Abstract
Background:
Suicide is the second-leading cause of death among adolescents and young adults in the United States, with rates rising over much of the last decade. The design, testing, and implementation of interventions to prevent suicide in this population is a public health priority. This manuscript outlines the design and methods for a research study that compares two interventions aimed at reducing suicide and suicide attempts in youth.
Methods:
We will enroll 300 youth aged 12–24 at high risk for suicide in this randomized controlled parallel group superiority trial. Participants will be randomly assigned to one of two study arms: (1) Zero Suicide Quality Improvement (ZSQI) implemented within the Kaiser Permanente Northwest (KPNW) health system, or (2) ZSQI plus a stepped care intervention for suicide prevention (SC-SP), where the services offered (including care management and dialectical behavior therapy [DBT]) increase based on risk level. Outcomes will be assessed at baseline, as well as 3-, 6-, and 12-months post randomization. The study was conceptualized and designed collaboratively by investigators at UCLA and KPNW.
Results:
To be reported in future manuscripts.
Conclusion:
The main objective of the study is to determine whether the SC-SP intervention is superior to ZSQI with regard to lowering rates of fatal and nonfatal suicide attempts. Interventions that incorporate the latest research need to be designed and tested under controlled conditions to make progress toward the goal of achieving zero suicide. The results from this trial will directly inform those efforts.
Trial Registration:
clinicaltrials.gov, NCT03092271, https://clinicaltrials.gov/ct2/show/NCT03092271https://clinicaltrials.gov/ct2/show/NCT01379027.
Keywords: Suicide, Self-Injurious Behavior, Dialectical Behavior Therapy, Adolescents, Young Adults, Randomized Controlled Trial
Suicide is the second-leading cause of death in the U.S. among those aged 10–34 years.1 Compared to earlier childhood, the onset of fatal and nonfatal suicide attempts (SAs), plans, and ideation begin to increase at age 12–13,2 with rates of suicide deaths more than doubling from adolescence to young adulthood: 6.9 vs. 16.3 per 100,000 for ages 12–17 and 18–24, respectively.3 Consequently, the adolescent and young adult years offer a developmental window when early intervention may prevent deadly behaviors from becoming established.
Zero suicide (ZS), a belief that suicide deaths are preventable within health systems, is a national priority in the U.S.4,5 Similar to system approaches that “design for zero” to address other health threats (e.g., infections, wrong-site surgery),6 health system approaches that strive towards ZS have been associated with impressive reductions in suicide deaths.4 However, a major challenge is the development of scalable strategies that can be widely disseminated and sustained. A “stepped care” approach provides one framework for addressing this issue and has been used to generate treatment models for a variety of health conditions.7–11 In recent years, there has also been a move toward adopting a stepped care approach to suicide prevention.12–14
Here, we describe the design and methods for an NIH-funded study (R01MH112147) that compares two approaches: (1) a health system quality improvement approach using ZS best practices (ZSQI) and (2) the ZSQI approach combined with a stepped care intervention for suicide prevention (SC-SP). The SC-SP intervention adds evidence-based suicide prevention care to usual health system practices, offering treatment services based on level of assessed risk.15 These services range from care management and digital health interventions at the lowest risk level, to individual and group psychotherapy at the highest risk level. The SC-SP intervention emphasizes dialectical behavior therapy (DBT) and other DBT-informed and cognitive behavioral therapy (CBT) treatments16,17 based on their demonstrated efficacy for reducing self-harm and suicidal behavior.18–20
The study has two primary aims. The first is to conduct an RCT to test the hypothesis that the SC-SP arm, when compared to ZSQI, will be associated with lower risk of fatal and nonfatal SAs over time. The second aim is to evaluate cost-effectiveness from the societal and health system perspective, including direct and indirect patient costs. We hypothesize that incremental cost-per-suicide-event-free day (SEFD) and incremental cost-per-depression-free-day (DFD) will be lower for the SC-SP group when compared to ZSQI only.
Methods
Setting
This study is being conducted at Kaiser Permanente Northwest (KPNW), a large nonprofit health system in the U.S. with more than 615,000 members. The study will be implemented by the Center for Health Research (CHR), an independent organization embedded within KPNW. The KPNW Institutional Review Board (FWA# 00002344, IRB# 00000405) has approved the study (Project# 1394006). They will review all research activities and monitor study progress.
Study Design
After confirming eligibility, participants will be enrolled in this two-arm randomized controlled parallel group superiority trial and assigned to either the ZSQI control condition or SC-SP experimental condition. Participants in both arms will receive treatment as usual in the health care system, informed by the health plan’s ZS quality improvement efforts. Participants in the SC-SP condition will also receive the 12-month SC-SP intervention, which includes 6 months of acute treatment using the stepped-care model and an additional 6 months of continuation treatment. SC-SP will have continued access to treatment as usual services in the health care system. Outcomes will be assessed at baseline and 3-, 6-, and 12-months post randomization.
Participants
We will enroll 300 participants at high risk for suicide. Inclusion criteria are (1) age 12–24 years; (2a) past year SA, interrupted SA, aborted SA, preparatory behavior, or active suicidal ideation (SI) with method and intent, or (2b) past year depression with history of SA and/or recurrent self-harm (SH; i.e., at least 3 distinct episodes of non-suicidal self-injury [NSSI]); (3) ability to participate in study activities, and (4) mental health coverage through KPNW. Exclusion criteria are (1) active psychosis, significant drug dependence, or other mental/behavioral conditions that might significantly interfere with participation (see additional detail below in Stage 1 Screening section); (2) life-threatening medical illness; or (3) other situations that would impede study participation (e.g., plans to move out of the area during the study).
Recruitment
We will utilize a two-stage screening process to (1) generate a recruitment pool of potential participants and (2) conduct an initial assessment of study eligibility. Those that meet screening criteria at both stages will be consented and scheduled for a baseline assessment to confirm eligibility.
Stage 1 Screening
We will apply a case-finding algorithm to the EHR to identify youth who are likely to qualify for the study. The algorithm will screen for (a) diagnosis codes to include (e.g., self-inflicted injury); (b) diagnosis codes to include but note for further screening (e.g., bipolar spectrum, attention deficit, autism spectrum, depressive, eating, and substance use disorders); (c) diagnosis codes to exclude (e.g., schizophrenia spectrum disorder); (d) administration of relevant survey measures (e.g., PHQ-9); (e) specific services (e.g., ED visits, psychiatric hospitalizations, emergency crisis line calls); and (f) suicide and SH variables (e.g., lifetime SAs, NSSI history). The algorithm generates a register of potential participants, and a random selection from those cases will be pulled as needed and added to the recruitment list. A secure EHR message will be sent to the primary care and/or mental health provider to elicit any objections to recruitment of any individual. A recruitment packet will also be mailed to each person on the list.
