days.
4.3. Reagents
Human bimagrumab (MedChemExpress, HY-P99355) or recombinant human IgG1 (SydLabs, PA007125) were administered by subcutaneous injection at 20 mg/kg once weekly. Semaglutide (Peptide Sciences) or equal volume vehicle control (0.9 % saline) were administered at 120 ug/kg once daily.
4.4. Histology and image analysis
eWAT and iWAT depots were fixed in 4 % paraformaldehyde overnight, dehydrated in ethanol, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin. Images were taken at 10x magnification on a Keyence inverted microscope. The Adiposoft plugin for ImageJ was used to quantify adipocyte area [41]. Four animals per group and two images per animal were quantified (n = 8 per group) and average adipocyte size was calculated. TA muscles were fixed in 10 % buffered formalin overnight, dehydrated in ethanol, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin. Images were taken at 20x magnification on a Keyence inverted microscope. Individual muscle fibers were manually selected and cross-sectional area was quantified using ImageJ. Two animals per group and two images per animal were quantified (n = 4 per group) and average fiber cross-sectional area was calculated.
4.5. Immunoblotting
Muscles were snap frozen in liquid nitrogen before being transferred to RIPA lysis buffer supplemented with protease inhibitor cocktail tablets (Roche) and phosphatase inhibitor cocktails I and II (Sigma) and homogenized using a handheld tissue homogenizer (Fisherbrand 150). Samples were centrifuged, and the lysate supernatant was used to determine total protein levels (BCA Protein Assay, Pierce). Samples were diluted with sample buffer and equal amounts were loaded onto SDS gels (Biorad 4561084). The following antibodies were used (all from Cell Signaling Technology): pan-Akt (Cat# 4691), Akt2 (Cat# 2964), p-Akt (Ser473) (Cat# 4060), p-Akt2 (Ser474) (Cat# 8599), Hsp90 (Cat# 4874).
4.6. Serum insulin, glycerol, and free fatty acids
Serum insulin levels were measured using ultra-sensitive Mouse Insulin ELISA Kit (Crystal Chem 90080). Any value below the limit of detection was given the value 0.1 ng/mL and marked in red. Serum glycerol content was measured using Free Glycerol Reagent (Sigma-Aldrich F6428-40 ML). Free fatty acids were measured using Wako HR Series NEFA-HR(2) assay (Fujifilm Wako).
4.7. Luminex
Mouse adiponectin was measured by absorbance sandwich ELISA (EMD Millipore, Billerica MA). Mouse MCP-1, IL-6, and leptin were measured by multiplex (madkmag-71k-04, EMD Millipore, Billerica MA). Beads were read on Luminex MAGPIX and data analysis was performed on Luminex Exponent4.2 (Luminex, Austin TX).
4.8. Exercise performance protocol
Mice were run in a 4-lane metabolic treadmill (Columbus Instruments, Columbus Ohio). Gas analysis tubing from the treadmills were connected directly to a Promethion CORE system (Sable Systems International, North Las Vegas, NV) for metabolic measurement. Two treadmill lanes were connected to each Promethion gas analyzer, flow rate was increased to 2500 LPM allowing for more frequent sampling of oxygen consumption (VO2) by indirect calorimetry, and data was processed using the 60-second macro (OneClickMacroV.2.53.2-slice1min). Animals were allowed to acclimate to the metabolic treadmills for 1 h to obtain a baseline measurements prior to the VO2 max test. The treadmills were then switched on for 30 min of pre-running at 10 m/min with no incline (0°). The following VO2 max protocol began immediately after pre-running with 2 min segments of each of the following speeds and inclines: 15 m/min at 0°, 20 m/min at 0°, 20 m/min at 5°, 20 m/min at 10°, 20 m/min at 15°, 20 m/min at 20°, 20 m/min at 25°, 25 m/min at 25°, 30 m/min at 25°. The VO2 max test was ended when a mouse stayed on the shock pad for 10 s, and the shock pad was then turned off to begin the 1 h recovery measurement. Two treadmill runs were completed sequentially in a day and the experiment was spread over two consecutive days to accommodate all 16 mice. Mice were not fasted. VO2 max was calculated as the average VO2 of the last 3 min of the VO2 max protocol, and normalized via ANCOVA (http://vassarstats.net/) for lean body mass, obtained on a LUNAR PIXimus DEXA scanner (Madison, WI).
