Abstract
Sex differences in AKI continue to be identified. Generally, women are protected from AKI when compared to men. Much of the protection exhibited in women is diminished after menopause. These sex and age effects have also been noted in animal models of AKI. Gonadal hormones, as modifiers of incidence, severity, and progression of AKI, have been offered as likely contributors to this sex and age effect. In animal models of AKI, estrogen and testosterone seem to modulate susceptibility. Questions remain however regarding cellular and molecular changes that are initiated by modulation of these hormones because both estrogen and testosterone have effects across cell types that play a role in AKI. Although findings have largely been informed by studies in males, molecular pathways that are involved in the initiation and progression of AKI may be modulated by gonadal hormones. Compounding the hormone-receptor effects are developmental effects of sex chromosomal complement and epigenetic influences that may confer sex-based baseline differences in gene and protein expression, and gene dosage effects of X inactivation and escape on molecular pathways. Elucidation of sex-based protection may afford a more complete view of AKI and potential therapeutic interventions. Furthermore, the effect on susceptibility to AKI in transgender patients, who receive life-altering and essential gender-affirming hormone therapy, requires greater attention. In this review, several potential contributors to the sex differences observed in humans and animal models are discussed.
Keywords: acute kidney failure, women's health
Introduction
AKI is defined by an abrupt loss in GFR leading to ischemia, tubular epithelial cell death and derangement, endothelial cell loss and damage, immune cell infiltration, and inflammatory responses to the cellular debris and hypoxia. Loss of epithelium through apoptosis, necrosis, and ferroptosis impedes the transepithelial transport mechanisms that rely on segregation of ions to drive transport. Oxidative stress, which induces reactive oxygen and nitrogen species, damages proteins and DNA. Finally, an inflammatory cascade results in the influx of immune cells and a cytokine storm affecting tubule and vascular responses.
Clinical care for AKI remains limited with no robust interventions beyond the supportive care of dialysis. Given the notable sex differences in kidney physiology and in pathophysiologic responses to AKI, the ability to advance novel treatments may benefit from a better understanding of sex differences in AKI. Resistance to AKI seen in both women and female rodents in multiple animal models suggests that unique responses may occur in females that are not found in males. These unique features might be exploited to define novel approaches to therapy.
Sex differences in incidence, severity, and recovery in AKI have been described for various etiologies, but the mechanistic insights into these differences are not well defined. The incidence of AKI in women is less than that in men in most cases.1 The exceptions of intensive care unit-based AKI and those due to cardiac surgeries were noted to be at least in part due to the older age and sicker nature of the women afflicted. Our understanding of these sex differences has been hindered by studies that include mostly or exclusively men2,3 or are confounded by inclusion of women with broad age ranges crossing pre-, peri-, and postmenopausal stages. Furthermore, clinical studies using small numbers of women, statistically age-matched for analysis, may be underpowered to establish sex differences. Animal modeling of AKI similarly has largely been conducted on males.4–6 When females have been used, a sex difference is frequently identified,7 which may suggest that women and females have unique physiology that confers protection.
A multitude of pathways are altered in AKI in a sex-dependent manner, and numerous proteins or genes have been explored in preclinical models using knockout, knockdown, and pharmacologic inhibition strategies.8,9 Piecing together these individual phenomena into an integrated view of how sex modulates AKI remains challenging. In this review, clinical and preclinical literature will be evaluated to highlight several important physiologic and pathophysiologic findings that inform our understanding of sex differences in AKI (Figure 1).
Figure 1.
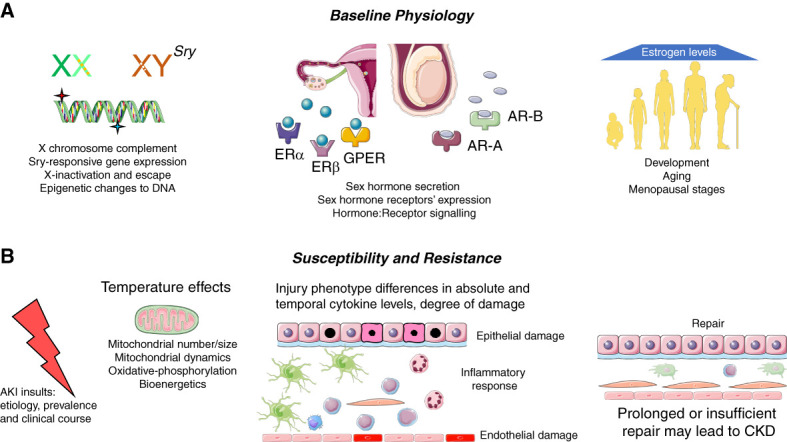
Potential sources of sex differences in AKI. Sex differences may be conferred in the baseline physiologic framework and can be seen in responses to injury and repair. (A) Baseline physiology. Chromosomal complement, X inactivation, and epigenetic changes leading to X-inactivation escape may establish different gene expression profiles. The presence of gonadal tissues and thus the ratios of sex hormones released lead to hormone-receptor interaction and subsequent gene and protein expression changes. The sex-based differences in density and localization of hormone receptors along the nephron may give rise to different capability to respond to physiologic and pathophysiologic signals. Changes occurring in hormonal abundance and additional molecular changes may lead to different outcomes in development, puberty, adulthood, and aging. (B) Susceptibility and resistance. Differences in responses between men and women may occur with different etiologies of AKI, and decreased susceptibility in women may lead to lower prevalence and different clinical severity or temporal courses, including transition to CKD. Differences in mitochondrial number, size, and dynamics, including mitochondrial fission and fusion, as well as differences in mitochondrial bioenergetics and induction of oxidative phosphorylation may be based on the basis of sex. Mitochondrial functional aspects may be altered by ambient temperatures. Temporal differences and extent of damage to epithelia and endothelia, and in the inflammatory response, may underlie sex hormonal or chromosomal influence. Degree and timing of cellular repair may differ between men and women or males and females. AR-A, androgen receptor A; AR-B, androgen receptor B; ERα, estrogen receptor α; ERβ, estrogen receptor β; GPER, G-protein–coupled estrogen receptor; Sry, sex-determining region Y. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
