Abstract
Background
Serum aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR) is one of the most frequent indicators to discriminate fibrosis and cirrhosis. However, the results remained controversial. The aim of this study was to evaluate the predictive effect of AAR on hepatitis B virus (HBV)-related cirrhosis development.
Method
A retrospective cohort study was conducted based on 1754 chronic HBV-infected patients. Clinical variables at their initial visit and follow-up data were collected. Cox proportional hazards model was constructed to evaluate the predictive value of AAR on cirrhosis risk, and its discrimination accuracy was determined by receiver operating characteristic (ROC). The time-dependent effect was assessed by a Fine and Gray competing risk model.
Results
Compared to patients with lower AAR, those with elevated AAR level had higher risk of cirrhosis development by adjusting for host characteristics (dichotomized analyses: hazard ratio = 2.77, P = 8.25 × 10-4; tertile analyses: hazard ratio = 2.95, P = 1.61 × 10-3), with an increasing risk trend (P trend = 4.56 × 10-4). The effect remained prominent when ALT or AST was abnormal, while no significant risk was observed when AST and ALT were simultaneously normal. Time-dependent effect analysis demonstrated a persistently higher risk, with the average hazard ratio equivalent to 1.92. AAR level could improve the discrimination efficacy of host variables with area under the curve increased from 0.684 to 0.711 (P = 0.039).
Conclusion
Higher AAR was significantly associated with increased risk of HBV-related cirrhosis, and might be a potential predictor of cirrhosis development.
Keywords: aspartate aminotransferase, alanine aminotransferase, cirrhosis, cohort study, hepatitis B virus
Background
Cirrhosis is the final stage of liver fibrosis, which is characterized by extensive degeneration and necrosis of hepatocytes, diffuse proliferation of fibrous tissue, as well as formation of regenerative nodules. It can be identified as a perpetuated wound-healing process after undergoing any chronic liver injury, in which chronic HBV infection play a dominate role. It is reported that there are nearly 257 million people suffering from hepatitis B virus (HBV) infection over the world, and approximately 444 000 people die from cirrhosis in 1 year [1].Cirrhosis has become a greater burden on the individual and on public health, which was estimated to be responsible for 560.4 age-standardized deaths per 100 000 population globally in 2019. And meanwhile it will give rise to growing financial burden [2]. The presence of cirrhosis is also the main factor associated with the prognosis and management of chronic liver diseases (CLDs), triggering for surveillance programmes for hepatocellular carcinoma and esophageal varices inpatients [3,4].
Accordingly, it is highly of great importance and cost-effectiveness for early identification on cirrhosis. Liver biopsy which has been considered the standard for cirrhosis has been limited in clinical application owing to its invasion, unclear accuracy and costly procedure [5]. Thus, various non-invasive approaches, based on the detection of individual serum markers, displaying either the deposition or the removal of extracellular matrix in the liver or simple routine blood test were proposed [6]. Aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR) was one of the most extensively explored variables for its risk association with cirrhosis but harvesting no consistent finding.
Herein, we sought to conduct a prospective study to thoroughly evaluate the risk association of AAR level with cirrhosis development in hospitalized patients, in order to determine whether AAR can be used as a reliably predictive index. To the best of our knowledge, this is one of the first prospective analysis performed to evaluate the predictive role of AAR on cirrhosis in hospitalized CHB patients.
