Abstract
Background
Sarcopenia is commonly observed in patients with cardiovascular diseases. However, studies on the association between sarcopenia and atrial fibrillation and their causal relationships are limited. We performed cross‐sectional and longitudinal analyses to investigate the association between sarcopenia and atrial fibrillation among community‐dwelling older adults.
Methods
A total of 2225 participants from the Korean Frailty and Aging Cohort Study (KFACS) from 2016 to 2017 were included in this cross‐sectional analysis. Sarcopenia was defined according to the Asian Working Group for Sarcopenia 2019 consensus. Atrial fibrillation was diagnosed on the basis of electrocardiographic findings. We investigated whether atrial fibrillation increased the risk of incident sarcopenia 2 years later and whether sarcopenia, in turn, increased the 2‐year risk of developing atrial fibrillation using KFACS data from 2018 to 2019.
Results
Of the 2225 participants (54.2% women; mean age 76.0 ± 3.9 years), 509 (22.9%) had sarcopenia at baseline. In the cross‐sectional analysis, sarcopenia was associated with atrial fibrillation after multivariate adjustment [odd ratio (OR), 2.127; 95% confidence interval (CI), 1.240–3.648; P = 0.006]. Among the sarcopenia components, low physical performance was associated with atrial fibrillation (OR, 1.872; 95% CI, 1.123–3.120; P = 0.016). During the 2‐year follow‐up period, atrial fibrillation was not associated with new‐onset of sarcopenia (OR, 1.483; 95% CI, 0.597–3.685; P = 0.396), and sarcopenia also did not significantly increase the risk of incident atrial fibrillation (OR, 1.120; 95% CI, 0.384–3.264; P = 0.836).
Conclusions
Although we found a significant association between sarcopenia and atrial fibrillation in a cross‐sectional analysis, we could not establish a causal relationship between the two based on 2 years of follow‐up. Further research with long‐term follow‐up is required to identify causal relationship between atrial fibrillation and sarcopenia.
Keywords: Atrial fibrillation, Longitudinal analysis, Older adults, Sarcopenia
Introduction
Sarcopenia, a geriatric syndrome, is defined as the loss of skeletal muscle mass and function with age. 1 The prevalence of sarcopenia was estimated to be approximately 10% in a meta‐analysis of 58 404 community‐dwelling older adults. 2 As the population ages, sarcopenia is expected to accelerate multiple adverse outcomes, such as falls, physical frailty, hospitalization and mortality. 3 Hence, there is an urgent need to determine the association with these age‐related comorbidities to improve outcomes.
Cardiovascular disease (CVD), the leading cause of death and disability worldwide, 4 becomes more frequent with advancing age. 5 Recently, there has been increasing research into the relationship between CVDs and geriatric conditions. 6 , 7 , 8 Atrial fibrillation, one of the CVDs, is associated with multimorbidity 9 and increases the risk of other serious CVDs such as stroke, heart failure and myocardial infarction. 10 In addition, patients with atrial fibrillation are associated with frailty, including reduced grip strength and slower walking speed. 11 A systematic review and meta‐analysis showed that 39.7% of patients with atrial fibrillation were frail and the frail group had a poorer prognosis than the robust group. 12 Although there has been evidence that CVDs cause muscle wasting, 13 , 14 , 15 , 16 studies on the association between atrial fibrillation and sarcopenia are still lacking. Xia et al. reported that sarcopenia was associated with atrial fibrillation in overweight/obesity patients. 17 However, in the aforementioned study, only low muscle mass was defined as sarcopenia, and as it was a cross‐sectional study, a causal relationship between the two was not established.
We hypothesized that sarcopenia and atrial fibrillation were closely interrelated and that atrial fibrillation could cause sarcopenia, which in turn increases the risk of atrial fibrillation. Therefore, this study aimed to investigate the association between sarcopenia and atrial fibrillation cross‐sectionally and longitudinally in community‐dwelling older adults.
Methods
Study population
The present study was based on the Korean Frailty and Aging Cohort Study (KFACS). The KFACS is a nationwide multicentre, longitudinal cohort study with a baseline survey performed in 2016–2017. Sex‐ and age‐stratified community‐dwelling older adults aged 70–84 years were recruited and followed up every 2 years. Further details of the KFACS protocol have been published previously. 18 Baseline and 2‐year follow‐up data from the KFACS were analysed in this study. Of the 3014 enrolled participants, 611 who had their appendicular skeletal muscle mass (ASM) measured using bioelectrical impedance analysis and 178 who had myocardial infarction, heart failure or cerebrovascular accident, which are CVDs associated with sarcopenia, were excluded. 15 , 19 , 20 Finally, 2225 participants were included in the cross‐sectional analysis. The longitudinal study was divided into two parts. First, we investigated the association of atrial fibrillation at baseline with the incidence of sarcopenia 2 years later. Participants who were lost to follow‐up (n = 370), had missing or incomplete data on sarcopenia (n = 12), or had sarcopenia at baseline (n = 405) were excluded, and 1438 participants were finally included for this first longitudinal analysis. Second, for the analysis of the association of sarcopenia and its components at baseline with incident atrial fibrillation after 2 years, we excluded participants who had no follow‐up electrocardiography (ECG) (n = 368) or had atrial fibrillation at baseline (n = 63). Details of the study flows are shown in Figure 1.