Stage 2 Screening
Stage 2 will involve an initial screening, with two separate sets of questions being asked. The first set will include general eligibility and availability questions. Those not excluded after these questions will be asked to provide verbal consent before a second set of more sensitive questions is asked. These questions will further assess inclusion and exclusion criteria (e.g., inquiring about any diagnosis codes recorded during case-finding screening). Individuals meeting preliminary criteria after this screening will be scheduled for a baseline assessment to determine final eligibility.
Consenting Procedures
Prior to the baseline assessment, the youth—and parent, if the participant is a minor—will be given a copy of the assent/consent and HIPAA authorization forms. Study staff will review these forms and answer any questions. The youth (and parent/guardian, if applicable) will then be asked to sign both forms.
Randomization
Participants will be randomized 1:1 to one of the two study arms, stratified by self-reported gender, age, and SH risk criteria (see Supplementary Material for additional detail). We will use permuted blocks within strata (randomized block sizes of 2 or 4, masked from staff) and generate the randomization schema using the PROC PLAN procedure in SAS version 9.4. SAS PROC PLAN results will be stored in the relational database of the study tracking system and will be used to assign participants to the next available record based on stratification variable values, resulting in study arm assignment. After study eligibility is confirmed through the baseline assessment, study interventionists will contact the youth (and parent, if applicable). Once youth willingness to participate is confirmed, intervention staff will select the “randomize” button in the study tracking system to reveal arm assignment and inform participants of the outcome. Assessment staff will remain blinded to study arm assignment.
Intervention Arms
Zero Suicide Quality Improvement (ZSQI)/Control Arm
This condition consists of the healthcare system’s ZSQI effort, following the guidelines generated by the Clinical Care and Intervention Task Force of the National Action Alliance for Suicide Prevention.4 This effort was initiated by the health plan independent of this research project, and will continue throughout the project. The ZSQI approach includes systematic initial and follow-up screening for suicide risk; identification and refinement of suicide prevention interventions; facilitation of referral and adherence to these suicide interventions; tracking/surveillance of suicide event outcomes; and continuous QI focused on the ZS goal. In addition to their other usual care, participants will have access to KPNW Emergency Psychiatric Services by telephone 24 hours a day, 7 days a week.
ZSQI Plus Stepped Care for Suicide Prevention (SC-SP) / Experimental Arm
Within the context of health system ZSQI, the SC-SP approach aims to deliver the most effective yet least resource-intensive treatment first, and then “step up” to more intensive services as needed. This method emphasizes alternatives to inpatient care, which is costly and may yield more negative outcomes than intensive outpatient approaches.21 Figure 1 shows the flow of events for participants randomized to the SC-SP intervention arm.
Figure 1.
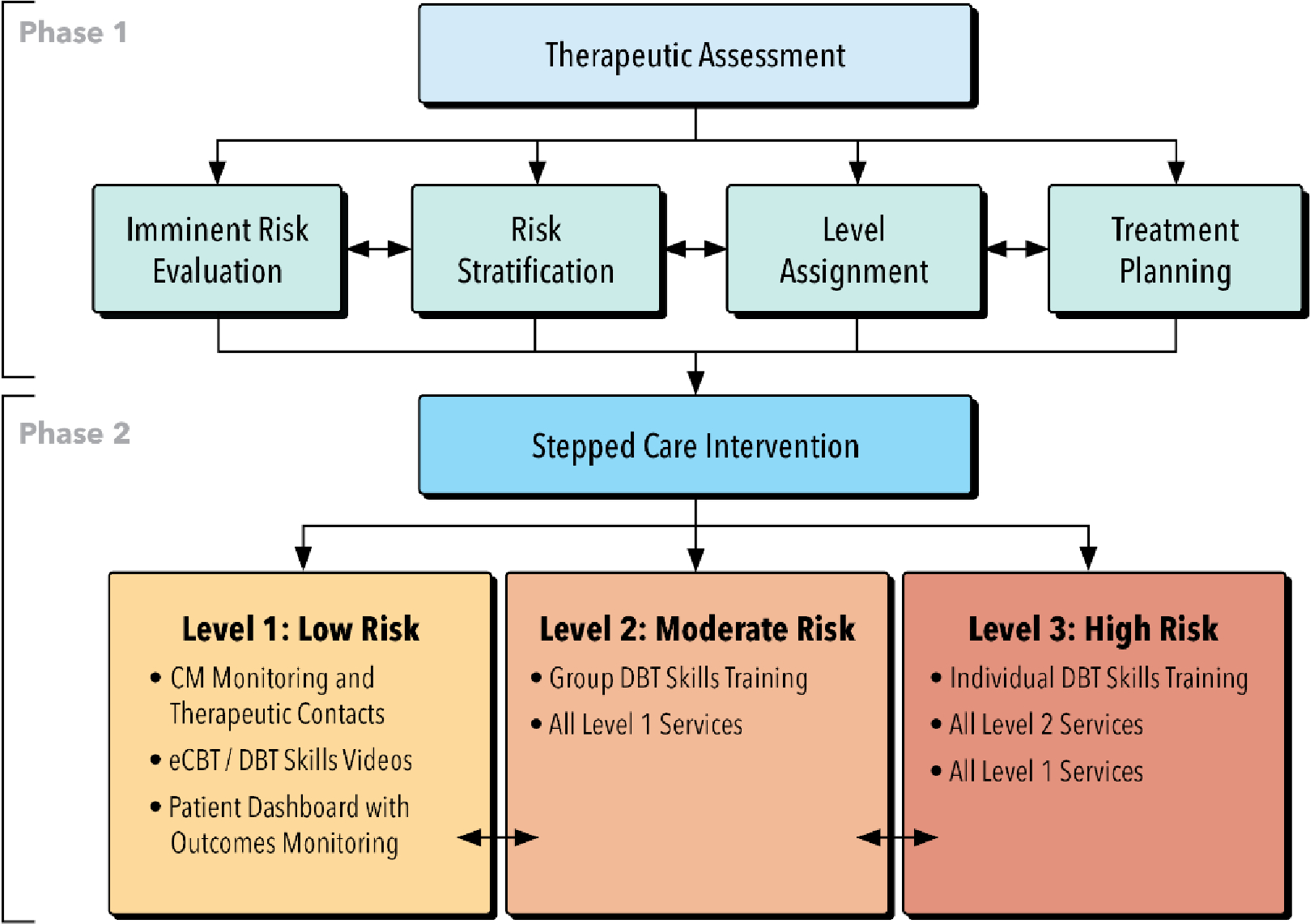
Event flow for participants randomized to the SC-SP condition.