AKI in Women and Females
Recent data continue to confirm that acute insults to the kidney result in a lower incidence of AKI in women than men. In a study of community-acquired AKI, women were shown to have a survival advantage, as well as less severe disease.10 AKI requiring dialysis also shows a reduced incidence in women relative to men.11 In sepsis-associated AKI, studies suggest that women are less susceptible,12 and they exhibit a survival advantage.13 In a meta-analysis, lesser incidence in women relative to men was seen in AKI, including hospital-associated AKI, disease-associated AKI, and AKI due to general surgery. A lack of sex difference was noted in the incidence of AKI in the setting of the intensive care unit. A higher odds ratio for AKI in women after cardiac surgery may be due to the older age and more advanced disease noted in women relative to men.14 Consistently, age has been shown to increase the incidence of AKI in men and women.15,16
Preclinical studies also show a more robust resistance to models of AKI in females than males. Several models exist within which variations of time, dose, etc., are used. In mice, ischemia-reperfusion injury (IRI) using the same ischemic time results in a more blunted rise in systemic indicators of injury (e.g., serum creatinine, BUN) and reduced morphological evidence of damage in females relative to males.2,17,18 When longer ischemia times are used in females to reflect comparable serum creatinine rise seen in males, similar levels of other injury markers are noted, although some temporal differences in injury markers may remain.19 Utilization of cisplatin-induced AKI shows more variability in sex differences than IRI, although head-to-head comparisons between males and females have been limited. Different doses of cisplatin, ages of mice, or animal species may underlie this variability.7,20,21 Other preclinical models of toxin-induced AKI, while more limited, consistently show a lesser degree of injury in females relative to males. As with patients, age conferred greater susceptibility in preclinical studies.7,22 Protein and gene profiles have been shown to be modulated in AKI in a sex-dependent fashion, including pathways involved in cell death, inflammation, and recovery among others.9,23 Integration of these diverse findings mechanistically remains challenging.
Transgender Physiology and AKI
Although sex has had some attention, gender has had little examination as a modifier of AKI. Sex is a biologic parameter, male, female, or intersex, which is defined by the chromosomal complement, external anatomy, reproductive tissue, and the concomitant hormonal milieu derived from these tissues. Gender identity is one's internal sense of being a man, a woman, or another gender. Examples of other gender identities include nonbinary, two-spirit, agender, and genderqueer, as well as a variety of other self-described identities. Transgender individuals have a gender identity that differs from their assigned sex at birth. For example, a transgender man was assigned female at birth, but his gender identity is that of a man. Transgender people often desire to pursue gender-affirming hormone therapy (GAHT). Such therapy can be life preserving by reducing gender dysphoria and aligning their appearance with their identity. Importantly, sex-defined measures of kidney function and unclear reference ranges for laboratory parameters contribute to a more challenging definition of AKI in transgender patients.24–27 Limited case studies available also highlight the complex considerations for transgender patients in their clinical care course,28,29 including, for example, the limited mechanistic understanding of delayed graft function after transplantation.28 In a small study from our group, we identified that AKI prevalence in transgender patients was highest in transfeminine individuals not receiving GAHT as compared to transfeminine individuals receiving GAHT, or transmasculine individuals regardless of GAHT.30 These data are consistent with the concept that the presence of estrogenic hormones resists induction of AKI, but further studies are required to understand the molecular mechanisms of susceptibility that may be altered in this setting by GAHT, especially in the presence of intact endogenous hormonal systems.
Baseline Physiologic Differences
Several fundamental characteristics of the kidney have been shown to differ by sex. Structural differences in anatomic structures revealed by morphometric analysis have demonstrated sex-based differences in cell heights and volumes in various nephron regions, which may have implications for intracellular concentrations of toxins delivered to induce AKI.31 Sex differences in the expression of a variety of transporters in the nephron have been noted in rodents and may underpin fundamental physiologic differences between the sexes32,33 and thereby their response to injury. Molecular characterization of the nephron demonstrates sex-based differences in gene expression in the S3 segment of the proximal tubule,34 a critical site of cellular damage and functional derangement in response to hypoxia and toxins. Cellular organelles such as mitochondria have been noted to exhibit sex differences.33 Development and maintenance of these sex differences may be due to multiple inputs from the chromosomal level, the gonadal level, and/or an interplay between the two, and each of these may be subsequently altered by the induction of AKI.