Figure 1.
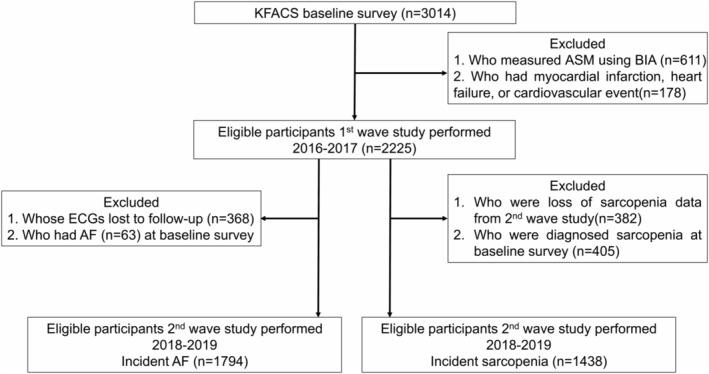
Flowchart of the participants in this study.
The study was conducted in accordance with the ethical standards of the Declaration of Helsinki, and all procedures were approved by the Institutional Review Board (IRB) of the Clinical Research Ethics Committee of the Kyung Hee University Medical Center (IRB number: 2015‐12‐103). Written informed consent was obtained from all participants.
Assessment of sarcopenia status
Sarcopenia was defined as ASM index (ASM/height2 < 7.0 kg/m2 for men and <5.4 kg/m2 for women), low muscle strength (handgrip strength <28 kg for men and <18 kg for women), and/or low physical performance (defined for both men and women as a low score in at least one of three physical performance measures: usual gait speed <1.0 m/s; sit‐to‐stand time ≥12 s; or the Short Physical Performance Battery (SPPB) score ≤9), according to the Asian Working Group for Sarcopenia 2019 consensus. 21 Muscle mass was measured using DXA (GE Healthcare Lunar, Medison, WI, USA; and Hologic DXA Systems, Hologic Inc., Bedford, MA, USA), and ASM was calculated as the sum of the lean mass in both arms and legs. Handgrip strength was assessed using a digital grip strength dynamometer (Takei TKK 5401; Takei Scientific Instruments, Tokyo, Japan). The handgrip strength of each hand was tested twice alternatively, and the maximum of the four measures was used for analysis. The SPPB consisted of a 4‐m usual gait speed test, five‐time sit‐to‐stand test and three standing balance tests. The 4‐m gait speed was measured using an automatic timer (Gaitspeedmeter, Dynamic Physiology, Dajeon, Korea) with acceleration and deceleration phases of 1.5 m each. The test was repeated twice, and the average was used for the analysis. The five‐time sit‐to‐stand test measured the time taken to stand up from a chair and return to the seated position five times with arms folded across the chest. In the standing balance test, participants were first asked to balance in the standing position with their feet side‐by‐side, semi‐tandem and fully tandem for 10 s each. Each SPPB test was scored from 0 to 4, and the total score ranged from 0 to 12.
Electrocardiography
Each participant underwent a standard 12‐lead ECG at rest in the supine position (1 mV = 10 mm). Electrocardiographic parameters including PR interval, RR interval, QRS duration and corrected QT interval were recorded digitally and analysed using the interpretation programs of the ECG machine. Based on ECG changes, left ventricular hypertrophy was diagnosed using Sokolow–Lyon or Cornel voltage criteria. 22 Right ventricular hypertrophy was defined according to Sokolow–Lyon or Myers et al. 23 Various arrhythmias, including atrial fibrillation, supraventricular premature beat, ventricular premature beat, atrioventricular block, 24 left bundle branch block, 25 right bundle branch block and left anterior fascicular block were diagnosed based on typical changes in ECG patterns.