Abbreviations. CM: care manager; eCBT: electronic (web-based) cognitive behavioral therapy; DBT: dialectical behavioral therapy.
Phase 1: Therapeutic Assessment.
All participants randomized to the SC-SP condition will take part in a therapeutic assessment designed to enhance safety and obtain initial data to inform stepped care level assignment and treatment planning.22,23 This initial phase is modeled after the SAFETY-Acute protocol developed and tested in emergency departments (EDs), community agencies, and outpatient treatment settings for evaluating and reducing imminent risk. 17,24–26 Within the context of the study SC-SP protocol, this intervention phase will span 2–3 sessions with a study Care Manager (CM) and include four components: imminent risk evaluation, risk stratification, level assignment, and treatment planning. An overview of these components is provided in Table 1.
Table 1.
Therapeutic assessment components.
| Imminent Risk Evaluation | Risk Stratification | Level Assignment | Treatment Planning |
|---|---|---|---|
| • Care manager will evaluate: ∘ Ability of youth/parent to identify strengths in the youth and family environment ∘ Ability of the youth to consider their pattern of emotional regulation, using a “feeling thermometer” ∘ Willingness and ability of youth to develop and use a safety plan that identifies behavioral, cognitive, and interpersonal strategies ∘ Ability of youth to commit to safety plan use rather than self-harm for a specific duration (at least until next scheduled session) • Imminent risk determination: ∘ If imminent risk is evident, youth will be triaged to emergency services and recontacted to continue the SC-SP intervention when the youth is evaluated as safe for outpatient care ∘ For youth evaluated as safe to continue with outpatient care, care manager will continue to risk stratification |
• Additional assessment of: ∘ Mental health symptoms ∘ Psychosocial functioning ∘ Protective processes ∘ Prior treatment ∘ View of treatment • Assessment guided by the “Stress & Distress Brochure”1 • Information used to: ∘ Determine appropriate assignment of stepped care intervention services ∘ Develop initial case conceptualization and treatment plan/goals |
• Stepped care level assigned based on degree of recent suicidal ideation, suicidal behavior, or NSSI reported • Level 1 (low risk) ∘ No reported SI, SA, or NSSI in the past 3 months, and ∘ PHQ-9 score <10 • Level 2 (moderate risk) ∘ PHQ-9 score ≥10, or ∘ Passive SI with no intent or plan in the past 3 months • Level 3 (high risk) ∘ Active SI with intent or plan in past 3 months, or ∘ SA in past 3 months, or ∘ ≥3 episodes of NSSI, or 2 NSSI episodes (one of which required medical attention) within the past 3 months |
• Intervention service options presented based on stepped care level • “Stress & Distress Brochure”1 used to guide development of collaborative treatment plan |
Modeled after the “Stress and Your Mood Brochure” used in the Youth Partners in Care Trial.27
Phase 2: Stepped Care Intervention.
Participants will be offered services based on assigned risk level. The three stepped care levels build in intensity, with higher levels including all services offered at lower levels (Table 2).
Table 2.
Treatment services offered by level assignment.
| Level 1 (low risk) | Level 2 (moderate risk) | Level 3 (high risk) |
|---|---|---|
| • Monthly therapeutic care management contacts1 ∘ Brief risk assessment ∘ Motivational interviewing around accessing/participating in treatment services ∘ Review and use of personal safety plan ∘ Problem-solving support ∘ Facilitating connection with interventionist and health system • Self-directed treatment options (as recommended by care manager) ∘ Internet-based cognitive behavioral therapy (eCBT)2 ∘ Dialectical Behavioral Therapy (DBT) skills videos3 |
• Group DBT sessions4 ∘ 18 total sessions over 3 modules focused on DBT skills training ∘ Modules (6 sessions each): Emotion regulation, interpersonal effectiveness, distress tolerance ∘ 90-minutes each session • + Level 1 treatment services ∘ Care management contact will also include support around use of skills taught in group |
• Individual DBT sessions5 ∘ 10 sessions6 ∘ 60-minutes each session • + Level 2 treatment services |
These contacts will be initiated by phone, with follow up as needed using the participant’s preferred mode of contact (phone or email) to increase success in maintaining connections with participants.
Participants will be offered access to Internet-based Cognitive Behavioral Therapy (eCBT)28 targeting depression, which provides self-directed tutorials on behavior activation and cognitive restructuring, along with the opportunity for participants to monitor and track mood over time.
Participants will be referred to select DBT skills videos on the "Now Matters Now" website.29 Skills taught through these videos emphasize skills taught in DBT and other suicide prevention interventions, such as mindfulness, emotion regulation, distress tolerance, and reducing access to lethal means.
Two DBT groups will be offered: either a multi-family group with youth/parent dyads (for participants < 18 years old) or a young adult group (for participants 18 years and older).
The individual treatment that will be offered in this trial is based on established DBT treatment and uses: regular tracking of patient mood and self-harm urges and behavior; structuring the session agenda based on life-threatening targets first, therapy-threatening targets second, and quality-of-life threatening targets third; chain and solution analyses of any self-harm behavior or significant increase in suicidal urges; integration of DBT skills and strategies; availability of additional CBT modules targeting depression and insomnia; and strategies for enhancing family protection and support.28,30–34 Therapists will also have the freedom to incorporate other CBT treatment strategies, as well as motivational enhancement techniques, to meet the needs of participants.
Additional individual sessions were possible if requested by the care manager and approved by an intervention supervisor.
Non-Study Treatment.
As the study site offers DBT and other psychotherapy as part of usual care, non-study treatments will be considered when developing the treatment plan, with participants permitted to continue existing treatment. If participants elect to continue usual care treatments, redundant stepped care elements will not be added (e.g., Level 2 participant who continues non-study DBT group will not be added to stepped care DBT group but will be offered Level 2 services such as therapeutic care management contacts, eCBT, and DBT-video options).
Level Changes.
Participants initially assigned to Levels 1 or 2 who report enduring increases in NSSI, SI, SA, or depressive symptoms (i.e., two weeks of elevated PHQ-9 scores) will be evaluated for a level change using the risk stratification protocol. If stepped care level is increased, additional services will be offered to participants per the study protocol.
Acute vs. Continuation Intervention Phases.
Months 1–6 post-randomization are conceptualized as the acute intervention phase, during which time all Level 1, 2 and 3 stepped care services will be offered (Table 2). The continuation phase, months 7–12, will wrap up DBT group/individual contacts and emphasize care management with the goal of transferring ongoing care back to the health system. During the continuation phase, services will include monitoring for increased risk; monthly therapeutic care management contacts; supporting the continued use of skills helpful for decreasing risk and enhancing functioning; and motivational enhancement strategies that encourage linkage to, and participation in, needed care. For participants deemed low risk by the CM, monthly contacts will be reduced to every other month. If increased risk is detected during this phase, the treatment plan will be revised to resume monthly care management contacts and encourage linkage to needed services within the health system.