Chromosomal Contributions to Sex Differences
Male and female sexual features are conferred by the X and Y chromosomes, with the presence of the sex-determining region Y (Sry) gene on the Y chromosome defining development of testes. Sry gene expression outside the testes has been shown, including in the kidney. Sry expression modulates BP and may alter expression of components of the renin-angiotensin-aldosterone system because Sry-binding sites have been identified in the promoters of renin-, angiotensinogen-, and angiotensin-converting enzyme.35
In females, one X chromosome is inactivated to prevent gene dosage effects. X inactivation is a complex process that involves epigenetic changes to the DNA that inhibits transcription of specific genes (reviewed by Migeon36). Chromatin location within the nucleus may also contribute to X inactivation. As each cell may inactivate either the maternally or paternally derived X chromosome randomly, X inactivation results in cell-to-cell mosaicism. Recent investigations identify that “escape” from X inactivation involving certain genes may occur and may happen differently in different tissues, in different cells within an organ, and at different times in development and aging, creating an intricate regulation of sex-based gene expression. Complex epigenetic changes on the inactive X chromosome may result in complete or partial X-inactivation escape which differs by tissue, and which may lead to reduced, but not eliminated expression levels. Furthermore, genes expressed on both the X and Y chromosomes may show different effects of escape than genes expressed only on the X chromosome. Species differences in escape genes are noted because mice have fewer escape genes than humans. Tissue-specific X-inactivation escape is not well understood at the molecular level and has been minimally studied in the kidney. Notably, some genes that escape X inactivation in the spleen are important in immune cell function,37 which may have important implications in sex differences in AKI.
Novel technological advances have resulted in animal models in which the source of sex-based effects can be segregated between the presence of gonadal tissue, and thus hormonal influence, and sex chromosomal content. One such mouse model, the 4-core genotype,38 has the Sry gene removed from the Y chromosome and placed on an autosome. Gonadal males with XX or XY chromosomes and gonadal females carrying the XX or XY chromosomes result. In addition, the gonadal tissue in these mice can be excised to better understand the influence of sex chromosome complements in the absence of gonadal hormones. Relevant to the kidney, the 4-core genotype mice exhibit increased gene expression levels of angiotensin II receptor type 2 and Mas1 receptor, the Ang(1-7) receptor, in the kidney in XX relative to XY regardless of gonadal tissue present,39 suggesting a more prominent chromosomal effect. In a study of cardiac injury using these mice, the presence of the second X chromosome increased the infarct size whether ovaries or testes were present.40 These latter findings are counter to the “female protection” previously observed in unaltered mice and highlight the complex interplay between chromosomal complement and gonadal hormonal influences on injury. No studies have used this unique animal model to examine AKI.
Gonadal Hormone Effects on AKI
Gonadal hormones are believed to modulate susceptibility, wherein testosterone is deleterious and estrogen is protective. Studies that combine women at ages that cross the pre-, peri-, and postmenopausal stages may confound the results. Studies that do stratify women into age ranges consistent with these menopausal stages demonstrate that younger, premenopausal women have a lower incidence of AKI. In the setting of transplantation, male kidneys implanted into individuals of either sex, or men receiving a kidney from either a woman or a man, show an increased rate of delayed graft function,2 which may suggest a complex interplay between kidney and systemic effects of gonadal hormones. Estrogen exerts its effects through the classical estrogen receptors, ERα and ERβ, and through the G-protein–coupled estrogen receptor, all of which show sex-defined expression patterns in the kidney.41 In fact, among nongonadal tissues, the kidney is recognized as the most estrogenic.42 Expression of estrogen receptors have been described in epithelia, vasculature, dendritic cells, macrophages, and mesenchymal cells, each of which has been shown to play a role in modifying severity of AKI.
Examination of estrogen deficiency, through pharmacologic inhibition or knockdown of the gene, has demonstrated the importance of estrogen signaling in susceptibility and lethality of AKI. Receptor blockade or ovariectomy increases kidney disfunction and damage. Administration of estrogen or an estrogen metabolite, with or without previous ovariectomy, restores or enhances the protective effects of estrogen in AKI. Furthermore, studies using ERα knockout mice in a model of IRI demonstrated lethality in the absence of ERα, but survival in wild-type mice.2 The presence of G-protein–coupled estrogen receptor also may facilitate protection.43
Testosterone effects on AKI have been less well studied. Studies differ in response to testosterone in AKI, in some cases exhibiting deleterious effects18 and in others conferring a protective response,44 perhaps due to the timing of administration. Orchiectomy decreases susceptibility, and replacement of testosterone after orchiectomy returns the susceptibility to that of intact mice.18,44 Testosterone receptors, including androgen receptors A (AR-A) and AR-B, are expressed throughout the nephron41 and thus may affect sex-based responses to the gonadal hormones. Urinary biomarkers used to define AKI show modulation by the presence or absence of testosterone.45 Epidermal growth factor receptor, a tyrosine kinase that is important in regulating cell proliferation, is expressed at higher levels in male mice and men than in female mice and women, and increases or decreases to its kinase activity lead to modulation of susceptibility to AKI in males only.46,47
Sex Differences in Inflammation
Inflammation is a hallmark of AKI. The importance of a cascade of immune cells in modulating both insult and recovery has been established in both rodent models and patients with AKI.48,49 Only a few studies have examined sex differences specifically (reviewed by Lima-Posada and Bobadilla8). Immune cells present in rodent kidneys in models of AKI show sex-specific differences that are modulated by the presence or absence of gonadal hormones.17 F4/80+ macrophage influx to the kidney was elevated in AKI, decreased by castration, but returned to similar levels after AKI when testosterone was replaced. Females also show influx of F4/80+ cells with AKI, but ovariectomy increases this influx, while estrogen replacement returns cell levels to that of intact animals with AKI. Testosterone also modulates inflammation in AKI by decreasing T-cell infiltration and shifting the cytokine profile to a more antiinflammatory state.50 Females demonstrate higher levels of regulatory T cells in inflammatory states, which may be protective in AKI. Macrophage shift from an M1 to M2 phenotype may also be different in males and females. Cytokine expression after a cisplatin-induced AKI shows both sex and age effects, with young females having the least inflammatory profile.7 Elevations in TNFα after IRI in intact males are reversed with testosterone supplementation or with aromatase inhibition to preserve levels of testosterone.44 Patient studies have been performed predominantly in men and with age ranges that reside near the menopausal stage in women or including age ranges that are substantial. While these facts highlight the resistance to AKI in women, a better understanding of the role of immune cells in sex differences in AKI clinically awaits further study.