Methods
Study population
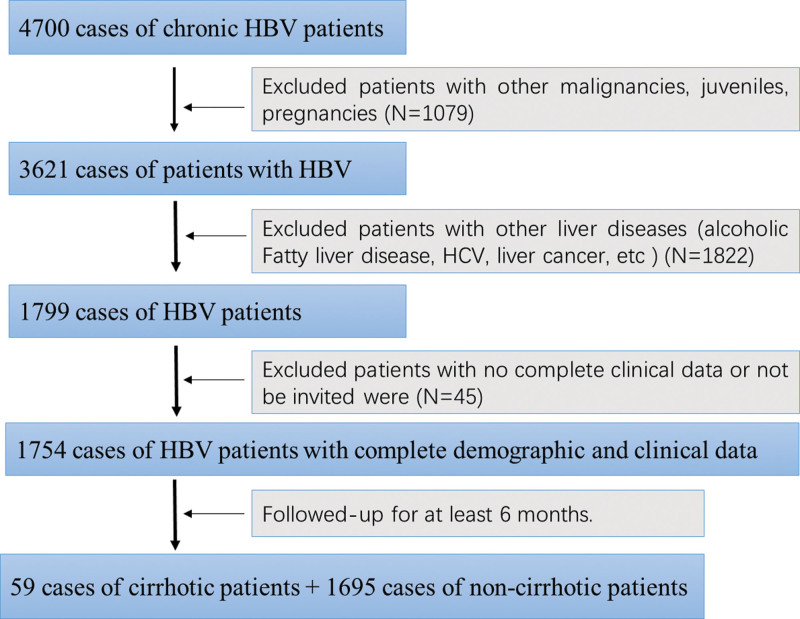
All subjects in this study were identified from a hospital-based cohort, which was comprised of hospitalized patients who visited the Ganzhou Fifth People’s Hospital and underwent treatment from 2016 to 2021.There was a total of 4700 chronic HBV patients enrolled in this cohort. In final, as shown in Fig. 1, we selected 1754 cases of chronic HBV patients by exclusive of the ones who did meet for the following criteria: (i)patients who were confirmed as liver cancer or other malignancies, or combined with other diseases such as hepatitis C virus (HCV), non-alcoholic fatty liver (NAFL), HIV, etc, so as to eliminate the confounding effects from other illness etiologies. (ii) patients who were pregnancies, juveniles or mental disordered. (iii) patients with severe diseases in other systems; (iv) patients with no complete clinical data or follow-up information, or whose follow-up time was shorter than 6 months. The study was approved by Ethics Committee of Ganzhou Fifth People’s Hospital and strictly comfort to the guidelines referring to administrate human subject-based research. The informed consents were obtained from each patient.
Fig. 1.
Enrollment diagram of study population.
Data collection
Demographic, clinical data and laboratory test were acquired for each patient by medical chart review and/or consultation with the competent physicians. Demographic parameters were collected such as gender, age, smoking status and drinking status. Clinical variables included whether to receive antiviral treatment (including Oral Entecavir/ tenofovir dipivoxil, or Injecting interferon α-2b), imaging examinations [computed tomography (CT) or MRI, etc, presentations (such as ascites, encephalopathy, and gastrointestinal bleeding), etc. Laboratory test with the records of albumin (ALB), total bilirubin, AST, ALT, HBV antigen and antibody detection] at the initial visit during this study. For each patient, hepatitis viral infection status was clinically determined before or at enrollment. The CHB patients were defined as being infected by HBV viral for at least 6 months. The albumin–bilirubin (ALBI) score, a simple index reflecting the underlying liver function, was calculated for each patient by the following formula based on the albumin and bilirubin levels: ALBI score=0.66×log10 bilirubin – 0.085×albumin, where bilirubin is in μmol/L and albumin in g/L. ALBI grade was assessed according to ALBI score, when ALBI score ≤-2.60 it was determined as ALBI grade 1, and grade 2 when ranged (-2.60,1.39), grade 3 when ALBI score>1.39. The AAR was defined as AST divided by ALT using the baseline laboratory test data. Liver cirrhosis was diagnosed mainly through imaging studies, which was charactered by medial segment atrophy of the left lobe, caudate lobe hypertrophy, or liver nodularity for early-stage disease, and images of portal hypertension such as varices, splenomegaly, patent paraumbilical vein, or ascites in advanced stage disease. Other evidences were adopted to support imaging results, consisting of clinical presentations (such as ascites, encephalopathy, and gastrointestinal bleeding), laboratory examination (such as thrombocytopenia, serum albumin, and prolonged prothrombin time), and liver biopsy.
After their first hospitalization, patients were followed up every 3 months. Endpoint of this study was liver cirrhosis development. Time to cirrhosis development was generated by the date difference from the study entry to the date of cirrhosis development during follow-up, or the date of last follow-up if the patients were alive or dead without cirrhosis. Patients free of cirrhosis at the whole follow-up period were censored for analysis. All baseline data were obtained at the first hospitalization period.