Care Manager Training and Quality Assurance
CMs will be master’s trained counselors with at least two years of clinical experience. Training will include detailed manuals and readings; 40–60 hours of training with DBT experts that include modeling and skill building role-plays; and ongoing review of recorded sessions to discuss clinical decisions, difficulties, and opportunities. To maintain treatment integrity, extensive quality assurance procedures will be implemented (see Supplementary Material for adherence protocol).
Assessment
Youth Assessments
Participants will be asked to complete four assessments. The first assessment collects baseline data and confirms study eligibility. Three follow-up assessments will occur at 3-, 6- and 12-months post randomization. Each assessment will include both self-report and interviewer-administered measures (Table 3). Baseline assessments will generally be administered in person but may be conducted by telephone if necessary. All follow-up assessments will be conducted by telephone, and participants will be asked to complete the self-report measures in REDCap prior to the interview portion of the assessment. The parents of all minors, and of some young adult participants who consent to parent participation, will also complete a baseline assessment.
Table 3.
Assessment measures by study time point, respondent, and administration type.
| Measure | Timepoint | Respondent | Administration | ||||
|---|---|---|---|---|---|---|---|
| BL | 3mo | 6mo | 12mo | INT | SR a | ||
| Primary & Secondary Outcomes | |||||||
| Columbia Suicide Severity Rating Scale (C-SSRS) | X | X | X | X | Y | X | X b |
| Suicide Attempt Self-Injury Interview (SASII) | X | X | X | X | Y | X | |
| Harkavy-Asnis Suicide Scale (HASS) | X | X | X | X | Y | X | |
| Center for Epidemiologic Studies Depression Scale (CES-D) | X | X | X | X | Y | X | |
| Mental & Behavioral Health | |||||||
| Alcohol Use Disorders Identification Test, Consumption Subscale (AUDIT-C) | X | X | X | Y | X | ||
| Brief Agitation Measure | X | X | X | Y | X | ||
| Drug Use Scale (DUS) | X | X | X | X | Y | X | |
| Generalized Anxiety Disorder Scale (GAD-7) | X | X | X | Y | X | ||
| Impulsive Premeditated Aggression Scale (IPAS) | X | X | X | Y | X | ||
| Kiddie Schedule for Affective Disorder & Schizophrenia (KSADS) | X | Y | X | ||||
| Patient Health Questionnaire – Depression (PHQ-9) | X | X | X | X | Y | X | |
| Personality Diagnostic Questionnaire (PDQ-4-BPD) | X | X | X | Y | X | ||
| Primary Care Posttraumatic Stress Disorder (PC-PTSD) Screen | X | Y | X | ||||
| PROMIS Fatigue | X | X | X | Y | X | ||
| PROMIS Sleep Disturbance | X | X | X | X | Y | X | |
| UPPS Impulsive Behavior Scale, Urgency Subscale | X | X | X | Y | X | ||
| Youth Risk Behavior Survey | X | X | X | Y | X | ||
| Other Suicide/SH Risk and Protective Factors | |||||||
| Connectedness Scale | X | X | X | Y | X | ||
| Difficulties in Emotion Regulation Scale, Short Form (DERS-SF) | X | X | X | X | Y | X | |
| Interpersonal Needs Questionnaire (INQ-R), Burdensomeness Scale | X | X | X | X | Y | X | |
| Life Events Scale, Adapted | X | X | X | X | Y | X | |
| National Firearm Survey, Gun Access | X | Y | X | ||||
| Parent Depression (PHQ-9) | X | P | X | ||||
| Parent Suicide History | X | Y | X | ||||
| Economic Analysis | |||||||
| All Services Outside KPNW (CASA Youth Report)c | X | X | X | X | Y | X | X |
| All Services Outside KPNW (CASA Parent Report) | X | P | X | ||||
| Sociodemographic and Other Characteristics | |||||||
| Demographics Youth | X | Y | X | X | |||
| Demographics Parent | X | P | X | ||||
| Everyday Discrimination Scale | X | X | Y | X | |||
| Family Acceptance Scale | X | X | Y | X | |||
| Homophobia Questionnaire | X | X | Y | X | |||
| Outness Inventory | X | X | Y | X | |||
| Sexual Identity Behavior and Attraction Scale | X | X | Y | X | |||
| Online Resource Use and Satisfaction | |||||||
| Mental Health Resources | X | Y | X | ||||
| Treatment Satisfaction | X | X | X | Y | X | ||
| Telehealth Treatment Satisfaction | X | Y | X | ||||
Abbreviations: BL=baseline; INT=interviewer-administered measure; SR=self-report measure; Y=youth; P=parent
If self-report measure not completed by youth/parent, then interviewer would administer at the time of assessment.
This measure was primarily interviewer-administered, but there were a small number of participants that completed a self-report version.
This measure was interviewer-administered at baseline and self-report at follow-up assessments.
Parent Assessment
Participants who are minors at enrollment will be required to have a parent or guardian participate. Participants aged 18 and older may invite a parent/guardian to take part, but parent participation will not be required. Participating parents will be asked to complete one assessment at baseline (see Table 3 for measures administered).
Incentives
Participants will receive Amazon gift card codes for completing assessments: $30 for the baseline assessment, $40 for the 3-month follow-up, $50 for the 6-month follow-up, and $60 for the 12-month follow-up. Parents will not receive incentives.
Measures
Table 3 lists all measures by study time point, respondent, and administration type. The primary and secondary outcome measures that will be used for primary analyses are described below. See Supplementary Material for additional measures that will be used in secondary analyses (see Analysis Plan section below for additional detail); these include measures to assess depression, anxiety, substance use, functioning, stressful events/environment, treatment received, demographics, and other suicide-related risk and protective variables.
Primary and Secondary Outcome Measures.
The primary outcome of interest is SAs (fatal, non-fatal, interrupted). Secondary outcomes of interest include (a) suicide events (SEs; defined as any SA, SA planning/preparatory acts, or active suicidal ideation, (b) SH episodes (SAs, NSSI), (c) severity of suicidality, and (d) depression symptomology. These outcomes will be captured using the measures described below. See section on Missing Data in Analysis Plan below and Supplementary Material section titled Data Source Rationale for detail regarding events included in primary models.