Covariates
Height, body weight and waist circumference were measured to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated as the body weight (kg) in kilograms divided by the height squared (kg/m2). Data on sociodemographic and health‐related variables were acquired using self‐report questionnaires. Sociodemographic variables included age, sex, education (elementary school and below, middle and high school, and college and above) and marital status (married and single/divorced/widowed). Health‐related factors included current smoking (yes or no) and alcohol consumption more than five times a week (yes or no). Physical activity was assessed using the International Physical Activity Questionnaire. The unit of physical activity was the metabolic equivalent task (MET), which refers to the total energy expenditure calculated by multiplying the duration and frequency of physical activities. 26 Participants were classified into three levels of physical activity: low (<600 MET‐min/week), moderate (600–2999 MET‐min/week) and high activity (≥3000 MET‐min/week). Resistance training was defined as exercise using weight machines (barbells, dumbbells and/or elastic bands) or exercise with free weights (push‐ups, sit‐ups and/or squats) at least three times per week. Nutritional status was evaluated using the Mini‐Nutritional Assessment Short Form (MNA‐SF), which consists of six items. 27 A medical history of hypertension, dyslipidaemia, diabetes mellitus and renal disease was obtained. Blood samples were obtained at approximately 8 am after an overnight fast of at least 8 h and were analysed as previously described. 18
Statistical analysis
Baseline characteristics were compared in accordance with the sarcopenia status using the Mann–Whitney U test for continuous variables after normality and the chi‐squared test for categorical variables. Logistic regression models were used to investigate the association between sarcopenia and atrial fibrillation. Model 1 estimated the unadjusted odds ratios (ORs) and 95% confidence intervals (CIs). Model 2 was adjusted for age and sex. In addition to the covariates in Model 2, Model 3 was adjusted for BMI, physical activity, current smoking, MNA‐SF and diabetes mellitus. We performed further analyses using multivariable logistic regression models with Firth's penalized likelihood method to address small sample size issue in the longitudinal analyses. 23 All analyses were performed using the SPSS software Statistics 23.0 package (IBM Corp., Armonk, NY, USA) and SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). Statistical significance was set as P < 0.05.
Results
Characteristics of participants
Table 1 shows the participants' characteristics according to the presence of sarcopenia at baseline. The mean age of the participants was 76.0 ± 3.9 years, and 1207 (54.2%) were females. Among the 2225 participants, 509 (22.9%) had sarcopenia. Compared with the non‐sarcopenia group, the sarcopenia group was older, had a lower BMI, and higher proportions of men, and current smokers. Total physical activity levels and nutritional status were higher in non‐sarcopenic participants; the proportions of participants performing moderate to high level physical activity and resistance training at least three times per week were significantly lower in the sarcopenia group. Diabetes mellitus was more frequent in the sarcopenia group. The sarcopenia group had significantly higher levels of creatinine, white blood cells, platelets and C‐reactive protein.
Table 1.
Baseline characteristics of participants according to the presence of sarcopenia (n = 2225)
| Non‐sarcopenia (n = 1716) | Sarcopenia (n = 509) | P value | |
|---|---|---|---|
| Age, years | 75.5 ± 3.8 | 77.5 ± 3.9 | <0.001 |
| Sex | <0.001 | ||
| Men | 739 (43.1) | 279 (54.8) | |
| Women | 977 (56.9) | 230 (45.2) | |
| Height, cm | 157.7 ± 8.5 | 158.4 ± 8.3 | 0.117 |
| Weight, kg | 61.9 ± 9.3 | 57.0 ± 8.6 | <0.001 |
| BMI, kg/m2 | 24.9 ± 2.9 | 22.7 ± 2.7 | <0.001 |
| Underweight | 16 (0.9) | 32 (6.3) | <0.001 |
| Normal weight | 910 (53.0) | 376 (73.9) | |
| Obese | 790 (46.0) | 101 (19.8) | |
| Waist circumference, cm | 88.3 ± 8.3 | 85.1 ± 8.7 | <0.001 |
| Current smoker | 78 (4.5) | 44 (8.6) | <0.001 |
| Alcohol consumption | 291 (17.0) | 94 (18.5) | 0.429 |