Statistical analysis
Statistical analysis was performed using the R software version 4.1.2. The risk of HBV-related cirrhosis with patients’ characteristics and AAR were estimated using the Cox proportional hazards regression model representing by hazards ratio (HR) and 95% confidence interval (CI), adopting univariate as well as multivariate analyses adjusting for age, gender, smoking status, drinking status, ALBI grade and AAR value. The AAR value was expressed asa categorical variable grouping by the median and the interquartile cutoff. The P for trend between AAR and cirrhosis risk was analyzed by setting AAR value as a continuous variable. Kaplan–Meier analysis was conducted to distinguish the cumulative incidence of cirrhosis development in patients with different AAR value, with log-rank test being used to examine the statistical significance. Receiver operating characteristic (ROC) curves were constructed to evaluate the area under the curve (AUC), the specificity and sensitivity of predicting cirrhosis by respectively using AAR value, demographic variables and the combination of AAR and demographic variables, which were correspondingly called AAR model, demo model and full model. An ROC test was carried out to compare the AUC difference between demo model and full model by the method of 2000 bootstrap resampling. In order to explore the time-dependent effects of AAR on cirrhosis risk during follow-up time, we constructed a Fine and Gray competing risk model to present the dynamic HR value. All statistical tests were two-sided, and the difference was statistically significant as the threshold of P-value was <0.05.
Results
Characteristics of the study population
There were a total of 1754 cases of chronic HBV patients enrolled in this study, who were all followed up over a 1-year exclusion window. During a median follow-up time of 2.6 (interquartile range: 1.8–3.6) years, 59 patients developed to cirrhosis (incidence rate, 3.36%). As summarized in Table 1, the majority of patients were male (89.83%), never smokers (50.85%), ever drinkers (61.02%), ALBI Grade 2 and 3 (76.27%), and accepted anti-virus treatment (71.19%). Older age (HR = 1.97, 95%CI 1.11–3.51) and receiving anti-virus treatment (HR = 1.82, 95%CI 1.03–3.22) were the most significant risk factors for cirrhosis development. Although significantly increased cirrhosis risks were also observed in ever smokers, and among patients with ALBI grade 2 and 3 in the univariate analysis, the associations became nonsignificant in the multivariate analysis.
Table 1.
The association between patients’ characteristics and HBV-induced cirrhosis risk
| Variables | Total (%) | Cirrhosis (%) | Univariate | Multivariatea | ||
|---|---|---|---|---|---|---|
| (n = 1754) | (n = 59) | HR (95%CI) | P-value | HR (95% CI) | P-value | |
| Age (years old) | ||||||
| <37 | 909 (51.82) | 18 (30.51) | 1.00 | 1.00 | ||
| ≥37 | 845 (49.28) | 41 (69.49) | 2.49 (1.43–4.33) | 1.34 × 10-3 | 1.97 (1.11–3.51) | 0.021 |
| Gender | ||||||
| Female | 355 (20.24) | 6 (10.17) | 1.00 | 1.00 | ||
| Male | 1399 (79.76) | 53 (89.83) | 2.26 (0.97–5.27) | 0.058 | 1.74 (0.71–4.27) | 0.224 |
| Smoking status | ||||||
| Never smokers | 1239 (70.64) | 30 (50.85) | 1.00 | 1.00 | ||
| Ever smokers | 515 (29.36) | 29 (49.15) | 2.33 (1.39–3.91) | 1.27 × 10-3 | 1.69 (0.96–2.98) | 0.070 |
| Drinking status | ||||||
| Never drinkers | 918 (52.34) | 23 (38.98) | 1.00 | 1.00 | ||
| Ever drinkers | 836 (47.66) | 36 (61.02) | 1.69 (1.00–2.86) | 0.051 | 1.24 (0.71–2.6) | 0.443 |
| ALBI Grade | ||||||
| 1 | 617 (35.18) | 14 (23.73) | 1.00 | 1.00 | ||
| 2 and 3 | 1137 (64.82) | 45 (76.27) | 1.88 (1.03–3.43) | 0.040 | 1. 56 (0.84–2.89) | 0.160 |
| Receiving antiviral treatment | ||||||
| Yes | 1456 (83.01) | 42 (71.19) | 1.00 | 1.00 | ||
| No | 298 (16.99) | 17 (28.81) | 1.83 (1.04–3.22) | 0.037 | 1.82 (1.03–3.22) | 0.039 |
Adjusted for age, gender, smoking and drinking status, ALBI grade, antiviral treatment.