Columbia Suicide Severity Rating Scale (C-SSRS).35
The C-SSRS will be administered to assess suicidal ideation and suicidal behavior. Scoring is derived from 10 categories related to SI and suicidal behavior, each with a binary (yes/no) response scale.36 The first five categories are subtypes of ideation, and the remainder are subtypes of suicidal behavior. The categories of suicidality increase in severity, and the C-SSRS score represents the highest category endorsed. The baseline administration of the C-SSRS will ask participants about suicidal ideation and behavior within the past year and suicidal behavior in their lifetime. At follow-up, participants will be asked about suicidal ideation and behavior since their last assessment.
Suicide Attempt Self-Injury Interview (SASII).
For youth endorsing any SH on the C-SSRS, the SASII will be administered to provide information on the timing/date, method, intent, expectations for death, and lethality/medical consequences of suicidal and non-suicidal self-harm episodes. The SASII is an interviewer-administered, semi-structured measure that has been found to have good inter-rater reliability and sufficient external validity.37,38 At baseline, total number of lifetime SAs, NSSI episodes, and the number of these events leading to medical treatment will be recorded with additional detail obtained for (1) the five most recent SAs, (2) NSSI during the past six months, and (3) the NSSI episode that resulted in the most serious injury, if this episode is not captured in the past six months. At follow-up, the SASII will assess the interval between assessments.
Harkavy-Asnis Suicide Scale (HASS).39
We will administer the 17-item HASS self-report scale that has been validated for use with youth.40 Items assess the frequency of suicidal ideation (both passive and active) and suicidal behavior (including attempts) in the past month and are rated on a 5-point Likert scale ranging from 0 (never) to 4 (daily). Total score is calculated by summing the ratings across all items, with higher scores indicating greater severity of suicidal ideation and behavior.
Center for Epidemiologic Studies Depression Scale (CES-D).41,42
This 20-item measure assesses current depression symptomatology based on self-reported frequency of symptoms in the past week. Each item has four response options ranging from 0 (rarely or none of the time [less than 1 day]) to 3 (all of the time [5–7 days]). Total score is calculated by summing the points for the endorsed response options after four of the items are reverse-coded.
Other Data Sources
Electronic Health Record (EHR) Data Extraction
Healthcare services will be extracted from the EHR, including pharmacotherapy, inpatient and outpatient use, diagnostic tests, etc. EHR data related to suicide, other causes of death, suicide attempts, other self-inflicted injury and injury method codes, hospitalizations, ED visits, and emergency psychiatric calls will also be obtained. EHR data covering a 4-year period (two years after enrollment and two years prior to enrollment) will be extracted and summarized at the end of the study for analysis.
Therapy Process Data
Data on intervention implementation and dose will be obtained from CM entries into the study tracking system and will include contact type (e.g., group or individual session, care management contact); delivery mode (in-person, phone, video); specific skills/content areas addressed during contact (e.g., crisis survival, problem solving, relapse prevention); session date, time, and duration; parent involvement; topics covered (e.g., treatment planning, safety plan review/adjustment, referrals); and when administered, participant PHQ-9 and C-SSRS scores.
Analysis Plan
RCT Analyses (Aim 1)
Overview.
Descriptive statistics and graphical summaries will be obtained for all demographic, clinical, and outcome measures to characterize the sample, compare the intervention groups at baseline, determine the advisability of scale transformations or non-parametric methods, and identify missing values, outliers, or other influential features. While primary analyses will be unadjusted,43 factors that show significant differences and are correlated with the primary outcomes will be included as covariates in follow-up sensitivity analyses to verify the robustness of observed intervention effects. We will also plot longitudinally obtained measures to identify trends and examine the timing of self-harm/suicide events relative to individual changes in suicidality and depression symptoms.
General Analytic Approach.
We will collect three primary types of data: binary outcomes with a date stamp (e.g., SAs), continuous outcomes (e.g., depression symptom scales) measured at each study assessment, and count variables (e.g., number of SH/NSSI events) aggregated across the intervention period. Correspondingly, our primary analytic tools will be survival analysis for dated outcomes and generalized linear mixed models (GLMMs) for continuous and count variables. Survival models appropriately handle censoring of time to event data, while GLMMs accommodate multiple outcome types via use of appropriate link functions (logistic, Poisson, identity). They include cross-sectional and longitudinal models in a common framework, allow both fixed and time-varying covariates and automatically handle missing data, producing unbiased estimates when observations are missing at random.
Time to Event Data.
We will compare the rates of SAs over time between the ZSQI and SC-SP groups using non-parametric Kaplan-Meier estimates of the survival functions (for time to first event in observation window). We will use the log-rank test to compare the curves overall and will also test for group differences at major follow-up points (3, 6, 12 months). Our primary interest is the difference in rates at the end of treatment, which, absent censoring due to early dropout, would be equivalent to a two-sample test of proportions (see missing data section below). In principle, estimates of the median times to event will be biased downwards due to censoring as the 12-month rates of SAs are anticipated to be below 50% in both groups; however, the curves will provide some information about the relative timing of events. Cox proportional hazards models can be used to adjust for covariates, if necessary, although underlying assumptions and model stability will need to be checked, especially if the event rate is extremely low in the SC-SP group. Parallel analyses will be performed for the secondary and exploratory time-to-event outcomes.
Repeated Measures Data.
For continuous outcomes we will fit GLMMs with intervention group (ZSQI, SC-SP), time (0, 3, 6, 12 months), and a group by time interaction as the primary predictors, along with random effects (intercepts and, if necessary, slopes) to account for the correlations induced by repeated measurements within subjects. When differential treatment effects over time (interactions) are significant, post-hoc contrasts can be used to compare the groups at individual time points and to examine the magnitude of within-group changes. The base model will treat time as continuous.
However, if the longitudinal plots suggest an alternative trajectory form (e.g., leveling off after the active treatment period), then time can be treated as a factor and a more complex covariance structure employed. The relative performance of these models can be compared using a likelihood ratio chi-squared test.
Count Outcomes.
To compare ZSQI and SC-SP groups on number of SH incidents, we will use a Poisson regression model treating length of follow-up as an offset variable. Our primary interest is in the 12-month comparison, but equivalent models can be fit at 3 and 6 months to determine whether treatment differences emerge earlier. We will test for over-dispersion and if present, consider the inclusion of covariates (to reduce heterogeneity) or alternative methods including negative binomial regression and zero-inflated models. If enough subjects have multiple SAs, we will run parallel models for these outcomes.
Missing Data and Multiple Testing Issues.