Sex Differences in Mitochondrial Biology
Mitochondria play a central role in induction and recovery from AKI, and sex-based differences in mitochondria are known.51 Females have higher mitochondrial number, larger size, and more robust function.51,52 Mitochondrial energetic derangement has been identified to alter injury in AKI.53,54 Intraarterial injection of mitochondria in female Yorkshire pigs undergoing an IRI model conferred reduced measures of kidney injury.55 Mitochondrial transplantation in male rats undergoing IRI56 or gentamicin-induced toxicity57 was protective; in the latter case, this protection was more pronounced when mitochondria were from females. Given the importance of gonadal hormones, it is noteworthy that estrogen receptors have been identified in mitochondria,58,59 and mitochondrial energetics are altered by manipulation of estrogen levels.60
Knockout of sirtuin-3, a protein involved in several mitochondrial processes, results in increased susceptibility to AKI. Testosterone decreases the mitochondrial expression of sirtuin-3, particularly in males.61 Activation of the adapter protein p66shc leads to mitochondrial dysfunction and increased reactive oxygen species elaboration. Males express higher levels of p66shc, which is modulated by testosterone.62
Peroxisome proliferator-activated receptors γ coactivator-1α (PGC-1α), a master regulator of several mitochondrial-based mechanisms, plays a role in mitochondrial biogenesis and thermoregulation and regulates turnover of mitochondria through mitophagy, a process to ensure mitochondrial quality. PGC-1α and one of its downstream effectors in thermoregulation, uncoupling protein 1, are downregulated in AKI,63 and elevated levels of PGC-1α correlate with reduced delayed graft function in patients.64 PGC-1α knockout animals exhibit kidney inflammation at baseline, which is enhanced in folic acid–induced injury in female mice, with decreased survival and increased injury noted.65 PGC-1α also regulates the expression of heme oxygenase-1 (HO-1),66 a cytoprotective enzyme that also shows a temporally different pattern of expression after AKI in males and females.7 Induction of HO-1 is protective both by its enzymatic role to breakdown heme with the elaboration of antioxidant molecules and by its transcriptional activity. Specific genetic polymorphisms in HO-1 confer a greater odds of having AKI, with the strongest association seen in women undergoing cardiac surgery.67 HO-1 also may enhance mitochondrial function and biogenesis by preventing mitochondrial fragmentation and promoting mitochondrial biogenesis. Importantly, HO-1 expression is regulated by estrogen.68,69
Ambient Temperature and AKI
Over the last couple of decades, nephropathy due to excess heat exposure has come to light as has a recognition that global warming may contribute to a rising incidence of this condition.70 Originally described in populations in Central America leading to the nomenclature of Mesoamerican nephropathy (MeN), this condition has been documented subsequently world-wide in hot climates. Although CKD due to MeN has been diagnosed, now referred to as CKD of unknown or nontraditional origin, studies also suggest that the resulting CKD is due to repeated events of AKI in the acute phase of the condition.71,72 The AKI is believed to result from rhabdomyolysis and systemic inflammation with a potentially complex pathophysiology.73 Repeated episodes of dehydration induce chronic vasopressin secretion, activation of the renin-angiotensin-aldosterone system, and activation of the polyol pathway that leads to endogenous fructose production, which in turn leads to a more proinflammatory, prooxidant milieu.74 CKD of unknown/CKD of nontraditional/MeN disproportionately affects young men, but whether this sex difference is due to different biology or to the disproportionate number of men in these affected industries has not been clarified. Exposure to hot environments increases incidence of kidney diseases, including AKI, and men are more likely than women to present at emergency departments with kidney disease in hot climates.75,76
Studies of heat stress in rodents have largely been conducted in males77–79; thus, understanding effects of sex or gender remains underexplored. Studies using more modest changes in temperature have not examined the kidney. Thermoneutral temperatures are those temperatures at which excess energy through mitochondrial shunting to heat generation, with a concomitant loss in ATP production, is not required to maintain core body temperatures. Animals housed under these conditions show a loss of sex differences in other organ systems. In models of nonalcoholic fatty liver disease and atherosclerosis, resistance to injury in female rodents was eliminated.80,81 Thermoneutral housing of mice exacerbates graft-versus-host disease82 and alters inflammatory and immune cell responses in the liver,80 lung,83 and heart.81 Several proteins and pathways are altered by thermoneutrality housing,84 as well as extreme housing temperature shifts to induce hypothermia or hyperthermia.85 In the latter case, the heat shock protein induction alters outcomes in AKI.86,87 Notably, altered ambient temperature results in different mobilization or activation of immune cells, including monocytes and T cells.83,88,89
In addition, estrus and pregnancy result in different thermoregulation which may have an effect on susceptibility to AKI.90 Circadian rhythm differences in peak and nadir body temperature vary in different species of male mice,91 although no information is available for females. This loss of sex differences in acute injuries with the modulation of ambient temperature warrants further study in the kidney.