Prospective association of AAR with cirrhosis risk in HBV patients
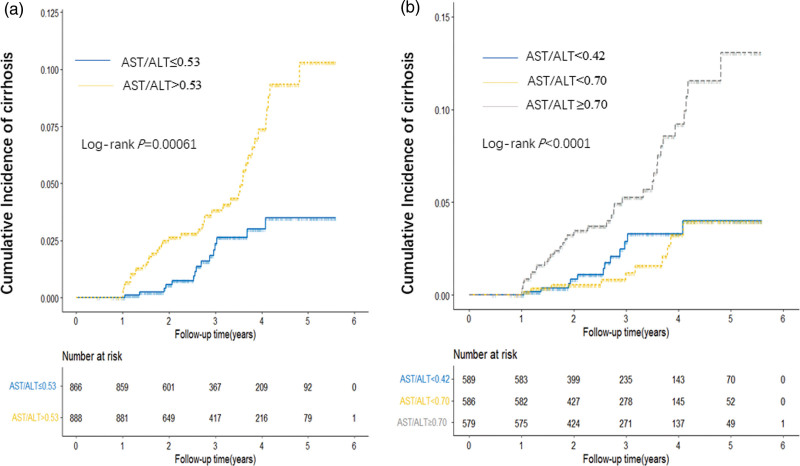
We analyzed the association between baseline value of AAR and cirrhosis risk using univariate and multivariate Cox model by categorizing the ratio into two levels (dichotomization analysis using a cutoff of the median AAR in all subjects) and three levels (tertile analysis using AAR cutoffs of tri-sectional quantiles in all subjects) (Table 2), using the lowest AAR value as reference. As shown in Table 2, a significant increasing risk between dichotomized value of AAR and cirrhosis were observed by both of univariate analysis and multivariate analysis. An increasing risk trend was exhibited with the increase of AAR according to quartile analyses (P trend = 4.56 × 10-4). Consistent with the Cox analyses, cumulative incidences of cirrhosis assessed by the Kaplan–Meier method and Log-rank test were significantly higher in patients with higher AAR in both dichotomization and tertile analyses (Fig. 2).
Table 2.
The association between AAR value and cirrhosis risk
| AAR value | Total (%) | Cirrhosis(%) | Univariate | Multivariatea | Log-rank P | ||
|---|---|---|---|---|---|---|---|
| (n = 1754) | (n = 59) | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| By median | |||||||
| ≤0.53 | 866 (49.37) | 15 (25.42) | 1.00 | 1.00 | |||
| >0.53 | 888 (50.63) | 44 (74.58) | 2.68 (1.49–4.83) | 9.97 × 10-4 | 2.77 (1.53–5.04) | 8.25 × 10-4 | 1.00 × 10-6 |
| By tertile | |||||||
| <0.42 | 589 (33.58) | 12 (20.34) | 1.00 | 1.00 | |||
| <0.70 | 586 (33.41) | 10 (16.95) | 0.78 (0.34–1.81) | 0.57 | 0.92 (0.40–2.14) | 0.85 | |
| ≥0.70 | 579 (33.01) | 37 (62.71) | 2.89 (1.50–5.55) | 1.47 × 10-3 | 2.95 (1.51–5.78) | 1.61 × 10-3 | 2.00 × 10-7 |
| P trend | 2.68 × 10-4 | 4.56 × 10-4 | |||||
Adjusted for age, gender, smoking and drinking status, ALBI grade, and antiviral treatment.
Fig. 2.
Cumulative incidence of HBV-related cirrhosis. The cumulative incidence of cirrhosis risk was derived using the Kaplan–Meier method and log-rank test based on a cutoff of median and tertile AAR. a by median; and b by tertile.