The survival and GLMM models naturally handle partially and fully missing outcome values provided the missingness is non-informative. Moreover, we will capture some deaths and non-fatal SAs through the EHR and state cause of death (COD) registries even for those individuals who drop out of the study or miss follow-up assessments. Thus, we do not anticipate that missing data will be a significant problem. Nonetheless, we will use logistic regression to identify factors related to early dropout and, if needed, perform follow-up sensitivity analyses, rerunning the primary models, adding values obtained through the EHR and state registries, and/or covarying for factors related to study withdrawal (see Supplementary Material for data source rationale). In the case of missing covariates or outcomes available only at the end of treatment, we will use the fully conditional specification approach44–49 handling each assessment point and treatment arm separately to avoid contamination of intervention-effect estimates. We are aware of multiple testing issues and will protect against Type I errors by carefully specifying primary outcomes and contrasts within each aim.50 For secondary and exploratory outcomes we will use the false discovery rate approach51 to correct for the number of tests within each domain; a Bonferroni correction would be over-conservative since many of the secondary outcomes are overlapping and the tests will consequently be correlated.
Power and Sample Size.
We plan to enroll 150 participants per arm and allow for 20% attrition by 12 months, leaving a minimum of 240 participants with complete data to achieve 80% power for two-sided significance tests. See Supplementary Material for additional detail.
Cost Effectiveness Analyses (Aim 2)
We will conduct patient-level intent-to-treat cost-effectiveness analyses (CEA). We do not anticipate that costs will be normally distributed, thus we plan to use the net benefit regression method52 with ordinary least squares regression analyses. The robustness of the parametric tests will be confirmed using non-parametric bootstrapping with a single model with 1000 replications using the bias corrected and accelerated method.53–55 Adjusted differences between the conditions will be estimated using ordinary least squares regression models with bootstrap interval estimates, and all analyses will be adjusted for baseline characteristics. We will conduct a base-case CEA from the health system perspective, and sensitivity analyses to examine uncertainty around key variables, such as unit cost estimates. We plan to implement a variety of methods to minimize the loss of clinical outcome and health services data for all study participants. However, we expect there will be data missing at some time points on some measures for some subjects. Missing data will be imputed using the multiple imputation by chained equations approach.44,56 Sample sizes comparable to ours have been sufficient for CEA using similar techniques.57
Discussion
This research study compares two strategies for preventing suicide and SAs among youth in a health system that uses an integrated EHR and provides both medical and behavioral health care. We focus on adolescents and young adults, an age group for whom suicide is currently the second-leading cause of death in the United States. Because first onset of suicidal behavior often occurs during this developmental period, and rates of suicide and SAs continue to increase as this group ages, documenting an effective suicide prevention strategy has the potential to reduce suicide and SAs during adolescence, young adulthood, and later life.
Barriers to suicide prevention need to be identified and addressed. Prior SH is the most robust predictor of suicide deaths and attempts.58–61 While providing effective depression treatment can reduce a major suicide risk factor, RCTs evaluating current youth depression treatments have not shown reductions in suicide or SA rates relative to control conditions.61–63 A recent meta-analysis indicated a small significant effect for current therapeutic interventions in reducing SH, with the strongest effects for DBT, CBT for suicidality and substance abuse, and mentalization based therapy.64 Additional evidence from two independent RCTs showed that DBT was associated with lower levels of SH relative to comparator conditions,20,65 which has made DBT the first intervention to meet Level 1 standards and be considered a well-established treatment for SH in adolescents.66,67
Given the high cost of inpatient care, along with limited evidence on the efficacy of inpatient psychiatric hospitalization for suicidal patients,4 effective outpatient approaches are needed. A DBT-informed stepped care approach has potential for improving access to effective suicide prevention services. Evaluation of this stepped care approach within a healthcare system that has already committed to ZSQI provides a unique opportunity, as the current strategy can be compared directly with a stepped care approach intended to enhance ZSQI efforts.
Supplementary Material
Acknowledgements
We would like to thank Shelley Reetz for her contributions to the development of the study protocol. We would also like to thank Jill Pope for editing this manuscript in preparation for submission.
Funding Source
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH112147. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures
The authors have no competing interests to declare.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Institute of Health (NIH). CDC Suicide Statistics. Accessed August 23rd, 2021, https://www.nimh.nih.gov/health/statistics/suicide
- 2.Nock MK, Green JG, Hwang I, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. Mar 2013;70(3):300–10. doi: 10.1001/2013.jamapsychiatry.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office of Disease Prevention and Health Promotion. Mental Health Statistics. Accessed August 24th, 2021, https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Mental-Health/data
- 4.Clinical Care and Intervention Task Force. Suicide Care in Systems Framework. National Action Alliance for Suicide Prevention. Accessed August 24, 2021, https://theactionalliance.org/sites/default/files/clinicalcareinterventionreport.pdf Published 2011. [Google Scholar]
- 5.Office of the Surgeon General National Action Alliance for Suicide Prevention. 2012 National Strategy for Suicide Prevention: Goals and Objectives for Action: A report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention. 2012. [PubMed]
- 6.May EL. The power of zero: Steps toward high reliability healthcare. Healthc Exec. Apr 2013;28(2):16–8, 20, 22 passim. [PubMed] [Google Scholar]