| Physical activity, MET | 3181.9 ± 3798.0 | 2609.5 ± 3485.9 | <0.001 |
| <600 MET/week | 255 (14.9) | 112 (22.0) | |
| 600–3000 MET/week | 855 (49.8) | 261 (51.3) | |
| >3000 MET/week | 606 (35.3) | 136 (26.7) | |
| Resistance training | 526 (30.7) | 126 (24.8) | 0.010 |
| MNA‐SF | 13.0 ± 1.4 | 12.3 ± 1.8 | <0.001 |
| Educational level | 0.139 | ||
| Elementary school and below | 756 (44.1) | 200 (39.3) | |
| Middle, high school | 628 (36.6) | 198 (38.9) | |
| College and above | 330 (19.3) | 111 (21.8) | |
| Living alone | 404 (23.5) | 107 (21.0) | 0.235 |
| Married | 583 (34.0) | 151 (29.7) | 0.069 |
| Co‐morbidity | |||
| Hypertension | 962 (56.1) | 290 (57.0) | 0.715 |
| Dyslipidaemia | 566 (33.0) | 154 (30.3) | 0.248 |
| Diabetes mellitus | 350 (20.4) | 126 (24.8) | 0.035 |
| Renal disease | 22 (1.3) | 7 (1.4) | 0.871 |
| Sarcopenic components | |||
| SMI, kg/m2 | 6.58 ± 0.95 | 5.66 ± 0.78 | <0.001 |
| Grip strength, kg | 26.9 ± 7.6 | 24.2 ± 6.3 | <0.001 |
| Gait speed, m/s | 1.2 ± 0.3 | 1.0 ± 0.2 | <0.001 |
| 5‐time STS, s | 10.9 ± 3.7 | 13.2 ± 4.0 | <0.001 |
| SPPB | 11.0 ± 1.5 | 10.1 ± 1.7 | <0.001 |
| Timed up and go, s | 10.1 ± 2.5 | 11.3 ± 2.6 | <0.001 |
| Biochemical variables | |||
| Fasting glucose | 104.0 ± 22.9 | 103.1 ± 24.7 | 0.106 |
| HbA1C | 6.01 ± 0.81 | 6.02 ± 0.83 | 0.895 |
| Total cholesterol | 176.0 ± 35.8 | 175.6 ± 36.2 | 0.657 |
| Triglyceride | 121.7 ± 62.4 | 117.5 ± 57.7 | 0.198 |
| HDL | 52.6 ± 13.9 | 53.9 ± 14.9 | 0.122 |
| LDL | 109.7 ± 33.1 | 107.9 ± 33.2 | 0.315 |
| BUN | 16.3 ± 4.9 | 17.0 ± 6.0 | 0.166 |
| Creatinine | 0.83 ± 0.29 | 0.89 ± 0.38 | <0.001 |
| WBC | 5.7 ± 1.5 | 6.1 ± 1.8 | <0.001 |
| RBC | 4.4 ± 0.4 | 4.4 ± 0.5 | 0.737 |
| Platelets | 228.7 ± 62.2 | 233.4 ± 64.5 | 0.022 |
| CRP | 1.37 ± 2.37 | 1.85 ± 3.23 | 0.037 |
Note: Data are presented as mean ± standard deviation (SD) for continuous variables, and numbers (%) for categorical variables.
Abbreviations: 5‐time STS, 5‐times sit‐to‐stand; BMI, body mass index; BUN, blood urea nitrogen; CRP, C‐reactive protein; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MNA‐SF, Mini‐Nutritional Assessment Short Form; RBC, red blood cell; SMI, skeletal muscle mass index; SPPB, short physical performance battery; WBC, white blood cell.
Cross‐sectional association between sarcopenia and atrial fibrillation
In the cross‐sectional study, atrioventricular block was the most frequent ECG finding, followed by left ventricular hypertrophy, right bundle branch block, atrial fibrillation, premature beat, left anterior fascicular block, left bundle branch block and right ventricular hypertrophy (Table 2). Sarcopenia was associated with a higher prevalence of atrial fibrillation (2.9% vs. 5.3%, P = 0.005). Figure S1a presents the ORs and 95% CIs for the association between sarcopenia and abnormal ECG findings in multivariable logistic regression model. Among ECG findings, atrial fibrillation remained significantly associated with sarcopenia even after adjusting for covariates with an OR of 2.127 (95% CI, 1.240–3.648; P = 0.006) (Table 3). When we investigated the association of atrial fibrillation with sarcopenia components, only low physical performance was significantly associated with a higher prevalence of atrial fibrillation (OR, 1.872; 95% CI, 1.123–3.120; P = 0.016) (Table 3).
Table 2.
Prevalence of abnormal ECG findings at baseline
| Total | Non‐sarcopenia (n = 1597) | Sarcopenia (n = 509) | P value | |
|---|---|---|---|---|
| Ventricular hypertrophy | ||||
| LVH | 157 (7.1%) | 119 (7.5%) | 38 (7.5%) | 0.681 |
| RVH | 11 (0.5%) | 9 (0.6%) | 2 (0.4%) | 0.710 |
| Supraventricular/ventricular premature beat | 26 (1.2%) | 20 (1.3%) | 6 (1.2%) | 0.980 |
| AV block | 164 (7.4%) | 128 (8.0%) | 36 (7.1%) | 0.769 |
| LBBB | 12 (0.5%) | 10 (0.6%) | 2 (0.4%) | 0.608 |
| RBBB | 119 (5.3%) | 90 (5.6%) | 29 (5.7%) | 0.690 |
| LAFB | 18 (0.8%) | 11 (0.7%) | 7 (1.4%) | 0.104 |
| Atrial fibrillation | 74 (3.3%) | 47 (2.9%) | 27 (5.3%) | 0.005 |
Note: Data are presented as numbers (%) for categorical variables.
Abbreviations: AV block, atrioventricular block; LAFB, left anterior fascicular block; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; RBBB, right bundle branch block; RVH, right ventricular hypertrophy.
Table 3.