Risk of AAR associated with cirrhosis stratified by liver function
Furthermore, we stratified all CHB patients by liver function condition, the ones with elevated AST (>40 U/L) or ALT (>50 U/L) were recognized as liver dysfunction. As shown in Table 3, higher risk of cirrhosis associated with higher AAR value was observed when ALT or AST was abnormal, while no significant risk was observed when AST and ALT were simultaneously normal.
Table 3.
The cirrhosis risk associated with AAR stratified by AST and ALT status
| Status | AAR | Total | cirrhosis | Univariate | Multivariatea | ||
|---|---|---|---|---|---|---|---|
| (n = 1754) | (n = 59) | HR(95%CI) | P-value | HR (95% CI) | P-value | ||
| Normal AST + normal ALT | |||||||
| ≤median | 96 (49.23) | 3 (23.08) | 1.00 | 1.00 | |||
| > median | 99 (50.77) | 10 (76.92) | 3.04 (0.82–11.24) | 0.095 | 2.27 (0.58–8.92) | 0.241 | |
| ASTb or ALTc abnormal | |||||||
| ≤median | 780 (50.03) | 15 (32.61) | 1.00 | ||||
| > median | 779 (49.97) | 31 (67.39) | 2.06 (1.11–3.82) | 0.022 | 1.91 (1.01–3.61) | 0.047 | |
Adjusted for age, gender, smoking and drinking status, ABLI grade, and antiviral treatment.
AST abnormal: AST > 40U/L.
ALT abnormal: ALT > 50U/L.
Time-dependent effect of AAR value on cirrhosis risk
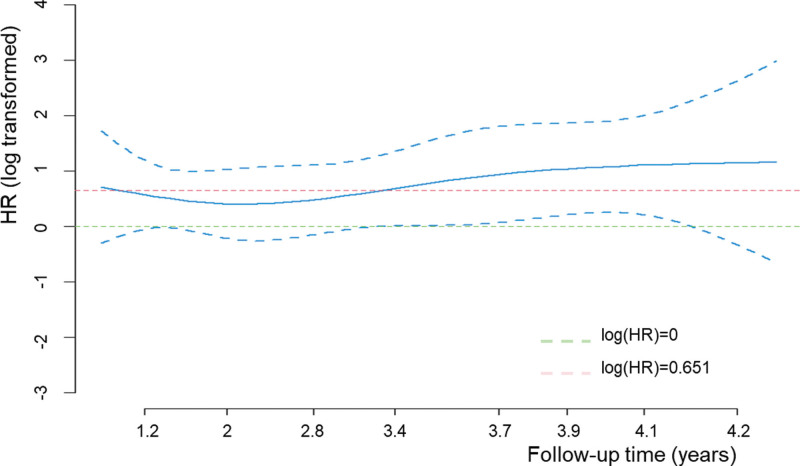
To evaluate the association between AAR and cirrhosis risk over time, we adopted a Fine and Gray competing risk model to explore the time-dependent effects of AAR on cirrhosis risk during the whole follow-up time, by adjusting for host variables (Fig. 3). The line was fluctuated up and down with the line of HR = 1.92. Increased risk of cirrhosis conferred by AAR was decreased with increasing of follow-up time, and arrived a lowest point at 2.1 years. Then the HR value remained constantly increased.
Fig. 3.
Time-dependent effect of AAR value on cirrhosis risk. Time-dependent effects of AAR on cirrhosis risk were explored by a Fine and Gray competing risk model. The blue solid line, hazard ratios; the blue dotted line, 95% confidence intervals.