- 7.Abrams DB, Orleans CT, Niaura RS, Goldstein MG, Prochaska JO, Velicer W. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: A combined stepped-care and matching model. Ann Behav Med. 1996;18(4):290–304. doi: 10.1007/BF02895291 [DOI] [PubMed] [Google Scholar]
- 8.McFarland KF. Type 2 diabetes: Stepped-care approach to patient management. Geriatrics. Oct 1997;52(10):22–6, 35, 39. [PubMed] [Google Scholar]
- 9.Von Korff M, Tiemens B. Individualized stepped care of chronic illness. West J Med. Feb 2000;172(2):133–7. doi: 10.1136/ewjm.172.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Straten A, Hill J, Richards DA, Cuijpers P. Stepped care treatment delivery for depression: A systematic review and meta-analysis. Psychol Med. Jan 2015;45(2):231–46. doi: 10.1017/S0033291714000701 [DOI] [PubMed] [Google Scholar]
- 11.Edmond SN, Moore BA, Dorflinger LM, et al. Project STEP: Implementing the Veterans Health Administration’s Stepped Care Model of Pain Management. Pain Med. Sep 1 2018;19(suppl_1):S30–S37. doi: 10.1093/pm/pny094 [DOI] [PubMed] [Google Scholar]
- 12.Jobes DA, Gregorian MJ, Colborn VA. A stepped care approach to clinical suicide prevention. Psychol Serv. Aug 2018;15(3):243–250. doi: 10.1037/ser0000229 [DOI] [PubMed] [Google Scholar]
- 13.Jobes DA, Chalker SA. One Size Does Not Fit All: A Comprehensive Clinical Approach to Reducing Suicidal Ideation, Attempts, and Deaths. Int J Environ Res Public Health. Sep 26 2019;16(19)doi: 10.3390/ijerph16193606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobes DA. Managing Suicidal Risk: A Collaborative Approach. 2nd ed. The Guilford Press; 2016. [Google Scholar]
- 15.Treatment for Suicidal Ideation, Self-Harm, and Suicide Attempts Among Youth (samhsa.gov). Accessed November 29, 2021, https://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP20-06-01-002.pdf
- 16.Asarnow JR, Hughes JL, Babeva KN, Sugar CA. Cognitive-behavioral family treatment for suicide attempt prevention: A randomized controlled trial. J Am Acad Child Adolesc Psychiatry. Jun 2017;56(6):506–514. doi: 10.1016/j.jaac.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asarnow JR, Baraff LJ, Berk M, et al. An emergency department intervention for linking pediatric suicidal patients to follow-up mental health treatment. Psychiatr Serv. Nov 2011;62(11):1303–9. doi: 10.1176/ps.62.11.pss6211_1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Anci KE, Uhl S, Giradi G, Martin C. Treatments for the Prevention and Management of Suicide: A Systematic Review. Ann Intern Med. Sep 3 2019;171(5):334–342. doi: 10.7326/M19-0869 [DOI] [PubMed] [Google Scholar]
- 19.DeCou CR, Comtois KA, Landes SJ. Dialectical Behavior Therapy Is Effective for the Treatment of Suicidal Behavior: A Meta-Analysis. Behav Ther. Jan 2019;50(1):60–72. doi: 10.1016/j.beth.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 20.McCauley E, Berk MS, Asarnow JR, et al. Efficacy of Dialectical Behavior Therapy for Adolescents at High Risk for Suicide: A Randomized Clinical Trial. JAMA Psychiatry. Aug 1 2018;75(8):777–785. doi: 10.1001/jamapsychiatry.2018.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huey SJ Jr., Henggeler SW, Rowland MD, et al. Multisystemic therapy effects on attempted suicide by youths presenting psychiatric emergencies. J Am Acad Child Adolesc Psychiatry. Feb 2004;43(2):183–90. doi: 10.1097/00004583-200402000-00014 [DOI] [PubMed] [Google Scholar]
- 22.Giles L, Shepard L, Asarnow J, Keeshin BR. Implementation of a trauma-informed suicide prevention intervention for youth presenting to the emergency department in crisis. Evid Based Pract Child Adolesc Ment Health. 2021;6(3):343–353. doi: 10.1080/23794925.2021.1961643 [DOI] [Google Scholar]
- 23.Goldston DB, Asarnow JR. Quality improvement for acute trauma-informed suicide prevention care: Introduction to special issue. Evid Based Pract Child Adolesc Ment Health. 2021;6(3):303–306. doi: 10.1080/23794925.2021.1961645 [DOI] [Google Scholar]
- 24.Rotheram-Borus MJ, Piacentini J, Cantwell C, Belin TR, Song J. The 18-month impact of an emergency room intervention for adolescent female suicide attempters. J Consult Clin Psychol. Dec 2000;68(6):1081–93. doi: 10.1037//0022-006x.68.6.1081 [DOI] [PubMed] [Google Scholar]
- 25.Rotheram-Borus MJ, Bradley J. Triage model for suicidal runaways. Am J Orthopsychiatry. Jan 1991;61(1):122–7. doi: 10.1037/h0079228 [DOI] [PubMed] [Google Scholar]
- 26.Asarnow JR, Berk MS, Baraff LJ. Family intervention for suicide prevention: A specialized emergency department intervention for suicidal youths. Prof Psychol Res Pr. 2009;40(2):118–125. doi: 10.1037/a0012599 [DOI] [Google Scholar]
- 27.Asarnow JR, Jaycox LH, Duan N, et al. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: A randomized controlled trial. JAMA. 2005;293(3):311–319. doi: 10.1001/jama.293.3.311 [DOI] [PubMed] [Google Scholar]
- 28.Clarke G, Kelleher C, Hornbrook M, Debar L, Dickerson J, Gullion C. Randomized effectiveness trial of an Internet, pure self-help, cognitive behavioral intervention for depressive symptoms in young adults. Cogn Behav Ther. 2009;38(4):222–34. doi: 10.1080/16506070802675353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteside U, Richards J, Huh D, et al. Development and Evaluation of a Web-Based Resource for Suicidal Thoughts NowMattersNow.org. J Med Internet Res. May 2 2019;21(5):e13183. doi: 10.2196/13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linehan M. DBT skills training manual. 2nd ed. 2014. [Google Scholar]
- 31.Asarnow JR, Berk M, Hughes JL, Anderson NL. The SAFETY Program: a treatment-development trial of a cognitive-behavioral family treatment for adolescent suicide attempters. J Clin Child Adolesc Psychol. 2015;44(1):194–203. doi: 10.1080/15374416.2014.940624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asarnow J, Jaycox L, Rea M, et al. Stress and your mood: A manual for individuals. UCLA School of Medicine; 2003. [Google Scholar]
- 33.Asarnow J, Berk MS, Navarro M, Huey SJ Jr., Langer D. The Safety Program: Ecological cognitive-behavioral intervention for adolescent suicide attempters. UCLA Semel Institute; 2006. doi: 10.1080/15374416.2014.940624. [DOI] [Google Scholar]
- 34.Clarke G, DeBar LL, Ludman E, Asarnow JR, Jaycox L, Firemark A. STAND project intervention manual: Collaborative care, cognitive-behavioral program for depressed youth in a primary care setting. Kaiser Permanente Center for Health Research; 2006. [Google Scholar]
- 35.The Columbia Protocol for Research. The Columbia Lighthouse Project. Accessed September 13, 2021, https://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-research/
- 36.Nilsson ME, Suryawanshi S, Gassmann-Mayer C, Dubrava S, McSorley P, Jiang K. Columbia–suicide severity rating scale scoring and data analysis guide. Accessed September 13, 2021, https://cssrs.columbia.edu/wp-content/uploads/ScoringandDataAnalysisGuide-for-Clinical-Trials-1.pdf