Cross‐sectional association of sarcopenia and its components with atrial fibrillation (n = 2225)
| No. of atrial fibrillation/no. of participants | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Sarcopenia | 27/509 (5.3%) | 1.989 (1.226–3.228) | 0.005 | 1.765 (1.070–2.913) | 0.026 | 2.127 (1.240–3.648) | 0.006 |
| Low muscle mass a | 33/813 (4.1%) | 1.415 (0.887–2.256) | 0.145 | 1.079 (0.668–1.743) | 0.756 | 1.309 (0.773–2.218) | 0.316 |
| Low muscle strength b | 20/475 (4.2%) | 1.381 (0.818–2.330) | 0.227 | 1.305 (0.758–2.248) | 0.337 | 1.279 (0.731–2.238) | 0.389 |
| Low physical performance c | 43/1094 (3.9%) | 1.452 (0.908–2.322) | 0.120 | 1.836 (1.115–3.023) | 0.017 | 1.872 (1.123–3.120) | 0.016 |
| Low muscle mass alone d | 6/304 (2.0%) | 0.673 (0.283–1.600) | 0.370 | 0.484 (0.200–1.170) | 0.107 | 0.665 (0.264–1.675) | 0.386 |
Note: Model 1: unadjusted; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, BMI, physical activity, current smoking, Mini‐Nutritional Assessment Short Form score, and diabetes mellitus. Bold means statistically significance (P < 0.05).
Abbreviations: CI, confidence interval; OR, odd ratio.
Low muscle mass defined by SMI (adjusted by height2), men <7.0 kg/m2, women <5.4 kg/m2.
Low muscle strength defined by grip strength, men <28 kg and women <18 kg.
Low physical performance defined by gait speed <1.0 m/s or 5‐time sit‐to‐stand ≥12 s or SPPB ≤9.
Low muscle mass alone defined by low skeletal muscle mass index (SMI, adjusted by height2), men <7.0 kg/m2, women <5.4 kg/m2 with neither low muscle strength nor low physical performance.
Longitudinal association of atrial fibrillation with incident sarcopenia
During the 2‐year follow‐up period, 184 new cases of sarcopenia were identified among 1438 non‐sarcopenia participants at baseline. Figure S1b shows the association between ECG findings at baseline and incident sarcopenia. Participants with atrial fibrillation at baseline were more likely to develop new‐onset sarcopenia than those without; however, the results were not statistically significant (15.8% vs. 12.7%, P = 0.576, not shown). After multivariate adjustment, OR for the longitudinal association between atrial fibrillation and incident sarcopenia was 1.483 (95% CI, 0.597–3.685; P = 0.396) (Table 4), and the OR calculated by Firth's logistic regression was 1.566 (95% CI, 0.644–3.814; P = 0.323) (Table S1), neither of which showed statistical significance.
Table 4.
Longitudinal association of atrial fibrillation at baseline with incident sarcopenia after 2 years (n = 1438)
| No. of incident sarcopenia/No. of participants | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Non‐atrial fibrillation | 178/1400 (12.7%) | Reference | Reference | Reference | |||
| Atrial fibrillation | 6/38 (15.8%) | 1.287 (0.531–3.122) | 0.576 | 1.225 (0.499–3.011) | 0.658 | 1.483 (0.597–3.685) | 0.396 |
Note: Model 1: unadjusted; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, BMI, physical activity, current smoking, Mini‐Nutritional Assessment Short Form score, and diabetes mellitus.
Abbreviations: CI, confidence interval; OR, odd ratio.
Longitudinal association of sarcopenia with incident atrial fibrillation
After 2‐year of follow‐up, 23 cases of incident atrial fibrillation were identified among 1794 participants without atrial fibrillation at baseline. We performed a longitudinal analysis to investigate the association of sarcopenia and its components with incident atrial fibrillation and there was no significant association of sarcopenia and its components with incident atrial fibrillation (Table 5 and Table S2).
Table 5.
Longitudinal association of sarcopenia and its components at baseline with incident atrial fibrillation after 2 years (n = 1794)
| No. of atrial fibrillation/no. of participants | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Sarcopenia | 5/384 (1.3%) | 1.020 (0.376–2.766) | 0.969 | 0.883 (0.317–2.460) | 0.811 | 1.120 (0.384–3.264) | 0.836 |
| Low muscle mass a | 9/647 (1.4%) | 1.142 (0.491–1.652) | 0.758 | 1.071 (0.450–2.548) | 0.877 | 1.600 (0.629–4.072) | 0.324 |
| Low muscle strength b | 3/351 (0.9%) | 0.613 (0.181–2.076) | 0.432 | 0.504 (0.145–1.757) | 0.282 | 0.491 (0.140–1.726) | 0.268 |
| Low physical performance c | 10/827 (1.2%) | 0.898 (0.392–2.059) | 0.800 | 0.778 (0.324–1.865) | 0.573 | 0.687 (0.279–1.691) | 0.414 |
| Low muscle mass alone d | 4/263 (1.5%) | 1.250 (0.408–3.828) | 0.696 | 1.364 (0.424–4.384) | 0.603 | 1.887 (0.541–6.586) | 0.320 |
Note: Model 1: unadjusted; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, BMI, physical activity, current smoking, Mini‐Nutritional Assessment Short Form score, and diabetes mellitus.