An integrated theory of the mechanisms discussed is challenging because of the complexity of input and the interplay of these various mechanisms with gonadal hormones and chromosomal complements. The multitude of downstream effects directed by estrogen and testosterone may suggest that sex hormones can alter both kidney-centric and systemic effects. Similarly, emerging evidence of a role for the sex chromosomes in modulating molecular pathways independently of gonadal hormones is expanding our understanding of sex differences. Fundamental morphological and molecular expression differences in males versus females, coupled with the modulation of key pathways in AKI by gonadal hormones or sex chromosomes, may result in very different effects in AKI between the sexes. A better scientific understanding of the differences between the male and female kidney, and their different responses to AKI, awaits further studies.
Disclosures
L.M. Curtis reports the following: employer: self and spouse: University of Alabama at Birmingham; consultancy: spouse: DynaMed; ownership interest: spouse: Creegh Pharmaceuticals and Goldilocks Therapeutics, Inc.; research funding: spouse: Genzyme/Sanofi Fabry Fellowship Award; honoraria: spouse: Mayo Clinic, University of Maryland, University of Toledo, and University of Virginia; patents or royalties: spouse: pending patent that describes small molecule inducers of heme oxygenase-1 for the treatment of acute and chronic kidney disease; advisory or leadership role: self: Women in Nephrology Executive Council; past president: spouse: editorial board for American Journal of Physiology Renal Physiology, Laboratory Investigation, and Kidney International; advisory board of Alpha Young, LLC, Angion, Creegh Pharmaceuticals, Goldilocks Therapeutics, and Zydus; medical advisory boards of Alpha Young, Creegh Pharmaceuticals, and Vexev; and other interests or relationships: self: Women in Nephrology Executive Council, past president (2023).
Funding
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases from R01 DK129239-01 (L.M. Curtis), DK137307, 1U24DK137318, R01 DK118932.
Author Contributions
Conceptualization: L.M. Curtis.
Data curation: L.M. Curtis.
Formal analysis: L.M. Curtis.
Writing – original draft: L.M. Curtis.
Writing – review & editing: L.M. Curtis.
References
- 1.Neugarten J, Golestaneh L. Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol. 2018;19(1):314. doi: 10.1186/s12882-018-1122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aufhauser DD Jr. Wang Z Murken DR, et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest. 2016;126(5):1968–1977. doi: 10.1172/jci84712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garovic VD, August P. Sex differences and renal protection: keeping in touch with your feminine side. J Am Soc Nephrol. 2016;27(10):2921–2924. doi: 10.1681/ASN.2016040454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spandou E Tsouchnikas I Karkavelas G, et al. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant. 2006;21(2):330–336. doi: 10.1093/ndt/gfi177 [DOI] [PubMed] [Google Scholar]
- 5.Lee S Huen S Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. doi: 10.1681/ASN.2009060615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei Q, Xiao X, Fogle P, Dong Z. Changes in metabolic profiles during acute kidney injury and recovery following ischemia/reperfusion. PLoS One. 2014;9:e106647. doi: 10.1371/journal.pone.0106647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis LM. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol. 2017;313(3):F740–F755. doi: 10.1152/ajprenal.00049.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima-Posada I, Bobadilla NA. Understanding the opposite effects of sex hormones in mediating renal injury. Nephrology. 2021;26(3):217–226. doi: 10.1111/nep.13806 [DOI] [PubMed] [Google Scholar]
- 9.Nemours S Castro L Ribatallada-Soriano D, et al. Temporal and sex-dependent gene expression patterns in a renal ischemia-reperfusion injury and recovery pig model. Sci Rep. 2022;12(1):6926. doi: 10.1038/s41598-022-10352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adrah R, Golestaneh L. Female Sex Protects Against Mortality After an Episode of Acute Kidney Injury (AKI). NKF Spring Clinical Meetings; 2018. [Google Scholar]
- 11.Neugarten J, Golestaneh L, Kolhe NV. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol. 2018;19(1):131. doi: 10.1186/s12882-018-0937-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26(2):167–172. doi: 10.1007/s001340050041 [DOI] [PubMed] [Google Scholar]
- 13.O'Brien Z Cass A Cole L, et al. Sex and mortality in septic severe acute kidney injury. J Crit Care. 2019;49:70–76. doi: 10.1016/j.jcrc.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 14.Neugarten J, Sandilya S, Singh B, Golestaneh L. Sex and the risk of AKI following cardio-thoracic surgery: a meta-analysis. Clin J Am Soc Nephrol. 2016;11(12):2113–2122. doi: 10.2215/CJN.03340316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue JL Daniels F Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142. doi: 10.1681/ASN.2005060668 [DOI] [PubMed] [Google Scholar]
- 16.Gokalp C Ilgen U Otman E, et al. Serum estradiol level predicts acute kidney injury in medical intensive care unit patients. Intern Emerg Med. 2022;17(8):2253–2260. doi: 10.1007/s11739-022-03077-8 [DOI] [PubMed] [Google Scholar]
- 17.Kang KP Lee JE Lee AS, et al. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol Med Rep. 2014;9(6):2061–2068. doi: 10.3892/mmr.2014.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279(50):52282–52292. doi: 10.1074/jbc.M407629200 [DOI] [PubMed] [Google Scholar]