Cirrhosis risk prediction models incorporating AAR
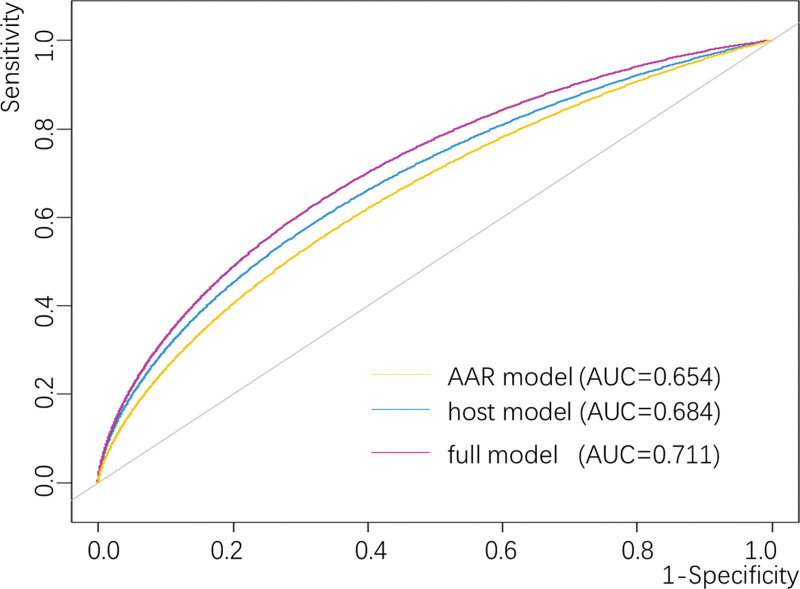
A time-dependent ROC curve was constructed, in order to evaluate their discrimination accuracy by calculating and comparing the AUC in different models (Fig. 4). 3 curves were drawn to exhibit the predictive accuracy of various models, including AAR only, host variables (age, gender, smoking status, drinking status, ALBI Grade) only (host model), and AAR plus demographic variables (Full model), with the AUC was 0.654, 0.684 and 0.711 respectively. The added AUC from Demo to full model was statistically significant (P = 0.039) by bootstrap resampling method.
Fig. 4.
Discrimination accuracy of the prediction models. Discrimination accuracy for predicting cirrhosis risk after initial sample collection was assessed by constructing ROC of different models. The AAR-only model was based on baseline of AAR as continuous variable. Host model was based on demographic variables including age, gender, smoking status, drinking status, ALBI Grade and antiviral treatment. Full model was based on the combination of AAR and host variables. P values for the differences between Full and host models were calculated by 2000 bootstrap resampling.
Discussion
In this study, we evaluated the predicting role of AAR value on developing cirrhosis in a prospectively enrolled cohort of hospitalized CHB patients by collecting their clinical and follow-up data after at least 6 months. We arrived at the conclusion that patients with higher AAR value exhibited significantly increasing risk of cirrhosis development independent of other host characteristics (gender, age, drinking history, smoking history and ALBI grade). Moreover, the effect remained prominent with the increasing follow-up time.
Cirrhosis is a multi-stage process involving many pathological changes, manifested as extensive liver parenchyma damage, liver cell necrosis, fibrous tissue proliferation, normal liver structure disorder and hard texture [7]. Consequently, it is accomplished with abnormal liver function at the most time. Hepatic function produces a marked effect on predicting the development and prognosis of patients with cirrhosis and other liver diseases [8,9]. Serum ALT and AST are two common liver enzymes that are routinely detected in the clinical setting, which was originated from damaged hepatocytes [10]. Their activities have been widely considered the commonest marker to reflect the severity of liver injury [11], so as to monitor CLD prognosis and treating response, as well as in aiding decisions for clinical strategy. For example, normalized levels of enzymes for a prolonged period are a prompting to discontinue treatment in patients with hepatitis B, autoimmune hepatitis and overlap syndrome [12,13]. AAR value is one main effective hallmark to reflect extent of liver damage and relevant complication [14], by comprehensively considering the characteristics of AST and ALT released into the blood. According to previous study, AAR was mainly used as a marker to identify fibrosis in patients with NAFLD(non-alcoholic fatty liver disease) [15,16], and performed good discrimination accuracy in advanced alcoholic liver disease (ALD) [17]. Furthermore, AAR value was also revealed to be correlated with increased risks for development of CHC-related cirrhosis [18]. Although numerous clinical data were conducted to identify the clinical value of AAR on liver diseases, non-ignorable disputation was manifested [19–21].