Abbreviations: CI, confidence interval; OR, odd ratio.
Low muscle mass defined by SMI (adjusted by height2), men <7.0 kg/m2, women <5.4 kg/m2.
Low muscle strength defined by grip strength, men <28 kg and women 18 kg.
Low physical performance defined by gait speed <1.0 m/s or 5‐time sit‐to‐stand ≥12 s or SPPB ≤9.
Low muscle mass alone defined by low skeletal muscle mass index (SMI, adjusted by height2), men <7.0 kg/m2, women <5.4 kg/m2 with neither low muscle strength nor low physical performance.
Discussion
This study aimed to investigate whether sarcopenia and atrial fibrillation were related in community‐dwelling older adults. We found that atrial fibrillation was independently associated with sarcopenia in cross‐sectional analysis. However, among the sarcopenia components, the aforementioned association was only significant for low physical performance. Moreover, there was no significant association between sarcopenia and incident atrial fibrillation, or between atrial fibrillation and incident sarcopenia in the 2‐year longitudinal analysis.
Both skeletal and cardiac muscles are striated muscle, suggesting the existence of a corresponding pathological mechanism between sarcopenia and heart failure. 28 In a previous study, sarcopenia was closely related to myocardial mass, 29 and a Singapore study described ‘Cardio‐Sarcopenia’. 30 We found an association between sarcopenia and atrial fibrillation in the cross‐sectional analysis, but could not show a bidirectional causal relationship in the longitudinal analysis; sarcopenia did not increase the risk of incident atrial fibrillation, and atrial fibrillation, in turn, did not lead to the development of sarcopenia. This might be due to the short follow‐up interval of 2 years and the low incidence of atrial fibrillation (1.3%) and sarcopenia (12.8%) in the follow‐up period.
Several previous studies have shown that CVD is associated with poor physical function. 31 , 32 , 33 , 34 Along with the context, we revealed that among sarcopenia components, low physical performance was associated with atrial fibrillation. Sarcopenia is thought to result in cardiac dysfunction, mediated by reduced cardiorespiratory function and physical fitness. Another mechanism is that a subclinical CVD can negatively affect the left ventricular filling and cardiac output, thereby reducing physical performance.
Atrial fibrillation causes several uncomfortable symptoms, such as palpitations, dyspnoea and fatigue, which can lead to decreased mobility and subsequently frailty. There is growing evidence of the association between atrial fibrillation and physical frailty in older adults. 11 , 31 , 35 Notably, atrial fibrillation drives the development of a frailty phenotype. 36 However, studies on the association between atrial fibrillation and sarcopenia are limited. In the only comparable study reported by Xia et al., atrial fibrillation was associated with sarcopenia defined by height‐adjusted ASM only in overweight/obese participants. 17 Our finding that the odds of atrial fibrillation increased in participants with sarcopenia supports those of previous studies. The relationship between sarcopenia and atrial fibrillation might be attributed to underlying mechanisms including age‐related changes in the cardiac conduction system, such as loss of atrial cardiomyocytes, increased interstitial fibrosis, and the altered distribution and function of ion channels, which may predispose individuals to atrial fibrillation. 37
This study had some limitations. First, to clarify the association between atrial fibrillation itself and sarcopenia, we excluded those who had a self‐report history of CVDs which are frequently associated with sarcopenia. 7 , 38 Accordingly, it is possible that a significant number of participants who actually had CVDs but were unaware of them were included, which might have affected the results of this study. However, because in real life these factors are complexly intertwined and influence each other, we performed further analyses including participants with CVDs, which were found to be consistent with the main results (Tables S3–S5). Second, as atrial fibrillation was diagnosed based on a single ECG evaluation in this study, paroxysmal atrial fibrillation may have been overlooked, which resulting in an underestimation of the prevalence. In fact, those who have paroxysmal atrial fibrillation are known to account for 25% of total atrial fibrillation patients. 39 Third, the number of participants in this cohort was relatively small and they were ambulatory community‐dwelling Korean older adults. Therefore, the study may have limited generalizability. Finally, in the longitudinal analysis to show the causal relationship, the follow‐up period was 2 years, which was shorter than that in other studies. 36 , 40 Thus, the incidence of sarcopenia and atrial fibrillation was low, which could be the reason for the lack of statistical significance in longitudinal analysis.