- 19.Dixon E, Wu H, Muto Y, Wilson P, Humphreys B. Spatially resolved transcriptomic analysis of acute kidney injury in a female murine model. J Am Soc Nephrol. 2022;33(2):279–289. doi: 10.1681/ASN.2021081150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Gender difference in cisplatin-induced nephrotoxicity in a rat model: greater intensity of damage in male than female. Nephrourol Mon. 2013;5(3):818–821. doi: 10.5812/numonthly.10128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Q, Wang M-H, Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol. 2005;25(5):491–499. doi: 10.1159/000088171 [DOI] [PubMed] [Google Scholar]
- 22.Nath KA Garovic VD Grande JP, et al. Heme oxygenase-2 protects against ischemic acute kidney injury: influence of age and sex. Am J Physiol Renal Physiol. 2019;317(3):F695–F704. doi: 10.1152/ajprenal.00085.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viñas JL Porter CJ Douvris A, et al. Sex diversity in proximal tubule and endothelial gene expression in mice with ischemic acute kidney injury. Clin Sci (Lond). 2020;134(14):1887–1909. doi: 10.1042/cs20200168 [DOI] [PubMed] [Google Scholar]
- 24.Fadich SK, Kalayjian A, Greene DN, Cirrincione LR. A retrospective analysis of creatinine-based kidney function with and without sex assigned at birth among transgender adults. Ann Pharmacother. 2022;56(7):791–799. doi: 10.1177/10600280211050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Imborek KL, Krasowski MD. Challenges in transgender healthcare: the pathology perspective. Lab Med. 2016;47(3):180–188. doi: 10.1093/labmed/lmw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maheshwari A, Dines V, Saul D, Nippoldt T, Kattah A, Davidge-Pitts C. The effect of gender-affirming hormone therapy on serum creatinine in transgender individuals. Endocr Pract. 2022;28(1):52–57. doi: 10.1016/j.eprac.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 27.Allen AN, Jiao R, Day P, Pagels P, Gimpel N, SoRelle JA. Dynamic impact of hormone therapy on laboratory values in transgender patients over time. J Appl Lab Med. 2021;6(1):27–40. doi: 10.1093/jalm/jfaa192 [DOI] [PubMed] [Google Scholar]
- 28.Ramadan OI Naji A Levine MH, et al. Kidney transplantation and donation in the transgender population: a single-institution case series. Am J Transplant. 2020;20(10):2899–2904. doi: 10.1111/ajt.15963 [DOI] [PubMed] [Google Scholar]
- 29.Collister D, Saad N, Christie E, Ahmed S. Providing care for transgender persons with kidney disease: a narrative review. Can J Kidney Health Dis. 2021;8:2054358120985379. doi: 10.1177/2054358120985379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckenrode HE, Gutierrez OM, Osis G, Agarwal A, Curtis LM. Kidney disease prevalence in transgender individuals. Clin J Am Soc Nephrol. 2022;17(2):280–282. doi: 10.2215/CJN.04660421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris AN Lee H-W Osis G, et al. Differences in renal ammonia metabolism in male and female kidney. Am J Physiol Renal Physiol. 2018;315(2):F211–F222. doi: 10.1152/ajprenal.00084.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veiras LC Girardi ACC Curry J, et al. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol. 2017;28(12):3504–3517. doi: 10.1681/ASN.2017030295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosszu A, Fekete A, Szabo AJ. Sex differences in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2020;319(2):F149–F154. doi: 10.1152/ajprenal.00099.2020 [DOI] [PubMed] [Google Scholar]
- 34.Ransick A Lindström NO Liu J, et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell. 2019;51(3):399–413.e7. doi: 10.1016/j.devcel.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner ME, Ely D, Prokop J, Milsted A. Sry, more than testis determination? Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R561–R571. doi: 10.1152/ajpregu.00645.2010 [DOI] [PubMed] [Google Scholar]
- 36.Migeon BR. Migeon BR: X inactivation, female mosaicism, and sex differences in renal diseases. J Am Soc Nephrol. 2008;19(11):2052–2059. doi: 10.1681/ASN.2008020198 [DOI] [PubMed] [Google Scholar]
- 37.Raznahan A Parikshak NN Chandran V, et al. Sex-chromosome dosage effects on gene expression in humans. Proc Natl Acad Sci U S A. 2018;115(28):7398–7403. doi: 10.1073/pnas.1802889115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itoh Y Mackie R Kampf K, et al. Four Core Genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8(69):69. doi: 10.1186/s13104-015-0986-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dadam FM Cisternas CD Macchione AF, et al. Sex chromosome complement involvement in angiotensin receptor sexual dimorphism. Mol Cell Endocrinol. 2017;447:98–105. doi: 10.1016/j.mce.2017.02.041 [DOI] [PubMed] [Google Scholar]
- 40.Li J Chen X McClusky R, et al. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc Res. 2014;102(3):375–384. doi: 10.1093/cvr/cvu064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimont A, Bloch-Faure M, El Abida B, Crambert G. Mapping of sex hormone receptors and their modulators along the nephron of male and female mice. FEBS Lett. 2009;583(10):1644–1648. doi: 10.1016/j.febslet.2009.04.032 [DOI] [PubMed] [Google Scholar]
- 42.Jelinsky SA Harris HA Brown EL, et al. Global transcription profiling of estrogen activity: estrogen receptor alpha regulates gene expression in the kidney. Endocrinology. 2003;144(2):701–710. doi: 10.1210/en.2002-220728 [DOI] [PubMed] [Google Scholar]