4.9. Statistical analysis
All data presented as means +/− SEM. Analysis using ordinary one-way ANOVA followed by Tukey's multiple comparisons test was performed when more than two groups at one time point were compared. Analysis using two-way ANOVA followed by Tukey's multiple comparisons test was performed when more than two groups at more than one time point were compared. P < 0.05 was considered significant.
Author contributions
Elizabeth Nunn: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Natasha Jaiswal: Data curation, Formal analysis, Writing – review & editing. Matthew Gavin: Data curation, Investigation. Kahealani Uehara: Formal analysis, Investigation. Megan Stefkovich: Formal analysis, Investigation. Karima Drareni: Data curation, Formal analysis, Investigation. Ryan Calhoun: Formal analysis, Investigation. Michelle Lee: Data curation, Formal analysis. Corey D. Holman: Formal analysis, Investigation. Joseph A. Baur: Investigation, Writing – review & editing. Patrick Seale: Writing – review & editing. Paul M. Titchenell: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None declared.
Acknowledgements
We thank the University of Pennsylvania Diabetes Research Center (DRC) for use of the RIA Biomarker Core (P30-DK19525), and the University of Pennsylvania Rodent Metabolic Phenotyping Core (RRID:SCR_022427), supported in part by NIH grant S10-OD025098, for use of the EchoMRI to perform body composition measurements and for performing metabolic exercise performance experiments. This work was supported by National Institutes of Health Grants DK123252 (P.M.T.), Cox Research Institute and internal funds from the University of Pennsylvania (P.M.T.)
Data availability
Data will be made available on request.
References
- 1.N.d. World Obesity Federation . 2023. World obesity atlas.https://data.worldobesity.org/publications/?cat=19 [Google Scholar]
- 2.Bray G.A., Ryan D.H. Evidence-based weight loss interventions: individualized treatment options to maximize patient outcomes. Diabetes Obes Metabol. 2021;23(Suppl 1):50–62. doi: 10.1111/dom.14200. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum M., Foster G. Differential mechanisms affecting weight loss and weight loss maintenance. Nat Metab. 2023;5(8):1266–1274. doi: 10.1038/s42255-023-00864-1. [DOI] [PubMed] [Google Scholar]
- 4.Campbell J.E., Müller T.D., Finan B., DiMarchi R.D., Tschöp M.H., D'Alessio D.A. GIPR/GLP-1R dual agonist therapies for diabetes and weight loss—chemistry, physiology, and clinical applications. Cell Metabol. 2023 doi: 10.1016/j.cmet.2023.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 6.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 7.Jastreboff A.M., Kaplan L.M., Frías J.P., Wu Q., Du Y., Gurbuz S., et al. Triple-hormone-receptor agonist retatrutide for obesity - a phase 2 trial. N Engl J Med. 2023;389(6):514–526. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 8.Wilding J.P.H., Batterham R.L., Davies M., Van Gaal L.F., Kandler K., Konakli K., et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the <scp>STEP</scp> 1 trial extension. Diabetes Obes Metabol. 2022;24(8):1553–1564. doi: 10.1111/dom.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christoffersen B.Ø., Sanchez-Delgado G., John L.M., Ryan D.H., Raun K., Ravussin E. Beyond appetite regulation: targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity (Silver Spring, Md. 2022;30(4):841–857. doi: 10.1002/oby.23374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartori R., Romanello V., Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021;12(1):330. doi: 10.1038/s41467-020-20123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibel R.L., Rosenbaum M., Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum M., Vandenborne K., Goldsmith R., Simoneau J.-A., Heymsfield S., Joanisse D.R., et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kim D., Lee J., Park R., Oh C., Moon S. Association of low muscle mass and obesity with increased all-cause and cardiovascular disease mortality in US adults. Journal of Cachexia, Sarcopenia and Muscle. 2023 doi: 10.1002/jcsm.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han H.Q., Zhou X., Mitch W.E., Goldberg A.L. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45(10):2333–2347. doi: 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Akpan I., Goncalves M.D., Dhir R., Yin X., Pistilli E.E., Bogdanovich S., et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes. 2009;33(11):1265–1273. doi: 10.1038/ijo.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lach-Trifilieff E., Minetti G.C., Sheppard K., Ibebunjo C., Feige J.N., Hartmann S., et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34(4):606–618. doi: 10.1128/MCB.01307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goncalves M.D., Pistilli E.E., Balduzzi A., Birnbaum M.J., Lachey J., Khurana T.S., et al. Akt deficiency attenuates muscle size and function but not the response to ActRIIB inhibition. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heymsfield S.B., Coleman L.A., Miller R., Rooks D.S., Laurent D., Petricoul O., et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.33457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaiswal N., Gavin M.G., Quinn W.J., Luongo T.S., Gelfer R.G., Baur J.A., et al. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol Metabol. 2019;28:1–13. doi: 10.1016/j.molmet.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaiswal N., Gavin M., Loro E., Sostre-Colón J., Roberson P.A., Uehara K., et al. AKT controls protein synthesis and oxidative metabolism via combined mTORC1 and FOXO1 signalling to govern muscle physiology. Journal of Cachexia, Sarcopenia and Muscle. 2022;13(1):495–514. doi: 10.1002/jcsm.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Rønne J., Alanentalo T., Baquero A.F., et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6) doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 23.Cava E., Yeat N.C., Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8(3):511–519. doi: 10.3945/an.116.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villareal D.T., Chode S., Parimi N., Sinacore D.R., Hilton T., Armamento-Villareal R., et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPherron A.C., Lee S.-J. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109(5):595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo T., Jou W., Chanturiya T., Portas J., Gavrilova O., McPherron A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4(3) doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullican S.E., Tomaru T., Gaddis C.A., Peed L.C., Sundaram A., Lazar M.A. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol Endocrinol. 2013;27(1):127–134. doi: 10.1210/me.2012-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav H., Quijano C., Kamaraju A.K., Gavrilova O., Malek R., Chen W., et al. Protection from obesity and diabetes by blockade of TGF-β/smad3 signaling. Cell Metabol. 2011;14(1):67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsurutani Y., Fujimoto M., Takemoto M., Irisuna H., Koshizaka M., Onishi S., et al. The roles of transforming growth factor-β and Smad3 signaling in adipocyte differentiation and obesity. Biochem Biophys Res Commun. 2011;407(1):68–73. doi: 10.1016/j.bbrc.2011.02.106. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.-J., Lehar A., Liu Y., Ly C.H., Pham Q.-M., Michaud M., et al. Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A. Proc Natl Acad Sci USA. 2020;117(49):30907–30917. doi: 10.1073/pnas.2019263117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adam R.C., Pryce D.S., Lee J.S., Zhao Y., Mintah I.J., Min S., et al. Activin E–ACVR1C cross talk controls energy storage via suppression of adipose lipolysis in mice. Proc Natl Acad Sci USA. 2023;120(32) doi: 10.1073/pnas.2309967120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barchetta I., Cimini F.A., Ciccarelli G., Baroni M.G., Cavallo M.G. Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. J Endocrinol Invest. 2019;42(11):1257–1272. doi: 10.1007/s40618-019-01052-3. [DOI] [PubMed] [Google Scholar]
- 33.Kirichenko T.V., Markina Y.V., Bogatyreva A.I., Tolstik T.V., Varaeva Y.R., Starodubova A.V. The role of adipokines in inflammatory mechanisms of obesity. Int J Mol Sci. 2022;23(23) doi: 10.3390/ijms232314982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cnop M., Havel P.J., Utzschneider K.M., Carr D.B., Sinha M.K., Boyko E.J., et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 35.Sindhu S., Thomas R., Shihab P., Sriraman D., Behbehani K., Ahmad R. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latres E., Mastaitis J., Fury W., Miloscio L., Trejos J., Pangilinan J., et al. Activin A more prominently regulates muscle mass in primates than does GDF8. Nat Commun. 2017;8(1) doi: 10.1038/ncomms15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morvan F., Rondeau J.-M., Zou C., Minetti G., Scheufler C., Scharenberg M., et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc Natl Acad Sci USA. 2017;114(47):12448–12453. doi: 10.1073/pnas.1707925114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro T., Ebert S.M., Adams C.M., Rasmussen B.B. Amino acid sensing in skeletal muscle. Trends Endocrinol Metabol. 2016;27(11):796–806. doi: 10.1016/j.tem.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safety and efficacy of bimagrumab and semaglutide in adults who are overweight or obese. NCT05616013. U.S. National Library of Medicine; 2023. https://Clinicaltrials.Gov/Study/NCT05616013 [Google Scholar]
- 41.Galarraga M., Campión J., Muñoz-Barrutia A., Boqué N., Moreno H., Martínez J.A., et al. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res. 2012;53(12):2791–2796. doi: 10.1194/jlr.D023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.