In conclusion, we demonstrated a significant association between sarcopenia and atrial fibrillation in community‐dwelling older adults. However, we could not establish a causal relationship between sarcopenia and atrial fibrillation. Further long‐term follow‐up of the causal effect of sarcopenia on atrial fibrillation is required, which can help to identify patients with potential risk factors to implement timely interventions.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI15C3153) and the Research Program funded by the National Institute of Health, Korea Disease Control and Prevention Agency (2021‐ER0605‐00).
Conflict of interest
The authors report no potential conflict of interest relevant to this article. The manuscript complies with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 41
Supporting information
Table S1. Longitudinal association of atrial fibrillation at baseline with incident sarcopenia after 2 years*
Table S2. Longitudinal association of sarcopenia and its components at baseline with incident atrial fibrillation after 2 years*
Table S3. Cross‐sectional association of sarcopenia and its components with atrial fibrillation including participants with MI, HF, or CVD (n = 2403).
Table S4. Longitudinal association of atrial fibrillation at baseline with incident sarcopenia after 2 years including participants with MI, HF, or CVD (n = 1558).
Table S5. Longitudinal association of sarcopenia and its components at baseline with incident atrial fibrillation after 2 years including participants with MI, HF, or CVD (n = 1930).
Figure S1. Odd ratios and 95% confidence intervals of EGC findings and sarcopenia in (a) cross‐sectional analysis and (b) longitudinal analysis.
Shim GY, Kim M, Won CW. Cross‐sectional and longitudinal association between atrial fibrillation and sarcopenia: Findings from the Korean frailty and aging cohort study. Journal of Cachexia, Sarcopenia and Muscle 2023; 10.1002/jcsm.13401
References
- 1. Cruz‐Jentoft AJ, Sayer AAJTL. Sarcopenia. Lancet (North American ed) 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 2. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐ analysis of general population studies. J Diabetes Metab Disord 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta‐analysis. PLoS ONE 2017;12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collaborators GBDCoD . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990‐2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scherbakov N, Pietrock C, Sandek A, Ebner N, Valentova M, Springer J, et al. Body weight changes and incidence of cachexia after stroke. J Cachexia Sarcopenia Muscle 2019;10:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen YY, Chen WL, Peng TC, Liaw FY, Chao YP, Kao TW. Relationship between sarcopenia and cardiovascular disease risk among taiwanese older adults. Public Health Nutr 2022;25:1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Tou NX, Gao Q, Gwee X, Wee SL, Ng TP. Frailty and risk of cardiovascular disease and mortality. PLoS ONE 2022;17:e0272527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu J, Nadarajah R, Nakao YM, Nakao K, Wilkinson C, Mamas MA, et al. Temporal trends and patterns in atrial fibrillation incidence: a population‐based study of 3.4 million individuals. Lancet Reg Health Eur 2022;17:100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016;50:e1–e88. [DOI] [PubMed] [Google Scholar]
- 11. Yang M‐T, Wu Y‐W, Chan D‐C, Chien M‐Y. The relationship between atrial fibrillation and frailty in community‐dwelling older adults. Arch Gerontol Geriatr 2020;90:104103. [DOI] [PubMed] [Google Scholar]
- 12. Proietti M, Romiti GF, Raparelli V, Diemberger I, Boriani G, Dalla Vecchia LA, et al. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: A systematic review and meta‐analysis of 1,187,000 patients. Ageing Res Rev 2022;79:101652. [DOI] [PubMed] [Google Scholar]
- 13. Ochi M, Kohara K, Tabara Y, Kido T, Uetani E, Ochi N, et al. Arterial stiffness is associated with low thigh muscle mass in middle‐aged to elderly men. Atherosclerosis 2010;212:327–332. [DOI] [PubMed] [Google Scholar]
- 14. Jun JE, Choi MS, Park SW, Kim G, Jin SM, Kim K, et al. Low skeletal muscle mass is associated with the presence, incidence, and progression of coronary artery calcification. Can J Cardiol 2021;37:1480–1488. [DOI] [PubMed] [Google Scholar]
- 15. Sato R, Akiyama E, Konishi M, Matsuzawa Y, Suzuki H, Kawashima C, et al. Decreased appendicular skeletal muscle mass is associated with poor outcomes after ST‐segment elevation myocardial infarction. J Atheroscler Thromb 2020;27:1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fulster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013;34:512–519. [DOI] [PubMed] [Google Scholar]
- 17. Xia MF, Chen LY, Wu L, Ma H, Li XM, Li Q, et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: a cross‐sectional study. Clin Nutr 2021;40:571–580. [DOI] [PubMed] [Google Scholar]
- 18. Won CW, Lee S, Kim J, Chon D, Kim S, Kim CO, et al. Korean frailty and aging cohort study (KFACS): cohort profile. BMJ Open 2020;10:e035573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, et al. Sarcopenia and heart failure. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced sarcopenia: muscle wasting and disability after stroke. Int J Cardiol 2013;170:89–94. [DOI] [PubMed] [Google Scholar]