- 43.Gohar EY, Almutlaq RN, Fan C, Balkawade RS, Butt MK, Curtis LM. Does G protein-coupled estrogen receptor 1 contribute to cisplatin-induced acute kidney injury in male mice? Int J Mol Sci. 2022;23(15):8284. doi: 10.3390/ijms23158284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soljancic A Ruiz AL Chandrashekar K, et al. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Regul Integr Comp Physiol. 2013;304(11):R951–R958. doi: 10.1152/ajpregu.00360.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuji S, Sugiura M, Tsutsumi S, Yamada H. Sex differences in the excretion levels of traditional and novel urinary biomarkers of nephrotoxicity in rats. J Toxicol Sci. 2017;42(5):615–627. doi: 10.2131/jts.42.615 [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Chen J-K, Wang S-W, Moeckel G, Harris RC. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol. 2003;14(12):3147–3154. doi: 10.1097/01.ASN.0000098681.56240.1a [DOI] [PubMed] [Google Scholar]
- 47.Zhang M-Z Sasaki K Li Y, et al. The role of the EGF receptor in sex differences in kidney injury. J Am Soc Nephrol. 2019;30(9):1659–1673. doi: 10.1681/ASN.2018121244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dellepiane S, Leventhal JS, Cravedi P. T cells and acute kidney injury: a two-way relationship. Front Immunol. 2020;11:1546. doi: 10.3389/fimmu.2020.01546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30(3):268–277. doi: 10.1016/j.semnephrol.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patil CN Wallace K LaMarca BD, et al. Low-dose testosterone protects against renal ischemia-reperfusion injury by increasing renal IL-10-to-TNF-α ratio and attenuating T-cell infiltration. Am J Physiol Renal Physiol. 2016;311(2):F395–F403. doi: 10.1152/ajprenal.00454.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan JS, Sheikh-Hamad D. Mitochondrial dysfunction in acute kidney injury and sex-specific implications. Med Res Arch. 2019;7(2). doi: 10.18103/mra.v7i2.1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Justo R Frontera M Pujol E, et al. Gender-related differences in morphology and thermogenic capacity of brown adipose tissue mitochondrial subpopulations. Life Sci. 2005;76(10):1147–1158. doi: 10.1016/j.lfs.2004.08.019 [DOI] [PubMed] [Google Scholar]
- 53.Jassem W, Heaton ND. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int. 2004;66(2):514–517. doi: 10.1111/j.1523-1755.2004.761_9.x [DOI] [PubMed] [Google Scholar]
- 54.Lan R Geng H Singha PK, et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol. 2016;27(11):3356–3367. doi: 10.1681/ASN.2015020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doulamis IP Guariento A Duignan T, et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am J Physiol Renal Physiol. 2020;319(3):F403–F413. doi: 10.1152/ajprenal.00255.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jabbari H Roushandeh AM Rostami MK, et al. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim Biophys Acta Mol Basis Dis. 2020;1866(8):165809. doi: 10.1016/j.bbadis.2020.165809 [DOI] [PubMed] [Google Scholar]
- 57.Arjmand A, Shiranirad S, Ameritorzani F, Kamranfar F, Seydi E, Pourahmad J. Mitochondrial transplantation against gentamicin-induced toxicity on rat renal proximal tubular cells: the higher activity of female rat mitochondria. In Vitro Cell Dev Biol Anim. 2023;59(1):31–40. doi: 10.1007/s11626-022-00743-1 [DOI] [PubMed] [Google Scholar]
- 58.Velickovic K Cvoro A Srdic B, et al. Expression and subcellular localization of estrogen receptors α and β in human fetal brown adipose tissue. J Clin Endocrinol Metab. 2014;99(1):151–159. doi: 10.1210/jc.2013-2017 [DOI] [PubMed] [Google Scholar]
- 59.Yang SH Liu R Perez EJ, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101(12):4130–4135. doi: 10.1073/pnas.0306948101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereira-Simon S, Xia X, Catanuto P, Elliot S. Oxidant stress and mitochondrial signaling regulate reversible changes of ERα expression and apoptosis in aging mouse glomeruli and mesangial cells. Endocrinology. 2012;153(11):5491–5499. doi: 10.1210/en.2012-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen H Holliday M Sheikh-Hamad D, et al. Sirtuin-3 mediates sex differences in kidney ischemia-reperfusion injury. Transl Res. 2021;235:15–31. doi: 10.1016/j.trsl.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 62.Reed DK, Arany I. p66shc and gender-specific dimorphism in acute renal injury. In Vivo. 2014;28(2):205–208. PMID: 24632974. [PubMed] [Google Scholar]
- 63.Portilla D Dai G McClure T, et al. Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int. 2002;62(4):1208–1218. doi: 10.1111/j.1523-1755.2002.kid553.x [DOI] [PubMed] [Google Scholar]
- 64.Drury ER, Zsengeller ZK, Stillman IE, Khankin EV, Pavlakis M, Parikh SM. Renal PGC1α may be associated with recovery after delayed graft function. Nephron. 2018;138(4):303–309. doi: 10.1159/000485663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fontecha-Barriuso M Martín-Sánchez D Martinez-Moreno JM, et al. PGC-1α deficiency causes spontaneous kidney inflammation and increases the severity of nephrotoxic AKI. J Pathol. 2019;249(1):65–78. doi: 10.1002/path.5282 [DOI] [PubMed] [Google Scholar]
- 66.Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat. 2016;125:8–18. doi: 10.1016/j.prostaglandins.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leaf DE Body SC Muehlschlegel JD, et al. Length polymorphisms in heme oxygenase-1 and AKI after cardiac surgery. J Am Soc Nephrol. 2016;27(11):3291–3297. doi: 10.1681/ASN.2016010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baruscotti I Barchiesi F Jackson EK, et al. Estradiol stimulates capillary formation by human endothelial progenitor cells: role of estrogen receptor-{alpha}/{beta}, heme oxygenase 1, and tyrosine kinase. Hypertension. 2010;56(3):397–404. doi: 10.1161/hypertensionaha.110.153262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu J-T Kan W-H Hsieh C-H, et al. Mechanism of estrogen-mediated attenuation of hepatic injury following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. J Leukoc Biol. 2007;82(4):1019–1026. doi: 10.1189/jlb.0607355 [DOI] [PubMed] [Google Scholar]