A stratified analysis by liver enzymes AST and ALT status was also demonstrated to reveal that higher AAR level conferred a significant risk at the case of abnormal liver enzymes, but no risk observed at the normal status. It followed that the predictive effect of AAR value on cirrhosis had been covered up by normal liver enzymes. And the population with abnormal AST or ALT were the beneficiary whose higher AAR value could acquire the best clinical utility in predicting cirrhosis. At the other aspect, the result might be brought about perhaps due to the small number of cirrhosis patients in the sub-cohort of normal liver enzymes. As similar to the result, Fredrik Åberg developed adynamic AAR model (dAAR) based on age, ALT and AAR to discover that dAAR performed well in predicting incident severe liver disease [22]. A hint was given to us that AAR added with other useful variables may provide a better prospective performance.
Another potentially important finding of our study was that AAR had a performance with AUC value of 0.654 in predicting cirrhosis development, which significantly improve the accuracy of host variables. As Nyblom H’s study suggested, AAR seemed to be a hint to the diagnosis of cirrhosis in patients with primary biliary cirrhosis [23]. It was also reported that AAR had a predictive performance in development of esophageal varices (EVs)among cirrhotic patients with the AUC value of 0.726, and high-risk EVs with AUC of 0.648 [24]. Based on the result of Behnaz Amernia’s study, AAR appeared to perform the ability of differentiating fibrosis in NAFLD patients with AUC value of 0.720[15].AAR was also applied to construct a scoring system with age, hyperglycemia, BMI, platelet count and albumin, which could accurately distinguish advanced fibrosis from NAFLD patients [25]. Although the discrimination performance of AAR only on cirrhosis prediction was not very well, it could be a potential indicator to predict cirrhosis development. The result might be caused by the small number of cirrhosis patients.
Several strengths were exhibited in our study. The first one was that a relatively large population was included with 1754 cases of CHB patients from a single hospital. The second superiority was that the variable we focused on for the comprehensive analysis was the single one, so as to eliminate the multiple comparison issue. Last but not least, the research was based on a respective cohort, which had a stronger credibility of causality than previous cross-sectional studies. Nevertheless, the present study had several limitations. First, as a retrospective study, there was an inevitable data bias. Second, the patient population was limited to hospitalized patients with CHB, whose clinical symptoms were fairly severe or with a prolong course. In addition, we only assessed the risk of a proportion of demographic and clinical variables which led to a comparatively low discriminative accuracy. More parameters should be incorporated into the model.
In summary, a prospective hospital-based cohort study was undertaken to ascertain the correlation between AAR value and cirrhosis development. Elevated AAR value seemed to be of clinic value to predict the development of cirrhosis in patients with chronic hepatitis B, though the performance appeared to be not very well. Further studies are warranted to validate this finding and test its clinical applicability in prediction of developing cirrhosis. We propose that other effective risk factors may be evaluated and combined with AAR to build a cirrhosis risk assessment model that can be utilized in the clinical settings.
Conclusion
A prospective hospital-based cohort study that included a large number of CHB patients was adopted to reveal the predictive role of higher AAR level on the risk of HBV-related cirrhosis, especially in patients with abnormal liver function. In addition, the correlation remained prominent over time, and the adding of AAR could make the predictive performance of cirrhosis better on the basis of demographic variables. Therefore, higher AAR might be a potential predictor of cirrhosis development.
Acknowledgements
The authors would like to thank the Ganzhou Fifth People’s Hospital for support in the successful conduction of this study, including granting authorization, patients’ recruition, data collection, etc. And else, we would express our appreciate to the patients for their cooperation in follow-up procedure. Finally, efforts taken by all authors would be grated for completing this passage.
This work was supported by Science and Technology Innovation Outstanding Young Talents Training Program of Jiangxi Province (Grant No. 20192BCBL23017), and The Youth Jinggang Scholars Program in Jiangxi Province.
XL contributed to the study design and data collection. HC contributed to the study design, data analysis and manuscript writing. XD contributed to the data analysis. GZ contributed to the data collection and manuscript writing. DL contributed to the data collection. FX contributed to the data collection. HL contributed to the data collection. YL contributed to the data collection. HL contributed to the study design. SW contributed to the study design and manuscript writing.