- 21. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–307 e302. [DOI] [PubMed] [Google Scholar]
- 22. Zhang W, Zhou Y, Bai B, Yu S, Xiong J, Chi C, et al. Consistency of left ventricular hypertrophy diagnosed by electrocardiography and echocardiography: the Northern Shanghai Study. Clin Interv Aging 2019;14:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sokolow M, Lyon TP. The ventricular complex in right ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;38:273–294. [DOI] [PubMed] [Google Scholar]
- 24. Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007;40:228–234. e228. [DOI] [PubMed] [Google Scholar]
- 25. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:992–1002. [DOI] [PubMed] [Google Scholar]
- 26. Chun MY. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med 2012;33:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short‐form mini‐nutritional assessment (MNA‐SF). J Gerontol A Biol Sci Med Sci 2001;56:M366–M372. [DOI] [PubMed] [Google Scholar]
- 28. Wang M, Hu S, Zhang F, Liu J, Mao Y. Correlation between sarcopenia and left ventricular myocardial mass in chronic heart failure patients. Aging Med (Milton) 2020;3:138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pela G, Tagliaferri S, Perrino F, Bussolati G, Longobucco Y, Zerbinati L, et al. Interaction of skeletal and left ventricular mass in older adults with low muscle performance. J Am Geriatr Soc 2020;69:148–154. [DOI] [PubMed] [Google Scholar]
- 30. Keng BMH, Gao F, Teo LLY, Lim WS, Tan RS, Ruan W, et al. Associations between skeletal muscle and myocardium in aging: a syndrome of “cardio‐sarcopenia”? J Am Geriatr Soc 2019;67:2568–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donoghue OA, Jansen S, Dooley C, de Rooij S, van der Velde N, Kenny RA. Atrial fibrillation is associated with impaired mobility in community‐dwelling older adults. J Am Med Dir Assoc 2014;15:929–933. [DOI] [PubMed] [Google Scholar]
- 32. Magnani JW, Wang N, Benjamin EJ, Garcia ME, Bauer DC, Butler J, et al. Atrial fibrillation and declining physical performance in older adults: the health, aging, and body composition study. Circ Arrhythm Electrophysiol 2016;9:e003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Imran TF, Orkaby A, Chen J, Selvaraj S, Driver JA, Gaziano JM, et al. Walking pace is inversely associated with risk of death and cardiovascular disease: the physicians' health study. Atherosclerosis 2019;289:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shih YL, Shih CC, Chen JY. The association between walking speed and risk of cardiovascular disease in middle‐aged and elderly people in Taiwan, a community‐based, cross‐sectional study. PLoS ONE 2020;15:e0235277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koca M, Yavuz BB, Tuna Doğrul R, Çalışkan H, Şengül Ayçiçek G, Özsürekçi C, et al. Impact of atrial fibrillation on frailty and functionality in older adults. Ir J Med Sci 2020;189:917–924. [DOI] [PubMed] [Google Scholar]
- 36. Richard G, O'Halloran AM, Doody P, Harbison J, Kenny RA, Romero‐Ortuno R. Atrial fibrillation and acceleration of frailty: findings from the Irish longitudinal study on ageing. Age Ageing 2022;51. 10.1093/ageing/afab273 [DOI] [PubMed] [Google Scholar]
- 37. Pugh KG, Wei JY. Clinical implications of physiological changes in the aging heart. Drugs Aging 2001;18:263–276. [DOI] [PubMed] [Google Scholar]
- 38. Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, et al. Association between sarcopenia and cardiovascular disease among middle‐aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine 2022;44:101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zoni‐Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orkaby AR, Kornej J, Lubitz SA, McManus DD, Travison TG, Sherer JA, et al. Association between frailty and atrial fibrillation in older adults: the Framingham heart study offspring cohort. J Am Heart Assoc 2021;10:e018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Haehling S, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update. J Cachexia Sarcopenia Muscle 2021;2021:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Longitudinal association of atrial fibrillation at baseline with incident sarcopenia after 2 years*
Table S2. Longitudinal association of sarcopenia and its components at baseline with incident atrial fibrillation after 2 years*
Table S3. Cross‐sectional association of sarcopenia and its components with atrial fibrillation including participants with MI, HF, or CVD (n = 2403).
Table S4. Longitudinal association of atrial fibrillation at baseline with incident sarcopenia after 2 years including participants with MI, HF, or CVD (n = 1558).
Table S5. Longitudinal association of sarcopenia and its components at baseline with incident atrial fibrillation after 2 years including participants with MI, HF, or CVD (n = 1930).
Figure S1. Odd ratios and 95% confidence intervals of EGC findings and sarcopenia in (a) cross‐sectional analysis and (b) longitudinal analysis.