- 37.Linehan MM, Comtois KA, Brown MZ, Heard HL, Wagner A. Suicide Attempt Self-Injury Interview (SASII): Development, reliability, and validity of a scale to assess suicide attempts and intentional self-injury. Psychol Assess. Sep 2006;18(3):303–12. doi: 10.1037/1040-3590.18.3.303 [DOI] [PubMed] [Google Scholar]
- 38.Asarnow JR, Bai S, Babeva KN, et al. Sleep in youth with repeated self-harm and high suicidality: Does sleep predict self-harm risk? Suicide Life Threat Behav. Dec 2020;50(6):1189–1197. doi: 10.1111/sltb.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman JMH, Asnis GM. Assessment of suicidal behavior: A new instrument. Psychiatr Ann. 1989;19(7):382–387. doi: 10.3928/0048-5713-19890701-11 [DOI] [Google Scholar]
- 40.Asarnow J, McArthur D, Hughes J, Barbery V, Berk M. Suicide attempt risk in youths: Utility of the Harkavy-Asnis suicide scale for monitoring risk levels. Suicide Life Threat Behav. Dec 2012;42(6):684–98. doi: 10.1111/j.1943-278X.2012.00122.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): A systematic review with meta-analysis. PLoS One. 2016;11(5):e0155431. doi: 10.1371/journal.pone.0155431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 43.Kraemer HC. A source of false findings in published research studies: Adjusting for covariates. JAMA Psychiatry. Oct 2015;72(10):961–2. doi: 10.1001/jamapsychiatry.2015.1178 [DOI] [PubMed] [Google Scholar]
- 44.Potthoff RF, Tudor GE, Pieper KS, Hasselblad V. Can one assess whether missing data are missing at random in medical studies? Stat Methods Med Res. Jun 2006;15(3):213–34. doi: 10.1191/0962280206sm448oa [DOI] [PubMed] [Google Scholar]
- 45.Little RJ, Rubin DB. Statistical analysis with missing data. 2 ed. John Wiley & Sons; 2002. [Google Scholar]
- 46.National Research Council. The prevention and treatment of missing data in clinical trials. The National Academies Press; 2010. doi: 10.17226/12955. [DOI] [PubMed] [Google Scholar]
- 47.O’Neill RT, Temple R. The prevention and treatment of missing data in clinical trials: An FDA perspective on the importance of dealing with it. Clin Pharmacol Ther. 2012;91(3):550–4. doi: 10.1038/clpt.2011.340 [DOI] [PubMed] [Google Scholar]
- 48.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7(2):147–77. doi: 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- 49.Raghunathan T, Lepkowski J, Hoewyk JV, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Accessed December 21, 2021, https://www.researchgate.net/profile/James-Lepkowski/publication/244959137_A_Multivariate_Technique_for_Multiply_Imputing_Missing_Values_Using_a_Sequence_of_Regression_Models/links/543509d30cf294006f737dca/A-Multivariate-Technique-for-Multiply-Imputing-Missing-Values-Using-a-Sequence-of-Regression-Models.pdf
- 50.Little RJ, Wang J, Sun X, et al. The treatment of missing data in a large cardiovascular clinical outcomes study. Clin Trials. Jun 2016;13(3):344–51. doi: 10.1177/1740774515626411 [DOI] [PubMed] [Google Scholar]
- 51.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. Jul 1990;9(7):811–8. doi: 10.1002/sim.4780090710 [DOI] [PubMed] [Google Scholar]
- 52.Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: A framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002;11(5):415–430. doi: 10.1002/hec.678 [DOI] [PubMed] [Google Scholar]
- 53.O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: An introduction to statistical issues and methods. Stat Methods Med Res. Dec 2002;11(6):455–68. doi: 10.1191/0962280202sm304ra [DOI] [PubMed] [Google Scholar]
- 54.O’Brien BJ, Drummond MF, Labelle RJ, Willan A. In search of power and significance: Issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care. Feb 1994;32(2):150–63. doi: 10.1097/00005650-199402000-00006 [DOI] [PubMed] [Google Scholar]
- 55.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320(7243):1197–1200. doi: 10.1136/bmj.320.7243.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Royston P Multiple imputation of missing values. Stata Journal. 2004;4(3):227–241. doi: 10.1177/1536867X0400400301 [DOI] [Google Scholar]
- 57.Lynch FL, Dickerson JF, Clarke G, et al. Incremental cost-effectiveness of combined therapy vs medication only for youth with selective serotonin reuptake inhibitor-resistant depression: Treatment of SSRI-resistant depression in adolescents trial findings. Arch Gen Psychiatry. Mar 2011;68(3):253–62. doi: 10.1001/archgenpsychiatry.2011.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawton K, Bergen H, Kapur N, et al. Repetition of self-harm and suicide following self-harm in children and adolescents: Findings from the multicentre study of self-harm in England. J Child Psychol Psychiatry. 2012;53(12):1212–9. doi: 10.1111/j.1469-7610.2012.02559.x [DOI] [PubMed] [Google Scholar]
- 59.Hawton K, Bergen H, Cooper J, et al. Suicide following self-harm: Findings from the multicentre study of self-harm in England, 2000–2012. J Affect Disord. 2015;175:147–51. doi: 10.1016/j.jad.2014.12.062 [DOI] [PubMed] [Google Scholar]
- 60.Finkelstein Y, Macdonald EM, Hollands S, et al. Long-term outcomes following self-poisoning in adolescents: A population-based cohort study. Lancet Psychiatry. Jun 2015;2(6):532–9. doi: 10.1016/S2215-0366(15)00170-4 [DOI] [PubMed] [Google Scholar]
- 61.Brent DA, McMakin DL, Kennard BD, Goldstein TR, Mayes TL, Douaihy AB. Protecting adolescents from self-harm: A critical review of intervention studies. J Am Acad Child Adolesc Psychiatry. Dec 2013;52(12):1260–71. doi: 10.1016/j.jaac.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA. Feb 27 2008;299(8):901–913. doi: 10.1001/jama.299.8.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. Aug 18 2004;292(7):807–20. doi: 10.1001/jama.292.7.807 [DOI] [PubMed] [Google Scholar]
- 64.Ougrin D, Tranah T, Stahl D, Moran P, Asarnow JR. Therapeutic interventions for suicide attempts and self-harm in adolescents: Systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. Feb 2015;54(2):97–107 e2. doi: 10.1016/j.jaac.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 65.Mehlum L, Ramleth RK, Tormoen AJ, et al. Long term effectiveness of dialectical behavior therapy versus enhanced usual care for adolescents with self-harming and suicidal behavior. J Child Psychol Psychiatry. Oct 2019;60(10):1112–1122. doi: 10.1111/jcpp.13077 [DOI] [PubMed] [Google Scholar]
- 66.Glenn CR, Franklin JC, Nock MK. Evidence-based psychosocial treatments for self-injurious thoughts and behaviors in youth. J Clin Child Adolesc Psychol. 2015;44(1):1–29. doi: 10.1080/15374416.2014.945211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asarnow JR, Mehlum L. Practitioner review: Treatment for suicidal and self-harming adolescents - advances in suicide prevention care. J Child Psychol Psychiatry. Oct 2019;60(10):1046–1054. doi: 10.1111/jcpp.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