Ethics approval was obtained from Ethics Committee of Ganzhou Fifth People’s Hospital, and all methods were conducted in accordance with the Declaration of Helsinki. Consent to participation was also signed from each patient.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Xiaohuan Lai and Haiyan Chen contributed equally to the writing of this article.
References
- 1.Ginzberg D, Wong RJ, Gish R. Global HBV burden: guesstimates and facts. Hepatol Int 2018; 12:315–329. [DOI] [PubMed] [Google Scholar]
- 2.Jepsen P, Younossi ZM. The global burden of cirrhosis: a review of disability-adjusted life-years lost and unmet needs. J Hepatol 2021; 75:S3–S13. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63:743–752. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018; 69:182–236. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM. Liver biopsy reliability in clinical trials: thoughts from a liver pathologist. J Hepatol 2020; 73:1310–1312. [DOI] [PubMed] [Google Scholar]
- 6.Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: past, present and future. J Hepatol 2022; 76:1362–1378. [DOI] [PubMed] [Google Scholar]
- 7.Smith A, Baumgartner K, Bositis CC. Diagnosis and management. Am Fam Physician 2019; 100:759–770. [PubMed] [Google Scholar]
- 8.Wei L, Ye Z, Bao Z, Xu X, Lin X, Chen L. Application of acoustic radiation force impulse elastography combined with serum markers in Child-Pugh grading. Clinics (Sao Paulo) 2020; 75:e1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hann HW, Wan S, Myers RE, Hann RS, Xing J, Chen B, et al. Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PLoS One 2012; 7:e47687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int 2013; 33:1398–1405. [DOI] [PubMed] [Google Scholar]
- 11.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000; 342:1266–1271. [DOI] [PubMed] [Google Scholar]
- 12.Komori A. Recent updates on the management of autoimmune hepatitis. Clin Mol Hepatol 2021; 27:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol 2020; 26:261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai XY, Zheng YY, Tang JN, Wang W, Guo Q-Q, Yin S-S, et al. Alkaline phosphatase-to-albumin ratio as a novel predictor of long-term adverse outcomes in coronary artery disease patients who underwent PCI. Biosci Rep 2021; 41:BSR20203904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amernia B, Moosavy SH, Banookh F, Zoghi G. FIB-4, APRI, and AST/ALT ratio compared to FibroScan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar Abbas, Iran. BMC Gastroenterol 2021; 21:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the enhanced liver fibrosis test vs fibrotest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 2018; 154:1369–1379. [DOI] [PubMed] [Google Scholar]
- 17.Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2004; 39:336–339. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Wang L, Gao P. Chronic hepatitis C virus infection: relationships between inflammatory marker levels and compensated liver cirrhosis. Medicine (Baltim) 2019; 98:e17300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caballería L, Pera G, Arteaga I, Rodríguez L, Alumà A, Morillas RM, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol 2018; 16:1138–1145.e5. [DOI] [PubMed] [Google Scholar]
- 20.Guéchot J, Boisson RC, Zarski JP, Sturm N, Calès P, Lasnier E; ANRS HCEP 23 Fibrostar Group. AST/ALT ratio is not an index of liver fibrosis in chronic hepatitis C when aminotransferase activities are determinate according to the international recommendations. Clin Res Hepatol Gastroenterol 2013; 37:467–472. [DOI] [PubMed] [Google Scholar]
- 21.Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev 2013; 34:117–130. [PMC free article] [PubMed] [Google Scholar]
- 22.Åberg F, Danford CJ, Thiele M, Talbäck M, Rasmussen DN, Jiang ZG, et al. A dynamic aspartate-to-alanine aminotransferase ratio provides valid predictions of incident severe liver disease. Hepatol Commun 2021; 5:1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyblom H, Bjornsson E, Simren M, Aldenborg F, Almer S, Olsson R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int 2006; 26:840–845. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AL, Hayward KL, Patel P, Horsfall LU, Cheah AEZ, Irvine KM, et al. Predicting liver-related outcomes in people with nonalcoholic fatty liver disease: the prognostic value of noninvasive fibrosis tests. Hepatol Commun 2022; 6:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854. [DOI] [PubMed] [Google Scholar]