- 70.Johnson RJ Sánchez-Lozada LG Newman LS, et al. Climate change and the kidney. Ann Nutr Metab. 2019;74(suppl 3):38–44. doi: 10.1159/000500344 [DOI] [PubMed] [Google Scholar]
- 71.Fischer RSB Vangala C Mandayam S, et al. Clinical markers to predict progression from acute to chronic kidney disease in Mesoamerican nephropathy. Kidney Int. 2018;94(6):1205–1216. doi: 10.1016/j.kint.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer RSB Vangala C Truong L, et al. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int. 2018;93(3):681–690. doi: 10.1016/j.kint.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 73.Hansson E Glaser J Jakobsson K, et al. Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients. 2020;12(6):1639. doi: 10.3390/nu12061639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madero M, García-Arroyo FE, Sánchez-Lozada LG. Pathophysiologic insight into MesoAmerican nephropathy. Curr Opin Nephrol Hypertens. 2017;26(4):296–302. doi: 10.1097/MNH.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 75.Qu Y Zhang W Boutelle A-YM, et al. Associations between ambient extreme heat exposure and emergency department visits related to kidney disease. Am J Kidney Dis. 2023;81(5):507–516.e1. doi: 10.1053/j.ajkd.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 76.Borg M, Bi P, Nitschke M, Williams S, McDonald S. The impact of daily temperature on renal disease incidence: an ecological study. Environ Health. 2017;16(1):114. doi: 10.1186/s12940-017-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen B Yang B Zhu J, et al. Hsp90 relieves heat stress-induced damage in mouse kidneys: involvement of antiapoptotic PKM2-AKT and autophagic HIF-1α signaling. Int J Mol Sci. 2020;21(5):1646. doi: 10.3390/ijms21051646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goto H Nakashima M Nakashima H, et al. Heat acclimation ameliorated heat stress-induced acute kidney injury and prevented changes in kidney macrophages and fibrosis. Am J Physiol Renal Physiol. 2022;323(3):F243–F254. doi: 10.1152/ajprenal.00065.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sánchez-Lozada LG García-Arroyo FE Gonzaga G, et al. Kidney injury from recurrent heat stress and rhabdomyolysis: protective role of allopurinol and sodium bicarbonate. Am J Nephrol. 2018;48(5):339–348. doi: 10.1159/000494663 [DOI] [PubMed] [Google Scholar]
- 80.Giles DA Moreno-Fernandez ME Stankiewicz TE, et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med. 2017;23(7):829–838. doi: 10.1038/nm.4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giles DA Ramkhelawon B Donelan EM, et al. Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol Metab. 2016;5(11):1121–1130. doi: 10.1016/j.molmet.2016.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leigh ND Kokolus KM O'Neill RE, et al. Housing temperature-induced stress is suppressing murine graft-versus-host disease through β2-adrenergic receptor signaling. J Immunol. 2015;195(10):5045–5054. doi: 10.4049/jimmunol.1500700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao W Zhou L Zhao X, et al. Thermoneutral housing temperature regulates T-regulatory cell function and inhibits ovabumin-induced asthma development in mice. Sci Rep. 2017;7(1):7123. doi: 10.1038/s41598-017-07471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eng JWL, Reed CB, Kokolus KM, Repasky EA. Housing temperature influences the pattern of heat shock protein induction in mice following mild whole body hyperthermia. Int J Hyperthermia. 2014;30(8):540–546. doi: 10.3109/02656736.2014.981300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan L-J, Rajasekaran NS, Sathyanarayanan S, Benjamin IJ. Mouse HSF1 disruption perturbs redox state and increases mitochondrial oxidative stress in kidney. Antioxid Redox Signal. 2005;7(3–4):465–471. doi: 10.1089/ars.2005.7.465 [DOI] [PubMed] [Google Scholar]
- 86.Fekete A Vannay Á Vér A, et al. Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2006;291(4):F806–F811. doi: 10.1152/ajprenal.00080.2006 [DOI] [PubMed] [Google Scholar]
- 87.Sreedharan R, Chen S, Miller M, Haribhai D, Williams CB, Van Why SK. Mice with an absent stress response are protected against ischemic renal injury. Kidney Int. 2014;86(3):515–524. doi: 10.1038/ki.2014.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kokolus K, Spangler H, Povinelli B, Farren M, Lee K, Repasky E. Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naïve and tumor-bearing mice. Front Immunol. 2014;5:23. doi: 10.3389/fimmu.2014.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams JW Elvington A Ivanov S, et al. Thermoneutrality but not UCP1 deficiency suppresses monocyte mobilization into blood. Circ Res. 2017;121(6):662–676. doi: 10.1161/CIRCRESAHA.117.311519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gordon CJ. Temperature Regulation in Laboratory Rodents. Cambridge University Press; 1993. [Google Scholar]
- 91.Connolly MS, Lynch CB. Circadian variation of strain differences in body temperature and activity in mice. Physiol Behav. 1981;27(6):1045–1049. doi: 10.1016/0031-9384(81)90368-1 [DOI] [PubMed] [Google Scholar]


